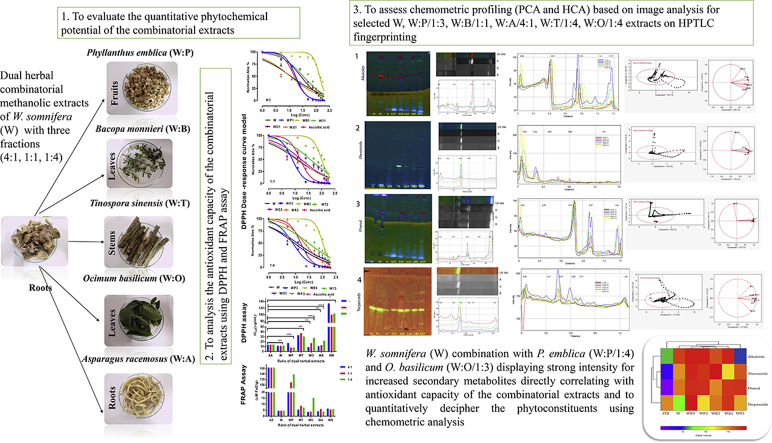
Abstract
Background
Traditional medicine adequately emphasis plant resources for addressing a wide variety of human ailments by utilizing the naturally occurring phytoconstituents; in particular medicinal plants or parts of plants in combination have prodigious antioxidant potentials.
Objective
The present study aims to analyze methanolic extract of W. somnifera (W) individually, and in dual combination with five Rasayana herbs P. emblica - (W:P), B. monnieri - (W:B), T. sinensis - (W:T), O. basilicum - (W:O), A. racemosus - (W:A) in three dual ratios [4:1, 1:1, and 1:4]. The efficacy of the combinations is assessed based on their chemometric profiling.
Material and methods
A total of 15 dual combinatorial methanolic extracts together with W. somnifera were evaluated for their preliminary phytochemical profiles, antioxidant potentials using DPPH and FRAP assays. Five dual samples were selected and analyzed for High-Performance Thin-Layer Chromatography (HPTLC) image-based chemometric profiling followed by Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)-Heatmaps.
Results
Qualitative phytochemical analysis of combinatorial extracts exhibited a richness for a variety of phytoconstituents. The antioxidant activity was significantly higher for DPPH IC50 (μg/mL): W = 11.56 ± 3.69; W:P/1:4 = 7.89 ± 1.52; W:O/1:4 = 8.995 ± 2.64 and FRAP (μM FeE/g): W = 4.56 ± 0.54; W:P/1:4 = 138.34 ± 9.25; W:O/1:4 = 15.32 ± 1.64. Chemometric data acquisition displayed improved secondary metabolite close cluster combination with W:O/1:4 and W:P/1:4 than W. somnifera (W) alone.
Conclusion
The dual herbal combinatorial study revealed that the methanolic combinatorial extracts phytoconstituents correlated with an increase in the antioxidant potential and would serve as a promising source for phytomedicine.
Keywords: Withania somnifera, Antioxidants, Chemometric, Secondary metabolites, Dual herbal combination
Graphical abstract
Highlights
-
•
Dual herbal combination of W. somnifera (W) in ratios (4:1, 1:1, 1:4) with P. emblica (W:P), B. monnieri (W:B), T. sinensis (W:T), O. basilicum (W:O), A. racemosus (W:A).
-
•
Combinatorial extracts expressed different levels of antioxidant capacity.
-
•
Strong intensity, better clustering pattern for combinatorial extracts.
-
•
Antioxidant potential correlates with improved characterization.
1. Introduction
Plants and plant-derived metabolites are treasured resources for the production of new drugs that possess unique properties for a variety of medicinal purposes. Recently, plant-derived drugs have led to a shift from the universal trend of synthetic medicines to herbal medicines [1]. WHO (World Health Organisation) 2019 monograph refers Traditional medicine (TM)/Complementary and Alternative Medicine (CAM) to both indigenous medicines and Indian Ayurveda that includes herbal medicines in the form of herbs, herbal materials, herbal preparations, and finished herbal products, that contains parts of the plant, plant materials or combinations thereof as active ingredients [2]. Free radicals and reactive oxygen species (ROS) are chemical species produced by chemical reactions and metabolic processes in the cells. Free radicals can initiate oxidation of biomolecules leading to cell injury and can persuade numerous diseases in humans [3]. Naturally, rich antioxidant phytochemicals protect the cells from the harmful effects of free radicals and other oxidants hence serving as preventive molecules [[4], [5], [6]]. Medicinal plants synthesize characteristic active antioxidants in the form of bioactive substances like alkaloids, terpenoids, carbohydrates, tannins, steroids, phenol, and flavonoids which produce definite biological action against certain human ailments [7,8].
Withania somnifera, the revered herb of Indian Ayurvedic medicine known as “Rasayana”, nervine tonic acts as a major adaptogen among the medicinal plants [9]. The chemical characterization of roots depicts 12 alkaloids, 40 withanolides, sitoindosides (VII, VIII, IX, X), Ashwagandhanolide which are reported as active markers [[10], [11], [12]]. Ashwagandha is used as anthelmintic, diuretic, astringent, narcotic, thermogenic, aphrodisiac [13]. Tinospora sinensis (Malabar Gulbel) is defined by big deciduous climber reported to have two new lignan glucosides namely tinosposides A and tinosposides B from the stems and in combination with other herbs to heal muscle rigidity, alleviating pain, and as well as tranquilizing the mind [14]. Traditionally the stem and leaves juice is used against ulcerated wounds, piles, and chronic rheumatism [15]. Phyllanthus emblica is known as Indian gooseberry, the fruits tonic restores the body’s energy and vigor. Amla is highly nutritious and rich in vitamin C, tannins and flavonoids [[16], [17], [18]], alkaloids like Emblicanin A and B, gallic acid, phyllatine, phyllatidine. They act against astringent, dyspepsia, colitis, hemorrhoids, hematuria, hepatoprotective, antiaging, gastroprotective [19], and also acts as an anti-platelet aggregator, vasodilator, and antiatherogenic [20]. Bacopa monneri suitably termed as ‘Medhya Rasayana’, well known as nootropic [21], this wonder plant is a brain tonic to increase the memory and relive in epileptic disorders and effective against neurological disorders from ancient times [22] and contain bacoside A & B, brahmin as the main alkaloid, nicotine, herpestine [23,24]. Ocimum basilicum in French ‘Herbe royale’ named for its peculiar pleasant smell [25] has a group of 20 monoterpenes, triterpenes, sesquiterpenes, flavonoids, phenols, and steroids [26,27] that acts as hepatoprotective, antihyperglycemic [28]. Asparagus racemosus, “queen of herbs” is an amazing herb which promotes cellular vitality and the roots are reported as antidiabetic, galactagogue, a nutritive tonic that contains 10 different steroidal groups like shatavaroside, alkaloids like Asparagamine A, and some flavonoids [29,30] and traditionally indicated in ‘Vata’, hypertension, cardiac disorders [31]. Shatavari improves stress-mediated reproductive health complications as well as prevent ageing, longevity, impart immunity and improves mental functions [32]. Shatavari root tonic is mostly prescribed to females as a uterine tonic which nourishes and strengthens the female reproductive system [33,34]. It is evident that the above-mentioned herbs hold a superior place in the Ayurvedic classics as “Rasayana” rejuvenator with a wide range of health benefits. Specific parts of the plants are selected in terms of their potential availability of phytoconstituent content that acts as cytoprotective, anticancer, anti-inflammatory, immunomodulatory and immunoadjuvant capacity. The traditional herb-based remedy of the reported plants are ingredients in many formulations and has been involved in In vitro and In vivo models proving scientific bases for their pharmacognosy and therapeutic applications. Even though, several reports and information regarding health benefits of antioxidant are available for the former herbs there is a call for systematic combinatorial study to improve the plethora of chemical constituents to enhance the body’s defence against cell mediated immunity.
The current investigation compares the methanolic extracts of three (4:1, 1:1, and 1:4) fractional dual herbal combination of roots of W. somnifera (L.) Dunal. - Ashwagandha (W) as a sole entity and in combination with five diverse most powerful and medically treasured plants namely fruits of P. emblica - Amla (W:P), leaves of Bacopa monnieri - Brahmi (W: B), the stem of T. sinensis -Guduchi (W:T), leaves of O. basilicum -Sweet basil (W:O), roots of A. racemosus - Shatavari (W:A) to analyze preliminary phytochemical constituents, antioxidant activities, and High-Performance Thin-Layer Chromatography (HPTLC) image-based chemometric profiling.
