Abstract
BACKGROUND:
Arterial stiffness is thought to contribute to the pathophysiology of heart failure with preserved ejection fraction (HFpEF). We sought to examine arterial stiffness in HFpEF and hypertension and investigate associations of arterial and left ventricular hemodynamic responses to exercise.
METHODS:
A total of 385 symptomatic individuals with EF ≥ 50% underwent upright cardiopulmonary exercise testing with invasive hemodynamic assessment of arterial stiffness and load (aortic augmentation pressure, augmentation index, systemic vascular resistance index, total arterial compliance index, effective arterial elastance index, and pulse pressure amplification) at rest and during incremental exercise. An abnormal hemodynamic response to exercise was defined as a steep increase in pulmonary capillary wedge pressure relative to cardiac output (ΔPCWP/ΔCO > 2 mmHg/L/min). We compared rest and exercise measures between HFpEF and hypertension in multivariable analyses.
RESULTS:
Among 188 HFpEF participants (age 61±13, 56% women), resting arterial stiffness parameters were worse compared to 94 hypertensive participants (age 55 ± 15, 52% women); these differences were accentuated during exercise in HFpEF (all p≤0.0001). Among all participants, exercise measures of arterial stiffness correlated with worse ΔPCWP/ΔCO. Specifically, a 1-SD higher exercise augmentation pressure was associated with 2.15-fold greater odds of abnormal LV hemodynamic response (95% CI 1.52–3.05, p<0.001). Further, exercise measures of systemic vascular resistance index, elastance index, and pulse pressure amplification correlated with lower peak VO2.
CONCLUSIONS:
Exercise accentuates elevated arterial stiffness in HFpEF, which in turn correlate with left ventricular hemodynamic responses. Unfavorable ventricular-vascular interactions during exercise in HFpEF may contribute to exertional intolerance and inform future therapeutic interventions.
Keywords: Arterial stiffness, arterial load, HFpEF, exercise capacity, heart failure, hypertension
Graphical Abstract:
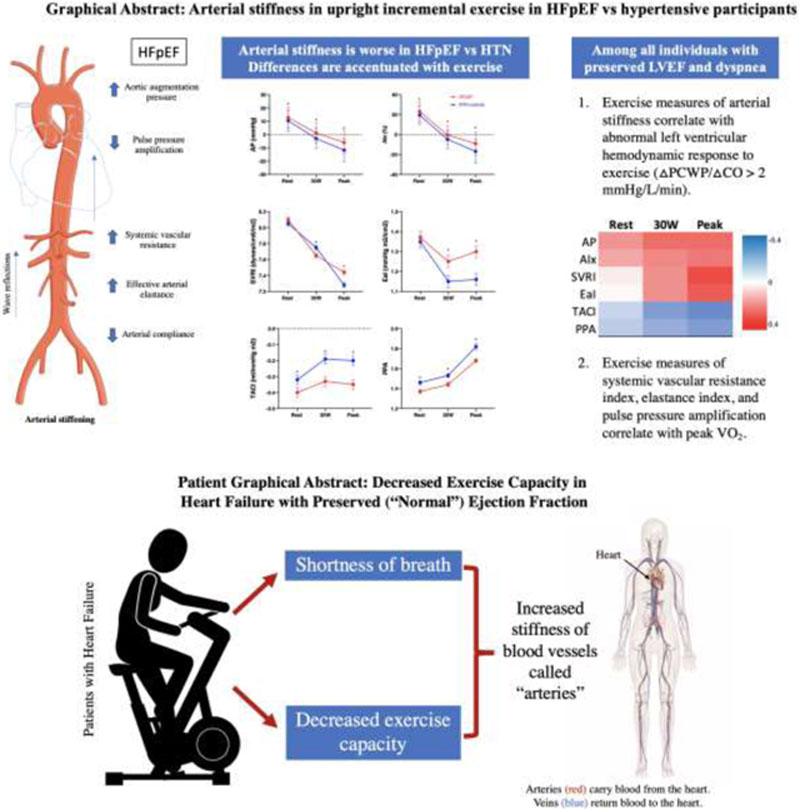
Arterial stiffness and load are accentuated with exercise in heart failure with preserved ejection fraction compared with hypertensive participants and correlate with left ventricular hemodynamic responses to upright incremental exercise. Patients with heart failure exercised on a stationary bicycle while we measured their heart and lung function and their blood pressure. Compared with patients with high blood pressure, patients with heart failure had stiffer blood vessels. Stiffer blood vessels may be related to the shortness of breath and difficulty exercising experienced by patients with heart failure.
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is an increasingly common condition with no known effective treatments, in no small part due to substantial phenotypic heterogeneity that challenges broad therapeutic approaches.(1–3) A hallmark of HFpEF is exertional intolerance. The pathophysiology of reduced exercise capacity in HFpEF is multifactorial, with contributions from chronotropic incompetence, impaired stroke volume augmentation, abnormal ventricular-arterial coupling and impaired peripheral oxygen extraction during exercise.(4–10) None of these putative mechanisms of exercise intolerance are adequately characterized during rest alone, though repeated measurements during exercise, in contrast, permit quantification of the deficits and their contribution to exercise intolerance. Several recent studies have shown that exercise may uncover abnormal physiologic responses such as elevated cardiac filling pressures that are not evident at rest.(11,12) Increased arterial stiffness and arterial load, out of proportion from aging or chronic hypertension alone, have been recognized as important contributors to the hemodynamic abnormalities observed in HFpEF.(13–16)
The extent to which measures of arterial stiffness and load are increased in HFpEF during exercise and their relationship to continuous invasive cardiac hemodynamic measures during exercise remains unclear. While a prior study correlated supine submaximal exercise to invasive hemodynamics in the HFpEF population, no studies to date have evaluated whether arterial waveform abnormalities during continuous upright exercise relate to exercise capacity.(15) Utilizing a comprehensively phenotyped sample of patients undergoing cardiopulmonary exercise testing (CPET) with continuous invasive arterial waveform assessment and hemodynamic monitoring, we sought to 1) examine resting and exercise arterial waveform measures that reflect arterial stiffness and pulsatile and non-pulsatile arterial load among symptomatic HFpEF patients and hypertensive controls and 2) determine the association of arterial waveform measures during exercise with left ventricular (LV) hemodynamic responses to exercise and exercise capacity. We hypothesized that abnormalities in measures of arterial stiffness at rest would be accentuated during exercise provocation in relation to hypertensive controls. Further, we also hypothesized that abnormal arterial stiffness and load during exercise would be directly associated with worse exercise capacity and abnormal rise in LV filling pressures during exercise, suggesting unfavorable ventricular-vascular interaction as a potential mechanism underlying exertional intolerance in HFpEF.
