ABSTRACT
It is well known that electrical signals are deeply associated with living entities. Much of our understanding of excitable tissues is derived from studies of specialized cells of neurons or myocytes. However, electric potential is present in all cell types and results from the differential partitioning of ions across membranes. This electrical potential correlates with cell behavior and tissue organization. In recent years, there has been exciting, and broadly unexpected, evidence linking the regulation of development to bioelectric signals. However, experimental modulation of electrical potential can have multifaceted and pleiotropic effects, which makes dissecting the role of electrical signals in development difficult. Here, I review evidence that bioelectric cues play defined instructional roles in orchestrating development and regeneration, and further outline key areas in which to refine our understanding of this signaling mechanism.
KEY WORDS: Bioelectric signaling, Development, Competence, Induction, Morphogenetic fields
Summary: This Review discusses how understanding the relationship between electrical signaling and developmental systems requires the definition of hierarchical levels of action provided by bioelectric signaling.
Introduction
Ever since Volta and Galvani discovered electrical properties innate to biological systems in the late 18th century, we have been entranced by the electrical property of life. The concept of electrical activity within tissues was confirmed with the findings that electrical impulses across both nerves and muscles act to regulate key properties of their function. However, we now know that electrical potential is intrinsic to the normal function of all cells, organelles and molecules. Electrical potential is a by-product, as well as a regulator, of diverse essential properties across multiple levels of biological organization. For example, electrical potential drives respiration, and shapes pH and redox state; it also mediates cell-cell communication, cell migration and tissue repair. Recently, enticing data suggest that changes in both cell and tissue excitability, as well as broader electrical fields across tissues, are important for the development and physiology of organisms as well as their capacity for repair. Given that patterns of electrical activity exist within developing tissues, and when disrupted can lead to distinct phenotypes, the role of such electrical signaling in instructing development and regeneration has become a topic of great interest.
However, to say that electrical signaling is essential for development is a truism and is not particularly informative. As an example, the first act in development – the block of polyspermy in fertilization – highlights the complexity underlying changes in electrical potential. In this event, a triggered wave of oocyte membrane polarization is necessary for assuring reproductive success (Jaffe, 1976; Jaffe and Cross, 1986). This depolarization results from the specific action of ion channels causing a rise in intracellular calcium. However, this intracellular calcium change in turn affects specific proton pumps altering cellular pH, leading to increased hydrostatic pressure and polymerization of actin subunits that aid acrosome entry into the cytoplasm. Thus, even in this specific cascade, the indirect effects of shifts in electrical activity of a cell are large, many resulting in a dependent response of other channels and pumps in the cell, which in turn result in changes in the local cellular environment and intracellular signaling responses (Jaffe, 2018). Within this context, a statement of the essential role of electrical signaling in development, although true, is not truly useful. However, an ancillary statement can be made: that changes in electrical signaling or potential, within and among levels of biological organization, such as cell, tissues and organs, have instructional roles in development such that differential character states (types) are formed. This hypothesis is a rephrasing of a more broad ‘bioelectric code’ hypothesis that instructs development and regeneration (Levin, 2012 compare with Jaffe, 1981); however, it does not specifically rely on providing graded information. Working within this conceptual framework of differential bioelectrical character states, one can pursue targeted, empirical approaches to distill generalized properties of bioelectric signaling within development and regeneration.
When considering such properties, it is important to assess the effect of bioelectric activity on a particular level of biological organization to separate the effects comprising different logical types (see Glossary, Box 1) of action or regulation. Changes in electrical potential across cellular membranes regulate a myriad of effects on the cellular environment, as well as specific effects on integrated membrane proteins and enzymes within the cell. These changes may drive cell autonomous phenotypes, such as migration, proliferation and apoptosis, that do not represent a linear cause and effect of the component changes. As cells can be electrically coupled (discussed below), cells may show correlated responses to changes in bioelectric signaling that provide a different context of response of the tissue, removed from the downstream action of electrical signaling on the individual cellular components. Such shifts in the regulation of phenotype or level of analysis, from molecular to cellular, from cellular to tissue, or from tissue to organism, represent different logical types in mechanism leading to phenotype (Bateson, 1979). This attention to the level at which bioelectrical signals affect development provides a platform to identify aspects of signaling that are unique to bioelectric signaling, as well as those that are in common with more well-characterized canonical signaling cascades. With this platform in mind, I focus here on how cells and tissues use changes in resting electrical potential to inform decisions, both in the context of development and during regeneration. Through this discussion, I argue that an appreciation of the unique role of electrical signaling requires not only an analysis of immediate, or first-order, actions, but also of emergent properties that orchestrate development across tissues. The local and extended effects of electrical signaling implicate bioelectricity as a unique and essential property in development that has distinct biomedical implications in the regulation of repair and regeneration.
Box 1. Glossary.
Capacitance. The ability of a system to store electrical charge
Competence. The ability of a system (cell/tissue) to respond to a specific inductive signal
Induction. Imparting the particular action of another cell or tissue; also referred to as an evocator of a particular response in the sense that it is retained in the responding tissue
Logical type. Different character states of a system that are not directly a property of the parts (see Bateson, 1979)
Morphogenetic field. A region of developmental potency, defined as the range of differentiative capabilities that can be expressed in a given tissue under a variety of experimental conditions (Slack, 1983; compare with Jacobson and Sater, 1988)
Quorum sensing. The ability to detect and respond to the extent of a collective, such as cell number, size or state; property of a single unit to a character state of a group
Resilience. The ability of a developmental system to respond to shifting perturbations
Vmem. Resting membrane potential or electromotive force
The generation, regulation and coordination of electrical potential
One of the key properties that drives electrical signaling is the differential gradient of ions across the semipermeable membranes of cells and organelles. The resting membrane potential, Vmem (see Glossary, Box 1), in a cell is the electrical potential across the plasma membrane. This resting membrane potential results from the regulation of potassium (K) and sodium (Na) concentrations on each side of the membrane (Wright, 2004). The extensive repertoire of ion channels, the differential expression of channels and isoforms with unique response characteristics and ion affinities, as well as post-translational modification of channels, all contribute to maintaining both the steady state Vmem as well as dynamic responses to alterations in Vmem due to environmental and other stimuli. As I discuss below, Vmem can play a role in regulating the proliferative potential and potency of cells, and it can also be coordinated across cells to generate tissue-level behaviors and patterns.
The link between resting membrane potential, proliferation and developmental potency
Vmem varies widely in and between cells. Overall, a general correlation has been established between membrane polarization and proliferative potential (Fig. 1A) (Binggeli and Weinstein, 1985; Blackiston et al., 2009; Cone, 1971; Levin, 2012). Metastatic cancer cells, for example, show hypopolarization on average (Fraser et al., 2005; Payne et al., 2019), while differentiated cell types tend to be more polarized. Similar trends are exhibited by differentiating myoblasts and developing neural crest cells, which begin differentiation at relatively low resting voltages (−10 to −35 mV) and shift to higher polarity (−55 to −75 mV) as they mature (reviewed by Bauer and Schwarz, 2001). It is unclear whether hypopolarized cells retain broader potency for developmental fates, although it has been reported that adult stem cells in general have a lower Vmem (Blackiston et al., 2009).
Fig. 1.
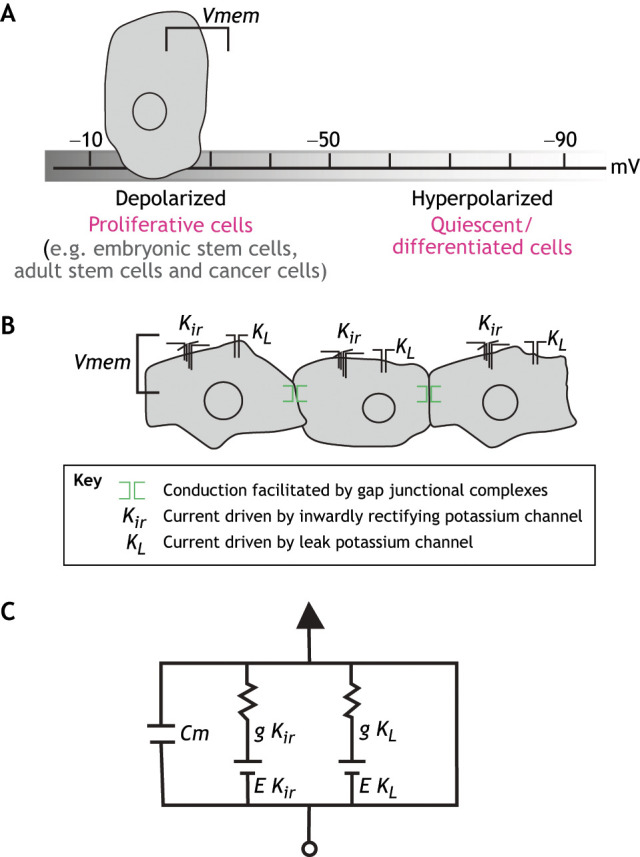
Changes in Vmem within and among cells in a network. (A) Vmem is a measure of the resting membrane potential across a plasma membrane of a cell. It is the product of a suite of pumps and channels that regulate the movement of ions across the plasma membrane. Resting Vmem can vary within a cell and during phases of the cell cycle. However, the resting Vmem is generally associated with the proliferative state of a cell, becoming hyperpolarized with progressive differentiation. (B) When cells are coupled in a series, voltage differential is minimized through ionic currents flowing through gap junctions. (C) Recent work has shown that key properties of Vmem regulation can be modeled through a simple integrated feedback circuit that considers the additive effects of inwardly rectifying (Kir) and leak (KL) potassium channels. Cm, membrane capacitance; g, conductance of channel; E, electromotive force of channel. Image in C is modified, with permission, from McNamara et al. (2020).
