Abstract
Introduction
Emerging heated tobacco products (HTPs) were designed to reduce exposure to toxicants from cigarette smoke (CS) by avoiding burning tobacco and instead heating tobacco. We studied the effects of short-term inhalation of aerosols emitted from HTP called IQOS, on lung damage and immune-cell recruitment to the lungs in mice.
Methods
Numerous markers of lung damage and inflammation including albumin and lung immune-cell infiltrates, proinflammatory cytokines, and chemokines were quantified in lungs and bronchoalveolar (BAL) fluid from IQOS, CS, or air-exposed (negative control) mice.
Results
Importantly, as a surrogate marker of lung epithelial-cell damage, we detected significantly increased levels of albumin in the BAL fluid of both HTP- and CS-exposed mice compared with negative controls. Total numbers of leukocytes infiltrating the lungs were equivalent following both IQOS aerosols and CS inhalation and significantly increased compared with air-exposed controls. We also observed significantly increased numbers of CD4+IL-17A+ T cells, a marker of a T-cell immune response, in both groups compared with air controls; however, numbers were the highest following CS exposure. Finally, the numbers of CD4+RORγt+ T cells, an inflammatory T-cell subtype expressing the transcription factor that is essential for promoting differentiation into proinflammatory Th17 cells, were significantly augmented in both groups compared with air-exposed controls. Levels of several cytokines in BAL were significantly elevated, reflecting a proinflammatory milieu.
Conclusions
Our study demonstrates that short-term inhalation of aerosols from IQOS generates damage and proinflammatory changes in the lung that are substantially similar to that elicited by CS exposure.
Implications
Exposure of mice to IQOS, one of the candidate modified-risk tobacco products, induces inflammatory immune-cell accumulation in the lungs and augments the levels of proinflammatory cytokines and chemokines in the BAL fluid. Such an exacerbated pulmonary proinflammatory microenvironment is associated with lung epithelial-cell damage in IQOS-exposed mice, suggesting a potential association with the impairment of lung function.
Introduction
Cigarette smoking is recognized to be a significant risk factor for the development of chronic obstructive pulmonary disease, several cancers, cardiovascular disease, and oral disease.1–5 Cigarette smoke (CS) triggers inflammation and other physiological changes in the airway epithelium causing pulmonary damage and suppression of both innate and adaptive immunity resulting in repeated infections.6–9 Evidence from animal models shows that cessation of exposure to CS results in a reduction in pulmonary inflammation and at least partially restores immune function.9 Pulmonary damage can result from a direct impact on lung tissue or an indirect effect as a consequence of the generation of a proinflammatory milieu comprising cytokines and chemokines made by immune cells recruited to the lung.
In an effort to reduce tobacco-related harm and keep and expand their customer base, the tobacco companies attempted to develop “safer cigarettes” since the 1960s.10 Recently, Philip Morris International (PMI) developed a heated tobacco product (HTP), also called “Heat-not-Burn” product, that is currently marketed under the brand name IQOS. With a sleek, technologically forward design, IQOS was initially launched in 2014 in Nagoya, Japan and Milan, Italy, and was gradually rolled out in other countries and was authorized for sale in the United States in 2019.11,12 On July 7, 2020, the FDA has accepted- modified-risk tobacco product application for IQOS based on scientific studies that switching completely from conventional cigarettes to the IQOS system significantly reduces the body’s exposure to harmful or potentially harmful chemicals.13
PMI has published several studies comparing the effects of exposure to aerosols from their HTP to CS. In vitro studies conducted by PMI demonstrated that, at similar nicotine concentrations, HTP aerosols elicited inflammatory processes and cellular stress responses to a lower degree than CS.14 Based on a study conducted in mice, long-term inhalation of aerosols from HTP had a lower impact on lung inflammation than exposure to CS.15 These studies also demonstrated that switching exposures from chronic CS to HTP aerosols considerably impeded the progression of CS-induced emphysematous and atherosclerotic changes.15 PMI in a recently completed clinical study concluded that by switching from menthol cigarettes to menthol THS significant reduction in the exposure to harmful and potentially harmful constituents were observed to levels approaching those detected in subjects who abstained from smoking, and this was associated with the overall improvements in biomarkers of potential harm.16,17 However, an industry-independent study demonstrated that acute exposures to IQOS aerosols impaired arterial flow-mediated dilation, a measure of vascular endothelial function, in a similar way to CS.18 Furthermore, upon reviewing PMI’s application to assess the toxicities associated with IQOS use, Moazed et al. concluded that there was evidence of severe pulmonary inflammation and immune toxicities in rats exposed to IQOS aerosols.19 Their review further confirmed that among human users, there was no evidence of improvement in lung inflammation or pulmonary function in cigarette smokers who had switched over to IQOS. One of the major limitations of studies conducted by PMI was that they failed to evaluate measurements of lung-specific inflammatory immune responses in their human studies.
In this study, we report on the impact of acute inhalation exposure to IQOS aerosols and CS on pulmonary inflammation as reflected by changes in immune-cell infiltrates, the profiles of proinflammatory cytokines/chemokines in the bronchoalveolar (BAL) and in the levels of albumin in the BAL which served as a surrogate marker of lung epithelial-cell damage.
