Abstract
Introduction
Ketamine treatment is capable of significant and rapid symptom improvement in adults with treatment-resistant depression (TRD). A limitation of ketamine treatment in TRD is the relatively short duration of time to relapse (e.g., median 2–4 weeks). The objective of the systematic review herein is to identify strategies capable of prolonging the acute efficacy of ketamine in adults with TRD.
Methods
PubMed/MEDLINE databases were searched from inception to December 2020 for clinical studies written in English using the following key terms: ketamine, prolong, and depression. A total of 454 articles were identified from the literature search which included all clinical studies regarding prolonging the antidepressant effects of ketamine. Twenty-two articles were included: ten randomized controlled trials (RCTs), eight prospective open-label trials, one retrospective chart review, and three case reports. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for data extraction. The primary outcome was prolonged effect, defined as statistically significant antidepressant effects following acute ketamine treatment.
Results
A total of 454 articles were identified, and 22 articles were included. Different treatment modalites including pharmacological interventions, manualized-based psychotherapies, electroconvulsive therapy, transcranial magnetic stimulation, and intravenous monotherapy were examined to determine their impact on the prolongation of antidepressant effects following acute ketamine treatment. No treatment modality, other than repeat-dose IV ketamine, has demonstrated ability to significantly prolong the acute efficacy of IV ketamine in TRD.
Conclusion
Hitherto, available open-label data and controlled trial data support repeat administration of IV ketamine as an effective strategy to prolong the efficacy of ketamine’s antidepressant effects (although not the focus of the study herein, maintenance repeat-dose esketamine treatment is proven effective in esketamine responders). There is a need to identify multimodality strategies that are safe and capable of prolonging the efficacy of ketamine in adults with TRD.
Keywords: Anxiety, Bipolar disorder, Depression, Esketamine, Ketamine, Major depressive disorder, Suicidality, Treatment-resistant depression
Key Summary Points
| Up to 40% of patients with major depressive disorder fail to achieve syndromal and functional recovery with conventional monoamine-based antidepressants. Prolonging the rapid significant antidepressants effects of IV ketamine in persons with treatment resistant depression is a priority research vista. Esketamine has demonstrated both acute and maintenance efficacy in adults with treatment resistant depression. |
| We explored strategies to prolonging ketamine’s efficacy in adults with treatment-resistant depression. |
| We identified 454 articles within our literature search and included 22 articles for data extraction. |
| No modality of treatment has demonstrated ability to sufficiently prolong the acute efficacy of ketamine in treatment resistant depression; however, some modalities of treatment showed promising results. |
| There remains a need to identify multimodality strategies that are safe and capable of prolonging efficacy of ketamine. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14291405.
Introduction
Major depressive disorder (MDD) is a common mental disorder that causes severe functional impairment affecting approximately 10–20% of individuals at some time in their life [1]. Notwithstanding the availability of disparate psychopharmacological and psychological therapies for treating MDD, up to 40% of patients fail to achieve syndromal and functional recovery [2, 3]. For example, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study reported that approximately half of patients with MDD failed to achieve remission of depressive symptoms following two sequential courses of conventional treatment [4]. In addition to suboptimal acute treatment outcomes in MDD, monoamine-based treatments require 2–3 weeks before a clinically significant effect may be observed [5, 6]. The foregoing therapeutic inertia contributes to ongoing disability, psychological dysfunction and risk for suicide [3, 5].
During the past two decades, the rapid antidepressant effects of ketamine have been reported in adults with treatment-resistant depression (TRD) [7]. Racemic ketamine, as well as esketamine, have demonstrated significant and rapid symptom improvement as well as anti-suicidality effects in adults with TRD [8–10]. Notwithstanding the efficacy of ketamine in adults with TRD, a major limitation is the relatively short duration to relapse (e.g., median 2–4 weeks) [11].
Multiple clinical studies have reported the maintenance antidepressant effects of repeat sub-anesthetic dose intravenous (IV) ketamine for up to 6 weeks, with replicated large effect sizes [9, 12–14]. Compared to conventional antidepressants, IV ketamine is differentiated by (1) efficacy in patients with TRD, (2) a relatively rapid onset of therapeutic effect, and (3) preliminary evidence for reducing suicidality [10, 15]. From a tolerability and safety perspective, an additional, albeit not treatment-limiting concern with ketamine is the potential for dissociation, as well as vasopressor effects [16–18]. Evaluating the long-term effectiveness of ketamine is limited by a dearth of long-term controlled trial data [13, 19].
The objective of this systematic review is to identify strategies that are capable of prolonging efficacy of IV ketamine in adults with TRD. The focus is on IV racemic ketamine rather than esketamine, as esketamine has demonstrated maintenance of efficacy as part of a randomized controlled trial (RCT) wherein repeat-dose esketamine was associated with a prolonged time to relapse when compared to placebo. Thus, the best evidence for prolonging the efficacy of esketamine is to continue repeat-dose esketamine [20].
Herein, we focused on identifying strategies that prolong the antidepressant effects of IV ketamine in adults with TRD. Further, we specifically study the possible synergistic effects and evaluate evidence for (1) prolonged ketamine antidepressant effect combined with psychopharmacological continuation therapy, (2) prolonged ketamine antidepressant effect combined with a psychological therapy, and (3) efficacy of repeated IV ketamine treatment.
Methods
Our search adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and examined ways of prolonging the antidepressant effect of ketamine in patients with TRD.
Studies were identified by searching PubMed/MEDLINE from inception to December 2020 for clinical studies (e.g., case reports, chart reviews, clinical trials). Included studies discussed methods of prolonging the antidepressant effects of IV ketamine administration in individuals with TRD. The following search terms were used: ((ketamine OR esketamine OR s-ketamine OR r-ketamine) AND (treatment-resistant depress* OR TRD OR resistant OR treatment refract* depress* OR MDD OR depress* OR major depress*) AND (prolong* OR continu* OR repeat* OR maintenance OR sustain*)). “Prolonged effects” were defined as statistically significant (P < 0.05) antidepressant effects following acute IV ketamine treatment known to last up to 7 days [21] and “repeated IV ketamine treatment” defined as more than one instance of IV ketamine treatment beyond acute treatment. All studies were written in English and were conducted in human subjects. Further relevant articles were identified manually through a search of article reference lists. Different studies within this systematic review were compared across a variety of categories including antidepressant effects, safety and tolerability, and limitations. As a result of differences in treatment design, dosage, and treatment frequency, the statistical power of this systematic review remains limited.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Search Results
The initial search yielded 454 records in PubMed/MEDLINE, and 399 records after the removal of duplicates (Fig. 1). After title and abstract screening, 362 records were excluded and 37 full-text articles were assessed for eligibility. We included 22 articles in this review: 10 RCTs, 8 prospective open-label trials, 1 retrospective chart review, and 3 case reports (1–28 cases per article). A summary of the 18 prospective studies and 4 retrospective studies (i.e., case reports, chart reviews) can be found in Tables 1 and 2, respectively.
Fig. 1.
