Abstract
Due to advances in understanding the immune microenvironment of colorectal cancer (CRC), microsatellite classification (dMMR/MSI‐H and pMMR/MSS) has become a key biomarker for the diagnosis and treatment of CRC patients and therefore has important clinical value. Microsatellite status is associated with a variety of clinicopathological features and affects drug resistance and the prognosis of patients. CRC patients with different microsatellite statuses have different compositions and distributions of immune cells and cytokines within their tumor microenvironments (TMEs). Therefore, there is great interest in reversing or reshaping CRC TMEs to transform immune tolerant "cold" tumors into immune sensitive "hot" tumors. This requires a thorough understanding of differences in the immune microenvironments of MSI‐H and MSS type tumors. This review focuses on the relationship between CRC microsatellite status and the immune microenvironment. It focuses on how this relationship has value for clinical application in diagnosis and treatment, as well as exploring the limitations of its current application.
Keywords: colorectal cancer, diagnosis and treatment, immune microenvironment, microsatellite status
The main differences of TME among different MSI types.
1. INTRODUCTION
The incidence of colorectal cancer (CRC) ranks third in the world among malignant tumors, and CRC is also the second leading cause of cancer‐related deaths. There are about 1.8 million new cases of CRC and 881,000 deaths annually. 1 It is estimated that by 2030, the global burden of CRC will increase by 60%. 2 At present, early detection technology has greatly assisted early diagnosis and intervention of CRC, but about 25% of patients are nonetheless diagnosed as stage IV. 3 In the past 15 years, treatment strategies for metastatic colorectal cancer (mCRC) have improved, but the 5‐year overall survival rate (OS) is still only 14%, 4 posing a serious threat to public health. Immunotherapy is a new emerging tumor treatment method following surgical resection, chemoradiotherapy, and biological targeted therapy. Immunotherapy can eliminate tumor cells and inhibit tumor growth and metastasis by activating the immune system and exerting the immune capacity of the tumor microenvironment (TME). Immunotherapy is highly specific, which can not only damage normal cells but can also stimulate immune memory. Immunotherapy has become the focus of CRC treatment research in recent years. Immune checkpoint inhibitors (ICIs) are the most widely used form of immunotherapy.
At present, the FDA has approved ICI treatment for patients with mismatch repair‐deficient (dMMR) / microsatellite instability‐high (MSI‐H) mCRC 5 ; however, this represents only 15% of all CRC patients and only 2%–4% of stage IV mCRC patients. 6 Most patients with proficient MMR (pMMR) / microsatellite‐stable (MSS) CRC cannot benefit from ICI treatment alone. 7 , 8 In recent years, the TME has emerged as an important source of potential therapeutic targets. The TME has an extraordinarily complex regulatory network, which plays a key role in the occurrence, progression, and treatment of tumors. 9 Reversing the inhibitory immune microenvironment of pMMR/MSS CRC and improving patient responses to ICIs have become urgent tasks. 5 , 10 Recent research has focused on predicting the behavior of cancer as well as studying its response to treatment by MSI detection and immune markers; however, many genetic and epigenetic factors as well as environmental and lifestyle factors can also affect immune cells, microbiota, tumor development and behavior, and response to treatment. Therefore, there are still limitations in the current application of this prediction and evaluation method, and thus, a need for further studies to be conducted in this area.
2. MICROSATELLITE STATUS AND CRC IMMUNE MICROENVIRONMENT
dMMR occurs due to changes in MMR genes, which result in the loss of the repair function of one or several MMR proteins (MLH1, MSH2, MSH6, and PMS2), which leads to pairing errors during DNA replication. 11 These changes may be sporadic or hereditary. 12 Microsatellite instability (MSI) is the result of the accumulation of nucleotide insertions or deletions in the genome. 13 MSI can be divided into microsatellite instability‐high (MSI‐H), microsatellite instability‐low (MSI‐L), and microsatellite‐stable (MSS). At present, CRC patients are generally divided into two groups, dMMR / MSI‐H type, and pMMR / MSS or MSI‐L type (hereinafter referred to as pMMR/MSS type). 6
It is well established that immune cells and cytokines in the TME can play dual roles in antagonizing or promoting tumors. 14 The body mainly achieves immune surveillance through three stages of immune elimination, immune balance, and immune escape. 15 Studies have shown that immune dysfunction caused by immunosuppression or autoimmune disease is associated with the high incidence of various cancers. 16 In addition, the infiltration of immune cells in the TME is an important factor affecting tumor heterogeneity and prognosis. 17 , 18 , 19 , 20 , 21 The same types of tumors have different biological characteristics and different immune microenvironments, as is the case for colorectal and rectal cancers. 22 These differences directly affect responses to ICI treatment. 23 , 24 , 25 , 26 The main differences in the tumor microenvironments (TMEs) of dMMR/MSI‐H, and pMMR/MSS CRC patients are described below (Figure 1).
FIGURE 1.
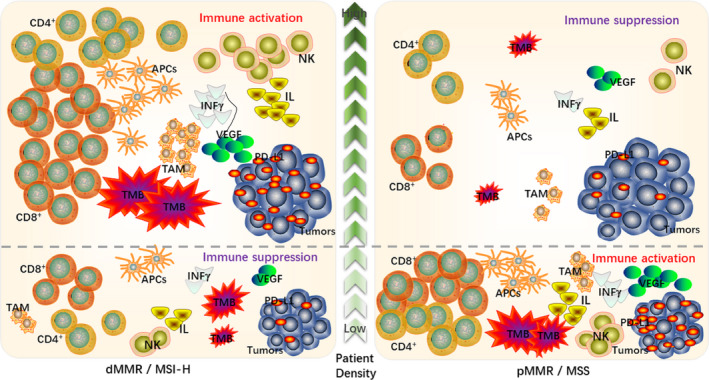
The main differences of TME among different MSI types
2.1. Tumor mutation burden
According to the latest classification of CRC consensus molecular subtypes (CMSs), MSI‐H belongs to the CMS1 type and accounts for 14% of CRC cases. 27 , 28 Several studies have shown high tumor mutation burden (TMB) in the TME of dMMR / MSI‐H CRC. 6 Common mutations include widespread hypermethylation, BRAF mutations, and mutations in genes encoding DNA mismatch repair proteins. These unique highly mutated genomic structures can be regarded as new antigens; this makes them more sensitive to ICI therapy regardless of the cancer tissue type. 29 , 30 , 31 In addition, high TMB can stimulate the presentation efficiency of antigen‐presenting cells (APCs), increase the diversity of MHC phenotypes, and affect the prognoses of patients. 32 , 33 Among these, increased expression of MHC‐I molecules can promote the differentiation of CD8+ T cells into CD8+ cytotoxic T lymphocytes (CTLs) and directly produce tumor cell killing effects. 34 Furthermore, upregulation of MHC‐II can induce CD4+ T helper cells to indirectly activate other immune cells. 35 , 36 Therefore, a high TMB dMMR / MSI‐H phenotype has become an important biomarker to suggest that ICI treatment will be effective. 12 , 37
pMMR/MSS type CRC accounts for about 95% of all CRC cases. The level of TMB in the TME of MSS patients is significantly lower than that in MSI‐H patients; this greatly limits MHC expression on the surface of APCs, preventing an effective anti‐tumor immune response and limiting the effectiveness of ICIs. 38 , 39 However, the response of pMMR / MSS patients to ICI treatment is very heterogeneous. There are some MSS patients with TMB levels close to MSI‐H CRC patients, and these patients have more abundant T cell antigen receptors (TCRs), which may activate anti‐tumor immune responses by regulating the TCR‐MHC signaling pathway. These patients have better prognoses than other MSS patients. 40
2.2. Tumor infiltrating lymphocytes
CRC patients with different MSI types have different compositions and distributions of immune cells and cytokines within their TMEs. 6 MSI‐H type tumors have significantly increased recruitment of tumor infiltrating lymphocytes, including activated cytotoxic T lymphocytes (CTLs), Th1 cells, and CD4+ T cells, as well as NK cells and macrophages. 41 Local tumor infiltration of CTLs is a prerequisite for response to ICIs. 42 In addition, MSI‐H type tumors also have increased secretion of tumor necrosis factor, perforin, granzyme, IL‐1, IL‐6, IFN‐γ, and other related cytokines in the TME, 6 , 43 and these cytokines regulate the TME immune "activation" or "inhibition" state. 44 , 45 At the same time, a variety of inflammatory mediators infiltrate to form an inflammatory TME, and continuous inflammatory stimulation leads to exhaustion of T lymphocytes, which upregulate inhibitory receptors such as PD‐1, CTLA‐4, TIM3, and LAG‐3. 46 , 47 These immunosuppressive receptors bind to the corresponding ligands in the TME and regulate the anti‐tumor immune response. 33 It has been shown that increased interferon expression is associated with better prognosis and can induce the secretion of chemokines and induce adaptive immune responses. 6 , 33 , 48 Thus, high TIL concentrations in MSI‐H CRC patients indicate a better survival outcome.
