Abstract
Excessive consumption of fructose, the sweetest of all naturally occurring carbohydrates, has been linked to worldwide epidemics of metabolic diseases in humans, and it is considered an independent risk factor for cardiovascular diseases. We provide an overview about the features of fructose metabolism, as well as potential mechanisms by which excessive fructose intake is associated with the pathogenesis of metabolic diseases both in humans and rodents. To accomplish this aim, we focus on illuminating the cellular and molecular mechanisms of fructose metabolism as well as its signaling effects on metabolic and cardiovascular homeostasis in health and disease, highlighting the role of carbohydrate-responsive element–binding protein in regulating fructose metabolism.
Keywords: Fructose, Metabolic diseases, Pathogenesis
Introduction
In the past decades, dietary patterns have changed remarkably. In conjunction with the advent of sedentary lifestyles in both industrialized and developing countries, this trend of changing dietary patterns is highly associated with the increasing epidemic of obesity, non-alcoholic fatty liver disease (NAFLD), type 2 diabetes, and metabolic syndrome.[1,2] The rapid increase in pediatric obesity and NAFLD has become one of the major public health concerns globally. Sugars in the form of sucrose (half fructose) or high-fructose corn syrup (HFCS, about 45–55% fructose) are major sweeteners added in food and beverages, which comprise a large proportion of the modern diet, in particular in children, adolescents, and young adults. In Western diets, added sugars account for about 14% to 17% of the total caloric intake, which is above the recommended level of 10% of the total caloric intake according to the World Health Organization's guidelines.[3] In China, the past decades have seen a great increase in the consumption of sugar-sweetened beverages (SSB), according to the China National Nutrition Surveys (CNNS).[4] It is well recognized that excessive consumption of added sugars is a risk factor for cardiometabolic diseases, and has been a big public health problem.[5,6] Therefore, recommendations for the restriction of sugar consumption attract significant attention.
As the major component of added sugar, fructose is a monosaccharide with a similar formulary to glucose. However, its metabolism pathway is quite different from glucose in vivo.[7–9] There is substantial evidence that excessive fructose intake has detrimental effects on multiple metabolic diseases. It causes visceral fat accumulation and leads to obesity, hyperlipidemia, insulin resistance, hypertension, and hyperuricemia, which are associated with development of diabetes, fatty liver disease, cardiovascular disease, and gout.[5,6,10] Even for those in the “normal” range of fructose consumption, fructose can still rapidly impair intermediate physiological endpoints like circulating lipids and insulin sensitivity in humans.[11] Therefore, further understanding fructose metabolism and its role in the development of metabolic diseases will provide fundamental insights into pathogenic mechanisms, which assists to develop new diagnostic, preventative, and therapeutic strategies for metabolic disease. In this review, we aim to illuminate the physiological and biochemical characteristics of fructose metabolism as well as its association with metabolic diseases.
Fructose Metabolism and Homeostasis
Intestinal fructose absorption
Dietary fructose is predominantly absorbed from the intestinal lumen and transported across apical membrane, which is mediated by the facilitative glucose transporter (GLUT) family members.[9,12] Both GLUT5 and GLUT2 are able to facilitate fructose transportation, but GLUT5 shows higher affinity for fructose. Fructose absorption occurs mainly in the brush border of the lower part of the duodenum and jejunum via GLUT5 and translocated into the circulation through GLUT2.[13] In intestinal epithelial cells, fructose induces GLUT5 expression and activation to facilitate its translocation in an energy-independent manner. This sensibility of GLUT5 for fructose thereby triggers intracellular signaling in response to nutrient concentrations.[12] As a consequence, GLUT5 deficiency leads to intestinal fructose malabsorption as well as intestinal dysfunction.
Endogenous fructose production
The vast majority of fructose in vivo is derived from the dietary source of sugar, while it can also be synthesized endogenously through sorbitol (polyol) pathway. This metabolic route exists in a wide range of tissues and is regulated by two enzymes: aldose reductase and sorbitol dehydrogenase. Glucose is first converted to sorbitol by aldose reductase, and then oxidized to fructose by sorbitol dehydrogenase.[10] Physiologically, endogenous fructose is produced as a source of energy mainly for sperm and fertility as well as for fetus development. In contrast to the ubiquitous expression of sorbitol dehydrogenase, aldose reductase expression is largely limited to the hypertonic areas of the inner medulla and papilla in the kidney; therefore, the sorbitol pathway was once considered to be inactive in the majority of tissues other than those in kidney. As a result, the locally produced and accumulated sorbitol helps maintain osmotic pressure for a proper urinary concentrating mechanism.[14] Therefore, circulating fructose levels are much lower than that of glucose.[15] The estimated blood concentration of fructose in human and laboratory rodents ranges from 6 μmol/L to 1500 μmol/L, as determined by mass spectrometry detection (only ∼1/1000 of glucose level in circulation).[16,17] Recent studies suggest that the sorbitol pathway can also be active in brain.[18–20] In this regard, researchers have reported the fructokinase expression and fructose metabolism in the brain, as well as the endogenous fructose generation in the hypothalamus, for the production and release of vasopressin.[18] In addition, the endogenous fructose and activated sorbitol pathways are also considered to be a contributor to diabetic microvascular complication. There is some evidence supporting the view that endogenous fructose production and ketohexokinase (KHK) activation within the kidney contribute to the development of diabetic nephropathy.[21] Meanwhile, glucose activates the sorbitol pathway both in humans and mice, which could be a mechanism for the exacerbated cardiometabolic risks by severe hyperglycemia.[22] The question of whether endogenous fructose is involved in the pathogenesis of metabolic diseases needs to be addressed by further investigation.
Fructolysis and fructose disposal
Cellular glucose is catalyzed by glucokinase to produce glucose-6-phosphate (G-6-P) and eventually enters the glycolysis pathway after a series of catalytic reactions. Remarkably, fructose metabolism occurs via a divergent pathway with distinctive metabolic consequences.[7] The canonical pathway of fructose metabolism is fructolysis, that is initiated by KHK to produce fructose-1-phosphate (F-1-P). F-1-P is then cleaved by aldolase B into dihydroxyacetone phosphate and glyceraldehyde. Glyceraldehyde is phosphorylated by triokinase to generate glyceraldehyde 3-phosphate (GAP). GAP and other triose phosphates are resynthesized into glucose via gluconeogenesis or converged with glycolytic pathway to enter the lipogenesis or oxidation pathways.[6] Incontrast to glucose, intracellular fructose is rapidly phosphorylated by KHK due to the lack of negative feedback of substrate, which depletes the cells of adenosine triphosphate (ATP) and phosphoric acids stores after intake of excessive fructose. The excessive cellular consumption of ATP stimulates adenosine monophosphate (AMP) deaminase activation to catalyze AMP to hypoxanthine and eventually increases uric acid levels via purine pathway. Uric acid can be degraded by uricase in rodents. However, humans lack uricase, and that leads to the elevation of circulating uric acid after excessive fructose consumption, which might contribute as an evolutionary benefit[23] [Figure 1].
