ABSTRACT
The predictive ability of metabolic conditions, such as hypercholesterolemia, on the outcome of cancer patients to immune-checkpoint inhibitors (ICIs) therapy, has been recently explored. The reasons for their value in this setting are to be searched in the individual himself more than in his tumor, as the target of the immune-checkpoint blockade is the immune system. The efficacy of ICIs on the tumor may be based on two simple premises: 1) the physiological immune function has been blocked, and 2) the tumor progression (mainly) depends on this block. The metabolic syndrome may represent the epiphenomenon of an “inflamed patient,” no longer able of physiological functions required to prevent chronic inflammatory events. The metabolic dysfunction could represent merely “a biomarker” of the patient who satisfies both the two premises reported above. Suggestions from preclinical and translational researches should be transferred in the clinical setting, implementing randomized clinical trials with observational endpoints such as the effect of concomitant drug medications and the impact of blood cholesterol levels and other metabolic conditions on the outcome of ICI treatment.
KEYWORDS: Cholesterol, obesity, paradox index, metabolic syndrome, cancer, immune system, immune-checkpoint inhibitors, outcome, immunotherapy, inflammation
In recent years, metabolomics reached immuno-oncology. According to the NCEP ATP III definition, metabolic syndrome is present if three or more of the following five criteria are met: waist circumference over 40 inches (men) or 35 inches (women), blood pressure over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dl, fasting high-density lipoprotein (HDL) cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women) and fasting blood sugar over 100 mg/dl.1 The predictive ability of such metabolic conditions, especially with regard to hypercholesterolemia and obesity, for the outcome of cancer patients to immune-checkpoint inhibitors (ICIs) therapy, has been recently explored, providing fascinating evidences of correlations between metabolic syndrome and T-cell-mediated immune response.2 Even beyond the cell-mediated mechanisms, the metabolic pathways are involved in a complex interplay with other immunologic compartments, especially those enriching the tumor micro-environment (TME). The TME includes stromal cells, extracellular matrix, adipocytes, mesenchymal stem cells, blood vessels, macrophages, T cells, B cells, cytokines, exosomes, and metabolites.3
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that have acquired immunosuppressive activities, including the overexpression of PD-L1.3,4 In turn, the PD-L1 overexpression by tumor cells or by the immune cell compartment is known as a positive predictor of the therapeutic efficacy of immune-checkpoint blockade in cancer patients.5
Hypercholesterolemia stimulates the proliferation of hematopoietic precursor cells in the bone marrow and gives rise to peripheral MDSCs through a mechanism known as “emergency granulo-monocytopoiesis.”4,6,7 This could render the hypercholesterolemic patient more prone to have high circulating MDSCs availability. Generally, MDSCs and tumor-associated macrophages (TAMs) are enriched in the TME, given the ability of the tumor itself to secreting cytokines that increase MDSCs and TAMs mobilization and infiltration within the tumor mass.4 During emergency granulo-monocytopoiesis, terminal differentiation and M2 polarization of TAMs occur, sustaining the immune-suppression mediated by the tumor itself.4,8 M2 macrophages accumulate in the TME and diminish T cell anti-tumor immune responses.9,10
In a previous research, we hypothesized that hypercholesterolemia, as a low-grade inflammatory condition, may facilitate the proliferation and the migration of TAMs and MDSCs to the TME.11 Although TAMs and MDSCs have immunosuppressive activities, based on our data of improved ICI efficacy in hypercholesterolemic patients, we speculated that the tumor infiltration with MDSCs overexpressing PD-L1 could render ICIs more effective.11 Nevertheless, until today, the presence of MDSCs and TAMs has been associated with the poor outcome with ICIs therapy, 8 because they release many suppressive factors. A major limitation of the current evidence is constituted by the fact that the two specific compartments (peripheral and tumor) have not been investigated separately with respect to MDSC presence and function. At this moment, clear phenotypic characterization of MDSC in human (and mouse) tissues by immunohistochemistry is lacking, thus preventing the clarification of the true impact of these cells in the TME when compared to their circulating counterpart.6,7
The crossroads between immunity and cholesterol is probably more complex, considering the role of dendritic cells (DCs) in decreasing the cholesterol plasma levels, preventing the atherosclerotic plaque progression even in the case of a proatherogenic signature.12 In addition, cholesterol is the generator of cholecalciferol (vitamin D3), which is produced from cholesterol precursors in the skin and activated to calcitriol (1,25[OH]2D) through hydroxylation in the liver and kidney. The immunological function of calcitriol and its receptor (vitamin D receptor, VDR) is crucial for T-cell differentiation and its effector function, providing a further wire passing from cholesterol to vitamin D and possibly contributes to anticancer immunotherapy efficacy.13 Finally, the literature about cholesterol seems consistent with respect to cell-mediated immunity: the enhancement of T-cell activation has been associated with proatherogenic effect in vivo, suggesting that hypercholesterolemia could be the epiphenomenon of an easily triggerable T-cell compartment, predicting the likeliness of immune response to checkpoint blockade.12,14 In turn, the inhibition of cholesterol metabolism and esterification in T-cells is able of potentiating the efficiency of the CD8+cell mediated antitumor response in mice.15 Avasimibe, an inhibitor of the cholesterol esterification enzyme acetyl-CoA acetyltransferase (ACAT) 1, was developed, demonstrating to obtain enhanced proliferation as well as effector function of CD8+T cells.15 Basing on these evidences, an increased level of blood cholesterol, if interpreted as the result of the inefficiency of its metabolism, may even represent the signal of a causative event contributing to T-cell responses and activation against the tumor.