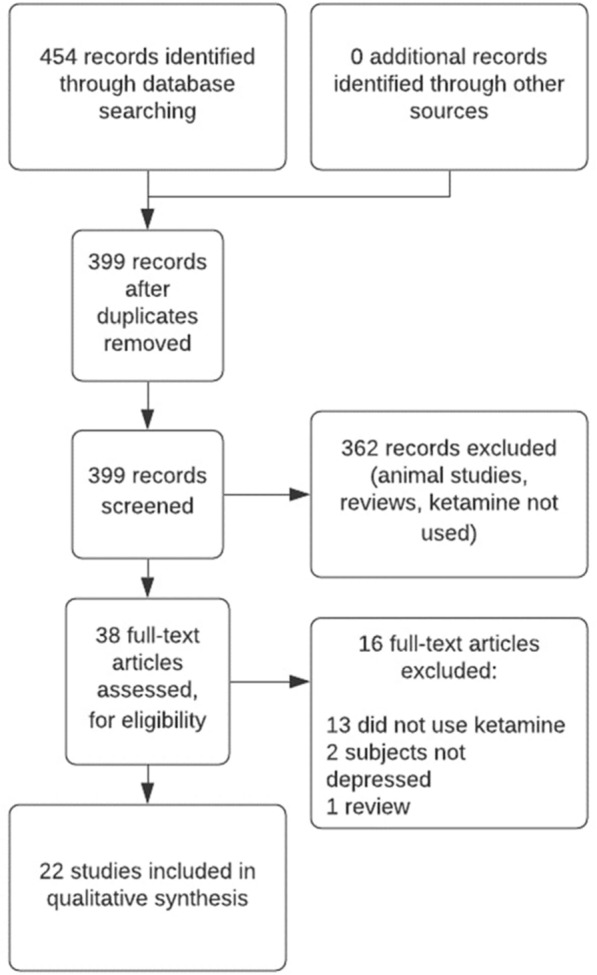
Flowchart outlining the systematic literature research using PubMed/MEDLINE
Table 1.
Summary of prospective studies, including RCTs and open-label studies
| Study | Design | Inclusion criteria | Intervention groups | Antidepressant effects | Response rate | Relapse rate | Safety and tolerability | Limitations | Prolonged | TRD |
|---|---|---|---|---|---|---|---|---|---|---|
| aan het Rot et al. [22] | Single arm, open-label, 12 days |
Aged over 18 years Previously responded to a single IV ketamine dose (≥ 50% reduction in MADRS) MDD, lack of response to ≥ 2 antidepressants trials in current MDE |
Ketamine 0.5 mg/kg IV 6 times over 12 days (n = 10 starting, n = 9 completing) No control group |
For participants who completed the study (9/10; 1 withdrew for nonresponse) by dose 6 all had significant improvement in depressive symptoms (mean reduction in MADRS of 85%); no significant changes in peak BPRS+ scores (χ2(5) = 3.41, P = 0.6) were seen across the 6 doses |
Response criteria: ≥ 50% reduction in MADRS score from the pre-ketamine baseline Response rate 90% (9/10) |
88.9% (8/9) relapsed on average 19 days after sixth infusion (range 6–45 days) |
Mild, transient AEs with significant dissociative effects in n = 3 Most common AEs: abnormal sensations, headache, and weakness or fatigue |
Lack of formal cognitive testing Compared efficacy of single IV ketamine dose to other pre-selected samples with favorable responsivity No control group |
Y | Y |
| Abdallah et al. [33] | RCT, 2 week |
Aged 21–65 years MDD with ongoing MDE (MADRS ≥ 18) Lack of response to ≥ 1 antidepressant trial in current trial |
Rapamycin 6 mg or placebo 2 h prior to ketamine 0.5 mg/kg IV (n = 23) | Significant prolongation of antidepressant effects observed after week 2 by time interaction (F(8,245) = 2.02, P = 0.04). A higher response rate (41%) and remission rates (29%) following rapamycin + ketamine versus placebo + ketamine (13%, P = 0.04, and 7%, P = 0.003) |
Response criteria: 50% reduction in MADRS score Response rate 41% (n = 7/17) |
Not reported in study |
Mild, transient AEs with significant dissociative effects Most common AEs: fatigue, headaches, nausea, and pain |
Lacked treatment by time interaction which may result in limited statistical power Unclear method for monitoring AEs (suicide ideation) |
Y | Y |
| Chen et al. [23] | RCT, 6 week |
Aged 21–65 years MDD or BP disorder or MDE (HAM-D ≥ 16) Response (≥ 50% reduction of HAM-D) after single ketamine dose Lack of response to ≥ 2 antidepressant trials |
Ketamine 0.5 mg/kg IV twice, then increasing DCS daily (250–1000 mg) (n = 16) vs ketamine 0.5 mg/kg IV twice with placebo (n = 16) | Significant antidepressant effect observed on day 4 after two doses of ketamine (P = 0.003); no significant HAM-D reduction in follow-up between DCS or placebo (P = 0.30) |
Response criteria: ≥ 50% reduction of HAMD Response rate 65.6% (21/32) |
Not reported in study |
Mild, transient non-significant AEs in DCS group include dizziness, sedation, hand tremor, and itching No significant difference in AEs between groups |
Lacked independent study of DCS treatment, other antidepressant treatments were not discontinued Unclear method for monitoring AEs (suicide ideation) All participants had responded to ketamine; unclear if extrapolation to those with severe suicidal symptoms is possible |
Y | Y |
| Costi et al. [24] | RCT, 2 week |
Aged 21–65 years MDD with ongoing MDE (QIDS-SR ≥ 14; CGI-S ≥ 4) Lack of response to ≥ 2 antidepressant trials (≤ 50% improvement) |
Ketamine 0.5 mg/kg IV once, then increasing lithium daily (600–1200 mg) (n = 18) vs ketamine 0.5 mg/kg IV twice with placebo (n = 16) | No significant difference in depression severity between treatment groups at week 2 (P = 0.91); also, no difference between treatment groups in continuing the antidepressant effect of ketamine | Not reported in study | Relapse rate at end of 2-week study was 25% (2/8) |
Mild, transient AEs with significant dissociative effects No significant difference in AEs between groups |
Low lithium treatment dosages Too short a study duration to see full potential effects TRD sample limits generalizability to broader MDD population |
Y | Y |
| Cusin et al. [25] | Single arm, open-label, 3 week |
Aged 18–65 years MDD with ongoing MDE (HAM-D ≥ 20) Lack of response to ≥ 3 antidepressant trials in current trial Suicide ideation for > 3 months |
Ketamine 0.5–0.75 mg/kg IV 6 times (n = 14 starting, n = 12 completing) No control group |
Significant antidepressant effect observed after week 3 (response rate 41.7%; remission rate 16.7%) Improvements in HAM-D scores approached statistical significance at week 3 (Cohen d = 0.48; P = 0.052); however, significant differences in HAM-D scores were observed after week 6 (Cohen d = 1.01; P = 0.002). 80% (4/5) of responders had prolonged antidepressant effects for 2 weeks, and 1/5 for 6 weeks |
Response criteria: ≥ 50% reduction of HDRS Response rate 35.7% (5/14) |
Relapse rate at 2-week FU 80% (4/5) |
Mild, transient AEs with no significant dissociative effects Most common: headache and nausea |
No control group Unclear method for monitoring AEs Unclear if symptom improvement between doses 1–6 were related to increased dosage or cumulative effect |
Y | Y |
| Ibrahim et al. [26] | RCT, 4 week |
Aged 18–65 MDD with ongoing MDE (MADRS ≥ 22) Lack of response to ≥ 2 antidepressant trials (≤ 25% MADRS) |
Ketamine single dose 0.5 mg/kg IV then riluzole 100–200 mg daily vs ketamine single dose 0.5 mg/kg IV (n = 21) then placebo once daily (n = 21) |
Significant antidepressant effect was observed immediately after ketamine dose prior to randomization (response rate 62%); no significant improvements in MADRS were observed between treatment groups after week 4 Relapse times were not significant with the ketamine–riluzole group taking 17.2 days and the ketamine–placebo group 9.8 days |
Response criteria: ≥ 50% reduction of MADRS Response rate 61.9% (26/42) |
Relapse rate at 2-week FU 73.1% (19/26) |
Mild, transient AEs with no significant dissociative effects (during ketamine IV) No differences in AEs or overall discontinuation between groups |
Unclear method of monitoring AEs Medically unwell population; unclear if extrapolation to general population is possible |
Y | Y |
| Ionescu et al. [32] | RCT, 3 week |
Aged 18–65 years MDD with ongoing MDE (HDRS ≥ 20, HDRS suicide item score ≥ 2) SI for ≥ 3 months Lack of response to ≥ 3 antidepressant trials in current episode |
Patients were randomized to receive ketamine 0.5 mg/kg IV (n = 13) or saline placebo (n = 13) twice weekly for 2 weeks (n = 26 starting, n = 19 completing) |
