Abstract
Recent data show an interaction between COVID-19 and nicotine and indicate the need for an assessment of transdermal nicotine use in non-smokers. Assessments have been conducted into the short-term cognitive effects of nicotine and into diseases such as Parkinson's, Tourette syndrome, ADHD or ulcerative colitis.
Methods
Analyses of nicotine administration protocols and safety were conducted after reviewing Medline and Science Direct databases performing a search using the words [transdermal nicotine] AND [non-smoker] AND selected diseases.
Results
Among 298 articles identified, there were 35 reviewed publications reporting on 33 studies of non-smokers receiving transdermal nicotine for > 48 hours. In the 16 randomized trials, 7 crossover, 1 case/control and 9 open studies patients received an initial nicotine dose of between 2.5 mg and 15 mg/day. In 22 studies, daily doses increased by 2 to 7 steps in 3 to 96 days until the dose was between 5 mg and 105 mg/day. The target nicotine dose was 19.06 ± 20.89 mg/day. The 987 non-smokers (534 never-smokers, 326 ex-smokers and 127 classified as “non-smokers”) received or did not receive nicotine. The most common side-effects were nausea and skin itching. Forty-three (7.1%) non-smokers stopped treatment because of an adverse event of nicotine. No hospitalization related to nicotine side-effects were reported.
Conclusion
Despite a relatively safe tolerance profile, transdermal nicotine therapy in non-smokers can only be used in clinical trials. There is a lack of formal assessment of the potential risk of developing a tobacco addiction. This review offers baseline data to set a transdermal nicotine protocol for non-smokers with a new purpose.
Keywords: Transdermal nicotine, Non-smoker, Side-effects, Clinical trial, COVID-19
1. Introduction
Recent data suggest an interaction between COVID-19 and nicotine [1], [2], [3], [4], [5].
Studies show that the penetration of SARS-CoV-2 into upper respiratory tract, bronchial and pulmonary cells involve transmembrane receptor ACE2 [6], which probably interacts with acetylcholine nicotinic receptors of the α7 subtype [7]. The mechanism of the interactions remains hypothetical [7], [8].
Excessive secondary cytokine reaction plays a role in the mortality associated with COVID [7], [8]. One of the hypotheses to explain the effect of nicotine on the occurrence of severe forms of COVID [8] and death is based on the loss of the downregulation of the parasympathetic nervous system, which exerts an inhibitory effect on cytokine storm, especially in the lung and digestive tract. The α 7-type nicotinic receptors [8] are part of this chain of reaction. Nicotine intake may reduce the onset of a “cytokine storm” in the lung or other organs [7], [8].
These pathophysiological hypotheses, these unexpected epidemiological data [2], [4], [5] and these pathophysiological hypotheses lead us to consider the use of transdermal nicotine in non-smokers, in controlled clinical trials for non-smokers, to assess the efficacy, the safety and the risk of developing nicotine addiction.
Transdermal nicotine is used in current medical practice for smoking cessation. When used with this indication, it has been the subject of numerous publications on smokers, with or without underlying disease. The safety of this treatment is established by Cochrane reviews [9]. Although some case studies suggest a link between the use of nicotine patches and cardiovascular events, their use has been shown to be safe in patients with cardiac disease and is not associated with a risk of myocardial infarction among smokers and ex-smokers [10]. In non-smokers, the use of nicotine replacement therapy has been assessed to treat Alzheimer's-related diseases and cognitive disorders in the elderly and post-cancer chemotherapy [11], [12], [13], [14], [15], [16], [17], autism [18], Attention-Deficit Hyperactivity Disorder (ADHD) [19], [20], major depressive disorder [21], [22], [23], Parkinson's disease [24], [25], [26], [27], [28], [29], Tourette syndrome [30], [31], [32], [33] and ulcerative colitis [34], [35], [36], [37], [38], [39], [40], [41], [42]. Biological inflammatory effects of nicotine replacement treatment have been tested at least 2 days in healthy non-smoker volunteers in three studies [43], [44], [45].
We have not identified general reviews on transdermal nicotine use for non-smokers. Reviews [46], [47] covered the use of transdermal nicotine and nicotine enemas in ulcerative colitis [48].
The analysis of the clinical or biological effects or benefits of transdermal nicotine on very heterogeneous diseases or the biological facts gleaned from our analysis is not the objective of this systematic review, which brings together many studies, often with no structured report.
The objective of the present review is to conduct a systematic analysis of the nicotine doses used, the duration of use, the progressive increase or decrease of the doses, the side-effects leading, or not, to an interruption of the treatment, and the risk of developing a nicotine addiction in non-smokers.
2. Material and methods
2.1. Selection of articles
The literature search was conducted in June 2020, using two databases (PubMed, ScienceDirect), and the search terms: [‘transdermal nicotine’] AND [‘non-smoker’] with or without AND ‘colitis’ OR ‘ADHD’ OR ‘Parkinson’ OR ‘Tourette’ OR ‘depression’]. We also verified the absence of studies meeting the selection criteria in the references from the first selection of articles and in the Cochrane reviews and clinicaltrial.org data bases.
The articles used for the analysis were selected at first by their title and then by their abstracts and were reviewed by two independent medical doctors.
