Abstract
Background:
Aphasia is a common, debilitating consequence of stroke, and speech therapy is often inadequate to achieve a satisfactory outcome. Neuromodulation techniques have emerged as a potential augmentative treatment for improving aphasia outcomes. Most studies have targeted the cerebrum, but there are theoretical and practical reasons that stimulation over the cerebral hemispheres might not be ideal. On the other hand, the right cerebellum is functionally and anatomically linked to major language areas in the left hemisphere, making it a promising alternative target site for stimulation.
Objective:
To provide preliminary effect sizes for the ability of a short course of anodal transcranial direct current stimulation (tDCS) targeted over the right cerebellum to enhance language processing in individuals with chronic poststroke aphasia.
Method:
Ten individuals received five sessions of open-label anodal tDCS targeting the right cerebellum. The effects of the tDCS were compared to the effects of sham tDCS on 14 controls from a previous clinical trial. In total, 24 individuals with chronic poststroke aphasia participated in the study. Behavioral testing was conducted before treatment, immediately following treatment, and at the 3-month follow-up.
Results:
Cerebellar tDCS did not significantly enhance language processing measured either immediately following treatment or at the 3-month follow-up. The effect sizes of tDCS over sham treatment were generally nil or small, except for the mean length of utterance on the picture description task, for which medium-to-large effects were observed.
Conclusion:
These results may provide guidance for investigators who are planning larger trials of tDCS for individuals with chronic poststroke aphasia.
Keywords: aphasia, neuromodulation, cerebellum, tDCS
Approximately 20% of stroke survivors experience chronic poststroke aphasia or difficulties communicating on a daily basis. It is estimated that there are >1 million aphasic stroke survivors in the United States, with 80,000 new cases added every year (Ellis et al, 2010). The standard treatment for aphasia is speech-language therapy, which involves practicing communication activities with a speech-language therapist one-on-one or in small groups. Although intensive speech-language therapy can enhance these individuals’ language processing (Bhogal et al, 2003; Brady et al, 2016; Breitenstein et al, 2017), many individuals do not have access to the services necessary to reliably improve their outcomes, and not all individuals show gains after therapy (Gottesman and Hillis, 2010). Moreover, regardless of the intensity of the therapy, individuals with aphasia rarely achieve satisfactory outcomes from speech-language therapy alone and are often left with a persistent communication disability.
Transcranial Direct Stimulation
In recent years, neuromodulation techniques, such as transcranial direct current stimulation (tDCS), have been used to potentially enhance the recovery of, and improve outcomes in, individuals with chronic poststroke aphasia (Bucur and Papagno, 2019; Elsner et al, 2019; Hao et al, 2013; Turkeltaub, 2015). TDCS is a safe, noninvasive method of inducing prolonged changes in cellular excitability by passing a constant low level of direct electrical current through targeted brain tissue in order to modulate neuronal activity (Zaghi et al, 2010). Within the motor system, the effect has often been observed to be polarity dependent, where anodal (positive) stimulation increases cortical excitability and cathodal (negative) stimulation decreases cortical excitability (Nitsche and Paulus, 2000). However, this is an oversimplification, as the relationship between polarity and excitability has not been observed consistently in studies using tDCS to modulate individuals’ cognitive skills (Jacobson et al, 2012).
Trials of tDCS on individuals with aphasia have focused primarily on improving lexical retrieval, which is measured using picture naming (eg, Baker et al, 2010; Monti et al, 2008). To date, studies investigating the use of tDCS to augment speech-language therapy and enhance outcomes have produced some evidence that tDCS can improve the ability of an individual with chronic poststroke aphasia to correctly name nouns (Elsner et al, 2019).
The neural mechanisms and optimal stimulation parameters of tDCS for chronic poststroke aphasia treatment are poorly understood (Hamilton et al, 2011), and it is not clear which brain regions should be excited or inhibited in order to achieve enhanced clinical outcomes. Naturally, choices of stimulation site and polarity have been motivated by theories of aphasia recovery. For example, a majority of studies have conducted anodal stimulation over the left hemisphere (Baker et al, 2010; Fridriksson et al, 2018b; Spielmann et al, 2018) based on the observation that optimal language recovery involves the functional re-recruitment of the remaining left-hemisphere tissue (Fridriksson, 2010; Fridriksson et al, 2010; Heiss et al, 1999; Meinzer et al, 2008; Saur et al, 2006). Other studies have conducted cathodal stimulation over the right hemisphere (Kang et al, 2011; Da Silva et al, 2018; You et al, 2011) based on the theory that right-hemisphere recruitment is maladaptive or ineffective via a mechanism such as interhemispheric disinhibition (Richter et al, 2008).
Targeting the Cerebral Hemispheres
There are, however, disadvantages to targeting either cerebral hemisphere, and both of the abovementioned approaches have yielded mixed results. A major disadvantage to stimulating the left cerebral hemisphere is that individuals with chronic stroke aphasia often have large regions of encephalomalacia that are filled with CSF at the site of their stroke, which is expected to provide a low-resistance conduit for electrical current. This low resistance allows the electrical current to shunt through nonviable tissue, thereby reducing exposure of the targeted perilesional tissue to stimulation (Datta et al, 2011). One approach to overcoming this challenge has been to individualize electrode placement on the basis of a pretreatment fMRI scan so that the stimulation targets the residual functional tissue (Baker et al, 2010; Fridriksson et al, 2018b). However, the effects of the lesion on the electrical field are unpredictable without using complicated electrical field modeling methods (Dmochowski et al, 2013), the accuracy of which remains largely unverified. This issue thus makes the selection of optimal electrode locations in the left hemisphere difficult.
A major disadvantage to applying cathodal stimulation to inhibit right-hemisphere regions is that it may be counterproductive in some cases because compensatory recruitment of the right hemisphere has been found in regions that are homotopic to the stroke (Leff et al, 2002; Musso et al, 1999; Ohyama et al, 1996; Saur et al, 2006; Skipper-Kallal et al, 2017a, 2017b; Xing et al, 2016), despite evidence that the right hemisphere may be less computationally efficient for language compared with the left hemisphere (Heiss et al, 1999, 2003).
