Abstract
Pain is common among patients with cirrhosis, yet managing pain in this population is challenging. Opioid analgesics are thought to be particularly high risk in patients with cirrhosis, and their use has been discouraged. We sought to understand patterns of opioid use among inpatients with cirrhosis and the risks of serious opioid‐related adverse events in this population. We used the Vizient Clinical Database/Resource Manager, which includes clinical and billing data from hospitalizations at more than 500 academic medical centers. We identified all nonsurgical patients with cirrhosis hospitalized in 2017‐2018 as well as a propensity score‐matched cohort of patients without cirrhosis. Inpatient prescription records defined patterns of inpatient opioid use. Conditional logistic regression compared rates of use and serious opioid‐related adverse events between patients with and without cirrhosis. Of 116,146 nonsurgical inpatients with cirrhosis, 62% received at least one dose of opioids and 34% had regular inpatient opioid use (more than half of hospital days), rates that were significantly higher than in patients without cirrhosis (adjusted odds ratio [AOR] for any use, 1.17; 95% confidence interval [CI], 1.13‐1.21; P < 0.001; AOR for regular use, 1.07; 95% CI, 1.02‐1.11; P = 0.002). Compared with patients without cirrhosis, patients with cirrhosis more often received tramadol (P < 0.001) and less commonly received opioid/acetaminophen combinations (P < 0.001). Rates of serious opioid‐related adverse events were similar in patients with and without cirrhosis (1.6% vs. 1.9%; AOR, 0.96; P = 0.63). Conclusion: Over half of patients with cirrhosis have pain managed with opioids during hospitalization. Patterns of opioid use differ in patients with cirrhosis compared with patients without cirrhosis, although rates of serious adverse events are similar. Future studies should further explore the safety and efficacy of opioids in patients with cirrhosis, with the goal of improving pain management and quality of life in this population.
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- AOR
adjusted odds ratio
- CDB/RM
Vizient Clinical Data Base/Resource Manager
- CI
confidence interval
- ICD‐10‐CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- IQR
interquartile range
Pain is reported in up to 80% of outpatients with cirrhosis and is associated with poor health‐related quality of life and increased health care utilization( 1 , 2 , 3 ); yet, there is a paucity of published data about safe and effective pain management options for patients with cirrhosis, resulting in lack of consensus guidelines, variable physician prescribing patterns, and chronic undertreatment of pain in this population.( 4 , 5 , 6 ) Opioid analgesics, in particular, although commonly used to treat pain in the general population, have been the subject of increased scrutiny over the last several years due to increasing rates of opioid dependence and overdose deaths in the United States.( 7 ) Patients with cirrhosis are thought to be particularly susceptible to opioid overdose due to impaired drug metabolism, and opioids specifically are thought to exacerbate hepatic encephalopathy.( 8 ) As a result, opioid use is discouraged as a pain management option in this population.( 4 )
Management of pain presents a particular challenge among hospitalized patients; a national focus on the assessment and management of inpatient pain has resulted in pain becoming the “fifth vital sign” during hospitalization and has led to high rates of opioid use among both medical and surgical inpatients.( 9 , 10 ) Patients who receive opioids during hospitalization are also at risk for inpatient opioid‐related adverse events as well as subsequent opioid dependence and overdose after discharge.( 11 , 12 ) Inpatients with cirrhosis in particular, who are frequently hospitalized with medical conditions causing acute pain on top of preexisting chronic pain and may have acute worsening of impairment of drug clearance in the setting of acute hepatic decompensation, are particularly vulnerable to opioid‐related harm and have also been shown to have frequent inpatient opioid use.( 13 ) Therefore, a more complete understanding of the risks of inpatient opioid analgesics in this specific population is warranted. In the current study, we used a propensity score‐matched contemporary national hospitalization cohort to help understand the frequency and patterns of opioid use among inpatients with and without cirrhosis and the risks of serious opioid‐related adverse events in this population.
Patients and Methods
Source of Data
This was a retrospective cohort study using de‐identified inpatient data from the Vizient Clinical Data Base/Resource Manager (CDB/RM). Vizient is a consortium of 3,000 hospitals across the United States; more than 150 academic medical centers and over 400 affiliate hospitals participate in the CBD/RM, which provides clinical, discharge, procedure, cost, and outcome data for each hospital encounter among member institutions. Data are extracted from hospital billing systems approximately 30 days after discharge and finalized after cleaning and validation steps are completed by Vizient. All data were de‐identified to conform with requirements of the Health Insurance Portability and Accountability Act.
Study Population
The study population consisted of all inpatient nonsurgical admissions in the Vizient CDB/RM with hospital discharges from January 1, 2017, to December 31, 2018, for patients ≥18 years old at admission (Fig. 1). Surgical admissions were excluded because of the high likelihood for acute postsurgical pain among this cohort that results in differing analgesic requirements. Excluded surgical admissions included operating room‐based procedures, defined using the Agency for Healthcare Research and Quality’s (AHRQ) Procedure Class Definitions for International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) procedure codes.( 14 , 15 ) To ensure all surgical admissions were removed, we also excluded admissions in which the attending of record was a surgeon. Given high rates of appropriate opioid use at the end of life, we excluded admissions if patients died during hospitalization or if they were discharged with hospice care. However, we performed a sensitivity analysis that included these patients because opioid‐related adverse events may have contributed to death. We also removed admissions in which length of stay was <1 or over 365 days. Admissions were excluded if they represented a 30‐day readmission following an index hospitalization in the Vizient database to ensure as many unique patients as possible.
FIG. 1.
