Abstract
Recent attention to consequences of head trauma among former professional American-style football players has increased the likelihood that former players and their healthcare providers attribute neurocognitive effects to these exposures. In addition to head trauma, however, many potentially modifiable risk factors are associated with cognitive impairment. We examined the association of self-reported risk factors for cognitive impairment (e.g., cardiovascular health, sleep, pain, depression, anxiety, smoking, physical impairment, and physical activity) with cognition-related quality of life, measured by the Quality of Life in Neurological Disorders, Applied Cognition-General Concerns (Neuro-QOL) among 3803 former National Football League (NFL) players. We examined the prevalence of risk factors among men who had experienced a high number of concussion symptoms during playing years, comparing men with good current cognition-related QOL, the “healthy concussed,” to men with poor cognition-related QOL, the “unhealthy concussed.” Physical functioning, pain, depression, and anxiety were very strongly associated with poor cognitive-related QOL (risk ratio range, 2.21–2.70, p < 0.0001 for all). Short sleep duration and low physical activity were also strongly associated (RR = 1.69 and 1.57, respectively, p < 0.0001 for both). The largest differences between healthy and unhealthy concussed were in chronic pain (72.0% vs. 21.2%), depressive symptoms (50.3% vs. 6.3%), anxiety symptoms (53.4% vs. 11.6%), and physical impairment (52.4% vs. 12.5%). Substantial differences also existed in prevalence of sleep apnea, short sleep duration, high-intensity exercise, weight training, high blood pressure, and body mass index ≥35 kg/m2 (all differences >10 percentage points). We identified cognitive risk factors, including chronic pain, mood problems, sleep problems, obesity, and lack of exercise, that were commonly present in former football players with cognition-related impairment. Better treatment for these factors may reduce cognitive problems in this population.
Keywords: concussion, depression, pain, physical function, professional American-style football, sleep
Introduction
Head trauma, including both concussive events and repeated subconcussive events, have been associated with cognitive impairment in former professional American-style football players, combat veterans, and the general public.1–3 These findings are derived mostly from retrospective clinical assessments. To the degree that animal models can simulate neurocognitive decline, however, controlled experiments are suggestive.4,5 The recent attention to the consequences of repeated head trauma among former American-style football players has increased the likelihood that former players, their health care providers, families, and other stakeholders attribute even mild neurocognitive effects to these exposures.
In addition to head trauma, however, there are many potentially modifiable or treatable risk factors that are associated with cognitive impairment. These include sleep apnea,6, short sleep duration,7,8 obesity,9 hypertension,10 cardiovascular disease,9 diabetes,11 chronic pain,12,13 depression,11,14 anxiety,15,16 smoking,9 physical impairment,17,18 and physical activity.19 If mitigated, these potentially modifiable factors may reduce risk of cognitive impairment even in persons with a history of head trauma or improve cognitive function among those already showing cognitive impairments.
In the present study, we examine the association of potentially modifiable risk factors for cognitive impairment with cognition-related quality of life (QOL) among former American-style football players. We sought to identify the potentially modifiable or treatable risk factors most prevalent in former players and most strongly associated with poor cognition-related QOL, because these risk factors may be good targets for intervention to improve the health of these men.
To further explore the possible role of intervening on potentially modifiable risk factors to improve cognition-related QOL among former American-style football players, we examined prevalence of these cognitive related risk factors among former players who experienced large numbers of concussion symptoms during their playing career, but nonetheless reported good current cognition-related QOL—the “healthy concussed.” We compared prevalence of cognitive risk factors among these healthy concussed men with men who experienced concussion symptoms during their playing career and report poor current cognition-related QOL—the “unhealthy concussed.” We hypothesized that the healthy concussed would have fewer potentially modifiable other cognitive risk factors, while the unhealthy concussed would have an excess of these risk factors.
Methods
The Football Players Health Study sought to enroll former players who participated in the National Football League (NFL) since 1960, the year the transition to helmets with hard plastic shells was essentially complete. We obtained home or e-mail addresses from the NFL Players Association for 14,906 players, of which 2415 were invalid or inactive. The remaining 12,491 players were mailed or e-mailed a questionnaire. At the time of this analysis, 3913 players had responded. The study was approved by the Harvard T.H. Chan School of Public Health and the Beth Israel Deaconess Medical Center Institutional Review Boards. Participants provided informed consent.
