Abstract
Recent updates in the diagnosis and management of chronic inflammatory conditions can be brought together to better understand autoimmune diseases (ADs). With organ-specific or organ-limited and systemic ADs, physicians often are faced with a dilemma when making a diagnosis and may feel a kind of embarrassment when a more distinct nosological entity cannot be found. ADs often overlap with other diseases and good diagnostic procedures for ADs only become evidence-based when refined histopathologic, immunopathologic, and general laboratory analyses are available. Immunofluorescence analyses, Western blotting, CUT & RUN technology allow localization of the site of autoantibody-reactivity on the relevant DNA sequence. The Polymerase chain reaction technology and CRISPR-Cas9, the new gene editor using pools of synthetic non-coding RNAs in screening experiments, are expected to lead to advances in the diagnosis of ADs. The current use of mRNA as a vaccine against COVID-19 has increased confidence in the use of mRNA or long non-coding RNAs in the treatment strategy for ADs. The integration of new knowledge about innate immunity, the complement system, vaccinology, and senescence into the care of patients with ADs expands the therapeutic arsenal of disease-modifying drugs and allows for the repurposing of anti-cytokine monoclonal/biosimilar antibodies, originally designed for chronic inflammatory diseases, for ADs. This review article brings together some of the most relevant ideas; a case report included in this review highlights the difficulty of distinguishing between ADs, chronic inflammation, and/or granular disease.
Keywords: Innate immunity, Cytokine, Complement, Monoclonal antibodies, Biosimilars I
Graphical abstract

Abbreviations/glossary
- ACE2
Angiotensine converting enzyme
- ADs
Autoimmune diseases
- AIHA
Autoimmune hemolytic anemia
- ANA
Antinuclear antibody
- ANCA
Autoantibody neutrophilic cytoplasmic antigen
- C
Complement system C1 – C9, control proteins C1INH, H, I
- CAR-T
Chimeric antigen receptor of T cells
- CAD
Cold agglutinin disease
- Cas 9
CRISPR-associated protein
- CRISPR
Clustered regularly interspaced short palindromic repeats
- CUT & RUN
Cleavage under targets & release using nuclease
- DAMP
Damage‐associated molecular pattern molecules
- FAMH
Foederatio Analyticorum Medicorum Helveticorum (www.famh.ch)
- FAP
Fibroblast activation protein, a cell membrane dipeptidyl peptidase
- FKBP
FK506- binding protein
- FLC
Free light chains
- IC
Immune complex
- ICAP
International consensus on antinuclear antibody pattern
- ICD
International classification of diseases
- IMID
Immune-mediated inflammatory diseases
- IL
Interleukin
- IRF
IFN regulatory factor
- IFN
Interferon
- Lectin
Carbohydrate-binding proteins, activators of C
- MAPK
Mitogen-activated protein kinase
- MEK
MAPK/extracellular signal-regulated kinase
- NHL
Non-Hodgkin Lymphoma
- PAMP
Pathogen‐associated molecular pattern molecules
- PBC
Primary biliary cirrhosis
- PD
Programmed cell death protein
- PNH
paroxysmal nocturnal hemoglobinuria
- SAD
Systemic autoimmune diseases
- TLR
Toll-like receptor
- VHH domains
Camelid VHH domain, from heavy-chain-only antibodies
1. Introduction
In the International Statistical classification of disease-related Health Problems (ICD), autoimmune diseases (ADs) form a substantial group on the recently published list. The group contains various ADs with single nosological entities [1].
The derailment of the immune system affects hardly only a single organ. However, when it does, the targeted organ suffers from disparate histological and functional damage. In severe situations, and in patients with a genetic presupposition,an AD can spread from the initially targeted organ and becomes non-organ specific (NOS). This evolution into a systemic AD often happens on the grounds of chronic inflammation or immunosenescence. Immunosenescence leads to an incident peak of ADs in the group of people over 80 years ([2,3]. Nevertheless, most ADs locate to a beforehand peak of onset, except few diseases such as giant cell arteritis or primary biliary cholangitis [4]. With more profound insight into innate immunity, cells carrying markers such as Toll-like receptors (TLR) or with endosome enlightening, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) become operative. Derived from microorganisms, PAMPs drive inflammation whereby DAMPs on host cells, including tumor-, dead- or dying cells, and specific released cell (i.e. RNA) respond to various (auto-) signals such as hypoxia [5].
Both types of receptors bind to the corresponding TLR thereby, opening the path to ADs if RNA derived from infectious agents or damaged cells come into the process [6]. Overlapping effector functions are an embarassment entangling those who search to ascribe distinct functions to distinct cell types. Identification of subsets of lymphocytes is pivotal to developing targeted therapies in immune-mediated inflammatory diseases (IMIDs) and recently, murine studies involving FAPa+THY1+ marker positive fibroblasts distinct from destructive FAPa+THY1- clone allowed identification of a «clean » cell subset with IMID-damage property [7]. The immune system as a primary target for ADs is rarely concerned, when so, it shows up as an immunohematological disorder [8]. When suffering from a B-cell mediated ADs, patients are at increased risk to develop indolent non-Hodgkin lymphoma [9] and immune-related diseases, e.g. rheumatoid arthritis, systemic lupus erythematosus (SLE) and Crohn's disease, lymphoma, including indolent NHL [10].
The autoantibody-identification technology in the search for organ specificity - for decennaries the state-of-the-art diagnostic tool of ADs diagnostics - is now being completed by a series of new approaches: (i) fluorescent Western Blotting, (ii) CUT & RUN technology: a recombinant protein A-microccocal nuclease (pA-MN) fusion assay [11] in which unfixed permeabilized cells are incubated with antibody, thus allowing to study the very site of autoantibody-reactivity at the concerned DNA sequence [12] revealed by the in vitro IC level indeed. The polymerase chain reaction (PCR) technology and the CRISPR-Cas9, the newfangled gene editor [13]using pools of synthetic cRNAs in screening experiments also lines up for advancements in diagnosis of ADs. This review will also update refined assays of the complement system [14,15] and therapeutic options derived therefrom.
Once ADs is diagnosed, therapy becomes an issue: early approaches made usage of low-dose alkylating agents and/or steroids – whereby, astonishing enough at the time of this writing, many clinicians still start with combinations of these to switch to modern regimens involving immunosuppressants/rapalogues (sirolimus) and monoclonal antibodies/biosimilars later on. The advances in med lab analytics now allow us to draw patient charts with many parameters from which we will look more closely at the cytokine and complement system profiles [16,17]. The idea of using immunotherapy in cancer treatment can be ascribed to Thomas and Burnet who proposed the theory of cancer immunosurveillance in 1957. They suggested that lymphocytes acted as sentinels to identify and eliminate somatic cells transformed by spontaneous mutations. Approaching cancer with immunotherapy (CAR-T, PD-1) [18] also stimulated therapy of ADs and vice versa. When Borel/Stähelin described cyclosporine, a new chapter of measured immunosuppression making organ transplantation possible opened up. Indeed, the experiences accumulated here led to a deeper insight into treating ADs, now refined with rapalog [19]. A neologism termed immune checkpoint wants to delineate cancer treatment possibly with concomitant ADs [20,21].