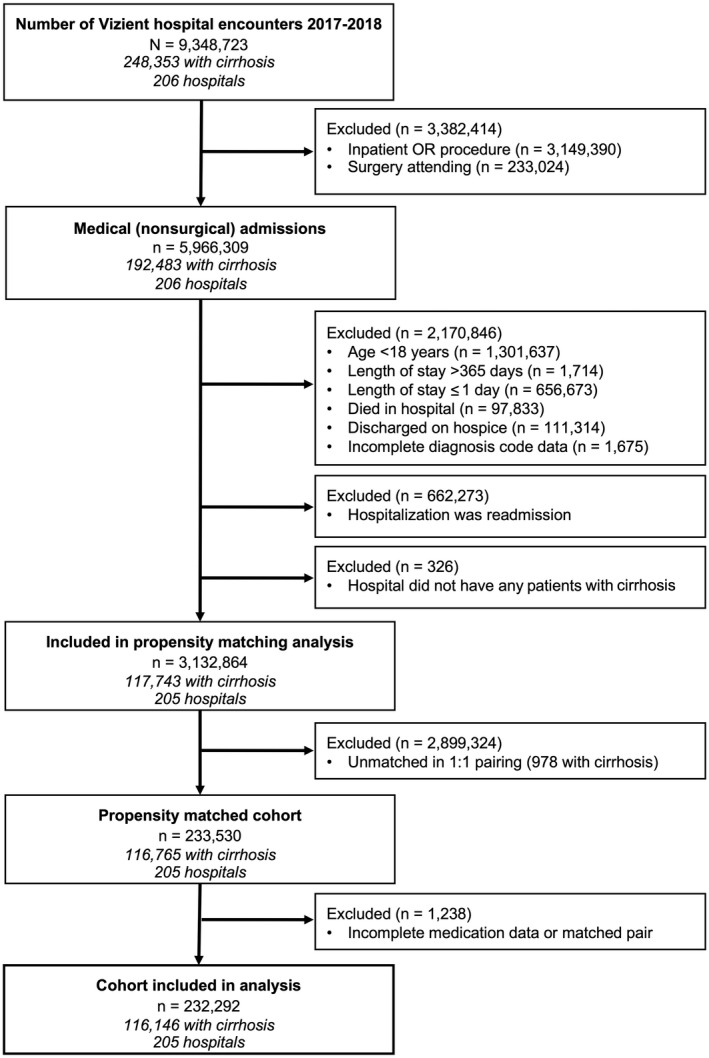
Study cohort flow diagram. Abbreviation: OR, operating room.
Patients with cirrhosis were identified from within this medical cohort by the presence of one of nine ICD‐10‐CM diagnosis codes for cirrhosis, whether principal or secondary. A subset of seven of these ICD‐10‐CM codes (Supporting Table S1) has been validated previously, with a >90% positive predictive value for identifying patients with cirrhosis.( 16 , 17 ) Two additional ICD‐10‐CM codes were added (K72.10, chronic hepatic failure with coma; K72.11, chronic hepatic failure without coma) to increase sensitivity of identifying patients with cirrhosis.
Covariate Definitions
We were interested in demographic, clinical, and hospitalization factors associated with inpatient opioid use and serious opioid‐related adverse events among patients with and without cirrhosis. Demographic factors included in the Vizient data and tested as covariates in our analyses included sex, patient age group (precise age is not provided to ensure data remains de‐identified), race/ethnicity, primary payer, and hospital region.
ICD‐10‐CM diagnosis codes were used to determine comorbidities and cirrhosis‐related complications. We used the administrative data‐derived Charlson Comorbidity Index as a proxy for patient comorbidity.( 16 , 17 ) Cirrhosis complications were defined as ascites, varices, variceal bleed, hepatic encephalopathy, hepatorenal syndrome (HRS), and spontaneous bacterial peritonitis (SBP) (see Supporting Table S2). Codes for ascites and esophageal varices have been validated previously, with positive predictive value >90%.( 18 ) Hepatic encephalopathy was defined as either having an ICD‐10‐CM code for liver disease “with coma” or at least one inpatient charge for both lactulose and rifaximin (medication charges are available in the Vizient CDB/RM, as described below). While lactulose and rifaximin can each be used for nonhepatic encephalopathy indications, use of both medications is suggestive of treatment for hepatic encephalopathy. HRS and SBP are each defined using single specific ICD‐10‐CM codes. In an effort to use the most sensitive definition of hepatic decompensation, a diagnosis code for any one of these cirrhosis‐related complications categorized a patient as having decompensated cirrhosis. To minimize confounding by decompensation status, we performed secondary analyses in which we stratified patients as noncirrhosis, compensated cirrhosis, or decompensated cirrhosis.
Hospitalization‐specific covariates included admission status (emergency, urgent, elective, trauma), admission source (emergency room, transfer, other), length of stay, and intensive care unit stay, all of which were variables in the Vizient CDB/RM. Physician specialty was defined as the specialty of the primary discharging provider, categorized as general medicine, liver/gastroenterology specialist, and other. Hospital characteristics, including size, teaching status, and liver transplant center, were available in the database.
Prevalence and Patterns of Opioid Use
In order to identify patients who received opioids during hospitalization, we used inpatient prescription charges by hospital day, which were available in the Vizient CDB/RM. The database included drug formulation information, which we used to define parenteral and oral administration. A list of medications categorized as opioids is shown in Supporting Table S3. A similar list has been used in administrative data set studies of inpatient opioid use.( 12 , 19 ) Opioid combinations with other analgesics were included. Buprenorphine was excluded as it is most commonly used as treatment for opioid dependence, but methadone was included as it is also used for the treatment of chronic pain. We performed a sensitivity analysis excluding methadone in case its exclusion biased our results.
We identified inpatient opioid use in the following two ways: (1) any charge for an opioid on at least one day of hospitalization (any opioid use) and (2) any charge for an opioid on more than half of hospital days (regular inpatient opioid use). “Regular inpatient opioid use” was used as our primary exposure for all multivariable models, although sensitivity analyses were performed using “any opioid use” as an alternate exposure definition. We also evaluated rates of opioid administration within 24 hours of admission and on the day of discharge as well as the types of opioids used during hospitalization and routes of administration. We identified covariates that were associated with these particular patterns of opioid use in patients with cirrhosis compared with patients without cirrhosis.
