Abstract
Background
Lipid-rich plaques (LRP) in the non-culprit lesions (NCL) in patients with the acute coronary syndrome may trigger lesion-related, adverse cardiovascular events. Aggressive lipid-lowering therapy may stabilize LRP; however, the times of stabilization remain undefined.
Case summary
A 60-year-old man presented with unstable angina. Coronary angiography revealed a severely stenotic lesion (culprit lesion) in the left descending artery, and another non-obstructive lesion in the distal left main trunk artery. Near-infrared spectroscopy (NIRS) imaging showed LRP with a maximum lipid core burden index (LCBI)4mm of 422. Optical coherence tomographic (OCT) imaging showed the vulnerable plaque as a thin cap fibroatheroma with a thickness of 50 µm. We prescribed aggressive lipid-lowering treatment with a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, and serially observed this lesion for 24 months. The NIRS imaging showed that the LCBI gradually decreased over time (max LCBI4mm of 422, 417, 318, 265, and 106 conducted at index percutaneous coronary intervention, 3, 8, 12, and 24 months, respectively). As plaque regression and stabilization of high-risk LRP were observed, we promptly discontinued treatment with the PCSK9i inhibitor.
Discussion
During the long-term, 24-month, follow-up using serial NIRS–IVUS imaging, we observed the gradual decrease in LCBI over time, due to aggressive lipid-lowering therapy. Compared with the lowering of low-density lipoprotein cholesterol, the stabilization of vulnerable plaques may require longer times of about 2 years. Evaluation of NCL-related adverse cardiac events by serial intravascular imaging over time, using NIRS–IVUS or OCT, may be warranted in such cases.
Keywords: Case report, Near-infrared spectroscopy, Optical coherence tomography, Lipid-rich plaque, Vulnerable plaque, Non-culprit lesion
Learning points
Aggressive and early lipid-lowering therapy may effectively cause lipid-rich plaque (LRP) regression; however, it may take 2 years of such treatment to decrease the lipid core.
There is a delay of several months between the decrease of low-density lipoprotein cholesterol and the regression of LRP.
Follow-up by near-infrared spectroscopy–intravascular ultrasound imaging such as 8 months is not sufficient to assess LRP, and longer-term follow-up such as 24 months will be needed in future large-scale studies.
Introduction
Patients with the acute coronary syndrome (ACS) face substantial risks of future adverse cardiac events, including recurrent ACS, even after revascularization of culprit lesions.1 This risk is partly attributable to the presence of vulnerable plaques or lipid-rich plaques (LRP) in non-culprit lesions (NCLs). In the PROSPECT study, major cardiovascular events were equally attributed to the recurrences at both culprit lesions and NCLs. Additionally, the NCLs responsible for cardiovascular events were frequently angiographically mild.2 Coronary imaging, including optical coherence tomography (OCT), intravascular ultrasound (IVUS), and near-infrared spectroscopy (NIRS), may detect vulnerable plaques, such as thin cap fibroatheromas (TCFA), larger plaque burden, and higher lipid core burden index (LCBI) that trigger lesion-related myocardial infarction.2,3 However, the treatment strategy for vulnerable plaques in angiographically mild NCLs is not well known. Conversely, lipid-lowering therapy, with statins, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, or both, reduced the risks of cardiovascular events.4 Additionally, this treatment also reduced the plaque volumes in patients with ACS in the serial IVUS study.5
Here, we report the case of a patient who underwent serial assessment of vulnerable LRP in NCLs, using NIRS–IVUS and OCT, and was treated with early administration of aggressive lipid-lowering therapy with a PCSK9 inhibitor.
