Abstract
Diets high in saturated fat (HFD) disrupt dopamine neurotransmission, whereas fasting alters tonic and phasic dopamine release to drive motivation and food consumption. However, functional compartments in the nucleus accumbens (NAc) influencing these effects are not well characterized, and sex comparisons have not been made. This study sought to determine whether consumption of a HFD, sex, or being fed versus fasted altered baseline dopamine release and reuptake throughout NAc subregions. Male and female C57BL/6 mice were fed a control diet or nutrient matched HFD for six weeks. Ex-vivo fast-scan cyclic voltammetry revealed females had significantly slower dopamine reuptake in the NAc core than males when fed ad lib control diet. Fasting enhanced dopamine release and reuptake in the NAc core but not the medioventral shell. Further, being fasted versus fed significantly increased dopamine release throughout the NAc core in control males but specifically promoted release and reuptake in only the ventrolateral core of HF-fed males, effects which were lacking in females. Finally, fasting promoted dopamine release and reuptake in the rostral NAc core of controls and more caudally in HFD groups. These data support that dopamine neurotransmission is heterogeneous in NAc subregions and suggest the ventrolateral core is responsive to energy state. Furthermore, a rostrocaudal gradient in the NAc core might control valence responses to fasting that could promote overeating after chronic HFD consumption.
Keywords: High-fat diet, fasting, dopamine, nucleus accumbens, sex comparisons, voltammetry
1. Introduction
Diet-induced obesity is exacerbated by foods with high energy density from fat and sugar (i.e. palatable foods) that disrupt energy homeostasis and dampen dopamine release and reuptake within the nucleus accumbens (NAc)1–3. The NAc integrates environmental cues with homeostatic signals, ultimately responding to midbrain neurons that release dopamine4–7. Moreover, there are distinct circuits in the NAc that communicate energy status and hedonic value with the hypothalamus to influence food intake5,8. This downstream satiety circuit resides in the medial NAc shell and core where medium spiny neurons (MSNs) send GABA afferents to the hypothalamus. GABAergic MSNs in the NAc are regulated by dopamine binding to dopamine D1-type receptors (D1Rs) and D2Rs followed by synaptic clearance of dopamine by the dopamine transporter (DAT)4–6,9,10. Physiological energy state (i.e. being fed ad lib versus fasted) uniquely impacts NAc dopamine neurotransmission that influences GABA signaling, motor output, and decision making8,11–16, which initiates food seeking and feeding in the fasted state and promotes satiety to inhibit feeding in a sated state. However, whereas consumption of palatable foods acutely stimulates dopamine release in the NAc17, long-term intake of diets high in saturated fat (HFD) may disrupt dopamine tone and capacity for phasic release and dopamine reuptake by the DAT1–3,18–20. Therefore, long-term intake of HFD alters dopamine neurotransmission which may disrupt homeostatic roles engaged by feeding and food deprivation to promote overeating.
Circulating nutrients and appetitive hormones released from the digestive system and adipose tissue convey energy status and control circuits from hypothalamic feeding centers to the ventral tegmental area (VTA) and NAc4. Energy state directly impacts dopamine neurotransmission, as appetitive hormones target dopamine cell bodies in the VTA21 and dopamine terminal fields in the NAc1,2,20. Moreover, early work in rats showed eating after 20 hours of food deprivation increased NAc dopamine turnover compared to being maintained on a fast or fed ad lib11. Access to palatable foods augmented this relationship, as 36-hour food deprivation after exposure to sucrose versus chow reduced NAc dopamine12. However, conditioned cues for sucrose and food pellets increased NAc core dopamine release in an 18-hour fasted but not sated state13. Interestingly, overnight food deprivation and short-term exposure to HFD both promoted overeating via altered plasticity of NAc shell circuits8 that innervate the lateral hypothalamus to gate feeding5. A 24–36 hour fast also reduced midbrain DAT expression and striatal dopamine reuptake14. Overall, energy restriction reduces dopamine tone and circulating insulin which may prime D1Rs, D2Rs, DATs, and hormone sensitivity in the VTA and NAc2,14–16,20 to heighten phasic dopamine neurotransmission that drives motivation and consumption upon presentation with salient stimuli. This contrasts with diminished dopaminergic responses with ad lib availability of palatable foods.
Chronic consumption of a HFD and obesity disrupt appetitive hormones that influence dopamine neurotransmission1,2,20. However, while effects of energy state and palatable food consumption have been well-explored with males, there remains a sparsity of sex comparisons. Additionally, the striatum is composed of heterogeneous striosome and matrix compartments with alternate dopamine neurotransmission and protein expression22–24, though these differences are not fully understood. However, immunofluorescence showed the ventrolateral NAc core had striosomal patches and high DAT density22. Therefore, the purpose of this study was to examine sex differences in NAc dopamine neurotransmission due to a HFD in fasting or fed energy states, specifically characterizing if changes in dopamine were anatomically homogeneous or heterogeneous throughout the NAc. Our data show that specific striatal subregions responded differently to energy state and diet based on spatial and anatomic recording locations within the NAc. Importantly, we highlight how sex differentially alters dopamine neurotransmission in response to a fed versus fasted state.