Figure 1.
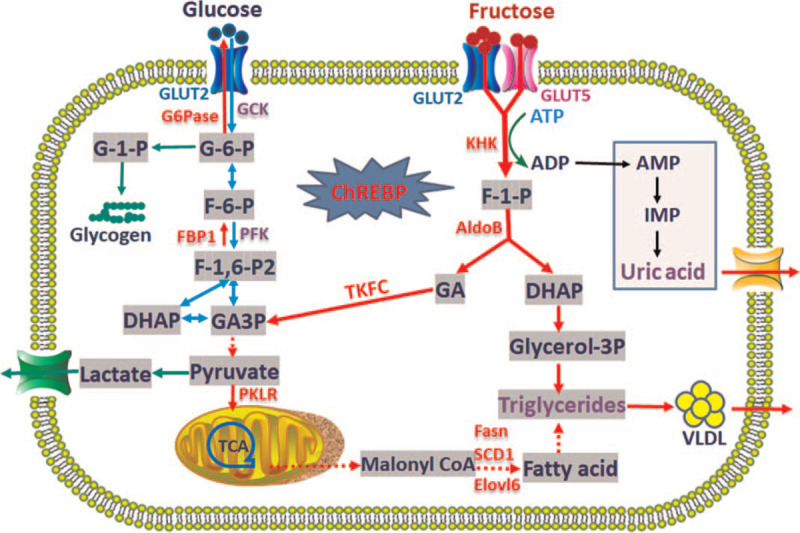
Schematic demonstration for ChREBP-regulated fructose metabolism pathway and its effects on glucose metabolism. Fructose enters cells via GLUT2 or GLUT5, and undergoes fructolysis without negative-feedback, which leads to the activation of ChREBP and its target genes encoding the key enzymes involved in fructolysis, gluconeogenesis, glycolysis, and lipogenesis (as labeled in red). As a result, large amount of fructose uptake and fructolysis promotes uric acid production. AMP: Adenosine monophosphate; ATP: Adenosine triphosphate; ChREBP: Carbohydrate-responsive element-binding protein; Elovl6: Very-long-chain fatty acid elongase 6; F-1,6-P2: Fructose-1,6-bisphosphate; F-1-P: Fructose-1-phosphate; F-6-P: Fructose-6-phosphate; FBP1: Fructose-1,6-bisphosphatase 1; G-1-P: Glucose 1-phosphate; G-6-P: Glucose-6-phosphate; GA: Glyceraldehyde; GCK: Glucokinase; GLUT: Glucose transporter; KHK: Ketohexokinase; PFK: Phosphofructokinase; SCD1: Stearoyl coenzyme A desaturase 1; TKFC: Triokinase; VLDL: Very-low-density lipoprotein.
Fructose concentrations are at a low level of 0.04 mmol/L in peripheral blood. After dietary fructose intake, it rapidly increases about 10 fold then returns to fasting levels within 2 h.[10] Dietary fructose metabolism is initiated in small intestine, which is a primary organ for fructose clearance.[8] Low doses of fructose are about 90% cleared by the intestine, with only trace fructose but extensive fructose-derived glucose, lactate, and glycerate entering the portal blood. Therefore, the small intestine not only provides a place for the absorption and metabolism of fructose, but also acts as a barrier to prevent a large amount of fructose from pouring into the liver, with potential toxic effects. The capacity to absorb fructose in healthy adults ranges from <5 g to >50 g.[10] When fructose ingestion exceeds intestinal clearance capacity, the extra fructose is released into portal circulation at the disposal of the liver.
The liver is a central metabolic processor. It can sense and integrate peripheral nutrient status, thereby coordinating energy storage and consumption. It is also considered to be the major organ to dispose fructose in circulation.[10,24] Hepatic fructose uptake from portal circulation is mediated by GLUT2, which then undergoes further metabolism to produce substrates for multiple metabolic pathways, affecting both glucose and lipid homeostasis, and consequently contributing to the pathogenesis of metabolic disorders.[25] Kidney is also an important organ for fructose metabolism. Physiologically, fructose can be converted into glucose by renal gluconeogenesis. Although renal gluconeogenesis may result from classic substrates such as lactate, glutamine, alanine, and pyruvate, fructose appears to be the preferred substrate, based on the speed and efficiency of the reaction.[26] The proximal straight tubule, where the GLUT5 is expressed on the apical side of the cell membrane, is a primary site for urinary fructose uptake and subsequent metabolism by fructokinase. In addition, the proximal convoluted tubule is also found to express fructokinase and aldolase B, which can be rapidly induced by fructose. Aldolase B deficiency in the patients with hereditary fructose intolerance causes F-1-P accumulation in the proximal convoluted tubule. On the other hand, activation of fructose by fructokinase requires a phosphate, which decreases intracellular phosphate levels and depletes ATP. This process activates AMP deaminase and stimulates uric acid via purine pathway.[27] Therefore, an excessive amount of fructose, either from diet or as a result of endogenous production, leads to intracellular uric acid accumulation, which increases the risk of developing gout.
Fructose intolerance
The development of fructose intolerance is closely related to abnormal expression and function of the enzymes critically involved in fructose absorption and metabolism.[28] Hereditary fructose intolerance, caused by catalytic deficiency of aldolase B, results in a serious defect of fructose metabolism along with an severe accumulation of F-1-P, which leads to hypoglycemic and severe abdominal symptoms as well as liver and kidney injury after fructose consumption.[29] Another hereditary fructose intolerance is related to fructose bisphosphatase 1 (FBP1), which has long been recognized as a key component to control the rate of hepatic gluconeogenesis in response to energy status. Individuals with FBP1 deficiency present with hypoglycemia and metabolic acidosis after fructose consumption, due to impaired gluconeogenesis.[30] The hereditary fructose intolerance is often fatal and increases mortality; however, the precise mechanism is not entirely clear so far.
Fructose intolerance is also caused by impaired fructose absorption. The ability of a healthy adult to absorb fructose daily is in the range of 5 to 50 g.[9] Unabsorbed fructose in the intestine can be excreted through feces or utilized by intestinal flora; the latter could lead to impaired intestinal flora homeostasis and intestinal barrier function, thereby causing gastrointestinal symptoms. Deletion of GLUT5 in mice reduces fructose absorption by 75% and causes fructose malabsorption with symptoms such as cecum and colon dilatation, and gas accumulation.[31] Intestinal GLUT5 expression levels are low prenatally and rapidly increase after weaning, which then can be further induced by diets containing fructose.[32] In humans, fructose malabsorption is prevalent in infants, toddlers, and young children compared with that in adults; this is due to their low expression pattern of intestinal GLUT5.[33] Therefore, GLUT5 has been considered as a potential pharmacologic candidate for the prevention of fructose-induced diseases.
