Abstract
Effects of dietary P level on the oral bioavailability of Pb present in soil were examined in a mouse model. Adult female C57BL/6 mice had free access to AIN-93G purified rodent diet amended with Pb as a soluble salt, Pb acetate, or in a soil matrix (NIST SRM 2710a). In these studies, the basal diet contained P at a nutritionally sufficient level (0.3% w/w) and the modified diets contained P at a lower (0.15%) or a higher (1.2%) level. For either dietary Pb source (Pb acetate or NIST SRM 2710a), low dietary P level markedly increased accumulation of Pb in bone, blood, and kidney. Tissue Pb levels in mice fed a high P in diet were not different from mice fed the basal P diet. Dietary P and Pb interacted to affect body weight change and feed efficiency in mice. The relative contribution of different Pb species in diet and feces was also affected by dietary P level. Differences in Pb species between diet and feces indicated that transformation of Pb species can occur during gastrointestinal tract transit. These interactions between Pb and P that alter Pb speciation may be important determinants of the bioavailability of Pb ingested in soil.
Graphical Abstract
Introduction
Exposure to Pb in early life has profound and long-lasting health effects.(1–3) Widespread use of this toxic metal has resulted in extensive and persistent contamination of soil and dust.(4,5) Because hand to mouth activity in children can be an important pathway for ingestion of Pb-contaminated soil and dust ingestion, these media are major sources for exposure of children to Pb.(6–8) Quantifying Pb exposure through soil and dust ingestion requires estimation of cumulative intake of these materials and estimation of the bioavailability of Pb present in these materials. Operationally, bioavailability is defined as the amount of a contaminant absorbed into the body following skin contact, ingestion, or inhalation (https://www.epa.gov/superfund/soil-bioavailability-superfund-sites). Although the divalent metal transporter 1 ultimately mediates absorption of ingested Pb across the gastrointestinal barrier,(9–12) bioavailability also depends on interactions between Pb and other dietary components. For example, interactions between Pb and P, an essential nutrient are of particular interest.(13) Variation in dietary P level has been shown to affect the uptake and systemic kinetics of ingested soluble Pb compounds. In rats consuming a Pb-amended diet, lower dietary P levels increased tissue levels of orally administered Pb; in contrast, higher dietary P levels reduced tissue levels of orally administered Pb(14,15) and reduced the toxicity of ingested Pb.(16) Effects of dietary P on the kinetic and dynamic behavior of ingested Pb have generally been considered in terms of formation of poorly soluble Pb–P complexes. The role of P in control of solubility of Pb has been examined in both aqueous environments and in soils.(17,18) Because oxyanionic P forms poorly soluble complexes and sorption products with Pb, application of inorganic phosphate to Pb-contaminated soils is a common strategy for soil remediation.(19–21) The efficacy of P treatment of Pb-contaminated soils to reduce the bioavailability of Pb has been demonstrated in rats and mice.(22,23)
The work reported here extends our understanding of the role of Pb–P interactions by examining the effect of dietary P level on tissue distribution of Pb in a mouse model developed to provide estimates of the relative bioavailability (RBA) of Pb present in soil.(24) This study also examined interactions between Pb and P in the mouse gastrointestinal tract to provide insights into P-dependent processes that could affect soil Pb bioavailability. A better understanding of interactions between Pb and P that occur in the environment or in the gastrointestinal tract may contribute to the development of effective strategies to reduce Pb exposure and accumulation in children.
Methods
Test Materials
The test soil evaluated in mouse assays was NIST Standard Reference Material 2710a (Montana I soil, SRM 2710a, www-s.nist.gov/srmors/certificates/2710a.pdf) with a certified Pb concentration of 5520 mg kg−1. Lead acetate trihydrate (Sigma-Aldrich, St. Louis, MO) was the soluble lead reference compound for mouse assays.
Test Diets
AIN-93G is a purified rodent diet formulated to meet nutritional requirements of rodents during growth, pregnancy, and lactation.(25) In work reported here, Dyets, Inc. (Bethlehem, PA) prepared AIN-93G diets that differed in P concentration. On a weight basis, basal AIN-93G rodent diet contained 0.3% P with approximately equal amounts of P contributed by potassium phosphate in the diet’s mineral mix and by phosphoproteins in casein, the diet’s protein source. Low P AIN-93G rodent diet that contained 0.15% P was prepared by omission of potassium phosphate from the mineral mix. Hence, in the low P diet, casein phosphoproteins were the sole source of P. High P AIN-93g rodent diet (1.2% P) was prepared by increasing the amount of potassium phosphate in the mineral mix. Total P levels in diets were confirmed by elemental analysis (data not shown).
Mouse Assay
The mouse assay for estimation of the RBA of soil-borne Pb has been described.(24) An important element of assay design was selection of dietary Pb levels (usually 4 dosage levels) that minimized nonlinear relations between cumulative Pb dosage and tissue Pb levels and did not produce overt toxic responses in mice. For studies reported here, addition of test materials, Pb acetate or NIST SRM 2710a, to test diets yielded dietary Pb levels between about 3 to about 30 mg kg−1 (Supporting Information Figure S1).
Female C57BL/6 mice received from Charles River (Raleigh, NC) at 4 weeks of age were acclimatized for 12 to 14 days in a 12-h light–12-h dark photocycle at 20–22 °C with free access to Prolab RMH 3000 rodent diet (Lab Diet, St. Louis, MO) and drinking water. During the 9-day assay, mice were housed in groups of 3 in metabolic cages (Lab Products, Seaford, DE) with free access to an AIN-93G rodent diet amended with a test material and drinking water. A standard assay consisted of 3 metabolic cages with the cage as the unit of observation and analysis. Environmental conditions were identical throughout acclimation and assay stages of studies. For each cage, daily food and water consumption were recorded. Cumulative food intake was the sum of daily food consumption. Combined body weights of mice in each cage were determined before transfer into metabolic cages and at assay termination. At termination, mice were euthanized with CO2 and a heparinized blood sample and kidneys were collected. After evisceration and pelt removal, carcasses were defleshed in dermestid beetle cultures. This procedure provided a nearly complete skeleton for Pb determination. Samples from 3 mice in each metabolic cage were pooled by tissue type before being processed for Pb determination.
To complement the studies of the effects of dietary P level on Pb bioavailability, mouse assays were also conducted under identical conditions in mice that received unamended AIN-93G rodent diets with different P levels. These studies provided comparative data on effects of dietary P levels on cumulative food consumption, relative weight gain, and feed efficiency.