2. Materials and methods
2.1. Reagents, chemicals, and reference standards
1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-Tri (2-pyridyl)-s-triazine (TPTZ), FeSO4·7H2O, DMSO, FeCl3, AlCl3, Benedict’s reagent, Fehling’s reagent A&B, Analytical grade MeOH solvent was purchased from Sigma–Aldrich, India; Catechin (CA), Colchicine, Oleanolic acid (OA), Quercetin, Ascorbic acid (AA) were obtained from Merck, Mumbai, India.
2.2. Collection and authentication of plant materials
The roots of Ashwagandha (Amukkura), Stems of Giloy (Potchindil), and roots of Shatavari (Thannirvittan) procured from Siddha medicinal plants garden (SMPG), Central Council for Research in Siddha (CCRS), Ministry of AYUSH, Govt. of India, Mettur Dam. Leaves of Brahmi (Nirbrahmi) and Sweet basil leaves (Thiruneetrupachai) were collected from CIMH, Kanjikode. Amla fruits (Nellikai) were collected from a farm site at Coimbatore. All the plant samples were collected between Aug–Dec 2018. Medicinal plant species include B. monnieri (L.)-Wettest. - PLANTAGINACEAE, W. somnifera (L.) Dunal-SOLANACEAE, O. basilicum L.- LAMIACEAE, P. emblica L. PHYLLANTHACEAE, A. racemosus Willd - ASPARAGACEAE and T. sinensis (Lour.) MERR. -MENISPERMACEAE. Plants species were authenticated by the Botanical Survey of India (BSI), Southern Regional Center, Coimbatore. The voucher number of the specimens are BSI/SRC/5/23/2019/Tech/3219-3224 (Supplementary Table S1).
2.3. Preparation of combinatorial methanolic extracts
The selected parts of the plants were shade dried, electrically blended, powdered, and sieved using clean muslin cloth individually and stored at room temperature until further analysis. The powdered W. somnifera (W) (20 g) alone and in dual herbal combination involving three different ratios 4:1, 1:1, and 1:4 with P. emblica (W:P), B. monnieri (W:B), T. sinensis (W:T), O. basilicum (W:O), A. racemosus (W:A) (16:4 g, 10:10 g, 4:16 g) respectively were taken for further process (Supplementary Table S2). The samples were successively extracted with 250 mL of methanol in a Soxhlet apparatus at 60 °C. The course was tracked for an overall 24 h and the filtrate was evaporated using a Rotary thin-film evaporator and the extracts were stored at 4 °C until further analysis. The percentage yield for the methanolic combinatorial extracts was calculated (Supplementary Table S3).
2.4. Qualitative phytochemical analysis of combinatorial extracts
For the Phytochemical screening, methanolic combinatorial extracts of 1 mg/mL were dissolved in analytical grade DMSO to qualitatively analyze the important families of secondary metabolites based on their precipitation and coloring reactions following standard procedures of Trease and Harborne [35,36]. The extracts were subjected to qualitative analysis to different chemical tests for the detection of alkaloids [Dragendroff’s (Potassium bismuth iodide) test, Mayer’s (Potassiomercuric iodide) test, Wagner’s (Iodo potassium iodide) test], flavonoids (1% dil. Ammonia solution test, 1% Aluminium chloride test), tannins (5% Ferric chloride test, 10% Lead Sub acetate test, 10% Gelatin test), carbohydrates (Molisch test, Fehling’s test, Benedict’s test), cardiac glycosides (Keller kiliani test, Legal test), Steroids/Terpenoids (Salkowski test, Liebermann Burchadt test), glycoside test, saponins (Froth test), amino acids (Ninhydrin test), Anthraquinone glycosides (Borntrager test), protein (Biuret test), organic acid (Oxalic acid), Inorganic test (Sulphate test), Phenol (Ellagic acid) and Coumarin test.
2.5. Determination of the antioxidant activities of combinatorial extracts
2.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The antiradical activity of the dual herbal combinatorial extracts was evaluated using the free radical DPPH assay with minor modifications in Brand-Williams et al., protocol [37]. For each sample, different concentrations (5–200 μg/mL) were mixed with 1.5 mL of methanol and with freshly prepared 1.5 mL of 6 × 10−5 M DPPH in methanol solution. After vigorous shaking, the mixtures were incubated for 30 min at room temperature in the dark and the absorbance value was read at 517 nm against the methanol blank. Ascorbic acid (1 mg/mL) was used as the reference standard. The percentage inhibition for DPPH activity was calculated using the following formula:
| Inhibition (%) = [(AC−AS)/ AC] x 100. |
where AC is the absorbance of the control, and AS is the absorbance of the sample.
2.5.2. Ferric Reducing Antioxidant Power (FRAP) assay
The Ferric reducing antioxidant power of the combinatorial extracts was estimated according to the modified method of Benzie and Strain [38]. A volume of 1.5 mL of the combinatorial extracts (100 μg/mL) was mixed with 1.5 mL freshly prepared FRAP reagent containing 300 mM acetate buffer at pH 3.6 (3.1 g CH3COONa in 16 mL glacial acetic acid), 10 mM TPTZ solution in 40 mM HCl and 20 mM Fecl3.6H2O solution (10:1:1, v/v/v). Further, the reaction mixture was incubated in a water bath for 30 min at 37°C and the absorbance was measured at 593 nm. The ferric reducing power of the combinatorial extracts was analyzed relating to the standard calibration curve for Ascorbic acid and FeSO4 (2–200 μM) and results were expressed in μM FeE/g (Supplementary Fig. S1). Higher value absorbance of the combinatorial mixture indicates a greater ferric reducing capacity.
2.5.3. Statistical analysis
Results were expressed as mean ± SD (n = 3). The non-linear regression analysis was performed for DPPH analysis to calculate the dose–response relation of methanolic dual herbal combinatorial extracts and the Pearson, r two-tailed was evaluated to find out the correlation coefficient and the value p < 0.0001 which was considered to be significant. The FRAP capacity was subjected to a standard nonlinear calibration curve. The statistical and graphical evaluations were performed by GraphPad Prism version 6.0, USA.
2.6. Explorative phytoconstituents data analysis of the combinatorial extract
2.6.1. High-performance thin-layer liquid chromatography (HPTLC) fingerprint processing
Densitometric HPTLC analysis was performed for alkaloids (Colchicine), flavonoids (Quercetin), phenol (Catechin), terpenoids (Oleanolic acid) for the development of the characteristic fingerprinting profile. The methanolic extracts were dissolved with HPTLC grade methanol and a concentration of 10 μg of the sample and 10 μg standard solutions were loaded using a Hamilton syringe to form a 6.0 mm band length on 10 × 10 cm TLC plate pre-coated with Silica gel 60 F254 (E. Merck, Mumbai, India) in a semiautomatic CAMAG LINOMAT 5 instrument. Linear rising progression was done using 20 × 10 cm CAMAG Twin tank with their respective mobile phases solvent mixtures for former mentioned (v/v/v/v): Ethyl acetate: MeOH: water (10:1.35:1); MeOH: GAA: Formic acid: Water (3:0.9:0.9:0.5); Toluene: Ethyl acetate: MeOH: Formic acid (6:6:1:0.1); Toluene: ethyl acetate: MeOH: Acetone (14:4:1:1) up to 70.0 mm for 20 min at room temperature. The plates were oven-dried at 60 °C for 5 min and post derivatization agents were sprayed as formerly mentioned: Anisaldehyde sulphuric acid; 1% Ethanolic AlCl3; 5% Alcoholic FeCl2; anisaldehyde sulfuric acid reagents respectively and oven-dried at 120 °C for 20 min. The images were documented using CAMAG Visualizer at UV 254 nm, 366 nm, and visible light. The Spectro densitometric analysis was done by CAMAG TLC scanner 3 linked with WinCATS software.
2.6.2. Fingerprint image analysis using chemometric techniques
Chemometric techniques are used to explore the chemical profile of alkaloids, flavonoids, terpenoids, and phenolic compounds present in the combinatorial extracts according to the process described by Ristivojević et al., [39]. The image of the HPTLC plate was subsequently handled by the Image J program, inbuilt FIJI version 1.52v (NIH, Wisconsin, USA) public image processing program by java platform [40,41]. The image is split into three filter channels: primary colors red, green, and blue filter channels were deionized, baseline drift removed and rectangular selection tool used to outline the image. Finally, the line profile plots were achieved and the plots were combined to draw a single-channel 2D graph, intensity (x-axis) by pixel distance (y-axis) with distance along the fixed-line. The images were combined alongside with their plot profiles with Rf values as independent variables for each object considered.
Principal component analysis (PCA) was employed to reduce the dimensionality of data hyperspace and Hierarchical Cluster analysis (HCA) and Heatmaps for sample clustering based on their chemical fingerprint variabilities. The data matrix was constructed with Microsoft Excel and PCA was performed using the JMP 15.1.0 statistical discovery software (SAS Institute Inc., NC, USA). The Agglomerative hierarchical clustering analysis (HCA) and heat maps were performed by NCSS 2020 20.0.1 (Utah, USA).