Our contribution here is an update among these lines bringing the ADs avatar more closely to current insights and, through presenting modern med lab diagnostic tools, opening unprecedented therapeutic options.
2. Role of innate immunity in AD
The innate arm of the immune system including granulocytes, monocytes, NK cells, cells, group 2 lymphoid cells (ILC2s) [22], dendritic cells and macrophages has received limited attention in ADs management. NK cells have recently been identified as accessible for therapeutic measures [23]. Profiling of immune performance now begins to include innate immune cells [24]. Maybe this delay comes from the longstanding view that this arm of the immune system is a prerogative of the adaptive immunity and completely shielded from any adaptation during the course of an autoimmune or infectious attack. The contribution of this arm was held inert to vaccinations and autoimmune derailments. As yet, trainded innate immunity and contribution to tolerance may occur: genetic studies provide for hints [25] to ADs determining disease severity and outcome exploited using genomics and high-resolution single-cell analyses [[26], [27], [28]]. The current way of living in the XXIth century, trained immunity of innate kind may become part of age-related chronic inflammation; it is conceivable, that DAMPs ligands could overshoot to drive ADs: epigenetics might thus be responsible for excessive reactions which, if they fall on the ground of genetic predisposition, will induce ADs. The cytokines, e.g. IFN- stimulate immune cells and depend on IFN-resposive genes which, on their turn, throw a bridge between innate and acquired immunity [29].
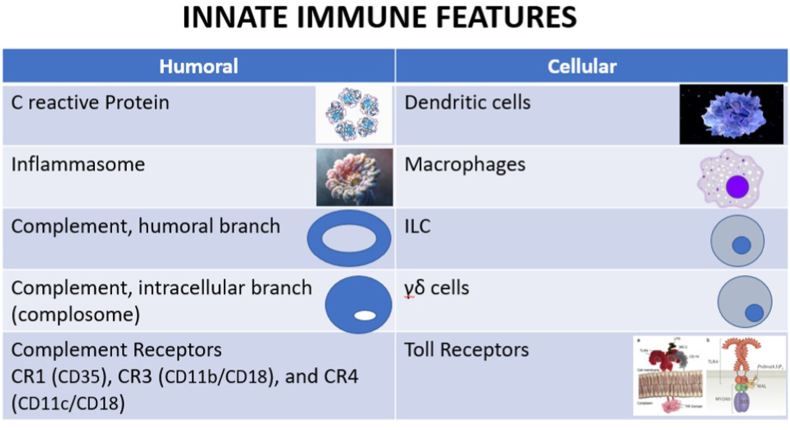
On the humoral side, the complement system and archaic proteins like protein C participate in ADs; inlammasomes as a multiprotein collection are at the origin of interleukin (IL) production, namely (IL)-1 and IL-18, both necessary instructors of adaptive immunity [30]. Moreover, complement activation through the doors of classical, alternative and lectin pathways yields biologically active molecules, e.g. C3a, C5a, SC5b-9. Autoantibodies targeting complement proteins can play an equilibrium between activation and inhibition of C. The disease resulting from the formation of C autoantibodies depends on which component becomes targeted [31]. The hyper immunoglobulin D (HIDS) syndrome constitutively features mevalonate kinase deficiency [32] pointing to HIDS as an autoinflammatory disorder; HIDS patients suffer from recurrent fever, lymphadenopathy, arthralgia, hepatosplenomegaly and skin rash (Raynaud syndrome) [33] (Fig. 1).
Fig. 1.
Innate Immunity and Autoimmune Diseases. The figure shows major innate immune players which (could) participate in ADs. The protein-oligomer inflammasomes are receptors/sensors that regulate the activation of caspase-1 (not shown) and induce inflammation in response to infectious microbes and molecules derived from host proteins - autoantigens. They have been implicated in chronic inflammatory disorders and other systemic ADs. The role of complosome – intracellular C components – is currently delved in more details.
3. Role of acquired immunity in autoimmune disease
Mainstay of immune systems’ attack against self. It is the derailment of humoral immunity which has been diagnosed at the origin of ADs diagnosis. Single-domain heavy-chain-only antibodies (sdAbs) have now opened up the study of antigen-binding properties of conventional antibodies (Abs), including bispecific Abs where the specificity of the very antigen-binding site differs within the same molecule. Camelids encompass a unique repertoire of functional Abs which are naturally devoid of light chains. Discovered in the early 90s, the use of their pared-down version, i.e. sdAbs or VHH ligands, because of their small size (12–15 kD) enables them to reach epitopes unavailable for conventional IgGs. In addition, these ligands bind their targets with strong affinity and selectively. We can now access remote and/or hidden antigenic epitopes using camelid heavy chain, single domain Abs [34]. For drug development, or for diagnostic precision, sdAbs are used to bring the autoantibody detection to an unprecedented precision and informative value. The creation of unique VHH domains will continue to encompass autoreactive sdAbs, providing for opening new opportunities to treat and understand ADs [35].
3.1. Routine laboratory in autoimmune diagnostics
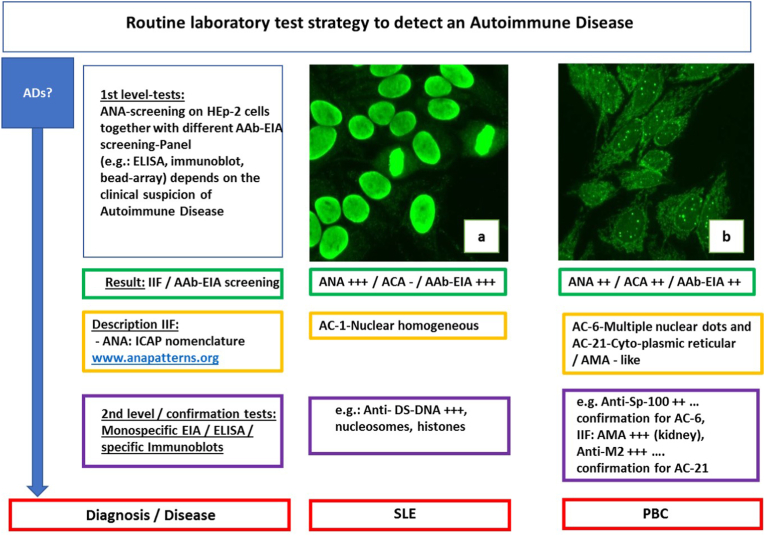
In addition to the medical history, a basic physical examination and a special examination with equipment adapted to the clinical picture of the disease, such as extended imaging (e.g. CT), the ADs diagnostics in the medical laboratory benefits from numerous particular assays of importance for the diagnosis in clinical immunology. Here, a selection of different indirect immunofluorescence tests, EIA (enzyme immunoassay), ELISA and immunoblots (line or dot blots), are available. One of the screening classics in rheumatology is the determination of anti-nuclear antibodies (ANA) on Hep2 cells by indirect immunofluorescence. The microscopic evaluation of these ANA patterns has been based on the ICAP nomenclature (patterns AC-0 to AC-29, ICAP (International Consensus on Antinuclear Antibody Pattern (ICAP, www.anapatterns.org) for several years.
