Abstract
Lipid perturbations contribute to detrimental outcomes in obesity. We previously demonstrated that nervonic acid, a C24:1 ω-9 fatty acid, predominantly acylated to sphingolipids, including ceramides, are selectively reduced in a mouse model of obesity. It is currently unknown if deficiency of nervonic acid-sphingolipid metabolites contribute to complications of obesity. Mice were fed a standard diet, a high fat diet, or these diets supplemented isocalorically with nervonic acid. The primary objective was to determine if dietary nervonic acid content alters the metabolic phenotype in mice fed a high fat diet. Furthermore, we investigated if nervonic acid alters markers of impaired fatty acid oxidation in the liver. We observed that a nervonic acid-enriched isocaloric diet reduced weight gain and adiposity in mice fed a high fat diet. The nervonic acid enrichment led to increased C24:1-ceramides and improved several metabolic parameters including blood glucose levels, and insulin and glucose tolerance. Mechanistically, nervonic acid supplementation increased PPARα and PGC1α expression and improved the acylcarnitine profile in liver. These alterations indicate improved energy metabolism through increased β-oxidation of fatty acids. Taken together, increasing dietary nervonic acid improves metabolic parameters in mice fed a high fat diet. Strategies that prevent deficiency of, or restore, nervonic acid may represent an effective strategy to treat obesity and obesity-related complications.
Keywords: ceramide, fatty acid oxidation, obesity, omega-9, sphingolipids
1 |. INTRODUCTION
The significance of changes in lipids, including specific fatty acids within specific lipid classes, in both normal metabolic homeostasis and in obesity, metabolic syndrome, and diabetes is gaining recognition.1–3 Obese individuals have excess circulating free fatty acids (FFAs), and lipid composition within most tissues is altered. These alterations have detrimental consequences contributing to insulin resistance, inflammation, and lipotoxicity. A particular lipid class of interest are the sphingolipids that have been implicated to contribute to metabolic dysfunction and insulin resistance4–7
A major type of sphingolipids are bioactive ceramides. Ceramides contain a sphingoid backbone coupled to fatty acyl-chains of different lengths through an amide linkage. A family of six known ceramide synthases (CerS) catalyze the fatty acid acylation step. CerS isoforms differ in tissue distribution and fatty acyl-CoA specificity.8 Emerging evidence suggests that the acyl-ceramide composition influences biological responses.8 Deletion of specific CerS in animals with consequent changes in ceramide and sphingolipid fatty acid composition leads to both biophysical and biochemical membrane perturbations. Specifically relevant to obesity, deletion of the C16 (palmitate)-ceramide generating CerS59 or CerS610 protects against diet-induced obesity and associated complications. Furthermore, haploinsufficiency of the predominantly C22-C24-ceramide generating CerS2 lead to elevations in C16-ceramides and consequently increased susceptibility to diet-induced steatohepatitis and insulin resistance in mice.11 Surprisingly, the physiological or pathophysiological consequences of altering ceramide species other than C16-ceramides have not been rigorously investigated.
We previously published that C24:1(nervonate)-ceramides are reduced in various tissues in models of type 1 diabetes and in a diet-induced mouse model of obesity.12 Though the consequences of elevated C16-ceramides have received attention, the roles of reduced very-long-chain ceramides, such as C24:1-ceramides, are largely unknown. A few studies in human demonstrating reduced circulating nervonic acid (NA) in obesity and metabolic syndrome supported our preclinical models.13–15 A negative correlation between circulating NA and obesity-related risk factors has been observed.13 Plasma NA was significantly lower in obese compared to lean participants and was inversely correlated with BMI.14 Furthermore, a study with Japanese males found that subjects with metabolic syndrome demonstrated reduced NA in serum lipids compared to subjects without metabolic syndrome.15 To extend beyond these correlative studies, we herein describe studies investigating how restoration of NA through the diet affects obesity and related metabolic complications.
2 |. RESEARCH DESIGN AND METHODS
2.1 |. Animals
Animal experiments were approved by the Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were group housed in standard cages with up to five mice/cage with a 12 hours dark/light cycle. All mice were weighed once per week. Weekly food consumption was measured by averaging the amount consumed per cage among the mice in the cage. Body composition was determined using an EchoMRI-100H Body Composition Analyzer (EchoMRI LLC, Houston, TX).
2.2 |. Diets
Diets were obtained from Research Diets (New Brunswick, NJ). The low-fat chow was D12450Ji (10 kcal% fat) and the high-fat chow was D12492 (60 kcal% fat). The two chows were reformulated, replacing a portion of dietary fat with an ethyl ester of nervonic acid from Nu-Chek Prep (Elysian, MN), to produce isocaloric diets with nervonic acid contents of 6 g/kg diet (0.6%). For the three most prevalent fatty acids in these diets: C16 comprised 14.4%, 11.7%, 18.5%, and 18.1%; C18:1 comprised 27.4%, 22.8%, 32%, and 31.4%; and C18:2 comprised 39.6%, 36.2%, 26.9%, and 26.5% of the fatty acid composition of the Cnt, Cnt + NA, HFD, and HFD + NA diets, respectively. The full fatty acid profile is shown in Figure S1. Mice were started on diets at ~8 weeks of age and given ad libitum access.
2.3 |. Insulin and glucose tolerance tests (ITT and GTT) and analysis of blood glucose and plasma insulin
ITT and GTT were performed as described previously.16 Blood glucose levels were determined by a glucometer (Contour Next EZ Blood Glucose Monitoring System, Bayer, Leverkusen, Germany) at indicated time points after injection. Plasma insulin levels were determined using a Stellux Chemi Rodent Insulin ELISA kit (Cat. No. 80-INSMR-CH01, APLCO, (Salem, NH)) or an Ultrasensitive Rat/Mouse Insulin ELISA kit low range (Cat. No. 90060, Crystal Chem, (Downers Grove, IL)). The luminescence assay was conducted using a Victor2 Plate Reader (Perkin Elmer, Waltham, MA).
2.4 |. Determination of energy expenditure, respiratory exchange ratio, and locomotion
For determination of energy expenditure, respiratory exchange ratios, and activity, mice were placed in an Oxymax metabolic chamber system (Comprehensive Laboratory Animal Monitoring System, CLAMS, from Columbus Instruments (Columbus, OH)). Oxygen consumption (V̇O2), carbon dioxide production (V̇CO2), and ambulatory activity were determined for each mouse over 72 hours Only the last two complete light and dark cycles (spanning 48 hours) were used for data analysis. Feces were collected during the metabolic cage studies and shipped to the Mouse Metabolic Phenotyping Core at the University of Texas Southwestern for bomb calorimetry analysis using a Parr 6200 Isoperibol Calorimeter.
2.5 |. Cell culture
HEK293 cells were grown in DMEM containing 10% of FBS. Cells were transfected with Silencer Select siRNA to CerS2 (s26789) or Silencer Select negative control No. 1 using Lipofectamine 3000 following the manufacturer’s protocol. All culture reagents were obtained from Thermo Fisher Scientific (Waltham, MA). 48 hours posttransfection, cells were treated with vehicle or 1 μM nervonic acid for 24 hours.
2.6 |. Transcript analysis
RNA was isolated from mouse liver using TRIzol reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. Total RNA underwent DNase I treatment (New England Biolabs, Ipswich, MA) and cDNA was made using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCR was carried out using Bio-Rad SYBR green probes (PPARα qMmuCID0005156, SIRT1 qMmuCID0015511, PGC1α qMmuCID0006032). The TATA binding protein (qMmuCID0040542) gene was used to normalize target gene abundance. Biological samples were run in triplicate.