In the entire cohort, HDRS scores improved across infusions with no statistical differences between treatment groups; no differences in depression severity or suicide ideation between groups were observed (P = 0.47, P = 0.32, respectively) At 2 weeks 25% (3/12) in the ketamine group and 33% (4/12) in the placebo group responded; similarly, 17% (2/12) in the ketamine group and 8% (1/12) in the placebo group remitted; there was no significant difference between groups in remittance (P ≥ 0.05) |
Response criteria: ≥ 50% improvement on the HDRS Response rate (ketamine) 3/12 (25%) Response rate (placebo) 4/12 (33%) |
Relapse rate (ketamine) (at 3-month FU) 83.3% (10/12) Relapse rate (placebo) (at 3-month FU) 83.3% (10/12) |
Not mentioned |
No active control Limited statistical power due to small sample size Unclear method for monitoring AEs Restriction to outpatients |
Y | Y |
| Lenze et al. [35] | RCT, 8 week |
Aged 18–65 years MDD with ongoing MDE (MADRS ≥ 22) Lack of response to ≥ 2 antidepressant trials in current episode |
96-h ketamine IV 0.6 mg/kg and clonidine 0.6 mg orally daily (n = 10) vs 40 min ketamine 0.5 mg/kg IV and clonidine 0.6 mg orally daily (n = 10) | No significance difference in MADRS changes at treatment completion (P = 0.35) nor during 8 weeks of FU (P ≤ 0.05), both groups had sustained reduction in depressive symptoms compared to baseline. No significant difference in sustained reduction of depression (sustained reduction rate 40% vs 20%) |
Response criteria: ≥ 50% reduction in MADRS Total response rate 40% (4/10) in 96-h group at 2 weeks to 20% (2/10) at 8 weeks 20% (2/10) in 96-h group at 2 weeks to 10% (1/10) at 8 weeks |
Not reported in study |
Mild, transient AEs with no significant dissociative effects, no AEs attributable to clonidine No differences in AEs or overall discontinuation between groups |
Clonidine dose was higher in 96-h arm Limited statistical power due to small sample size Unclear method for monitoring dissociative AEs |
Y | Y |
| Loo et al. [27] | Placebo-controlled, open-label, 5 week |
Aged ≥ 18 years MDD with ongoing MDE (MADRS ≥ 20) Lack of response to ≥ 1 antidepressant trials in current episode |
Sequential cohorts (total n = 15) received either IV ketamine (n = 4), IM (n = 5) or SC (n = 6) at five weekly ascending doses (0.1–0.5 mg/kg) with midazolam 0.01 mg/kg randomly inserted |
No significance difference in MADRS between 0.1 mg/kg vs 0.0 mg/kg at week 1 (P = 0.066); however, there was a significant improvement in MADRS at week 2 vs 0.0 mg/kg (P = 0.001). All routes of ketamine administration resulted in comparable MADRS scores; 50% of patients (5/10) achieved remission status The midazolam condition resulted in no significant changes in BPRS+ or CADSS. The mean time to relapse was 23.2 days |
Response criteria: ≥ 50% improvement in MADRS Response rate 80% (12/15) |
Relapse rate 80% (8/10) at end of 5-week study |
Mild, transient AEs with dose-related dissociative effects (ketamine), fewest AEs were noted with SC administration (ketamine) Most common AEs (ketamine) fatigue, lightheadedness, dizziness, blurred vision, and emotional lability |
Treatment assignment was sequential rather than randomized Treatment effect used ascending doses rather than randomized dose design Limited statistical power due to small sample size |
Y | Y |
| Mathew et al. [34] | RCT, 32 days |
Aged 21–70 years MDD with ongoing MDE (IDS-C30 ≥ 32) Lack of response to ≥ 2 antidepressant trials in current episode |
Patients were randomized to lamotrigine (300 mg) or placebo (n = 26), then each group was given ketamine 0.5 mg/kg IV; responders (n = 14) were then randomized into riluzole continuation (100–200 mg/day) |
Significant MADRS response (≥ 50% reduction from baseline) was seen in 65% and 54% of patients at the 24-h and 72-h time points Riluzole showed no significance difference in time-to-relapse compared to placebo group (24.4 days vs 22.0 days, P = 0.68), with a relapse rate of 80% vs 50% of riluzole and placebo trial completers, respectively; the RCT was terminated early. Lamotrigine did not significantly improve MADRS scores post-ketamine (P = 0.36) |
Response criteria: ≥ 50% reduction in MADRS score at 24 h relative to the previous day's baseline Response rate (72 h post-ketamine) 53.8% (14/26) |
Relapse rate (riluzole) 80% (4/5) at end of 32 days study Relapse rate (placebo) 50% (4/8) at end of 32-day study |
Mild to moderate, transient AEs with dissociative effects No significant difference in AEs between groups |
Failed to collect blood levels of lamotrigine Limited statistical power due to small sample size |
Y | Y |
| Murrough et al. [28] | Single arm, open-label, 12 days |
MDD with ongoing MDE (IDS-C30 ≥ 32) Lack of response to ≥ 2 antidepressant trials in current episode No antidepressants in past 2 weeks |
Ketamine 0.5 mg/kg IV up to six times over a 12-day period (n = 24 starting, n = 21 completing) No control group |
Significant mean improvement in MADRS observed at 2 h after first ketamine dose (P ≤ 0.001), these effects were largely sustained for rest of treatment period; study end response was strongly predicted by response at 4 h (94% sensitive, 71% specific); overall response rate was 70.8% The median time for responders to relapse after last ketamine dose was 18 days |
Response criteria: ≥ 50% improvement in MADRS Response rate 70.8% (17/24) |
Relapse rate (at end of FU) 83.3% (20/24) | Mild, transient AEs with significant dissociative effects |
No control group Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
| Phillips et al. [29] | RCT, 6 weeks |
Aged 18–65 years MDD with ongoing MDE (MADRS ≥ 25) Lack of response to ≥ 2 antidepressant trials in current episode |
Patients were randomized to receive midazolam 30 µg/kg IV or ketamine 0.5 mg/kg IV (n = 41); after relapse of depressive symptoms patients received ketamine 0.5 mg/kg IV 6 times over 2 weeks (n = 41 starting, n = 39 completing), responders then received 4 doses of ketamine 0.5 mg/kg IV once weekly (n = 23) | Compared to midazolam, ketamine had a significant improvement in MADRS 24 h post-infusion (P = 0.03); MADRS scores were maintained with no significant changes with once-weekly maintenance infusions (P = 0.49). Response rate at end of repeated infusions was 59% |
Response criteria (ketamine): ≥ 50% decrease in MADRS total score from baseline Response rate (ketamine) 58.9% (23/39) |
Not reported in study | Mild, transient AEs with significant dissociative effects |
Lack of dissociative side effects with active placebo (midazolam) No active control in repeated or maintenance phases |
Y | Y |
| Rasmussen et al. [13] | Single arm, open-label, 2 week |