Regulation of Fructose Metabolism
Fructose metabolism is self-regulated in a flux-dependent manner. Under physiological condition, fructose clearance is accomplished through fructolysis and gluconeogenesis, which are initiated from intestinal absorption and metabolism, with the circulating fructose cleared in the liver and kidney. Each component of fructose metabolism pathway, which includes transporters and metabolic enzymes, controls the metabolic flux as well as the amount of intermediate metabolites to maintain metabolic homeostasis.[10] Therefore, the activity and expression of these components are likely to have robust and sustained effects on fructose metabolism. At the transcriptional level, the expression of genes critically involved in fructose metabolism is coordinately regulated by carbohydrate-responsive element binding protein (ChREBP).
ChREBP
Carbohydrate-responsive element binding protein, a basic helix-loop-helix leucine zipper transcription factor, was initially found to recognize and bind to the carbohydrate response element within the promoter of the gene encoding liver-type pyruvate kinase (LPK).[34,35] It has two isoforms, ChREBP-α and ChREBP-β, due to alternative usage of the promoters and the first exons. Each isoform is complexed with Mlx forms heterodimer to transcriptionally regulate expression of the target genes. The β isoform is expressed at extremely low levels; however, its transcriptional activity is much more potent than that of the α isoform.[36] ChREBP is abundantly expressed in both liver and intestine,[37,38] and regulates transcription of many genes involved in monocarbohydrate transport, glycolysis, fructolysis, and de novo lipogenesis (DNL),[37,39–41] thereby contributing to glucose and lipid homeostasis. The target genes regulated by ChREBP are very extensive with different physiological significance. Among them, some are related to glucose metabolism such as LPK, G6P, GLUT4, glycerol-3-phosphate dehydrogenase (GPDH), and glucokinase regulatory protein (GKRP), whereas some are related to lipid metabolism including fatty acid synthesis, acetyl coenzyme A carboxylase, very-long-chain fatty acid elongase 6 (Elovl6), stearoyl coenzyme A desaturase 1, and microsomal triglyceride transfer protein . It is particularly noteworthy to observe that the gene encoding fibroblast growth factor 21 (FGF21), an important factor regulating glucose and lipid metabolism, is also a target of ChREBP.[42]
To a lesser extent, ChREBP is also expressed in adipose tissue. We recently found that ChREBP-β overexpression in brown adipocytes downregulates the expression of genes involved in mitochondrial biogenesis, autophagy, and respiration, as well as thermogenesis (eg, Dio2, UCP1), which suggests that ChREBP acts as a negative regulator of thermogenesis.[43] Thus, ChREBP plays an important role in sensing nutrient metabolism and maintaining energy homeostasis upon environmental changes.
ChREBP and fructose metabolism
Although ChREBP was initially identified as a glucose-responsive factor, phenotypical characterization of its global knockout mice first demonstrates that ChREBP is required for fructose tolerance.[37] Upon fructose or sucrose ingestion, ChREBP-deficient mice exhibit high mortality, which was once linked to the diminished expression of enzymes required for fructolysis, such as aldolase B and KHK in the liver.[38,44] Given the consistent phenotype as manifested by a severely distended cecum along with proximal colon full of both gas and fluid contents, and severe diarrhea upon fructose ingestion, the relevance of intestinal ChREBP in fructose tolerance has been established in recent years. In the small intestine, ChREBP expression is robustly induced by high-fructose diet (HFrD), which is required for the subsequent activation of GLUT5 and the genes involved in fructolysis and gluconeogenesis.[45] In the absence of ChREBP, insufficient induction of intestinal GLUT5 could be the main reason for fructose intolerance. Therefore, as an important site for fructose absorption and metabolism, small intestine is highly regulated by ChREBP in response to dietary fructose stimulation.
The liver is the major organ for the disposal of circulating fructose; however, the role of ChREBP in liver fructose metabolism is controversial. Fructose can produce multiple intracellular carbohydrate metabolites that activate hepatic ChREBP independently of hepatic insulin signaling.[38,46,47] In both rats and mice, high fructose feeding increases hepatic carbohydrate metabolites as well as the activity of ChREBP and its target genes.[38,48] Another recent study also indicates that fructose-activated ChREBP stimulates hepatic FGF21 expression and secretion, which participates in an adaptive response to fructose consumption.[49] Although it has been observed that HFrD activates hepatic lipogenesis in a ChREBP-dependent manner, ChREBP-null mice chronically fed with a high fructose diet exhibit severe steatohepatitis and early signs of fibrosis.[50] This unexpected finding complexes the role of fructose-activated ChREBP in hepatic lipid metabolism. Jois et al[51] generated a liver-specific ChREBP-α knockout mice with the deletion of exon 1a, while the alternative promoter and expression cassette of ChREBP-β was intact. They demonstrated that deletion of hepatic ChREBP caused insulin resistance, but did not change liver glycogen contents or the expression of ChREBP target genes in liver glycolysis and lipogenesis. Using another liver-specific knockout mice model, Linden et al[52] show an approximately two-fold increase in liver glycogen contents and a mild increase in plasma alanine aminotransferase levels in HFrD-fed mutant mice, without significant morphological abnormality in the liver. In contrast, our recent study demonstrates that liver-specific ablation of ChREBP causes severe transaminitis and hepatomegaly with massive glycogen-overload in mice fed with an HFrD without significant inflammation, cell death, or fibrosis in the liver, suggesting that liver ChREBP protects mice against fructose-induced hepatotoxicity by regulating liver glycogen metabolism and ATP homeostasis.[53] These substantial evidences demonstrate a protective role of liver ChREBP in fructose-induced hepatic toxicity. The marked difference in fructose-induced liver response in the above-mentioned knockout mouse model is most likely caused by the difference of gene targeting and mouse genetic background. Nevertheless, the mechanism and biological significance of differential responsiveness of the ChREBP target genes to fructose requires further investigation [Figure 2].
Figure 2.