2.7 |. Lipid measurements by liquid chromatography-mass spectrometry
Liver samples from rats fed a low- or high-fat diet were obtained from Dr Scot Kimball’s laboratory at Pennsylvania State University (Hershey, PA) and analyzed for sphingolipids as described previously.17 Mouse liver samples were analyzed with modifications as described previously.18 Glycerol-based lipids were extracted the same way as for sphingolipids. Acylcarnitines were quantified as butyl esters adapted from.19 Extracts were analyzed by LC-MS/MS on an I-class Acquity with a 2.1 mm × 15 cm C18 BEH 1.7 μm particle size column coupled to an in-line TQ-S mass spectrometer using multiple reaction monitoring. Calibration curves were generated from standard mixtures of acylcarnitines from Cambridge Isotope Laboratories.
2.8 |. Data analysis
Statistical analysis was performed with GraphPad Prism version 6 or 7 software (GraphPad Software, San Diego, CA). Statistical significance was determined by one-way ANOVA with Tukey’s or Sidak’s posttest for multiple comparison. Two-way ANOVA was utilized to test for interactions between diets and NA. Growth curves were analyzed using permutation tests of differences between groups of growth curves with the growth curve function of statmod in R. The number of permutations was set to 10,000. Thresholds of significance are indicated in figure legends and represent adjusted p values. All data are expressed as mean ± SEM with the number of replicates given in figure legends.
3 |. RESULTS
3.1 |. A high fat diet (HFD) reduces very-long-chain sphingolipids in rodent models of obesity
We initially investigated the ceramide profile of livers of mice on a high fat diet (HFD) with 60% of calories from fat (soybean oil supplemented with lard) or a low-fat chow (10 kcal% fat) (Figure 1A). We demonstrate that C22:1 and C24:1 ceramide species were significantly decreased by 51% and 43% by the HFD, replicating a prior experiment, reported in Ref. 12, that used a 55% HFD with corn oil supplemented with vegetable shortening. In the current expanded study, we also report dichotomous increases in C18, C20, and C22 ceramides (Figure 1A). Two additional mouse groups were fed either an isocaloric normal or a HFD both enriched in an ethyl ester form of nervonic acid (NA, 0.6% by diet weight). In both the normal and HFD fed groups, NA-enrichment led to increases in C24:1-ceramides, with concomitant decreases in other ceramides C20-C26. The sum of the amounts of these individual ceramide species (Figure 1A inset) demonstrates no significant differences between diets. The HFD also reduced levels of C24:1-hexosylceramide (Figure 1B) and C24:1-sphingomyelin (Figure 1C) with dietary NA increasing these on both diets. As expected, only small amounts of NA-containing glycerolipids were detected, but a similar pattern was observed. The HFD reduced C24:1-lysophosphatidylcholine (Figure 1D), diacylglycerides C16:0/C24:1 (Figure 1E) and C18:1/C24:1 (Figure 1G), and NA-enriched control and HFDs elevated these species, including diacylglycerides C18:0/C24:1 (Figure 1F) and C18:2/C24:1 (Figure 1H). C24:1-acylation was undetectable in other lipid classes (eg, phosphatidic acid, phosphatidylglycerol). As we observed previously,12 the HFD reduced C24:1-ceramides in plasma (Figure 1I). Dietary NA-enrichment led to significant increases in plasma C24:1-ceramides on both the control and HFDs. Furthermore, siRNA-mediated knockdown of CerS2 in HEK293 cells demonstrated a similar pattern as observed in the liver. Cers2 knockdown diminished C24:1-ceramide amounts and reduced NA acylation into ceramide (Figure 1J).
FIGURE 1.
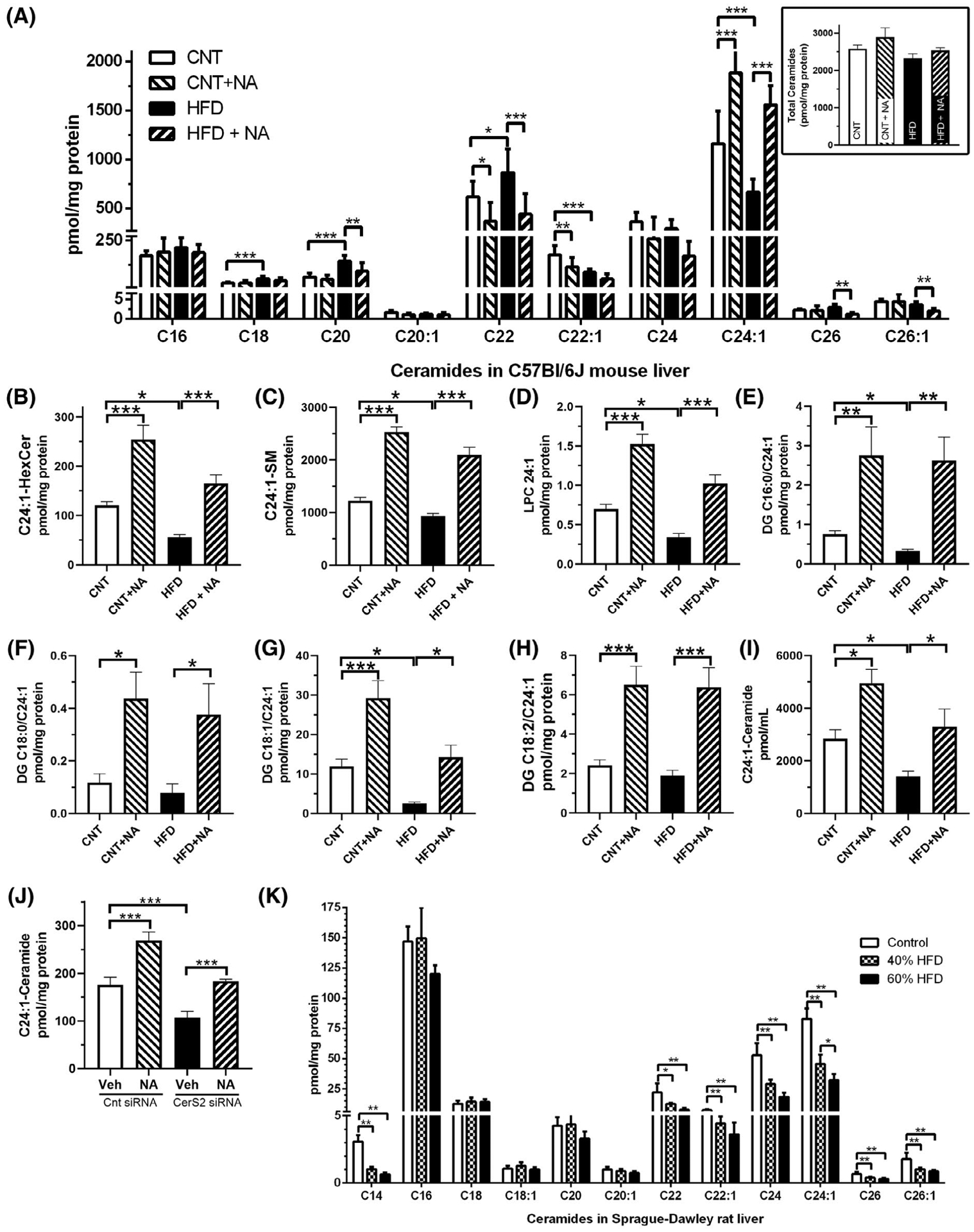