METHODS:
Study Sample
The study sample included 483 consecutive patients with exertional dyspnea (New York Heart Association class II-IV symptoms) and left ventricular ejection fraction (LVEF) ≥50% who underwent clinically indicated cardiopulmonary exercise testing to maximal effort (defined as respiratory exchange ratio >1.0) with invasive hemodynamic monitoring and arterial stiffness measurements at Massachusetts General Hospital between 2009 to 2017. Participants were excluded if they had one or more of the following: pulmonary arterial hypertension in the absence of left heart disease (n=8), history of heart or lung transplantation (n=9), complex adult congenital heart disease (n=2), mitochondrial disease (n=13), undergoing evaluation for lung transplant (n=7), moderate or greater aortic or mitral valve disease or prior valve replacement (n=39), or oxygen-dependent lung disease (n=18), leaving 385 participants for analysis. Informed consent was obtained from all participants, and the Massachusetts General Hospital Institutional Review Board approved the study.
Cardiopulmonary Exercise Testing
All participants underwent insertion of a pulmonary artery catheter via the internal jugular vein and systemic arterial catheter via the radial artery, followed by maximal upright cycle ergometry at 5 to 15 W/min continuous ramp after an initial 3-minute period of unloaded exercise as previously described.(17) Serial gas exchange (MedGraphics, St. Paul, MN) and minute-by-minute hemodynamic measures were assessed during exercise. Hemodynamic measures included pulmonary capillary wedge pressure (PCWP), pulmonary artery (PA) pressure, and cardiac output calculated utilizing the direct Fick method. Pulmonary artery catheter measurements were obtained at end-expiration.
HFpEF was defined as impaired peak VO2 (<80% predicted) with either (1) elevated PCWP at rest (supine PCWP ≥ 15mmHg) or (2) abnormally steep increase in PCWP relative to cardiac output (CO) during exercise (ΔPCWP/ΔCO slope > 2.0 mmHg/L/min with peak PCWP ≥ 15mmHg, calculated using multi-point measures of PCWP and CO during CPET, average of 10±2 measures per individual) as previously described.(18,19) The hypertensive control group included individuals with normal rest PCWP, exercise ΔPCWP/ΔCO slope ≤ 2.0 mmHg/L/min, and elevated resting blood pressure (≥140/90mmHg) or treatment with anti-hypertensive medication. Participants who were not classified as either HFpEF (n=188) or HTN (n=94) were included in analyses examining the association of arterial stiffness parameters with PCWP/CO slope and peak VO2 among the entire cohort of 385 participants with exertional dyspnea. In sensitivity analyses, we reclassified HFpEF using the following hemodynamic criteria: PCWP/CO slope >2 mmHg/L/min and peak PCWP ≥25mmHg as per previous studies(15,20).
Arterial Stiffness and Load Assessment
Peripheral arterial pressure measures were ascertained using invasive radial arterial catheter. Central aortic pressure waveforms at rest, 30 Watts, and peak exercise were derived from invasive radial artery pressure tracings using a mathematical transfer function as previously described and validated at rest and during exercise (SphygmoCor, Atcor Medical).(21,22) Pressure tracings were additionally manually reviewed for quality control. Surrogate measures of arterial stiffness were calculated using simultaneously measured hemodynamic parameters. Higher values of augmentation pressure (AP) and augmentation index (Aix) indicate greater wave reflection and late-systolic load.(23) Non-pulsatile load, or the steady component of vascular resistance, was quantified by systemic vascular resistance index (SVRI=80*[mean arterial pressure – right atrial pressure]/cardiac index). Further parameters measured included: pulse pressure amplification (PPA=peripheral-to-central PP ratio; decreased with increased aortic wave reflection), total arterial compliance index (TACI = stroke volume index/central pulse pressure; a metric of aortic stiffness), and effective arterial elastance index (EaI = end systolic pressure/stroke volume index; dependent on resistive load and heart rate).(24,25) Worse overall arterial stiffness is indicated by a higher effective arterial elastance index and lower total arterial compliance index and pulse pressure amplification. We lump these parameters together as “arterial stiffness” for ease of description, acknowledging that overall left ventricular afterload is composed of a steady resistive component and a pulsatile component described by parameters including systemic vascular resistance, arterial compliance, impedance, and wave reflection amplitude.(23)
Clinical and Biomarker Assessment:
At the time of CPET, participants underwent history and physical examination, measurement of body mass index (BMI), and fasting blood draw, including N-terminal pro-B type natriuretic peptide (Roche, NT-proBNP, intra-assay coefficient of variation 2.4–3.8%) and high-sensitivity C-reactive protein (Roche, hsCRP, intra-assay coefficient of variation 0.4–8.4%). Blood specimens were immediately processed and stored at −80°C. Echocardiography within one year of CPET was utilized for abstraction of LVEF and presence of structural heart disease (left ventricular hypertrophy, left atrial enlargement, and diastolic dysfunction).(26)
Statistical Analysis:
Baseline clinical characteristics were summarized for the total sample. HFpEF and HTN subgroups were compared using t-tests or Chi2 tests as appropriate. Arterial stiffness parameters at rest, 30W, and peak exercise were summarized using raw means and adjusted means accounting for age, sex, BMI, and presence of diabetes mellitus (DM). Aortic augmentation pressure and index were additionally adjusted for heart rate.(27) We examined the association of arterial stiffness parameters with peak VO2, and PCWP/CO slope as a measure of LV hemodynamic response using partial correlation coefficients first adjusting for age and sex (and heart rate for augmentation pressure and index), then further adjusting for BMI and DM as covariates. Our primary outcomes were augmentation pressure and index at rest and 30W of exercise between HFpEF and HTN, yielding four comparisons with a Bonferroni correction for significance of p=0.0125. In secondary analyses, we examined associations of arterial stiffness with abnormal PCWP/CO slope. We evaluated whether HFpEF status modifies the association of arterial stiffness with outcomes at peak exercise (PCWP/CO slope and peak VO2) utilizing an interaction term (HFpEF*arterial stiffness parameter) in multivariable Cox models. Data were log-transformed where necessary. Analyses were conducted using STATA v. 15.1 (College Station, TX). A p value of < 0.05 was considered significant.
RESULTS
Of 385 individuals with exertional dyspnea and LVEF≥50% who underwent CPET with arterial stiffness assessment, the mean age was 56±15 years, and 58% were women (Table 1). Among these individuals, 188 had HFpEF (age 61±13 years, 56% women), and 94 met criteria for the hypertensive control group (age 55±15 years, 52% women). Participants with HFpEF had a greater burden of comorbid conditions, including diabetes mellitus (21%), obesity (53%) and prior cardiovascular hospitalization (39%) compared with hypertensive controls (P<0.05 for all). Compared with hypertensive controls, the HFpEF group had a higher median NT-proBNP concentration (99 pg/mL [46–260 pg/mL] vs 42 pg/mL [23–113 pg/mL], p=0.0001).
Table 1.