Materials and Methods
Tobacco Products
IQOS devices (model 2.0) and HEETS Red Label inserts (PMI) were purchased from an IQOS flagship store in Toronto, ON, Canada. Reference tobacco cigarettes 3R4F were supplied by the Kentucky Tobacco Research and Development Center (University of Kentucky, Lexington, KY). Prior to testing, all HEETS inserts and tobacco cigarettes were conditioned at 22 ± 1°C with a relative humidity of 60 ± 3% for a period of 48 hours following ISO 3402.20 Both tobacco products used in the study (conventional tobacco cigarettes and Heetsticks for IQOS) were commercially available products containing different tobacco fillers. Both products were used as purchased without any modification as intended for consumer use. Reference tobacco cigarettes were light using an electric lighter, whereas the Heetsticks were placed in IQOS heater device (holder). Tobacco was not removed from cigarettes and placed in IQOS.
Mice
Eight-week-old male and female C57BL/6NCr mice were procured from Charles River and housed under specific pathogen-free conditions in the Department of Laboratory Animal Resources (Roswell Park Comprehensive Cancer Institute, Buffalo, NY) with light/dark cycle of 12/12 hours. Number of animals per group in each experiment was n = 10 (5 males and 5 females). All experiments involving mice were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at Roswell Park Comprehensive Cancer Center (Buffalo, NY) and complied with all state, federal, and National Institutes of Health (NIH) regulations.
Animal Exposure System
Mice were placed in a modified 15-L exposure chamber (Vet Equip Inc., Livermore, CA) with two sections separated with a 0.5-cm steel wire mesh (Supplementary Figure 1). Each set of mice (male and female) was rotated once per day between cage positions to ensure uniform exposure to emissions from tobacco products. The exposure chamber was connected to a smoking machine using a Tygon tubing. During exposure to HTP, animal exposure chambers were connected to a vacuum-operated smoking machine developed in-house and customized for IQOS device (see Supplementary Materials). During exposure to CS, animal exposure chambers were connected to JB2090 automatic smoking machine (CH Technologies, Westwood, NJ).
Exposure Protocol
Mice were exposed for a total of 5 hours/day for 2 weeks to emissions from 20 HEETS or 20 tobacco cigarettes. Each day, 20 series of puffs (12 puffs/series from IQOS and 8 puffs/series from tobacco cigarettes) were generated every 15 minutes following the Health Canada Intensive puffing regime of 55-mL puff every 30 seconds. The puff durations for IQOS and tobacco cigarettes were 5 and 2 seconds, respectively. IQOS device was activated manually before each series of puffs and placed in a charger during breaks between puff series. During intervals between individual puffs and breaks between puff series, animals were exposed to filtered air. Mice were euthanized 16 hours after the final exposure.
Monitoring of Exposure Conditions
The concentration of particulates (PM2.5) was constantly monitored during experiments using SidePak AM510 monitor (TSI, Shoreview, MN) connected directly to a sampling port in the exposure chamber. SidePak Buddy software21 was used to analyze temporal and time-weighted average PM2.5 concentrations inside the exposure chambers. Calibration factors of 1.00 and 0.32 were used for IQOS and CS, respectively. We also collected air samples from inside exposure chambers to measure airborne nicotine concentration. Nicotine was sampled every day using an active sampling technique on XAD-4 sorbent tubes (SKC Inc., Eighty Four, PA) with a flow rate of 1.7 L/min. Sorbent tubes were processed following NIOSH 2551 protocol,22 and nicotine was analyzed using gas chromatography as described in Supplementary Materials. Samples of animal fur and wipes of wall surface area inside the exposure chamber were also collected to measure potential deposition of nicotine. Detailed descriptions of the sampling procedures, and analytical methods used are provided in Supplementary Materials. Temperature, atmospheric pressure, and humidity sensors placed inside the exposure chamber were used for continuous monitoring of exposure conditions during experiments.
Analysis of Blood Samples
Blood samples were collected at the end of weeks 1 and 2 immediately after the exposure. Serum was separated, and samples (100 µL) were analyzed for cotinine, a primary metabolite of nicotine, using liquid chromatography method with tandem mass spectrometry as described in Supplementary Material.
BAL Fluid and Lung Tissue Collection and Isolation of Lung Leukocytes
Mice were euthanized, and tracheae were cannulated to collect BAL fluid by injecting 0.75 mL ice cold 1% bovine serum albumin solution in phosphate-buffered saline twice using IV catheters. Lungs were harvested and subjected to collagenase IV/DNase I digestion.9 The resulting single-cell suspension was passed through a 40-μm filter to remove debris and undigested tissue, then underlaid with Ficoll-Paque and centrifuged with brake off. Leukocytes at the interface were collected, washed, and counted using trypan blue staining.
ELISA
Albumin levels in the BAL were quantified by ELISA using reagents from Bethyl Laboratories (Montgomery, TX), and plates were developed with 3,3′,5,5′-tetramethylbenzidine from eBioscience Inc. (San Diego, CA), and absorbance was read at 450 nm as described previously.9
Flow Cytometry
Immune cells were stained with cell type-specific antibodies (Supplementary Materials) for FACS analysis to determine the numbers and phenotype of various immune subsets as described previously.9 Samples were acquired using LSRII-A flow cytometer (BD), and data were analyzed using FlowJo 10.03 software (Tree Star Inc., Ashland, OR). Gating strategy followed was exactly as described previously9 and summarized in Supplementary Figure 2.