It should be noted in the nomenclature that not only nuclear immunofluorescence (e.g., ICAP AC1, homogeneous pattern) but also immunofluorescence of the cytoplasm of Hep2 cells (e.g., ICAP AC21 mitochondria-like) is assessed (Fig. 2). This has a great advantage for patient care. In fact, both partial assessments (nucleus and cytoplasm) supplement each other and give clues in which diseases a specific AC pattern may occur. As in the case of ICAP AC21 (mitochondria-like), there is an indication of a possible primary biliary cholangitis [36].
Fig. 2.
Routine laboratory test strategy to detect an autoimmune disease, (examples). AD (Autoimmune disease), IIF (indirect immunofluorescence), AAb-EIA (Autoantibody-enzyme-immuno-assay), ANA (Anti-nuclear antibodies), ACA (anti-cytoplasmic antibodies), AMA (Anti-mitochondrial antibody. panels a, b: ANA on HEp-2 cells (Euroimmun, Lübeck, Germany), images from laboratory Dr. Risch, SLE (Systemic lupus erythematosus), PBC (Primary biliary cholangitis).
Further confirmatory analysis for anti-mitochondrial antibodies using indirect immunofluorescence and a specific EIA for M2 antibodies can confirm the suspected diagnosis of autoimmune hepatitis [37].
Very challenging to the certified laboratory specialist on validation duty are these ANA-ICAP AC patterns when they display combined mixed patterns, frequently at different titer levels with reactive cytoplasmic set up.
3.2. Extension to non-immunological lab assays
The clinical immunology department is often associated with classical routine laboratory analysis i.e. hematology, clinical chemistry, infectious serology/microbiology [38] and depends on additional aspects, namely genetic clarification HLA typing none the least. Clinics and specialized centers for ADs additionally offer histological sampling and classical clarifications such as capillary microscopy and functional tests (e.g. Schirmer test).
The combined mosaic of all laboratory tests performed substantiates the clinician's tentative diagnosis and thus expands the basis for an adapted and optimized autoimmune therapy.
The transcription factor IFN regulatory factor 5 (IRF5) is a central mediator of innate and adaptive immunity throwing a bridge among them. Although the mechanism (-s) by which IRF5 contributes to disease pathogenesis remain unclear, much of the data point to its role in regulating the expression of proinflammatory cytokines, including IFN-α, IL-6, IL-7, TNF-α, and IL-12, as well as pathogenic autoantibody production [39]. Free light chains kappa (FLC - K) and lambda (FLC-K) assessed individually and/or computed by ratio may help to distinguish systemic (SAD) from single-organ shot of ADs [40] FK506 binding proteins (FKBP) are part of the highly conserved immunophilin family [41]. Its members have essential roles in regulating signaling pathways involved in inflammation, adaptive immune responses, cancer, and developmental biology. The original member of this family, FKBP12, a physiologic regulator protein of the cell cycle, is a well-known binding partner for the immunosuppressive drugs tacrolimus (FK506) and sirolimus (RapamycinR).
3.3. Immune complexes
The designation “immune complexes” is used to describe antibody-binding to antigen in different contexts. Often the clinician understands soluble ICs, circulating in blood or suspended in various bodily fluids, such as synovial fluids, exudates like pleural fluid or abscesses. In sharp contrast, tissue-bound ICs occur when soluble ICs become stuck in a tissue or when ICs are formed locally. Many lab assays are based on quantitating an antigen detected by a specific antibody used as a laboratory reagent. Finally, cell surface markers are revealed by labelled antibodies.
SLE, the systemic ADs par excellence, remains characterized by elevated levels of circulating antinuclear autoantibodies (ANAs) and severe immune dysregulation [42]. Immune dysregulation may be conferred by genetic susceptibility and/or environmental triggers. Circulating SLE-ICs often containing as antigen (viral?) DNA [43] is a hallmark of SLE [44]. During decennaries clinicians have neglected ICs, albeit their dosage in the med lab is accessible and commercial tests are abound. Immune Complex detection include Panels utilizing, Raji cell immune complex and C1q binding assays.
ICs are important in monitoring disease activity and treatment schedules. Decorated with C components, ICs hook up to a series of receptors on immune cells and initiate cell activation; B cell uptake impacts germinal center reactions [45] and prolong/intensify exposure to antigen. The B cell follicle features a dense network of cytoplasmic extensions of follicular dendritic cells keeping antigens, often in immune complexed form in contact.
Circulating immune complexes are usually searched for using panels containing both Raji cell immune complex and C1q binding assays. In the past 50 years, only few new drugs have been approved for the treatment of SLE, like Golimumab, a human IgG1κ monoclonal antibody specific for human tumor necrosis factor alpha (TNFα) - global immunosuppression to control disease activity remains the standard of care. Molecular mimicry is implied in ADs which presumably arise upon infection with virus (SARS-CoV-2) [46] or with bacteria, e.g. Campylobacter jejuni [47]. One new target could be IFN regulatory factor 5 (IRF5), a member of the IRF family of transcription factors. Subsequent studies revealed essential important roles for IRF5 in innate and adaptive immunity, macrophage polarization, cell growth regulation, and apoptosis [39]. IRF5 is now identified as an autoimmune susceptibility gene: IRF5 polymorphisms associate with autoimmune and inflammatory conditions indeed, including inflammatory bowel disease, primary biliary cirrhosis, rheumatoid arthritis, SLE, and systemic sclerosis [39].
3.4. Risk assessment in cohorts
To gain deeper insight, a series of biological phenomena are now forwarded to analyze the risk of disease, in other words to predict health and longevity more precisely than using chronological age as a measure. These clocks include DNA methylation patterns [[48], [49], [50]] inflammaging [51], gene expression constellations [52], frailty indices [53], serum protein levels [54] and IgG glycosylation [55]. Defense against immunopathological damage, these features might as well be seen as natures’ resistance against development of ADs [56].
Aging is associated with a state of chronic inflammation (“inflammaging”) followed by an increased likelihood of developing ADs. Epigenetic changes in non-dividing and dividing cells, including immune cells, due to environmental factors might contribute to the inflammation and autoimmunity both at work in diseases of aging. In senescent cells, innate immune signaling pathways will also become alerted: cyclic GMP-AMP synthase is a DNA sensor which will translocate to the endoplasmic reticulum where it acts as a stimulator of interferon genes [57]. There are few doubts, that such pathways interfere in ADs striking the elderly.
4. Microbial impingement to decide between health and disease
The remission of a Hodgkin lymphoma upon COVID 19 in a 61-year old man has rocked the news [58]. In a triangle between lymphoproliferative diseases, ADs and viral disease, the interrelationship that governs the human immunopathology becomes evident. In this patient, the SARS-CoV-2 virus likely triggered an anti-tumor immune response.
At the Bern University, CRISPR screening was used to identify clinically approved immunosuppressants that could treat coronavirus infections. The team around Volker Thiel found that several autophagy-related genes were common host defense factors required to replicate both endemic and emerging SARS-CoV-2 virus. The team concluded that inhibition of the immunophilins with the clinically-approved drugs Cyclosporine A and Alisporivir could result in a dose-dependent reduction of SARS-CoV-2 virus replication in primary human nasal epithelial cells [59]. CRISPR is a therapeutic opening to treat ADs by impinging on T cells: one may dissect the regulatory circuitry governing activation from differentiation of T helper type 2 (Th2) cells. Thus, one can distinguish cell activation versus differentiation in a quantitative balance [60]; new approaches using CRISPR in fact do not edit genes directly, but the scissors are used to prevent genes from being expressed: gene-silencing therapy [61].