2. Materials and Methods
Animals & Diet
Six-week old male and female C57BL/6 mice (n=74 total) from seven experimental cohorts staggered over 18 months were used for this retrospective analysis of NAc dopamine neurotransmission in fed (ad lib) or fasted (12h) physiological states (Experimental Design, Figure 1A). A 12h fast was chosen to simulate a physiological state similar to a prolonged overnight fast. All mice were purchased from Jackson Laboratories (Bar Harbor, ME), maintained on a 12h light/dark cycle with free access to water and given nutrient matched DIO purified diets (Research Diets Inc.; New Brunswick, NJ) with high saturated fat (HF: 60% kcals from fat, D12492; n=36) or low saturated fat (Control: 10% kcals from fat: D12450J; n=38) for six weeks. Body weight and food intake were collected weekly. Three cohorts had constant access to their respective Control or HF diets, while four cohorts were fasted for 12h before proceeding to fast scan cyclic voltammetry (FSCV). Our final experimental groupings were as follows: Control Males and Females (n=8 ad lib; n=11 fasted, each sex), and HF Males and Females (n=7 ad lib; n=11 fasted, each sex). Importantly, fasted and ad lib fed cohorts were part of two separate studies using different pharmacological agents during FSCV. For this reason, here, we report baseline comparisons of dopamine release and maximal rate of reuptake. These specific cohorts were selected because each contained males and females with identical feeding paradigms. All experiments were approved by the UNC Greensboro Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
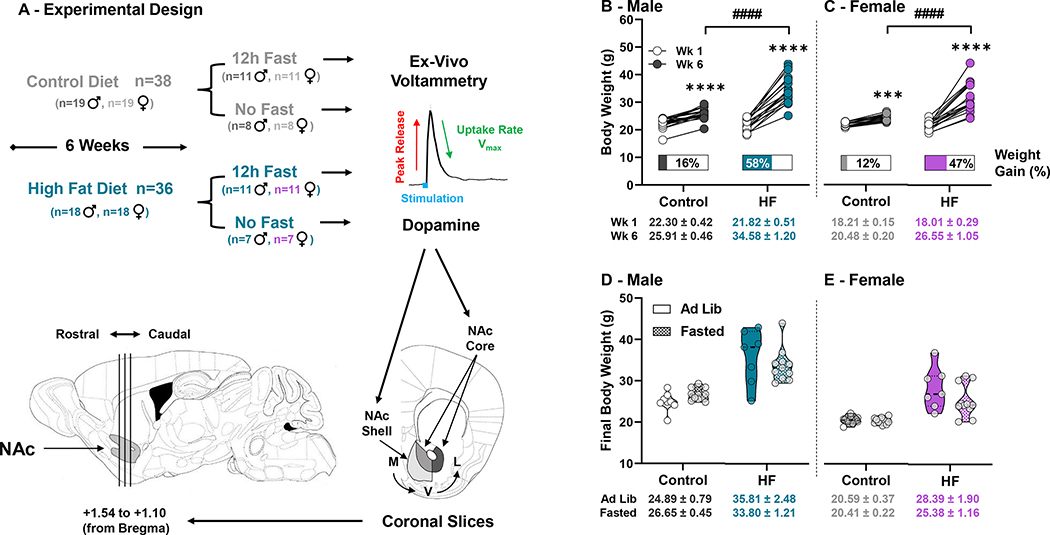
Figure 1: Experimental Design and Body Weight –
(A) After consuming the control of HF diet for 6-weeks, a subset of mice from each group underwent a 12h fast just prior to ex-vivo voltammetry. Dopamine was measured in medial (M) to ventral (V) or ventral to lateral (L) subregions in the NAc Core and Shell from brain slices collected between +1.54 and +1.10 mm (from bregma). All recordings were cataloged and grouped by rostral to caudal orientation. Body weights in grams in week-1 (Wk 1) and week-6 (Wk 6) of feeding for males (♂) (B) and females (♀) (C), with the mean percent weight gain displayed under pre- and post-feeding data points for each mouse and the average body weight ± SEM for each group under the X axis. Final body weights between ad lib fed and fasted conditions in both diet groups for males (D) and females (E) did not differ. (* denotes a significant effect of time within group, # denotes significant differences between diet groups) (***, p<0.001; ****, p<0.0001; ####, p<0.0001)
Fast Scan Cyclic Voltammetry (FSCV)
We used FSCV to measure dopamine release and reuptake within the NAc shell and core ~3h into the dark cycle, as previously described3. Mice were anesthetized using 5% isoflurane, decapitated, with the brain removed and submerged in ice cold artificial cerebral spinal fluid (aCSF) (in mM: 126 NaCl, 25 NaHCO3, 11 D-glucose, 2.5 KCl, 2.4 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 0.4 L-ascorbic acid, pH adjusted to 7.4) for slicing on a compresstome (Precisionary Instruments; Greenville, NC). Next, 300 μm brain slices containing the NAc (from +1.54 to +1.10 mm from bregma) equilibrated for 60 min at 32 °C in oxygenated (95% O2/5% CO2) aCSF flowing at 100 mL/min. Dopamine was recorded using a triangular waveform applied to a glass capillary-pulled carbon-fiber working microelectrode (70–100 μm length, 7 μm diameter). The microelectrode was maintained at a potential of −0.4 V versus an Ag/AgCl reference electrode and subsequently ramped up to +1.2 V and back to −0.4 V at a scan rate of 400 V/s every 100 ms. Dopamine release was evoked with a single electrical pulse (4 ms pulse width, 350 μA stimulation amplitude) from a bipolar stimulating electrode (Plastics One, Roanoke, VA, 8IMS3033SPCE) every 3 min until stable. Anatomical placement of each recording was catalogued and cross-referenced with Franklin and Paxinos mouse brain atlas25. Next, dopamine release was evoked using 5-pulse stimulations at 20 Hz to model “phasic” dopamine release. Dopamine signals were acquired and modelled using Demon Voltammetry Software (Wake Forest School of Medicine; Winston-Salem, NC), based on Michaelis−Menten kinetics using a Km of 160 nM for dopamine26. Dopamine current was converted to concentration by calibrating each electrode with 3 μM dopamine after each experiment.
Statistical Analysis
Graph Pad Prism v.8.30 (La Jolla, CA) was used for statistical analyses. To determine data normality between cohorts, D’Agostino & Pearson, Shapiro-Wilk, and Kolmogorov-Smirnov tests and one-way analysis of variance (ANOVA) were conducted. Once normality and cohort homogeneity of experimental variables were confirmed, experimental groups from each cohort were combined. For body weight, two-way repeated measures ANOVA was used to identify significant differences within subjects and between treatment groups over time. Two-tailed t-tests were used to identify sex differences for each dopamine neurochemistry parameter for each diet. Following appraisal of sex as a biological variable on baseline parameters, two-way ANOVAs were used to determine main effects of diet and fed/fasted state on dopamine release and reuptake. Three-way ANOVAs were used to identify the effects of diet, fed/fasted state, and recording location within the NAc core/shell (medial, ventral, or lateral of the anterior commissure) and for rostral to caudal anatomical orientation of recordings. Tukey’s or Sidak’s post-hoc analyses were used for two-way and three-way ANOVAs to identify significant differences between groups. Statistical significance was set at p≤0.05.