3. Results
3.1. Qualitative phytochemical analysis of the methanolic combinatorial extract
Preliminary phytochemical analysis of the dual herbal combination of methanolic extracts are summarized in (Table 1). W. somnifera (W) shows a high presence for carbohydrates, cardiac glycosides, amino acids, organic acid, coumarins whereas it possesses a moderate presence for alkaloids, flavonoids, steroids, and saponins. Combinatorial methanolic extracts of W:B/1:1 and W:O/1:4 showed a high presence for alkaloids, amino acids but a low level of representation in the other combinations. Tannins and phenol were remarkably high for all W:P samples, W:B/1:1 showed high for tannins while moderate presence was seen in W:O/1:4 samples. Flavonoids were high for all 1:1 samples and moderate presence was shown in other combinations. Cardiac glycosides, terpenoids, saponins, proteins were present strong for W:O/1:4 samples. W:T/1:4 showed a high presence for phenol constituents. The combinatorial extract W:A/4:1 showed a high presence for flavonoids and coumarins. Anthraquinone glycosides were absent for all of the samples.
Table 1.
Phytochemical analysis of methanolic extracts of combinatorial samples.
| S. No | Phyto constituents Analysed | W | WP |
WB |
WT |
WO |
WA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WP1 | WP2 | WP3 | WB1 | WB2 | WB3 | WT1 | WT2 | WT3 | WO1 | WO2 | WO3 | WA1 | WA2 | WA3 | |||
| 1. | Alkaloid test | ||||||||||||||||
| 1a. | Dragendroff | ++ | ++ | ++ | + | ++ | +++ | ++ | ++ | ++ | + | ++ | + | −− | ++ | ++ | + |
| 1b. | Mayer’s test | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− |
| 1c. | Wagner’s test | + | ++ | ++ | + | ++ | + | +++ | ++ | + | + | ++ | + | +++ | ++ | + | + |
| 2. | Tannins test | ||||||||||||||||
| 2a. | FeCl3 test | + | +++ | +++ | +++ | + | +++ | ++ | + | + | + | −− | + | ++ | −− | + | −− |
| 2b. | Lead sub acetate | −− | +++ | +++ | +++ | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− |
| 2c. | Gelatin test | −− | +++ | +++ | −− | + | +++ | ++ | + | −− | −− | −− | + | + | + | −− | −− |
| 3. | Flavonoids test | ||||||||||||||||
| 3a. | Ammonia | ++ | ++ | −− | ++ | ++ | −− | −− | ++ | +++ | ++ | + | −− | −− | + | −− | −− |
| 3b. | AlCl3 test | −− | ++ | +++ | + | ++ | +++ | ++ | ++ | +++ | ++ | + | +++ | −− | +++ | −− | −− |
| 4. | Carbohydrate test | ||||||||||||||||
| 4a. | Molisch test | ++ | ++ | +++ | ++ | +++ | +++ | +++ | + | ++ | + | +++ | +++ | +++ | +++ | +++ | ++ |
| 4b. | Fehling’s test | +++ | +++ | + | +++ | −− | −− | −− | ++ | +++ | ++ | ++ | + | ++ | ++ | +++ | ++ |
| 4c. | Benedict’s test | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | +++ | +++ | + | ++ | +++ | + | +++ | + |
| 5. | Cardiac glycoside | ||||||||||||||||
| 5a. | Keller-killiani | −− | ++ | +++ | + | −− | + | + | −− | −− | −− | −− | −− | +++ | + | +++ | ++ |
| 5b. | Legal test | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | + | +++ | ++ | ++ | −− |
| 6. | Steroids/Terpenes | ||||||||||||||||
| 6a. | Salkowski | + | ++ | +++ | + | +++ | + | +++ | ++ | ++ | ++ | + | +++ | +++ | ++ | +++ | ++ |
| 6b. | Liebermann Burch | ++ | −− | −− | + | + | + | ++ | + | + | ++ | + | + | +++ | + | ++ | + |
| 7. | Glycoside test | + | +++ | −− | ++ | −− | −− | −− | + | −− | −− | −− | + | ++ | −− | −− | −− |
| 8. | Anthraquinone | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− | −− |
| 9. | Saponins Frothing | ++ | −− | + | −− | ++ | +++ | +++ | + | −− | −− | −− | −− | −− | −− | ++ | +++ |
| 10. | Amino Ninhydrin | +++ | −− | −− | −− | +++ | −− | +++ | + | −− | + | −− | −− | +++ | +++ | −− | +++ |
| 11. | Protein Biuret | −− | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | + | + | +++ | +++ | −− | +++ | −− |
| 12. | Phenol Ellagic test | −− | ++ | +++ | +++ | −− | ++ | −− | −− | + | +++ | −− | + | ++ | −− | ++ | −− |
| 13. | Organic test | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 14. | Inorganic test | −− | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 15. | Coumarin | +++ | + | ++ | + | + | ++ | + | + | +++ | ++ | ++ | ++ | −− | +++ | +++ | + |
+++ High,++ Moderate,+ low, −− Absent; the experiments were conducted in triplicates, and classification was based on the intensity of color and amount of precipitate formed. W. somnifera (W) along with combination [4:1(1), 1:1(2), 1:4(3)] of P. emblica W:P (WP), B. monnieri W:B (WB), T. sinensis W:T (WT), O. basilicum W:O (WO), and A. racemosus W:A (WA).
3.2. Antioxidant assay determination for methanolic combinatorial extracts
3.2.1. DPPH radical scavenging activity
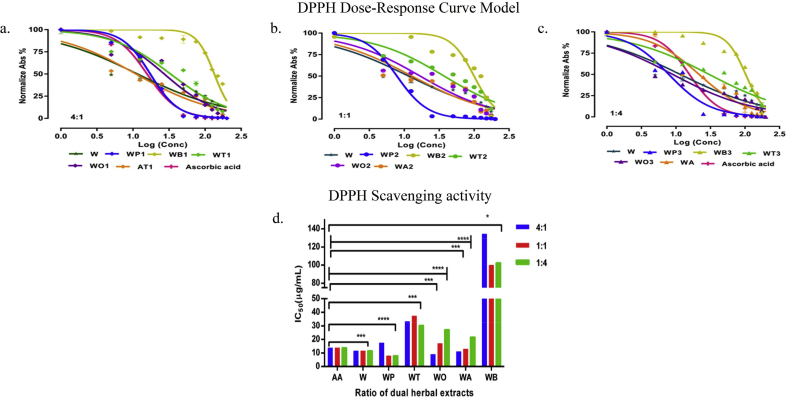
The antioxidant activity of methanolic extracts obtained from W. somnifera (W) and combinatorial extracts along with the standard ascorbic acid are visualized in Fig. 1a-d The low IC50 value indicates maximum scavenging activity for W:P/1:4 (7.89 μg/mL) and W:O/1:4 (8.99 μg/mL) and W:A/4:1 (11.01 μg/mL) in comparison with W. somnifera (W) (11.56 μg/mL) and standard Ascorbic acid (15.68 μg/mL). The IC50 value indicating moderate scavenging ability was noticed for W:T/1:4 (30.27 μg/mL) and W:B/1:1 (99.87 μg/mL) respectively. According to their fractional combination for each antioxidant concentration tested the reaction kinetics were plotted and represented in Fig. 1. The Pearson correlation coefficient showed a good correlation between ascorbic acid standard and the combinatorial extracts. Out of the all extracts, significant correlation (p < 0.0001) was exhibited for [W:P/1:4] R2 = 0.9152; [W:O/1:4] R2 = 0.8480 and [W] R2 = 0.7517 respectively (Fig. 1d).
Fig. 1.
a, b, c Graphical representation of Dose-response fit for the methanolic combinatorial extracts with std ascorbic acid. The disappearance of DPPH radical as a function of dose-response inhibition to evaluate antioxidant activity for methanolic combinatorial extracts ratios (a) 4:1 (1) (b) 1:1 (2) (c) 1:4 (3) and of W. somnifera (W) in combination with P. emblica W:P (WP), B. monnieri W:B (WB), T. sinensis W:T (WT), O. basilicum W:O (WO) and A. racemosus W:A (WA) with reference to standard ascorbic acid. mean ± SD (n=3). d. DPPH scavenging IC50 bar graph representation, denoted with Pearson’s correlation coefficient, r of combinatorial extracts: ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 in comparison with standard ascorbic acid. (Supplementary Table S4).