Timeline
| 2017 September |
|
| 2017 December |
|
| 2018 May |
|
| 2019 September |
|
Case presentation
A 60-year-old man presented to our hospital with chest pain at rest. Physical examination revealed no abnormality (heart rate 70 b.p.m.; blood pressure 128/64 mmHg; respiratory rate 14 breaths/min; oxygen saturation 98% in ambient room air). The patient had hypertension and dyslipidaemia and, he was only on dietary intervention. Electrocardiography revealed sinus rhythm and inverted T wave in leads I, aVL, and V1–V4 without prior Q-wave. A chest X-ray showed no active lung lesion. Transthoracic echocardiography at the emergency department revealed an ejection fraction of almost 60% and no segmental wall motion abnormality. His laboratory tests showed normal levels of serum creatine phosphokinase at 115 (normal range 47–200) mg/dL with MB fraction at 1 (0–12) mg/dL, and normal levels of troponin-T at 0.012 (0.000–0.100) ng/mL. The patient was diagnosed with unstable angina pectoris, and he was transferred to our catheterization laboratory. We performed an urgent coronary angiogram (CAG), which showed 90% narrowing in the mid-portion of the left descending artery (LAD), and 50% narrowing in the distal left main trunk artery (LMT). We performed primary percutaneous coronary intervention (PCI) in the LAD for the culprit lesion using 2.5 mm × 30 mm Resolute Onyx (Medtronic Inc., Santa Rosa, CA, USA). Using intravascular NIRS–IVUS and OCT imaging, we evaluated the lesion with 50% narrowing in the distal LMT as an NCL. These images showed plaque vulnerability. The NIRS-IVUS images showed extensive amounts of lipidic materials as LRP. Maximum LCBI4mm was 422. The OCT showed LRP with a thin cap and thickness of 50 µm. Minimum lumen area (MLA) was 5.00 mm2. The patient had high levels of low-density lipoprotein cholesterol (LDL-C) on admission (LDL-C 153 [70–139] mg/dL; high-density lipoprotein cholesterol 62 [45–75] mg/dL; triglycerides, 243 [30–149] mg/dL; and haemoglobin A1C 5.5 [4.6–6.2]%). We prescribed strict observation and aggressive lipid-lowering therapy using rosuvastatin (10 mg at index PCI) and alirocumab (75 mg), a PCSK9 inhibitor, every 2 weeks to rapidly reduce the levels of LDL-C, in addition to aspirin 100 mg, clopidogrel 75 mg, olmesartan 20 m, and bisoprolol 1.25 mg.
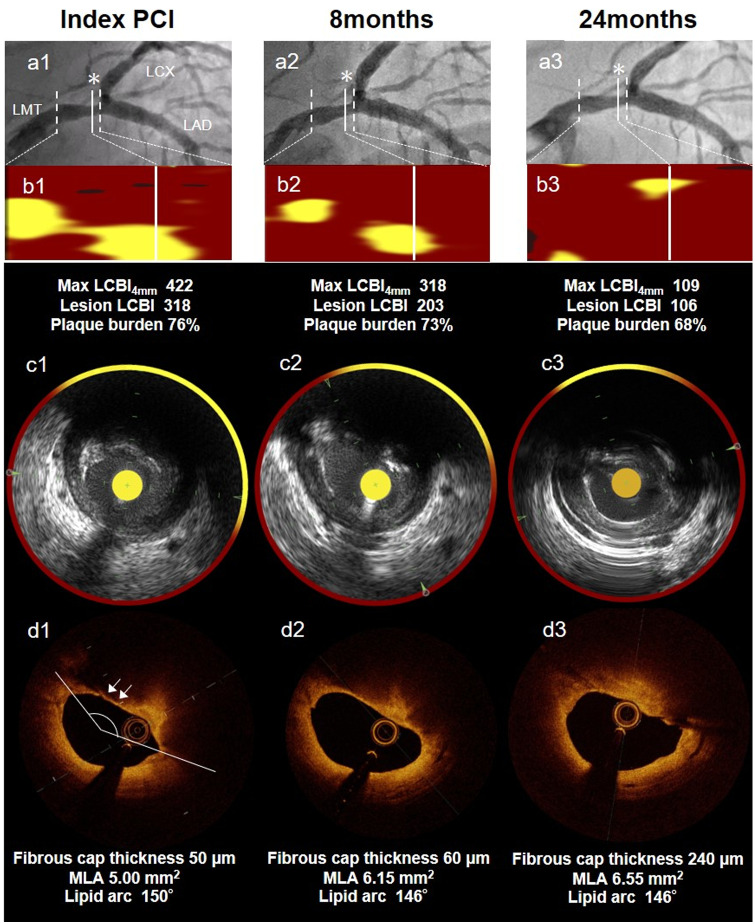
We performed serial NIRS–IVUS and OCT measurements in the distal LMT. Three months later, CAG showed a similar 50% narrowing in the distal LMT, without remarkable changes. MaxLCBI4mm remained high at 417. The OCT showed LRP with a thin cap of 60 µm. We continued aggressive lipid-lowering therapy and serial observation of this lesion for 24 months. The results are shown in Figure 1. After 24 months, we re-performed CAG, OCT, and NIRS–IVUS imaging after obtaining patient’s consent. The CAG showed a similar 50% stenosis in the distal LMT. Although the OCT showed an increase in fibrous cap thickness at 240 µm, the changes in attenuation angle and low-intensity signal with irregular borders seen on the OCT and grayscale IVUS images were unremarkable. Notably, NIRS–IVUS revealed a significant reduction in LCBI (max LCBI4mm 106). The clinical course of the patient was uneventful. The treatment with the PCSK9 inhibitor was suspended once. The stabilized NCL will be followed up by non-invasive imaging such as coronary computed tomography angiography (CCTA).