3. Results
A HFD increased body weight and altered dopamine release and reuptake similarly in males and females
Mice fed the HFD consumed significantly more kcals per day than mice receiving the control diet (11.4 ± 0.3 vs. 15.6 ± 0.8 kcals/day, Control and HF males respectively; p=0.007) (9.4 ± 0.2 vs. 14.5 ± 0.8 kcals/day, Control and HF females respectively; p=0.002). Similarly, males and females fed HF showed significantly higher percent weight gain than controls (males: HF 58% vs. control 16%; p<0.0001) (females: HF 47% vs. control 12%; p<0.0001) (Figure 1). In males, main effects of diet (F(1,33)=19.49; p<0.0001), time (F(1,33)=245.3; p<0.0001), and subject (F(33,33)=2.944; p=0.0013) with a diet x time interaction (F(1,33)=75.05; p<0.0001) were found, and Sidak’s multiple comparisons test revealed significantly increased body weight in control (p<0.0001) and HF-fed (p<0.0001) males (Figure 1B). Females similarly had main effects of diet (F(1,33)=20.73; p<0.0001), time (F(1,33)=195.5; p<0.0001), and subject (F(33,33)=2.510; p=0.0049) with a diet x time interaction (F(1,33)=60.23; p<0.0001), and Sidak’s multiple comparisons test indicated significantly increased body weight in control (p=0.0002) and HF-fed (p<0.0001) females (Figure 1C). No difference in final body weights were observed between ad lib and fasted male or female mice (Figure 1D,E).
We also examined if biological sex impacted dopamine release and reuptake in the NAc core and shell (Table 1). No statistically significant differences were observed between HF males and females for any dopamine parameter measured in the ad lib or fasted state. In control mice, significantly slower dopamine reuptake rate (Vmax) in the NAc core of ad lib fed females compared to males (2.12 ± 0.19 vs. 2.73 ± 0.15, respectively) was observed (p=0.017).
Table 1: Sex Effects on Dopamine Release and Reuptake in ad lib and Fasted States –
Baseline dopamine release in μM and maximal rate of reuptake (Vmax) in μM/s were directly compared between males and females within control and HFD groups and within ad lib fed or fasted states. Displayed are 1-pulse (1p) and 5-pulse (5p) electrical stimulations in the nucleus accumbens (NAc) core, and 5p stimulations in the NAc shell. Data represent group means ± SEM, with p values for sex comparisons from each measurement.
| NAc Shell | Control | High Fat | ||||
|
Male
|
Female
|
Male
|
Female
|
|||
| Ad Lib | (p) | (p) | ||||
| 5p Release (μM) | 1.17 ± 0.16 | 1.11 ± 0.25 | (0.836) | 1.15 ± 0.17 | 1.03 ± 0.29 | (0.701) |
| Vmax (μM/s) | 1.59 ± 0.19 | 1.52 ± 0.17 | (0.789) | 1.42 ± 0.18 | 1.39 ± 0.22 | (0.921) |
| Fasted | ||||||
| 5p Release (μM) | 1.37 ± 0.19 | 1.36 ± 0.19 | (0.973) | 1.23 ± 0.15 | 1.20 ± 0.24 | (0.932) |
| Vmax (μM/s) | 1.65 ± 0.12 | 1.68 ± 0.15 | (0.891) | 1.44 ± 0.15 | 1.43 ± 0.19 | (0.977) |
| NAc Core | Control | High Fat | ||||
|
Male
|
Female
|
Male
|
Female
|
|||
| Ad Lib | (p) | (p) | ||||
| 1p Release (μM) | 1.15 ± 0.09 | 1.17 ± 0.16 | (0.925) | 1.15 ± 0.19 | 1.26 ± 0.22 | (0.717) |
| 5p Release (μM) | 1.99 ± 0.21 | 2.28 ± 0.34 | (0.481) | 2.07 ± 0.30 | 2.03 ± 0.43 | (0.937) |
| Vmax (μM/s) | 2.73 ± 0.15 | 2.12 ± 0.19 | (0.017)* | 1.99 ± 0.19 | 2.36 ± 0.27 | (0.273) |
| Fasted | ||||||
| 1p Release (μM) | 2.07 ± 0.15 | 1.92 ± 0.20 | (0.551) | 2.13 ± 0.23 | 2.24 ± 0.22 | (0.754) |
| 5p Release (μM) | 3.96 ± 0.25 | 3.52 ± 0.30 | (0.273) | 4.05 ± 0.44 | 3.99 ± 0.39 | (0.925) |
| Vmax (μM/s) | 2.93 ± 0.18 | 3.19 ± 0.23 | (0.381) | 3.00 ± 0.21 | 3.41 ± 0.25 | (0.272) |
Fasting increased dopamine release and reuptake rate in the NAc core, but not the shell
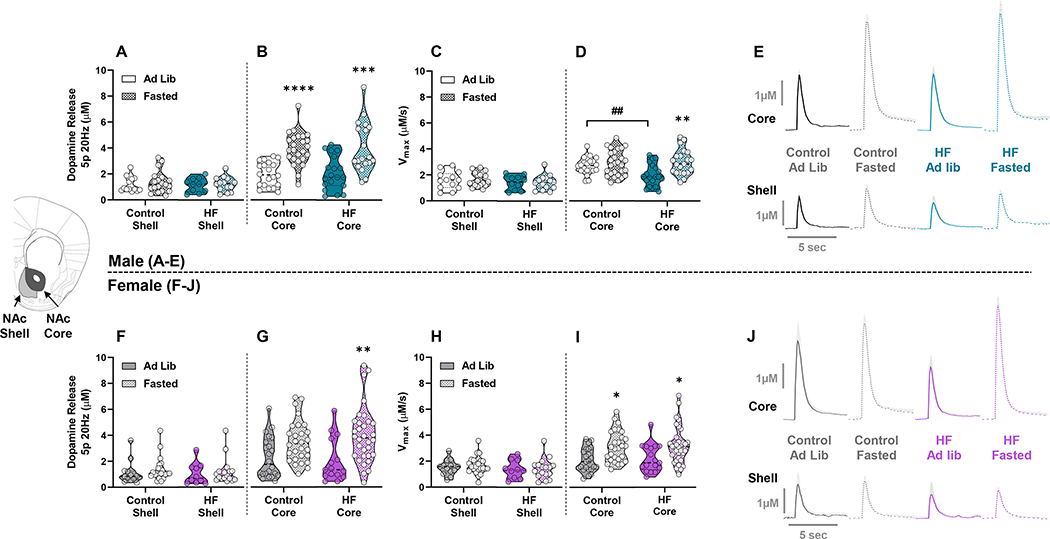
Next, we examined the effect of diet and a 12h fast on dopamine release and reuptake in the NAc shell and core. No effect of diet or fasting was observed on dopamine release or reuptake in the NAc shell of either sex (Figure 2A,C,F,H). In the NAc core of male mice, there was a main effect of fasting on dopamine release (F(1,79)=41.91; p<0.0001), with post-hoc analysis indicating a significant increase in fasted control (p<0.0001) and HF (p<0.001) groups compared to ad lib counterparts (Figure 2B). There was also a main effect of fasting on Vmax (F(1,85)=10.22; p=0.0019) in male mice, and a diet x fasting interaction (F(1,85)=4.581; p=0.0352) indicating the HF ad lib group had reduced Vmax compared to ad lib controls (p=0.004) and fasting significantly increased Vmax only in the HF group (p=0.0026) (Figure 2D). In female mice, there was a main effect of fasting on dopamine release (F(1,93)=17.28; p<0.0001), however, post-hoc analysis showed that only females from the HF group had significantly increased dopamine release (p=0.0051) when fasted (Figure 2G). Similarly, there was a main effect of fasting on Vmax (F(1,98)=17.54; p<0.0001) in female mice, with post-hoc analysis identifying significantly elevated Vmax in control (p=0.0166) and HF (p=0.0303) groups (Figure 2I). Overall, these data show that a 12h fast augments dopamine release and speeds the rate of dopamine reuptake in the NAc core but not shell. Fasting had a larger effect on dopamine release in males and a greater stimulatory effect of dopamine reuptake in females.