Multiplex Cytokine/Chemokine Assay
Cytokine concentrations in the BAL were measured by performing Luminex multiplex cytokine/chemokine assay using Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad, #M60009RDPD, Lot# 64209360) following the manufacturer’s instructions. The kit assayed for IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17A, Eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α. Data acquisition was performed on FLEXMAP 3D (Luminex Corp., Austin, TX). During data analysis, limit of detection was used for any data points with values less than limit of detection.
Statistical Analysis
Statistically significant differences between the mean values of different groups were determined by two-way ANOVA with Tukey’s post-test comparisons by GraphPad Prism 8 software (GraphPad, La Jolla, CA). In each experiment, n = 10 mice were used (five males and five females). The differences between the two groups were considered statistically significant when p values were <.05.
Results
Exposure Conditions
The exposure protocol used in our study resulted in comparable concentrations of particulates inside the chambers (197.0 ± 47.0 µg/m3 from IQOS vs 269.0 ± 94.0 µg/m3 from CS; p > .05; Table 1). Although significantly higher airborne nicotine concentrations were measured inside cages after exposure to IQOS compared with CS (432.5 ± 260.8 vs 174.7 ± 106.2 µg/m3; p < .05; Table 1), exposure to both tobacco products resulted in comparable cotinine concentrations in the serum of exposed animals (29.5 ± 19.7 vs 35.6 ± 17.8 ng/mL, respectively; Table 1). Low levels of nicotine were detected on chamber walls after exposure to IQOS, whereas no nicotine was detected on chamber walls after exposure to CS. No nicotine was detected on animal fur after exposure to both tobacco products. Environmental conditions inside the chambers did not differ significantly except for slightly higher temperature recorded after exposure to IQOS (22.5 ± 0.2°C vs 21.7 ± 0.5°C; p < .05; Table 1).
Table 1.
Exposure Conditions Inside Chambers Recorded During Experiments (Mean ± SD)
| Heated Tobacco Product, IQOS | Tobacco cigarette | ||
|---|---|---|---|
| Particulates PM2.5 (µg/m3) | Time-weighted average/day | 197 ± 47 | 269 ± 94 |
| Airborne nicotine (µg/m3) | Average/day | 432.5 ± 260.8* | 174.7 ± 106.2 |
| Serum cotinine (ng/mL) | Weeks 1 and 2 | 29.5 ± 19.7 | 35.6 ± 17.8 |
| Nicotine deposited on chamber walls (µg/cm2) | Average/day | 8.0 ± 2.2* | Below LOQ |
| Nicotine deposited on animal fur (µg/cm2) | Average/10 mice | Below LOQ | Below LOQ |
| Atmospheric pressure (mBar) | Average/day | 991.2 ± 9.1 | 993.5 ± 2.7 |
| Temperature (°C) | Average/day | 22.5 ± 0.2* | 21.7 ± 0.5 |
| Relative humidity (%) | Average/day | 70.5 ± 2.9 | 66.0 ± 7.1 |
All results are for n = 10 (10 samples collected every day), except for serum cotinine which are for n = 20 (10 mice serum samples per week). LOQ = limit of quantitation.
*A significant difference between IQOS and CS exposure conditions (p ≤ .05).
Acute Exposure to IQOS Aerosols or CS Induces Lung Epithelial Cell Damage
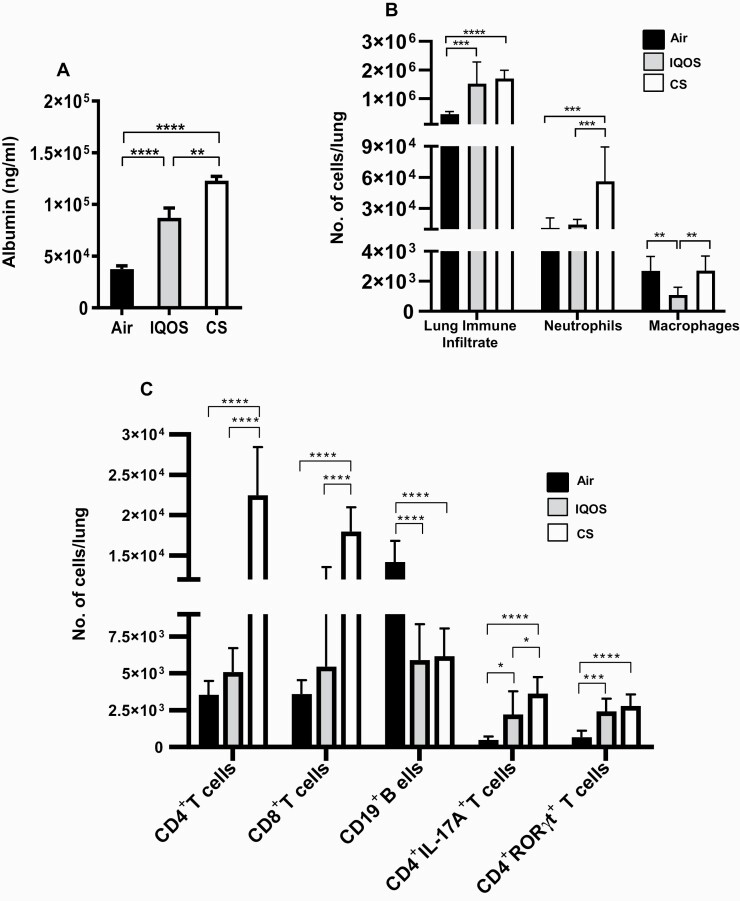
Albumin levels in the BAL of mice were quantified as a surrogate marker of lung epithelial-cell damage. We observed that acute exposures to IQOS aerosols or CS induced significantly increased levels of albumin in the BAL of mice compared with air-exposed controls, indicating smoke/aerosol-induced lung epithelial-cell damage in mice (Figure 1A). However, lung epithelial cell damage was significantly higher in mice acutely exposed to CS than aerosols from IQOS. It is noteworthy that the albumin levels in the BAL after either IQOS aerosol or CS inhalation were equivalent in mice of both sexes (Supplementary Figure 3), demonstrating that the lung damage induced by these exposures is not sex biased.