The approach might become used to silence genomics behind the ‘cytokine storm' at work in heavy COVID-19; those interested in ADs recognized a resemblance of COVID-19 to ADs [62,63]) [64].
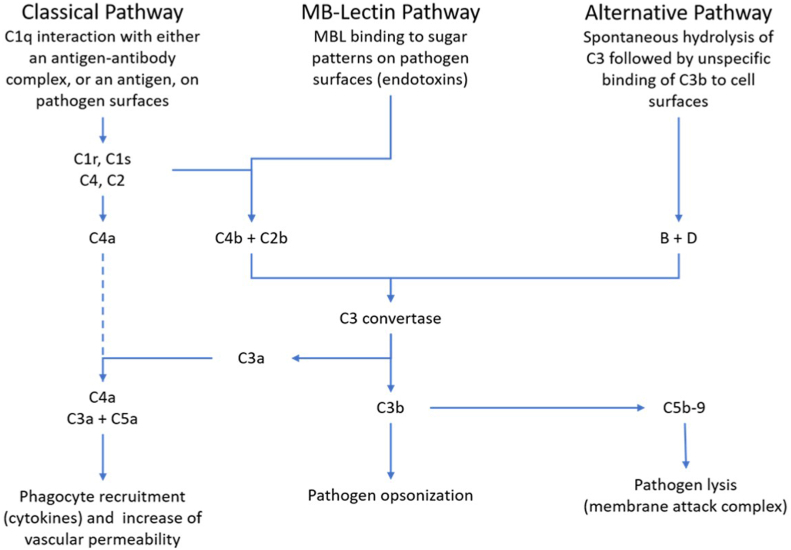
5. The role of the complement system in ADs
The C system (Fig. 3) is an essential member of defense – it opsonizes and lyses targets-, maintaining the hosts integrity, and on the other side, its activity may turn towards the host; we have recently reviewed this activity elsewhere [38].
Fig. 3.
The complement system as part of a network mazing immunopathological events in ADs. It is evident, that C activation producing anaphylatoxins, C3a, C5a and the SC5b-9 complex will activate cytokine-producing cells hence becoming responsible, at least in part, for cytokine storm.
The breakthrough to include C in clinical care came with Eculizumab, a mAb drug that made plasma exchange superfluous in the treatment of most patients suffering from hemolytic uremic syndrome (HUS) ([65]. This mAb against C5 was initially found helpful in treating paroxysmal nocturnal hemoglobinuria (PNH), under the continued line of sight for novel approaches such as by targeting C3 by a pegylated peptide [66]. The interest of clinicians into the so far as esoteric divulged C system rose. The story currently goes on with avocapan, a C5a receptor inhibitor to treat patients with ANCA-positive vasculitis [67]and with sutimlimab, a humanized mAb targeting C1s to treat cold agglutinin disease (CAD) [68]; in this study, activity of the classical C pathway was inhibited, with CH50 going down as well. Sutimlimab, did not touch the level of C1q, known to occasionally interfering with ADs. Some authors propose 4-cycle bendamustine plus rituximab to achieve durable remission in AIHA [69].
Of course, rare C component deficiencies are treated with purified components (e.g. C1INH in angioneurotic edema [65]). For ophthalmologists, the C system is an important tool in the evaluation of degenerative rear sight disease [8]. The deep knowledge of the C has given ideas/production of new therapeutic approaches [70] not only to tame C but to treat ADs. The importance of the C system in SARS-CoV-2 infection has recently been underlined while discovering the ch3 cluster rs 11385942 variant on the 3p21.31 locus giving rise to increased SC5b-9 levels in humoral fluids [71]. A special issue of Frontiers in Immunology focused on C and its role in COVID-19 and ADs is under way at the time of this writing [72].
6. Vaccines and the role of adjuvants
6.1. Disease prevention shifts to therapy
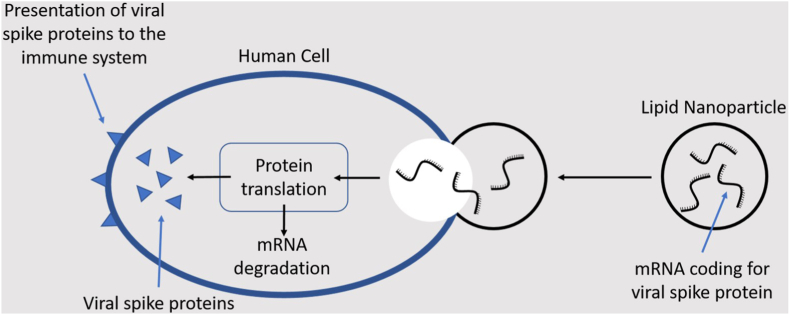
Liposomal formulations of mRNA vaccines (mRNA-LPX) (Fig. 4) optimized for the systemic delivery of RNA-encoded antigen information are now in the limelight to prevent COVID -19 disease.
Fig. 4.
This sketch displays the fast and efficient function of a potent vaccine based on liposome embedded mRNA. Such an approach shows promise in ADs as anticipated using a mouse model of multiple sclerosis [73]. Dendritic cells (see also Fig. 1) are antigen-presenting cells recognized by T helper cells. The figure inspires application of lnc_mRNA to treat ADs.
Notwithstanding the paucity of animal models, mRNA vaccines will transform into broader field of application including ADs [74]. It remains doubtful that either mRNA vaccines induce ADs. The daily accumulation of knowledge about the pathophysiology of COVID-19 spills over to the field of ADs which features cytokine imbalance alike [75]. Next-generation sequencing has provided a picture of the transcriptome to identify long noncoding RNAs (lncRNAs) the role of which rises hope to treat ADs and cancer [76]. Liposomal formulations of mRNA vaccines containing CH3-pseudouridine can induce antigen-specific tolerance and in mice; such mRNA prevented experimental autoimmune encephalomyelitis [73] or abrogated disease progression: personalization of vaccine at the doorstep.
6.2. Untoward effects
When Israeli immunologists assumed adjuvants of vaccines to induce ADs many clinics pursued the diagnosis (Shoenfeld-Syndrome) [77]. Nevertheless, none of these vaccines became withheld from use.
Therapeutic antibodies are still growing in their application for the treatment of cancer, autoimmune and inflammatory diseases. In our view, the very beginning of treating ADs with polyclonal polyspecific IgG dates back to the early 1980ies [78].
When Emil von Behring realized that a compound in our species remained active after infectious attack to protect us against subsequent invasions by the same predator he barely realized what this discovery would bear as consequences. The discovery of a balanced anti-idiotypic network, to a large part, came as an answer to the question: how do IVIG work? [79]. IVIG not only substitute immunodeficient patients, but modulate regulatory networks failing in ADs [78]. The role antibodies play in nature exceeds the meaning of the prefix « anti –«. These proteins are endowed with regulatory capacities. The immunoglobulin therapy is but one consequence. But we are now at the dawn of checkpoint inhibitors (PD 1 inhibitors), capable of downregulating PD 1, a negative regulator of T cell activity.