Clinical characteristics and CPET parameters of HFpEF and hypertensive control participants
| Total sample n=385 | HFpEF subgroup n=188 | HTN control subgroup n=94 | P-value* | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Women, n (%) | 224 (58) | 106 (56) | 49 (52) | 0.50 |
| Age, years | 56 ± 15 | 61 ± 13 | 55 ± 15 | 0.001 |
| BMI, kg/m2 | 29.5 ± 6.5 | 31.3 ± 6.7 | 27.1 ± 5.1 | <0.0001 |
| Hypertension, n (%) | 313 (81) | 170 (90) | 94 (100) | 0.002 |
| Diabetes Mellitus, n (%) | 62 (16) | 40 (21) | 9 (9) | 0.01 |
| Current Smoker, n (%) | 13 (4) | 4 (2) | 4 (4) | 0.31 |
| Prior MI, n (%) | 17 (4) | 10 (5) | 4 (4) | 0.70 |
| Prior CV admission, n (%) | 121 (31) | 74 (39) | 21 (22) | 0.004 |
| Prior HF admission, n (%) | 23 (6) | 14 (7) | 4 (4) | 0.14 |
| Diuretic, n (%) | 109 (28) | 76 (40) | 14 (15) | <0.001 |
| Beta blocker use, n (%) | 121 (31) | 79 (42) | 26 (28) | <0.001 |
| Nitrate use, n (%) | 18 (6) | 16 (9) | 2 (2) | 0.02 |
| Calcium channel blocker use, n (%) | 58 (15) | 29 (16) | 20 (21) | 0.008 |
| ACEi or ARB use, n (%) | 104 (27) | 60 (32) | 28 (30) | <0.001 |
| NT-proBNP, pg/mL, median (IQR) | 67 (30–167) | 99 (46–260) | 42 (23–113) | 0.0001 |
| CRP, pg/mL, median (IQR) | 1.8 (0.8–4.9) | 3.0 (1.2–5.9) | 1.3 (0.5–3.0) | 0.0001 |
| Echocardiographic characteristics | ||||
| LVEF, % | 65 ± 7 | 65 ± 8 | 65 ± 6 | 0.40 |
| LV hypertrophy | 20% | 28% | 17% | 0.047 |
| Left atrial enlargement | 32% | 40% | 27% | 0.05 |
| Diastolic dysfunction | 32% | 40% | 26% | 0.04 |
| CPET characteristics | ||||
| Peak VO2, ml/kg/min | 17.2 ± 5.2 | 15.1 ± 3.7 | 20.7 ± 4.8 | 0.0001 |
| % pred VO2, Wasserman, % | 76.7 ± 16.3 | 75.1 ± 15.5 | 84.8 ± 14.1 | 0.0001 |
| VE/VCO2 slope | 36.2 ± 8.3 | 36.8 ± 8.6 | 34.9 ± 6.6 | 0.10 |
| Max work, watts | 107 ± 38 | 98 ± 35 | 126 ± 37 | 0.0001 |
| Respiratory exchange ratio | 1.15 ± 0.1 | 1.15 ± 0.1 | 1.15 ± 0.08 | 0.73 |
| Stroke volume reserve | 1.35 + 0.78 | 1.38 + 1.05 | 1.35 + 0.28 | 0.11 |
| PCWP/CO slope, mmHg/L/min | 2.1 ± 1.6 | 3.1 ± 1.7 | 1.2 ± 0.4 | <0.0001 |
Table displays mean ± standard deviation unless otherwise specified
P-value for HFpEF vs HTN control comparison
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CO, cardiac output; CRP, C-reactive protein; CV, cardiovascular; HTN, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCWP, pulmonary capillary wedge pressure; RER, respiratory exchange ratio; VE/VCO2 slope, ventilator efficiency; and VO2, oxygen consumption.
Exercise capacity is worse in HFpEF compared to hypertensive controls
HFpEF participants had lower peak VO2 (15.1±3.7 ml/kg/min) compared with hypertensive controls (20.7±4.8 ml/kg/min), with correspondingly lower % predicted peak VO2 and maximum work achieved (Table 1). There were no differences between HFpEF and hypertensive control groups in resting heart rate, resting or peak systolic blood pressure, or cardiac output (Table 2). Peak heart rate was lower among HFpEF participants (130±25 beats/minute) compared to hypertensive controls (147±21 beats/min). As expected, individuals with HFpEF had steeper PCWP/CO slopes (3.1±1.7 mmHg/L/min) compared to hypertensive controls (1.2±0.4 mmHg/L/min, P<0.05).
Table 2.
Exercise parameters among HFpEF and hypertensive participants
| HFpEF | HTN control | |||||
|---|---|---|---|---|---|---|
| Rest | 30W | Peak | Rest | 30W | Peak | |
| Hemodynamic parameters | ||||||
| SBP, mmHg | 152 ± 22 | 168 ± 28 | 192 ± 30 | 150 ± 19 | 161 ± 25 | 192 ± 22 |
| DBP, mmHg | 77 ± 12* | 81 ± 14 | 91 ± 18 | 80 ± 10 | 83 ± 12 | 92 ± 13 |
| HR, bpm | 75 ± 14 | 96 ± 18 | 126 ± 25* | 78 ± 14 | 96 ± 16 | 142 ± 23 |
| PCWP, mmHg | 15 ± 6* | 15 ± 6* | 25 ± 7* | 10 ± 3 | 9 ± 3 | 17 ± 5 |
| CO, L/min | 5.2 ± 1.5* | 8.1 ± 2.2 | 11.0 ± 3.2* | 5.5 ± 1.5 | 8.3 ± 1.9 | 13.3 ± 2.9 |
denotes p<0.05 difference between HFpEF and HTN controls. Table displays raw values.
Abbreviations: CO, cardiac output; DBP, diastolic blood pressure; HR, heart rate; HTN, hypertension; PCWP, pulmonary capillary wedge pressure; and SBP, systolic blood pressure.
Exercise unmasks greater arterial stiffness in HFpEF compared with hypertensive controls
Rest and exercise hemodynamic and arterial stiffness parameters are displayed in Tables 2 and 3 and Figure 1. Figure 1 and Table 3 present adjusted means after accounting for age, sex, BMI, and DM (and heart rate where applicable) of primary outcomes augmentation pressure and index at rest and 30W and secondary outcomes of compliance index, systemic vascular resistance index, elastance index and pulse pressure amplification throughout exercise. At rest, there were no differences in SVRI or EaI between HFpEF participants and hypertensive controls (P>0.05), but other resting measures of arterial stiffness (augmentation pressure and index, compliance index, and pulse pressure amplification) were significantly worse amongst HFpEF participants (P<0.05 for all in multivariable-adjusted analyses, Table 3). Progression to 30W and peak exercise further delineated differences between the two groups, with more abnormal measures of all parameters of arterial stiffness (P≤0.0001 for all at peak) among patients with HFpEF. Specifically, at peak exercise, adjusted augmentation pressure was 5.75 mmHg higher among HFpEF patients vs hypertensive controls, with an absolute difference of 7.7% in augmentation index. In sensitivity analyses using PCWP/CO slope >2 mmHg/L/min and peak PCWP ≥25mmHg to define HFpEF (79% of original HFpEF definition), we found no substantive differences when compared with primary results. Specifically, we again show worse arterial stiffness among HFpEF patients at 30W and peak exercise, with no differences among resting measures (Supplemental Table 1).