In an effort to understand whether patients were likely to receive opioids because of chronic or outpatient use, we identified the proportion of patients with one of four ICD‐10‐CM diagnosis codes for chronic pain that have been validated in the literature.( 20 ) We also identified patients with one of 143 opioid‐related ICD‐10‐CM diagnosis codes either for opioid abuse or opioid poisoning, as defined by the AHRQ.( 21 )
Serious Opioid‐Related Adverse Events
Our primary outcome was serious opioid‐related adverse events, which were defined as either naloxone exposure or an opioid‐related adverse event diagnosis code, a definition that has been used in the literature with high‐positive predictive value.( 19 , 22 ) We defined naloxone exposure as at least one charge for naloxone, excluding charges on the day of admission as this could represent opioid overdose before presentation. Opioid‐related adverse event diagnosis codes included one of 102 ICD‐10‐CM codes for opioid poisoning (see above), as defined by the AHRQ, that was not indicated as present on admission.( 21 )
Statistical Analysis
To ensure that baseline characteristics were similar between patients with and without cirrhosis, we used a propensity score model to match patients on clinically relevant demographic and clinical characteristics, defined a priori, which were hypothesized to be associated with in‐hospital opioid use and opioid‐related adverse events. These included age group, sex, race, primary payer, Charlson Comorbidity Index, and chronic pain diagnosis. Propensity scores were calculated for each subject to identify the conditional probability of having cirrhosis, using a logistic regression model with cirrhosis as the dependent variable, stratified by hospital. One hospital that did not admit any patients with cirrhosis over the 2‐year study period was excluded. Patients with and without cirrhosis were then matched in a 1:1 ratio using caliper matching without replacement with a caliper size of 0.1 times the pooled standard deviation of the logit of the propensity score. The psmatch2 program was applied for matching,( 23 ) and the pstest command was used to evaluate the success of matching by comparing the differences in baseline characteristics before and after matching. For each covariate from the propensity score model, an absolute value of the standardized difference <0.25 and a variance ratio between 0.5 and 2.0 (inclusive) indicated adequate sample balance. The double‐adjustment method was used for all matching covariates given a large sample size.( 24 )
Categorical variables were presented as percentages and compared between unmatched groups by χ2 and Fisher’s exact tests. Continuous variables were presented as medians with interquartile ranges (IQRs) and compared between unmatched groups by Wilcoxon rank‐sum tests, given non‐normal distributions. Unconditional logistic regression clustered by hospital was used for subgroup analyses to identify patient and hospitalization factors associated with the regular inpatient opioid use outcome within each of the cirrhosis and noncirrhosis subgroups. Conditional logistic regression models grouped by matched pairs and clustered by hospital were used to identify associations between cirrhosis and our outcomes. Using the covariates from the cirrhosis subgroup multivariable model in a model including patients with and without cirrhosis, we tested for the interaction between a cirrhosis diagnosis and all other covariates to determine how predictors of our outcomes differed in patients with cirrhosis compared with patients without cirrhosis. In all analyses after including all baseline factors that were thought to be possible confounders, backward selection was used to develop models. Terms with significance <0.1 were selected for inclusion in the multivariable models. Covariates not reaching a significance of P < 0.05 were sequentially eliminated. Two‐sided P < 0.05 was considered statistically significant. All P values reported below are from double‐adjusted conditional logistic regression models, unless otherwise specified. Analyses were performed using Stata/MP 16.1 statistical software (College Station, TX).
Results
Patient Demographics and Clinical Characteristics
Of 9,348,723 hospitalizations in the 2017‐2018 Vizient CDB/RM, 5,966,309 (63.8%) were among nonsurgical patients (Fig. 1). Of these, 192,483 (3.2%) had a primary or secondary diagnosis of cirrhosis. Overall, 117,743 with cirrhosis and 3,015,121 without cirrhosis met our inclusion criteria. Selected demographic and clinical characteristics of the cohort before propensity matching are shown in Table 1. Overall, 55.8% of patients were women, 65.7% were white, and 40.0% were 65 years or older. Among patients with cirrhosis, 61.1% had decompensated cirrhosis; of these, 43.6% had ascites, 23.3% had hepatic encephalopathy, and 26.1% had varices. Patients with cirrhosis were less likely to be women (40.1% vs. 56.5%; P < 0.001), less likely to have private insurance (19.2% vs. 23.8%), and had more comorbidities (median Charlson Index 4 vs. 2) than patients without cirrhosis. They were also more likely to have a chronic pain diagnosis (10.7% vs. 7.5%). Following propensity matching and exclusion of 1,238 additional patients either with incomplete medication data or their matched pairs, a final cohort with 232,292 patients was identified. Characteristics of the propensity‐matched cohort and individual covariate sample balance diagnostics are shown in Table 2, and a plot of propensity scores before and after propensity matching by cirrhosis diagnosis is shown in Supporting Fig. S1.
Table 1.
Baseline Characteristics of Cohort Before Propensity Score Matching
| Characteristic | Total | No cirrhosis | Cirrhosis | P Value |
|---|---|---|---|---|
| N = 3,132,864 | n = 3,015,121 (96.2%) | n = 117,743, (3.8%) | ||
| Female sex | 55.80% | 56.50% | 40.10% | <0.001 |
| Age group (years) | <0.001 | |||
| 18‐30 | 14.60% | 15.10% | 2.30% | |
| 31‐50 | 22.00% | 22.10% | 21.30% | |
| 51‐64 | 23.30% | 22.50% | 44.50% | |
| 65+ | 40.00% | 40.40% | 31.80% | |
| Race | <0.001 | |||
| White | 65.70% | 65.50% | 70.30% | |
| Black | 22.30% | 22.60% | 14.90% | |
| Asian | 2.30% | 2.30% | 2.00% | |
| Hispanic | 7.80% | 7.70% | 11.20% | |
| Other | 1.80% | 1.80% | 1.60% | |
| Region | <0.001 | |||
| Midwest | 35.80% | 36.00% | 31.50% | |
| Northeast | 30.20% | 30.30% | 26.70% | |
| South | 22.80% | 22.70% | 26.70% | |
| West | 11.20% | 11.10% | 15.00% | |
| Primary payer | <0.001 | |||
| Private/commercial | 23.60% | 23.80% | 19.20% | |
| Medicaid | 21.80% | 21.60% | 27.00% | |
| Medicare | 47.80% | 47.90% | 45.00% | |
| Other | 6.70% | 6.70% | 8.70% | |
| Charlson Comorbidity Index | 2.0 (0.0‐4.0) | 2.0 (0.0‐4.0) | 4.0 (3.0‐6.0) | <0.001 |
| Chronic pain ICD‐10‐CM code | 7.60% | 7.50% | 10.70% | <0.001 |
| Admission status | <0.001 | |||
| Emergency | 71.90% | 71.60% | 78.30% | |
| Urgent | 17.70% | 17.70% | 17.80% | |
| Elective | 9.60% | 9.90% | 3.20% | |
| Other | 0.80% | 0.80% | 0.70% | |
| Transfer | 12.50% | 12.30% | 18.60% | <0.001 |
| Teaching hospital | 73.50% | 73.20% | 81.00% | <0.001 |
| Cirrhosis complications | ||||
| Decompensated cirrhosis | ‐ | 61.10% | ||
| Ascites | ‐ | 43.60% | ||
| Hepatic encephalopathy | ‐ | 23.30% | ||
| Varices | ‐ | 26.10% | ||
| Variceal bleed | ‐ | 5.60% | ||
| Spontaneous bacterial peritonitis | ‐ | 4.00% | ||
| Hepatorenal syndrome | ‐ | 3.80% |
Data are presented as percent or median (IQR).