Endogenous anti‐tumor T‐cell immunity is mainly achieved by CTLs with high PD‐1 expression. Compared with most MSI‐H CRCs, the TME of MSS type patients usually conveys an immune rejection or immune desert phenotype. It is manifested by low TIL infiltration and lack of CTLs or insufficient CTL activity 38 , 39 ; this is also an important potential mechanism of resistance to PD‐1/PD‐L1 inhibitors. Some studies have found that the level of TIL infiltration in the TME directly affects the recruitment of CTLs and the ability to recognize malignant cells, and high levels of TILs are usually conducive to ICI treatment. 49 , 50 Pamplona et al. also showed that CD8A expression (an indicator of TIL infiltration) can be used as a biomarker to evaluate the prognosis of patients with MSS tumors. 51 Recently, Dahna et al. found that pembrolizumab can not only restore the cytotoxic function of T lymphocytes but can also promote the recruitment of other immune cells to tumor sites by blocking the interaction between PD‐1 and PD‐L1. 52 Therefore, reshaping the TME and increasing the degree of TIL infiltration may be important new directions for the treatment of MSS CRC patients.
2.3. PD‐L1 expression
Korehisa et al 53 found that 5.4% of CRC tumor cells in MSS patients and 36.1% of CRC tumor cells in MSI‐H patients were PD‐L1 positive, and 27% of stromal cells in MSS patients and 72.2% of stromal cells in MSI‐H patients were PD‐L1. Expression of PD‐L1 in MSI‐H type patients in both tumor cells and stromal cells is much higher than in MSS type patients, which indicates that PD‐1 / PD‐L1 blockers have more targets and higher sensitivity in the TME of MSI‐H CRC patients. In addition, when MSI‐H CRC is about to invade and metastasize, the expression of PD‐L1 on tumor cells in the TME and CD68 / CD163+(M2) macrophages in the stroma is upregulated, which induces immune escape. 54 , 55 This suggests that PD‐1 / PD‐L1 inhibitors have great potential in the treatment of MSI‐H CRC patients with high PD‐L1 expression and can effectively inhibit tumor progression in these patients.
PD‐L1 expression in most MSS CRC patients is significantly lower than in MSI‐H CRC patients, but this is not absolute. Llosa et al. showed that some patients with pMMR / MSS tumors have TMEs similar to dMMR/MSI‐H patients. For example, the TMEs of some patients have high PD‐L1 expression and high infiltration of PD‐1+ CD8� cytotoxic lymphocytes without inhibitory Th17 cells; these factors relate to the benefit of patients receiving pembrolizumab. 56 Recently, Nicolas et al. also showed that dMMR/MSI‐H CRC is not the only subgroup that benefits from ICI treatment. Anti PD‐1 combined with anti CTLA‐4 therapy can enhance the immunogenicity of some pMMR / MSS CRC tumors, thus activating the anti‐tumor immune response and improving the patient's prognosis and survival. 7 Therefore, the use of checkpoint blockade therapy—to save effector T cells from exhaustion or induce Treg depletion—can help prevent PD‐1/PD‐L1 binding, reverse the TME in MSS patients, and inhibit immune escape. 5 , 13 , 57
2.4. VEGF expression
Vascular endothelial growth factor (VEGF) is the strongest and most specific pro‐angiogenic growth factor. It can stimulate tumor growth and metastasis by stimulating the growth of tumor microvessels. 58 Miyamoto et al 59 reported that MSI‐H, and MSS tumors utilize different carcinogenic pathways, including the abnormal expression of angiogenesis‐related genes. Sun et al 60 also found that MSI‐H CRC, and MSS CRC may use two different angiogenesis pathways. High VEGF expression in CRC patients is associated with blood metastasis, lymph node metastasis, advanced TNM stage and depth of invasion. Lower VEGF expression in the TME of MSI‐H tumors is associated with lower invasion and better prognosis.
VEGF expression in the TME of most MSS type CRC patients is upregulated, which leads to increased recruitment of myeloid‐derived suppressor cells (MDSC), downregulation of IL‐12 and upregulation of IL‐10 in macrophages, and M1 macrophage polarization into M2 macrophages 61 ; these events induce the formation of the inhibitory TME, which is conducive to the growth, invasion, and metastasis of tumors. 62 Therefore, VEGF inhibitors combined with PD‐1 / PD‐L1 inhibitors can exert a synergistic effect, simultaneously blocking the activation of VEGF‐related pathways and PD‐1 / PD‐L1‐related pathways in the TME and reducing tumor neovascular density. This combination can reduce the occurrence of immune escape, improving the prognosis of patients with MSS CRC.
3. APPLICATION OF MICROSATELLITE STATUS IN THE DIAGNOSIS AND TREATMENT OF CRC
CRC is a molecularly heterogeneous disease characterized by three carcinogenic pathways, including chromosome instability (CIN), microsatellite instability (MSI), and CpG island methylation phenotype (CIMP). 63 Studies have shown that about 85%–90% of hereditary non‐polyposis CRC and about 10%–15% of sporadic CRC patients have high expression of MSI‐H. 64 , 65 Moreover, it has been shown that MMR gene deletions in CRC patients are mainly caused by gene mutation or promoter methylation; of these, MSH2 and MLH1 gene mutations account for more than 90% of all gene mutations. 66 In 2018, the National Comprehensive Cancer Network (NCCN) guidelines recommended that MSI status should be considered in CRC patients regardless of tumor type, especially in stage II patients. Thus, the classification of the CRC microsatellite status is significant for the clinical diagnosis and treatment of patients.
3.1. Guiding Lynch syndrome screening
Lynch syndrome (LS) is the most common hereditary CRC syndrome. 67 It is a familial disease of autosomal dominant inheritance, which is clinically similar to sporadic MSI‐H CRC. In contrast to sporadic CRC patients, LS carriers or family members usually develop CRC or other Lynch‐related tumors when they are young. 68 CRC and endometrial cancers are the most common cancer affecting LS patients. When the patient is diagnosed, LS increases the lifetime risk of CRC to about 80%. 69 Parag et al 70 performed a retrospective meta‐analysis studying the significance of MSI detection of colorectal adenomas for LS screening and found that 69.5% of patients in the LS cohort could be diagnosed through detection of MSI status of their routine adenomas. Thus, the dMMR/MSI‐H adenoma phenotype is a risk factor for CRC among LS patients, and MSI detection has especially important application value for early LS screening.
3.2. Guiding the evaluation of prognosis
The prognosis of CRC patients is closely related to the age of diagnosis, gender, disease stage, tumor location, degree of differentiation, pathological type and other characteristics, while the invasion, metastasis, and prognosis of CRC are significantly related to the classification of the status of the microsatellite. 5 , 71 Many studies have shown that sporadic CRC with the MSI‐H phenotype is more common in women and may be related to estrogen secretion. 72 , 73 Hormone replacement therapy can reduce the risk of MSI‐H CRC. 74 , 75 The increased DNA methylation caused by MSI‐H is also related to the age of onset, and menopausal women have a higher risk of developing sporadic MSI‐H CRC. In addition, most patients with MSI‐H CRC have primary tumors located in the proximal colon 76 ; which accounts for 15% of stage II‐III tumors and 4%–5% of stage IV tumors. 77 Moreover, MSI‐H tumors are usually poorly differentiated or mucinous adenocarcinoma with characteristic lymphocytic infiltration. 78 However, Watanabe et.al have confirmed that CRC‐specific survival was significantly better in patients with MSI cancer than in those with MSS (p = .02). They also found that MSI was strongly associated with a decreased likelihood of lymph node and distant organ metastases at diagnosis (all p < .001). 79 Therefore, dMMR / MSI‐H CRC may indicate a better prognosis, which may be related to high infiltration of lymphocytes and high sensitivity to immunotherapy.
