Abstract
Objective:
Because the treatment of intermittent claudication (IC) is elective, good short- and long-term outcomes are imperative. The objective of the present study was to examine the outcomes of endovascular management of IC reported in the Vascular Quality Initiative and compare them with the Society for Vascular Surgery guidelines for IC treatment to determine whether real-world results are within the guidelines.
Methods:
Patients undergoing peripheral vascular intervention for IC from 2004 to 2017 with complete data and >9 month follow-up were included. The primary outcome measures were IC recurrence and repeat procedures performed ≤2 years after the initial treatment.
Results:
A total of 16,152 patients met the inclusion criteria, with a mean age of 66 years. Of the 16,152 patients, 61% were men, 45% were current smokers, and 28% had been discharged without antiplatelet or statin medication. Adjusted analyses revealed that treatment of more than two arteries was associated with a shorter time to IC recurrence (hazard ratio [HR], 1.19; 95% confidence interval [CI], 1.09–1.31) and a shorter time to repeat procedures (HR, 1.25; 95% CI, 1.09–1.45). The use of atherectomy was also associated with a shorter time to IC recurrence (HR, 1.29; 95% CI, 1.08–1.33) and a shorter time to repeat procedures (HR, 1.31; 95% CI, 1.13–1.52). Discharge with antiplatelet and statin medications was associated with a longer time to IC recurrence (HR, 0.84; 95% CI, 0.78–0.91) and a longer time to repeat procedures (HR, 0.77; 95% CI, 0.69–0.87). Life-table analysis at 2 years revealed that only 32% of patients were free from IC recurrence, although 76% had not undergone repeat procedures. Stratified by anatomic treatment level, 37% of isolated aortoiliac interventions, 22% of aortoiliac and femoropopliteal interventions, 30% of isolated femoropopliteal interventions, and 20% of femoropopliteal and tibial interventions had remained free from IC recurrence at 2 years.
Conclusions:
Most patients treated with an endovascular approach to IC did not meet the Society for Vascular Surgery guidelines for long-term freedom from recurrent symptoms of >50% at 2 years. Many lacked preprocedure optimization of medical management. The use of atherectomy and treatment of more than two arteries were associated with poor outcomes after peripheral vascular intervention for IC, because only 32% of these patients were free from recurrent symptoms at 2 years. Even when risk factor modification is optimized before the procedure, vascular specialists should be aware of the association between atherectomy and multivessel interventions with poorer long-term outcomes and counsel patients appropriately before intervention. (J Vasc Surg 2021;73:1693-700.)
Keywords: Claudication, Peripheral arterial disease, Endovascular, Peripheral vascular interventions
Peripheral arterial disease (PAD) affects 8 to 12 million patients in the United States.1 The most common symptom of PAD is intermittent claudication (IC),2 which has been defined in the Society for Vascular Surgery (SVS) practice guidelines for atherosclerotic occlusive disease of the lower extremities as “reproducible discomfort in a specific muscle group that is induced by exercise and then relieved with rest.”3 Patients with IC can present with a wide range of symptoms and varying degrees of limitations in daily function.4 The current data have suggested that the management of IC should be individualized, with risk factor modification, including smoking cessation,5 medical therapy,6 and a supervised exercise program,7 before consideration of invasive treatment.8
IC will progress to rest pain, tissue loss, and amputation in <5% of patients.9,10 Thus, any invasive treatment for this lifestyle-limiting condition should primarily be considered for patients with disabling symptoms who have complied with medical management and for whom the potential benefits of a procedure outweigh the risks.
The SVS practice guidelines have recommended that any treatment modality offered for IC should provide a reasonable likelihood of a sustained benefit to the patient (>50% likelihood of clinical efficacy for ≥2 years).8 Given these recommendations, we analyzed the real-world practice patterns and outcomes of endovascular procedures to treat IC in the Vascular Quality Initiative (VQI) to determine whether they met the SVS IC guidelines.
METHODS
Dataset.
The VQI peripheral vascular intervention (PVI) module was queried for all unique patients who had undergone endovascular interventions for the indication of IC from 2004 to 2017. The VQI is a national quality improvement registry designed to improve the quality, safety, effectiveness, and cost of vascular healthcare (available at: www.vascularqualityinitiative.org/). All data are self-reported by the participating institutions. The VQI requires at least one entry of follow-up data between 9 and 21 months after a procedure. The primary outcome measures were IC recurrence in the ipsilateral limb (assessed with a yes vs no data entry in the VQI PVI follow-up module) and the performance of repeat procedures on the ipsilateral limb during follow-up.
Patient cohort and variables.
Patients were excluded from the analysis if they had undergone an emergency procedure or had had an indication for intervention other than IC. Only patients with complete data and follow-up data for >9 months were included. Patients with incomplete data were omitted from the analysis. Patient demographics, comorbidities, medications, and procedure details were recorded according to the VQI data definitions.
Statistical analysis.
Cox proportional hazards models were used to examine the factors independently associated with time to IC recurrence and repeat procedures and account for the different follow-up periods among the patients. We selected covariates for inclusion in the multivariable models that were significant on univariate analysis and clinically relevant to the outcomes examined. Factors that correlated highly with each other were not included in the same model to preserve model stability. Thus, age, sex, race, and comorbidities were used as covariates in all models. Smoking, the number of arteries treated, use of angioplasty, stenting, and/or atherectomy (Appendix; Supplementary Table I, online only), and discharge antiplatelet agents and statins were included as covariates that might be associated with the outcomes. Kaplan-Meier survival curves were used to assess the freedom from IC recurrence and time to repeat procedure (Supplementary Figs 1 and 2, online only). In the case of patients who had not reached the endpoint of IC recurrence or repeat procedure, they were censored at the last entry.
To evaluate the effect of the different lengths of patient follow-up on the overall results, we performed a sensitivity analysis (Supplementary Tables II to IV, online only) by repeating all analyses with the inclusion of only those centers that had reported follow-up data for ≥9 months for ≥70% of patients treated. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Demographics
A total of 54,849 unique patients who had undergone an index limb-first endovascular intervention for the indication of IC were identified. Of these patients, 29,068 were excluded because of previous aneurysm repair, previous bypass, endarterectomy, or PVI, previous open or endovascular inflow treatment, or previous open or endovascular infrainguinal treatment. After these exclusions, 25,781 patients were available for inclusion, of whom 16,152 patients had long-term follow-up data. Of this final cohort, 79% of these patients had had complete follow-up data entered at 18 months. The lower extremity treated during the index operation was considered the ipsilateral limb, and any contralateral limb interventions were not captured on the follow-up form and were excluded.