Experimentally altering electrical potential of cells through modulation of exogenous electrical fields, or experimentally altering resting membrane potential, suggests that cell Vmem is an integral regulator of proliferative potential (Blackiston et al., 2009). The specific mechanisms underlying this association, however, are not clear; there is no clear functional link, or epistasis, starting with changes in Vmem, that explains how variation in electrical potential regulates the cell cycle. It is possible that the compartmentalized logic of linear pathways typically used to describe cellular signaling may not easily apply to the regulatory action of electrical signaling. Proliferative potential can be thought of as an emergent character state of the cell – in this discussion, a state arising from the concerted action of the expressed membrane channel constituents and feedback through cell cycle machinery and checkpoints. Changes in bioelectric state may act by setting the context for proliferation such that a myriad of cellular events occur within a set space and time, allowing for coordination of signaling and progression through cell cycle or differentiation programs.
Mediation of cell-to-cell coordination
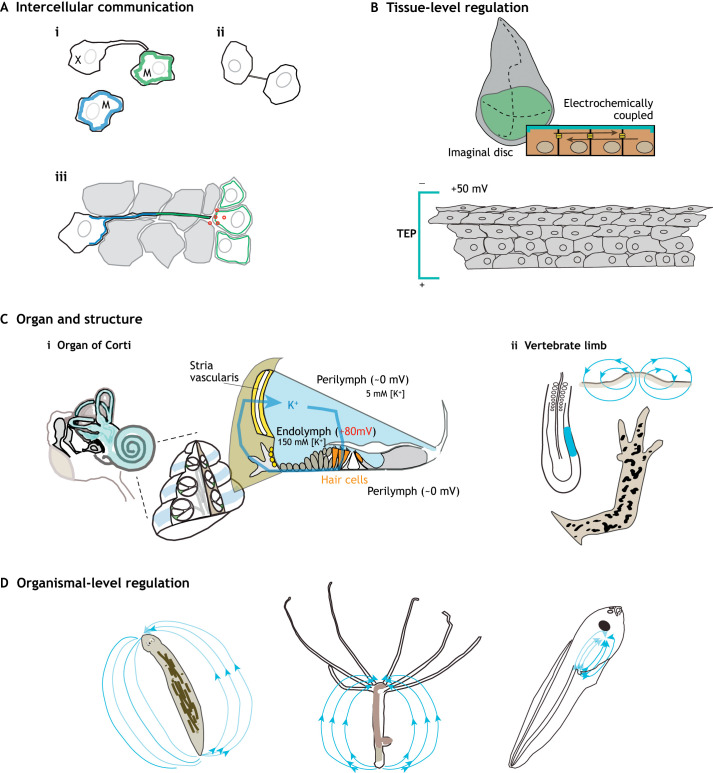
A singular property of electrical activity as a signaling agent is its propensity to impart information about cell state among, and between, cells. Electrical states are transferable signals that can spread across tissues through specialized gap junction channels that form between cells and allow transport of small charged molecules and ions (Fig. 1B). These channels can vary in affinity, and they can be tuned and developmentally regulated, thus biasing the direction and nature of electrochemical signals across tissues. Through these channels, communication can occur dynamically and over long distances through cell collectives or broad cellular extensions. Indeed, specialized cellular structures termed tunneling nanotubes and cytonemes have been shown to mediate cell-to-cell signaling via the transport of signals though long cytoplasmic bridges or cellular processes (Ariazi et al., 2017). In tunneling nanotubes, the signals can even be gated by differential gap junction regulation (Wang et al., 2010), modulating the directionality of signaling. A striking example of such long-distance electrically coupled signaling comes from studies of pigment patterning. The interplay between specialized pigment cells, termed xanthophores and melanocytes, in zebrafish determines the refinement of stripe patterning. Specifically, an analysis of isolated pigment cells showed contact-mediated cell repulsion and changes in Vmem between cells of different fates through extended cell projections (Inaba et al., 2012) (Fig. 2A). Although a function for gap junctions was not shown in this study, inhibition of gap junctions in melanocytes and xanthophores has been shown to interrupt normal segregation of zebrafish pigment cell types, leading to dispersed spotty pigment patterns (Usui et al., 2019).
Fig. 2.
Bioelectrical control of cellular and tissue level processes in development. Overview of some pertinent developmental roles regulated by bioelectrical signals at different levels of organization. (A) Intercellular communication. (i) Transmission of state between cells can occur via long cellular processes termed nanotubes and cytonemes, e.g. between zebrafish melanocytes (M) and xanthophores (X). This process is dependent on potassium channel-mediated regulation of Vmem and gap junctions. (ii) Nanotubes and cytonemes also facilitate the transmission of small molecules and ions between cells. This process can require gap junctional complexes. (iii) Long-distance targeted signaling can also occur through regional release of growth factors (red dots) in a process that is dependent on potassium channel function. Blue and green indicate different character states of cells induced by differential electrical signaling. (B) Tissue-level regulation. The Drosophila imaginal disc epithelium is electrically coupled and bounded, causing localized coordination of developmental signals. A trans-epithelial potential (TEP) exists across the stratified epithelia. (C) Signaling within and across organ and structures. (i) In the organ of Corti of the inner ear, the differential expression of potassium channels and gap junctions across the structure supports the generation and maintenance of large electrical potential in the endolymph, providing an electrical gradient for signaling after hair cell activation. (ii) Early development of the vertebrate limb is marked by regional shifts in currents and the creation of electrical fields demarcating a limb field in early limb buds with specific limb-forming potential (blue). (D) Signaling among and across tissues comprising organismal-level regulation. Many organisms show local as well as global electrical fields. For example, planaria, hydra and Xenopus show variation of bioelectric fields across their anterior to posterior axes.
The generation of pattern
Considerable work has focused on understanding how bioelectric signaling and electrical connectivity among cells may influence patterning in developing systems (e.g. Cervera et al., 2016). Recently, it was demonstrated that, given a simple integrated feedback circuit of potassium channels and gap junction communication among adjacent cells, resting quasi-stable states of membrane potential could be generated (McNamara et al., 2020). In this model (Fig. 1C), potassium regulation is controlled by a simple additive effect of inward rectifying potassium channels (Kir) and a ‘leak’ channel (KL). Connection between cells with gated gap junction channel function leads to non-cell autonomous function and dynamic regulation of Vmem (McNamara et al., 2020). This relationship implies that pattern can arise from the dynamics of a simple bioelectric circuit. Importantly, this phenomenon does not require pre-patterned states of gene expression, providing asymmetry that can then serve as a template for differential gene regulation. The dynamics outlined in this model serve as a tool with which to relate phenotypic response to genetic changes observed in bioelectric regulators.
Genetic evidence for bioelectric signaling in development
The clearest mechanistic evidence for bioelectric regulation of development is grounded in genetic analyses, whereby specific genetic alterations are measured for their effects on phenotype. Such analyses fall into two distinct classes of changes defined by the resultant effect on the system: loss of normal function of a component or extended gain of function within the system. The combination of both loss- and gain-of-function approaches provides a powerful means to reveal functional integration and potential within a system; however, it is important to integrate the type of effect of a given perturbation when interpreting particular experimental findings.
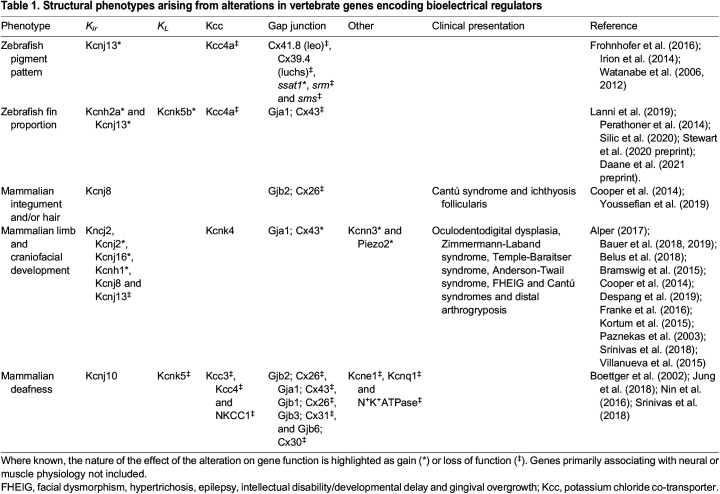
Below, I provide an overview of the genetic evidence for bioelectric signaling through a focus on structural phenotypes arising in development (summarized in Table 1). This focus on structural phenotypes allows a refined phenotypic analysis that is distinct from that used to study the known regulation of electrical signaling in ‘excitable’ tissues, such as muscle, and neural activity. A recent analysis of channelopathies across animal models and human disease has also been performed and is more inclusive than the discussion provided here (Srivastava et al., 2021). One important trend that arises from these analyses is that channels mediating potassium conductance as well connexins (which are gap junction components) are commonly disrupted. Many of these cases are thought to represent loss of normal function, but some cases, such as KCNJ2 mutations underlying Cooks syndrome (Franke et al., 2016), represent gain-of-function effects due to misexpression or neofunctionalization of the channel. Potassium channels and connexins are diverse and multifaceted regulators of membrane potential that harbor signaling properties independent of their conductive abilities. As potassium is a primary regulator of resting membrane potential (Wright, 2004), its prominence in developmental disorders supports the notion that Vmem itself may be normally required to regulate development distinct from its indirect function on canonical signaling.
Table 1.