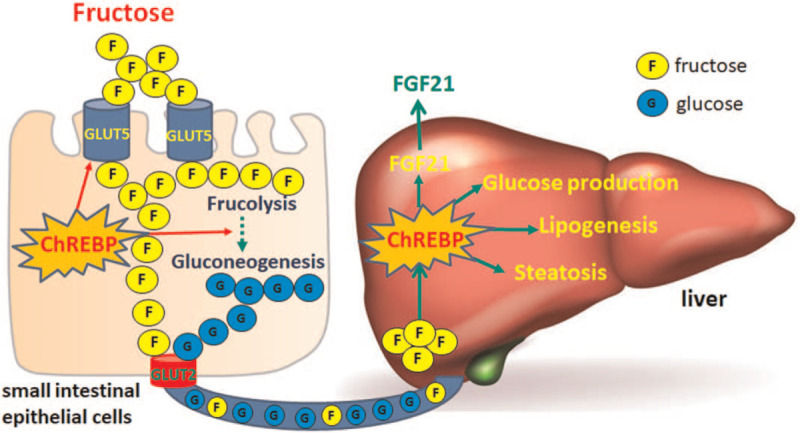
ChREBP regulates fructose metabolism in intestine and liver. Dietary fructose gets into absorptive enterocytes in small intestine through GLUT5 located at brush border, and is translocated into circulation via GLUT2. In enterocytes, fructose also undergoes fructolysis and gluconeogenesis, and consequently is absorbed into circulation in the form of glucose. As a positive feedback mechanism, dietary fructose activates ChREBP pathway, and thereby promotes fructose absorption and glucogenesis in enterocytes. In the liver, fructose-activated ChREBP promotes glucose production, lipogenesis, and FGF21 production and secretion. Long-term fructose overconsumption promotes the pathogenesis of hepatic steatosis due to excessive activation of ChREBP pathway. ChREBP: Carbohydrate-responsive element–binding protein; FGF21: Fibroblast growth factor 21; GLUT: Glucose transporter.
Regulation of ChREBP pathway
The expression and activity of ChREBP are tightly regulated by carbohydrate in the liver.[44] At the post-translational level, its nuclear translocation and transcriptional activity depend on glucose-modulated phosphorylation and acetylation.[54,55] At the transcriptional level, ChREBP-α is activated by glucose,[56,57] possibly through its metabolites, the identity of which remains subject to much uncertainty.[47,58] It is worth noting that ChREBP-α has been identified as one of the direct target genes of liver X receptor and thyroid hormone receptor β (TRβ) in the liver.[59–61] Although these nuclear receptors are involved in transcriptional regulation of carbohydrate and lipid metabolism,[62–64] neither of them is necessary for the induction of ChREBP expression by glucose or refeeding.[56,61] So far, the molecular mechanism for the regulation of ChREBP expression is still unknown. Our previous study identified a new member of C2H2 subfamily of zinc finger proteins, named as zinc finger and BTB domain-containing protein 20 (ZBTB20),[65] which is responsive to carbohydrate stimulation and capable of binding to and activating ChREBP-α gene, thus regulating the expression of ChREBP-driven metabolic genes in the liver.[66] ZBTB20 is abundantly expressed in adult hepatocytes. Tissue-specific deletion of ZBTB20 results in a marked decrease in ChREBP-α expression and a dramatic decline of ChREBP-β expression levels upon fructose stimulation, protecting the mice against fructose-induced steatosis. Our finding points to ZBTB20 as a top-level critical regulator in the hierarchy of ChREBP-regulated fructose metabolism pathway in the liver, providing a novel insight into the transcriptional regulation of ChREBP. However, ZBTB20 is hardly expressed in the intestinal epithelial cells. Therefore, the regulation of ChREBP pathway in different tissues needs further investigation.
Effects of Fructose on Metabolism
Energy storage as fat and glycogen
In humans, dietary fructose functions as an energy source that can be deposited as fat and glycogen. Hepatocytes are able to convert fructose-derived carbon into glucose, glycogen, lactate, lipid, carbon dioxide, and/or other metabolites.[67] Isotope-labeled metabolic tracer techniques provide a novel way to quantitatively evaluate the conversion and oxidation of ingested fructose, even in human bodies. In healthy subjects, after acute fructose ingestion, about 29% to 54% fructose is converted to glucose and then further incorporated into glycogen, 28% fructose is converted to lactate, and a small trace enters DNL pathway.[24] Fructose-derived metabolites enter the triose-phosphate pool, bypassing the phosphofructokinase (PFK)-restricted glycolytic flux, which results in unrestricted hepatic fructolysis. Thus, fructose-overloads lead to large, rapid expansions in the hexose- and triose-phosphate pools, providing increasing substrate for multiple metabolic pathway, including glycogen and lipogenesis for energy storage.[10] Very recently, Zhao et al[68] report that dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate, indicating a potential role of microbiota in the pathogenesis of fructose-induced metabolic disease.
Fructose has been regarded as a potent adipogenic nutrient. Chronic fructose intake increases the adipogenic potential on adipocyte precursor cells.[69] Although glucose and fructose consumption exhibit similar effects on weight gain, fructose-sweetened, but not glucose-sweetened, beverages increase visceral adiposity, induce dyslipidemia, and impair insulin sensitivity in overweight/obese humans.[70] The precise molecular mechanism of fructose-induced insulin resistance is related to proinflammatory changes and endoplasmic reticulum stress in visceral adipose tissue (VAT), as well as adiponectin resistance.[71] According to the study by Marek et al,[71] excessive fructose causes not only visceral fat accumulation but also macrophage infiltration and production of proinflammatory cytokines in the VAT, including TNF-α and MCP-1. This low-grade inflammation status in the adipose tissue in obesity is widely recognized as a major cause of insulin resistance and cardiovascular risk. In addition, they also found the impairment of endoplasmic reticulum (ER) function caused by fructose load, as evidenced by XBP1 activation and decreased Ero-1α expression. Their evidence may provide an important mechanism for pathogenesis of insulin resistance and progression of type 2 diabetes and cardiovascular disease that are associated with increasing consumption of fructose-sweetened foods in modern societies.