Aged ≥ 18 years MDD or BP with ongoing MDE (MADRS ≥ 25) Lack of response to ≥ 2 antidepressant trials in current episode |
Ketamine 0.5 mg/kg IV up to four times twice weekly (n = 10) No control group |
For participants who completed the study (n = 10), by end of treatment had significant improvement in depressive symptoms (mean reduction in MADRS of 71% (P = 0.0009); no significant improvements in BPRS or YMRS. Of the remitting patients (n = 5), 2/5 sustained improvement during 4 weeks FU |
Response criteria: ≥ 50% decrease in MADRS total score from baseline Response rate 80% (8/10) |
Relapse rate 60% (3/5) | Mild, transient AEs with significant dissociative effects |
No control group Limited statistical power due to small sample size |
Y | Y |
| Shiroma et al. [30] | Single arm, open-label, 12 days |
Aged 18–70 years MDD with ongoing MDE (HDRS ≥ 14) Lack of response to ≥ 2 antidepressant trials in current episode |
Ketamine 0.5 mg/kg IV up to six times over a 12-day period (n = 14 starting, n = 12 completing) No control group |
For participants who completed six infusions (12/14), significant antidepressant was observed after 12 days (response rate 91.7%; remission rate 66.6%); the mean time in patients who relapsed (6/10) was 16 days (range 7–28 days) |
Response criteria: ≥ 50% improvement MADRS Response rate 91.7% (11/12) |
Relapse rate (at 4 weeks FU) 54.5% (6/11) | Mild, transient AEs with significant dissociative effects |
No control group Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
| Singh et al. [37] | RCT, 4 weeks |
Aged 18–64 years MDD with ongoing MDE (IDS-C30 ≥ 34) Lack of response to ≥ 1 antidepressant trial in current episode |
Patients were randomized into four arms to receive either: ketamine 0.5 mg/kg IV twice weekly (n = 18 starting, 12 completing), ketamine 0.5 mg/kg IV thrice weekly (n = 17 starting, 11 completing), saline placebo twice weekly (n = 17 starting, 1 completing) or saline placebo thrice weekly (n = 16 starting, 1 completing) | Significant improvement in MADRS score from baseline at 15 days was observed in both ketamine frequency groups compared to the placebo (P ≤ 0.001, P ≤ 0.001 respectively). Similarly, the mean MADRS change continued to improve from baseline to 29 days in both ketamine frequency groups. No statistical difference was seen between the ketamine groups throughout the study to 18 days FU |
Response criteria: ≥ 50% from baseline in MADRS score Response rate (ketamine 2 times/week) 92.3% (12/13) *excluded discontinued patients for reasons other than lack of efficacy Response rate (ketamine 3 times/week) 84.6% (11/13) *excluded discontinued patients for reasons other than lack of efficacy |
Not reported in study |
Mild, transient AEs with significant dissociative effects No significant difference in AEs between similar treatment groups |
No active control Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
| Vande Voort et al. [12] | Single arm, open-label, 2 weeks |
Aged 18–64 years MDD or BP with ongoing MDE Lack of response to ≥ 2 antidepressant trials in current episode |
Ketamine 0.5 mg/kg IV up to 6 times thrice weekly (n = 12 starting, n = 7 completing) No control group |
The entire cohort (n = 12) including non-remitters (7/12), by end of treatment had improvement in depressive symptoms (mean reduction in MADRS of 41.5%; this improvement in MADRS was more significant in remitters MADRS of 79.1% (P ≤ 0.001) No significant improvements in YMRS. All remitting patients, (5/5) sustained improvement during 4-week FU; overall response rate of 58.3% |
Response criteria: ≥ 50% reduction from baseline in MADRS total score Response rate 58.3% (7/12) |
Relapse rate (at 4 weeks FU) 80% (4/5) | Mild, transient AEs with significant dissociative effects |
No control group Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
| Wilkinson et al. [31] | Open-label, 10 week |
Aged 18–65 years MDD with ongoing MDE (IDS-C30 ≥ 34) Lack of response to ≥ 2 antidepressant trials in current episode |
Ketamine 0.5 mg/kg IV up to 4 times over 2 weeks concurrent with 12 sessions of CBT over 10 weeks (n = 16) No control group |
Significant antidepressant effect was observed after 2 weeks (response rate 50%; remission rate 43.8%); the median time for responders who relapsed (5/8) was 12 weeks following ketamine exposure Nonresponders showed significant improvement in MADRS until week 3, then showed no difference; among remitters, (3/7) maintained remission until 4 weeks, and (2/7) until 8 weeks |
Response criteria: ≥ 50% in MADRS score from baseline Response rate 50% (8/16) |
Relapse rate (at 8 weeks post-ketamine) 25% (2/8) | Not mentioned |
No control group Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
| Yoosefi et al. [36] | RCT, 2 weeks |
Aged 20–50 years MDD with ongoing MDE (HAM-D ≥ 18) History of treatment resistance to previous antidepressant trials |
Patients were randomized to receive thiopental 2–3 mg/kg IV (n = 14) or ketamine 1–2 mg/kg IV (n = 15); patients then received ECT thrice weekly for 2 weeks (n = 29 starting, n = 27 completing) |
Significant antidepressant effect was observed 48 h after first ECT only in ketamine group (P = 0.002); there was no significant difference between HAM-D improvements in treatment groups at end of study Patients in the ketamine group showed significant improvement in cognitive function using a paired t test analysis of MMSE scores (P = 0.004); this was not significant in the thiopental group (P = 0.37) |
Response criteria: 60% reduction in the score of the baseline HAM-D Response rate not reported in study |
Not reported in study | None |
No control group Unable to record seizure duration by EEG Limited statistical power due to small sample size Unclear method for monitoring AEs |
Y | Y |
TRD the study sample was treatment resistant to depression, MADRS Montgomery–Asberg Depression Rating Scale, MDD major depressive disorder, MDE major depressive episode, IV intravenous, BPRS+ Brief Psychiatric Rating Scale, AE adverse effect, Y yes (meets criteria for satisfaction), RCT randomized controlled trial, BP bipolar disorder, HAM-D Hamilton Depression Rating Scale, DCS d-cycloserine, QIDS-SR Quick Inventory of Depressive Symptomatology, Clinician Rating, CGI-S Clinical Global Impression Scale, FU follow-up, IM intramuscular injection, SC subcutaneous injection, CADSS Clinician-Administered Dissociative States Scale, IDS-C30 Inventory of Depressive Symptomatology Clinician Rating, HDRS Hamilton Depression Rating Scale, MMSE Mini-Mental State Examination, SI suicidal ideation
*Intravenously administered racemic ketamine was used unless otherwise specified
Table 2.