Structural phenotypes arising from alterations in vertebrate genes encoding bioelectrical regulators
Experimental modulation of genetic models reveal integration of biological signaling
Combining genetic with experimental/chemical approaches has been crucial for understanding the molecular foundations of bioelectric signaling in developmental processes. Much of the experimental evidence for a role for bioelectric signaling in development is founded on gain-of-function experiments; the upregulation and mis-expression of activated or dominant-negative forms of channels or channel regulators results in an increased or new function of particular gene products and their effect on electrical properties of the cell such as Vmem. Although these types of data make it difficult to define specific mechanisms at a cellular or developmental level, at face value they do tell you what the system can achieve in response to changes in bioelectric signals. Interesting cases have been identified in which loss-of-function genetic data in combination with experimental gain-of-function approaches provide insights into mechanisms and epistasis. For example, blocking connexin signaling using pharmacological agents in the context of activated channel function demonstrates the necessity for electrocoupling to resolve pigmentation phenotypes in zebrafish (Usui et al., 2019). Likewise, treatment of regenerating zebrafish fins either with the pharmaceutical compound FK506 (which binds to calcineurin and alters potassium channel activity) or by altering activity of the potassium chloride co-transporter Kcc4a results in overgrowth phenotypes (Kujawski et al., 2014; Lanni et al., 2019). These growth responses, however, are dependent on the function of the potassium channel Kcnk5b (Daane et al., 2018; Lanni et al., 2019). Thus, through combinatorial experimental analysis in defined genetic backgrounds, our understanding of bioelectric signaling and its role can be refined and interrogated.
Lessons inferred
Generating a comprehensive understanding of bioelectric signaling as a whole based on current findings proves challenging, as each channel component is expressed in many tissues during development and often has both conductive and classical signaling roles. Furthermore, in cases of misexpression during ontogeny, the altered context of the signaling created may mask or mimic other functions not yet identified. However, as I highlight below, some general trends can be distilled.
Coupling is an essential aspect of bioelectric signaling during development
Electrically coupled cells can form extended, functional units that can facilitate regionally bounded, non-cell-autonomous developmental signaling (Levin, 2007). Several examples of such coupled units exist (Fig. 2). Epithelial cells of the developing Drosophila wing imaginal disc are locally coupled, allowing ionic transfer within a bounded compartment coincident with anterior-posterior lineage restriction (Weir and Lo, 1982). Likewise, cells of the anterior pituitary of fishes are electrically coupled to facilitate coordinated hormone release (Levavi-Sivan et al., 2005). Cells of the limb ectoderm (including the apical ectodermal ridge; Laird et al., 1992), as well as early limb mesenchymal cells, are also electrically coupled (Allen et al., 1990; Coelho and Kosher, 1991) as are early placodes of feathers (Serras et al., 1993) (Fig. 3A). Accordingly, loss of gap junction function in tissues often disrupts normal development and leads to dysmorphology, as seen in both experimental models as well as human disorders (Srinivas et al., 2018) (Table 1).
Fig. 3.
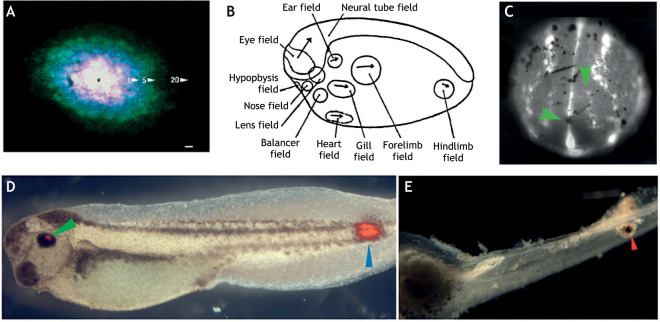
Bioelectric association with and modulation of regional developmental fields. (A) Regional transmission of electrically charged lucifer yellow within the feather tract placodal epidermis of chick embryos. An overlay of images taken at 1, 5 and 20 min after injection of charged dye into a single placodal cell (marked with an asterisk) reveals bounded coupling within the feather placode during development. Image reproduced from Serras et al. (1993). (B) Fate map of regional morphogenic fields in a developing Xenopus embryo. Image reproduced, with permission, from Huxley and De Beer (1936). (C) Staining of a developing Xenopus embryo showing regionalization of voltage-sensitive dyes; brighter areas represent relatively hyperpolarized cells (green arrowheads), whereas dimmer staining indicates relatively depolarized cells. Image reproduced, with permission, from Adams et al. (2016). (D,E) Ectopic eye formation in Xenopus non-neurogenic tissue after ectopically altering potassium channel activity during early development. Red staining marks tissue positive for b-crystallin, which is found in normal eye tissue (green arrowhead) and in ectopic tissue formed in caudal regions of the embryo (blue arrowhead); E indicates a further example of pigmented eye tissue forming in caudal domains. Images reproduced from Pai et al. (2012).
Although the details of such coupling are not known, the developmental consequence of coupling would impart a regulation of signaling – away from the cellular to a more pan-cellular, or even organismal, level (Fig. 2C,D). Indeed, gap junction-mediated coupling between cells of a tissue could impart unique character states to cell assemblages such that morphogenetic signaling can be temporally and spatially coordinated across cells. This character state is a different level of regulation – or logical type – than the action of individual cells. Intriguingly, such higher-order morphogenetic properties could provide a mechanistic foundation for classical embryological concepts such as competence and induction (discussed below). Coupling mediated via the expression of connexins with different functional characteristics can also allow regional heteromeric assemblages of gap junctional complexes that exhibit unidirectional/biased transport. Such asymmetry underlies the potential for the generation of electrochemical gradients across electrically coupled cells and thus the generation of pattern (e.g. McNamara et al., 2020).
Bioelectric modules may be leveraged during development
In many traits and tissues, the pairing of potassium channels with particular connexin gap junctional components that affect similar phenotypic outcomes suggests that these genes are functionally interrelated and may work via the same general mechanism. Pigment patterning in zebrafish provides an illustration of how changes in individual channel components suggest the presence of correlated modules. Gain-of-function mutations in the potassium channel Kcnj13 result in broadened pigment patterns, whereas loss-of-function mutations in the gap junction channel connexin 41.8 (Cx41.8) lead to disruption of stripes (Watanabe et al., 2006). In addition, overexpression of the gap junction suppressor Ssat or, alternatively, mutations in spermidine synthase affecting polyamine-dependent regulation of gap junctional conductance lead to abrogation of stripes (Frohnhofer et al., 2016; Usui et al., 2019; Watanabe et al., 2012). In the context of the zebrafish fin, gain-of-function mutations in kcc4a (slc12a4), kcnj13 or kcnk5b, all of which encode potassium channels, lead to fin proportional overgrowth (Perathoner et al., 2014; Silic et al., 2020), whereas loss of the connexin Cx34 results in proportionally short fins. Furthermore, cx34 loss specifically abrogates the fin overgrowth phenotype caused by increased potassium channel function (Perathoner et al., 2014; Sims et al., 2009). Interestingly, recent comparative genetic analyses of natural variation in pigmentation and fin length show that these modules are shaped in evolution (Podobnik et al., 2020; Schartl et al., 2020).
The examples discussed here highlight the potential for a generalized module of bioelectric regulation. A recently reported regulatory circuit (McNamara et al., 2020) (Fig. 1C) builds on this idea and details the sufficiency of regulation of three classes of channels in establishing unique patterning dynamics within cellular systems. This simple circuit involves varied expression and differential gating of Kir, KL (e.g. K2P) and gap junction channels (Fig. 1B,C). Current experimental data fit nicely to this model, such that phenotypes in pigment patterning and fin size arise due to overactive Kir such as Kcnj13 and Kcnh2a (Silic et al., 2020; Stewart et al., 2020 preprint; Daane et al. 2021 preprint). These phenotypes are affected by alterations in the gap junction components Cx41.8 and Cx43, respectively (Iovine and Johnson, 2000; Irion et al., 2014), seemingly to have specific pairing with tissue specificity of Kir. Further evidence of such a circuit comes from the fact that Kcnk5b, a KL, is necessary (Daane et al., 2018; Lanni et al., 2019) and sufficient (Perathoner et al., 2014) to alter these signaling pathways (Harris et al., 2020).
Levels matter
The readout of biological signaling is affected not only by which particular channels and modulators are expressed, but also by their levels. Simply increasing the expression level, or the activity, of a wide set of potassium channels and regulators can drive development in predictable and patterned outcomes. For example, zebrafish fin size can be modulated by increases in ion conductance in potassium channels through modulation of the conductance as well as increased levels of wild-type channels (Perathoner et al., 2014; Silic et al., 2020). Similarly, craniofacial dysmorphology in Xenopus can be triggered by mis-expression and over-expression of wild-type and variant potassium channels (Adams et al., 2016). In both cases, a general principle emerges in that multiple causes of changes in Vmem can affect similar developmental outcomes. Interestingly, in line with the circuit model of McNamara (McNamara et al., 2020), specific classes of ion channel (inward rectifying and leak channels versus other potassium channel types) are associated with particular outcomes, whereas others do not seem to be sufficient (Silic et al., 2020).
The gain-of-function effects of potassium channels in experimental treatments are mirrored in the types of genetic alterations seen in mutant screens of model organisms and in human channelopathies. In many instances, mutations in potassium channels exhibit a dose-dependent effect, such that homozygous carriers have enhanced presentation of the phenotype. In channelopathies, structural defects can arise from gain-of-function mutations of channel genes, leading to increased, mis-localized or novel regulation of the gene product (Table 1). For example, individuals with Cooks syndrome have shortened digits and aplasia of the nails attributed to increased expression and mis-regulation of Kcnj2/16 in the autopod during development (Despang et al., 2019; Franke et al., 2016). Deficiencies in cardiac physiology and behavior have also been linked to copy number variations in potassium channel gene loci or their direct modifiers (Maussion et al., 2017; Tsai et al., 2016), again suggesting that gene dose may contribute to altered regulation. The identification of developmental disorders caused by loss-of-function variants in channels further suggests that balance among potassium channel function and/or non-conductive based signaling of channels is essential (e.g. George et al., 2019). The contrast of the different classes of channels in regulating phenotypes may prove to be informative for highlighting different signaling mechanisms or for identifying compensatory shifts in activity among co-expressed channels in the system (e.g. Dahal et al., 2012, 2017).
