Introduction
Patients with cancer have a unique susceptibility to coronavirus disease 2019 (COVID-19) [1], [2]. Multiple reports have assessed the impact of anti-neoplastic therapies on COVID-19 outcomes in these patients [3], [4], [5], [6], and although radiotherapy (RT) has been investigated [1], [4], [6], the exact impact of RT dose to the cardiopulmonary system on COVID-19 outcomes is largely unknown.
We investigated the effect of RT and underlying medical comorbidities on COVID-19 outcomes in patients treated at a tertiary cancer center located in the New York City metropolitan area, an early epicenter of the U.S. COVID-19 pandemic. We hypothesized that the RT dose to cardiopulmonary structures would be associated with COVID-19 outcomes.
Methods
This study was completed under the approved institutional review board protocol #20-146. All patients with a positive SARS-CoV2 RT-PCR test from the first U.S. case identified on March 8, 2020 to May 6, 2020 and who were previously treated with RT at the Memorial Sloan Kettering Cancer Center were assessed (C.f. Supplemental material; Methods for further details on data extraction, treatment, and analysis). Demographics, primary cancer histology, comorbidities, date of COVID-19 diagnosis, history of RT (up to 3 years prior to COVID-19 diagnosis) were extracted from the electronic medical records. The three outcomes studied were hospitalization, life-threatening disease (ICU-level acuity), and death.
Cardiopulmonary dose was represented by fractionation-corrected mean heart dose (MHD) and mean lung dose (MLD). Thirteen variables were explored as predictors for the three studied outcomes: history of acute renal injury (ARI; defined as azotemia prior to COVID-19 diagnosis) age, gender, asthma, body mass index (BMI), coronary artery disease (CAD), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), diabetes, hypertension (HTN), MHD, and MLD, and days between RT end and COVID-19 diagnosis (ΔTRT COVID). In the multivariate analysis, a TRIPOD type 2a model [7], which includes a training and validation subset (cf. Supplemental material; Methods), was developed.
Results
We identified 350 patients with prior RT who were subsequently diagnosed with COVID-19 from March 8th, 2020 to May 6th, 2020 (Tables S1 and S2; Figs. S1A and B). Of these, 195 patients had some extent of MHD and MLD. Among the 350 patients, 39% required hospital admission, 17% had life-threatening disease, and there were 48 deaths due to COVID-19 (Table S1).
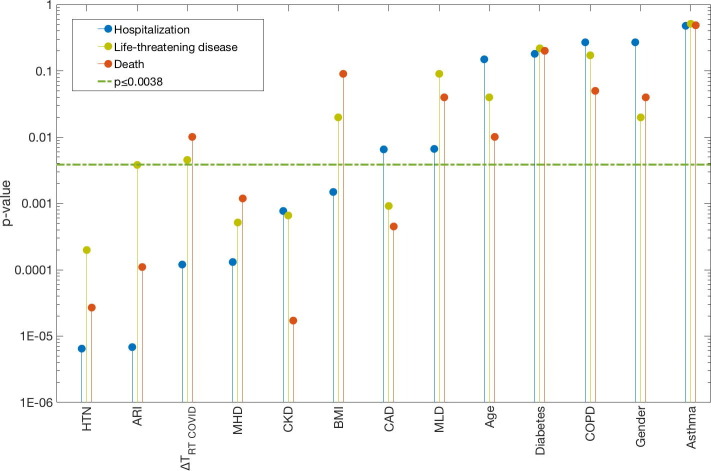
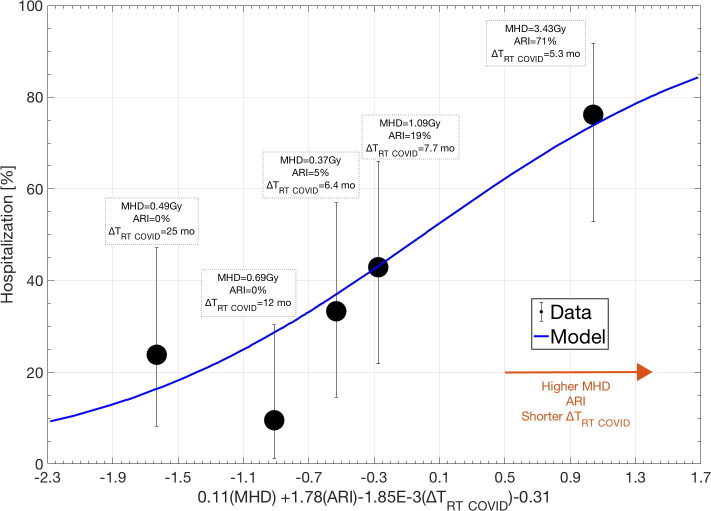
History of acute renal injury (ARI), CKD, HTN and MHD were significantly associated with all three COVID-19 outcomes: p-value range: 6.8E-6 to 3.8E-3 (Fig. 1 , Table S3). In addition, BMI and ΔTRT COVID were candidate predictors for hospitalization (p-value: 1.5E−3, 1.2E−4), and CAD was a candidate predictor for both life-threatening disease and death (p-value: 9.1E−4, 4.5E−4). For hospitalization, two candidate multivariate models were identified: (1) ARI, MHD and ΔTRT COVID or (2) HTN, MHD and ΔTRT COVID (model frequency: 19%, 13%). The model that included ARI, MHD and ΔTRT COVID was considered the final multivariate model since it was generalizable in the validation subset (AUC = 0.73; p = 3.85E−5; pHL = 0.53; Table S3) while the other model was not (p = 0.22). The dose–response curve in Fig. 2 indicates the agreement between the observed and predicted risk of hospitalization and suggests that a higher risk of hospitalization is likely with the presence of ARI, increased MHD and a shorter ΔTRT COVID. The scatterplot in Fig. S2 further illustrates that a larger portion of patients that were hospitalized received a higher MHD compared to the non-hospitalized patients. For life-threatening disease and death from COVID-19, no multivariate association was established (cf. Supplemental material; Results).