Summary of retrospective studies, including case series and case reports on prolonging ketamine treatment
| Study | No. of cases | Dose and duration | Description | Prolonged | TRD |
|---|---|---|---|---|---|
| Best et al. [2] | 28 |
Frequency of treatment was dependent on patient responsiveness (10–30 sessions) CTK included pre-treatment with rTMS, ketamine 0.4–2.3 mg/kg IV until patient stiffened, and then TMS was administered |
Chart review of patients with TRD who received combination therapy with TMS and ketamine (CTK) to determine the efficacy of the combined antidepressant therapies. A significant mean reduction in CGI severity following CTK treatment completion was observed (P ≤ 0.0001). This reduction was sustained for 2 years following treatment completion (P ≤ 0.0001). CTK allowed for greater TMS intensities than would otherwise be tolerated. CTK was well tolerated, with no treatment-limiting adverse outcomes throughout the study | Y | Y |
| Messer et al. [38] | 2 |
Patient A: 6 doses of ketamine 0.5 mg/kg IV (on days 1, 3, 5, 7, 9, 11) Patient B: 2 doses of 0.5 mg/kg IV (on days 1, 7) and 4 saline infusions (on days 3, 5, 9, 11) |
A 50-year-old man with a history of depression and concomitant suicidal ideation with comorbid sleep apnea and obesity was treated with 11 medication trials and seven right unilateral ECTs and reported no change in mood. He received six doses of ketamine intravenously every other day during a 12-day period. He achieved a sustained clinical response (BDI ≤ 18) on day 4 with only mild, transient adverse effects; his response lasted until day 39. Repeated treatments of ketamine every other day for 12 days produced sustained remission of his depressive symptoms A 45-year-old man with a history of treatment-resistant major depressive disorder with comorbid hypertension was treated with 9 medication trials and 105 ECT treatments, producing short-term and long-term memory loss, and an incomplete recovery from depression. He also received implantation of a vagal nerve stimulator 5 years prior. He received two doses of ketamine intravenously on days 1 and 7 within the 12-day period, and saline on days 3, 5, 9, and 11. His MMSE score was 30 at baseline. He achieved a sustained clinical response (BDI ≤ 18) on day 2 with only mild, transient adverse effects; his response lasted until day 24. Repeated treatments of ketamine twice produced sustained remission of his depressive symptoms |
Y | Y |
| Messer et al. [39] | 1 | Three doses of ketamine 0.5 mg/kg IV every other day for 5 days, followed by 3 series of 6 doses of ketamine 0.5 mg/kg IV given over 16 weeks, followed by a maintenance dose of ketamine 0.5 mg/kg IV given every 3 weeks over 15 months | A 46-year-old woman with a history of treatment-resistant major depressive disorder was treated with 24 medication trials and 273 ECT treatments, producing short-term and long-term memory loss and an incomplete recovery from depression. All interventions have produced only short-lived remission. Her long-term use of ECT caused significant problems with memory loss and focused attention. She received ketamine 0.5 mg/kg IV which led to a dramatic improvement in her depressive symptoms (DBI 22 to 6). She then received three additional infusions every other day for 5 days. This produced a remission lasting for 17 days after the last infusion. Three series of six infusions were given on alternating business days over the following 16 weeks. These three series produced remissions lasting 16, 28, and 16 days, respectively. A maintenance dose of ketamine 0.5 mg/kg IV was then established at a 3-week inter-dose interval. She then remained in remission for > 15 months with only mild, transient adverse effects typical of ketamine | Y | Y |
| Szymkowicz et al. [40] | 3 | Up to 6 doses of ketamine 0.5 mg/kg IV with doses occurring every other day followed by an individualized maintenance dose of ketamine 0.5 mg/kg IV | Three cases of TRD with suicide ideation are reported in which repeated doses of ketamine IV were provided. The first patient had rapid and moderately sustained depression symptom relief 4 h post-infusion 1 (MADRS ≤ 8). The second patient only achieved MADRS scores of a moderate level of depression 4 h post-infusion 4. The third patient also only achieved MADRS scores of a moderate level of depression 4 h post-infusion 4; however, significant functional improvement was observed. The total number of ketamine treatments varied from 16, 34, and 32, respectively. Dissociative effects of worsened cognitive difficulties and insomnia post-ketamine treatment in patient 2; however, no other adverse effects were noted | Y | Y |
TRD the study sample was treatment resistant to depression, rTMS repetitive transcranial magnetic stimulation, TMS transcranial magnetic stimulation, CGI the Clinical Global Impression Scale, Y yes (meets criteria for satisfaction), ECT electroconvulsive therapy, BDI Beck Depression Inventory, MMSE Mini-Mental State Examination, MADRS Montgomery–Asberg Depression Rating Scale
*Intravenously administered racemic ketamine was used unless otherwise specified
Prospective Studies Assessing Prolonging Ketamine’s Antidepressant Effects in MDD
A total of ten RCTs and eight open-label trials (Table 1) were identified that assessed the extension of racemic IV ketamine’s antidepressant effect using a pharmacological intervention (i.e., clonidine, d-cycloserine, lamotrigine, lithium, rapamycin, riluzole; k = 5), psychotherapy (i.e., cognitive behavioral therapy [CBT]; k = 1), electroconvulsive therapy (ECT) (k = 1), or IV ketamine monotherapy (k = 9). The majority of prospective studies (12/18) included repeat ketamine infusions [12, 13, 22–25, 27–32].
Of the six studies involving oral agents concurrent with ketamine (pooled n = 177), all studies, except one, reported no statistically significant effect of the adjunct intervention compared to ketamine alone. The exception was a RCT completed by Abdallah et al. [33], where a single dose of rapamycin (6 mg) prior to a single dose of IV ketamine showed significantly higher response and remission rates after 2 weeks. Between the rapamycin and placebo trail completers, higher response rates (41%) and remission rates (29%) were reported in the preinfusion rapamycin treatment group compared to the response rates (13%) and remission rates (7%) in the placebo arm (P = 0.04, P = 0.003 respectively).
In a 32-day RCT (n = 26) by Mathew et al. [34], daily riluzole continuation (100–200 mg/day) did not significantly prolong post-ketamine relapse after 1 month [34]. Time-to-relapse was the primary outcome measure. During the 32-day trial, riluzole did not demonstrate a significant difference in time-to-relapse compared to the placebo group, who only received 0.5 mg/kg IV ketamine and a placebo add-on (24.4 days vs 22.0 days; P = 0.68), with a relapse rate of 80% vs 50% for the riluzole and placebo trial completers, respectively. As a result of the foregoing relapse rates, the RCT was terminated prematurely [34]. Furthermore, no significant improvements were found in Montgomery–Asberg Depression Rating Scale (MADRS) scores post-ketamine treatment (P = 0.36) [34].
While no other studies were terminated prematurely, similar, non-significant outcomes were observed in some studies, including no statistically significant differences in sustained reduction of depression (sustained reduction rate 40% vs 20%) in an 8-week RCT (n = 20) by Lenze et al. [35]; no Hamilton Depression Rating Scale (HAM-D) reductions between d-cycloserine (DCS) or placebo in follow-up (P = 0.30) in a 6-week RCT (n = 32) by Chen et al. [20]; and no differences between treatment groups in continuing the antidepressant effect of IV ketamine in a 2-week RCT (n = 34) by Costi et al. [24].
In a 4-week RCT (n = 42) by Ibrahim et al. [26], no significant improvements in MADRS score were observed between treatment groups after week 4. Moreover, no significant difference in days-to-relapse were observed, with 17.2 and 9.8 days to relapse in the ketamine–riluzole (a single dose of 0.5 mg/kg IV ketamine then 100–200 mg daily of riluzole) and the ketamine–placebo groups, respectively (0.5 mg/kg IV ketamine then placebo once daily).
