SUMMARY
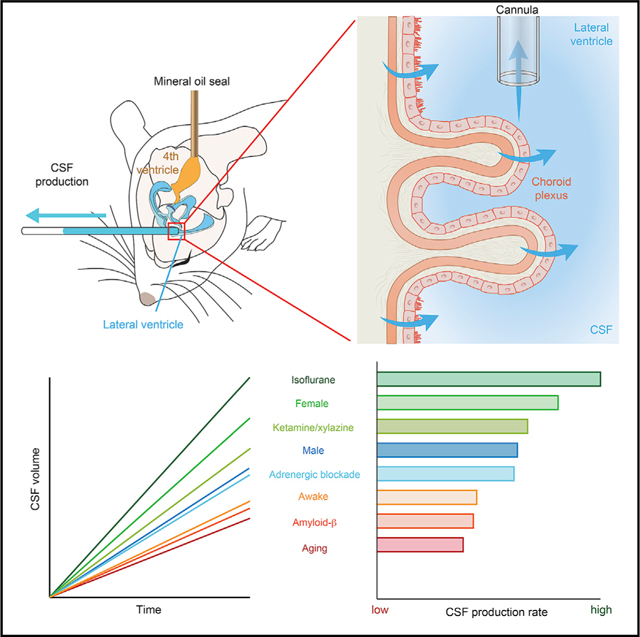
The emerging interest in brain fluid transport has prompted a need for techniques that provide an understanding of what factors regulate cerebrospinal fluid (CSF) production. Here, we describe a methodology for direct quantification of CSF production in awake mice. We measure CSF production by placing a catheter in a lateral ventricle, while physically blocking outflow from the 4th ventricle. Using this methodology, we show that CSF production increases during isoflurane anesthesia, and to a lesser extent with ketamine/xylazine anesthesia, relative to the awake state. Aged mice have reduced CSF production, which is even lower in aged mice overexpressing amyloid-β. Unexpectedly, CSF production in young female mice is 30% higher than in age-matched males. Altogether, the present observations imply that a reduction in CSF production might contribute to the age-related risk of proteinopathies but that the rate of CSF production and glymphatic fluid transport are not directly linked.
In Brief
Liu et al. develop a method for direct quantification of cerebrospinal fluid (CSF) production in awake mice. Using this method, the authors evaluate the effect of brain states, ages, sex, anesthetic types, and amyloid-β burden on CSF production.
Graphical Abstract
INTRODUCTION
Cerebrospinal fluid (CSF) production has emerged in recent years as an important and largely undetermined factor that may influence glymphatic/lymphatic CSF transport in the central nervous system (CNS) (Aspelund et al., 2015; Iliff et al., 2012; Louveau et al., 2015). CSF is principally produced by the choroid plexus, located in the roof of the two lateral ventricles and of the 3rd and 4th ventricles (Johanson, 2008; Lun et al., 2015). Extrachoroidal sources consisting of filtration or secretion of fluid across capillary walls may also contribute to total CSF production (Oresković and Klarica, 2010). CSF flows from the 4th ventricle into the subarachnoid space (SAS) (Johanson, 2008), where it is shunted out of the intracranial cavity by meningeal lymphatic vessels and by lymphatic drainage along cranial and spinal nerves (Louveau et al., 2017). CSF can from here enter the brain by glymphatic transport along the perivascular spaces surrounding penetrating arteries (Rasmussen et al., 2018). The glymphatic/lymphatic system plays an important role in clearance of proteins implicated in neurodegenerative diseases (Benveniste et al., 2019; Nedergaard and Goldman, 2020). Several lines of work have provided evidence for a role in amyloid-β clearance; indeed, as much as 60% of soluble cerebral amyloid-β is cleared from the brain by glymphatic transport (Iliff et al., 2012). Manipulations of glymphatic/lymphatic transport that cause increased amyloid-β accumulation include elimination of meningeal lymphatic vessels (Da Mesquita et al., 2018), ligation of cervical lymphatic vessels (Wang et al., 2019), and genetic deletion of aquaporin-4 (AQP4) water channels (Xu et al., 2015). Conversely, local application of vascular endothelial growth factor-C (VEGF-C) enhanced meningeal lymphatic drainage, which improved learning and memory performance of a transgenic mouse model of Alzheimer’s disease with amyloid-β overexpression (Da Mesquita et al., 2018). The magnitude of glymphatic/lymphatic CSF transport in mice peaks in adolescence, declines with normal aging (Antila et al., 2017; Munk et al., 2019), and is suppressed in the APP/PS1 murine model of Alzheimer’s disease (Peng et al., 2016).
These findings prompt several questions with regard to the physiology of CSF production, which has been a neglected topic in recent decades. For example, does normal aging reduce CSF production? Does genetic overexpression of amyloid-β itself antagonize CSF production (Balusu et al., 2016)? Similarly, it remains an open question whether CSF production contributes to the state-dependent regulation of the glymphatic system. One of the most intriguing aspects of the glymphatic system is that it is nearly inactive during wakefulness but is facilitated by slow-wave activity during sleep (Cai et al., 2020; von Holstein-Rathlou et al., 2018; Xie et al., 2013), which implies a role in the restorative function of sleep. However, the causality of these associations is unclear because sleep-wake regulation of glymphatic flow might be a downstream consequence of CSF production. Also, certain anesthetic regimens like ketamine/xylazine (K/X) facilitate perivascular CSF inflow and boost glymphatic transport, whereas isoflurane is ineffective (Hablitz et al., 2019).
The present lack of basic information regarding the quantitation of CSF production in mice primarily reflects technical limitations. Current methodologies use indirect dye dilution methods that interfere with basal CSF production and yield variable results depending on the rate and volume of dye infusion (Oresković and Klarica, 2014; Oreskovic et al., 2003). We have herein adapted a technique originally developed for the measurement of CSF production in anesthetized rats to the study of awake mice (Karimy et al., 2015, 2017). This approach affords access to common transgenic models, such as the murine models of Alzheimer’s disease, while avoiding the confounders arising from earlier methods of CSF measurement.