Table 2.
Baseline Characteristics of Propensity Score‐Matched Cohort
| Matching Covariates | Total | No cirrhosis | Cirrhosis | Standardized Difference or P Value* |
|---|---|---|---|---|
| N = 232,292 | n = 116,146 (50%) | n = 116,146 (50%) | ||
| Female sex | 40.3% | 40.4% | 40.3% | 0.002 |
| Age group (years) | ||||
| 18‐30 | 2.2% | 2.0% | 2.3% | 0.02 |
| 31‐50 | 21.1% | 20.8% | 21.3% | 0.01 |
| 51‐64 | 44.7% | 45.0% | 44.5% | 0.01 |
| 65+ | 32.1% | 32.2% | 31.9% | 0.006 |
| Race | ||||
| White | 70.5% | 70.7% | 70.3% | 0.009 |
| Black | 15.1% | 15.4% | 14.9% | 0.01 |
| Asian | 1.9% | 1.8% | 2.0% | 0.01 |
| Hispanic | 10.9% | 10.7% | 11.2% | 0.02 |
| Other | 1.5% | 1.4% | 1.6% | 0.02 |
| Primary payer | ||||
| Private/commercial | 19.3% | 19.3% | 19.4% | 0.003 |
| Medicaid | 26.9% | 26.8% | 27.0% | 0.002 |
| Medicare | 45.5% | 45.8% | 45.2% | 0.01 |
| Other | 8.3% | 8.2% | 8.5% | 0.01 |
| Charlson Comorbidity Index | 4.0 (3.0‐6.0) | 4.0 (3.0‐6.0) | 4.0 (3.0‐6.0) | 0.008 |
| Chronic pain ICD‐10‐CM code | 10.5% | 10.3% | 10.8% | 0.02 |
| Other covariates | ||||
| Admission status | <0.001 | |||
| Emergency | 77.4% | 76.6% | 78.2% | |
| Urgent | 17.6% | 17.3% | 17.9% | |
| Elective | 4.3% | 5.4% | 3.2% | |
| Other | 0.8% | 0.8% | 0.7% | |
| Transfer | 17.7% | 16.7% | 18.6% | <0.001 |
| Teaching hospital | 80.9% | 80.9% | 80.9% | 0.98 |
| Cirrhosis complications | ||||
| Decompensated cirrhosis | ‐ | 61.1% | ||
| Ascites | ‐ | 43.6% | ||
| Hepatic encephalopathy | ‐ | 23.4% | ||
| Varices | ‐ | 26.1% | ||
| Variceal bleed | ‐ | 5.5% | ||
| Spontaneous bacterial peritonitis | ‐ | 4.0% | ||
| Hepatorenal syndrome | ‐ | 3.8% |
Data are presented as percent or median (IQR).
Standardized difference of means presented for matched covariates and P values for other covariates that were not used in the propensity‐matching model.
Patterns of Inpatient Opioid Use
Inpatient opioid use was common among our cohort; nearly two thirds of our cohort (59.6%) received at least one opioid dose during hospitalization and one third (33.2%) had regular inpatient opioid use (i.e., on over half of hospital days). Among those who received at least one opioid dose, 68.5% received at least one dose of an intravenous opioid, 80.4% received an opiate within 24 hours of admission, and 48.8% received opioids on the day of discharge. Among those patients with regular inpatient opioid use, over 95% received their first dose within the first 24 hours of hospitalization (see Supporting Fig. S2). The most commonly used types of opioids overall were fentanyl, oxycodone, and morphine, as shown in Fig. 2A. Distribution by opioid type was similar between patients with and without cirrhosis. On conditional logistic regression with double adjustment, patients without cirrhosis were significantly more likely to receive morphine, hydromorphone, hydrocodone, and opioid/acetaminophen combinations, while patients with cirrhosis were significantly more likely to receive tramadol and methadone. Fentanyl was much less commonly used among patients with regular inpatient opioid use (Fig. 2B); oxycodone was most frequently used in this subset of patients, with similar rates of oxycodone use in regular opioid users with and without cirrhosis (P = 0.3). Differences in patterns of use between patients with and without cirrhosis were similar to those observed in patients with regular inpatient opioid use. When stratified by decompensation status, patients with decompensated cirrhosis were significantly less likely to receive any type of opioid except tramadol, which was significantly more likely to be used in patients with decompensated cirrhosis (P < 0.001 for regular and any inpatient opioid use).
FIG. 2.
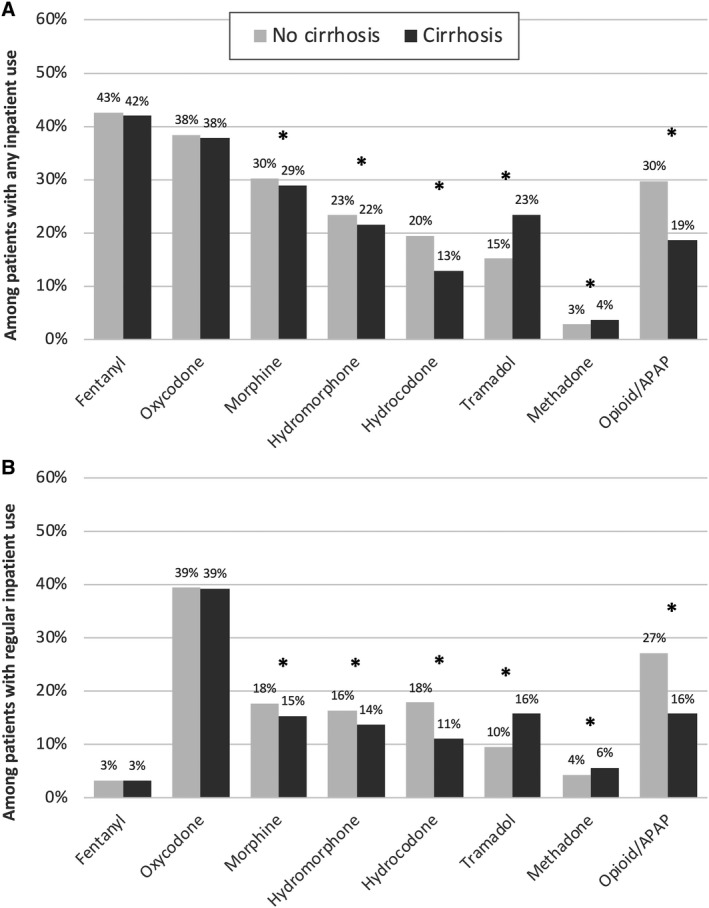
Rates of inpatient opioid use by type among patients with and without cirrhosis. (A) Any inpatient use; (B) regular inpatient use (over half of hospital days). *P < 0.01, on conditional logistic regression with double adjustment. Abbreviation: APAP, acetaminophen.
