Abstract
We conducted a comparative retrospective study to quantify the impact of coronavirus disease 2019 (COVID-19) on patient safety. We found a statistically significant increase in central-line–associated bloodstream infections and blood culture contamination rates during the pandemic. Increased length of stay and mortality were also observed during the COVID-19 pandemic.
Central-line–associated bloodstream infections (CLABSIs) pose a significant burden to healthcare systems. CLABSIs are associated with an increased length of stay (LOS) by 14 days, increased morbidity and mortality by 12%–25%, and $46,000 in excess cost per case.1 To reduce CLABSIs, hospitals have implemented prevention bundles that focus on central venous catheter (CVC) insertion and maintenance practices. The available data indicate that such endeavors have been tremendously successful.2,3
Although hospitals have attempted to maintain best infection control practices, the coronavirus disease 2019 (COVID-19) pandemic has presented unique challenges, such as continuously changing recommendations, patient surges, and resource shortages.4 Research evaluating the consequences of the COVID-19 pandemic on infection control metrics is limited. In an attempt to quantify the impact, we compared CLABSI rates, blood culture contamination rates, mortality rates, and LOS during the pandemic to those before the crisis.
Methods
Study design
We conducted a comparative retrospective cohort study at an academic tertiary-care center in Detroit, Michigan, to examine blood culture contamination and CLABSI rates between a pre–COVID-19 cohort and a “during” COVID-19 cohort. The pre–COVID-19 period was defined as January–May 2019 and the COVID-19 period was defined as January–May 2020. Patients aged <18 years were excluded. Our institutional review board approved this study.
Data collection
TheraDoc Infection Control Surveillance System identified positive blood cultures and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test results. The Infection Control Department reviewed all positive blood cultures alongside the National Healthcare Safety Network (NHSN) Patient Safety Manual to determine whether a patient with a positive blood culture met CLABSI criteria.5 CLABSI rates were calculated as CLABSIs per 1,000 central-line days. The following information collected for patients with a confirmed CLABSI by medical record review: demographics, SARS-CoV-2 nasopharyngeal test results, Charlson comorbidity index,6 LOS, and cause of death. Additionally, the NHSN standardized infection ratio model was used to compare 2019 CLABSI rates to those of hospitals with similar profiles.5
Blood cultures that only grew Bacillus (excluding B. anthracis), Corynebacterium (excluding C. diphtheria), Cutibacterium acnes, coagulase-negative Staphylococcus, or α-hemolytic Streptococcus (excluding S. pneumoniae) without a repeat blood culture positive for the same organism in the subsequent 4 days were considered contaminated.
Analysis
Patients were divided into cohorts based on the date of infection relative to COVID-19. We used the Fisher exact test and 2-tailed Wilcoxon signed-rank test for analysis. A P value < .05 was considered statistically significant. SAS software (SAS Institute, Cary, NC) was used for calculations.
Results
Blood culture contamination rate
Blood culture contamination rates increased from 3.2% in the pre–COVID-19 cohort (546 of 16,984) to 3.8% (689 of 18,344) in the COVID-19 cohort (P < .01). The blood culture contamination rate peaked at 4.4% in April 2020, coinciding with greatest number of COVID-19 patients (Supplementary Figs. 1 and 2 online).
CLABSI rate
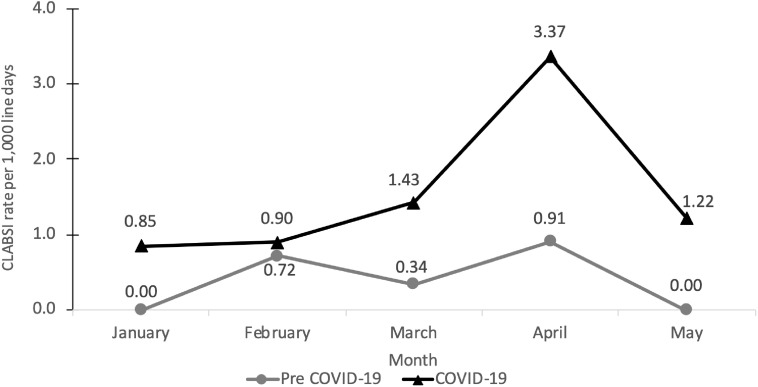
Of the 36 patients who developed CLABSIs, 6 patients (17%) were in the pre–COVID-19 cohort and 30 patients (83%) were in the COVID-19 cohort. The average monthly CLABSI rate increased from 0.40 before COVID-19 to 1.7 during COVID-19 (P < .01) (Fig. 1). Increases in the CLABSI rate correlated with increases in the COVID-19 case load. April 2020 had both the highest CLABSI rate and the highest incidence of COVID-19 patients (Fig. 1 and Supplementary Fig. 2 online). Notably, resistance patterns of organisms isolated from blood cultures associated with CLABSIs did not share similar patterns among patients. Additionally, when comparing the 2019 CLABSI rate to similar hospitals using the NHSN model, the data indicated that rates were 25% lower than expected. The NHSN has not published 2020 data.7
Fig. 1.