The response and relapse rate in six studies studying oral agents (i.e., clonidine, d-cycloserine, lamotrigine, lithium, rapamycin, and riluzole) concurrent with IV ketamine were widely reported (k = 5/6 [20, 26, 33–35]; k = 3/6 [24, 26, 34], respectively). The total mean response rate for oral agents in these RCTs was 56.6% (72/127). Furthermore, the foregoing studies reported a total relapse rate of 64.1% (25/39) at the end of their study durations.
Within the foregoing six studies, the primary aim was to prolong the efficacy of ketamine using an oral agent as continuation therapy. All six oral continuation trials were well tolerated without any significant safety concerns reported. Three trials had no significant reported dissociative effects [20, 26, 35]; however, the remaining three trials [24, 33, 34] did report significant, although mild and transient dissociative effects.
An open-label trial (n = 16) by Wilkinson et al. [31] evaluated repeat-dose ketamine infusions of 0.5 mg/kg (up to four infusions) administered over 2 weeks, concurrent with 12 sessions of CBT over 10 weeks. In this sample all patients were diagnosed with MDD, ongoing major depressive episode (MDE), and treatment resistance, defined as a lack of response to at least two antidepressant trials in their current episode. A significant antidepressant effect was observed following 2 weeks of IV ketamine treatment with 50% (8/16) of patients as responders and 43.8% (7/16) of patients as remitters. For responders (5/8), the median time to relapse following ketamine treatment was 12 weeks, whereas nonresponders only had significant improvement in MADRS scores until week 3 and showed no further improvement thereafter. Remitters (3/7) maintained remission until week 4, with some patients (2/7) maintaining clinical remission until 8 weeks post-ketamine treatment. Responders (2/8) reported a relapse at week 8 post-ketamine treatment. Adverse effects were not recorded in the study [31].
In a smaller RCT (n = 29), Yoosefi et al. [36] assessed the efficacy of IV ketamine with thiopental in patients receiving ECT (primary outcome). Patients were randomized to receive either 2–3 mg/kg of IV thiopental or 1–2 mg/kg of IV ketamine, followed by thrice weekly ECT for 2 weeks. In patients diagnosed with MDD, a history of treatment resistance to previous antidepressant trials and an ongoing MDE with moderate-to-severe symptoms based on a Hamilton Depression Rating Scale (HDRS) of 18 or higher was associated with significant antidepressant effects within 48 h of the ECT in only the ketamine group (P = 0.002).
It was reported that no significant differences between HAM-D improvements in treatment groups were observed at the end of the study. Subjects in the ketamine group demonstrated significant improvement in cognitive function, as indicated by a paired samples t test analysis of Mini-Mental State Examination (MMSE) scores (P = 0.004). Conversely, the improvement in cognitive function as measured by the MMSE scores was not significant in the thiopental group (P = 0.37). No adverse effects from either the ketamine or the ECT treatment were reported.
Nine studies ranging in duration from 12 days to 6 weeks analyzed antidepressant effects in IV ketamine monotherapy and time to relapse [12, 13, 22, 25, 27–30, 32]. The included patients were diagnosed with MDD and were treatment resistant, defined as a lack of response to varying numbers of antidepressant trials. Of these prospective studies, eight were open-label trials [12, 13, 22, 25, 27, 28, 30, 32] and two were RCTs [29, 32] with a total of 166 patients receiving repeated ketamine monotherapy. As a result of the disparate dosing intensity as well as frequency of ketamine administration, the mean response rate reported in those that completed all ketamine infusions also varied from 25% [32] to 92% [30], both in open-label trials. The total mean response rate for these repeat-ketamine trials was 64.2% (95/148). The reported relapse rate was 77.8% (63/81). Similarly, the mean time until relapse following the final ketamine infusion varied from 16 days [30] to 24 days [34], both in open-label trials. All nine IV ketamine monotherapy trials were reported with good profiles of tolerability and safety, with no significant differences in adverse effects or discontinuation between treatment groups. Moreover, most reported adverse events were transient and mild in dissociation [12, 13, 22, 25, 27, 28, 30, 32].
The criteria for response varied within the selected studies; for example, most of the prospective studies (14/18) used the definition of at least 50% reduction in MADRS scores compared to the baseline or the previous ketamine infusion [12, 13, 21, 22, 26–31, 33–35, 37]. Four studies, all involving up to six repeat-ketamine infusions over a period of 12–14 days, recorded depressive symptoms using MADRS scores [12, 22, 28, 30]. The foregoing studies reported similar baseline scores with a mean MADRS baseline score of 30.85 ± 1.66. Consequently, MADRS score between these studies improved significantly within 24 h, with aan het Rot et al. [22] reporting 6.9 ± 2.8 and Murrough et al. [28] reporting 18.8 ± 5.5. At study endpoint, MADRS scores showed further variation observed at 29.7 ± 6.4, 18.9 ± 6.6, and 5.4 ± 3.4, respectively [12, 22, 28]. Of the trials reporting study endpoint MADRS scores, the duration of ketamine infusions also varied from 40 to over 100 min [12, 22, 28].
Other studies (4/18) using the HDRS defined response as at least 50% improvement in HDRS scores [20, 25, 32, 36] (Table 1). Two similar studies, both using repeat ketamine with a study duration of 3 weeks, recorded depressive symptoms using HDRS scores [25, 32]. The two other studies [25, 32] included participants with moderate-to-severe symptom severity on the HDRS at baseline (i.e., HDRS scores of 31.6 ± 5.2 and 28.6 ± 4.8, respectively). The antidepressant effects observed at week 3 did not endure, with each study group reporting relapse rates of 83.3% (10/12) and 80% (4/5), respectively [25, 32].
Retrospective Studies Assessing Prolonging Ketamine’s Antidepressant Effects for MDD
Retrospective studies including one chart review (28 cases) [2] and three case reports (with 1–3 cases per article) [38–40] were identified. A total of four patients received psychopharmacological and psychological therapies, and 28 patients received transcranial magnetic stimulation (TMS), to prolong the antidepressant effects of IV ketamine (Table 2). All patients enrolled in the retrospective studies were diagnosed with TRD and reported suicidal ideation [2, 38–40]. Best et al. [2] reported (n = 28) on the efficacy of combination TMS with ketamine (CTK). The course of treatment involved 10–30 sessions of coincident treatment of 0.4–2.3 mg/kg IV ketamine (20 min) and high-output TMS (30 min). A significant mean reduction in Clinical Global Impression-Severity (CGI-S) following CTK treatment was observed (P ≤ 0.0001), along with a sustained effect 2 years post-CTK treatment (α = 0.01; t = 27.36; P < 0.0001). Long-term efficacy was reported with a mean difference in CGI-S scores from baseline to 2 years following completion of treatment of 4.68 ± 0.47 [2].
In the three case studies, patients were given repeat-dose ketamine infusions, followed by maintenance ketamine infusions if deemed necessary [38–40]. Overall, IV ketamine infusions were well tolerated and deemed safe [2, 38–40], with significant dissociative effects only reported in one case study [40], and other mild, transient dissociative effects reported in all studies [2, 38–40] (Table 2). The antidepressant effects reported by Best et al., demonstrate a promising mean reduction in the CGI-S group value from 6.1 ± 0.8 to 1.7 ± 0.7 [2]. The prolongation of ketamine’s initial antidepressant effect was observed in all four retrospective studies [2, 38–40], with sustained effects varying greatly from 17 days to 2 years with varying adjunct treatment types, dosages, and frequency of dosages [38]. All studies included patients that had TRD, with some patients being unsuccessfully treated previously with upwards of 24 medication trials and 273 ECTs [39].
