Abstract
Non-alcoholic fatty liver disease (NAFLD), which includes the subtype non-alcoholic steatohepatitis (NASH), is a major complication of type 2 diabetic mellitus (T2DM), even among non-obese patients. However, the exact cause of NAFLD/NASH in non-obese patients with T2DM is unclear. We studied a non-obese mouse model of T2DM created through the malnourishment of embryos by culture in vitro for 48 h in α-minimum essential medium (MEM) at the two-cell stage. We compared the development of steatohepatitis in these MEM mice with control mice that were similarly cultured in standard potassium simplex-optimized medium (KSOM). We also studied the effects of 10 weeks of consumption of barley, which contains large amounts of the soluble fiber β-glucan, on the steatohepatitis of the adult MEM mice. The size of lipid droplets, the area of fibrosis, and the mRNA expression of the transforming growth factor beta (Tgfb) gene in the liver were higher in adult MEM mice fed a rice-based diet than in KSOM mice fed the same diet. However, barley consumption reduced the area of fibrosis and TGFB expression in MEM mice. In conclusion, adult mice that are cultured in MEM at the two-cell embryo stage develop steatohepatitis and T2DM, accompanied by higher hepatic TGFB expression, than KSOM controls. Furthermore, the consumption of barley during adulthood ameliorates the steatohepatitis and reduces the TGFB expression.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Barley, Minimal essential medium, Fibrosis, Transforming growth factor beta
Graphical abstract
Highlights
-
•
The mouse preimplantation embryos cultured in αMEM in vitro developed steatohepatitis.
-
•
Dietary barley suppressed diabetic steatohepatitis with fibrosis.
-
•
Barley intake also reduced gene and protein expression of TGFB in livers of MEM mice.
Abbreviations
- αMEM
α minimum essential medium
- ALT
alanine aminotransferase
- AST
aspartate aminotransaminase
- Col1a1/2
collagen type I alpha 1/2 chain
- CVD
cardiovascular disease
- Cyc1
cytochrome c-1
- ICR
Institute of Cancer Research
- IGT
impaired glucose tolerance
- HTF
human tubal fluid
- IL
interleukin
- IUGR
intrauterine growth restriction
- KC
mice induced by culturing embryos in
- KSOM
medium and subsequently fed a rice-based diet
- KSOM
potassium simplex-optimized medium
- MDA
malondialdehyde
- MEM
α-minimum essential medium
- MMP
matrix metalloproteinase
- MB
MEM-cultured mice fed a barley-based diet
- MR
MEM-cultured mice fed a rice-based diet
- NAFL
non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NEFA
non-esterified fatty acid
- OGTT
oral glucose tolerance testing
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- PVDF
polyvinylidene fluoride
- ROS
reactive oxygen species
- SDS
odium dodecyl sulfate
- SEM
standard error of the mean
- TGFB/B1
transforming growth factor beta/beta 1
- TIMP
tissue inhibitor of metalloproteinase
- Tnfa
tumor necrosis factor alpha
- T2DM
type 2 diabetes mellitus
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a liver disorder that initially manifests as non-alcoholic fatty liver (NAFL), but can progress to steatohepatitis and cirrhosis. It is considered to be a substantial risk factor for the development and progression of type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD), and cirrhosis [1]. When NAFL progresses to feature inflammation, ballooning degeneration, and fibrosis, it is then classified as non-alcoholic steatohepatitis (NASH).
Recently, it has been discovered that the maternal nutritional environment during fetal development predisposes toward NAFL/NASH in later life. For example, the offspring of rat mothers fed a 25% food-restricted diet during gestation showed higher levels of liver triglycerides and cholesterol 3 weeks after birth than controls [2]. Similarly, maternal mice who were subjected to 40% caloric restriction during gestation had offspring that developed NASH by 17 weeks of age after consuming a high-fat diet from 9 weeks of age [3,4]. However, in these studies, the animals did not exhibit clinical signs of, nor were they assessed for, the development of T2DM and/or hepatic fibrosis. In a previous study, we demonstrated that mice derived from embryos cultured in α-minimal essential medium (MEM) at the two-cell stage and then transplanted back into the uterus (MEM mice) showed marked hyperglycemia and were slightly overweight [5]. MEM mice also exhibited higher expression of genes encoding proinflammatory molecules, including the integrins Cd11a, Cd11b, Cd11c and Cd18, interleukin (IL)-1 beta (Il1b), S100a6, and tumor necrosis factor alpha (Tnfa), in peripheral leukocytes [6]. It has also been reported that the serum concentration of myeloperoxidase-DNA, a marker of neutrophil extracellular traps that indicates the aggregation of inflammatory proteins in the blood, was higher in patients with NASH than in healthy individuals [7]. In addition, NASH patients who had been treated with cenicriviroc, an inhibitor of hepatic macrophages, which secrete large amounts of proinflammatory cytokines, showed an amelioration of their histopathologic signs, including hepatic fibrosis [8]. Recent animal studies have demonstrated that proinflammatory cytokines such as transforming growth factor beta (TGFB) [9] and IL18 are involved in the development of NAFLD [10]. However, it is still unclear whether mouse models of NASH, featuring liver fibrosis, show high expression of these proinflammatory cytokines.
Human and animal studies have indicated that barley consumption may help prevent steatohepatitis in patients with T2DM. Acarbose ameliorates postprandial hyperglycemia by inhibiting delaying carbohydrate digestion, in a similar manner to barley, and has been shown to ameliorate NASH in rats [11], and to reduce triglyceride levels and body mass in patients with T2DM [12]. However, it has not been determined whether barley consumption ameliorates steatohepatitis in patients with T2DM or animal models. Barley contains the abundant soluble dietary fiber β-glucan, which reduces postprandial blood glucose concentration. In a randomized, double-blind, placebo-controlled, crossover study conducted in healthy individuals, the circulating glucose concentrations of individuals that consumed barley flour bread containing 2.5 g of β-glucan were lower than those of individuals that consumed wheat flour bread 30 min after the meal [13]. It has also been reported that the supplementation of a high-fat diet with 10% germinated barley for 14 weeks reduces liver fibrosis in rats with steatohepatitis but no T2DM [14]. However, it has not been determined whether steatohepatitis with fibrosis would also be ameliorated by barley intake in a relatively lean animal model of T2DM.
We conducted the present study to explore the hypothesis that steatohepatitis in adult MEM mice would be accompanied by high expression of proinflammatory cytokines, including TGFB and IL18, and that barley intake by the MEM mice would prevent the development of steatohepatitis and the upregulation of TGFB and IL18.