Figure 1.
Case: A 60-year-old-man presenting with unstable angina pectoris. (A1–3) Coronary angiogram reveals mild stenosis in the distal portion of left main trunk artery seen as a non-culprit lesion at index primary percutaneous coronary intervention, 8 months later, and 24 months later. (B1–3) Chemogram at index percutaneous coronary intervention, 8 months later, and 24 months later. (*)minimum lumen area site. (C1–3, D1–3) Near-infrared spectroscopy-intra vascular ultrasound and optical coherence tomography images with minimum lumen area site in non-culprit lesion segment. B1–3 show that yellow pixels were significantly and gradually reduced during the follow-up duration of 24 months. Max LCBI4mm decreased from 422 to 106. The optical coherence tomography at index percutaneous coronary intervention detected a thin-cap fibroatheroma with a thickness of 50 µm (white arrow). This thickness increased to 240 µm at 24 months. UAP, unstable angina pectoris; LMT, left main trunk artery; PCI, percutaneous coronary intervention; OCT, optical coherence tomography; NIRS-IVUS, near-infrared spectroscopy-intra vascular ultrasound; MLA, minimum lumen area.
Discussion
Patients with vulnerable plaques are at increased risk of adverse cardiovascular events. The LRP study demonstrated that the rates of non-culprit- and culprit-related major adverse cardiovascular events were similar, and 9% of patients had subsequent non-culprit events within 2 years.3 Particularly, vulnerable plaque rupture may directly lead to death or serious cardiac outcomes, such as in cases with an LM lesion. The treatment of vulnerable plaques is important; however, the strategies remain undefined.
The PROSPECT study demonstrated that a TCFA, a plaque burden of 70% or more, and an MLA of 4.0 mm2 or less in NCL was associated with adverse cardiac events.2 Notably, plaque burden of 70% or more showed the strongest association. The VIVA and AtheroRemo IVUS studies confirmed these findings.6,7 In the LRP NIRS–IVUS study, a cut-off of 400 for max LCBI4mm was an independent predictor for subsequent cardiac events.3 NIRS-IVUS imaging needed to be validated for the detection of LRP. Morphologically, rupture-prone plaques are usually TCFA, including the lipid-rich necrotic core covered by a thin fibrous cap (<65 µm).8 Cap thickness measured by OCT was associated with the prevalence of plaque rupture.9 In our case, LRP was detected by NIRS-IVUS (max LCBI4mm 400 or more) and had a large lipid arc in NCL at the index primary PCI. Additionally, our case had TCFA with a fibrous cap of 50 µm. Therefore, this NCL might have triggered subsequent serious cardiac events, such as death or serious myocardial injury. Conversely, Stone et al.10 demonstrated that PCI of angiographically mild lesions with large plaque burden was safe and was associated with favourable long-term outcomes. Percutaneous coronary intervention induced the formation of a neo-cap as a barrier between the necrotic core and lumen, which stabilized the plaque, and reduced the amount of lipid core either due to plaque translation or embolization. However, in LMT, the advantages of performing revascularization of vulnerable NCLs with mild coronary stenosis remain unclear.
Plaque vulnerability is partly attributable to elevated LDL-C levels. Previous clinical trials have shown that the risk is lower among patients who receive statin therapy to lower the LDL-C levels than among those who receive a placebo.11 In the ESTABLISH study, early high-intensity statin therapy significantly reduced the plaque volumes in patients with ACS.5 PCSK9 inhibitors significantly reduced LDL-C levels, when administered either alone or with a statin.12 They also reduced the risk of adverse cardiac events among such patients.4 Recently, the PARADIGM study reported that the use of statins was associated with the decreased progression of rupture-prone plaques and plaque stability, as evident from a serial evaluation using CCTA.13
The patient’s treatment strategy was determined according to the following reasons. First, revascularization is generally deferred in Asian patients if the MLA on IVUS MLA is ≥4.5–4.8 mm2, because Asians generally have smaller hearts than Caucasians.14 The patient’s MLA in the LM lesion at index PCI was 5.0 mm2, indicating that myocardial perfusion was maintained. Second, we would have had to use a stent for the bifurcation of the distal LMT to seal the vulnerable plaque; however, there was a concern of triggering critical adverse coronary events, such as stent thrombosis. Therefore, we decided to defer revascularization and to perform strict observation with aggressive lipid-lowering therapy. In our clinical practice, non-invasive imaging such as positron emission tomography and CCTA are able to predict atheroma progression.15,16 However, these non-invasive modalities have a low spatial resolution, making it difficult to distinguish a change in lipid core burden from fibrous cap thickness of the non-flow limiting and vulnerable rupture-prone plaque lesion in LMT. Consequently, even though this case was not in the context of a clinical trial or other research study, we selected serial evaluation by intravascular imaging, which allows for a more reliable evaluation of the risk of plaque rupture and the degree of plaque stabilization than non-invasive imaging, until the vulnerable plaque had been stabilized.