By vaccines and intrinsic means, i.e. using anti-PD-1 antibodies one can now boost T cell activity against tumor cells. Some clones of anti-PD1 antibodies stimulate, others can inhibit PD 1 function i n vitro [80]. With a humanized mouse model, it was discovered possible to see Ig gene segments undergoing de novo V(D)J recombinations to fret antibody clones – somatic hypermutation as background drivers not excluded. We assume that such checkpoint antibody therapy will not remain restrained to oncology but that it will also become a means to help patients suffering from ADs. Diagnostic tools are on their way to update possibilitiy to identifiy the binding capacity of autoantibodies. Approaches based on radiolabelled binding partners, e.g. cells opened the door of molecular imaging [81]. Electron tomograpy follows on the steps but these techniques are so sensitive, that they raise questions about what health and disease is. To develop new drugs a recently exploited method named « DeepBAR» (Bennett acceptance ratio method) seems to us promising [82] since it allows to feel out the receptor/ligand region of antibodies with their epitopes; indeed, DeepBAR uses calculations of free energy using generative models between host-guest, i.e. receptor-ligand interactions.
The procedure might be valuable for computing standard binding free energy used in drug design, such as in biosimilars [83].
With the mAb technology stepping from animal studies into human therapy [84] and the ensuing pooling of more than one specificities [85] the approach to treat ADs with antibodies has gained momentum and raised the phantasy of researchers to cover the epitopes with non-antibodies Small proteins called affimers with comparable affinities to target molecules lie antibodies, show advantages over current antibody-based detection systems. None the least, because they do not elicit anti-idiotypic antibodies accused to inhibit their expression [86]. Affimer reagents, which selectively bind to the therapeutic antibody idiotype can target the calibration curves met by National regulatory agencies (US) criteria. The dynamic ranges compared favorably with commercially available reagents. Therefore, affimer proteins therefore represent promising anti-idiotypic reagents that are simple to select and manufacture. They also offer the sensitivity, specificity and consistency required for pharmacokinetic assays [87].
Anti-idiotypic affimer proteins that bind therapeutic antibodies are introduced as alternative affinity reagents to traditional antibodies. DeepBAR combines chemistry and machine learning to speed up calculations to design drug molecules’ binding affinity to proteins (see below). A nonbridging ELISA assay for pharmacokinetic analysis of these biotherapeutics in serum is developed. ACE2, and TMPRSS2 protease promote SARS-CoV-2 infectivity, while inflammatory cytokines IL-6, or G-CSF worsen COVID-19 severity. More recently, MEK inhibitors (MEKi) VS-6766, trametinib and selumetinib were shown to reduce ACE2 expression in human cells hence dampening devastating activities of the cytokine network [23]. MEK inhibitors can suppress inflammatory cytokines while helping NK cells to keep abreast of disease progression [23].
The cyclophosphamide- (followed by azathioprine-) or rituximab-as well as steroid-regimens have become standard approaches during the last decades for most ADs. This changed with the availability of mAbs and biosimilars. It is from the side of therapeutics and with those diseases responding to particular - experimental at the outset – regimens that a deeper insight into the immunopathology becomes possible.
7. Case report
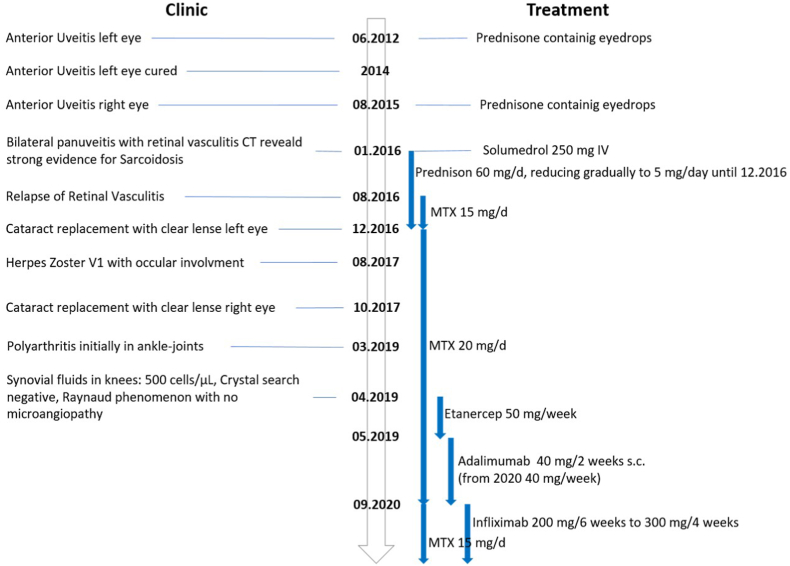
We here illustrate the problem to delineate ADs with a case report of systemic sarcoidosis stage II diagnosed in a middle-aged women [88,89] a diagnosis at the brink of inflammatory and ADs, immune complexes present or not [90,91], the disease involves lungs, eyes and joints. It started in June 2012 with an anterior uveitis treated with prednisone-containing eyedrops. 2014, the uveitis was cured to relapse August 2015 in the opposite (right): eye-drop therapy. In 2015 the patient lost 10 kg and developed a dry cough and thoracic pain. An albeit transitory one-month right cheek parotitis completed the clinical picture along 2015. January 2016: blurred vision left eye, bilateral pan uveitis with retinal vasculitis (Fig. 5, Fig. 6).
Fig. 5.
Flow Chart Case Report of a case diagnosed for sarcoidosis type II; evolution over 7 years. Please note the instauration of an actual ADs therapeutic regimen for a non-ADs.
Fig. 6.
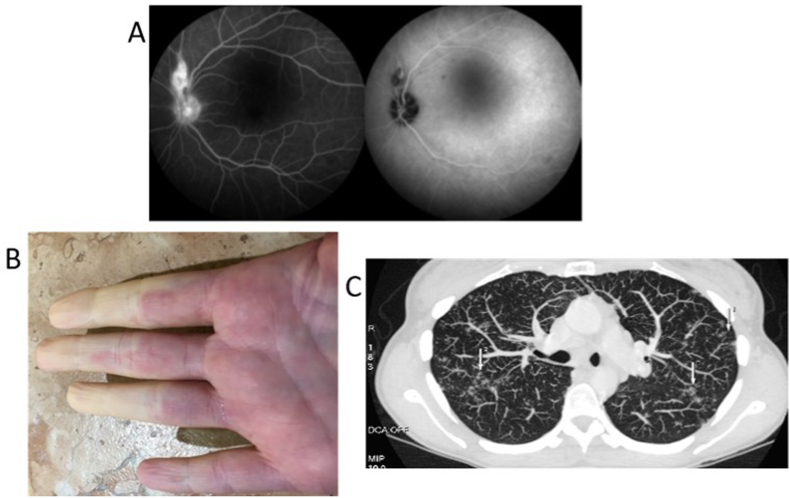
Iconography of the Case Report from Fig. 5. In (A) retinal granuloma above the optic papilla. (Heidelberg retinal tomography). In (B) Raynaud phenomenon (right hand). In (C) thoracic CT cut displaying lymph node and lung participation of the sarcoidosis.