Table 3.
Adjusted arterial stiffness and load parameters during exercise among HFpEFand hypertensive participants
| HFpEF | HTN control | P-value | |
|---|---|---|---|
| AP (mmHg) | |||
| Rest | 12.80 (7.8) | 10.39 (7.9) | p=0.05 |
| 30w | 1.15 (7.6) | −3.01 (7.6) | p=0.0003 |
| Peak | −6.00 (8.6) | −11.75 (8.6) | p=0.0001 |
| Aix (%) | |||
| Rest | 23.14 (9.2) | 19.55 (9.3) | p=0.01 |
| 30w | 0.45 (9.3) | −4.83 (9.3) | p=0.0002 |
| Peak | −9.06 (11.6) | −16.81 (11.6) | p=0.0001 |
| SVRI (dynes-sec*m2/cm5) | |||
| Rest | 8.10 (0.03) | 8.05 (0.03) | p=0.14 |
| 30w | 7.65 (0.03) | 7.75 (0.03) | p=0.005 |
| Peak | 7.44 (0.03) | 7.28 (0.03) | p<0.0001 |
| EaI (mmHg*m2/ml) | |||
| Rest | 1.37 (0.03) | 1.35 (0.03) | p=0.61 |
| 30w | 1.25 (0.03) | 1.15 (0.03) | p=0.004 |
| Peak | 1.30 (0.03) | 1.16 (0.03) | p=0.0001 |
| TACI ml/mmHg*m2) | |||
| Rest | −0.40 (0.03) | −0.32 (0.03) | p=0.03 |
| 30w | −0.33 (0.03) | −0.19 (0.03) | p=0.0002 |
| Peak | −0.35 (0.03) | −0.20 (0.04) | p<0.0001 |
| PPA (mmHg) | |||
| Rest | 1.37 (0.02) | 1.46 (0.02) | p=0.001 |
| 30w | 1.44 (0.02) | 1.53 (0.02) | p=0.0006 |
| Peak | 1.68 (0.02) | 1.82 (0.03) | p<0.0001 |
Values are expressed as mean (standard error), and values are adjusted for age, sex, body mass index, and presence of diabetes mellitus. AP and Aix also are adjusted for heart rate. Abbreviations: Aix, augmentation index; AP, aortic augmentation pressure; EaI, elastance index; HTN, hypertension; PPA, pulse pressure amplification; SVRI, systemic vascular resistance index; and TACI, total arterial compliance index.
Figure 1.
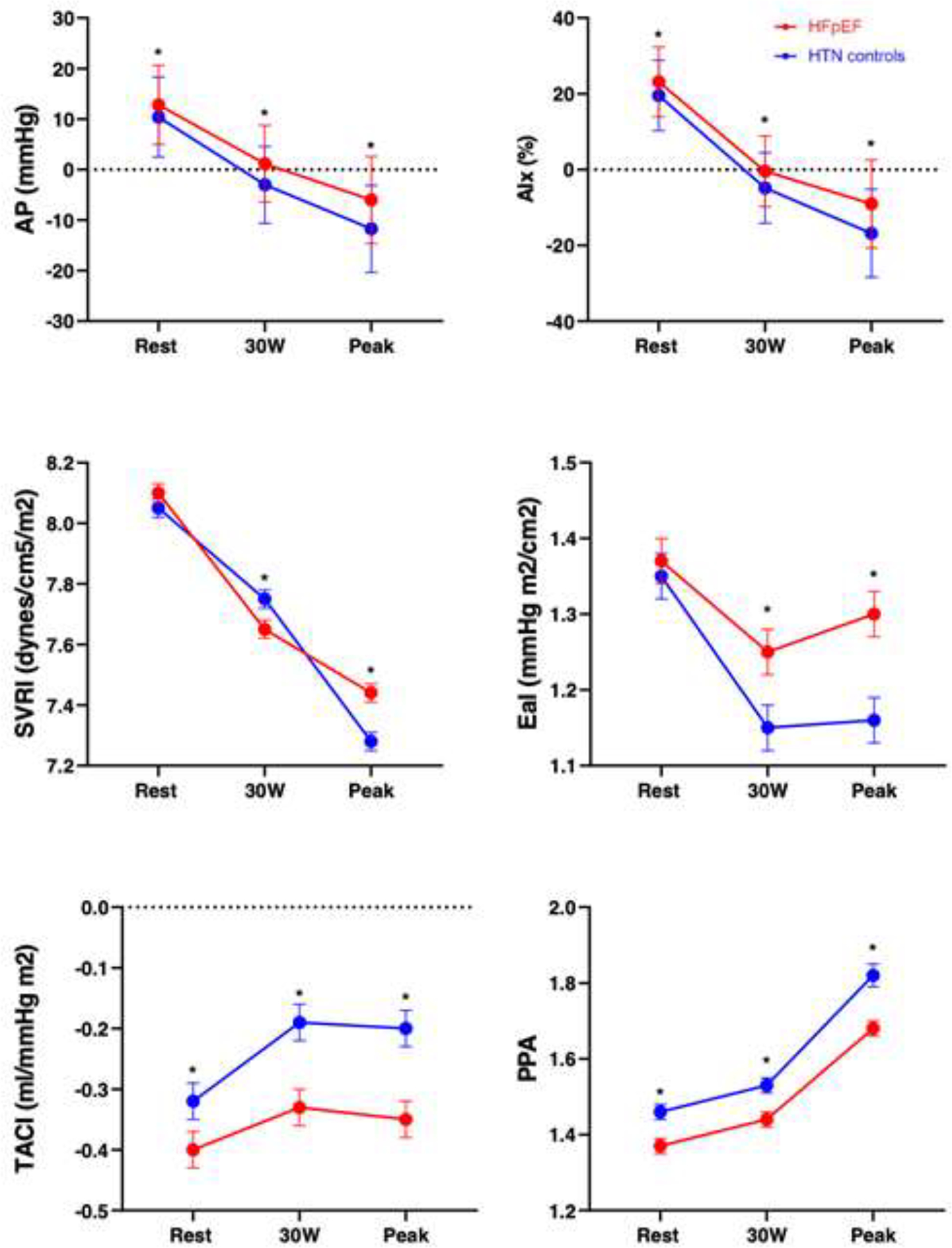
Arterial stiffness and load parameters at rest, 30W, and peak exercise in HFpEF participants and hypertensive controls. * denotes p<0.05 difference between HFpEF and hypertensive controls.
Abbreviations: Aix, augmentation index; AP, aortic augmentation pressure; EaI, elastance index; HTN, hypertension; PPA, pulse pressure amplification; SVRI, systemic vascular resistance index; and TACI, total arterial compliance index.