Measures
The short form of the Quality of Life in Neurological Disorders, Applied Cognition-General Concerns (Neuro-QOL) was used to assess cognition-related QOL.20 Eight items queried past seven-day cognitive difficulties (e.g., “I had to read something several times to understand it”). Response options were: 0: never, 1: rarely (once), 2: sometimes (2–3 times), 3: often (once/day), or 4: very often (several times/day). Responses were summed to create a continuous measure. A United States (US) population sample (N = 1109) was used to create standardized T-scores with mean = 50 and standard deviation (SD) = 10.20 Based on published guidelines, we created an indicator of “moderate or severe symptoms or impairment” using a score ≤40, which corresponds to ≤1 SD below the US population mean.21 The short form had excellent internal consistency in our data (Cronbach alpha = 0.97). Ten men did not respond to Neuro-QOL questions and were excluded from analyses.
We queried 10 football-related concussion symptoms during playing years with, “While playing or practicing football, did you experience a blow to the head, neck, or upper body followed by: headaches, nausea, dizziness, loss of consciousness, memory problems, disorientation, confusion, seizure, visual problems, and feeling unsteady on your feet.” Response options for each symptom were: no, once, 2–5, 6–10, or 11+ times. We coded these as 0, 1, 3.5, 8, and 13 and calculated the mean across all 10 items. We calculated quartiles of the concussion score. Men who did not respond to the concussion symptom questions (N = 104) were excluded from analyses.
Potentially modifiable cognitive risk factors
The Patient Health Questionnaire (PHQ)-4 assessed symptoms of depression and anxiety. Responses were summed separately for depression and anxiety and dichotomized per scoring recommendations to indicate high depressive symptoms or high anxiety symptoms. In a meta-analysis of four studies, the recommended cutoff had sensitivity = 0.76 and specificity = 0.81.22,23 Pain interference in daily life was measured with the six-item Patient Reported Outcomes Measurement Information System (PROMIS) pain interference scale (e.g., “In the past 7 days, how much did pain interfere with your enjoyment of life?”). Physical functioning in daily life was measured with the six-item PROMIS physical functioning scale (e.g., “Are you able to go up and down stairs at a normal pace?”). Moderate or severe impairment related to pain or physical functioning was defined according to published guidelines as ≥1 SD below the US population mean.24
Current exercise was queried with the average number of hours spent each week walking, jogging, running, in other aerobic activity (e.g., bicycling), low intensity exercise (e.g., yoga), and weight training. Response options for each type of exercise included zero, <1, 1–5, 6–10, or >10 h. We combined jogging, running, and other aerobic activity as “high intensity.” In addition, we calculated metabolic equivalent task hours (METS) per week and divided this measure into quartiles for analysis. Men in the lowest quartile did very little exercise (median = 1.5 mets/week, equivalent to walking 0.5 h/week), while men in the highest quartile did substantial exercise (median = 48 mets/week, equivalent to running 6 h/week).
Current smoking, weight, and height, and medical provider recommendation or prescription of medication for high blood pressure, heart failure, heart rhythm problems, and diabetes were by self-report. We calculated body mass index (BMI) from self-reported weight and height, and created an indicator for class 2 (“moderate-risk,” BMI 35 to 39.9 kg/m2) or class 3 (“high-risk,” BMI ≥40.0 kg/m2) obesity.25 Lifetime medical provider's diagnosis of stroke, heart attack, and sleep apnea was by self-report, as was heart surgery (bypass, angioplasty, or stent placement). Heavy drinking was defined as ≥14 drinks/week. Participants were asked how many hours of sleep they get on an average weekday. Low sleep duration was defined as <5 h/night7. Race/ethnicity was coded as: Black, White, or other races. Age at questionnaire was in years.
Analyses
We examined the prevalence of cognitive risk factors by football-related concussion symptoms. We calculated the association of each cognitive risk factor with moderate or severe impairment in cognition-related QOL by fitting models with cognition-related QOL as the dependent variable and each risk factor as the independent variable in separate models, adjusted for age at questionnaire and race/ethnicity. As poor cognition-related QOL and cognitive risk factors (e.g., pain) may be associated because they are common sequelae of concussion, we further adjusted for concussion symptoms in each model, including squared and cubed terms, because this was the best-fitting model by quasi-likelihood under the independence model criterion (QIC).26 We estimated risk ratios with PROC GENMOD (SAS 9.4), using the modified Poisson regression.27
The prevalence of potentially modifiable or treatable cognitive risk factors among the healthy concussed was compared with the unhealthy concussed (men who experienced the highest quartile of concussion symptoms during their playing career and either did not report or did report current impairment in cognition-related QOL). Similarly, we examined the prevalence of cognitive risk factors among men in the study who experienced the fewest concussion symptoms during their playing career (the lowest quartile), separately examining men with and without impairment in cognition-related QOL. In addition we conducted a sensitivity analysis restricted to men age 35 years or younger at the time of the questionnaire, who may have had more accurate recall of their concussion exposure during their playing years than older men in the study.