Central-line–associated bloodstream infections per 1,000 central-line days between January through May. The pre–COVID-19 cohort had 6 central-line bloodstream infections (CLABSIs) per 15,026 central-line days, and the COVID-19 cohort had 30 CLABSIs per 18,106 central-line days. The change in the CLABSI rate was statistically significant at P < .05.
Length of stay and mortality
Patients within the pre–COVID-19 cohort who developed a CLABSI had a median LOS of 19 days compared to patients of the COVID-19 cohort, who had a median LOS of 27 days (P = .12) (Table 1). Among the 30 patients with CLABSIs identified within the COVID-19 cohort, 16 (53.3%) died, compared to 2 of 6 (33.3%) in the pre–COVID-19 period (P = .66) (Table 1).
Table 1.
Baseline Patient Characteristics Between Two Cohorts
| Characteristics | Pre–COVID-19 Cohort (N=6) |
COVID-19 Cohort (N=30) |
P Value |
|---|---|---|---|
| Age, median (IQR) | 60.3 (17.3) | 62.6 (26.1) | 0.92 |
|
Race, no. (%)
Black White Other/Unknown |
4 (66.7) 1 (16.7) 1 (16.7) |
18 (60.0) 6 (20.0) 6 (20.0) |
1.0 |
| Females, no. (%) | 3 (50.0) | 17 (56.7) | 1.0 |
| Expired, no. (%) | 2 (33.3) | 16 (53.3) | 0.66 |
| Sepsis as primary cause of death, no. (%) | 2 (100.0) | 6 (37.5) | 0.18 |
|
Charlson comorbidity index, no. (%) 0–1 2–3 4–5 >5 |
1 (16.7) 3 (50.0) 1 (16.7) 1 (16.7) |
9 (30.0) 7 (23.3) 4 (13.3) 10 (33.3) |
0.64 |
|
Type of central venous catheter
a, no. (%) Peripherally inserted central venous catheter Internal jugular Mediport Femoral Subclavian |
2 (33.3) 4 (66.7) 0 (0.0) 0 (0.0) 0 (0.0) |
5 (15.6) 13 (40.6) 1 (3.1) 7 (21.9) 6 (18.8) |
0.48 |
|
Central venous catheter insertion location, no. (%) Emergency department Floor (acute care or intensive care unit) Interventional radiology or operating room Present on admission |
0 (0.0) 4 (66.7) 2 (33.3) 0 (0.0) |
4 (13.3) 14 (46.7) 11 (36.7) 1 (3.3) |
0.87 |
| Intensive care unit, no. (%) | 3 (50.0) | 21 (70.0) | 0.38 |
| Vasopressors, no. (%) | 3 (50.0) | 22 (73.3) | 0.34 |
| Ventilator, no. (%) | 2 (33.3) | 21 (70.0) | 0.16 |
| Bilevel positive air pressure, no. (%) | 2 (33.3) | 4 (13.3) | 0.26 |
| Length of stay, median (IQR) | 19.0 (9.0) | 27.0 (33.0) | 0.12 |
| Causative organism from blood culture associated with CLABSI, no. (%) | 0.22 | ||
| Fungal Gram negative Gram positive |
4 (66.7) 0 (0.0) 2 (33.3) |
8 (26.7) 6 (20.0) 16 (53.3) |
|
Note. COVID-19, coronavirus 2019; IQR, interquartile range; CLABSI, central-line–associated bloodstream infection.
2 patients had multiple central venous catheters.
Discussion
Blood culture contamination rates were 19% higher during the COVID-19 period. Even though the blood culture policy did not change, several nurses acknowledged the following lapses in infection control practices: (1) using skin disinfectant for less time than the manufacturer’s recommendation, (2) collecting serial cultures from the same site, and/or (3) failing to collect multiple blood cultures. Moreover, staff disclosed that specimens were frequently obtained from CVCs to decrease time in patient rooms as collecting cultures through a CVC is easier and quicker. Studies demonstrate that such collection practices are associated with lower-quality cultures and higher contamination rates.8 The most common reason cited by staff for deviations from best practices was limited time secondary to staffing shortages.