We selected peer-reviewed studies concerning human, published in French or English, and published after 1992 (date of the start of marketing of transdermal nicotine) in which non-smokers used transdermal nicotine for at least two days.
Duplicate publications and studies which did not concern transdermal nicotine and short-term treatment (less than 48 h), and involved subjects who were other than non-smokers, were excluded from the analysis.
2.2. Analysis of the articles
Selected studies were focused on the characteristics of the populations (never smokers or ex-smokers), how nicotine was administered: initial dose, target dose, gradual increase of dose procedure, reduction of dose in case of poor tolerance, interruption due to adverse event and the characteristics of side-effects.
In cases where a study was published twice, the second publication was considered only if it provided useful data that was not included in the first study (follow-up or more precise analysis of adverse event).
The selected studies were classified according to their methods: prospective controlled studies (randomized or not) versus placebo or other comparators, crossover studies in which patients received transdermal nicotine or placebo in a random order, or studies without comparator.
The daily nicotine dose was identified at the first day in all studies. The number of steps to increase nicotine dose, the duration of the gradual rise of dose in days, and the final target of transdermal nicotine daily doses were analysed in case of progressive increase of the nicotine dose.
Reported side-effects were analysed with a focus on those leading to discontinuation of treatment, reported in most of the studies. Detailed analysis of side-effects (nausea, itching, abnormal dreams, cardiovascular effects, neurological disturbances) is very inconsistently reported in the articles. Eleven of the 33 studies did not report side-effects. Due to the poor quality of adverse event reports in most studies, we took the decision to perform a specific retrospective analysis in the subgroup of 13 studies that provided a clear report of adverse events in each treatment group with quantification of nausea, itching and skin irritation, abnormal dreams and sleep disorders and neurological disturbances, and a report of dropouts from treatment because of side effects.
We also looked for direct or indirect signs of an induction of nicotine dependence or initiation of smoking.
Originally, we had planned to conduct a separate analysis of never smokers (less than 100 cigarettes in their lifetime) and former smokers (smoker who stopped smoking more than three months earlier), but the available data were insufficient to conduct such an analysis.
We also checked the disclosures of interest and links to pharmaceutical and tobacco industries (see Excel file in additional material section).
3. Results
3.1. Studies selected
3.1.1. Number of studies
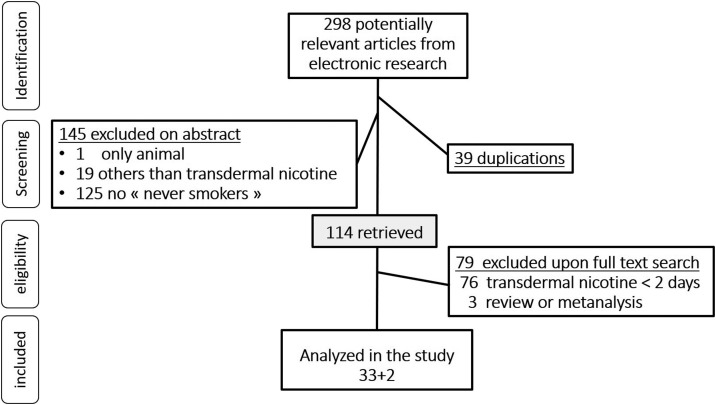
Among the 298 articles selected for our analysis, 35 covered 33 studies about the use of transdermal nicotine for more than 48 hours for non-smokers. Two of the studies were published twice, to incorporate new follow-up information or new side effects. The process of identification, screening and selection of included articles is illustrated in the PRISMA flow diagram (Fig. 1 ).
Fig. 1.
Flow diagram of the selected studies for the systematic review of transdermal nicotine in non-smokers according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).
3.1.2. Typology of the selected studies
All the studies are prospective. More half of the studies included two comparative groups of patients. Seventeen of these comparative studies (regrouping 758 of 987 patients) were randomized; one was a prospective case control study [38] (Table 1 ). One of these studies compared two doses of transdermal nicotine (7 mg/day and 14 mg/day) with a reference group that received no nicotine [11]. Two of the studies compared transdermal nicotine to prednisone [35], [36], [42]. One study compared transdermal nicotine to fluoxetine [22]. Seven studies, which included 86 patients, were designed as a double-blind crossover between nicotine and placebo. Nine prospective studies were open and non-comparative and included 100 patients.
Table 1.
Main characteristics of the 33 studies on the use of transdermal nicotine in non-smokers included in this analysis.