RESULTS
Direct Measurement of CSF Production by Blocking the Aqueduct of Sylvius
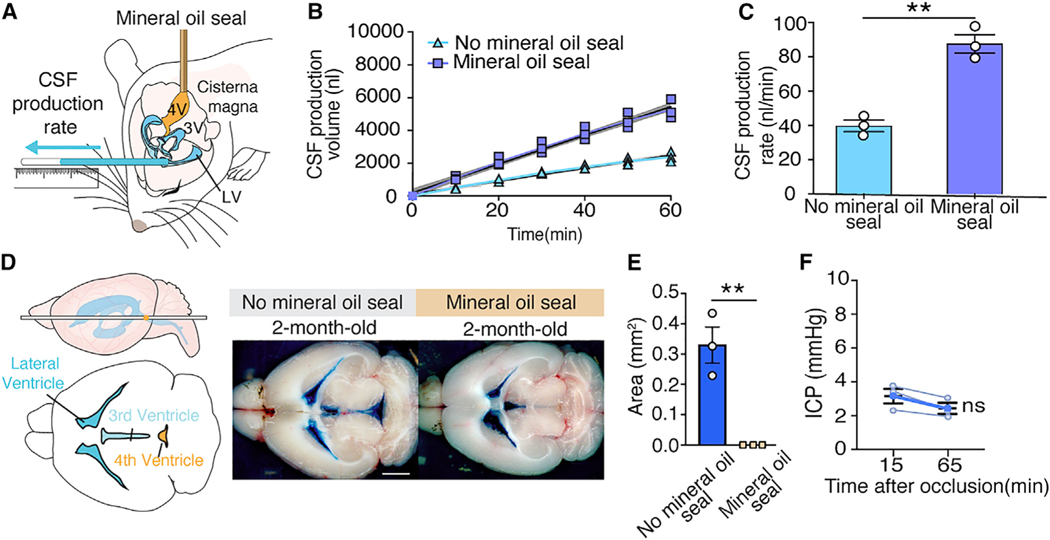
To enable direct measurements of CSF production, we first anesthetized adult male C57BL/6J mice with K/X. When they became unresponsive to tail pinch, we retracted the scalp and drilled a small burr hole over the right cerebral hemisphere, inserted a 30-gauge needle into the lateral right ventricle, and secured it in place with dental cement. The mouse head was then rotated 90° downward to expose the atlanto-occipital mem brane. We then occluded the 4th ventricle by inserting a separate 30-gauge needle into the 4th ventricle and infusing mineral oil (1 μL) (Figure 1A). Sealing the 4th ventricle sharply increased the rate of CSF collection from the lateral ventricle. CSF production in mice with blockage of the 4th ventricle (87.6 ± 3.0 nL/min) versus without seal 39.8 ± 1.8 nL/min; p = 0.0016, unpaired t test, n = 3 (Figures 1B and 1C). To assess the effectiveness of the mineral oil seal, we next injected 0.5% Evans blue (0.5 μL/ min, 0.5 μL) by the cannula inserted into the lateral ventricle. Evans blue was trapped in the lateral and 3rd ventricles in mice with the mineral oil seal and only reached the 4th ventricle in control mice that had a patent aqueduct at 60 min (Figures 1D and 1E). Upon blocking the normal egress route by the aqueduct of Sylvius, CSF efflux diverted toward the cannula inserted into the lateral ventricle. Of note, the occlusion of the 4th ventricle did not increase intracranial pressure (ICP) when the CSF could exit by the lateral ventricle cannula (Figure 1F).
Figure 1. Assessment of the Patency of 4th Ventricular Mineral Oil Seal.
(A) To measure CSF production in mice, a small burr hole was made over the right lateral ventricle (anterior-posterior [AP]: −0.10 mm, medial-lateral [ML]: 0.85 mm from bregma) in C57BL/6J male mice anesthetized with ketamine/xylazine (K/X). A 30-G needle connected to PE-10 tubing was lowered 2.00 mm through the burr hole. The atlantooccipital membrane was surgically exposed and the head rotated 90 degrees downward. A 30-G needle connected to PE-10 tubing filled with mineral oil was inserted into the 4th ventricle, and mineral oil (1 μL, 1 μL/min) was infused, thus occluding the aqueduct of Sylvius. After closure of the aqueduct of Sylvius, CSF was obliged to exit by the needle inserted in the lateral right ventricle. The position of CSF in the PE-10 tube was marked every 10 min for a total of 60 min. The cannula was not inserted in the 4th ventricle in control mice without the mineral oil seal.
(B) CSF production was measured in young male mice under K/X anesthesia with and without injection of mineral oil seal in the 4th ventricle. The best fit lines from the linear regression (colored line) with 95% confidence intervals (shaded region) are plotted. The linear regression in mice without the mineral oil seal is R2 = 0.99, p < 0.0001 and in mice with the mineral oil seal is R2 = 0.99, p < 0.0001.
(C) Comparison of the rates of CSF production with and without the mineral oil seal. CSF production rate is the slope from the linear regression in (B). **p < 0.01, unpaired t test. Bar graphs represent mean ± SEM.
(D) To assess the efficacy of the mineral oil seal on CSF outflow, Evans blue (0.5%, 0.5 μL, 0.5 μL/min, 60-min circulation) was infused into the right lateral ventricle. The brain was harvested, and the distribution of Evans blue was documented by macroscopic imaging. Evans blue remained trapped in the lateral and the 3rd ventricles when the aqueduct of Sylvius was blocked. In contrast, when Evans blue (0.5%, 0.5 μL, 0.5 μL/min, 60-min circulation) was infused in mice with a patent aqueduct of Sylvius, Evans blue was abundantly present in the 4th ventricle (scale bar: 2 mm).
(E) Comparison of Evans blue coverage area in 4th ventricle in mice with (n = 3) or without (n = 3) a mineral oil seal. **p < 0.01, unpaired t test. Bar graphs represent mean ± SEM.
(F) ICP at 15 and 65 min after direct CSF production measurement in mice with mineral oil seal of the 4th ventricle. Paired t test; ns, not significant.
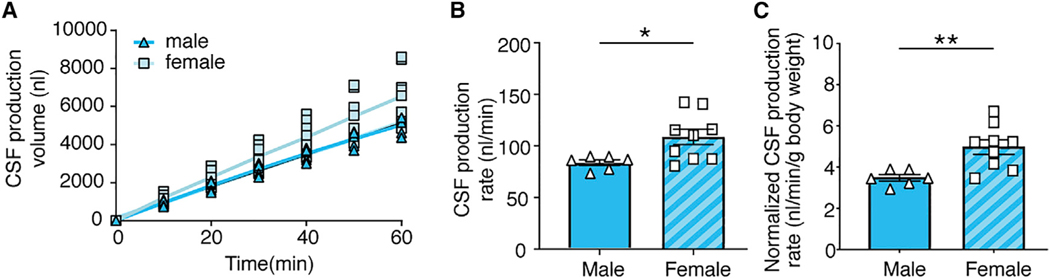
We next used this approach to compare CSF production in young (2-month-old) female and male mice under K/X anesthesia. CSF was collected for 60 min after aqueductal occlusion, and volume measurements were obtained every 10 min. Surprisingly, females exhibited a significantly higher rate of CSF production than males (108.0 ± 7.6 nL/min versus 83.9 ± 2.8 nL/min, p = 0.027) (Figures 2A and 2B). Normalization of CSF production to body weight accentuated the higher CSF production in females (5.0 ± 0.4 nL/min/g versus 3.5 ± 0.2 nL/min/g body weight, p = 0.0059) (Figure 2C). To our knowledge, these are the first observations pointing to the existence of a sex difference in CSF production. Because a recent study indicated that glymphatic CSF tracer influx is comparable in young, middle-aged, and aged C57BL/6J wild-type (WT) mice of both sexes (Giannetto et al., 2020), our observation suggests that glymphatic fluid transport rate is not simply a downstream effect of CSF production.
Figure 2. The Effect of Sex on CSF Production.
(A) CSF production volume was plotted as a function of time in young male and female mice. The linear regression (colored line) was plotted with 95% confidence intervals (shaded region). Results from the linear regression in male mice are R2 = 0.998, p < 0.0001 and in female mice are R2 = 1.000, p < 0.0001.
(B) Comparison of the rate of CSF production in male and female mice. CSF production rate is the slope from the linear regression in (A). *p < 0.05, unpaired t test. Bar graphs represent mean ± SEM.
(C) Comparison of CSF production rate in young male and female mice normalized to body weight. **p < 0.01, unpaired t test. Bar graphs represent mean ± SEM.