Figure 2: Effect of Fasting on Dopamine Release and Reuptake within the NAc Core and Shell –
Dopamine release (μM) and maximal rate of reuptake (Vmax) (μM/s) with 5-pulse stimulations at 20 Hz for males (A-D) and females (F-I). Dopamine release and reuptake were compared between ad lib fed and fasted states within control or HFD groups. Data include all recordings collected in the NAc shell and NAc core. Aggregated line traces of dopamine curves are shown for males (E) and females (J), with peak height indicating the magnitude of dopamine release and the slope of the descending curve representing Vmax. (* denotes a significant effect of fasting within group, # denotes significant differences between diet groups) (*, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; ##, p<0.01).
Subregions of the NAc core respond differently to fasting and HFD
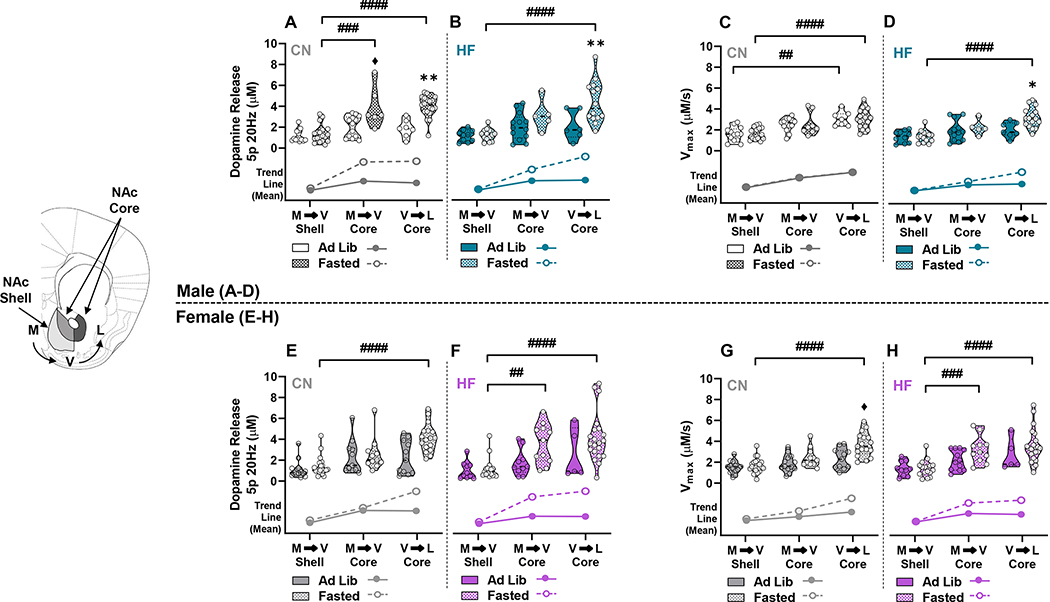
To identify whether the NAc responds in a heterogeneous or homogeneous manner to HFD during ad lib fed or fasting states, we analysed our data according to anatomical subregions. The subgroups were comprised of the medial to ventral (M/V) shell, M/V core, and ventral to lateral (V/L) core (Figure 3). A 3-way ANOVA was conducted to identify effects of diet, ad lib or fasted state, and sub-anatomical regions on dopamine release and Vmax in each sex. In males, there were main effects of region (F(2,127)=33.76; p<0.0001), fasting (F(1,127)=34.55; p<0.0001), and a region x fasting interaction (F(2,127)=9.146; p=0.0002) on dopamine release (Figure 3A,B). Tukey’s post-hoc analysis revealed significantly increased release only in the fasted state within diet groups. Fasted male controls had significantly greater dopamine release in the M/V (3.91 ± 0.79 μM) and V/L (3.98 ± 0.24 μM) core compared to M/V shell (1.37 ± 0.19 μM) (p=0.0004 and p<0.0001, respectively) (Figure 3A), but significantly increased dopamine release was only observed between the M/V shell (1.23 ± 0.15 μM) and V/L core (4.38 ± 0.54 μM) (p<0.0001) in fasted HF males (Figure 3B). Additionally, release in the V/L core was significantly increased by fasting in control (1.88 ± 0.32 μM ad lib, 3.98 ± 0.24 μM fasted; p=0.0037) and HF (2.11 ± 0.57 μM ad lib, 4.38 ± 0.54 μM fasted; p=0.005) groups (Figure 3A,B). A near significant increase in release (p=0.06) was observed in M/V core of fasted (3.91 ± 0.79 μM) compared to ad lib (2.05 ± 0.28 μM) controls. With respect to Vmax, there were main effects of region (F(2,133)=35.06; p<0.0001) and diet (F(1,133)=5.750; p=0.0179), with post-hoc analysis identifying within group differences between the M/V shell and V/L core in both ad lib (1.59 ± 0.19 μM M/V, 3.07 ± 0.27 μM V/L, p=0.0043) and fasted states (1.65 ± 0.12 μM M/V, 3.08 ± 0.20 μM V/L; p<0.0001) in the control group (Figure 3C,D). There was also a significant increase in Vmax between the M/V shell (1.4 ± 0.15 μM) and V/L core (3.22 ± 0.24 μM) of fasted HF males (p<0.0001), and fasting significantly elevated Vmax in the V/L core compared to ad lib (2.07 ± 0.27 μM ad lib, 3.22 ± 0.24 μM fasted; p=0.05) (Figure 3D). In female mice there were main effects of region (F(2,147)=24.63; p<0.0001) and fasting (F(1,147)=15.24; p=0.0001) with a region x fasting interaction (F(2,147)=3.131; p=0.0466) on dopamine release (Figure 3E,F). In contrast with male mice, post-hoc analysis in females showed no effects between ad lib or fasted groups in any NAc subregion of control or HF mice, but there were significant differences between subregions within fasted or ad lib states. Fasted female controls had significantly elevated release in the V/L core (4.14 ± 0.32 μM) compared to the M/V shell (1.36 ± 0.19 μM) (p<0.0001) (Figure 3E). Fasted HF females had significantly increased dopamine release between the M/V shell (1.20 ± 0.24 μM) and both the M/V (3.63 ± 0.57 μM) and V/L (4.18 ± 0.51 μM) core (p=0.0032 and p<0.0001, respectively) (Figure 3F). With respect to Vmax in female mice, main effects of region (F(2,153)=27.62; p<0.0001) and fasting (F(1,153)=13.63; p=0.0003) were observed (Figure 3G,H). Similar to dopamine release, post-hoc analysis showed significantly increased Vmax in the fasted state between the M/V shell and V/L core of both control (1.68 ± 0.17 μM M/V shell, 3.67 ± 0.27 μM V/L core; p<0.0001) and HF (1.43 ± 0.19 μM M/V shell, 3.50 ± 0.33 μM V/L core; p<0.0001) groups, as well as significantly faster Vmax in the V/M core (3.22 ± 0.38 μM) of the HF group compared to the M/V shell (1.43 ± 0.19 μM) (p=0.0007) (Figure 3G,H). Elevated Vmax in the fasted (3.67 ± 0.27 μM) compared to ad lib state (2.34 ± 0.29 μM) was also observed in the V/L core of control females, nearing significance (p=0.051) (Figure 3G). These findings indicate that dopamine release and reuptake increased from the M/V shell to the V/L core after fasting. Further, being fasted versus fed increased dopamine release throughout the NAc core of control males but specifically increased release and restored reuptake only in the V/L core of HF-fed males. Interestingly, the subregion effects of fasting were lacking between control and HF-fed females.
Figure 3: Differences in Dopamine Release and Reuptake Grouped by Anatomical Position within the NAc Core and Shell -.
Dopamine release and Vmax in ad lib and fasted mice from control (CN) and HF groups were grouped anatomically in the medial to ventral (M→V) shell, M→V core, and ventral to lateral (V→L) core in males (A-D) and females (E-H). Trend lines below the violin plots show the relative difference between means during ad lib and fasted states within anatomical subregions. (* denotes a significant effect of fasting within anatomical subregions for CN or HF groups, # denotes significant differences between anatomical subregions within each diet group) (♦, near significant, p=0.06; *, p<0.05; **, p<0.01; ##, p<0.01; ###, p<0.001; ####, p<0.0001).
Fasting impacts dopamine in the caudal NAc core of HF-fed mice, but the rostral NAc core of controls
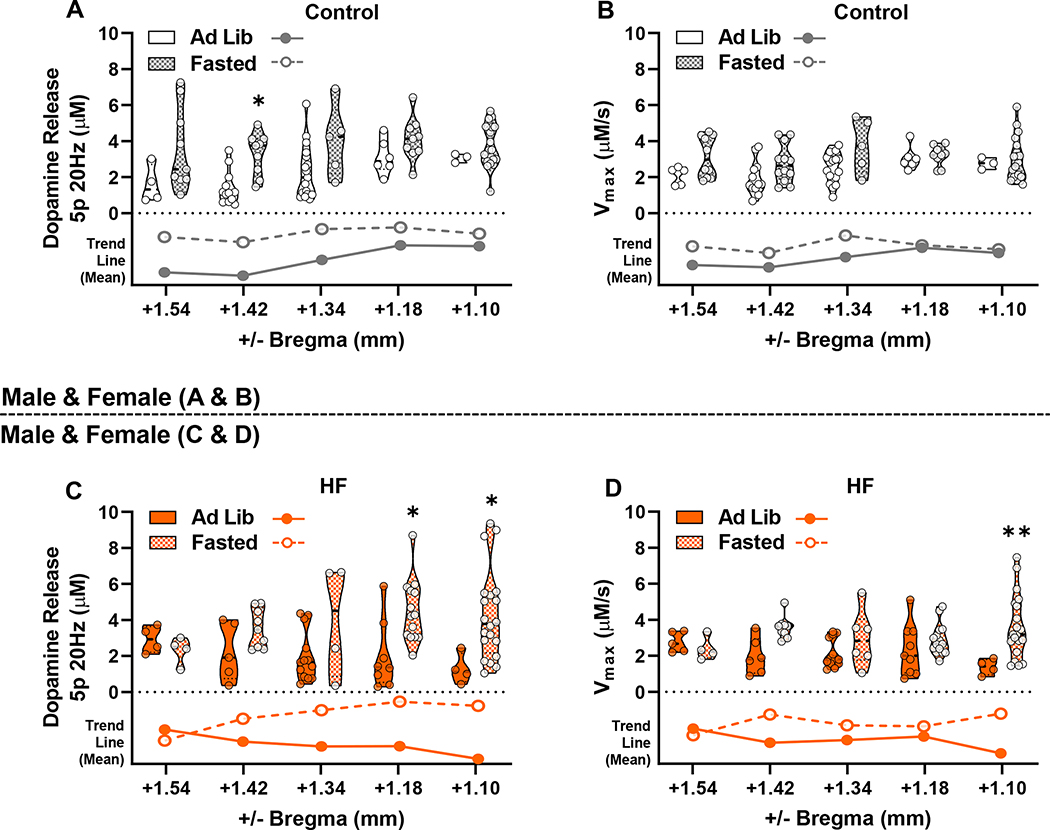
Finally, we sought to determine if diet or fasting affected dopamine neurotransmission within the NAc core from a rostral to caudal anatomical orientation spanning from +1.54 to +1.10 mm from bregma, respectively. Males and females were combined for these analyses to create a complete rostrocaudal data set for each diet group. In controls, mean dopamine release in ad lib mice trended from low to high (rostral to caudal) while the HF group trended high to low (rostral to caudal) (Figure 4A); however, no main effect of diet was observed on dopamine release or Vmax in recordings from the five coronal cross-sections in either ad lib (Figure 4A,B) or fasted states (Figure 4C,D). A main effect of fasting was found on dopamine release within the control (F(1,84)=18.97; p<0.0001) (Figure 5A) and HF (F(1,75)=11.74; p=0.0010) (Figure 5C) groups. Post-hoc analysis showed that fasting significantly increased dopamine release in rostral slices from the control group (+1.42 mm from bregma, p=0.0198) and in caudal slices in the HF group (+1.18 and +1.10 mm from bregma, p=0.0172 and p=0.0232, respectively). There were also main effects of fasting on Vmax in the control (F(1,89)=8.762; p=0.0039) and HF (F(1,81)=11.43; p=0.0011) diet groups (Figure 5B,D). Post-hoc analysis identified significantly faster Vmax in the fasted HF group compared to ad lib in the most caudal slice (+1.10 mm from bregma) (p=0.0049) (Figure 5D). Therefore, whereas diet had little effect during the same energy state, being fasted versus fed promoted NAc core dopamine release rostrally in controls and promoted release and reuptake caudally in HF-fed mice.