Targeting the Cerebellar Hemispheres
Recently, the right posterolateral cerebellum has been proposed as an alternative site for tDCS in individuals with chronic poststroke aphasia (Sebastian et al, 2016; Turkeltaub et al, 2016). The right cerebellum is typically spared in individuals with chronic poststroke aphasia, which most frequently results from damage to the left cerebral hemisphere. The right posterolateral cerebellar hemisphere exhibits functional connectivity with temporal and frontoparietal association areas in the contralateral cerebral cortex, including the left frontal language areas (Buckner et al, 2011).
While the cerebellum has traditionally been associated with motor function, it is now thought to have a role in multiple aspects of speech and language (Mariën et al, 2014). Indeed, functional neuroimaging has shown that right posterolateral cerebellar activation is often present during a wide range of language tasks (Stoodley and Schmahmann, 2009). Moreover, our group’s previous work (D’Mello et al, 2017; Turkeltaub et al, 2016) showed that anodal tDCS over the right cerebellum improves verbal fluency and impacts resting-state connectivity in the cerebral language networks of healthy adults, suggesting that the cerebellum is a viable candidate for neuromodulation in individuals with aphasia.
Although there are several potential mechanisms that might underlie the effect of cerebellar tDCS to improve language processing, our previous work (D’Mello et al, 2017; Turkeltaub et al, 2016) suggested that tDCS enhances connectivity between the cerebellum and language-associated cortical regions in order to facilitate internal linguistics models for language processing at multiple levels, including sentence processing and phonemic fluency.
Based on these findings from our previous work, we hypothesized that tDCS targeted over the right posterolateral cerebellum would improve language performance (relative to sham stimulation) in individuals with chronic poststroke aphasia. To explore preliminary effect sizes of cerebellar tDCS for individuals with aphasia, we conducted an open-label pilot study of anodal cerebellar tDCS targeting the right cerebellum and compared changes in language test performance of individuals with aphasia versus changes of a sham control group from a previous tDCS clinical trial that used an identical intervention (ClinicalTrials.gov Identifier: NCT01709383).
METHOD
Participants
Twenty-four individuals who had experienced a left-hemisphere stroke (22 ischemic, 2 hemorrhagic) participated in the study. Ten individuals received active stimulation, and 14 individuals received sham stimulation. Participants in the active stimulation group were recruited from a convenience sample consisting of sequentially referred patients, mainly from the aphasia clinic at MedStar National Rehabilitation Hospital in Washington, DC. Inclusion criteria matched those from the aforementioned clinical trial. Individuals scoring >97 on the Western Aphasia Battery—Revised (WAB–R; Kertesz and Raven, 2007) at baseline and without apparent difficulty on any baseline testing were not enrolled in the study.
For the historical sham control group, we included the entire sham group from the previous tDCS clinical trial. The sham group was enrolled as a sequentially referred series of aphasic patients, mainly referred from the aphasia clinic at MedStar National Rehabilitation Hospital.
All 24 participants were at least 6 months post stroke (chronicity = 43.4 ± 39.9 months [range 6.8–177.6 months]); age = 60.2 ± 10.5 years [range 42–81 years]; 16 male, 8 female; 21 right-handed, 3 left-handed; education = 16.6 ± 2.7 years [range 12–21 years]). Aside from the stroke events, the study participants had no history of psychiatric or other neurologic conditions.
The study protocol was approved by the institutional review board of Georgetown University and was performed according to the ethical guidelines of the Declaration of Helsinki and its later amendments. All individuals provided informed written consent before enrolling in the study. As an exploratory pilot study to assess effect sizes that could be used to motivate a larger trial, the sample was not powered to detect significant effects.
Behavioral Testing
We administered the same behavioral tests to the active stimulation group that had been administered in the previous clinical trial to the sham group. In both instances, the tests were administered once before treatment, once at 24 hours post treatment, and once at the 3-month follow-up. Four additional tasks that are thought to engage the cerebellum (Mariën et al, 2014) were administered to the active stimulation group only. We administered these additional tests at two baseline time points before treatment in an attempt to distinguish practice effects from genuine treatment effects. Because this was an exploratory study, we did not pre-specify a single primary outcome measure.
Tests Given to Both Groups
We administered five behavioral tests, for which we had data available from the historical sham control group, to the active stimulation group. We used the Aphasia quotient from the WAB–R to assess the active stimulation group’s response to treatment, the Picnic Scene picture description task to assess speech production at the sentence level, a 60-item version of the Philadelphia Naming Test (PNT; Roach et al, 1996) to assess lexical retrieval, and both a category fluency task and a letter fluency task to assess fluency. In the two fluency tasks, participants were administered three categories (ie, animals, things you buy at the supermarket, and things you wear) or three letters (F, A, and S) and were asked to produce as many exemplars as possible in 60 seconds. Responses were scored online; we also video-recorded the responses so that scoring could be confirmed offline.
Tests Given to Only the Active Stimulation Group
Four tasks that had not been administered to the historical sham control group were administered to the active treatment group. These tasks included cloze sentence completion, verb generation, verb naming, and motor speech production. The cloze sentence completion task consists of 50 declarative statements that are presented visually and auditorily by a computer, with the final word unspoken and represented by a blank (eg, “The American flag is red, white, and _____.”). Participants are given 6 seconds to say aloud a word that fills in the blank. We included the cloze sentence completion task because cerebellar tDCS has previously been observed to modulate the cerebellar circuitry for the semantic prediction component of the task, which is thought to rely on the forward modeling supported by the cerebellum (D’Mello et al, 2017). We video-recorded the responses so that scores could be figured and RTs could be determined offline.
The verb generation task consists of 50 written names of objects that are presented on a computer. Participants are given 6 seconds to say aloud an “action word” that matches each presented object (eg, boat → row). No verb generation stimuli overlapped with any of the items that had been trained during treatment. Responses were video-recorded and were scored offline for acceptability and measured for response times.
The verb naming task consists of 30 images that are presented on a computer. Each image consists of a person performing an action. Participants are given 20 seconds to say aloud the verb that best describes the action depicted. We included the verb generation and verb naming tasks because recent work on cerebellar tDCS in individuals with chronic poststroke aphasia found improvements in verb generation but not verb naming during stimulation (Marangolo et al, 2018).