Most patients were men (61%) and white (85%). Preoperatively, 60.4% of the patients were taking antiplatelet agents and a statin. Regarding preoperative statin use, 2% of the patients were reported to not be taking a statin medication for “medical reasons.” In addition, 28% of the patients were discharged without an antiplatelet or a statin agent, and 45% of the patients were current smokers at the time of intervention (Table I). The mean follow-up for the overall cohort was 11.4 months (range, 3–65 months). During the study period, 6.2% of the patients had progressed to tissue loss, with 2.7% of the patients progressing to rest pain. Balloon angioplasty was used as the sole treatment modality in 16.7% of patients, stenting only in 17.9%, atherectomy alone in 0.49%, with the most frequent pattern being balloon angioplasty and stenting in 52.8% of the cohort (Supplementary Table I, online only). Atherectomy was used in 12% of the cohort (2021 patients), with the following anatomic distribution. Of the 2021 patients, 59 (2.9%) had undergone aortoiliac atherectomy, 1435 (71%) had undergone femoropopliteal atherectomy only, 258 (12.8%) had undergone aortoiliac and femoropopliteal atherectomy, 189 (9.4%) had undergone femoropopliteal and tibial atherectomy, and 67 patients (3.32%) had undergone tibial atherectomy only.
Table I.
Demographics and preoperative comorbidities
| Variable | Mean ± SD or No. (%) |
|---|---|
| Age, years | 66 ± 10.4 |
| Gender | |
| Male | 9852 (61) |
| Female | 6299 (39) |
| Race | |
| White | 13,729 (85) |
| Black/other | 2422 (15) |
| Coronary artery disease | 4361 (27) |
| Current smoking | 7268 (45) |
| Diabetes | 5976 (37) |
| Discharge without antiplatelet/statin | 4522 (28) |
| Hypertension | 13,567 (84) |
| More than two arteries treated | 2261 (14) |
| Atherectomy | 1938 (12) |
SD, Standard deviation.
Outcomes by multivariable analysis
IC recurrence.
The Kaplan-Meier estimate demonstrated that 32% of patients were free from recurrent symptoms at 2 years (Fig 1). The Kaplan-Meier estimates of freedom from IC (Supplementary Fig 1, online only) at 2 years of follow-up stratified by anatomic level of treatment were as follows: 5595 (43.8%), isolated aortoiliac intervention with 37% freedom from IC; 1110 (8.7%), aortoiliac and femoropopliteal intervention with 22% freedom; 5358 (41.9%), isolated femoropopliteal intervention with 30% freedom; and 554 (4.3%), femoropopliteal and tibial intervention with 20% freedom (Fig 2).
Fig 1.
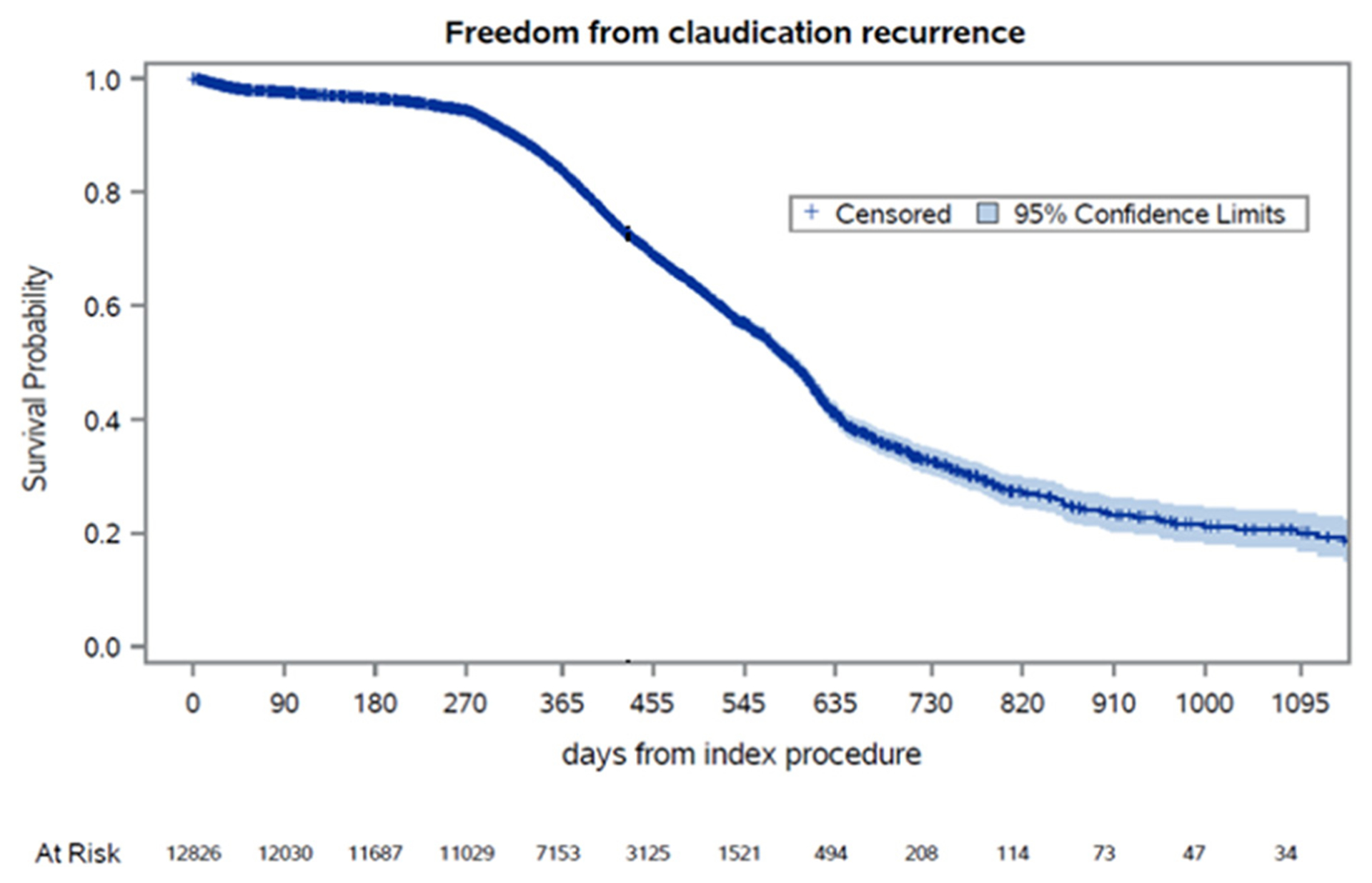
Kaplan-Meier estimates for freedom from intermittent claudication (IC) recurrence over time. Standard error did not exceed 10%.