The cruciality of the TME has been highlighted in several recent research, being able of rendering the tumor “hot” or “cold” basing on its composition, and suggesting that its modulation could improve the sensitivity to immunotherapy. Some procedures with the objective of lighting up the fire in cold tumors, making them more sensitive to ICI immunotherapy, have been recently investigated.16,17
Nevertheless, in the modern era of cancer immunotherapy, the researcher in oncology should have acquired the concept of targeting the patient instead of prosecuting to targeting the tumor.18 With this standpoint, the reasons for the crosstalk between the response to ICI and the metabolic status of the individuals should be searched even more in the individual himself than in his tumor, as the direct target of the immune-checkpoint blockade is no longer the tumor cell but the T-effector cell. Along this line, the TME modifications must be interpreted merely as the secondary consequence of a systemic reaction provided by the host, dependent on his immune system activation or suppression.
Immunotherapy recently revolutionized the therapeutic approach to solid tumors from a cytotoxic and immunosuppressive perspective (e.g., chemotherapy) to an immune-restoring attempt, rely on the normalization of the immunological functions. Indeed, the immune-checkpoint inhibition limits its effects in removing a block, allowing the recovery of the normal operation of the immune system, more than enhancing its activation.19 For this reason, the efficacy of ICI on the tumor may be based on two simple premises: 1) the physiological immune function has been blocked, and 2) the tumor progression (mainly) depends on this block. Otherwise, ICI immunotherapy could not make the big difference that has been reached in terms of the overall survival of patients when compared to the old standard treatments.20–28 As a consequence, systemic metabolic conditions such as high blood cholesterol, obesity, hyperglycemia, and diabetes mellitus, atherosclerosis and hypertension may represent the epiphenomena of an “inflamed patient,” no longer able of physiological functions required to prevent such events (Figure 1). Moreover, after the establishment of such conditions, the inflamed patient, despite being rich in cytokines and pro-inflammatory mediators (both in the innate and adaptive compartments), is even less immunocompetent, as suggested by the M2 markers upregulation happening in the adipose tissue macrophages in a subcutaneous depot in the case of obesity.29 Despite the apparent hyperactivation of immunity and the putative pro-inflammatory activation attributed to obesity, the immune mechanism at the cell level has been clearly shown to be defective in obese individuals, sometimes also with protective feedbacks. For example, the obese visceral adipose tissue does not generate a more inflammatory environment compared with the lean tissue in mouse models, and a M1-to-M2 shift seems happening in the atherosclerotic plaque, promoting its regression.14,29
Figure 1.