2. Materials and methods
2.1. Animals and sample collection
In a previous study, we demonstrated that mice derived from embryos cultured in MEM exhibit T2DM and high postprandial inflammatory gene expression in peripheral leukocytes, compared to mice derived from embryos cultured in potassium simplex-optimized medium (KSOM) in vitro [6]. Furthermore, the expression of some inflammatory genes in peripheral leukocytes was reduced by feeding these mice a diet containing barley. In the present study, we used same MEM and KSOM mice produced by the Kiwa Laboratory Animal Co., Ltd. (Wakayama, Japan) to analyze the development of steatohepatitis. The MEM mouse model and KSOM control mice have been described previously [5,6]. Briefly, two-cell stage embryos were collected from the oviducts of female Institute of Cancer Research (ICR) mice after mating with male ICR mice, and cultured in either MEM (135–15175, Wako Pure Chemical Industries, Ltd., Osaka, Japan) or KSOM (ARK Resource, Kumamoto, Japan) control medium for 48 h. The compositions of the MEM and KSOM are shown in Table S1. At the morula stage, the embryos were transferred to the oviducts of pseudopregnant recipients. After birth, the offspring were fed a normal diet until the age of 8 weeks, after which they were fed a high-sugar, high-fat (Western-style) diet (Table S2) for 58 days (weeks 10–16). Twenty-four MEM/ICR and eight KSOM/ICR male mice, aged 19–25 weeks, were then transferred to the University of Yamanashi, where they were provided with water and food ad libitum, housed two to a cage, and maintained under controlled conditions (temperature 23 ± 2 °C; humidity 50% ± 0%; 12-h light/12-h dark cycle). MEM mice were randomly allocated to two groups of similar age and body mass: one group was fed a high-rice based diet (Niigata Flour Milling Co., Ltd., Niigata, Japan; n = 12; MR) and the other a diet containing barley powder (Hakubaku Co., Ltd., Yamanashi, Japan; n = 12; MB). KSOM control mice (n = 8) were fed a high-rice based diet (KC). The compositions of the diets, which were mixed and solidified by Oriental Yeast Co., Ltd. (Tokyo, Japan), are shown in Table S2. Because this was an exploratory study, we did not calculate required sample sizes or perform the study blind. The β-glucan content of the barley diet averaged 1.06 g/100 g barley (n = 2), as determined by Hakubaku Co., Ltd. The body masses and food intakes of the mice were measured every 2 weeks. The study protocol was approved by the Ethics Committee of the University of Yamanashi (approval number A30-24) and was performed according to institutional animal experiment regulations.
After 9 weeks of diet feeding, oral glucose tolerance testing (OGTT) was performed after 6 h of fasting. A glucose load (2 g/kg body mass) was administered to the mice and subsequently blood glucose concentration was measured in blood samples collected from a tail vein 0, 15, 30, 60, and 120 min later using capillary tubes containing heparin (#02-668-10, Thermo Fisher Scientific Inc, Waltham, MA). The samples were centrifuged, and the supernatants were collected and stored at −80 °C until use.
After 10 weeks of diet feeding, the body masses of the non-fasted mice were measured, then they were decapitated and trunk blood was collected using tubes containing heparin (CJ-2HL, Terumo Corp., Tokyo, Japan) between 10:00 and 14:00 h. Liver, mesenteric adipose tissue, and epididymal adipose tissue samples were collected, weighed, and then immediately snap-frozen and stored at −80 °C until use. The mean dissection time for each group was similar. Blood samples were subjected to two rounds of centrifugation at 800×g for 20 min at 4 °C and plasma was collected and stored at −80 °C until use.
After measuring the total liver mass, a second liver lobe sample was collected for histologic analysis in tubes containing 4% paraformaldehyde and allowed to stand at 4 °C for 24 h. The paraformaldehyde was replaced with phosphate-buffered saline (PBS), then the samples were transferred to 70% ethanol, and paraffin-embedded histopathologic samples were prepared by New Histo Science Laboratory Co., Ltd. (Tokyo, Japan).
2.2. Staining of sections
Sections were Azan-stained by KAC Co., Ltd. (Shiga, Japan), then the perivascular regions of the liver were examined under a light microscope (CX41LF, Olympus Corp., Tokyo, Japan) and 10 images/mouse were obtained for the analysis of the area of lipid deposits, lipid droplet diameter, and area of fibrosis using ImageJ software (Image Processing and Analysis in Java, NIH, Bethesda, MD, USA).
Preparation of liver homogenates and biochemical analysis of liver and plasma factors Approximately 0.1 g of each frozen liver sample was homogenized in RIPA buffer (1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 20 mM Tris-HCl [pH 8.0], 5 mM EDTA, 150 mM NaCl, 1 mM Na3VO4, 0.1 mM Na2MoO4, 10 mM NaF) containing protease inhibitor cocktail tablets (cOmplete™, Roche Diagnostics K.K., Risch-Rotkreuz, Switzerland). Fifty-μL aliquots of each homogenate were collected and stored for the determination of triglyceride content. The remainder of each homogenate was incubated with rotation at 4 °C for 30 min, centrifuged at 17,800×g for 15 min at 4 °C, and stored at −80 °C for the subsequent determination of malondialdehyde (MDA) content and western blotting. Liver and plasma triglyceride content was quantified using a Triglyceride E Test Kit (432–40201, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). Liver MDA concentration was determined using a NWLSSTM Malondialdehyde Assay (Northwest Life Science, Vancouver, WA, USA). Plasma non-esterified fatty acid (NEFA) concentration was quantified using a NEFA C Test Kit (279–75401, Fujifilm Wako Pure Chemical Corporation). Plasma aspartate aminotransaminase (AST) and alanine aminotransferase (ALT) activities were determined using a Transaminase CII Test Kit (431–30901, Fujifilm Wako Pure Chemical Corporation). Each test was performed by following the manufacturer's protocol.
2.3. Quantitative real-time RT-PCR (qRT-PCR)
RNA was extracted from liver samples using the method described by Chomczynski and Sacchi [15], then converted to cDNA using SuperScript III reverse transcriptase (Thermo Fisher Scientific Inc), according to the manufacturer's instructions. LightCycler 480 Universal Probes Master and LightCycler System (Roche Diagnostics K.K.) were used to perform qRT-PCR, according to the manufacturer's protocols. The primer sequences and Universal Probe Library numbers are shown in Table S3. The expression of each target gene mRNA, relative to that of a reference gene (cytochrome c-1, Cyc1), was calculated using a formula based on the delta-delta Ct (threshold cycle) method: 2(Ct Cyc1 − Ct target gene) [16]. The methods used for qRT PCR have been described previously [17].