3.3. Guiding diagnosis of post‐colonoscopy CRC
Colorectal endoscopy is considered to be the gold standard for the diagnosis of CRC, but it is not infallible. Post‐colonoscopy CRC (PCCRC) is defined as a CRC diagnosed 6–36 months after a negative result from a colonoscopy. Although the number of these patients is very small, it is very important for clinics. 80 Arain et al 81 reported that, after adjusting for tumor location, MSI‐H was independently associated with PCCRC (odds ratio: 2.7; 95% CI: 1.1–6.8). Sawhney et al 82 also showed that the probability of MSI in PCCRC was 3.7 times higher than that in noninterval / detected cancers. In 2019, Samadder et al 83 found that MSI was observed in 32% of PCCRC and only 13% of detected CRC (p = .005) in a cross‐sectional study based on CRC cases in Utah, and they concluded that PCCRC was associated with MSI (odds ratio was 4.20; 95% CI was 1.58–11.14). Therefore, MSI detection also has important application value in the field of CRC diagnosis.
3.4. Guiding adjuvant chemotherapy
Many studies have found that when 5‐fluorouracil (5‐FU) is used in patients with MSI‐H and MSS type CRCs, 5‐FU adjuvant chemotherapy for MSI‐H is unfavorable to the survival of patients. 84 However, patients with MSI‐H CRC seem to respond well to irinotecan treatment. 85 , 86 It has been suggested that the difference in patient response to 5‐FU and irinotecan treatment may be due to the fact that in MSI‐H patients, cell death induced by 5‐FU treatment requires the MMR system to function, whereas irinotecan induced DNA damage can be lethal directly. 87 In addition, some scholars have found that MSI‐H tumors highly express thymidylate synthase, which may also lead to resistance to 5‐FU. 88 However, in a multicenter international trial (MOSAIC) study on the efficacy of oxaliplatin/fluorouracil/calcium leucovorin in adjuvant treatment of colon cancer, researchers analyzed the MSI status of CRC patients and followed up for 10 years; they found FOLFOX4 adjuvant chemotherapy can improve OS in patients with dMMR / MSI‐H type III CRC. 89 The MSI status may affect the effectiveness of adjuvant chemotherapy in patients with CRC, but additional clinical trials are needed to determine the role of MSI classification in the selection of an adjuvant chemotherapy regimen.
3.5. Guiding targeted therapy
Some studies have shown that promoter methylation and genome amplification or mutation of HER2, MET, PTEN, or PIK3CA are common in MSI‐H tumors. These lead to decreased expression of EGFR ligands, decreased efficacy of EGFR inhibitors, and resistance to EGFR therapy. 90 , 91 For example, cetuximab treatment of MSI‐H CRC usually has adverse reactions, while bevacizumab can reduce immunosuppressive cells and enhance anti‐tumor immune responses by inhibiting angiogenesis and promoting vascular normalization. The CALGB/SWOG 80405 study compared the efficacy of first‐line (FOLFOX or FOLFIRI) combined with bevacizumab or cetuximab in the treatment of mCRC. The results showed that the median OS of the MSI‐H group and the MSS group was 30 months versus 11.9 months, and the median OS of MSS mCRC patients treated with cetuximab and bevacizumab was similar (n = 586; median OS: 30.7 months vs. 30.3 months). 92 In addition, a subgroup analysis of the NASBP C‐08 study found that MSI‐H patients in stage II‐III CRC who received FOLFOX+bevacizumab had better outcomes than those treated with chemotherapy alone. 93 Recently, Zaanan et al. retrospectively analyzed data of 128 patients with MSI‐H / dMMR mCRC who received first‐line chemotherapy alone or combined with anti‐EGFR treatment from 2007 to 2017; they found that the addition of anti‐EGFR to chemotherapy significantly improved progression‐free survival (PFS) in patients with familial mCRC. 94 Therefore, in the era of precision treatment, MSI testing for patients with sporadic or hereditary CRC can help guide patients in choosing a suitable targeted therapy plan and predicting the benefits of targeted therapy.
3.6. Guiding immunotherapy
The long‐term clinical efficacy of ICIs in treating refractory malignant solid tumors has revolutionized cancer treatment. 95 , 96 In five clinical trials of prembrolizumab for CRC, KEYNOTE‐016 97 、KEYNOTE‐164 98 、KEYNOTE‐012 99 、KEYNOTE‐028, 100 and KEYNOTE‐158, 101 a higher overall remission rate was observed, which further indicates that pembrolizumab is effective in treating MSI‐H tumors. 102 Although anti‐PD‐1/PDL‐1 immunotherapy is generally ineffective for CRC, 37 , 103 , 104 definite clinical responses have been observed in patients with dMMR / MSI‐H CRC. 105 , 106 Although not all MSI‐H CRC patients can respond to immunotherapy, 37 , 97 PD‐1 / PD‐L1 blockade therapy reactivates effector T cells, inhibits immune escape, and shapes the activated immune TME. 107 These factors make PD‐1/PD‐L1 blockade therapy likely to become an important CRC treatment modality in the future. Therefore, MSI detection is not only a directional "landmark" for immunotherapy in CRC patients, but it is also a predictive marker for the efficacy of ICI treatment. 108
4. MICROSATELLITE STATUS AND APPLICATION PROGRESS OF ICIS
Samstein et al. showed that patients with CRC in the MSS/MSI‐L group are not sensitive to ICI treatment 95 ; however, due to the complexity of anti‐tumor immune responses and the heterogeneity between tumor and metastasis, dMMR / MSI‐H status alone may not be enough to accurately identify those responsive to ICI treatment. 109 In recent years, to further clarify the effectiveness immunotherapy for CRC, many studies on ICI treatment have been carried out worldwide. Recent preclinical and clinical studies have shown that ICIs combined with chemotherapy, molecular targeted therapy, radiotherapy, or new immunomodulators can act synergistically and extend the application of ICIs to MSS type CRC. 7 , 110 , 111
4.1. dMMR/MSI‐H type CRC and ICIs
4.1.1. Single drug research
The most recent KEYNOTE 177 study 112 is an international, randomized, open phase III clinical trial of MSI‐H / dMMR mCRC, comparing the role of pembrolizumab and chemotherapy in the first‐line treatment of MSI‐H / dMMR stage IV CRC. This clinical trial is estimated to be completed in December 2021. The latest follow‐up results show that compared to those treated with chemotherapy, patients receiving pembrolizumab as a first‐line treatment had significant improvements in PFS. This may change the first‐line treatment for patients with dMMR mCRC. 113 , 114
4.1.2. Combined targeting
A phase II clinical trial, CheckMate‐142 (NCT02060188), 115 is studying the efficacy and safety of nivolumab (3 mg/kg every 2 weeks) or nivolumab combined with ipilimumab (1 mg/kg every 3 or 6 weeks) in the first‐line treatment of dMMR / MSI‐H CRC. This study is expected to be completed in July 2022. Follow‐up results thus far have been encouraging. 29 , 106 , 116 , 117
4.1.3. Combined chemotherapy ± targeting treatment
A phase III randomized COMMIT study (NCT02997228) 118 is also in progress. At present, 347 patients with MSI‐H / dMMR MCRC have been randomly assigned to mFOLFOX6 / bevacizumab combined with or without atezolizumab or atezolizumab combined with chemotherapy as the first‐line treatment. 78 This clinical trial is still in progress and is expected to be completed in April 2022. The results are highly anticipated.
4.2. pMMR/MSS type CRC and ICIs
Because pMMR/MSS CRC patients respond poorly to single‐agent ICIs, current research mainly focuses on combination therapy.
4.2.1. Combined radiotherapy
The ongoing PEMREC trial (NCT04109755) 119 evaluates the feasibility of a neoadjuvant regimen without chemotherapy for patients with locally advanced pMMR CRC. The enrolled patients use radiotherapy combined with pembrolizumab as a neoadjuvant treatment regimen.