Fig 2.
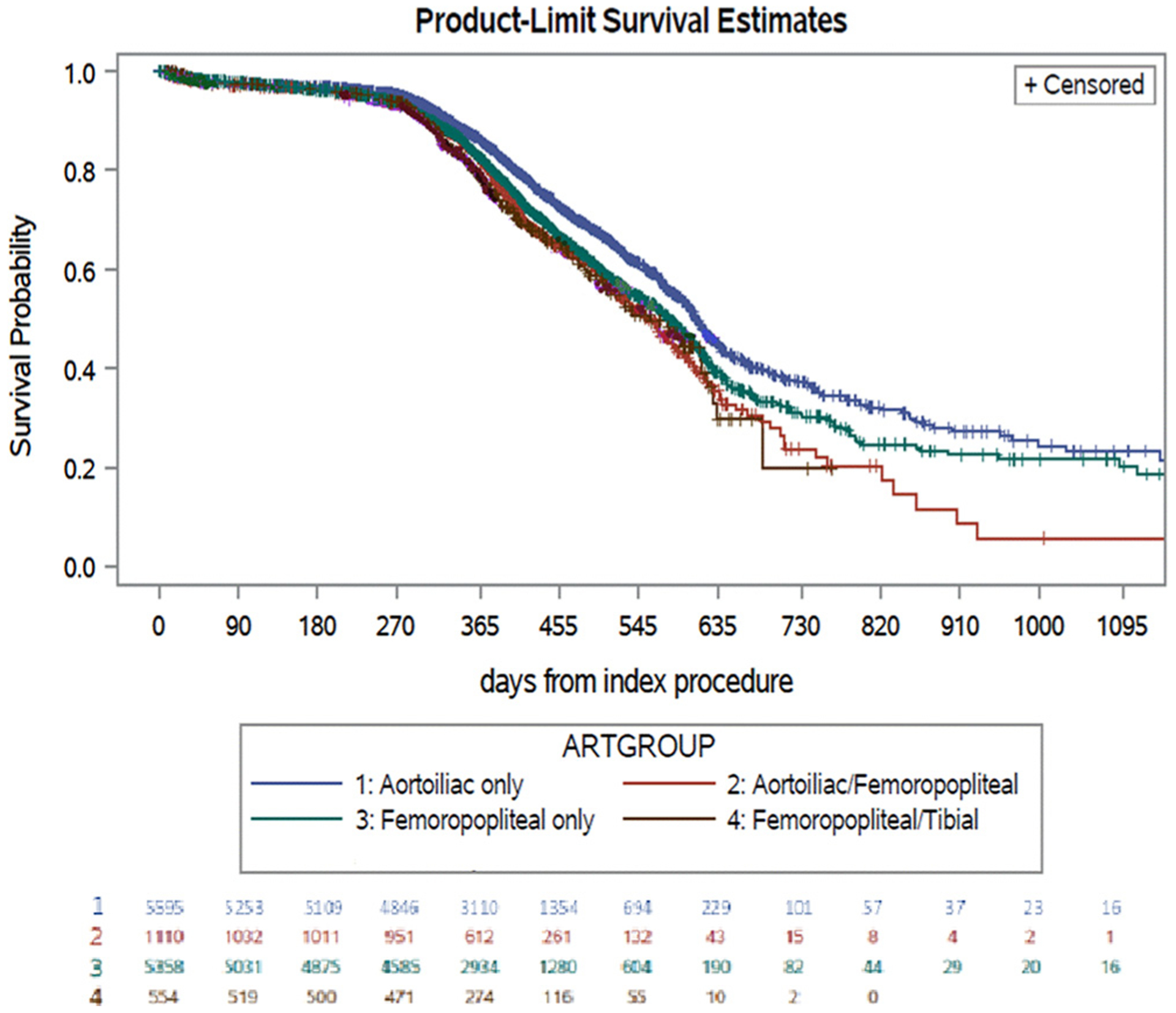
Kaplan-Meier estimates for freedom from intermittent claudication (IC) recurrence over time. Standard error of 10% for each group: aortoiliac only, 1161 days; aortoiliac/femoropopliteal, 718 days; femoropopliteal only, 954 days; femoropopliteal/tibial, 614 days; tibial only, 426 days.
A multivariable model for the time to IC recurrence demonstrated that treatment of more than two arteries (hazard ratio [HR], 1.19; 95% confidence interval [CI], 1.09–1.31) and atherectomy use (HR, 1.29; 95% CI, 1.08–1.33; Table II) were associated with a decreased time to IC recurrence. The use of antiplatelet agents and a statin on discharge was associated with an increased time to IC recurrence (HR, 0.84; 95% CI, 0.78–0.91), as was any use of stenting (HR, 0.82; 95% CI, 0.75–0.88).
Table II.
Adjusted analysis for claudication recurrence
| Survival model results | P value | ||
|---|---|---|---|
| Variable | HR | 95% CI | |
| Smoking | 1.06 | 0.98–1.13 | .15 |
| More than two arteries treated | 1.19 | 1.09–1.31 | .0001 |
| Atherectomy | 1.29 | 1.08–1.33 | .0003 |
| Antiplatelet/statin combined | 0.84 | 0.78–0.91 | .0001 |
| Stenting | 0.82 | 0.75–0.88 | <.0001 |
CI, Confidence interval; HR, hazard ratio.
Repeat procedures.