Ceramides are altered in models of diet-induced obesity. (A) Ceramide composition was assessed by LC-MS/MS of liver samples derived from male C57Bl6/J mice (n = 10/group) fed a control or 60% high-fat chow, or isocaloric diets supplemented with nervonic acid (0.6%). The sum of the reported ceramide species is shown in the inset. Other NA-containing lipids were also quantified, including (B) C24:1-hexosylceramide, (C) C24:1-sphingomyelin, (D) C24:1-lysophosphatidylcholine (LPC), and (E–H) diacylglycerides (DG) (E) C16:0/C24:1, (F) C18:0/C24:1, (G) C18:1/C24:1, and (H) C18:2/C24:1. (I) Plasma C24:1-ceramides were assessed after 72 hours on the indicated diets (n = 4/group). For (A–I), two-way ANOVA did not reveal significant interactions between the diets and NA. (J) CerS2 knockdown decreased C24:1-ceramide, which is only partially restored by 1 μM NA treatment for 24 hours in HEK293 cells (n = 3), CerS2 siRNA diminished mRNA by 70% (data not shown). (J) Ceramide composition was determined from male Sprague-Dawley rats (n = 5/group) fed a low fat, a 40%, or a 60% fat containing diet. One-way ANOVA was utilized to assess differences between groups (*P < .05, **P < .01, ***P < .001)
To assess the specificity of the findings in mice, we also determined the ceramide composition in livers of rats fed a 40% or 60% HFD (Figure 1K). We observed decreases in steady-state C14, C22, C22:1, C24, C24:1, C26, and C26:1-ceramides in both 40% and 60% HFD fed rats. More specifically, we report an approximate 55% reduction in C24:1- and C24-ceramides on the 40% HFD and an approximate 65% reduction, on the 60% HFD. Taken together, in two distinct rodent models of obesity, we observe perturbations in very-long-chain fatty acid-containing ceramides, with diminished C24:1-ceramide being consistent in both models. Our findings also demonstrate that a NA-enriched diet modulates ceramide profiles and increases C24:1-lipids in mice on either diet.
3.2 |. Dietary enrichment with nervonic acid reduces weight gain in mice on a HFD
Mice were either fed normal, a 60% high-fat, or NA-supplemented isocaloric normal or high-fat chows and body weights were monitored over a three month period (Figure 2A). As expected, the HFD fed mice gained more body weight than control diet fed mice. Interestingly, mice fed a HFD enriched with NA (HFD + NA) demonstrated significantly less body weight gain than the HFD fed mice, and body weights were not significantly different from mice on the control diet. Even mice fed a NA-enriched control diet demonstrated significantly less body weight gains over time than mice on the control diet.
FIGURE 2.
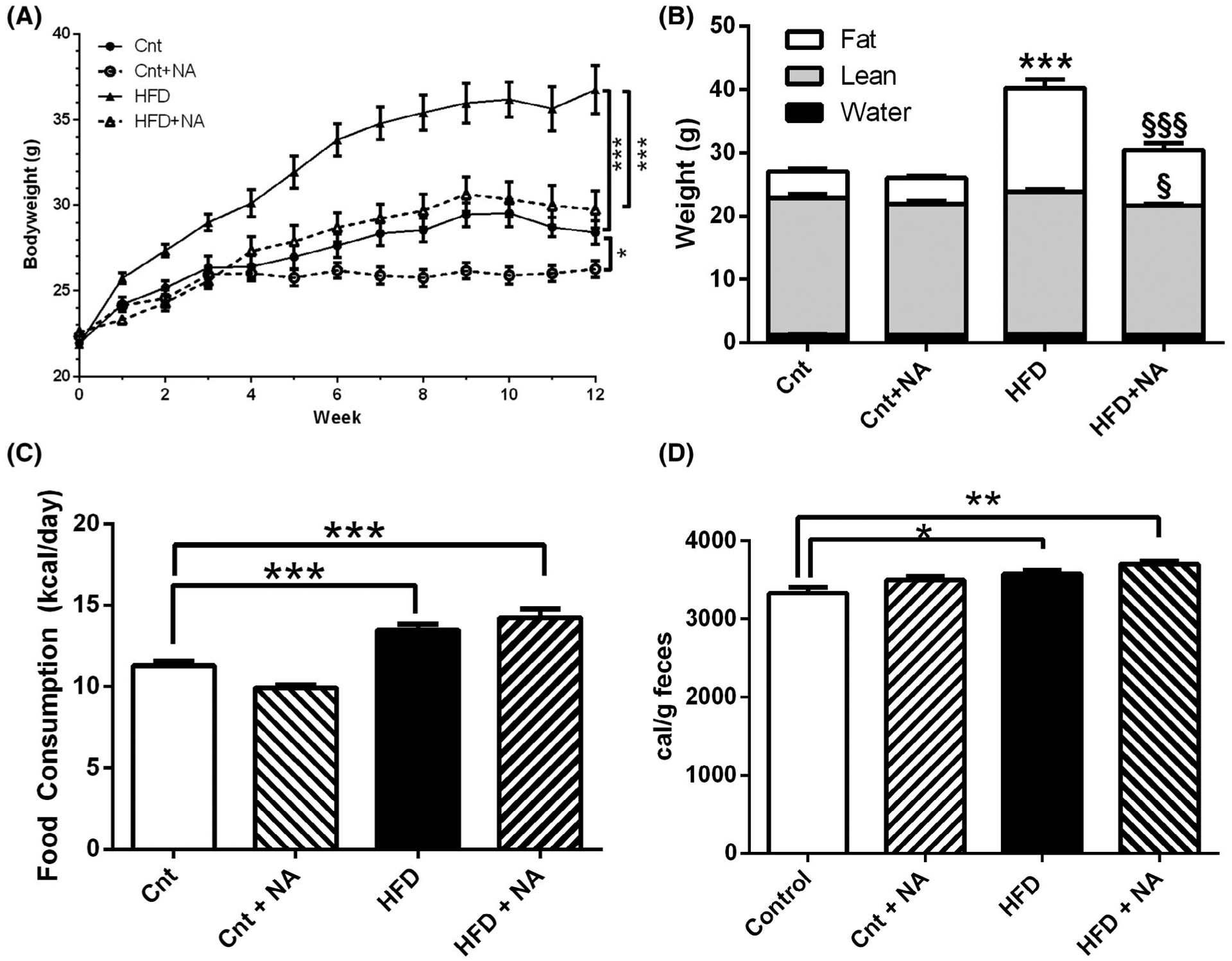
Nervonic acid prevents diet-induced body weight gain. (A) Male C57BL/6J mice were fed a control or high-fat diet supplemented with or without nervonic acid and body weights were followed over 12 weeks. Pairwise comparisons between groups using a permutation test was performed (n = 10–12 mice/group *P < .05, ***P < .001). (B) Body composition was assessed from the same mice by NMR-MRI. One-way ANOVA was utilized to assess differences between groups (n = 10–12 mice/group ***P < .001 from control, §§§P < .001 from HFD, §P = .025 from HFD). Two-way ANOVA demonstrates a significant interaction of diet with NA for fat mass (P < .001) (C) Average food consumption was measured over 12 weeks (n = 10–12 mice/group ***P < .001). (D) Bomb calorimetry was utilized to quantify fecal caloric content (n = 10–11 mice/group *P < .05)
Body composition analysis revealed that the difference in weight gain between mice on the HFD and HFD + NA can be attributed to a large extent to a reduction in fat mass in HFD + NA fed mice (Figure 2B). At the time of sacrifice, HFD and HFD + NA fed animals showed average body fat contents of 16.44 g/mouse and 9.48 g/mouse, respectively. Lean mass was slightly, but significantly, reduced in the HFD + NA versus the HFD group, though the lean mass of the HFD + NA group was not significantly different to the lean mass of control or control + NA groups. The lean mass was also not significantly different between the control and control + NA diet groups. Water mass was similar for all experimental groups.
