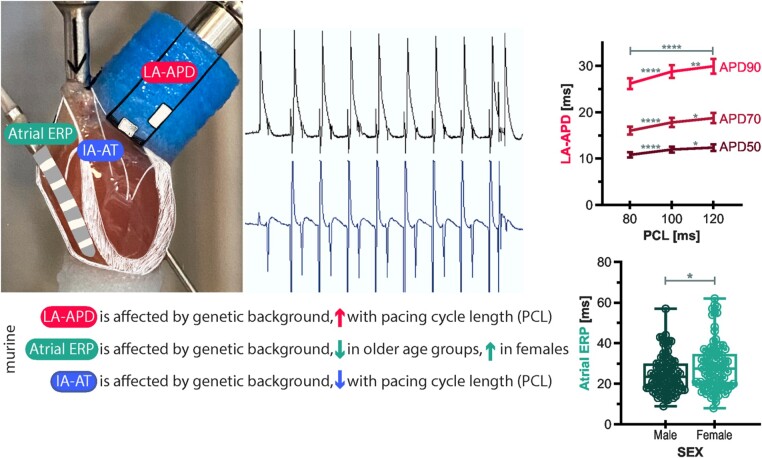
Abstract
Aims
Genetically altered mice are powerful models to investigate mechanisms of atrial arrhythmias, but normal ranges for murine atrial electrophysiology have not been robustly characterized.
Methods and results
We analyzed results from 221 electrophysiological (EP) studies in isolated, Langendorff-perfused hearts of wildtype mice (114 female, 107 male) from 2.5 to 17.7 months (mean 7 months) with different genetic backgrounds (C57BL/6, FVB/N, MF1, 129/Sv, Swiss agouti). Left atrial monophasic action potential duration (LA-APD), interatrial activation time (IA-AT), and atrial effective refractory period (ERP) were summarized at different pacing cycle lengths (PCLs). Factors influencing atrial electrophysiology including genetic background, sex, and age were determined.
LA-APD70 was 18 ± 0.5 ms, atrial ERP was 27 ± 0.8 ms, and IA-AT was 17 ± 0.5 ms at 100 ms PCL. LA-APD was longer with longer PCL (+17% from 80 to 120 ms PCL for APD70), while IA-AT decreased (−7% from 80 to 120 ms PCL). Female sex was associated with longer ERP (+14% vs. males). Genetic background influenced atrial electrophysiology: LA-APD70 (−20% vs. average) and atrial ERP (−25% vs. average) were shorter in Swiss agouti background compared to others. LA-APD70 (+25% vs. average) and IA-AT (+44% vs. average) were longer in 129/Sv mice. Atrial ERP was longer in FVB/N (+34% vs. average) and in younger experimental groups below 6 months of age.
Conclusion
This work defines normal ranges for murine atrial EP parameters. Genetic background has a profound effect on these parameters, at least of the magnitude as those of sex and age. These results can inform the experimental design and interpretation of murine atrial electrophysiology.
Keywords: Atrial, Action potential duration, Effective refractory period, Activation time, Murine, Genetic background
Graphical Abstract
What’s new?
Genetic background markedly affects murine left atrial action potential duration (LA-APD), atrial effective refractory period (ERP), and interatrial activation time (IA-AT) and should be considered during experimental design.
Murine LA-APD is longer at longer pacing cycle length (PCL) and murine IA-AT decreases with longer PCL in Langendorff-perfused isolated beating hearts.
Murine atrial ERP was shorter at older age and prolonged in female hearts independent of age.
This study provides a robust normal range of ex vivo beating heart atrial electrophysiological parameters in mice based on over 200 individual experiments.
Introduction
The burden of disease associated with atrial fibrillation (AF) and its recently identified genomic basis make research into the genetic, genomic, and molecular mechanisms a priority for cardiac electrophysiologists.1 Sex and age shape the presentation and outcome of cardiovascular diseases including AF.2–4
Isolated, Langendorff-perfused hearts from wildtype (WT) and genetically altered murine models are widely studied to understand the electrophysiological (EP) effects of specific genetic mutations.5 Functional findings can be correlated with signalling studies and the effects of pharmacological interventions within the same model.
Systematic comparisons have found that genetic background, sex, and age affect murine ventricular action potential duration (APD), activation time (AT), and effective refractory period (ERP) in WT murine hearts.6 Whether similar differences exist in atrial EP parameters is unknown. Here, we report the range of normal atrial EP parameters in isolated murine WT hearts and present the effects of genetic background, sex, and age on these measurements.
Methods
Experiment selection for analysis
This is a retrospective analysis of experimental data from a range of atrial EP studies in the isolated beating Langendorff heart (see Figure 1 for workflow). Isolated, Langendorff-perfused murine WT atrial electrophysiology experiments were carried out by our research groups at the University of Münster (2005–2011) and at the University of Birmingham (2011–2019) and collated for analysis. Animals were fed standard chow diet and kept under controlled conditions (12 h light cycle, 22 °C room temperature, 55% humidity).
Figure 1.
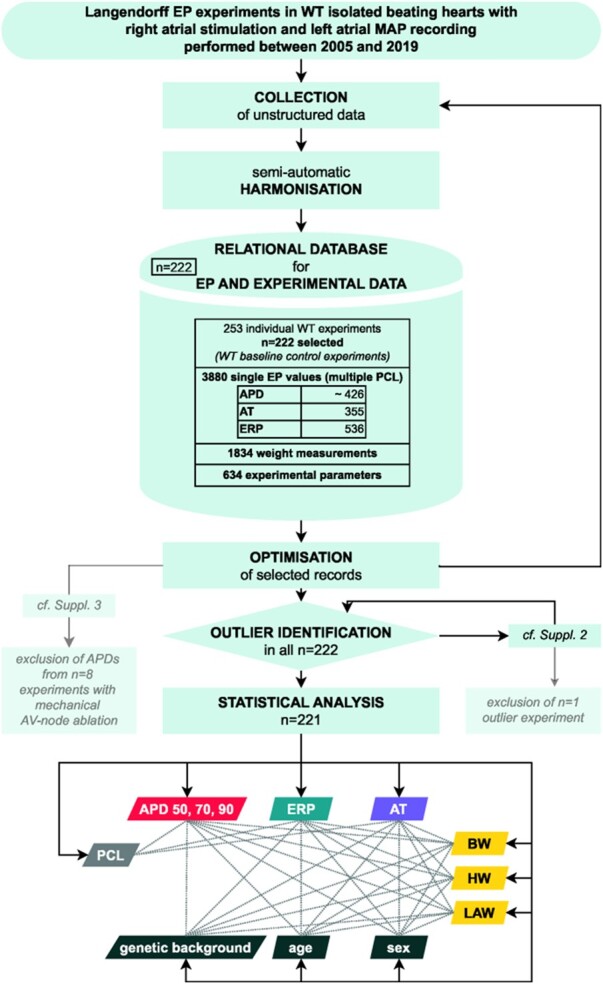
Analysis workflow. APD50, APD70, APD90, action potential duration at 50%, 70%, and 90% repolarization; AT, activation time; BW, body weight; EP, electrophysiological; ERP, effective refractory period; HW, heart weight; LAW, left atrium weight; MAP, monophasic action potential; PCL, pacing cycle length; WT, wildtype.