| Type of studies | Randomized studies | Case/control study | Cross over studies | Non comparative studies | Total |
|---|---|---|---|---|---|
| Study (n) | 16 | 1 | 7 | 9 | 33 |
| Subject (n) | 758 | 43 | 86 | 100 | 987 |
| Mean age (year ± sd) | 42.50 ± 18.04 | 43.00 | 55.86 ± 27.08 | 40.44 ± 22.50 | 44.79 ± 21.25 |
| Sex ratio (M/F) | 388/262 | ND | 45/23 | 69/25 | 502/410 |
| Never smoker (n) | 400 | 43 | 26 | 65 | 534 |
| Former smoker (n) | 293 | 0 | 4 | 29 | 326 |
| Non-smoker with no precision (n) | 65 | 0 | 56 | 6 | 127 |
| Nicotine arm (n) | 404 | 20 | 86 | 100 | 608 |
| Placebo (or other comparator arm) (n) | 356 | 23 | 86 | 0 | 465 |
| Mean target dose (mg ± sd) | 19.00 ± 19.66 | 15.00 | 13.86 ± 6.82 | 24.78 ± 30.42 | 19.36 ± 20.90 |
| Mean initial dose (mg ± sd) | 5.38 ± 2.75 | 0 | 11.00 ± 7.37 | 6.67 ± 3.42 | 7.21 ± 4.84 |
| Mean number of step (n) | 3.06 | 1.00 | 1.57 | 2.89 | 2.64 |
| Mean gradual rise of dose duration (day ± sd) | 13.19 ± 18.90 | NA | 2.43 ± 3.31 | 17.44 ± 30.73 | 11.67 ± 20.95 |
| Duration of active treatment (days ± sd) | 7.81 ± 79.53 | 162.00 | 15.43 ± 11.91 | 37.89 ± 57.86 | 56.73 ± 69.94 |
3.1.3. Disease concerned by selected studies
Trials with transdermal nicotine of non-smokers covered Attention-Deficit Hyperactivity Disorder, or ADHD (n = 2), autism (n = 1), cognitive impairment in the elderly (n = 6), post cancer chemotherapy (n = 1), major depressive disorder (n = 3), Parkinson's disease (n = 5), Tourette disease (n = 4), ulcerative colitis (n = 8) and healthy volunteers (n = 3) (see supplemental electronic files materiel).
3.2. Protocols of administration of nicotine
3.2.1. Dose of transdermal nicotine assessed in studies
The daily target dose of transdermal nicotine varied from 5 mg/day to 105 mg/day in different studies.
Daily target doses were 10 mg/day maximum for patients with autism, ADHD and Tourette syndrome. The highest target dose (105 mg/day) was in a study for patients with Parkinson's disease (34), but the median target dose for Parkinson study was less than half that, at 50 mg/day.
Seven studies proposed a target nicotine dose of > 20 mg/day for non-smokers with a mean age over 55 years old. Target dose varied from 5 mg/day to 7 mg/day for patients under 25 years old.
The first-day nicotine dose varied from 2.5 mg to 22 mg/day (mean = 7.28 ± 4.78 mg/day).
The mean target dose of nicotine in the 33 studies was 19.06 ± 20.89 mg/day. Twenty studies used patches delivering nicotine in 16 hours with a mean target nicotine daily dose of 22.25 ± 26.08 mg/day (1.39 mg/h). Thirteen studies used patches delivering nicotine in 24 hours with a mean daily target dose of nicotine of 14.15 ± 6.58 mg/day (0.59 mg/h).
3.2.2. Gradual rise of dose to reach the target of nicotine delivered
In 11 studies, full dose was provided the first day without gradual increase (11.36 ± 5.89 mg/day). In 22 studies, daily dose was progressively increased until 7 mg/day to 105 mg/day (mean dose = 23.36 ± 24.47 mg/day) in 2 to 7 steps for a duration from 3 to 96 days.
At the end of the plateau treatment period, a gradual decrease of nicotine dose was set up in 11 of the 33 studies.
3.2.3. Duration of transdermal nicotine delivery
The mean duration of transdermal nicotine treatment was 56.61 ± 71.10 days with a large range from 2 to 238 days. The duration of the treatment was less than 10 days for patients suffering from ADHD, autism, Tourette syndrome and for the healthy subjects. Treatment lasting over 100 days concerned only patients with Parkinson's disease or with major depressive disorder.
3.3. Patients included
3.3.1. Population of the study
Patients studied were aged 10 to 80 years old, with a mean age in the nicotine group of 44.79 ± 21.25 years, with important variations depending on the disease studied. The 987 patients were non-smokers, including 326 ex-smokers (stopped smoking 3 months to over 8 years earlier, according to the studies); 534 never-smokers and 127 who did not specific the subcategory of non-smoker. Table 1 presents the main characteristics of these studies, including the transdermal treatment applied in each type of study. A total of 608 non-smokers were assigned to receive nicotine and 465 were assigned to receive a transdermal placebo or other comparator for a minimum period of 2 days. Among them, 86 non-smokers were assigned to receive transdermal nicotine and transdermal placebo in cross-over studies, and 465 non-smokers were assigned to receive placebo or other comparator in non-crossover studies.
3.3.2. Treatment cessation because of the side-effect
The rates of discontinuation of treatment reported for side-effects are 43/608 (7.1%) for non-smokers receiving transdermal nicotine and 9/465 (1.9%) for non-smokers receiving transdermal placebo or another comparator.
There are no changes or only trends to variation of dropping out rate of the treatment according to age, nicotine dose, gradual rise of dose and disease. No hospitalizations or deaths were reported as adverse events due to transdermal nicotine.
3.3.3. Side effects associated with transdermal nicotine treatment or comparator
A total of 320/612 patients of nicotine group reported side effects. In the 612 patients of the nicotine group, 648 side-effects occurred (Ratio 1.07 episode of SE/patient). Ratios of 0.25 episode of nausea, 0.04 sleep or dream disorders, 0.22 itching or other skin disorders were reported in patients receiving nicotine.