Tissue Processing and Pb Analysis
Pooled kidneys and skeletons from each cage were homogenized with a model 6850 freezer mill (Spex, Metuchen, NJ). Aliquots of tissue homogenates were digested in ultrahigh purity nitric acid (SCP Science, Champlain, NY) using a closed vessel microwave reaction system (CEM Microwave, Matthews, NC). Before analysis, digested samples were diluted with deionized water to 5 to 10% nitric acid concentration. Samples of test diets were acid digested as described for tissue samples. Pb concentrations in mouse tissues and diet samples were determined by inductively coupled plasma-mass spectrometry using a X-Series II ICP/MS (SCP Science). Quality control samples analyzed with each digestion batch included reagent blanks, blank spikes, matrix spikes, and NIST SRM 2710a (Montana I soil), SRM 955c (caprine blood), SRM 1577c (bovine liver), and SRM 1486 (bone meal). Pb levels in pooled blood samples from each mouse in a metabolic cage were determined by anodic stripping voltammetry (LeadCare Ultra, Magellan Diagnostics, North Billerica, MA). Performance of the blood Pb analysis has been reported.(23)
Pb Speciation Analysis
X-ray absorption spectroscopy data for Pb species in diet and feces were collected at the DuPont-Northwestern-Dow Collaborative Access Team Sector 5, beamline 5BM-D, at the Advanced Photon Source of the Argonne National Laboratory, Lemont, IL. Data collection was conducted at the Pb LIII-edge (13035 eV) in fluorescence mode with two Vortex-ME4 four-element silicon drift detectors for the samples. All sample and standard spectra were calibrated to a Pb foil on the same energy grid, averaged, and normalized, and the background was removed by spline fitting. Various Pb standards were used as reference spectra for linear combination fitting analyses of the sample spectra to determine distribution of Pb phases. Additional information on Pb speciation analysis is presented in Supporting Information.
Data Analysis
Formulas used to calculate experimental variables evaluated in this study are provided in Supporting Information Table S1. All statistical analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows. Tissue-specific RBAs (bone, blood, kidney) were estimated for NIST SRM 2710a relative to Pb acetate, for each dietary P level (low, basal, high). RBA was estimated as the ratio of linear regression slopes (m) for the relationship between cumulative Pb dose (mg) and tissue Pb level (mg/kg of bone or kidney, mg/L of blood)
where mtest and mref are linear slopes for the test soil (NIST SRM 2710a) and reference (Pb acetate) groups, respectively.(26) Regression slopes were estimated by simultaneously fitting test and reference group tissue data to linear regression models sharing common intercepts using SAS PROC REG.(27) Confidence intervals for RBAs and heteroskedastic-consistent covariances for parameter estimates were obtained as previously described.(26,28) A point estimate for the RBA was calculated as the average of tissue-specific RBA values.(26) Confidence intervals on the point estimate were estimated from Monte Carlo simulation, which consisted of averaging repeated random draws from probability distributions of each tissue-specific RBA (normal: mean, SE), with equal probability for each tissue.(26) Mean RBA values were considered to be significantly different if the confidence intervals on the means did not overlap. Linear regression slopes for tissue Pb as a function of cumulative Pb intake, from different dietary P groups, were compared using SAS PROC GLM with inclusion of an interaction term for dietary P and cumulative Pb intake. Slopes for dietary P groups were considered to be significantly different if the interaction term was significant (P ≤ 0.05). Data on food intake, body weight change, feed efficiency, or tissue Pb levels were analyzed using analysis of variance (SAS PROC ANOVA or GLM). Means were compared using Dunnett’s test or Tukey’s HSD test, with p ≤ 0.05 considered significant. Stepwise linear regression of these variables was conducted (SAS PROC REG) with p < 0.15 for entry into the model. Studentized residuals (>3 or ←3) were used to identify and exclude statistical outliers.