Tracer Leaks into the Neuropil when the Indirect Approach Is Used for Measuring CSF Production
Ventriculo-cisternal perfusion of tracers is the conventional, albeit indirect, approach for estimating CSF production in experimental animals (Oshio et al., 2005). This approach entails injecting a tracer into the lateral ventricle and subsequently measuring the time course of tracer concentration changes in CSF collected at the cisterna magna. Several prior reports have noted that this procedure is subject to inaccuracies and provides variable results (Oresković and Klarica, 2010, 2014). We hypothesized that the loss of tracer to the parenchyma during intraventricular infusion would yield artifactually high CSF production measurements. To test this hypothesis, we assessed the dispersion of commonly used CSF tracers into the neuropil following their intraventricular injection. CSF containing 0.03% Evans blue (0.96 kDa) and 0.5% tetramethylrhodamine isothiocyanate (TRITC)-dextran (155 kDa) was infused (0.7 μL/min, 42 μL) into the left lateral ventricle to replicate prior studies (Oshio et al., 2003; Oshio et al., 2005; Steffensen et al., 2018). Of note, ICP did not change discernibly during the 60-min tracer infusion (Figures S1A and S1B). Mice were decapitated at 5 and 60 min after the infusion, and the brains were processed for microscopic inspection. Imaging of freshly prepared slices revealed diffusion of tracers into the subependymal regions, as well as along the injection tract at 60 min, but not after 5 min (Figures S1C and S1D). This progressive loss of tracers into the neuropil would skew quantitative results toward higher CSF production values.
Wakefulness Suppresses CSF Production, which Is Counteracted by Pan-adrenergic Receptor Antagonists
The level of brain activity or arousal state affects the rate of glym phatic CSF transport (Xie et al., 2013), but the effects of arousal state on CSF production are unknown. Mice underwent surgical installation of head plates and were trained for several days to tolerate head fixation prior to CSF measurements. Of note, the stress neuromodulator norepinephrine does not increase upon immobilization in well-trained mice (Xie et al., 2013). On the day of the experiment, a ventricular cannula was inserted into the craniotomy under isoflurane anesthesia, and mineral oil was injected in the 4th ventricle as above and the mice allowed to recover for 30 min (Figure 3A). Wakefulness was attested by spontaneous whisking, visual tracking of moving objects, and paw withdrawal when touched. Under these post-operative waking conditions, the mean rate of CSF production was 59.7 ± 4.6 nL/min. Pan-adrenergic inhibition was obtained by intraperitoneal (i.p.) administration of a cocktail of α1- (prazosin), α2- (atipamezole), and non-selective β-adrenergic (propranolol) receptor antagonists, after which CSF production was significantly higher in a separate group of awake mice (81.6 ± 6.4 nL/ min, p = 0.019) (Figures 3B and 3C), consistent with the idea that adrenergic signaling inhibits CSF production (Lindvall et al., 1978b). Because the type of anesthesia is an important determinant of glymphatic activity (Benveniste et al., 2017; Hablitz et al., 2019; Lilius et al., 2019), we next compared two commonly used anesthetic regimens: K/X and isoflurane. The analysis showed that CSF production was 33% higher with isoflurane (133.2 ± 8.7 nL/min) than with K/X anesthesia (89.8 ± 2.4 nL/min, p = 0.0009) (Figures 3D and 3E). These two sets of observations are the first evidence of state- and anesthesia-dependent changes in CSF production. The opposing relative effects of the two anesthetic regimens on CSF production versus glymphatic transport add further support to the conclusion that brain fluid transport is not simply a function of CSF production.
Figure 3. Pan-inhibition of Noradrenergic Receptors Increases CSF Production in Awake Mice, but K/X and to a Lesser Degree Isoflurane Anesthesia Are More Effective.
(A) Schematic diagram of the method used for measuring CSF production in awake mice, with and without pan-inhibition of Noradrenergic (NE) receptors.
(B) CSF production was measured in awake mice after receiving a cocktail of NE receptor antagonists or vehicle. Plots of the linear regression (colored line) with 95% confidence interval (shaded region): awake mice, R2 = 0.9889, p < 0.0001; NE receptor antagonists, R2 = 0.991, p < 0.0001.
(C) Comparison of the rate of CSF production in awake mice receiving a cocktail of NE receptor antagonists or vehicle. CSF production rate is the slope from the linear regression in (B). *p < 0.05, unpaired t test. Bar graphs represent mean ± SEM.
(D) CSF production was measured in awake, K/X, and isoflurane-anesthetized mice. Plots of the linear regression (colored line) with 95% confidence interval (shaded region): awake, R2 = 0.850, p < 0.0001; K/X, R2 = 0.988, p < 0.0001; isoflurane, R2 = 0.953, p < 0.0001.
(E) Comparison of the rate of CSF production in awake, K/X, and isoflurane groups. CSF production rate is the slope from the linear regression in (D). ****p < 0.0001, ***p < 0.001, *p < 0.05; one-way ANOVA with post hoc Tukey’s test. Bar graphs represent mean ± SEM.
Aging, as Well as Overexpression of Amyloid-β, Suppresses CSF Production
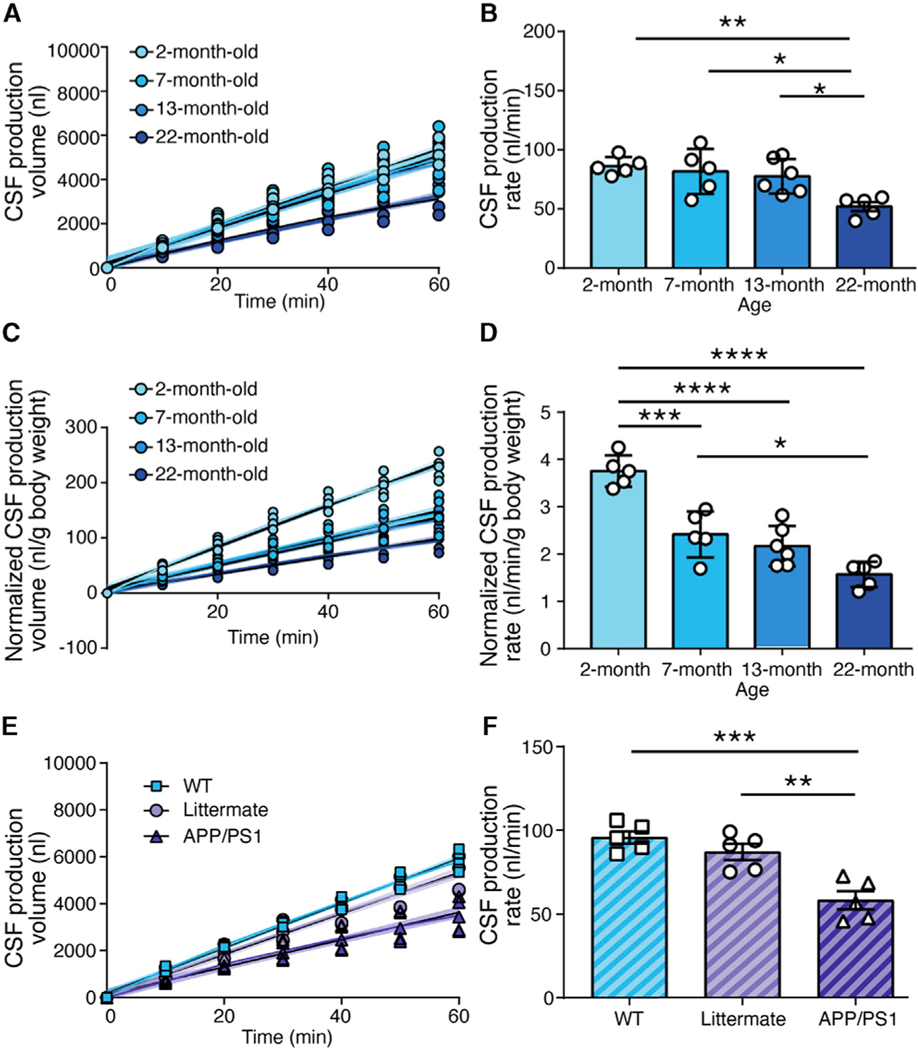
Prior studies have shown that CSF production in the human and rat brain declines with age (Chiu et al., 2012; May et al., 1990), whereas it remains unknown whether aging similarly affects CSF production in mice. We quantified CSF production in 2-, 7-, 13-, and 22-month-old male C57BL/6J mice anesthetized with K/X (Figures 4A and 4B). The analysis disclosed an insignificant trend toward a decline in CSF production from 2 to 7 months (~5%, p = 0.95) and from 7 to 13 months (~5%, p = 0.96), followed by a steep decline from 13 to 22 months of age (~33%, p = 0.01). To account for changes in body size as the animals aged, we also normalized the CSF production to the body weight in the 2-, 7-, 13-, and 22-month-old K/X-anesthetized mice (Figures 4C and 4D). Normalization of CSF production to body weight accentuated the age-dependent decline in CSF production.