The Kaplan-Meier estimates demonstrated that 76% of patients treated with endovascular procedures for IC were free from repeat procedures at 2 years (Fig 3). The Kaplan-Meier estimates of freedom from repeat procedures (Supplementary Fig 2, online only) at 2 years of follow-up, stratified by the anatomic level of treatment, were as follows: 5796 (43.7%), isolated aortoiliac intervention with 86% freedom from repeat procedures; 1126 (8.5%), aortoiliac and femoropopliteal intervention with 71% freedom; 5607 (42.3%), isolated femoropopliteal intervention with 67% freedom; 565 (4.3%); and femoropopliteal and tibial intervention with 50% freedom (Fig 4; Supplementary Fig 2, online only). During the postoperative period, three patients had required an ipsilateral major amputation.
Fig 3.
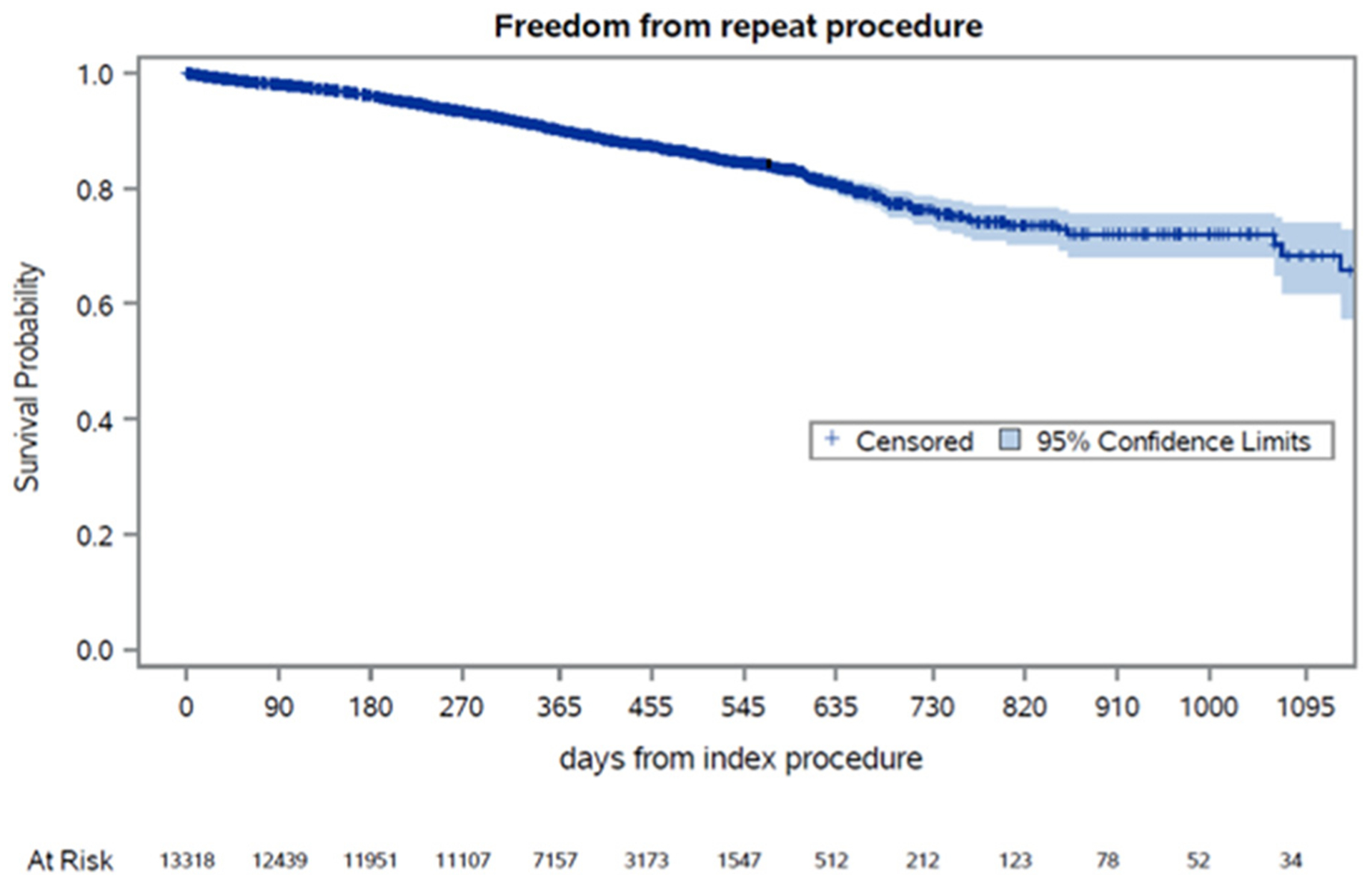
Kaplan-Meier estimates for freedom from repeat procedures over time. Standard error did not exceed 10%.
Fig 4.