2.4. Western blotting
Tissue lysates were prepared and SDS-polyacrylamide gel electrophoresis (PAGE) was performed as described previously [18]. Briefly, tissue samples lysed in RIPA were quantified using the Lowry method [19], then lysates containing 46.3 μg of protein were heat-denatured at 65 °C for 20 min, then separated by SDS-PAGE. The separated proteins were then wet-transferred to polyvinylidene fluoride (PVDF) membranes (Immobilon-P, EMD Millipore Corporation, Billerica, MA, USA) in tris/glycine transfer buffer at 60 V for 30 min. The membranes were blocked for 1 h in 3%–5% skim milk in PBS containing 0.5 M NaCl and 0.05% Tween 20 (PBS-T) and then incubated with a primary antibody against all subtypes of TGFB (rabbit polyclonal antibody, #3711, Cell Signaling Technology, Beverly, MA, USA) or IL18 (mouse monoclonal antibody, M157-3, Medical & Biological Laboratories Co., Ltd., Nagoya, Japan) at 4 °C overnight. After three washes with PBS-T, the membranes were incubated with the secondary antibodies anti-rabbit IgG (RPN1004, biotinylated species-specific whole antibody, GE Healthcare, Little Chalfont, UK) or anti-mouse IgG (RPN1001, biotinylated species-specific whole antibody, GE Healthcare) at 4 °C overnight. The membranes were washed again with PBS-T three times, then incubated with horseradish peroxidase-linked anti-biotin antibody (1:2000; #7075, Cell Signaling Technology) in PBS-T containing skim milk at 4 °C overnight. Specific bands were detected using enhanced chemiluminescence (PerkinElmer, Waltham, MA, U.S.A.) and Chemidoc™ Universal Hood II (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The TGFB signals on membranes were normalized to the total amounts of protein transferred, identified by staining with 0.125% Coomassie brilliant blue (Sigma-Aldrich, St. Louis, MO, USA) in 20% methanol and 10% acetic acid, and quantified using a ChemiDoc XRS Plus System (Bio-Rad Laboratories, Inc.), as described previously [20,21]. Samples were randomly selected for analysis using Microsoft Excel because the number of wells available for SDS-PAGE was limited.
2.5. Statistical analysis
All experimental data are presented as means ± standard errors of the mean (SEM). Comparisons were made between mice that were incubated as embryos in αMEM or KSOM, and between those who consumed rice or barley-based diets using Student's t-test. Differences between groups were considered to be statistically significant when p < 0.05. All statistical analyses were performed using Excel Statistics 2010 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
3. Results
3.1. Basic body parameters and food intake
Before the consumption of the test diets (rice or barley), the body mass of the MR group was higher than that of the KC group, but did not differ between the MR and MB mice (Table 1). After 10 weeks of consumption of the test diets, the body masses of the MR and KC mice were similar, as were those of the MR and MB mice. Daily food intake during the experimental period was higher in the MR than in the KC mice, but did not differ between the MR and MB mice. There were no significant differences in blood glucose concentration between the MR and KC or the MR and MB mice after 10 weeks of diet-feeding, either before at the 120-min time point of OGTT. The masses of the liver and mesenteric and epididymal adipose tissue depots also did not significantly differ between the groups. After 10 weeks of consumption of the test diets, the non-fasting liver triglyceride and MDA concentrations were similar in the MR and KC mice and in the MR and MB mice. There were no differences in non-fasting plasma triglyceride, NEFA, AST, or ALT between the MR and KC mice or between the MR and MB mice (Table 1).
Table 1.
Effects of 10 weeks of barley feeding on body composition and food consumption in mice.
| KSOM |
αMEM |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rice diet | Rice diet | Barley diet | |||||||
| Age at administration of test diet (weeks) | 19 | ± | 0.0 | 21 | ± | 0.6* | 21 | ± | 0.7 |
| Age after 10 weeks of feeding (weeks) | 28 | ± | 0.0 | 30 | ± | 0.6* | 30 | ± | 0.7 |
| Body weight at administration of test diet (g) | 59 | ± | 1.2 | 75 | ± | 2.9* | 75 | ± | 3.3 |
| Body weight after 10 weeks of feeding (g) | 70 | ± | 3.5 | 81 | ± | 4.2 | 84 | ± | 3.8 |
| Blood glucose, fasting (mg/dL) | 178 | ± | 13 | 271 | ± | 31 | 300 | ± | 58 |
| Blood glucose after glucose loading (mg/dL) | 281 | ± | 34 | 420 | ± | 59 | 422 | ± | 40 |
| Blood glucose after 10 weeks (mg/dL) | 191 | ± | 7 | 334 | ± | 73 | 290 | ± | 71 |
| Liver weight (g) | 4.1 | ± | 0.3 | 5.4 | ± | 0.4 | 5.8 | ± | 0.6 |
| Mesenteric fatty tissue weight (g) | 1.4 | ± | 0.2 | 1.4 | ± | 0.2 | 1.5 | ± | 0.1 |
| Testicular fatty tissue weight (g) | 3.7 | ± | 0.4 | 3.0 | ± | 0.4 | 2.7 | ± | 0.3 |
| Liver triglyceride (mg/dL) | 339 | ± | 37 | 331 | ± | 54 | 396 | ± | 52 |
| Liver MDA (μM) | 1.9 | ± | 0.4 | 1.3 | ± | 0.2 | 1.8 | ± | 0.3 |
| Plasma triglyceride (mg/dL) | 179 | ± | 17 | 184 | ± | 27 | 199 | ± | 22 |
| Plasma NEFA (mEq/L) | 0.82 | ± | 0.07 | 0.71 | ± | 0.05 | 0.70 | ± | 0.08 |
| Plasma AST (IU/L) | 105 | ± | 10 | 106 | ± | 5 | 113 | ± | 9 |
| Plasma ALT (IU/L) | 27 | ± | 6 | 39 | ± | 8 | 42 | ± | 11 |
| Food consumption (g/day) | 4.74 | ± | 0.04 | 7.22 | ± | 0.46* | 8.56 | ± | 1.08 |
MDA, malondialdehyde; NEFA, non-esterified fatty acid; AST, aspartate aminotransferase; ALT, alanine aminotransferase; αMEM, α-minimum essential medium mice; KSOM, potassium simplex optimized medium mice. Data are expressed as means ± standard error of the mean (8–12 mice). Statistical analyses were performed by Student's t-test. *P < 0.05 compared with KSOM mice.