Throughout the duration of this study, average food consumption was determined (Figure 2C). Mice on high fat diets demonstrated significant increases in kcal per day consumed compared to controls, while the average food consumption between HFD and HFD + NA was similar over the 12 weeks of the study. Food consumption of mice on the control + NA diet was also not significantly different from control mice. To evaluate whether food calories were not equally well absorbed, fecal caloric measurements were determined by bomb calorimetry (Figure 2D). Comparing gross heat of combustion, small but significant increases in fecal caloric content was observed in mice on HFD and HFD + NA diets compared to controls. No differences in fecal caloric content were observed between control and control + NA or HFD and HFD + NA diets. Therefore, similar to food consumption, dietary NA enrichment did not have an apparent effect on diet absorption. Taken together, these data demonstrate that animals on a HFD supplemented with NA exhibit reduced body weight gain with reduced adiposity that is not due to reduced food consumption or absorption.
3.3 |. Dietary nervonic acid alters energy balance parameters, but not activity, in mice on a HFD
As food consumption or assimilation did not appear to be responsible for differences in body weight gains, we next evaluated if mice on the HFD + NA when compared to the HFD had changes in energy expenditure (Figure 3). Using metabolic cages, we found elevated oxygen consumption (VO2) (Figure 3A) in mice on the HFD compared to controls in both the light and dark cycle. Mice on the HFD + NA had VO2 levels that were significantly lower than in mice on a HFD and similar to mice on control diets in both the light and dark cycle. Carbon dioxide production (VCO2) in mice on the HFD was not significantly different from mice on control diets, but mice on the HFD + NA showed a significant reduction to control and HFD groups (Figure 3B). When calculating respiratory exchange ratios (RER = VCO2/VO2) for both the light and dark cycle (Figure 3C), we found that mice on the HFD and HFD + NA showed similar RERs and that these were significantly lower than for mice on control diets. These data indicate increased fat utilization for energy production in mice on high fat diets as expected. No statistical changes of RER was observed for mice on control + NA compared to the control diet for the light and dark, cycles. Heat production was highest in mice on HFD (Figure 3D), while mice in the HFD + NA and control diet groups had similar lower values in both the light and dark cycles. No differences were observed between control and control + NA diets. Activity measurements were determined and graphed as beam breaks per hour (Figure 3E) and cumulatively beam breaks over the 12 hours light and 12 hours dark cycles (Figure 3F). Mice on a HFD demonstrated reduced ambulatory activity compared to mice on control diets. Ambulatory activities were similar for mice on the HFD and HFD + NA diets, and for mice on the control and control with NA diets. Taken together, these data suggest that animals on a HFD, enriched with NA, have decreased energy expenditure and heat production, but exhibit similar ambulatory activity when compared to mice on the HFD. This data thus cannot explain the reduced body weight and adiposity in mice on the HFD + NA diet.
FIGURE 3.
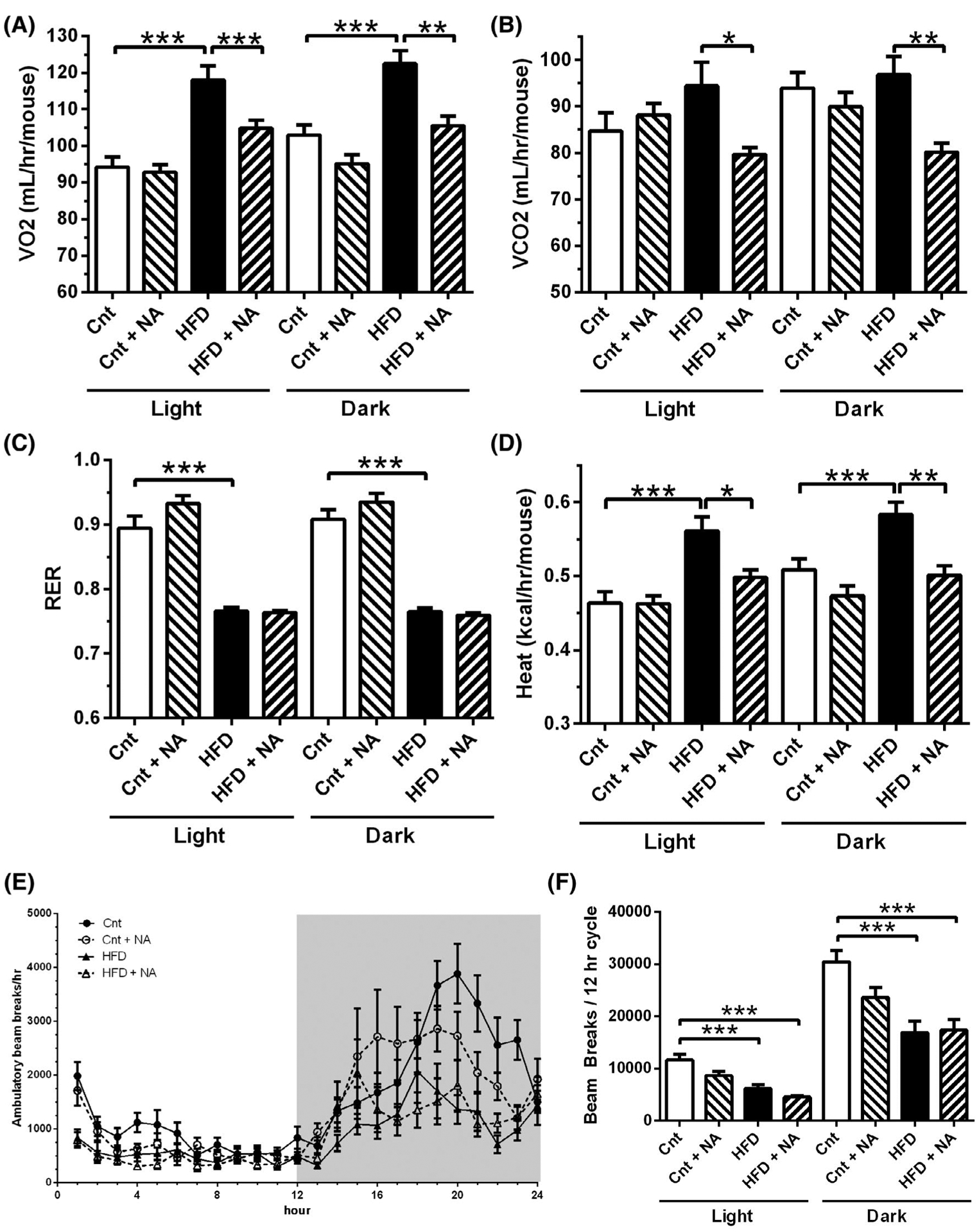
Nervonic acid-enrichment of a HFD leads to energy expenditure values similar to control mice. (A) VO2, (B) VCO2, (C) RER, (D) Heat, (E & F) locomotion were assessed by placing mice on indicated diets in metabolic cages. Shaded region in (E) represents the dark cycle (n = 10–12 mice/group **P < .01, ***P < .001). Two-way ANOVA demonstrates a significant interaction of diet with NA for VO2, VCO2, and heat in the light cycle (P < .05)
3.4 |. Dietary nervonic acid enrichment improves glycemic control in mice on a HFD
To determine metabolic consequences of dietary NA enrichment, we assessed circulating glucose and insulin levels under fasting and random-fed conditions and performed glucose and insulin tolerance tests. After 8-weeks, the HFD led to increased fasting (Figure 4A) and random-fed (Figure 4B) blood glucose levels when compared with control. Mice on HFD + NA demonstrated significant reductions in blood glucose levels compared to their HFD fed counterparts under both random-fed and fasted conditions. Blood glucose levels of mice on the HFD + NA diet were not significantly different from mice on control diets under random fed or fasted conditions. No significant differences were observed between mice on a control and mice on a control with NA diet.
