Abstract
To flourish, cancers greatly depend on their surrounding tumor microenvironment (TME), and cancer-associated fibroblasts (CAFs) in TME are critical for cancer occurrence and progression because of their versatile roles in extracellular matrix remodeling, maintenance of stemness, blood vessel formation, modulation of tumor metabolism, immune response, and promotion of cancer cell proliferation, migration, invasion, and therapeutic resistance. CAFs are highly heterogeneous stromal cells and their crosstalk with cancer cells is mediated by a complex and intricate signaling network consisting of transforming growth factor-beta, phosphoinositide 3-kinase/AKT/mammalian target of rapamycin, mitogen-activated protein kinase, Wnt, Janus kinase/signal transducers and activators of transcription, epidermal growth factor receptor, Hippo, and nuclear factor kappa-light-chain-enhancer of activated B cells, etc., signaling pathways. These signals in CAFs exhibit their own special characteristics during the cancer progression and have the potential to be targeted for anticancer therapy. Therefore, a comprehensive understanding of these signaling cascades in interactions between cancer cells and CAFs is necessary to fully realize the pivotal roles of CAFs in cancers. Herein, in this review, we will summarize the enormous amounts of findings on the signals mediating crosstalk of CAFs with cancer cells and its related targets or trials. Further, we hypothesize three potential targeting strategies, including, namely, epithelial–mesenchymal common targets, sequential target perturbation, and crosstalk-directed signaling targets, paving the way for CAF-directed or host cell-directed antitumor therapy.
Subject terms: Drug development, Cancer microenvironment
Introduction
Cancer, as a major public health problem worldwide, is the second leading cause of death with an estimated 10.0 million globally in 2020.1,2 Majority of cancer deaths from cancers are caused by local recurrence and/or distant organ/tissue metastasis.3,4 If the cancers are identified in the early stage and occur in the original lesion site, the total 5-year relative survival rate of the ten most common cancers is ~34.2–100%, with a local recurrence rate of <16.1% after surgery, radiation, and/or chemotherapy, while the total 5-year relative survival rate drops to 2.5–30.2% for advanced cancers with frequent recurrences and/or metastasis,5,6 thereby requiring more aggressive treatments, including immunotherapy, biological therapy, or targeted therapy, etc. However, the recurrent or metastatic cancers can exhibit quick progression and/or become resistant to therapeutic strategies, which are exclusively or mainly aimed at cancer cells. One of the main reasons for the failure of cancer treatment is that the tumor microenvironment (TME) is fully or partially ignored in the development of antitumor therapy.7 Since cancer progression is highly associated with the physiological state of TME, targeting nonneoplastic stromal components that substantially contribute to tumor progression are considered for cancer treatment.8
Cancer-associated fibroblasts (CAFs), as the major components of TME, have been extensively explored and are known to be involved in diverse cellular processes, including cell differentiation, proliferation, and stemness; extracellular matrix (ECM) remodeling; and cell migration and apoptosis, all of which can exert critical roles in tumor biological behaviors, including tumorigenesis, tumor growth, energy metabolism, tumor immunity, angiogenesis, tumor progression, recurrence, and metastasis.9–11 The biological activities of CAFs are mediated by various intracellular and extracellular factors, especially those in signaling pathways closely related to cancer progression, which might be targeted for anticancer therapy. Since CAFs exert molecular and functional heterogeneity in different cancers and even in different stages of the same type of tumor and because of the specific crosstalk between CAFs and cancer cells,12 any therapeutic strategies developed should exploit the specificity and diversity of CAFs to optimize treatment efficacy for targeted therapy. To better understand the nature of CAFs, herein, we summarize historic milestones of the basic research and clinical studies on CAFs, especially those focused on precursors of CAFs and CAFs isolation, heterogeneity, signaling pathways, and involvement in cancer therapy and therapy resistance, and suggest new potential therapeutic strategies.
Definition of fibroblasts
Fibroblasts were firstly identified in the 1850s as spindle-shaped cells in connective tissue that can synthesize collagen13 and originated mostly from the primitive mesenchyme of the mesoderm,14 and, in some cases, partially from the neural crest of the ectoderm.15,16 Indeed, the definition of fibroblasts is constantly changing. It is widely accepted that fibroblasts in normal tissues are generally embedded within fibrillar ECM as single resting mesenchymal cells.17 They are also defined by their cell morphology, tissue location, and lack of the lineage markers that are expressed by other cells, such as epithelial cells and leukocytes.15 Currently, vimentin is widely used to compare fibroblasts with cells expressing epithelial markers, and fibroblast‐specific protein 1 (FSP1, also known as S100A4) is considered as a reliable marker for quiescent fibroblasts.18 However, vimentin and FSP1 are also expressed by cells in mesenchymal lineages in addition to fibroblasts; thus, cellular shape and location are frequently combined for the identification of fibroblasts,19–23 demonstrating that details on the lineage of fibroblasts remain to be determined.
Quiescent fibroblasts in the interstitial space are the major producers of ECM under normal physiological conditions and can be reversibly activated to facilitate repair and regeneration in response to tissue damage.24 Preceding their functioning in the regeneration stage, quiescent fibroblasts are activated into myofibroblasts and then accumulate at the sites of repair for wound healing.25 In these cases, activated fibroblasts secrete transforming growth factor-beta (TGF-β) and acquire a contractile phenotype via the expression of α-smooth muscle actin (α-SMA), thereby effectively closing wounds.26 In addition, fibroblasts are also critical for the homeostasis of adjacent epithelial cells, acting in an indirect paracrine manner, similar to that of growth factors27,28 or via direct mesenchymal–epithelial cell interactions.29,30 In angiogenesis with increased production of vascular endothelial growth factor A (VEGFA),31 the immune response, and keratinocyte proliferation, fibroblasts play roles by secreting cytokines and chemokines.27,32 Further, ECM development mediated by fibroblasts in lymph nodes acts as a “highway” to transport potential antigens and contributes to the migration of leukocytes,33 indicating that the structural roles of fibroblasts allow effective immune responses. Interestingly, when wounds heal, activated fibroblasts are restored to the quiescent phenotype owing to apoptosis,34 indicating that reversibility is a hallmark feature of fibroblasts associated with tissue repair.
From fibroblasts to CAFs in TME
Cellular sources and heterogeneity of CAFs
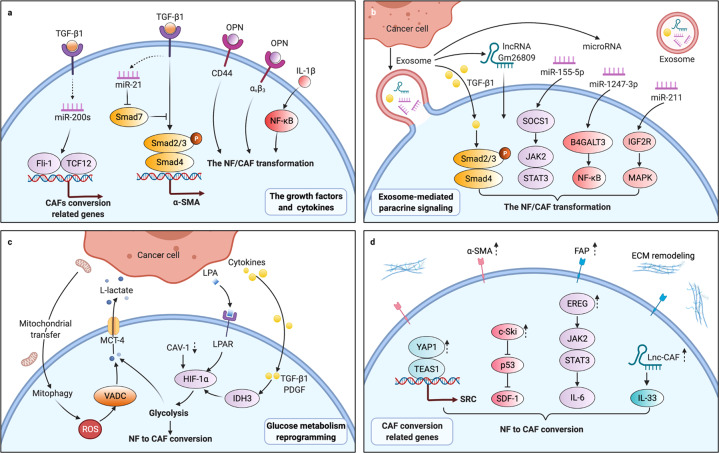
Cancers, as ongoing and unabated injurious stimuli, initiate fibroblasts irreversibility transition, driving acquisition of cancer-associated phenotypes (Fig. 1). The irreversibility transitions could be driven in a variety of ways. First, TME as a reservoir of growth factors, cytokines, and other factors signals to resident fibroblasts contributing to the transformation of normal fibroblasts (NFs) to CAFs. A diverse set of factors, including TGF-β1, osteopontin (OPN), and interleukin-1β (IL-1β), etc., which are released from cancer cells and/or immune cells,35 induce the transition of stromal fibroblasts to CAFs by regulating the TGF-β and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways.36–39 Then, exosomes also play essential roles in cellular communications, promoting fibroblasts to acquire new receptors or even genetic material from the cancer cells. Cancer-derived exosomes shuttling cargos such as microRNAs (miRNAs), long noncoding RNA (lncRNA) Gm26809, or TGF-β1 to reprogram NFs into CAFs via the downstream mitogen-activated protein kinase (MAPK), NF-κB, signal transducers and activators of transcription 3 (STAT3), or TGF-β signaling cascades.38,40–42 Also, a shift in energy metabolism such as aerobic glycolysis is potentially considered as a priming event in the conversion of NFs into CAFs. Lysophosphatidic acid (LPA), TGF-β1, or platelet-derived growth factor (PDGF) from cancer cells are able to induce aerobic glycolysis of fibroblasts via hypoxia-inducible factor-1α (HIF-1α) pathway; fibroblasts can also be metabolically reprogrammed via caveolin-1 (CAV-1) downregulation or cancer cell-derived mitochondrial transfer.43–46 In addition, various evidence has proven that the conversion of NFs into CAFs is accompanied by changes in the self-expression of certain components. For instance, Yes-associated protein 1 (YAP1) in NFs, as a transcriptional coactivator, modulates the transcription of SRC by forming a protein compound with TEA domain transcription factor-1 (TEAD1), resulting in cytoskeletal protein activation and ultimately transformation into CAFs.47 Overall, induced by cancer cells and TME, etc., NFs are activated to CAFs. Compared to resting NFs, CAFs acquire enhanced proliferative and secretory capabilities, which contribute to ECM remodeling, autocrine activation, and immunomodulatory function. Activated CAFs are characterized by different markers that are expressed at low levels or not expressed in NFs (Table 1). Among them, all or part of a combination with α-SMA, fibroblast-activated protein (FAP), and PDGF receptor α/β (PDGFRα/β) could be used to distinguish CAFs from NFs in cancers. Some markers, since CAFs are suggested to represent a heterogeneous population of cells,48 are required to characterize this heterogeneity.
Fig. 1.
Conversion from normal fibroblasts (NFs) to cancer-associated fibroblasts (CAFs). a Grow factors and cytokines such as transforming growth factor-beta 1 (TGF-β1), osteopontin (OPN), and IL-1β combined with their reporters in NFs, then activated the downstream effector including miRNAs and CD44, etc. to regulate the targeted gene expression of CAFs through TGF-β/Smads and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways. b Cancer-derived exosomes shuttling cargos such as miRNAs and lncRNAs transformed NFs to CAFs via the downstream signals including TGF-β/Smads, Janus kinase/signal transducers and activators of transcription (JAK/STAT), NF-κB and mitogen-activated protein kinase (MAPK) cascades. c NF-CAF conversion was driven by glucose metabolism reprogramming and hypoxia-inducible factor-1α (HIF-1α) signaling pathway was implicated in this glycolysis. d Changes in cellular homeostasis triggered the self-propelled conversion by regulating the cytoskeletal proteins activation and secreted phenotype through the JAK/STAT and p53 signaling pathways. Fli-1 leukemia integration 1, TCF12 transcription factor 12, SOCS1 suppressor of cytokine signaling 1, B4GALT3 β-1,4-galactosyltransferases III, IGF2R insulin-like growth factor 2 receptor, LPA lysophosphatidic acid, VDAC voltage-dependent anion channel, YAP1 Yes-associated protein 1, TEAD1 TEA domain transcription factor-1, SDF-1 stromal cell-derived factor-1, also known as CXCL12, EREG epiregulin, ROS reactive oxygen species
Table 1.
