Abstract
Introduction
Cigarette smoking remains the leading preventable cause of death in the United States. Recent efforts have explored the potential health and policy benefits of reducing nicotine, an addictive component, in combustible cigarettes. To date, an experimental, prospective analysis directly comparing the effects of varying regulatory environments on purchases of multiple products has yet to be conducted. The present study compared real purchasing of conventional cigarettes, reduced-nicotine cigarettes, and a variety of other nicotine and tobacco products across a range of regulatory environments.
Methods
Participants were assigned to one of five groups, each associated with a different nicotine level (mg of nicotine to g of tobacco) in SPECTRUM investigational cigarettes (15.8, 5.2, 2.4, 1.3, and 0.4 mg/g). Across sessions, participants made real purchases for nicotine/tobacco products in an Experimental Tobacco Marketplace. Each session corresponded with a distinct regulatory environment wherein different nicotine/tobacco products were available for purchase.
Results
Our results suggest that the primary drivers of cigarette and nicotine purchasing are regulatory environment and the presence/absence of alternative nicotine and tobacco products. Perhaps surprisingly, nicotine level does not appear to be such a driver of purchasing behavior under these experimental conditions. Investigational cigarette purchasing is lowest when other preferred combustible products are available and highest when investigational cigarettes are the only combustible product available for purchase.
Conclusions
If a reduced-nicotine policy is implemented, great care should be taken in determining and making available less-harmful nicotine/tobacco products as the availability of preferred combustible products may result in undesirable levels of purchasing.
Implications
This is the first experimental study investigating different potential regulatory effects related to a reduced-nicotine policy by examining purchasing across a range of nicotine/tobacco products. Our results suggest the presence of affordable, highly preferred combustible products is likely to maintain tobacco purchasing at undesirable levels. To promote switching to less-harmful products, affordable alternate nicotine and tobacco products should be readily available. Finally, our results suggest that the availability of noncigarette products, not cigarette nicotine level, will most likely affect purchasing of reduced-nicotine cigarettes.
Introduction
Despite a declining smoking rate, cigarette smoking remains the leading preventable cause of death and costs the U.S. healthcare industry billions of dollars each year.1 Cigarettes contain nicotine, which is addictive and contributes to the maintenance of smoking behavior.2 In 2009, the U.S. Food and Drug Administration (FDA) was given purview to set limits on maximum nicotine levels in cigarettes via the Tobacco Control Act.3 Recently, a number of studies have evaluated the potential impact of a reduced-nicotine standard for cigarettes, including a recent special issue in Nicotine & Tobacco Research.4 Overall, these studies have generally found abrupt transitions to reduced-nicotine cigarettes5 and low nicotine levels (~0.4-mg nicotine per g of tobacco) would be beneficial in promoting smoking abstinence and reducing dependence and toxin exposure.6 General recommendations from these studies suggest a successful nicotine product standard would incorporate noncombustible alternatives, promoting less-harmful products, and informing the public on the relative risks of nicotine products.5
Behavioral economics is one framework for understanding the behavioral motivations and tendencies toward drugs such as nicotine.7,8 Indeed, a number of studies have evaluated nicotine reductions using this framework.5,9,10 Importantly, in recent years, behavioral economics has been expanded to experimentally investigate purchasing decisions mimicking a real tobacco marketplace. The Experimental Tobacco Marketplace (ETM) is an online storefront where researchers manipulate product variety, availability, and price, and participants purchase and receive actual products.11–14 Results from these studies support the use of the ETM in modeling potential regulatory decisions.
To our knowledge, no study has prospectively estimated the impact of a nicotine reduction policy in an experiential arrangement mimicking the real, complex tobacco marketplace where costs are incurred in exchange for receipt of tobacco products. Any comprehensive nicotine policy should aim to minimize intake of harmful tobacco products (eg, cigarettes), while promoting harm-reduction alternatives (eg, nicotine replacement therapy). Thus, the goal of the present study was to leverage the ETM to prospectively evaluate tobacco purchasing across a variety of regulatory contexts, including the availability of reduced-nicotine investigational cigarettes, in a way that participants incurred actual monetary costs to purchase products and received the products they purchased.
Three primary comparisons were investigated across a range of investigational cigarette nicotine levels. First, we compared behavioral economic measures of intensity and change in price sensitivity of investigational cigarette purchasing across regulatory environments. We hypothesized purchasing would be greatest when investigational cigarettes were the only product available, lower when alternative nicotine/tobacco products were available for purchase, and lowest when conventional (usual-brand) cigarettes were available in the marketplace. Second, we compared the same behavioral economic measures of usual-brand cigarette purchasing across regulatory environments when investigational cigarettes were and were not available. We hypothesized that conventional cigarette purchasing would be greater when investigational cigarettes were absent from the marketplace. Third, we evaluated purchasing of noncombustible nicotine/tobacco products across regulatory environments including when conventional and investigational cigarettes were and were not available. We hypothesized that alternate tobacco purchasing would be highest when only investigational cigarettes were available, lower when usual-brand cigarettes were available, and lowest when both cigarettes were available for purchase.