All experiments in Birmingham were conducted according to the Animals (Scientific Procedures) Act 1986 and approved by the Home Office (Home Office References 30/2967 and PFDAAF77F) and the institutional review board at the University of Birmingham. All experiments in Münster were approved by the regional authority (licence numbers G61/99, G83/2004).
Heart isolation, preparation, and electrophysiological recordings
Experimental details have been described previously.7–9 Deep terminal anaesthesia was achieved by intraperitoneal injection of either urethane (2 g/kg body weight (BW)) or sodium pentobarbital (Euthatal; 100 µL at 200 µg/µL) or by inhalation of 5% isoflurane with an O2 flow rate of 3 L/min. Murine hearts were extracted under heparinization, cannulated via the ascending aorta and mounted on a vertical Langendorff-apparatus (Hugo Sachs/Harvard Apparatus, Germany) for retrograde perfusion of the aorta with Krebs-Henseleit (KH) buffer.9–12 Protocols aimed for constant aortic pressure (100 ± 5 mmHg), coronary flow rate (4 ± 1 mL/min), and temperature (36 ± 1 °C). Preparation time, defined as time from extraction to start of perfusion, was logged and aimed to be ≤5 min in 4°C KH solution or ≤2 min at room temperature. Deviation from these norms led to experimental exclusion. Before starting the EP study, stimulation and measurements, extracted hearts were given time to adjust to KH buffer perfusion and temperature.
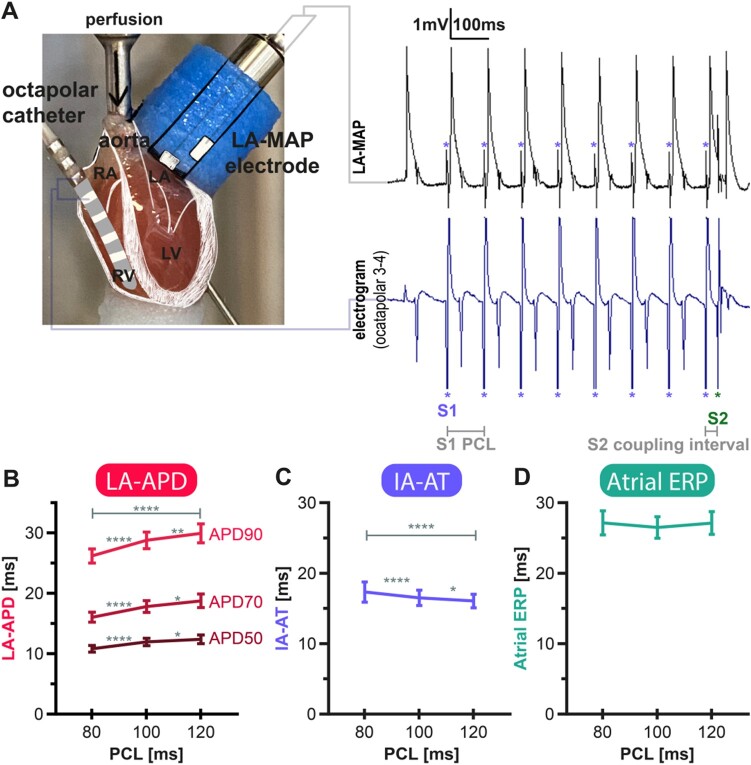
Stimulation was conducted via an octapolar EP catheter inserted through the vena cava or a small hole into the right atrium (RA, see Figure 2 and Graphical Abstract). Atrial monophasic action potentials (MAPs) were recorded using a left atrial epicardial spring-loaded MAP-catheter. Pre-amplified (Model 2000, EP Technologies Inc., CA, USA) MAP signals were analyzed semi-automatically under manual quality control using either a custom-made (based on LabVIEW, National Instruments, TX, USA) or commercially available software (ecgAUTO, EMKA Technologies, France).
Figure 2.
Isolated Langendorff murine heart electrophysiological study and left atrial monophasic action potential (MAP) cycle length dependency. (A) Setup of the Langendorff experiment and original murine left atrial MAPs at a train-of-eight S1-paced beats (purple asterisk) with an S1 pacing cycle length (PCL) of 100 ms and one coupled extra stimulus (S2; green asterisk) with a coupling interval of 35 ms. Below the MAP signals, the bipolar trace between atrial and ventricular electrodes of the octapolar EP catheter are shown. Asterisk indicates stimulation artefacts examplarily (*). (B) Left-atrial action potential duration (LA-APD) at 50%, 70%, and 90% repolarization plotted over steady state PCL. LA-APD at all stages of repolarization is PCL-dependent leading to longer APDs at longer PCLs. (C) Interatrial activation time (IA-AT) was steady state PCL-dependent and decreased with higher PCL. (D) PCL-function of atrial effective refractory period (Atrial ERP). Atrial ERP did not adapt to S1-PCL within the investigated range. Data derived from the pooled cohort of all genetic backgrounds, sexes, and ages from experiments in which data points at each PCL were available. Plotted are means with 95% confidence intervals of the mean. *P < 0.05, **P < 0.01, ****P < 0.001.
Stimulation protocols and analysis of electrophysiological parameters
Isolated hearts were paced from the RA using two electrodes of the EP-catheter at different pacing cycle lengths (PCLs) of 80, 100, and 120 ms. Left atrial APD (LA-APD), atrial ERP, and interatrial AT (IA-AT) were analyzed.
LA-APD was measured at different constant steady state S1-PCLs as the time from the fastest upstroke to 30%, 50%, 70%, and 90% (APD30, APD50, APD70, and APD90, respectively) repolarization, with 0% defined as the peak of the MAP upstroke. Full (100%) repolarization was defined as return to the mean baseline diastolic electrical activity on the LA-MAP electrode. During review, MAPs were quality coded according to standard criteria and experiments were excluded if less than three criteria were fulfilled. Standard criteria were: stable baseline, stable MAP morphology, fast upstroke without inflections, upstroke duration ˂2 ms, MAP amplitude >1 mV, and rapid repolarization without early plateau.
