Abstract
The gangliosidoses are lysosomal storage disorders caused by accumulation of GM1 or GM2 gangliosides. GM1 gangliosidosis has both central nervous system and systemic findings; while, GM2 gangliosidosis is restricted primarily to the central nervous system. Both disorders have autosomal recessive modes of inheritance and a continuum of clinical presentations from a severe infantile form to a milder, chronic adult form. Both are devastating diseases without cure or specific treatment however, with the use of supportive aggressive medical management, the lifespan and quality of life has been extended for both diseases. Naturally occurring and engineered animal models that mimic the human diseases have enhanced our understanding of the pathogenesis of disease progression. Some models have shown significant improvement in symptoms and lifespan with enzyme replacement, substrate reduction, and anti-inflammatory treatments alone or in combination. More recently gene therapy has shown impressive results in large and small animal models. Treatment with FDA-approved glucose analogs to reduce the amount of ganglioside substrate is used as off-label treatments for some patients. Therapies also under clinical development include small molecule chaperones and gene therapy.
Keywords: GM1 gangliosidosis, GM2 gangliosidosis, Tay Sachs disease, Animal models, Treatments
Background
The sphingolipidoses are rare, autosomal recessive neurodegenerative disorders resulting from accumulation of sphingolipid metabolites due to deficiencies in the catabolic enzymes required for their degradation (Figure 1). GM1 gangliosidosis is caused by mutations in GLB1 (chromosome 3p21.33) leading to decreased activity of β-galactosidase (β-GAL), and storage of GM1 ganglioside (Figure 2). When enzyme activity is decreased, sphingolipid metabolites accumulate in the lysosome and, thus, interfere with appropriate functioning of the organelle. The hallmark of GM1 gangliosidosis is progressive neurodegeneration and includes phenotypes that range from mild to severe based on the amount of residual enzyme activity as determined by the specific GLB1 mutations (1). A second disorder, mucopolysaccharidosis IVB (Morquio B disease) is also caused by mutations in GLB1 due to accumulation of keratan sulfate, a second substrate for β-GAL. Individuals with Morquio B have progressive skeletal changes but are cognitively normal and will not be further considered in this review (2).
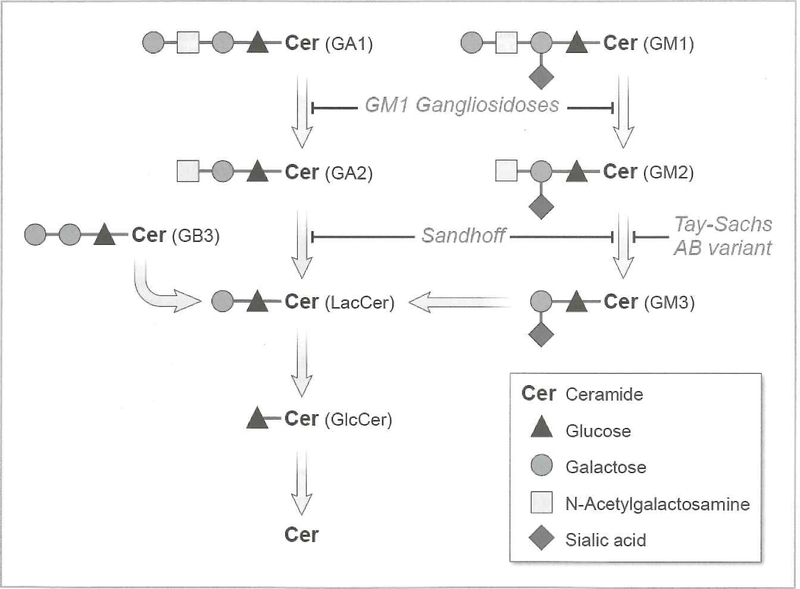
Figure 1. Sphingolipid Catabolism.
GM1 ganglioside is metabolized by β-galactosidase to form GW2 ganglioside. Abnormalities of this metabolic step lead to accumulation of GM1 ganglioside and GM1 gangliosidosis. GM2 ganglioside is converted to GM3 ganglioside by the action of β-hexosaminidase A. Deficiencies of this enzyme result in the accumulation of GM2 ganglioside and Tay-Sachs or Sandhoff disease
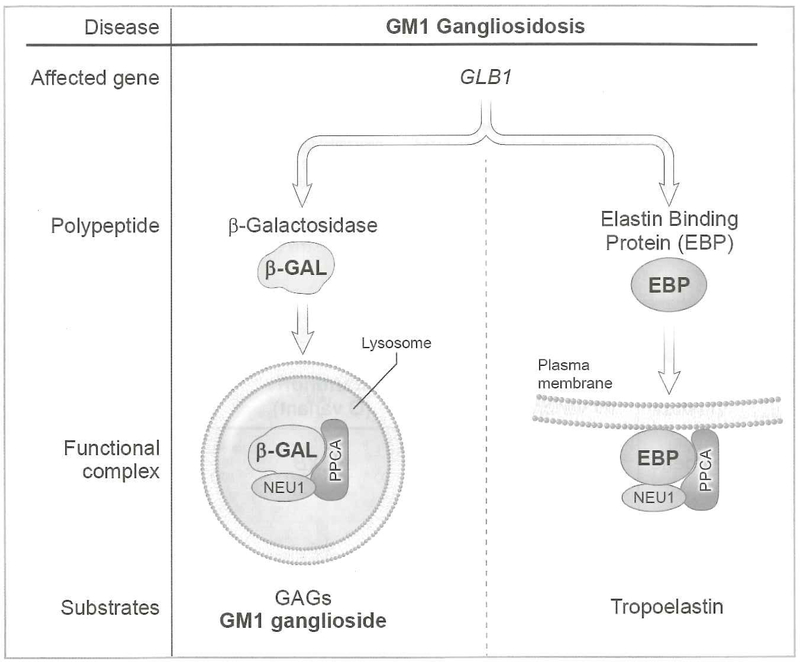
Figure 2. GM1 Gangliosidosis.