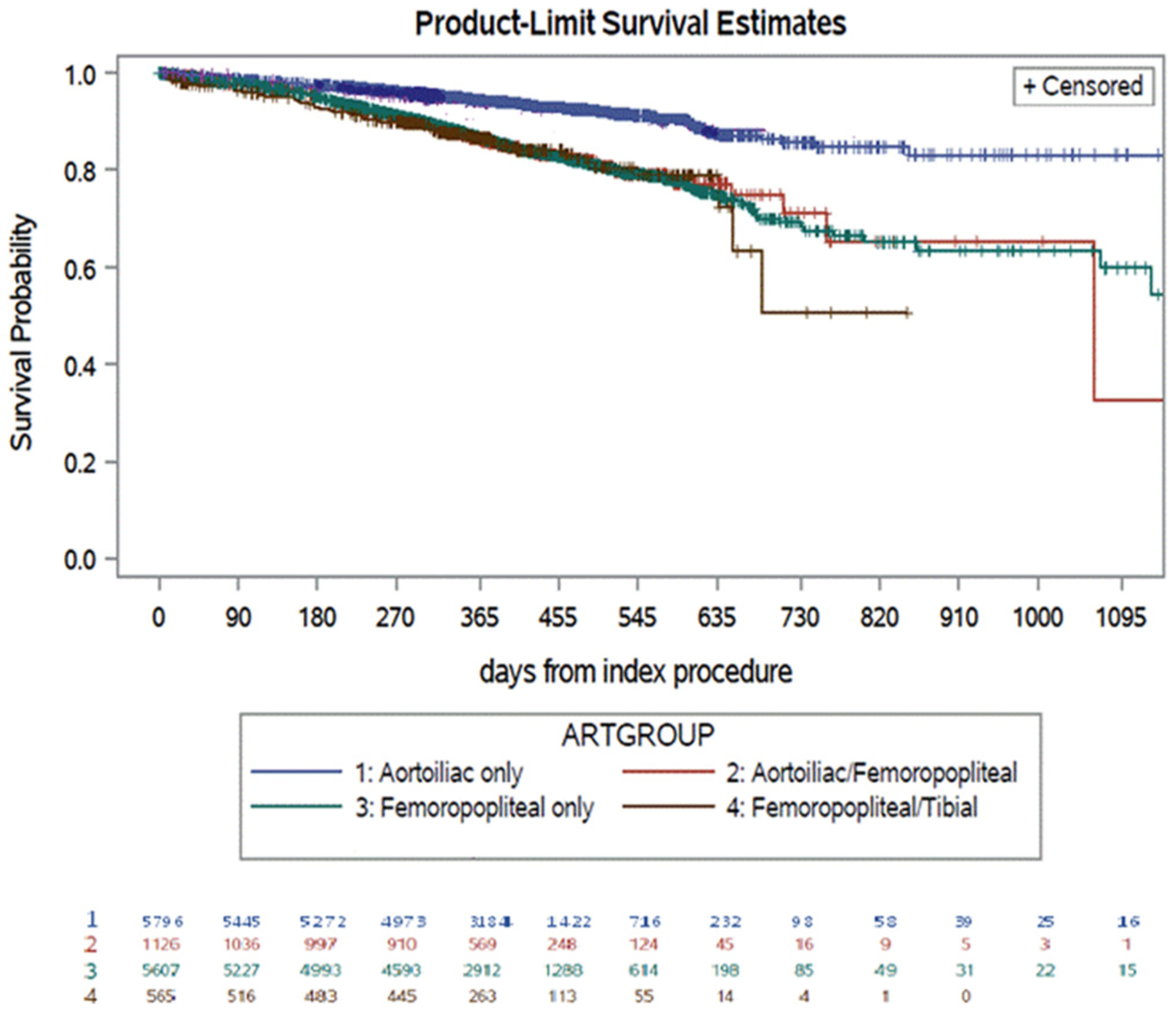
Kaplan-Meier estimates for freedom from repeat procedures over time. Standard error of 10% for each group: aortoiliac only, 1650 days; aortoiliac/femoropopliteal, 1006 days; femoropopliteal only, 1129 days; femoropopliteal/tibial, 650 days; tibial only, 450 days.
Of the repeat procedures, these had occurred most commonly to the same anatomic segment in 40.2%, more proximal arteries in 6.7%, more distal arteries in 3.1%, and both proximal and distal arteries in 0.03%.
On multivariable analysis, the treatment of more than two arteries (HR, 1.25; 95% CI, 1.09–1.45) and atherectomy use (HR, 1.31; 95% CI, 1.13–1.52) were associated with a decreased time to a repeat procedure. The prescription of antiplatelet agents and a statin at discharge was associated with an increased time to a repeat procedure (HR, 0.77; 95% CI, 0.69–0.87), as was the use of any stenting during the index procedure (HR, 0.79; 95% CI, 0.70–0.89; Table III).
Table III.
Adjusted analysis for repeat procedures
| Variable | Survival model results | P value | |
|---|---|---|---|
| HR | 95% CI | ||
| Smoking | 1.05 | 0.93–1.17 | .39 |
| More than two arteries treated | 1.25 | 1.09–1.45 | .001 |
| Atherectomy | 1.31 | 1.13–1.52 | .0003 |
| Antiplatelet/statin combined | 0.77 | 0.69–0.87 | <.0001 |
| Stenting | 0.79 | 0.70–0.89 | .0002 |
CI, Confidence interval; HR, hazard ratio.
Sensitivity analysis (long-term follow-up group)
Restricting the overall dataset to centers with >70% follow-up at ≥9 months identified 4607 patients. The demographics for these 4607 patients were similar to those of the overall group (Supplementary Table II, online only).
Cox proportional hazards modeling demonstrated that treatment of more than two arteries (HR, 1.31; 95% CI, 1.10–1.59; P = .002) led to a reduced time to IC recurrence, with antiplatelet and statin therapy on discharge increasing the time to IC recurrence (HR, 0.84; 95% CI, 0.72–0.97; P = .02). Similarly, the point estimates for smoking and atherectomy use were in a direction and magnitude similar to those for the overall group. The Kaplan-Meier estimates of freedom from IC recurrence at 2 years for the long-term follow-up group, stratified by the anatomic level of treatment, were similar to the estimates for the overall cohort (Supplementary Fig 1, online only).
Cox proportional hazards modeling demonstrated that treatment of more than two arteries was associated with a reduced time to repeat procedures (HR, 1.61; 95% CI, 1.26–2.06; P = .0001), again with similar direction and magnitude of the point estimates for atherectomy use and discharge prescription of antiplatelet agents and statins.
The Kaplan-Meier estimates of freedom from repeat procedures at 2 years for the long-term follow-up group, stratified by anatomic level of treatment, were also similar to the estimates for the overall group.
DISCUSSION
In the present large VQI-based registry study of patients who had undergone PVI for IC, compliance with guideline-based optimal medical management was moderate and symptom recurrence in the treated limb was soberingly high (68% reporting ipsilateral recurrent IC within 2 years of treatment). Medical therapy, level of disease treated, number of arteries treated, and use of atherectomy were all significant predictors of both symptom recurrence and limb reintervention. These data are cause for concern, given the increasing number of interventions for IC and the limited comparative effectiveness evidence existing in the field. Recent data from both the EUCLID trial (a study comparing cardiovascular effects of ticagrelor and clopidogrel in patients with peripheral artery disease)11 and a regional longitudinal cohort study12 have also highlighted the potential down-stream risks of early revascularization for IC in terms of acute limb ischemia and major amputation. These contemporary VQI results suggest significant opportunities for improvement in preprocedural care, patient selection, and durability of the chosen intervention for IC.