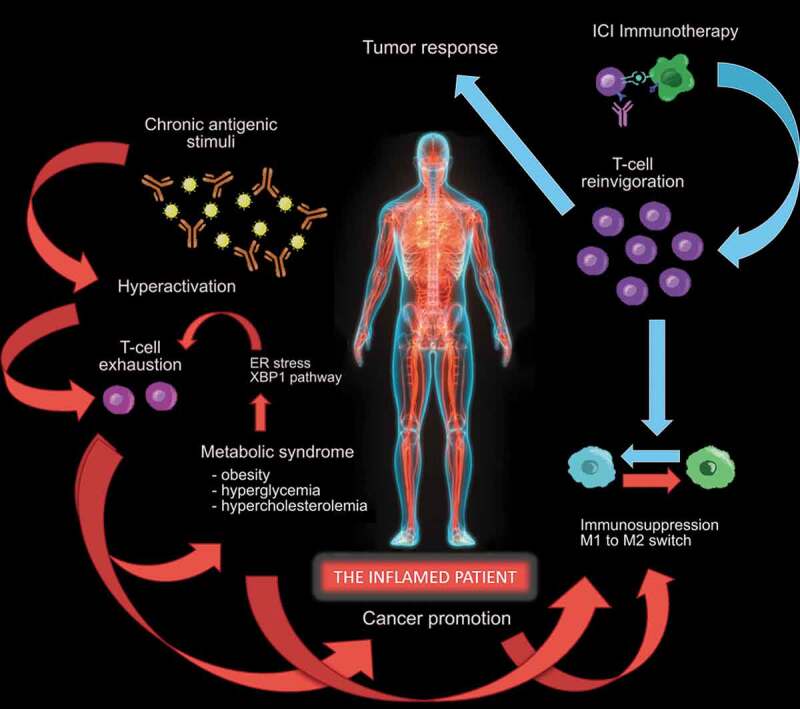
The “inflamed patient” is an individual whose immune system is dysfunctional, due to the exhaustion of T cells, that initially acquired effector functions, undergoing persistent antigen exposure. T-cell exhaustion is associated with inefficient control of inflammation and carcinogenesis, mediated by sustained upregulation of multiple inhibitory receptors (including PD-1), constituting the basis for promotion of cancer and metabolic syndrome, representing the epiphenomena of an inflamed patient. The manifestation of the metabolic syndrome includes obesity, hyperglycemia and hypercholesterolemia, the latter in turn favoring the CD8 + T-cell exhaustion in an endoplasmic-reticulum (ER)-stress-XBP1-dependent manner in the tumor microenvironment (TME). These phenomena trigger a potential vicious circle, rendering the patient even more “inflamed”, and favoring the M1 to M2 shift both in peripheral tissues and in the TME, furtherly diminishing T cell anti-tumor immune responses. The reversion of the initiating event, represented by T-cell exhaustion, can be reached with immune-checkpoint inhibitor (ICI) therapy, blocking the PD-1/PD-L1 axis and obtaining T-cell reinvigoration. This favors the recovery of the immune homeostasis, shifting the balance from immune-suppression to immune-functionality and promoting the T-cell response against the tumor
The T-cell exhaustion found in the case of cancer, may have characterized the patient even before the carcinogenesis, potentially being responsible for both the promotion of cancer and other co-existing conditions, such as the metabolic syndrome. Considering this, the metabolic dysfunction could represent merely “a biomarker” of the patient who satisfies both the two premises reported above.
The opportunity of reversing the immunological energy should be exploited not only at the tumor level but above all at the patient level, modulating systemic conditions in favor of an immune normalization. If on a hand generating even more inflammation to light up the fire in the case of energy can work,16,17 on the other hand, it could be a wrong strategy in the case of exhaustion, mainly derived from previous (aberrant) hyperactivation. Exhausted T cells (TEX) arise from cells that initially acquired effector functions, but then become dysfunctional due to chronic antigenic stimuli. The hope is in the concept that TEX is not terminally dysfunctional, but they could be reinvigorated: it is now well established that PD-1/PD-L1 pathway blockade can at least partially reverse the exhausted condition and improve immunity in cancer patients.30
Of note, the lack of these “metabolic biomarkers” does not exclude the presence of the first premise, not always derived from exhaustion but often generated by T-cell anergy, a hypo-responsiveness state where T cells fail to acquire effector functions.
In the case of the metabolic syndrome, the exhaustion means that T-cells were previously able of effector function and that their exhaustion can have, in part, contributed to the immune-dependent progression of the tumor, consequently marking a patient more likely to benefit from the T-cell reinvigoration given by immune-checkpoint blockade.
Obesity is another disorder included in the metabolic syndrome. Recent studies have demonstrated that overweight cancer patients treated with ICIs had prolonged overall survival and progression-free survival compared to those of non-overweight patients.31–33 We previously interpreted such correlation as possibly due to the capacity of white adipose tissue to modulate the immune response.33 It is also well known that obesity is associated with an increased risk of developing tumors, such as clear cell renal-cell carcinoma, but it is paradoxically associated with improved oncological outcomes in the same cancer type.34–36 Transcriptomic differences have been found in the primary tumor and peritumoral adipose tissue depending on body-mass index (BMI), possibly contributing to the apparent survival advantage in obese patients with clear cell RCC compared with patients at a normal weight.37 The peritumoral adipose tissue microenvironment might have clinical relevance, constituting the peripheral side of the TME, in continuous crosstalk with the systemic immunity.