Measures of arterial stiffness correlate with left ventricular hemodynamic response to exercise and exercise capacity
We next examined the association of arterial stiffness with LV hemodynamic responses with exercise among all individuals with exertional dyspnea and LVEF≥50% who underwent CPET (n=385) (Table 4, Figure 2). Resting measures of arterial stiffness (augmentation pressure and index, compliance index, and pulse pressure amplification) were associated with PCWP/CO slope (augmentation pressure, r=0.17, p=0.001; augmentation index, r=0.14, p=0.008; compliance index, r=−0.10, p=0.04; pulse pressure amplification, r=−0.12, p=0.02), whereas no association was found with systemic vascular resistance index or elastance index. Compared to resting assessments, both low-level exercise (30W) and peak exercise revealed consistently stronger correlations between all measures of arterial stiffness with PCWP/CO slope (at peak: augmentation pressure, r=0.24; augmentation index, r=0.22; systemic vascular resistance index, r=0.30, elastance index, r=0.27, compliance index, r=−0.27, pulse pressure amplification, r=−0.23; p<0.0001 for all). In exploratory analyses, we found that the association of exercise arterial stiffness and PCWP/CO slope was apparent among individuals with HFpEF, whereas these associations were not significant among hypertensive controls (Supplemental Table 2, Supplemental Figure 1). In the test for interaction of HFpEF status with arterial stiffness parameter, at peak exercise, HFpEF status modified the association of augmentation index, systemic vascular resistance index, elastance index, and compliance index with PCWP/CO slope (p ≤0.02 for all).
Table 4.
Association of arterial stiffness and load with exercise cardiac performance, including PCWP/CO slope and peak VO2
| Rest r (P-value) | 30W r (P-value) | Peak r (P-value) | |
|---|---|---|---|
| PCWP/CO slope | |||
| AP | 0.17 (0.001) | 0.24 (<0.0001) | 0.24 (<0.0001) |
| Aix | 0.14 (0.008) | 0.19 (0.003) | 0.22 (<0.0001) |
| SVRI | 0.03 (0.60) | 0.18 (0.001) | 0.30 (<0.0001) |
| EaI | 0.01 (0.78) | 0.18 (0.0007) | 0.27 (<0.0001) |
| TACI | −0.10 (0.04) | −0.23 (<0.0001) | −0.27 (<0.0001) |
| PPA | −0.12 (0.02) | −0.19 (0.0003) | −0.23 (<0.0001) |
| Peak VO2 | |||
| AP | −0.05 (0.29) | −0.05 (0.30) | −0.08 (0.14) |
| Aix | −0.09 (0.09) | −0.07 (0.22) | −0.07 (0.15) |
| SVRI | −0.10 (0.06) | −0.08 (0.17) | −0.29 (<0.0001) |
| EaI | −0.11 (0.04) | −0.13 (0.02) | −0.13 (0.01) |
| TACI | 0.10 (0.049) | 0.12 (0.03) | 0.12 (0.03) |
| PPA | 0.04 (0.42) | 0.02 (0.70) | 0.23 (<0.0001) |
Values are expressed as partial correlation coefficient, r, (p-value). Values are adjusted for age, sex, body mass index, and presence of diabetes mellitus. AP and Aix also are adjusted for heart rate. Abbreviations: Aix, augmentation index; AP, aortic augmentation pressure; CO, cardiac output; EaI, elastance index; HTN, hypertension; PCWP, pulmonary capillary wedge pressure; PPA, pulse pressure amplification; SVRI, systemic vascular resistance index; TACI, total arterial compliance index; and VO2, oxygen consumption.
Figure 2.
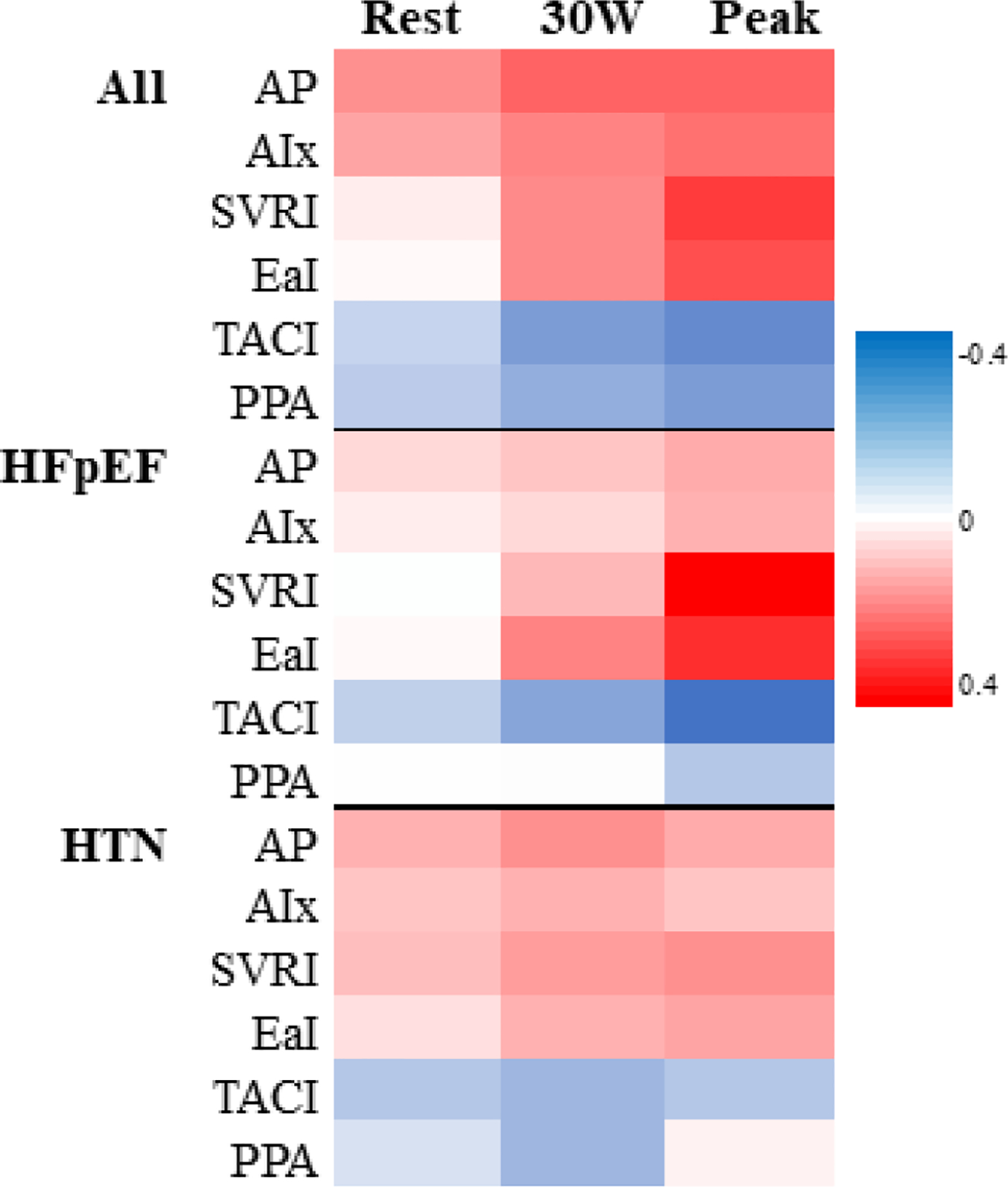
Arterial stiffness and load and PCWP/CO slope correlations during exercise. Analyses are adjusted for age, sex, body mass index, and presence of diabetes mellitus. Augmentation pressure and index are also adjusted for heart rate. The red and blue shading represent positive and negative correlations, respectively.