Results
In general, cognitive risk factors were more prevalent in men who reported more concussion symptoms at the time of football head injury (Table 1). There were particularly large differences in prevalence of pain interference in daily life (11.8 vs. 61.1%), high depressive symptoms (5.4 vs. 40.9%), high anxiety symptoms (5.5 versus 44.5%), physical impairment (10.1 versus 43.8%), short sleep duration (3.9 vs. 18.3%), and history of sleep apnea (15.5 vs. 29.3%).
Table 1.
Prevalence of Cognitive Risk Factors by Concussion Symptoms at Time of Football Head Injury, Harvard Football Players Health Study, 2015–2019 (N = 3803)
| |
|
Concussion symptom quartile |
|||
|---|---|---|---|---|---|
| |
All players |
Q1 (0–10, fewest) |
Q2 (10.5–23) |
Q3 (23.5–43) |
Q4 (43.5–130, most) |
| |
N = 3803 |
n = 928 |
n = 979 |
n = 924 |
n = 972 |
| % (n) | % (n) | % (n) | % (n) | ||
| Poor cognition-related QOL | 47.3 (1798) | 17.2 (160) | 35.9 (351) | 56.6 (523) | 78.6 (764) |
| Demographic factors | |||||
| Age, years, mean (SD) | 52.1 (14.3) | 54.0 (15.6) | 53.0 (14.8) | 51.2 (13.2) | 50.2 (13.1) |
| Race, Black | 37.3 (1419) | 36.6 (340) | 34.2 (335) | 39.3 (363) | 39.2 (381) |
| Race, White | 58.7 (2231) | 59.1 (549) | 62.4 (611) | 57.4 (530) | 55.7 (541) |
| Risk factors | |||||
| Sleep apnea history | 22.2 (845) | 15.5 (144) | 20.1 (197) | 23.7 (219) | 29.3 (285) |
| Sleep duration, <5 hrs | 9.8 (372) | 3.9 (36) | 5.8 (57) | 10.9 (101) | 18.3 (178) |
| BMI | |||||
| ≥30 < 35 kg/m2 | 34.7 (1320) | 31.4 (291) | 34.9 (342) | 35.1 (325) | 37.2 (362) |
| ≥35 kg/m2 | 18.4 (701) | 17.0 (158) | 16.6 (162) | 19.2 (177) | 21.0 (204) |
| Smoking, current | 3.2 (120) | 2.3 (21) | 2.8 (27) | 3.8 (35) | 3.8 (37) |
| Physical activity | |||||
| Low-intensity, none | 49.2 (1870) | 49.9 (463) | 51.8 (507) | 47.1 (435) | 47.8 (465) |
| High-intensity, none | 32.7 (1242) | 28.2 (262) | 31.1 (305) | 32.5 (300) | 38.6 (375) |
| Walking, none | 25.2 (957) | 24.9 (231) | 23.7 (232) | 24.1 (223) | 27.8 (271) |
| Weight training, none | 34.8 (1323) | 32.7 (303) | 33.8 (331) | 35.8 (331) | 36.8 (358) |
| Total, lowest quartile, ≤5 mets/week | 25.6 (972) | 21.4 (199) | 24.0 (235) | 25.5 (236) | 31.1 (302) |
| Cardiovascular disease history | 18.3 (697) | 17.8 (165) | 19.2 (188) | 18.5 (171) | 17.8 (173) |
| Stroke history | 2.7 (103) | 1.8 (17) | 2.7 (26) | 3.0 (28) | 3.3 (32) |
| High blood pressure history | 37.1 (1409) | 37.4 (347) | 34.2 (335) | 38.2 (353) | 38.5 (374) |
| Diabetes history | 8.6 (328) | 7.3 (68) | 10.1 (99) | 8.8 (81) | 8.2 (80) |
| Pain interference in daily life, high | 34.2 (1299) | 11.8 (109) | 22.3 (218) | 40.9 (378) | 61.1 (594) |
| High depressive symptoms | 20.8 (789) | 5.4 (50) | 12.8 (125) | 23.5 (217) | 40.9 (397) |
| High anxiety symptoms | 22.3 (847) | 5.5 (51) | 13.3 (130) | 25.4 (234) | 44.5 (432) |
| Alcohol consumption, heavy | 13.2 (501) | 11.1 (103) | 11.9 (116) | 15.7 (145) | 14.1 (137) |
| Physical impairment | 24.7 (938) | 10.1 (93) | 18.4 (180) | 25.9 (239) | 43.8 (426) |
| Total number of risk factors, mean (SD)* | 3.8 (2.8) | 2.8 (2.2) | 3.3 (2.6) | 4.0 (2.9) | 5.0 (3.1) |
QOL, quality of life; SD, standard deviation.