Phenotype and heterogeneity of CAF
| Tissue type | Phenotype | Markers | Origin and/or function | Ref. |
|---|---|---|---|---|
| BC (human) | CAF-S1 |
CD29Med, FAPHi, FSP1Med, α-SMAHi, PDGFRβMed-Hi, CAV-1Low |
Regulatory of cancer invasion and immune response | 58 |
| CAF-S2 |
CD29Low, FAPNeg, FSP1Neg-Low, α-SMANeg, PDGFRβNeg, CAV-1Neg |
ND | ||
| CAF-S3 | CD29Med, FAPNeg, FSP1Med-Hi, α-SMANeg-Low, PDGFRβMed, CAV-1Neg-Low | ND | ||
| CAF-S4 |
CD29Hi, FAPNeg, FSP1Low-Med, α-SMAHi, PDGFRβLow-Med, CAV-1Low |
Regulatory of actin cytoskeleton and oxidative metabolism | ||
| OSCC (human) | CAF-N | KGF | High fibroblast motility | 60 |
| CAF-D | TGF-β1 | Low fibroblast motility | ||
| NF | HGF, MMP3 | Lower tumor incidence | ||
| LC (human) | Cluster 1 | COL10A1 | Showing a strong EMT signals | 65 |
| Cluster 2 | COX4I2, ACTA2, MEF2C | Regulatory of myogenesis and angiogenesis | ||
| Cluster 3 | ND | Upregulating collagen and ECM molecules expression | ||
| Cluster 4 | PLA2G2A | Similar to cluster 1 | ||
| Cluster 5 | MMP3 | Low myogenesis and high mTOR expression | ||
| Cluster 6 | FIGF | Representing nonmalignant fibroblasts | ||
| Cluster 7 | ND | Similar to cluster 4 but differing in the glycolysis pathway | ||
| CRC (human) | CAF-A | MMP2, DCN, COL1A2 | Regulatory of ECM remodeling and express FAP | 66 |
| CAF-B | ACTA2, PDGFA, TAGLN | Activation of cytoskeletal gene | ||
| NF | MGP (ND) | ND | ||
| HNSCC (human) | CAF1 | CTHRC1, COL1A1, POSTN, TPM4, MFAP2 (ND) | Promoting cancer metastasis | 67 |
| CAF2 | CFD, APOD, CXCL12, GPC3, SEPP1 (ND) | |||
| NF | Depleted of markers for myofibroblasts and CAFs | Resting fibroblasts | ||
| OC (human) | FAP-high CAF | FAP, TGF-β, COL11A1, SULF1, IL-6, CXCL12 | Regulatory of cancer invasion and immune regulation | 345 |
| FAP-low CAF | DLK1, TCF21, COLEC11 | Regulatory of glucose homeostasis, lipid metabolism, etc. | ||
| NF | COMP, SFRP2, GJB2 (ND) | ND | ||
| BC (mouse) | mCAF | Fibulin-1, PDGFRα | From resident fibroblast/regulatory of tumor immune response | 57 |
| vCAF | Nidogen-2 | From vasculature/promoting vascular development | ||
| cCAF | Ki-67 | Representing the proliferative segment of vCAF | ||
| dCAF | SCRG1 | From malignant cell/locating on tumor–stroma boundary | ||
| PDAC (mouse) | myCAF | α-SMA | Adjacent to tumor cells and promoting desmoplasia | 61 |
| iCAF | IL-6, LIF | Locating away within stroma and promotes tumor progression | ||
| NF | ND | Pancreatic stellate cells | ||
| BC (mouse) | Cluster 0 | Ly6c1 | From resident fibroblasts/promoting cancer progression and immune evasion | 68 |
| Cluster 1 | α-SMA | Promoting cancer development and progression | ||
| Cluster 2 | Cdk1 | Identifying as dividing cells | ||
| Cluster 3 | Cd53 | High transcriptional enrichment for desmin | ||
| Cluster 4 | Crabp1 | From Ly6c1high fibroblasts | ||
| Cluster 5 | Cd74 | Expressing MHC class II and regulatory of immune-modulatory | ||
| HCC (ND) | Activated myofibroblast phenotype | α-SMA, FAP, vimentin, vollagen 1α, PDGFRα, FN | Maintaining and enhancing the stemness of HCC cells | 398 |
| Mesenchymal stromal cell phenotype | CD90, CD73, CD105, CD29, CD44, CD166 | Regulatory of immunosuppression |
α-SMA alpha-smooth muscle actin, ACTA2 actin alpha 2, APOD apolipoprotein D, BC breast cancer, CAF cancer-associated fibroblast, CD clusters of differentiation, CAV-1 caveolin-1, Cdk1 cyclin-dependent kinases 1, CFD complement factor D, COL1 collagen type I, COLEC11 human collectin subfamily member 11, COX4I2 cytochrome c oxidase subunit 4I 2, Crabp1 cellular retinol-binding protein-I, CRC colon adenocarcinoma, CTHRC1 collagen triple helix repeat-containing protein 1, CXCL12 C–X–C motif chemokine 12, DCN decorin, DLK1 delta-like 1, ECM extracellular matrix, ELK3 ETS-domain protein, EMT epithelial–mesenchymal transition, FAP fibroblast activation protein, FIGF c-fos-induced growth factor, FN fibronectin, FOXO1 forkhead box protein O1, FSP1 fibroblast activation protein 1, GPC3 glypican-3, HCC hepatocellular carcinoma, HNSCC head and neck squamous cell carcinoma, HOXB2 homeobox 2, IL interleukin, KGF keratinocyte growth factor, LC lung cancer, LIF leukemia inhibitory factor, MEF2C myocyte enhancer factor 2C, MFAP2 microfibrillar associated protein 2, MMP matrix metalloproteinase, ND not determined, OC ovarian cancer, OSCC oral squamous cell carcinoma, PDAC pancreatic ductal adenocarcinoma, PDGF platelet-derived growth factor, PLA2G2A phospholipase A2 group IIA, POSTN periostin, SEPP1 selenoprotein P1, SCRG1 scrapie responsive protein 1, SULF1 sulfatase1, TAGLN transgelin, TCF21 transcription factor 21, TGF transforming growth factor, TPM4 tropomyosin-4
It is becoming clear that there are subpopulations of CAFs for distinct functional states, raising the question of what determines the CAFs’ heterogeneity. Overwhelming evidence suggests that CAFs’ heterogeneity includes different organs/tissues, sources, functions, secretion types, and others.49,50 The alterations in CAFs show a remarkable spectrum of organs/tissues specificity. For example, CAV-1 was found to induce glycometabolic reprogramming in breast CAFs,51 while CAV-1-induced aerobic glycolysis was not completely verifiable in oral CAFs.52 Therefore, some alterations in CAFs appear only in cancers from one or a few tissue types, instead of a pan-cancer genome and transcriptome commonalities. The heterogeneity of CAFs in the same organ or tissue is likely held to depend on their precursor fibroblasts.53 Generally, CAFs are derived from the activated local tissue-resident fibroblasts, fibrocytes recruited from bone marrow, mesenchymal stem cells (MSCs) and stellate cells, or are the products of the mesenchymal transition of epithelial and endothelial cells, and the transdifferentiation of pericytes, smooth muscle cells, and adipocytes.54,55 Depending on their origin, the functions, and markers of CAF subtypes are diverse and unique. The CAF subtypes from local tissue-resident fibroblasts are similar to myofibroblasts with high expression of cytoskeletal proteins like α-SMA for cell contraction, while the CAF subtypes are derived from perivascular cells might be associated with metastasis. However, drawing definitive conclusions on the cellular origins of CAFs is difficult because currently there is no available means to track the conversion between cell states directly or to collect longitudinal samples from the same lesion in human tissue. Mouse models with well-characterized disease progression have been created to shed light on the origin of CAFs.56 In a mouse model of breast cancer, three transcriptionally diverse subpopulations of CAFs were defined via various lineage sources.57 In addition, the cues emanating from molecular phenotypes or secretion phenotypes might also determine the CAFs’ heterogeneity. Recently, single-cell RNA-sequencing and conventional RNA-sequencing of human tissues have allowed better unbiased assessment of heterogeneous CAFs.20,58,59 By analyzing a combination of classical markers, such as FAP and PDGFRβ, CAFs in breast cancer were distinguished by levels of marker expression.58 Another classic way to identify CAFs involves analyzing the different secretory phenotypes exhibited in different subtypes. For instance, elastin and collagen levels are distinctively expressed in CAFs of the lung TME.60
Accordingly, the high heterogeneity in CAFs raises an interesting question: If CAFs would switch in distinct functional states or subtypes? As an answer to this question, it has been suggested that IL-1 signaling induces the generation of inflammatory CAFs, and TGF-β antagonizes CAF switching from an inflammatory phenotype to a myofibroblast phenotype.61,62 Taken together, all these evidences show that the discovery of the heterogeneity of CAFs revealed a remarkably complex and diverse portrait.
Methods for isolation and culture of CAFs
Progression in heterogeneity studies requires more accurate methods for isolation and culture of CAFs. Without question, fibroblasts are easily isolated and cultured on plastic, e.g., human skin, mouse ears, and tail tips can be used as sources to isolate fibroblasts that can be digested and cultured in a medium.63 Using the typical curettage method combined with trypsinization or enzyme digestion methods for CAF primary cell culture, this model is unnecessary to purify cells prior to culture because of their rapid initial proliferation of fibroblasts. Antibiotics and additional washing steps are usually included in the culture process to prevent infections with bacterial and/or mycoplasma. Epithelial cells growing either in groups or scattered among the CAFs can be easily removed because of differences in adhesion ability and tolerance to trypsin of these two cell types, greatly contributing to further research on CAFs.64 In 2006, our group separated the CAFs from human oral cancer tissues using this curettage method.22 However, in these studies involving CAFs, caveats were included to suggest subtle variations in various subtypes requiring the need for new markers. CAF subtypes can be identified through multicolor flow cytometry (fluorescence-activated cell sorting). After tissue digestion, lineage markers are used to exclude hematopoietic, epithelial, and endothelial cells, and various combinations of CAF markers are used for CAF subtype identification.58 CAF subtypes can also be identified through single-cell transcriptomics and mass cytometry methods.57,65–68 Although the α-SMA and FAP staining for distinction CAFs from NFs are available, putative CAF subtype identification methods still require more reproducibility, validation, and repeated optimization.
In primary cell cultures, early passaged and immortalized CAFs have functions that can be directly investigated in vitro, and it is crucial to replicate the TME considering the intricate interactions among tumor cells, CAFs, and other stromal cells. The crosstalk of CAFs with cancer cells has been evaluated through various culture patterns. Cells can be directly cocultured and indirectly cocultured in Transwell chambers or conditioned medium (CM).69,70 Indeed, we extended the two-dimensional (2D) culture of fibroblasts from oral precancerous lesions with the addition of Candida.71 Furthermore, techniques differ for cell culture in 2D and 3D, with the latter allowing patterns of growth in vitro to better mimic that of the tissue architecture in vivo.72 One type of 3D coculture is implemented through the use of reconstituted matrices. The solid porous scaffold is based on a range of natural and synthetic materials and serves as a membrane providing a platform that can be added to a mixture of different cells, including CAFs. For instance, this scaffold‐based technology can be used to replicate tissue architecture, which is composed of alternate layers of cells, and especially for tumors of the epithelium with CAFs.73,74 Aggregate culture platforms of particular interest are scaffold‐free systems, also referred to as spheroids or organoids, in which heterogeneous populations can be evaluated for drug resistance and sensitivity or can be used to establish hypoxic cancer models.72,75,76 However, organoids commonly contained only epithelial cells and lack fibroblasts and types of other cells, such as immune and endothelial cells.77 To overcome these limitations of organoids, a multilayer bladder called an “assembloids” has been created by reconstituting tissue stem cells with stromal components representative of an organized architecture.78 In sum, either classical 2D/3D cultures or assembloids will benefit functional studies of CAFs in the context of the gradually accepted importance of TME.
Major signaling pathways and targeted therapies in CAFs
Many signaling pathways have been explored extensively in CAF-mediated cancer progression for their roles in carcinogenesis, tumor growth, cell migration and invasion, energy metabolism, and cancer recurrence and metastasis. Various endogenous and exogenous factors in CAFs, including biomarkers, cytokines, chemokines, miRNAs, and lncRNAs, are involved in the regulation of these signaling pathways. Several major signal cascades affect not only the biological behaviors of CAFs themselves but also the crosstalk between CAFs and cancer cells. Therefore, in this section, we will discuss how signaling pathways regulate the CAFs, the crosstalk of CAFs with cancer cells, and the targeted therapies.
TGF-β signaling pathway
TGF-β signaling pathway in CAFs and its targeted therapy
Were it not for the fibroblasts, TGF-β would more likely be discovered many years later, because TGF-β was initially identified by its ability to stimulate the growth of rat fibroblasts.79 In the canonical TGF-β signaling pathway, one group of TGF-β superfamily ligands, including the TGF-β/Activin/Nodal, bind to TGF-β type II receptor (TGF-βRII), which phosphorylates TGF-βRI. The binding of TGF-βRII and TGF-βRI propagates signaling by phosphorylating Smad2/3, while Smad1/5/8/9 are mediated by another group of TGF-β ligands, such as bone morphogenetic protein (BMP), through binding of BMP-RII and BMP-RI. Phosphorylated Samd2/3 heterotrimerize with Smad4 and translocate into the nucleus as a transcription factor complex, subsequently regulating the transcription of TGF-β target genes. Inhibitory Samd6/7 binds to activated type I receptors and then inhibit signal transduction of the TGF-β family.80,81 In the noncanonical TGF-β signaling pathway, TGF-β superfamily ligands can activate Rho, extracellular signal-regulated kinase (ERK), Janus kinase/STAT3, and phosphoinositide 3-kinase (PI3K)/AKT pathways in CAFs.51,82,83
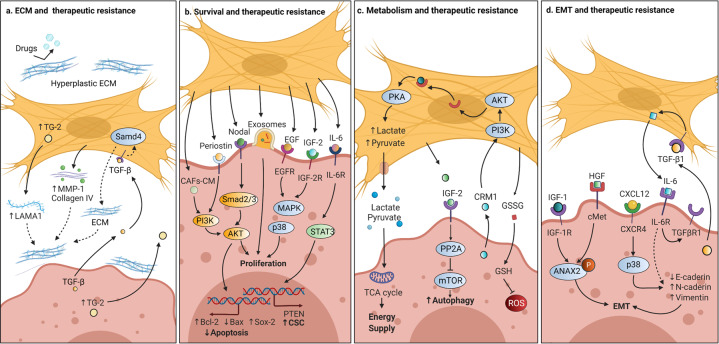
Over the past nearly four decades, TGF-β has been further explored and found to be widely produced by nearly all cell types including CAFs, and the TGF-β signaling pathway has been found to have pleiotropic effects on CAF behaviors through autocrine and paracrine mechanisms.15,38,84 Resident NFs can be induced to transition into CAFs by TGF-β1 in various tumors, including bladder, breast, colorectal, and pancreatic cancer,38,85,86 indicating that TGF-β1-driven CAF generation is a common event during cancer development. Mechanistically, TGF-β1 alters the target gene expression of stromal fibroblasts through the canonical TGF-β signaling pathway, leading to differential gene expressions such as α-SMA and FAP in CAFs.37,87 After treatment with TGF-β1, MSCs were induced to differentiate into CAFs through the activation of the JAK/STAT3 signaling cascade, and inhibition of TGF-β/Smads signaling pathway reduced the transformation.84,88,89 These data suggest that both canonical and noncanonical TGF-β pathways exhibit roles in promoting CAFs generation. In addition, CAF proliferation was attenuated by a TGF-β receptor inhibitor (LY2109761) in hepatocellular carcinoma (HCC),90 and CAF migration was enhanced by TGF-β1 through overexpression of the tight junction protein occludin in colon cancer.83 Paracrine TGF-β caused the activation of noncanonical TGF-β/RhoA/ROCK axis signaling, as well as the TGF-β canonical pathway that induced transcriptional regulation of Snail1 and Twist1 target genes to increase CAFs contractility and ECM remodeling.82 Of note, since the cellular biological behaviors are driven by energy, as a hallmark of cancer, metabolic reprogramming of CAFs is defined as reverse Warburg effect (RWE), characterized by increased lactate, glutamine, nucleotides, fatty acids, and pyruvate derived from aerobic glycolysis.51,91 Recently, studies have supported the supposition that TGF-β signaling pathway plays a critical role in RWE mainly through metabolic reprogramming-related proteins, including CAV-1 and isocitrate dehydrogenase-3α (IDH3α).92,93 Mechanistically, CAV-1 interacted with the TGF-βRI, and induced its degradation, and then suppressed TGF-β-dependent Smad2 phosphorylation and nuclear translocation.94 TGF-β overexpression in CAFs decreased mitochondrial activity and increased glycolysis via CAV-1 downregulation in breast cancer, and TGF-β1-induced CAFs switched metabolic programming from oxidative phosphorylation to aerobic glycolysis by downregulating IDH3α in colon cancer.92,93 Importantly, CAF-specific endoglin (TGF-β family coreceptor) targeted by a neutralizing antibody (TRC105) decreased the metastatic spread of colorectal cancer cells to the liver in vivo.37 Summary, not only the components of canonical and noncanonical TGF-β signaling pathways in CAFs could be targeted for antitumor therapy but also the biomarkers such as CAF-derived CAV-1 and endoglin, etc., have great potential to be targeted in cancer treatment.