CT exam, including lungs, reveals substantial evidence of sarcoidosis: steroid therapy is instigated, 60 mg/d reducing down to 5 mg/d gradually. August 2016: relapse of retinal vasculitis led to treatment with methotrexate (MTX) 15 mg/d, later 20 mg/d mandatory – excellent response-- > stop prednisone. March 2019: polyarthritis initially treated in vain with etanercept, replaced by adalimumab (Humira ™), which lost efficiency on its own to be replaced by infliximab in September 2020 (Remicade™) on the grounds of continuing MTX; stop adalimumab. Please note, that infliximab, a chimeric mAb primarily developed to treat ADs, began improving patient health. The recurrent anterior uveitis (left eye) responded to topical glucocorticoid drops to relapse in the right eye now involving the retina. Four years after disease onset an insufficient response to 5 mg/d prednisone, MTX first 20 mg/d later 15 mg/d was added to the regimen with good response early on. A steroid-induced cataract was replaced by a clear lens on the left eye in December 2016; on the right eye in October 2017. In March 2019 a polyarteritis took hold initially in the ankle-joints, knees – synovial fluid 500 cells/ml – cristal search negative. Quite soon the hands, the right elbow and both knees became painful; left nervus tibialis and right nervus medianus were affected by neuropathy; there was also a transitory herpes zoster episode with ocular involvement. The condition is occasionally overshadowed by a fatigue syndrome. The evolution was supplemented by a Raynaud phenomenon, but capillary microscopy showed no microangiopathy. Under 300 mg infliximab iv (Remicade™ x4/weekly), the subjective feeling of this sarcoidosis comes and goes at the time of this writing. The respective medications involving Etanercept, Humira (adalimumab) and Infliximab are listed in Fig. 5.
This case serves as an example of repurposing drugs to treat diseases for which they were initially not envisaged. In this case infliximab originally developed to treat rheumatoid arthritis, was prescribed to treat sarcoidosis. Immune-mediated diseases appear to clump in families. In populations of patients with sarcoidosis, relative risk estimates of Sjögren's syndrome, SLE, autoimmune hepatitis, ankylosing spondylitis, multiple sclerosis (MS), coeliac disease, autoimmune thyroid disease, and ulcerative colitis, may vary as much as between 2.1 and 11.6. As yet in relatives of patients with sarcoidosis, relative risk estimates varied between 1.3 and 5.8 for sarcoidosis, Multiple Sclerosis, coeliac disease, type 1 diabetes, Graves' disease, rheumatoid arthritis, Crohn's disease, and ulcerative colitis [88].
Wrapping up, it has to be stated that the term ADs denominates a large group of nosological entities sharing the presence of autoreactive cells. Current diagnostic tools and response to (experimental and/or repurposed) treatments provide for deeper insights we hope to have outlined here.
Informed consent was obtained for publication of the case report, including iconography. The privacy rights of the patient are observed.
None of the authors has competing interests to declare.
Individual contributions: TL and UN conceptualized the review, BS and AH cured linguistics, AH & BS and MN cared for figure design, LR and MR provided resources and validated the review editing. BS is our linguistic expert (English mother tongue).
Submission declaration and verification
This Review is not under consideration for publication elsewhere and is approved by all authors and by the responsible authorities where the work was carried out, and if accepted, it will not be published elsewhere in the same form in English or in any other language, including electronically without the written consent of the copyright holder.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The constructive discussions on senescence exchanged with Pasquale Di Cesare are kindly acknowledged. The project was funded by an inhouse grant. No external funding body was involved.
Contributor Information
Thomas Lung, Email: thomas.lung@risch.ch.
Benjamin Sakem, Email: benjamin.sakem@risch.ch.
Andreas Hemmerle, Email: andreas.hemmerle@risch.ch.
Michèle Nydegger, Email: michele.nydegger@triemli.zurich.ch.
Martin Risch, Email: martin.risch@ksgr.ch.
Lorenz Risch, Email: lorenz.risch@risch.ch.
Urs Nydegger, Email: urs.nydegger@risch.ch.
References
- 1.Scherlinger M., Mertz P.…Arnaud L. doi: arj: worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun. Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102531. [DOI] [PubMed] [Google Scholar]
- 2.Lleo A., Leung P.S.C., Hirschfield G.M., Gershwin E.M. The pathogenesis of primary biliary cholangitis: a comprehensive review. Semin. Liver Dis. 2020;40(1):34–48. doi: 10.1055/s-0039-1697617. [DOI] [PubMed] [Google Scholar]
- 3.Evert J., Lawler E., Bogan H., Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58(3):232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 4.Watad A., Bragazzi N.L., Shoenfeld Y. Autoimmunity in the elderly: insights from basic science and clinics - a mini-review. Gerontology. 2017;63(6):515–523. doi: 10.1159/000478012. [DOI] [PubMed] [Google Scholar]
- 5.Tang D., Kang R.…Lotze M.T. PAMPs and DAMPs: signals that spur autophagy and immunity. Immunol. Rev. 2012;249(1) doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoh T., Akira S. Toll-like receptor signaling and its inducible proteins. Microbiol. Spectr. 2016;4(6) doi: 10.1128/microbiolspec.MCHD-0040-2016. [DOI] [PubMed] [Google Scholar]
- 7.Croft A.P., Campos J., Buckley C.D. Distinct fibroblast subsets drive inflammation and damage arthritis. Nature. 2019;570(7760):246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swinkels M., Zhang J.H., Clark S.J. C-reactive protein and pentraxin-3 binding of factor H-like protein 1 differs from complement factor H: implications for retinal inflammation. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-017-18395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S.S., Vajdic C.M., Smedby K.E. Associations of non-Hodgkin Lymphoma (NHL) risk with autoimmune conditions according to putative NHL loci. Am. J. Epidemiol. 2015;181(6):406–421. doi: 10.1093/aje/kwu290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallah M., Liu X.… K. H: autoimmune diseases associated wih non-Hodkin lymphome: a nationwide cohort study. Ann. Oncol. 2014;25(10):2025–2030. doi: 10.1093/annonc/mdu365. [DOI] [PubMed] [Google Scholar]
- 11.Hainer S.J. TG. F: high-resolution chromatin profiling using CUT&RUN. Curr Protoc Mol Biol. 2019;126(1) doi: 10.1002/cpmb.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meers M.P., Bryson T.D., Henikoff J.G., Henikoff S. Improved CUT&RUN chromatin profiling tools. Elife. 2019 doi: 10.7554/eLife.46314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simeonov D.R. A. M: CRISPR-based tools in immunity. Annu. Rev. Immunol. 2019;37:571–597. doi: 10.1146/annurev-immunol-042718-041522. [DOI] [PubMed] [Google Scholar]