Two measures of oral diadochokinesis were used to assess motor speech. The first measure was sequential motion rate, in which participants are asked to repeat single CV (consonant–vowel) syllables (eg, puh, tuh, and kuh) as quickly and steadily as possible for 5 seconds. The second measure was alternating motion rate, in which participants are asked to repeat an ordered sequence of CV syllables (eg, puh + tuh + kuh) as quickly and steadily as possible for 5 seconds. We included the motor speech exam because motor speech programming is one aspect of communication to which the cerebellum is thought to contribute (Mariën et al, 2014).
Treatment Procedure
Study Design
The 24 study participants received 5 consecutive days of treatment that involved multimodal speech therapy targeting anomia paired with anodal tDCS targeting the right posterolateral cerebellum. We tested the active stimulation participants on four occasions: twice before treatment, once immediately post treatment, and at the 3-month follow-up. The historical sham control group had been tested on the last three occasions during the previous control trial.
Speech Therapy
The multimodal speech therapy targeted anomia by engaging the participants’ productive language systems during 5 consecutive days of tDCS administration. We based the number of sessions on a prior study by Lindenberg et al (2010) that had demonstrated that 5 consecutive days of tDCS could improve arm motor function after a stroke. Based on the hypothesis that tDCS would modulate language networks in the brain, the speech therapy was designed primarily to engage the language network broadly during stimulation rather than to consolidate learning on specific training items.
We used an explicit written protocol to standardize procedures across participants. Ten words depicted on picture cards were trained per session (50 items total over the 5-day treatment period). Based on each individual’s baseline PNT score, he or she received items from a hard word list (PNT accuracy ≥ 20 out of 60) or an easy word list (PNT accuracy < 20 out of 60). Thirty items were shared between the two lists. Five of the historical sham control participants had received the easy list; all of the other participants received the hard list.
Each session began with picture naming, supported by phonemic and semantic cuing. The participants generated semantic features of each item, produced sentences containing each item, and wrote each item using pencil and paper. Each session lasted a total of 60 minutes, including setup and takedown; the treatment itself lasted ~45 minutes. In order to allow as direct a comparison of outcomes as possible, intervention for the active stimulation group was kept identical to what had been delivered to the historical sham control group. The treatment materials are available as supplemental digital content 1 and supplemental digital content 2.
Cerebellar tDCS
In the active condition, the tDCS was applied using a custom-designed CT stimulator (Soterix Medical) consisting of a current source powered by 9-volt batteries and 5×5-cm electrode sponges with saline solution to make contact with the scalp. The anode was placed over the right posterolateral cerebellum (estimated to be over lobule VII), measured 1 cm down and 4 cm to the right of the inion. The cathode was placed on the upper right bicep. The current was ramped up to 2 mA over 30 seconds, applied during the first 20 minutes of speech therapy, and then ramped down over 30 seconds.
In the sham condition, the tDCS setup was applied using a Soterix HD-tDCS- CT unit consisting of a current source powered by 9-volt batteries and AgCl-coated electrodes and conductive gel. Two anodes were placed at the left inferior frontal gyrus (F7 and F5) and two cathodes were placed at the right-hemisphere homolog (F6 and F8). The current was ramped up to 1.5 mA over 30 seconds, followed immediately by a 30-second ramp-down. No current was delivered until 19 minutes later, when the current was again ramped up to 1.5 mA over 30 seconds, followed by a 30-second ramp-down. The ramp up and down is supposed to simulate the change in sensation that individuals sometimes report feeling during active tDCS as the current is ramped up at the start of treatment and ramped down at the end.
Statistical Analysis
Modeling of Treatment Effects
For the tests that were administered to both the active stimulation group and the historical sham control group, a one-way ANCOVA was used to model the treatment effects immediately after treatment and at the 3-month follow-up. Specifically, for each outcome measure, two models were estimated. The first model measured the change from pretreatment to immediate post treatment, and the second model measured the change from pretreatment to the 3-month follow-up. In each model, the immediate post treatment (or 3-month follow-up) score was measured as a function of study arm (active, sham), controlling for baseline score and lesion volume. A significant effect of the factor of arm was interpreted as an effect of stimulation.
For the tests that were administered to only the active stimulation group, a one-way ANCOVA was again used to model the treatment effects immediately post treatment and at the 3-month follow-up. Again, for each outcome measure, two models were estimated. The first model measured the change from pretreatment to immediate posttreatment, and the second model measured the change from pretreatment to the 3-month follow-up. In each model, test score performance was measured as a function of time. The pretreatment scores were entered as the average of the two baseline measurements. A significant effect of time was interpreted as an effect of stimulation and speech therapy. Lesion volume was included as a covariate in this model.
Measuring Individual Responders
Because of heterogeneity in participant characteristics, we anticipated that some individuals in the active stimulation group might show a gain post treatment even if the group as a whole did not. To measure these individual responders, for each test with data from the historical sham control group, we quantified gains for each individual in the active stimulation group by calculating a z score for treatment gain scores relative to the historical sham control group’s treatment gains.
RESULTS
Stimulation and Sham Groups
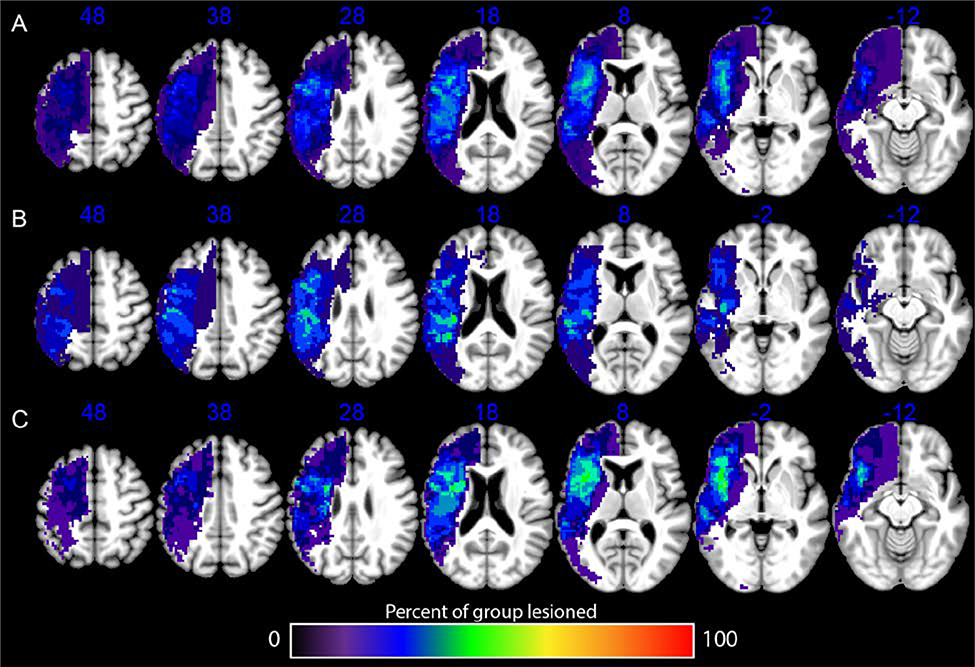
As shown in Table 1, the active stimulation group and the historical sham control group did not differ significantly in age, time since stroke, lesion volume, years of education, or WAB–R Aphasia quotient as measured at the start of the study. Both groups’ strokes encompassed the middle cerebral artery distribution, with some strokes extending into the anterior and posterior cerebral artery territories (Figure 1). Over the course of the study, there were no serious adverse events such as seizure, hospitalization, or death. All of the participants completed all of the treatment and testing sessions. Individual participant data for the demographics and all test scores are available in supplemental digital content 3.