3.2. Liver lipid droplets and fibrosis
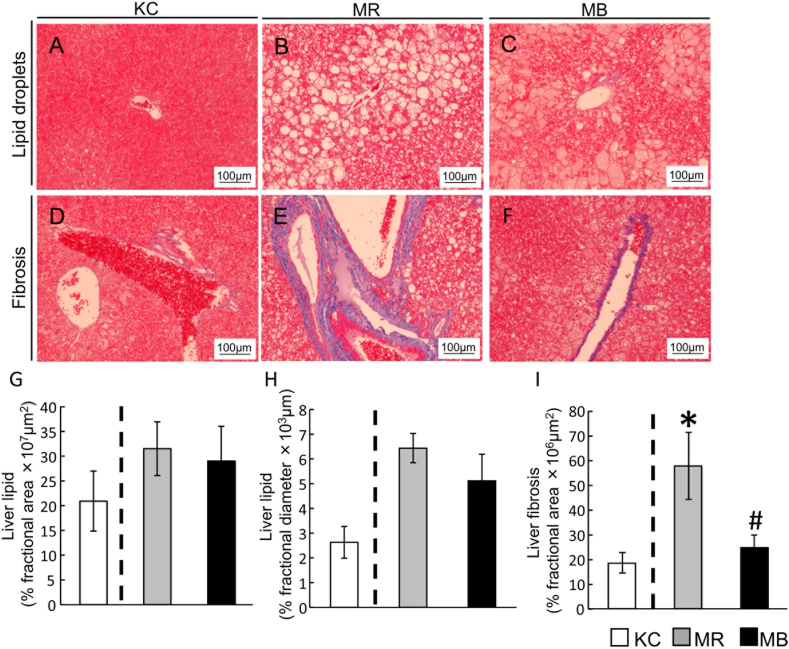
The area and diameters of lipid droplets (Fig. 1A–C), and the area of perivascular fibrosis (Fig. 1D–F) were quantified using Azan-stained liver sections. Although there were no differences in lipid droplet area between the MR and KC mice or between the MR and MB mice (Fig. 1G), the diameter of the lipid droplets was larger in the MR than in the KC mice (Fig. 1H). In addition, the area of fibrosis was larger in the MR than in the KC mice, and was smaller in the MB than in the MR mice (Fig. 1I).
Fig. 1.
Histologic sections of the liver showing the lipid droplets and areas of fibrosis after 10 weeks of consumption of the experimental diets. Lipid droplets (A–C) and fibrosis (D–F) in Azan-stained sections (scale bar, 100 μm) in the three groups of mice. Quantification of lipid droplet area (G) and diameter (H) and the area of fibrosis (I). MB, minimum essential medium (MEM) mice fed a barley-based diet; MR, MEM mice fed a rice-based diet; KC, potassium simplex-optimized medium control mice fed a rice-based diet. Data are expressed as means ± SEMs for 8–12 animals and were analyzed using Student's t-test. *P < 0.05 vs. the KC group; #P < 0.05 vs. the MR group.
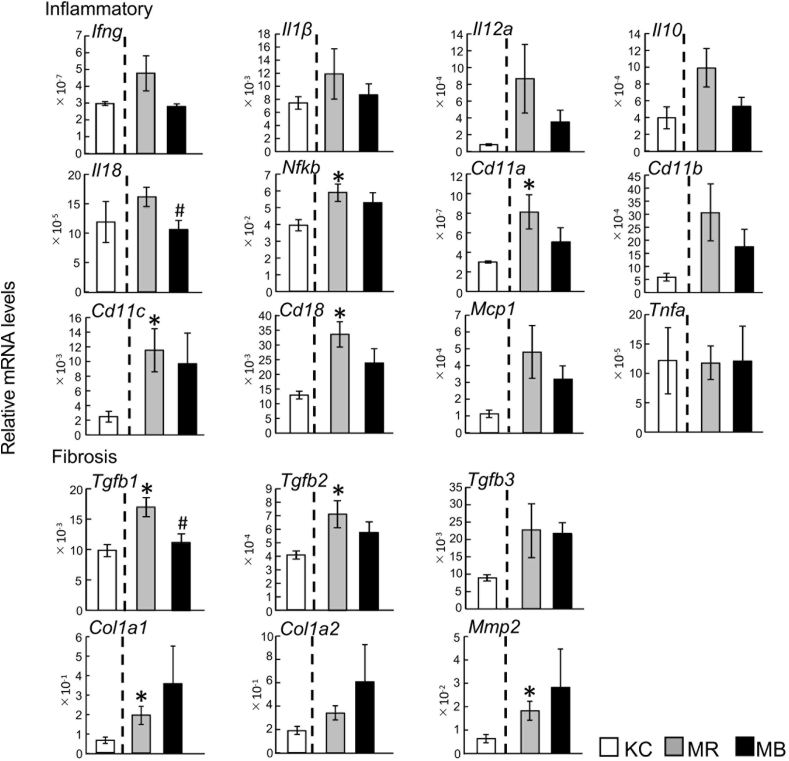
3.3. Hepatic mRNA expression of genes expressing inflammation and fibrosis-related factors
After 10 weeks, the mRNA expression in the non-fasting state of several genes that encode proinflammatory factors (nuclear factor kappa B (Nfkb), integrin alpha L (Cd11a), integrin alpha X (Cd11c), and integrin beta 2 (Cd18)) was higher in the MR group than in the KC group. (Fig. 2). The mRNA expression of Il18 was lower in the MB than in the KC mice. There were no differences between the KC and MR mice, or between the MR and MB mice, in the mRNA expression of the following genes: interferon gamma (Ifng), IL-1 beta (Il1b), IL-12a (Il12a), IL-10 (Il10), integrin alpha M (Cd11b), monocyte chemoattractant protein-1 (Mcp1), and Tnfa. The mRNA expression of two fibrosis-related genes, transforming growth factor beta 1 and 2 (Tgfb1 and Tgfb2), was higher in the MR mice than in the KC mice, and the expression of Tgfb1 was lower in the MB mice than in the MR mice. The mRNA expression of transforming growth factor beta 3 (Tgfb3) did not differ between the KC and MR mice or the MR and MB mice. The mRNA expression of collagen type I alpha 1 chain (Col1a1) and matrix metalloproteinase 2 (Mmp2) was higher in the MR mice than in the KC mice, but there were no differences in the mRNA expression of collagen type I alpha 2 chain (Col1a2) between the KC and MR mice or between the MR and MB mice (Fig. 2).