Results and Discussion
Effect of Dietary P Level on Pb Accumulation in Tissues
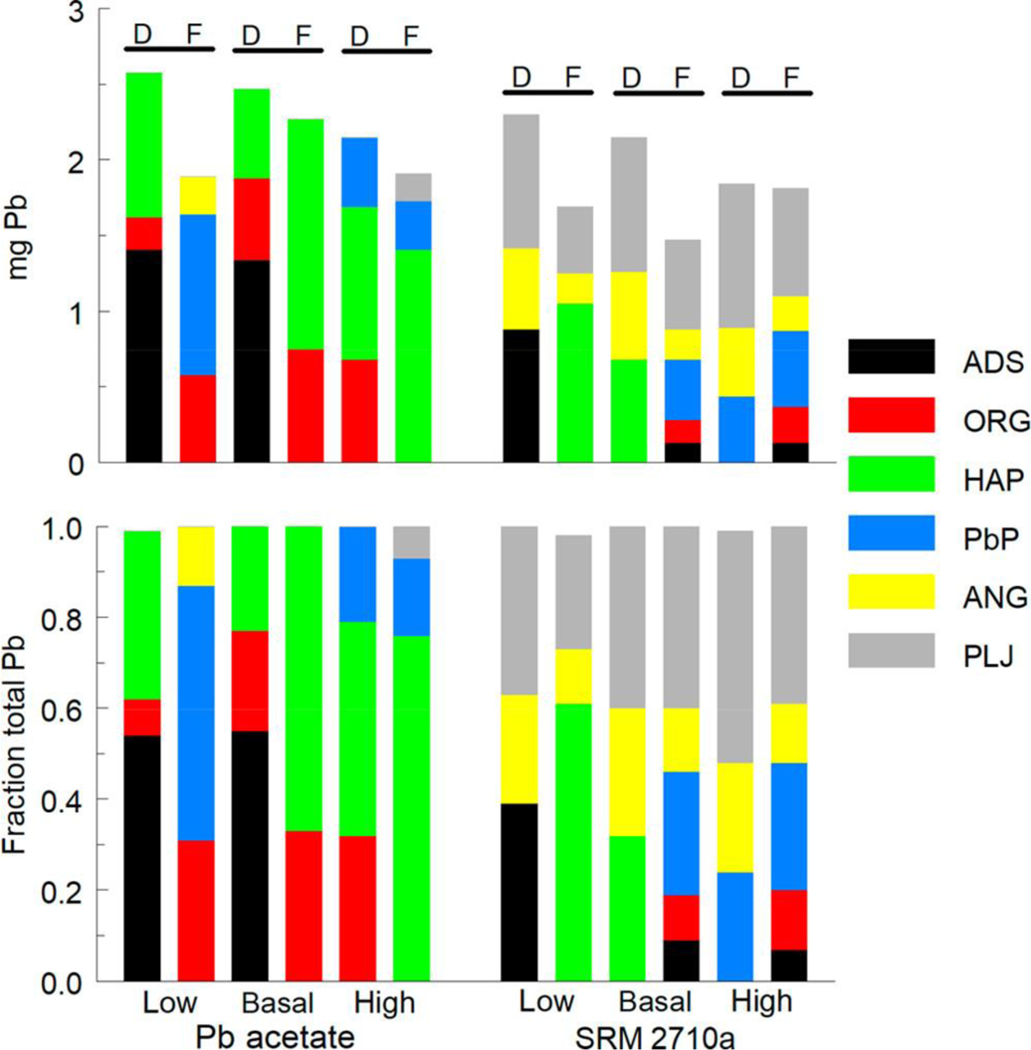
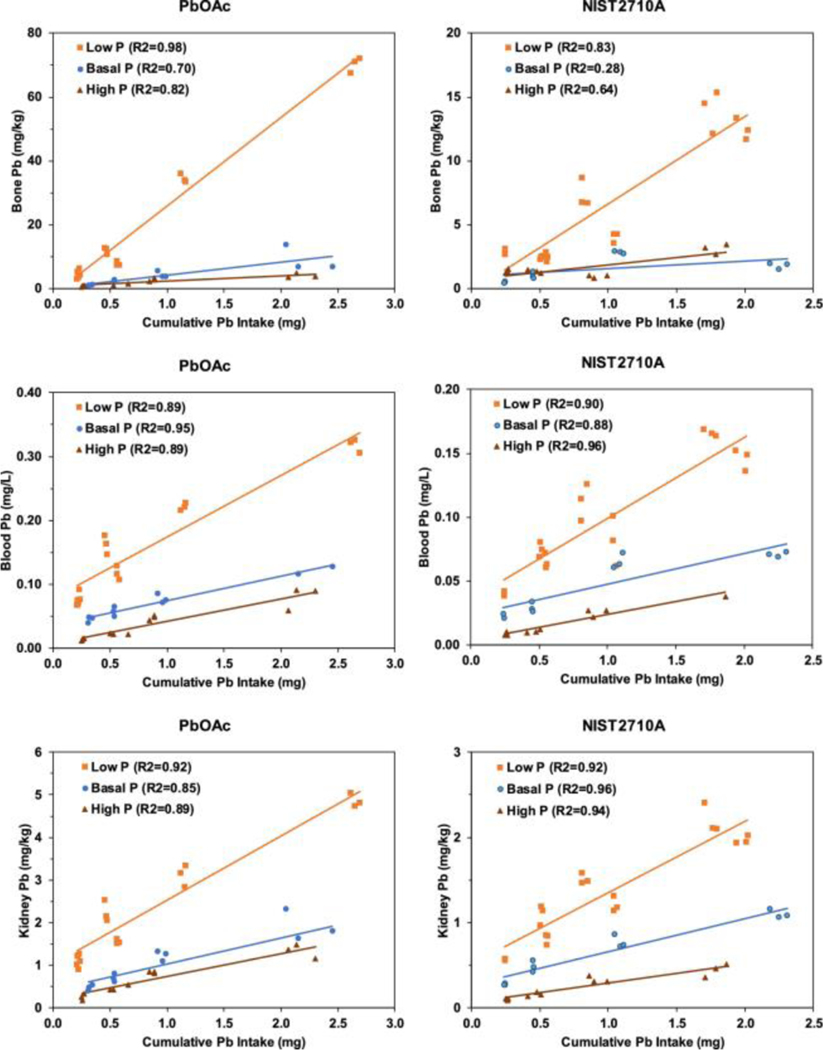
Figure 1 shows effects of dietary P level on the relation between cumulative dietary intake of Pb and tissue Pb concentrations in mice that consumed diets amended with Pb acetate or NIST SRM 2710a. For either test material, highest to lowest rank order of tissue Pb concentrations was bone, kidney, and blood. Irrespective of Pb source (NIST SRM 2710a or Pb acetate) or cumulative Pb intake, consumption of a low P diet significantly increased tissue Pb levels relative to basal and high P diets (Figure 1). For both Pb acetate and NIST SRC 2710a groups, slopes for tissue Pb (bone, blood, or kidney) as a function of cumulative Pb intake were significantly higher (P < 0.001) in mice maintained on the low P diet compared to mice maintained on the basal P or high P diet.
Figure 1.
Absolute (upper) and relative (lower) levels of Pb species in diet and feces. Cumulative amounts of Pb species ingested from diet (D) and excreted in feces (F) shown for two Pb sources and three dietary P levels. ADS, adsorbed Pb; ORG, organic material-associated Pb; HAP, hydroxyapatite-associated Pb; PbP, trilead diphosphate; ANG, anglesite; PLJ, plumbojarosite.
In mice that consumed diets amended with Pb acetate or NIST SRM 2710a, low dietary P levels resulted in a 2- to 5-fold increase in mean tissue Pb levels compared to those found in mice that consumed basal P diets. Increased tissue Pb levels in rodents have been consistently reported in rodents that consume low P diets.(14,15) Notably, effects of low P diet on tissue Pb accumulation occurred when Pb was delivered in either a soluble form or in a soil matrix. Thus, factors that account for increased uptake and accumulation of Pb in mice consuming low P diets must be independent of physical or chemical properties of the Pb source. Because processing of milk to produce casein removes soluble P,(29) phosphoproteins are the sole source of P in the low P AIN-93G diet. Although intestinal endopeptidases release P from casein phosphoproteins to meet the nutritional requirement for this element,(30) it is unclear whether P in intact phosphoproteins readily interacts with Pb. Binding of Pb to casein through interactions with cysteinyl thiols has been described;(31) however, interactions of phosphoprotein P and Pb have not been characterized. Thus, in terms of Pb–P interactions, there may be qualitative differences between P present in diets that contain casein as a sole source of P and in diets which contain P as a soluble salt. These putative differences in accessibility and reactivity of P from different dietary sources could affect Pb–P interactions. Expressing dietary P levels on the basis of P mass (% of diet weight) may not adequately reflect differences in availability of dietary P to interact with Pb.
Tissue Pb levels were typically lower in mice that consumed high P diets than in mice that consumed basal P diets (Figure 1). Tissue Pb slopes for mice maintained on the high P diet were significantly lower (P < 0.03) than slopes for mice maintained on the basal diet for all tissues except for bone Pb in the NIST SRM 2710a group (P = 0.11). Effects of high dietary P level were relatively modest; tissue Pb levels in mice receiving high P diets ranged from 0.3- to 1.1-fold of those found in tissue of mice that consumed basal P diet. These effects on tissue Pb accumulation were consistent with earlier findings in rodent studies of the effect of high P intake on tissue Pb levels. In a single dose model, gastrointestinal Pb absorption was reduced in rats that received both Pb and a high phosphate solution orally.(32) Whole body retention of Pb was reduced by increased dietary P levels in rats that consumed Pb acetate-containing diets.(14) The modifying effects of high dietary P levels on tissue Pb accumulation were seen with both Pb sources. Thus, the effects of P on Pb absorption and retention did not depend on physical and chemical properties of the Pb source.