FIGURE 4.
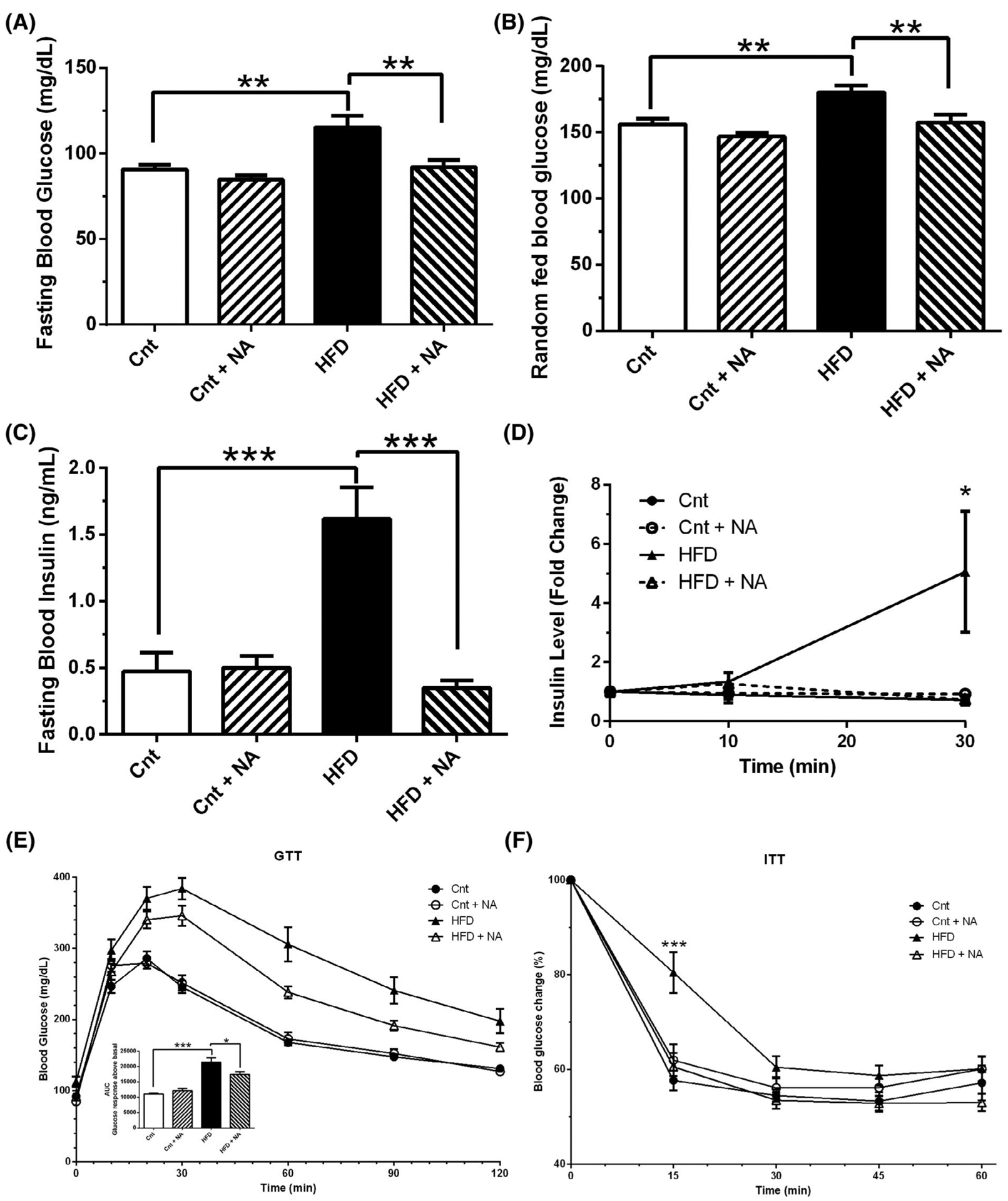
Nervonic acid improves glucose tolerance and insulin sensitivity in mice on a high-fat diet. (A) Fasting and (B) random-fed blood glucose, (C) fasting insulin, and (D) insulin levels after IP injection of glucose at indicated time points were measured in mice after 8 weeks on different diets. (E) GTT and (F) ITT were performed on mice after 10 and 11 weeks on diets, respectively. One-way ANOVA was utilized to assess differences between groups (n = 10–12 mice/group *P < .05, **P < .01, ***P < .001). Two-way ANOVA demonstrates a significant interaction of diet with NA for fasting blood glucose (P < .05), fasting insulin (P < .001), glucose-stimulated insulin production (P < .05), GTT (P < .01), and ITT (P < .005)
Fasting plasma insulin levels were measured after 8 weeks on diets (Figure 4C). In the control diet, NA did not affect insulin levels. Mice on the HFD demonstrated a significant increase in basal insulin levels, whereas in mice on the HFD + NA insulin levels, when compared to HFD mice, were significantly reduced to levels not significantly different from mice on control diets. Insulin levels were also assessed after intraperitoneal administration of glucose (Figure 4D). Mice on the HFD showed a significant increase in insulin after 30 minutes, while mice on the HFD + NA showed no such increase. No discernable differences in insulin levels were observed at any of the time points analyzed in the control groups.
Glucose tolerance tests were performed after 10 weeks on diets (Figure 4E). Impaired glucose tolerance was observed in mice on both high fat diets compared to control diets. However, mice on the HFD + NA showed significantly improved glucose tolerance over HFD. To assess insulin sensitivity, insulin tolerance tests were performed at 11 weeks on diets (Figure 4F). Mice on the HFD showed a diminished response to lower blood glucose levels after insulin injection, whereas mice on the HFD + NA responded similarly to mice on control diets. Mice on the control diet containing NA responded similar to mice on the control diet alone. Taken together, the HFD enriched with NA normalized blood glucose and insulin levels, and improved glucose tolerance and insulin sensitivity when compared to the HFD alone.