Nausea was the most common adverse event and the most common nicotine-related disorder. It was reported 3.02 times more frequently in the nicotine group than in comparator groups.
The second most frequent adverse event was itching under the patches. It was 1.85 times more frequent with nicotine than with placebo patches.
Various neurological effects (dizziness, feeling of empty headedness, headache) were too poorly described to provide a quantitative analysis from the outcomes of the studies. Sleep disorders were infrequent (6.39% with nicotine, 5.22% with placebo patches) but most studies with comparative assessment between groups used a 16 h nicotine patch (7/12) with no nicotine delivery during the night.
No severe cardiovascular side-effects were reported apart from some non-attributable bradycardia [40] and orthostatic hypotension when nicotine was used at remarkably high doses in non-smokers (90 mg/day) [28]. Even in an elderly population at risk of cardiovascular events, no severe side-effects were reported in studies of mild cognitive impairments [12], major depressive disorder [21] or Parkinson’ disease in which patients received very high nicotine doses [28].
In the 9 studies reporting cardiovascular monitoring [11], [15], [17], [18], [21], [23], [43], [44], [45], hemodynamic parameters were mostly stable; however, a slight drop in systolic blood pressure [15] and slight transient increase of blood pressure [13] were observed. One study, which used Holter monitoring, reported an increase in heart rate of 5 beats per minute for patients receiving transdermal nicotine [15].
In the subgroup of 13 studies (519 patients) with a detailed report of the side-effects described in the methods chapter, the treatment dropout rate is 18/266 (6.77%) for non-smokers receiving transdermal nicotine and 5/252 (1.98%) for non-smokers receiving a transdermal comparator (Table 2 ).
Table 2.
Side-effects report in the 13/33 comparative studies who report adverse event in booth groups: nicotine and comparator (10 placebo,1 prednisolone, 2 no comparator).
| Transdermal nicotine | Comparator (placebo or other) | Ratio of percentage of AE | |
|---|---|---|---|
| Population | 266 | 252 | |
| Adverse event (n) | 504 | 243 | |
| Ratio adverse event/subjects | 1,89 | 0,96 | 1.96 |
| Nausea n (%) | 102 (38.35%) | 19 (7.53%) | 4.94 |
| Itching n (%) | 82 (30.83%) | 43 (17.06%) | 1.70 |
| Anormal dream n (%) | 24 (9.02%) | 16 (6.35%) | 1.01 |
| Drop out for adverse event n (%) | 18 (6.77%) | 5 (1.98%) | 3.42 |
The 266 patients assigned to transdermal nicotine groups presented 504 side-effects, i.e. 1.89 side-effects per patient. In the control groups, consisting of 252 patients, the number of side-effects reported was 243, i.e. 0.96 side-effects per patient.
3.3.4. Side-effects in never smokers compared to ex-smokers
Side-effects were recorded separately for never smokers and former smokers (no cigarette in the last 3 months to 8 years) in only five studies; four studies reported figures. More side-effects were reported in the group of never smokers (ratio: 25/29 = 0.86) than in the group of former smokers (ratio: 23/49 =0.47) in the four studies.
3.3.5. Assessment of nicotine dependence after the use of the transdermal nicotine
No withdrawal symptoms or associated addictive behaviour was reported in non-smokers in the 33 clinical trials with nicotine patches. For non-smokers, no trial reported a craving at the end of the use of the patch, and none reported the use of cigarettes or other nicotine sources at the end of the trials, including during an extended follow-up of 12 weeks after cessation of transdermal nicotine [37].
3.3.6. Efficacy of transdermal nicotine in study
In 5 of the 33 studies, the objective was only to analyse biological changes.
In 28 studies, there was an objective to improve clinical parameters and treat disease. No significant deleterious effect of nicotine on disease or clinical signs explored were reported. Six studies reported no effects of transdermal nicotine; 12 studies reported a trend toward improvement and 10 a significant improvement attributed to transdermal nicotine.
The 4 studies on Tourette disease displayed a significant improvement of tics. The positive effect persisted for 2 to 8 weeks after discontinuation of transdermal nicotine [31], [32].
The study on autism and ADHD showed a trend to improvement (n = 1) or improvement (n = 1).
In ulcerative colitis, studies and metanalyses reported no benefits compared to standard treatment [47].
In other diseases, the efficacy is poor of absent and results heterogeneous (Excel file in additional material section).
4. Discussion
4.1. Summary of the main results
4.1.1. Protocols of transdermal nicotine
The initial doses were between 2.5 mg/24 hrs to 22 mg/24 hrs. Target doses varied from 7 mg/24 hrs to 105, but only two studies, involving Parkinson's disease, used the highest doses. The duration of treatment varied from 2 to 240 days in the studies (Excel large file in additional material section).
4.1.2. Safety of transdermal nicotine use by non-smoker
Only 13 out of 33 studies included detailed reporting on side effects. In these 13 studies, the number of side effects per patient was 2 times higher with nicotine (1.89/0.96), and nausea was nearly 5 times more frequent in nicotine groups. Skin reactions were 1.7 times more frequent with nicotine patches than with placebo patches.