3.2.2. Ferric Reducing Antioxidant Power (FRAP) assay
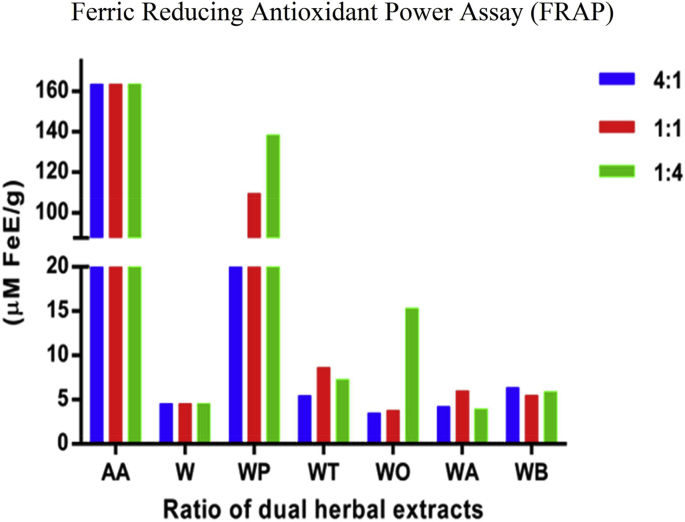
The FRAP potential of methanolic extracts of W. somnifera (W) and combinatorial extracts in comparison with standard Ascorbic acid are visualized in Fig. 2. The results are expressed in the FeSO4 equivalent calibration curve R2 = 0.9839, and Ascorbic acid R2 = 0.9638 (Supplementary Fig. S1). The outcomes exhibited a greater rate of ferric reducing capacity for all the P. emblica combinatorial W:P samples and especially high for the W:P/1:4 (138.34 μM FeE/g) sample followed by a moderate rate for W:O/1:4 (15.32 μM FeE/g) sample. Ascorbic acid showed the highest ferric reducing antioxidant ability 163.48 μM FeE/g while W. somnifera (W) showed low quantity to reduce Fe3+ ions with 4.56 μM FeE/g respectively (Supplementary Table S2).
Fig. 2.
Bar Graph representation of Frap assay for the methanolic combinatorial extracts ratios 4:1 (1), 1:1 (2), 1:4 (3) and of W. somnifera (W) in combination with P. emblica W:P (WP), B. monnieri W:B (WB), T. sinensis W:T (WT), O. basilicum W:O (WO) and A. racemosus W:A (WA) with reference to standard ascorbic acid. mean ± SD (n = 3). Supplementary Table S4; Fig. S1.
3.3. HPTLC – chemometric analysis of selected combinatorial extracts
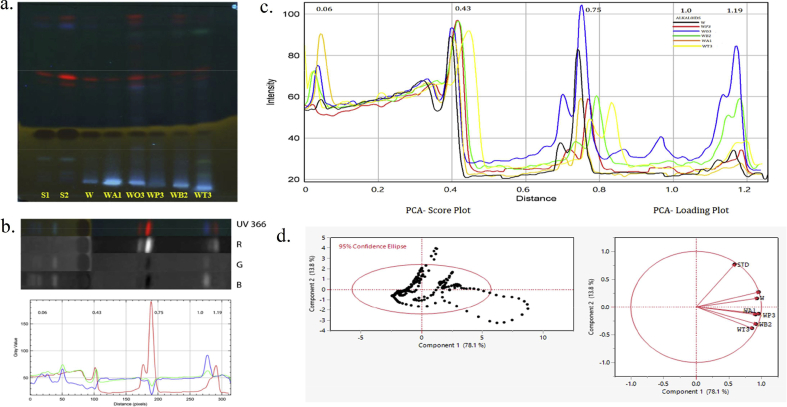
HPTLC analysis for the selected combinatorial extracts (W: P/1:4, W:O/1:4, W:B/1:1, W:A/4:1, W:T/1:4) demonstrated a promising antioxidant potential within their combination along with W. somnifera (W) with reference to their respective standards for secondary metabolites like alkaloids (Colchicine), flavonoids (Quercetin), phenol (Catechin) and terpenoids (Oleanolic acid) visualized at UV 366 nm (Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7). The HPTLC image J software processed are depicted in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7 [Reference standard - similarly interpreted for samples (Supplementary Figs. S3–S6)] to generate Red (R), Green (G), and Blue (B) filter channel line profile plots for each sample chromatograms in track order to perform full chemometric analysis by refining their selectivity for the spots that are equivalent to their fluorescence color. The best filter channel was selected from each of the secondary metabolites based on their observed bands in a display for alkaloids (red), flavonoids (blue), phenol (blue), and terpenoids (red) to combine and compare the line profile plots of the samples.
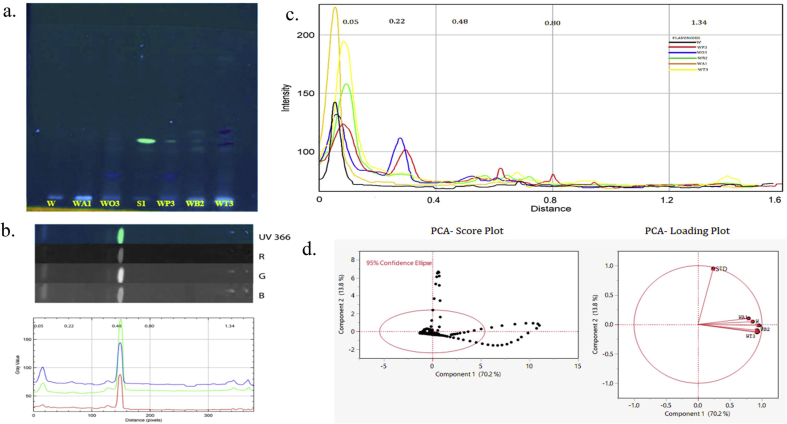
Fig. 3.
Chemometric analysis HPTLC alkaloids fingerprint for methanolic combinatorial extracts along with their respective standard a. HPTLC Chromatogram (UV 366 nm) tracks: Alkaloids S1, S2 (Std): Colchicine and W. somnifera (W); A. racemosus WA1 (4:1) ; O. basilicum WO3 (1:4); P. emblica WP3 (1:4); B. monnieri WB2 (1:1); T. sinensis WT3 (1:4) methanolic combinatorial extracts. b. 2D line profile of STD (samples are interpreted in same manner displayed in Supplementary data Fig S3) filter channels red (R), green (G) and blue (B). c. Superimposed line profile plot for comparing the methanolic combinatorial extracts with Rf distance (Black- W; Red- WP3; Blue-WO3; Green-WB2; Orange-WA1; Yellow-WT3). d. PCA Score plot of PC1 & PC2 mined from data analysis of the extracts rendering to the intensities of the pixels and PCA Loading plot for the projection of samples chromatogram for their secondary metabolites data variance.
Fig. 4.
Chemometric analysis HPTLC flavonoids fingerprint for methanolic combinatorial extracts along with their respective standard a. HPTLC chromatogram (UV 366 nm) tracks: Flavonoids S1 (Std): Quercetin ; and W. somnifera (W); A. racemosus WA1 (4:1); O. basilicum WO3 (1:4); P. emblica WP3 (1:4); B. monnieri WB2 (1:1); T. sinensis WT3 (1:4) methanolic combinatorial extracts. b. 2D line profile of STD (samples are interpreted in same manner displayed in Supplementary data Fig S4) filter channels red (R), green (G) and blue (B). c. Superimposed line profile plot for comparing the methanolic combinatorial extracts with Rf distance (Black- W; Red- WP3; Blue-WO3; Green-WB2; Orange-WA1; Yellow-WT3). d. PCA Score plot of PC1 & PC2 mined from data analysis of the extracts rendering to the intensities of the pixels and PCA Loading plot for the projection of samples chromatogram for their secondary metabolites data variance.
Fig. 5.