Other studies on the safety and efficacy of radiotherapy combined with pembrolizumab in MSS type mCRC, especially patients with liver metastases, such as NCT02837263 120 and NCT02437071 121 are under active development.
4.2.2. Combined radiotherapy ± targeting
A phase II clinical trial of neoadjuvant radiotherapy for rectal cancer combined with atozolizumab and bevacizumab, the Tarzan trial (NCT04017455), 122 is currently recruiting patients and is expected to be completed in August 2024.
4.2.3. Combined targeting
At present, NCT03442569 trial 123 is exploring the potential synergistic effect of anti‐EGFR and ICIs, as well as the safety and efficacy of the combined application of panitumumab, nivolumab, and ipilimumab in KRAS / NRAS / BRAF wild‐type MSS MCRC.
REGONIVO(EPOC1603) study is an open‐label, dose‐escalation, dose‐expansion, and phase Ib study for patients with advanced gastric cancer or CRC. A total of 25 CRC patients (24 MSS and 1 MSI‐H) were enrolled in the CRC cohort to receive regorafenib combined with nivolumab. The results of this study showed that eight (33%) of the 24 patients with MSS mCRC achieved objective remission, which indicated that regorafenib 80 mg combined with nivolumab is controllable and has encouraging anti‐tumor activity in patients with MSS. 124
In addition, a phase I / II clinical trial (NCT03657641 125 and NCT03797326 126 ) on regorafenib or lenvatinib in combination with anti‐PD‐1/PD‐L1 in mCRC patients will study whether regorafenib or levatinib interacts synergistically with anti‐PD‐1/PD‐L1 treatment. 127
4.2.4. Combined immunotherapy
In a phase II trial to explore the combination of a cancer vaccine (GVAX colon vaccine) with pembrolizumab and cyclophosphamide in the treatment of pMMR / MSS type CRC, biochemical reactions were observed in 41% of patients. This indicates that GVAX can regulate the anti‐tumor immune response. 128
Cibisatamab (CEA‐CD3‐TCB; RG7802, RO6958688) is a T cell bispecific antibody (TCB). In an ongoing study, the activity of CEA‐TCB combined with atezolizumab was enhanced and the toxicity was controllable. In the clinical treatment of pMMR / MSS mCRC patients, ORR was 18% and DCR was 82%, and the results were encouraging. 129
The niche study (NCT03026140 130 ) carried out by Chalabi et al. identified dMMR and pMMR in patients with early‐stage colon cancer and divided them into two groups. The results showed that 4 / 15 (27%; 95% CI: 8%–55%) of pMMR tumors showed pathological responses, with three MPRs and one partial response. After treatment, the infiltration of CD8+ T cells significantly increased, indicating that the anti‐tumor immune function was partially activated.
5. LIMITATIONS OF MSI STATUS AND IMMUNE MARKERS IN PREDICTING CANCER BEHAVIOR AND TREATMENT RESPONSE
At present, MSI status and immune markers are widely used as the foundation of immunotherapy, especially for CRC due to its molecular characteristics. Not only can this predict the prognosis of patients as well as their response to certain intervention measures, but it can also help identify causal relationships and optimize prevention strategies by examining the relationship between a certain etiology and different molecular subtypes. 131 However, in the process of clinical application we often find that the predictive power of MSI detection and immune marker detection (such as PD‐L1) varies between patients. 132 Therefore, scholars have increasingly suggested that, in addition to the molecular characteristics and immune markers of a tumor, various other factors might also affect tumor evolution and response to treatment. The development, behavior, and response of tumor cells to TME treatment might be affected by the following factors: genetic and epigenetic factors, environmental factors and lifestyle, dietary habits, microbial factors, and the application of some anti‐inflammatory drugs. 133 This also explains why some PD‐L1 + tumor patients do not respond to ICIs while some PD‐L1 – tumor patients respond strongly to ICIs. 134
5.1. Genetic and epigenetic factors
Colorectal cancer is a heterogeneous disease with different genetic and epigenetic variations in which genetic factors (such as SNP or family history) have a significant impact on the tumor antigen landscape. 135 Nonsynonymous mutations and insertions as well as deletions in protein coding genes are both the main sources of tumor new antigens (TNA) and important targets of tumor specific CD8 + cytotoxic T lymphocytes (CTLs). 136 Therefore, gene sequence changes caused by genetic factors have been shown to affect the clinical outcomes in CRC patients.
Epigenetic changes are also closely related to the prognosis of patients. 131 , 137 Previous studies have found that there is a specific tumor phenotype, CpG island methylator phenotype (CIMP), which is characterized by widespread CpG island hypermethylation, and is affected by a variety of factors that lead to the poor prognosis of a tumor. 138 Other studies have found that DNA hypomethylation at the LINE‐1 repeat element can also lead to poor prognosis of colon cancer. LINE‐1 hypomethylation may provide alternative promoter activation [215] and help regulate the expression of noncoding RNA in many genes. 139 Additionally, one carbon metabolism plays a major role in DNA synthesis and methylation. 140 DNA demethylation activated retrotransposons may transpose throughout the genome, leading to gene destruction and chromosomal instability (CIN). Although these epigenetic changes are usually reversible, they can be passed on to cell progeny and affect the proliferation, invasion, and metastasis of tumor cells as well as their resistance to treatment. 141
5.2. Environmental factors and lifestyle
Traditional epidemiological studies have confirmed that the formation of a tumor is a complex and multifactorial process in which environmental factors are also involved. People will form different lifestyle habits due to their local environmental factors (such as weather, terrain, etc.). 131 There are currently some studies that analyze how lifestyle factors (such as physical exercise, smoking, and obesity) are related to the occurrence and prognosis of CRC. 142 In 2008, Ogino s et al. studied the interaction between obesity and fatty acid synthetase (FASN) and its impact on the prognosis of colon cancer. It was found that obesity had an adverse effect on the prognosis of patients with FASN positive colon cancer, but obesity did not affect the prognosis of patients with FASN negative colon cancer. 143 These data suggest that excessive energy in obese patients may promote the growth and proliferation of tumor cells through FASN activation. It was also found that energy balance has a relationship with many signal transduction pathways impacting tumor invasion such as activation of STMN1, PI3 K, and Wnt. 144 , 145
5.3. Dietary factors
It is known that regular consumption of red processed meat, low dietary fiber intake, excessive alcohol consumption, and vitamin B and D deficiency increase the risk of CRC. 146 , 147 However, the exact mechanisms by which these items increase this risk remains to be clarified. 148 As for preventive dietary items, the potential for fish oil or omega‐3 polyunsaturated fatty acids (PUFA) to prevent cancer has been debated. 149 The results of Song, et al 150 showed that high omega‐3 PUFA intake can reduce the risk of CRC in patients with a high Foxp3 + regulatory T cell (Treg) count, but does not reduce the risk of cancer in patients with a low Foxp3 + Treg count. Some studies have also found that certain diets can reduce the risk of CRC by inhibiting Fusobacterium nucleatum. The so‐called “cautious diet” which is rich in whole grains and fiber is associated with a low risk of CRC. The level of Clostridium nucleatum can be detected, but it is not associated with low cancer risk. 151 Trans fatty acids and salt in one's diet can also affect inflammation. Researchers have shown that high levels of salt can make macrophages display a pro‐inflammatory phenotype and promote the differentiation of CD4 + T cells into Th‐17 cells. 152 Ultimately, drastic dietary changes can lead to detectable changes in the structure of the intestinal microbial community over a relatively short period of time. These changes can serve to regulate and affect metabolism and the immune system response and thus affect the prognosis of the tumor and the efficacy of different anti‐tumor treatments. 153
5.4. Microbiological factors
Evidence from many preclinical models and population cohorts shows that the diversity and composition of intestinal flora plays an important role in both the pathogenesis of tumors in the gastrointestinal tract as well as in other parts of the body. 154 , 155 Host microbiota factors may affect patients’ responses to different forms of cancer treatment and treatment‐related toxicity. 156 , 157 This points to the potential use of intestinal flora as a biomarker of cancer treatment response. 158
At present, Salmonella typhi and Helicobacter pylori in cholangiocarcinoma as well as H. pylori in gastric cancer have been identified as carcinogenic enterobacteria. 159 , 160 F. nucleoatum has been proven to play a role in the formation and progression of colon serrated adenoma and colon cancer. 161 It can also be detected in lymph nodes and distant metastasis in patient samples, 162 which is related to many clinicopathological and molecular characteristics. The metabolic components of F. nucleoatum, including Fad adhesion complex (FadAc), can activate the Wnt /β‐Catenin signaling pathway in human colon cancer cell lines and induce changes in carcinogenic transcription. 163
Some microorganisms produce metabolites that can promote the growth of tumors, while some compounds or metabolites derived from microorganisms can play the role of tumor inhibitors and immune regulators and thus can be used in the treatment of tumors. A number of studies have reported that there is a strong relationship between the gut microbiota and the response to immune checkpoint blocking therapy. 164 , 165 , 166 Regulating the gut microbiota can enhance the therapeutic response, and it can also regulate the drug toxicity in anti‐tumor therapy. For example, the common diarrhea response to Irinotecan (topoisomerase I inhibitor) is mediated by the symbiotic bacterial β – glucuronidase. Selective enzyme inhibition may protect the microbiota from the toxicity induced by Irinotecan. 167 Ultimately, changing the microbial community may be a new direction for targeted therapy to explore in the future.