Abbreviations: Aix, augmentation index; AP, aortic augmentation pressure; EaI, elastance index; HTN, hypertension; PPA, pulse pressure amplification; SVRI, systemic vascular resistance index; and TACI, total arterial compliance index.
There were only modest correlations between rest measures of compliance and elastance indices with peak VO2 (compliance index, r=0.10, p=0.049; elastance index, r=−0.11, p=0.04). By contrast, exercise measures of systemic resistance and arterial stiffness were significantly associated with peak VO2 (systemic vascular resistance index, r=−0.29, p<0.0001; elastance index, r=−0.13, p=0.01; compliance index, r=0.12, p=0.03; pulse pressure amplification, r=0.23, P<0.0001). There were no significant correlations between augmentation pressure and index throughout exercise and peak VO2. HFpEF status only modified the association of systemic vascular resistance index with peak VO2 at peak exercise (p=0.006); tests for interaction with other arterial stiffness parameters were not significant.
In exploratory analyses, we also demonstrate correlations between all measures of arterial stiffness other than PPA and peak PCWP and delta PCWP (peak PCWP – rest PCWP); these correlations similarly strengthen from rest to low-level (30W) to peak exercise (Supplemental Table 3). Resting parameters of all measures of arterial stiffness correlate with stroke volume reserve (stroke volumepeak/stroke volumerest).
Arterial stiffness predictors of an abnormal PCWP/CO response to exercise
Among the whole sample (n=385 participants), we next examined the association of arterial stiffness with abnormal LV hemodynamic response to exercise, defined as an abnormally steep PCWP/CO slope > 2mmHg/L/min (Supplemental Table 4, Figure 3).(18,28) We found that most rest measures of arterial stiffness, other than elastance index and systemic vascular resistance index, were associated with abnormal PCWP/CO response to exercise. Specifically, a 1-SD higher augmentation pressure was associated with a 2-fold increased odds of having abnormally steep PCWP/CO response to exercise (multivariable-adjusted OR 1.98, 95% CI 1.42, 2.75, P=<0.001). Moreover, we found that arterial stiffness measures ascertained at 30W and peak exercise displayed increased odds of an abnormal PCWP/CO response for each individual parameter (P<0.001 for all). For example, a 1-SD higher systemic vascular resistance index at 30W and peak exercise was associated with 1.8- and 2.2-fold increased odds of abnormal PCWP responses to exercise, respectively (OR 1.8, 95% CI 1.35–2.38, p<0.001 at 30W; and OR 2.16, 95% CI 1.58–2.96, p<0.001 at peak exercise).
Figure 3.
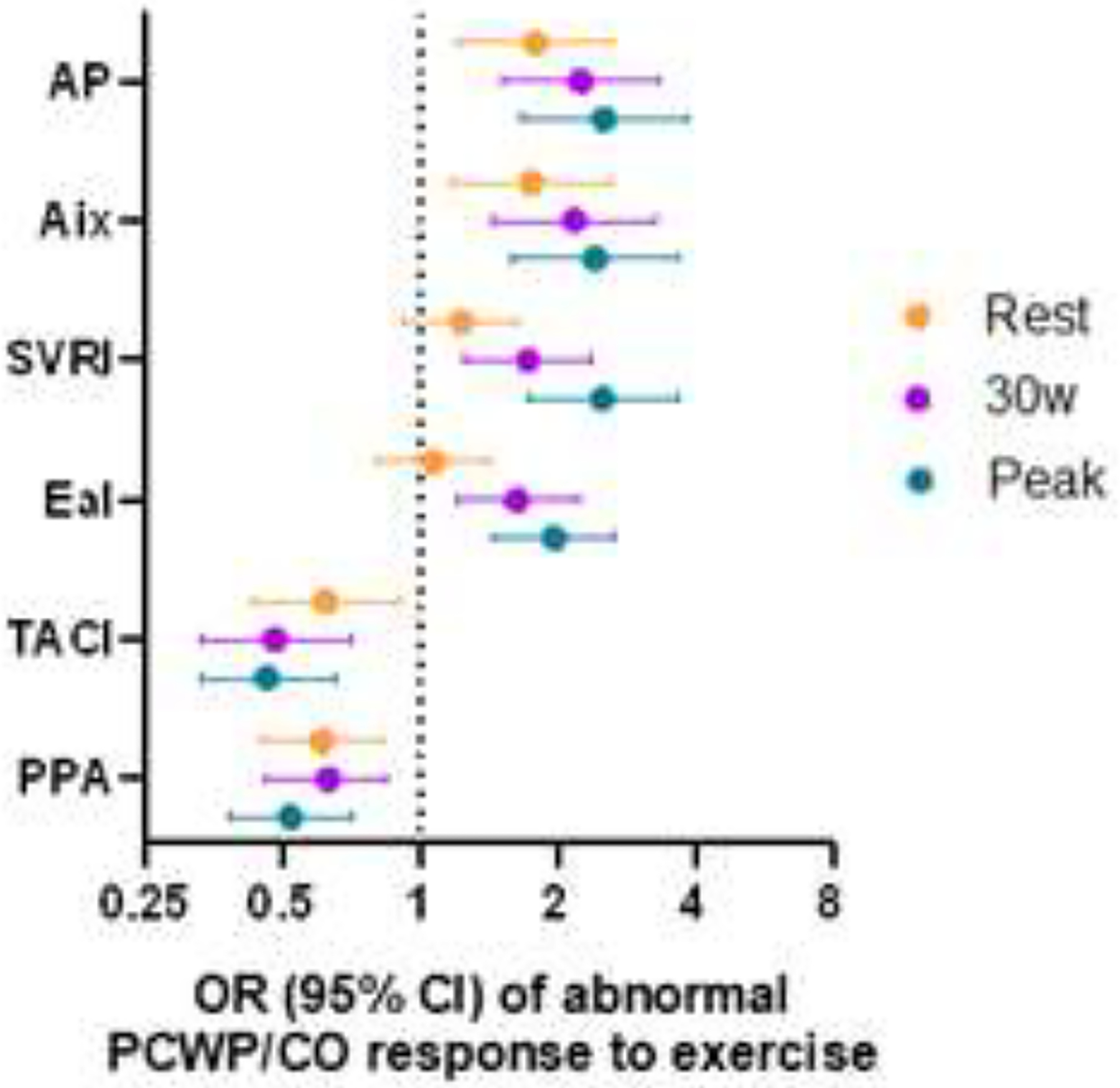
Parameters of arterial stiffness and arterial load are associated with abnormal PCWP/CO response to exercise. Odds ratio (OR) is per one-standard deviation increase in predictor variable.