Figure 1.
(A) Acute exposure to IQOS aerosols or combustible cigarettes (CS) induces lung epithelial-cell damage. At the end of the exposures, mice were euthanized, bronchoalveolar lavage (BAL) harvested, and the levels of albumin in the BAL were quantified by ELISA as described in Materials and Methods. (B–C) Acute exposure to IQOS aerosols or smoke from CS modulates airway immune-cell infiltration. Total number of leukocytes, CD11b+Ly6G+ neutrophils, and CD11b+Ly6G-CD68+ macrophages (B), and numbers of CD4+ T cells, CD8+ T cells, and CD19+ B cells and CD4+IL17A+ and CD4+RORγt+ inflammatory T cells (C) in the lungs of mice exposed to air, IQOS aerosols, or CS were determined by flow cytometry using specific markers and following a gating strategy as described previously9 and shown in Supplementary Figure 2. Data are mean ± SE. *p ≤ .05, **p ≤ 0.01, ***p ≤ .005, ****p ≤ .0001, two-way ANOVA with Tukey’s post-test comparisons by GraphPad Prism 8 software (GraphPad, La Jolla, CA). In each experiment, n = 10 mice were used.
Acute inhalation of IQOS aerosols or CS augments pulmonary immune-cell infiltration and modulates the numbers of innate immune cells in the lungs.
Exposure of mice to emissions from IQOS and CS resulted in markedly augmented infiltration of total immune cells to the lungs compared with exposure to filtered air (Figure 1B). Immunophenotypic analysis of the infiltrating cells in the lungs revealed significantly increased numbers of neutrophils in mice following CS inhalation compared to both air and IQOS aerosol inhalation (Figure 1B). There was a marked reduction in the numbers of macrophages in the lungs of mice as a result of IQOS aerosol inhalation compared with air or CS exposures (Figure 1B).
We evaluated the role of sex on the differential responses to inhalation of IQOS aerosols or CS and noted that acute exposure to CS induced higher immune-cell infiltration to the lungs of male compared with female mice. Sex did not have a differential impact on the total immune-cell infiltration to the lungs in mice exposed to IQOS aerosols (Supplementary Figure 4A). Additionally, acute exposure to CS induced a greater accumulation of neutrophils when evaluated by sex (83 013 neutrophils/lung in males vs 29 570/lung in females, p < .01) but did not influence the numbers of macrophages in the lungs of male versus female mice (Supplementary Figure 4B and C). Accumulation of neither neutrophils nor macrophages in the lungs of mice exposed to IQOS aerosols was affected by sex (Supplementary Figure 4B and C).
Acute Exposure to IQOS Aerosols or CS Modulates Adaptive Immune Cell Accumulation in the Lungs
Acute exposure of mice to CS but not aerosols from IQOS significantly enhanced the numbers of CD4+ and CD8+ T cells in the lungs when compared with exposure to filtered air (Figure 1C). In contrast, lower numbers of CD19+ B cells were found in the lungs following inhalation of both IQOS aerosols and CS versus air-exposed mice (Figure 1C). Although acute IQOS exposure induced greater infiltration of T and B cells in male mice than female mice (9849 CD4+ T cells in males vs 4863 cells in females; 24 709 CD8+ T cells in males vs 2604 cells in females; 10 946 CD19+ B cells in males vs 5124 cells in females), the differences were not significant (Supplementary Figure 4D–F). Furthermore, infiltration of T and B cells to the lungs in response to acute exposure to CS was equivalent in mice of both sexes (Supplementary Figure 4D–F).
Proinflammatory Cell Accumulation in the Lungs Following Acute Exposure to IQOS Aerosol or Cigarette Smoke
We assessed the influence of acute exposure to IQOS aerosols and CS on the accumulation of inflammatory T cells and found exposure to both IQOS aerosols and CS induced increased infiltration of CD4+IL-17A+ T cells into the lungs compared with air-exposed controls; however, the response was higher in the CS group (Figure 1C). Acute exposure to both IQOS aerosols and CS equivalently enhanced the infiltration of CD4+RORγt+ T cells (Figure 1C). The abundance of these inflammatory T cells is strongly associated with several diseases having inflammation as an underlying component (Table 3). There were no sex-specific differences seen in infiltration of CD4+IL-17A+ and CD4+RORγt+ T cells in the lungs (Supplementary Figure 4G and H).