Figure 4: Rostrocaudal Comparison of Dopamine Release and Reuptake Between Diet Groups –
Dopamine release (A) and Vmax (B) were compared between coronal slices from ad lib control and HF mice, with recordings grouped in a rostral to caudal anatomical orientation (+1.54, +1.42, +1.34, +1.18, and +1.10 mm from bregma). Similarly, fasted dopamine release (C) and Vmax (D), grouped in a rostrocaudal fashion, were compared between control and HF groups. Voltammetry recordings from both sexes were combined. Trend lines show relative differences between means of control and HF groups within rostrocaudal positions below the violin plots.
Figure 5: Rostrocaudal Comparison of Dopamine Release and Reuptake Between Fasted/ad lib States Within Diet Groups –
Dopamine release (A) and Vmax (B) were compared between ad lib and fasted groups for mice fed the control diet with recordings grouped by anatomical slices from +1.54 (most rostral), +1.42, +1.34, +1.18, and +1.10 (most caudal) mm from bregma. Similarly, dopamine release (C) and Vmax (D) are shown for mice fed HF. Voltammetry recordings from both sexes were combined. Trend lines below violin plots show relative differences between means during ad lib and fasted states within rostrocaudal positions. (* denotes significance between ad lib and fasted states within rostrocaudal slice groupings) (*, p<0.05; **, p<0.01).
4. Discussion
Here we show NAc dopamine release and maximal rate of reuptake (Vmax) were increased in fasted male and female mice. When examining the NAc as a homogeneous structure, few sex differences in dopamine neurotransmission are apparent. However, when examined within anatomical subregions of the NAc, we report an increase in dopaminergic signaling from the M/V shell to the V/L core that is more pronounced in males. Specifically, fasted HF males had enhanced dopamine release and Vmax in the V/L core, while fasted control males showed significantly elevated dopamine release in the M/V and V/L core. Females showed a general increase in dopamine release from medial to lateral recording sites but lacked significant subregion differences, which indicates that subregion heterogeneity of dopamine neurotransmission is sex- and diet-dependent. Further, fasting-induced increases in dopamine neurotransmission occurred at caudal recording sites for HF-fed males and females but more rostrally in controls. Overall, our data show anatomical heterogeneity of dopamine neurotransmission in the NAc and highlight how shifts in dopaminergic signaling caused by a HFD may impact circuits projecting from the NAc that have discrete anatomical origins within the NAc core or shell.
The NAc has distinct functions within core and shell subregions. For example, presentation of a conditioned stimulus and cue-induced consumption of a palatable treat increased extracellular dopamine in the NAc core, while unconditioned food intake increased dopamine in the shell27. Both the core and shell have been implicated in incentive salience (i.e. wanting)28,29, but the medial shell specifically determines hedonic valuation (i.e. liking) during consumption29. However, we did not report a major effect of diet or fed/fasting state in the medial shell. Conversely, the NAc core is necessary for associative learning and cue-initiated motivation7,28 and may be more relevant for discerning physiological stimuli. Our results suggest negative energy states, particularly after chronic palatable food consumption, could promote salience for food via phasic dopamine release in the V/L core. DAT expression was recently shown to be dense in the V/L NAc, and electrically-evoked dopamine release was greater in striosomes than matrix in the NAc22. These concepts were functionally replicated by our data that showed increased fasting-induced dopamine reuptake occurred most consistently in the V/L NAc core. In support of this, psychostimulants robustly increased dopamine concentrations in the NAc core versus shell after food restriction but not ad lib feeding30, implicating increased NAc core DAT expression/function in response to negative energy states. Functionally segregated appetitive compartments likely exist throughout the NAc with unique control over the DAT, and our data support heightened dopamine release and reuptake in the V/L NAc core that could promote food attention and seeking after fasting. Seminal tracing studies showed the NAc core is largely innervated by the VTA, cortical, subthalamic, and amygdalar nuclei and sends projections to the ventral pallidum, substantia nigra, and globus pallidus31,32. Conversely, the medial NAc shell receives inputs from the VTA, lateral hypothalamus, and brain stem and sends projections to the ventral pallidum, VTA, and lateral hypothalamus31,32. Thus, the medial NAc shell is central to a homeostatic circuit between the lateral hypothalamus and medial ventral pallidum whereas the lateral core may have a greater impact on behavioral seeking. However, we report fasting-induced increases in dopamine release in the M/V core of control males, possibly suggesting a homeostatic functional gradient where the shell overlaps with the medial core. Additional differences exist between NAc striosome and matrix compartments, as there are rich patches of μ-opioid receptors, substance P, D1Rs, and pro-dynorphin while matrix co-expresses calbindin, enkephalin, D2Rs, and cholinergic markers23. There is dense calbindin and enkephalin co-staining in rostral dorsolateral NAc core matrix24 and dense striosomal patches in the rostroventral striatum33. While the rostral NAc may form adaptive responses to negative energy states, our data suggest that a HFD may desensitize this dopaminergic response. Further, GABA inactivation of the caudal core robustly reduced approach towards conflicting cues and inhibited appetitive place preference but potentiated aversive place avoidance34, which suggested the caudal core is integral to motivated approach decision making. We report that fasting promoted dopamine release and reuptake in the core rostrally in controls and caudally in the HFD group. Therefore, it is possible being deprived of food represents greater negative valence to mice fed HFD that could motivate cue-initiated food seeking by signaling in the caudal core. However, few studies have examined functional rostrocaudal gradients in the core, and further work is required to explore why control mice have greater fasting-induced dopamine release in the rostral core.