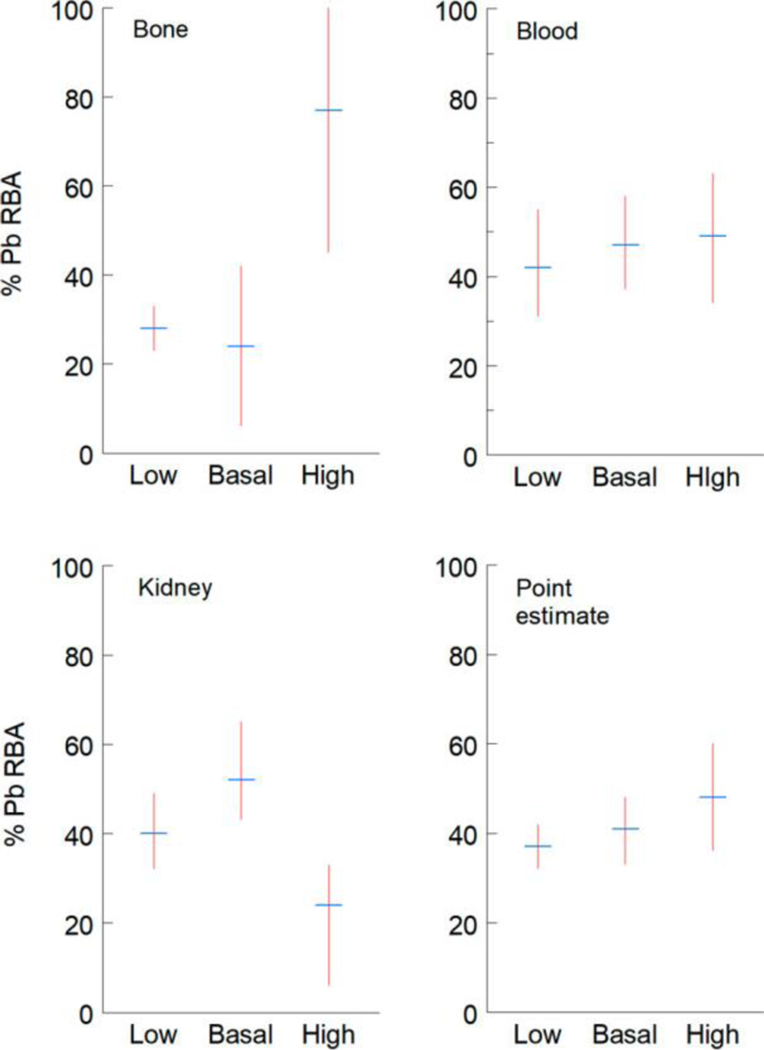
Effect of Dietary P Level on Estimated RBA for Lead in NIST SRM 2710a
As described in Methods, ratios of linear regression slopes (m) for relations between cumulative Pb doses (mg) and tissue Pb levels were used to calculate tissue-specific and point estimates of Pb RBA in NIST SRM 2710a in diets that contained low, basal, or high P (Figure 2, data summary in Table S10). Tissue-specific and point estimates of Pb RBA obtained in mice that consumed NIST SRM 2710a-amended AIN-93G diet containing basal P were similar to those previously reported: bone: 34% (95% CL: 28–39), blood 42% (36, 47), kidney 60% (53, 69).(24) Soil Pb RBA estimates derived from bone, blood, and kidney data were not significantly different in mice consuming either low or basal P diet (i.e., 95% confidence intervals overlapped), even though bioavailability was substantially different as indicated from the slopes of the cumulative Pb dose-tissue level regression models (Figure 1). A change in bioavailability will not necessarily result in a change in RBA because RBA is the ratio the bioavailability of Pb in soil to that of Pb in Pb acetate. For example, if bioavailability of Pb from soil and from Pb acetate are similarly depressed by dietary P, the RBA will not change. In contrast, RBA estimates derived from bone or kidney data from mice consuming high P diet were discordant with estimates for other dietary groups. Point estimates of Pb RBA for all dietary P levels were not significantly different.
Figure 2.
Estimates of percentage relative bioavailability (% RBA) of Pb in NIST SRM 2710a in mice that consumed AIN-93G rodent diet with low, basal, or high P levels. Mean and 95% confidence intervals shown for % RBAs derived from bone, blood, and kidney data. Point estimate % RBA derived from mean of all tissue estimates.
Discordance among RBA estimates derived from Pb levels in kidney and bone in mice consuming high P diets versus low or basal P diets indicates that high dietary P differentially affected the dose-kidney and dose-bone slopes for Pb acetate and NIST SRM 2710a. In addition to enhanced formation of poorly soluble Pb–P complexes in the gastrointestinal tract of mice that consume high P diets, reduced Pb uptake into bone and kidney in animals that consumed high P diet could reflect effects of excess dietary P on these tissues. In female rats, high (1.5%) dietary P level reduced bone mineral density, an effect likely related to changes in calcium (Ca) metabolism produced by changes in dietary Ca/P ratio.(33) Interactions between Ca and P present in diet includes formation of poorly soluble complexes that can affect uptake of both elements across the gastrointestinal barrier. In mice, low Ca/P molar ratios in diet reduced uptake of Ca and higher molar ratios reduced uptake of P.(34) Similarly, there is evidence that high levels of P in diet can affect renal structure and function in rodents. In rats, abnormal Ca accumulation in kidney (nephrocalcinosis) and attendant effects on renal function have been linked to dietary P level and to the ratio of Ca and P in diet.(35,36) Basal P AIN-93G rodent diet contains 3 g of P and 5 g of Ca per kg (1.3 Ca/P molar ratio). This formulation meets requirements for these essential nutrients for normal growth and development(37) and reduces the potential of diet-induced nephrocalcinosis in rodents.(25,38) Alteration of the Ca/P ratio of high P AIN-93G rodent diet by addition of inorganic P to diet may result in pathophysiological effects associated with low Ca/P molar ratios.
If dietary P levels or Ca/P molar ratios are critical determinants of Pb absorption and distribution, then standardization of P and Ca levels in diets used in mouse assays used to estimate Pb RBA merits consideration. In natural ingredient rodent diets prepared from grains and other plant and animal products, P and Ca levels can vary widely. As shown in Figure S1, P levels in natural ingredient rodent diets range from about 0.5 to 1.5% and Ca levels range from about 0.5 to 2.4%. By comparison, P and Ca levels are 0.3 and 0.5%, respectively, in basal P AIN-93G rodent diet; these concentrations yield the optimal molar ratio of these nutrients.(25) Evaluation of Pb RBA estimates obtained in earlier studies in rodents should be examined with respect to dietary P and Ca levels and their molar ratio. For example, earlier studies of soil Pb bioavailability in rats have used both a natural ingredient diet(39) and a purified diet (AIN-76A) with a higher P level.(39,40) In analyses reported here, RBA estimates for Pb in NIST SRM 2710a used data obtained from mice that consumed diets with identical P levels. Discordant levels of P in diets which affected the slope of regression lines would affect the calculation of the ratio used to estimate RBA. Given the potential effect of dietary P and Ca levels on estimation of Pb RBA, comparisons of tissue Pb levels or of RBA estimates obtained in studies in which mice consumed diets that differed in composition (natural ingredient vs purified) and in P and Ca levels should be made with caution.