Table 3.
Biomarkers of Lung Pathologies Having Inflammation as a Causal Factor
| Cellular and secretory markers associated with lung inflammation and pulmonary diseases | |||
|---|---|---|---|
| Biomarker | Clinical relevance | References | |
| 1 | CD4+IL-17A+ T cells | Respiratory infection, inflammation, lung adenocarcinoma | 8,9,33 |
| 2 | CD4+RORγt+ T cells | Respiratory infection, inflammation, autoimmune diseases, IBD | 8,9,34 |
| 3 | IFN-γ | Respiratory infection, inflammation, Influenza virus infection, tuberculosis | 35–37 |
| 4 | TNF-α | Respiratory viral infection, COPD | 38–40 |
| 5 | IL-17A | Respiratory infection, inflammation, IBD, rheumatoid arthritis, psoriasis, COPD | 8,9,41–43 |
| 6 | MIP-1a | Respiratory infection, RSV infection, and lung inflammation | 44–46 |
| 7 | MIP-1β | RSV infection, inflammation | 44,47,48 |
| 8 | IL-6 | COPD, respiratory infection, asthma | 40,49 |
| 9 | RANTES | RSV infection, asthma, sarcoidosis | 50, 51 |
| 10 | IL-2 | COPD, asthma, respiratory tract infection | 52, 53 |
| 11 | IL-5 | Allergic asthma, COPD | 52, 54 |
| 12 | KC | COPD, lung inflammation | 55-57 |
| 13 | Eotaxin | COPD, asthma | 58, 59 |
| 14 | G-CSF | COPD, cystic fibrosis | 60, 61 |
| 15 | IL-13 | Respiratory infection, inflammation, asthma | 62–66 |
Table depicts various cellular and secretory biomarkers and their association with lung diseases having pulmonary inflammation as an underlying factor. IBD = inflammatory bowel disease; COPD = chronic obstructive pulmonary disease; RSV = respiratory syncytial virus.
IQOS Aerosols and CS Induce Proinflammatory Cytokines and Chemokines in the BAL of Mice Following Acute Exposure
We observed that the levels of various cytokines and chemokines associated with inflammation were augmented after exposure to IQOS aerosols and CS. Acute exposure to IQOS aerosols elevated the levels of Th1 cytokines IL-2, IFN-γ, TNF-α, Th2 cytokines IL-13, IL-5, and Th17-related cytokine IL-17A in the BAL (Table 2 and depicted with histograms in Supplementary Figure 5). Furthermore, IQOS aerosol exposure significantly elevated the levels of various inflammation-associated chemokines in the BAL of exposed mice. Levels of RANTES, KC, and Eotaxin, the signature chemokines of an inflammatory milieu, were markedly augmented in the BAL of mice exposed to IQOS aerosols (Table 2 and Supplementary Figure 5). We also detected elevated levels of macrophage inflammatory proteins (MIP), MIP-1α and MIP-1β, which belong to the family of chemotactic cytokines known as chemokines and are crucial for immune responses toward infection and inflammation in the BAL of mice exposed to IQOS aerosols (Table 2 and Supplementary Figure 5). Overall, the levels of proinflammatory cytokines and chemokines induced were similar following both IQOS and CS exposures. Association of various proinflammatory cytokine/chemokine biomarkers with various lung pathologies is given in Table 3.
Table 2.
Two-Week Exposure to IQOS Aerosols or CS Augments Inflammatory Cytokine/Chemokine Profile in the Bronchoalveolar Fluid
| Cytokine/ chemokine (pg/mL) | Air | IQOS | CS |
|---|---|---|---|
| IL-2 | 1.19 ± 0.14 | 2.79 ± 0.28*** | 2.32 ± 0.21** |
| IFN-γ | 24.60 ± 4.94 | 72.53 ± 12.02** | 54.92 ± 9.14 |
| TNF-α | 32.87 ± 18.50 | 157.94 ± 32.64* | 147.15 ± 31.13* |
| IL-6 | 0.67 ± 0.09 | 0.92 ± 0.088 | 1.06 ± 0.11* |
| IL-9 | 26.50 ± 4.10 | 133.35 ± 14.18**** | 136.36 ± 15.42**** |
| IL-13 | 21.50 ± 3.76 | 43.62 ± 2.75*** | 27.91 ± 2.39** |
| IL-5 | 1.48 ± 0.25 | 3.84 ± 0.37*** | 2.74 ± 0.14* |
| IL-17A | 4.22 ± 1.29 | 8.94 ± 1.68 | 6.98 ± 1.60 |
| G-CSF | 9.25 ± 1.94 | 8.71 ± 0.99 | 15.92 ± 1.91* ,† |
| RANTES | 13.39 ± 2.79 | 44.31 ± 6.12* | 30.12 ± 3.62* |
| KC | 83.29 ± 14.54 | 352.6 ± 53.11*** | 284.27 ± 29.75* |
| Eotaxin | 83.38 ± 19.77 | 386.81 ± 62.90*** | 257.45 ± 28.22* |
| MIP-1α | 11.16 ± 1.82 | 18.61 ± 3.04 | 32.27 ± 5.11** ,† |
| MIP-1β | 17.51 ± 5.52 | 121.08 ± 31.55* | 141.13 ± 33.29* |
Levels of various inflammation-associated cytokines and chemokines in the bronchoalveolar of mice after 2 wk of exposure to air, IQOS aerosols, or CS were quantified by Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad USA; Cat # M60009RDPD) as described in Materials and Methods. Data are mean ± SE. In each experiment, n = 10 mice were used.