The findings from the present study support the importance of medical management of IC before and after intervention. Good evidence has shown that antiplatelet agents and statin medications improve the outcomes from a primary and secondary stroke, myocardial infarction, and death standpoint for patients with atherosclerotic disease.13–18 These same medications have also been shown to have beneficial effects on patency after peripheral interventions.19–21 Despite the well-recognized benefits, 28% of patients undergoing PVIs for IC in the VQI had not been prescribed antiplatelet agents or statin medications at discharge. Although the stain intolerance rate has been an estimated at 10% to 20% in the general population22,23 and patients might have declined these medications on discharge, it is more likely that antiplatelet agents and statins were simply not prescribed after PVI. Given that 2% of the patients were classified as not taking statin medications for medical reasons suggests that statin intolerance in this population is low. These data highlight a significant opportunity for optimization of modifiable risk factors before elective intervention for IC. Furthermore, the treatment of these patients is not consistent with the grade IA recommendations from the SVS practice guidelines for antiplatelet and statin therapy for patients with symptomatic PAD.8
In the present study population, 86% of patients were free from repeat procedures after aortoiliac intervention at 2 years. However, the proportion of patients undergoing isolated aortoiliac intervention who were free from IC at 2 years was much lower at only 37%. The rate of freedom from repeat procedures was similar to the rate of patients who had lost primary patency in the COBEST (covered vs balloon expandable stent trial) for aortoiliac occlusive disease.24 However, the rate of symptom recurrence was in contrast to much of the literature on isolated aortoiliac interventions for IC, which have been described as providing excellent results, even for TASC (TransAtlantic Inter-Society Consensus) D lesions, at ≤5 years of follow-up.24–28 We believe that IC recurrence is a much more clinically relevant outcome than patency, given that the ultimate goal of treatment is the durable relief of symptoms from the patient’s perspective. Therefore, that only 32% of the patient cohort reported being free from IC recurrence at 2 years is very suggestive of poor patient-centered outcomes after PVI for IC. This is arguably more clinically important than target lesion revascularization, patency, and lesion recurrence, which are all, essentially, proxy measures for a perceived benefit for the patient at a specific time point. Any outcome related to improving the quality of life of a patient should be measured by a validated quality of life questionnaire, such as the peripheral artery questionnaire,29 which at present, is not captured in a validated manner in the VQI.
Tsai et al30 reported data on PVI outcomes from a community-based integrated healthcare system. They estimated the incidence of target limb reintervention after PVI for IC (n = 934) at 16.1% and 24.4% at 1 and 3 years, respectively, notably similar to the data from our study. However, they did not report outcomes specific to the level of disease treated, nor did they report on IC symptom recurrence or functional outcomes. Our data highlight the large discrepancy between symptom recurrence and repeat intervention for IC and expose the limited relevance of outcomes such as target lesion and target limb reintervention, which have been commonly used in clinical device studies. Moreover, the lack of data on anatomic patency of the index intervention meant that we could not discern whether the symptom recurrence was related to anatomic failure at the treated artery or other unrelated causes. More robust longitudinal registry data, incorporating anatomic patency, reintervention, and functional and patient-reported outcomes, are sorely needed to better characterize the true effectiveness of interventions for IC.
Atherectomy use was associated with an increased incidence of IC recurrence and repeat procedures in the present study. The CONFIRM (coronary CT angiography evaluation for clinical outcomes: an international multi-center registry) I, II, and III Registries, which were a series of prospectively maintained registries of orbital atherectomy from 2009 to 2011 reported the pooled experience of 62 iliac atherectomies and 1570 superficial femoral artery atherectomies in a total cohort of 3135 patients.31 This corresponded to a rate of 1.9% for iliac atherectomy and 50% for superficial femoral artery atherectomy, similar to the data reported in the present study of 2.9% for aortoiliac atherectomy and 61% for femoropopliteal atherectomy.
A Cochrane review comparing atherectomy vs angioplasty for both IC and critical limb ischemia indications demonstrated inferior results with atherectomy.32 This was postulated to be due to distal embolization. The initial wave of enthusiasm for debulking plaque and attaining similar long-term patency without the need for adjunctive stenting led to a surge in atherectomy procedures.33,34 More recently, atherectomy has been used in combination with drug-coated balloon angioplasty.33,35–37 Nonetheless, evidence for the clinical effectiveness of atherectomy is lacking compared with existing balloon angioplasty and stenting. Investigators have suggested that the greater reimbursement for atherectomy procedures might be associated with the increased use.33,34,38 The VQI lacks robust data, however, regarding specific known associated risk factors such as severe calcification, length and degree of stenosis or occlusion, and other vessel features. Therefore, confounding factors could have been present that led to the use of atherectomy, in particular, unfavorable lesions, and should be remembered when evaluating these data. A targeted evaluation of atherectomy and IC outcomes is very challenging to study in a retrospective cohort and a randomized prospective trial would be best, which is a consideration for future study.
The SVS practice guidelines espouse grade IA recommendations for multidisciplinary comprehensive smoking cessation interventions for patients with IC.8 Despite this recommendation, 45% of the cohort were active smokers at the time of intervention. It is possible that this was related to the unclear effect of smoking cessation on IC in reported studies. Smoking cessation has been shown to improve IC and ankle pressures after exercise compared with active smoking.39 Active smoking has also been associated with more severe IC pain that occurred sooner during exercise and required longer to subside compared with nonsmokers.40 However, a meta-analysis of physical training, smoking cessation, and pharmacologic adjuncts for IC concluded that the evidence was weak regarding the usefulness of smoking cessation on pain-free and total walking distances.41
A positive correlation between the level of invasiveness of the procedure and smoking cessation has been described previously.42,43 The perioperative period is a “window of opportunity” to improve the rates of smoking cessation. Patients undergoing endovascular approaches are most at risk of continued smoking owing to the minimally invasive nature of the procedure. As such, the onus falls on the physician considering an elective procedure for IC to ensure that every attempt to facilitate smoking cessation has been exhausted, including withholding the intervention until enrollment in a smoking cessation program has been pursued.
Study limitations.
VQI data are self-reported and subject to reporting bias. The effects of IC and the treatment are not objectively scored, and no validated measures of symptoms or quality of life, such as the peripheral artery questionnaire, are included.29 Given that the interventions for IC treat a lifestyle limitation, it is important that future studies include an objective measure of the degree of lifestyle limitation and the effect of the interventions. Furthermore, regarding nonoperative interventions for IC, such as medical therapy, supervised exercise therapy, and smoking cessation programs, these are not captured in the VQI PVI module in the present iteration and would be of clinical interest in further editions of the PVI module.