How might bioelectric signaling orchestrate development?
Given the integral nature of bioelectric processes as an emergent property of organelles, cells, tissues and organs, how can one discern a specific role for bioelectricity during development?
On a bioelectric ‘code’ in development
The bioelectric code model has been stated as information stemming from temporal and regional patterns of signaling instructing development (Levin, 2012); this hypothesis includes both instructional and permissive signals of developmental fate decisions through regional control of differential electrical signaling across non-excitable tissues. More recently, by extending a postulate of Jaffe (Jaffe, 1981) of electrical ‘leaks’ providing positional value in a developing and regenerative system, the notion of a bioelectric ‘code’ has been extended, or shaped, to impart positional signaling within a system, such that a threshold-based readout of electrical signals may provide instructive signals for differentiation and morphogenesis (Levin and Martyniuk, 2018; Pietak and Levin, 2017). This extended bioelectric ‘code’ hypothesis would be ancillary to a broader ‘instructional’ definition, as the latter would include binary decisions between systems rather than necessitating graded or differential information. The differential read out of bioelectric signaling that leads to graded, differentiated states (Levin, 2014) remains unsubstantiated.
Developmental hallmarks as a measure of bioelectric regulation
Morphogenesis requires tissue interactions that are regionally specified both in time and space. The morphogenetic cascade encompasses ‘first order’ cellular processes, such as cell proliferation and differentiation, but also broader processes that reflect instructional or permissive functions of cell and tissue assemblages. Such characteristics require coordinated activity and responses to developmental signals that are not the simple summated response of individual cells within tissues. Indeed, properties such as competence, induction and morphogenetic fields have been defined as fundamental character states of tissues during development. An impediment towards our ability to understand these higher order processes stems from the coordination and nonautonomous activity of cells. This coordination imparts properties to tissues that arise from coupling of their response (see below). I argue that it is in these tissue-level properties that we may find a fuller understanding of the role of bioelectricity in development. This hypothesis is not intended to limit the integrated activity of bioelectrical signaling in cellular and biochemical functions but rather to emphasize that higher-order properties may impart unique regulatory roles of bioelectrical signaling in development. Below, I highlight recent work that supports a role for bioelectricity in regulating these crucial developmental properties and states. This does not to serve as a global review of the nature of these phenomenon, but rather aims to place emphasis on bioelectric signaling in these processes.
Competence
Signaling between tissues requires that a tissue is capable to respond to and integrate a signal. A bead loaded with FGF placed in the mesenchyme of the flank of a developing chick induces a supernumerary limb, whereas placement of the bead in non-appendage-forming fated tissue does not elicit outgrowth (Cohn et al., 1995). This ability to respond to a specific inductive signal is called competence (see Glossary, Box 1) (Waddington, 1940). This property can be autonomous to a cell or to a tissue. During development, competence is regionally specified. An example of regional competence can be seen in neurogenic placodes of the head, in which defined regions of the anterior ectoderm respond to external developmental signals by developing sensory and neurogenic structures (Streit, 2007). Even during late developmental periods, regional competence shapes form. For example, integumentary structures such as hair/vibrissa in mammals, or feathers and scales in birds, form in defined tracks and, in many cases, the intra-tract ectoderm is not responsive to appendage-inducing signals (Fig. 3A) (Sengel, 1971). This state of regionalization sets up a temporally and spatially dynamic area of potency – a morphogenetic field (discussed below). Importantly, competence is not a passive state, but an actively acquired condition (Gilbert, 2000); it requires tissues to attain regional coordination of an altered state, which can be accomplished by regulation of differential gene expression. Competence has been described as a poised resting state of tissues, ‘a complex of reactions between substances which form an unstable mixture, which may at certain times have two or more alternative modes of change’ (Waddington, 1939). This unstable state can respond to signaling by resolving to one equilibrium or another. Thus, competence can be thought of as an emergent property of a collection of equi-potential progenitors that provides context for signals to effect a phenotypic state.
Evidence for a role for bioelectricity in competence exists but is scattered and limited. This is in part due to the lack of genetically encoded sensors to track the dynamics of Vmem during development. Nonetheless, the use of voltage-sensitive dyes has been successful in observing differential patterns of dye accumulation in developmentally active regions (Fig. 3C) (Adams and Levin, 2012a,b). For example, a voltage differential is observed during early neurulation in Xenopus embryos and mirrors known developmentally active domains (Fig. 3B). Upon disruption by potassium channel expression, these areas change gene expression profiles, leading to altered developmental trajectories (Pai et al., 2012) and craniofacial dysmorphology (Adams et al., 2016). Intriguingly, these broad dominant-negative (gain-of-function) approaches led to the ability to make eye tissue in non-anterior neurogenetic tissues (Pai et al., 2012) (Fig. 3D,E). These findings are difficult to integrate with specific causal mechanisms but suggest enhanced ability of tissue(s) to respond to inductive signals or a shift in competence.
Induction
Induction refers to the process of transmitting a signal to a receiving cell or tissue to cause a change in state (see Glossary, Box 1). Inductive signals can be instructional or permissive, informing or evoking a response within a tissue, respectively. Recent data point to a role for bioelectric signaling in induction. For example, long distance signaling during appendage development is controlled by bioelectric-mediated release of signaling factors. Specifically, altering the function of kcnj2/kir2.1 or of the Drosophila orthologs Irk1, Irk2 and Irk3 leads to differential release of extracellular vesicles at target sites, causing a change in BMP/Dpp signaling in responding tissues; this results in altered patterning of the craniofacial and appendicular skeleton in mice (Dahal et al., 2017, 2012), or of adult wings in Drosophila (Dahal et al., 2012). Similarly, in the process of zebrafish stripe formation, melanocytes can induce repulsive behavior and state change in xanthophores through contact-mediated changes to their resting membrane potential (Inaba et al., 2012). It is unclear whether changes in Vmem caused by alterations in channel function contain specific information or are simply permissive. However, as ion channels have different specificities and gating characteristics, and are sensitive to post-translational modification, they are well suited to mediate transfer of specific information.
Morphogenetic fields
One of the key transformative concepts within developmental biology is that of the morphogenetic field (reviewed by Gilbert et al., 1996; see also Glossary, Box 1). Within a developing embryo, localized areas – defined as tracts or fields – serve as regional areas competent to form different structures (Fig. 3B). Notable examples of such areas include the defined neurogenic placodes of the anterior ectoderm, the tracts of integumentary appendages and the milk lines in register along the body axis. Within competent tissue, the differential response of equi-competent cells to a graded signal can lead to positional information and individuation. Studies on morphogens have shown the power of differential signaling to impart cues that inform cell fates within a tissue. It has been proposed that bioelectric signaling through Vmem could also serve as such a gradient through the electro-coupling of cells (Adams and Levin, 2013).
For bioelectric signaling to play such a role would require a readout to differences in Vmem to be instructive of specific programs, thus providing a basis for determination of type. Although we know that the differentiation states of individual cells are associated with resting Vmem (as discussed above), there is little evidence for a correlation between tissue differentiation and graded responses to Vmem across a tissue. Within regenerating planaria, alteration of Vmem and bioelectric signaling leads to transformation of regional identity along the primary body axis (Beane et al., 2011; Durant et al., 2016) such that anterior structures are formed due to early hypopolarization (Durant et al., 2019). Although this experimental evidence does not provide clear observation of graded output, the binary axial information that is activated upon injury clearly suggests modulation of polarity and activation of different developmental programs. Extending beyond the variation of Vmem across tissue, the early work of Burr and Northrop integrated the dynamics of electrical fields with classic morphogenetic fields (Burr and Northrop, 1955). This idea is attractive and has driven the work of many influential groups; however, we still have limited understanding of the dynamics of electrical fields across tissues and structures during development, and further empirical analysis is required.
Properties of bioelectric signaling that could provide tissue-level regulation of development
Bioelectric signaling can act within and across cells, affecting specific signaling events as well as the general physiological context. These intrinsic properties of bioelectric signaling impart characteristics in signaling that may underlie unique properties of tissues in development. I highlight below several properties, or character states, of developing systems and the potential for bioelectric signaling in their action.
An analogy of capacitance
Formally, capacitance (see Glossary, Box 1) is the change in electric charge in a system relative to the corresponding change in potential (V). Similar signals could therefore elicit varied levels and extents of responses based on differential capacitance within cells and tissues. Capacitance can also lead to build-up of charge in a system. Depending on the release kinetics of local regulators, release of electrical charge can be maintained over time as a battery and thus buffer temporal fluctuations. An intriguing aspect of the notion of developmental capacitance is that the relative response to perturbation is generally a function of geometry. As differential Vmem is regulated in part through spatial and temporal regulation of gene expression throughout development, the response of different systems can be modulated by the territory of connectivity. Although capacitance has been included within conceptual models of how tissues integrate bioelectric signaling (Ferreira et al., 2016; McNamara et al., 2020), this property of bioelectric signaling in modulating developmental decisions remains to be assessed.
Signal amplitude
Signal amplitude refers to the level of signal transmitted. It is clear that, in bioelectric signaling, levels can influence the extent of a response. A unique and emergent property of bioelectric signaling during development may reside in the ability to refine or amplify signals within regional areas of tissues by building up Vmem or electromotive force (EMF). In response to signals, such as growth factors, or to mechanical perturbation, an amplification of a voltage differential could potentiate a coordinated response within a tissue. Varied capacitance of electro-coupled cell assemblages could also promote amplification of differential signaling across a tissue. Indeed, dye studies often show very distinct shifts in polarization across a tissue (Fig. 3C), suggesting regionalization of Vmem during development (Adams et al., 2016) and in regeneration (Beane et al., 2011). The varied amplitude of signals could impart the capability to encode differential responses depending on the sensitivity of responding cells, thus providing information on type.