4.1.3. Risk of initiation of nicotine addiction
The number of nicotinic acetylcholine receptors is low in a nicotine naive subject. A never smoker who tries to smoke a first cigarette presents signs of overdose after 4 to 6 puffs. Nicotine tolerance appears quickly with the repetition of nicotine intake and produces a desensitization and a multiplication of the number of nicotinic receptors.
With cessation of smoking, the number of receptors on the surface of the cells usually decreases in 3 months, but with new exposure to nicotine, tolerance to nicotine appears much faster in a former smoker than in a never smoker. The risk is greater when nicotine is taken in a form which induces sudden peaks of nicotine, as with cigarette puffs. The transdermal route provides nicotine on a much more regular and slower basis, without peaks of nicotine, and therefore with much less desensitization and multiplication of the number of receptors.
Most of the studies did not formally assess the risk of initiation of dependence, but no cases of addiction to tobacco or nicotine were reported in the 608 non-smokers who received transdermal nicotine.
A formal study on withdrawal symptoms and addiction to nicotine at least one month after cessation of transdermal nicotine is needed to assess such risks in never smokers and in ex-smokers.
4.1.4. Efficacy of transdermal nicotine use for non-smokers
The efficacy of transdermal nicotine in the 33 studies is absent or marginal and anecdotal. Transdermal nicotine use in non-smokers is not recommended as a routine treatment in any disease.
4.2. Discussion of the results in view of other data on the targeting of nicotinic receptors
Nicotinic receptors are acetylcholine receptors that include a natural ligand acetylcholine, which is an essential substance for human life. For the acetylcholine receptors, other ligands can be found, such as muscarine for a subtype of receptor or nicotine for another subtype. Other substances, such as snake venom, can contain ligands of acetylcholine nicotinic receptors.
Motor symptoms in Tourette disease, dysfunction of the dopaminergic system is the main system involved, but there is also a dysfunction of the striatal cholinergic system. A down regulation of striatal interneuron transcripts and a reduction of the number of cholinergic interneurons has been demonstrated in Tourette's syndrome [43], [49]. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioural manifestations of Tourette syndrome [50]. Acetylcholine nicotinic receptor subtypes are involved in the beneficial effects of nicotine on Tourette syndrome, including acetylcholine nicotinic receptors α4β2, α6β2 and α7.
Ulcerative colitis is the only disease where meta-analysis has been conducted. Cochrane review reports meta-analysis efficacy and the side-effects of nicotine (all forms) in non-smokers in this situation [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]. The 2006 meta-analysis assessing transdermal and enema nicotine treatment (46) reported, in comparison to placebo, a non-significant trend of increased remissions (RR (95% CI) 1.40 (0.63–3.12)) but a significant increased risk of side effects with nicotine (RR = 1.95 (1.38–2.78)) and a trend of withdrawal from the study due to side effects (RR = 3.44 (0.71–16.71))). However, nicotine was shown to be less effective than corticosteroid.
But in 2021, transdermal nicotine is not recommended by any guidelines in non-smokers. Our COVID-19 hypothesis is not an invitation for a clinical use outside of studies.
4.3. Strengths and limitations of the study
The number of non-smokers who received nicotine in the 33 studies is only 609. The highest number of patients included in a random study [11] was 102 and the highest number of patients receiving transdermal nicotine in a study was 77. Only 5 out of 33 studies [11], [17], [18], [21], [29] were registered as clinical trials with an NCT number.
Our main objective was to specify the protocols using transdermal nicotine for non-smokers: dose, gradual increase of dose, adaptation of the dose to improve the tolerance, duration of the treatment and the decrease of the dose at the end of the treatment. These safety parameters were poorly or not described in some studies.
In addition, doses causing side-effects were non-exhaustively reported in certain studies, with global analysis of the relation between side-effects and dosage excluded. The most consistently reported outcome was the number of dropouts from studies due to side-effects. A minority of studies (14/33) differentiated for analysis the never smokers from the ex-smokers and reported the figures for each population. Only 3 studies presented some data about repartition of adverse events in each category of non-smoker. It was not possible to differentiate the doses used in relation to the side-effects observed in the groups of never smokers compared to the groups of ex-smokers.
4.4. Design of transdermal delivery protocol COVID-19 trials
To design a protocol for transdermal nicotine delivery to prevent and treat COVID-19, we set up three trials–one for prevention, and others for patients with COVID-19 and in intensive care units) and performed the same review as used on the 33 studies.
In those 33 studies, the duration of treatment was dependent on the target of the study. For our transdermal nicotine in COVID study we set a duration of 82 days of full dose for the preventive study, the duration of hospital stay + 7 days for in-patients (maximum 35 days) and the duration of ventilation + 2 days for intensive care patients (maximum 30 days).
We used 24-hour nicotine patches (used in 11/33 of the studies we analyzed),
Ten out of 33 studies analyzed used less than 14 mg/day of transdermal nicotine, 10/33 used > 15 mg/day and 13/33 studies used 14 or 15 mg/day of transdermal nicotine. For our study, we chose the median dose, 14 mg/day, as our target.