TABLE 1.
Demographics of the Active Stimulation and Historical Sham Control Groups
| Group | Active | Sham | Comparison | P |
|---|---|---|---|---|
| n | 10 | 14 | - | - |
| Male/Female | 7/3 | 9/5 | - | - |
| Age (years) | 60.36 (13.17) | 60.13 (8.63) | t(14.43) = 0.05 | 0.96 |
| Time since stroke (months) | 42.72 (55.06) | 43.96 (26.90) | t(12.09) = –0.07 | 0.95 |
| Lesion volume (mm3) | 53,308 (59,047) | 50,191 (60,386) | t(19.81) = 0.13 | 0.90 |
| Education (years) | 17.00 (2.31) | 16.29 (2.92) | t(21.70) = 0.67 | 0.51 |
| Aphasia quotient | 79.2 (15.44) | 65.1 (26.77) | t(22) = 1.49 | 0.15 |
Data are presented as M ± SD unless otherwise noted.
FIGURE 1.
Overlay showing lesion overlap for all study participants (A), only individuals in the active treatment group (B), and only individuals in the historical sham control group (C).
Behavioral Results
Tests Given to Both Groups
There was no significant effect of tDCS on scores or subscores from the WAB–R Aphasia quotient, the picture description task, the PNT (accuracy), or the category or letter fluency tasks, either immediately post treatment or at the 3-month follow-up relative to the baseline scores. Statistics for these measures are shown in Table 2.
TABLE 2.
Statistics for the Tests Given to Both Groups
| Measure | Pre-score (Sham; Active) M (SD) | Post-score (Sham; Active) M (SD) | Time Point | Adjusted Mean Difference | 95% CI | F (df) | P | |
|---|---|---|---|---|---|---|---|---|
| Western Aphasia Battery—Revised | ||||||||
| Aphasia Quotient | 65.1 (26.7); 79.2 (15.4) | 65.6 (27.3); 80.2 (16.8) | F1 | 0.43 | −2.2 – 3.1 | 0.12 (1,20) | 0.74 | 0.006 |
| 66.6 (26.8); 82.3 (15.6) | F2 | 1.9 | −0.76 – 4.5 | 2.19 (1,20) | 0.15 | 0.099 | ||
| Spontaneous Speech | 12.3 (6.4); 16.0 (3.6) | 12.3 (6.3); 16.2 (3.6) | F1 | 0.5 | −0.54 – 1.3 | 0.78 (1,20) | 0.39 | 0.038 |
| 12.7 (6.1), 16.5 (3.4) | F2 | 0.4 | −0.67 – 1.5 | 0.62 (1,20) | 0.44 | 0.03 | ||
| Auditory Verbal Comprehension | 8.1 (1.8); 8.5 (1.6) | 7.8 (1.9); 8.3 (2.0) | F1 | 0.025 | −0.51 – 0.56 | 0.01 (1,20) | 0.92 | 0.00047 |
| 8.1 (1.9); 8.7 (1.5) | F2 | 0.22 | −0.18 – 0.61 | 1.29 (1,20) | 0.27 | 0.061 | ||
| Repetition | 6.1 (3.0); 7.5 (1.4) | 6.2 (3.1); 7.9 (1.6) | F1 | 0.24 | −0.34 – 0.81 | 0.73 (1,20) | 0.40 | 0.035 |
| 6.2 (3.0); 7.9 (1.7) | F2 | 0.28 | −0.45 – 1.00 | 0.64 (1,20) | 0.43 | 0.031 | ||
| Naming and Word Finding | 6.1 (3.0); 7.6 (1.7) | 6.6 (3.0); 7.8 (2.1) | F1 | −0.2 | −0.81 – 0.42 | 0.44 (1,20) | 0.51 | 0.022 |
| 6.3 (3.0); 8.1 (1.9) | F2 | 0.37 | −0.29 – 1.00 | 1.35 (1,20) | 0.26 | 0.063 | ||
| Picture Description Task | ||||||||
| Mean length of utterance | 4.43 (2.75); 5.58 (2.76) | 4.04 (2.65); 6.05 (2.75) | F1 | 1.2 | −0.03 – 2.4 | 4.15 (1,19) | 0.056 | 0.18 |
| 4.27 (2.41); 5.82 (2.70) | F2 | 0.61 | −0.22 – 1.4 | 2.37 (1,19) | 0.14 | 0.11 | ||
| Speech rate (wpm) | 46.4 (40.1); 67.9 (40.5) | 52.0 (52.6); 68.1 (36.5) | F1 | −0.94 | −24 – 22 | 0.01 (1,19) | 0.93 | 0.00 |
| 41.9 (50.1), 70.1 (42.9) | F2 | 9.6 | −11 – 20 | 0.98 (1,19) | 0.33 | 0.05 | ||
| # of unique real words | 51.5 (45.8); 80.6 (60.0) | 56.5 (63.5); 88.0 (70.6) | F1 | 5.1 | −17 – 27 | 0.24 (1,19) | 0.63 | 0.01 |
| 59.9 (58.0); 94.3 (71.2) | F2 | 1.9 | −12 – 16 | 0.08 (1,19) | 0.78 | 0.00 | ||
| # of unique nouns | 17.5 (14.3); 29.5 (17.6) | 22.2 (19.4), 31.1 (19.6) | F1 | −1.2 | −8.9 – 6.6 | 0.1 (1,19) | 0.76 | 0.01 |
| 21.0 (16.5); 31.7 (17.9) | F2 | −0.009 | −6.3 – 6.3 | 0 (1,19) | 1.00 | 0.00 | ||
| # of unique verbs | 10.2 (7.9); 10.2 (8.4) | 9.8 (11.1); 12.9 (12.2) | F1 | 4.6 | 0.06 – 9.1 | 4.5 (1,19) | 0.05 | 0.19 |
| 13.5 (11.9); 14.9 (13.4) | F2 | 2.3 | −1.9 – 6.5 | 1.29 (1,19) | 0.27 | 0.06 | ||
| # of total words | 103 (116); 186 (163) | 134 (213); 222 (237) | F1 | 22 | −70 – 120 | 0.26 (1,19) | 0.62 | 0.01 |
| 131 (172); 226 (209) | F2 | 9.0 | −51 – 69 | 0.1 (1,19) | 0.76 | 0.01 | ||
| Philadelphia Naming Test | ||||||||
| Philadelphia Naming Test (% accuracy) | 53.6% (39.2); 78.5% (19.2) | 57.1% (39.1); 80.0% (20.3) | F1 | −0.019 | −0.08 – 0.04 | 0.39 (1,20) | 0.54 | 0.02 |