TGF-β signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
The CAF-mediated TGF-β pathway contributes to cancer progression by regulating many physiological processes, including cancer cell proliferation, migration, invasion, and metastasis.95 Previous studies showed that TGF-β-activated CAFs secreted growth factors, including TGF-β, fibroblast growth factor 2/7 (FGF2/7), VEGF, PDGF, and hepatocyte growth factor (HGF), to promote cancer cell proliferation.96 CAFs stimulated gastric cancer cell migration and invasion, which were attenuated by Smad2 small interfering RNA (siRNA) and anti-TGF-β-neutralizing antibody.97 TGF-β-activated ECM remodeling in CAFs created biochemical and mechanical stimuli for the invasion of cancer cells.92,98–100 Intriguingly, HCC cells were found to have high levels of connective tissue growth factor (CTGF) as a consequence of elevated TGF-β1 expression, and LY2109761 (a TGF-β receptor inhibitor) not only suppressed CAF proliferation but also alleviated CTGF expression, thereby reducing tumor growth and dissemination,90 indicating that TGF-βR-targeted therapy seems to have good efficacy in terms of antitumor metastasis. Consistently, CAFs contributed to the “education” of cancer cells, changing their behavior from indolent or nonaggressive into that of an invasive and metastatic phenotype.101,102 However, in our preliminary studies and the data from Wang lab, TGF-βRI mutation was detected in ~19% of head and neck squamous cell carcinoma (HNSCC) with metastasis and decreased or abrogated TGF-βRII/TGF-βRIII expression was evident in 35.3% of human oral squamous cell carcinoma (OSCC) on the protein level and in >70% of human HNSCC at the messenger RNA levels.103–105 These data have illustrated that TGF-βR-targeted therapy exerts strict indications, and its mutation needs to be detected before targeting TGF-βR. Notably, the nutrients recycled through the RWE via CAF-derived CAV-1 could be transferred into adjacent tumor cells to promote cancer progression in a paracrine fashion,94 while loss of CAV-1 in the tumor–stroma led to activated TGF-β signaling to trigger the epithelial–mesenchymal transition (EMT) of cancer cells,92,106,107 demonstrating that CAF-derived CAV-1 plays a paradoxical role in tumor progression, and any targeted strategies used to exploit the dual roles of CAV-1 in TGF-β signaling pathway should be developed with consideration of the ability of CAV-1 to transition from acting as a tumor promotor to acting as a suppressor to optimize treatment efficacy.
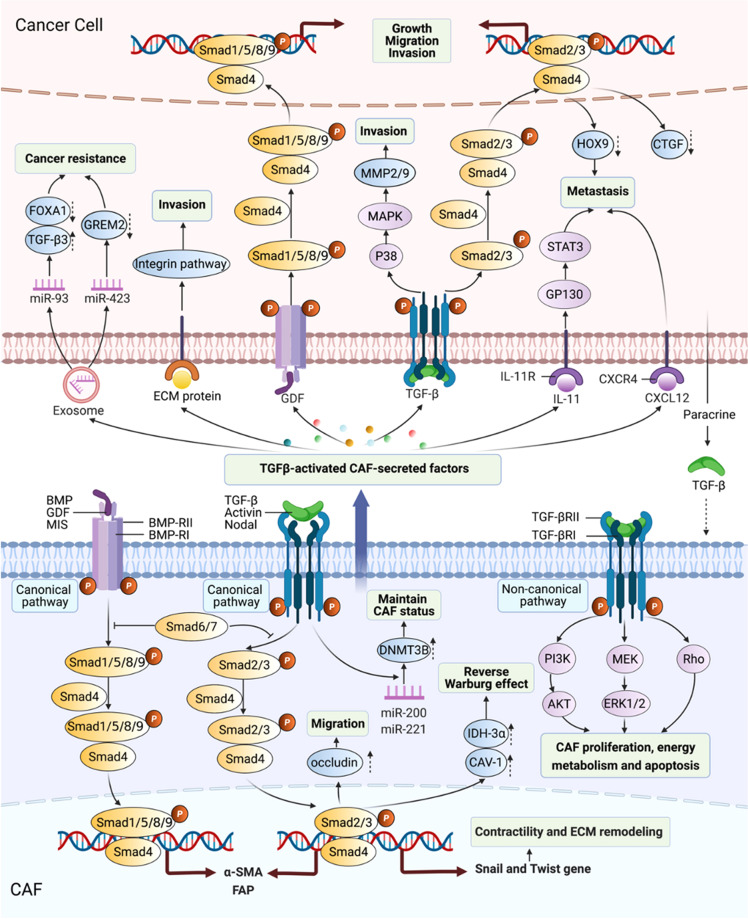
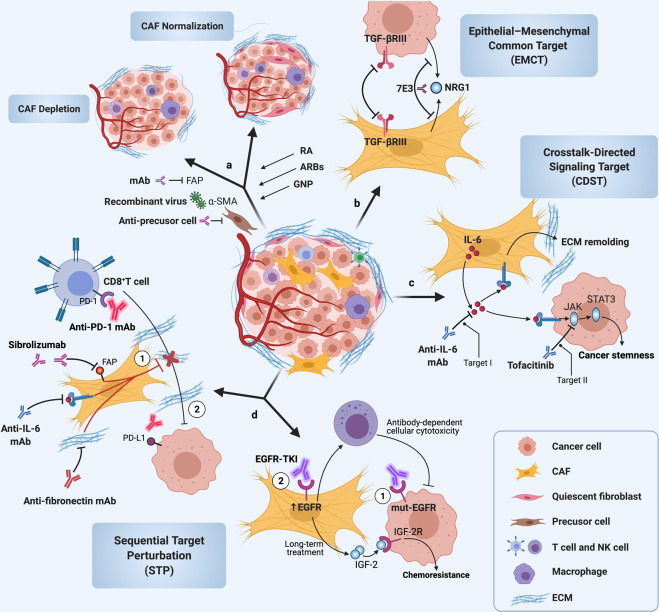
Indeed, the effect of autocrine TGF-β signaling on CAFs remained unclear until a study demonstrated that the establishment of TGF-β autocrine signaling pathways induced CAF formation during breast cancer progression,108 indicating that tumor-derived TGF-β, in a positive-feedback loop, could affect the biological characteristics of CAFs and that the crosstalk between CAFs and cancer cells is not unidirectional but bidirectional (Fig. 2). For instance, tumor-derived TGF-β was likely to recruit CAFs affiliated with the invasive front and at the bone metastatic disease to promote tumor development.102 Cancer cell-induced reactive oxygen species (ROS) promoted the loss of CAV-1 in CAFs via autophagy and then activated HIF-1α under ROS-induced pseudohypoxic conditions.45,109 In ovarian cancer through TGF-β signaling pathway, tumor-derived lysophosphatidic acid and exosomes promoted the differentiation of MSCs to CAFs,17,102,110–113 and cancer-derived TGF-β stimulated the expression of IL-6, C–X-C motif chemokine 12 (CXCL12), and VEGFA in CAFs to induce metastasis.114 Accumulating evidence suggests that abundant miRNAs in CAFs have regulatory roles in tumor progression (Table 2); for instance, targeting miR-101 attenuated TGF-β signal transduction by downregulating TGF-βR1 and Smad2 in HCC cells to suppress vascular mimicry (VM) formation.115 In fact, since the bidirectional crosstalk between the CAFs and cancer cells, any therapeutic strategy targeted CAFs or cancer cells might not obtain optimize efficacy. Thus, using a systems biology strategy, we combined experimental and computational analyses for the prediction of epithelial targets in an interactive network of proteins and found that TGF-βRIII would be targeted as an epithelial–mesenchymal common target (EMCT) in OSCC.116 In summary, the paracrine and autocrine TGF-β signaling pathway contributes extensively to the crosstalk of CAFs and cancer cells, and EMCTs show great potential for use in targeted therapy, while more studies are still needed to determine which TGF-β signaling component can serve as a common target in antitumor therapy.
Fig. 2.
TGF-β signaling pathway-mediated crosstalk between CAFs and cancer cells. The canonical TGF-β signaling pathways consist of TGF-β/Activin/Nodal-Smads pathway and bone morphogenetic protein/growth differentiation factor/Müllerian-inhibiting substance (BMP/GDF/MIS)-Smads pathway. Noncanonical pathways represent those that activate TGF-βR, but induce no-Smads pathway. Within tumor microenvironment (TME), a large number of TGF-β protein secreted by cancer cells mediated the transformation of NFs into CAFs supporting cancer progression by activation TGF-β signaling pathway, particularly canonical pathway. Activated CAFs can be orchestrated by TME to maintain their status and promote their proliferation and migration. In turn, these adaptations would also be contributed to the formation of tumor-promoting TME. CAF-secreted factors regulated extracellular matrix (ECM) remodeling to accelerate cancer invasion and metastasis indirectly. On the other hand, most factors derived from CAFs can directly mediate intricate regulation on the cancer cells. Most proteins, such as ECM proteins, GDF, TGF-β, IL-11, and CXCL12, could activate the pathway of cancer cells to exert biological functions, including promoting cancer growth, migration, invasion, and metastasis, through receptor–ligand binding. Genetic information would also transfer from CAFs into cancer cells by extracellular vehicles. CAF-derived exosomal miR-93 and miR-423 would be endocytosed by cancer cells and then promoted cancer chemoresistance and radioresistance. FOXA1 forkhead box protein A1, MMP2 matrix metalloproteinase 2, GP130 glycoprotein 130, CTGF connective tissue growth factor, IDH3α isocitrate dehydrogenase-3α, CAV-1 caveolin-1
Table 2.