Eating behavior
The sweetness of added sugar increases the palatability of food and beverages, thereby stimulating appetite and encouraging overeating. In rats, both glucose and fructose increase caloric intake, resulting in increased serum leptin, decreased serum peptide tyrosine tyrosine (PYY) and hypothalamic neuropeptide Y (NPY). However, fructose vs. glucose increases circulating levels of the hunger hormone ghrelin as well as hypothalamic levels of endocannabinoids and cannabinoid receptor CB1 mRNA.[72,73] In humans, fructose relative to glucose results in a smaller increase in systemic glucose, insulin, and glucagon-like polypeptide 1 (GLP-1) levels, with an insufficient induction of satiety. Functional magnetic resonance imaging based studies show that consumption of fructose relative to glucose resulted in greater hypothalamic blood flow and activation of brain regions that are involved in attention and reward processing, which may promote feeding behavior.[74,75]
Fructose in the Pathogenesis of Metabolic Diseases
Although the question remains controversial as to whether fructose consumption is a major contributor to the epidemics of metabolic syndrome,[76–78] SSB have been considered as an independent risk factor for metabolic diseases.[79] Studies in both young people and adults have suggested that excessive fructose consumption may lead to adverse metabolic effects, such as dyslipidemia, increased visceral adiposity, and fatty liver disease,[80,81] which in turn increases the risk of cardiovascular diseases. On the other hand, short-term fructose restriction decreases liver fat, VAT, and DNL, and improves insulin kinetics in children with obesity.[82]
Effect of fructose on obesity and insulin resistance
There are plenty of studies which support the contention that fructose consumption is closely associated with weight gain or obesity due to increased caloric intake.[83–85] On the other hand, fructose induces adipogenesis, oxidative stress, inflammation, and glucocorticoid activation to promote adiposity.[86,87] For example, fructose stimulates 11-beta-hydroxysteroid dehydrogenase (HSD) – expression and activity, thereby promoting the adipogenic effects of glucocorticoids.[86] In addition, fructose-induced leptin resistance may be a contributing factor to dysregulated energy metabolism and weight gain,[88] given the key role of leptin in satiety response and energy expenditure.[89]
It is worthy to note that there are divergent effects of fructose and glucose on metabolic disorders due to their distinct metabolic pathways. Kuzma et al[90] reported that short-term overconsumption of high glucose-sweetened beverages significantly increases fasting plasma insulin levels in healthy humans. Rather than fructose, glucose promotes blood glucose levels as well as insulin secretion, more directly. Glucose-stimulated glycemic load may be a determinant of fasting insulin concentrations. Previous reports suggest that consumption of foods with a high glycemic index is a risk factor for the development of insulin resistance,[91] and pure glucose, by definition, has the highest glycemic index. Therefore, in a short time, glucose beverage was sufficient to induce chronic hyperglycemia and concomitant hyperinsulinemia. Although fructose intake seems not to stimulate insulin secretion, chronic fructose stimulation renders pancreatic β-cells hyper-responsive to glucose-stimulated insulin secretion through extracellular ATP signaling.[92] Moreover, it readily induces hyperinsulinemia in both rodent models and human subjects,[10,93] most likely as a result of insulin resistance.[25] There is some evidence to support the role of fructose-induced hepatic lipogenesis in insulin resistance, which may be associated with diacylglycerol accumulation, protein kinase C ε activation, and impaired insulin-mediated Akt2 activation.[94,95] However, the precise role and mechanism of fructose in insulin resistance need to be intensively explored.
Effect of fructose on NAFLD and hyperlipidemia
Excessive fructose consumption may have significant effects on lipid metabolism, contributing to steatosis and hypertriglyceridemia.[10] Even in the setting of insulin resistance, it stimulates hepatic lipid accumulation through increasing hepatic DNL because fructose metabolism does not require insulin. Fructose consumption activates lipogenic program immediately and then contributes to increased triglyceride-rich very-low-density lipoprotein secretion,[96,97] whereas it acutely suppresses hepatic fatty acid oxidation.[98] Thus, fructose stimulates hepatic triglyceride production both by providing substrate for fatty acid and triglyceride synthesis and by activating signaling systems to enhance lipid production.[10] Meanwhile, fructose can increase transcriptional regulation of DNL by activating key transcription factors, including sterol regulatory element–binding protein 1c (SREBP1c) and ChREBP.[25,99] This fructose-induced DNL has been strongly correlates with NAFLD and hypertriglyceridemia.[100–102]
It is well established that fructose enhances hepatic lipogenesis; however, so far the association between fructose and cholesterol metabolism has been poorly appreciated. Based on a cross-sectional study among 6113 US adults in 1999–2006, there was a statistically significant correlation between dietary added sugars and unfavorable blood lipid levels, namely lower high-density lipoprotein cholesterol levels and higher triglycerides levels in fructose consumers, and higher low-density lipoprotein (LDL)-cholesterol levels in women.[103] A short-term (3.5 inpatient days) intervention study showed that HFCS intake with 10% to 25% of energy requirement increased postprandial triglyceride, and 17.5% to 25.0% of energy requirement increased fasting and/or postprandial LDL cholesterol.[11] Recent animal studies show that high fructose diet induces elevation of hepatic cholesterol, which can be abolished by deletion of hepatic ChREBP in mice.[25] It is further confirmed by us that hepatic-specific ChREBP knockout mice show decreasing level of total and free cholesterol in the liver.[53] These data indicate a potential role of ChREBP in fructose-regulated cholesterol metabolism. Since multiple organs are involved in cholesterol absorption, synthesis, transport, clearance, and biotransformation, it is worthwhile to clarify the precise molecular mechanism of excess fructose-mediated cholesterol metabolism disorder.
Effect of fructose on hyperuricemia and hypertension
A large body of evidence revealed the association of excessive consumption of fructose with hyperuricemia in both animals and humans, and for this reason, fructose has been recognized to be a potential risk for developing hyperuricemia and gout.[104–106] Despite the conflicting studies on the relationship between fructose consumption and hyperuricemia, it is undeniable that excessive fructose intake is potentially related to the risk of hyperuricemia. The National Health and Nutrition Examination Survey (NHANES) 1999–2004 databases and a logistic regression model reveal no significant correlation between fructose intake and hyperuricemia risk.[107] There is a meta-analysis showing an undesirable association between SSB and fruit juice intake with the risk of gout, but no association with fruit intake.[108] In fact, the majority of studies support that high fructose intake can lead to hyperuricemia. In a meta-analysis involving 125,299 participants, it is shown that high fructose consumption corresponds with an increased risk of gout.[105] Also, a substantial correlation between SSB consumption and the elevated risk of gout and hyperuricemia in adults is indicated in a systematic review and meta-analysis.[109] In addition, a cross-sectional survey based on the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) baseline data (2008–2010) reports a substantially positive correlation of a high fructose intake with hyperuricemia.[104] A recent meta-analysis including 244 rats with diverse fructose feeding indicates a significant link of hyperuricemia to high fructose consumption.[106] Fructose rapidly generates F-1-P to push the fluxes of trioses for lipogenesis that causes depletion of ATP store and degradation of AMP. This process contributes to production of uric acid by purine pathway.[6] As a result, uric acid activates the renin-angiotensin system and inhibits endothelial NO, thereby increasing blood pressure.[110,111] A systematic review of 18 prospective cohort studies demonstrate that incidence of hypertension increases by 13% per 1 mg/dL increment in serum uric acid level.[112] In addition to uric acid, fructose ingestion induces several other physiologic responses that contribute to the pathogenesis of hypertension, which include gastrointestinal sodium absorption, renal sodium reabsorption, RAS system, and renal sympathetic nervous system.[113] Therefore, restriction of sugar consumption may be a practical means to prevent cardiovascular disease.