Chronic Pain and Opioid‐Related Diagnoses
Among patients with regular inpatient opioid use, only 19.5% had an ICD‐10‐CM diagnosis of chronic pain, with similar rates in patients with and without cirrhosis. Even fewer patients, 10.8% of regular inpatient opioid users, had an opioid‐related diagnosis code (i.e., opioid abuse or poisoning), which was slightly higher among patients with cirrhosis compared to patients without cirrhosis (11.3% vs. 10.3%, respectively), although this was not statistically significant on conditional logistic regression (P = 0.4). When stratified by decompensation status, this clinical difference was completely explained by higher rates of opioid‐related diagnosis codes among those with compensated cirrhosis (13.7% in compensated vs. 9.6% in decompensated cirrhosis).
Risk Factors For Inpatient Opioid Use
Patients with regular inpatient opioid use in our cohort were more likely to be women (43.7% vs. 38.6%, P < 0.001), less than 65 years old (78.4% vs. 62.8%, P < 0.001), and non‐Hispanic white (73.1% vs. 69.3%, P < 0.001). Patients with cirrhosis had a higher rate of regular inpatient opioid use than patients without cirrhosis (34.0% vs. 32.4%) and had 7% higher odds of inpatient opioid use on multivariable logistic regression (adjusted odds ratio [AOR] 1.07; 95% confidence interval [CI], 1.02‐1.08; P = 0.002). Among patients with cirrhosis, age under 65 years was associated with greater than twice the odds of opioid use compared with patients 65 years and over on unconditional multivariable analysis (AOR, 2.32; 95% CI, 2.22‐2.43) (Table 3). Other demographic factors most strongly associated with opioid use in patients with cirrhosis included: female sex (AOR, 1.27; 95% CI, 1.21‐1.29), non‐Asian race (AOR, 1.87; 95% CI, 1.60‐2.19), and having public insurance (e.g., Medicare or Medicaid) (AOR, 1.42; 95% CI, 1.34‐1.49). In addition to a chronic pain diagnosis (AOR, 3.46; 95% CI, 3.17‐3.74), having compensated cirrhosis was associated with higher rates of opioid use; specifically, the presence of varices or hepatic encephalopathy was associated with decreased odds of opioid use (AOR, 0.88; 95% CI, 0.84‐0.92; and AOR, 0.80; 95% CI, 0.75‐0.86). Patients with a general medicine provider during hospitalization were also more likely to have regular inpatient opioid use (AOR, 1.15; 95% CI, 1.07‐1.23). Risk factors for opioid use were similar for patients with and without cirrhosis, as shown in Fig. 3, although on interaction analysis, public insurance was more strongly associated with regular opioid use in patients with cirrhosis compared with patients without cirrhosis and age under 65 years was more strongly associated with opioid use in patients without cirrhosis (interaction P value for public insurance/cirrhosis and age/cirrhosis were both <0.001). On sensitivity analysis using any opioid use as our outcome, a cirrhosis diagnosis was also independently associated with increased odds of opioid use (AOR, 1.17; 95% CI, 1.13‐1.21).
Table 3.
Risk factors For Regular Inpatient Opioid Use Among Patients With Cirrhosis (n = 116,146)*
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | AOR | 95% CI | P value | |
| Female sex | 1.23 | 1.19‐1.26 | <0.001 | 1.25 | 1.21‐1.29 | <0.001 |
| Age group (years) | ||||||
| 18‐30 | REF | REF | ||||
| 31‐50 | 0.93 | 0.84‐1.04 | <0.001 | 0.89 | 0.80‐0.98 | <0.001 |
| 51‐64 | 0.79 | 0.71‐0.88 | 0.71 | 0.64‐0.78 | ||
| 65 and over | 0.41 | 0.37‐0.46 | 0.31 | 0.28‐0.35 | ||
| Race | ||||||
| Asian/Pacific Islander | REF | REF | ||||
| White | 2.37 | 2.02‐2.79 | <0.001 | 1.95 | 1.66‐2.28 | <0.001 |
| Black | 2.49 | 2.09‐2.96 | 1.95 | 1.65‐2.31 | ||
| Hispanic | 1.78 | 1.26‐2.07 | 1.25 | 1.23‐1.69 | ||
| Primary payer | ||||||
| Private | REF | REF | ||||
| Medicaid | 1.48 | 1.39‐1.58 | <0.001 | 1.34 | 1.25‐1.43 | <0.001 |
| Medicare | 1.05 | 0.99‐1.11 | 1.49 | 1.39‐1.60 | ||
| Other | 1.1 | 0.95‐1.26 | 1.07 | 0.93‐1.25 | ||
| Charlson Comorbidity Index | 1.01 | 0.99‐1.01 | 0.14 | |||
| Chronic pain diagnosis | 3.66 | 3.37‐3.97 | <0.001 | 3.45 | 3.17‐3.74 | <0.001 |
| Compensated cirrhosis | 1.17 | 1.12‐1.23 | <0.001 | |||
| Ascites | 0.98 | 0.94‐1.03 | 0.47 | |||
| Varices | 0.85 | 0.82‐0.89 | <0.001 | 0.88 | 0.84‐0.92 | <0.001 |
| Hepatic encephalopathy | 0.83 | 0.78‐0.88 | <0.001 | 0.8 | 0.75‐0.86 | <0.001 |
| Outside hospital transfer | 1.05 | 0.99‐1.12 | 0.1 | |||
| Admission status | ||||||
| Emergency | REF | |||||
| Urgent | 0.98 | 0.90‐1.05 | 0.39 | |||
| Elective | 1.04 | 0.92‐1.18 | ||||
| Other | 1.2 | 0.93‐1.54 | ||||
| Physician specialty | ||||||
| Internal medicine | REF | REF | ||||
| GI/liver specialist | 0.78 | 0.70‐0.87 | 0.86 | 0.77‐0.97 | <0.001 | |
| Critical care | 0.85 | 0.76‐0.95 | <0.001 | 0.85 | 0.76‐0.96 | |
| Other | 0.86 | 0.80‐0.92 | 0.89 | 0.83‐0.95 | ||
| Transplant center | 0.92 | 0.82‐1.04 | 0.2 | |||
| Teaching hospital | 0.84 | 0.76‐0.94 | 0.002 | 0.83 | 0.74‐0.93 | 0.001 |
All analyses clustered by hospital.