For atrial ERP measurements, the RA was stimulated with a train of eight S1 stimuli at a constant PCL (80, 100, and 120 ms) followed by a single extra stimulus (S2) with an initial S2 coupling interval of 50 ms. The interval was decreased by 1 ms after each series. Atrial ERP was defined as the shortest S2 interval which still resulted in depolarization of the left atrium.
IA-AT was defined as the time from the right atrial stimulus artefact to the steepest upstroke of the MAP signal. The time of the stimulus artefact was captured either together with the MAP itself as a farfield artefact, or on the intracardial electrocardiogram recorded by the electrodes of the EP-catheter located in the RA.
Data storing, archiving, querying, outlier handling, and statistical analysis
Data analysis workflow is illustrated in Figure 1. A relational database scheme was developed to structure and harmonize all murine model parameters and to ensure correct linkage of these parameters to the same isolated heart experiment. Harmonization of unstructured raw data was performed semi-automatically depending on raw data format. With knowledge of existing experiments, the data collection was optimized, exemplarily by manual search for obviously missing data. During this optimization process, acquired data was again injected into the semi-automatic harmonization process and stored in the database. SQL-based queries were developed to obtain data in different arrangements for analysis and for consistency checks. A statistic-based outlier analysis identified outlier data points. Related experiments were re-analyzed by a second observer (Supplementary material online, S1).
Descriptive, explorative, and inductive statistical analysis was performed semi-automatically in SPSS 26 (IBM, CA, USA) and using common data science packages in Python 3.8 (Pandas, NumPy, SciPy, ResearchPy). Data were visualized using Seaborn in Python 3.8, as well as GraphPad Prism 8 and Adobe Illustrator 24.
Normal distribution was evaluated via Q–Q plots and Shapiro–Wilk testing. If grouped data were not normally distributed, square root or logarithmic transformation was applied to normalize data. If data were normally distributed or could be normalized, paired t-tests and repeated-measures analysis of variance (ANOVA) were employed to compare experiments in which multiple EP parameters were obtained at different conditions, e.g. APD at different PCL. Unpaired t-tests and one-way ANOVA were employed when comparing different age groups, sex categories, or genetic backgrounds. Bonferroni correction was used for post hoc testing after one-way ANOVA. Welch’s correction was used if variances were heterogeneous. Kruskal–Wallis tests with Dunn’s tests for post hoc analysis were used if data could not be transformed into Gaussian distributions. In this case, medians and interquartile range are reported in text and tables. If not stated otherwise, values in graphs, text, and tables report means ± standard error of the mean.
Ethics approval
All experiments in Birmingham were conducted according to the Animals (Scientific Procedures) Act 1986 and approved by the Home Office (Home Office References 30/2967 and PFDAAF77F) and the institutional review board at the University of Birmingham. All experiments in Münster were approved by the regional authority (Licence numbers G61/99, G83/2004). All experimental protocols conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The manuscript does not contain clinical studies or patient data.
Results
Animals and experimental conditions
This report encompasses intact isolated beating heart ‘ex vivo’ atrial EP studies from 221 WT murine hearts, 114 females and 107 males, from five different genetic backgrounds [C57BL/6 (n = 76), FVB/N (n = 23), MF1 (n = 33), 129/Sv (n = 69), and Swiss agouti (n = 20)] between 2 and 18 months (11 and 76 weeks) of age. Murine age distribution was not different between sex categories. The sex distribution was similar in most genetic backgrounds. Table 1 summarizes the murine characteristics.
Table 1.
Characteristics of included mice and background distribution
| Total | C57BL/6 | FVB/N | MF1 | 129/Sv | Swiss agouti | ||
|---|---|---|---|---|---|---|---|
| N | 221 | 76 | 23 | 33 | 69 | 20 | |
| Sex | Female | 114 (52%) | 32 (42%) | 16 (70%) | 19 (58%) | 40 (58%) | 7 (35%) |
| Male | 107 (48%) | 44 (58%) | 7 (30%) | 14 (42%) | 29 (42%) | 13 (65%) | |
| Age (weeks) | Total | 28.1 ± 1.1 | 25.6 ± 1.3 | 16.9 ± 1 | 24.3 ± 4.2 | 34.4 ± 2.1 | 32.9 ± 1.8 |
| Female | 27.9 ± 1.5 | 24.3 ± 1.6 | 17.2 ± 1.5 | 21 ± 4.6 | 37 ± 2.7 | 32.9 ± 2.8 | |
| Male | 28.3 ± 1.5 | 26.7 ± 2.0 | 16.2 ± 0.6 | 29.7 ± 8.2 | 30.8 ± 3.2 | 32.9 ± 2.4 | |
| Body weight (g) *median/IQR | Total | 29.4 ± 10.4 | 29.3 ± 11.4 | 26.9 ± 3.0 | 36.6 ± 7.5 | 27.2 ± 7.1 | 36 ± 6.5 |
| Female | 26.5 ± 8.1 | 24.3 ± 4.0 | 26 ± 2.7 | 34.2 ± 3.4 | 24.8 ± 4.0 | 33.7 ± 4.6 | |
| Male | 33.2 ± 9.6 | 31.4 ± 6.6 | 30.6 ± 2.6 | 39.6 ± 4.6 | 29.2 ± 7.6 | 39.4 ± 5.7 | |
| Heart weight (mg) | Total | 197.9 ± 3.8 | 184.8 ± 6.0 | 163.4 ± 5.9 | 246.2 ± 9.9 | 192 ± 6.4 | 224.6 ± 8.4 |
| Female | 171.4 ± 3.8 | 159.4 ± 5.9 | 155.2 ± 6.5 | 211 ± 8.2 | 161.5 ± 4.8 | 203.5 ± 10.4 | |
| Male | 221.6 ± 5.2 | 203.8 ± 8.1 | 176.4 ± 9.8 | 281.4 ± 9.9 | 224 ± 6.3 | 235.9 ± 10.6 | |
| Heart weight/body weight ratio (mg/g) | Total | 6.5 ± 0.1 | 6.3 ± 0.2 | 6.1 ± 0.2 | 6.6 ± 0.3 | 6.9 ± 0.1 | 6.2 ± 0.2 |
| Female | 6.3 ± 0.1 | 6.3 ± 0.2 | 6.2 ± 0.3 | 6.1 ± 0.5 | 6.5 ± 0.1 | 5.9 ± 0.3 | |
| Male | 6.6 ± 0.1 | 6.3 ± 0.2 | 5.9 ± 0.4 | 7.1 ± 0.3 | 7.3 ± 0.2 | 6.3 ± 0.2 | |
| LA weight (mg) *median/IQR | Total | 3.7 ± 2.4 | 2.9 ± 1.2 | 4.0 ± 1.3 | 6.0 ± 2.2 | 3.5 ± 1.4 | 4.8 ± 2.5 |
| Female | 3.2 ± 1.8 | 2.6 ± 0.9 | 3.9 ± 1.3 | 5.4 ± 1.7 | 2.8 ± 1.3 | 4.5 ± 2.3 | |
| Male | 4.1 ± 2.4 | 3.3 ± 1.7 | 4.1 ± 1.8 | 6.8 ± 2.3 | 3.7 ± 1.6 | 5.1 ± 2.3 | |
Stated are means with percentages for sex distributions, means ± standard error of the mean for age, heart weight, and heart weight-to-body weight ratio (HWBW ratio), and median and interquartile range (IQR) for body weight and LA weight, respectively.