Perspective
Excessive fructose is an independent risk factor for metabolic diseases; therefore, restriction of dietary fructose intake is an important approach to preventing cardiometabolic diseases, and intervention of endogenous fructose production also receives increasing attention. While intestine, liver, and kidney are the major organs involved in fructose metabolism, the physiology and significance of brain in fructose metabolism need intensive investigation to better understand the effect of fructose on sweet taste preference and eating behavior. While there is some evidence to indicate that excessive fructose consumption affects cholesterol homeostasis, the underlying cellular and molecular mechanism needs to be clarified. ChREBP is the master regulator of fructose metabolism; the means by which ChREBP senses fructose stimulation is still an enigma. Obtaining a better understanding of the biochemical regulatory mechanism of ChREBP pathway will definitely provide new insights to the regulation of fructose metabolism and its metabolic effects. Future advances in this field will benefit our efforts to achieve better cardiometabolic health.
Funding
The work was supported by the grants from National Natural Science Foundation and the National Key R&D Program of China (Nos. 2019YFA0802500, 91857203, 2018YFA0800602, and 31730042).
Conflicts of interest
None.
Footnotes
How to cite this article: Shi YN, Liu YJ, Xie Z, Zhang WJ. Fructose and metabolic diseases: too much to be good. Chin Med J 2021;134:1276–1285. doi: 10.1097/CM9.0000000000001545
References
- 1.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol 2019; 15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 3.Powell ES, Smith-Taillie LP, Popkin BM. Added sugars intake across the distribution of US children and adult consumers: 1977-2012. J Acad Nutr Diet 2016; 116:1543–1550. doi: 10.1016/j.jand.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Li Y, Yang X, Hemler EC, Fang Y, Zhao L, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: A cross-sectional population-based study. Lancet Diab Endocrinol 2019; 7:540–548. doi: 10.1016/S2213-8587(19)30152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019; 139:2113–2125. doi: 10.1161/CIRCULATIONAHA.118.037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taskinen MR, Packard CJ, Boren J. Dietary fructose and the metabolic syndrome. Nutrients 2019; 11:1987.doi: 10.3390/nu11091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest 2017; 127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 2018; 27:351–361. e3. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merino B, Fernandez-Diaz CM, Cozar-Castellano I, Perdomo G. Intestinal fructose and glucose metabolism in health and disease. Nutrients 2020; 12:94.doi: 10.3390/nu12010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest 2018; 128:545–555. doi: 10.1172/JCI96702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015; 101:1144–1154. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferraris RP, Choe JY, Patel CR. Intestinal absorption of fructose. Annu Rev Nutr 2018; 38:41–67. doi: 10.1146/annurev-nutr-082117-051707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones HF, Butler RN, Brooks DA. Intestinal fructose transport and malabsorption in humans. Am J Physiol Gastrointest Liver Physiol 2011; 300:G202–G206. doi: 10.1152/ajpgi.00457.2010. [DOI] [PubMed] [Google Scholar]
- 14.Andres-Hernando A, Johnson RJ, Lanaspa MA. Endogenous fructose production: what do we know and how relevant is it? Curr Opin Clin Nutr Metab Care 2019; 22:289–294. doi: 10.1097/Mco.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francey C, Cros J, Rosset R, Crézé C, Rey V, Stefanoni N, et al. The extra-splanchnic fructose escape after ingestion of a fructose-glucose drink: an exploratory study in healthy humans using a dual fructose isotope method. Clin Nutr ESPEN 2019; 29:125–132. doi: 10.1016/j.clnesp.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Cousineau C, Xing G, Raha N, Clemens S, Mofikoya M, et al. Development and validation of a quantitative ultra performance LC((R)) hydrophilic interaction liquid chromatography MS/MS method to measure fructose and sorbitol in human plasma. Bioanalysis 2019; 11:407–425. doi: 10.4155/bio-2018-0286. [DOI] [PubMed] [Google Scholar]
- 17.Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WN. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin Biochem 2010; 43:198–207. doi: 10.1016/j.clinbiochem.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Roncal-Jimenez CA, Lanaspa-Garcia MA, Oppelt SA, Kuwabara M, Jensen T, et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J Neurophysiol 2017; 117:646–654. doi: 10.1152/jn.00781.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JJ, Jiang L, Hamza M, Dai F, Belfort-DeAguiar R, Cline G, et al. The human brain produces fructose from glucose. JCI Insight 2017; 2:e90508.doi: 10.1172/jci.insight.90508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oppelt SA, Zhang W, Tolan DR. Specific regions of the brain are capable of fructose metabolism. Brain Res 2017; 1657:312–322. doi: 10.1016/j.brainres.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 2014; 25:2526–2538. doi: 10.1681/Asn.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013; 4:2434.doi: 10.1038/Ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RJ, Stenvinkel P, Andrews P, Sanchez-Lozada LG, Nakagawa T, Gaucher E, et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med 2020; 287:252–262. doi: 10.1111/joim.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SZ, Empie MW. Fructose metabolism in humans - what isotopic tracer studies tell us. Nutr Metab 2012; 9:89.doi: 10.1186/1743-7075-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab 2016; 27:719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa T, Johnson RJ, Andres-Hernando A, Roncal-Jimenez C, Sanchez-Lozada LG, Tolan DR, et al. Fructose production and metabolism in the kidney. J Am Soc Nephrol 2020; 31:898–906. doi: 10.1681/Asn.2019101015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 2005; 1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 28.Buziau AM, Schalkwijk CG, Stehouwer CDA, Tolan DR, Brouwers MCGJ. Recent advances in the pathogenesis of hereditary fructose intolerance: Implications for its treatment and the understanding of fructose-induced non-alcoholic fatty liver disease. Cell Mol Life Sci 2020; 77:1709–1719. doi: 10.1007/s00018-019-03348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouteldja N, Timson DJ. The biochemical basis of hereditary fructose intolerance. J Inherit Metab Dis 2010; 33:105–112. doi: 10.1007/s10545-010-9053-2. [DOI] [PubMed] [Google Scholar]