Abbreviations: GI, gastrointestinal; OR, odds ratio; REF, reference.
FIG. 3.
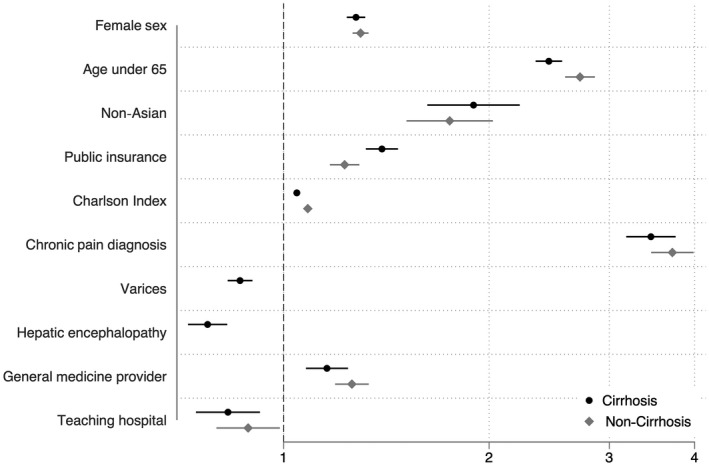
Risk factors for regular inpatient opioid use on multivariable logistic regression clustered by hospital in patients with and without cirrhosis.
Serious Opioid‐Related Adverse Events
Among the 77,096 patients with regular inpatient opioid use, 660 (0.9%) had a charge for naloxone use during hospitalization, excluding naloxone charges on the day of admission. In addition, 775 patients (1.0%) had an ICD‐10‐CM code for opioid poisoning that was not present on admission, resulting in a total of 1,354 patients (1.8%) with a serious opioid‐related adverse event during hospitalization. Patients with cirrhosis had a lower rate of serious opioid‐related adverse events than patients without cirrhosis (1.6% vs. 1.9%), but this difference was not statistically significant on multivariable logistic regression (AOR, 0.96; 95% CI, 0.81‐1.13; P = 0.63). Similarly, among the 138,817 patients with any opioid use, patients with cirrhosis had a lower rate of serious opioid‐related adverse events (1.4% vs. 1.5%); again this difference was not statistically significant on multivariable conditional logistic regression (AOR, 0.91; 95% CI, 0.82‐1.00; P = 0.05). In sensitivity analysis excluding patients who received methadone, overall adverse event rates were lower (0.9%) among regular opioid users and statistically higher among patients without cirrhosis compared to those with cirrhosis (1.4% vs. 0.8%; P = 0.002). Additionally, when including patients who died in the hospital or were discharged on hospice, serious opioid‐related adverse event rates were similar between patients with and without cirrhosis (P = 0.09). Rates of serious opioid‐related adverse events by decompensation status are shown in Fig. 4. Patients with decompensated cirrhosis had lower rates of serious opioid‐related adverse events, but this was only statistically significant when compared to patients without cirrhosis in the cohort of patients with any opioid use during hospitalization (P = 0.003).
FIG. 4.
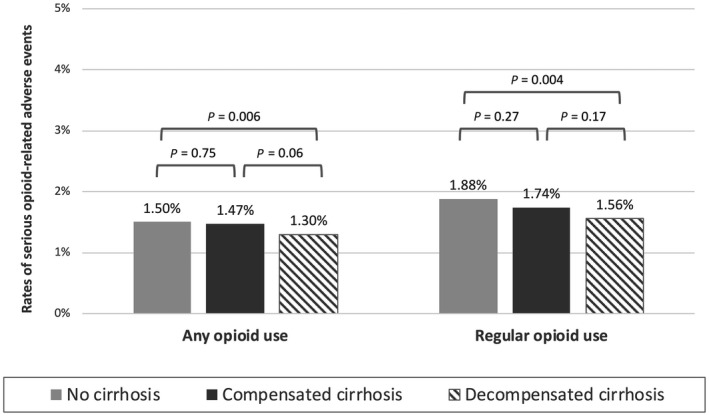
Rates of serious opioid‐related adverse events by hepatic decompensation status.
Discussion
In a large national representative cohort of medical inpatients, we found that opioid use was very common; nearly two thirds of hospitalized patients received at least one dose of opioids during hospitalization, and over one third received opioids regularly throughout admission. In our propensity‐matched cohort, overall rates of opioid use were slightly higher among patients with cirrhosis, although types of opioids given differed, with patients with cirrhosis more likely to receive tramadol and less likely to receive opioid/acetaminophen combinations. Among patients with regular inpatient opioid use, rates of serious opioid‐related adverse events were low and patients with cirrhosis appeared to have similar rates of these adverse events compared with patients without cirrhosis, with slightly lower risk of adverse events in those with decompensated disease.