Due to strict quality standards and variability in protocols, not all measurements (LA-APD, atrial ERP, and IA-AT) were consistently available from all experiments. The number of conducted experiments in different genetic backgrounds and sex categories can be retrieved from Table 1. Table 2 summarizes all EP parameters and states numbers of available measurements for each parameter.
Table 2.
Reference values for left atrial action potential duration (LA-APD), atrial effective refractory period (ERP) and interatrial activation time (IA-AT) for five different genetic backgrounds
| PCL (ms) |
Left atrial action potential duration (LA-APD) (ms) |
Atrial effective refractory period (Atrial ERP) (ms) |
Interatrial activation time (IA-AT) (ms) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APD50 |
APD70 |
APD90 |
||||||||||||||
| 120 | 100 | 80 | 120 | 100 | 80 | 120 | 100 | 80 | 120 | 100 | 80 | 120 | 100 | 80 | ||
| Genetic background | C57BL/6 | 13 ± 0.4 | 12 ± 0.3 | 11 ± 0.3 | 19 ± 0.6 | 18 ± 0.5 | 16 ± 0.5 | 29 ± 0.9 | 29 ± 0.7 | 25 ± 0.7 | 26 ± 1.0 | 25 ± 0.9 | 26 ± 1.0 | 15 ± 0.4 | 15 ± 0.5 | 15 ± 0.6 |
| n = 76 | ||||||||||||||||
| FVB/N | 12 ± 1.2 | 11 ± 0.9 | 10 ± 0.8 | 22 ± 1.9 | 20 ± 1.9 | 16 ± 1.5 | 33 ± 2.5 | 29 ± 2.2 | 29 ± 1.6 | 36 ± 3.2 | 36 ± 2.9 | 36 ± 3.1 | 16 ± 2.3 | 17 ± 2.0 | 17 ± 2.0 | |
| n = 23 | ||||||||||||||||
| MF1 | 11 ± 0.8 | 10 ± 0.5 | 10 ± 0.5 | 17 ± 1.3 | 16 ± 0.9 | 16 ± 0.8 | 29 ± 2.0 | 27 ± 1.5 | 26 ± 1.3 | 30 ± 2.2 | 30 ± 2.3 | 30 ± 2.6 | 16 ± 0.8 | 17 ± 1.3 | 18 ± 1.3 | |
| n = 33 | ||||||||||||||||
| 129/Sv | 17 ± 1.6 | 16 ± 1.7 | 13 ± 1.4 | 23 ± 2.5 | 22 ± 2.3 | 19 ± 1.9 | 36 ± 3.3 | 36 ± 3.7 | 28 ± 2.7 | 27 ± 1.4 | 26 ± 1.4 | 26 ± 1.5 | 23 ± 1.8 | 24 ± 2.1 | 28 ± 3.6 | |
| n = 69 | ||||||||||||||||
| Swiss agouti | 9 ± 0.7 | 10 ± 0.8 | 14 ± 1.0 | 14 ± 1.0 | 24 ± 2.1 | 25 ± 2.1 | 19 ± 1.8 | 20 ± 2.2 | 17 ± 3.8 | 15 ± 1.4 | 16 ± 1.3 | 14 ± 2.5 | ||||
| n = 20 | ||||||||||||||||
| Total | Mean | 12 | 12 | 11 | 19 | 18 | 16 | 30 | 29 | 26 | 27 | 27 | 27 | 16 | 17 | 17 |
| SEM | 0.4 | 0.3 | 0.3 | 0.6 | 0.5 | 0.4 | 0.8 | 0.7 | 0.6 | 0.8 | 0.8 | 0.9 | 0.5 | 0.5 | 0.7 | |
| SD | 4.1 | 3.6 | 2.9 | 6.3 | 5.7 | 4.5 | 8.7 | 7.7 | 6.2 | 10.5 | 10.6 | 11.3 | 5.1 | 6.1 | 7.2 | |
| N | 122 | 132 | 116 | 124 | 135 | 115 | 119 | 123 | 109 | 166 | 186 | 168 | 110 | 122 | 100 | |
Mean values ± standard error of the mean for LA-APD50, LA-APD70, and LA-APD90, as well as atrial ERP and IA-AT are displayed in milliseconds for pacing cycle lengths (PCL) 80 ms, 100 ms, and 120 ms and five different genetic backgrounds and in summary, respectively.
Experimental conditions were constant over the timespan of data collection. Mean coronary flow rate was 4.0 ± 0.1 mL/min and temperature was 36.4 ± 0.1 °C without changes over time. Despite the decade long timespan for these experiments performed by different staff in two institutions, EP parameters did not vary over time. Slight variations in preparation time, flow rate or temperature did not significantly affect atrial electrophysiology in this analysis.
PCL-adaptation of murine LA-APD and characteristics of atrial ERP and IA-AT
Murine LA-MAPs showed a typical triangular shape with a steep upstroke during depolarization and a fast, early depolarization lacking a plateau (Figure 2A).13 Mean APD at 70% repolarization in the left atrium (LA-APD70) was 16 ± 0.4 ms at 80 ms PCL, 18 ± 0.5 ms at 100 ms PCL, and 19 ± 0.6 ms at 120 ms PCL (Table 2). APD (LA-APD50, LA-APD70, and LA-APD90) was prolonged at longer PCL (Figure 2B). Atrial APD70 prolonged by 11% (P < 0.0001) from 80 ms to 100 ms PCL, while prolongation from 100 ms to 120 ms PCL was only 5% (P < 0.0001) suggesting a non-linear PCL-adaptation of APD (Figure 2B, Supplementary material online, S2).