Figure 4. The Effect of Age and Overexpression of Amyloid-β on CSF Production.
(A) CSF production was measured in 2-, 7-, 13-, and 22-month-old K/X-anesthetized male mice. The linear regressions (colored line) and 95% confidence intervals (shaded region) are plotted for each set of data: 2-month-old mice, R2 = 0.970, p < 0.0001; 7-month-old mice, R2 = 0.886, p < 0.0001; 13-month-old mice, R2 = 0.916, p < 0.0001; 22-month-old mice, R2 = 0.915, p < 0.0001.
(B) Comparison of the rate of CSF production in 2-, 7-, 13-, and 22-month-old mice. CSF production rate is the slope from the linear regression in (A). **p < 0.01, *p < 0.05; one-way ANOVA with post hoc Tukey’s test. Bar graphs represent mean ± SEM.
(C) CSF production volume normalized to body weight in male mice of the four ages. The linear regression (colored line) and 95% confidence interval (shaded region) are plotted for each set of data: normalized 2-month-old mice, R2 = 0.968, p < 0.0001; normalized 7-month-old mice, R2 = 0.908, p < 0.0001; normalized 13-month-old mice, R2 = 0.911, p < 0.0001; normalized 22-month-old mice, R2 = 0.906, p < 0.0001.
(D) Comparison of the normalized rate of CSF production in male mice of the four ages. Normalized CSF production rate is the slope from the linear regression in (C). ****p < 0.0001, ***p < 0.001, *p < 0.05; one-way ANOVA with post hoc Tukey’s test. Bar graphs represent mean ± SEM.
(E) CSF production was measured in 6-month-old wild-type, littermate, and APP/PS1 female mice with K/X anesthesia. Linear regression (colored line) and 95% confidence intervals (shaded region) are plotted: wild-type mice, R2 = 0.986, p < 0.0001; littermate controls, R2 = 0.956, p < 0.0001; APP/PS1 mice, R2 = 0.898, p < 0.0001.
(F) Comparison of the rates of CSF production in wild-type, littermate, and APP/PS1 female mice. CSF production rate is the slope from the linear regression in (E). ***p < 0.001, **p < 0.01; one-way ANOVA with post hoc Tukey’s test. Bar graphs represent mean ± SEM.
To assess the effects of amyloid-β overproduction on CSF production, we compared 6-month-old female APP/PS1 mice with age-matched female littermates on a (C57BL/6xC3H)F2 background (Jankowsky et al., 2001). The CSF production rate was significantly lower in APP/PS1 mice than in their WT littermates (~33%, p = 0.0025). We then included a second control group consisting of 6-month-old C57BL/6J female mice and found that CSF production did not differ significantly between C57BL/6J and C57BL/6xC3HF2 backgrounds, at least when comparing 6-month-old females (Figures 4E and 4F). The age-dependent decline in CSF production and the exacerbated decline in APP/PS1 mice both match prior observations of reduced glymphatic transport in aging (Kress et al., 2014), as well as findings in mice overexpressing amyloid-β (Da Mesquita et al., 2018; Peng et al., 2016). The ventricular volume increased slightly during aging, raising the possibility that an incomplete seal of the larger 4th ventricle could contribute to the reduced CSF production measurements. However, a critical assessment of the patency of the mineral oil seal showed that 1 μL mineral oil is sufficient to occlude the 4th ventricle in young and aged mice (Figures S2 and S3).
Foramen of Magendie Is Closed in Mice
Interestingly, real-time imaging of the exposed cisterna magna revealed that Evans blue injected into the lateral ventricle exited the lateral foramina of Luschka, but not the medially positioned foramen of Magendie in four control mice. This observation extends the prior report that rat pups lack an open median aperture (Brocklehurst, 1979; Jones, 1980; Jones and Sellars, 1982; Jones et al., 1993) by showing that the foramen of Magendie is also closed in adult mice (Figure S2C; Video S1).
Brain State or the EEG Architecture Does Not Predict CSF Production
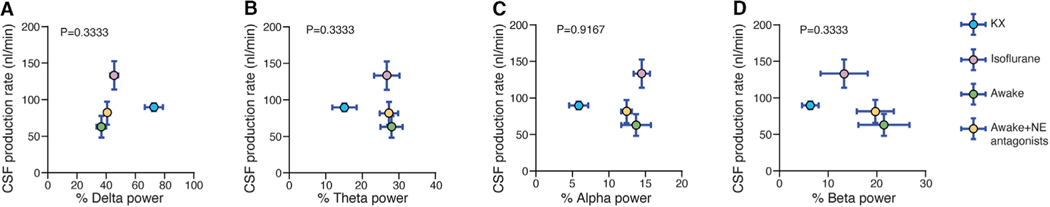
To determine whether brain state could modulate CSF production, we correlated CSF production rate with electroencephalogram (EEG) traces recorded in a separate group of animals (awake, pan-inhibition of adrenergic receptors, K/X or isoflurane anesthesia) that were not head restrained. EEG is the gold standard for determining brain state, but an analysis of EEG architecture, i.e., power in the delta (1 to 4 Hz), beta (13 to 20 Hz), theta (4 to 8 Hz), and alpha bands (8 to 13 Hz), did not reveal any correlations with CSF production rate across the four treatment groups (Figure 5). This observation is significant because several studies have demonstrated that delta and beta power are two of the strongest predictors of glymphatic inflow (Hablitz et al., 2019; Xie et al., 2013). The absence of any association between delta power and CSF production adds additional weight to our contention that glymphatic activity and CSF production are not covariant.
Figure 5. CSF Production Does Not Correlate with EEG Architecture.
Scatterplots depicting the correlation between CSF production rate and delta (A), theta (B), alpha (C), and beta (D) power in the EEG. Comparisons were made across awake controls and awake mice with pan-inhibition of noradrenergic (NE) receptors, K/X, and isoflurane-anesthetized mice. Each dot represents the group mean (whiskers, SD). Correlations were calculated using group means; p values are displayed for each correlation (R2 = 0.64 for delta, theta, and beta power; R2 = 0.04 for alpha power). K/X, n = 5 animals for CSF production rate and n = 7 animals for EEG; isoflurane, n = 5 animals for CSF production rate and n = 8 animals for EEG; NE antagonists, n = 6 animals for CSF production rate and n = 8 animals for EEG; awake, n = 7 animals for CSF production rate, n = 8 animals for EEG.