Previous studies have established high rates of outpatient opioid prescriptions among patients with cirrhosis, even in comparison to patients with other chronic diseases.( 25 , 26 ) Our study describes patterns of opioid use in inpatients with cirrhosis and suggests that opioids are used in this population at rates that are similar to or higher than in inpatients without cirrhosis. We found this to be true using multiple definitions of inpatient opioid use and even after adjustment for multiple other predictors of analgesic use. Our findings are consistent with other recent publications that have described patterns of inpatient opioid use in the general population (both surgical and nonsurgical), suggesting that approximately 50% of all inpatients receive opioids during admission.( 12 , 19 ) Also similar to prior studies, we found that many patients who received opioids during hospitalization received their first doses in the emergency room or within the first day of hospitalization and that the medications were continued throughout their stay. This was true in our cohort despite fewer than 20% of patients having documented chronic pain diagnoses and only 10% having opioid‐related diagnoses. Overall, these findings suggest that inpatients with cirrhosis have pain requiring analgesics at least as frequently as inpatients without cirrhosis, as expected based on prior studies of outpatients with cirrhosis. When stratified by decompensation status, however, patients with decompensated disease had similar rates of opioid use overall but were less likely to have regular opioid use compared with patients with compensated cirrhosis, likely due to provider concerns regarding opioid safety in these patients. As it is unlikely that patients with decompensated cirrhosis had lower rates of pain than patients with compensated disease or the general population, these findings suggest pain may be undertreated in those with hepatic decompensation.
Patterns of opioid use also differed in patients with cirrhosis compared to the general population. In particular, our finding that tramadol is more commonly used among patients with cirrhosis (particularly those with decompensated cirrhosis) further suggests that providers are attempting to minimize opioid‐related side effects in this population; tramadol has been suggested to be less likely to cause sedation and respiratory depression than other opioids.( 27 , 28 ) In doing so, however, they may be using a less effective analgesic option, further highlighting possible undertreatment of pain in this vulnerable population.( 29 , 30 ) Additionally, tramadol has been shown to be associated with an increased risk of hypoglycemia and hyponatremia compared to other opioids, adverse effects that may be particularly concerning among patients with decompensated cirrhosis but which have not been studied in this population specifically.( 31 , 32 ) Our findings of increased tramadol use in combination with decreased rates of opioid/acetaminophen combinations in patients with cirrhosis may also reflect provider concerns about adverse effects of other analgesics, such as acetaminophen or nonsteroidal anti‐inflammatories, in patients with cirrhosis.( 4 )
Do high rates of inpatient opioid use in patients with cirrhosis translate to increased risk of harm in this vulnerable population? We found that during hospitalization, rates of serious opioid‐related adverse events, defined as naloxone use after admission or an opioid poisoning ICD‐10‐CM code not present on admission, were similar among patients with cirrhosis compared to patients without cirrhosis. Our serious adverse event rates of 1%‐2% are consistent with a large 2014 study of hospitalized nonsurgical patients.( 19 ) Other studies have suggested overall rates of opioid‐related adverse events may be as high as 14%,( 33 , 34 , 35 ) but these studies used different methods that would detect complications (e.g., constipation or urinary retention specifically caused by opioids), which are not easily detected in administrative data. While it is possible that rates of such minor adverse events differ between patients with and without cirrhosis, these may not necessarily be contraindications to opioid use for treatment of severe pain.
Our study has several limitations. First, because the Vizient CDB/RM is an administrative data set, it lacks clinical and laboratory information tailored specifically to patients with cirrhosis, which may allow for better characterization of risk factors for adverse events in this population. However, given our large sample size, propensity‐matched cohort, and adjustment for multiple covariates in all our models, even small differences between patients with and without cirrhosis should have been detected in our analyses. The lack of clinical details (i.e., reliance on ICD‐10‐CM codes) also prevented the evaluation of important cirrhosis‐specific opioid‐related adverse events, such as development of new hepatic encephalopathy or progressive hepatic decompensation during hospitalization. Second, as with all analyses of administrative data sets, several of our outcomes of interest (i.e., opioid‐related adverse events) are based on ICD‐10‐CM codes, which are subject to coding variability by provider. However, there is no reason why coding would systematically differ between patients with and without cirrhosis. Third, the lack of preadmission outpatient medications precludes analysis of which patients were receiving opioids as an outpatient and which were newly started on opioids during their hospitalization, and this may be an important variable in analyzing adverse events, although we used valid ICD‐10‐CM codes for chronic pain, chronic opioid use, and opioid use disorder. Finally, this analysis did not include dosage information; it is possible that patients with cirrhosis receive lower doses of opioids, resulting in similar adverse event rates compared with patients without cirrhosis. Regardless, our findings suggest that there are patterns of opioid use that can be safe in this population, including in those with decompensated disease, and may not need to be avoided completely.
Our study has important implications not only for clinicians struggling to make appropriate analgesic choices in this high‐risk population but also for national and international organizations seeking to guide appropriate analgesic use in patients with cirrhosis. Our findings confirm that pain and the need for pain control is common among inpatients with cirrhosis. However, patients with cirrhosis, particularly those with decompensated disease, may be at risk for undertreatment of their pain, likely because physicians feel handcuffed by possible risks of multiple classes of analgesics. Additionally, our findings suggest that there may in fact be a subset of patients with cirrhosis in whom opioids can be safe analgesic options. Future studies with data sets with additional clinical detail should be used to further characterize this population and to elucidate population‐specific efficacy and risks, including additional clinical and health resource utilization outcomes, of opioid analgesics in patients with cirrhosis, with the ultimate goal of reducing pain and improving quality of life among patients with chronic liver disease.
Supporting information
Supplementary Material
Supported by the National Institute on Aging (Research Project Grant R01AG059183 to J.C.L.), National Institute of Diabetes and Digestive and Kidney Diseases (National Research Service Award Hepatology Training Grant T32DK060414 to J.B.R.; Center Core Grant P30DK026743 to J.C.L, A.M.S.), and American Association for the Study of Liver Diseases (2020 Anna S. Lok Advanced/Transplant Hepatology Award to J.B.R.).
Potential conflict of interest: Nothing to report.