While LA-APD adapted to longer steady state PCL by LA-APD prolongation, atrial ERP was not PCL-dependent in the range of PCL tested and therefore ERP/APD90-ratio varied between 1.2 ± 0.1 at 80 ms and 1.0 ± 0.05 at 120 ms PCL (P < 0.0001, Figure 2B and D). Mean atrial ERP was 26 ± 1.0 ms at 80 ms PCL, 25 ± 0.9 ms at 100 ms PCL, and 26 ± 1.0 ms at 120 PCL.
IA-AT at 80 ms PCL was 17 ± 0.7 ms, 17 ± 0.5 ms at 100 ms PCL, and 16 ± 0.5 ms at 120 ms PCL (Table 2). IA-AT decreased by 5% from 80 ms to 100 ms PCL and by another 3% to 120 ms PCL, suggesting a linear correlation between PCL and IA-AT. IA-AT total decrease between 80 ms and 120 ms PCL was 7% (P < 0.0001, Figure 2C). Neither heart weight (HW, 6.5 ± 0.1 mg, Table 1) nor LA weight (LAW, 3.7 ± 2.4 mg, Table 1) showed significant effects on IA-AT in this study.
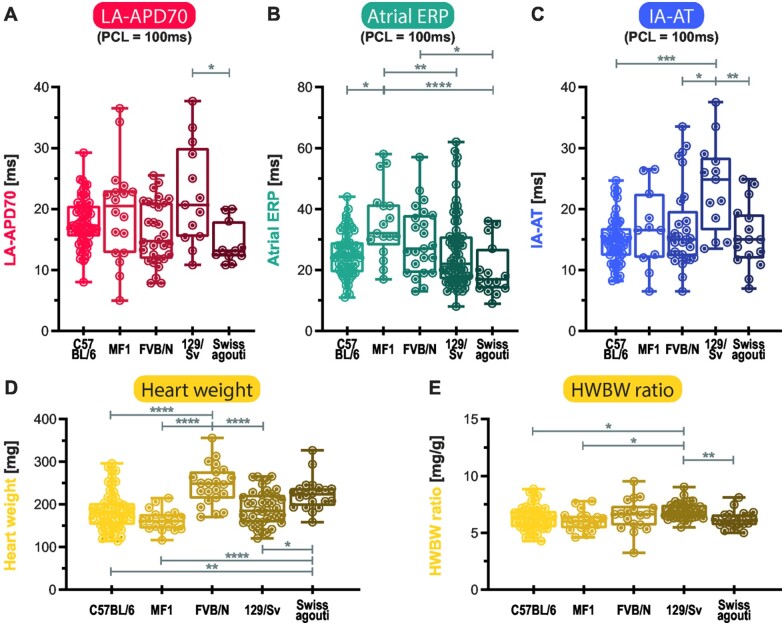
Murine genetic background affects atrial electrophysiology
Genetic background influenced atrial APD70 (P < 0.01). In Swiss agouti, atrial APD70 at 100 ms PCL was between 11% (vs. MF1, ns (not significant)) and 36% (vs. 129/Sv, P < 0.05) shorter compared to other genetic backgrounds (20% shorter vs. C57BL/6, ns, 27% shorter vs. FVB/N, ns, Figure 3A). Atrial ERP at 100 ms PCL was significantly different between genetic backgrounds (P < 0.0001) and shorter in Swiss agouti compared to FVB/N (−44%, P < 0.0001) and MF1 (−33%, P < 0.05). Atrial ERP was 37% longer in FVB/N compared to 129/Sv (P < 0.01) and 42% longer compared to C57BL/6 mice (P < 0.05, Figure 3B). IA-AT was longer in 129/Sv compared to other genetic backgrounds, +60% vs. C57BL/6 (P < 0.001), +53% vs. Swiss agouti (P < 0.01), and +41% vs. MF1 (P < 0.05, Figure 3C).
Figure 3.
Effects of genetic background on electrophysiological parameters and weights. Left atrial action potential duration at 70% repolarization (LA-APD70) (A), atrial effective refractory period (Atrial ERP) (B), interatrial activation time (IA-AT) (C), each at 100 ms pacing cycle length (PCL), as well as heart weight (D) and heart weight-to-body weight ratio (HWBW ratio) (E), are shown for each background as boxplots with min-to-max-whiskers and statistically tested dependent on whether they were in or could be transformed to Gaussian distribution by one-way ANOVA or alternatively Kruskal–Wallis test. All tested electrophysiological parameters, as well as mouse and heart weights were background-dependent. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Genetic background also influenced BW, HW, and LAW (all P < 0.0001, Table 1): MF1 and Swiss agouti mice were between 17% and 51% heavier than C57BL/6, FVB/N, and 129/Sv mice and hearts, respectively (Figure 3D). LAW was higher in MF1 mice compared to C57BL/6 (107%, P < 0.0001), FVB/N (52%, P < 0.01), 129/Sv (71%, P < 0.0001), and higher in Swiss agouti compared to C57BL/6 (66%, P < 0.01). In contrast, heart weight/body weight (HWBW) ratios were much more stable between different genetic backgrounds: only 129/Sv mice had slightly higher HWBW ratios compared to C57BL/6 (+9%, P < 0.05), FVB/N (+14%, P < 0.05), and Swiss agouti (+12%, P < 0.01) genetic backgrounds (Figure 3E).
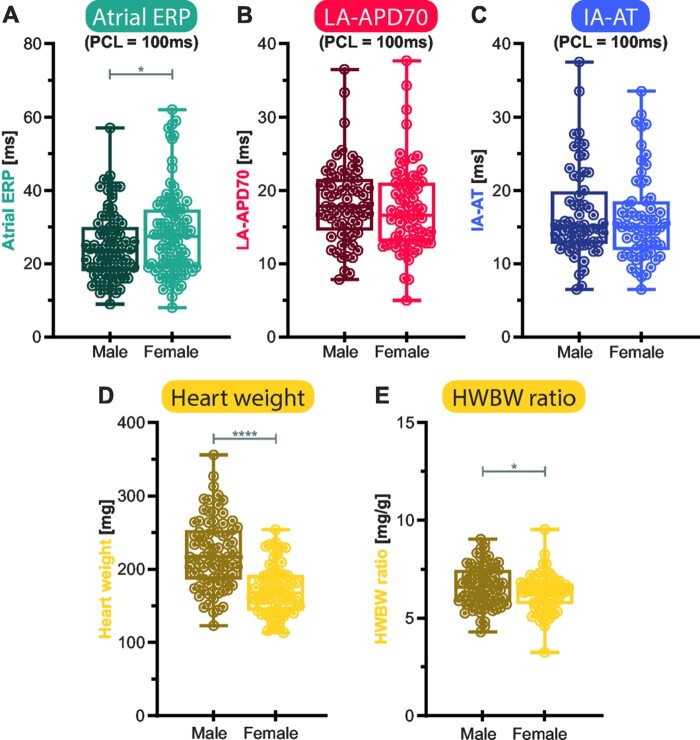
Sex affects atrial ERP
Considering the whole dataset using a univariate approach, mean atrial ERP at 100 ms PCL was 25 ± 1 ms in male mice compared to 28 ± 1 ms in females, and therefore 14% longer in females (P < 0.05, Figure 4A). This effect was tested independent of genetic background and age. LA-APD and IA-AT were not affected by sex in the range of PCL tested in this study (Figure 4B and C).