- 14.West E.E., Kolev M., Kemper C. Complement and the regulation of T cell responses. Annu. Rev. Immunol. 2018;36:309–338. doi: 10.1146/annurev-immunol-042617-053245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo M.W., Kemper C., Woodruff T.M. COVID-19: complement, coagulation, and collateral damage. J. Immunol. 2020;205(6):1488–1495. doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conigliaro P., Triggianese P.…Chimenti M.S. Complement, infection, and autoimmunity. Curr. Opin. Rheumatol. 2019;31(5):532–541. doi: 10.1097/BOR.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 17.Ling M., Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin. Lab. Med. 2019;39(4) doi: 10.1016/j.cll.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai S., Togashi Y.… H. N: the PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020;21(11) doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber K.H., Apelo Si Arriola.… DW. L, . NCJd: a novel rapamycin analog is highly selective for mTORC1 in vivo. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S., Gerber D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin. Canc. Biol. 2020;64:93–101. doi: 10.1016/j.semcancer.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fässler M., Diem S.…Flatz L. Antibodies as biomarker candidates for response and survival to checkpoint inhibitors in melanoma patients. J Immunother Cancer. 2019;7(1) doi: 10.1186/s40425-019-0523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkel P., Xia W., Jefferies W.A. Beyond unconventional: what do we really know about group 2 innate lymphoid cells? J. Immunol. 2021;206(7):1409–1417. doi: 10.4049/jimmunol.2000812. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L., Huntington K.…El-Deiry W.S. Natural Killer cell activation, reduced ACE2, TMPRSS2, cytokines G-CSF, M-CSF and SARS-CoV-2-S pseudovirus infectivity by MEK inhibitor treatment of human cells. bioRxiv. 2020, aug 3 [Google Scholar]
- 24.Rendeiro A.F., Casano J.…Inghirami G. Profiling of immune dysfunction in COVID-19 patients allows early prediction of disease progression. Life Science Alliance. 2020 doi: 10.26508/lsa.202000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajishengalis G., Xiaofei L.…Chavakis T. Trained innate immunity and its implications for mucosal immunityand inflammation. Adv. Exp. Med. Biol. 2019;1197:11–26. doi: 10.1007/978-3-030-28524-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua B.A., Van Der Werf I., Jamieson C., Signer R.A. Post-transcriptional regulation of homeostatic, stressed, and malignant stem cells. Cell Stem Cell. 2020;26(2) doi: 10.1016/j.stem.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulte-Schrepping J., Reusch N.… (DeCOI). SLDC-OI: severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182(6):1419–1440. doi: 10.1016/j.cell.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Made C.I., Hoischen A.…Ikeno Y. Primary immunodeficiencies in cytosolic pattern-recognition receptor pathways: toward host-directed treatment strategies. Immunol. Rev. 2020;297(1):247–272. doi: 10.1111/imr.12898. [DOI] [PubMed] [Google Scholar]
- 29.Combes A.J., Courau T.…Krummel M.F. Global absence and targeting of protective immune states in severe COVID-19. Nature. 2021;591:124–130. doi: 10.1038/s41586-021-03234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin J.I., Lee K.H.… Kronbichler A: inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J Autoimmmun. 2019;103 doi: 10.1016/j.jaut.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Defendi F., Thielens N.M.…Dumestre-Pérard C. The immunopathology of complement proteins and innate immunity in autoimmune disease. Clin. Rev. Allergy Immunol. 2020;58:229–251. doi: 10.1007/s12016-019-08774-5. [DOI] [PubMed] [Google Scholar]
- 32.Bekkering S., Arts R.J.W.…Netea M.G. Metabolic induction of trained immunity through the mevalonate pathway. Cell. 2018;(1–2):135–146. doi: 10.1016/j.cell.2017.11.025. J.W. [DOI] [PubMed] [Google Scholar]
- 33.van der Meer J.W., Simon A. doi: ro: the challenge of autoinflammatory syndromes: with an emphasis on hyper-IgD syndrome. Rheumatology. 2016:ii23–ii29. doi: 10.1093/rheumatology/kew351. [DOI] [PubMed] [Google Scholar]
- 34.Wrapp D., De Vlieger D., McLellan J.S. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(5) doi: 10.1016/j.cell.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoey R.J., Eom H., Horn J.R. Structure and development of single domain antibodies as modules for therapeutics and diagnostics. Exp. Biol. Med. 2019;244(17):1568–1576. doi: 10.1177/1535370219881129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EASL European Association for the Study of the Liver. Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. JHepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Lung T., Sakem B.…Nydegger U. The complement system in liver diseases: evidence-based approach and therapeutic options. J Transl Autoimmun. 2019 doi: 10.1016/j.jtauto.2019.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nydegger U., Lung T. T. B: inflammation thread runs across medical laboratory specialities. Mediat. Inflamm. 2016;2016:4121837. doi: 10.1155/2016/4121837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S., De S., Barners B. Inhibition of IRF5 hyperactivation protects from lupus onset and severity. J. Clin. Invest. 2020;130(12):6700–6717. doi: 10.1172/JCI120288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettacchioly E, Legaffric C, .., Renaudineau Y: An elevated polyclonal free light chain level reflects a strong interferon signature in patients with systemic autoimmune diseases. J Transl Autoimmun 2021, 4(100090). [DOI] [PMC free article] [PubMed]
- 41.Annett S., Moore G., Robson T. FK506 binding proteins and inflammation related signalling pathways; basic biology, current status and future prospects for pharmacological intervention. Pharmacol. Ther. 2020 doi: 10.1016/j.pharmthera.2020.107623. [DOI] [PubMed] [Google Scholar]
- 42.Stojan G., Petri M. Anti-C1q in systemic lupus erythematosus. Lupus. 2016;25(8):873–877. doi: 10.1177/0961203316645205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdanos D.P., Sakkas L.I. From microbiome to infectome in autoimmunity. Curr. Opin. Rheumatol. 2017;29(4):369–373. doi: 10.1097/BOR.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 44.Nydegger U.E., Lambert P.H., Gerber H., Miescher P.A. Circulating immune complexes in the serum in systemic lupus erythematosus and in carriers of hepatitis B antigen. Quantitation by binding to radiolabeled C1q. J. Clin. Invest. 1974;54:297–309. doi: 10.1172/JCI107765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arulraj T., Binder S., Meyer-Hermann M. Rate of immune complex cyclingg in follicular dendritic cells determines the extent of protecting antigen integrity and availability to germinal center B cels. J. Immunol. 2021;206(7):1436–1442. doi: 10.4049/jimmunol.2001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Rumeileh S., Abdelhak A.…Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2020:1–30. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soltani E.Z., Rahmani F., Rezaei N. Autoimmunity and cytokines in Guillain-Barre syndrome revisited: review of pathomechanisms with an eye on therapeutic options. Eur. Cytokine Netw. 2019;30(1):1–14. doi: 10.1684/ecn.2019.0424. [DOI] [PubMed] [Google Scholar]
- 48.Levine M.E., Lu A.T., Horvath S. doi: a-: an epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10(4):573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu A.T., Quach A., Wilson J.G., Reiner A.P., Aviv A., Raj K., Hou L., Baccarelli A.A., Li Y., Stewart J.D. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 2019;11(2):303–327. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Wilson R.… H. B: DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat. Commun. 2017;17(8) doi: 10.1038/ncomms14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alpert A., Yishai Pickman Y., Shen-Orr S.S. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019;25(3):487–495. doi: 10.1038/s41591-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mamoshina P., Zhavoronkov A. edn. vol. 10. Moskalev A; 2019. Deep integrated biomarkers of aging. (Biomarkers of Human Aging, Healthy Aging and Longevity). [Google Scholar]