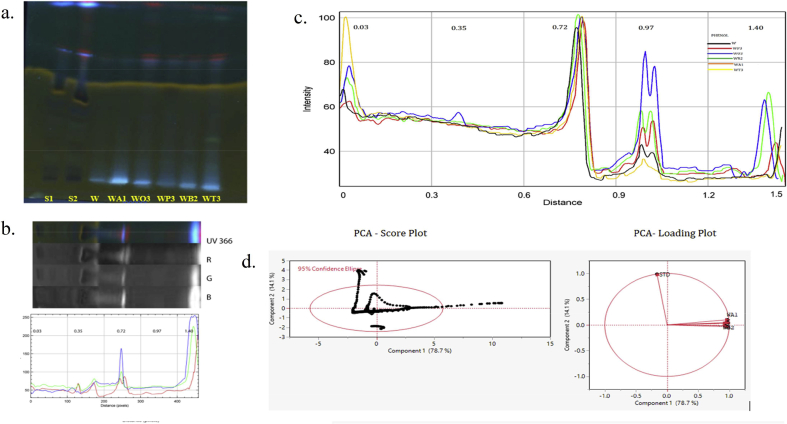
Chemometric analysis HPTLC phenol fingerprint for methanolic combinatorial extracts along with their respective standard. a. HPTLC chromatogram (UV 366 nm) tracks: Phenol S1, S2: Catechin; and W. somnifera (W); A. racemosus WA1 (4:1); O. basilicum WO3 (1:4); P. emblica WP3 (1:4); B. monnieri WB2 (1:1); T. sinensis WT3 (1:4) methanolic combinatorial extracts. b. 2D line profile of STD (samples are interpreted in same manner displayed in Supplementary data Fig S5) filter channels red (R), green (G) and blue (B). c. Superimposed line profile plot for comparing the methanolic combinatorial extracts with Rf distance (Black- W; Red- WP3; Blue-WO3; Green-WB2; Orange-WA1; Yellow-WT3). d. PCA Score plot of PC1 & PC2 mined from data analysis of the extracts rendering to the intensities of the pixels and PCA Loading plot for the projection of samples chromatogram for their secondary metabolites data variance.
Fig. 6.
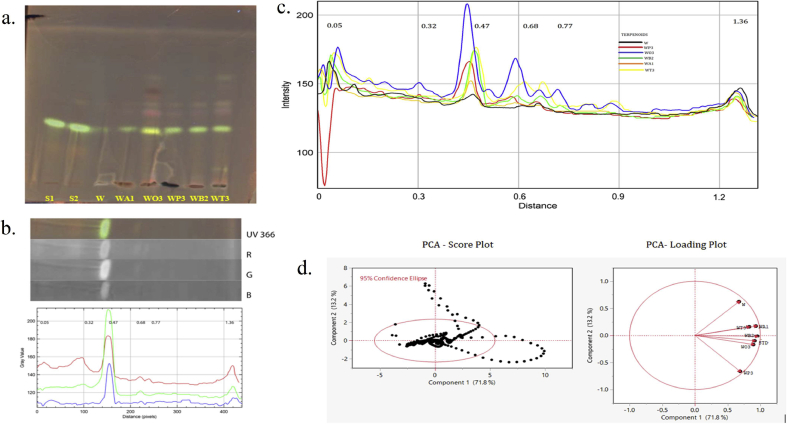
Chemometric analysis HPTLC terpenoids fingerprint for methanolic combinatorial extracts along with their respective standard. a. HPTLC chromatogram (UV 366 nm) tracks: Terpenoids S1, S2 (Std): Oleanolic acid; and W. somnifera (W); A. racemosus WA1 (4:1) ; O. basilicum WO3 (1:4) ; P. emblica WP3 (1:4); B. monnieri WB2 (1:1); T. sinensis WT3 (1:4) methanolic combinatorial extracts. b. 2D line profile of STD (samples are interpreted in same manner displayed in Supplementary data Fig S6) filter channels red (R), green (G) and blue (B). c. Superimposed line profile plot for comparing the methanolic combinatorial extracts with Rf distance (Black- W; Red- WP3; Blue-WO3; Green-WB2; Orange-WA1; Yellow-WT3). d. PCA Score plot of PC1 & PC2 mined from data analysis of the extracts rendering to the intensities of the pixels and PCA Loading plot for the projection of samples chromatogram for their secondary metabolites data variance.
Fig. 7.
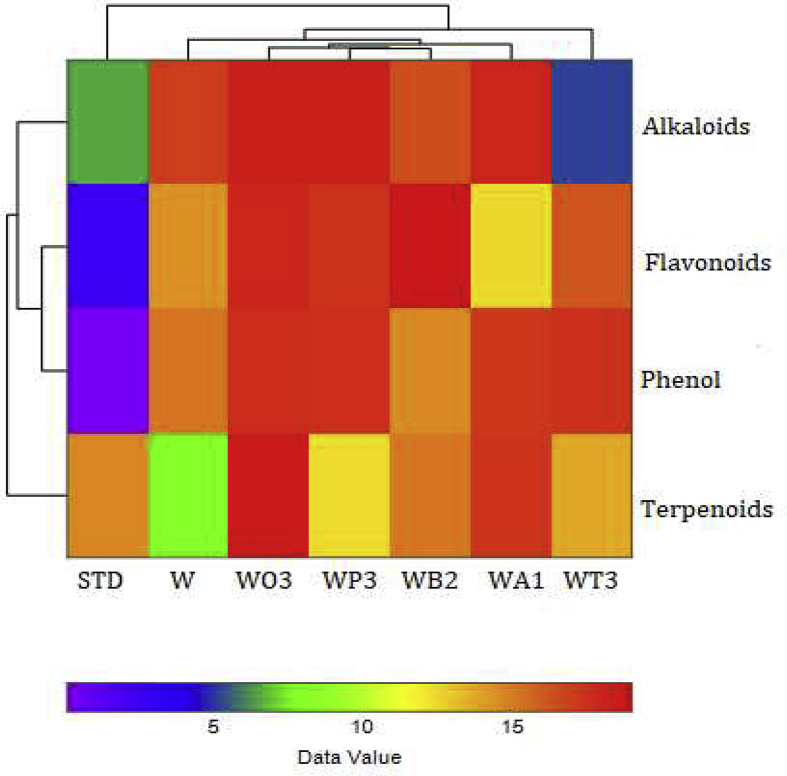
Hierarchical Clustered Heat-maps for methanolic combinatorial plant extracts. HCA Heatmaps for HPTLC datasets of W. somnifera (W) alone and along with P. emblica W:P/1:4 (WP3), O. basilicum W:O/1:1 (WO2), A. racemosus W:A/4:1 (WA1), B. monnieri W:B/1:1 (WB2), T. sinensis W:T/1:4 (WT3) with their respective standard (STD) based on their peak area of characteristic bands at UV 366 nm. An increase in metabolites concentration is indicated by color code change ranging from blue to red. The clustered dendrogram shows the linkage between the samples’ secondary metabolites. (Supplementary Table 9 & 10).
The explorative study revealed that the first two Principal Components (PCs) of all sources of variability (score plot and loading plot of data matrix) showed easier viewing of differences between the methanolic combinatorial extracts, W. somnifera (W), and phytoconstituent standards. PC’s three dimensional (3D) graphical representation of the coordinates shows an optimal conception of the distribution, relative to 95% confidence ellipses as represented in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7.
HPTLC chromatogram mean pixel intensity and mean values were calculated for each zone of all samples and standards, alkaloids (7 samples x 306 variables), flavonoids (7 samples x 347 variables), phenol (7 samples x 477 variables), and terpenoids (7 samples x 440 variables) are used as the dataset for chemometric analysis. The mutual projection of factor scores for the most important component (PC1) followed by PC2 represented up to 78.1% (PC1) and 13.8% (PC2) for alkaloid, 70.2% (PC1) and 13.8% (PC2) for flavonoids, 78.7% (PC1) and 14.1% (PC2) for phenol, 71.8% (PC1) and 13.2% (PC2) for terpenoids respectively.
The corresponding loading plots are displayed according to each component variables Rf value that contributed to the highest variances among the samples. The line profile plots of Rf values constituted in Fig. 3c is 0.06, 0.43, 0.75, 1.0, 1.19 for alkaloids have the most impact on this PC that distinguishes W:O/1:4 sample from other methanolic combinatorial extracts fingerprints and 0.43 appeared to be standard colchicine which rooted high for W:P/1:4 and W:B/1:1. The formatted loading matrix was high for PC1 in the order W:O/1:4 > W:P/1:4 > W > W:B/1:1 > W:A/4:1 > W:T/1:4 > STD and PC2 represented their negative and low variability impact are shown in (Supplementary Table S5).
Similarly, Rf values portrayed in Fig. 4c is 0.05, 0.22, 0.48, 0.80, 1.34 for flavonoids revealed greater influence for W:O/1:4 combinatorial extracts, Rf 0.48 standard quercetin content was meager for W. somnifera (W) in comparison with other samples. The formatted loading matrix for PC1 of the pixel intensities was in the order W:O/1:4 > W:B/1:1 > W:P/1:4 > W:T/1:4 > W > W:A/4:1 > STD and PC2 of flavonoids data matrix represented negative impacts are shown in (Supplementary Table S6).
For phenolic components, the Fig. 5c describes Rf 0.03, 0.35, 0.72, 0.97, 1.40 values displayed high intensities for all the combinatorial extracts but specifically distinguishing Rf 0.97 for W:O/1:4 sample from other extracts. Standard catechin represents PC1 with the negative value among the other combinatorial extracts. The formatted loading plot for PC1 for phenol variabilities represented in the hierarchical order that differed less in their intensities mean values W:B/1:1 > W:T/1:4 > W:P/1:4 > W:A/1:4 > W > W:O/1:4 > STD and PC2 represented high for the standard are shown in (Supplementary Table S7).