NEU1, PCCA, and β-GAL assemble in the lysosome. This complex is required for β-GAL to convert GM1 to GM2 ganglioside. Alternate splicing of BGAL leads to formation of EBP. This forms a membrane-associated complex with PCCA and NEU1 to metabolize tropoelastin to elastin fibers
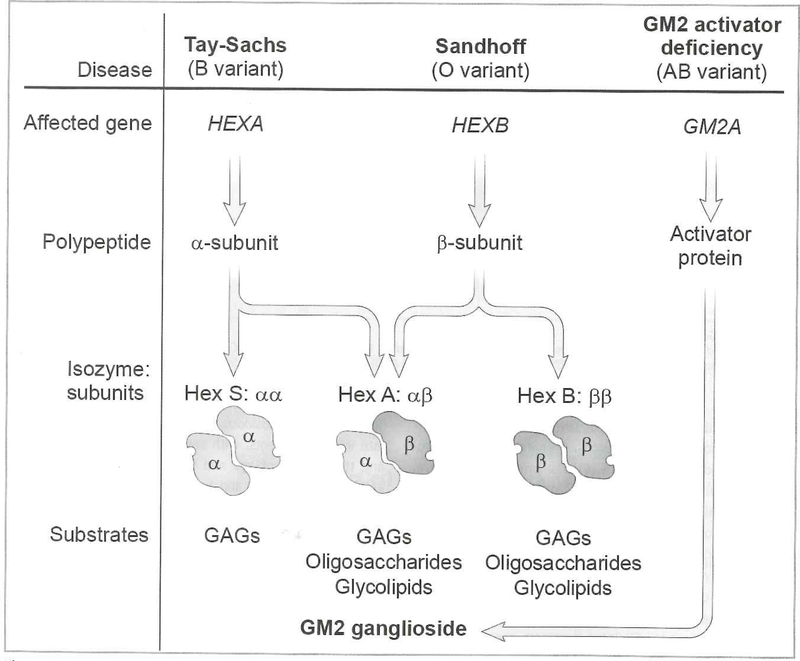
Progressive accumulation of GM2 ganglioside secondary to deficiency of the enzyme β-hexosaminidase A (Hex A) is the underlying cause of both Tay Sachs (TSD) and Sandhoff (SD) diseases (Figure 3). Hex A is a heterodimer composed of α and β subunits. TSD is caused by mutations in the HEXA gene (chromosome 15q24.1), encoding the α subunit. SD is caused by mutations in the HEXB gene (chromosome 5q13), causing deficiency of the β subunit (3). β-hexosaminidase B enzyme (HexB), a homodimer of the β subunit, also degrades glycosaminoglycans. A third protein, the GM2 activator, encoded by GM2A (chromosome 5q33.1) is also required for degradation of GM2 ganglioside. This lipid transport protein is required for extraction of GM2 ganglioside from the membrane for presentation to Hex A. Its deficiency results in a disorder clinically indistinguishable from infantile TSD.
Figure 3. GM2 Gangliasidoses.
Deficiencies in β-hexosaminidase A resulting from mutations in either HEX A (β subunit) or HEX B (β subunit) lead to Tay Sachs or Sandhoff disease respectively. Mutations in GM2A (GM2 activator protein) lead to GM2 activator deficiency.
GM1 Gangliosidosis Clinical Description and Natural History
Prospective natural history studies for GM1 gangliosidosis are lacking; however, several case series have been published (4,5), as has extensive documentation of more than 200 GM1 patients (6) (table 1).
Table 1.
Clinical and laboratory features of GM1 and GM2 gangliosidoses
| GM1 Gangliosidosis (incidence 1:100,000–1:200,000) | GM2 Gangliosidosis (incidence TSD 1:3,500–250,00; SD 1:300,000; GM2 activator <1:300,000) | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical Features | Infantile (I) | Late Infantile (IIA) | Juvenile (IIB) | Adult (III) | Infantile (I) | Juvenile (II) | Adult (III) | GM2 Activator Deficiency |
| Age of onset | 3–6 mo. | 1–3 yrs. | 3–5 yrs. | 10+ yrs. | 4–6 mo. | 2–10 yrs. | >10 yrs. | 4–6 mo. |
| Ophthalmologic | CRM | CC | CC | +/−CC | CRM | − | − | CRM |
| Motor decline | +++ | ++ | + | +/− | +++ | ++ | + | +++ |
| Cognitive decline | +++ | ++ | + | +/− | +++ | ++ | +/− | +++ |
| Skeletal abnormalities | + | + | +/− | + | − | − | − | − |
| Hepatosplenomegaly | + | + | +/− | − | −TSD/+SD | − | − | − |
| Cardiomyopathy | + | +/− | +/− | +/− | − | − | − | − |
| Psychiatric symptoms | − | − | − | − | − | − | +/− | − |
| Ethnic predisposition | B | R, B | R | J | AJ, FC, Caj | − | AJ | − |
| Population Carrier Screening | − | − | − | − | + | − | + | − |
| Mutations documented | GLB1=160 (not including Morquio B mutations)* | HEXA=176, HEXB=88* | GM2A=7* | |||||
| Animal Models | M, C, D, B, S | M,F,S | M | |||||
Incidence given as the number of patients per live births. Ranges are noted for TSD and GM1 gangliosidosis due to populations with higher incidence rates. The overall incidence is the lower population frequency.
HGMD Professional 2014.4 accessed 1/22/2015
CRM=cherry red macula
CC=corneal clouding
R=Roma
J=Japanese
AJ=Ashkenazi Jewish
FC=French Canadian
Caj=Cajun
B=Brazilian
M=Mouse
C=Cat
S=Sheep
F=Flamingo
B=Bear
D=Dog
Type I (infantile) GM1 is the most severe form with onset of symptoms prior to age 12 months. Prenatal manifestations can also include hydrops fetalis (6). The primary findings are severe central nervous system (CNS) dysfunction with early developmental delay, hypotonia and an exaggerated startle response, followed by spasticity and rapid regression. At the end of the first year, most infants are blind and deaf with severe CNS dysfunction leading to decerebrate rigidity (7). Some infants can have cardiomyopathy, hepatosplenomegaly, and most have poor feeding. Seizures and coarsened facial features are common. Skeletal dysplasia can be seen at diagnosis, is progressive, and leads to morbidity including restrictive lung disease in this group of patients. Death ensues at 2 to 4 years often due to aspiration pneumonia (1).
Type II GM1 gangliosidosis has been further sub-divided into late infantile patients, with onset of symptoms between one and three years of age and life expectancy between five and ten years of age, and juvenile, with onset of symptoms between ages three and ten years with life expectancy into the third decade. Disease progression is notable for plateauing of motor and cognitive development followed by slow developmental regression. The juvenile form often includes skeletal dysplasia but of variable severity. The earliest symptoms are often slurred speech and difficulty with ambulation. Patients have a slow, but unremitting, regression of milestones in both sub-populations of Type II disease (1).
Type III (adult/chronic) GM1 gangliosidosis has been best characterized in the Japanese population. Onset of symptoms is in late childhood to the third decade, typically presenting with generalized dystonia leading to unsteady gait and speech disturbance (7). However, within a short period of time, most patients (64%) have extrapyramidal signs including akinetic-rigid parkinsonism. The symptom cluster is similar to the extrapyramidal signs in Parkinson disease, a common misdiagnosis (8). The natural history of the disease is related to the level of neurological impairment and is often noted late in the disease. The vast majority of patients (95%) have skeletal abnormalities such as short stature, kyphosis, and scoliosis which are rarely life threatening but can cause significant morbidity. The life span of adults with Type III disease is shorter than their unaffected relatives (7).