Figure 4.
Sex-specific differences in left atrial electrophysiology, heart weight, and heart weight-to-body weight ratio (HWBW ratio). Atrial effective refractory period (ERP), (A), Left atrial action potential duration (LA-APD70), (B) and interatrial activation time (IA-AT), (C) as electrophysiological parameters, as well as heart weight (HW), (D) and HWBW ratio (E) were compared between both sex-categories by unpaired Student’s t-test. Atrial ERP varied between female and male animals with a 14.5% prolongation in females. The other tested electrophysiological values did not vary significantly between sex categories in this study. HW was higher in males. Displayed are boxplots with min-to-max-whiskers. *P < 0.05, ****P < 0.0001.
Male HW and LAW was 29% and 28% (P < 0.0001 and P < 0.05) heavier than in females respectively, whilst BW of males was 25% larger (P < 0.0001, Figure 4D). Sex differences in HW were most pronounced in the 129/Sv background (39% higher in males), whilst HW differences were small in FVB/N mice (14% higher). HWBW ratio was different between male and female mice (5%, P < 0.05, Figure 4E), while LAW/BW ratio was not.
Age affects atrial ERP
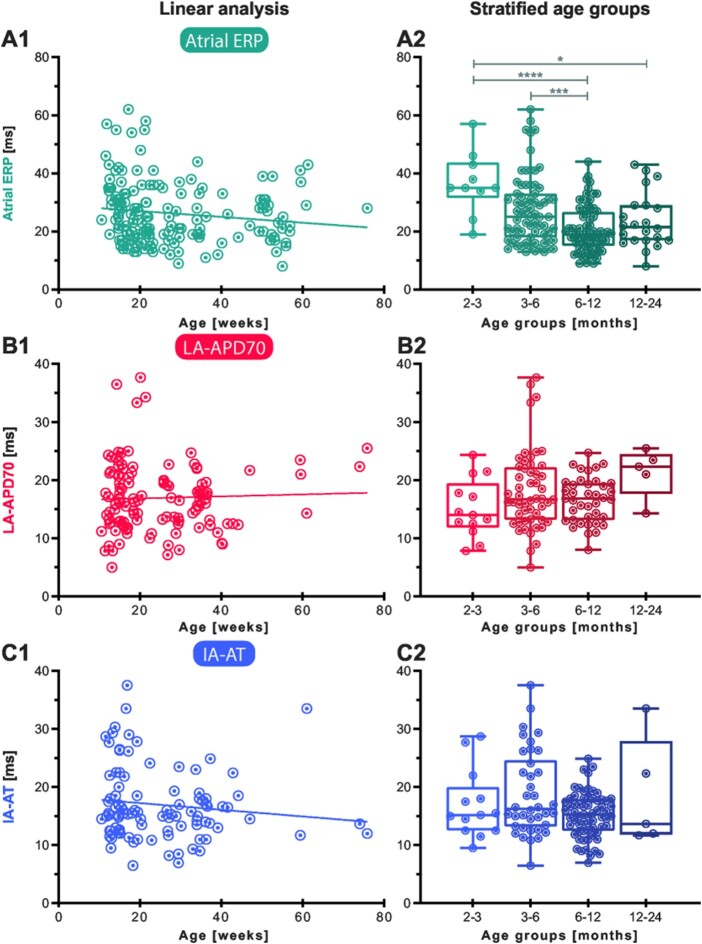
With age as a continuous variable, neither IA-AT, LA-APD, nor atrial ERP showed a clear linear correlation in this cohort. However, there was a trend towards shorter atrial ERP in older mice (Figure 5A1). For further analysis, experiments were clustered in four age groups: hearts from young adult (2–3 months), mature adult (3–6 months), middle-aged (6–12 months), and old mice (12–24 months). Stratified, the dataset revealed age-dependent shortening of atrial ERP at 100 ms PCL comparing young mice between 2 and 3 months of age to older groups (P < 0.0001 vs. 6–12 months and P < 0.05 vs. 12–24 months, Figure 5A2). Also, mature adults had higher atrial ERPs compared to middle-aged mice (P < 0.001, Figure 5A2). LA-APD and IA-AT were neither affected by age in a linear correlation nor categorical age groups in this study (ANOVA P > 0.05, Figure 5B and C).
Figure 5.
Continuous and stratified effects of age on left atrial action potential duration (LA-APD), atrial effective refractory period (ERP), and interatrial activation time (IA-AT). Graphs in the left column show atrial ERP (A1), LA-APD70 (B1), and IA-AT (C1), each at 100 ms pacing cycle length (PCL), plotted over mouse age in weeks at the time of experiment and results of linear regression. Linear regression was not statistically significant for all three parameters. Mice were then stratified in age groups (A2, B2, C2) and atrial ERP was found to be affected by age group (A2; ANOVA P < 0.0001). Age group results are shown as boxplots with min-to-max-whiskers. In Bonferroni-corrected post hoc testing, atrial ERP at 100 ms PCL was increased in younger groups (<3 months and 3–6 months) compared to older groups (6–12 and 12–24 months). *P < 0.05, ***P < 0.001, ****P < 0.0001.
Discussion
The analysis provides a robust reference for normal murine atrial EP parameters based on a dataset of over 200 isolated ex vivo Langendorff-perfused beating WT hearts from five different genetic strains (Table 2). Experiments were performed over a decade without systematic changes in parameters, confirming the robustness of the experimental setup. Detailed interrogation of the dataset identified the following findings:
Different genetic backgrounds have a distinct EP footprint: C57BL/6 and Swiss agouti show a smaller APD/ERP ratio. 129/Sv show markedly longer IA-AT and longer LA-APDs.
Pacing at shorter PCL shortens LA-APD.
IA-AT increases with shorter atrial PCL, suggesting reduced conduction velocity at shorter PCL.
Female hearts show longer atrial ERPs than male hearts.
Atrial ERP is shorter in old hearts.