*p ≤ .05, **p ≤ .01, ***p ≤ .001, ****p ≤ .0001, IQOS and CS versus air, or †p ≤ .05, IQOS versus CS. Ordinary one-way ANOVA with Tukey’s post-test comparisons by GraphPad Prism 8 software (GraphPad, La Jolla, CA).
Discussion
We performed a side-by-side comparison of the potential impact of IQOS aerosol and CS exposure on the recruitment of immune cell infiltrates to the lung, the levels of cytokines and chemokines, and lung epithelial-cell damage using our preclinical animal model of aerosol and cigarette smoke inhalation. Our findings reveal that acute exposure to IQOS aerosols induces a proinflammatory microenvironment comprising inflammatory immune-cell accumulation as well as multiple proinflammatory cytokines/chemokines in the lungs. Furthermore, this acute inhalatory exposure to IQOS aerosols induced a proinflammatory lung microenvironment, which promotes lung damage. These proinflammatory changes induced by IQOS aerosols could potentially lead to further outcomes including increased susceptibility to respiratory infections and suppressed responses to prophylactic vaccinations, conclusions that can be drawn from our previous studies on tobacco smoking and immunity.7,9
Although short-term inhalation of IQOS aerosols, like CS, augmented total immune-cell infiltration to the lungs of mice, nevertheless, we did not observe significant increase in the total numbers of neutrophils and macrophages. These findings corroborate with PMI’s report on a mouse model of long-term exposure to CS and IQOS, which demonstrated a lower impact of IQOS aerosols on the absolute numbers of neutrophils and macrophages when compared with CS-exposed mice.15 Recent in vitro studies have demonstrated that IQOS exposure is as detrimental as CS and concluded that inhalation of IQOS aerosols has the potential to induce oxidative stress and inflammation, increase infections, airway remodeling, and initiate epithelial–mesenchymal transition-related changes in the airways of users of these devices.23 IQOS-aerosol-induced detrimental effects could potentially be attributed to high exposure to nicotine that was comparable to exposure achieved from CS. It is also possible that other toxicants emitted from IQOS could cause the observed detrimental effects. Tobacco inserts used in IQOS devices have been shown to emit numerous toxicants, including respiratory irritants and cardiovascular toxicants such as tobacco-specific nitrosamines, metals, volatile organic compounds, phenolic compounds, polycyclic aromatic hydrocarbons, as well as minor tobacco alkaloids and organic solvents, although typically at levels lower than those found in CS.24–27 Additionally, inhalation risks of large doses of humectants used in IQOS and other HTPs, for example, propylene glycol and vegetable glycerin, are currently not well characterized.
Our observation that exposure to CS but not emissions from IQOS caused a significant enhancement in the numbers of CD4+ and CD8+ T cells in the lungs is supported by an early study that showed higher numbers of these cells in the BAL of mice exposed to CS than IQOS aerosols.15 However, in the present study, the proinflammatory nature of IQOS aerosols is highlighted by our observation of the increased numbers of CD4+IL-17A+ and CD4+RORγt+ T cells in addition to increased total immune cells that infiltrated the lungs of IQOS-aerosol-exposed mice similar to CS-exposed mice. The abundance of these cells has been strongly associated with diseases having inflammation as an underlying factor (Table 3). However, we also acknowledge that the chemical composition of IQOS emissions is different than CS exposure and this difference might play a major role in the differential accumulation or recruitment of various immune cells in the lungs after exposure. Our conclusion of a proinflammatory lung microenvironment induced by IQOS is further supported by the differential changes in the profiles of inflammatory cytokines and chemokines induced in the BAL. Although proinflammatory cytokines such as TNF-α, IFN-γ, and KC were significantly increased, cytokines related to allergic asthma conditions, such as IL-5 and IL-13, were also increased in the BAL fluid, indicating Th2 differentiation. Furthermore, the elevated levels of IL-9, RANTES, and Eotaxin suggest the potential for increased mast cells, eosinophilia, and mucin upregulation. Th1 cytokines, particularly IFN-γ, acting in concert with Th2 cytokines, suggest allergic inflammation in the lung with acute exposure to IQOS. Our cytokine data are consistent with the flow cytometric analysis showing augmented numbers of inflammatory T cells in the lungs of mice exposed to IQOS aerosol, implicating elicited inflammatory response and immune regulation.
In contrast, PMI studies reported an abundance of proinflammatory cytokines and chemokines in the BAL of mice that inhaled cigarette smoke, but not in the BAL of mice that inhaled IQOS aerosols.15 Additionally, in our study, the elevated levels of albumin quantitated in the BAL as a surrogate marker of lung epithelial-cell damage substantiate the inflammatory milieu induced by the inhalation of IQOS aerosol. These detrimental effects could be due to the fact that IQOS aerosol contains harmful constituents.25 A review of the data from PMI’s application submitted to the FDA reported that biomarkers of potential harm in human smokers including measures of inflammation, oxidative stress, and lung function were not detectably different after inhalation of IQOS aerosol compared with CS.19,28
Limitations of our study include the lack of experiments to demonstrate the long-term effect of IQOS aerosol inhalation on pulmonary inflammation and immune dysfunction and that differences could result from experiments done using nose-only exposure systems. It is also possible that long-term exposure may reveal different results. Furthermore, we recognize that our study is limited to a one-time point investigation following 2-week IQOS aerosol inhalation and lacks the histopathological evaluation of lung tissue following the exposures.