GMI 1 Gangliosidosis Pathologic Basis of Disease/Animal Models
β-GAL is part of a 3-enzyme lysosomal multienzyme complex that also includes the carboxypeptidase protein protective protein/cathepsin A (PPCA) and the lysosomal sialidase neuraminidase-1 (NEU1) (Figure 2). Deficiencies of these proteins give rise to the rare autosomal recessive disorders galactosialidosis and sialidosis, respectively. NEU1 and β-GAL associate with the precursor form of PPCA in an early biosynthetic compartment and are subsequently chaperoned in complex with PPCA to lysosomes where they acquire their full catalytic activity. Another related enzyme complex bound to the cell surface elastin receptor has been identified at the plasma membrane of a number of human cell types. This complex is composed of PPCA, NEU1, and elastin binding protein (EBP) (9). EBP, a β-GAL-specific lectin, is the product of alternative splicing of GLB1 mRNA that shares most of its amino acid sequence with β-GAL but is catalytically inactive and does not localize to lysosomes (10). EBP functions as a chaperone for tropoelastin and facilitates the extracellular deposition of elastic fibers onto the microfibrillar scaffold. Mutations in GLB1 that disrupt the binding function of EBP in addition to decreasing enzyme activity of β-GAL have been reported in GM1 patients with cardiomyopathy (11).
Study of the knockout mouse model of GM1 gangliosidosis (β-gal −/− mouse) has been particularly helpful in elucidating the pathogenesis of neurodegeneration in GM1 gangliosidosis due to its similarity with the human disease. The mice develop severe neurodegeneration with tremors, ataxia, and gait abnormalities and subsequent paralysis of hind limbs. The mice have massive and progressive accumulation of GM1 ganglioside throughout the brain and spinal cord. This accumulation is associated with loss of motor function and widespread CNS inflammation. The brain and spinal cord undergo morphological changes due to lysosomal distention and GM1 accumulation and extensive apoptosis is visualized in the CNS as early as one month of age (12,13).
The accumulation of GM1 ganglioside at the membrane of the endoplasmic reticulum (ER) induces ER stress. This leads to activation of the unfolded protein response (UPR) and mitochondria-mediated neuronal apoptosis due to impaired cellular Ca2+ homeostasis within glycosphingolipid-enriched microdomains at the interface between ER and mitochondrial membranes (14,15).
Several naturally occurring animal models of GM1 gangliosidosis have been reported, including cats, dogs, brown bears, and sheep (16–19). One of the best characterized is the cat where the specific mutations impair transit of β-GAL protein to lysosomes (20). In feline fibroblasts, abnormal protein folding was observed, consistent with the data from the mouse model and suggesting that the UPR and subsequent apoptotic signaling contributes to pathogenesis and disease progression in GM1 gangliosidosis.
GM2 Gangliosidosis Clinical Description / Natural History
Several key natural history studies in infantile (21) and juvenile (22) patients have helped to characterize this devastating disease. Although rare in the general population, infantile TSD is better known than GM1 gangliosidosis due to its historically high incidence and increased carrier frequency in the Ashkenazi Jewish, French Canadian, and Cajun populations. Effective carrier screening programs have dramatically decreased the number of infants born with TSD in these populations. Based on retrospective analysis of parent surveys, historical databases, and review of the literature, Bley et al. characterized infantile TSD and SD patients with developmental arrest, exaggerated startle response, and hypotonia. The average age of symptom onset was 5 months, with average age at diagnosis of 13 months (21). Interestingly, infants gained early developmental milestones at the expected ages, then abruptly plateaued and began to regress. Historical databases from the National Tay-Sachs and Allied Diseases Association indicated a lifespan of less than 3 years, as compared with a greater than four year lifespan in more recently surveyed patients indicating that more aggressive medical interventions such as gastrostomy placement and vigorous pulmonary toilet improve lifespan (21). The finding of a cherry red macula is often the first key to diagnosis of infantile TSD and SD although it is not specific to GM2 gangliosidosis.
The natural history of 21 juvenile GM2 gangliosidosis patients (15 TSD, 6 SD) was described (22). The average age of symptom onset was 5.3 years with gait disturbance and incoordination as the most common findings. Juvenile SD patients were more likely to have psychiatric and neuropathic findings than the TSD patients; while TSD patients had more dysphagia, incontinence and disordered sleep. Most juvenile GM2 patients succumb to their illness by the second decade (23).
Late-Onset Tay-Sachs (LOTS) patients have been described in multiple small studies with initial symptoms of gait disturbance and balance beginning in the late teens (24–26). Difficulty climbing stairs is a common initial finding. Psychiatric illness can also be a presenting feature and an ongoing co-morbidity in many patients with LOTS (23). LOTS patients are often misdiagnosed until well into their 3rd or 4th decade. The level of cognitive disability is variable and primarily affects processing speed, visual sequencing and set shifting (27). Abnormalities in saccadic eye movements were found in two small cohorts (28). Neuroimaging showed atrophy and decreased N-acetyl aspartate by magnetic resonance spectroscopy (29,30).
GM2 Gangliosidosis Pathologic Basis of Disease/Animal Models
Naturally occurring animal models exist for the three major forms of GM2 gangliosidosis. Cats (31) and golden retriever dogs (32), have been described with Hex B deficiency and serve as models of Sandhoff disease. Hex A deficiency has been described in the Japanese Chin dog (33), Jacobs sheep (34), and flamingo (35). GM2 activator deficiency has been identified in the Japanese spaniel (36).
Models of the GM2 gangliosidoses have been established in the mouse by gene targeting technologies. Knockout of the HEXA gene yielded models with some phenotypic similarities to TSD including virtually no Hex A enzyme activity, GM2 ganglioside storage and neuronal pathology, but surprisingly was without early acute neurologic manifestations (37,38). In contrast, gene targeted mice with a null HEXB gene, characteristic of Sandhoff disease, showed an early neurologic phenotype that included tremors, ataxia, paralysis and early demise generally between 3 to 4 months of age (38,39). Brains from the mice contained large amounts of stored GM2 ganglioside as well as GA2 glycolipid (asialo-GM2). Storage pathology was widespread throughout the CNS. At late stages of the disease, apoptotic neurons were present in the CNS particularly in the spinal cord, thalamus and brain stem (38,40). Prior to the onset of neuronal death, a vigorous innate immune reaction was apparent characterized by elevation of pro-inflammatory cytokines, activated microglia and infiltrating monocytes (40,41). An astrogliosis reaction was also prominent (42).
The large phenotypic difference between the HEXA and HEXB gene knockout mice was unexpected given the early onset of acute neurodegeneration characteristic of both infantile TSD and SD patients. The basis for the absence of an acute neurodegenerative phenotype in the HEXA knockout mice was found to be due to a “by-pass” pathway for the degradation of GM2 ganglioside in mice that is not prominent in humans (39).