Even immune-related adverse events (irAEs) have been significantly correlated with obesity (irrespective of the treatment dose of the ICI, frequently weight-based in the first ICI regimens), confirming their nature as the upside-down of efficacy of immunotherapy.38–40 Despite the hypothesis of a tumor role in the pathogenesis of irAEs, mediated by the cross-reactivity between tumor- and self-antigens,41,42 we postulate the likeliness of a systemic etiopathogenesis, closely related to the host and to his immune system more than to the tumor itself. In support of this version, we previously showed how individuals with prior autoimmune disorders are more prone to develop irAEs, even outside the affected organ.43,44
Despite intriguing potential interpretations in favor of a possible causality relationship between metabolic conditions and ICI response, a simple statistical correlation between independent variables, subtended by the same immunological background, is honestly more plausible.
Notwithstanding, the likely “collateral nature” of metabolic epiphenomena should not discourage attempts to modulating these factors to synergize with immunotherapy. It has already been suggested that modulating cholesterol may be an effective strategy to improve the antitumor efficacy of ICIs.15,45 According to our data on a retrospective population, the use of statins was positively related to the outcome of advanced cancer patients to ICI at the multivariate analysis.11 Moreover, statins have been reported to reduce T-cell exhaustion in patients with HIV-1 infection.46 Such suggestions from preclinical and translational researches should be transferred in the clinical setting, firstly investigating patient populations enrolled in prospective-randomized trials, implemented with observational endpoints such as the effect of concomitant drug medications and the impact of blood cholesterol levels and other metabolic conditions on the outcome to ICI treatment, when compared to the control arm with standard therapy, in order to confirm their predictiveness aside from their value in prognostication.
Disclosure of potential conlficts of interest
Melissa Bersanelli received funding for research (institutional) by Seqirus, Roche S.p.A., Pfizer and Novartis and honoraria as a speaker at scientific events (personal fees) by Astra Zeneca, Bristol-Myers Squibb (BMS), Novartis and Pfizer; as a consultant for the advisory role (personal fees) by Novartis, BMS and Pfizer. Sebastiano Buti received honoraria as a speaker at scientific events and advisory role by BMS, Pfizer; MSD, Ipsen, Roche S.p.A., Eli-Lilly, AstraZeneca and Novartis; he also received research funding from Novartis. Alessio Cortellini reports grants from AstraZeneca, grants from MSD, grants from BMS, grants from Roche, grants from Novartis, outside the submitted work.
References
- 1.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–37. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H-Y, Wu C-Y, Powell JD, Lu K-L. Manipulation of metabolic pathways and its consequences for anti-tumor immunity: a clinical perspective. Int J Mol Sci. 2020. June 4;21(11):4030. doi: 10.3390/ijms21114030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7(1):12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safari E, Ghorghanlu S, Ahmadi-Khiavi H, Mehranfar S, Rezaei R, Motallebnezhad M. Myeloid-derived suppressor cells and tumor: current knowledge and future perspectives. J Cell Physiol. 2019;234(7):9966–81. doi: 10.1002/jcp.27923. [DOI] [PubMed] [Google Scholar]
- 5.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015. April;14(4):847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 6.Porta C, Marino A, Consonni FM, Bleve A, Mola S, Storto M, Riboldi E, Sica A. Metabolic influence on the differentiation of suppressive myeloid cells in cancer. Carcinogenesis. 2018;39(9):1095–104. doi: 10.1093/carcin/bgy088. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Strauss L. Energy metabolism drives myeloid-derived suppressor cell differentiation and functions in pathology. J Leukoc Biol. 2017;102(2):325–34. doi: 10.1189/jlb.4MR1116-476R. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–89. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 11.Perrone F, Minari R, Bersanelli M, Bordi P, Tiseo M, Favari E, Sabato R, Buti S. The prognostic role of high blood cholesterol in advanced cancer patients treated with immune checkpoint inhibitors. J Immunother. 2020. Jul/Aug;43(6):196–203. doi: 10.1097/CJI.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 12.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009. May 5;119(17):2367–75. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 13.Bersanelli M, Leonetti A, Buti S. The link between calcitriol and anticancer immunotherapy: vitamin D as the possible balance between inflammation and autoimmunity in the immune-checkpoint blockade. Immunotherapy. 2017. November;9(14):1127–31. [DOI] [PubMed] [Google Scholar]