miRNAs in CAF
| miRNAs (expression) | Effects on cancer cells and mechanism of action | Potential targeting therapy | Ref. |
|---|---|---|---|
| Lung cancer | |||
| miR-1 (↓) | Proliferation, chemoresistance by NF-κB and Bcl-xl pathwaya | Pathway | 399 |
| miR-101 (↓) | Growth, metastasis by CXCL12 and PI3K-Akt pathwaya | Restoring miR-101 | 400 |
| miR-210 (↑) | Migration, proliferation, invasion, EMT by PI3K/AKT pathwaya | Exosomal mRr-210 | 401 |
| miR-1/206 (↓) | Angiogenesis, TAMs accumulation, growth, metastasis by FOXO3a/VEGF/CCL2 signaling | Delivery of pre-miR-1/206 and anti-miR-31 | 402 |
| miR-31 (↑) | |||
| Breast cancer | |||
| miR-1-3p (↓) | Progression, metastasis by GLIS1 gene | MiR-1-3p EVs | 403 |
| miR‐22 (↑) | Chemoresistance by PI3K‐AKT pathway | Nanoparticles | 404 |
| miR-26b (↓) | Migration, invasion via TNKS1BP1/CPSF7/COL12A1a | MiR-26b | 405 |
| miR-29b (↓) | Growth, chemoresistance, migration by p38-STAT1 pathway | Suppressor miR-29b | 354 |
| miR-92 (↑) | PD-L1; migration, proliferation by LATS2 of Hippo pathway | MiR-92 inhibitor | 406 |
| miR-200b/c (↓) | Growth, active mobility, invasion by NF-κB pathway | Pathway | 407 |
| miR-200s (↓) | Invasion, metastasis via transcription factors Fli-1 and TCF12 | MiR-200s | 408 |
| miR-205 (↓) | Angiogenesis by targeting YAP1 through STAT3 pathway | Pathway | 409 |
| miR-221 (↑) | Growth, migratory by A20/c-Rel/CTGF signalinga | LNA-i-miR-221 | 272 |
| miR-320 (↓) | Proliferation, invasion by PI3K/AKT pathway | MiR-320 agents | 410 |
| miR-181d-5p (↑) | EMT via transcription factor CDX2 and HOXA5a | Exosomal miR-181d-5p | 411 |
| miR-3613-3p (↑) | Proliferation, metastasis by SOCS2 gene expressiona | Exosomal miR-3613-3p | 412 |
| miR-4516 (↓) | Proliferation by targeting FOSL1 genea | MiR-4516 agents | 413 |
| miR-16/148a (↑) | Migration, metastasis by FAK pathway | Pathway | 414 |
| miR-141 (↓) | MiR-200b/c/miR-221/DNMT3B feedback loop influencing TGF-β1 expression, and TGF-β1/DNMT3B/miR-141 axis enhancing TCF12 in CAF to promote cancer cell proliferationa | Regulatory loop/axis | 415 |
| miR-221 (↑) | |||
| miR-200b/c (↓) | |||
| Prostate cancer | |||
| miR-15/16 (↓) | Proliferation and capability of CAF by FGF2 and FGFR1 | Restoring miR-15/16 | 416 |
| miR-146a-5p (↓) | Metastasis, invasion by EGFR/ERK pathway | Exosomal miR-146a-5p | 417 |
| miR-409 (↑) | Tumorigenesis, EMT, and stemness by tumor suppressor genes | MiR-409 | 418 |
| miR-423-5p (↑) | Chemoresistance by the TGF-β signaling pathway | MiR-423-5p inhibitor | 419 |
| Colorectal cancer | |||
| miR-21 (↑) | Motility and invasion by MMP inhibitor RECKa | MiR-21 | 420 |
| miR-31 (↑) | Radiosensitivity via genes Beclin-1, ATG, DRAM, and LC3a | MiR-31 | 421 |
| miR-92a-3p (↑) | Stemness, EMT, metastasis, and chemoresistance by activating Wnt/β-catenin pathway and inhibiting mitochondrial apoptosis | Inhibiting exosomal miR-92a-3p | 422 |
| miR-93-5p (↑) | Radioresistance by TGF-β signaling pathway | Exosomal miR-93-5p | 367 |
|
miR-17/192 (↓) miR-200c (↓) |
Invasion by regulating ECM target genes on the protein levela | Restoring miR-17/192, and/or miR-200c | 423 |
| Gastric cancer | |||
| miR-34 (↓) | Proliferation and invasion by targeting 16 genes | Exosomal miR-34 | 424 |
| miR-106b (↑) | Migration and invasion by PTEN-mediated signaling pathwaya | MiR-106b | 425 |
| miR-139 (↓) | Growth and metastasis by downregulating MMP11 | Exosomal miR-139 | 308 |
| miR-149 (↓) | EMT and stem-like properties by COX-2/PGE2 signaling | MiR-149 | 426 |
| miR-214 (↓) | Migration and invasion by EMT and targeting FGF9a | MiR-214/FGF9 | 427 |
| miR-522 (↑) | Suppressing ferroptosis by targeting ALOX15 and blocking lipid-ROS accumulation, chemotoxicity promoting miR-522 secretion by activating USP7/hnRNPA1 pathway | Blocking miR-522 packaging into exosomes | 9 |
| Hepatocellular carcinoma | |||
| miR-29b (↓) | Invasion, migration, and apoptosis by DNMT3ba | MiR-29b mimic | 428 |
| miR-101 (↓) | Vascular mimicry formation by SDF-1 signaling | Signaling networks | 115 |
| miR-320a (↓) | Proliferation, migration, and metastasis by MAPK pathway | Transfer of miR-320a | 429 |
| miR-1247-3p (↑) |
Stemness, EMT, chemoresistance, and tumorigenicity by IL-6/8; lung metastasis by β1-integrin-NF-κB pathway |
Tumor–stromal crosstalk | 430 |
| Cholangiocarcinoma | |||
| miR-15a (↓) | Migration by regulating PAI-2 expressiona | MiR-15a/PAI-2 axis | 431 |
| Cervical and squamous cell carcinoma | |||
| miR-10a-5p (↑) | Angiogenesis and tumorigenicity by Hedgehog pathway | MiR-10a-5p evs | 432 |
| Pancreatic cancer | |||
| miR-21(↑) | Desmoplasia, drug resistance, and CAF activation by PDCD4 gene | MiR-21 | 433 |
| miR-106b (↑) | Chemoresistance by directly targeting TP53INP1 genea | Exosomal miR-106b | 309 |
| miR-146a (↑) | Proliferation and survival by gemcitabine-induced Snail pathway | Exosomal inhibitors | 70 |
| Head and neck cancer | |||
| miR-7 (↑) | Proliferation and migration via RASSF2 and decreasing PAR-4a | Inactivation of the RASSF2-PAR-4 axis | 434 |
| miR-34a-5p (↓) | Proliferation and metastasis by AKT/GSK3β/β-catenin pathway | MiR-34a-5p/AXL axis | 136 |
| miR-196a (↑) | Proliferation and resistance by regulating CDKN1B and ING5a | Exosomal miR-196a | 435 |
| miR-3188 (↓) | Proliferation and apoptosis by targeting BCL-2 | Exosomal miR-3188 | 436 |
| Melanoma | |||
| miR-155 (↑) | Angiogenesis by SOCS1/JAK2/STAT3 signaling pathway | Exosomal miR-155 | 437 |
| Osteosarcoma | |||
| miR-1228 (↑) | Migration and invasion by endogenous SCAI mRNA and proteina | Exosomal miR-1228 | 438 |
| Ovarian cancer | |||
| miR-21 (↑) | Motility, invasion, lowering chemosensitivity and apoptosis by binding to APAF1 coding sequence | Inhibiting miR-21 | 439 |
| miR-98-5p (↑) | Cisplatin resistance by downregulating CDKN1A | Exosomal miR-98-5p | 440 |
| miR-31/214 (↓) | Recruitment and growth by regulating CCL5 | MiRNAs | 441 |
| miR-155 (↑) | |||
| Endometrial cancer | |||
| miR-31 (↓) |
Motility and invasion by targeting partially the SATB2 homeobox gene |
MiR-31/SATB2 signal | 442 |
| miR-148a (↓) | Invasion by decreasing WNT10B in WNT/β-catenin pathwaya | Restoring miR-148a | 443 |
| miR-148b (↓) | EMT by relieving the suppression of gene DNMT1 | Transfer of miR-148b | 444 |
ALOX15 arachidonate lipoxygenase 15, AKT protein kinase B, APAF1 apoptotic protease-activating factor-1, Bcl-xL B cell lymphoma-extra large, CAF cancer-associated fibroblasts, CCL C–C chemokine ligand, CDKN cyclin-dependent kinase inhibitor, CDX2 caudal-related homeobox 2, COX-2 cyclooxygenase-2, CTGF connective tissue growth factor, CXCL C–X–C chemokine ligand, DNMT DNA methyltransferase, EGFR epidermal growth factor receptor, ECM extracellular matrix, EMT epithelial–mesenchymal transition, ERK extracellular signal-related kinase, EV extracellular vesicles, FAK focal adhesion kinase, FGF fibroblast growth factors, Fli-1 friend leukemia integration 1, FOXO3a Forkhead box O3, GLIS1 Gli-similar 1, GSK glycogen synthase kinase, hnRNPA1 heterogeneous nuclear ribonucleoprotein A1, HOXA5 homeobox A5, IL interleukin, ING5 inhibitor of growth 5, JAK Janus kinase, MAPK mitogen-activated protein kinases, MMP matrix metalloproteinases, NF-κB nuclear factor kappa-B, PAI-2 plasminogen activator inhibitor 2, PGE2 prostaglandin E2, PTEN phosphate and tensin homolog, PI3K phosphatidylinositol-3-kinase, ROS reactive oxygen species, SCAI suppressor of cancer cell invasion, SDF-1 stromal cell-derived factor-1, SOCS suppressor of cytokine signaling, STAT signal transducer and activator of transcription, TAMs tumor-associated macrophages, TCF12 transcription factor 12, TGF transforming growth factor, USP7 ubiquitin-specific protease 7, VEGF vascular endothelial-derived growth factor
aWithout xenograft models
PI3K/AKT/mTOR signaling pathway
PI3K/AKT/mTOR signaling pathway in CAFs and its targeted therapy
PI3K, as an intracellular phosphatidylinositol kinase, encompasses p85 and p110.117 Transmembrane growth factor receptors include epidermal growth factor receptor (EGFR), G-protein-coupled estrogen receptor (GPER), VEGF, and insulin growth factor receptor 1, etc. and can activate PI3K and then phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3).118,119 Then, PIP3 binds to the phosphoinositide-dependent kinase 1 (PDK1) and PDK2, and subsequently recruits AKT to the plasma membrane and phosphorylates the threonine/serine (Thr308/Ser473) phosphorylation site to activate AKT.117–119 Activated AKT can phosphorylate and activate its substrate mammalian target of rapamycin (mTOR) via direct and indirect pathways.120–122 Phosphatase and tensin homolog (PTEN), as a tumor suppressor that dephosphorylates PIP3 into PIP2 for inactivation of AKT and PDK1, negatively regulating the PI3K/AKT/mTOR signaling pathway.117,123
The PI3K/AKT/mTOR signaling pathway is crucial to many aspects of cell differentiation, growth, apoptosis, and mobility.124–126 Ample evidence has concluded that PI3K/AKT pathway mainly promotes the differentiation of diverse cells into CAFs (Fig. 3). For instance, tumor-derived exosomal miRNA-21, which directly targets PTEN, drove hepatic stellate cell differentiation into CAFs by downregulating PTEN and activating PDK1/AKT signaling pathway.55 BMP2 activated the PI3K/AKT and MEK/ERK signaling pathways and induced the transition from pericytes to CAFs, and Noggin (BMP signaling pathway inhibitor) inhibited PI3K/AKT and MAPK signaling pathways and reversed the pericyte–CAFs transition.127 The Notch signaling pathway also promoted CAF differentiation from human bone MSCs via AKT pathway.128 In addition, there could be a potential correlation between CAF survival and AKT signaling pathway. B7-H3 has been recognized as a co-stimulatory molecule in immune responses.129 In renal cell carcinoma, B7-H3 silencing increased apoptosis and prevented the cell cycle process and simultaneously inhibited AKT phosphorylation,130 suggesting that AKT pathways might play a role in promoting CAF proliferation and in inhibiting the apoptosis induced by B7-H3. In another study, overexpression of Noggin in CAFs decreased CAF proliferation.131 Further, the PI3K/AKT signaling pathway affected the CAF motility. GW4064, as an activator of farnesoid X receptor (FXR), significantly reduced cell migration, and this inhibition was also found in cells expressing wild-type AKT.132–135 Interestingly, the PI3K/AKT inhibitor LY294002 significantly potentiated the inhibitory effects mediated by GW4064,132 illustrating that PI3K/AKT signaling pathway was involved in the CAF motility mediated by FXR.
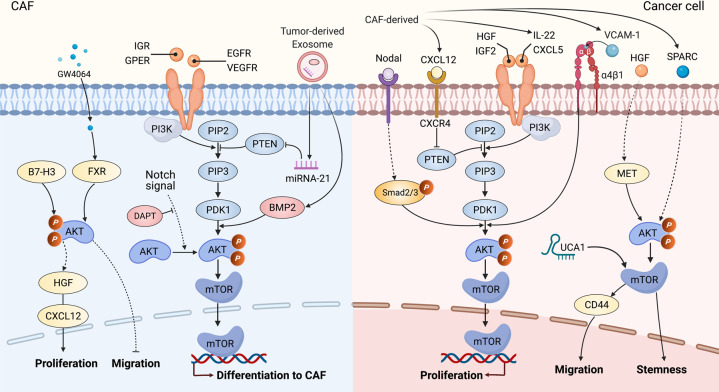
Fig. 3.