In this ‘by-pass” pathway, GM2 ganglioside is acted upon by a sialidase to yield GA2 glycolipid (asialo-GM2 ganglioside), which can be subsequently degraded by the Hex B still present in the HEXA knockout mice. In humans, this “by-pass” degradation of GM2 must not operate at an appreciable rate because of the very high levels of GM2 storage in TSD brain.
A mouse model of the GM2 activator deficiency (AB variant of GM2 gangliosidosis) was also established by gene disruption (43). These GM2 activator deficient mice displayed a phenotype that was of intermediate severity between the mildly affected HEXA knockout mice and the very severely affected HEXB knockout mice. The GM2 activator knockout mice, like the HEXA knockout mice showed neuronal storage that was more regionally restricted than the HEXB knockout mice, but with additional significant storage in the cerebellum. The majority of the lipid storage was GM2 ganglioside with lesser amounts of GA2 glycolipid. Consistent with storage in cerebellum, the GM2 activator knockout mice exhibited defects in motor function not found in similarly aged HEXA knockout mice. However, the GM2 activator knockout mice, unlike the HEXB knockout mice, survived for greater than a year.
Models of late onset Tay-Sachs (LOTS) disease, have been derived from HEXA knockout mice (44,45). After one year of age the majority of HEXA knockout mice were found to exhibit some clinical signs of neurological disease. However, 100% of female mice could be induced to a symptomatic condition after having at least four litters. The symptoms of the late onset phenotype included hind limb weakness, tremors, impaired motor coordination and balance, and ataxia.
A novel model of GM2 gangliosidoses was derived by crossing the HEXA and HEXB knockout mice to produce double knockout mice with a total lysosomal β-hexosaminidase deficiency (absence of β-hexosaminidase A, B and S) (46,47). Surprisingly, these mice, in addition to gangliosidosis, also displayed phenotypic, biochemical and cellular features of mucopolysaccharidosis illustrating a crucial role for the lysosomal β-hexosaminidases in the degradation of glycosaminoglycans.
Therapy for GM1 and GM2 Gangliosidosis
Animal Models
Therapy for the GM1 and GM2 gangliosidoses in animal models and human patients has proceeded along similar lines and will be treated collectively. Pathology in murine models of both GM1 and GM2 gangliosidosis shows widespread glycosphingolipid storage throughout the brain as well as inflammation as demonstrated by microglial activation and increased cytokine production by immunohistochemical staining and apoptosis (40,48–50). Improvement in CNS pathology and/or increased survival has been demonstrated in murine models of Sandhoff disease (40,51) and GM1 gangliosidosis (50), respectively, following bone marrow transplantation (BMT), although an earlier study in the GM1 canine did not show improvement (52). Intracranial transplant of neuronal stem cells in Sandhoff disease mice showed preserved motor function, improved survival, reduced ganglioside storage, increased Hex A activity, and diminished activation of microglial cells (53). Likewise, intracerebral cell transplantation of fetal brain cells and/or mesenchymal stem cells into GM1 mice resulted in temporary engraftment and decrease in GM1 ganglioside (54). Direct intraventricular injection of highly mannosylated Hex A enzyme improved motor function, increased longevity and decreased substrate accumulation in SD mice (55). Using intracerebroventricular injection of a novel chimeric Hex B subunit containing amino acid substitutions from the α-subunit, critical for binding to the GM2 activator protein, Matsuoka et al (56) showed restoration of enzyme activity, and decrease in storage of GM2 ganglioside in SD mice. The authors suggest that chimeric enzyme could be used as a less antigenic enzyme replacement therapy in Tay-Sachs disease patients.
By partially inhibiting glycosphingolipid biosynthesis, substrate reduction therapy (SRT) with glucose analog, N-butyldeoxynorjirimycin (NB-DNJ), improved neurologic function, reduced brain ganglioside, and increased survival in SD mice (57,58), and was synergistic in SD mice undergoing BMT (59). SRT using the galactose analog N-butyldeoxygalactonorjirimycin (NB-DGJ) showed greater efficacy and fewer side effects than NB-DNJ (60). Likewise in the GM1 mouse model, treatment with NB-DNJ and NB-DGJ both resulted in improved survival and behavioral outcomes; NB-DGJ was better tolerated but NB-DNJ showed greater improvement possibly due to greater mitigation of CNS inflammation (61,62). Treatment of SD mice with non-steroidal anti-inflammatory drugs indomethacin and aspirin resulted in a small incremental increase in lifespan and was synergistic when administered in combination with NB-DNJ (63).
Pharmacologic chaperones are low molecular weight compounds that are both substrate analogs and competitive inhibitors of lysosomal hydrolases that stabilize the folding of mutant proteins and facilitate their transport to lysosomes. In transgenic mice expressing the GLB1 common missense mutation R201C, chaperone N-octyl-4-epi-beta-valienamine increased β-GAL activity in the CNS, decreased brain GM1 ganglioside accumulation, improved neurologic function, and prolonged survival (64,65).
Early attempts at gene therapy for neurodegenerative disorders were limited by lack of tropism to the CNS. More recently, prevention of neurodeterioration with long term survival of SD mice was achieved using stereotactic intracerebral injections of recombinant adeno-associated virus (rAAV), rAAV2, containing both α and β subunits of Hex A (66,67). Further studies documented the requirement for pre-symptomatic or early symptomatic initiation of therapy for optimal outcomes (68). Intravenous injection of rAAV 9-Hex B, a viral strain tropic to the CNS, into SD mouse neonates resulted in improved neurologic function and increased survival (69). Scaling intracerebral injection gene therapy to a larger brain, Bradbury (70) showed greatly improved function and long term survival in SD cats receiving rAAVrh8 containing both α and β subunits of feline Hex A. In a similar fashion, AAV-mediated delivery of β-GAL by intracerebroventricular injection into neonatal GM1 mice resulted in widely distributed β-GAL enzyme and normalization of glycosphingolipid levels and cholesterol distribution (71). Direct thalamic infusion and injection into deep cerebellar nuclei of AAV2/1- β-GAL vector in adult GM1 mice resulted in distribution of enzyme activity throughout the brain and complete reduction of GM1 storage in all regions except the spinal cord (partial reduction) (72). Scaling of this technique to the larger GM1 feline brain has resulted in symptom-free survival beyond 38 months compared to 8 months for untreated animals (73).
Human Patients
GM1 and GM2 gangliosidoses are uniformly fatal neurodegenerativo disorders with no proven effective therapy. Based on findings in the animal models, a number of interventions have been undertaken in single cases or small cohorts in order to mitigate the relentless progression of disease. Bone marrow transplantation was not successful in treating the neurologic complications in case reports of juvenile GM1 or GM2 gangliosidosis (74,75). Cord blood transplantation in infantile GM2 gangliosidosis was likewise unsuccessful (76). Substrate reduction therapy with the imino sugar, NB-DNJ (miglustat), had the same safety and side effect profile at the approved dose as type 1 Gaucher disease (77,78). No improvement in neurologic impairment was documented in infantile (79), juvenile (77,80,81) or late onset GM2 gangliosidosis (78) although temporary stabilization of disease was seen in some patients. Chaperone therapy with pyrimethamine in late onset GM2 patients demonstrated increases in Hex A activity in plasma (82) and lymphocytes (83) but clinical improvement was either variable or not evaluated. Deep brain stimulation in a case of type III GM1 gangliosidosis showed functional improvement of dystonia but no change in disease progression (84).