Signal buffering
Variation in the spatial and temporal aspects of signaling across cells is an innate source of stochasticity in developing systems (Eldar and Elowitz, 2010), and buffering of this variation across cells can provide robustness to patterning mechanisms (Ladbury and Arold, 2012). Electrical coupling provides a rapid temporal and spatial averaging of changes in cell state. As such, electro-coupling of cells within a tissue could provide a mechanism for ‘smoothing’ signals across a tissue that, when integrated with developmental patterning networks, could suppress heterogeneity and increase robustness.
Integration of such electro-dynamic fluxes across a tissue can also shape responsiveness to morphogen signaling during development. Recent work on the processing and activation of Sonic hedgehog (SHH) signaling by cholesterol has shown that this modification affecting the signaling potential of SHH ligands is driven by ion-specific gradients of the cell plasma membrane (Petrov et al., 2020). In particular, activation of SHH signaling by inhibition of PTCH1 through cholesterol-bound SHH ligand is driven by a potassium gradient. Likewise, promotion of cholesterol modification of SHH requires a contrasting sodium gradient. Interestingly, mutation of predicted residues of PTCH1 thought to be key for this regulation is associated with cancer caused by hyperactive SHH signaling.
Sensitivity
The generation of spatially refined electric potentials can impart resting capacity to respond to developmental signals within a cell, tissue or organ. As an analogy, the regulation of ionic potential in the inner ear is generated, in part, through the specific regulation of regional expression of potassium channels and connexins in the cochlear epithelium (Fig. 2C). The function of these channels is to permit the generation of localized endocochlear potential (of +80 mV) that locally drives the sensitized function of hair cells (Nin et al., 2016). This spatial regulation of channels permits a broad circuit within the cochlea and generates and maintains broad electrical potential in the organ (Kikuchi et al., 2000; Nin et al., 2016). When hair cells are triggered, the potassium gradient permits generation of an action potential and translation of sound into neural output. Accordingly, loss of potassium channel function, or of gap junctional complexes, leads to acquired deafness in humans and mice (Kikuchi et al., 2000). Although this is a physiological phenotype, cochlear endolymph potential requires the function of channels such as Kcnk5 or Kcc4, which also regulate structural phenotypes during development (Boettger et al., 2002; Cazals et al., 2015). Thus, transmission of signal via differential regulation of bioelectric signaling within a system is an apt analogy for building sensitization within tissues through local creation and modulation of differential electrical potential. The generation of such sensitization may be a foundation for competence in tissues.
The regulative nature of development and bioelectric signaling
Development is a robust process – it is able to accommodate variation yet still unfolds in a predictable fashion. The regulative capacity of developing systems has been an area of considerable study, and is mediated in part due to feedback regulation within and between structures and systems. This integration among parts of the whole defines developmental modules (Gilbert et al., 1996). Because of their unique properties of collective signaling, electrically coupled cell populations and electrical fields are attractive candidates for defining and establishing developmental modules. A module in this case would be a collection of cells or tissues connected such that, given a certain size or connectivity, a state change occurs and acquisition of a new identity or behavior is acquired across and within the tissue. This is similar to notions of quorum sensing (see Glossary, Box 1) within tissue or cellular structures, such that state changes require a threshold response among components within a regional area (e.g. Widelitz and Chuong, 2016). The conceptual definition presented here adds the property of a functional collective, acting as a unit due to shared bioelectric signaling. This property could contribute to, for example, the scaling of growth of individual elements and organs, which is essential for obtaining relative tissue proportions and sizes. It has been shown that activation of Kcnk5b in zebrafish disrupts scaling of adjacent paired lepidotrichial rays, which are elements that are normally maintained at equal lengths to make up the predominant skeletal elements of the fins (Murciano et al., 2007; reviewed by Harris et al., 2020). Such higher-order regulation may be essential to explain patterning phenotypes arising from mis-regulated potassium channels.
Bioelectric signaling also has the capability to provide resilience (see Glossary, Box 1) to the formation of a structure such that variations in signaling or behavior in part of the system are buffered (as discussed above). Indeed, resiliency in the face of variation within a ‘physio-chemical’ system has been postulated as a fundamental facet of electrical signaling (Burr and Northrop, 1955). Of note, such regulative properties have been attributed to morphogenetic fields and may act in similar ways during development. Harrison famously demonstrated the existence of regionally defined, self-regulating areas of the embryo capable of forming specific organ systems including the limb (Fig. 3B). Importantly, if these areas were cut, divided or transplanted, they still retained the ability to regulate pattern and type (De Robertis et al., 1991). Bioelectric signaling would not necessarily need to provide instructional cues within bioelectric fields – such attributes that provide binary information about the state of a regional group of cells would be sufficient.
A role for bioelectric signaling in regeneration
The processes of regeneration and development are subject to similar regulatory mechanisms. It is therefore no surprise that bioelectrical signaling has also been linked to regeneration. Tissues and organs have a particular electrical signature, and it has been known for over 150 years that damage to a tissue, such as a lesion or fracture, can cause localized and heightened shifts in these signatures. Based on pioneering work by Matthews (1903), which was extended by Jaffe and Borgens among others (reviewed by McLaughlin and Levin, 2018), it is clear that there are endogenous electrical fields present across developing systems as well as in post-embryonic and adult tissues that are responsive to change in that system (e.g. damage or differentiation). When these fields are experimentally altered, these changes can lead to a change in the phenotypic qualities of the system, such as repair, regenerative capacity and developmental patterning (Borgens et al., 1977; Levin, 2003). This topic has been extensively reviewed in several recent articles (McLaughlin and Levin, 2018; Tyler, 2017). As such, I focus here on core functions of bioelectric signaling as a unique signaling property that initiates and integrates development in the process of a regenerative response.
After damage in animals as well as in plants, bioelectric flux across a tissue acts as an initial cue for repair (Burr et al., 1938; Tyler, 2017). As in development, the bioelectric changes in tissues after damage are tied to classic signaling cascades, such as those triggered by secondary signaling mediators (e.g. PI3KOH; Zhao et al., 2006) or by correlated changes in redox status leading to the generation of reactive oxygen species (Ferreira et al., 2016). Cells are also known to use electrical currents to instruct migration to wound sites. This directional migration, or galvanotropism, due to electric fields is thought to mediate early cell migration in wound healing and regeneration (Reid and Zhao, 2014). It is tempting to argue that galvanotopism may be a prevalent force that directs cell migration during normal development; however, this hypothesis has not yet been clearly proven.
Response to damage can be a component of a broader regenerative response within newly formed tissue to reform lost structures. Interestingly, a current flux is correlated with regenerative potential; highly regenerative animals exhibit a distinct current reversal after amputation, whereas non-regenerative animals generally lack this characteristic (Reid et al., 2009; Reid and Zhao, 2014; Tyler, 2017). As in development, such changes in electrical current in regenerating systems have broad cellular effects linked to differential signaling within cells. In earthworms undergoing regeneration of their bodies, each segment has a specific electric potential, and growth ceases upon re-attaining the field potential of a normally patterned worm (Kurtz and Schrank, 1955; Moment, 1949). This observation has led many to ask whether electrical signals might provide information concerning normal patterning, function or state that is leveraged in regenerative programs (Burr and Northrop, 1955; Jaffe, 1981; Levin, 2009). Amputated limbs and fins grow back to their previous sizes. Could modulation of external electrical field dynamics as well as shifts in tissue-level Vmem instruct this reformation? As field dynamics are an electrical state imparted by the geometric organization of structures, could this resting electrical state change also be a trigger for loss?
Blocking or disrupting Vmem through expression/misexpression of components of bioelectric signaling or exogenous voltage application can lead to altered wound repair and disrupted or arrested regeneration of lost structures (reviewed by McLaughlin and Levin, 2018). The evidence that application of exogenous fields to wounds leads to increased wound healing (reviewed by Tyler, 2017) or enhanced regenerative responses (e.g. Tseng and Levin, 2013; Tseng et al., 2010) has led many to ask whether such treatments can enhance recovery in damage and disease. Even in cases of persistent ulceration and refractory wounds, the application of electrostimulation shows clinical promise when performed in tandem with standard treatment (Gould et al., 2015; Kloth, 2014; Koel and Houghton, 2014). Application of electrical stimulation across skeletal fractures also shows evidence of increased healing (e.g. de Haas et al., 1980), while alterations to the endogenous electrical field in limb or tail amputations can lead to increased growth and patterning of the otherwise non-regenerative structures (Leppik et al., 2016; Oliveira et al., 2019; Tseng et al., 2010). Thus, similar to findings of genetic alterations in many channelopathies, modulation of the levels or the direction of the electrical field across a tissue can cause gain-of-function effects on tissue response parameters, in this context exposing sufficiency of electrical signaling to drive repair of damaged structures. This broader epimorphic role of electrical signaling for informing and/or initiating a regenerative response is attractive although not understood.
Conclusions and perspectives
It is clear that bioelectric signaling can influence development and regeneration, albeit through multifaceted mechanisms occurring at different levels of biological organization. This fact makes empirical analyses of bioelectric signaling difficult and often clouds interpretations of the data. This has led to calls for revised experimental approaches in bioelectricity research that can prove causation (Levin, 2020; Pezzulo and Levin, 2015). However, as discussed here, a refocus of bioelectric research, centering on and defining different levels of function, can parse inter-dependent roles of bioelectric regulation and permit empirical analyses extending beyond broad teratology. In particular, an overlay of bioelectric signaling analyses onto a framework of developmental processes and concepts, such as induction, competence, capacitance and morphogenic fields, may provide important insights into the unique role bioelectric signaling plays in organizing life, while still retaining hypotheses that are addressable in reductionist analyses. Thus, once broken down to the component levels of analysis – cell, compartment, tissue, organ/structure and organism – the study of bioelectric signaling can be embraced by the notion of developmental mechanics, or ‘Entwicklungsmechanick’, as an empirical approach (Dupont, 2017; Maienschein, 1991). The emergent properties imparted via bioelectric signaling, which allow it to coordinate functions within cells and tissues and across structures, may represent a fundamentally unique property of development that stands apart from, and integrates, canonical signaling networks.