- 30.Moey LH, Azize NAA, Yakob Y, Leong HY, Keng WT, Chen BC, et al. Fructose-1, 6-bisphosphatase deficiency as a cause of recurrent hypoglycemia and metabolic acidosis: clinical and molecular findings in Malaysian patients. Pediatr Neonatol 2018; 59:397–403. doi: 10.1016/j.pedneo.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, et al. Slc2a5 (GLUT5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 2009; 284:5056–5066. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J 2001; 360:265–276. doi: 10.1042/Bj3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol-London 2013; 591:401–414. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Luis O, Valero MC, Jurado LA. WBSCR14, a putative transcription factor gene deleted in Williams-Beuren syndrome: Complete characterisation of the human gene and the mouse ortholog. Eur J Hum Genet 2000; 8:215–222. doi: 10.1038/sj.ejhg.5200435. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U SA 2001; 98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012; 484:333–U366. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A 2004; 101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest 2016; 126:4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Robinson LN, Towle HC. ChREBP center dot Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem 2006; 281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 40.Jeong YS, Kim D, Lee YS, Kim HJ, Han JY, Im SS, et al. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One 2011; 6:e22544.doi: 10.1371/journal.pone.0022544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poungvarin N, Chang B, Imamura M, Chen JS, Moolsuwan K, Sae-Lee C, et al. Genome-wide analysis of ChREBP binding sites on male mouse liver and white adipose chromatin. Endocrinology 2015; 156:1982–1994. doi: 10.1210/en.2014-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab 2013; 24:257–268. doi: 10.1016/j.tem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Wei C, Ma X, Su K, Qi S, Zhu Y, Lin J, et al. ChREBP-beta regulates thermogenesis in brown adipose tissue. J Endocrinol 2020; 245:343–356. doi: 10.1530/Joe-19-0498. [DOI] [PubMed] [Google Scholar]
- 44.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab 2006; 4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Kim M, Astapova II, Flier SN, Hannou SA, Doridot L, Sargsyan A, et al. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight 2017; 2:e96703.doi: 10.1172/jci.insight.96703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arden C, Tudhope SJ, Petrie JL, Al-Oanzi ZH, Cullen KS, Lange AJ, et al. Fructose 2,6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem J 2012; 443:111–123. doi: 10.1042/Bj20111280. [DOI] [PubMed] [Google Scholar]
- 47.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci U S A 2003; 100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koo HY, Miyashita M, Cho BHS, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. Biochem Biophys Res Commun 2009; 390:285–289. doi: 10.1016/j.bbrc.2009.09.109. [DOI] [PubMed] [Google Scholar]
- 49.Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab 2017; 6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D, Tong X, VanDommelen K, Gupta N, Stamper K, Brady GF, et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J Clin Invest 2017; 127:2855–2867. doi: 10.1172/JCI89934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jois T, Chen W, Howard V, Harvey R, Youngs K, Thalmann C, et al. Deletion of hepatic carbohydrate response element binding protein (ChREBP) impairs glucose homeostasis and hepatic insulin sensitivity in mice. Mol Metab 2017; 6:1381–1394. doi: 10.1016/j.molmet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linden AG, Li S, Choi HY, Fang F, Fukasawa M, Uyeda K, et al. Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice. J Lipid Res 2018; 59:475–487. doi: 10.1194/jlr.M081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi JH, Lu JY, Chen HY, Wei CC, Xu XF, Li H, et al. Liver ChREBP protects against fructose-induced glycogenic hepatotoxicity by regulating L-type pyruvate kinase. Diabetes 2020; 69:591–602. doi: 10.2337/db19-0388. [DOI] [PubMed] [Google Scholar]
- 54.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A 2001; 98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bricambert J, Miranda J, Benhamed F, Girard J, Postic C, Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest 2010; 120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denechaud PD, Bossard P, Lobaccaro JMA, Millatt L, Staels B, Girard J, et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Investig 2008; 118:956–964. doi: 10.1172/JCI34314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dentin R, Pegorier JP, Benhamed F, Foufelle F, Ferre P, Fauveau V, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem 2004; 279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 58.Dentin R, Tomas-Cobos L, Foufelle F, Leopold J, Girard J, Postic C, et al. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J Hepatol 2012; 56:199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis - The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 2007; 282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto K, Ishida E, Matsumoto S, Okada S, Yamada M, Satoh T, et al. Carbohydrate response element binding protein gene expression Is positively regulated by thyroid hormone. Endocrinology 2009; 150:3417–3424. doi: 10.1210/en.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gauthier K, Billon C, Bissler M, Beylot M, Lobaccaro JM, Vanacker JM, et al. Thyroid hormone receptor beta (TR beta) and liver X receptor (LXR) regulate carbohydrate-response element-binding protein (ChREBP) expression in a tissue-selective manner. J Biol Chem 2010; 285:28156–28163. doi: 10.1074/jbc.M110.146241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, et al. The nuclear receptor LXR is a glucose sensor. Nature 2007; 445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 63.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 2003; 9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 64.Blennemann B, Leahy P, Kim TS, Freake HC. Tissue-specific regulation of lipogenic mRNAs by thyroid hormone. Mol Cell Endocrinol 1995; 110:1–8. doi: 10.1016/0303-7207(95)03509-6. [DOI] [PubMed] [Google Scholar]
- 65.Xie Z, Zhang H, Tsai W, Zhang Y, Du Y, Zhong J, et al. Zinc finger protein ZBTB20 is a key repressor of alpha-fetoprotein gene transcription in liver. Proc Natl Acad Sci U S A 2008; 105:10859–10864. doi: 10.1073/pnas.0800647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu G, Zhou L, Zhang H, Chen R, Zhang Y, Li L, et al. Regulation of hepatic lipogenesis by the zinc finger protein Zbtb20. Nat Commun 2017; 8:14824.doi: 10. 1038/Ncomms14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iizuka K. The role of carbohydrate response element binding protein in intestinal and hepatic fructose metabolism. Nutrients 2017; 9:181.doi: 10.3390/Nu9020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020; 579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zubiria MG, Alzamendi A, Moreno G, Rey MA, Spinedi E, Giovambattista A. Long-term fructose intake increases adipogenic potential: Evidence of direct effects of fructose on adipocyte precursor cells. Nutrients 2016; 8:198.doi: 10.3390/Nu8040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009; 119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marek G, Pannu V, Shanmugham P, Pancione B, Mascia D, Crosson S, et al. Adiponectin resistance and proinflammatory changes in the visceral adipose tissue induced by fructose consumption via ketohexokinase-dependent pathway. Diabetes 2015; 64:508–518. doi: 10.2337/db14-0411. [DOI] [PubMed] [Google Scholar]
- 72.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept 2008; 150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Erlanson-Albertsson C, Lindqvist A. Fructose affects enzymes involved in the synthesis and degradation of hypothalamic endocannabinoids. Regul Pept 2010; 161:87–91. doi: 10.1016/j.regpep.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Luo S, Monterosso JR, Sarpelleh K, Page KA. Differential effects of fructose versus glucose on brain and appetitive responses to food cues and decisions for food rewards. Proc Natl Acad Sci U S A 2015; 112:6509–6514. doi: 10.1073/pnas.1503358112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Page KA, Chan O, Arora J, Belfort-DeAguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013; 309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 2016; 53:52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan TA, Sievenpiper JL. Controversies about sugars: Results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur J Nutr 2016; 55:S25–S43. doi: 10.1007/s00394-016-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horst KWT, Serlie MJ. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 2017; 9:981.doi: 10.3390/Nu9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of nonalcoholic fatty liver disease. J Hepatol 2018; 68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang YH, An T, Zhang RC, Zhou Q, Huang Y, Zhang J. Very high fructose intake increases serum LDL-cholesterol and total cholesterol: a meta-analysis of controlled feeding trials. J Nutr 2013; 143:1391–1398. doi: 10.3945/jn.113.175323. [DOI] [PubMed] [Google Scholar]