DISCUSSION
This study presents a direct measurement of CSF production in mice without confounders due to tracer infusion and investigates its modulation by a number of relevant physiological variables. We report that wakefulness is associated with a relatively low CSF production rate, which is partly antagonized by pan-inhibition of adrenergic receptors. Anesthesia effectively increased CSF production—isoflurane more so than K/X. On the other hand, CSF production declined by 58% over the mouse lifespan after correction for increases in body weight. A murine model of Alzheimer’s disease, the APPswe/PS1dE9 (APP/PS1) mouse, exhibited 33% lower CSF production than age-matched WT littermates. Perhaps the most surprising observation was that CSF production in healthy young female mice was almost 30% higher than that in age-matched males. Plotting CSF production against the EEG power spectrum revealed no significant correlations, in contrast to findings for glymphatic flow. The present results are of direct importance toward understanding brain fluid transport in the context of the newly discovered glymphatic and meningeal lymphatic systems (Mestre et al., 2017). The methodology introduced here enables direct CSF measurements in awake mice and will be useful in future studies aiming to elucidate the effect of pharmacological, behavioral, or genetic manipulations on CSF production.
Prior Measurements of CSF Production in Human and Animals
Before elaborating upon the biological significance of present observations, it is important to place this study in the context of the existing methodological literature. In humans, CSF production is most commonly studied by magnetic resonance imaging (MRI), which provides a quantitative, non-invasive measurement of CSF outflow through the cerebral aqueduct. This approach suffers from the shortcoming that it misses any CSF production arising from the 4th ventricle and from extrachoroidal sources. MR-based estimation of CSF production rate in adult human brain is ~0.3–0.7 mL/min (~430–700 mL/day) (Brinker et al., 2014; Huang et al., 2004). Similar methods applied in awake volunteers showed a nightly peak of 0.7 mL/h at 2:00 a.m., versus an evening nadir of only 0.2 mL/min at 6:00 p.m. (Nilsson et al., 1992b). A later MR study reported CSF flow to be 0.6 mL/min (864 mL/day) through the cerebral aqueduct, with considerably higher CSF flow from the foramen magnum of 2.7 mL/min (3.9 L/day) (Piechnik et al., 2008). Thus, available neuroimaging data suggest that CSF production in humans is under circadian control and that extrachoroidal CSF production may be a significant contributor to the total rate of CSF production. Both observations require independent confirmation, preferably using an alternate approach.
In animals, CSF production is traditionally measured by the indirect tracer dilution method originally developed by Pappenheimer (Heisey et al., 1962), who observed CSF production in awake goats with chronically implanted intraventricular and intracisternal cannulas. The indirect tracer dilution method suffers from the limitation that a large volume of fluid is injected into the lateral ventricles, causing a mechanical disturbance of the choroid plexus or lowering the CSF temperature, any of which effects might perturb CSF production. The osmolality of the infused fluid is also of critical importance; if the injected fluid is just a few milliosmoles lower than CSF, tissue water absorption will occur prior to fluid collection in the cisterna magna, thus altering the apparent dilution. Conversely, excessive osmolarity will yield an overestimation of the CSF production. Furthermore, the tracers will tend to diffuse into the periventricular tissue (Figure S1C), also skewing the results toward overestimation of the CSF production rate. Perhaps the most important argument against the perfect validity of indirect CSF measurements is that quantitative results depend on the flow rate of the infused fluid (Oreskovic et al., 2003). Yet, all published work on CSF production in experimental animals has hitherto relied on the indirect approach, given the lack of alternate approaches.
The direct approach for CSF production measurements originally developed by Karimy et al., (2015) presents distinct advantages over the classical technique in that it avoids fluid and tracer injection and gives direct quantitation in real time (Karimy et al., 2015), albeit without capturing the contribution of the 4th ventricle to CSF production, similar to MRI-based measurements of CSF flow through the cerebral aqueduct in human subjects. How much of a problem does this represent? Although the choroid plexus in the 4th ventricle accounts for about one-third of the total choroid plexus tissue, it may be responsible for 40%–60% of the total CSF production (Welch, 1963). However, the contribution of either the choroid plexus in the 4th ventricle or extrachoroidal sources might be substantially larger. In rats, the CSF production rate was 3.38 μL/min, as measured by the indirect tracer dilution measurement method, with collection of CSF in cisterna magna (Chodobski et al., 1998), but was only 0.39–1.40 μL/min when the direct approach was used, with collection in the lateral ventricle (Karimy et al., 2015). Similarly, 2-month-old female mice had a CSF production of ~0.325 μL/min according to the indirect measurement method (Rudick et al., 1982), whereas we observed a production rate of only 0.108 μL/min by the present direct method (Figure 2). Future studies should aim to put CSF production measurements on a firmer footing by resolving these discrepancies.
Effects of Sex, Adrenergic Tone in Brain, Aging, and Overproduction of Amyloid-β
We found that female mice produced CSF at a rate ~30% higher than their age-matched male counterparts, which has not been reported earlier (Figures 2A and 2B). The choroid plexus expresses sex hormone receptors (Lindvall-Axelsson and Owman, 1990; Nilsson et al., 1992a; Skipor and Thiery, 2008), including estrogen receptors (Hong-Goka and Chang, 2004). One clinical study reported that premenstrual women exhibit a greater total CSF volume than men, but it is uncertain how this observation might relate to CSF production (Teasdale et al., 1988). The larger variance in mean CSF production seen in female mice may reflect day-to-day changes in circulating sex hormones because we did not do experiments in phase with the 4- to 5-day mouse estrous cycle (Byers et al., 2012). Of note, a recent report based on more than 400 mice documented that the activity of the glymphatic system (as distinct from CSF production) is comparable between male and female mice (Giannetto et al., 2020). So, we conclude that there must be a dissociation between sex differences in CSF production rate versus CSF transport kinetics.
The choroid plexus is densely innervated by sympathetic nerve fibers (Edvinsson et al., 1975; Edvinsson and Lindvall, 1978; Nilsson et al., 1992a). Sympathetic denervation or pharmacological inhibition of adrenergic receptors increased CSF production in rodents (Lindvall et al., 1978b, 1979), whereas cholinergic parasympathetic inhibition reduced CSF production (Lindvall et al., 1978a). Our data confirmed that administration of a noradrenaline receptor antagonist cocktail increased CSF production in awake mice. In humans, CSF production is higher at night (Nilsson et al., 1992b), possibly contributing to the slight physiological elevation of ICP at night, along with effects of body position (Andresen et al., 2016).
It is possible that the effect of anesthesia and pan-inhibition of noradrenergic receptors on CSF production reflect an influence on the sympathetic nervous system. Alternately, the pharmacological effects of pan-inhibition may include changes in cerebral perfusion. Isoflurane is known to increase cerebral blood flow and volume (Li et al., 2014), whereas K/X anesthesia has less hemodynamic effects. Thus, higher CSF production rates under isoflurane anesthesia may be secondary to perfusion changes, despite the relatively high noradrenergic tonus.
CSF production was 50% lower in a group of elderly human subjects (67 to 84 years) than in young subjects (21 to 36 years) (May et al., 1990). In mice aged 22 months, which are close to their maximum lifespan, we observed 40% lower CSF production than that in 2-month-old mice (Figures 4A and 4B) (Flurkey et al., 2007). Patients with moderate-to-severe Alzheimer’s disease had reduced CSF production compared to healthy aged controls (Silverberg et al., 2001), which is supported by the observation that CSF production is 33% lower in APP/PS1 mice than in unaffected littermates and WT controls (Figures 4E and 4F).