Twenty-two out of 33 studies used dose escalation. We chose to do the same to improve tolerance. The duration of dose escalation for patients 14-15 mg/day of transdermal nicotine varied from 2 to 28 days. We chose to use 14 days for the preventive protocol, but in the ICU group, where an optimal dose was needed as soon as possible, we gradually increased the dose every 8 hours to achieve target dose in one day (from 3.5 to 7 to 10.5 mg/day until the target of 14 mg/day was reached).
Doses were decreased at the end of protocol in only 8 of 33 studies, for 1 to 84 days. To minimize the risk of withdrawal symptom we gradually decreased the dose over 21 days.
5. Conclusions
This review provides a first synthesis of available data on the practical administration of transdermal nicotine and its safe use in non-smokers.
A protocol for the use of nicotine patches with an initial dose of 3.5 mg/day and gradual increases in dose of up to 14 mg/day over two weeks for those who tolerate it, could be proposed for 3 protocols evaluating nicotine and COVID-19 link [51], [52], [53].
Funding
No funding was received for this study.
Authors’ contributions
BD participated in conception of the study, collection and selection of articles, editing and validation of the manuscript. AL participated in the collection and selection of articles, editing and validation of the manuscript. MA participated in the editing and validation of the manuscript. RG participated in conception of study, the collection and selection of articles, editing and validation of the manuscript.
Disclosure of interest
BD attended a tobacco cessation meeting in 2018, sponsored by Novartis Pharma SA and provided training for pulmonologists sponsored by Chiesi and Novartis in 2019 on e-cigarette and smoking cessation.
AL declares that she has no competing interest.
MA attended medical training for physicians on smoking cessation, supported by Bouchara and Janssen. She has worked as an expert for a scientific advisory board for Pfizer and Johnson and Johnson Santé Beauté France.
RG has received compensation as a member of the scientific advisory board of Janssen, Lundbeck, Roche, SOBI, Takeda. He has served as a paid consultant and/or speaker for Astra Zeneca, Boehringer-Ingelheim, Pierre Fabre, Lilly, Lundbeck, MAPREG, Otsuka, Pileje, SANOFI, Servier, LVMH, and has received research support from Servier. He is a co-founder and stock shareholder in Regstem.
Acknowledgements
Maija Lenormand, project assistant and Karin Zeitvogel journalist for assistance finalizing the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.resmer.2021.100844.
Online Supplement. Supplementary data
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Grant R., Besombes C., Jolly N., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons D., Shahab L., Brown J., Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7) Addiction. 2020 doi: 10.1111/add.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infectious Diseases. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyara M, Tubach F, Pourcher V, Morelot-Panzini C, Pernet J, Haroche J, et al. Low incidence of daily current tobacco smoking in patients with symptomatic COVID-19. (submitted) https://www.qeios.com/read/WPP19W.3.1 10.32388/WPP19W.3.
- 5.Zureik M., Baricault B., Vabre C., Semenzato L., Drouin J., Cuenot F., et al. Nicotine-replacement therapy, as a surrogate of smoking, and the risk of hospitalization with Covid-19 and all-cause mortality: a nationwide, observational cohort study in France. (submitted) MedRxiv. 2020 doi: 10.1101/2020.07.28.20160630. [.07.28.20160630] [DOI] [Google Scholar]
- 6.Berlin I., Thomas D., Le Faou A.L., Cornuz J. COVID-19 and smoking. 2020;22:1650–1652. doi: 10.1093/ntr/ntaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Changeux J.P., Amoura Z., Rey F.A., et al. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications Académie des sciences. Comptes-rendus biologies. 2020;343 doi: 10.5802/crbiol.8. [preprint] [DOI] [PubMed] [Google Scholar]
- 8.Bonaz B., Sinniger V., Pellissier S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19? Bioelectron Med. 2020;6:15. doi: 10.1186/s42234-020-00051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindson N., Chepkin S.C., Ye W., Fanshawe T.R., Bullen C., Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2019;4 doi: 10.1002/14651858.CD013308. [CD013308] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel S.E., Berlin J.A., Miles C., Jaskowiak J., Carson J.L., Strom B. Risk of acute first myocardial infarction and use of nicotine patches in a general population. J Am Coll Cardiol. 2001;37:1297–1302. doi: 10.1016/s0735-1097(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 11.Howe M.N., Price I.R. Effects of transdermal nicotine on learning memory verbal fluency concentration and general health in a healthy sample at risk for dementia. Int Psychogeriatr. 2001;13:465–475. doi: 10.1017/s1041610201007888. [DOI] [PubMed] [Google Scholar]