Fig. 2.
Quantitative real-time PCR data showing the relative mRNA expression of inflammation and fibrosis-related genes in non-fasting liver samples from mice fed the experimental diets for 10 weeks. MB, minimum essential medium (MEM) mice fed a barley-based diet; MR, MEM mice fed a rice-based diet; KC, potassium simplex-optimized medium control mice fed a rice-based diet. Data are expressed as means ± SEMs for 8–12 animals and were analyzed using Student's t-test. *P < 0.05 vs. the KC group; #P < 0.05 vs. the MR group.
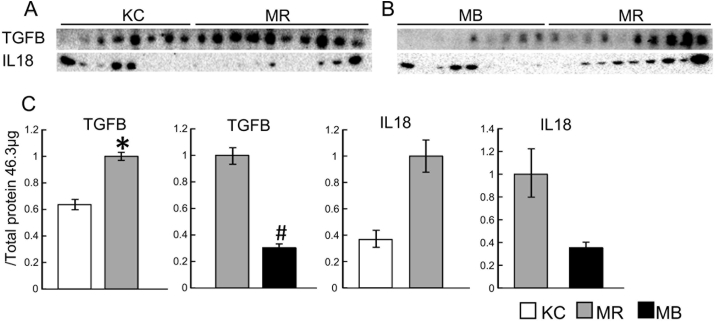
3.4. Hepatic protein levels of TGFB and IL18
The protein expression of TGFB (all the subtypes combined) was higher in the MR mice than in the KC mice, and lower in the MB mice than in the MR mice (Fig. 3A–C), but there were no differences in IL18 protein expression. Images showing the staining of the PVDF membranes for total protein, used for the normalization of expression, are provided in Fig. S7.
Fig. 3.
Protein levels of transforming growth factor beta (TGFB) and interleukin (IL)18 in liver samples from mice fed the experimental diets for 10 weeks. Sample western blots and their quantification are shown. MB, minimum essential medium (MEM) mice fed a barley-based diet; MR, MEM mice fed a rice-based diet; KC, potassium simplex-optimized medium control mice fed a rice-based diet. Data are expressed as means ± SEMs for 8–11 animals and were analyzed using Student's t-test. *P < 0.05 vs. the KC group; #P < 0.05 vs. the MR group.
4. Discussion
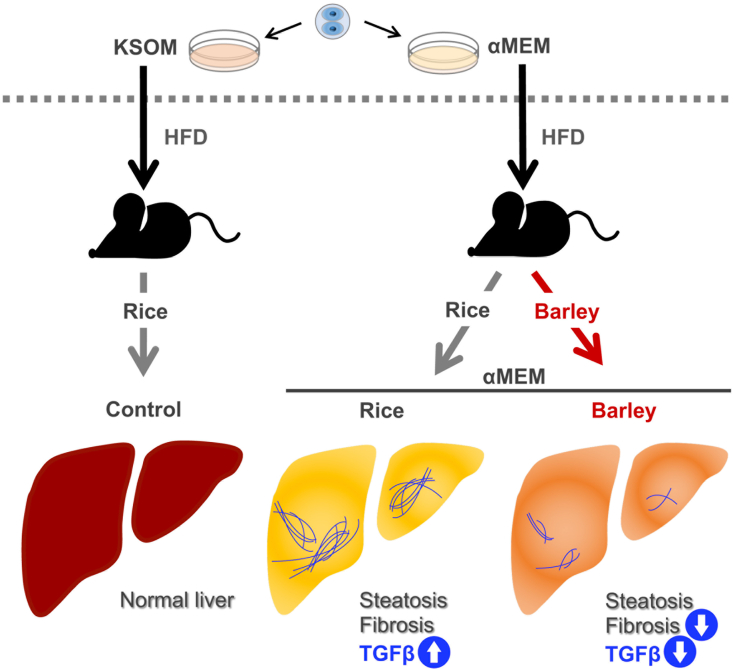
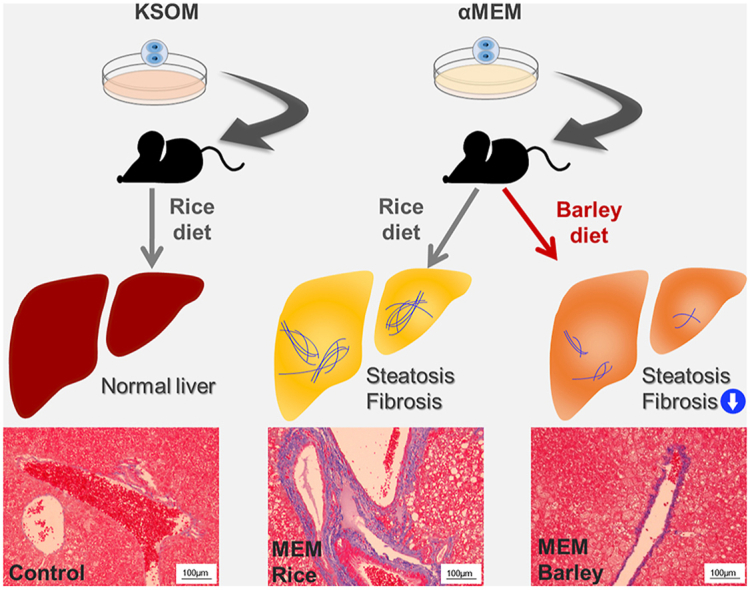
In the present study, we have shown that adult mice derived from embryos cultured in MEM in vitro at the two-cell stage had larger areas of perivascular fibrosis and lipid droplets in their livers than KSOM control mice fed the same rice-based diet for 10 weeks (Fig. 4). There have been few reports of the use of MEM to culture early embryos in vitro, but it has been demonstrated that mice conceived by in vitro fertilization (IVF) and cultured in regular medium (human tubal fluid [HTF] medium supplemented with 10% serum substitute), then fed a diet including the long-chain fatty acid palmitic acid, have high liver triglyceride levels [22]. In this study, the control mice, which were the offspring of mothers treated by controlled ovarian hyperstimulation, were fed the same diet as the IVF mice but did not develop steatohepatitis [22]. HTF medium is considered to be comparable with KSOM medium because both are used to culture early embryos for IVF. Thus, taking these results together with the present findings, it is possible that the in vitro culture of embryos in regular medium increases their risk of steatohepatitis, and that culture in MEM further increases this risk. The results of this study, in which culture in MEM and KSOM were compared, suggest that additional comparisons between other types of medium used to culture two-cell embryos, such as HTF, should be performed, to compare their potential effects on the development of T2DM and steatohepatitis.