Implications for Glymphatic/Lymphatic Transport
Our motivation for developing a new method to study CSF production in awake mice arose from the need to determine whether glymphatic fluid transport is a downstream function of CSF production. Multiple lines of evidence presented here suggest that CSF production rate is not a major determinant of glymphatic activity. The finding arguing most strongly for the lack of an association between glymphatic flow and CSF production is our observation that CSF production is highest under isoflurane anesthesia, even though isoflurane strongly suppresses glymphatic CSF inflow compared to other anesthetic regimens (Hablitz et al., 2019). A disconnect between CSF production and glymphatic transport was also noted when analyzing sex differences. We found that CSF production is 30% in higher in young female mice than in males, and yet there were no sex differences in glymphatic fluid transport between the two sexes in groups of young, middle-aged, and aged C57BL/6J WT mice (Giannetto et al., 2020).
The present correlational analysis supports the notion that the glymphatic system and CSF production are under independent regulation. Although the distinct regulatory mechanisms remain to be elucidated, the methodology introduced here shall support diverse studies of CSF production in the mouse brain.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
RESOURCE AVAILABILITY
Lead Contact
Future information and requests regarding reagents and resources should be directed to and will be fulfilled by the Lead Contact, Maiken Nedergaard (Maiken_Nedergaard@urmc.rochester.edu).
Materials Availability
This study did not generate new unique reagents. Commercially available reagents are indicated in the Key Resources Table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Propranolol hydrochloride | Sigma-Aldrich | Cat#P8688 |
| Prazosin | Sigma-Aldrich | Cat#P7791 |
| Atipamezole | Sigma-Aldrich | Cat#A9611 |
| Evans blue | Sigma-Aldrich | Cat#E2129 |
| TRITC-dextran | Sigma-Aldrich | Cat# T1287 |
| Experimental Models: Organisms/Strains | ||
| Mouse strain: C57BL/6 | Charles River Laboratories | Strain code: 027 |
| Mouse strain: APPswe/PS1dE9 (APP/PS1) | The Jackson Laboratory | Stock No: 34829-JAX |
| Software and Algorithms | ||
| Fiji software | Freely available online | https://imagej.net/Fiji |
| MATLAB 9.3 | MathWorks | https://www.mathworks.com/products/compiler/matlab-runtime.html |
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
Data and Availability
Datasets are available to readers and there are no restrictions on any of the data or materials presented in this paper. MATLAB code to analyze EEG recordings is available from Lead Contact without restriction.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Two-month old male and female C57BL/6J mice acquired from Charles River Laboratories (Wilmington, MA, USA) and 7, 13, and 22-month old male C57BL/6J mice acquired from the National Institute on Aging (Bethesda, MD, USA) were utilized for all experiments, except for the analysis of the effect of amyloid-β overexpression. For this, female APPswe/PS1dE9 (APP/PS1) mice (The Jackson Laboratory, stock No. 34829-JAX), littermate controls, and WT mice were all compared at 6 months of age (Lok et al., 2013). All mice were maintained on a 12-hour light/dark cycle. All efforts were taken to minimize the number of animals used and all experiments were approved by the University Committee on Animal Resources of the University of Rochester.
METHOD DETAILS
Anesthesia
The mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/mL) (K/X) administered by intraperitoneal (i.p.) injection and 2% isoflurane. If a mouse became responsive to toe pinch during the experiments under K/X, an additional 1/10 of the initial ketamine dosage was administered. Isoflurane was always delivered with 100% oxygen. For measuring CSF production during wakefulness, the mice were anesthetized with 2% isoflurane during cannula implantation. CSF production rate was measured when the mice were awake, 30 min after recovery from anesthesia. At the end of the experiment, mice were re-anesthetized and killed by decapitation.
Surgical procedure and quantification of the rate of CSF production
The mouse head was fixed in a stereotactic apparatus, the skull exposed, and a small burr hole was made over the right lateral ventricle (AP = −0.1 mm, ML = 0.85 mm), whereupon the dural membrane was exposed. A hubless sharp 30G needle (BD, Cat#305106) connected to polyethylene tubing (Intramedic, PE-10) was lowered through the burr hole to a depth of 2.00 mm DV. The needle was fixed to the skull with dental cement and the opposite end of the polyethylene tubing was sealed by high-temperature cautery (Bovie Medical Corporation, Cat# AA01). The mouse head was then flexed on the ear bars at 90 degrees, so that the nose faced downward. Another hubless blunt 30G needle (Exel International, Cat#26562) connected to a segment of PE-10 tubing filled with mineral oil (Sigma-Aldrich, Cat#M5904) was inserted into the cisterna magna (CM) and advanced gently 2 mm through the foramina into the 4th ventricle. One microliter of mineral oil was infused at a rate of 1 μL/min over 1 min with a syringe pump (Harvard Apparatus), and the sealed tip of the cannula exiting the right ventricle was severed to allow for egress of CSF. CSF was allowed to flow freely out of the right ventricle and into the cannula for 5 minutes to allow stabilization and equilibration before quantification of CSF flow rate. Following the first 5 minutes, a second experimenter blind to the experimental condition marked the position of CSF within the PE-10 tube placed in the right lateral ventricle at 10 min intervals to quantify CSF production. We tested the accuracy of the method by injecting 1 μl CSF into PE-10 tubes. The length of tube filled with CSF was 14.42 ± 0.06 mm (range 14.36 to 14.48 mm). The volume of CSF was calculated as follows: CSF volume = π ∙ Radius2 ∙ length. The internal diameter of PE-10 is 0.28 mm. The rate of CSF production (nL/min) was calculated as the slope of the volume-time relationship calculated by linear regression. If the needle was obstructed with blood or tissue, there was no CSF drainage from the tube in the lateral ventricle, and such mice were excluded from the study. We utilized a total of 92 animals in this study, of which 14 animals were excluded. Thus, the failure rate was 15.2%. The failure rate was evenly distributed across the groups and did not correlate with the mean CSF production rate (p = 0.65).
Measurements in awake mice
Mice were anesthetized with K/X and placed in a stereotactic frame. Their scalp was shaved and the skin was cleaned with a chlorhexidine swab followed by an alcohol wipe to remove the chlorhexidine, and an iodine solution was applied and left to dry. The scalp was opened and retracted and the exposed skull was irrigated with sterile saline and cleaned by applying sterile cotton swabs. A sterilized stainless-steel and light-weight head plate was attached to the mouse skull using a mixture of dental cement with cyanoacrylate glue (Sweeney et al., 2019). The head plate is made of 0.9 mm thick stainless steel, measuring 19 × 12 mm, and equipped with a round hole of 9.0 mm diameter at the center. The mice received Banamine (1.1 mg/kg) subcutaneously before the procedure and for three days post-surgery as an analgesic. The mice were trained to tolerate positioning in the head plate stand (Cat# MAG-1, Narishige International USA Inc), as well as a restraint tube in three daily training sessions, each lasting 30 min for three days post-surgery. The restraint tube is an open-ended acrylic cylinder, measuring 9 cm long × 3.5 cm in diameter. Following training, mice were anesthetized again using 2% isoflurane for implantation of the cannulae in the lateral ventricle and the 4th ventricle, as described above. All incisions were infiltrated with 0.25% bupivacaine topical anesthetic to prevent the animal from experiencing post-surgical pain. Once the cannulae were in place, the neck was flexed 90 degrees and the headplate was attached to the head stand. The mouse’s body was kept motionless by confinement in the restraint tube. Anesthesia was discontinued and CSF production was measured after a 30 min recovery period in head-fixed mice.
Drug administration
To determine the effect of pan-adrenergic receptor blockade on the rate of CSF production, a cocktail of propranolol (10 mg/kg, non-selective β-receptor antagonist), prazosin (10 mg/kg, α1 receptor antagonist), and atipamezole (1 mg/kg, α2 receptor antagonist) was prepared in 0.9% saline (Xie et al., 2013). All drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA). The cocktail was administered i.p. 15 min before measuring the rate of CSF production.
