How does membrane voltage control cellular proliferation? This is a key but poorly understood step in understanding how dysregulation of the electrical balance in a cell can lead to uncontrolled proliferation and, eventually, to tumor development. Although the phenomenon is well established (1–3), the underlying mechanisms have been unclear. On page 873 of this issue, Zhou et al. (4) show that persistent changes in the resting membrane potential (the voltage across the membrane of a cell), caused by the uncontrolled expression of ion channels, can cause negatively charged lipid in the inner membrane leaflet to cluster and attract the signaling protein K-Ras, enhancing its ability to promote cell proliferation.
Ion channels control the rapid movement of ions across cellular membranes (5). They are best known as the gatekeepers of excitatory cellular processes such as neuronal firing, muscle contraction, and heartbeat. They also control ion homeostasis and set the membrane potential of all resting or non-excitable cells. Less well understood is their role in regulating cell division (mitosis). It has been known for nearly 40 years that cells undergoing mitosis are more depolarized—less negatively charged on the inside—than their quiescent counterparts (6–8) and that, as cells transition through the different states of the cell division cycle, their membrane voltage changes (9) in concert with the expression of many different ion channels (3). Furthermore, overexpression of K+, Na+, Ca2+, and Cl— channels has been observed in numerous tumors (2), and their pharmacological inhibition can restore normal proliferative behavior (2, 10).
In addition to their role in tumorigenesis, ion channels are also involved in other aspects of cancer biology such as cell adhesion, cell volume regulation, programmed cell death (apoptosis), and angiogenesis (1, 8). It is therefore not surprising that ion channels are viewed as highly promising targets for cancer treatment, especially given the great variety of readily available compounds that specifically target various channel types and can serve as scaffolds for the development of anticancer drugs (10). However, efforts in these directions have been stymied by a poor understanding of the cellular signaling pathways that are affected by channel overexpression and by the ensuing alterations in the resting membrane potential.
Zhou et al. investigated whether changes in membrane potential might alter the lateral spatiotemporal distribution of charged lipids in the membrane, which in turn might affect the distribution of membrane-bound signaling proteins, such as the Ras family of guanosine triphosphatases (GTPases). The human genes encoding H-, N-, and K-Ras are among the most commonly occurring mutated oncogenes. Mutations that constitutively activate K-Ras are found in nearly 25% of all human tumors (11). Positively charged residues in the C termini of Ras proteins interact with negatively charged lipids that sequester these proteins into spatially localized assemblies called nanoclusters. Such aggregation is essential for K-Ras-induced activation of the RAF-mitogen-activated protein kinase (MAPK) cascade (12).
Using an elegant combination of electron microscopy, electrophysiological recordings, and fluorescence imaging, Zhou et al. show that membrane depolarization specifically and reversibly promotes clustering of two types of negatively charged lipids, phosphatidylserine and phosphatidylinositol 4,5-bisphosphate (PIP2). Upon depolarization, nanoclustering of phosphatidylserine and K-Ras increased with closely matching spatiotemporal and voltage dependencies. This activated the RAF-MAPK cascade, thereby promoting cell proliferation (see the figure). By contrast, hyperpolarizing potentials had the opposite effect of reducing phosphatidylserine and K-Ras clustering and decreasing RAF-MAPK signaling.