BMI ≥35 and heavy alcohol consumption are considered the risk levels in the count of total risk factors. Physical activity in metabolic equivalent (METS) hours/week was calculated from reported frequency of five types of exercise, then divided into quartiles.
Nearly every cognitive risk factor was associated with cognition-related QOL in age- and race-adjusted models (Table 2). Associations of physical functioning, pain, depression, and anxiety with impairment in cognitive-related QOL were very strong (RR range, 2.21–2.70, p < 0.0001 for all). Associations of short sleep duration and low physical activity were also strong (RR range, 1.57–1.69, p < 0.0001 for all). In models further adjusted for concussion symptoms, associations were attenuated but still statistically significant for all factors except alcohol consumption and BMI.
Table 2.
Association of Potentially Modifiable or Treatable Risk Factors with Moderate or Severe Impairment in Cognition-Related Quality of Life, Harvard Football Players Health Study, 2015–2019 (N = 3803)*
| |
Age- and race-adjusted |
Further adjusted for concussion symptoms |
|---|---|---|
| Risk Ratio (95% confidence interval) | ||
| Risk factors | ||
| Current smoking | 1.50 (1.34, 1.68)*** | 1.34 (1.20, 1.50)*** |
| Alcohol consumption, heavy vs. moderate | 1.14 (1.04, 1.26)** | 1.07 (0.98, 1.17) |
| Physical activity, lowest vs. highest quartile | 1.57 (1.42, 1.73)*** | 1.37 (1.27, 1.51)*** |
| Sleep apnea history | 1.46 (1.37, 1.56)*** | 1.23 (1.15, 1.30)*** |
| Sleep duration, <5 vs. ≥5 h/night | 1.69 (1.58, 1.81)*** | 1.25 (1.18, 1.33)*** |
| Body mass index, ≥35 vs. <25 kg/m2 | 1.33 (1.11, 1.59)** | 1.12 (0.96, 1.30) |
| Cardiovascular disease history | 1.18 (1.08, 1.29)*** | 1.11 (1.02, 1.19)** |
| Stroke history | 1.38 (1.18, 1.61)*** | 1.18 (1.02, 1.35)* |
| High blood pressure history | 1.23 (1.14, 1.32)*** | 1.14 (1.07, 1.21)*** |
| Diabetes history | 1.22 (1.11, 1.35)*** | 1.17 (1.08, 1.28)*** |
| Physical impairment | 2.21 (2.08, 2.34)*** | 1.62 (1.53, 1.73)*** |
| High pain interference in daily life | 2.70 (2.52, 2.88)*** | 1.96 (1.82, 2.10)*** |
| High depressive symptoms | 2.39 (2.26, 2.52)*** | 1.72 (1.62, 1.82)*** |
| High anxiety symptoms | 2.46 (2.32, 2.60)*** | 1.77 (1.67, 1.87)*** |
| History of football-related concussion symptoms, highest vs. lowest quartile | 4.55 (3.94, 5.26)*** | – |
Each risk factor was entered as the independent variable in separate models adjusted for age at questionnaire and race/ethnicity. For variables with more than one level, including alcohol, body mass index, and concussion symptoms, only the most severe is shown. Physical activity in metabolic equivalent (METS) hours/week was calculated from reported frequency of five types of exercise, then divided into quartiles.