EEG measurement
Mice under 2% isoflurane anesthesia were placed in a stereotactic frame, for installation of a head plate on the mouse skull, as described above. Small burr holes were drilled in the skull 2.5 mm lateral and 2 mm posterior to bregma on either side of the midline. EEG wire leads were then inserted into the burr holes on either side of the midline between the skull and underlying dura. The animals recovered for seven days before the EEG was measured. All EEG recordings were obtained by commercial telemetric electrodes (Pinnacle Technology). The EEG signal was recorded for ten min for KX, isoflurane, wakefulness or pan-adrenergic antagonist treat- ment using Clampx10.2 (1000 Hz sampling rate) (Ding et al., 2016; Hablitz et al., 2019). EEG data were analyzed using a customized program in MATLAB. The Chronux toolbox (http://chronux.org/) was used to calculate relative and absolute power of each band (delta, 1 to 4 Hz; theta, 4 to 8 Hz; alpha, 8 to 13 Hz; and beta, 13 to 20 Hz).
Intraventricular CSF tracer infusion
Burr holes 0.5 mm in diameter were drilled above both lateral ventricles (AP: −0.1mm, ML: ± 0.85 mm and DV: +2.0 mm from bregma). One 30G needle, connected to PE-10 tubing filled with the tracer, 0.03% Evans blue (MW 0.96 kDa, Sigma-Aldrich E2129) and 0.5% TRITC-dextran (MW 155 kDa, Sigma-Aldrich, Cat#T1287) was inserted into the left lateral ventricle. The other 30G needle, connected to PE-10 tubing filled with aCSF, was inserted into the right lateral ventricle to monitor ICP during CSF tracer infusion through the left lateral ventricle. The last 30G needle connected to PE-10 tubing, was inserted into the cisterna magna to drain CSF during tracer infusion. The CSF tracer was infused at a rate of 0.7 μl/min with a syringe pump (Harvard Apparatus). After CSF tracer infusion for either five (control baseline) or 60 min, the animals were killed by decapitation, and the brains removed and immediately sliced into 200 μm thick coronal sections using a calibrated vibratome (VT1200S, Leica). The entry of the tracers into the brain was evaluated by epifluorescence macroscopy (MVX10, Olympus). Single-channel images were acquired using MetaMorph Basic software (Molecular Devices) at 2× magnification. Exposure times were determined based on findings in control brains and kept constant for both groups. Tracer influx was quantified independently by blinded investigators using FIJI (ImageJ) software. Regions of interest (ROIs) were drawn around the coronal slice, as well as within the ventricles, so that only the tissue was measured for mean pixel intensity (MPI). The average fluorescence was computed for six sections taken from each animal.
Assessment of 4th ventricle occlusion
To determine the effect of the occlusion of the 4th ventricle with mineral oil, the surgical procedure was as described above for quantification of the rate of CSF production, with additional removal of the dura above the cisterna magna and insertion of a 30G needle, connected to PE-10 tubing filled with 0.5% Evans blue (MW 0.96 kDa, Sigma-Aldrich E2129) into the left lateral ventricle through a burr hole (AP: −0.1mm, ML: 0.85 mm and DV: +2.0 mm from bregma). The Evans blue (0.5 μl, 0.5 μl/min) and the mineral oil (1 μl, 1 μl/min) were injected simultaneously into the left ventricle and the cisterna magna with two independently controlled syringe pumps (Harvard Apparatus). Imaging was done using a surgery microscope (MZ8, Leica) at 5× magnification. After 65 min, the animals were killed by decapitation, the brains removed, and immediately sliced using a calibrated vibratome (VT1200S, Leica) without immersion in aCSF. The slices were imaged using a light macroscope (SZX12, Olympus).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were performed on GraphPad Prism 8 (GraphPad Software) and MATLAB 9.3. An unpaired t test was used for comparisons for two groups and one-way ANOVA for comparisons for more than two groups. Linear least-squares regression was used for calculation of correlations between group averages of EEG band powers and CSF production rate. p < 0.05 was considered statistically significant. All bar graph data is presented as mean ± standard error of the mean (SEM).
Supplementary Material
Highlights.
CSF production in mice is lower than previously reported using indirect techniques
Wakefulness, aging, and overexpression of amyloid-β suppress CSF production
Isoflurane, adrenergic inhibition, and female sex increase CSF production
CSF production does not correlate with brain state measured using EEG
ACKNOWLEDGMENTS
This work was supported by the National Institute of Neurological Disorders and Stroke grant R01NS100366 (the National Institutes of Health, United States); the National Institute on Aging RF1AG057575 (the National Institutes of Health, United States), the US Army Research Office grant MURI W911NF1910280HT (United States), Foundation Leducq Transatlantic Networks of Excellence Program, Novo Nordisk and Lundbeck Foundations, and the EU Horizon 2020 research and innovation program grant 666881 (SVDs@target, Denmark). The European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme grant 742112 (Denmark). We would like to thank Dan Xue for assistance with the illustrations and Prof. Paul Cumming for comments on the manuscript. We also thank Professors Hazel Jones and Kjeld Møllgård for discussions.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108524.
REFERENCES
- Andresen M, Hadi A, and Juhler M. (2016). Evaluation of Intracranial Pressure in Different Body Postures and Disease Entities. Acta Neurochir. Suppl. (Wien) 122, 45–47. [DOI] [PubMed] [Google Scholar]
- Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, et al. (2017). Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, and Alitalo K. (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balusu S, Brkic M, Libert C, and Vandenbroucke RE (2016). The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen. Res. 11, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, Probst S, Nedergaard M, Stein EA, and Lu H. (2017). Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology 127, 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, and Wardlaw J. (2019). The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 65, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker T, Stopa E, Morrison J, and Klinge P. (2014). A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst G. (1979). The significance of the evolution of the cerebrospinal fluid system. Ann. R. Coll. Surg. Engl. 61, 349–356. [PMC free article] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, and Taft RA (2012). Mouse estrous cycle identification tool and images. PLoS One 7, e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Qiao J, Kulkarni P, Harding IC, Ebong E, and Ferris CF (2020). Imaging the effect of the circadian light-dark cycle on the glymphatic system in awake rats. Proc. Natl. Acad. Sci. USA 117, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, Johanson CE, and Silverberg GD (2012). Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS 9, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Szmydynger-Chodobska J, and Johanson CE (1998). Vasopressin mediates the inhibitory effect of central angiotensin II on cerebrospinal fluid formation. Eur. J. Pharmacol. 347, 205–209. [DOI] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, and Nedergaard M. (2016). Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, and Lindvall M. (1978). Autonomic vascular innervation and vasomotor reactivity in the choroid plexus. Exp. Neurol. 62, 394–404. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Håkanson R, Lindvall M, Owman Ch., and Svensson K-G (1975). Ultrastructural and biochemical evidence for a sympathetic neural influence on the choroid plexus. Exp. Neurol. 48, 241–251. [DOI] [PubMed] [Google Scholar]
- Flurkey KC, Currer JM, and Harrison DE (2007). The Mouse in Aging Research. In The Mouse in Biomedical Research, 2nd Edition (Elsevier; ). [Google Scholar]