Effect of Dietary P Level on Pb Speciation in Diet and Feces
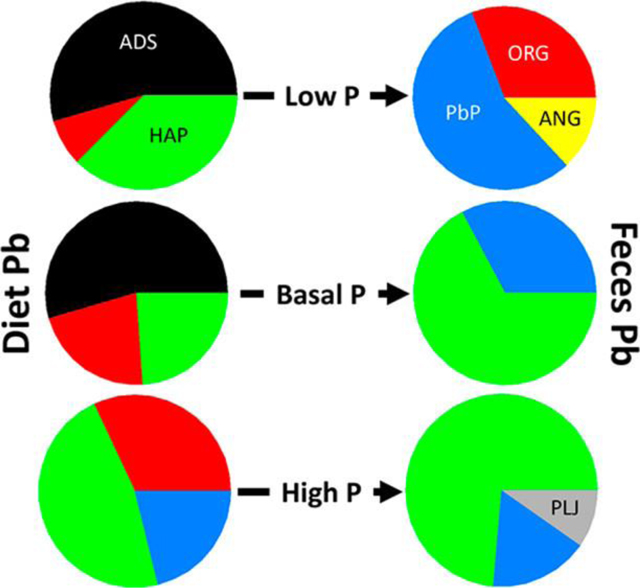
Chemical transformation of Pb during transit of the mouse gastrointestinal tract can be monitored by determining Pb species present in diets and in feces. Figure 3 shows both absolute amounts of different Pb species ingested from Pb-amended diets and excreted in feces and fractional contributions of these species to ingested and excreted Pb. In Pb acetate-amended diets, dietary P level affected the predominant Pb species. In low and basal P diets, adsorbed Pb was the predominant form; in the high P diet, hydroxyapatite was the predominant process (microbial activity) that affected Pb speciation in the gastrointestinal tract.(41–43) Changes in Pb species that occur in the gastrointestinal tract could affect the bioavailability of ingested Pb species. For NIST SRM 2710a-amended diets, irrespective of dietary P level, plumbojarosite, a poorly soluble species, predominated. However, dietary P level did affect relative contributions of other Pb species (adsorbed, anglesite, hydroxyapatite) to NIST SRM 2710a-amended diets.
Figure 3.
Effect of dietary P level on relations between cumulative Pb intake and tissue Pb concentrations in mice. Mice ingested AIN-93G rodent diet amended with Pb acetate (PbOAc) or NIST SRM 2710a. Dietary P levels were 0.15% (low P), 0.3% (basal P), and 1.2% (high P). Regression lines for tissue Pb concentrations as functions of cumulative Pb intake and R2 values shown for bone, blood, and kidney.
Comparisons of Pb species in diets and in feces produced by mice that consumed these diets provided insight into effects of dietary P level on changes in Pb speciation during transit of the gastrointestinal tract. Feces from mice that consumed a low P diet amended with Pb acetate did not contain adsorbed Pb or hydroxyapatite, components of the diet, but did contain a larger fraction of organic-associated Pb. Anglesite and trilead diphosphate, species absent from the low P diet, were present in feces. Feces of mice that consumed Pb acetate-amended diet with a basal P level had higher fractional levels of hydroxyapatite and organic-associated Pb. Adsorbed Pb, a component of the basal P diet, was absent from feces. Organic-associated Pb was absent, and the fractional contribution of hydroxyapatite and trilead diphosphate to fecal Pb increased and decreased, respectively, in feces of mice that consumed Pb acetate-amended diet with a high P level. Notably, feces from these mice contained plumbojarosite, a species absent from Pb acetate-amended diet with a high P level.
Similar comparison of Pb species in NIST SRM 2710a-amended diet and in feces of mice that consumed these diets found that dietary P level affected speciation. Compared to diet, feces from mice consuming low P diet lacked adsorbed Pb, had lower fractional contributions of anglesite and plumbojarosite, and contained hydroxyapatite. Feces from mice that consumed diet with a basal P level contained trilead diphosphate, organic-associated Pb, and adsorbed Pb; notably, these species were absent from diet. In feces, fractional contribution of anglesite was smaller and the fractional contribution of plumbojarosite was similar to that found in diet. Hydroxyapatite, a component of diet, was absent from feces. The fractional contributions of plumbojarosite in diet and feces were similar. Feces from mice that consumed NIST SRM 2710a-amended diet with a high P level contained organic-associated Pb and adsorbed Pb as novel species. Although the fractional contribution of trilead diphosphate was similar in diet and feces, fractional contributions of plumbojarosite and anglesite were lower in feces than in diet.
Pb–P Interactions in the Mouse Model
As part of our evaluation of the effects of Pb–P interactions on soil Pb RBA, we examined effects of dietary P level on food consumption, relative weight gain, and feed efficiency in the mouse model. Table 1 summarizes effects of dietary P levels on cumulative food intake, % body weight change, and % feed efficiency in mice consuming an unamended or a Pb-amended AIN-93G rodent diet. For an unamended diet, means for cumulative food intake, % body weight change, or % feed efficiency were not significantly different for different dietary P levels. In mice consuming diets amended with either Pb source, cumulative food intake was significantly lower for either low or high dietary P levels than for mice consuming a Pb-amended basal P diet. For either Pb source, a high dietary P level resulted in significantly lower cumulative food intake than that found in mice consuming Pb-amended low or basal P diets. In mice fed a Pb acetate-amended diet with a high P level, % body weight change and % feed efficiency were significantly lower than those found in mice fed low or basal P diets amended with this Pb source. Compared to mice that consumed the basal P 2710a-amended diet, mice fed a low P 2710a-amended diet had significantly increased % body weight change without an effect on % feed efficiency. In contrast, % body weight change and % feed efficiency were both significantly lower in mice that consumed a high P diet that was amended with NIST SRM 2710a. Additional analyses of the effects of dietary amendments and P levels on response in mice are summarized in Supporting Information (Tables S2–S5).
Table 1.