References
- 1. Madan A, Barth KS, Balliet WE, Hernandez‐Tejada MA, Borckardt JJ, Malcolm R, et al. Chronic pain among liver transplant candidates. Prog Transplant 2012;22:379‐384. [DOI] [PubMed] [Google Scholar]
- 2. Rogal SS, Bielefeldt K, Wasan AD, Lotrich FE, Zickmund S, Szigethy E, et al. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:1009‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng J‐K, Hepgul N, Higginson IJ, Gao W. Symptom prevalence and quality of life of patients with end‐stage liver disease: a systematic review and meta‐analysis. Palliat Med 2019;33:24‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandok N, Watt KDS. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85:451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J, et al. Use of over‐the‐counter analgesics in patients with chronic liver disease: physicians' recommendations. Drug Saf 2008;31:261‐270. [DOI] [PubMed] [Google Scholar]
- 6. Weersink RA, Taxis K, Drenth JPH, Houben E, Metselaar HJ, Borgsteede SD. Prevalence of drug prescriptions and potential safety in patients with cirrhosis: a retrospective real‐world study. Drug Saf 2019;42:539‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid‐involved overdose deaths ‐ United States, 2010‐2015. MMWR Morb Mortal Wkly Rep 2016;65:1445‐1452. [DOI] [PubMed] [Google Scholar]
- 8. Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:319‐331. [DOI] [PubMed] [Google Scholar]
- 9. Whelan CT, Jin L, Meltzer D. Pain and satisfaction with pain control in hospitalized medical patients: no such thing as low risk. Arch Intern Med 2004;164:175‐180. [DOI] [PubMed] [Google Scholar]
- 10. Deng LX, Patel K, Miaskowski C, Maravilla I, Schear S, Garrigues S, et al. Prevalence and characteristics of moderate to severe pain among hospitalized older adults. J Am Geriatr Soc 2018;66:1744‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calcaterra SL, Yamashita TE, Min S‐J, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med 2016;31:478‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donohue JM, Kennedy JN, Seymour CW, Girard TD, Lo‐Ciganic W‐H, Kim CH, et al. Patterns of opioid administration among opioid‐naive inpatients and associations with postdischarge opioid use: a cohort study. Ann Intern Med 2019;171:81‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moon AM, Jiang Y, Rogal SS, Becker J, Barritt AS. In inpatients with cirrhosis opioid use is common and associated with length of stay and persistent use post‐discharge. PLoS One 2020;15:e0229497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agency for Healthcare Research and Quality . Clinical classifications software refined (CCSR) for ICD‐10‐CM diagnoses. www.hcup‐us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp. Updated November 30, 2020. Accessed January 2021.
- 15. Agency for Healthcare Research and Quality . Procedure Classes for ICD‐10‐PCS (beta version). https://www.hcup‐us.ahrq.gov/toolssoftware/procedureicd10/procedure_icd10.jsp. Updated November 12, 2020. Accessed January 2021.
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 17. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 18. Mapakshi S, Kramer JR, Richardson P, El‐Serag HB, Kanwal F. Positive predictive value of International Classification of Diseases, 10th Revision, codes for cirrhosis and its related complications. Clin Gastroenterol Hepatol 2018;16:1677‐1678. [DOI] [PubMed] [Google Scholar]
- 19. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med 2014;9:73‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orhurhu V, Olusunmade M, Urits I, Viswanath O, Peck J, Orhurhu MS, et al. Trends of opioid use disorder among hospitalized patients with chronic pain. Pain Pract 2019;19:656‐663. [DOI] [PubMed] [Google Scholar]
- 21. Moore BJ, Barrett ML. Case study: exploring how opioid‐related diagnosis codes translate from ICD‐9‐CM to ICD‐10‐CM. https://www.hcup‐us.ahrq.gov/datainnovations/ICD‐10CaseStudyonOpioid‐RelatedIPStays042417.pdf. Published April 24, 2017. Accessed August 2019.
- 22. Nwulu U, Nirantharakumar K, Odesanya R, McDowell SE, Coleman JJ. Improvement in the detection of adverse drug events by the use of electronic health and prescription records: an evaluation of two trigger tools. Eur J Clin Pharmacol 2013;69:255‐259. [DOI] [PubMed] [Google Scholar]
- 23. Leuven E, Sianesi B. PSMATCH2: stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. https://ideas.repec.org/c/boc/bocode/s432001.html. Published 2003. Accessed August 2020.
- 24. Austin PC. Double propensity‐score adjustment: a solution to design bias or bias due to incomplete matching. Stat Methods Med Res 2017;26:201‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konerman MA, Rogers M, Kenney B, Singal AG, Tapper E, Sharma P, et al. Opioid and benzodiazepine prescription among patients with cirrhosis compared to other forms of chronic disease. BMJ Open Gastroenterol 2019;6:e000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogal SS, Beste LA, Youk A, Fine MJ, Ketterer B, Zhang H, et al. Characteristics of opioid prescriptions to veterans with cirrhosis. Clin Gastroenterol Hepatol 2019;17:1165‐1174.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotb HIM, Fouad IA, Fares KM, Mostafa MG, Abd El‐Rahman AM. Pharmacokinetics of oral tramadol in patients with liver cancer. J Opioid Manag 2008;4:99‐104. [DOI] [PubMed] [Google Scholar]
- 28. Rakoski M, Goyal P, Spencer‐Safier M, Weissman J, Mohr G, Volk M. Pain management in patients with cirrhosis. Clin Liv Dis (Hoboken) 2018;11:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turturro MA, Paris PM, Larkin GL. Tramadol versus hydrocodone‐acetaminophen in acute musculoskeletal pain: a randomized, double‐blind clinical trial. Ann Emerg Med 1998;32:139‐143. [DOI] [PubMed] [Google Scholar]
- 30. Toupin April K, Bisaillon J, Welch V, Maxwell LJ, Jüni P, Rutjes AWS, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev 2019;5:CD005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fournier J‐P, Azoulay L, Yin H, Montastruc J‐L, Suissa S. Tramadol use and the risk of hospitalization for hypoglycemia in patients with noncancer pain. JAMA Intern Med 2015;175:186‐193. [DOI] [PubMed] [Google Scholar]
- 32. Fournier J‐P, Yin H, Nessim SJ, Montastruc J‐L, Azoulay L. Tramadol for noncancer pain and the risk of hyponatremia. Am J Med 2015;128:418‐425.e5. [DOI] [PubMed] [Google Scholar]
- 33. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid‐related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother 2013;27:62‐70. [DOI] [PubMed] [Google Scholar]
- 34. Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid‐based postsurgical pain control using administrative claims data from a large health system: opioid‐related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy 2013;33:383‐391. [DOI] [PubMed] [Google Scholar]
- 35. Shafi S, Collinsworth AW, Copeland LA, Ogola GO, Qiu T, Kouznetsova M, et al. Association of opioid‐related adverse drug events with clinical and cost outcomes among surgical patients in a large integrated health care delivery system. JAMA Surg 2018;153:757‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