| 56.9% (38.5); 82.7% (16.4) | F2 | 0.021 | −0.034 – 0.076 | 2.19 (1,20) | 0.62 | 0.03 | ||
| Fluency Tasks | ||||||||
| Category fluency (words) | 21.17 (20.57); 33.40 (15.54) | 24.08 (23.07); 35.5 (17.19) | F1 | −2.1 | 5.6 – 1.3 | 1.7 (1,19) | 0.21 | 0.086 |
| 23.42 (22.46); 35.4 (14.58) | F2 | −0.37 | −3.6 – 2.8 | 0.06 (1,19) | 0.81 | 0.003 | ||
| Letter fluency (words) | 9.42 (11.67); 16.00 (13.65) | 10.42 (12.29); 18.90 (16.25) | F1 | 1.8 | −3.2 – 6.9 | 0.58 (1,19) | 0.46 | 0.031 |
| 9.67 (13.32), 17.90 (12.57) | F2 | 2.2 | −2.1 – 6.5 | 1.16 (1,19) | 0.30 | 0.16 | ||
Data are presented as M ± SD unless otherwise noted. Results are shown for measurements immediately following treatment (F1) and at the 3-month follow-up (F2).
Notably, this study was exploratory and was not powered to find significant effects of treatment across the groups. The primary goal was to examine effect sizes, irrespective of statistical significance. The most promising effect was observed for mean length of utterance on the picture description task, for which there was a large effect of tDCS immediately post treatment ( = 0.18, approximately equivalent to Cohen’s d = 0.94) and a medium effect of tDCS at the 3-month follow-up ( = 0.11, approximately equivalent to Cohen’s d = 0.70). The number of unique verbs on the picture description task showed a similar pattern ( = 0.19 immediately post treatment and 0.06 at the 3-month follow-up, approximately equivalent to Cohen’s d of 0.97 and 0.50, respectively). For some of the other measures, medium to large effects were observed at one time point (eg, immediately post treatment for category fluency and at the 3-month follow-up for Aphasia quotient and letter fluency), but no effects were observed at the other time point.
Tests Given to Only the Active Stimulation Group
There was no significant effect of speech therapy paired with tDCS on six measures from the tests with tests that were given to only the active stimulation group, including cloze sentence completion accuracy and response time, verb generation accuracy and response time, verb naming accuracy, and motor speech exam performance (mean alternating motion rate and sequential motion rate, measured as repetitions per second). These scores and their associated statistics are shown in Table 3.
TABLE 3.
Test Scores and Statistics Associated With 6 Measures From the Tests Given to Only the Active Stimulation Group
| Task | Measure (units) | Baseline Scores | Time point | Posttreatment Score M (SD) | Adjusted Mean Difference | 95% CI | F (df) | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| M 1 (SD) | M 2 (SD) | |||||||||
| Cloze sentence completion | Accuracy (%) | 64.6% (21.6) | 64.8% (21.0) | F1 | 70.2% (20.0) | 5.6 | −24 – 12 | 0.44 (1,17) | 0.52 | 0.025 |
| F2 | 67.6 (22.76) | −3 | −22 – 16 | 0.12 (1,17) | 0.74 | 0.007 | ||||
| RT (seconds) | 1.45 (0.87) | 1.26 (1.04) | F1 | 1.17 (0.94) | 0.18 | −0.56 – 0.93 | 0.27 (1,17) | 0.61 | 0.016 | |
| F2 | 1.26 (1.07) | 0.06 | −0.70 – 0.88 | 0.06 (1,17) | 0.81 | 0.004 | ||||
| Verb generation | Accuracy (%) | 46.0% (40.29) | 49.6% (42.28) | F1 | 53.2% (42.38) | 5.4 | −41 – 30 | 0.10 (1,17) | 0.75 | 0.006 |
| F2 | 55.8% (40.72) | −8 | −43 – 27 | 0.24 (1,17) | 0.63 | 0.014 | ||||
| RT (seconds) | 2.14 (1.02) | 1.78 (0.92) | F1 | 1.36 (0.56) | 0.56 | −0.24 – 1.4 | 2.23 (1,15) | 0.16 | 0.13 | |
| F2 | 1.72 (0.87) | 0.23 | −0.73 – 1.2 | 0.26 (1,15) | 0.62 | 0.26 | ||||
| Verb naming | Accuracy (%) | 14.3% (7.59) | 15.4% (7.57) | F1 | 14.8% (7.27) | 0.05 | −5.2 – 5.3 | 0 (1,17) | 0.98 | 0.001 |
| F2 | 17.0% (7.77) | −2.1 | −7.8 – 3.5 | 0.65 (1,17) | 0.43 | 0.037 | ||||
| Motor speech exam | Mean AMR SMR (rps) | 2.78 (1.13) | 3.25 (0.98) | F1 | 3.17 (1.09) | −0.15 | −1 – 0.74 | 0.13 (1,17) | 0.72 | 0.008 |
| F2 | 3.29 (0.99) | −0.28 | −1 – 0.47 | 0.62 (1,17) | 0.44 | 0.035 | ||||
Data are presented as M ± SD unless otherwise noted. Results are shown for measurements immediately following treatment (F1) and at the 3-month follow-up (F2).