Discussion
As part of this systematic review, ten RCTs (N = 266 patients receiving ketamine), eight prospective open-label trials (N = 115 patients receiving ketamine), and four retrospective studies (total N = 34 patients receiving ketamine) were included, for a total of 415 patients receiving IV ketamine for TRD. The quality of studies varied and were characterized by a lack of control groups, lack of blinding, small sample sizes (i.e., limited generalizability), and heterogenous methodology for ascertaining and recording adverse effects. Many of the RCTs were of moderate to high quality. Taken together, the evidence base is insufficient with respect to concluding that any specific modality of treatment (other than repeat-dose IV ketamine or intranasal esketamine) has demonstrated safety and maintenance efficacy in acute ketamine responders. Numerous themes emanated and surrounded our analysis herein.
The extant literature contains numerous studies that utilize repeat-dose IV ketamine monotherapy as a means of sustaining the antidepressant effects following ketamine infusion. The most effective frequency of 0.5 mg/kg dosing was deemed to be twice weekly [37]. All studies included in this systematic review were delimited to ketamine in their study protocol, except for Loo et al. [27], and recorded ketamine infusions about two or three times weekly. There remains, however, a lack of sufficient data with respect to IV ketamine dosing intensity and frequency; a priority for future research.
Moreover, preliminary data suggests that neurostimulatory and/or psychotherapeutic strategies may possibly prolong the antidepressant efficacy of ketamine [42, 43]. Regarding psychotherapy which includes but is not limited to modalities like CBT, the initial outcome of the open-label trial by Wilkinson et al. [31] where up to four infusions of IV ketamine over 2 weeks were administered, concurrent with 12 sessions of CBT over 10 weeks, produced a median relapse time of 12 weeks in the initial responders to ketamine and offered some degree of symptomatic benefit. The lack of control group, however, precludes decisive conclusions on the adjunctive effects of ketamine and CBT.
Best et al. [2] reported that rTMS combined with ketamine resulted in a sustained reduction of CGI severity for up to two years. TMS was well tolerated with no treatment-limiting adverse outcomes from this neurostimulation throughout their study [2, 42]. It was also reported that ECT was well tolerated with a suggestion of greater efficacy in combination with ketamine. The foregoing benefit was not limited by significant cognitive impairment [36, 38, 39].
Another theme that arose from the current systematic review was the absence of studies evaluating whether oral agents could prolong the antidepressant effects of IV ketamine. Oral agents tested were clonidine [35], d-cycloserine [20], lamotrigine [34], lithium [24], rapamycin [33], and riluzole [26, 34], many of which had significant limitations within their study protocol [35]. It is also reported that lithium dosed to achieve higher peripheral levels may provide a more robust and/or more sustained antidepressant effect [24]. The overall response and relapse rates (i.e., 59.1% and 64.1%, respectively) do not appear significantly different from what is reported from repeat-dose IV ketamine monotherapy (i.e., 64.2% and 77.8%, respectively).
There are several methodological aspects that affect inferences and interpretations of our findings. The overarching limitation is the lack of large, replicated, rigorous, randomized control trials with well-characterized populations and standardized outcome measures. A second major limitation is the heterogeneity of populations that were studied as well as lack of sufficient data with respect to patient characteristics and the degree of treatment resistance/prior longitudinal course before study entry [4]. Additional limitations include but are not limited to the absence of a traditional trial design that would re-randomize “enriched acute responders” with appropriate survival statistics.
Conclusions
These results indicate that repeat-dose ketamine monotherapy, albeit insufficiently studied with RCT and placebo, does demonstrate efficacy prolongation in persons with TRD who initially respond to IV ketamine. Moreover, ECT, CBT, and rapamycin concurrent with ketamine treatment for patients with TRD also demonstrate preliminary evidence of efficacy. Most oral agents (i.e., clonidine, d-cycloserine, lamotrigine, lithium, and riluzole) studied concurrently or following a single ketamine infusion were not found to have significant effect in prolonging efficacy in adults with TRD.
Repeat-dose IV ketamine has demonstrated preliminary evidence for maintaining efficacy, and RCT evidence supports repeat-dose esketamine as a maintenance treatment strategy in TRD (this review focused primarily on ketamine rather than esketamine) [20]. Intravenous ketamine treatment for TRD still remains a safe and tolerable short-term intervention; however, further large-scale research is needed on the long-term efficacy and safety of ketamine within this patient population [20].
Acknowledgements
Funding
The authors have received no funding for writing this review. No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Eric P. McMullen: data curation, investigation, project administration, visualization, writing – original draft. Yena Lee: writing – review and editing. Orly Lipsitz: writing – review and editing. Leanna M.W. Lui: writing – review and editing. Maj Vinberg: writing – review and editing. Roger Ho: writing – review and editing. Nelson B. Rodrigues: writing – review and editing. Joshua D. Rosenblat: writing – review and editing. Bing Cao: writing – review and editing. Hartej Gill: writing – review and editing. Kayla M. Teopiz: writing – review and editing. Danielle S. Cha: writing – review and editing. Roger S. McIntyre: conceptualization, project administration, visualization, supervision, writing – review and editing.