Methods
Participants
A total of 231 participants were recruited via physical flyers and online advertisements from the area surrounding Roanoke, VA, during 2017–2019. Eligibility criteria included self-reported smoking an average of between 5 and 40 cigarettes per day during the past 30 days, breath carbon monoxide (CO) level of ≥10 ppm at intake, not pregnant/lactating, and no immediate plans to quit smoking cigarettes or move out of the area. An a priori power analysis specifying a medium effect size (f = 0.25) of alternate product substitution with four repeated measures, type 1 error rate of α = .05, and 90% power yielded a total sample size of 150 participants. This sample size yielded sufficient power to detect a small effect size (f = 0.14) of a nicotine level by regulatory environment interaction. After 23 participants were excluded, the final, randomized sample included 208 participants, of which 151 were included in the final analyses (see Supplementary Materials for CONSORT checklist; see Supplementary Figure S1 for CONSORT diagram).
Procedure
For their first session, participants completed a battery of behavioral assessments. This session took approximately an hour to complete and there were no minimum CO requirements after eligibility was assessed for any session. After this first session, participants completed an additional four sessions (see Experimental Tobacco Marketplace section) relevant to the current study. Relevant to the current paper, participants completed the Fagerström Test of Nicotine Dependence (FTND15), a six-item questionnaire providing an overall measure of nicotine dependence, and Timeline Followback (TLFB16), capturing daily usage of tobacco products during the previous 30 days. Participants were then randomized to one of five cigarette conditions (ie, nicotine level), each corresponding to a different level of nicotine in SPECTRUM investigational cigarettes (mg of nicotine to g of tobacco): 15.8, 5.2, 2.4, 1.3, and 0.4 mg/g, with the 15.8 mg/g cigarette serving as the control for flavor and brand differences between the SPECTRUM and usual-brand cigarettes. Nicotine-level assignment was determined in a double-blind fashion with neither the participant nor the research assistants aware of each participant’s assigned concentration. At the end of the session, participants were provided a 5-day supply of their assigned cigarette in the flavor (tobacco or menthol) matching their usual-brand cigarette. This 5-day supply was individualized per participant and based on their average cigarettes smoked per day during the past 30 days (multiplied by 5). To gain experience with their double-blind randomly assigned cigarette concentration, participants were instructed to only smoke the investigational cigarettes for the next 5 days and to refrain from smoking their own cigarettes. Although smoking adherence was not incentivized, participants were told: not to share or give away any cigarettes, to return any unused cigarettes, and they had 2 days following the 5-day sampling to resume their typical smoking patterns. No specific instructions were provided in the event participants ran out of cigarettes to reduce the likelihood they would insincerely report their cigarette use. During the first session following sampling, participants were encouraged to honestly report all cigarettes smoked (including nonstudy cigarettes) and told there were no consequences for reporting nonstudy cigarette use. For the 2 days following this sampling period, participants could resume their typical smoking patterns without limitations in place regarding which nicotine products to consume. Participants returned after these 7 days to complete the first of four ETM sessions (~30–45 min each), with approximately 7 days between each session (5 days each of consumption period for purchased products, followed by 2 days of unrestricted access to nicotine products). Thus, the study is a between-subject, double-blind experimental design, where randomized allocation is the between-subject factor, and repeated measurements over time is the within-subject factor.
Experimental Tobacco Marketplace
The ETM is akin to an online storefront (eg, Amazon) displaying a variety of tobacco/nicotine products including a stock image, price per unit, and brief product description. The available products differed based on experimental condition (ie, regulatory environment; see below). Alternate products included combustible cigarettes, electronic nicotine delivery devices, snus/dip, and nicotine replacement therapies (see Supplementary Materials for product listing, Supplementary Figure S2 for the ETM interface). Generally, in ETM experiments, as participants add the desired quantities of each product, total costs are subtracted from their budget, which is statically displayed at the top of the screen. Once purchases are finalized, participants check out and depending on the trial block are presented with another purchasing scenario with different product prices. In the current experiment, participants were provided a budget to purchase products for the 5 days following each purchase session. This allowance was calculated in accordance with previous studies by multiplying the average number of cigarettes smoked per day, based on the 30-day TLFB per-day average, by $0.25 (approximate average cost of a single cigarette in Roanoke, VA) and multiplying that amount by 5 to yield a 5-day allowance.14,17 In each regulatory environment, the price of one product (either the participant’s usual-brand cigarette or assigned investigational cigarette) was manipulated across six independent pricing scenarios (presented in escalating order). The prices of all other products remained constant across these scenarios and were consistent with local market prices of each product.
At each ETM session, participants first completed a TLFB for tobacco products used since their last session. Participants were then seated at a computer and provided instructions relating to the products available during that day’s regulatory environment. Across four regulatory environments (counterbalanced across participants), different combinations of products were displayed and available for purchase. The Current Marketplace included the participant’s usual-brand cigarette and all other products (except the investigational cigarette). The Experimental Control included only the investigational cigarette. The Proposed Marketplace was identical to the Current Marketplace, except the participant’s usual-brand cigarette was replaced with their assigned investigational cigarette. The Combined Marketplace consisted of all products including both usual-brand and investigational cigarettes.
Each regulatory environment (except the Combined Marketplace) consisted of six trials corresponding to the following ascending prices: $0.13, $0.25, $0.50, $1.00, $2.00, and $4.00 per cigarette. In the Combined Marketplace (UB), six trials corresponded to increasing price of the participant’s usual-brand cigarette, during which the investigational cigarette cost $0.25 per cigarette. For the other six trials (Combined Marketplace [IC]), the investigational cigarette increased in price while the usual-brand cigarette cost $0.25 per cigarette. The order in which the different cigarettes increased in price was counterbalanced across participants and all other alternative nicotine/tobacco products remained at a fixed price (Supplementary Table S1). In order for participants to realize and experience their purchases from one of the assessed prices, at the conclusion of the ETM session participants drew a random poker chip out of a bag. Each chip in the bag was associated with one price experienced during that session’s regulatory environment. Participants received all the products they purchased (and any unspent money) associated with the price from the chip they pulled.