- 53.Rockwood K., Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Lehallier B., Gate D., Schaum N., Nanasi T., Lee S.E., Yousef H., Moran Losada P., Berdnik D., Keller A., Verghese J. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019;25(12):1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vučković F., Krištić J.… G. L: association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheum. 2015;67(11):2978–2989. doi: 10.1002/art.39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohli J., Veenstra I., Demaria M. The struggle of a good friend getting old:cellular senescence in viral responses and therapy. EMBO Rep. 2021 doi: 10.15252/embr.202052243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Challenor S., Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br. J. Haematol. 2021;(3):192. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 59.Vk Ph, Annika Kratzel A., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henriksson J., Chen X., Teichmann S.A. Genome-wide CRISPR screens in T helper cells reveal pervasive crosstalk between activation and differentiation. Cell. 2019;176(4):882–896. doi: 10.1016/j.cell.2018.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreno AM, Aleman F, .., Mali P: Long-lasting analgesia via targeted in situ repression of Nav 1.7 in mice. Sci. Transl. Med. 2021, 13(584). [DOI] [PMC free article] [PubMed]
- 62.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lung T., Kazatchkine M., Risch L., Risch M., Nydegger U. A consideration of convalescent plasma and plasmaderivatives in the care of severely- ill patients with COVID 19. Transfus. Apher. Sci. 2020;59(5) doi: 10.1016/j.transci.2020.102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMillan P., Dexhiemer T.…Uhal B.D. COVID-19 - a theory of autoimmunity against ACE-2 explained. Front. Immunol. 2021 doi: 10.3389/fimmu.2021.582166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patriquin C., Kuo K.H.M. Eculizumab and beyond: the past, present, and future of complement therapeutics. Transfus. Med. Rev. 2019;33(4):254–265. doi: 10.1016/j.tmrv.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Hillmen P., Szer J., de la Tour P. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2021;384:1028–1037. doi: 10.1056/NEJMoa2029073. [DOI] [PubMed] [Google Scholar]
- 67.Jayne D.R.W., Merkel P.A.…Bekker M.D. Avocapan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 2021;384(feb 18):599–609. doi: 10.1056/NEJMoa2023386. [DOI] [PubMed] [Google Scholar]
- 68.Röth A., Barcellini W.…Berentsen S. Sutimlimab in cold agglutinin disease. N. Engl. J. Med. 2021;384:1323–1334. doi: 10.1056/NEJMoa2027760. [DOI] [PubMed] [Google Scholar]
- 69.Berentsen S. How I treat cold agglutinin disease. Blood. 2021;137(10):1295–1303. doi: 10.1182/blood.2019003809. [DOI] [PubMed] [Google Scholar]
- 70.Zelek W., Xie L., Morgan B.P., Harris C.L. Compendium of curreent complement therapeutics. Ml Immunol. 2019;114:341–352. doi: 10.1016/j.molimm.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 71.Valenti L., Griffini S., Cugno M. Chromosome 3 cluster rs11385942 variant links complement activation with severe COVID-19. J. Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102595. al e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prohaszka Z.…Frazer-Abel A. Complement analysis in the era of targeted therapeutics. Mol. Immunol. 2018;102:84–88. doi: 10.1016/j.molimm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Flemming A. mRNA vaccine shows promise in autoimmunity. YNat Rev Immunol. 2021, Jan 12 doi: 10.1038/s41577-021-00504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krienke C., Kolb L.… U. S: a noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021, Jan 8;(371):145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 75.Caso F., Costa L.…Scarpa R. Could SARS-CV-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robinson E.K., Covarrubias S., Carpenter S. The how and why of lncRNA function:an innate immune perspective. Biochim Biophys Acta Gene Regul Mech. 2020;(4):1863. doi: 10.1016/j.bbagrm.2019.194419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen Tervaert J.W. Autoinflammatory/autoimmunity syndrome induced by adjuvants (ASIA; Shoenfeld's syndrome): a new flame. Autoimmun. Rev. 2018;17(12):1259–1264. doi: 10.1016/j.autrev.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Imbach P., Barandun S.…Wagner H.P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpra in childhood. Lancet. 1981;i:1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 79.Sultan Y., Kazatchkine M.D., Maisonneuve P., Nydegger U.E. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet. 1984;8406:765–768. doi: 10.1016/s0140-6736(84)90701-3. [DOI] [PubMed] [Google Scholar]
- 80.Tian M., Cheng H.L.…Alt F.W. An in vivo method for diversifying the functions of therapeutic antibodies. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(10) doi: 10.1073/pnas.2025596118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Signore A., Erba P.A. Molecular imaging of inflammation/infection: the future of disease management. Curr. Pharmaceut. Des. 2018;24(7):741–742. doi: 10.2174/138161282407180514095417. [DOI] [PubMed] [Google Scholar]
- 82.Ding X., Zhang B. DeepBAR: a fast and exact method for binding free energy computation. J. Phys. Chem. Lett. 2021;12:2509–2515. doi: 10.1021/acs.jpclett.1c00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vulto A.G., Jaquez O.A. The process defines the product: what really matters in biosimilar design and production? Rheumatology. 2017;56:iv14–iv29. doi: 10.1093/rheumatology/kex278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajewsky K. The advent and rise of monoclonal antibodies. Nature. 2019:47–49. doi: 10.1038/d41586-019-02840-w. [DOI] [PubMed] [Google Scholar]
- 85.Weinreich D.M., Sivapalasingam S., Norton T. Investigators T: REGN-COV2, a neutralizing antibody cocktail. N. Engl. J. Med. 2020;17 dec doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohler H., Anastas Pashov A., Kieber-Emmons T. The promise of anti-idiotype revisited. Front. Immunol. 2019 doi: 10.3389/fimmu.2019.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adamson H., Nicholl A.T.… DC. T: affimers as anti-idiotypic affinity reagents for pharmacokinetic analysis of biotherapeutics. Biotechniques. 2019:261–269. doi: 10.2144/btn-2019-0089. [DOI] [PubMed] [Google Scholar]
- 88.Terwiel M., Grutters J.C., van Moorsel C.H.M. Clustering of immune-mediated diseases in sarcoidosis. Curr. Opin. Pulm. Med. 2019;25(5):539–553. doi: 10.1097/MCP.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 89.Llanos O., Hamzeh N. Sarcoidosis. Med. Clin. 2019;103(3):523–534. doi: 10.1016/j.mcna.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 90.Starshinova A., Zinchenko Y., Yablonskiy P. Specific features of immune complexes in patients with sarcoidosis and pulmonary tuberculosis. Immunol. Res. 2018;66(6):737–743. doi: 10.1007/s12026-018-9052-1. [DOI] [PubMed] [Google Scholar]
- 91.Starshinova A.A., Malkova A.M.…Yablonskiy P.K. Sarcoidosis as an autoimmune disease. Front. Immunol. 2020:10. doi: 10.3389/fimmu.2019.02933. [DOI] [PMC free article] [PubMed] [Google Scholar]