PI3K/AKT/mTOR signaling pathway in CAFs and the crosstalk of CAFs with cancer cells. In the CAFs, by the receptor–ligand binding, activated phosphatidylinositol-3-kinase (PI3K) can phosphorylate phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3), while miRNA-21 could attenuate the inhibition of phosphatase and tensin homolog (PTEN) on PIP3. As a result, PIP3 activated phosphoinositide-dependent kinase 1 (PDK1)/AKT signaling cascade to transfer the rapamycin target protein (mTOR) into the nuclei, subsequently regulating the expression of targeted genes associated with differentiation into CAFs and motility, etc. Notch signaling pathway was also involved in CAF differentiation via AKT signaling pathway. B7-H3 promoted AKT phosphorylation for proliferation in CAFs, while AKT phosphorylation was involved in the inhibitory effects on the migration mediated by GW4064. Similarly, CAF-derived HGF, IGF-2, IL-22, and CXCL5 can activate PI3K/AKT/mTOR signaling axis, while CXCL12 can inhibit PTEN. Nodal-induced activation of Smad2/3 could activate AKT phosphorylation and lncRNA UCA1 collaborated with mTOR. Consequently, CAF-mediated PI3K/AKT signaling pathway regulated the cell proliferation, migration, and stemness in cancer cells. FXR farnesoid X receptor, HGF hepatocyte growth factor, IGR insulin growth factor receptor, GPER G-protein-coupled estrogen receptor, EGFR epidermal growth factor receptor, VEGFR vascular endothelial growth factor, DAPT, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester, VCAM-1 vascular cell adhesion molecule-1, SPARC secreted protein acidic and rich in cysteine
PI3K/AKT/mTOR signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
Many studies on the PI3K/AKT/mTOR pathway in CAFs have shown that activation of this cascade promoted various cancer behaviors, especially cell proliferation (Fig. 3). The fact that PI3K/AKT signaling pathways regulated CAF-mediated cancer cell proliferation in oral,136 lung,137,138 gastric,139 colon,140 endometrial,141 and anal142 cancers. Mechanistically, in gastric cancer, a neutralizing antibody against Nodal attenuated CAF-induced cancer cell proliferation through Nodal-induced activation of the Smad2/3/AKT signal axis.139 In another study, blockade of PTEN phosphorylation by siRNA led to the promotion of colon cancer cell proliferation upon stimulation with CXCL12 through the activation of PI3K/AKT signaling pathway.140 In contrast, Subramaniam et al. found that a specific PI3K inhibitor (LY294002) reversed the CAF-mediated cell proliferation in endometrial cancer.141 These findings suggest that the role of AKT signaling axis in various cancers seems to be tumor/tissue-specific and/or that different inhibitors affect different signal transduction pathways to promote cell proliferation. These observations raise the question: Do different inhibitors attenuate CAF-mediated proliferation via PI3K/AKT signaling pathway in the same type of tumor? In general, blocking vascular cell adhesion molecule-1 (VCAM-1) by siRNA138 and inhibiting IL-22 with an anti-IL-22 antibody,137 CAF-CM-promoted proliferation was attenuated via factors downstream of PI3K/AKT signaling cascade in the same type of lung cancer.
In addition, in colorectal cancer, CAFs increased the adhesion of cancer cells to endothelial cells and the migration of cancer cells in liver or lung metastasis by upregulating CD44 through HGF/MET/AKT signal pathway.143 VM was reported to be facilitated by the cancer cells with sufficient plasticity to form vascular networks for the perfusion of rapidly growing tumors and metastases.144,145 Kim et al. provided data showing that CAF-CM-induced VM was closely associated with a high level of erythropoietin-producing human hepatocellular receptor A2 (EphA2).146 Interestingly, both an EphA2 inhibitor (siRNA) and a PI3K inhibitor (LY294002) decreased VM induced by CAF-CM and suggested that the EphA2/PI3K or HGF/PI3K signaling pathway was involved in CAF-CM-mediated VM,146,147 implying that both EphA2 and HGF might be potential therapeutic targets for cancer anti-vascular treatment in gastric cancer. Of note, downregulation of CAF-derived secreted protein acidic and rich in cysteine (SPARC) can lead to dedifferentiation of gastric cancer cells to CD44+/CD24− cancer stem cell (CSC)-like cells, and the AKT/mTOR and MEK/ERK signaling pathways might be involved in these processes,148 indicating that CAF-derived SPARC maintained tumor stemness through the AKT/mTOR signaling pathway. Further, both miRNAs and lncRNAs, the two most studied classes of noncoding RNAs (ncRNAs), are crucial regulators of gene expression and interact closely with the PI3K/AKT/mTOR pathway during oncogenesis.136,149–151 For instance, in colorectal cancer, CAFs upregulated lncRNA UCA1 in cancer cells and collaborated with mTOR to suppress the miR-143, thereof leading to an increase in KRAS protein and resulting in regulation of the EMT and cell invasion and migration.152 Similar to the EMCT discussed above, Ogier et al. provided data showing that 7E3 blocked neuregulin 1 (NRG1)-mediated HER3 and AKT/MAPK signals to inhibit tumor growth in pancreatic cancer,153 demonstrating that NRG1 expressed by CAFs and cancer cells is an EMCT candidate. Overall, the CAF-mediated PI3K/AKT signaling pathway regulated cell proliferation, migration, VM, and stemness, and both miRNAs and lncRNAs were involved in this signal cascade. Although various PI3K/AKT inhibitors have been used in many studies, maximizing their utility in CAF-targeted therapy remains challenging. Optimization of the tumor-type selection strategies, the EMCT, and combinatory approaches will help to improve the efficacy of these agents.
MAPK signaling pathway
MAPK signaling pathway in CAFs and its targeted therapy
MAPK signaling pathways comprises signaling cascades involving three major kinases: ERK, c-Jun-N-terminal kinase (JNK), and p38 (MAPK14).154,155 Components of the MAPK pathways respond to various input signals, including cytokines, chemokines, growth factors, and stress, etc., signals. Therefore, the MAPK pathway is divided into mitogen- and stress-activated MAPK pathways, with classical representatives being ERK as the mitogen-responsive MAPKs and JNK and p38 as the stress-responsive MAPKs.156,157 Once the phosphorylation of ERK1/2, JNK1/2/3, or p38 is induced by an upstream cascade, these kinases are translocated into the nucleus where they activate transcription factors, subsequently leading to the regulation of gene expression.157,158
First, it was reported that miR-211 directly targeted the insulin-like growth factor 2 receptor to activate the MAPK signaling pathway, resulting in CAF generation.40 Gastric cancer cell-derived exosomes induced pericytes to form CAFs by activating PI3K/AKT and MEK/ERK pathways; however, BMP pathway inhibition reversed the cancer exosome-induced CAF transition.127 Surprisingly, CAFs could utilize lipids endogenously synthesized by a gold nanoparticle to induce the expression of lipogenesis genes such as fatty acid synthase (FASN), sterol response element-binding protein 2, and fatty acid-binding protein 3, and thus maintain a quiescent phenotype.159 In addition, Ando et al. showed that eicosapentaenoic acid, a polyunsaturated fatty acid, decreased the expression of IL-6 and VEGF in CAFs by inhibiting the ERK pathway, thereby reducing the cancer angiogenesis in vitro.160–162 Indeed, fatty acids are necessary for the basic functions of nearly all cell types including CAFs,163 and FASN is a key lipogenic enzyme in the biogenesis of fatty acids that generates palmitate from malonyl-CoA and acetyl-CoA in the presence of nicotinamide adenine dinucleotide phosphate.164,165 Intriguingly, 17β-estradiol (E2) and G1 upregulated FASN involved in the metabolism of fatty acids in CAFs via EGFR/ERK signaling cascade.166 In fact, MAPK signal was found to be involved not only in the metabolism of fatty acids but also in glycolysis in CAFs. We have found that CAF-lncRNA H19 regulated the levels of phosphorylated ERK, JNK, and p38 and further promoted glycolysis reprogramming in OSCC,52 demonstrating that the activated MAPK signaling may contribute to glucose metabolism in CAFs. In human lung cancer, CAFs displayed significantly higher migration activity in response to PDGF-BB than fibroblasts derived from noncancerous tissues and were presumed to be more dependent on ERK1/2 signaling for enhanced migration activity.167 Similarly, in another study, Eck et al. found that compared to the NFs in the mammary tissue, CAFs expressed increased CXCR4 and that AMD3100 (a CXCR4 inhibitor) suppressed the phosphorylation of ERK1/2 caused by CXCL12, subsequently leading to less invasive and migratory CAF phenotypes.168 In addition, it was reported that tissue inhibitor of metalloproteinase-1 (TIMP-1) could enhance prostate CAFs proliferation and migration in vitro and activate the ERK1/2 signaling pathway in CAFs.169 However, TIMP-1 significantly promoted CAF proliferation and motility but not the proliferation of tumor cells in prostate cancer,169 suggesting that the TIMP-1-mediated accumulation of prostate CAFs likely resulted from both enhanced infiltration and expansion of prostate CAFs within the tumors. With regard to ECM, upregulated Snail1 was found in CAFs, and it was required for the fibrogenic response of CAFs exposed to a stiff matrix.170 Mechanistically, increased ERK2 activity augmented the nuclear accumulation of Snail1 to decrease cytosolic proteasome degradation, and Snail1 affected the expression and activity of YAP1 in CAFs exposed to a stiff matrix.170
MAPK signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
MAPK signaling pathways, as ubiquitous signal transduction, regulate almost all aspects of cellular function in cancers.157,171 For cell proliferation mediated by CAFs in MAPK signal, endometrial cancer cell proliferation was prompted significantly by CAF-CM compared to NF-CM through the phosphorylated ERK, and it could be reversed by U0126 (an ERK selective inhibitor).141 Similarly, CAF-derived epiregulin significantly enhanced cancer cell proliferation through a downstream effector of ERK, and ERK inhibitors U0126 and PD98059 counteracted epiregulin-induced promotion of tumor growth in colitis-associated cancer.172 These findings suggest that MAPK/ERK signaling pathway is evolutionarily conserved and that U0126 is a highly effective depressant of this cascade. Notably, it was shown that Twist1 exhibited a dual role in CAFs and cancer cells in the EMT process: on the one hand, Twist1 promoted the expression and secretion of CXCL12 from CAFs, and its knockdown in CAFs inhibited tumor growth; on the other hand, activated CXCL12/CXCR4 signaling promoted EMT process through ERK/AKT-Twist1-MMP1/E-cadherin pathway in esophageal cancer cells.173
Of note, one of the most important characteristics of the MAPK pathway in the crosstalk between CAFs and cancer cells (Fig. 4), we propose, is the extensive cross-signaling between MAPK pathways and other cascades, such as PI3K/AKT signal, JAK/STAT cascade, and TGF-β pathway, in various cancers.127,147,174 For instance, blocking VCAM-1 suppressed proliferation and invasion of CAF-CM-treated cancer cells by activating the MAPK/AKT signaling pathway.138 Consistently, CAFs secreted urokinase plasminogen activator (uPA) to promote cancer cell proliferation, migration, and invasion through PI3K/AKT and MAPK/ERK signaling pathways in esophageal squamous cell carcinoma (ESCC).175 In another study, CAFs promoted the viability of neuroblastoma cells by increasing their proliferation and inhibiting their apoptosis through co-activation of the JAK2/STAT3 and MEK/ERK1/2 signaling pathways.176 In a mouse model of neuroblastoma, inhibition of JAK2/STAT3 and MEK/ERK/1/2 by ruxolitinib and trametinib treatment, respectively, potentiated the tumor response to etoposide and suppressed tumor progression.176 In summary, MAPK signaling pathways have great potential as targets in cancer therapy, and currently, the most extensively studied MAPK signal is the ERK pathway. An alternative approach, unlike the EMCT, we suspect, which is supported by the observation with CAF-derived epiregulin,172 CAF-derived periostin177 or CAF-secreted uPA,175 is that the dual targeting of the key biomarker in CAFs and its vital downstream effector of MAPK signaling axis in cancer cells may optimize the efficacy of blocking the crosstalk between CAFs and cancer cells in targeted therapy.
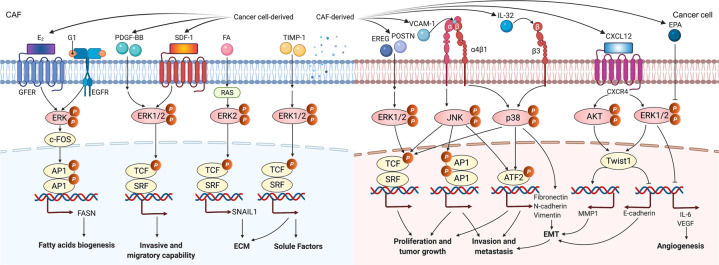
Fig. 4.
MAPK signaling pathway in CAFs and the crosstalk of CAFs with cancer cells. In CAFs, by E2 and G1, the EGFR/ERK signaling upregulated FASN expression for the metabolism of fatty acids. The PDGF-BB and SDF-1 could stimulate the higher invasive and migratory capability of CAFs via ERK1/2 phosphorylation. FA activated RAS upregulating SNAIL1 via ERK2 signaling, which mediated the fibrogenic response of CAFs. TIMP-1 enhanced CAF proliferation and migration and activated ERK1/2 signaling pathway in CAFs by the production of soluble factors. In the crosstalk between CAFs and cancer cells, CAF-derived EREG and POSTN could enhance the cancer cell proliferation and tumor growth by the downstream effector of ERK1/2. VCAM-1 and CAF-derived IL-32 increased the proliferation, invasion, metastasis, and EMT in cancer cells by activating p38/MAPK signaling pathway. Activated CXCL12/CXCR4 signal promoted EMT process through ERK/AKT-Twist1-MMP1 pathway. EPA decreased the expression of IL‑6 and VEGF secretion in CAFs by inhibition of ERK phosphorylation, thereof affecting angiogenesis. E2 17β-estradiol, G1 1-(4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)-ethanone, 1-[(3aS,4R,9bR-rel)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone, PDGF-BB platelet-derived growth factor-BB, FA focal adhesions, TIMP-1 tissue inhibitor of metalloproteinase-1, AP1 activating protein 1, FASN fatty acid synthase, SRF serum response factor, TCF ternary complex factor, POSTN periostin, EPA eicosapentaenoic acid, ECM extracellular matrix, EMT epithelial–mesenchymal transition
In addition, MAPK/p38 or MAPK/JNK signaling pathway also plays important roles in the crosstalk of CAFs with cancer cells (Fig. 4). Blockage of MAPK/p38 pathway diminished IL-32-induced EMT markers, cell invasion, and metastasis in breast cancer.178 Li et al. found that DSF/Cu increased cellular ROS levels and activated the apoptosis-related MAPK pathway without inducing a significant change in JNK or p38 expression.179 However, stress-activated MAPK pathways, including JNK cascade and p38 pathway, continued to exert the complementary functions in CAF-targeted MAPK signaling pathways in cancer treatment.