Summary of Effects of Dietary P Level and Dietary Amendment on Cumulative Food Intake, Relative Growth, and Feed Efficiency in Mice Consuming Unamended and Pb-Amended AIN-93G Rodent Dieta
| Dietary Amendment | Dietary P Level | ||||
|---|---|---|---|---|---|
| Low | Basal | High | All P Levels | ||
| Cumulative food intake (g) | Unamended | 81.8 ± 7.4a x | 73.9 ± 2.4a x | 70.9 ± 2.1a x | 75.4 ± 6.4x |
| PbAc | 77.2 ± 3.6a x | 83.8 ± 5.0b y | 72.6 ± 5.3c x | 77.8 ± 6.2x | |
| 2710a | 77.6 ± 4.2a x | 82.7 ± 4.8b y | 72.9 ± 4.4c x | 77.7 ± 5.6x | |
| Diet P Mean | 77.8 ± 4.4a | 81.6 ± 5.9b | 72.5 ± 4.5c x | ||
| % Body Weight Change | Unamended | 6.04 ± 3.58a x | 7.85 ± 3.75a x | 4.52 ± 3.67a x | 6.27 ± 3.65x |
| PbAc | 7.72 ± 2.49a x | 7.76 ± 2.84a x | –0.42 ± 2.46b y | 5.41 ± 4.50x | |
| 2710a | 7.03 ± 3.42a x | 6.54 ± 2.43b x | 3.42 ± 3.28a x | 5.94 ± 3.45x | |
| Diet P Mean | 7.23 ± 3.04a | 7.27 ± 2.81a | 1.93 ± 3.58b | ||
| % Feed Efficiency | Unamended | 3.71 ± 1.97a x | 5.38 ± 2.38a x | 3.16 ± 2.58a x | 4.18 ± 2.36x |
| PbAc | 5.24 ± 1.66a x | 4.94 ± 1.53a x | –0.30 ± 1.84b y | 3.57 ± 2.97x | |
| 2710a | 4.66 ± 2.17a x | 4.32 ± 1.63a x | 2.39 ± 2.24b x | 3.96 ± 2.24x | |
| Diet P Mean | 4.81 ± 1.96a | 4.76 ± 1.72a | 1.35 ± 2.52b | ||
Stepwise linear regression was used to evaluate potential variables to explain variance in % body weight change and % feed efficiency. Variables evaluated were dietary Pb level, dietary P level, dietary Pb source, cumulative Pb intake, cumulative P intake, cumulative food intake, and two interaction terms (cumulative food intake*dietary P level) and (dietary P level*dietary Pb source). The final models for % body weight change and % feed efficiency were similar; parameters for the feed efficiency model are shown in Table 2 (both models are presented in Tables S6 and S7). Cumulative food intake, dietary Pb source, (cumulative food intake*dietary P level), and (dietary P level*dietary Pb source) were retained as significant predictors of % body weight change (adjusted r2 = 0.50) and % feed efficiency (adjusted r2 = 0.48). The strongest association was with cumulative food intake. In the full model, this variable explained about one-half of the explained variance in % body weight change (partial r2 = 0.27) and % feed efficiency (partial r2 = 0.22). The interaction term, (cumulative food intake*dietary P level) also explained a relatively large portion of the variance in % body weight change (partial r2 = 0.15) and % food efficiency (partial r2 = 0.16).
Table 2.
Linear Regression Model for Percent Feed Efficiency
| Parameter | Estimate (mean ± SE) | Partial r2 | P |
|---|---|---|---|
| Intercept | –10.00 ± 2.61 | 0.0002 | |
| Cumulative food intake | 0.23 ± 0.03 | 0.22 | <0.0001 |
| Cumulative food intake*dietary P level | –0.030 ± 0.004 | 0.16 | <0.0001 |
| Dietary Pb source | –2.38 ± 0.70 | 0.02 | 0.0010 |
| Dietary P level*dietary Pb source | 1.51 ± 0.34 | 0.10 | <0.0001 |
| Model adjusted r2 = 0.48 | |||
Changes seen in the current study were consistent with evidence that dietary P levels can modify physiological responses in rodents. In rats, some studies have linked higher dietary P levels with reduced food intake and reduced weight gain.(44–47) However, other studies have not linked high dietary P levels to altered food consumption and ponderal growth in rats.(15,48) In mice, increased dietary P level has been associated with diminished weight gain with female mice more responsive than male mice.(49,50) Reduced food intake and lower % feed efficiency in mice consuming high P diets may be due to increased satiety and increased postprandial thermogenesis associated with increased P intake.(51)
Notably, these analyses did not illuminate the nature of the interaction between the dietary Pb source and the dietary P level that resulted in effects on body weight change and feed efficiency. Pb levels in bone, the major depot for absorbed Pb in the mouse model, in Pb acetate-treated mice were about 10-fold higher in mice that consumed a low P diet compared to mice that consumed a high P diet. Thus, effects on body weight change and feed efficiency cannot be attributed to higher tissue levels of Pb in mice that consumed high P diets. Furthermore, when included in the final regression models, bone Pb level was not a significant explanatory variable for body weight change or feed efficiency and r2 values for the full models were not improved by the addition of bone Pb. These findings suggest that the interaction between dietary P level and Pb source was not explained by the different absorbed Pb doses in mice dosed with Pb acetate. An alternative explanation is that the interactions between P and Pb occur in the gastrointestinal tract. These interactions may contribute to the observed changes in Pb RBA produced by changes in dietary P levels. Interactions between P and Pb in the gastrointestinal tract that affect the bioavailability of Pb have been described in mice that received both Pb and P by gavage.(41) Results from the present study suggest that the interactions between Pb and P in the gastrointestinal tract occur with concurrent ingestion of Pb and P in food.
This study found that lowering the level of P in the diet markedly increased the gastrointestinal uptake and tissue accumulation of Pb, when mice ingested either a soluble form of Pb (Pb acetate) or soil-borne Pb. Patterns of changes in Pb speciation during gastrointestinal transit differed as functions of the source of dietary Pb and the dietary P level, suggesting that interactions between Pb and P may affect uptake across the gastrointestinal barrier. The modest effects of high dietary P levels on Pb uptake and accumulation suggest that increased P intake would provide little benefit in terms of reducing the risk of exposure to Pb. In addition, clinical and epidemiological evidence of a linkage between high dietary P intake and renal disease indicates that increased P intake is not a useful strategy for mitigating Pb exposure.(13,52,53)
Supplementary Material
Acknowledgments
Part of this work was performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. Portions of this work were funded by U.S. Environmental Protection Agency Office of Superfund Remediation and Technology Innovation (OSRTI) under contract EP-W-09–031. This document has been subjected to review by the National Exposure Research Laboratory (NERL) and approved for publication. Approval does not signify that the contents reflect the views of the Agency nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