3.5 |. Nervonic acid improves biomarkers of energy metabolism in the liver
We next assessed markers of energy metabolism in the liver by RT-qPCR (Figure 5A). The HFD significantly reduced transcript levels of PGC1α and SIRT1, but not PPARα, while the HFD + NA increased mRNA levels of PPARα, and restored PGC1α and SIRT1 back to control levels. As these genes regulate the expression of genes involved in fatty acid β-oxidation and are major regulators of metabolism, we next analyzed acylcarnitine levels as a functional readout of altered cellular metabolism by a liquid chromatography-mass spectrometry approach. The graphs of short-(Figure 5B), medium-(Figure 5C), and long-chain acylcarnines (Figure 5D) depict alterations in hydroxyl- and non-hydroxyl-acylcarnitines species from C0-C20 due to the diets. The most marked differences observed between the control and HFD-fed mice were significant increases in many medium and long-chain (C10-C20) acylcarnitine species (Figure 5C,D). These changes are consistent with impaired fatty acid oxidation and may be a marker of insulin resistance.20,21 In contrast, mice on a HFD + NA showed significant decreases in these long-chain acylcarnitines compared to the HFD. Furthermore, the NA enrichment, in both the control diet and the HFD, led to increases in free carnitine (C0), acetyl-carnitine (C2) and several hydroxyl-fatty acylcarnitines (C4-OH. C6-OH, and C10-OH) (Figure 5B,C). Increases in hydroxy-carnitines, an intermediate in fatty acid oxidation, may reflect increased higher lipid flux, with increases in C2-carnitine being an indication of elevated fatty acid oxidation. Taken together, these results support that NA-enriched diets may increase fatty acid utilization via β-oxidation, possibly as a function of increased, or restored, PPARα/PGC1α/SIRT1 transcription.
FIGURE 5.
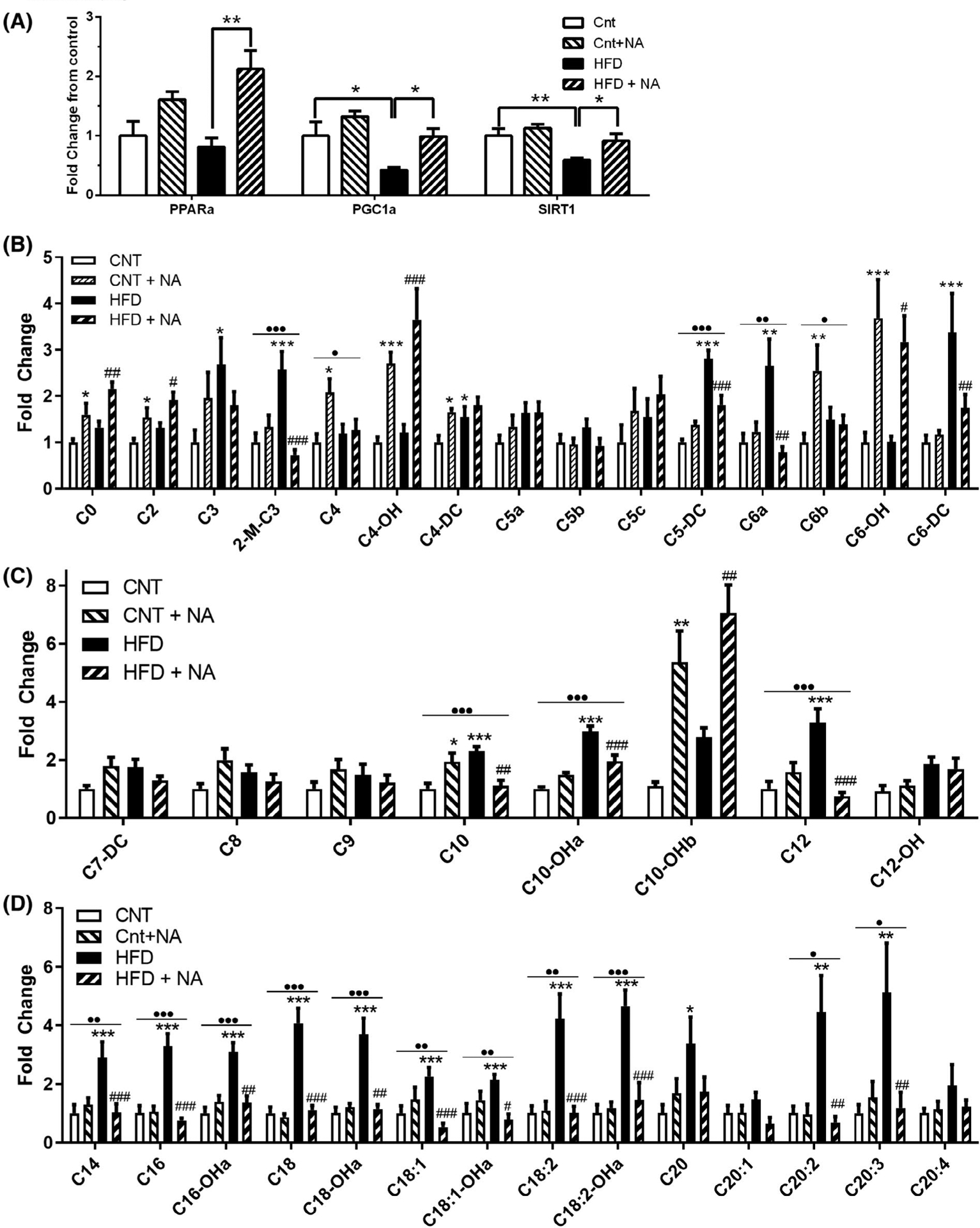
Nervonic acid enrichment improves markers of liver fatty acid oxidation. (A) RT-qPCR was utilized to assess transcript levels of PPARα, PGC1α, and SIRT1 (n = 8 mice/group *P < .05, **P < .01). (B) Short, (C) Medium, and (D) Long-chain acylcarnitine levels were assessed by LC-MS/MS (n = 10–11 mice/group *P < .05, **P < .01, ***P < .001 when compared to control, #P < .05, ##P < .01, ###P < .001 for HFD to HFD + NA comparisons). Where significant, results from two-way ANOVA indicating an interaction between the diet and NA are indicated above each acylcarnitine (•P < .05, ••P < .01, •••P < .001)
4 |. DISCUSSION
Our studies demonstrate that dietary NA supplementation provides protection against HFD-induced obesity and associated metabolic complications. NA and other very-long-chain fatty acid containing ceramides are reduced in the liver of mice and rats on a HFD (Figure 1) and HFD + NA reverses these changes. Simultaneously, HFD + NA decreases body weight gains and reduces adiposity, while not changing food intake or absorption (Figure 2). Mice on a HFD enriched with NA demonstrate energy expenditure and heat production similar to control mice but have similar RER and activity levels as HFD-fed counterparts (Figure 3). Improved body composition with decreased adiposity in HFD + NA mice is associated with better glycemic control and increased insulin sensitivity (Figure 4). Better energy utilization in HFD + NA mice is suggested by increased liver expression of PPARα, PGC1α, SIRT1, and improved fatty acid oxidation is supported by altered acylcarnitine profiles in mice on the NA-supplemented HFD (Figure 5). This is the first evidence of a link between NA levels in liver and the development of obesity and related metabolic complications.