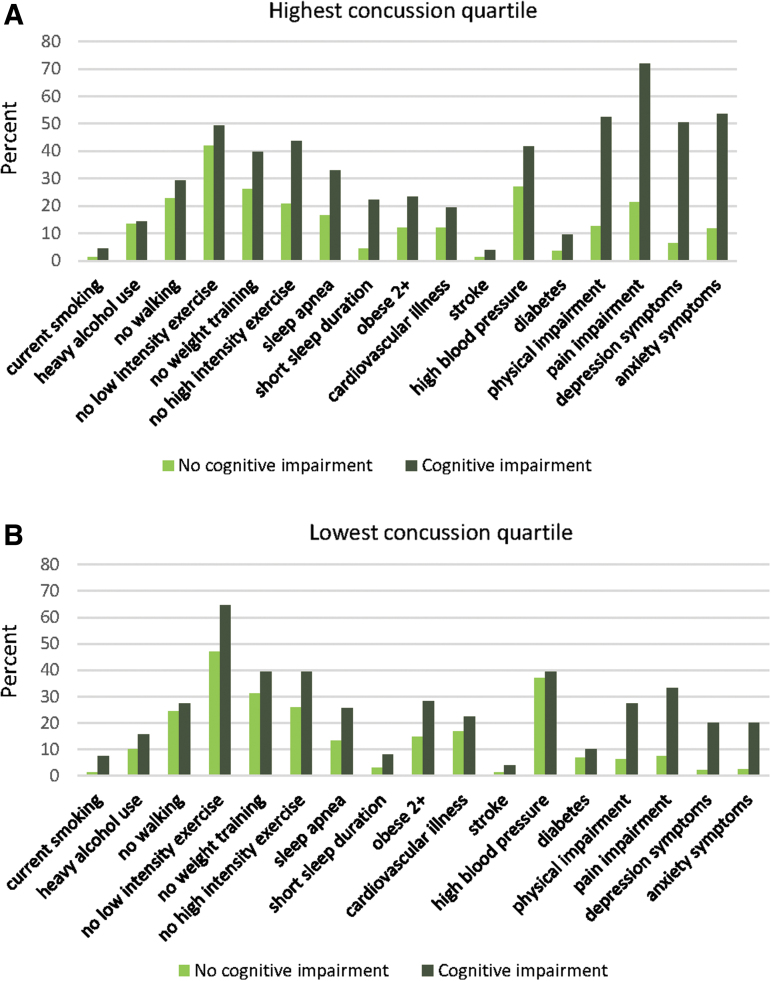
Prevalence of each cognitive risk factor was higher among the unhealthy concussed than the healthy concussed, with the exception of stroke, heavy drinking, no walking, and no low intensity exercise (Fig. 1A, all p < 0.05). The greatest differences were in high pain interference in daily life (72.0 vs. 21.2%), high depressive symptoms (50.3 vs. 6.3%), high anxiety symptoms (53.4 vs. 11.6%), and physical impairment (52.4 vs. 12.5%). There were also substantial differences in prevalence of sleep apnea, short sleep duration, no high-intensity exercise, no weight training, high blood pressure, and BMI ≥35 kg/m2 (all differences >10 percentage points).
FIG. 1.
Prevalence (%) of potentially treatable or modifiable risk factors among men with the most and the fewest concussion symptoms at time of football injury (highest and lowest quartile) and cognition-related quality of life, Harvard Football Players Health Study, 2015–2019 (n = 1900).
The healthy concussed had mean = 2.5 (SD = 1.9) cognitive risk factors, while the unhealthy concussed had mean = 5.6 (SD = 3.0) risk factors. Similarly, among men with the fewest football-related concussion symptoms (lowest quartile), men with impairment in cognition-related QOL had greater prevalence of each risk factor compared with men with no impairment (all p < 0.05), with the exception of no walking, diabetes, high blood pressure, and cardiovascular illness (Fig. 1B), although differences were less pronounced than among men in the highest quartile of concussion symptoms. Results were very similar in the subsample of men age 35 years or younger at the time of the questionnaire (Supplementary Table S1).
Because physical impairment and pain were far more prevalent among unhealthy versus healthy concussed, we hypothesized that more bodily injury during football playing years among the unhealthy concussed might contribute to their poorer cognition-related QOL. To explore this hypothesis, we examined the occurrence of seven types of surgical procedures (Table 3) during football playing years, as an indicator of football-related bodily injury. We did not find notable differences in occurrence of these surgeries when comparing the healthy concussed with the unhealthy concussed group, either for specific kinds of surgeries (Table 3, all chi-square p > 0.05) or for number of types of surgeries (healthy concussed, mean = 1.7 types of surgeries, SD = 1.4; unhealthy concussed, mean = 1.7, SD = 1.3, t test p = 0.64).
Table 3.
Surgery during Playing Years among Men in the Highest Quartile of Concussion Symptoms, by Prevalence of Moderate or Severe Cognition-Related Quality of Life, Harvard Football Players Health Study, 2015–2019 (N = 972)
| |
Healthy concussed* |
Unhealthy concussed |
|---|---|---|
| n = 208 | n = 764 | |
| Type of surgery | ||
| Neck | 7.2 | 7.1 |
| Back | 7.2 | 9.2 |
| Knee | 54.8 | 55.6 |
| Ankle | 20.7 | 19.0 |
| Shoulder | 29.3 | 28.7 |
| Hand | 26.4 | 24.5 |
| Anterior cruciate ligament | 24.5 | 21.3 |
| Number of types of surgery | 1.7 | 1.7 |
The table is restricted to men in the top quartile of self-reported concussion symptoms at the time of football injury. “Unhealthy concussed” are men who reported current moderate or severe impairment in cognition-related quality of life. “Healthy concussed” men did not report moderate or severe impairment. No difference between the groups is statistically significant at p < 0.05.