Fig. 4.
Graphical abstract. Mice grown in α-minimum essential medium in vitro as embryos develop diabetes and steatohepatitis. The consumption of barley ameliorates this steatohepatitis and reduces the high transforming growth factor β (TGFB) expression. αMEM, minimum essential medium; HFD, high-fat diet; KSOM, potassium simplex-optimized medium.
Many previous studies have shown that TGFB plays critical roles in the progression of NASH. It activates hepatic stellate cells and induces hepatic fibrosis by increasing collagen production [23]. Kupffer cells, the resident macrophages in the liver, are induced to produce reactive oxygen species (ROS), which leads to TGFB production by hepatic stellate cells, promoting this progression [9]. Indeed, it was previously reported that transgenic mice that overexpress Tgfb1 develop hepatic fibrosis and have higher hepatic mRNA expression of procollagen I than control mice [24]. In addition, the mRNA expression of Tgfb1 in the liver is higher in patients with NASH and fibrosis than in those with simple steatosis [25]. A study of a mouse model of NASH that was generated by feeding a methionine- and choline-deficient diet for 8 weeks showed that ROS exposure in the liver further increases the expression of Tgfb1 and TGFB1 protein during the progression of NASH [26]. In the present study, we have demonstrated that the mRNA expression of Tgfb1 and Tgfb2, and TGFB protein levels, are higher in the livers of MEM mice fed a rice-based diet than in KSOM mice fed the same diet. In contrast, barley consumption reduced the mRNA expression of Tgfb1 and Tgfb3 and TGFB protein levels of MEM mice. These results indicate that MEM mice develop steatohepatitis and fibrosis, and that this is closely associated with higher TGFB expression. However, the mRNA expression of the collagen type I genes Col1a1 and Col1a2, a collagen protease Mmp2, and the protease inhibitors Timp1, Timp2, and Timp3 did not change in response to barley consumption by MEM mice (Fig. S10). However, the areas of Azan staining of collagen fibers were larger in MEM mice fed a rice-based diet than those in KSOM mice fed the same diet, and the areas in MEM mice were reduced by the consumption of a barley-based diet. These results indicate that the high collagen fiber content in the livers of MEM mice fed a rice-based diet and the lower content in the livers of MEM mice fed a barley-based diet are not caused by differences in the mRNA expression of collagen genes.
Several previous studies have shown that the degradation of the extracellular matrix, which includes collagen, by enzymes such as matrix metalloproteinase (MMP), is important for the development of hepatic fibrosis [27,28]. Immunostaining showed that MMP proteins are activated in mouse livers as NASH develops and fibrosis progresses [29], which suggests that MMPs may limit fibrosis. Although it has been reported that TGFB inhibits MMP production [30], we did not find any differences in MMP mRNA expression between MEM and KSOM mice fed the rice-based diet, or between rice-fed and barley-fed MEM mice. In addition, tissue inhibitor of metalloproteinase (TIMP) is known to be an MMP inhibitor, and the treatment of human hepatic stellate cells with TGFB induces an imbalance in MMP/TIMP by respectively down- and upregulating these molecules [31]. However, we did not detect any differences in the mRNA expression of collagens, MMP, or TIMPs among the groups (Fig. S10). Therefore, the mechanism underlying the induction of hepatic fibrosis in MEM mice and the prevention of this by barley consumption remains unclear.
Interestingly, we found a significant positive association between TGFB protein expression and lipid droplet area, but a weaker association between TGFB protein and the area of fibrosis. A previous study has demonstrated that lipid droplets are prominent in severe steatohepatitis [32]. Taking the present results together with those of previous studies, it may be that TGFB is expressed in livers with large lipid droplets, and it subsequently induces hepatic fibrosis. Future studies should determine which collagen fibers in the liver of adult mice are induced by culturing embryos in MEM using global proteomic analysis and subsequent characterization of the distribution of candidate proteins on tissue sections. In addition, it should be determined whether TGFB expression in MEM mice fed a rice-based diet correlates with the severity of the subsequent hepatic fibrosis.
In the present study, we used an antibody against all the subtypes of TGFB, because the expression of all the subtypes was higher or tended to be higher in the MR mice than in the KC mice, and it was lower or tended to be lower in the MB mice than in the MR mice. Although TGFB1 is well known to be associated with fibrosis, several previous studies have shown that TGFB2 and TGFB3 are also involved in fibrosis. One previous study demonstrated that the induction of TGFB2 in mice by CCl4, which induces acute liver injury, closely correlated with collagen 1a1 mRNA expression [33]. In addition, it has been reported that the distribution of TGFB3 protein in the human liver before allografting mirrors that of tenascin, which is an extracellular matrix component that is involved in the development of liver fibrosis at an early stage, suggesting that TGFB3 may also be involved in early hepatic fibrosis [34]. Therefore, further studies are required to determine the contributions of each TGFB subtype to the development of fibrosis in the livers of MEM mice.
We found that barley consumption reduces the expression of Il18 at the mRNA level, but not at the protein level. IL18 is known to be expressed by parenchymal cells in tissues including the liver, as well as by leukocytes, and its expression is induced by inflammasome activation caused by the escalation of oxidative stress [35]. Lana et al. reported that the knockout of the Il18 gene in mice reduces the hepatic fat accumulation and other pathology associated with steatosis [36]. Taken together with the present findings, this suggests that IL18 may be involved in the development of hepatic fibrosis and its amelioration by barley consumption in MEM mice. However, the exact role of IL18 in the progression of NASH is unclear, and this hypothesis should be further tested.