BW, HW, or left atrial weight do not affect atrial EP parameters in WT murine hearts when mice were kept on normal chow diet.
Genetic background strain modifies murine LA-APD, atrial ERP, and IA-AT
We observed a distinct atrial EP profile for each different murine genetic background concordant to findings in ventricular myocardium. Similar to our report of left atrial parameters, ventricular APD was longer in FVB/N mice compared to C57BL/6 in an earlier study.6 Comparisons of cardiac electrophysiology between different genetic backgrounds are still limited. However, several studies found inter-strain differences in cardiac electrophysiology.6,14,15
Expression differences in ultra-rapid delayed rectifier K+ currents between male CD-1 and male C57BL/6 mice were suggested to cause differences in repolarization between these two strains and are thought to be caused by different testosterone levels.16 The occurrence of ventricular arrhythmias varied in a comparison between four inbred strains and were postulated to be caused by different beta-adrenergic responses as observed by others,17 intracellular calcium handling, ion channel expression, and phosphorylation.14 Of note, our study was conducted in isolated beating hearts ex vivo. Therefore, differences in tone of the autonomic nervous system are unlikely to account for genetic background-related differences.
In a study comparing strain-specific differences in sodium currents in cardiomyocytes, sodium currents in neonatal cardiomyocytes varied between C57BL/6, FVB/N, and 129/Sv genetic background.18 Remme et al.19 found an mRNA and protein reduction of the cardiac sodium channel beta subunit in ventricles from the 129 genetic background compared to FVB/N associated with changes in activation kinetics. A trend to differential atrial expression of Kcnc4 between MF1, Swiss agouti, and CD-1 strains was observed.20
Our study provides systematic data on genetic background-dependent differences in atrial EP parameters measured is isolated hearts ex vivo, providing a reference for their interpretation and highlighting the importance of genetic background for the design and interpretation of research in murine models. Further research into the molecular mechanisms is warranted.
Age- and sex-dependency of atrial murine electrophysiological parameters
This study found shorter atrial ERP at older age (Figure 5). While the distribution of sex categories was balanced within each age group, the different genetic backgrounds were not. As genetic background also influenced atrial ERP in isolated testing (Figure 3B), it cannot be excluded as a confounder of the observed effect. Multifactorial testing was not possible due to the limited number of experiments available. It was recently postulated that atrial APs would be prolonged by age based on differences in ion channel expression in human atrial tissue.21 Our study did not find relevant age-dependent changes in LA-APD in the range of PCLs studied. Longer ventricular APDs and ERPs in female mice6 have been attributed to different concentrations of repolarizing ion channels related to sex hormone concentrations.16 In line with this, progesterone was shown to elicit an AP-prolonging effect on hearts of ovariectomized mice.22 In our study, female mice had a longer atrial ERP. It is not unlikely that a small effect of sex on LA-APD was masked by the greater variability of LA-APD compared to atrial ERP (Figure 4).
We analyzed combined effects of genetic background and sex on LA-APD70, atrial ERP, and IA-AT at 100 ms PCL in a two-way ANOVA with the knowledge that subgroups within this large dataset are still limited in size to perform robust multifactorial analysis. Two-way ANOVA was significant for all variables when combining genetic background and sex within the model as described in detail in Supplementary material online, S3. It is likely that combinations of the factors genetic background, sex, and age have effects beyond those found in univariate analysis. Proof of this hypothesis would require verification in an even larger dataset.
Cycle-length-dependency of murine LA-APD and IA-AT
Murine left atrial APs have not been characterized in a dataset as large as this before. However, different sites of the heart, even within the same chamber, have distinct EP profiles and functions and therefore require separate characterization in health and disease.5,11 The durations seen in this analysis [e.g. LA-APD50 (12 ± 0.4 ms), LA-APD70 (18 ± 0.6 ms), and LA-APD90 (29 ± 0.8 ms) at 120 ms PCL] are in the same range as found in a previous study on 21 isolated, murine Langendorff-perfused hearts of unknown genetic background in a perpendicular setup using miniature contact MAP electrodes at a PCL of 130 ms13 and are comparable to APDs recorded using optical mapping.10–12 The mean values for IA-AT and atrial ERP presented here are also comparable to those reported from in vivo transvenous stimulation recordings.23
BW, HW, and LAW, or weight ratios, did not affect atrial EP parameters in this WT cohort with only minor variations in normal weight on a standard chow diet. Thus, normal ranges of HW and BW do not induce electrical signs of atrial AP-prolonging effects of hypertrophy.6 In contrast, obesity has been associated with changes in atrial electrophysiology and found to cause atrial remodelling.24
Rate-dependent shortening of APD and slowing of conduction velocity is a well-known feature of the myocardium and is vital to retain a sufficient diastole at faster heart rates. However, alterations in rate adaptation are hypothesized to be implicated in genesis of cardiac arrhythmias.25 Shortening of APDs at shorter PCL as observed in this study in the murine left atrium was also seen in other studies.11,12 A constant atrial ERP/APD ratio over a range from 300 ms to 700 ms PCL was reported in human RA,26 while this study observed adaptation of LA-APD, but not atrial ERP, to PCL in the range of PCL tested, leading to slight variation in ERP/APD-ratios. The lack of murine left atrial ERP shortening with shorter PCL in this study may be caused by the observed increase in IA-AT from right atrial pacing site to LA MAP recording site with shorter PCL or by the short range of tested PCLs in the current atrial experimental setting. Of note, a similar flat PCL-ERP relationship was observed in murine ventricles over a range of PCLs from 100 to 300 ms.6
Limitations
This study reports a resource for murine left atrial EP parameters based on a large dataset and is therefore relatively robust. Influences of age, sex, and genetic background on EP parameters were found in univariate analysis and influences of animal weights were reported earlier.6 Although containing over 200 experiments, the dataset was too small to detect minute differences and to perform a robust multifactorial multivariate analysis and regression models.
Effects of BW and HW on atrial electrophysiology were smaller than those found on ventricular electrophysiology using a similar approach.6 Although this dataset is limited by only few animals being studied after senescence, shortening effects of age on atrial ERP were observed. To make robust statements of influences of ageing on left atrial electrophysiology, a study including animals from a large and continuous age range would be ideal.
While the EP isolated heart investigation offers a wide range of opportunities partially described in this article and has been lauded for animal welfare aspects, the EP study can also be performed in vivo27 and it may be particularly intriguing to perform an EP study in vivo and subsequently in the isolated heart.
This study did not require further sacrifice of animals, as the dataset is based on our group’s previously collected data comparing effects of disease modelling genetic mutations to WT littermate controls.