AMR = alternating motion rate. rps = repetitions per second. RT = response time. SMR = sequential motion rate.
Nevertheless, there was a medium-sized effect of tDCS immediately post treatment on verb generation response time ( = 0.13, approximately equivalent to Cohen’s d = 0.77) and a large effect of tDCS at the 3-month follow-up ( = 0.26, approximately equivalent to Cohen’s d = 1.18). For some of the other measures, medium to large effects were observed at one time point (eg, at the 3-month follow-up for verb naming and motor speech exam), but no effects were observed at the other time point.
Individual Responders
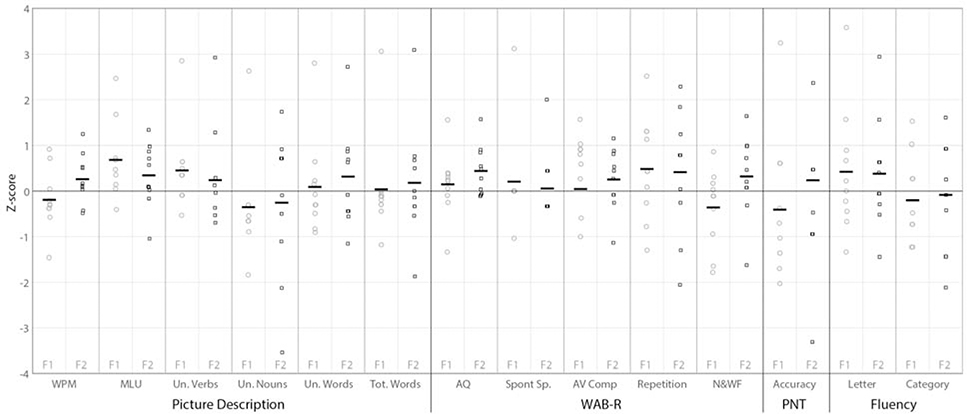
Although a few individuals in the active stimulation group showed high z scores (>2) on individual tests, there were none who clearly responded more than the historical sham control group across multiple tests, and no tests on which there were multiple clear responders in the active stimulation group (Figure 2). On mean length of utterance, which showed the most reliable effect across the group analyses, 9 of the 10 individuals in the active stimulation group had positive z scores compared with the historical sham control group (ie, an improvement greater than the sham group mean) immediately post treatment, and 8 had positive z scores at the 3-month follow-up. Only one individual had a z score >2 immediately post treatment, and none did at the 3-month follow-up. Results were more variable for the number of different verbs on the picture description task, with 6 of the 10 individuals in the active stimulation group having positive z scores immediately post treatment and 4 at the 3-month follow-up. In this case, the effect sizes at the group level were largely driven by a single individual with a z score of 2.9 both immediately post treatment and at the 3-month follow-up.
FIGURE 2.
For each test with data from a historical sham control group, gains for each individual in the active stimulation group were calculated as a z score of their treatment gain scores relative to the historical sham control group’s treatment gains. For each test measure, individual participant z scores are shown for immediately post treatment (F1, gray circles) and at the 3-month follow-up (black squares, F2). AV Comp = auditory verbal comprehension. AQ = Aphasia quotient. F1 = immediately post treatment. F2 = 3-month follow-up. MLU = mean length of utterance. N&WF = naming and word finding. PNT = Philadelphia Naming Test. SpontSp = spontaneous speech. Un = unique. WAB–R = Western Aphasia Battery–Revised.
DISCUSSION
In this open-label pilot study, we tested whether anodal tDCS targeted over the right posterolateral cerebellum could enhance language processing in individuals with chronic stroke aphasia. In our sample, individuals in the active stimulation group did not achieve statistically significant posttreatment behavioral gains in test scores compared with those of a historical sham control group. In addition, compared to scores measured at two baseline sessions, the active stimulation group did not demonstrate significant gains on measures that are thought to engage the cerebellum.
The lack of statistically significant treatment effects is not surprising given that the study was exploratory and was not powered to establish the efficacy of cerebellar tDCS. For this reason, we also examined the effect sizes of the comparison between active cerebellar tDCS and sham tDCS. If we detected promising effect sizes, then we would be well-positioned to design a larger study that was adequately powered to measure effects of tDCS across the active treatment group. Of the several measures examined, only mean length of utterance on the picture description task showed a promising effect of cerebellar tDCS. Mean length of utterance is one of the main measures of fluency of narrative speech production, which is commonly reduced in individuals with chronic poststroke aphasia. Improving speech fluency would be clinically important for many individuals with chronic post-stroke aphasia and would differentiate cerebellar tDCS, which may improve speech fluency, from approaches that so far have been shown to improve noun naming only.
We also examined the effect sizes of the comparison between pretreatment and posttreatment scores (immediately post treatment and at the 3-month follow-up) for measures that were administered to only the active cerebellar tDCS group and not the historical sham control group. Of the several measures examined, only the response time on the verb generation task showed a promising effect of cerebellar tDCS. These findings should be interpreted cautiously given the number of measures that were examined and the open-label design; however, the findings might warrant follow-up in future investigations of the use of cerebellar tDCS for treating individuals with chronic poststroke aphasia.
Our study sample included heterogeneous lesion locations, behavioral severities, and stroke chronicities. Although a few of the individuals in the active group did show gains above the control group on individual measures, the improvement was small and was not consistent enough across multiple related measures to clearly demonstrate that the effects were not random. Thus, we did not find clear evidence that there were individual participants in the active group who were particularly responsive to cerebellar tDCS. Given the small sample size of this pilot study, it remains possible that cerebellar tDCS is effective for individuals with lesions in specific parts of the neural circuitry, with certain types or degrees of behavioral deficit, at certain times during the recovery process, or for individuals with specific genetic characteristics (Fridriksson et al, 2018a).