Wnt signaling pathway
Wnt signaling pathway in CAFs and its targeted therapy
Wnt signaling pathway includes 19 Wnt ligands and more than 15 receptors, which can be classified into canonical and noncanonical signaling pathways.116,180 In the canonical cascade, in the absence of Wnt ligands, cytoplasmic β-catenin combines with Axin complex and phosphorylates by glycogen synthase kinase 3β (GSK3β), leading to β-catenin degradation in the cytoplasm via β-TrCP200 ubiquitination.181,182 Conversely, in the presence of Wnt ligands, including Wnt1, Wnt2, and Wnt3a, the ligands combine with Fzd/LRP (LDL-receptor-related protein) receptors, and then, LRP receptors are phosphorylated by GSK3β, thereby causing the release of β-catenin from the Axin complex and translocation from the cytoplasm into the nucleus for targeted gene expression, including CD44, c-Myc, and cyclin D1.183 β-Catenin is not involved in the noncanonical Wnt signaling process. Through the binding of FZD receptors or ROR1/ROR2/RYK coreceptors, Wnt/RCP and Wnt/Ca2+ signaling cascades are activated for transcriptional responses and/or cytoskeletal rearrangement.184,185
In Wnt/β-catenin signaling, CAF-derived β-catenin became a major concern, as it is seemed to be a relatively early-stage event in carcinogenesis. For instance, many CAFs infiltrated into and/or around invasive tissue in the presence of high β-catenin levels in human melanoma.186 Through a new conditional gene knockout system (Col1α2-CreER mouse), β-catenin was depleted in dermal fibroblasts, causing cell cycle arrest and suppressing cell proliferation and chemical factor and ECM protein production.186 Similarly, in colorectal cancer, Mosa et al. generated a Wnt3HA/HAAPCmin/+ mouse model and demonstrated a direct role of Wnt signaling in fibroblast activation, contractility, and CAF phenotypic plasticity.187 Importantly, β-catenin ablation reduced the expression of PDGFRα and FSP1 with no obvious cytoskeletal rearrangement in stromal fibroblasts,188 noting that other signaling pathways might be implicated in the process of cytoskeletal rearrangement mediated by noncanonical Wnt signaling cascade. It has been shown that β-catenin also forms a β-catenin/E-cadherin complex that contributes to the motility and migration of fibroblasts.189 In HNSCC, periostin is highly produced and secreted by CAFs.190 CAF-derived periostin was found to be a potential ligand for protein tyrosine kinase 7 (PTK7) and was correlated with Wnt/β-catenin signal activation.190 In addition, unlike DKK1/2/4, which suppressed the Wnt/β-catenin signaling cascade, DKK3 neither interacted with LRP5/6 nor fulfilled the antagonistic role of a bona fide member of the DKK family in the canonical Wnt signaling pathway.191,192 However, DKK3 decreased the stability of Kremen to increase LRP6 membrane localization and stabilization of β-catenin.191,193 Interestingly, in CAFs, heat-shock factor-1 interacted with the DKK3 locus and upregulated the expression of DKK3,194 indicating that DKK3 might be a target for blocking of the Wnt/β-catenin signaling.
Wnt signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
Wnt signaling pathway is aberrantly activated in various cancers, including melanoma,186 esophageal,195 head and neck,190,196 breast,193 gastric,197 liver,198 ovarian,193 and colorectal cancers,193,199 and its genetic alterations are frequent, at ~66.55%, in cancers.200 What distinguishes the Wnt signaling pathway in CAFs from other pathways? Notably, in contrast to the studies on mutations in APC, RNF43, ZNRF3, AXIN1/2, and CTNNB1 detected in human colorectal adenocarcinoma,201 endometrial cancer,202 HCC,203 and gastric cancer,204 few studies have been published related to their alterations of these genes in CAFs. To further address the role of cancer cell mutations in CAFs, using a 3D coculture model, Zhou et al. found that melanoma growth was suppressed by CAF deactivation induced by β-catenin ablation, which led to the reduced production of paracrine factors and ECM proteins.188 Similarly, CAF-derived periostin promoted the CSC phenotype, tumor progression, and metastasis via canonical Wnt/β-catenin signaling pathway in HNSCC.190 Mechanistically, CAF-derived periostin bound to PTK7 on the cancer cell membrane and transferred the signals to disheveled proteins by LRP6, thereby inducing the phosphorylation of GSK3β and the hypophosphorylation of β-catenin, leading to the translocation of β-catenin from the cytoplasm to the nuclei.190 However, it was reported that β-catenin-mediated Wnt signaling was dispensable for the function of CAFs in ECM remodeling and promoting cell proliferation and invasion in breast cancer.193 This suggests that Wnt/β-catenin signaling pathway affects the crosstalk of CAFs and cancer cells in highly specific tumor types. Interestingly, by generating Wnt-independent tumor organoids, which secreted the Wnt antagonist Sfrp1, Mosa et al. found that Sfrp1 or genetic depletion of β-catenin strongly decreased the number of cancer-associated myofibroblasts (myCAFs: α-SMA+/Acta2+).187 Coculture of this tumor organoid with inflammatory CAFs (iCAFs: IL-6+/Tnfa+/IL-1a+) resulted in the upregulation of Vim and Zeb1, while myCAFs reverse this upregulation,187 indicating that the EMT process could be induced by Sfrp1 and that tumor behaviors were differentially regulated via Wnt signaling pathway in specific CAF subtypes.
In addition, CAF-derived Wnts can lead to cell growth and other biological functions of cancer cells (Fig. 5). For example, Wnt2 protein secreted by CAFs enhanced cell invasion and migration in colorectal cancer205 and angiogenesis by shifting the balance towards proangiogenic signaling in colon cancer.206 It is likely that treatment with CAF-CM and an elevated autophagy rate augmented the levels of β-catenin and P-GSK3β, which are the key proteins in the Wnt/β-catenin pathway, thereby promoting tumor progression.207 Notably, the upregulation of Wnt proteins in CAFs was explainable with both intrinsic and extrinsic aspects. On the one hand, Wnt5a was enriched by the loss of H3K27me3 in CAFs, and inhibition of secreted Wnt5a from CAFs suppressed cancer cell growth and migration in gastric cancer.208 On the other hand, Taxotere treatment enhanced Wnt16 expression in CAFs and this in turn might have contributed to the proliferation, invasion, and chemoresistance of breast cancer cells.209 Taken together, these findings show that the attenuated Wnt signaling cascade in CAFs could contribute as a suppressor of tumor progression, in a manner similar to that described for reduction in tumor cell-intrinsic Wnt signaling activity; however, more studies are required to dissect the underlying potential mechanisms and genetic factors related to CAF-mediated Wnt signaling in cancer progression.
Fig. 5.
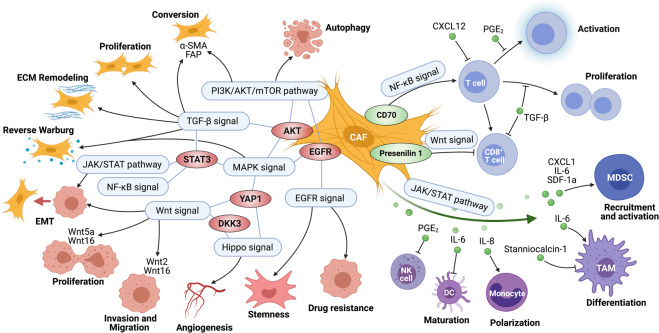
Crosstalk of different signaling pathways among CAFs, cancer cells, and immune cells. A reservoir of biological behaviors of CAFs, including CAFs generation, proliferation, ECM remodeling, and energy metabolism, etc. were regulated by several major signals like TGF-β and PI3K/AKT/mTOR signaling pathways. Importantly, CAF-mediated signaling pathways like JAK/STAT, Wnt, Hippo, MAPK, EGFR, and NF-κB signal were widely involved in cancer cells proliferation, stemness, invasion, migration, metastasis, angiogenesis, epithelial–mesenchymal transition (EMT) process, and therapeutic resistance. CAF-mediated signaling pathways did not always display with individual effects, but commonly crossed to each other to form a signaling network in cancer progression by the cross-connections such as STAT3, AKT, and YAP1. As the great source of cytokines, chemokines, and growth factors, CAF-secreted factors, including TGF-β1, IL-6, IL-8, CXCL1, CXCL12, and PGE2, etc., affect proliferation and activation of T cell, recruitment and activation of myeloid-derived suppressor cells (MDSCs), differentiation, and polarization of monocytes/macrophages, etc. PGE2 prostaglandin E2
JAK/STAT signaling pathway
JAK/STAT signaling pathway in CAFs and its targeted therapy
JAK/STAT signaling pathway is a signal cascade stimulated by many kinds of cytokines and consists of a host of ligands and several tyrosine kinase-related receptors with four tyrosine kinase JAK and seven transcription factor STAT family members, suppressors of cytokine signaling proteins, and multiple STAT-dependent operons.210,211 JAK enzymes share a common domain structure consisting of seven JAK-homology domains.212 Typically, cytokines, chemokines, and/or growth factors integrate with tyrosine kinase-related receptors, and the latter recruits JAK, activating receptor and JAK. The phosphorylated tyrosine on the receptor molecule binds with the SH2 site of STAT.213 STAT binding to the receptor triggers the tyrosine phosphorylation of STAT, leading to STAT dimer formation and its translocation into the nuclei where it targets gene expression.214
JAK/STAT signaling pathway is constitutively activated in CAFs. In TME, CAF-derived cytokines, including IL-6, IL-10, IL-11, and IL-22, act as ligands for JAK/STAT signal cascade (Table 3). Intriguingly, IL-6, as a pro-inflammatory cytokine, partnered with GP130 to activate STAT3, while IL-10, as an anti-inflammatory cytokine, did not interact GP130 but promoted the phosphorylation of STAT3,215–217 demonstrating that different cytokines are likely to activate the same STAT protein. Actomyosin contractility plays a key role in ECM remodeling by CAFs to permit cell migration. GP130-IL6ST signaling influenced JAK1-derived actomyosin-mediated contractility through the phosphorylation of MLC2 in CAFs and promoted ECM remodeling.218,219 Consistently, cytokine oncostatin M not only promoted actomyosin-mediated contractility and ECM remodeling by CAFs through GP130-IL6ST, JAK1, and ROCK signal axes but also induced CAF generation through the JAK/STAT signaling pathway.220 In addition, aberrant DNA methylation contributed to the maintenance of the phenotype of CAFs via the JAK/STAT cascade.221 Since STAT3 acetylation caused the epigenetic modification-dependent loss of SRC homology phosphatase-1 (SHP-1) and dephosphorylates JAK1, SHP-1 knockout led to the sustained constitutive phosphorylation of JAK1 and STAT3, which maintained the contractility- and invasion-promoting properties of CAFs.221,222 To attenuate the effect of specific cytokines on JAK/STAT signal, therapeutic approaches, including blocking cytokine antibodies or inhibitors, are warranted to identify the tumor-promoting roles of CAFs. Targeted inhibition, such as that induced by 5-azacytidine and ruxolitinib treatment, resulted in the sustained abrogation of JAK1/STAT3 phosphorylation and rescued SHP-1 expression, thereby inhibiting the tumor-promoting invasive phenotypes of CAFs.223,224 In an analysis of miRNAs in CAFs through JAK/STAT signaling pathway, miR-210 increased the expression of matrix metalloproteinase 9 (MMP9), FGF2, and VEGFA by activating the JAK2/STAT3 signaling pathway for proangiogenesis and ten-eleven translocation 2 was identified as the target of miR-210 in CAFs, which was implicated in proangiogenic switching.225 In addition, p53 was reported to regulate the CAF properties through STAT3 signaling, and CAF activation, migration, and invasion could be clearly inhibited by Stattic (Y705), an inhibitor of STAT3.226
Table 3.