In conclusion, we have demonstrated that IQOS aerosol inhalation is detrimental, recruiting proinflammatory cells to the lung, inducing a microenvironment mediated by proinflammatory cytokines and chemokines and lung epithelial-cell damage. Since sustained smoke, allergic, or environmental-triggered inflammation induces airway remodeling that plays a pivotal role in airflow limitation and lung damage in disorders such as asthma and chronic obstructive pulmonary disease,29–32 long-term inhalation of IQOS aerosols could potentially also be detrimental to pulmonary health. Importantly, alterations in the lung epithelial cells and immune impairment after long-term exposure to IQOS aerosols may be severe, thereby warranting more extensive investigations before IQOS could be confidently considered a safe alternative to combustible cigarette smoking.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
TAB, MLG, and YMT contributed to the conception of the work. All authors contributed to investigation, methodology, data curation, resources, visualization, and analysis and were involved in original drafting and/or reviewing/editing of the manuscript, and all authors approved the final version of the manuscript. YMT and MLG have full access to all study data and take responsibility for the integrity of the data and accuracy of the data analysis.
[Note: References 51–66 are available in Supplementary Material.]
Funding
Research reported in this publication was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health (RO1HL142511) to YT and MLG, a Roswell Park Institute Alliance Foundation award, and NCI Grant P30CA016056 involving the use of Roswell Park Core facilities.
Declaration of Interest
MLG reports research grant from Pfizer and personal fees from Johnson & Johnson, outside of this work. Others report none.
References
- 1. Vellappally S, Fiala Z, Smejkalová J, Jacob V, Somanathan R. Smoking related systemic and oral diseases. Acta Med (Hradec Kralove). 2007;50(3):161–166. [PubMed] [Google Scholar]
- 2. Thun MJ, Carter BD, Feskanich D, et al. 50-Year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Bookshelf ID: NBK179276. [Google Scholar]
- 4. Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Facts Sheet-Tobacco. 2019. http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed April 2020.
- 6. Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. [DOI] [PubMed] [Google Scholar]
- 7. Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, Thanavala Y. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol. 2014;192(11):5226–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(suppl 2):S169–S175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhat TA, Kalathil SG, Bogner PN, et al. Secondhand smoke induces inflammation and impairs immunity to respiratory infections. J Immunol. 2018;200(8):2927–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glantz, SA, Slade J, Bero LA, Hanauer P, and Barnes DE. Chapter 4: the search for a safe cigarette. In: The Cigarette Papers. Berkeley, CA: University of California Press; 1996. http://ark.cdlib.org/ark:/13030/ft8489p25j/. [Google Scholar]
- 11. Vape Ranks. Phillip Morris Launches New Type of Smokeless Cigarette; 2014. http://vaperanks.com/phillipmorris-launches-new-type-of-smokeless-cigarette/.
- 12. U.S. Food & Drug Administration. FDA Permits Sale of IQOS Tobacco Heating System through Premarket Tobacco Product Application Pathway. 2019. https://archive.ph/mA2Ik. Accessed December 2019.
- 13. FDA Authorizes Marketing of IQOS Tobacco Heating System with “Reduced Exposure” Information. 2020. https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information.
- 14. Iskandar AR, Mathis C, Schlage WK, et al. A systems toxicology approach for comparative assessment: biological impact of an aerosol from a candidate modified-risk tobacco product and cigarette smoke on human organotypic bronchial epithelial cultures. Toxicol In Vitro. 2017;39:29–51. [DOI] [PubMed] [Google Scholar]
- 15. Phillips B, Szostak J, Titz B, et al. A six-month systems toxicology inhalation/cessation study in ApoE−/− mice to investigate cardiovascular and respiratory exposure effects of modified risk tobacco products, CHTP 1.2 and THS 2.2, compared with conventional cigarettes. Food Chem Toxicol. 2019;126:113–141. [DOI] [PubMed] [Google Scholar]
- 16. Haziza C, de La Bourdonnaye G, Donelli A, et al. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the Menthol Tobacco Heating System 2.2 for 3 months (part 1). Nicotine Tob Res. 2020;22(4):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haziza C, de La Bourdonnaye G, Donelli A, et al. Favorable changes in biomarkers of potential harm to reduce the adverse health effects of smoking in smokers switching to the Menthol Tobacco Heating System 2.2 for 3 months (part 2). Nicotine Tob Res. 2020;22(4):549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nabavizadeh P, Liu J, Havel CM, et al. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control. 2018;27(suppl 1):s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moazed F, Chun L, Matthay MA, Calfee CS, Gotts J. Assessment of industry data on pulmonary and immunosuppressive effects of IQOS. Tob Control. 2018;27(suppl 1):s20–s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ISO 3402:1999. Tobacco and Tobacco Products – Atmosphere for Conditioning and Testing. Geneva, Switzerland: International Organization for Standardization (ISO). [Google Scholar]