5.5. Use of anti‐inflammatory drugs
The inflammatory microenvironment of tumor cells can change the tissue homeostasis to build an internal environment suitable for tumor growth and metastasis. 168 It is known that frequent use of aspirin or non‐steroidal anti‐inflammatory drugs can affect the development of malignant tumors. The effect of these drugs on the anti‐tumor immune process of the immune system has shown to be especially important in the prevention of CRC. 169 , 170 Randomized trials have confirmed that frequent use of aspirin or other PTGS2 inhibitors reduces the risk of colorectal adenoma as well as increases levels of hpgd mRNA expression in adjacent normal colon tissues. 171 , 172 , 173 There is also experimental evidence that the overexpression of PTGS2 (COX‐2) is related to the aggressive behavior of tumor. PTGS2 plays an important role in the development of CRC. Regular aspirin use in patients with confirmed CRC can significantly reduce the mortality rate of PTGS2 positive cancer patients. 174 Other research found that regular aspirin use was associated with longer survival in patients with the PIK3CA mutation but not in patients with wild‐type PIK3CA. This suggests that the PIK3CA mutation may be a predictive biomarker of aspirin response, which may be related to the interaction between phosphatidylinositol‐4,5‐diphosphate 3‐kinase (PI3 K) and the PTGS2 pathway. 175 Additionally, many experimental results have shown that the incidence rate and survival rate of patients with CRC diagnosed with low expression of TIL and CD274 (PD‐L1) were strongly correlated with aspirin use. This correlation may be due to the fact that anti‐inflammatory drugs are another means of changing microbial composition and reducing microbial diversity. 176 , 177 Some studies have found that the use of antibiotics may reduce the anti‐tumor effect of immune checkpoint inhibitors. 178 , 179
6. APPLICATION STATUS AND PROSPECT OF MOLECULAR PATHOEPIDEMIOLOGY
Molecular pathological epidemiology (MPE), first proposed by Ogino, is a relatively new research field based on the molecular typing of cancer. 180 It integrates molecular pathology, immune response, and clinical results of cancer, using epidemiological research design methods to analyze the impact of lifestyle habits and changes in an individual's molecular environment have on disease development, prognosis, and outcome. MPE is widely used as a combination of pathology and epidemiology. 181 Not only can it be used to enhance the etiology and heterogeneity of almost all human diseases, but it can also be combined with other multidisciplinary fields. 182 For example, MPE can be integrated into immunology, life cycle epidemiology, microbiology, pharmacology, and social sciences to assess intermediate biomarkers that can predict future disease outbreaks. 183 , 184 , 185 , 186
Currently, genome‐wide association studies (GWAS) and immunology are combined with MEP studies to form GWAS‐MPE or Immune‐MPE, respectively. These multidisciplinary studies evaluate the effects of exogenous and endogenous factors on carcinogenesis. 137 , 187 , 188 The development of these comprehensive fields can fill the research gaps between tumor genetics, immunology, and epidemiology, and can also help us clarify the carcinogenic mechanisms of some exposure factors. 186 , 189 The study of MPE can provide a reasonable explanation for the differences in prognosis and treatment responses among patients. Further research in this field can also further improve the accuracy of evaluating the prognosis and the prediction to the response to treatment, which are the future directions of tumor research with far‐reaching significance.
7. CONCLUSION
The development and gradual maturation of immunotherapy has revolutionized CRC treatment. Microsatellite status has also played an indispensable role in the clinical diagnosis and treatment of patients and is especially useful as a predictor immunotherapy effectiveness. Previous studies have found that ICIs can bring long‐term survival benefits to patients with MSI‐H tumors, but they are not the only group to benefit. In this paper, the differences between MSI‐H and MSS were discussed, including expression of TMB, TILs, PD‐L1, and VEGF in the TME of CRC patients; these are important factors affecting patient response to ICI treatment. Although MSI status along with some immune markers detection results are used as the premise of immunotherapy as well as other anti‐tumor treatments globally, this method of prediction has its limitations and can be affected by genetic and epigenetic factors, environmental and lifestyle factors, dietary habits, microbiological factors, and drug factors. But despite these limitations, MPE offers a new horizon of possibilities for further improving the accuracy of prognosis. It is thus necessary to use GWAS‐MPE or Immuno‐MPE combination methods to develop an in‐depth understanding of molecular mechanisms of CRC immunoreactivity and how they relate to cancer treatment. Furthermore, it is imperative to develop effective TME regulatory drugs or better combination therapies, overcome primary and secondary drug resistance in CRC treatment, and achieve truly individualized precision therapies.
CONFLICT OF INTEREST
None of the authors has any financial support or relationships that may pose a conflict of interest.
AUTHOR CONTRIBUTIONS
Chen Hongsheng made substantial contributions to the conception and design of this study. Bai Junge wrote the first draft of the manuscript. All authors made substantial contributions to the acquisition or analysis of data used in this article. Bai Xuefeng revised the manuscript for the purpose of important intellectual content.