Data Analysis
We first calculated bivariate correlations between demographic variables of interest (FTND, cigarettes smoked per day, age, education, monthly income). To examine how target-product cigarette purchasing decreased as a function of increasing price, demand for usual-brand and investigational cigarettes was calculated using the following exponentiated equation18:
| (1) |
where Q represents consumption at each price point, Q0 is the amount of consumption at free price, k is a parameter signifying the range of consumption in logarithmic units (2), α is the rate of change in elasticity across the entire curve, and C is price per cigarette. In Equation 1, smaller values of α (ie, more negative log[α]) reflect relatively greater valuation. Logarithmically transformed demand parameters were estimated at the group level via nonlinear least squares regression and Extra Sum-of-Squares F-tests evaluated whether a single log(Q0) (ie, derived intensity) and a single log(α) (ie, price sensitivity) adequately described demand curves across regulatory environments. Demand curves were fit to unaggregated, untransformed data unlike some approaches that fit to preprocessed averaged data. Whereas parameter estimates are the same between these two methods, the latter provide artificially high R2 values.
Estimates of alternative, fixed-price product purchasing (including usual-brand and investigational cigarettes) were evaluated using linear mixed-effects modeling where the intercept reflects estimated purchasing when the target product is free and the slope represents increases/decreases in purchasing as the target product’s price increases. Generalized estimating equations (GEE19,20) were used for comparisons of observed individual-specific purchasing at the lowest price ($0.13/cigarette) and overall raw purchasing quantities. Similar to mixed-effects models, GEE was chosen to account for the repeated measurements across individuals and these models are relatively robust against covariance structure misspecification. For both mixed effects and GEE, an exchangeable covariance structure was specified. Pairwise comparisons based on the mixed-effects models report estimated marginal means with Kenward–Roger21 adjustments to degrees of freedom and Benjamini–Hochberg22 false discovery rate adjustments to p-values. All analyses were performed in R v.3.6.123 using the following packages: beezdemand,24lme4,25geepack,20tidyverse,26tableone,27 and sjPlot.28
Results
Participant Demographics
A total of 151 participants were included in the final analyses, except for analyses including monthly income, which was missing for one participant (Supplementary Figure S1). Participant demographics are presented in Table 1. Except for education, no significant differences were observed among the groups on any demographic variables. Supplementary Table S2 shows Pearson bivariate correlations among several of the demographic variables measured.
Table 1.
Participant Demographics
| Nicotine level (mg nicotine/g of tobacco; n) | |||||||
|---|---|---|---|---|---|---|---|
| 0.4 (n = 32) | 1.3 (n = 29) | 2.4 (n = 30) | 5.2 (n = 31) | 15.8 (n = 29) | Overall (n = 151) | p | |
| Numeric variablesa: median [IQR] | |||||||
| Age | 41.00 [32.75, 46.25] | 37.00 [28.00, 49.00] | 46.00 [34.00, 51.00] | 42.00 [36.00, 51.00] | 39.00 [34.00, 48.00] | 41.00 [33.00, 50.00] | .608 |
| Monthly income | 707.00 [0.00, 891.00] | 600.00 [0.00, 1275.00] | 513.00 [0.00, 1050.75] | 500.00 [0.00, 1354.00] | 430.00 [0.00, 1000.00] | 563.00 [0.00, 1100.00] | .995 |
| Numeric variablesb: mean (SD) | |||||||
| Education | 11.75 (1.57) | 11.86 (1.90) | 12.37 (1.71) | 13.19 (2.48) | 12.62 (2.27) | 12.36 (2.06) | .037 |
| FTND | 6.75 (1.81) | 6.62 (1.84) | 6.63 (2.01) | 6.48 (2.23) | 6.72 (2.00) | 6.64 (1.96) | .987 |
| Cigarettes per day | 21.36 (9.63) | 20.18 (7.26) | 20.11 (7.84) | 20.81 (8.24) | 21.90 (7.09) | 20.87 (8.02) | .900 |
| Categorical variablesc: [reference] n (%) | |||||||
| Sex [male] | 16 (50.0) | 17 (58.6) | 14 (46.7) | 16 (51.6) | 16 (55.2) | 79 (52.3) | .907 |
| Ethnicityd [Hispanic] | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (10.3) | 4 (2.6) | .050 |
| Usual-brand cigarette flavor [tobacco] | 14 (43.8) | 8 (27.6) | 13 (43.3) | 13 (41.9) | 12 (41.4) | 60 (39.7) | .688 |
| Multiuser [multiuser] | 13 (40.6) | 7 (24.1) | 9 (30.0) | 11 (35.5) | 10 (34.5) | 50 (33.1) | .717 |
| Raced | .734 | ||||||
| White | 21 (65.6) | 19 (65.5) | 20 (66.7) | 24 (77.4) | 17 (58.6) | 101 (66.9) | |
| African American | 7 (21.9) | 9 (31.0) | 9 (30.0) | 7 (22.6) | 10 (34.5) | 42 (27.8) | |
| American Indian | 1 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | |
| More than one race | 3 (9.4) | 1 (3.4) | 1 (3.3) | 0 (0.0) | 2 (6.9) | 7 (4.6) |
FTND = Fagerström test of nicotine dependence; IQR = interquartile range.
aNonparametric Kruskal–Wallis test. bParametric ANOVA. cChi-square test of independence. dFisher’s exact test.