Summary
The gangliosidoses are inherited, uniformly fatal neurodegenerativo disorders of variable onset and disease progression. Detailed natural history studies to characterize disease progression and identify relevant biomarkers have been limited since the site of pathology is primarily the brain. Pathogenesis in animal models has uncovered avenues for therapeutic intervention. Concurrently, biomarker and imaging studies to identify outcome measures for human clinical trials are ongoing in many centers. Recent reports in small and large animal models utilizing small molecules and targeted gene therapy are encouraging. Convergence of therapeutic studies in model systems and natural history studies in human patients are expected to lead to clinical trials in the near future. Designs for such trials in GM1 and GM2 patients are currently in progress.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Human Genome Research Institute (DR and CT) and National Institute of Diabetes and Digestive and Kidney Diseases (RP). A.d’A. holds the Jewelers for Children Endowed Chair in Genetics and Gene Therapy. A.d’A research was funded in part by NIH grants GM60905 and DK52025, the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities (ALSAC) and the National Tay-Sachs & Allied Disease Association (NTSAD).
Abbreviations:
- NEU1
Alpha neuraminidase
- β-GAL
β-galactosidase enzyme
- CNS
Central nervous system
- EBP
Elastin binding protein
- ER
Endoplasmic reticulum
- Hex A
β-hexosaminidase A
- Hex B
β-hexosaminidase B
- LOTS
Late onset Tay Sachs disease
- NB-DGJ
N-butyldeoxygalactonorjirimycin
- NB-DNJ
N-butyldeoxynorjirimycin
- PPCA
Protective protein cathepsin A
- rAAV
Recombinant adeno-associated virus
- SD
Sandhoff disease
- SRT
Substrate reduction therapy
- UPR
Unfolded protein response
- TSD
Tay Sachs disease
Footnotes
Disclosure
The authors have no conflicts of interest to declare
References
- 1.Regier DS, Tifft CJ. GLB1-Related Disorders. In: GeneReviews(R). Edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Smith RJH, Stephens K. Seattle (WA) 2013 [Google Scholar]
- 2.Regier DS, Oetgen M, Tanpaiboon P. Mucopolysaccharidosis Type IVA. In: GeneReviews(R). Edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K. Seattle (WA) 2015 [Google Scholar]
- 3.Schulze H, Sandhoff K. Sphingolipids and lysosomal pathologies. Biochim Biophys Acta 2014;1841:799–810 [DOI] [PubMed] [Google Scholar]
- 4.Hofer D, Paul K, Fantur K, Beck M, Roubergue A, Vellodi A, Poorthuis BJ, Michelakakis H, Plecko B, Paschke E. Phenotype determining alleles in GM1 gangliosidosis patients bearing novel GLB1 mutations. Clinical Genetics 2010;78:236–246 [DOI] [PubMed] [Google Scholar]
- 5.Caciotti A, Garman SC, Rivera-Colon Y, Procopio E, Catarzi S, Ferri L, Guido C, Martelli P, Parini R, Antuzzi D, Battini R, Sibilio M, Simonati A, Fontana E, Salviati A, Akinci G, Cereda C, Dionisi-Vici C, Deodato F, d’Amico A, d’Azzo A, Bertini E, Filocamo M, Scarpa M, di Rocco M, Tifft CJ, Ciani F, Gasperini S, Pasquini E, Guerrini R, Donati AAA, Morrone A. GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochimica et Biophysica Acta 2011;1812:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetti-Pierri N, Scaglia F. GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Molecular Genetics and Metabolism 2008;94:391–396 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y NE, Matsuda J, Higaki K, Oshima A. The Online Metabolic and Molecular Bases of Inherited Disease. β-Galactosidase Deficience (β-Galanctosidosis): GM1 Gangliosidosis and Morquio B Disease. Edited by Valle B, Vogelstein Kinzler, Antorakis Ballabia, Scriver Childs, Sly 2008 [Google Scholar]
- 8.Roze E, Paschke E, Lopez N, Eck T, Yoshida K, Maurel-Ollivier A, Doummar D, Caillaud C, Galanaud D, Billette de Villemeur T, Vidailhet M, Roubergue A, Dystonia and parkinsonism in GM1 type 3 gangliosidosis. Movement Disorders: Official Journal of the Movement Disorder Society 2005;20:366–1369 [DOI] [PubMed] [Google Scholar]
- 9.Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem 2006;281:3698–3710 [DOI] [PubMed] [Google Scholar]
- 10.Morreau H, Galjart NJ, Gillemans N, Willemsen R, van der Horst GT, d’Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem 1989;264:20655–20663 [PubMed] [Google Scholar]
- 11.Caciotti A, Donati AAA, Boneh A, d’Azzo A, Federico A, Parini R, Antuzzi D, Bardelli T, Nosi D, Kimonis V, Zammarchi E, Morrone A. Role of beta-galactosidase and elastin binding protein in lysosomal and nonlysosomal complexes of patients with GM1-gangliosidosis. Human Mutation 2005;25:285–292 [DOI] [PubMed] [Google Scholar]
- 12.Bonten EJ, Annunziata I, d’Azzo A. Lysosomal multienzyme complex: pros and cons of working together. Cellular and molecular life sciences: CMLS 2014;71:2017–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Azzo A, Tessitore A, Sano R. Gangliosides as apoptotic signals in ER stress response. Cell Death and Differentiation 2006;13:404–414 [DOI] [PubMed] [Google Scholar]
- 14.Tessitore A, del PMM, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, d’Azzo A. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Molecular Cell 2004;15:753–766 [DOI] [PubMed] [Google Scholar]
- 15.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d’Azzo A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Molecular Cell 2009;36:500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamato O, Ochiai K, Masuoka Y, Hayashida E, Tajima M, Omae S, lijima M, Umemura T, Maede Y. GM1 gangliosidosis in shiba dogs. The Veterinary record 2000;146:493–496 [DOI] [PubMed] [Google Scholar]
- 17.Prieur DJ, Ahern-Rindell AJ, Murnane RD. Ovine GM-1 gangliosidosis. The American Journal of Pathology 1991;139:1511–1513 [PMC free article] [PubMed] [Google Scholar]