INFORMED CONSENT
Informed consent is not required for this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68 394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66 683‐691. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. [DOI] [PubMed] [Google Scholar]
- 4. Lumpkins CY, Philp A, Nelson KL, Miller LM, Greiner KA. A road map for the future: an exploration of attitudes, perceptions, and beliefs among African Americans to tailor health promotion of cancer‐related genetic counseling and testing. J Genet Couns. 2020;29:518‐529. [DOI] [PubMed] [Google Scholar]
- 5. Emambux S, Tachon G, Junca A, Tougeron D. Results and challenges of immune checkpoint inhibitors in colorectal cancer. Expert Opin Biol Ther. 2018;18:561‐573. [DOI] [PubMed] [Google Scholar]
- 6. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch‐repair tumours. Gastroenterol Rep (Oxf). 2020;8:11‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A. Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol. 2018;9:160‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz M, Salamero‐Boix A, Niesel K, Alekseeva T, Sevenich L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front Immunol. 2019;10:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Lv J, Guo F, et al. Gut microbiome influences the efficacy of PD‐1 antibody immunotherapy on MSS‐type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Link JT, Overman MJ. Immunotherapy progress in mismatch repair‐deficient colorectal cancer and future therapeutic challenges. Cancer J. 2016;22:190‐195. [DOI] [PubMed] [Google Scholar]
- 13. Kloor M, von Knebel DM. The immune biology of microsatellite‐unstable cancer. Trends Cancer. 2016;2:121‐133. [DOI] [PubMed] [Google Scholar]
- 14. Lei X, Lei Y, Li JK, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126‐133. [DOI] [PubMed] [Google Scholar]
- 15. Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60 319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang K, Taggart MW, Reyes‐Uribe L, et al. Immune profiling of premalignant lesions in patients with lynch syndrome. JAMA Oncol. 2018;4 1085‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGranahan N, Favero F, de Bruin EC, Birkbak NJ, Szallasi Z, Swanton C. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med. 2015;7(283):283ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jerby‐Arnon L, Shah P, Cuoco MS, et al. A cancer cell program promotes T Cell exclusion and resistance to checkpoint blockade. Cell. 2018;175(4):984–997.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bao X, Shi R, Zhang K, et al. Immune landscape of invasive ductal carcinoma tumor microenvironment identifies a prognostic and immunotherapeutically relevant gene signature. Front Oncol. 2019;9:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bao X, Shi R, Zhao T, Wang Y. Mast cell‐based molecular subtypes and signature associated with clinical outcome in early‐stage lung adenocarcinoma. Mol Oncol. 2020;14 917‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao X, Shi R, Zhao T, Wang Y. Immune landscape and a novel immunotherapy‐related gene signature associated with clinical outcome in early‐stage lung adenocarcinoma. J Mol Med (Berl). 2020;98 805‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol. 2020;11:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inaguma S, Lasota J, Wang Z, Felisiak‐Golabek A, Ikeda H, Miettinen M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD‐L1)‐positive colorectal carcinomas. Mod Pathol. 2017;30:278‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JH, Park HE, Cho NY, Lee HS, Kang GH. Characterisation of PD‐L1‐positive subsets of microsatellite‐unstable colorectal cancers. Br J Cancer. 2016;115 490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD‐1 and PD‐L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenbaum MW, Bledsoe JR, Morales‐Oyarvide V, Huynh TG, Mino‐Kenudson M. PD‐L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor‐infiltrating lymphocytes. Mod Pathol. 2016;29:1104‐1112. [DOI] [PubMed] [Google Scholar]
- 27. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21 1350‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thanki K, Nicholls ME, Gajjar A, et al. Consensus molecular subtypes of colorectal cancer and their clinical implications. Int Biol Biomed J. 2017;3 105‐111. [PMC free article] [PubMed] [Google Scholar]
- 29. Overman MJ, Lonardi S, Wong K, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair‐deficient/microsatellite instability‐high metastatic colorectal cancer. J Clin Oncol. 2018;36 773‐779. [DOI] [PubMed] [Google Scholar]
- 30. Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, Hashemy SI. PD‐1/ PD‐L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110 312‐318. [DOI] [PubMed] [Google Scholar]
- 31. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357 409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson DB, Estrada MV, Salgado R, et al. Melanoma‐specific MHC‐II expression represents a tumour‐autonomous phenotype and predicts response to anti‐PD‐1/PD‐L1 therapy. Nat Commun. 2016;7:10582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov. 2015;5 43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu L, Pan K, Zheng HX, et al. IL‐17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother. 2013;36 451‐458. [DOI] [PubMed] [Google Scholar]
- 35. Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19 9‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non‐small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019;18 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18 1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res. 2016;22 1865‐1874. [DOI] [PubMed] [Google Scholar]
- 39. Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front Immunol. 2020;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanz‐Pamplona R, Melas M, Maoz A, et al. Lymphocytic infiltration in stage II microsatellite stable colorectal tumors: a retrospective prognosis biomarker analysis. PLoS Medicine. 2020;17:e1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44 698‐711. [DOI] [PubMed] [Google Scholar]
- 42. Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24 1550‐1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kloor M, Staffa L, Ahadova A, von Knebel DM. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. 2014;399:23‐31. [DOI] [PubMed] [Google Scholar]
- 44. Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune‐cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15 857‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salem ME, Bodor JN, Puccini A, et al. Relationship between MLH1, PMS2, MSH2 and MSH6 gene‐specific alterations and tumor mutational burden in 1057 microsatellite instability‐high solid tumors. Int J Cancer. 2020;147 2948‐2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zarour HM. Reversing T‐cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22 1856‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8 1069‐1086. [DOI] [PubMed] [Google Scholar]
- 48. Kistner L, Doll D, Holtorf A, Nitsche U, Janssen KP. Interferon‐inducible CXC‐chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget. 2017;8 89998‐90012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143‐158. [DOI] [PubMed] [Google Scholar]
- 50. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanz‐Pamplona R, Gil‐Hoyos R, López‐Doriga A, et al. Mutanome and expression of immune response genes in microsatellite stable colon cancer. Oncotarget. 2016;7:17711‐17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coupez D, Hulo P, Touchefeu Y, Bossard C, Bennouna J. Pembrolizumab for the treatment of colorectal cancer. Expert Opin Biol Ther. 2020;20 219‐226. [DOI] [PubMed] [Google Scholar]
- 53. Korehisa S, Oki E, Iimori M, et al. Clinical significance of programmed cell death‐ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer. 2018;142 822‐832. [DOI] [PubMed] [Google Scholar]
- 54. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD‐L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203 883‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Llosa NJ, Luber B, Tam AJ, et al. Intratumoral adaptive immunosuppression and type 17 immunity in mismatch repair proficient colorectal tumors. Clin Cancer Res. 2019;25 5250‐5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ozcan M, Janikovits J, von Knebel DM, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology. 2018;7:e1445453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176 1248‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miyamoto N, Yamamoto H, Taniguchi H, et al. Differential expression of angiogenesis‐related genes in human gastric cancers with and those without high‐frequency microsatellite instability. Cancer Lett. 2007;254 42‐53. [DOI] [PubMed] [Google Scholar]
- 60. Sun Guogang SX. Correlation of microsatellite instability, vascular endothelial growth factor expression and pathological characteristics and prognosis in patients with colorectal cancer. Zhejiang J Traumatic Surg. 2016;21 615‐618. [Google Scholar]
- 61. Trinchieri G. Interleukin‐12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133‐146. [DOI] [PubMed] [Google Scholar]
- 62. Arai H, Battaglin F, Wang J, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev. 2019;81:101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Harada S, Morlote D. Molecular pathology of colorectal cancer. Adv Anat Pathol. 2020;27:20‐26. [DOI] [PubMed] [Google Scholar]
- 64. Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19‐26. [DOI] [PubMed] [Google Scholar]
- 65. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121 3281‐3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073‐2087.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lynch HT, Shaw MW, Magnuson CW, Larsen AL, Krush AJ. Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med. 1966;117:206‐212. [PubMed] [Google Scholar]
- 68. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138 2044‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919‐932. [DOI] [PubMed] [Google Scholar]