In the present study, we have revealed for the first time that barley consumption ameliorates the hepatic fibrosis and steatosis of a mouse model of T2DM. Barley has previous been reported to reduce postprandial hyperglycemia, an effect that is due to its substantial β-glucan content [37]. A previous study also demonstrated that a barley sprout extract reduces lipid accumulation in the liver of mice, thereby alleviating severe alcoholic steatohepatitis [38]. Another study demonstrated that, in mice with moderately impaired glucose tolerance (IGT) induced by high-fat diet-feeding, the addition of 4% barley β-glucan for 12 weeks ameliorates the excess weight gain, hepatic lipid accumulation, and insulin resistance [39]. However, the reductions in lipid droplet accumulation and fibrosis in MEM mice that were identified in the present study occurred in the absence of an effect of barley consumption on their IGT, assessed using OGTT and the measurement of non-fasting blood glucose concentration. This discrepancy in findings may be explained by the relatively low barley content of the present diet (approximately 12% w/w). In addition, we did not assess whether the diet affected postprandial hyperglycemia. Another possibility is that changes in the microbiota are induced by the barley-based and that these influence the liver phenotype, because barley β-glucan is metabolized by the microbiota to produce short-chain fatty acids [40] and hydrogen [41]. Further research should be performed to determine whether IGT and postprandial hyperglycemia are reduced and whether the microbiota is altered by the feeding of a barley-based diet.
In summary, we have revealed that adult mice derived from embryos cultured in MEM at the two-cell stage develop diabetic steatohepatitis and hepatic fibrosis. The fibrosis and expression of the fibrosis marker TGFB are reduced by barley consumption later in life. This discovery may help with the elucidation of the molecular mechanism whereby NASH develops in individuals with T2DM but without obesity, and aid the search for food-derived substances and drugs that can ameliorate NASH in such patients.
Credit author statement
Shiori Ishiyama: Validation, Formal analysis, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Mayu Kimura: Validation, Investigation, Resources. Nodoka Umihira: Validation, Investigation, Resources. Sachi Matsumoto: Validation, Investigation, Resources. Atsushi Takahashi: Validation, Investigation, Resources. Takao Nakagawa: Investigation, Resources. Teruhiko Wakayama: Methodology, Writing - Review & Editing. Satoshi Kishigami: Conceptualization, Methodology, Resources, Writing - Review & Editing, Funding acquisition. Kazuki Mochizuki: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition.
Funding
This work was supported by the Mishima Kaiun Memorial Foundation, a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [20H04103], and the Adaptable and Seamless Technology Transfer Program through target-driven R&D (A-STEP) of the Japan Science and Technology Agency (JST).
Acknowledgments
We thank Mark Cleasby, PhD, from Edanz Group (https://www.jp.edanz.com/ac) for editing a draft of this manuscript. We also thank Hakubaku Co., Ltd for donating the barley powder.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101029.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis, Nat. Rev. Gastroenterol. Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 2.Choi G.Y., Tosh D.N., Garg A., Mansano R., Ross M.G., Desai M. Gender-specific programmed hepatic lipid dysregulation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 2007;196 doi: 10.1016/j.ajog.2007.02.024. 477.e1-477.e7. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu-Kato K., Itoh H., Kohmura-Kobayashi Y., Ferdous U.J., Tamura N., Yaguchi C., Uchida T., Suzuki K., Hashimoto K., Suganami T., Ogawa Y., Kanayama N. Undernourishment in utero primes hepatic steatosis in adult mice offspring on an obesogenic diet; involvement of endoplasmic reticulum stress. Sci. Rep. 2015;5 doi: 10.1038/srep16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urmi J.F., Itoh H., Muramatsu-Kato K., Kohmura-Kobayashi Y., Hariya N., Jain D., Tamura N., Uchida T., Suzuki K., Ogawa Y., Shiraki N., Mochizuki K., Kubota T., Kanayama N. Plasticity of histone modifications around Cidea and Cidec genes with secondary bile in the amelioration of developmentally-programmed hepatic steatosis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-52943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.University of Yamanashi. S. Kishigami, K. Mochizuki, T. Wakayama. The Method for Producing Diabetic Animal Model and the Diabetic Animal Model. P2020-31551A. 2020-03-05. (Japanese).
- 6.Ishiyama S., Kimura M., Umihira N., Matsumoto S., Takahashi A., Nakagawa T., Wakayama T., Kishigami S., Mochizuki K. Mice derived from in vitro-αMEM cultured preimplantation embryos exhibit postprandial hyperglycemia and higher inflammatory gene expression in peripheral leukocytes. Biosci. Biotechnol. Biochem. 2021 doi: 10.1093/bbb/zbab023. [DOI] [PubMed] [Google Scholar]
- 7.van der Windt D.J., Sud V., Zhang H., Varley P.R., Goswami J., Yazdani H.O., Tohme S., Loughran P., O'Doherty R.M., Minervini M.I., Huang H., Simmons R.L., Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347–1360. doi: 10.1002/hep.29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krenkel O., Puengel T., Govaere O., Abdallah A.T., Mossanen J.C., Kohlhepp M., Liepelt A., Lefebvre E., Luedde T., Hellerbrand C., Weiskirchen R., Longerich T., Costa I.G., Anstee Q.M., Trautwein C., Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 9.Liu W., Baker R.D., Bhatia T., Zhu L., Baker S.S. Pathogenesis of nonalcoholic steatohepatitis. Cell. Mol. Life Sci. 2016;73:1969–1987. doi: 10.1007/s00018-016-2161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borai I.H., Shaker Y., Kamal M.M., Ezzat W.M., Ashour E., Afify M., Gouda W., Elbrashy M.M. Evaluation of biomarkers in egyptian patients with different grades of nonalcoholic fatty liver disease. J. Clin. Transl. Hepatol. 2017;5:109–118. doi: 10.14218/JCTH.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber C.S., Leo M.A., Mak K.M., Xu Y., Cao Q., Ren C., Ponomarenko A., DeCarli L.M. Acarbose attenuates experimental non-alcoholic steatohepatitis. Biochem. Biophys. Res. Commun. 2004;315:699–703. doi: 10.1016/j.bbrc.2004.01.116. [DOI] [PubMed] [Google Scholar]
- 12.Yamagishi S., Nakamura K., Inoue H. Acarbose is a promising therapeutic strategy for the treatment of patients with nonalcoholic steatohepatitis (NASH) Med. Hypotheses. 2005;65:377–379. doi: 10.1016/j.mehy.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka T., Tsuchida A., Yamaji A., Kurosawa C., Shinohara M., Takayama I., Nakagomi H., Izumi K., Ichikawa Y., Hariya N., Yamashita S., Mochizuki K. Consumption of a meal containing refined barley flour bread is associated with a lower postprandial blood glucose concentration after a second meal compared with one containing refined wheat flour bread in healthy Japanese: a randomized control trial. Nutrition. 2020;72 doi: 10.1016/j.nut.2019.110637. [DOI] [PubMed] [Google Scholar]