- 12.Newhouse P., Kellar K., Aisen P., White H., Wesnes K., Coderre E., et al. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology. 2012;78:91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snaedal J., Johannesson T., Jonsson J.E., Gylfadottir G. The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer's disease. Dementia. 1996;7:47–52. doi: 10.1159/000106852. [DOI] [PubMed] [Google Scholar]
- 14.White H.K., Levin E.D. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacology (Berl) 1999;143:158–165. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A.L., Langley L.K., Monley J., Bauer T., Rottunda S., McFalls E., et al. Nicotine patches in Alzheimer's disease: pilot study on learning memory and safety. Pharmacol Biochem Behav. 1995;51:509–514. doi: 10.1016/0091-3057(95)00043-v. [DOI] [PubMed] [Google Scholar]
- 16.White H.K., Levin E.L. Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl) 2004;171:465–471. doi: 10.1007/s00213-003-1614-8. [DOI] [PubMed] [Google Scholar]
- 17.Vega J.N., Albert K.M., Mayer I.A., Taylor W.D., Newhouse P.A. Nicotinic treatment of post-chemotherapy subjective cognitive impairment: a pilot study. J Cancer Surviv. 2019;13:673–686. doi: 10.1007/s11764-019-00786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis A.S., van Schalkwyk G.I., Lopez M.O., Volkmar F.R., Picciotto M.R., Sukhodolsky D.G., et al. An Exploratory Trial of Transdermal Nicotine for Aggression and Irritability in Adults with Autism Spectrum Disorder. J Autism Dev Disord. 2018;48:2748–2757. doi: 10.1007/s10803-018-3536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gehricke J.G., Hong N., Whalen C.K., Steinhoff K., Wigal T.L. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychol Addict Behav. 2009;23:644–655. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- 20.Shytle R.D., Silver A.A., Wilkinson B.J., Sanberg P.R. A pilot-controlled trial of transdermal nicotine in the treatment of attention deficit hyperactivity disorder. World J Biol Psychiatry. 2002;3:150–155. doi: 10.3109/15622970209150616. [DOI] [PubMed] [Google Scholar]
- 21.Gandelman J.A., Kang H., Antal A., Albert K., Boyd B.D., Conley A.C., et al. Transdermal Nicotine for the Treatment of Mood and Cognitive Symptoms in Nonsmokers With Late-Life Depression. J Clin Psychiatry. 2018;79(5) doi: 10.4088/JCP.18m12137. [18m12137] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haro R., Drucker-Colín R. Effects of long-term administration of nicotine and fluoxetine on sleep in depressed patients. Arch Med Res. 2004;35:499–506. doi: 10.1016/j.arcmed.2004.11.010. [PMID: 15631874] [DOI] [PubMed] [Google Scholar]
- 23.McClernon F.J., Hiott F.B., Westman E.C., Rose J.E., Levin E.D. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind placebo-controlled trial. Psychopharmacology (Berl) 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [Epub 2006 Sep 15. PMID: 16977477] [DOI] [PubMed] [Google Scholar]
- 24.Kelton M.C., Kahn H.J., Conrath C.L., Newhouse P.A. The effects of nicotine on Parkinson's disease. Brain Cogn. 2000;43:274–282. [PubMed] [Google Scholar]
- 25.Lemay S., Blanchet P., Chouinard S., Masson H., Soland V., Bedard M.-A. Poor tolerability of a transdermal nicotine treatment in Parkinson's disease. Clin Neuropharmacology. 2003:26–227. doi: 10.1097/00002826-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lemay S., Chouinard S., Blanchet P., Masson H., Soland V., Beuter A., et al. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:31–39. doi: 10.1016/S0278-5846(03)00172-6. [PMID: 14687854] [DOI] [PubMed] [Google Scholar]
- 27.Vieregge A., Sieberer M., Jacobs H., Hagenah J.M., Vieregge P. Transdermal nicotine in PD: a randomized double-blind placebo-controlled study. Neurology. 2001;57:1032–1035. doi: 10.1212/wnl.57.6.1032. [DOI] [PubMed] [Google Scholar]
- 28.Villafane G., Cesaro P., Rialland A., Baloul S., Azimi S., Bourdet C., et al. Chronic high dose transdermal nicotine in Parkinson's disease: an open trial. Eur J Neurol. 2007;14:1313–1316. doi: 10.1111/j.1468-1331.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 29.Villafane G., Thiriez C., Audureau E., Straczek C., Kerschen P., Cormier-Dequaire F., et al. High-dose transdermal nicotine in Parkinson's disease patients: a randomized open-label blinded-endpoint evaluation phase 2 study. Eur J Neurol. 2018;25:120–127. doi: 10.1111/ene.13474. [DOI] [PubMed] [Google Scholar]
- 30.Dursun S.M., Reveley M. The efficacy of a dose-escalated application of transdermal nicotine plus sulpiride in Tourette's syndrome. Eur Psychiatry. 1996;11:204–206. doi: 10.1016/0924-9338(96)88392-1. [DOI] [PubMed] [Google Scholar]
- 31.Dursun S.M., Reveley M.A., Bird R., Stirton F. Longlasting improvement of Tourette's syndrome with transdermal nicotine. Lancet. 1994;344:1577. doi: 10.1016/s0140-6736(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 32.Silver A.A., Shytle R.D., Philipp M.K., Wilkinson B.J., McConville B., Sanberg P.R. Transdermal nicotine and haloperidol in Tourette's disorder: a double-blind placebo-controlled study. J Clin Psychiatry. 2001;62:707–714. doi: 10.4088/jcp.v62n0908. [DOI] [PubMed] [Google Scholar]