CAF-secreted factors and their roles in cancers
| Factors | Cancer type | Recipient cell | Biological function | Activated pathway | Refs. |
|---|---|---|---|---|---|
| Cytokine | |||||
| IL-1β | OSCC | Cancer cell | Promotes cell growth | NF-κB pathway | 445 |
| IL-1β | OSCC, BC | Cancer cell | Promotes cell invasion | IL-1β/ IL-1R pathway | 446,447 |
| IL-6 | ESCA | Cancer cell | Promotes cell chemoresistance | STAT3/NF-κB pathway | 218,448 |
| IL-6 | LC, HCC, PRAD | Cancer cell | Promotes cell metastasis and chemoresistance | JAK2/STAT3 pathway | 219,449–451 |
| IL-6 | GC, BLCA, GBC, BC, UCEC | Cancer cell | Promotes cancer progression | JAK/STAT3 pathway | 230,448,452–456 |
| IL-6 | HNSC | Cancer cell | Promotes cancer progression | Integrin αvβ3/NF-κB pathway | 295 |
| IL-6 | CRC | Monocyte | Promotes cell adhesion | ERK1/2 pathway | 341 |
| IL-6 | HCC | Cancer cell | Promotes stem cell-like properties | STAT3/Notch pathway | 457 |
| IL-6 | HCC | Neutrophils | Regulates cell survival, activation, and function | STAT3/PD-L1 pathway | 458 |
| IL-8 | CRC | Monocyte | Recruits monocyte and promotes its polarization | IL-8/CXCR2 pathway | 341 |
| IL-8 | GC | Cancer cell | Promotes cisplatin resistance | NF-κB pathway | 459 |
| IL-11 | GC, LC | Cancer cell | Promotes cell chemoresistance and metastasis | JAK/STAT3 pathway | 174,460,461 |
| IL-17a | GC | Cancer cell | Promotes cell migration and invasion | JAK2/STAT3 pathway | 232 |
| IL-22 | GC | Cancer cell | Promotes cell invasion | STAT3 and ERK pathway | 462 |
| IL-23 | BC | Cancer cell | Promotes cell invasion and metastasis | p38/MAPK pathway | 178 |
| IL-25 | BC | Cancer cell | Suppresses cell metastasis | ND | 463 |
| IL-33 | HNSC | Cancer cell | Promotes cell migration and invasion | ND | 464 |
| Chemokine | |||||
| CXCL5 | CRC | Cancer cell | Promotes tumor immunosuppression | PI3K/AKT pathway | 465 |
| CXCL9 | OSCC | Cancer cell | Suppresses cell apoptosis | CXCL9/CXCR3 pathway | 466 |
| CXCL12 | CRC | Cancer cell | Promotes cell metastasis | PI3K/AKT pathway | 140 |
| CXCL12 | BC | Cancer cell | Promotes cell invasion | TGF-β pathway | 305 |
| CXCL12 | OC | Cancer cell | Promotes EMT and cisplatin resistance | Wnt/β-catenin pathway | 467 |
| CXCL12 |
LC, HNSC, PAAD, GC |
Cancer cell | Promotes cancer progression | CXCL12/CXCR4 pathway | 468–472 |
| CCL3 | PRAD | Cancer cell | Promotes cell migration and invasion | JAK/STAT3 pathway | 473 |
| CCL5 | GC | Cancer cell | Promotes cell progression | CCL5/CCR5 pathway | 474 |
| CXCL2 | LUAD | Cancer cell | Promotes cancer immunosuppression | ND | 475 |
| CXCL16 | BC | Monocyte | Promotes the recruitment of monocyte | ND | 476 |
| SDF-1 | PAAD, CRC | Cancer cell | Promotes cell gemcitabine resistance and metastasis | SDF-1/CXCR4 pathway | 477,478 |
| SDF-1 | UCEC | Cancer cell | Promotes cancer progression | PI3K/AKT and MAPK/ERK pathway | 479 |
| Growth factors | |||||
| CRC | Cancer cell | Promotes cell cetuximab resistance | MAPK pathway | 253 | |
| FGF1 | OC | Cancer cell | Promotes cancer progression | FGF/FGFR pathway | 480 |
| FGF2 | BC, LC, CRC | Cancer cell | Promotes cancer progression | FGF/FGFR pathway | 481–483 |
| FGF9 | GC | Cancer cell | Promotes cell invasion | ERK and AKT pathway | 484 |
| GPER | BC | Cancer cell | Promotes cell proliferation | GPER/EGFR/ERK pathway | 248 |
| GDF15 | PRAD | Cancer cell | Promotes cancer progression | TGF-β/GDF15 pathway | 485 |
| HGF | CRC, GC, HNSC | Cancer cell | Promotes cell progression and metastasis | HGF/c-Met pathway | 143,452,486 |
| HGF | GC | HUVEC | Promotes angiogenesis | PI3K/AKT and ERK1/2 pathway | 147 |
| HGF | HCC | Cancer cell | Promotes cell chemoresistance | MEK-ERK1/2 pathway | 487 |
| HGF | HCC | Cancer cell | Promotes cell plasticity | c-Met/FRA1/HEY1 pathway | 488 |
| HGF | LC | Cancer cell | Promotes cell chemoresistance | HGF/IGF-1/ANXA2 pathway | 489 |
| IGF-1 | |||||
| IGF-1 | BLCA | Cancer cell | Promotes cell chemoresistance | IGF-1/AKT pathway | 355 |
| IGF-1 | CRC | Cancer cell | Promotes cell survival | IGF-1/IGF1R pathway | 490 |
| IGF-2 | AC | Cancer cell | Promotes cell growth | PI3K/AKT/mTOR pathway | 142 |
| IGF-2 | LC | Cancer cell | Promotes cell chemoresistance | IGF-2/IGF‐1R pathway | 491 |
| TGF-β1 | BC, PRAD | Cancer cell | Promotes cell proliferation and migration | TGF-β/Smad pathway | 492,493 |
| VCAM-1 | LC | Cancer cell | Promotes cell growth and invasion | AKT and MAPK pathway | 138 |
| VCAM-1 | GC | Cancer cell | Promotes cell invasion | JAK/STAT1 pathway | 494 |
| Others | |||||
| ADAM17 | BC | Cancer cell | Promotes cell proliferation | EGFR, AKT, and ERK pathway | 495 |
| Activin A | CRC | Cancer cell | Promotes cell migration and invasion | ND | 496 |
| ANXA3 | LC | Cancer cell | Promotes cell cisplatin resistance | ANXA3/JNK pathway | 497 |
| Asporin | GC | CAF | Promotes CAF migration | ND | 498 |
| CD9, GAS6 | GC | Cancer cell | Promotes cell migration | ND | 499,500 |
| CDH-11 | BC | Cancer cell | Promotes cell migration | ND | 501 |
| CLEC3B | CRC | Cancer cell | Promotes cell migration | ND | 502 |
| Collagen | BC | Cancer cell | Promotes cell chemoresistance | PI3K/AKT pathway | 503 |
| Collagen | PDAC | Cancer cell | Promotes cell growth and migration | Integrin β1/FAK pathway | 504 |
| Fatty acids | CRC | Cancer cell | Promotes cell migration | ND | 505 |
| FN | PRAD | Cancer cell | Promotes cell migration | ND | 313 |
| Gal1 | GC | Cancer cell | Promotes cancer progression and metastasis | ND | 506 |
| Galectin-1 | GC | HUVEC | Promotes angiogenesis | ND | 507 |
| Grem1 | BC | Cancer cell | Promotes cancer progression | BMP/Smad pathway | 508 |
| HIAR | BC | Endothelial cell | Promotes angiogenesis and cell migration | VEGF/VEGFR pathway | 509 |
| HIC-5 | ESCA | Cancer cell | Promotes cell metastasis | ND | 510 |
| HMGB1 | BC | Cancer cell | Promotes cell stemness and tumourigenicity | HMGB1/TLR4 pathway | 511 |
| Lactate | BC | Cancer cell | Promotes cell invasion | TGF-β1/p38/MAPK pathway | 512 |
| LOX | GC | Cancer cell | Promotes cell growth | ND | 513 |
| LOXL2 | CRC | Cancer cell | Promotes cell invasion and metastasis | FAK pathway | 514 |
| Lumican | GC | Cancer cell | Promotes cell tumorigenesis and metastasis | Integrin β1/FAK pathway | 515 |
| M-CSF | PDAC | Monocyte | Promotes TAM phenotype | ND | 516 |
| MFAP5 | OSCC | Cancer cell | Promotes cell proliferation and migration | MAPK and AKT pathway | 517 |
| MK | OSCC | Cancer cell | Promotes cell cisplatin resistance | ND | 518 |
| MMP2 | CESC, OSCC | ECM | Promotes cancer invasion | ND | 519,520 |
| NAB2 | HNSC | Cancer cell | Promotes cell growth | ND | 521 |
| Notch3 | HCC | Cancer cell | Promotes cell self-renewal | ND | 522 |
| PAI-1 | ESCA | Cancer cell | Promotes cell cisplatin resistance | AKT and ERK1/2 pathway | 523 |
| POSTN | GC | Cancer cell | Promotes cell growth | ERK pathway | 177 |
| POSTN | HNSC | Cancer cell | Promotes cell stemness | PTK7-Wnt/β-catenin pathway | 190 |
| POSTN | CESC | Endothelial cell | Promotes cancer metastasis | FAK/SRC pathway | 524 |
| POSTN | OC, OAC | Cancer cell | Promotes cell cisplatin resistance and invasion | PI3K/AKT pathway | 525,526 |
| Pyruvate | Lymphoma | Cancer cell | Promotes cell survival | ND | 361 |
| RANKL | OSCC | Osteoclast | Promotes bone invasion | ND | 527 |
| SNAI1 | LC | Cancer cell | Promotes cell migration and invasion | ND | 528 |
| TNFSF4 | LUAD | Cancer cell | Promotes chemoresistance | NF-κB/BCL-XL pathway | 529 |
| uPA | ESCA | Cancer cell | Promotes cancer progression | PI3K/AKT and ERK pathway | 175 |
| WNT2 | CRC | Cancer cell | Promotes cell invasion and migration | ND | 205,206 |
AC anal cancer, ANXA3 annexin A3, BC breast cancer, BLCA bladder cancer, CAF cancer-associated fibroblast, CDH-11 cadherin-11, CESC cervical and endocervical cancer, CLEC3B c-type lectin domain family 3 member B, CRC colon adenocarcinoma, ECM extracellular matrix, EGF epidermal growth factor, EGFR epidermal growth factor receptor, ERK extracellular regulated protein kinases, ESCA esophageal carcinoma, FGF fibroblast growth factor, FN fibronectin, Gal1 galectin-1, GAS6 growth arrest specific protein 6, GBC gallbladder cancer, GC gastric cancer, GDF15 growth differentiation factor 15, GPER estrogen receptor, Grem1 gremlin 1, HCC hepatocellular carcinoma, HGF sepatocyte growth factor, HIAR hypoxia-induced angiogenesis regulator, HIC-5 hydrogen peroxide-inducible clone 5, HMGB1 high-mobility group box 1, HNSCC head and neck squamous cell carcinoma, HUVEC human umbilical vein endothelial cell, IGF insulin-like growth factor, IL interleukin, JAK janus tyrosine kinase, LC lung cancer,LOX lysyl oxidase, LOXL2 lysyl oxidase-like 2, LUAD lung adenocarcinoma, MAPK mitogen-activated protein kinase, MEK mitogen-activated protein kinase, MFAP5 microfibril associated protein 5, MK midkine, mTOR mammalian target of rapamycin, ND not determined, NF-κB nuclear factor kappa-B, OAC esophageal adenocarcinoma, OC oarian cancer, OSCC oral squamous cell carcinoma, PAAD pancreatic adenocarcinoma, PDAC pancreatic ductal adenocarcinoma, PAI-1 plasminogen activator inhibitor 1, PD-L1 programmed cell death 1 ligand 1, PI3K PI3 kinase, POSTN periostin, PRAD prostate adenocarcinoma, PTK tyrosine protein kinase, RANKL receptor activator of nuclear factor kappa-B ligand, SDF-1 stromal cell-derived factor-1, SPARC secreted protein acidic and rich in cysteine, STAT3 signal transducer and activator of transcription 3, TGF transforming growth factor, TNFSF4 tumor necrosis factor ligand superfamily member 4, UCEC uterine corpus endometrial carcinoma, VCAM-1 vascular cell adhesion molecule-1
JAK/STAT signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
Constitutive activation of JAKs and STATs was first identified as being associated with malignancy,227 and accumulating evidence has shown that CAF-mediated JAK/STAT signaling pathway is widely involved in human cancers, including prostate,228 lung,229 breast,230 colorectal,231 gastric,232 endometrial,233 liver,234 and oral235 cancers, through various tumor biological processes, including in increased cell plasticity, proliferation, migration, invasion, EMT, angiogenesis, and metastasis. Notably, IL-6 represents the most investigated cytokine regulating the crosstalk between CAFs and cancer cells (Table 3). In HCC, CAF-derived IL-6 facilitated HCC cell EMT, which in turn activated the IL-6/IL-6R/STAT3 axis in a positive-feedback loop to promote the expression of TG2 for the acquisition of EMT phenotypes.234 IL-6 binding with GP130 could trigger STAT3 activation, and this response could be suppressed by the inhibition of netrin-1.236 Netrin-1, as a multifunctional secreted glycoprotein, is highly expressed in colon CAFs, and its blocking antibody (Net1-mAb) inhibited cancer stemness, plasticity, and intercellular signaling between CAFs and cancer cells by suppressing the IL-6/JAK2/STAT3 signaling pathway.236
As the upstream of IL-6, epiregulin-induced CAF activation promote EMT by activating IL-6/JAK2/STAT3 signaling axis, which could be inhibited by a JAK2 inhibitor (AG490).235 Intriguingly, the migration of melanoma cells was dependent on GP130-IL6ST/JAK1-ROCK signaling; however, although it was not necessary in cancer cells, this signaling pathway was required for CAF-induced ECM remodeling to promote the invasion of squamous cell carcinoma (SCC),237 indicating that the targeted therapy of JAK/STAT signaling pathway for SCC invasion might not be the epithelium but CAFs. Further, cytokine signaling of GP130-IL6ST/JAK1 cascade mediated actomyosin contractility in cancer cells and CAFs to promote SCC invasion.237 Estrogen in CAF-CM promoted gastric cancer cell proliferation and invasion via IL-6/STAT3 signal axis, and these two processes could be inhibited by an IL-6-neutralizing antibody and STAT3 siRNA, respectively.236
In addition, CAF-derived IL-17a significantly promoted the migration and invasion of gastric cancer cells by activating the JAK2/STAT3 signaling pathway, and the effects of CAF-mediated cancer progression were inhibited significantly by treatment with an IL-17a-neutralizing antibody or JAK2 inhibitor (AG490).232 In addition, Heichler et al. found that IL-11 was frequently overexpressed in colorectal cancer and acted as a signal transducer and activator of STAT3, which was inversely correlated with patient prognosis.231 Taken together, therapeutic agents targeting the JAK/STAT signaling pathway, including blocking antibodies against Netrin-1, cytokines including IL-6, IL-11, and IL-17a, or inhibitors of JAK kinase such as AG490 or STAT activity such as STAT3 siRNA, could be useful agents in antitumor treatment.