- 18.Yamato O, Endoh D, Kobayashi A, Masuoka Y, Yonemura M, Hatakeyama A, Satoh H, Tajima M, Yamasaki M, Maede Y. A novel mutation in the gene for canine acid beta-galactosidase that causes GM1-gangliosidosis in Shiba dogs. Journal of Inherited Metabolic Disease 2002;25:525–526 [DOI] [PubMed] [Google Scholar]
- 19.Muthupalani S, Torres PA, Wang BC, Zeng BJ, Eaton S, Erdelyi I, Ducore R, Maganti R, Keating J, Perry BJ, Tseng FS, Waliszewski N, Pokras M, Causey R, Seger R, March P, Tidwell A, Pfannl R, Seyfried T, Kolodny EH, Alroy J. GM1-gangliosidosis in American black bears: clinical, pathological, biochemical and molecular genetic characterization. Molecular Genetics and Metabolism 2014;111:513–521 [DOI] [PubMed] [Google Scholar]
- 20.Martin DR, Rigat BA, Foureman P, Varadarajan GS, Hwang M, Krum BK, Smith BF, Callahan JW, Mahuran DJ, Baker HJ. Molecular consequences of the pathogenic mutation in feline GM1 gangliosidosis. Molecular Genetics and Metabolism 2008;94:212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bley AE, Giannikopoulos OA, Hayden D, Kubilus K, Tifft CJ, Eichler FS. Natural history of infantile G(M2) gangliosidosis. Pediatrics 2011;128:e1233–e1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maegawa GH, Stockley T, Tropak M, Banwell B, Blaser S, Kok F, Giugliani R, Mahuran D, Clarke JT. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics 2006;118:e1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaback MM, Desnick RJ. Hexosaminidase A Deficiency. In: GeneReviews(R). Edited by Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K. Seattle (WA) 2011 [Google Scholar]
- 24.Neudorfer O, Pastores GM, Zeng BJ, Gianutsos J, Zaroff CM, Kolodny EH. Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genetics in medicine: Official Journal of the American College of Medical Genetics 2005;7:119–123 [DOI] [PubMed] [Google Scholar]
- 25.Neudorfer O, Kolodny EH. Late-onset Tay-Sachs disease. The Israel Medical Association Journal. IMAJ 2004;6:107–111 [PubMed] [Google Scholar]
- 26.Frey LC, Ringel SP, Filley CM. The natural history of cognitive dysfunction in late-onset GM2 gangliosidosis. Arch Neurol 2005;62:989–994 [DOI] [PubMed] [Google Scholar]
- 27.Zaroff CM, Neudorfer O, Morrison C, Pastores GM, Rubin H, Kolodny EH. Neuropsychological assessment of patients with late onset GM2 gangliosidosis. Neurology 2004;62:2283–2286 [DOI] [PubMed] [Google Scholar]
- 28.Rucker JC, Shapiro BE, Han YH, Kumar AN, Garbutt S, Keller EL, Leigh RJ. Neuro-ophthalmology of late-onset Tay-Sachs disease (LOTS). Neurology 2004;63:1918–1926 [DOI] [PubMed] [Google Scholar]
- 29.Inglese M, Nusbaum AO, Pastores GM, Gianutsos J, Kolodny EH, Gonen O. MR imaging and proton spectroscopy of neuronal injury in late-onset GM2 gangliosidosis. AJNR Am J Neuroradiol 2005;26:2037–2042 [PMC free article] [PubMed] [Google Scholar]
- 30.Streifler JY, Gornish M, Hadar H, Gadoth N. Brain imaging in late-onset GM2 gangliosidosis. Neurology 1993;43:2055–2058 [DOI] [PubMed] [Google Scholar]
- 31.Muldoon LL, Neuwelt EA, Pagel MA, Weiss DL. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease). The American Journal of Pathology 1994;144:1109–1118 [PMC free article] [PubMed] [Google Scholar]
- 32.Yamato O, Matsuki N, Satoh H, Inaba M, Ono K, Yamasaki M, Maede Y. Sandhoff disease in a golden retriever dog. Journal of Inherited Metabolic Disease 2002;25:319–320 [DOI] [PubMed] [Google Scholar]
- 33.Sanders DN, Zeng R, Wenger DA, Johnson GS, Johnson GC, Decker JE, Katz ML, Platt SR, O’Brien DP. GM2 gangliosidosis associated with a HEXA missense mutation in Japanese Chin dogs: a potential model for Tay Sachs disease. Molecular Genetics and Metabolism 2013;108:70–75 [DOI] [PubMed] [Google Scholar]
- 34.Porter BF, Lewis BC, Edwards JF, Alroy J, Zeng BJ, Torres PA, Bretzlaff KN, Kolodny EH. Pathology of GM2 gangliosidosis in Jacob sheep. Veterinary Pathology 2011;48:807–813 [DOI] [PubMed] [Google Scholar]
- 35.Zeng BJ, Torres PA, Viner TC, Wang ZH, Raghavan SS, Alroy J, Pastores GM, Kolodny EH. Spontaneous appearance of Tay-Sachs disease in an animal model. Molecular Genetics and Metabolism 2008;95:59–65 [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa Y, Li SC, Wood PA, Li YT. Biochemical basis of type AB GM2 gangliosidosis in a Japanese spaniel. Journal of Neurochemistry 1987;48:860–864 [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka S, Johnson MD, Grinberg A, Westphal H, Crawley JN, Taniike M, Suzuki K, Praia RL. Targeted disruption of the Hexa gene results in mice with biochemical and pathologic features of Tay-Sachs disease. Proceedings of the National Academy of Sciences of the United States of America 1994;91:9975–9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang JQ, Trasler JM, Igdoura S, Michaud J, Hanal N, Gravel RA. Apoptotic cell death in mouse models of GM2 gangliosidosis and observations on human Tay-Sachs and Sandhoff diseases. Human Molecular Genetics 1997;6:1879–1885 [DOI] [PubMed] [Google Scholar]
- 39.Sango K, Yamanaka S, Hoffmann A, Okuda Y, Grinberg A, Westphal H, McDonald MP, Crawley JN, Sandhoff K, Suzuki K, Praia RL. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nature Genetics 1995;11:170–176 [DOI] [PubMed] [Google Scholar]
- 40.Wada R, Tifft CJ, Praia RL. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proceedings of the National Academy of Sciences of the United States of America 2000;97:10954–10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu YP, Praia RL. Deletion of macrophage-inflammatory protein 1 alpha retards neurodegeneration in Sandhoff disease mice. Proceedings of the National Academy of Sciences of the United States of America 2004;101:8425–8430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu YP, Mizugishi K, Bektas M, Sandhoff R, Praia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Human Molecular Genetics 2008;17:2257–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Hoffmann A, Grinberg A, Westphal H, McDonald MP, Miller KM, Crawley JN, Sandhoff K, Suzuki K, Praia RL. Mouse model of GM2 activator deficiency manifests cerebellar pathology and motor impairment. Proceedings of the National Academy of Sciences of the United States of America 1997;94:8138–8143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Miklyaeva, Dong W, Bureau A, Fattahie R, Xu Y, Su M, Fick GH, Huang JQ, Igdoura S, Hanai N, Gravel RA. Late onset Tay-Sachs disease in mice with targeted disruption of the Hexa gene: behavioral changes and pathology of the central nervous system. Brain Research 2004;1001:37–50 [DOI] [PubMed] [Google Scholar]