There are few sex comparisons exploring how energy state, diet, and obesity affect dopamine neurotransmission. Animal work showed females expressed greater striatal DAT density than males35, and dopamine release evoked by psychostimulants that target the DAT were sex-hormone dependent36. Therefore, increased DAT expression and shuttling in females may underly our findings that fasting tended to promote V/L NAc core dopamine reuptake in females. Conversely, males consuming ad lib HFD had slower dopamine reuptake in the NAc core when examined as a homogeneous structure, but fasting restored phasic dopamine release and reuptake specifically in the V/L core. However, fasting did not increase the reduced Vmax in the NAc core of control females compared to males, which indicates Vmax reduction in HF-fed ad lib males was diet dependent. We started feeding during late adolescence, and while sex differences in Vmax were not previously reported, male adolescent rats versus adult counterparts had reduced NAc core tonic and phasic dopamine release and hyperresponsivity of D2R autoreceptors, whereas females showed an opposite pattern37. In males, food restriction increased D1R- and D2R-induced activity, while ad lib feeding reduced D2R-induced activity, suggesting energy state altered pre- and postsynaptic D2Rs16. Further, long-term HFD intake induced striatal insulin resistance, inhibited dopamine release and reuptake and DAT shuttling, and increased sensitivity to quinpirole1,2,20,38, which occurred in males but not females38. Our reported HFD-induced reduction of dopamine clearance in males when fed ad lib could be due to diet-induced insulin resistance. Additionally, faster dopamine clearance in control males than females that occurred in the fed state could be due to heightened sensitivity of D2Rs, because both insulin receptors and D2R autoreceptors control surface expression of the DAT39. Chronic HFD consumption reduced NAc dopamine and VTA tyrosine hydroxylase expression in males and females18. Therefore, our reports that males had greater dopamine release when fasted likely occurred independent of dopamine synthesis. Conversely, fasted females experienced increased phasic release in the core only in the HF group, which was not significant within specific subregions. While HF males and females had greater dopamine release and reuptake in the V/L core compared to the shell when fasted, HF females also showed greater release and reuptake in the M/V core compared to the shell. Differences such as these likely depend on sexually dimorphic striatal development and diet-induced alterations to heterogeneous NAc compartments.
Food intake is a complex behavior dependent on energy state, sex hormones, environmental stimuli, and hedonic value of available foods1,4,7,13,20,21,36, which modulate dopamine neurotransmission in the NAc. Heterogeneous control over food intake occurs within specific NAc subregions16,27,30, and this organization responds to appetitive signals to reduce food intake when fed but increase salience of rewards and food intake when fasted. However, long-term consumption of palatable foods induces resistance to anorectic signals1,2,20 and alters tonic and phasic dopamine release1–3,19,20 that may promote fasting-induced hyperphagia but inhibit the ability to cease after satiation. Further determination of feeding behaviors that result from altered dopamine kinetics in rostral or caudal NAc subregions is warranted, but our data provide evidence that a HFD has heterogeneous effects on NAc dopamine that must be considered in future behavioral or circuit-based studies.
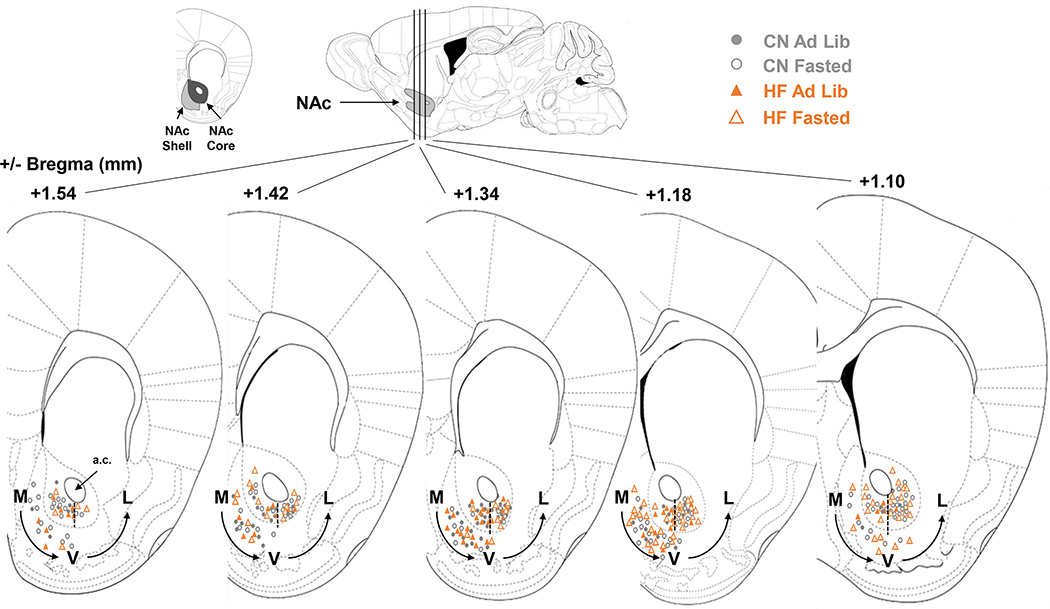
Figure 6: Electrode Placements -.
Recording sites in the NAc core and shell of male and female mice marked at the time of recording and referenced to Franklin & Paxinos mouse brain atlas. Medial (M), ventral (V), and lateral (L) designations are based from the anterior commissure (A.C.), with a dashed line as a landmark for where M→V and V→L groupings were separated.
Acknowledgments
The present study was supported by the Nutrition Department at the University of North Carolina at Greensboro.