Discussion
Depression, anxiety, pain, and physical impairment were very strongly associated with moderate or severe impairment in cognition-related QOL in this sample of former professional American-style football players. We also found low physical activity to be strongly associated with impairment in cognition-related QOL.
Men who had sustained high levels of concussion symptoms during football playing years and yet did not have cognition-related impairment (the healthy concussed) had far fewer additional cognitive risk factors than men who did have cognition-related impairment. Particularly notable were differences in pain and physical functioning in these two groups, despite our finding that the two groups did not differ in occurrence of seven types of surgical procedures during playing years. Among highly concussed men with cognition-related impairment, prevalence of pain interference in daily life (72%) and impairment in physical functioning (52%) were strikingly high.
Our findings raise the possibility that at least some cognition-related QOL difficulties in men who have experienced many concussions could be ameliorated with treatment of co-occurring cognitive risk factors. We cannot rule out the alternative, or possibly co-occurring possibility, that cognitive impairment may have caused increased prevalence of the risk factors. For example, cognitive impairment could lead to depression, anxiety, and physical impairment.28–30 It is also possible that some of the risk factors may be indicators of brain injury (e.g., depression, anxiety, sleep problems, pain).31–33 Even were this the case, however, treatment of these risk factors could still potentially improve cognitive well-being if presence of these factors (e.g., depression, anxiety) subsequently worsens cognitive function or cognition-related QOL.34
With a cross-sectional study, we have limited ability to differentiate among these possibilities. Thus, exploring these issues prospectively would be of value. In addition, our assessment of concussion symptoms at the time of football head injury was retrospective and may be subject to recall bias, although results were very similar in analyses restricted to men age 35 and younger. There could be concern that players with depression or anxiety (or any other of the health conditions we examine) may overreport their concussion symptom history. If this were occurring, though, it would mean that within groups of high or low concussion symptom players, those with depression (or any of the other conditions) would in reality have had fewer concussion symptoms. Thus, to the extent that concussions lead to increased cognitive impairment (or poor cognitive function leads to increased concussion symptom reporting), then that biased reporting would result in those conditions (e.g., depression) being associated with better cognitive health (because those with the health conditions would have in reality experienced fewer concussion symptoms), opposite to our findings.
Despite limitations, we nevertheless identify many cognitive risk factors, including pain, mood problems, sleep apnea, short sleep duration, obesity, and lack of exercise, that are commonly present in former professional American-style players with cognition-related impairment. This raises the possibility that better treatment for these health factors may reduce cognitive problems in this population of former professional athletes. This possibility should be explored further, because it offers potential interventions for men who might otherwise think their cognitive decline is inevitable.
A longitudinal study that can better determine the sequencing of these cognitive risk factors, a clinical trial to investigate potential benefits of intervening on these factors, or a trial to treat or prevent these risk factors in the transition from professional football career35 would be important next steps.
Supplementary Material
Funding Information
This study was funded by the National Football League (NFL) Players Association.
Author Disclosure Statement
Dr. Baggish has received funding from the National Football League Players Association, the American Heart Association, the American Society of Echocardiography and receives compensation for his role as team cardiologist from US Soccer, US Rowing, the New England Patriots, the Boston Bruins, the New England Revolution, and Harvard University. He also serves on the editorial board for Circulation, and serves as an associate editor for Medicine Science Sports & Exercise. Dr. Zafonte received royalties from: (1) Oakstone for authorship of an educational CD; (2) Demos Publishing for serving as co-editor of the text Brain Injury Medicine. Dr. Zafonte serves on the Scientific Advisory Board of Myomo, Oxeia Biopharma, ElMINDA, and Biodirection. He also evaluates patients in the MGH Brain and Body-TRUST Program, which is funded by the NFL Players Association (NFLPA). All other authors are either partially or fully supported by the Football Players Health Study at Harvard University, which is in turned sponsored by the NFLPA. Please note that the NFLPA had no role in: the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Other authors report no potential or actual conflicts of interest.