The chromatogram of terpenoids within Fig. 6c detailed Rf peaks 0.05, 0.32, 0.47, 0.68, 0.77, 1.36 exposed samples W:O/1:4 followed by W:T/1:4 then W:B/1:1 and Rf 0.05 showed lower negative PC2 for W:P/1:4 samples and standard Oleanolic acid Rf 0.47 was lower for sample W. somnifera (W). The formatted loading plot for terpenoids PC1 displayed in the hierarchical order W:B/1:1 > W:A/1:4 > STD > W:O/1:4 > W:T/1:4 > W:P/1:4 > W and PC2 was represented high for W. somnifera (W) for its low impacts of intensity are shown in (Supplementary Table S8). The PCA datasets of squared cosines of variables (normalized covariance interaction via cosines) for the secondary metabolites includes the differences of combinatorial extracts are compared and expressed in consecutive three PCs through the bar graph shown in (Supplementary Fig. S3-S6 and Table S5-S8).
An agglomerative hierarchical cluster algorithm (HCA)-Heatmaps was achieved in addition to PCA to cluster similar objects (secondary metabolites) more effortlessly with the samples and to further elucidate PCA results. The best results were obtained by implying group average and Euclidean distance to compare the hierarchical cluster analysis by cophenetic correlation for the variables (Fig. 7). The Cophenetic Correlation Coefficient for sample clusters are CPCC = 0.9829 and for phytoconstituents are CPCC = 0.9886 by comparing their dendrograms. It is obvious from the heatmap result that W:P/1:4 combinatorial sample shows close clusters with W:B/1:1 distance value (2.397), W:O/1:4 (2.867), W:A/4:1 (3.403), W (4.544) explaining their clustering pattern for the phytoconstituents. They were followed by W:T/1:4 (6.698) and STD (12.04) with distant clustering for secondary metabolites. The phytoconstituents phenol and flavonoids displayed close distance value around (2.689) whereas alkaloids (5.234) and terpenoids (6.295) where clustered distantly are shown in (Supplementary Tables S9 and S10).
4. Discussion
From the results, it is inferred that the methanolic extracts of the roots of W. somnifera (W) individually and in three fractions of dual combinations [4:1 (1), 1:1 (2), 1:4 (3)] with fruits of P. emblica (W:P), leaves of B. monnieri (W:B), the stem of T. sinensis (W:T), leaves of O. basilicum (W:O) and roots of A. racemosus (W:A) expressed different levels of antioxidant phytoconstituents within their combinations.
The action of a single herb does not usually meet the requirements for therapy in alleviating the severity of disease and typically, in Phytotherapy, preparations of the herbal combination shall provide a multi-targeted holistic therapeutical regime. Thus, the combination of herbal preparations shall provide synergistic biological effects for medicinal needs than mono herbal preparations. Most of the ayurvedic preparations take into consideration, not just the disease condition but also in instilling a generalized health benefit more preventive in nature and hence dual and multiple combinations are preferred. Furthermore, several combinations are more administered according to age, gender, genetics, and environmental factors during the therapy.
The identification of phytochemical constituents at all stages of extraction are equally important in the study of medicinal plants [42]. Methanol was identified as the most effective solvent for the extraction of phenolics, flavonoids, alkaloids, saponins on the selected medicinal plants and their respective parts from the previous studies conducted [[43], [44], [45], [46], [47]] and its efficiency in the extraction of the phytoconstituents.
The criteria as adaptogenic plants were fulfilled by the work carried out by Nirmala et al., on six herbs (Tinospora cordifolia, A. racemosus, Emblica officinalis, W. somnifera, P. longum, and Tinospora chebula) and evaluated against a variety of stressors in combination with cisplatin and each of the plants’ extracts [48]. Earlier studies on the combination of P. emblica, T. cordifolia, and Ocimum sanctum for treatment of Alzheimer’s disease dementia treatment on normal and memory-impaired rats demonstrating nootropic activity for formulations with combinations [49]. EUMil, a polyherbal formulation made of W. somnifera (L) Dunal, O. sanctum L, A. racemosus Willd and E. officinalis Gaertn., is used against chronic stress [50]. The initial studies and substantial work of single formulation based on W. somnifera in comparison with other adaptogenic herbs and polyherbal formulation have been used extensively [51]. Currently, the Ayurvedic intervention for COVID – 19 exposes the rationale for treating asymptomatic individuals with a decoction of the combination of herbs, T. cordifolia, Z. officinale, C. longa, O. sanctum, etc., [52].
Combinatorial bioactive phytocompounds with hydrophilic and lipophilic constituents; carotenoids and flavonoids; carotenoids and phenolic acids; or tocopherols and water-soluble vitamins improve the high antioxidative synergy [53]. Thus, reflecting the adaptogenic herb combination of alkaloid rich W. somnifera with the flavonoids, phenols, saponins rich herbal botanicals. To avoid inappropriate ratios, the dual herbs were combined in their high, equal, and low fractions so the participant compounds increase their capability to scavenge free radicals. Otherwise, it may lead to the formation of hydrogen bonds at active hydroxy groups leading to lower biological effects [54]. Thus, the evaluation of interactions between multiple bioactive compounds has gained interest and popularity [55,56].
Earlier reports of free radical scavenging properties of P. emblica, O. basilicum, B. monnieri, T. sinensis, A. racemosus, and W. somnifera divulges as primary antioxidants to protect cells from oxidative stress [16,29,[57], [58], [59], [60], [61]]. The stable free radical DPPH measures the chemical ability of the combinatorial extracts to get reduced by donating hydrogen to the radical [62]. The measured antioxidant activity for the combinatorial extracts compared with W. somnifera (W) is in the following order W:P/1:4 > W:O/1:4 > W:A/4:1 > W > W:T/1:4 > W:B/1:1. Thus, an increase in the phytoconstituents is directly proportional to the concentration of the antioxidant capacity, and decreased effect may be due to some antagonist behavior of the active compounds [63]. The Ferric reducing properties of the combinatorial extracts function by donating hydrogen or electron to Fe (III) [64]. The Ferric reducing potential was distinguishably higher for all P. emblica (W:P) methanolic combinatorial extracts explaining a strong correlation for phenolic compounds and revealing W:P/1:4 sample with increased redox contribution in determining the antioxidant potential [65].
Inclusive assessment with the above findings, HPTLC image-based chemometric analysis was performed for the potential antiradical combinatorial extracts from each of their combinations. The total variance among the phytoconstituents suggests that the digitalized chromatograms of the extracts contributed significantly high towards alkaloids, flavonoids, phenol, and terpenoids in the samples. Furthermore, the Principal Component Analysis (PCA) score plots revealed distinct groups that favor the PC1 axis for the phytocomponents that make compact clustering of the samples whereas remaining possible variabilities are fed in PC2 with low intensities and negative impact. Through close examination, the methanolic extract of W. somnifera (W) line profile plot explains its low impact on overall secondary metabolites fingerprint in comparison with other dual herbal combinatorial extracts of W:P/1:4 and W:O/1:4 showed high presence for the mentioned secondary metabolites, W:B/1:1 sample had low intensities for alkaloids, W:A/4:1 showed high values for terpenoids and W:T/1:4 displayed high for phenol explaining the importance of improved phytoconstituents content through herbal combinations. The study revealed the relationship of the Rf values representing maximum peak height for the compound directly representing the intensity of the compound.
Hierarchical Cluster analysis (HCA) on the other hand elucidated the cophenetic matrix of the combinatorial extracts exposed through a double dendrogram grouping pattern to find variation in the bioactive content. The lower phytoconstituent content for the extracts was placed farther in sub-cluster from other extracts. Thus, the clustering pattern for the Phenol group reveals the largest group of phytochemicals that account to have highest antioxidant activity, and flavonoids are the naturally occurring phenolic compounds that possess ideal structural chemistry for free radical scavenging activities. The alkaloids are biosynthesized from phenylalanine and tyrosine leading to a common share of the biosynthetic pathway and define the relative abilities of the compound to scavenge free radicals. Terpenes and alkaloids originate from the same prenyl units that construct terpenes skeletons [9] thus explaining the double dendrogram clustering. These findings confirm improvement in efficacy based holistic chemical profiling for dual herbal combinatorial methanolic extracts in the hierarchical order of W:P/1:4, W:O/1:4 displaying high concentrations and W:B/1:1 and W:A/4:1 revealing a moderate degree of bioactivity rather than the methanolic extract W. somnifera (W) indicating low presence for required bioactivity for W:T/1:4 and was clustered farther after W. somnifera (W) indicating less concentration of metabolite fingerprints followed by their respective standards.