The lack of robust treatment effects observed in our study is at odds with a case study by Sebastian et al (2016) that found that anodal cerebellar tDCS enhanced spelling in an individual with chronic poststroke aphasia. However, there are important differences between the two studies. We enrolled participants with chronic aphasia after a left-hemisphere stroke rather than bilateral lesions, and we did not assess spelling ability after treatment. In addition, Sebastian and colleagues (2016) employed a greater treatment intensity, lasting 15 training sessions rather than the 5 days in our study.
Our results are in line with previous findings that the use of cerebellar tDCS enhanced verb generation but not verb naming in individuals with chronic poststroke aphasia (Marangolo et al, 2018). Although the current study was not powered to detect differences between tasks, it does appear that verb generation improved more numerically than verb naming, and there was a medium-to-large effect on response time during verb generation. However, because of a lack of control group for these measures and the open-label design, it is difficult to know whether this effect was just a general effect of practice or a placebo effect.
Study Limitations
Although it is possible that cerebellar tDCS is not effective in improving language processing in individuals with chronic poststroke aphasia by modulating language networks, we cannot draw such a conclusion from a single study. Our trial was a pilot study, and it had several important limitations. First, our study employed an open-label design, meaning that individuals were not randomized to the treatment group and thus knew that they were receiving stimulation. However, while crossover designs are regarded as offering better internal validity, there are some limitations to crossover tDCS studies as well, including evidence that individuals can differentiate between real and sham stimulation (Kessler et al, 2012; O’Connell et al, 2012) and that the effects of tDCS may last for multiple months after stimulation, which can preclude an appropriate washout period (Vestito et al, 2014). Nevertheless, an open-label design is not optimal, and comparisons between the active and historical sham control groups should be interpreted with additional caution.
Second, our modest sample size naturally limited our statistical power to detect an effect of treatment across the two groups. However, except perhaps for mean length of utterance, the effect sizes do not suggest that a lack of effect was primarily related to low statistical power. In addition, treatment lasted only 1 week and involved general multimodal therapy targeted at improving anomia. This treatment was based on the hypothesis that tDCS would modulate language processing through alterations in brain networks rather than on the hypothesis that tDCS would enhance learning of specific items during therapy. A more intense treatment regimen with a greater dosage, or a regimen targeted at individualized speech-language deficits, might have had a different outcome. Another possibility is that the stimulation produced specific behavioral effects, such as changes to certain types of errors produced, that we did not measure in this study.
An additional limitation is that based on our study measures, we cannot say whether the tDCS enhanced learning of the items that were trained during therapy. Thus, it is also possible that tDCS enhanced performance on the trained items relative to the sham group, separate from alterations in language processing. In this light, it is possible that 1 week of treatment showed effects on trained items, but that treatment dosage or duration needs to be considerably greater in order to modulate language networks in a way that affects language processing more generally.
Finally, we used anodal stimulation, but as noted in the introduction, the neural mechanisms of tDCS-induced changes in language processing are not known. Some authors have claimed that cathodal, rather than anodal, stimulation of the cerebellum is preferable, under the hypothesis that cathodal stimulation inhibits Purkinje cells in cerebrocerebellar circuits to ultimately disinhibit the left frontal lobe resources that are responsible for behavioral enhancement (Marangolo et al, 2018; Pope and Miall, 2014). Thus, it is possible that cathodal stimulation would have produced a different outcome due to the mechanism of action of cerebellar stimulation.
CONCLUSION
This pilot study provided no clear evidence that anodal tDCS targeting the right cerebellum given daily for 5 days with multimodal speech therapy produces behavioral improvements in individuals with chronic stroke aphasia. Given observed effect sizes, additional work may be warranted to determine whether cerebellar tDCS has a future as an adjuvant to speech therapy in individuals with aphasia. Because the active stimulation group did show promising changes in mean length of utterance, future studies using cerebellar tDCS with individuals with chronic poststroke aphasia should consider whether cerebellar tDCS might specifically improve measures of fluency in spontaneous speech.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mackenzie Fama, PhD, Zainab Anbari, and Kate Spiegel of Georgetown University for data collection.
Supported in part by a grant (21012062) from the Doris Duke Charitable Foundation and two grants (R21DC014087 and R01DC014960) from the National Institute on Deafness and Other Communication Disorders to P.E.T., and two grants (KL2TR000102 and TL1TR001431) from the National Institutes of Health/National Center for Advancing Translational Sciences via the Georgetown–Howard Universities Center for Clinical and Translational Science and one grant (U10NS086513) from the National Institute of Neurological Disorders and Stroke to A.T.D.
Glossary
- PNT
Philadelphia Naming Test
- tDCS
transcranial direct current stimulation
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.cogbehavneurol.com.