Fig. 1.
Univariate analysis p-values (y-axis) between each of the 13 variables (x-axis) and each of the three endpoints (Hospitalization: blue; Life-threatening disease: yellow; Death: Orange). The green dash-dotted line is the significance level corrected for thirteen tests. Further details pertaining to the univariate and multivariate results are given in Table S2. The Abbreviations: ARI: Acute Renal Injury; BMI: Body Mass Index; HTN: Hypertension; MHD: Mean Heart Dose; MLD: Mean Lung Dose; ΔTRT COVID: Time between radiotherapy and COVID-19 diagnosis.
Fig. 2.
The dose–response curve for the multivariate hospitalization model in the validation data. The model included ARI, MHD and ΔTRT COVID. The solid blue line is the model; black quintiles represent data (error bars: 95% exact binomial confidence intervals). Above each quintile, the population average MHD and ΔTRT COVID are given along with the ARI rate.
Discussion
To date, this is the largest study evaluating the impact of RT on outcomes in COVID-19 patients by specifically assessing the role of cardiopulmonary dose. By using a rigorous modeling approach, we found that a history of acute renal injury (ARI), the time between RT and COVID-19 diagnosis (ΔTRT COVID) and mean heart dose (MHD) were associated with the risk of hospitalization due to COVID-19. A similar relationship was, however, not established with life-threatening COVID-19 disease or death. Based on data from 107 patients, an early analysis from another NYC-based hospital found MLD to be associated with COVID-19 mortality [8]. However, that study did not assess the impact of heart dose, patient comorbidities or other characteristics that have previously been established to impact COVID-19 outcomes. In summary, while the interactions between patient comorbidities, cancer therapies, and COVID-19 are complex, our results based on data from 350 patients suggest the need for close monitoring in patients with a history of recent thoracic radiation, but do not support withholding RT in patients with cancer to reduce the risk for life-threatening COVID-19 outcomes.
Conflict of interest statement
Funding: MSKCC Cancer Center Support Grant, Principal Investigator (Thompson). Agency: NIH/NCI, 5 P30 CA008748-54. National Institutes of Health [T32-CA009207 to JL, K30-UL1TR00457 to JL]; and the Conquer Cancer Foundation [Young Investigator Award to JL].
Disclosure: JL: Reports honoraria from Targeted Oncology and Physicians' Education Resource. DRG: Reports honoraria from Merck, Bristol Myers Squibb, AstraZeneca, Reflexion, Medscape, Vindico, US Oncology, and Varian. Reports research support from Merck, Bristol Myers Squibb, AstraZeneca and Varian. Serves on advisory board for AstraZeneca. All remaining authors have declared no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2021.06.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y.u., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garassino M.C., Whisenant J.G., Huang L.-C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee L.YW., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.A., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Rizvi H, Preeshagul IR, Egger JV, Hoyos D, Bandlamudi C, et al. COVID-19 in patients with lung cancer. Ann Oncol 2020:S0923-7534(20)39894-X. [DOI] [PMC free article] [PubMed]
- 7.Moons K.G.M., Altman D.G., Reitsma J.B., Ioannidis J.P.A., Macaskill P., Steyerberg E.W., et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162 doi: 10.7326/M14-0698. W1-73. [DOI] [PubMed] [Google Scholar]
- 8.Kabarriti R., Brodin N.P., Maron M.I., Tomé W.A., Halmos B., Guha C., et al. Extent of prior lung irradiation and mortality in COVID-19 patients with a cancer history. Adv Radiat Oncol. 2020;5:707–710. doi: 10.1016/j.adro.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.