- 81.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011; 96:E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwarz JM, Noworolski SM, Erkin-Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017; 153:743–752. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DiNicolantonio JJ, Mehta V, Onkaramurthy N, O’Keefe JH. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog Cardiovasc Dis 2018; 61:3–9. doi: 10.1016/j.pcad.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Bes-Rastrollo M, Schulze MB, Ruiz-Canela M, Martinez-Gonzalez MA. Financial conflicts of interest and reporting bias regarding the association between sugar-sweetened beverages and weight gain: a systematic review of systematic reviews. PLoS Med 2013; 10:e1001578.doi: 10.1371/journal.pmed.1001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morenga LAT, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014; 100:65–79. doi: 10.3945/ajcn.113.081521. [DOI] [PubMed] [Google Scholar]
- 86.Legeza B, Marcolongo P, Gamberucci A, Varga V, Banhegyi G, Benedetti A, et al. Fructose, glucocorticoids and adipose tissue: implications for the metabolic syndrome. Nutrients 2017; 9:426.doi: 10.3390/Nu9050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senesi S, Legeza B, Balazs Z, Csala M, Marcolongo P, Kereszturi E, et al. Contribution of fructose-6-phosphate to glucocorticoid activation in the endoplasmic reticulum: possible implication in the metabolic syndrome. Endocrinology 2010; 151:4830–4839. doi: 10.1210/en.2010-0614. [DOI] [PubMed] [Google Scholar]
- 88.Vasselli JR. Fructose-induced leptin resistance: Discovery of an unsuspected form of the phenomenon and its significance. Focus on “Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding,” by Shapiro et al. Am J Physiol Regul Integr Comp Physiol 2008; 295:R1365–R1369. doi: 10.1152/ajpregu.90674.2008. [DOI] [PubMed] [Google Scholar]
- 89.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000; 62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 90.Kuzma JN, Cromer G, Hagman DK, Breymeyer KL, Roth CL, Foster-Schubert KE, et al. Consuming glucose-sweetened, not fructose-sweetened, beverages increases fasting insulin in healthy humans. Eur J Clin Nutr 2019; 73:487–490. doi: 10.1038/s41430-018-0297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gower BA, Pearson K, Bush N, Shikany JM, Howard VJ, Cohen CW, et al. Diet pattern may affect fasting insulin in a large sample of black and white adults. Eur J Clin Nutr 2020; doi: 10.1038/s41430-020-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartley C, Brun T, Oberhauser L, Grimaldi M, Molica F, Kwak BR, et al. Chronic fructose renders pancreatic beta-cells hyper-responsive to glucose-stimulated insulin secretion through extracellular ATP signaling. Am J Physiol Endocrinol Metab 2019; 317:E25–E41. doi: 10.1152/ajpendo.00456.2018. [DOI] [PubMed] [Google Scholar]
- 93.Horst KW, Schene MR, Holman R, Romijn JA, Serlie MJ. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: A systematic review and meta-analysis of diet-intervention trials. Am J Clin Nutr 2016; 104:1562–1576. doi: 10.3945/ajcn.116.137786. [DOI] [PubMed] [Google Scholar]
- 94.Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 2009; 9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, Guigni BA, et al. Dissociation of inositol-requiring enzyme (IRE1 alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem 2012; 287:2558–2567. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sobrecases H, Le KA, Bortolotti M, Schneiter P, Ith M, Kreis R, et al. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab 2010; 36:244–246. doi: 10.1016/j.diabet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Hudgins LC, Parker TS, Levine DM, Hellerstein MK. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab 2011; 96:861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Softic S, Meyer JG, Wang GX, Gupta MK, Batista TM, Lauritzen H, et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab 2019; 30:735–753. e4. doi: 10.1016/j.cmet.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu X, Ren LP, Wang C, Zhu YJ, Xing HY, Zhao J, et al. Role of X-box binding protein-1 in fructose-induced de novo lipogenesis in HepG2 cells. Chin Med J 2018; 131:2310–2319. doi: 10.4103/0366-6999.241799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jegatheesan P, De Bandt JP. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017; 9:230.doi: 10.3390/Nu9030230. [Google Scholar]
- 101.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 2018; 68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taskinen MR, Soderlund S, Bogl LH, Hakkarainen A, Matikainen N, Pietilainen KH, et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J Intern Med 2017; 282:187–201. doi: 10.1111/joim.12632. [DOI] [PubMed] [Google Scholar]
- 103.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010; 303:1490–1497. doi: 10.1001/jama.2010.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siqueira JH, Mill JG, Velasquez-Melendez G, Moreira AD, Barreto SM, Bensenor IM, et al. Sugar-sweetened soft drinks and fructose consumption are associated with hyperuricemia: Cross-sectional analysis from the Brazilian Longitudinal Study of adult health (ELSA-Brasil). Nutrients 2018; 10:981.doi: 10.3390/Nu10080981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jamnik J, Rehman S, Mejia SB, de Souza RJ, Khan TA, Leiter LA, et al. Fructose intake and risk of gout and hyperuricemia: A systematic review and meta-analysis of prospective cohort studies. BMJ Open 2016; 6:e013191.doi: 10.1136/bmjopen-2016-013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sayehmiri K, Ahmadi I, Anvari E. Fructose feeding and hyperuricemia: A systematic review and meta-analysis. Clin Nutr Res 2020; 9:122–133. doi: 10.7762/cnr.2020.9.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun SZ, Flickinger BD, Williamson-Hughes PS, Empie MW. Lack of association between dietary fructose and hyperuricemia risk in adults. Nutr Metab 2010; 7:16.doi: 10.1186/1743-7075-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ayoub-Charette S, Liu Q, Khan TA, Au-Yeung F, Mejia SB, de Souza RJ, et al. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. Nutr Metabol Res 2019; 9:e024171.doi: 10.1136/bmjopen-2018-024171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: A systematic review and meta-analysis. Crit Rev Food Sci Nutr 2020; 60:1–10. doi: 10.1080/10408398.2018.1503155. [DOI] [PubMed] [Google Scholar]
- 110.Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 2009; 154:807–813. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 112.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res 2011; 63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komnenov D, Levanovich PE, Rossi NF. Hypertension associated with fructose and high salt: Renal and sympathetic mechanisms. Nutrients 2019; 11:569.doi: 10.3390/Nu11030569. [DOI] [PMC free article] [PubMed] [Google Scholar]