- 14.Poels K, van Leent MMT, Reiche ME, Kusters PJH, Huveneers S, de Winther MPJ, Mulder WJM, Lutgens E, Seijkens TTP. Antibody-mediated inhibition of CTLA4 aggravates atherosclerotic plaque inflammation and progression in hyperlipidemic mice. Cells. 2020. August 29;9(9):E1987. doi: 10.3390/cells9091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C. Potentiating the antitumor response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–55. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Z, Liu H, Xue Y, Lin J, Fu Y, Xia Z, Pan D, Zhang J, Qiao K, Zhang Z, et al. Reversing cold tumors to hot: an immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy. Bioact Mater. 2020. August 28;6(2):312–25. doi: 10.1016/j.bioactmat.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janji B, Hasmim M, Parpal S, De Milito A, Berchem G, Noman MZ. Lighting up the fire in cold tumors to improve cancer immunotherapy by blocking the activity of the autophagy-related protein PIK3C3/VPS34. Autophagy. 2020. September;5:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol. 2017. February 10;8(1):37–53. doi: 10.5306/wjco.v8.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanmamed MF, Chen L, Paradigm A. Shift in cancer immunotherapy: from enhancement to normalization. Cell. 2019. January 24;176(3):677. doi: 10.1016/j.cell.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM. Prolonged survival in stage iii melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016. November 10;375(19):1845–55. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015. October 22;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015. July 9;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018. April 5;378(14):1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017. October 5;377(14):1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016. November 10;375(19):1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 26.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016. November 10;375(19):1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015. November 5;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020. September 24;383(13):1218–30. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 29.Giròn-Ulloa A, Gonzàlez-Domìnguez E, Klimek RS, et al. Specific macrophage subsets accumulate in human subcutaneous and omental fat depots during obesity. Immunol Cell Biol. 2020. July 22. doi: 10.1111/imcb.12380. [DOI] [PubMed] [Google Scholar]
- 30.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015. April;36(4):265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young AC, Quach HT, Song H, Davis EJ, Moslehi JJ, Ye F, Williams GR, Johnson DB. Impact of body composition on outcomes from anti-PD1 ± anti-CTLA-4 treatment in melanoma. J Immunother Cancer. 2020. July;8(2):e000821. doi: 10.1136/jitc-2020-000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018. March;19(3):310–22. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, et al.. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–98. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami H-O, Lee JE, Lee HM. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132(3):625–34. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 36.Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, Mikklineni N, Fiegoli B, Kim PH, Voss MH. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105(24):1862–70. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez A, Furberg H, Kuo F, Vuong, L, Ged Y, Patil S, Ostrovnaya I, Petruzella S, Reising A, Patel P, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2019. December 20. published online. doi: 10.1016/S1470-2045(19)30797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M, Zoratto F, et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur J Cancer. 2020. March;128:17–26. [DOI] [PubMed] [Google Scholar]
- 39.Centanni M, Moes DJAR, Troco´niz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58(7):835e57. doi: 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018. 10:1758835918764628. doi: 10.1177/1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bomze D, Hasan Ali O, Bate A, Flatz L. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden [published online ahead of print, 2019 Aug 22]. JAMA Oncol. 2019;5(11):1633–35. doi: 10.1001/jamaoncol.2019.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer [published correction appears in JAMA Oncol. 2019 Jul 1;5(7):1070]. JAMA Oncol. 2019;5(7):1043–47. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, Bersanelli M, Michiara M, Grassadonia A, Brocco D. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist. 2019. June;24(6):e327–e337. doi: 10.1634/theoncologist.2018-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortellini A, Buti S, Agostinelli V, Bersanelli M. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019. Aug-Oct;46(4–5):362–71. Epub 2019 Nov 7. PMID: 31727344. doi: 10.1053/j.seminoncol.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Xingzhe M, Enguang B, Yong L, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J. Cholesterol induces CD8 + T cell exhaustion in the tumor microenvironment. Cell Metab. 2019. July 2;30(1):143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS. 2016. January;30(2):171–83. doi: 10.1097/QAD.0000000000000917. [DOI] [PubMed] [Google Scholar]