In the present era where ayurvedic preparations are being tried for several therapeutic interventions for a host of diseases and clinical conditions; there is an imminent need for an appropriate and scientific validation of the various combinatorial preparation that has been used since time immemorial. Chemometric profiling shall prove to be a gold standard tool in the analysis of various phytoconstituents that are incorporated in standardized ayurvedic preparations; which will bring to limelight the chemical nature of the preparations. This will further help in devising other downstream analysis for validating the combinatorial efficacies of several ayurvedic preparations which are in vogue for the treatment of ailments. For instance, molecular and cell culture-based validation studies can be sophisticated when the chemical identity of the preparation is evident. In these lines, the present study of chemometric profiling shall open up further avenues for an efficient and foolproof characterization method for several such ayurvedic Rasayanas. The AYUSH-64, an ayurvedic polyherbal formulation developed for treating malaria now has serious attention focused on COVID-19 cases with chosen herbs are W. somnifera, G. glabra, T. cordifolia, and P. longum for accelerating the recovery [66]. For such studies, the chemometric profiling shall be an added asset.
This present study is an innovative attempt to characterize the phytoconstituents by chemometric profiling. Erstwhile, very few studies were proposed on plants and plant constituents were not directly used in evaluating ayurvedic preparations. Hence, this study will be significant for deciphering the phytoconstituents through a more structured and analytical method. Furthermore, these findings confirm improvement in efficacy based holistic chemical profiling, while providing an approach for investigation of the dual herbal combinatorial methanolic fingerprint. The coherent chromatographic chemometric tool gave an intuitive pictorial image profiling for precise efficacy assessment of fingerprints of dual herbal extracts.
5. Conclusion
We are presenting an efficient and effective analytical method, the results on the comparison of the dual herbal combinatorial methanolic extracts of W. somnifera (W) with five diverse plant species P. emblica (W:P), B. monnieri (W:B), T. sinensis (W:T), O. basilicum (W:O), and A. racemosus (W:A) exhibiting comparatively better antioxidant activities due to efficient three fractional methanolic extraction of their phytochemicals. The ability to scavenge chain propagating radicals based on their antioxidant capacity possess most bioactive metabolites that support the results of HPTLC image-based chemometric fingerprint profiling of alkaloids, flavonoids, phenols, and terpenoids. The PCA and HCA-Heatmaps of the HPTLC data set revealed strong intensity and better clustering patterns for W:O/1:4, W:P/1:4, W:B/1:1, W:A/4:1, and W:T/1:4 in comparison with W. somnifera (W) based on their chemicals fingerprint. Naturally, the fractional combination revealed greater depth in differentiating the extracts based on their antioxidant effects by involving in their phytoconstituents contribution. Furthermore, our results showed that the methanolic dual herbal combinatorial extract might have good potential for an array of health-promoting antioxidant benefits leading to a curative path with its wide range of therapeutic applications. The present study has set a precedence for use of chemometric profiling for quantitatively deciphering phytoconstituents. This will be useful to several ayurvedic formulations which shall have a sound scientific background; hence the correlation of the outcomes of treatment procedures can become predictable and more personalized. In the current era of advancement in genomics; there can be an amalgamation of the chemometric profiling with the genetic data providing a wholesome picture of how several ayurvedic preparation/formulations may influence treatment regime to different subjects.
Source(s) of funding
None.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2020.10.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jain Chitra, Khatana Shivani, Vijayvergia Rekha. Bioactivity of secondary metabolites of various plants: a review. Int J Pharm Sci Res. 2019;10:494–504. doi: 10.13040/IJPSR.0975-8232.10(2).494-04. [DOI] [Google Scholar]
- 2.World Health Organization [WHO] 2019. WHO global report on traditional and complementary medicine; p. 2019. [Google Scholar]
- 3.Sharma G.N., Gupta G., Sharma P. A comprehensive review of free radicals, antioxidants, and their relationship with human ailments. Crit Rev Eukaryot Gene Expr. 2018;28:139–154. doi: 10.1615/CritRevEukaryotGeneExpr.2018022258. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 5.Chaudhari G.M., Mahajan R.T. Comparative antioxidant activity of twenty traditional Indian medicinal plants and its correlation with total flavonoid and phenolic content. Int J Pharmaceut Sci Rev Res. 2015;30:105–111. [Google Scholar]
- 6.Larson R.A. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. doi: 10.1016/0031-9422(88)80254-1. [DOI] [Google Scholar]
- 7.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceut J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parasuraman S., Thing G.S., Dhanaraj S.A. Polyherbal formulation: concept of ayurveda. Phcog Rev. 2014;8:73–80. doi: 10.4103/0973-7847.134229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N., Bhalla M., De Jager P., Gilca M. An overview on ashwagandha: a rasayana (rejuvenator) of ayurveda. Afr J Tradit, Complementary Altern Med. 2011;8:208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra C.L., Mehta V.L., Das P.K., Dhalla N.S. Studies on Withania-ashwagandha, Kaul. V. The effect of total alkaloids (ashwagandholine) on the central nervous system. Indian J Physiol Pharmacol. 1965;9:127–136. [PubMed] [Google Scholar]
- 11.Mishra L.C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alternative Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 12.J J. Therapeutic potential of Withania somnifera: a report on phyto-pharmacological properties. Int J Pharm Sci Res. 2014;5:2131–2148. doi: 10.13040/IJPSR.0975-8232.5(6).2131-48. [DOI] [Google Scholar]
- 13.Alam N., Hossain M., Khalil M.I., Moniruzzaman M., Sulaiman S.A., Gan S.H. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochemistry Rev. 2012;11:97–112. doi: 10.1007/s11101-011-9221-5. [DOI] [Google Scholar]
- 14.Haque M.A., Jantan I., Abbas Bukhari S.N. vol. 207. Elsevier Ireland Ltd; 2017. (Tinospora species: an overview of their modulating effects on the immune system). [DOI] [PubMed] [Google Scholar]
- 15.Hegde S., Jayaraj M. A review of the medicinal properties, phytochemical and biological active compounds of Tinospora sinensis (Lour.) merr. J Biol Act Prod Nat. 2016;6:84–94. doi: 10.1080/22311866.2016.1185968. [DOI] [Google Scholar]
- 16.Bhandari P., Kamdod M. Emblica officinalis (Amla): a review of potential therapeutic applications. Int J Green Pharm. 2012;6:257–269. doi: 10.4103/0973-8258.108204. [DOI] [Google Scholar]
- 17.Patel S., Goyal R.K. Emblica officinalis geart.: a comprehensive review on phytochemistry, pharmacology and ethnomedicinal uses. Res J Med Plant. 2012;6:6–16. doi: 10.3923/rjmp.2012.6.16. [DOI] [Google Scholar]
- 18.Variya B.C., Bakrania A.K., Patel S.S. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res. 2016;111:180–200. doi: 10.1016/j.phrs.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Krishnaveni M., Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21:93–105. doi: 10.1515/jbcpp.2010.21.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Hashem-Dabaghian F., Ziaee M., Ghaffari S., Nabati F., Kianbakht S. A systematic review on the cardiovascular pharmacology of Emblica officinalis Gaertn. J Cardiovasc Thorac Res. 2018;10:118–128. doi: 10.15171/jcvtr.2018.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deo Y.K. Critical review on pharmacological properties of Brahmi. Int J Ayurvedic Med. 2013;4:92–99. [Google Scholar]
- 22.Nemetchek M.D., Stierle A.A., Stierle D.B., Lurie D.I. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J Ethnopharmacol. 2017;197:92–100. doi: 10.1016/j.jep.2016.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacopa monniera. Monograph. Altern Med Rev. 2004;9:79–85. [PubMed] [Google Scholar]
- 24.Russo A., Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Khair-ul-Bariyah S., Ahmed D., Ikram M. Ocimum basilicum: a review on phytochemical and pharmacological studies. Pakistan J Chem. 2012;2:78–85. doi: 10.15228/2012.v02.i02.p05. [DOI] [Google Scholar]