Supporting Information
References
- 1.Surkan PJ; Zhang A; Trachtenberg F; Daniel DB; McKinlay S; Bellinger DC Neuropsychological function in children with blood lead levels < 10 microg/dL. NeuroToxicology 2007, 28, 1170–1117, DOI: 10.1016/j.neuro.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger DC The protean toxicities of lead: new chapters in a familiar story. Int. J. Environ. Res. Public Health 2011, 8, 2593–2628, DOI: 10.3390/ijerph8072593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason LH; Harp JP; Han DY Pb neurotoxicity: neuropsychological effects of lead toxicity. BioMed Res. Int. 2014, 2014, 840547, DOI: 10.1155/2014/840547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mielke HW; Laidlaw MA; Gonzales CR Estimation of leaded (Pb) gasoline’s continuing material and health impacts on 90 US urbanized areas. Environ. Int. 2011, 37, 248–257, DOI: 10.1016/j.envint.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 5.Datko-Williams L; Wilkie A; Richmond-Bryant J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ. 2014, 468–469, 854–863, DOI: 10.1016/j.scitotenv.2013.08.089 [DOI] [PubMed] [Google Scholar]
- 6.Xue J; Zartarian V; Moya J; Freeman N; Beamer P; Black K; Tulve N; Shalat S. A meta-analysis of children’s hand-to-mouth frequency data for estimating nondietary ingestion exposure. Risk Anal. 2007, 27, 411–420, DOI: 10.1111/j.1539-6924.2007.00893.x [DOI] [PubMed] [Google Scholar]
- 7.Laidlaw MA; Zahran S; Pingitore N; Clague J; Devlin G; Taylor MP Identification of lead sources in residential environments: Sydney Australia. Environ. Pollut. 2014, 184, 238–246, DOI: 10.1016/j.envpol.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Zartarian V; Xue J; Tornero-Velez R; Brown J. Children’s Lead Exposure: A multimedia modeling analysis to guide public health decision-making. Environ. Health Perspect. 2017, 125, 097009 DOI: 10.1289/EHP1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bannon DI; Portnoy ME; Olivi L; Lees PS; Culotta VC; Bressler JP Uptake of lead and iron by divalent metal transporter 1 in yeast and mammalian cells. Biochem. Biophys. Res. Commun. 2002, 295, 978–984, DOI: 10.1016/S0006-291X(02)00756-8 [DOI] [PubMed] [Google Scholar]
- 10.Bannon DI; Abounader R; Lees PS; Bressler JP Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol Cell Physiol. 2003, 284, C44–50, DOI: 10.1152/ajpcell.00184.2002 [DOI] [PubMed] [Google Scholar]
- 11.Bressler JP; Olivi L; Cheong JH; Kim Y; Bannona D. Divalent metal transporter 1 in lead and cadmium transport. Ann. N. Y. Acad. Sci. 2004, 1012, 142–152, DOI: 10.1196/annals.1306.011 [DOI] [PubMed] [Google Scholar]
- 12.Shawki A; Anthony SR; Nose Y; Engevik MA; Niespodzany EJ; Barrientos T; Öhrvik H; Worrell RT; Thiele DJ; Mackenzie B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol Gastrointest Liver Physiol. 2015, 309, G635–G647, DOI: 10.1152/ajpgi.00160.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang AR; Anderson C. Dietary Phosphorus Intake and the Kidney. Annu. Rev. Nutr. 2017, 37, 321–346, DOI: 10.1146/annurev-nutr-071816-064607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarterman J; Morrison JN The effects of dietary calcium and phosphorus on the retention and excretion of lead in rats. Br. J. Nutr. 1975, 34, 351–362, DOI: 10.1017/S0007114575000414 [DOI] [PubMed] [Google Scholar]
- 15.Barton JC; Conrad ME Effect of phosphate on the absorption and retention of lead in the rat. Am. J. Clin. Nutr. 1981, 34, 2192–2198, DOI: 10.1093/ajcn/34.10.2192 [DOI] [PubMed] [Google Scholar]
- 16.Spickett JT; Bell RR The influence of dietary phosphate on the toxicity of orally ingested lead in rats. Food Chem. Toxicol. 1983, 21, 157–161, DOI: 10.1016/0278-6915(83)90230-2 [DOI] [PubMed] [Google Scholar]
- 17.Sauve S; McBride M; Hendershot W. Lead Phosphate Solubility in Water and Soil Suspensions. Environ. Sci. Technol. 1998, 32, 388–393, DOI: 10.1021/es970245k [DOI] [Google Scholar]
- 18.Martínez CE; Jacobson AR; Mcbride MB Lead phosphate minerals: solubility and dissolution by model and natural ligands. Environ. Sci. Technol. 2004, 38, 5584–5590, DOI: 10.1021/es049617x [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Innovations in ground water and soil cleanup: From concept to commercialization; The National Academies Press: Washington, D.C, 1997; pp 80–166. 10.17226/5781. [DOI] [Google Scholar]
- 20.Scheckel KG; Diamond GL; Burgess MF; Klotzbach JM; Maddaloni M; Miller BW; Partridge CR; Serda SM Amending soils with phosphate as means to mitigate soil lead hazard: a critical review of the state of the science. J. Toxicol. Environ. Health, Part B 2013, 16, 337–380, DOI: 10.1080/10937404.2013.825216 [DOI] [PubMed] [Google Scholar]
- 21.Bolan N; Kunhikrishnan A; Thangarajan R; Kumpiene J; Park J; Makino T; Kirkham MB; Scheckel K. Remediation of heavy metal(loid)s contaminated soils--to mobilize or to immobilize?. J. Hazard. Mater. 2014, 266, 141–166, DOI: 10.1016/j.jhazmat.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 22.Hettiarachchi GM; Pierzynski GM; Oehme FW; Sonmez O; Ryan JA Treatment of contaminated soil with phosphorus and manganese oxide reduces lead absorption by Sprague-Dawley rats. J. Environ. Qual. 2003, 32, 1335–1345, DOI: 10.2134/jeq2003.1335 [DOI] [PubMed] [Google Scholar]
- 23.Bradham KD; Diamond GL; Nelson CM; Noerpel M; Scheckel KG; Elek B; Chaney RL; Ma Q; Thomas DJ Long-Term in Situ Reduction in Soil Lead Bioavailability Measured in a Mouse Model. Environ. Sci. Technol. 2018, 52, 13908–13913, DOI: 10.1021/acs.est.8b04684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradham KD; Green W; Hayes H; Nelson C; Alava P; Misenheimer J; Diamond GL; Thayer WC; Thomas DJ Estimating relative bioavailability of soil lead in the mouse. J. Toxicol. Environ. Health, Part A 2016, 79, 1179–1826, DOI: 10.1080/15287394.2016.1221789 [DOI] [PubMed] [Google Scholar]
- 25.Reeves PG; Nielsen FH; Fahey GC AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A. J. Nutr. 1993, 123, 1939–1951, DOI: 10.1093/jn/123.11.1939 [DOI] [PubMed] [Google Scholar]
- 26.Casteel SW; Weis CP; Henningsen GM; Brattin WJ Estimation of relative bioavailability of lead in soil and soil-like materials using young swine. Environ. Health Perspect. 2006, 114, 1162–1171, DOI: 10.1289/ehp.8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finney DJ 1978. Statistical methods in biological assay, 3rd ed.; Charles Griffin & Co.: London, UK. [Google Scholar]