Adherence During 5-Day Sampling
Previous research has suggested noncompliance issues with smokers consuming conventional cigarettes when assigned to reduced-nicotine cigarettes.29 We correlated the number of investigational cigarettes consumed (obtained via the TLFB) during the sampling period with the number of cigarettes participants received at the assessment session. Correlation coefficients ranged from .68 to .83, with the 15.8 and 0.4 mg/g groups displaying the greatest correspondence (r = .83), the 5.2 and 2.4 mg/g groups displaying medium correspondence (r = .79), and the 1.3 mg/g group displaying the lowest correspondence (r = .68).
Investigational Cigarette Demand Across Regulatory Environments
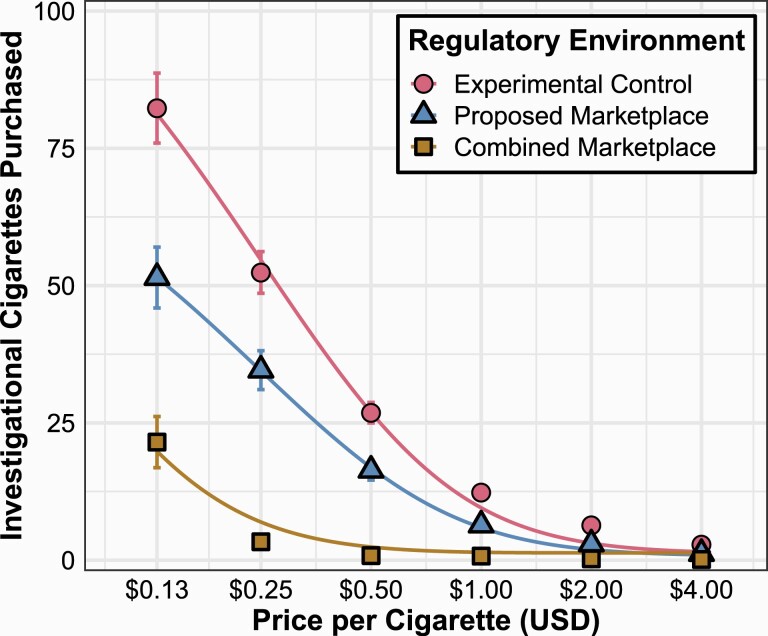
Our first aim compared behavioral economic measures of intensity and change in price sensitivity, where we hypothesized valuation would be greatest when investigational cigarettes were the only product available, lower when alternative nicotine/tobacco products were available, and lowest when usual-brand cigarettes were available. At the group level, the Extra Sum-of-Squares F-test indicated significantly different price sensitivity (log(α); F(2,2711) = 30.95, p < .001) values for investigational cigarettes across the three regulatory environments (Figure 1, Supplementary Tables S3 and S4; Experimental Control R2 = .35; Proposed Marketplace R2 = .22; Combined Marketplace R2 = .08), as well as differences in derived intensity (log(Q0); F(2,2711) = 4.89, p = .008). Derived intensity was marginally higher in the Experimental Control and Combined Marketplace compared with the Proposed Marketplace, whereas price sensitivity decreased systematically with the lowest value (highest valuation) when investigational cigarettes were the only product (ie, Experimental Control), intermediate values in the Proposed Marketplace, and highest (lowest valuation) in the Combined Marketplace where usual-brand cigarettes were available concurrently with investigational cigarettes. However, given the differences in the range of purchasing between the three regulatory environments (ie, substantially less purchasing in the Combined Marketplace) and that both parameters are highly influenced by the span parameter (k), estimates of derived intensity (ie, Q0) from Equation 1 is not readily interpretable. Therefore, we examined differences across condition and nicotine level in observed intensity (ie, purchasing at lowest price) at the individual level using GEE.
Figure 1.
Investigational cigarettes purchased across regulatory environment as a function of increasing price. Symbols and error bars indicate mean and SEM.
Results of the GEE suggested an orderly significant effect of regulatory environment and cigarettes per day in predicting observed intensity. Estimated marginal means indicated intensity values of 81.8 (SE = 5.68), 51.0 (SE = 5.11), and 21.0 (SE = 4.36) for the Experimental Control, Proposed Marketplace, and Combined Marketplace, respectively. Every additional cigarette smoked per day resulted in an increase of ~2.9 (SE = 0.82) in observed intensity. No significant main effect of nicotine level or regulatory environment by nicotine-level interaction was detected .
In some regulatory environments, many participants did not purchase any investigational cigarettes at any price. We used logistic GEE (binomial family and logit link function) to estimate the probability that no purchases were made at all prices within a given regulatory environment and nicotine level, and the model included additional subject-specific variables. For each regulatory environment and within nicotine-level assignment, data were coded as to whether no investigational cigarettes were purchased at any price or at least one cigarette was purchased at one or more prices. Results suggested only regulatory environment significantly predicted no purchasing . On average, compared with the Experimental Control, the odds of all zero investigational cigarette purchasing were seven times more likely to occur in the Proposed Marketplace and 36 times more likely in the Combined Marketplace (five times higher in the Combined Marketplace compared with the Proposed Marketplace). Nicotine level, usual-brand cigarette flavor, cigarettes per day, monthly income, or whether the participant was a multiuser were predictive of zero purchasing (Supplementary Table S5). The proportion of participants reporting all zero purchases in the Experimental Control, Proposed Marketplace, and Combined Marketplace were 0.06, 0.30, and 0.67, respectively.