Additionally, focusing on the patient-centered outcome of recurrent IC does not allow for differentiation between a de novo lesion causing IC vs failure of the treated index vascular territory. Thus, a patient could have undergone an iliac intervention only to develop a new superficial femoral artery lesion within 2 years that led to the development of symptoms of IC recurrence in the face of a patent iliac intervention. Participation in the VQI is voluntary and might not be representative of the general population. Patients could also have undergone reintervention at another institution outside the VQI and thus not have been included in the PVI module. Finally, the PVI dataset captures only the PVIs in the VQI. A matched comparison with open peripheral vascular procedures is planned in the future to attempt to compare the durability of these two approaches for IC.
The lack of robust follow-up data is an issue that could have the affected the results by skewing data toward patients with poor outcomes. Patients with recurrent symptoms might have been more likely to return for follow-up care. Thus, it is possible that centers with low follow-up rates were overrepresented by patients with symptom recurrence. This might result in falsely elevated rates of repeat procedures and symptom recurrence. The time to IC recurrence is a reflection of when the patient returned to the provider because of symptoms. Some patients might have delayed their return to the provider, which would have resulted in a bias toward a longer time to symptom recurrence. In contrast, the limited length of follow-up time is likely to have significantly underestimated the recurrence and reintervention rates actually experienced by patients.
This possible bias was investigated by performing a sensitivity analysis using only centers with >70% follow-up rates at >9 months after intervention. We reanalyzed this cohort, with more robust follow-up, using the same criteria as for the overall cohort. Although the results of the sensitivity analysis did not meet statistical significance compared with the overall cohort, that might have been related to the significantly smaller sample size of the sensitivity analysis relative to the overall sample.
CONCLUSIONS
In the VQI database, PVIs for IC were associated with poor 2-year relief of symptoms. Furthermore, most patients who had undergone intervention for IC had not been medically optimized before the intervention, with high rates of smoking and poor use of antiplatelet agents and statins. In the present study, we have demonstrated that PVIs for IC frequently fail to meet the recommendations from the SVS guidelines from a patient symptom recurrence and medical treatment perspective. These findings suggest areas for improvement, including in medical and lifestyle management, patient selection, and the technical and anatomic durability outcomes of the procedures. Given the financial incentives to treat patients, with particular emphasis on reimbursement for using technologies such as atherectomy, we must ensure that clinical decision-making for elective procedures for IC is evidence-based and undertaken only once risk factor modification has been aggressively pursued.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
A retrospective analysis of prospectively collected data from the Vascular Quality Initiative of intermittent claudication (IC)
Key Findings:
In a cohort of 16,152 patients who had undergone elective endovascular procedures for IC, a large proportion of patients were poorly optimized before undergoing an invasive procedure. Furthermore, symptom recurrence was seen in 78% of patients at 2 years and was more likely with treatment of more than two arteries (odds ratio [OR], 1.19) and atherectomy (OR, 1.29). The use of antiplatelet medications and statins was associated with a decreased odds of recurrent symptoms (OR, 0.85) and repeat procedures (OR, 0.77).
Take Home Message:
Patients undergoing elective procedures for IC should receive aggressive risk factor modification before invasive treatment, because symptom recurrence is high and associated with poor medical optimization.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented at the Forty-seventh Annual Symposium of the Society for Clinical Vascular Surgery, Boca Raton, Fla, March 17–20, 2019.
Additional material for this article may be found online at www.jvascsurg.org.
REFERENCES
- 1.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med 2008;13:209–15. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee D, Cho L. Peripheral arterial disease: considerations in risks, diagnosis, and treatment. J Natl Med Assoc 2009;101:999–1008. [DOI] [PubMed] [Google Scholar]
- 3.Society for Vascular Surgery Lower Extremity Guidelines Writing Group, MS Conte, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015;61(Suppl): 2S–41S. [DOI] [PubMed] [Google Scholar]
- 4.Malgor RD, Alahdab F, Elraiyah TA, Rizvi AZ, Lane MA, Prokop LJ, et al. A systematic review of treatment of intermittent claudication in the lower extremities. J Vasc Surg 2015;61(Suppl):54S–73S. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner KW, House AK, Castleden WM. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. Med J Aust 1983;1:217–9. [DOI] [PubMed] [Google Scholar]
- 6.Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med 2003;114:359–64. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conte MS, Pomposelli FB. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication: introduction. J Vasc Surg 2015;61(Suppl):1S. [DOI] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45(Suppl S):S5–67. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, Skinner JJ Jr, Schwartz MJ, Shurtleff D. Intermittent claudication: incidence in the Framingham study. Circulation 1970;41:875–83. [DOI] [PubMed] [Google Scholar]
- 11.Jones WS, Baumgartner I, Hiatt WR, Heizer G, Conte MS, White CJ, et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation 2017;135:241–50. [DOI] [PubMed] [Google Scholar]
- 12.Golledge J, Moxon JV, Rowbotham S, Pinchbeck J, Yip L, Velu R, et al. Risk of major amputation in patients with intermittent claudication undergoing early revascularization. Br J Surg 2018;105:699–708. [DOI] [PubMed] [Google Scholar]
- 13.Xie X, Wang X, Laskowitz DT, Zhao X, Miao Z, Liu L, et al. Effect of dual versus mono antiplatelet therapy on recurrent stroke modulated by activated partial thromboplastin time. Eur J Neurol 2019;26. 1168–e78. [DOI] [PubMed] [Google Scholar]