- Giannetto M, Xia M, Stæger FF, Metcalfe T, Vinitsky HS, Dang JAML, Xavier ALR, Kress BT, Nedergaard M, and Hablitz LM (2020). Biological sex does not predict glymphatic influx in healthy young, middle aged or old mice. Sci. Rep. 10, 16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN, Lilius TO, and Nedergaard M. (2019). Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 5, eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisey SR, Held D, and Pappenheimer JR (1962). Bulk flow and diffusion in the cerebrospinal fluid system of the goat. Am. J. Physiol. 203, 775–781. [DOI] [PubMed] [Google Scholar]
- Hong-Goka BC, and Chang FL (2004). Estrogen receptors alpha and beta in choroid plexus epithelial cells in Alzheimer’s disease. Neurosci. Lett. 360, 113–116. [DOI] [PubMed] [Google Scholar]
- Huang TY, Chung HW, Chen MY, Giiang LH, Chin SC, Lee CS, Chen CY, and Liu YJ (2004). Supratentorial cerebrospinal fluid production rate in healthy adults: quantification with two-dimensional cine phase-contrast MR imaging with high temporal and spatial resolution. Radiology 233, 603–608. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid b. Sci. Transl. Med. 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, and Borchelt DR (2001). Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol. Eng. 17, 157–165. [DOI] [PubMed] [Google Scholar]
- Johanson CE (2008). Choroid Plexus–Cerebrospinal Fluid Circulatory Dynamics: Impact on Brain Growth, Metabolism, and Repair (Humana Press; ). [Google Scholar]
- Jones HC (1980). Intracellular pores between the ependymal cells lining the roof of the fourth cerebral ventricle in mammalian fetuses. Z. Kinderchir. 31, 309–316. [Google Scholar]
- Jones HC, and Sellars RA (1982). The movement of fluid out of the ventricles in fetal and neonatal rats. Z. Kinderchir. 37, 130–133. [Google Scholar]
- Jones HC, Richards HK, Bucknall RM, and Pickard JD (1993). Local cerebral blood flow in rats with congenital hydrocephalus. J. Cereb. Blood Flow Metab. 13, 531–534. [DOI] [PubMed] [Google Scholar]
- Karimy JK, Kahle KT, Kurland DB, Yu E, Gerzanich V, and Simard JM (2015). A novel method to study cerebrospinal fluid dynamics in rats. J. Neurosci. Methods 241, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, Furey CG, Zhou X, Mansuri MS, Montejo J, et al. (2017). Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 23, 997–1003. [DOI] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Patel S, Wang DJ, and Zhang X. (2014). Effect of high dose isoflurane on cerebral blood flow in macaque monkeys. Magn. Reson. Imaging 32, 956–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilius TO, Blomqvist K, Hauglund NL, Liu G, Staeger FF, Baerentzen S, Du T, Ahlstrom F, Backman JT, Kalso EA, et al. (2019). Dexmedeto-midine enhances glymphatic brain delivery of intrathecally administered drugs. J. Control. Release 304, 29–38. [DOI] [PubMed] [Google Scholar]
- Lindvall M, Edvinsson L, and Owman C. (1978a). Reduced cerebrospinal fluid formation through cholinergic mechanisms. Neurosci. Lett. 10, 311–316. [DOI] [PubMed] [Google Scholar]
- Lindvall M, Edvinsson L, and Owman C. (1978b). Sympathetic nervous con- trol of cerebrospinal fluid production from the choroid plexus. Science 201, 176–178. [DOI] [PubMed] [Google Scholar]
- Lindvall M, Edvinsson L, and Owman C. (1979). Effect of sympathomimetic drugs and corresponding receptor antagonists on the rate of cerebrospinal fluid production. Exp. Neurol. 64, 132–145. [DOI] [PubMed] [Google Scholar]
- Lindvall-Axelsson M, and Owman C. (1990). Actions of sex steroids and corticosteroids on rabbit choroid plexus as shown by changes in transport capacity and rate of cerebrospinal fluid formation. Neurol. Res. 12, 181–186. [DOI] [PubMed] [Google Scholar]
- Lok K, Zhao H, Shen H, Wang Z, Gao X, Zhao W, and Yin M. (2013). Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci. Lett. 557, 84–89. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, and Kipnis J. (2017). Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 127, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun MP, Monuki ES, and Lehtinen MK (2015). Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Kaye JA, Atack JR, Schapiro MB, Friedland RP, and Rapoport SI (1990). Cerebrospinal fluid production is reduced in healthy aging. Neurology 40, 500–503. [DOI] [PubMed] [Google Scholar]
- Mestre H, Kostrikov S, Mehta RI, and Nedergaard M. (2017). Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. (Lond.) 131, 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk AS, Wang W, Bechet NB, Eltanahy AM, Cheng AX, Sigurdsson B, Benraiss A, Mae MA, Kress BT, Kelley DH, et al. (2019). PDGF-B Is Required for Development of the Glymphatic System. Cell Rep. 26, 2955–2969.e2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, and Goldman SA (2020). Glymphatic failure as a final common pathway to dementia. Science 370, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C, Lindvall-Axelsson M, and Owman C. (1992a). Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res. Brain Res. Rev. 17, 109–138. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Ståhlberg F, Thomsen C, Henriksen O, Herning M, and Owman C. (1992b). Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. 262, R20–R24. [DOI] [PubMed] [Google Scholar]
- Oresković D, and Klarica M. (2010). The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res. Brain Res. Rev. 64, 241–262. [DOI] [PubMed] [Google Scholar]
- Oresković D, and Klarica M. (2014). Measurement of cerebrospinal fluid formation and absorption by ventriculo-cisternal perfusion: what is really measured? Croat. Med. J. 55, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M, Vukic M, and Marakovic J. (2003). Evaluation of ventriculo-cisternal perfusion model as a method to study cerebrospinal fluid formation. Croat. Med. J. 44, 161–164. [PubMed] [Google Scholar]
- Oshio K, Song Y, Verkman AS, and Manley GT (2003). Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir. Suppl. (Wien) 86, 525–528. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, and Manley GT (2005). Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 19, 76–78. [DOI] [PubMed] [Google Scholar]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, et al. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 93, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechnik SK, Summers PE, Jezzard P, and Byrne JV (2008). Magnetic resonance measurement of blood and CSF flow rates with phase contrast—normal values, repeatability and CO2 reactivity. Acta Neurochir. Suppl. (Wien) 102, 263–270. [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Mestre H, and Nedergaard M. (2018). The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Zirretta DK, and Herndon RM (1982). Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J. Neurosci. Methods 6, 253–259. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, and Saul TA (2001). The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Skipor J, and Thiery JC (2008). The choroid plexus—cerebrospinal fluid system: undervaluated pathway of neuroendocrine signaling into the brain. Acta Neurobiol. Exp. (Warsz.) 68, 414–428. [DOI] [PubMed] [Google Scholar]
- Steffensen AB, Oernbo EK, Stoica A, Gerkau NJ, Barbuskaite D, Tritsaris K, Rose CR, and MacAulay N. (2018). Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat. Commun. 9, 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney AM, Pla V, Du T, Liu G, Sun Q, Peng S, Plog BA, Kress BT, Wang X, Mestre H, et al. (2019). In Vivo Imaging of Cerebrospinal Fluid Transport through the Intact Mouse Skull using Fluorescence Macroscopy. J. Vis. Exp. 10.3791/59774. 10.3791/59774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Grant R, Condon B, Patterson J, Lawrence A, Hadley DM, and Wyper D. (1988). Intracranial CSF volumes: natural variations and physiological changes measured by MRI. Acta Neurochir. Suppl. (Wien) 42, 230–235. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, Petersen NC, and Nedergaard M. (2018). Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett. 662, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, and Xiao M. (2019). Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 29, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. (1963). Secretion of Cerebrospinal Fluid by Choroid Plexus of the Rabbit. Am. J. Physiol. 205, 617–624. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, and Xiao M. (2015). Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Ab accumulation and memory deficits. Mol. Neurodegener. 10, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.