- 14.Al-Shali R.A., Ramadan W.S. Germinated barley downregulates hepatic stearoyl-CoA desaturase-1 enzyme gene expression in a hepatic steatohepatitis rat model. Anat. Sci. Int. 2020;95:489–497. doi: 10.1007/s12565-020-00546-y. [DOI] [PubMed] [Google Scholar]
- 15.Chomzynski P. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Farahzadi R., Fathi E., Vietor I. Mesenchymal stem cells could Be considered as a candidate for further studies in cell-based therapy of alzheimer's disease via targeting the signaling pathways. ACS Chem. Neurosci. 2020;11:1424–1435. doi: 10.1021/acschemneuro.0c00052. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T., Muramatsu T., Morioka K., Goda T., Mochizuki K. ChREBP binding and histone modifications modulate hepatic expression of the Fasn gene in a metabolic syndrome rat model. Nutrition. 2015;31:877–883. doi: 10.1016/j.nut.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/0922-338X(96)89160-4. [DOI] [PubMed] [Google Scholar]
- 20.Orchard R.C., Wilen C.B., Virgin H.W. Sphingolipid biosynthesis induces a conformational change in the murine norovirus receptor and facilitates viral infection. Nat. Microbiol. 2018;3:1109–1114. doi: 10.1038/s41564-018-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitler A.F., Gerrer K.H., Haas R., Jiménez-Soto L.F. Optimized semi-quantitative blot analysis in infection assays using the Stain-Free technology. J. Microbiol. Methods. 2016;126:38–41. doi: 10.1016/j.mimet.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang L.Y., Le F., Wang N., Li L., Liu X.Z., Zheng Y.M., Lou H.Y., Xu X.R., Chen Y.L., Zhu X.M., Huang H.F., Jin F. Alteration of fatty acid metabolism in the liver, adipose tissue, and testis of male mice conceived through assisted reproductive technologies: fatty acid metabolism in ART mice. Lipids Health Dis. 2013;12 doi: 10.1186/1476-511X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooley S., Ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanzler S., Lohse A.W., Keil A., Henninger J., Dienes H.P., Schirmacher P., Rose-John S., Meyer Zum Büschenfelde K.H., Blessing M. TGF-β1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;276 doi: 10.1152/ajpgi.1999.276.4.g1059. [DOI] [PubMed] [Google Scholar]
- 25.Cayón A., Crespo J., Mayorga M., Guerra A., Pons-Romero F. Increased expression of Ob-Rb and its relationship with the overexpression of TGF-β1 and the stage of fibrosis in patients with nonalcoholic steatohepatitis. Liver Int. 2006;26:1065–1071. doi: 10.1111/j.1478-3231.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomita K., Oike Y., Teratani T., Taguchi T., Noguchi M., Suzuki T., Mizutani A., Yokoyama H., Irie R., Sumimoto H., Takayanagi A., Miyashita K., Akao M., Tabata M., Tamiya G., Ohkura T., Hibi T. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–473. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- 27.Chan E.C., Jiang F., Peshavariya H.M., Dusting G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009;122:97–108. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Arthur M.J.P. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279 doi: 10.1152/ajpgi.2000.279.2.g245. [DOI] [PubMed] [Google Scholar]
- 29.Farrell G.C., Mridha A.R., Yeh M.M., Arsov T., Van Rooyen D.M., Brooling J., Nguyen T., Heydet D., Delghingaro-Augusto V., Nolan C.J., Shackel N.A., Mclennan S.V., Teoh N.C., Larter C.Z. Strain dependence of diet-induced NASH and liver fibrosis in obese mice is linked to diabetes and inflammatory phenotype. Liver Int. 2014;34:1084–1093. doi: 10.1111/liv.12335. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi H., Sakai T. Biological significance of local TGF-β activation in liver diseases. Front. Physiol. 2012;3:FEB. doi: 10.3389/fphys.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robert S., Gicquel T., Bodin A., Lagente V., Boichot E. Characterization of the MMP/TIMP imbalance and collagen production induced by IL-1 β or TNF-α release from human hepatic stellate cells. PloS One. 2016;11 doi: 10.1371/journal.pone.0153118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashek D.G. Hepatic lipid droplets: a balancing act between energy storage and metabolic dysfunction in NAFLD. Mol. Metab. 2020 doi: 10.1016/j.molmet.2020.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dropmann A., Dediulia T., Breitkopf-Heinlein K., Korhonen H., Janicot M., Weber S.N., Thomas M., Piiper A., Bertran E., Fabregat I., Abshagen K., Hess J., Angel P., Coulouarn C., Steven D., Meindl-Beinker N.M. TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget. 2016;7:19499–19518. doi: 10.18632/oncotarget.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demirci G., Nashan B., Pichlmayr R. Fibrosis in chronic rejection of human liver allografts: expression patterns of transforming growth factor-TGFβ1 and TGF-β3. Transplantation. 1996;62:1776–1783. doi: 10.1097/00007890-199612270-00016. [DOI] [PubMed] [Google Scholar]
- 35.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T.H., Brickey W.J., Ting J.P.Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lana J.P., Martins L.B., de Oliveira M.C., Menezes-Garcia Z., Yamada L.T.P., Vieira L.Q., Teixeira M.M., Ferreira A.V.M. TNF and IL-18 cytokines may regulate liver fat storage under homeostasis conditions. Appl. Physiol. Nutr. Metabol. 2016;41:1295–1302. doi: 10.1139/apnm-2016-0265. [DOI] [PubMed] [Google Scholar]
- 37.Fuse Y., Higa M., Miyashita N., Fujitani A., Yamashita K., Ichijo T., Aoe S., Hirose T. Effect of high β-glucan barley on postprandial blood glucose and insulin levels in type 2 diabetic patients. Clin. Nutr. Res. 2020;9:43. doi: 10.7762/cnr.2020.9.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y.H., Kim J.H., Kim S.H., Oh J.Y., Seo W.D., Kim K.M., Jung J.C., Jung Y.S. Barley sprouts extract attenuates alcoholic fatty liver injury in mice by reducing inflammatory response. Nutrients. 2016;8 doi: 10.3390/nu8070440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J.S., Kim H., Jung M.H., Hong S., Song J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- 40.Hughes S.A., Shewry P.R., Gibson G.R., McCleary B.V., Rastall R.A. In vitro fermentation of oat and barley derived β-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008;64:482–493. doi: 10.1111/j.1574-6941.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 41.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62:67–72. doi: 10.1079/pns2002207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.