- 26.Marwat S.K., Fazal-Ur-Rehman, Khan M.S., Ghulam S., Anwar N., Mustafa G. Phytochemical constituents and pharmacological activities of sweet Basil-Ocimum basilicum L. (Lamiaceae) Asian J Chem. 2011;23:3773–3782. [Google Scholar]
- 27.Ch M., Naz S., Sharif A., Akram M., Saeed M. Biological and pharmacological properties of the sweet basil (Ocimum basilicum) Br J Pharmaceut Res. 2015;7:330–339. doi: 10.9734/bjpr/2015/16505. [DOI] [Google Scholar]
- 28.Sestili P., Ismail T., Calcabrini C., Guescini M., Catanzaro E., Turrini E. The potential effects of Ocimum basilicum on health: a review of pharmacological and toxicological studies. Expet Opin Drug Metabol Toxicol. 2018;14:679–692. doi: 10.1080/17425255.2018.1484450. [DOI] [PubMed] [Google Scholar]
- 29.Singla R., Jaitak V. SHATAVARI (ASPARAGUS racemosus wild): a review on its cultivation, morphology, phytochemistry and pharmacological importance. Int J Pharm Sci Res. 2014;5:742–757. doi: 10.13040/IJPSR.0975-8232.5(3).742-57. [DOI] [Google Scholar]
- 30.Singh R. Asparagus racemosus : a review on its phytochemical and therapeutic potential. Nat Prod Res. 2016;30:1896–1908. doi: 10.1080/14786419.2015.1092148. [DOI] [PubMed] [Google Scholar]
- 31.Goyal R.K., Singh J., Lal H. Asparagus racemosus - an update. Indian J Med Sci. 2003;57(9):408–414. [PubMed] [Google Scholar]
- 32.Pandey A.K., Gupta A., Tiwari M., Prasad S., Pandey A.N., Yadav P.K. Impact of stress on female reproductive health disorders: possible beneficial effects of shatavari (Asparagus racemosus) Biomed Pharmacother. 2018;103:46–49. doi: 10.1016/j.biopha.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Hussain A., Ahmad P., Wahab S., Hussain S., Ali M. A review on pharmacological and phytochemical profile of Asparagus racemosus Willd. Pharmacologyonline. 2011;3:1353–1364. [Google Scholar]
- 34.Singh R., Kaur R., Arora S., Jaitak V. In-vitro anti-mutagenic activity of Asparagus racemosus: an ayurvedic medicinal plant. Am J Drug Discov Dev. 2013;3:286–292. [Google Scholar]
- 35.Trease G.E.E.W.C. vol. 15e. Saunders Co Ltd Singapore; 1989. (Trease and Evans’ pharmacognosy). [Google Scholar]
- 36.Harborne J.B. 3rd ed. Chapman Hall; 1998. Phytochemical methods A guide to modern tecniques of plant analysis. [DOI] [Google Scholar]
- 37.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 38.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 39.Ristivojević P., Trifković J., Vovk I., Milojković-Opsenica D. Comparative study of different approaches for multivariate image analysis in HPTLC fingerprinting of natural products such as plant resin. Talanta. 2017;162:72–79. doi: 10.1016/j.talanta.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Olech M., Komsta Ł., Nowak R., Cieśla Ł., Waksmundzka-Hajnos M. Investigation of antiradical activity of plant material by thin-layer chromatography with image processing. Food Chem. 2012;132:549–553. doi: 10.1016/j.foodchem.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 41.Alaerts G., Dejaegher B., Smeyers-Verbeke J., Vander Heyden Y. Recent developments in chromatographic fingerprints from herbal products: set-up and data analysis. Comb Chem High Throughput Screen. 2010;13:900–922. doi: 10.2174/138620710793360284. [DOI] [PubMed] [Google Scholar]
- 42.Nn A. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromatic Plants. 2015;4:3–8. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- 43.Ahmad M.P., Hussain A., Wahab S., Ansari A.A., Singh S., Mishra C. Pharmacognostical and phytochemical evaluation of root of Asparagus racemosus Willd. J Drug Deliv Therapeut. 2017;7:76–80. doi: 10.22270/jddt.v7i6.1524. [DOI] [Google Scholar]
- 44.Pretorius E., Oberholzer H.M., Becker P.J. Comparing the cytotoxic potential of Withania somnifera water and methanol extracts. Afr J Tradit, Complementary Altern Med. 2009;6:275–280. doi: 10.4314/ajtcam.v6i3.57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathew M., Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PloS One. 2014;9 doi: 10.1371/journal.pone.0086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parveen R., Shamsi T.N., Kumar H., Fatima S. Phytochemical analysis and in vitro biological characterization of aqueous and methanolic extract of Bacopa monnieri. Int J Pharm Pharmaceut Sci. 2016;8:90–96. doi: 10.22159/ijpps.2016v8i12.14739. [DOI] [Google Scholar]
- 47.Upadhyay N., Ganie S.A., Agnihotri R.K., Sharma R. Free radical scavenging activity of Tinospora cordifolia (Willd) miers. J Pharmacogn Phytochem. 2014;3:63–69. [Google Scholar]
- 48.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in ayurvedic medicine. Phyther Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275:AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 49.Malve H., Raut S., Marathe P., Rege N. Effect of combination of Phyllanthus emblica, Tinospora cordifolia, and Ocimum sanctum on spatial learning and memory in rats. J Ayurveda Integr Med. 2014;5:209–215. doi: 10.4103/0975-9476.146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muruganandam A.V., Kumar V., Bhattacharya S.K. Effect of poly herbal formulation, EuMil, on chronic stress-induced homeostatic perturbations in rats. Indian J Exp Biol. 2002;40:1151–1160. [PubMed] [Google Scholar]
- 51.Bhattacharya S.K., Bhattacharya A., Chakrabarti A. Adaptogenic activity of Siotone, a polyherbal formulation of Ayurvedic rasayanas. Indian J Exp Biol. 2000;38:119–128. [PubMed] [Google Scholar]
- 52.Rastogi S., Narayan D., Harsh R. 2020. COVID-19 pandemic : a pragmatic plan for ayurveda intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han R.-M., Li D.-D., Chen C.-H., Liang R., Tian Y.-X., Zhang J.-P. Phenol acidity and ease of oxidation in isoflavonoid/β-carotene antioxidant synergism. J Agric Food Chem. 2011;59:10367–10372. doi: 10.1021/jf202683n. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo M., Sánchez-Moreno C., de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121:691–696. doi: 10.1016/j.foodchem.2009.12.097. [DOI] [Google Scholar]
- 55.Britton E.R., Kellogg J.J., Kvalheim O.M., Cech N.B. Biochemometrics to identify synergists and additives from botanical medicines: a case study with hydrastis canadensis (goldenseal) J Nat Prod. 2018;81:484–493. doi: 10.1021/acs.jnatprod.7b00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Junio H.A., Sy-Cordero A.A., Ettefagh K.A., Burns J.T., Micko K.T., Graf T.N. Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis) J Nat Prod. 2011;74:1621–1629. doi: 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AJ U. Phytochemical evaluation of Ocimum basilicum (Sweet basil) leaves collected from Abakaliki-Nigeria, using Gas chromatography-Mass Spectrometry. Adv Biomed Pharm. 2016;3:46–58. doi: 10.19046/abp.v03i01.07. [DOI] [Google Scholar]
- 58.Nagababu E., Rifkind J.M., Boindala S., Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol Biol. 2010 doi: 10.1007/978-1-60327-029-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parveen R., Shamsi T.N., Singh G., Athar T., Fatima S. Phytochemical analysis and In-vitro Biochemical Characterization of aqueous and methanolic extract of Triphala, a conventional herbal remedy. Biotechnol Rep. 2018;17:126–136. doi: 10.1016/j.btre.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birla H., Rai S.N., Singh S Sen, Zahra W., Rawat A., Tiwari N. Tinospora cordifolia suppresses neuroinflammation in parkinsonian mouse model. NeuroMolecular Med. 2019;21:42–53. doi: 10.1007/s12017-018-08521-7. [DOI] [PubMed] [Google Scholar]
- 61.M R R., A S., G G. Phytochemical investigation on withania somnifera root marc generated IN ayurvedic industry. Int Res J Pharm. 2013;4:223–227. doi: 10.7897/2230-8407.04844. [DOI] [Google Scholar]
- 62.Baumann J., Wurm G., von Bruchhausen F. Prostaglandin synthetase inhibition by flavonoids and phenolic compounds in relation to their O2-scavenging properties. Arch Pharm (Weinheim) 1980;313:330–337. doi: 10.1002/ardp.19803130409. [DOI] [PubMed] [Google Scholar]
- 63.Surveswaran S., Cai Y.Z., Corke H., Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–953. doi: 10.1016/j.foodchem.2006.06.033. [DOI] [Google Scholar]
- 64.Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- 65.Jamuna K.S., Ramesh C.K., Srinivasa T., Raghu K.L. In vitro antioxidant studies in some common fruits. Int J Pharm Pharmaceut Sci. 2011;3:60–63. [Google Scholar]
- 66.Gundeti M.S., Bhurke L.W., Mundada P.S., Murudkar S., Surve A., Sharma R. AYUSH 64, a polyherbal Ayurvedic formulation in Influenza like Illness: results of a pilot study. J Ayurveda Integr Med. 2020 doi: 10.1016/j.jaim.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.