Supplementary Material
References
- 1. Baumgart, M., Snyder, H.M., Carrillo, M.C., Fazio, S., Kim, H., and Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 11, 718–726 [DOI] [PubMed] [Google Scholar]
- 2. DeKosky, S.T., Ikonomovic, M.D., and Gandy, S. (2010). Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 363, 1293–1296 [DOI] [PubMed] [Google Scholar]
- 3. Roberts, A.L., Pascual-Leone, A., Speizer, F.E., Zafonte, R., Baggish, A., Taylor, H., Nadler, L., Courtney, T.K., Connor, A., Grashow, R.G., Stillman, A., Marengi, D.A., and Weisskopf, M.G. (2019). Exposure to American football and neuropsychiatric health in former National Football League (NFL) players: findings from the Football Players Health Study Am. J. Sports Med. 47, 2871–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailes, J.E., Petraglia, A.L., Omalu, B.I., Nauman, E., and Talavage, T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 5. Dashnaw, M.L., Petraglia, A.L., and Bailes, J.E. (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus 33, E5. [DOI] [PubMed] [Google Scholar]
- 6. Osorio, R.S., Gumb, T., Pirraglia, E., Varga, A.W., Lu, S.E., Lim, J., Wohlleber, M.E., Ducca, E.L., Koushyk, V., Glodzik, L., Mosconi, L., Ayappa, I., Rapoport, D.M., and de Leon, M.J. (2015). Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84, 1964–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tworoger, S.S., Lee, S., Schernhammer, E.S., and Grodstein, F. (2006). The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis. Assoc. Disord. 20, 41–48 [DOI] [PubMed] [Google Scholar]
- 8. Keage, H.A., Banks, S., Yang, K.L., Morgan, K., Brayne, C., and Matthews, F.E. (2012). What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 13, 886–892 [DOI] [PubMed] [Google Scholar]
- 9. Debette, S., Seshadri, S., Beiser, A., Au, R., Himali, J.J., Palumbo, C., Wolf, P.A., and DeCarli, C. (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hassing, L.B., Hofer, S.M., Nilsson, S.E., Berg, S., Pedersen, N.L., McClearn, G., and Johansson, B. (2004). Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing 33, 355–361 [DOI] [PubMed] [Google Scholar]
- 11. Cooper, C., Sommerlad, A., Lyketsos, C.G., and Livingston, G. (2015). Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am. J. Psychiatry 172, 323–334 [DOI] [PubMed] [Google Scholar]
- 12. Berryman, C., Stanton, T.R., Bowering, K.J., Tabor, A., McFarlane, A., and Moseley, G.L. (2013). Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain 154, 1181–1196 [DOI] [PubMed] [Google Scholar]
- 13. Moriarty, O., McGuire, B.E., and Finn, D.P. (2011). The effect of pain on cognitive function: a review of clinical and preclinical research. Prog. Neurobiol. 93, 385-404 [DOI] [PubMed] [Google Scholar]
- 14. Geerlings, M.I., Schoevers, R.A., Beekman, A.T., Jonker, C., Deeg, D.J., Schmand, B., Adèr, H.J., Bouter, L.M., and Van Tilburg, W. (2000). Depression and risk of cognitive decline and Alzheimer's disease: results of two prospective community-based studies in the Netherlands. Br. J. Psychiatry 176, 568–575 [DOI] [PubMed] [Google Scholar]
- 15. Sinoff, G., and Werner, P. (2003). Anxiety disorder and accompanying subjective memory loss in the elderly as a predictor of future cognitive decline. Int. J. Geriatr Psychiatry 18, 951–959 [DOI] [PubMed] [Google Scholar]
- 16. DeLuca, A.K., Lenze, E.J., Mulsant, B.H., Butters, M.A., Karp, J.F., Dew, M.A., Pollock, B.G., Shear, M.K., Houck, P.R., and Reynolds, C.F. III, (2005). Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int. J. Geriatr Psychiatry 20, 848–854 [DOI] [PubMed] [Google Scholar]
- 17. Robertson, D.A., Savva, G.M., and Kenny, R.A. (2013). Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res. Rev. 12, 840–851 [DOI] [PubMed] [Google Scholar]