- 70. Dabir PD, Bruggeling CE, van der Post RS, et al. Microsatellite instability screening in colorectal adenomas to detect Lynch syndrome patients? A systematic review and meta‐analysis. Eur J Hum Genet. 2020;28 277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Di J, Liu M, Fan Y, et al. Phenotype molding of T cells in colorectal cancer by single‐cell analysis. Int J Cancer. 2020;146 2281‐2295. [DOI] [PubMed] [Google Scholar]
- 72. Tsai YJ, Huang SC, Lin HH, et al. Differences in gene mutations according to gender among patients with colorectal cancer. World J Surg Oncol. 2018;16 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johnson AA, Akman K, Calimport SR, Wuttke D, Stolzing A, de Magalhães JP. The role of DNA methylation in aging, rejuvenation, and age‐related disease. Rejuvenation Res. 2012;15 483‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288 321‐333. [DOI] [PubMed] [Google Scholar]
- 75. Slattery ML, Potter JD, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability‐positive colon cancer. Cancer Res. 2001;61 126‐130. [PubMed] [Google Scholar]
- 76. Kang S, Na Y, Joung SY, Lee SI, Oh SC, Min BW. The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors. Medicine (Baltimore). 2018;97:e0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moisio AL, Järvinen H, Peltomäki P. Genetic and clinical characterisation of familial adenomatous polyposis: a population based study. Gut. 2002;50 845‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun BL. Current microsatellite instability testing in management of colorectal cancer. Clin Colorectal Cancer. 2021;20(1):e12‐e20. [DOI] [PubMed] [Google Scholar]
- 79. Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite‐unstable colorectal cancer. Clin Cancer Res. 2007;13 3831‐3839. [DOI] [PubMed] [Google Scholar]
- 80. Rutter MD, Beintaris I, Valori R, et al. World endoscopy organization consensus statements on post‐colonoscopy and post‐imaging colorectal cancer. Gastroenterology. 2018;155(3):909‐925.e3. [DOI] [PubMed] [Google Scholar]
- 81. Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105 1189‐1195. [DOI] [PubMed] [Google Scholar]
- 82. Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700‐1705. [DOI] [PubMed] [Google Scholar]
- 83. Samadder NJ, Neklason D, Snow A, et al. Clinical and molecular features of post‐colonoscopy colorectal cancers. Clin Gastroenterol Hepatol. 2019;17(13):2731‐2739.e2. [DOI] [PubMed] [Google Scholar]
- 84. Alwers E, Jansen L, Bläker H, et al. Microsatellite instability and survival after adjuvant chemotherapy among stage II and III colon cancer patients: results from a population‐based study. Mol Oncol. 2020;14:363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219‐3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bohaumilitzky L, von Knebel DM, Kloor M, Ahadova A. Implications of hereditary origin on the immune phenotype of mismatch repair‐deficient cancers: systematic literature review. J Clin Med. 2020;9(6):1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jo WS, Carethers JM. Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark. 2006;2:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Okoń K, Klimkowska A, Wójcik P, Osuch C, Papla B, Stachura J. High thymidylate synthase expression is typical for sporadic MSI‐H colorectal carcinoma. Pol J Pathol. 2006;57:29‐33. [PubMed] [Google Scholar]
- 89. André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10‐year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33:4176‐4187. [DOI] [PubMed] [Google Scholar]
- 90. Cremolini C, Morano F, Moretto R, et al. Negative hyper‐selection of metastatic colorectal cancer patients for anti‐EGFR monoclonal antibodies: the PRESSING case‐control study. Ann Oncol. 2017;28:3009‐3014. [DOI] [PubMed] [Google Scholar]
- 91. Gallois C, Taieb J, Le Corre D, et al. Prognostic value of methylator phenotype in stage III colon cancer treated with oxaliplatin‐based adjuvant chemotherapy. Clin Cancer Res. 2018;24:4745‐4753. [DOI] [PubMed] [Google Scholar]
- 92. Innocenti F, Ou FS, Qu X, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37:1217‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pogue‐Geile K, Yothers G, Taniyama Y, et al. Defective mismatch repair and benefit from bevacizumab for colon cancer: findings from NSABP C‐08. J Natl Cancer Inst. 2013;105:989‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zaanan A, Henriques J, Cohen R, et al. Efficacy of anti‐EGFR in MSI metastatic colorectal cancer depending on sporadic or familial origin. J Natl Cancer Inst. 2020;113(4):496‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK‐3475; Anti‐PD‐1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286‐4293. [DOI] [PubMed] [Google Scholar]
- 97. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Le DT, Kim TW, Van Cutsem E, et al. Phase II open‐label study of pembrolizumab in treatment‐refractory, microsatellite instability‐high/mismatch repair‐deficient metastatic colorectal cancer: KEYNOTE‐164. J Clin Oncol. 2020;38:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncol. 2017;18:212‐220. [DOI] [PubMed] [Google Scholar]
- 100. O'Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti‐PD‐1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS ONE. 2017;12:e0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair‐deficient cancer: results from the phase II KEYNOTE‐158 study. J Clin Oncol. 2020;38:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Geantă M, Cioroboiu C. The FDA changed everything. Biomed Hub. 2017;2:52‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch‐repair deficiency and others. J Gastrointest Oncol. 2016;7:713‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kwak Y, Koh J, Kim DW, Kang SB, Kim WH, Lee HS. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7:81778‐81790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high‐level microsatellite instability (MSI‐H). Ann Oncol. 2014;25:1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lenz H‐JJ, Van Cutsem E, Limon ML, et al. Durable clinical benefit with nivolumab (NIVO) plus low‐dose ipilimumab (IPI) as first‐line therapy in microsatellite instability‐high/mismatch repair deficient (MSI‐H/dMMR) metastatic colorectal cancer (mCRC). Ann Oncol. 2018;29(Suppl):8.29087449 [Google Scholar]
- 107. Enamorado M, Iborra S, Priego E, et al. Enhanced anti‐tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun. 2017;8:16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability‐high as a predictor for anti‐PD‐1/PD‐L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vitiello GA, Miller G. Targeting the interleukin‐17 immune axis for cancer immunotherapy. J Exp Med. 2020;217(1):e20190456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hermel DJ, Sigal D. The emerging role of checkpoint inhibition in microsatellite stable colorectal cancer. J Pers Med. 2019;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen C, Liu Y, Cui B. Effect of radiotherapy on T cell and PD‐1 / PD‐L1 blocking therapy in tumor microenvironment. Hum Vaccin Immunother. 2021;1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Study of Pembrolizumab (MK‐3475) vs Standard Therapy in Participants With Microsatellite Instability‐High (MSI‐H) or Mismatch Repair Deficient (dMMR) Stage IV Colorectal Carcinoma (MK‐3475‐177/KEYNOTE‐177). Available online. https://clinicaltrials.gov/ct2/show/ NCT02563002. Accessed on 20 December 2020.
- 113. André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite‐instability‐high advanced colorectal cancer. N Engl J Med. 2020;383:2207‐2218. [DOI] [PubMed] [Google Scholar]
- 114. Cancer Discov. PD‐1 inhibitor bests chemo for colorectal cancer. 2020;10:OF2. [DOI] [PubMed] [Google Scholar]
- 115. An Investigational Immuno‐therapy Study of Nivolumab, and Nivolumab in Combination With Other Anti‐cancer Drugs, in Colon Cancer That Has Come Back or Has Spread (CheckMate142). Available online. https://clinicaltrials.gov/ct2/show/NCT02060188. Accessed on 20 December 2020.
- 116. Chalabi M, Fanchi LF, Van den Berg JG, et al. Neoadjuvant ipilimumab plus nivolumab in early stage colon cancer. Ann Oncol. 2018;29(Suppl):8.29087449 [Google Scholar]
- 117. Morse MA, Overman MJ, Hartman L, et al. Safety of nivolumab plus low‐dose ipilimumab in previously treated microsatellite instability‐high/mismatch repair‐deficient metastatic colorectal cancer. Oncologist. 2019;24:1453‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Combination Chemotherapy, Bevacizumab, and/or Atezolizumab in Treating Patients With Deficient DNA Mismatch Repair Metastatic Colorectal Cancer, the COMMIT Study. Available online: https://clinicaltrials.gov/ct2/show/ NCT02997228. Accessed on 20 December 2020.
- 119. Neo‐adjuvant Pembrolizumab and Radiotherapy in Localised MSS Rectal Cancer (PEMREC). Available online: https://clinicaltrials.gov/ct2/show/NCT04109755. Accessed on 20 December 2020.
- 120. PI Pembro in Combination With Stereotactic Body Radiotherapy for Liver Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02837263. Accessed on 20 December 2020.
- 121. Assess the Efficacy of Pembrolizumab Plus Radiotherapy or Ablation in Metastatic Colorectal Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT02437071. Accessed on 20 December 2020.
- 122. Neoadjuvant Treatment in Rectal Cancer With Radiotherapy Followed by Atezolizumab and Bevacizumab (TARZAN) (TARZAN). Available online: https://clinicaltrials.gov/ct2/show/ NCT04017455. Accessed on 20 December 2020.
- 123. PhII Trial Panitumumab, Nivolumab, Ipilimumab in Kras/Nras/BRAF Wild‐type MSS Refractory mCRC. Available online: https://clinicaltrials.gov/ct2/show/NCT03442569. Accessed on 20 December 2020.
- 124. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open‐label, dose‐escalation, and dose‐expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053‐2061. [DOI] [PubMed] [Google Scholar]
- 125. Regorafenib and Pembrolizumab in Treating Participants With Advanced or Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/ NCT03657641. Accessed on 20 December 2020.
- 126. Efficacy and Safety of Pembrolizumab (MK‐3475) Plus Lenvatinib (E7080/MK‐7902) in Previously Treated Participants With Select Solid Tumors (MK‐7902‐005/E7080‐G000‐224/LEAP‐005). https://clinicaltrials.gov/ct2/show/NCT03797326. Accessed on 20 December 2020.