Given the high proportion of all zero purchasing, and thus missing price sensitivity (α) values for the individual demand analysis, we modeled raw purchasing using GEE. Results suggested significant main effects of regulatory environment , price (log transformed; ), cigarettes per day , and monthly income , and a significant interaction effect between regulatory environment and price ; see Supplementary Table S6). Estimated marginal means indicated, on average, 30.41 (SE = 1.91), 18.64 (SE = 1.75), and 4.29 (SE = 0.91) cigarettes purchased in the Experimental Control, Proposed Marketplace, and Combined Marketplace, respectively. Nicotine level was not found to be a significant predictor either by itself or by interacting with regulatory environment or price .
Conventional Cigarette Demand Across Regulatory Environments
Our second aim compared behavioral economics measures of valuation for usual-brand cigarettes across regulatory environments, where the hypothesized demand would be greater when investigational cigarettes were absent from the marketplace. Comparing usual-brand cigarette purchasing at the group level, the Extra Sum-of-Squares F-test (Supplementary Figure S3; Supplementary Tables S7 and S8) indicated significantly greater price sensitivity (α) values for usual-brand cigarettes in the Combined Marketplace compared with the Current Marketplace (F(1,1807) = 9.9752, p = .002; Current Marketplace R2 = .51; Combined Marketplace R2 = .43), but no differences in derived intensity (Q0; F(1,1807) = 1.7176, p = .190) (Current Marketplace ; , Combined Marketplace ; ).
Similar to the analysis of intensity at the individual level for investigational cigarettes, GEE suggested a significant effect of regulatory environment , but no significant interaction or main effect of cigarette type . Estimated marginal means indicated intensity values of 98.4 (SE = 4.79) and 82.6 (SE = 5.23) for the Current Marketplace and Combined Marketplace, respectively. In this model, usual-brand cigarette flavor ; tobacco smoker’s intensity 17.5 higher than menthol), cigarettes per day , and monthly income were significant predictors of intensity. Sex was not a significant predictor of intensity .
Raw purchasing of usual-brand cigarettes was analyzed using GEE (Supplementary Table S9). These results suggested significant main effects of regulatory environment , price , cigarettes per day , and monthly income , and a significant interaction effect between regulatory environment and price . Nicotine level was not found to be a significant predictor either by itself or by interacting with regulatory environment or price . With all else in the model being equal, 6.1 more cigarettes were purchased in the Current Marketplace compared with the Combined Marketplace.
Cigarette and Alternative Product Substitution
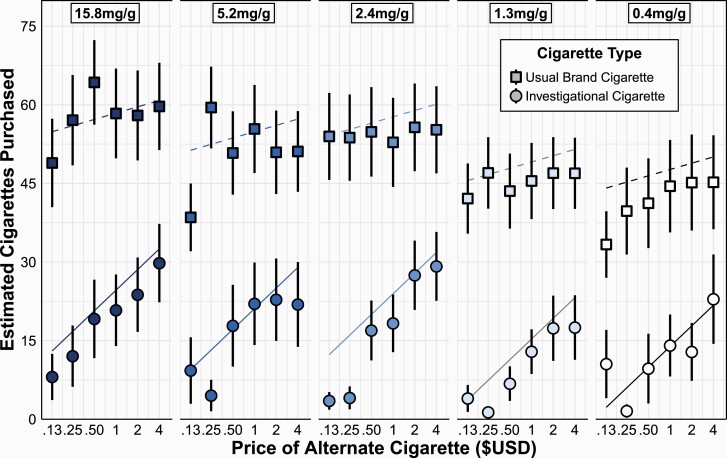
We used GEE to determine the degree to which investigational cigarettes substituted for usual-brand cigarettes and vice versa (Supplementary Table S10). Overall, regardless of price, usual-brand cigarettes tended to be purchased in greater quantities. This effect was observed even at low prices, which resulted in the substitution slopes for investigational cigarettes being higher than for usual-brand cigarettes (Figure 2). This pattern resulted in significant main effects of cigarette price (log transformed; ), cigarette type , monthly income , and cigarettes per day , as well as a significant cigarette by price interaction . Flavor , sex , investigational nicotine level , or a three-way interaction between nicotine level, cigarette type, and price were significant predictors .
Figure 2.
Substitution of investigational cigarettes for usual-brand cigarettes and vice versa in the Combined Marketplace across the different nicotine levels. Symbols and error bars indicate mean and SEM. Lines are derived from GEE models adjusted for covariates.