- 21. Klepeis NE, Ott WR, Switzer P. Real-time measurement of outdoor tobacco smoke particles. J Air Waste Manag Assoc. 2007;57(5):522–534. [DOI] [PubMed] [Google Scholar]
- 22. Nicotine: Method 2551, Issue 1, dated 15 January 1998. In: Ashley K and O’Connor PF, eds. NIOSH Manual of Analytical Methods (NMAM), 4th ed. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 1994. https://www.cdc.gov/niosh/docs/2003-154/pdfs/2551.pdf. [Google Scholar]
- 23. Sohal SS, Eapen MS, Naidu VGM, Sharma P. IQOS exposure impairs human airway cell homeostasis: direct comparison with traditional cigarette and e-cigarette. ERJ Open Res. 2019;5(1):00159–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis B, Williams M, Talbot P. iQOS: evidence of pyrolysis and release of a toxicant from plastic. Tob Control. 2019;28(1):34–41. [DOI] [PubMed] [Google Scholar]
- 25. Auer R, Concha-Lozano N, Jacot-Sadowski I, Cornuz J, Berthet A. Heat-Not-Burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177(7):1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salman R, Talih S, El-Hage R, et al. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob Res. 2019;21(9):1285–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leigh NJ, Palumbo MN, Marino AM, O’Connor RJ, Goniewicz ML. Tobacco-specific nitrosamines (TSNA) in heated tobacco product IQOS. Tob Control. 2018;27(suppl 1):s37–s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glantz SA. PMI’s own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes. Tob Control. 2018;27(suppl 1):s9–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD) – differences and similarities. Mater Sociomed. 2012;24(2):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Office of the Surgeon General U. S., Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US), and Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2010. [PubMed] [Google Scholar]
- 31. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909. [DOI] [PubMed] [Google Scholar]
- 32. Crotty Alexander LE, Shin S, Hwang JH. Inflammatory diseases of the lung induced by conventional cigarette smoke: a review. Chest. 2015;148(5):1307–1322. [DOI] [PubMed] [Google Scholar]
- 33. Bao Z, Lu G, Cui D, Yao Y, Yang G, Zhou J. IL-17A-producing T cells are associated with the progression of lung adenocarcinoma. Oncol Rep. 2016;36(2):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mickael ME, Bhaumik S, Basu R. Retinoid-related orphan receptor RORγt in CD4+ T-cell-mediated intestinal homeostasis and inflammation. Am J Pathol. 2020;190(10):1984–1999. [DOI] [PubMed] [Google Scholar]
- 35. Califano D, Furuya Y, Roberts S, Avram D, McKenzie ANJ, Metzger DW. IFN-γ increases susceptibility to influenza A infection through suppression of group II innate lymphoid cells. Mucosal Immunol. 2018;11(1):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208(11):2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee YM, Miyahara N, Takeda K, et al. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177(2):208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Damjanovic D, Divangahi M, Kugathasan K, et al. Negative regulation of lung inflammation and immunopathology by TNF-α during acute influenza infection. Am J Pathol. 2011;179(6):2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen TH, Maltby S, Simpson JL, et al. TNF-α and macrophages are critical for respiratory syncytial virus-induced exacerbations in a mouse model of allergic airways disease. J Immunol. 2016;196(9):3547–3558. [DOI] [PubMed] [Google Scholar]
- 40. Zheng J, Shi Y, Xiong L, et al. The expression of IL-6, TNF-α, and MCP-1 in respiratory viral infection in acute exacerbations of chronic obstructive pulmonary disease. J Immunol Res. 2017;2017:8539294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lorè NI, Bragonzi A, Cigana C. The IL-17A/IL-17RA axis in pulmonary defence and immunopathology. Cytokine Growth Factor Rev. 2016;30:19–27. [DOI] [PubMed] [Google Scholar]
- 42. Mikacenic C, Hansen EE, Radella F, Gharib SA, Stapleton RD, Wurfel MM. Interleukin-17A is associated with alveolar inflammation and poor outcomes in acute respiratory distress syndrome. Crit Care Med. 2016;44(3):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olszewska-Pazdrak B, Casola A, Saito T, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72(6):4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Preedy VR, Patel VB, eds. General Methods in Biomarker Research and Their Applications. Biomarkers in Disease: Methods, Discoveries and Applications. Netherlands: Springer; 2014. [Google Scholar]
- 46. Sugai K, Kimura H, Miyaji Y, et al. MIP-1α level in nasopharyngeal aspirates at the first wheezing episode predicts recurrent wheezing. J Allergy Clin Immunol. 2016;137(3):774–781. [DOI] [PubMed] [Google Scholar]
- 47. Bermejo-Martin JF, Garcia-Arevalo MC, De Lejarazu RO, et al. Predominance of Th2 cytokines, CXC chemokines and innate immunity mediators at the mucosal level during severe respiratory syncytial virus infection in children. Eur Cytokine Netw. 2007;18(3):162–167. [DOI] [PubMed] [Google Scholar]
- 48. Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8(9):1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Culley FJ, Pennycook AM, Tregoning JS, et al. Role of CCL5 (RANTES) in viral lung disease. J Virol. 2006;80(16):8151–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.