Disclosures
Dr. Roger McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Minerva, Intra-Cellular, Abbvie, and Eisai. Dr. Roger McIntyre is a shareholder and CEO of Champignon Brands, Inc. Dr Roger McIntyre is also a member of the journal’s Editorial Board. Dr. Joshua Rosenblat has received research grant support from the Canadian Cancer Society, Canadian Psychiatric Association, American Psychiatric Association, American Society of Psychopharmacology, University of Toronto, University Health Network Centre for Mental Health, Joseph M. West Family Memorial Fund and Timeposters Fellowship and industry funding for speaker/consultation/research fees from Janssen, Allergan, Lundbeck, Sunovion and COMPASS. He is the medical director of a private clinic providing intravenous ketamine infusions and intranasal esketamine for depression. Dr. Rosenblat has received research grant support from the Canadian Cancer Society, Canadian Psychiatric Association, American Psychiatric Association, American Society of Psychopharmacology, University of Toronto, University Health Network Centre for Mental Health, Joseph M. West Family Memorial Fund and Timeposters Fellowship and industry funding for speaker/consultation/research fees from Janssen, Allergan, Lundbeck, Sunovion and COMPASS. He is the medical director of a private clinic providing intravenous ketamine infusions and intranasal esketamine for depression. Dr. Maj Vinberg has received consultancy fees from Lundbeck, Sunovion and Janssen/Cilag within the last three years. Dr. Danielle Cha reports receiving royalties from Oxford University Press and Cambridge University Press outside the submitted work. Dr. Cha has also received honoraria from Lundbeck outside the submitted work. Eric McMullen, Yena Lee, Orly Lipsitz, Leanna Lui, Roger Ho, Nelson Rodrigues, Bing Cao, Hartej Gill and Kayla Teopiz have have no disclosures to make.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Nestler E, Gould E, Manji H. Preclinical models: status of basic research in depression. Biol Psychiat. 2002;52(6):503–528. doi: 10.1016/S0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 2.Best S, Pavel D, Haustrup N. Combination therapy with transcranial magnetic stimulation and ketamine for treatment-resistant depression: a long-term retrospective review of clinical use. Heliyon. 2019;5(8):e02187. doi: 10.1016/j.heliyon.2019.e02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre R, Filteau M, Martin L, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes B, Warden D, Trivedi M, Wisniewski S, Fava M, Rush A. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 5.Stassen H, Delini-Stula A, Angst J. Time course of improvement under antidepressant treatment: a survival-analytical approach. Eur Neuropsychopharmacol. 1993;3(2):127–135. doi: 10.1016/0924-977X(93)90264-M. [DOI] [PubMed] [Google Scholar]
- 6.Machado-Vieira R, Baumann J, Wheeler-Castillo C, et al. The timing of antidepressant effects: a comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010;3(1):19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanos P, Moaddel R, Morris P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621–660. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal G, Bandeira I, Correia-Melo F, et al. Intravenous arketamine for treatment-resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2020 doi: 10.1007/s00406-020-01110-5. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre R, Carvalho I, Lui L, et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576–584. doi: 10.1016/j.jad.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 10.Xiong J, Lipsitz O, Chen-Li D, et al. The acute antisuicidal effects of single-dose intravenous ketamine and intranasal esketamine in individuals with major depression and bipolar disorders: a systematic review and meta-analysis. J Psychiatr Res. 2021;134:57–68. doi: 10.1016/j.jpsychires.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Marcantoni W, Akoumba B, Wassef M, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009–January 2019. J Affect Disord. 2020;277:831–841. doi: 10.1016/j.jad.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Vande Voort J, Morgan R, Kung S, et al. Continuation phase intravenous ketamine in adults with treatment-resistant depression. J Affect Disord. 2016;206:300–304. doi: 10.1016/j.jad.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen K, Lineberry T, Galardy C, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 14.Cooper M, Rosenblat J, Cha D, Lee Y, Kakar R, McIntyre R. Strategies to mitigate dissociative and psychotomimetic effects of ketamine in the treatment of major depressive episodes: a narrative review. World J Biol Psychiatry. 2016;18(6):410–423. doi: 10.3109/15622975.2016.1139747. [DOI] [PubMed] [Google Scholar]
- 15.Lipsitz O, McIntyre R, Rodrigues N, et al. Early symptomatic improvements as a predictor of response to repeated-dose intravenous ketamine: results from the Canadian Rapid Treatment Center of Excellence. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110126. doi: 10.1016/j.pnpbp.2020.110126. [DOI] [PubMed] [Google Scholar]
- 16.Wan L, Levitch C, Perez A, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2014;76(03):247–252. doi: 10.4088/JCP.13m08852. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues N, McIntyre R, Lipsitz O, et al. Safety and tolerability of IV ketamine in adults with major depressive or bipolar disorder: results from the Canadian rapid treatment center of excellence. Expert Opin Drug Saf. 2020;19(8):1031–1040. doi: 10.1080/14740338.2020.1776699. [DOI] [PubMed] [Google Scholar]
- 18.Lipsitz O, Di Vincenzo J, Rodrigues N, et al. Safety, tolerability, and real-world effectiveness of intravenous ketamine in older adults with treatment-resistant depression: a case series. Am J Geriatr Psychiatry. 2021 doi: 10.1016/j.jagp.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Ng J, Lui L, Rosenblat J, et al. Ketamine-induced urological toxicity: potential mechanisms and translation for adults with mood disorders receiving ketamine treatment. Psychopharmacology. 2021 doi: 10.1007/s00213-021-05767-1. [DOI] [PubMed] [Google Scholar]
- 20.Daly E, Trivedi M, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiat. 2019;76(9):893. doi: 10.1001/jamapsychiatry.2019.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz J, Murrough J, Iosifescu D. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health. 2016;19(2):35–38. doi: 10.1136/eb-2016-102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.aan het Rot M, Collins K, Murrough J, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Cheng C, Gueorguieva R, et al. Maintenance of antidepressant and antisuicidal effects by d-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo–control study. Neuropsychopharmacology. 2019;44(12):2112–2118. doi: 10.1038/s41386-019-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costi S, Soleimani L, Glasgow A, et al. Lithium continuation therapy following ketamine in patients with treatment resistant unipolar depression: a randomized controlled trial. Neuropsychopharmacology. 2019;44(10):1812–1819. doi: 10.1038/s41386-019-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cusin C, Ionescu D, Pavone K, et al. Ketamine augmentation for outpatients with treatment-resistant depression: preliminary evidence for two-step intravenous dose escalation. Aust N Z J Psychiatry. 2016;51(1):55–64. doi: 10.1177/0004867416631828. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim L, DiazGranados N, Franco-Chaves J, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on Riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37(6):1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loo C, Gálvez V, O'Keefe E, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand. 2016;134(1):48–56. doi: 10.1111/acps.12572. [DOI] [PubMed] [Google Scholar]
- 28.Murrough J, Perez A, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips J, Norris S, Talbot J, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176(5):401–409. doi: 10.1176/appi.ajp.2018.18070834. [DOI] [PubMed] [Google Scholar]
- 30.Shiroma P, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson S, Wright D, Fasula M, et al. Cognitive behavior therapy may sustain antidepressant effects of intravenous ketamine in treatment-resistant depression. Psychother Psychosom. 2017;86(3):162–167. doi: 10.1159/000457960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ionescu D, Bentley K, Eikermann M, et al. Repeat dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord. 2019;243:516–524. doi: 10.1016/j.jad.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Abdallah C, Averill L, Gueorguieva R, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology. 2020;45(6):990–997. doi: 10.1038/s41386-020-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew S, Murrough J, aan het Rot M, Collins K, Reich D, Charney D. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13(1):71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenze E, Farber N, Kharasch E, et al. Ninety-six hour ketamine infusion with co-administered clonidine for treatment-resistant depression: a pilot randomised controlled trial. World J Biol Psychiatry. 2016;17(3):230–238. doi: 10.3109/15622975.2016.1142607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoosefi A, Sepehri A, Kargar M, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder. J ECT. 2014;30(1):15–21. doi: 10.1097/yct.0b013e3182a4b4c6. [DOI] [PubMed] [Google Scholar]
- 37.Singh J, Fedgchin M, Daly E, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173(8):816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 38.Messer M, Haller I, Larson P, Pattison-Crisostomo J, Gessert C. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22(4):442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- 39.Messer M, Haller I. Maintenance ketamine treatment produces long-term recovery from depression. Primary Psychiatry. 2010;17(4):48–50. https://sa1s3.patientpop.com/assets/docs/55882.pdf
- 40.Szymkowicz S, Finnegan N, Dale R. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J Affect Disord. 2013;147(1–3):416–420. doi: 10.1016/j.jad.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fava M, Freeman M, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) Mol Psychiatry. 2018;25(7):1592–1603. doi: 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pradhan B, Parikh T, Makani R, Sahoo M. Ketamine, transcranial magnetic stimulation, and depression specific yoga and mindfulness based cognitive therapy in management of treatment resistant depression: review and some data on efficacy. Depress Res Treat. 2015;2015:1–14. doi: 10.1155/2015/842817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, Brietzke E, Dodd S, Gorwood P, Ho R, Iosifescu DV, Lopez Jaramillo C, Kasper S, Kratiuk K, Lee JG, Lee Y, Lui LMW, Mansur RB, Papakostas GI, Subramaniapillai M, Thase M, Vieta E, Young AH, Zarate CA, Jr, Stahl S. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021 doi: 10.1176/appi.ajp.2020.20081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.