Abbreviations: Aix, augmentation index; AP, aortic augmentation pressure; EaI, elastance index; HTN, hypertension; PPA, pulse pressure amplification; SVRI, systemic vascular resistance index; and TACI, total arterial compliance index.
DISCUSSION:
We ascertained continuous invasive measures of arterial stiffness during exercise in a large sample that included patients with hemodynamically-confirmed HFpEF and hypertensive controls. We demonstrate the following: (1) at rest, measures of arterial stiffness are worse in HFpEF participants compared with hypertensive controls; (2) exercise provocation accentuates differences in arterial stiffness among patients with HFpEF compared to hypertensive controls, even at low-level exercise; and (3) measures of arterial stiffness ascertained during exercise are directly related to an abnormal increase in PCWP during exercise and correlate with a lower peak VO2. Our data provide the most direct evidence to date that abnormal vascular responses to exercise may contribute to impaired cardiac performance during exercise and overall exercise intolerance in HFpEF.
Arterial stiffening has been previously highlighted as an important component to the diverse range of hemodynamic and metabolic abnormalities that may be observed in patients with HFpEF.(13–16) Higher wave reflection amplitude with increased late-systolic load to the left ventricle has been shown to be associated with myocardial hypertrophy and fibrosis, heart failure, and cardiovascular mortality.(23,29) In prior studies, resting arterial stiffness parameters were similar between HFpEF and hypertensive participants without HF.(30) Less is known about exercise responses: one prior study suggested abnormal ventriculoarterial coupling during exercise in 23 patients with HFpEF compared with 15 controls using noninvasive measures of vascular stiffness.(31) In a study by Reddy et al. of invasively measured arterial stiffness among individuals with HFpEF vs hypertensive controls, there were no differences in aortic pulse pressure or augmentation index at rest or during exercise, although other measures of arterial stiffness were worse with exercise in HFpEF and directly related to increased supine PCWP during exercise.(15) We extend these prior reports by studying a larger sample of individuals with HFpEF and hypertensive controls with detailed assessment of invasively measured arterial stiffness and hemodynamics during upright exercise. Upright exercise permits greater exposure to exercise and repeated serial measures of arterial stiffness and hemodynamics while permitting patients to achieve true peak VO2 (i.e. peak VO2 was 15ml/kg/min in HFpEF, consistent with previous HFpEF studies of upright exercise,(32) compared to 8.6 ml/kg/min in the one other study of invasive arterial waveform analysis(15)).
Our study is also unique in that we examined direct associations of arterial stiffness with multi-point measurements of PCWP/CO slope. We acknowledge that prior studies involving exercise hemodynamics in HFpEF participants have utilized a PCWP during exercise ≥ 25 mmHg as cutoff(15,20). However, utilization of flow-corrected definitions of HFpEF during exercise, such as the PCWP/CO slope, allows for capture of a prognostically significant integrated hemodynamic measure of left heart performance, preferable to an isolated measurement of PCWP alone during exercise.(18,19,33)
Our findings underscore important interactions between cardiac performance and the systemic vasculature as previously noted.(34) In our study, we assess arterial elastance, Ea, as a lumped measurement of the mean resistive and pulsatile components of afterload. While Ea has been shown to be dominated by the nonpulsatile systemic vascular resistance and heart rate with negligible influence by pulsatile afterload,(35) pulsatile afterload has shown to be most strongly correlated with left ventricular diastolic relaxation.(36) In our study, we demonstrate that arterial stiffness, quantified by surrogates of wave reflection and late systolic load (augmentation pressure and index and pulse pressure amplification), metrics of stiffness (total arterial compliance index and effective arterial elastance index), and nonpulsatile load (systemic vascular resistance index), are independently associated with abnormal left ventricular hemodynamic response to exercise (the PCWP/CO slope), as well as the absolute peak PCWP and difference in PCWP between rest and peak exercise. These associations are unmasked or accentuated at low intensity exercise to 30W, providing opportunity for more widespread practical assessment of arterial stiffness as a diagnostic strategy in patients with dyspnea on exertion. Whether pulsatile or nonpulsatile components of arterial stiffness and load may identify high risk patients and guide therapies remains to be studied.
In addition, this is the first study to demonstrate that measures of arterial stiffness during exercise are related to peak VO2, even after adjustment for age, sex, BMI, and diabetes status. A prior study on exercise training in HFpEF demonstrated improvement in peak VO2 without change in flow-mediated arterial dilation or carotid artery distensibility (37), though other measures of arterial load were not directly assessed. As peak VO2 carries prognostic value in HFpEF(38), future studies may examine whether measuring surrogates of arterial stiffness similarly identifies patients with HFpEF at highest risk of adverse outcomes.
Beyond lending further insights into the pathophysiology of HFpEF, understanding contributions of arterial stiffness and load may inform therapeutic strategies.(39,40) Prior studies have highlighted that central pressures are higher in individuals with cardiovascular risk factors or disease,(41) respond differently to antihypertensive therapy,(42,43) and more strongly correlate with cardiovascular outcomes than peripheral pressures.(44–47) As a proof-of-concept, in patients with heart failure with reduced ejection fraction (HFrEF), aortic waveform-guided medical therapy as compared to standard brachial cuff pressures improved exercise capacity and functional status.(48,49) Whether these findings can be extrapolated to the HFpEF population has yet to be determined, though specific vasoactive agents are known to target large artery stiffness, including inorganic nitrites and nitrates in the HFpEF population (15,50) and phosphodiesterase type 5 inhibition, ivabradine, and sodium-glucose cotransport 2 inhibitors across broader samples (51–54). Whether phenotype-specific selection of HFpEF participants with the highest degree of arterial stiffness may help target vasoactive interventions in the future remains to be seen (40).
There are several limitations to this study. We included consecutive ambulatory participants referred for CPET in the context of chronic dyspnea on exertion. In this setting, we acknowledge that our HFpEF group may represent participants earlier in the disease course with hemodynamic abnormalities during exercise only (evidenced for example by only modest elevations in natriuretic peptide levels, only 40% diuretic use, and low prevalence of prior HF hospitalizations), and that generalizability to broader populations is unclear. Clinical heterogeneity among HFpEF samples may explain potential differences in findings of abnormal arterial stiffness during rest between HFpEF and hypertensive controls compared to prior studies. We did not adjust for use of antihypertensive agents which may have differential effects on ventricular arterial coupling.(55) We do not have advanced echocardiographic or magnetic resonance imaging data on these participants, limiting our ability to correlate arterial stiffness parameters to advanced non-invasive imaging parameters. It is also important to note that the associations of arterial stiffness and abnormal PCWP response to exercise and peak VO2 are not necessarily causal as ours is an observational study. Regardless of these limitations, one of the strengths of our study was the rigorous ascertainment of invasive arterial stiffness and hemodynamic measurements throughout exercise, facilitating multi-point analysis of PCWP/CO responses rather than a single static measurement.