Acknowledgements
This perspective and review of bioelectric signaling was fueled by discussions held at the workshop for Bioelectric signaling held at the 2019 Developmental Biology meeting in Boston, as well as the 119th Titisee conference in 2019 with presenters and discussants there. Additionally, the perspectives benefited from broader discussions with Dr Emily Bates, Michael Levin, Shigeru Kondo, Enrico Cohen and Ming Zao concerning their work; however, these views do not necessarily reflect their own. I also thank Dr Scott Gilbert for providing reference material and keeping history and concepts of experimental embryology alive and accessible to generations of new biologists. The work greatly benefited from review by Dr Jennifer Lanni, Katherine Woronowicz and Jacob Daane, prior to peer review.
Footnotes
Competing interests
The author declares no competing or financial interests.
Funding
The author's research is supported in part by a National Institutes of Health grant (R01HD084985). Deposited in PMC for release after 12 months.
References
- Adams, D. S. and Levin, M. (2012a). General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold. Spring Harb. Protoc. 2012, 385-397. 10.1101/pdb.top067710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, D. S. and Levin, M. (2012b). Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, 459-464. 10.1101/pdb.prot067702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, D. S. and Levin, M. (2013). Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 352, 95-122. 10.1007/s00441-012-1329-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, D. S., Uzel, S. G. M., Akagi, J., Wlodkowic, D., Andreeva, V., Yelick, P. C., Devitt-Lee, A., Pare, J.-F. and Levin, M. (2016). Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 594, 3245-3270. 10.1113/JP271930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, F., Tickle, C. and Warner, A. (1990). The role of gap junctions in patterning of the chick limb bud. Development 108, 623-634. [DOI] [PubMed] [Google Scholar]
- Alper, S. L. (2017). Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr. Top. Membr. 79, 97-134. 10.1016/bs.ctm.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Ariazi, J., Benowitz, A., De Biasi, V., Den Boer, M. L., Cherqui, S., Cui, H., Douillet, N., Eugenin, E. A., Favre, D., Goodman, S.et al. (2017). Tunneling nanotubes and gap junctions-their role in long-range intercellular communication during development, health, and disease conditions. Front. Mol. Neurosci. 10, 333. 10.3389/fnmol.2017.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson, G. (1979). Mind and Nature: A Necessary Unity. New York: Dutton. [Google Scholar]
- Bauer, C. K. and Schwarz, J. R. (2001). Physiology of EAG K+ channels. J. Membr. Biol. 182, 1-15. 10.1007/s00232-001-0031-3 [DOI] [PubMed] [Google Scholar]
- Bauer, C. K., Calligari, P., Radio, F. C., Caputo, V., Dentici, M. L., Falah, N., High, F., Pantaleoni, F., Barresi, S., Ciolfi, A.et al. (2018). Mutations in KCNK4 that affect gating cause a recognizable neurodevelopmental syndrome. Am. J. Hum. Genet. 103, 621-630. 10.1016/j.ajhg.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, C. K., Schneeberger, P. E., Kortüm, F., Altmüller, J., Santos-Simarro, F., Baker, L., Keller-Ramey, J., White, S. M., Campeau, P. M., Gripp, K. W.et al. (2019). Gain-of-function mutations in KCNN3 encoding the small-conductance Ca2+-activated K+ channel SK3 cause zimmermann-laband syndrome. Am. J. Hum. Genet. 104, 1139-1157. 10.1016/j.ajhg.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane, W. S., Morokuma, J., Adams, D. S. and Levin, M. (2011). A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem. Biol. 18, 77-89. 10.1016/j.chembiol.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belus, M. T., Rogers, M. A., Elzubeir, A., Josey, M., Rose, S., Andreeva, V., Yelick, P. C. and Bates, E. A. (2018). Kir2.1 is important for efficient BMP signaling in mammalian face development. Dev. Biol. 444 Suppl. 1, S297-S307. 10.1016/j.ydbio.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binggeli, R. and Weinstein, R. C. (1985). Deficits in elevating membrane potential of rat fibrosarcoma cells after cell contact. Cancer Res. 45, 235-241. [PubMed] [Google Scholar]
- Blackiston, D. J., McLaughlin, K. A. and Levin, M. (2009). Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle 8, 3527-3536. 10.4161/cc.8.21.9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger, T., Hübner, C. A., Maier, H., Rust, M. B., Beck, F. X. and Jentsch, T. J. (2002). Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416, 874-878. 10.1038/416874a [DOI] [PubMed] [Google Scholar]
- Borgens, R. B., Vanable, J. W., Jr. and Jaffe, L. F. (1977). Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. J. Exp. Zool. 200, 403-416. 10.1002/jez.1402000310 [DOI] [PubMed] [Google Scholar]
- Bramswig, N. C., Ockeloen, C. W., Czeschik, J. C., van Essen, A. J., Pfundt, R., Smeitink, J., Poll-The, B. T., Engels, H., Strom, T. M., Wieczorek, D.et al. (2015). ‘Splitting versus lumping’: temple-Baraitser and Zimmermann-Laband Syndromes. Hum. Genet. 134, 1089-1097. 10.1007/s00439-015-1590-1 [DOI] [PubMed] [Google Scholar]
- Burr, H. S. and Northrop, F. S. C. (1955). The electro-dynamic theory of life. Q. Rev. Biol. 10, 322-333. 10.1086/394488 [DOI] [Google Scholar]
- Burr, H. S., Harvey, S. C. and Taffel, M. (1938). Bio-electric correlates of wound healing. Yale J. Biol. Med. 11, 103-107. [PMC free article] [PubMed] [Google Scholar]
- Cazals, Y., Bévengut, M., Zanella, S., Brocard, F., Barhanin, J. and Gestreau, C. (2015). KCNK5 channels mostly expressed in cochlear outer sulcus cells are indispensable for hearing. Nat. Commun. 6, 8780. 10.1038/ncomms9780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera, J., Meseguer, S. and Mafe, S. (2016). The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci. Rep. 6, 35201. 10.1038/srep35201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho, C. N. D. and Kosher, R. A. (1991). A gradient of gap junctional communication along the anterior-posterior axis of the developing chick limb bud. Dev. Biol. 148, 529-535. 10.1016/0012-1606(91)90271-4 [DOI] [PubMed] [Google Scholar]
- Cohn, M. J., Izpisúa-Belmonte, J. C., Abud, H., Heath, J. K. and Tickle, C. (1995). Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell 80, 739-746. 10.1016/0092-8674(95)90352-6 [DOI] [PubMed] [Google Scholar]
- Cone, C. D.Jr. (1971). Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J. Theor. Biol. 30, 151-181. 10.1016/0022-5193(71)90042-7 [DOI] [PubMed] [Google Scholar]
- Cooper, P. E., Reutter, H., Woelfle, J., Engels, H., Grange, D. K., van Haaften, G., van Bon, B. W., Hoischen, A. and Nichols, C. G. (2014). Cantú syndrome resulting from activating mutation in the KCNJ8 gene. Hum. Mutat. 35, 809-813. 10.1002/humu.22555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daane, J. M., Lanni, J., Rothenberg, I., Seebohm, G., Higdon, C. W., Johnson, S. L. and Harris, M. P. (2018). Bioelectric-calcineurin signaling module regulates allometric growth and size of the zebrafish fin. Sci. Rep. 8, 10391. 10.1038/s41598-018-28450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daane, J. M., Blum, N., Lanni, J., Boldt, H., Iovine, M. K., Higdon, C. W, Johnson, S. L. and Harris, M. P. (2021). Modulation of bioelectric cues in the evolution of flying fishes. BioRxiv. 10.1101/2021.03.05.434157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal, G. R., Pradhan, S. J. and Bates, E. A. (2017). Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development 144, 2771-2783. 10.1242/dev.146647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal, G. R., Rawson, J., Gassaway, B., Kwok, B., Tong, Y., Ptacek, L. J. and Bates, E. (2012). An inwardly rectifying K+ channel is required for patterning. Development 139, 3653-3664. 10.1242/dev.078592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas, W. G., Watson, J. and Morrison, D. M. (1980). Non-invasive treatment of ununited fractures of the tibia using electrical stimulation. J. Bone Joint Surg. Br. 62-B, 465-470. 10.1302/0301-620X.62B4.6968752 [DOI] [PubMed] [Google Scholar]
- De Robertis, E. M., Morita, E. A. and Cho, K. W. (1991). Gradient fields and homeobox genes. Development 112, 669-678. [DOI] [PubMed] [Google Scholar]
- Despang, A., Schöpflin, R., Franke, M., Ali, S., Jerković, I., Paliou, C., Chan, W.-L., Timmermann, B., Wittler, L., Vingron, M.et al. (2019). Functional dissection of the Sox9-Kcnj2 locus identifies nonessential and instructive roles of TAD architecture. Nat. Genet. 51, 1263-1271. 10.1038/s41588-019-0466-z [DOI] [PubMed] [Google Scholar]
- Dupont, J.-C. (2017). Wilhelm His and mechanistic approaches to development at the time of Entwicklungsmechanik. Hist. Philos. Life Sci. 39, 21. 10.1007/s40656-017-0148-z [DOI] [PubMed] [Google Scholar]