- 18. Solfrizzi, V., Scafato, E., Seripa, D., Lozupone, M., Imbimbo, B.P., D'Amato, A., Tortelli, R., Schilardi, A., Galluzzo, L., Gandin, C., Baldereschi, M., Di Carlo, A., Inzitari, D., Daniele, A., Sabbà, C., Logroscino, G., Panza, F., Scafato, E., Farchi, G., Galluzzo, L., Gandin, C., Capurso, A., Panza, F., Solfrizzi, V., Lepore, V., Livrea, P., Motta, L., Carnazzo, G., Motta, M., Bentivegna, P., Bonaiuto, S., Cruciani, G., Postacchini, D., Inzitari, D., Amaducci, L., Di Carlo, A., Baldereschi, M., Gandolfo, C., Conti, M., Canal, N., Franceschi, M., Scarlato, G., Candelise, L., Scapini, E., Rengo, F., Abete, P., Cacciatore, F., Enzi, G., Battistin, L., Sergi, G., Crepaldi, G., Maggi, S., Minicuci, N., Noale, M., Grigoletto, F., Perissinotto, E., and Carbonin, P. (2017). Reversible cognitive frailty, dementia, and all-cause mortality. The Italian longitudinal study on aging. J. Am. Med. Dir. Assoc. 18, 89..e81–89.e88. [DOI] [PubMed] [Google Scholar]
- 19. Heyn, P., Abreu, B.C., and Ottenbacher, K.J. (2004). The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch. Phys. Med. Rehabil. 85, 1694–1704 [DOI] [PubMed] [Google Scholar]
- 20. Cella, D., Lai, J.S., Nowinski, C., Victorson, D., Peterman, A., Miller, D., Bethoux, F., Heinemann, A., Rubin, S., Cavazos, J., Reder, A.T., Sufit, R., Simuni, T., Holmes, G.L., Siderowf, A., Wojna, V., Bode, R., McKinnery, N., Podrabsky, T., Wortman, K., Choi, S., Gershon, R., Rothrock, N., and Moy, C. (2012). Neuro-QOL Brief measures of health-related quality of life for clinical research in neurology. Neurology 78, 1860–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. (2018). HealthMeasures: Transforming How Health is Measured. Northwestern University: Evanston, IL.. [Google Scholar]
- 22. Plummer, F., Manea, L., Trepel, D., and McMillan, D. (2016). Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen. Hosp. Psychiatry 39, 24–31 [DOI] [PubMed] [Google Scholar]
- 23. Kroenke, K., Spitzer, R.L., Williams, J.B., and Löwe, B. (2009). An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics 50, 613–621 [DOI] [PubMed] [Google Scholar]
- 24. HealthMeasures. PLEASE PROVIDE MORE INFORMATION. www.healthmeasures.net
- 25. Centers for Disease Control and Prevention (2017). Defining Adult Overweight and Obesity.www.cdc.gov
- 26. Pan, W. (2001). Akaike's information criterion in generalized estimating equations. Biometrics 57, 120–125 [DOI] [PubMed] [Google Scholar]
- 27. Zou, G. (2004). A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 [DOI] [PubMed] [Google Scholar]
- 28. Auyeung, T.W., Kwok, T., Lee, J., Leung, P.C., Leung, J., and Woo, J. (2008). Functional decline in cognitive impairment – the relationship between physical and cognitive function. Neuroepidemiology 31, 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuo, H.K., Leveille, S.G., Yu, Y.H., and Milberg, W.P. (2007). Cognitive function, habitual gait speed, and late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. Gerontology 53, 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beaudreau, S.A. and O'Hara, R. (2008). Late-life anxiety and cognitive impairment: a review. Am. J. Geriatr Psychiatry 16, 790–803 [DOI] [PubMed] [Google Scholar]
- 31. Verma, A., Anand, V., and Verma, N.P. (2007). Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 3, 357–362 [PMC free article] [PubMed] [Google Scholar]
- 32. Baumann, C.R., Werth, E., Stocker, R., Ludwig, S., and Bassetti, C.L. (2007). Sleep–wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 33. Bombardier, C.H., Fann, J.R., Temkin, N.R., Esselman, P.C., Barber, J., and Dikmen, S.S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fann, J.R., Uomoto, J.M., and Katon, W.J. (2001). Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics 42, 48–54 [DOI] [PubMed] [Google Scholar]
- 35. Isaacson, R.S., Hristov, H., Saif, N., Hackett, K., Hendrix, S., Melendez, J., Safdieh, J., Fink, M., Thambisetty, M., Sadek, G., Bellara, S., Lee, P., Berkowitz, C., Rahman, A., Meléndez-Cabrero, J., Caesar, E., Cohen, R., Lu, P.l., Dickson, S.P., Hwang, M.J., Scheyer, O., Mureb, M., Schelke, M.W., Niotis, K., Greer, C.E., Attia, P., Mosconi, L., and Krikorian, R. (2019). Individualized clinical management of patients at risk for Alzheimer's dementia. Alzheimers Dement. 15, 1588–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.