Conclusion
This study provides unparalleled, robust reference values for murine LA-APD, atrial ERP, and IA-AT based on 221 WT Langendorff-perfused murine hearts. The dataset identifies effects of genetic background, sex, and age on atrial EP parameters. The effects of genetic background are at least of the magnitude as those of age and sex in this model, illustrating the impact of genetic background on atrial electrophysiology. Reported findings can guide the planning of experimental design and interpretation of murine EP research.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors would like to thank Lisa Fortmüller, Nina Kreienkamp, and Daniela Volkery for expert help. The authors also thank Winnie Chua for statistical discussion of data.
Funding
This work was partially supported by the European Commission [633196 ‘CATCH ME’] to L.F. and P.K., Deutsche Forschungsgemeinschaft (DFG) [FA413] to L.F., British Heart Foundation (BHF) [FS/13/43/30324, AA/18/2/34218] to L.F. and P.K., and Leducq Foundation to P.K.; J.O. has received financial support for abroad studies within his scholarship of the Studienstiftung des deutschen Volkes (German Academic Scholarship Foundation).
Conflict of interest: L.F. has received institutional research grants from governmental and charity funding agencies (EU, DFG, MRC, and BHF). L.F. has received institutional research grants from industry in the past (Gilead). P.K. and L.E. have received research support from several drug and device companies active in AF and have received honoraria from several such companies in the past. L.F. and P.K. are listed as inventors on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 015140571, Markers for Atrial Fibrillation WO 2016012783).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A. et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 2. Yarnoz MJ, Curtis AB.. More reasons why men and women are not the same (gender differences in electrophysiology and arrhythmias). Am J Cardiol 2008;101:1291–6. [DOI] [PubMed] [Google Scholar]
- 3. Kloosterman M, Chua W, Fabritz L, Al-Khalidi HR, Schotten U, Nielsen JC. et al. Sex differences in catheter ablation of atrial fibrillation: results from AXAFA-AFNET 5. Europace 2020;22:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fabritz L, Crijns H, Guasch E, Goette A, Haeusler KG, Kotecha D. et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA Consensus Conference. Europace 2020; doi:10.1093/europace/euaa279. [DOI] [PubMed] [Google Scholar]
- 5. Riley G, Syeda F, Kirchhof P, Fabritz L.. An introduction to murine models of atrial fibrillation. Front Physiol 2012;3:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waldeyer C, Fabritz L, Fortmueller L, Gerss J, Damke D, Blana A. et al. Regional, age-dependent, and genotype-dependent differences in ventricular action potential duration and activation time in 410 Langendorff-perfused mouse hearts. Basic Res Cardiol 2009;104:523–33. [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld HH. et al. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 2011;4:123–33. [DOI] [PubMed] [Google Scholar]
- 8. Blana A, Kaese S, Fortmuller L, Laakmann S, Damke D, van Bragt K. et al. Knock-in gain-of-function sodium channel mutation prolongs atrial action potentials and alters atrial vulnerability. Heart Rhythm 2010;7:1862–9. [DOI] [PubMed] [Google Scholar]
- 9. Kirchhof P, Fabritz L, Fortmuller L, Matherne GP, Lankford A, Baba HA. et al. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol 2003;285:H145–153. [DOI] [PubMed] [Google Scholar]
- 10. Yu TY, Syeda F, Holmes AP, Osborne B, Dehghani H, Brain KL. et al. An automated system using spatial oversampling for optical mapping in murine atria. Development and validation with monophasic and transmembrane action potentials. Prog Biophys Mol Biol 2014;115:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmes AP, Yu TY, Tull S, Syeda F, Kuhlmann SM, O’Brien S-M. et al. A regional reduction in Ito and IKACh in the murine posterior left atrial myocardium is associated with action potential prolongation and increased ectopic activity. PLoS One 2016;11:e0154077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Syeda F, Holmes AP, Yu TY, Tull S, Kuhlmann SM, Pavlovic D. et al. PITX2 modulates atrial membrane potential and the antiarrhythmic effects of sodium-channel blockers. J Am Coll Cardiol 2016;68:1881–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz MR.. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol 2007;292:H614–621. [DOI] [PubMed] [Google Scholar]
- 14. Jelinek M, Wallach C, Ehmke H, Schwoerer AP.. Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of beta-adrenergic stimulation. Sci Rep 2018;8:2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI.. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol Genomics 2003;15:84–91. [DOI] [PubMed] [Google Scholar]
- 16. Brouillette J, Rivard K, Lizotte E, Fiset C.. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res 2005;65:148–57. [DOI] [PubMed] [Google Scholar]
- 17. Berthonneche C, Peter B, Schupfer F, Hayoz P, Kutalik Z, Abriel H. et al. Cardiovascular response to beta-adrenergic blockade or activation in 23 inbred mouse strains. PLoS One 2009;4:e6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mille M, Koenig X, Zebedin E, Uhrin P, Cervenka R, Todt H. et al. Sodium current properties of primary skeletal myocytes and cardiomyocytes derived from different mouse strains. Pflugers Arch Eur J Physiol 2009;457:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Remme CA, Scicluna BP, Verkerk AO, Amin AS, van Brunschot S, Beekman L. et al. Genetically determined differences in sodium current characteristics modulate conduction disease severity in mice with cardiac sodium channelopathy. Circ Res 2009;104:1283–92. [DOI] [PubMed] [Google Scholar]
- 20. Kahr PC, Piccini I, Fabritz L, Greber B, Scholer H, Scheld HH. et al. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One 2011;6:e26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biliczki P, Boon RA, Girmatsion Z, Bukowska A, Ordog B, Kaess BM. et al. Age-related regulation and region-specific distribution of ion channel subunits promoting atrial fibrillation in human left and right atria. Europace 2019;21:1261–9. [DOI] [PubMed] [Google Scholar]
- 22. Saba S, Zhu W, Aronovitz MJ, Estes NA 3rd, Wang PJ, Mendelsohn ME. et al. Effects of estrogen on cardiac electrophysiology in female mice. J Cardiovasc Electrophysiol 2002;13:276–80. [DOI] [PubMed] [Google Scholar]
- 23. Kaese S, Verheule S.. Cardiac electrophysiology in mice: a matter of size. Front Physiol 2012;3:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JP. et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 25. Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH. et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA 2000;97:6061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bode F, Kilborn M, Karasik P, Franz MR.. The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias. J Am Coll Cardiol 2001;37:920–5. [DOI] [PubMed] [Google Scholar]
- 27. Gehrmann J, Berul CI.. Cardiac electrophysiology in genetically engineered mice. J Cardiovasc Electrophysiol 2000;11:354–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.