- 28.Long JS; Ervin LH Using heteroskedasticity consistent standard errors in the linear regression model. Am. Stat. 2000, 54, 217–224, DOI: 10.1080/00031305.2000.10474549 [DOI] [Google Scholar]
- 29.Dalgleish DG; Corredig M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467, DOI: 10.1146/annurev-food-022811-101214 [DOI] [PubMed] [Google Scholar]
- 30.Guan D; Yoshioka M; Erickson RH; Heizer W; Kim YS Protein digestion in human and rat small intestine: role of new neutral endopeptidases. Am. J. Physiol. 1988, 255, G212–220, DOI: 10.1152/ajpgi.1988.255.2.G212 [DOI] [PubMed] [Google Scholar]
- 31.Srinivas S; Kaul P; Prakash V. Mechanism of interaction of Pb(II) with milk proteins: a case study of alpha-casein. J. Agric. Food Chem. 2007, 55, 9283–9238, doi.org/ DOI: 10.1021/jf070911t [DOI] [PubMed] [Google Scholar]
- 32.Aungst BJ; Fung HL Inhibition of oral lead absorption in rats by phosphate-containing products. J. Pharm. Sci. 1983, 72, 345–348, DOI: 10.1002/jps.2600720406 [DOI] [PubMed] [Google Scholar]
- 33.Koshihara M; Katsumata S; Uehara M; Suzuki K. Effects of dietary phosphorus intake on bone mineralization and calcium absorption in adult female rats. Biosci., Biotechnol., Biochem. 2005, 69, 1025–1028, DOI: 10.1271/bbb.69.1025 [DOI] [PubMed] [Google Scholar]
- 34.Masuyama R; Nakaya Y; Katsumata S; Kajita Y; Uehara M; Tanaka S; Sakai A; Kato S; Nakamura T; Suzuki K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J. Bone Miner. Res. 2003, 18, 1217–1226, DOI: 10.1359/jbmr.2003.18.7.1217 [DOI] [PubMed] [Google Scholar]
- 35.Cockell KA; L’Abbé MR; Belonje B. The concentrations and ratio of dietary calcium and phosphorus influence development of nephrocalcinosis in female rats. J. Nutr. 2002, 132, 252–256, DOI: 10.1093/jn/132.2.252 [DOI] [PubMed] [Google Scholar]
- 36.Cockell KA; Belonje B. Nephrocalcinosis caused by dietary calcium:phosphorus imbalance in female rats develops rapidly and is irreversible. J. Nutr. 2004, 134, 637–640, DOI: 10.1093/jn/134.3.637 [DOI] [PubMed] [Google Scholar]
- 37.National Research Council (US) Subcommittee on Laboratory Animal Nutrition. National Academies Press (US): Washington, DC, 1995, www.ncbi.nlm.nih.gov/books/NBK231918/#ddd00128. [Google Scholar]
- 38.Reeves PG; Rossow KL; Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J. Nutr. 1993, 123, 1923–1931, DOI: 10.1093/jn/123.11.1923 [DOI] [PubMed] [Google Scholar]
- 39.Aungst BJ; Fung HL The effects of dietary calcium on lead absorption, distribution, and elimination kinetics in rats. J. Toxicol. Environ. Health 1985, 16, 147–159, DOI: 10.1080/15287398509530726 [DOI] [PubMed] [Google Scholar]
- 40.Freeman GB; Johnson JD; Liao SC; Feder PI; Davis AO; Ruby MV; Schoof RA; Chaney RL; Bergstrom PD Absolute bioavailability of lead acetate and mining waste lead in rats. Toxicology 1994, 91, 151–63, DOI: 10.1016/0300-483X(94)90141-4 [DOI] [PubMed] [Google Scholar]
- 41.Freeman GB; Dill JA; Johnson JD; Kurtz PJ; Parham F; Matthews HB Comparative absorption of lead from contaminated soil and lead salts by weanling Fischer 344 rats. Toxicol. Sci. 1996, 33, 109–119, DOI: 10.1093/toxsci/33.1.109 [DOI] [PubMed] [Google Scholar]
- 42.Juhasz AL; Gancarz D; Herde C; McClure S; Scheckel KG; Smith E. In situ formation of pyromorphite is not required for the reduction of in vivo Pb relative bioavailability in contaminated soils. Environ. Sci. Technol. 2014, 48, 7002–7009, DOI: 10.1021/es500994u [DOI] [PubMed] [Google Scholar]
- 43.Topolska J; Latowski D; Kaschabek S; Manecki M; Merkel BJ; Rakovan J. Pb remobilization by bacterially mediated dissolution of pyromorphite Pb5(PO4)3Cl in presence of phosphate-solubilizing Pseudomonas putida. Environ. Sci. Pollut. Res. 2014, 21, 1079–1089, DOI: 10.1007/s11356-013-1968-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drewniak L; Skłodowska A; Manecki M; Bajda T. Solubilization of Pb-bearing apatite by bacteria isolated from polluted environment. Chemosphere 2017, 171, 302–307, DOI: 10.1016/j.chemosphere.2016.12.056 [DOI] [PubMed] [Google Scholar]
- 45.Quarterman J; Morrison JN; Humphries WR The influence of high dietary calcium and phosphate on lead uptake and release. Environ. Res. 1978, 17, 60–67, DOI: 10.1016/0013-9351(78)90061-0 [DOI] [PubMed] [Google Scholar]
- 46.Tani Y; Sato T; Yamanaka-Okumura H; Yamamoto H; Arai H; Sawada N; Genjida K; Taketani Y; Takeda E. Effects of prolonged high phosphorus diet on phosphorus and calcium balance in rats. J. Clin. Biochem. Nutr. 2007, 40, 221–228, DOI: 10.3164/jcbn.40.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsumata S; Matsuzaki H; Katsumata-Tsuboi R; Uehara M; Suzuki K. Effects of high phosphorus diet on bone metabolism-related gene expression in young and aged mice. J. Nutr. Metab. 2014, 2014, 1, DOI: 10.1155/2014/575932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammoud RU; Jabbour MN; Tawil AN; Ghattas H; Obeid OA Phosphorus supplementation mitigated food intake and growth of rats fed a low-protein diet. Curr. Dev Nutr. 2017, 1 (8), e000943 DOI: 10.3945/cdn.117.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suyama T; Okada S; Ishijima T; Iida K; Abe K; Nakai Y. High phosphorus diet-induced changes in NaPi-IIb phosphate transporter expression in the rat kidney: DNA microarray analysis. PLoS One 2012, 7 (1), e29483 DOI: 10.1371/journal.pone.0029483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eller P; Eller K; Kirsch AH; Patsch JJ; Wolf AM; Tagwerker A; Stanzl U; Kaindl R; Kahlenberg V; Mayer G; Patsch JR; Rosenkranz AR A murine model of phosphate nephropathy. Am. J. Pathol. 2011, 178, 1999–2006, DOI: 10.1016/j.ajpath.2011.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuzaki H; Katsumata S; Masuyama R; Uehara M; Nakamura K; Suzuki K. Sex differences in kidney mineral concentrations and urinary albumin excretion in rats given high-phosphorus feed. Biosci., Biotechnol., Biochem. 2002, 66, 1737–1739, DOI: 10.1271/bbb.66.1737 [DOI] [PubMed] [Google Scholar]
- 52.Bassil MS; Obeid OA Phosphorus Supplementation Recovers the Blunted Diet-Induced Thermogenesis of Overweight and Obese Adults: A Pilot Study. Nutrients 2016, 8, E801 DOI: 10.3390/nu8120801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Ac; [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.