- 33.Silver A.A., Shytle R.D., Philipp M.K., Sanberg P.R. Case study: long-term potentiation of neuroleptics with transdermal nicotine in Tourette's syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35:1631–1636. doi: 10.1097/00004583-199612000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Guslandi M., Tittobello A. Pilot trial of nicotine patches as an alternative to corticosteroids in ulcerative colitis. J Gastroenterol. 1996;31:627–629. doi: 10.1007/BF02355071. [DOI] [PubMed] [Google Scholar]
- 35.Guslandi M., Tittobello A. Outcome of ulcerative colitis after treatment with transdermal nicotine. Eur J Gastroenterol Hepatol. 1998;10:513–515. doi: 10.1097/00042737-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Guslandi M. Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis. 1999;14:261–262. doi: 10.1007/s003840050221. [DOI] [PubMed] [Google Scholar]
- 37.Pullan R.D., Rhodes J., Ganesh S., Mani V., Morris J.S., Williams G.T., et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [PMID: 8114833] [DOI] [PubMed] [Google Scholar]
- 38.Richardson C.E., Morgan J.M., Jasani B., Green J.T., Rhodes J., Williams G.T., et al. Effect of smoking and transdermal nicotine on colonic nicotinic acetylcholine receptors in ulcerative colitis. QJM. 2003;96:57–65. doi: 10.1093/qjmed/hcg007. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn W.J., Tremaine W.J., Offord K.P., Lawson G.M., Petersen B.T., Batts K.P., et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126:364–371. doi: 10.7326/0003-4819-126-5-199703010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Thomas G.A., Rhodes J., Mani V., et al. Transdermal nicotine as maintenance therapy for ulcerative colitis. N Engl J Med. 1995;332:988–992. doi: 10.1056/NEJM199504133321503. [DOI] [PubMed] [Google Scholar]
- 41.Thomas G.A., Davies S.V., Rhodes J., Russell M.A., Feyerabend C., Säwe U. Is transdermal nicotine associated with cardiovascular risk? J R Coll Physicians Lond. 1995;29:392–396. [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas G.A., Rhodes J., Ragunath K., Mani V., Williams G., Newcombe R.G., et al. Transdermal nicotine compared with oral prednisolone therapy for active ulcerative colitis. Eur J Gastroenterol Hepatol. 1996;8:769–776. [PubMed] [Google Scholar]
- 43.Madretsma S., Wolters L.M., van Dijk J.P., Tak C.J., Feyerabend C., Wilson, et al. In-vivo effect of nicotine on cytokine production by human non-adherent mononuclear cells. Eur J Gastroenterol Hepatol. 1996;8:1017–1020. doi: 10.1097/00042737-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini M.P., Newby D.E., Maxwell S., Webb D.J. Short-term effects of transdermal nicotine on acute tissue plasminogen activator release in vivo in man. Cardiovasc Res. 2001;52:321–327. doi: 10.1016/s0008-6363(01)00381-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Dijk A.P., Meijssen M.A., Brouwer A.J., Hop W.C., van Bergeijk J.D., Feyerabend C., et al. Transdermal nicotine inhibits interleukin 2 synthesis by mononuclear cells derived from healthy volunteers. Eur J Clin Invest. 1998;28:664–671. doi: 10.1046/j.1365-2362.1998.00344.x. [DOI] [PubMed] [Google Scholar]
- 46.Seidelin J.B., Nielsen O.H. Transdermal nikotinbehandling af colitis ulcerosa: gennemgang af et Cochrane-review [Transdermal nicotine treatment of ulcerative colitis: a survey of a Cochrane review] Ugeskr Laeger. 2006;168:674–677. [PubMed] [Google Scholar]
- 47.Nikfar S., Ehteshami-Ashar S., Rahimi R., Abdollahi M. Systematic review and meta-analysis of the efficacy and tolerability of nicotine preparations in active ulcerative colitis. Clin Ther. 2010;32:2304–2315. doi: 10.1016/j.clinthera.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Ingram J.R., Thomas G.A., Rhodes J., Green J.T., Hawkes N.D., Swift J.L., et al. A randomized trial of nicotine enemas for active ulcerative colitis. Clin Gastroenterol Hepatol. 2005;3:1107–1114. doi: 10.1016/s1542-3565(05)00849-9. [DOI] [PubMed] [Google Scholar]
- 49.Kataoka Y., Kalanithi P.S., Grantz H., Schwartz M.L., Saper C., Leckman J.F., et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M., Kobets A., Du J.C., Lennington J., Li L., Banasr M., et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioural manifestations of Tourette syndrome. Proc Natl Acad Sci U S A. 2015;112:893–898. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.APHP. Efficacy of Nicotine in Preventing COVID-19 Infection in Caregivers (NICOVID-PREV). ClinicalTrials NCT04583410. https://clinicaltrials.gov/ct2/show/NCT04583410.
- 52.APHP. Evaluation of the Efficacy of Nicotine Patches in SARS-CoV2 (COVID-19) Infection in Hospitalized Patients. ClinicalTrials NCT04608201. https://clinicaltrials.gov/ct2/show/NCT04608201.
- 53.APHP. Evaluation of the Efficacy of Nicotine Patches in SARS-CoV2 (COVID-19) Infection in Intensive Care Unit Patients (NICOVID-REA). ClinicalTrials NCT04598594. https://clinicaltrials.gov/ct2/show/NCT04598594.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.