In sum, we demonstrate that patients with HFpEF appear to have greater vascular stiffness compared with hypertensive controls, and that these differences in vascular stiffness are particularly pronounced during exercise provocation. We also show that worse arterial stiffness is associated with abnormal increases in PCWP relative to CO with exercise. We also demonstrate that systemic vascular resistance, pulse pressure amplification, and arterial elastance are correlated with lower peak VO2. Our findings support the clinical relevance of arterial stiffness as a contributor to the hallmark exertional intolerance and abnormal LV hemodynamic exercise responses in HFpEF. Future studies are needed to examine whether targeted therapeutic interventions against increased arterial stiffness may alter symptomatology and clinical outcomes in the HFpEF population.
Supplementary Material
Funding:
This work was supported by grants from the NIH/NHLBI R01-HL134893 (JEH), R01-HL140224 (JEH), K24-HL153669 (JEH), R01-HL131029 (GDL), the American Heart Association 15GPSGC24800006 (GDL) and the Heart Failure Research Innovation Fund (GDL).
Disclosures:
Dr. Ho has received research grants from Gilead Sciences and Bayer, and research supplies from EcoNugenics.
ABBREVIATIONS:
- Aix
aortic augmentation index
- AP
aortic augmentation pressure
- CO
cardiac output
- CPET
cardiopulmonary exercise testing
- EaI
effective arterial elastance index
- HFpEF
heart failure with preserved ejection fraction
- PCWP
pulmonary capillary wedge pressure
- PPA
pulse pressure amplification
- SVRI
systemic vascular resistance index
- TACI
total arterial compliance index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D et al. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail 2018;6:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho JE, Zern EK, Wooster L et al. Differential Clinical Profiles, Exercise Responses and Outcomes Associated with Existing HFpEF Definitions. Circulation 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlaug BA, Melenovsky V, Russell SD et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 5.Dhakal BP, Malhotra R, Murphy RM et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of Nondiastolic Factors to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2016;67:659–670. [DOI] [PubMed] [Google Scholar]
- 8.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010;56:855–63. [DOI] [PubMed] [Google Scholar]
- 9.Molina AJ, Bharadwaj MS, Van Horn C et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail 2016;4:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermann D, Kasner M, Steendijk P et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008;117:2051–60. [DOI] [PubMed] [Google Scholar]
- 11.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfs S, Zeh W, Hochholzer W et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 13.Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail 2009;15:658–64. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension 2013;61:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy YNV, Andersen MJ, Obokata M et al. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol 2017;70:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber T, Wassertheurer S, O’Rourke MF et al. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol 2013;61:1874–83. [DOI] [PubMed] [Google Scholar]
- 17.Houstis NE, Eisman AS, Pappagianopoulos PP et al. Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisman AS, Shah RV, Dhakal BP et al. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs G, Herve P, Barbera JA et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017;50. [DOI] [PubMed] [Google Scholar]
- 20.Reddy YNV, Obokata M, Wiley B et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001;38:932–7. [DOI] [PubMed] [Google Scholar]
- 22.Sharman JE, Lim R, Qasem AM et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006;47:1203–8. [DOI] [PubMed] [Google Scholar]
- 23.Townsend RR, Wilkinson IB, Schiffrin EL et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chemla D, Hebert JL, Coirault C et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 1998;274:H500–5. [DOI] [PubMed] [Google Scholar]
- 25.Kelly RP, Ting CT, Yang TM et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86:513–21. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Adamopoulos S, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525 Pt 1:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho JE, Zern EK, Wooster L et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated With Existing HFpEF Definitions. Circulation 2019;140:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang KL, Cheng HM, Sung SH et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 2010;55:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melenovsky V, Borlaug BA, Rosen B et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007;49:198–207. [DOI] [PubMed] [Google Scholar]
- 31.Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F, Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2012;59:455–61. [DOI] [PubMed] [Google Scholar]
- 32.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naeije R, Saggar R, Badesch D et al. Exercise-Induced Pulmonary Hypertension: Translating Pathophysiological Concepts Into Clinical Practice. Chest 2018;154:10–15. [DOI] [PubMed] [Google Scholar]
- 34.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 2008;4:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirinos JA, Rietzschel ER, Shiva-Kumar P et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension 2014;64:1022–31. [DOI] [PubMed] [Google Scholar]
- 36.Borlaug BA, Melenovsky V, Redfield MM et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 2007;50:1570–7. [DOI] [PubMed] [Google Scholar]
- 37.Kitzman DW, Brubaker PH, Herrington DM et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 2013;62:584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadruz W Jr., West E, Sengelov M et al. Prognostic Value of Cardiopulmonary Exercise Testing in Heart Failure With Reduced, Midrange, and Preserved Ejection Fraction. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senni M, Paulus WJ, Gavazzi A et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J 2014;35:2797–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah SJ, Kitzman DW, Borlaug BA et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEniery CM, Yasmin, McDonnell B et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension 2008;51:1476–82. [DOI] [PubMed] [Google Scholar]
- 42.Kampus P, Serg M, Kals J et al. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension 2011;57:1122–8. [DOI] [PubMed] [Google Scholar]
- 43.Williams B, Lacy PS, Thom SM et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006;113:1213–25. [DOI] [PubMed] [Google Scholar]
- 44.de Luca N, Asmar RG, London GM, O’Rourke MF, Safar ME, Investigators RP. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens 2004;22:1623–30. [DOI] [PubMed] [Google Scholar]
- 45.Jankowski P, Kawecka-Jaszcz K, Czarnecka D et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension 2008;51:848–55. [DOI] [PubMed] [Google Scholar]
- 46.Roman MJ, Devereux RB, Kizer JR et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197–203. [DOI] [PubMed] [Google Scholar]
- 47.Wang KL, Cheng HM, Chuang SY et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens 2009;27:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borlaug BA, Olson TP, Abdelmoneim SS et al. A randomized pilot study of aortic waveform guided therapy in chronic heart failure. J Am Heart Assoc 2014;3:e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wohlfahrt P, Melenovsky V, Redfield MM et al. Aortic Waveform Analysis to Individualize Treatment in Heart Failure. Circ Heart Fail 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chirinos JA, Londono-Hoyos F, Zamani P et al. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail 2017;19:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attina TM, Drummond ID, Malatino LS, Maxwell SR, Webb DJ. Phosphodiesterase type 5 inhibition improves arterial stiffness after exercise but not exercise capacity in hypertensive men. Am J Hypertens 2013;26:342–50. [DOI] [PubMed] [Google Scholar]
- 52.Hohneck AL, Fries P, Stroder J et al. Effects of heart rate reduction with ivabradine on vascular stiffness and endothelial function in chronic stable coronary artery disease. J Hypertens 2019;37:1023–1031. [DOI] [PubMed] [Google Scholar]
- 53.Reil JC, Hohl M, Reil GH et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J 2013;34:2839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Striepe K, Jumar A, Ott C et al. Effects of the Selective Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin on Vascular Function and Central Hemodynamics in Patients With Type 2 Diabetes Mellitus. Circulation 2017;136:1167–1169. [DOI] [PubMed] [Google Scholar]
- 55.Ikonomidis I, Aboyans V, Blacher J et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail 2019;21:402–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