- 45.Jeyakumar M, Smith D, Eliott-Smith E, Cortina-Borja M, Reinkensmeier G, Butters TD, Lemm T, Sandhoff K, Perry VH, Dwek RA, Platt FM. An inducible mouse model of late onset Tay-Sachs disease. Neurobiology of Disease 2002;10:201–210 [DOI] [PubMed] [Google Scholar]
- 46.Sango K, McDonald MP, Crawley JN, Mack ML, Tifft CJ, 5kop E, 5tarr CM, Hoffmann A, Sandhoff K, Suzuki K, Praia RL. Mice lacking both subunits of lysosomal beta-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nature Genetics 1996;14:348–352 [DOI] [PubMed] [Google Scholar]
- 47.Suzuki K, Sango K, Praia RL, Langaman C. Mice deficient in all forms of lysosomal beta-hexosaminidase show mucopolysaccharidosis-like pathology. Journal of Neuropathology and Experimental Neurology 1997;56:693–703 [PubMed] [Google Scholar]
- 48.Matsuda J, Suzuki O, Oshima A, Ogura A, Naiki M, Suzuki Y. Neurological manifestations of knockout mice with beta-galactosidase deficiency. Brain & Development 1997;19:19–20 [DOI] [PubMed] [Google Scholar]
- 49.Matsuda J, Suzuki O, Oshima A, Ogura A, Noguchi Y, Yamamoto Y, Asano T, Takimoto K, Sukegawa K, Suzuki Y, Naiki M. Beta-galactosidase-deficient mouse as an animal model for GM1-gangliosidosis. Glycoconjugate Journal 1997;14:729–736 [DOI] [PubMed] [Google Scholar]
- 50.Sano R, Tessitore A, Ingrassia A, d’Azzo A. Chemokine-induced recruitment of genetically modified bone marrow cells into the CNS of GM1-gangliosidosis mice corrects neuronal pathology. Blood 2005;106:2259–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norflus F, Tifft CJ, McDonald MP, Goldstein G, Crawley JN, Hoffmann A, Sandhoff K, Suzuki K, Praia RL. Bone marrow transplantation prolongs life span and ameliorates neurologic manifestations in Sandhoff disease mice. The Journal of Clinical Investigation 1998;101:1881–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brien JS, Storb R, Raff RF, Harding J, Appelbaum F, Morimoto S, Kishimoto Y, Graham T, Ahern-Rindell A, O’Brien SL. Bone marrow transplantation in canine GM1 gangliosidosis. Clin Genet 1990;38:274–280 [DOI] [PubMed] [Google Scholar]
- 53.Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee PJ, Baek RC, Clark D, Rose H, Fu G, Clarke J, McKercher S, Meerloo J, Muller FJ, Park Kl, Butters TD, Dwek RA, Schwartz P, Tong G, Wenger D, Lipton SA, Seyfried TN, Platt FM, Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nature Medicine 2007;13:439–447 [DOI] [PubMed] [Google Scholar]
- 54.Sawada T, Tanaka A, Higaki K, Takamura A, Nanba E, Seto T, Maeda M, Yamaguchi E, Matsuda J, Yamano T. Intracerebral cell transplantation therapy for murine GM1 gangliosidosis. Brain 6 Development 2009;31:717–724 [DOI] [PubMed] [Google Scholar]
- 55.Tsuji D, Akeboshi H, Matsuoka K, Yasuoka H, Miyasaki E, Kasahara Y, Kawashima I, Chiba Y, Jigami Y, Taki T, Sakuraba H, Itoh K. Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis. Ann Neurol 2011;69:691–701 [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka K, Tamura T, Tsuji D, Dohzono Y, Kitakaze K, Ohno K, Saito S, Sakuraba H, Itoh K. Therapeutic potential of intracerebroventricular replacement of modified human beta-hexosaminidase B for GM2 gangliosidosis. Molecular Therapy: The Journal of the American Society of Gene Therapy 2011; 19:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeyakumar M, Butters TD, Cortina-Borja M, Hunnam V, Praia RL, Perry VH, Dwek RA, Platt FM. Delayed symptom onset and increased life expectancy in Sandhoff disease mice treated with N-butyldeoxynojirimycin. Proc Natl Acad Sci USA 1999;96:6388–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arfi A, Zisling R, Richard E, Batista L, Poenaru L, Futerman AH, Caillaud C. Reversion of the biochemical defects in murine embryonic Sandhoff neurons using a bicistronic lentiviral vector encoding hexosaminidase alpha and beta. J Neurochem 2006;96:1572–1579 [DOI] [PubMed] [Google Scholar]
- 59.Jeyakumar M, Norflus F, Tifft CJ, Cortina-Borja M, Butters TD, Praia RL, Perry VH, Dwek RA, Platt FM. Enhanced survival in Sandhoff disease mice receiving a combination of substrate deprivation therapy and bone marrow transplantation. Blood 2001;97:327–329 [DOI] [PubMed] [Google Scholar]
- 60.Andersson U, Smith D, Jeyakumar M, Butters TD, Borja MC, Dwek RA, Platt FM. Improved outcome of N-butyldeoxygalactonojirimycin-mediated substrate reduction therapy in a mouse model of Sandhoff disease. Neurobiology of Disease 2004;16:506–515 [DOI] [PubMed] [Google Scholar]
- 61.Elliot-Smith E, Speak AO, Lloyd-Evans E, Smith DA, van der Spoel AC, Jeyakumar M, Butters TD, Dwek RA, d’Azzo A, Platt FM. Beneficial effects of substrate reduction therapy in a mouse model of GM1 gangliosidosis. Molecular Genetics and Metabolism 2008;94:204–211 [DOI] [PubMed] [Google Scholar]
- 62.Kasperzyk JL, d’Azzo A, Platt FM, Alroy J, Seyfried TN. Substrate reduction reduces gangliosides in postnatal cerebrum-brainstem and cerebellum in GM1 gangliosidosis mice. Journal of Lipid Research 2005;46:744–751 [DOI] [PubMed] [Google Scholar]
- 63.Jeyakumar M, Smith DA, Williams IM, Borja MC, Neville DC, Butters TD, Dwek RA, Platt FM. NSAIDs increase survival in the Sandhoff disease mouse: synergy with N-butyldeoxynojirimycin. Ann Neurol 2004;56:642–649 [DOI] [PubMed] [Google Scholar]