Additionally, the CLABSI rate during the pandemic increased 325%. Notably, 8 CLABSIs (26.7%) within the COVID-19 cohort occurred during the 5 days following insertion compared to 1 (16.7%) in the pre–COVID-19 cohort (P = 1.0) (Table 1). Although these results are not statistically significant, data exist to implicate timing of infection as clinically relevant. CLABSIs that develop within 5 days following CVC placement are likely caused by deviations from sterile technique during insertion and/or suboptimal site placement.9 Of the 6 CLABSIs in the pre–COVID-19 cohort, none were placed in the emergency department, compared to 4 (13.3%) in COVID-19 cohort (P = .87) (Table 1). CVCs inserted during emergent circumstances are less likely to be sterile because the urgency for lifesaving treatment exceeds aseptic technique.3 Moreover, 7 patients (21.9%) within the COVID-19 cohort had a femoral line, compared to none in the pre–COVID-19 cohort (P = .48) (Table 1). Physicians likely chose the femoral vein for placement to decrease time exposed to patients. Femoral CVC placement is quicker because ultrasound guidance is rarely required and the femoral vein is further away from the patient’s mouth and nose compared to internal jugular and subclavian veins. Although such variations were made in an effort to decrease the risk of SARS-CoV-2 exposure, national guidelines still discourage the use of femoral line placements.3
Notably, 18 (60%) patients from COVID-19 cohort tested positive for SARS-CoV-2, and studies show that patients with COVID-19 have an increased probability of developing secondary infections.10 However, if patients with COVID-19 were excluded from analysis, the CLABSI rate still increased from 0.40 to 0.77 per 10,000 central-line days, representing a 194% increase. Therefore, it is likely that increased demands placed on the healthcare system, regardless of inclusion of COVID-19 patients, contributed to the increased incidence of CLABSIs.
Our study has several limitations. First, our cohort size was small, especially for the period before the COVID-19 pandemic. Additionally, no audits were conducted on CVC insertion and maintenance to determine adherence to CLABSI prevention bundles; the lapses in infection control metrics were anecdotal evidence provided by clinicians. We were unable to compare our 2020 CLABSI rates to similar hospitals because the NHSN opted against collecting these data from January through June 2020 due to the COVID-19 pandemic.7
These data demonstrate higher rates of blood culture contamination and CLABSIs during the pandemic. Both rates reached peaks in April 2020, when the hospital’s COVID-19 case load was greatest. Reasons for such increases are likely attributed to stresses placed on the healthcare system, resource shortages, and consistent surges of high-acuity patients.4 The present report justifies greater investment in infection prevention to accommodate patient quality-care needs during a pandemic.
Acknowledgments
We are grateful to Barbara Washington for assistance with data collection.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2020.1335.
click here to view supplementary material
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Haddadin Y, Annamaraju P, Regunath H. Central-line–associated blood stream infections (CLABSIs). NCBI Resources website. https://www.ncbi.nlm.nih.gov/books/NBK430891/. Published 2020. Accessed July 25, 2020, 2020.
- 2. Furuya EY, Dick AW, Herzig CTA, Pogorzelska-Maziarz M. Central-line–associated bloodstream infection reduction and bundle compliance in intensive care units: a national study. Infect Control Hosp Epidemiol 2016;37:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Grady N, Alexander M, Dellinger PE, et al. Guidelines for the prevention of intravascular catheter related infections. Clin Infect Dis 2011;52:162–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adalja AA, Toner E, Inglesby TV. Priorities for the US health community responding to COVID-19. JAMA 2020;323:1343–1344. [DOI] [PubMed] [Google Scholar]
- 5. National Healthcare Safety Network (NHSN) Patient Safety Component Manual. Centers for Disease Control and Prevention website. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Published 2020. Accessed September 15, 2020.
- 6. Charlson M, Peterson J, Szatrowski TP, Mackenzie R, Gold J. Long-term prognosis after perioperative cardiac complications. J Clin Epidemiol 1994;47:1389–1400. [DOI] [PubMed] [Google Scholar]
- 7.Medicare and Medicaid Programs, Clinical Laboratory Improvement Amendments (CLIA), and Patient Protection and Affordable Care Act, additional policy and regulatory revisions in response to the COVID-19 public health emergency 2020. Government Information website. https://www.govinfo.gov/content/pkg/FR-2020-09-02/pdf/2020-19150.pdf. Published 2020. Accessed September 15, 2020.
- 8. Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control 2015;43:1222–1237. [DOI] [PubMed] [Google Scholar]
- 9. Davis J. Central-line–associated bloodstream infection: comprehensive, data-driven prevention. Patient Saf Advis 2011;8:100–105. [Google Scholar]
- 10. Langford BJ, So M, Raybardhan S, Leung V. Bacterial coinfection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2020.1335.
click here to view supplementary material