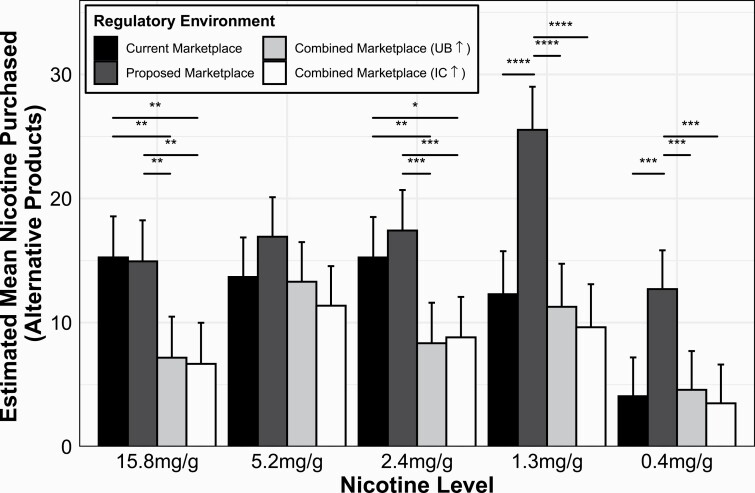
Our third aim evaluated purchasing of noncombustible nicotine/tobacco products across regulatory scenarios where we hypothesized alternate tobacco purchasing would be highest when investigational cigarettes were the only combustible product available, lower when usual-brand cigarettes were the only combustible product available, and lowest when both cigarettes were available. Comparing purchasing of alternative products was to determine the degree to which regulatory environment resulted in an overall switch from combustible tobacco products to non-combusted nicotine alternatives (Supplementary Table S11). Therefore, for each participant, we totaled the amount of nicotine purchased across noncombustible products for each cigarette price within each regulatory environment. We then examined the interaction of price, regulatory environment, and nicotine level on the resulting amount of nicotine purchased. The results of our mixed-effects model suggested a significant regulatory environment by nicotine level interaction (; see Figure 3). For all nicotine-level groups except the 5.2 mg/g group, mean nicotine purchased from alternative products was lower in both Combined Marketplaces compared with the Proposed Marketplace (ps < .01). For the 2.4 and 15.8 mg/g groups, significantly more nicotine was purchased in the Current Marketplace compared with the both Combined Marketplaces (ps ≤ .01). Finally, for the 0.4 and 1.3 mg/g groups, mean nicotine purchased was significantly higher in the Proposed Marketplace compared with the Current Marketplace (ps < .001). No significant pairwise comparisons were observed between nicotine levels for any given condition.
Figure 3.
Mean nicotine purchased (estimated marginal means; error bars indicate SEM) from alternate products in the various regulatory environments stratified by cigarette nicotine level. Combined Marketplace (UB ↑) indicates usual-brand cigarette price manipulated (investigational cigarette price fixed). Combined Marketplace (IC ↑) indicates investigational cigarette price manipulated (usual-brand cigarette price fixed). Statistical significance: *p < .05, **p < .01, ***p < .001, ****p < .0001.
These results should be evaluated in light of the total number of cigarettes purchased in each of the regulatory environments (Supplementary Figure S4). For all nicotine levels, the fewest number of cigarettes were purchased in the Proposed Marketplace . On average, slightly more usual-brand cigarettes were purchased in the Current Marketplace compared with investigational cigarettes purchased in the Experimental Control . Finally, the greatest number of cigarettes was purchased in the Combined Marketplaces where both cigarettes were available for purchase, both when the usual-brand cigarette increased in price (UB: ; IC: ) and when the investigational cigarette increased in price (UB: ; IC: ). When fitted in a GEE model, significant effects of cigarette type (ie, usual brand, investigational; ) and regulatory environment were found, but no effect of nicotine level or significant interaction between regulatory environment and nicotine level . That is, model estimated marginal means indicated an average of 29.2 fewer investigational cigarettes purchased at each price compared with usual-brand cigarettes .
Discussion
Given the recent proposals to reduce nicotine content in combustible cigarettes, the goal of the present study was to apply behavioral economic concepts to prospectively estimate the degree to which purchasing of tobacco and nicotine products would change across several regulatory environments. Our first aim was to characterize purchasing of reduced-nicotine cigarettes across regulatory environments. We found behavioral economic estimates of intensity and price sensitivity were highest when investigational cigarettes were the only product available and lower when alternative nicotine/tobacco products were available. Compared with the Experimental Control, we found participants were significantly more likely to purchase no investigational cigarettes in the Proposed Marketplace and even more likely in the Combined Marketplace. These findings have direct implications for tobacco policy such that restricting access to alternative, potentially less-harmful nicotine products (eg, NRT) may maintain higher levels of cigarette purchasing. Although we did not include combustible products other than the usual-brand and investigational cigarettes in the current experimental preparation, the magnitude of difference in how likely participants were to purchase no investigational cigarettes (seven times higher in the Proposed Marketplace, 36 times higher in the Combined Marketplace) suggests policy considerations for alternative combustible products (eg, preferred cigarette, cigarillos, little cigars) insofar as greater availability of combustible tobacco products will potentially compete with a reduced-nicotine cigarette. This is supported by our finding that, regardless of nicotine level, participants purchased relatively higher quantities of usual-brand cigarettes compared with the investigational cigarettes (see Figure 2).
Our second aim was to examine how usual-brand cigarette purchasing would be affected with the inclusion of reduced-nicotine cigarettes in a marketplace. Our results suggested minimal reductions in usual-brand cigarette purchasing when investigational cigarettes were available (Combined Marketplace) compared with when they were absent (Current Marketplace). When comparing total cigarettes purchased, the fewest total number were purchased in the Proposed Marketplace (investigational cigarettes), whereas the Combined Marketplace resulted in the most purchased with the vast majority representing usual-brand cigarettes.