- Durant, F., Lobo, D., Hammelman, J. and Levin, M. (2016). Physiological controls of large-scale patterning in planarian regeneration: a molecular and computational perspective on growth and form. Regeneration 3, 78-102. 10.1002/reg2.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant, F., Bischof, J., Fields, C., Morokuma, J., LaPalme, J., Hoi, A. and Levin, M. (2019). The role of early bioelectric signals in the regeneration of planarian anterior/posterior polarity. Biophys. J. 116, 948-961. 10.1016/j.bpj.2019.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar, A. and Elowitz, M. B. (2010). Functional roles for noise in genetic circuits. Nature 467, 167-173. 10.1038/nature09326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, F., Luxardi, G., Reid, B. and Zhao, M. (2016). Early bioelectric activities mediate redox-modulated regeneration. Development 143, 4582-4594. 10.1242/dev.142034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, M., Ibrahim, D. M., Andrey, G., Schwarzer, W., Heinrich, V., Schöpflin, R., Kraft, K., Kempfer, R., Jerković, I., Chan, W.-L.et al. (2016). Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature 538, 265-269. 10.1038/nature19800 [DOI] [PubMed] [Google Scholar]
- Fraser, S. P., Diss, J. K. J., Chioni, A.-M., Mycielska, M. E., Pan, H., Yamaci, R. F., Pani, F., Siwy, Z., Krasowska, M., Grzywna, Z.et al. (2005). Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin. Cancer Res. 11, 5381-5389. 10.1158/1078-0432.CCR-05-0327 [DOI] [PubMed] [Google Scholar]
- Frohnhöfer, H. G., Geiger-Rudolph, S., Pattky, M., Meixner, M., Huhn, C., Maischein, H.-M., Geisler, R., Gehring, I., Maderspacher, F., Nüsslein-Volhard, C.et al. (2016). Spermidine, but not spermine, is essential for pigment pattern formation in zebrafish. Biol. Open 5, 736-744. 10.1242/bio.018721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, L. F., Pradhan, S. J., Mitchell, D., Josey, M., Casey, J., Belus, M. T., Fedder, K. N., Dahal, G. R. and Bates, E. A. (2019). Ion channel contributions to wing development in Drosophila melanogaster. G3 9, 999-1008. 10.1534/g3.119.400028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, S. (2000). Developmental Biology (6th edn). Sunderland, MA: ): Sinauer Associates. [Google Scholar]
- Gilbert, S. F., Opitz, J. M. and Raff, R. A. (1996). Resynthesizing evolutionary and developmental biology. Dev. Biol. 173, 357-372. 10.1006/dbio.1996.0032 [DOI] [PubMed] [Google Scholar]
- Gould, L., Abadir, P., Brem, H., Carter, M., Conner-Kerr, T., Davidson, J., DiPietro, L., Falanga, V., Fife, C., Gardner, S.et al. (2015). Chronic wound repair and healing in older adults: current status and future research. Wound Repair. Regen. 23, 1-13. 10.1111/wrr.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M. P., Daane, J. M. and Lanni, J. (2020). Through veiled mirrors: fish fins giving insight into size regulation. Wiley Interdiscip. Rev. Dev. Biol. e381. 10.1002/wdev.381 [DOI] [PubMed] [Google Scholar]
- Huxley, J. and De Beer, G. (1936). The Elements of Experimental Biology. Cambridge: Cambridge University Press. [Google Scholar]
- Inaba, M., Yamanaka, H. and Kondo, S. (2012). Pigment pattern formation by contact-dependent depolarization. Science 335, 677. 10.1126/science.1212821 [DOI] [PubMed] [Google Scholar]
- Iovine, M. K. and Johnson, S. L. (2000). Genetic analysis of isometric growth control mechanisms in the zebrafish caudal Fin. Genetics 155, 1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion, U., Frohnhöfer, H. G., Krauss, J., Çolak Champollion, T., Maischein, H.-M., Geiger-Rudolph, S., Weiler, C. and Nüsslein-Volhard, C. (2014). Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. eLife 3, e05125. 10.7554/eLife.05125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, A. G. and Sater, A. K. (1988). Features of embryonic induction. Development 104, 341-359. [DOI] [PubMed] [Google Scholar]
- Jaffe, L. A. (1976). Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature 261, 68-71. 10.1038/261068a0 [DOI] [PubMed] [Google Scholar]
- Jaffe, L. F. (1981). The role of ionic currents in establishing developmental pattern. Philos. Trans. R. Soc. B Biol. Sci. 295, 553-566. 10.1098/rstb.1981.0160 [DOI] [PubMed] [Google Scholar]
- Jaffe, L. A. (2018). The fast block to polyspermy: New insight into a century-old problem. J. Gen. Physiol. 150, 1233-1234. 10.1085/jgp.201812145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, L. A. and Cross, N. L. (1986). Electrical regulation of sperm-egg fusion. Annu. Rev. Physiol. 48, 191-200. 10.1146/annurev.ph.48.030186.001203 [DOI] [PubMed] [Google Scholar]
- Jung, J., Choi, H. B., Koh, Y. I., Rim, J. H., Choi, H. J., Kim, S. H., Lee, J. H., An, J., Kim, A., Lee, J. S.et al. (2018). Whole-exome sequencing identifies two novel mutations in KCNQ4 in individuals with nonsyndromic hearing loss. Sci. Rep. 8, 16659. 10.1038/s41598-018-34876-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, T., Adams, J. C., Miyabe, Y., So, E. and Kobayashi, T. (2000). Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med. Electron Microsc. 33, 51-56. 10.1007/s007950070001 [DOI] [PubMed] [Google Scholar]
- Kloth, L. C. (2014). Electrical stimulation technologies for wound healing. Adv Wound Care 3, 81-90. 10.1089/wound.2013.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koel, G. and Houghton, P. E. (2014). Electrostimulation: current status, strength of evidence guidelines, and meta-analysis. Adv Wound Care 3, 118-126. 10.1089/wound.2013.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortüm, F., Caputo, V., Bauer, C. K., Stella, L., Ciolfi, A., Alawi, M., Bocchinfuso, G., Flex, E., Paolacci, S., Dentici, M. L.et al. (2015). Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat. Genet. 47, 661-667. 10.1038/ng.3282 [DOI] [PubMed] [Google Scholar]
- Kujawski, S., Lin, W., Kitte, F., Börmel, M., Fuchs, S., Arulmozhivarman, G., Vogt, S., Theil, D., Zhang, Y. and Antos, C. L. (2014). Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev. Cell 28, 573-587. 10.1016/j.devcel.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Kurtz, I. and Schrank, A. R. (1955). Properties of intact and regenerating earthworms, Eisenia foetida. Physiol. Zool. 28, 322-330. 10.1086/physzool.28.4.30152195 [DOI] [Google Scholar]
- Ladbury, J. E. and Arold, S. T. (2012). Noise in cellular signaling pathways: causes and effects. Trends Biochem. Sci. 37, 173-178. 10.1016/j.tibs.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, D. W., Yancey, S. B., Bugga, L. and Revel, J. P. (1992). Connexin expression and gap junction communication compartments in the developing mouse limb. Dev. Dyn. 195, 153-161. 10.1002/aja.1001950302 [DOI] [PubMed] [Google Scholar]
- Lanni, J. S., Peal, D., Ekstrom, L., Chen, H., Stanclift, C., Bowen, M. E., Mercado, A., Gamba, G., Kahle, K. T. and Harris, M. P. (2019). Integrated K+ channel and K+Cl- cotransporter functions are required for the coordination of size and proportion during development. Dev. Biol. 456, 164-178. 10.1016/j.ydbio.2019.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik, L. P., Froemel, D., Slavici, A., Ovadia, Z. N., Hudak, L., Henrich, D., Marzi, I. and Barker, J. H. (2016). Effects of electrical stimulation on rat limb regeneration, a new look at an old model. Sci. Rep. 5, 18353. 10.1038/srep18353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levavi-Sivan, B., Bloch, C. L., Gutnick, M. J. and Fleidervish, I. A. (2005). Electrotonic coupling in the anterior pituitary of a teleost fish. Endocrinology 146, 1048-1052. 10.1210/en.2004-1415 [DOI] [PubMed] [Google Scholar]
- Levin, M. (2003). Motor protein control of ion flux is an early step in embryonic left-right asymmetry. BioEssays 25, 1002-1010. 10.1002/bies.10339 [DOI] [PubMed] [Google Scholar]
- Levin, M. (2007). Gap junctional communication in morphogenesis. Prog. Biophys. Mol. Biol. 94, 186-206. 10.1016/j.pbiomolbio.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. (2009). Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin. Cell Dev. Biol. 20, 543-556. 10.1016/j.semcdb.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. (2012). Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays 34, 205-217. 10.1002/bies.201100136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. (2014). Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J. Physiol. 592, 2295-2305. 10.1113/jphysiol.2014.271940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, M. (2020). The biophysics of regenerative repair suggests new perspectives on biological causation. BioEssays 42, 1900146. 10.1002/bies.201900146 [DOI] [PubMed] [Google Scholar]
- Levin, M. and Martyniuk, C. J. (2018). The bioelectric code: An ancient computational medium for dynamic control of growth and form. Biosystems 164, 76-93. 10.1016/j.biosystems.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maienschein, J. (1991). The origins of Entwicklungsmechanik. In A Conceptual History of Modern Embryology (ed. GIbert S.), pp. 43-61. Baltimore: John Hopkins University Press. [Google Scholar]
- Matthews, A. P. (1903). Electrical polarity in the Hydroids. Am. J. Physiology 8, 294-299. 10.1113/jphysiol.2014.271940 [DOI] [Google Scholar]
- Maussion, G., Cruceanu, C., Rosenfeld, J. A., Bell, S. C., Jollant, F., Szatkiewicz, J., Collins, R. L., Hanscom, C., Kolobova, I., de Champfleur, N. M.et al. (2017). Implication of LRRC4C and DPP6 in neurodevelopmental disorders. Am. J. Med. Genet. A 173, 395-406. 10.1002/ajmg.a.38021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. A. and Levin, M. (2018). Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev. Biol. 433, 177-189. 10.1016/j.ydbio.2017.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, H. M., Salegame, R., Al Tanoury, Z., Xu, H., Begum, S., Ortiz, G., Pourquie, O. and Cohen, A. E. (2020). Bioelectrical domain walls in homogeneous tissues. Nat. Phys. 16, 357-364. 10.1038/s41567-019-0765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moment, G. B. (1949). On the relation between growth in length, the formation of new segments, and electric potential in an earthworm. J. Exp. Zool. 112, 1-12. 10.1002/jez.1401120102 [DOI] [PubMed] [Google Scholar]