A few human studies support our observation with preclinical animal models that reduced NA could contribute to obesity and the metabolic syndrome. A negative correlation between circulating NA levels and obesity-related risk factors has been described in a previous study, and the authors concluded that NA may prevent obesity-related metabolic disorders.13 Another group demonstrated that plasma NA levels were significantly lower in obese compared to lean participants and were inversely correlated with BMI.14 Furthermore, a study with Japanese males found that subjects with metabolic syndrome demonstrated reduced NA in serum lipids compared to subjects without metabolic syndrome.15 In contrast to these studies, a small study reported that obese adolescent females, but not males, had increased NA22 and in another small study, obese young male adults did not exhibit changes in NA-containing sphingomyelin or ceramides.23 An additional study reported that a low-calorie diet reduced NA in erythrocyte membranes of overweight/obese persons who lost at least 5% of their initial body weight.24 The discrepancies between these studies are not clear, though age, gender, sample type, and the patient population could have affected results. Regardless, these correlative studies largely point to a role of NA in regulating body composition.
Investigations into saturated and monounsaturated very-long-chain fatty acids (≥22 carbons), such as NA, have been fairly limited. It is presently unknown if the effect of NA in mice on a HFD is specific or if it extends to other very-long-chain fatty acids, such as C22:0, C22:1, and C24:0. Supporting our studies, behenic acid (C22:0), in triglyceride form (one molecule of C22 with two molecules of oleic acid), prevented obesity in rats via suppressed triglyceride absorption through pancreatic lipase inhibition.25 Yet, as we did not observe alterations in fecal caloric content, this mechanism may not apply to effects of the NA-enriched HFD used herein. Though it should be noted that bomb calorimetry does not differentiate between non-digestible and utilizable energy. We did not observe any overt toxicities in animals fed a NA-enriched diet (eg, ruffled fur, anorexia, cachexia, skin tenting, skin ulcerations, diarrhea, or death). However, a closely related fatty acid, erucic acid (C22:1), in rapeseed oil, was implicated in causing heart lesions in rats,26 though another group suggested linolenic acid as the culprit.27 Correlative studies suggested ω-9 fatty acids, including NA, increase the risk of all-cause or cardiovascular mortality.28 Interestingly, the Cowart laboratory further demonstrated that very-long-chain ceramides exhibit a lipotoxic effect in cardiomyocytes.29 Due to the controversies of ω-9 fatty acid supplementation, studies to investigate fatty acid specificity, dose responsiveness, fatty acid delivery formulations (free, ester, acylated), and toxicology of NA supplementation are ongoing.
The beneficial effect of NA may be exerted through re-acylation of dietary NA into sphingolipids to restore C24:1-ceramide levels (Figure 1A). Our data support that this restoration is through ceramide remodeling as total amounts of ceramides were consistent between diet groups (Figure 1A inset). While further studies are needed to demonstrate that acylation into ceramides is necessary for NA regulation of obesity, data suggest that very-long-chain fatty acids, such as NA, are selectively acylated into sphingolipids as opposed to glycerolipids (Figure 1A–H and Ref. 12,30). However, we cannot rule out effects of other lipid classes or NA as a circulating free fatty acid as contributing factors. The role of specific ceramide fatty acid composition, rather than total ceramide, has gained greater appreciation in disease states. C16-ceramide has been implicated in palmitate-induced insulin resistance and impaired fatty acid β-oxidation. C16-ceramides are significantly elevated in skeletal muscles and adipose tissues but not in livers of obese mice (Ref. 9,11 and Figure 1). Thus, effects may be tissue specific. Recently, it has been shown that the lipid enzyme CerS4, that generates C18- and C20-ceramides, contribute to hepatic insulin resistance.31 Consistently, in HFD-fed mice, we observed increases in hepatic C18-, C20-, and C22-ceramides and the NA-supplemented diet returned C20- and C22-ceramides back to control levels. Thus, indirectly, increasing NA could improve outcomes by decreasing detrimental effects of other ceramides in our mouse model. In vitro CerS2 knockdown data for C24:1-ceramide (Figure 1J) demonstrated a similar pattern to what is observed in liver upon NA addition (Figure 1A). Data from knockout mice supports an apparent lack of functional redundancy for CerS2 in generating C24 containing sphingolipids32,33 though we cannot rule out reverse ceramidase activity34 as a potential pathway for acylation in our studies. Independent of fatty acid composition, it was also recently demonstrated by the Summers’ laboratory that preventing the conversion of dihydroceramides to ceramides was sufficient to resolve hepatic steatosis and insulin resistance in obese mice.7 Furthermore, deoxysphingolipids that are generated when alanine is used instead of serine in de novo sphingolipid synthesis were also implicated in contributing to diabetes-related complications.35,36 The influence of NA on deoxysphingolipids will be pursued in future studies. Taken together, while long-chain fatty acid-derived ceramides have been implicated in detrimental effects of obesity and diabetes, our studies are the first to document that very-long-chain C24:1 species can lead to opposite effects. A similar dichotomy of the physiological actions of individual ceramide species is now being appreciated in cardiovascular and oncological diseases.37,38
Mechanistically, we demonstrate that NA supplementation increased PPARα and restored PGC-1α and SIRT1 expression levels in mice on a HFD. These targets have been implicated in obesity-related complications as they increase hepatic fatty acid oxidation and decrease the levels of circulating triglycerides responsible for adiposity.39,40 We observed evidence for increased fatty acid oxidation when we examined acylcarnitines. Mice on a HFD demonstrated a significant increase in several long-chain acylcarnitines that are largely restored to control levels when the HFD is enriched with NA. This observation implicates that impaired fatty acid β-oxidation in mice on a HFD is ameliorated with NA supplementation, which could then limit the detrimental effects of long-chain acylcarnitines, including insulin resistance, detergent effect, lipotoxicity, and reduced glucose utilization.20,21 Independent of diet, NA enrichment increased free carnitine, C2, C4-OH, C8-OH carnitines suggesting increased rates of hepatic fatty acid oxidation. Elevated C4-OH may also reflect increased ketogenesis41 and has been implicated in insulin resistance.42 Hydroxyl- and dicarboxyl-fatty acids are also products of fatty acid ω-oxidation, which can be elevated with a HFD.43,44 We observed reductions of the dicarboxyl-acylcarnitines C5 (C5-DC) and C6 (C6-DC) levels in mice on a HFD enriched with NA and improved glucose/insulin tolerance, suggesting that increased C4-OH predominantly reflects an intermediate in β-oxidation. With improved fatty acid oxidation, a likely consequence of upregulation of energy metabolism regulators, PPARα, PGC-1α, and SIRT1, the transcriptional mechanisms underlying this NA-induced upregulation is under active investigation.
We did not observe significant differences in whole body fatty acid oxidation (RER) or parameters of whole-body energy homeostasis that could explain the substantial decrease in body weight with HFD + NA. However, studies into energy expenditure are inherently underpowered.45 Our studies have about 80% power to detect a medium effect size. Consequently, small changes in parameters like energy expenditure and food intake may have been missed could still have a profound cumulative effect on body weight.
With this study, we established that a HFD specifically reduced very-long-chain sphingolipids, in particular the abundant C24:1 nervonic acid, and that restoring NA levels in mice on HFD reduced body weight gain and improved metabolic parameters. Although more investigation is needed into the mechanisms by which NA supplementation exerts effects, our study has possible clinical relevance for the treatment of metabolic disorders. Increasing dietary NA to restore HFD-induced reduction of very-long-chain fatty acids may be an effective way to improve the management of obesity and associated metabolic complications including diabetes.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Ruth Gordillo at UT Southwestern for bomb calorimetry studies, Dr. Scot Kimball at Penn State University for rat liver samples, and Pedro Costa-Pinheiro for RT-qPCR assistance. This project was supported by Intramural Funds (to TF) from the Research and Development Program at the University of Virginia and EY018336 from the National Institute of Health.