Our third aim was to determine the extent to which nicotine purchasing of noncombustible nicotine/tobacco products would differ across regulatory environments. We found for all but the 5.2 mg/g nicotine levels, mean nicotine purchased from alternate products was lower in the Combined Marketplace compared with the Proposed Marketplace. That is, alternative nicotine purchased was always higher when the investigational cigarette was the only combustible product in the marketplace (Proposed Marketplace). The makeup and composition of purchasing of alternate products is beyond the scope of the current article, although we note much of the nicotine purchased was from electronic nicotine delivery system devices, which in our current study was primarily from the disposable variety. Although, to our knowledge, this is the first study experimentally simulating various regulatory environments across a range of nicotine levels in investigational cigarettes, our results do seem at odds with other research suggesting lower nicotine levels would be more effective at reducing cigarette consumption compared to higher nicotine levels.
Somewhat surprisingly, we observed few differences in the above results across nicotine levels. In the models we constructed, we tested nicotine level as main and interaction effects with regulatory environment but failed to find any meaningful and significant results. Besides the fact the current study was powered to detect only a small effect of regulatory environment by nicotine-level interactions, one explanation for this finding could be that, anecdotally and consistent with other data obtained using these cigarettes,30 participants may have found these cigarettes to be similarly displeasing, regardless of nicotine content. If the FDA decides to limit the nicotine content via regulatory action, tobacco companies will likely develop and market cigarettes designed to maximize their taste and attractiveness to existing smokers. If and when feasible, future studies should replicate the current experimental methods using commercially available reduced-nicotine cigarettes.
Numerous strengths of the current study should be mentioned. First, our experiential purchasing procedures (ie, ETM) with a wide variety of nicotine/tobacco products allowed even greater approximation to potential real-world scenarios that may not otherwise be captured in other experimental designs where participants are provided free cigarettes. Rather, participants were provided a budget and incurred actual costs in their purchasing decisions. Second, we explored several regulatory environments, some of which served as experimental controls, giving us the ability to directly compare the effects of adding specific types of products. Finally, even though we detected few significant differences, we were able to explore the impact of regulatory environments across a wide range of nicotine levels (double-blind), which provided us the ability to conduct parametric analyses.
Several limitations and areas for future research are noted. First, the current study was specifically powered to detect substitution differences and a small effect of nicotine level by regulatory environment interactions. In a number of our analyses, we observed nicotine-level-dependent trends associated with purchasing, but these interaction effects did not meet the traditional statistical significant thresholds. With more participants, we would have been able to explore and interpret these interactions with increased confidence. Second, after the initial 5-day sampling period, participants were able to purchase as many or as few investigational cigarettes as they wanted. As such, participants may not have had enough exposure to the investigational cigarettes to regulate their purchasing based on nicotine content alone. Future research could examine the effects of longer-term exposure to these cigarettes prior to making purchasing decisions. Third, we relied on the use of retrospective TLFB measures to ascertain products used between each laboratory session, instead of a potentially more sensitive measure such as ecological momentary assessments or the collection of used nicotine and tobacco products. However, participants were free to purchase as many or as few products as they wanted and purchasing substantially more products than what participants would actually consume is unlikely as purchases in the present experiment incurred an actual monetary cost. Indeed, a major general theme emerging from the reduced-nicotine literature is a high level of noncompliance among participants who are asked to smoke these cigarettes (~12%–78%29). Strategies to mitigate, or at least lessen, noncompliance issues may include leveraging a more open economy31,cf32 and having those enrolled in the study acknowledge their responsibilities as a research participant to adhere to the research procedures. To open the economy, researchers could schedule specific days during the experiment where participants may use their own regular products and participants acknowledge they understand their responsibilities in the study. Fourth, we did not collect certain measures including health perceptions, withdrawal symptoms, or subjective ratings related to the different cigarettes. This information may be valuable for better understanding purchasing decisions; however, given the nature of participants engaging in actual purchasing decisions, we believe any differences in, for example, how much they liked the cigarettes would manifest themselves in purchasing patterns (eg, suppressed purchasing).
The current study sets the stage for further research questions exploring the regulatory impact of reduced-nicotine cigarettes. For example, we did not provide training or direct experience with the alternative products in the marketplace, and the products in the marketplace were limited to availability during the time the study was conducted. Future research could examine the likelihood of switching to alternative products after training or provided experience, as well as including new products such as current-generation electronic nicotine delivery system (eg, Juul). Interactions with other, nontobacco products such as alcohol and marijuana would be valuable avenues of inquiry.
The results of the present experiment suggest regulatory environment, and the availability of alternative nicotine/tobacco products, not necessarily specific nicotine level, has the greatest effect on purchasing of combustible cigarettes. We have shown how the ETM can aid in prospectively estimating the effects of tobacco public policy. Taken together, these results suggest promoting the availability of less-harmful alternative nicotine products in combination with lower nicotine-containing cigarettes may maximize the effectiveness of a comprehensive nicotine standard policy.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We thank Kelsey Stamborski, Alex Brown, and Elisa Crill for their assistance in data collection.
Funding
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (NIH) and Food and Drug Administration (FDA) Center for Tobacco Products (R01DA042535 awarded to M.N.K. and W.K.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or FDA.
Declaration of Interests
B.A.K., M.N.K., and C.T.F. have no conflicts of interest to report. Although the following activities/relationships do not create a conflict of interest pertaining to this manuscript, in the interest of full disclosure, W.K.B. report the following: W.K.B. is a principal of HealthSim, LLC; BEAM Diagnostics, Inc; and Red 5 Group, LLC. In addition, he conducts research supported by Indivior PLC, serves on the scientific advisory board for Sober Grid, Inc and is a consultant for Alkermes, Inc and Sandoz Inc.