- Murciano, C., Pérez-Claros, J., Smith, A., Avaron, F., Fernández, T. D., Durán, I., Ruiz-Sánchez, J., García, F., Becerra, J., Akimenko, M.-A.et al. (2007). Position dependence of hemiray morphogenesis during tail fin regeneration in Danio rerio. Dev. Biol. 312, 272-283. 10.1016/j.ydbio.2007.09.026 [DOI] [PubMed] [Google Scholar]
- Nin, F., Yoshida, T., Sawamura, S., Ogata, G., Ota, T., Higuchi, T., Murakami, S., Doi, K., Kurachi, Y. and Hibino, H. (2016). The unique electrical properties in an extracellular fluid of the mammalian cochlea; their functional roles, homeostatic processes, and pathological significance. Pflügers Arch. Eur. J. Physiol. 468, 1637-1649. 10.1007/s00424-016-1871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, K. M. C., Barker, J. H., Berezikov, E., Pindur, L., Kynigopoulos, S., Eischen-Loges, M., Han, Z., Bhavsar, M. B., Henrich, D. and Leppik, L. (2019). Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 9, 11433. 10.1038/s41598-019-47389-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, V. P., Aw, S., Shomrat, T., Lemire, J. M. and Levin, M. (2012). Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 139, 313-323. 10.1242/dev.073759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, S. L., Levin, M. and Oudin, M. J. (2019). Bioelectric control of metastasis in solid tumors. Bioelectricity 1, 114-130. 10.1089/bioe.2019.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas, W. A., Boyadjiev, S. A., Shapiro, R. E., Daniels, O., Wollnik, B., Keegan, C. E., Innis, J. W., Dinulos, M. B., Christian, C., Hannibal, M. C.et al. (2003). Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 72, 408-418. 10.1086/346090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perathoner, S., Daane, J. M., Henrion, U., Seebohm, G., Higdon, C. W., Johnson, S. L., Nüsslein-Volhard, C. and Harris, M. P. (2014). Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 10, e1004080. 10.1371/journal.pgen.1004080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov, K., Wierbowski, B. M., Liu, J. and Salic, A. (2020). Distinct cation gradients power cholesterol transport at different key points in the Hedgehog signaling pathway. Dev. Cell 55, 314-327.e7. 10.1016/j.devcel.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo, G. and Levin, M. (2015). Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr. Biol. 7, 1487-1517. 10.1039/C5IB00221D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietak, A. and Levin, M. (2017). Bioelectric gene and reaction networks: computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation. J. R. Soc. Interface 14, 20170425. 10.1098/rsif.2017.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podobnik, M., Frohnhöfer, H. G., Dooley, C. M., Eskova, A., Nüsslein-Volhard, C. and Irion, U. (2020). Evolution of the potassium channel gene Kcnj13 underlies colour pattern diversification in Danio fish. Nat. Commun. 11, 6230. 10.1038/s41467-020-20021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, B. and Zhao, M. (2014). The electrical response to injury: molecular mechanisms and wound healing. Adv. Wound Care 3, 184-201. 10.1089/wound.2013.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, B., Song, B. and Zhao, M. (2009). Electric currents in Xenopus tadpole tail regeneration. Dev. Biol. 335, 198-207. 10.1016/j.ydbio.2009.08.028 [DOI] [PubMed] [Google Scholar]
- Schartl, M., Kneitz, S., Ormanns, J., Schmidt, C., Anderson, J. L., Amores, A., Catchen, J., Wilson, C., Geiger, D., Du, K.et al. (2020). The developmental and genetic architecture of the sexually selected male ornament of swordtails. Curr. Biol. 31, 911-922.e4. 10.1016/j.cub.2020.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengel, P. (1971). The organogenesis and arrangement of cutaneous appendages in birds. Adv. Morphog. 9, 181-230. 10.1016/B978-0-12-028609-6.50009-1 [DOI] [PubMed] [Google Scholar]
- Serras, F., Fraser, S. and Chuong, C. M. (1993). Asymmetric patterns of gap junctional communication in developing chicken skin. Development 119, 85-96. [DOI] [PubMed] [Google Scholar]
- Silic, M. R., Wu, Q., Kim, B. H., Golling, G., Chen, K. H., Freitas, R., Chubykin, A. A., Mittal, S. K. and Zhang, G. (2020). Potassium channel-associated bioelectricity of the dermomyotome determines fin patterning in zebrafish. Genetics 215, 1067-1084. 10.1534/genetics.120.303390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, K., Jr., Eble, D. M. and Iovine, M. K. (2009). Connexin43 regulates joint location in zebrafish fins. Dev. Biol. 327, 410-418. 10.1016/j.ydbio.2008.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack, J. (1983). From Egg to EMbryo. DEterminative Events in Early DEvelopment . Cambridge: Cambridge University Press. [Google Scholar]
- Srinivas, M., Verselis, V. K. and White, T. W. (2018). Human diseases associated with connexin mutations. Biochim. Biophys. Acta (BBA) Biomembr. 1860, 192-201. 10.1016/j.bbamem.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, P., Kane, A., Harrison, C. and Levin, M. (2021). A meta-analysis of bioelectric data in cancer, embryogenesis, and regeneration. Bioelectricity 3, 42-67. 10.1089/bioe.2019.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, S., Le Bleu, H., Yette, G. A., Henner, A. L., Robbins, A. E., Braunstein, J. A. and Stankunas, K. (2020). longfin causes cis-ectopic expression of the kcnh2a ether-a-go-go K+ channel to autonomously prolong fin outgrowth. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit, A. (2007). The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 51, 447-461. 10.1387/ijdb.072327as [DOI] [PubMed] [Google Scholar]
- Tsai, C.-T., Hsieh, C.-S., Chang, S.-N., Chuang, E. Y., Ueng, K.-C., Tsai, C.-F., Lin, T.-H., Wu, C.-K., Lee, J.-K., Lin, L.-Y.et al. (2016). Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat. Commun. 7, 10190. 10.1038/ncomms10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, A. and Levin, M. (2013). Cracking the bioelectric code: probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 6, e22595. 10.4161/cib.22595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, A.-S., Beane, W. S., Lemire, J. M., Masi, A. and Levin, M. (2010). Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 30, 13192-13200. 10.1523/JNEUROSCI.3315-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, S. E. B. (2017). Nature's electric potential: a systematic review of the role of bioelectricity in wound healing and regenerative processes in animals, humans, and plants. Front. Physiol. 8, 627. 10.3389/fphys.2017.00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui, Y., Aramaki, T., Kondo, S. and Watanabe, M. (2019). The minimal gap-junction network among melanophores and xanthophores required for stripe pattern formation in zebrafish. Development 146, dev181065. 10.1242/dev.181065 [DOI] [PubMed] [Google Scholar]
- Villanueva, S., Burgos, J., López-Cayuqueo, K. I., Lai, K.-M. V., Valenzuela, D. M., Cid, L. P. and Sepúlveda, F. V. (2015). Cleft palate, moderate lung developmental retardation and early postnatal lethality in mice deficient in the Kir7.1 inwardly rectifying K+ channel. PLoS ONE 10, e0139284. 10.1371/journal.pone.0139284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, C. H. (1939). An introduction to Modern Genetics. New York: Macmillan. [Google Scholar]
- Waddington, C. H. (1940). Organisers and Genes. Cambridge: Cambridge University Press. [Google Scholar]
- Wang, X., Veruki, M. L., Bukoreshtliev, N. V., Hartveit, E. and Gerdes, H.-H. (2010). Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc. Natl. Acad. Sci. USA 107, 17194-17199. 10.1073/pnas.1006785107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M., Iwashita, M., Ishii, M., Kurachi, Y., Kawakami, A., Kondo, S. and Okada, N. (2006). Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 7, 893-897. 10.1038/sj.embor.7400757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M., Watanabe, D. and Kondo, S. (2012). Polyamine sensitivity of gap junctions is required for skin pattern formation in zebrafish. Sci. Rep. 2, 473. 10.1038/srep00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir, M. P. and Lo, C. W. (1982). Gap junctional communication compartments in the Drosophila wing disk. Proc. Natl. Acad. Sci. USA 79, 3232-3235. 10.1073/pnas.79.10.3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz, R. and Chuong, C.-M. (2016). Quorum sensing and other collective regenerative behavior in organ populations. Curr. Opin. Genet. Dev. 40, 138-143. 10.1016/j.gde.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S. H. (2004). Generation of resting membrane potential. Adv. Physiol. Educ. 28, 139-142. 10.1152/advan.00029.2004 [DOI] [PubMed] [Google Scholar]
- Youssefian, L., Vahidnezhad, H., Saeidian, A. H., Mahmoudi, H., Karamzadeh, R., Kariminejad, A., Huang, J., Li, L., Jannace, T. F., Fortina, P.et al. (2019). A novel autosomal recessive GJB2-associated disorder: Ichthyosis follicularis, bilateral severe sensorineural hearing loss, and punctate palmoplantar keratoderma. Hum. Mutat. 40, 217-229. 10.1002/humu.23686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M., Song, B., Pu, J., Wada, T., Reid, B., Tai, G., Wang, F., Guo, A., Walczysko, P., Gu, Y.et al. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442, 457-460. 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]