Abbreviations:
- CerS
ceramide synthase
- DG
diacylglycerides
- FFA
free fatty acid
- GTT
glucose tolerance test
- HFD
high fat diet
- ITT
insulin tolerance test
- LPC
lysophatidylcholine
- NA
nervonic acid
- PGC1α
peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PPARα
peroxisome Proliferator Activated Receptor Alpha
- RER
respiratory exchange ratio
- SIRT1
Sirtuin 1
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Hammad SS, Jones PJ. Dietary fatty acid composition modulates obesity and interacts with obesity-related genes. Lipids. 2017;52:803–822. [DOI] [PubMed] [Google Scholar]
- 2.Palomer X, Pizarro-Delgado J, Barroso E, Vazquez-Carrera M. Palmitic and oleic acid: the Yin and Yang of fatty acids in type 2 diabetes mellitus. TEM. 2018;29:178–190. [DOI] [PubMed] [Google Scholar]
- 3.Bandet CL, Tan-Chen S, Bourron O, Le Stunff H, Hajduch E. Sphingolipid metabolism: new insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci. 2019;20(3):479. 10.3390/ijms20030479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nature Rev Endocrinol. 2017;13:79–91. [DOI] [PubMed] [Google Scholar]
- 5.Lambert JM, Anderson AK, Cowart LA. Sphingolipids in adipose tissue: What’s tipping the scale? Adv Biol Regul. 2018;70:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox TE, Kester M. Therapeutic strategies for diabetes and complications: a role for sphingolipids? Adv Exp Med Biol. 2010;688:206–216. [DOI] [PubMed] [Google Scholar]
- 7.Chaurasia B, Tippetts TS, Mayoral Monibas R, et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochem Biophys Acta. 2014;1841:671–681. [DOI] [PubMed] [Google Scholar]
- 9.Gosejacob D, Jager PS, Vom Dorp K, et al. Ceramide synthase 5 is essential to maintain C16:0-ceramide pools and contributes to the development of diet-induced obesity. J Biol Chem. 2016;291:6989–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. [DOI] [PubMed] [Google Scholar]
- 11.Raichur S, Wang ST, Chan PW, et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. [DOI] [PubMed] [Google Scholar]
- 12.Fox TE, Bewley MC, Unrath KA, et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. J Lipid Res. 2011;52:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda E, Hatada K, Kimura J, Aizawa Y, Thanikachalam PV, Watanabe K. Relationships between serum unsaturated fatty acids and coronary risk factors: negative relations between nervonic acid and obesity-related risk factors. Int Heart J. 2005;46:975–985. [DOI] [PubMed] [Google Scholar]
- 14.Pickens CA, Sordillo LM, Comstock SS, et al. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins Leukot Essent Fatty Acids. 2015;95:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki Y, Kondo K, Maeba R, Nishimukai M, Nezu T, Hara H. Proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci. 2014;63:527–537. [DOI] [PubMed] [Google Scholar]
- 16.Lansey MN, Walker NN, Hargett SR, Stevens JR, Keller SR. Deletion of Rab GAP AS160 modifies glucose uptake and GLUT4 translocation in primary skeletal muscles and adipocytes and impairs glucose homeostasis. Am J Physiol-Endocrinol Metab. 2012;303:E1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2010;51:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson JM, Tan SF, Sharma A, et al. Ceramide analogue SACLAC modulates sphingolipid levels and MCL-1 splicing to induce apoptosis in acute myeloid leukemia. Molecular Cancer Research. 2020;18(3):352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giesbertz P, Ecker J, Haag A, Spanier B, Daniel H. An LC-MS/MS method to quantify acylcarnitine species including isomeric and odd-numbered forms in plasma and tissues. J Lipid Res. 2015;56:2029–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51:553–572. [DOI] [PubMed] [Google Scholar]
- 21.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson M, Marild S, Brandberg J, Lonn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity. 2006;14:1931–1939. [DOI] [PubMed] [Google Scholar]
- 23.Hanamatsu H, Ohnishi S, Sakai S, et al. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr Diabetes. 2014;4:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazzola R, Rondanelli M, Trotti R, Cestaro B. Effects of weight loss on erythrocyte membrane composition and fluidity in overweight and moderately obese women. J Nutr Biochem. 2011;22:388–392. [DOI] [PubMed] [Google Scholar]
- 25.Kojima M, Tachibana N, Yamahira T, et al. Structured triacylglycerol containing behenic and oleic acids suppresses triacylglycerol absorption and prevents obesity in rats. Lipids Health Dis. 2010;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulan HW, Kramer JK, Corner AH. Myocardial lesions in rats fed rapeseed oil. I. Influence of strain of rats. Can J Physiol Pharmacol. 1977;55:258–264. [DOI] [PubMed] [Google Scholar]
- 27.Dewailly P, Nouvelot A, Sezille G, Fruchart JC, Jaillard J. Changes in fatty acid composition of cardiac mitochondrial phospholipids in rats fed rapeseed oil. Lipids. 1978;13:301–304. [DOI] [PubMed] [Google Scholar]
- 28.Delgado GE, Kramer BK, Lorkowski S, Marz W, von Schacky C, Kleber ME. Individual omega-9 monounsaturated fatty acids and mortality—the Ludwigshafen risk and cardiovascular health study. J Clin Lipidol. 2017;11(1):126–135.e5. [DOI] [PubMed] [Google Scholar]
- 29.Law BA, Liao X, Moore KS, et al. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018;32:1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christophersen B, Krogstad T, Norseth J. Metabolism of erucic acid in adipocytes isolated from rat epididymal fat. Lipids. 1983;18:137–141. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaka T, Kuba M, Koyasu S, et al. Hepatocyte Elovl6 determines ceramide acyl-chain length and hepatic insulin sensitivity in mice. Hepatology. 2019;71:1609–1162. [DOI] [PubMed] [Google Scholar]
- 32.Imgrund S, Hartmann D, Farwanah H, et al. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. 2009;284:33549–33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pewzner-Jung Y, Park H, Laviad EL, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J Biol Chem. 2010;285:10902–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okino N, He X, Gatt S, Sandhoff K, Ito M, Schuchman EH. The reverse activity of human acid ceramidase. J Biol Chem. 2003;278:29948–29953. [DOI] [PubMed] [Google Scholar]
- 35.Mwinyi J, Bostrom A, Fehrer I, et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS One. 2017;12:e0175776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Othman A, Bianchi R, Alecu I, et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes. 2015;64:1035–1045. [DOI] [PubMed] [Google Scholar]
- 37.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51:50–62. [DOI] [PubMed] [Google Scholar]
- 38.Anroedh S, Hilvo M, Akkerhuis KM, et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J Lipid Res. 2018;59:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon M The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res. 2009;60:151–159. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CF, Ku HC, Lin H. PGC-1alpha as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hack A, Busch V, Pascher B, et al. Monitoring of ketogenic diet for carnitine metabolites by subcutaneous microdialysis. Pediatr Res. 2006;60:93–96. [DOI] [PubMed] [Google Scholar]
- 42.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. [DOI] [PubMed] [Google Scholar]
- 43.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol-Gastrointest Liver Physiol. 2006;290:G852–858. [DOI] [PubMed] [Google Scholar]
- 44.Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ. PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-hydroxylase (CYP4) isozymes: Implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009;2009:952734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschop MH, Speakman JR, Arch JR, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