Subsequently, LDL-C levels decreased rapidly. However, compared to the rapid decrease in LDL-C, stabilizing plaque vulnerability, observed as a decreased max LCBI4mm, needed more than 1 year (Figure 2). PCSK9 inhibitors are expensive drugs, and it is important to determine the duration of their use. Our case showed that LRP regression, defined by the LCBI value, requires a long term of 2 years. Therefore, long-term follow-up may be necessary when conducting large-scale studies, investigating lipid-lowering therapy and LRP regression in lesions.
Figure 2.
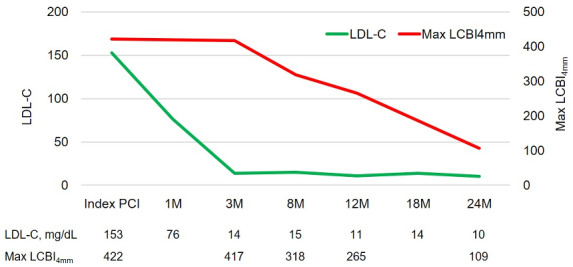
The line graph demonstrates that low density lipoprotein cholesterol levels decreased immediately, while the lipid core burden index value decreased gradually. LCBI, lipid core burden index; LDL-C, low density lipoprotein cholesterol.
Conclusions
Early aggressive lipid-lowering therapy with a PCSK9 inhibitor showed favourable outcomes for high-risk NCL LRPs in the LMT, as assessed by serial intravascular imaging, using NIRS–IVUS and OCT. As plaque stabilization of high-risk LRP may be required for at least several years, and this tends to lag behind the decrease in LDL-C levels, the serial assessment of high-risk LRP may be meaningful, in terms of treatment strategy.
Lead author biography

Norihito Takahashi received the MD degree from Juntendo University School of Medicine, Tokyo, Japan in 2013. He is medical doctor at the Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine in Japan. His research interest is mainly focused on ischemic heart disease in particular in intravascular imaging modalities.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that the written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient, in accordance with the COPE guidelines.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M.. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 2. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS. et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 3. Waksman R, Di Mario C, Torguson R, Ali ZA, Singh V, Skinner WH. et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: a prospective, cohort study. Lancet 2019;394:1629–1637. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R. et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 5. Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H. et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation 2004;110:1061–1068. [DOI] [PubMed] [Google Scholar]
- 6. Cheng JM, Garcia-Garcia HM, De Boer SPM, Kardys I, Heo JH, Akkerhuis KM. et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Eur Heart J 2014;35:639–647. [DOI] [PubMed] [Google Scholar]
- 7. Calvert PA, Obaid DR, O'Sullivan M, Shapiro LM, McNab D, Densem CG. et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease. JACC Cardiovasc Imaging 2011;4:894–901. [DOI] [PubMed] [Google Scholar]
- 8. Virmani R, Burke AP, Farb A, Kolodgie FD.. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13–C18. [DOI] [PubMed] [Google Scholar]
- 9. Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H. et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma. J Am Coll Cardiol 2014;63:2209–2216. [DOI] [PubMed] [Google Scholar]
- 10. Stone GW, Maehara A, Ali ZA, Held C, Matsumura M, Kjøller-Hansen L. et al. Percutaneous coronary intervention for vulnerable coronary atherosclerotic plaque. J Am Coll Cardiol 2020;76:2289–2301. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D. et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–1708. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M. et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 13. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A. et al. Effects of statins on coronary atherosclerotic plaques. JACC Cardiovasc Imaging 2018;11:1475–1484. [DOI] [PubMed] [Google Scholar]
- 14. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 15. Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H. et al. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J 2007;28:2243–2248. [DOI] [PubMed] [Google Scholar]
- 16. Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A. et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.