REFERENCES
- Baker JM, Rorden C, Fridriksson J. 2010. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 41:1229–1236. doi: 10.1161/STROKEAHA.109.576785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal SK, Teasell R, Speechley M. 2003. Intensity of aphasia therapy, impact on recovery. Stroke. 34:987–993. doi: 10.1161/01.STR.0000062343.64383.D0 [DOI] [PubMed] [Google Scholar]
- Brady MC, Kelly H, Godwin J, et al. 2016. Speech and language therapy for aphasia following stroke. Published online June 1. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Grewe T, Flöel A. 2017. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 389:1528–1538. doi: 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, et al. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. doi: 10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur M, Papagno C 2019. Are transcranial brain stimulation effects long-lasting in post-stroke aphasia? a comparative systematic review and meta-analysis on naming performance. Neurosci Biobehav Rev. 102:264–289. doi: 10.1016/j.neubiorev.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Da Silva FR, Mac-Kay APM, Chao JC, et al. 2018. Transcranial direct current stimulation: a study on naming performance in aphasic individuals. Published online August 30. Codas. 30:e20170240. doi: 10.1590/2317-1782/20182017242e [DOI] [PubMed] [Google Scholar]
- Datta A, Baker JM, Bikson M, et al. 2011. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 4:169–174. doi: 10.1016/j.brs.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Turkeltaub PE, Stoodley CJ. 2017. Cerebellar tDCS modulates neural circuits during semantic prediction: a combined tDCS-fMRI study. J Neurosci. 37:1604–1613. doi: 10.1523/JNEUROSCI.2818-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Huang Y, et al. 2013. Targeted transcranial direct current stimulation for rehabilitation after stroke. NeuroImage. 75:12–19. doi: 10.1016/j.neuroimage.2013.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Dismuke C, Edwards KK. 2010. Longitudinal trends in aphasia in the United States. NeuroRehabilitation. 27:327–333. doi: 10.3233/NRE-2010-0616 [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, et al. 2019. Transcranial direct current stimulation (tDCS) for improving aphasia in adults with aphasia after stroke. Published online May 21. Cochrane Database Syst Rev. 5:CD009760. doi: 10.1002/14651858.CD009760.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J 2010. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci. 30:11558–11564. doi: 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Baker JM, et al. 2010. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cereb Cortex. 20:1013–1019. doi: 10.1093/cercor/bhp160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Elm J, Stark BC, et al. 2018a. BDNF genotype and tDCS interaction in aphasia treatment. Brain Stimul. 11:1276–1281. doi: 10.1016/j.brs.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Elm J, et al. 2018b. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol. 75:1470–1476. doi: 10.1001/jamaneurol.2018.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Hillis AE. 2010. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 9:895–905. doi: 10.1016/S1474-4422(10)70164-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. 2011. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 118:40–50. doi: 10.1016/j.bandl.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Wang D, Zeng Y, et al. 2013. Repetitive transcranial magnetic stimulation for improving function after stroke. Published online May 31. Cochrane Database Syst Rev. 2013:CD008862. doi: 10.1002/14651858.CD008862.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, et al. 1999. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 45:430–438. doi: [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Kessler J, et al. 2003. Disturbance and recovery of language function: correlates in PET activation studies. NeuroImage. 20(suppl 1):S42–S49. doi: 10.1016/j.neuroimage.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. 2012. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 216:1–10. doi: 10.1007/s00221-011-2891-9 [DOI] [PubMed] [Google Scholar]
- Kang EK, Kim YK, Sohn HM, et al. 2011. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restor Neurol Neurosci. 29:141–152. doi: 10.3233/RNN-2011-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Raven JC. 2007. WAB–R: Western Aphasia Battery—Revised. San Antonio, Texas: PsychCorp. [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, et al. 2012. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 5:155–162. doi: 10.1016/j.brs.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, et al. 2002. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol. 51:553–558. doi: 10.1002/ana.10181 [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, et al. 2010. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 75:2176–2184. doi: 10.1212/WNL.0b013e318202013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangolo P, Fiori V, Caltagirone C, et al. 2018. Transcranial cerebellar direct current stimulation enhances verb generation but not verb naming in poststroke aphasia. J Cogn Neurosci. 30:188–199. doi: 10.1162/jocn_a_01201 [DOI] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, et al. 2014. Consensus paper: language and the cerebellum: an ongoing enigma. Cerebellum. 13:386–410. doi: 10.1007/s12311-013-0540-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, et al. 2008. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage. 39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, et al. 2008. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 79:451–453. doi: 10.1136/jnnp.2007.135277 [DOI] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, et al. 1999. Training-induced brain plasticity in aphasia. Brain. 122(pt 9):1781–1790. doi: 10.1093/brain/122.9.1781 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell NE, Cossar J, Marston L, et al. 2012. Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. Published online October 17. PLoS One. 7:e47514. doi: 10.1371/journal.pone.0047514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Senda M, Kitamura S, et al. 1996. Role of the nondominant hemisphere and undamaged area during word repetition in poststroke aphasics. a PET activation study. Stroke. 27:897–903. doi: 10.1161/01.str.27.5.897 [DOI] [PubMed] [Google Scholar]
- Pope PA, Miall RC. 2014. Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Published online April 2. Front Psychiatry. 5:33. doi: 10.3389/fpsyt.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Miltner WHR, Straube T. 2008. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 131:1391–1401. doi: 10.1093/brain/awn043 [DOI] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, et al. 1996. The Philadelphia Naming Test: scoring and rationale. Clinical Aphasiology. 24:121–133. [Google Scholar]
- Saur D, Lange R, Baumgaertner A, et al. 2006. Dynamics of language reorganization after stroke. Brain. 129(pt 6):1371–1384. doi: 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Sebastian R, Saxena S, Tsapkini K, et al. 2016. Cerebellar tDCS: a novel approach to augment language treatment post-stroke. Published online January 12, 2017. Front Hum Neurosci. 10:695. doi: 10.3389/fnhum.2016.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper-Kallal LM, Lacey EH, Xing S, et al. 2017a. Functional activation independently contributes to naming ability and relates to lesion site in post-stroke aphasia. Hum Brain Mapp. 38:2051–2066. doi: 10.1002/hbm.23504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper-Kallal LM, Lacey EH, Xing S, et al. 2017b. Right hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Published online January 12. Neural Plast. 2017:8740353. doi: 10.1155/2017/8740353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann K, van de Sandt-Koenderman WME, Heijenbrok-Kal MH, et al. 2018. Transcranial direct current stimulation does not improve language outcome in subacute poststroke aphasia. Stroke. 49:1018–1020. doi: 10.1161/STROKEAHA.117.020197 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2009. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 44:489–501. doi: 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE. 2015. Brain stimulation and the role of the right hemisphere in aphasia recovery. Published online November. Curr Neurol Neurosci Rep. 15:72. doi: 10.1007/s11910-015-0593-6 [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Swears MK, D’Mello AM, et al. 2016. Cerebellar tDCS as a novel treatment for aphasia? evidence from behavioral and resting-state functional connectivity data in healthy adults. Restor Neurol Neurosci. 34:491–505. doi: 10.3233/RNN-150633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestito L, Rosellini S, Mantero M, et al. 2014. Long-term effects of transcranial direct-current stimulation in chronic post-stroke aphasia: a pilot study. Published online October 14. Front Hum Neurosci. 8:785. doi: 10.3389/fnhum.2014.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Lacey EH, Skipper-Kallal LM, et al. 2016. Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain. 139(pt 1):227–241. doi: 10.1093/brain/awv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You DS, Kim DY, Chun MH, et al. 2011. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 119:1–5. doi: 10.1016/j.bandl.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, et al. 2010. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist. 16:285–307. doi: 10.1177/1073858409336227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.