References
- 1. Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48(3):326–333. doi: 10.1016/j.amepre.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benowitz N L, Drug therapy . Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med. 1988;319(20):1318–1330. doi: 10.1056/NEJM198811173192005 [DOI] [PubMed] [Google Scholar]
- 3. US Congress. Family Smoking Prevention and Tobacco Control Act. Pub L No. 111–31. 2009. [Google Scholar]
- 4. Stanton CA, Hatsukami DK. Nicotine standards in the United States. Nicotine Tob Res. 2019;21(suppl 1):S1–S4. doi: 10.1093/ntr/ntz191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith TT, Donny EC, Luo X, et al. . The impact of gradual and immediate nicotine reduction on subjective cigarette ratings. Nicotine Tob Res. 2019;21(suppl 1):S73–S80. doi: 10.1093/ntr/ntz158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White CM, Pickworth WB, Sved AF, Donny EC. Using product standards to render the most harmful tobacco products minimally addictive: maximum nicotine level, non-nicotine constituents, and scope. Nicotine Tob Res. 2019;21(suppl 1):S13–S15. doi: 10.1093/ntr/ntz121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aston ER, Cassidy RN. Behavioral economic demand assessments in the addictions. Curr Opin Psychol. 2019;30:42–47. doi: 10.1016/j.copsyc.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tidey JW, Cassidy RN, Miller ME, Smith TT. Behavioral economic laboratory research in tobacco regulatory science. Tob Regul Sci. 2016;2(4):440–451. doi: 10.18001/TRS.2.4.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. doi: 10.1001/jamapsychiatry.2017.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaplan BA, Pope DA, Dehart WB, Stein JS, Bickel WK, Koffarnus MN. Estimating uptake for reduced-nicotine cigarettes using behavioral economics. Tob Regul Sci. 2019;5(3):264–279. doi: 10.18001/trs.5.3.5 [DOI] [Google Scholar]
- 11. Pope DA, Poe L, Stein JS, et al. Experimental tobacco marketplace: Substitutability of e-cigarette liquid for cigarettes as a function of nicotine strength. Tob Control. 2019;28(2):206–211. doi: 10.1136/tobaccocontrol-2017-054024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quisenberry AJ, Koffarnus MN, Hatz LE, Epstein LH, Bickel WK. The experimental tobacco marketplace I: substitutability as a function of the price of conventional cigarettes. Nicotine Tob Res. 2016;18(7):1642–1648. doi: 10.1093/ntr/ntv230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pope DA, Poe L, Stein JS, et al. The experimental tobacco marketplace: Demand and substitutability as a function of cigarette taxes and e-liquid subsidies. Nicotine Tob Res. 2019;22(5):782–790. doi: 10.1093/ntr/ntz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bickel WK, Pope DA, Kaplan BA, DeHart WB, Koffarnus MN, Stein JS. Electronic cigarette substitution in the experimental tobacco marketplace: a review. Prev Med. 2018;117:98–106. doi: 10.1016/j.ypmed.2018.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, eds. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992:41–72. doi: 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- 16. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 17. Koffarnus MN, Wilson AG, Bickel WK. Effects of experimental income on demand for potentially real cigarettes. Nicotine Tob Res. 2015;17(3):292–298. doi: 10.1093/ntr/ntu139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koffarnus MN, Franck CT, Stein JS, Bickel WK. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol. 2015;23(6):504–512. doi: 10.1037/pha0000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.2307/2336267 [DOI] [Google Scholar]
- 20. Halekoh U, Højsgaard S, Yan J. The R Package geepack for generalized estimating equations. J Stat Softw. 2006;15(2):1–11. doi: 10.18637/jss.v015.i02 [DOI] [Google Scholar]
- 21. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3): 983–997. doi: 10.2307/2533558 [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 23. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.r-project.org/.
- 24. Kaplan BA, Gilroy SP, Reed DD, Koffarnus MN, Hursh SR. The R package beezdemand: behavioral economic easy demand. Perspect Behav Sci. 2019;42(1):163–180. doi: 10.1007/s40614-018-00187-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1548–7660):1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 26. Wickham H, Averick M, Bryan J, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 27. Yoshida K.. tableone: Create ‘Table 1’ to Describe Baseline Characteristics. R package version 0.10.0. https://CRAN.R-project.org/package=tableone. [Google Scholar]
- 28. Lüdecke D. sjPlot: Data Visualization for Statistics in Social Science. R package version 2.8.1. 2019. doi: 10.5281/zenodo.1308157 [DOI] [Google Scholar]
- 29. Berman ML, Glasser AM. Nicotine reduction in cigarettes: literature review and gap analysis. Nicotine Tob Res. 2019;21(supp 1):S133–S144. doi: 10.1093/ntr/ntz162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Veldheer S, Midya V, Lester C, et al. Acceptability of SPECTRUM research cigarettes among participants in trials of reduced nicotine content cigarettes. Tob Regul Sci. 2018;4(1):573–585. doi: 10.18001/TRS.4.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imam AA. Response-reinforcer independence and the economic continuum: a preliminary analysis. J Exp Anal Behav. 1993;59(1):231– 243. doi: 10.1901/jeab.1993.59-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith TT, Koopmeiners JS, White CM, et al. The impact of exclusive use of very low nicotine cigarettes on compensatory smoking: an inpatient crossover clinical trial. Cancer Epidemiol Biomarkers Prev. 2020;29(4):880–886. doi: 10.1158/1055-9965.EPI-19-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.