- 14.Montalescot G, Sabatine MS. Oral dual antiplatelet therapy: what have we learnt from recent trials? Eur Heart J 2016;37:344–52. [DOI] [PubMed] [Google Scholar]
- 15.Richman IB, Owens DK. Aspirin for primary prevention. Med Clin North Am 2017;101:713–24. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Manson JE. Aspirin for primary prevention of atherosclerotic cardiovascular disease: advances in diagnosis and treatment. JAMA Intern Med 2016;176:1195–204. [DOI] [PubMed] [Google Scholar]
- 17.Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley TR, Singh GD, Kokkinidis DG, Choy HK, Pham T, Amsterdam EA, et al. High-intensity statin therapy is associated with improved survival in patients with peripheral artery disease. J Am Heart Assoc 2017;6:e005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris SK, Roos MG, Landry GJ. Statin use in patients with peripheral arterial disease. J Vasc Surg 2016;64:1881–8. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell TFX, Deery SE, Darling JD, Shean KE, Mittleman MA, Yee GN, et al. Adherence to lipid management guidelines is associated with lower mortality and major adverse limb events in patients undergoing revascularization for chronic limb-threatening ischemia. J Vasc Surg 2017;66:572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stavroulakis K, Borowski M, Torsello G, Bisdas T; CRITISCH Collaborators. Association between statin therapy and amputation-free survival in patients with critical limb ischemia in the CRITISCH registry. J Vasc Surg 2017;66:1534–42. [DOI] [PubMed] [Google Scholar]
- 22.Guyton JR, Bays HE, Grundy SM, Jacobson TA. The National Lipid Association Statin Intolerance Panel. An assessment by the statin intolerance panel: 2014 update. J Clin Lipidol 2014;8(Suppl):S72–81. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med 2013;158:526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwipatayi BP, Sharma S, Daneshmand A, Thomas SD, Vijayan V, Altaf N, et al. Durability of the balloon-expandable covered versus bare-metal stents in the covered versus balloon expandable stent trial (COBEST) for the treatment of aortoiliac occlusive disease. J Vasc Surg 2016;64:83–94.e1. [DOI] [PubMed] [Google Scholar]
- 25.Humphries MD, Armstrong E, Laird J, Paz J, Pevec W. Outcomes of covered versus bare-metal balloon-expandable stents for aortoiliac occlusive disease. J Vasc Surg 2014;60:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorigo W, Piffaretti G, Benedetto F, Tarallo A, Castelli P, Spinelli F, et al. A comparison between aortobifemoral bypass and aortoiliac kissing stents in patients with complex aortoiliac obstructive disease. J Vasc Surg 2017;65:99–107. [DOI] [PubMed] [Google Scholar]
- 27.Kasemi H, Marino M, Dionisi CP, Di Angelo CL, Fadda GF. Seven-year approach evolution of the aortoiliac occlusive disease endovascular treatment. Ann Vasc Surg 2016;30:277–85. [DOI] [PubMed] [Google Scholar]
- 28.Pulli R, Dorigo W, Fargion A, Angiletta D, Azas L, Pratesi G, et al. Early and midterm results of kissing stent technique in the management of aortoiliac obstructive disease. Ann Vasc Surg 2015;29:543–50. [DOI] [PubMed] [Google Scholar]
- 29.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J 2004;147:301–8. [DOI] [PubMed] [Google Scholar]
- 30.Tsai TT, Rehring TF, Rogers RK, Shetterly SM, Wagner NM, Gupta R, et al. The contemporary safety and effectiveness of lower extremity bypass surgery and peripheral endovascular interventions in the treatment of symptomatic peripheral arterial disease. Circulation 2015;132:1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MS, Martinsen BJ, Hollowed J, Heikali D, Mustapha J, Adams G, et al. Acute procedural outcomes of orbital atherectomy for the treatment of iliac artery disease: sub-analysis of the CONFIRM registries. Cardiovasc Revasc Med 2018;19(Pt A):503–5. [DOI] [PubMed] [Google Scholar]
- 32.Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev 2014;3: CD006680. [DOI] [PubMed] [Google Scholar]
- 33.Mohan S, Flahive JM, Arous EJ, Judelson DR, Aiello FA, Schanzer A, et al. Peripheral atherectomy practice patterns in the United States from the Vascular Quality Initiative. J Vasc Surg 2018;68:1806–16. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee D, Hashemi H, Contos B. The disproportionate growth of office-based atherectomy. J Vasc Surg 2017;65:495–500. [DOI] [PubMed] [Google Scholar]
- 35.Milnerowicz A, Milnerowicz A, Kuliczkowski W, Protasiewicz M. Rotational atherectomy plus drug-coated balloon angioplasty for the treatment of total in-stent occlusions in iliac and infrainguinal arteries. J Endovasc Ther 2019;26:316–21. [DOI] [PubMed] [Google Scholar]
- 36.Sauguet A, Philippart R, Honton B. Directional atherectomy with antirestenotic therapy for the treatment of no-stenting zones. J Cardiovasc Surg (Torino) 2019;60:198–204. [DOI] [PubMed] [Google Scholar]
- 37.Sixt S, Carpio Cancino OG, Treszl A, Beschorner U, Macharzina R, Rastan A, et al. Drug-coated balloon angioplasty after directional atherectomy improves outcome in restenotic femoropopliteal arteries. J Vasc Surg 2013;58:682–6. [DOI] [PubMed] [Google Scholar]
- 38.Jones WS, Mi X, Qualls LG, Vemulapalli S, Peterson ED, Patel MR, et al. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. J Am Coll Cardiol 2015;65:920–7. [DOI] [PubMed] [Google Scholar]
- 39.Quick CR, Cotton LT. The measured effect of stopping smoking on intermittent claudication. Br J Surg 1982;69(Suppl):S24–6. [DOI] [PubMed] [Google Scholar]
- 40.Gardner AW. The effect of cigarette smoking on exercise capacity in patients with intermittent claudication. Vasc Med 1996;1:181–6. [DOI] [PubMed] [Google Scholar]
- 41.Girolami B, Bernardi E, Prins MH, Ten Cate JW, Hettiarachchi R, Prandoni P, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Intern Med 1999;159:337–45. [DOI] [PubMed] [Google Scholar]
- 42.Rajaee S, Cherkassky L, Marcaccio EJ Jr, Carney WI Jr, Chong TT, Garcia-Toca M, et al. Open revascularization procedures are more likely to influence smoking reduction than percutaneous procedures. Ann Vasc Surg 2014;28:990–8. [DOI] [PubMed] [Google Scholar]
- 43.Hoel AW, Nolan BW, Goodney PP, Zhao Y, Schanzer A, Stanley AC, et al. Variation in smoking cessation after vascular operations. J Vasc Surg 2013;57:1338–44. quiz: 44.e1–44.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