Funding
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R15DK119897 (SCF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- 1.Fordahl SC, Jones SR. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem Neurosci 2017;8(2):290–299. doi: 10.1021/acschemneuro.6b00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel JC, Stouffer MA, Mancini M, Nicholson C, Carr KD, Rice ME. Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur J Neurosci. 2019;49(6):794–804. doi: 10.1111/ejn.13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes CN, Wallace CW, Jacobowitz BS, Fordahl SC. Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat. Nutr Neurosci. 2020:1–13. doi: 10.1080/1028415X.2019.1707421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrario CR, Labouèbe G, Liu S, et al. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci. 2016;36(45):11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor EC, Kremer Y, Lefort S, et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015;88(3):553–564. doi: 10.1016/j.neuron.2015.09.038 [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 7.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10(8):1020–1028. doi: 10.1038/nn1923 [DOI] [PubMed] [Google Scholar]
- 8.Thoeni S, Loureiro M, O’Connor E, Luscher C. Depression of Accumbal to Lateral Hypothalamic Synapses Gates Overeating. Neuron. 2020;107:1–15. doi: 10.1016/j.neuron.2020.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, He Y, Tang J, Zong X, Hu M, Chen X. Molecular imaging of striatal dopamine transporters in major depression - A meta-analysis. J Affect Disord. 2015;174:137–143. doi: 10.1016/j.jad.2014.11.045 [DOI] [PubMed] [Google Scholar]
- 10.Soares-Cunha C, Coimbra B, Domingues AV, Vasconcelos N, Sousa N, Rodrigues AJ. Nucleus Accumbens Microcircuit Underlying D2-MSN-Driven Increase in Motivation. Eneuro. 2018;5(2):1–33. doi: 10.1523/ENEURO.0386-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffner TG, Hartman JA, Seiden LS. Feeding Increases Dopamine Metabolism in the Rat Brain. Science (80- ). 1980;208(4448):1168–1170. [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94(3):309–315. doi: 10.1016/j.physbeh.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aitken TJ, Greenfield VY, Wassum KM. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J Neurochem. 2016;136(5):1026–1036. doi: 10.1111/jnc.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68(1):11–20. doi: 10.1159/000054345 [DOI] [PubMed] [Google Scholar]
- 15.Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith MEA. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J Neurochem. 2017;140(5):728–740. doi: 10.1111/jnc.13930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119(4):1157–1167. doi: 10.1016/S0306-4522(03)00227-6 [DOI] [PubMed] [Google Scholar]
- 17.Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A high-fat meal, or intraperitoneal administration of a fat emulsion, increases extracellular dopamine in the nucleus accumbens. Brain Sci. 2012;2(2):242–253. doi: 10.3390/brainsci2020242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlin J, Hill-Smith TE, Lucki I, Reyes TM. Reversal of dopamine system dysfunction in response to high-fat diet. Obesity. 2013;21(12):2513–2521. doi: 10.1002/oby.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged High Fat Diet Reduces Dopamine Reuptake without Altering DAT Gene Expression. PLoS One. 2013;8(3):e58251. doi: 10.1371/journal.pone.0058251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6(8543):1–12. doi: 10.1038/ncomms9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mebel DM, Wong JCY, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. 2012;36(3):2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salinas AG, Davis MI, Lovinger DM, Mateo Y. Dopamine dynamics and cocaine sensitivity differ between striosome and matrix compartments of the striatum. Neuropharmacology. 2016;108:275–283. doi: 10.1016/j.neuropharm.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brimblecombe KR, Cragg SJ. The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem Neurosci. 2017;8(2):235–242. doi: 10.1021/acschemneuro.6b00333 [DOI] [PubMed] [Google Scholar]
- 24.Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: Immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium‐binding protein. J Comp Neurol. 1989;289(2):189–201. doi: 10.1002/cne.902890202 [DOI] [PubMed] [Google Scholar]
- 25.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. Amsterdam: Elsevier; 2008. [Google Scholar]
- 26.Yorgason JT, España RA, Jones SR. Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–164. doi: 10.1016/j.jneumeth.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89(3):637–641. doi: 10.1016/S0306-4522(98)00583-1 [DOI] [PubMed] [Google Scholar]
- 28.Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: Entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” J Neurosci. 2014;34(12):4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: Differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18(8):2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x [DOI] [PubMed] [Google Scholar]
- 31.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro‐gold. J Comp Neurol. 1993;338(2):255–278. doi: 10.1002/cne.903380209 [DOI] [PubMed] [Google Scholar]
- 32.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-Y [DOI] [PubMed] [Google Scholar]
- 33.Tajima K, Fukuda T. Region-specific diversity of striosomes in the mouse striatum revealed by the differential immunoreactivities for mu-opioid receptor, substance p, and enkephalin. Neuroscience. 2013;241:215–228. doi: 10.1016/j.neuroscience.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 34.Hamel L, Thangarasa T, Samadi O, Ito R. Caudal nucleus accumbens core is critical in the regulation of cue-elicited approach-avoidance decisions. eNeuro. 2017;4(1):1–14. doi: 10.1523/ENEURO.0330-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58(1):16–22. doi: 10.1159/000126507 [DOI] [PubMed] [Google Scholar]
- 36.Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204(2):361–372. doi: 10.1016/0006-8993(81)90595-3 [DOI] [PubMed] [Google Scholar]
- 37.Pitts EG, Stowe TA, Christensen B, Ferris MJ. Comparing dopamine release, uptake, and D2 autoreceptor function across the ventromedial to dorsolateral striatum in adolescent and adult male and female rats. Neuropharmacology. 2020;175(108163):1–9. doi: 10.1016/j.neuropharm.2020.108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos J, Hernandez-Casner C, Cruz B, Serafine KM. Sex differences in high fat diet-induced impairments to striatal Akt signaling and enhanced sensitivity to the behavioral effects of dopamine D2/D3 receptor agonist quinpirole. Physiol Behav. 2019;203:25–32. doi: 10.1016/j.physbeh.2017.11.014 [DOI] [PubMed] [Google Scholar]
- 39.Nash AI. Crosstalk between insulin and dopamine signaling: A basis for the metabolic effects of antipsychotic drugs. J Chem Neuroanat. 2017;83–84:59–68. doi: 10.1016/j.jchemneu.2016.07.010 [DOI] [PubMed] [Google Scholar]