- 64.Suzuki Y, Ichinomiya S, Kurosawa M, Matsuda J, Ogawa S, lida M, Kubo T, Tabe M, Itoh M, Higaki K, Nanba E, Ohno K. Therapeutic chaperone effect of N-octyl 4-epi-beta-valienamine on murine G(M1)-gangliosidosis. Molecular Genetics and Metabolism 2012;106:92–98 [DOI] [PubMed] [Google Scholar]
- 65.Suzuki Y, Ichinomiya S, Kurosawa M, Ohkubo M, Watanabe H, Iwasaki H, Matsuda J, Noguchi Y, Takimoto K, Itoh M, Tabe M, lida M, Kubo T, Ogawa S, Nanba E, Higaki K, Ohno K, Brady RO. Chemical chaperone therapy: clinical effect in murine G(M1)-gangliosidosis. Annals of Neurology 2007;62:671–675 [DOI] [PubMed] [Google Scholar]
- 66.Cachon-Gonzalez MB, Wang SZ, Lynch A, Ziegler R, Cheng SH, Cox TM. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc Natl Acad Sci USA 2006;103:10373–10378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cachon-Gonzalez MB, Wang SZ, McNair R, Bradley J, Lunn D, Ziegler R, Cheng SH, Cox TM. Gene transfer corrects acute GM2 gangliosidosis-potential therapeutic contribution of perivascular enzyme flow. Molecular Therapy: The Journal of the American Society of Gene Therapy 2012;20:1489–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higaki K, Ninomiya H, Suzuki Y, Nanba E. Candidate molecules for chemical chaperone therapy of GM1-gangliosidosis. Future Medicinal Chemistry 2013;5:1551–1558 [DOI] [PubMed] [Google Scholar]
- 69.Walia JS, Altaleb N, Bello A, Kruck C, LaFave MC, Varshney GK, Burgess SM, Chowdhury B, Hurlbut D, Hemming R, Kobinger GP, Triggs-Raine B. Long Term Correction of Sandhoff Disease Following Intravenous Delivery of rAAV9 to Mouse Neonates. Mol Ther 2015;23:414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bradbury AM, Cochran JN, McCurdy VJ, Johnson AK, Brunson BL, Gray-Edwards H, Leroy SG, Hwang M, Randle AN, Jackson LS, Morrison NE, Baek RC, Seyfried TN, Cheng SH, Cox NR, Baker HJ, Cachon-Gonzalez MB, Cox TM, Sena-Esteves M, Martin DR. Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy. Molecular Therapy: The Journal of the American Society of Gene Therapy 2013;21:1306–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Broekman ML, Baek RC, Comer LA, Fernandez JL, Seyfried TN, Sena-Esteves M. Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Molecular Therapy: The Journal of the American Society of Gene Therapy 2007;15:30–37 [DOI] [PubMed] [Google Scholar]
- 72.Baek RC, Broekman ML, Leroy SG, Tierney LA, Sandberg AAA, d’Azzo A, Seyfried TN, Sena-Esteves M. AAV-mediated gene delivery in adult GM1 -gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PloS One 2010;5:e13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCurdy VJ, Johnson AK, Gray-Edwards HL, Randle AN, Brunson BL, Morrison NE, Salibi N, Johnson JA, Hwang M, Beyers RJ, Leroy SG, Maitland S, Denney TS, Cox NR, Baker HJ, Sena-Esteves M, Martin DR. Sustained normalization of neurological disease after intracranial gene therapy in a feline model. Science Translational Medicine 2014;6:231ra248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs JF, Willemsen MA, Groot-Loonen JJ, Wevers RA, Hoogerbrugge PM. Allogeneic BMT followed by substrate reduction therapy in a child with subacute Tay-Sachs disease. Bone Marrow Transplantation 2005;36:925–926 [DOI] [PubMed] [Google Scholar]
- 75.Shield JP, Stone J, Steward CG. Bone marrow transplantation correcting beta-galactosidase activity does not influence neurological outcome in juvenile GM1-gangliosidosis. Journal of Inherited Metabolic Disease 2005;28:797–798 [DOI] [PubMed] [Google Scholar]
- 76.Martin PL, Carter SL, Kernan NA, Sahdev I, Wall D, Pietryga D, Wagner JE, Kurtzberg J. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation 2006;12:184–194 [DOI] [PubMed] [Google Scholar]
- 77.Maegawa GH, van Giersbergen PL, Yang S, Banwell B, Morgan CP, Dingemanse J, Tifft CJ, Clarke JT. Pharmacokinetics, safety and tolerability of miglustat in the treatment of pediatric patients with GM2 gangliosidosis. Molecular Genetics and Metabolism 2009;97:284–291 [DOI] [PubMed] [Google Scholar]
- 78.Shapiro BE, Pastores GM, Gianutsos J, Luzy C, Kolodny EH. Miglustat in late-onset Tay-Sachs disease: a 12-month, randomized, controlled clinical study with 24 months of extended treatment. Genetics in Medicine: Official Journal of the American College of Medical Genetics 2009;11:425–433 [DOI] [PubMed] [Google Scholar]
- 79.Bembi B, Marchetti F, Guerci VI, Ciana G, Addobbati R, Grasso D, Barone R, Cariati R, Fernandez-Guillen L, Butters T, Pittis MG. Substrate reduction therapy in the infantile form of Tay-Sachs disease. Neurology 2006;66:278–280 [DOI] [PubMed] [Google Scholar]
- 80.Masciullo M, Santoro M, Modoni A, Ricci E, Guitton J, Tonali P, Silvestri G. Substrate reduction therapy with miglustat in chronic GM2 gangliosidosis type Sandhoff: results of a 3-year follow-up. J Inherit Metab Dis 2010;33(Suppl 3):S355–S361 [DOI] [PubMed] [Google Scholar]
- 81.Maegawa GH, Banwell BL, Blaser S, Sorge G, Toplak M, Ackerley C, Hawkins C, Hayes J, Clarke JT. Substrate reduction therapy in juvenile GM2 gangliosidosis. Mol Genet Metab 2009;98:215–224 [DOI] [PubMed] [Google Scholar]
- 82.Clarke JT, Mahuran DJ, Sathe S, Kolodny EH, Rigat BA, Raiman JA, Tropak MB. An open-label Phase l/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants). Mol Genet Metab 2011;102:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osher E, Fattal-Valevski A, Sagie L, Urshanski N, Amir-Levi Y, Katzburg S, Peleg L, Lerman-Sagie T, Zimran A, Elstein D, Navon R, Stern N, Valevski A. Pyrimethamine increases beta-hexosaminidase A activity in patients with Late Onset Tay Sachs. Mol Genet Metab 2011;102:356–363 [DOI] [PubMed] [Google Scholar]
- 84.Roze E, Navarro S, Cornu P, Welter ML, Vidailhet M. Deep brain stimulation of the globus pallidus for generalized dystonia in GM1 Type 3 gangliosidosis: technical case report. Neurosurgery 2006;59:E1340; discussion E1340 [DOI] [PubMed] [Google Scholar]