- 127. Iwasa S, Okita N, Kuchiba A, et al. Phase II study of lenvatinib for metastatic colorectal cancer refractory to standard chemotherapy: the LEMON study (NCCH1503). ESMO Open. 2020;5(4):e000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yarchoan M, Huang C, Zhu Q, et al. A phase 2 study of GVAX colon vaccine with cyclophosphamide and pembrolizumab in patients with mismatch repair proficient advanced colorectal cancer. Cancer Med. 2020;9(4):1485‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Segal NH, Saro J, Melero I, et al. Phase I studies of the novel carcinoembryonic antigen T‐cell bispecific (CEA‐CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients (pts) with metastatic colorectal cancer (mCRC). Ann Oncol. 2017;28:v134. [Google Scholar]
- 130. Nivolumab, Ipilimumab and COX2‐inhibition in Early Stage Colon Cancer: an Unbiased Approach for Signals of Sensitivity (NICHE). Available online: https://clinicaltrials.gov/ct2/show/NCT03026140. Accessed on 20 December 2020.
- 131. Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27:212‐224. [DOI] [PubMed] [Google Scholar]
- 132. Zaretsky JM, Garcia‐Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med. 2016;375:819‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Aoki T, Chong LC, Takata K, et al. Single‐cell transcriptome analysis reveals disease‐defining T‐cell subsets in the tumor microenvironment of classic hodgkin lymphoma. Cancer Discov. 2020;10:406‐421. [DOI] [PubMed] [Google Scholar]
- 134. Kalbasi A, Ribas A. Tumour‐intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148‐1159. [DOI] [PubMed] [Google Scholar]
- 136. Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019;37:173‐200. [DOI] [PubMed] [Google Scholar]
- 137. Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rhee YY, Kim KJ, Kang GH. CpG island methylator phenotype‐high colorectal cancers and their prognostic implications and relationships with the serrated neoplasia pathway. Gut Liv. 2017;11:38‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Raptis S, Mrkonjic M, Green RC, et al. MLH1 ‐93G>A promoter polymorphism and the risk of microsatellite‐unstable colorectal cancer. J Natl Cancer Inst. 2007;99:463‐474. [DOI] [PubMed] [Google Scholar]
- 140. Stover PJ. One‐carbon metabolism‐genome interactions in folate‐associated pathologies. J Nutr. 2009;139:2402‐2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Patten DK, Corleone G, Győrffy B, et al. Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat Med. 2018;24:1469‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. van Zutphen M, Boshuizen HC, Kenkhuis MF, et al. Lifestyle after colorectal cancer diagnosis in relation to recurrence and all‐cause mortality. Am J Clin Nutr. 2021:13(5):370‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713‐5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jiang Y, Yan F, Feng Z, Lazarovici P, Zheng W. Signaling network of forkhead family of transcription factors (FOXO) in dietary restriction. Cells. 2020;9(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Benchoula K, Parhar IS, Wong EH. The crosstalk of hedgehog, PI3K and Wnt pathways in diabetes. Arch Biochem Biophys. 2021;698:108743. [DOI] [PubMed] [Google Scholar]
- 146. Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150:663‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wiseman MJ. Nutrition and cancer: prevention and survival. Br J Nutr. 2019;122:481‐487. [DOI] [PubMed] [Google Scholar]
- 148. Hamada T, Liu L, Nowak JA, et al. Vitamin D status after colorectal cancer diagnosis and patient survival according to immune response to tumour. Eur J Cancer. 2018;103:98‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shin A, Cho S, Sandin S, Lof M, Oh MY, Weiderpass E. Omega‐3 and ‐6 fatty acid intake and colorectal cancer risk in swedish women's lifestyle and health cohort. Cancer Res Treat. 2020;52:848‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Song M, Nishihara R, Cao Y, et al. Marine ω‐3 polyunsaturated fatty acid intake and risk of colorectal cancer characterized by tumor‐infiltrating T cells. JAMA Oncol. 2016;2:1197‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3:921‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Helmink BA, Khan M, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377‐388. [DOI] [PubMed] [Google Scholar]
- 153. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(548–563):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tsilimigras MC, Fodor A, Jobin C. Carcinogenesis and therapeutics: the microbiota perspective. Nat Microbiol. 2017;2:17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mima K, Ogino S, Nakagawa S, et al. The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol. 2017;26:368‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2019;30:2012. [DOI] [PubMed] [Google Scholar]
- 157. McQuade JL, Daniel CR, Hess KR, et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271‐285. [DOI] [PubMed] [Google Scholar]
- 159. Di Domenico EG, Cavallo I, Pontone M, Toma L, Ensoli F. Biofilm producing salmonella typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci. 2017;18(9):1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Carlosama‐Rosero YH, Acosta‐Astaiza CP, Sierra‐Torres CH, Bolaños‐Bravo HJ. Helicobacter pylori genotypes associated with gastric cancer and dysplasia in Colombian patients. Rev Gastroenterol Mex. 2021;S0375‐0906(21)00031‐8. [DOI] [PubMed] [Google Scholar]
- 161. Park CH, Han DS, Oh YH, Lee AR, Lee YR, Eun CS. Role of Fusobacteria in the serrated pathway of colorectal carcinogenesis. Sci Rep. 2016;6:25271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E‐cadherin/β‐catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kim D, Zeng MY, Núñez G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp Mol Med. 2017;49:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yang Y, Jobin C. Novel insights into microbiome in colitis and colorectal cancer. Curr Opin Gastroenterol. 2017;33:422‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science. 2018;359:104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chen C, Liu Y, Han P, Cui B. Research progress of preoperative FPR, FAR or AFR in patients with colorectal cancer. Cancer Manag Res. 2021;13:1791‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cao Y, Nishihara R, Wu K, et al. Population‐wide Impact of long‐term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15‐hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med. 2014;6:233re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Almendingen K, Larsen LN, Fausa O, Bratlie J, Høstmark AT, Aabakken L. Selective COX‐2 inhibition affects fatty acids, but not COX mRNA expression in patients with FAP. Fam Cancer. 2010;9:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Benamouzig R, Deyra J, Martin A, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one‐year results of the APACC trial. Gastroenterology. 2003;125:328‐336. [DOI] [PubMed] [Google Scholar]
- 174. Gray RT, Cantwell MM, Coleman HG, et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population‐based cohort study. Clin Transl Gastroenterol. 2017;8:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Paleari L, Puntoni M, Clavarezza M, DeCensi M, Cuzick J, DeCensi A. PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer. a systematic review and meta‐analysis of epidemiological studies. Clin Oncol (R Coll Radiol). 2016;28:317‐326. [DOI] [PubMed] [Google Scholar]
- 176. Cao Y, Nishihara R, Qian ZR, et al. Regular aspirin use associates with lower risk of colorectal cancers with low numbers of tumor‐infiltrating lymphocytes. Gastroenterology. 2016;151(879–892):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hamada T, Cao Y, Qian ZR, et al. Aspirin use and colorectal cancer survival according to tumor CD274 (programmed cell death 1 ligand 1) expression status. J Clin Oncol. 2017;35:1836‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non‐small‐cell lung cancer. Ann Oncol. 2018;29:1437‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kaderbhai C, Richard C, Fumet JD, et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37:3195‐3200. [DOI] [PubMed] [Google Scholar]
- 180. Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ogino S, Nowak JA, Hamada T, Milner DA Jr, Nishihara R. Insights into pathogenic interactions among environment, host, and tumor at the crossroads of molecular pathology and epidemiology. Annu Rev Pathol. 2019;14:83‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ogino S, Nishihara R, VanderWeele TJ, et al. Review article: the role of molecular pathological epidemiology in the study of neoplastic and non‐neoplastic diseases in the era of precision medicine. Epidemiology. 2016;27:602‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67:1168‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hamada T, Keum N, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nishi A, Milner DA Jr, Giovannucci EL, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn. 2016;16:11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lochhead P, Chan AT, Nishihara R, et al. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol. 2015;28:14‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Grizzi F, Basso G, Borroni EM, et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflamm Res. 2018;67:375‐389. [DOI] [PubMed] [Google Scholar]
- 188. Ogino S, Giannakis M. Immunoscore for (colorectal) cancer precision medicine. Lancet. 2018;391:2084‐2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18:19‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