Potential and proliferation.
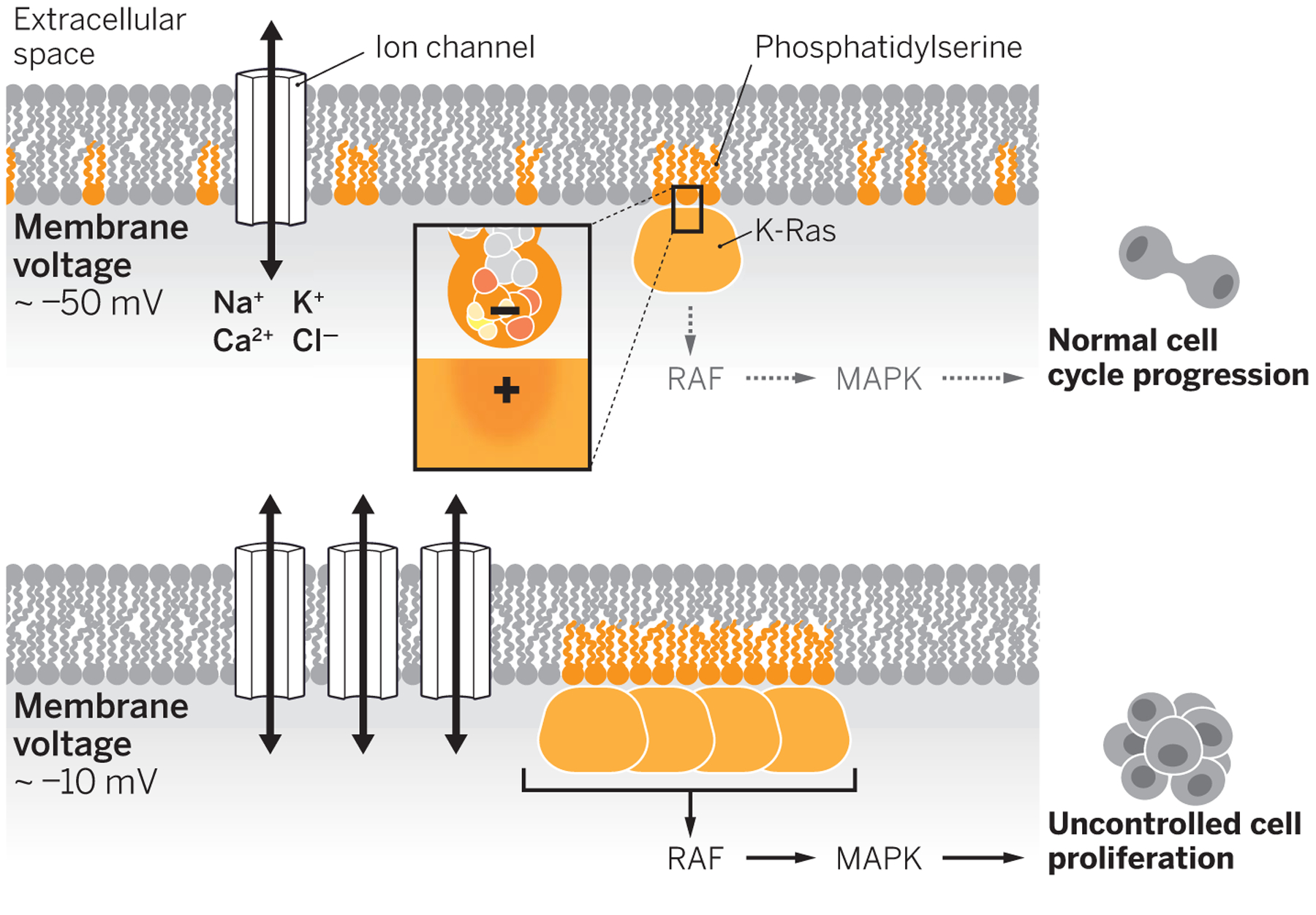
In a normal, nonproliferating cell, the resting membrane potential (Vm ≈ −50 mV) is set by ion channel activity. Phosphatidylserine lipids are in small clusters that localize with K-Ras, which leads to low activation of the RAF-MAPK pathway. Channel overexpression depolarizes the cell (Vm ≈ −10 mV), increasing the clustering of phosphatidylserine and K-Ras. This promotes RAF-MAPK signaling uncontrolled cell proliferation.
The study provides a long-awaited mechanism by which membrane voltage directly affects the cell division cycle, a breakthrough that should pave the way for developing strategies that silence oncogenic pathways. It also, perhaps, offers an accessible tuning knob—in the form of ion channel activity—that can be manipulated to influence cell signaling. Interestingly, the observed effects are highly specific in that only the distribution of phosphatidylserine and PIP2 is affected by membrane voltage, whereas that of other anionic lipids, such as phosphatidic acid or phosphatidylinositol 3,4,5-trisphosphate (PIP3), are unaffected. Similarly, K-Ras, unlike H- or N-Ras, responds to changes in phosphatidylserine distribution. The high selectivity of these effects among different Ras isoforms and similarly charged lipids could allow for the development of specifically targeted therapies. However, the molecular bases underlying the specificity of these effects remain unclear, and other unidentified proteins might be involved in these processes.
The results of Zhou et al. also raise the question of how the depolarization-induced lipid rearrangements affect signaling in excitable cells (neurons) that undergo repeated depolarization. It is possible that the millisecond time scale of an action potential is too fast to induce substantial lateral rearrangement of anionic lipids; the fastest rearrangement detected by Zhou et al. (using electron microscopy) in nonexcitable cells is 30 s, nearly four orders of magnitude slower than observed in excitable “firing” cells (those exhibiting bursts of repetitive and rapid changes in electrical potential). However, it may be that under particular conditions, such as during firing, there may not be enough time for lipids to reorganize back to a state that restores membrane potential to its baseline level. Over time, these small increments in clustering could accumulate, leading to a progressive activation of the MAPK pathway. If this were the case, it could represent a novel mechanism by which long-lasting effects of repeated firing could occur in excitable cells. ■
REFERENCES
- 1.Pardo LA, Stühmer W, Nat. Rev. Cancer 14, 39 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Rao VR et al. , Cancers 7, 849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litan A, Langhans SA, Front. Cell. Neurosci 10.3389/fncel.2015.00086 (2015). [DOI] [Google Scholar]
- 4.Zhou Y et al. , Science 349, 873 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hille B, Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA, ed. 3, 2001). [Google Scholar]
- 6.Cone CDJ Jr., Ann. N. Y. Acad. Sci 238, 420 (1974). [DOI] [PubMed] [Google Scholar]
- 7.Blackiston DJ et al. , Cell Cycle 8, 3527 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA, Philos. Trans. R. Soc. B 369, 20130094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stillwell EF et al. , Nat. New Biol 246, 110 (1973). [DOI] [PubMed] [Google Scholar]
- 10.Wulff H et al. , Nat. Rev. Drug Discov 8, 982 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downward J, Nat. Rev. Cancer 3, 11 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Tian T et al. , Nat. Cell Biol 9, 905 (2007). [DOI] [PubMed] [Google Scholar]