EGFR signaling pathway
EGFR signaling pathway in CAFs and its targeted therapy
EGFR belongs to the ErbB family of receptors, which includes ErbB1/EGFR/HER1, ErbB2/HER2/Neu, ErbB3/HER3, and ErbB4/HER4.238 ErbB family members can be activated by the following ligands: amphiregulin, betacellulin, EGF, heparin-binding EGF-like growth factor, TGF-α, epiregulin, epigen, and NRGs.239,240 Structurally, EGFR family members share a common domain arrangement that comprises a cysteine-rich extracellular domain, a hydrophobic transmembrane domain, and an intracellular tyrosine kinase domain. Specifically, the extracellular region of EGFR is subdivided into four domains, and the intracellular tyrosine kinase domain is highly conserved with variable phosphorylation sites.241,242 The EGFR signaling pathway is activated by ligand-induced receptor dimerization, in which the tyrosine residues in the intrinsic kinase domain of one receptor cross-phosphorylate specific residues in the C-terminal tail of the partnering receptor to recruit functional proteins.243,244
EGFR is expressed in almost all nonneoplastic cell types in TME, including CAFs, except mature cells in the lymphohematopoietic system.245,246 GPER was first reported as a GPCR gene in breast cancer,247 and Luo et al. found that GPER expression was abundant in breast CAFs.248 G15 (a selective GPER antagonist), AG (an inhibitor of EGFR), and U0126 (an inhibitor of ERK1/2) significantly inhibited GPER-mediated proliferation and cell cycle changes in breast CAFs induced by E2, G1, and tamoxifen,248 implying that GPER/EGFR/ERK signaling pathway was activated in this process. Of note, zinc chloride (ZnCl2) increased GPER-targeted CTGF in breast CAFs.249 Since CTGF was reported to have a role in the migration of different cell types,250 Pisano et al. found that ZnCl2-stimulated migration of CAFs was abolished by knockdown of GPER or CTGF, and this migratory response could be rescued by the addition of CTGF.249 In mammals, α1,6-fucosyltransferase (FUT8), as the only enzyme that catalyzes core α1,6-fucosylation (CF), was reported to be overexpressed in CAFs in 82% of lung adenocarcinoma cases, and upregulated FUT8 could prompt the CF modification at high levels.251 Downregulation of either EGFR or FUT8 reduced the phosphorylation of EGFR; however, the blockade of EGFR signaling was rescued by an EGFR activator in FUT8 down-regulated CAFs,251 demonstrating that EGFR activity in CAFs was regulated by this FUT8/CF treatment.
EGFR signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
Currently, EGFR tyrosine kinase inhibitors (EGFR-TKIs) are being effectively used for anticancer therapy, while CAF-derived survival signaling to cancer cells can modify EGFR-TKI efficacy.252–256 In HNSCC, it was reported that EGF-containing fibulin-like ECM protein-1 (FBLN3) was secreted by CAFs but not NFs, and CAF-derived FBLN3 could increase anchorage-independent growth and tumor sphere formation and maintain cancer stemness.257 Interestingly, targeting the EGFR signaling pathway with gefitinib effectively inhibited CAF-mediated cancer stemness,257 demonstrating that CAF-derived factors such as FBLN3 in the EGFR signal cascade promoted cancer stemness properties are potential therapeutic targets to effectively block CAF-promoted CSC niche formation. In another study, it was shown that FUT8/CF in CAFs prompted the proliferation of cancer cells through EGFR signal cascade in non-small-cell lung cancer,251 suggesting that EGFR signaling in CAFs exerted a catalytic effect on CAF-mediated cancer progression and could be regulated by the CF modification of EGFR.
In addition, the CAF-mediated EGFR signaling pathway plays promoting roles in tumor invasion and metastasis (Fig. 5). It was demonstrated that the collective invasion of SCC cells could be driven by the matrix-dependent mechano-sensitization to EGFR signaling.258 Because receptor tyrosine kinase (RTK) can interact exclusively with activated integrins, the ECM determines the type of RTK/integrin interaction proceeds on the cellular membrane, and this selectivity may change the intracellular location or conformation of EGFR.99,259 Given that the L-type calcium channel CAV1.1 functions with ECM stiffness and is triggered by EGFR signaling activation, calcium channel blockers may suppress SCC invasion and metastasis, and an EGFR blockade could trigger the EMT process in HNSCC.258 Notably, Gao et al. found that CAFs associated with high-grade serous ovarian cancer contributed to the formation of heterotypic spheroids in malignant ascites and that these CAF-centered spheroids recruited floating ovarian cancer cells, resulting in premetastatic niche formation at an early stage.260 In summary, these evidences support the supposition that the CAF-mediated EGFR signaling pathway is essential for several cellular functions, including maintenance of cancer stemness, cell proliferation and invasion, and metastasis. Importantly, in contrast to EGFR overexpression in tumor cells, which was positively correlated with the overall survival period of patients with several cancers,261 EGFR overexpression in CAFs had no significant relation to the prognosis of patients with colorectal cancer,246 indicating that EGFR in CAFs might not be an independent prognostic factor for survival evaluation in patients with cancers.
Hippo signaling pathway
Hippo signaling pathway in CAFs and its targeted therapy
Hippo signaling pathway was originally discovered to be an important regulator of organ size in Drosophila.262 In mammals, the canonical Hippo signaling cascade consists of mammalian sterile 20-like (MST) kinases, l (LATS) kinases, and adaptor proteins Salvador homolog 1 and Mps one binder kinase activator protein. Central to this cascade signals from MST1/2 to the oncogenic of transcriptional cofactors YAP1 and its paralog transcriptional coactivator with PDZ-binding motif (TAZ).263 The major target transcription factors regulated by YAP/TAZ are the TEAD family.263 Notably, in noncanonical Hippo signaling, for instance, phosphorylated YAP1/TAZ binds directly to an angiomotin family proteins, which is in the Crumbs complex, to α-catenin, β-catenin, PTPN14, and Scribble in adherens junctions and to ZO-1/2 in tight junctions, subsequently leading to the regulation of YAP1/TAZ localization and activity independently of LATS.264
In contrast to other classical signal transduction pathways, such as TGF-β or Wnt signal, the Hippo pathway does not seem to be involved in special extracellular ligands or transmembrane receptors, but is regulated by upstream signals and involved in cell morphology and polarity and cell–cell and cell–ECM adhesion.265–268 Studying prostate cancer, Shen et al. found that the YAP1/TEAD1 protein complex transformed NFs to CAFs by activating cytoskeletal proteins and actin by regulating SRC.47 In addition, the proliferation of CAFs was significantly inhibited by siYAP1 or the inhibitor verteporfin,47 indicating that YAP1 had multiple effects on CAFs. YAP activation in CAFs269,270 controlled the expression of several cytoskeletal regulators, including ANLN and CTGF, and regulated actomyosin contractility and ECM remodeling in CAFs via MYL9/myosin light chain-2,269 and in agreement, the gain of YAP function in CAFs was associated with reactivation of actomyosin contractility and SRC,193 supporting the supposition that YAP/TAZ activity of CAFs was primarily associated with its effect on ECM remodeling. In another study, Calvo et al. found that YAP depletion by siRNA caused weakening of the ability of CAFs to physically contract collagen-rich matrices, and fewer focal adhesions and fewer essential structures were formed for force transmission between the cytoskeleton and matrix; however, depletion of TAZ had little effect on these processes,269 indicating that TAZ is not the only downstream component of the YAP-mediated signaling pathway. Interestingly, in breast cancer, the activity of the upstream negative regulators MST1/2 was not different between NFs and CAFs, while the activity of LATS kinases and phosphorylated YAP was augmented in CAFs,269 suggesting that YAP was not activated by the attenuated activity of the canonical MST/LATS signaling pathway in CAFs.
Hippo signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
CAF-mediated Hippo signaling pathway tumor-promoting activities are mainly related to cell proliferation and invasion and angiogenesis (Fig. 5). In prostate cancer, CAFs with high YAP1 expression could prompt the proliferation of cancerous epithelial cells and were more likely to cause the distant metastasis.47 Similarly, Shen et al. found that knocking down YAP1 or SRC in CAFs attenuated the promotion of CM on the proliferation and invasion capacity of human prostate cancer cells,47 indicating that CAFs in prostate cancer could promote tumor cell proliferation and invasion, which was highly dependent on the paracrine activity of YAP1 and/or SRC in CAFs. Strikingly, we found that YAP expression in primary HNSCC cells was associated with Nodal-induced metastasis; however, YAP knockdown in HNSCC cells was not associated with changes in EMT.271 Notably, CAF-derived miR-221 could trigger proliferative and migratory effects on MDA-MB 231 and SkBr3 breast cancer cells by interfering with the A20/c-Rel/CTGF signaling pathway.272 MiR-221 was reported to be strongly upregulated and closely related to the invasion and advanced clinical stages of patients with breast cancer.273 In general, targeting miR-221 by specific inhibitors such as LNA-i-miR-221 might cause a suppressive effect on cancer progression, especially breast tumors.274,275 Consistently, the ability of CAFs to promote cancer cell invasion was significantly dependent on YAP function, and loss of YAP function reduced the formation of fibrous collagen networks by CAFs and suppressed angiogenesis in vivo.269 In addition, in a mouse model, treatment with Y27632 (a potent, ATP-competitive inhibitor of ROCK1 and ROCK2) reduced the nuclear localization of YAP in CAFs and inhibited angiogenesis,269 indicating that the metastatic function of YAP in HNSCC may not be a result of EMT.
With respect to the molecular mechanism of CAF-mediated YAP/TAZ signaling pathway in the crosstalk between CAFs and cancer cells, it was reported that DKK3 in CAFs potentiated the Wnt and Hippo signaling pathways.193 DKK3 knockdown in CAFs decreased the levels of unphosphorylated β-catenin and TAZ, reduced nuclear YAP/TAZ translocation, and inhibited β-catenin and YAP/TAZ transcriptional activity in breast, colorectal, and ovarian cancers.193 DKK3 reduced YAP/TAZ degradation through the Wnt/β-catenin signaling pathway, thus acting in parallel to YAP/TAZ regulation mediated through mechanotransduction.269,276 Although DKK3 could activate β-catenin in CAFs, the inhibition of β-catenin by RNA interference did not affect CAF-mediated ECM remodeling or cell growth or invasion in cancer,193 indicating that exogenous inhibitors targeted to Wnt/β-catenin signaling axis might not attenuate aggressive behaviors of CAFs and/or that Wnt/β-catenin signaling pathway might be one of the upstream regulators of the YAP/TAZ signaling cascade. In support of this notion, DKK proteins were reported to negatively affect the surface expression of Kremen, and DKK3 was shown to inhibit Wnt signaling by triggering LRP5/6 internalization through the formation of a ternary complex with Kremen1/2 receptors.191,192,277 In contrast, Ferrari et al. found that Kremen1, but not LRP6, immunoprecipitated with DKK3 in CAFs, while DKK3 silencing led to Kremen1 localization to the cell periphery,193 demonstrating that DKK3-mediated LRP6 regulation could activate β-catenin and YAP/TAZ, with the latter being the main mediator of DKK3 functions in the crosstalk between CAFs and cancer cells.
NF-κB signaling pathway
NF-κB signaling pathway in CAFs and its targeted therapy
As a ubiquitous transcription factor, NF-κB consists of five different proteins: RelA (p65), RelB, c-Rel, NF-κB1 (p50), and NF-κB2 (p52).278 Generally, core components of NF-κB signaling pathway are inhibitors of NF-κB (IκB) proteins, namely, the IκB kinase (IKK) complex and NF-κB transcription factor.279,280 In the canonical NF-κB pathway, activation is triggered by the binding of ligands (e.g., TNF-α, IL-1β) to their respective receptors (e.g., Toll-like receptors (TLRs), TNFR, and IL-1R), which leads to the phosphorylation and activation of IKK, thereof initiating the phosphorylation of IκB proteins.281,282 In the noncanonical NF-κB pathway, ligands such as CD40 bind to their cognate receptors. Then, this binding leads to activation of NF-κB by NF-κB-inducing kinase, which phosphorylates IKKα and facilitates IKKα phosphorylation of p100 for p52 generation. RelB/p52 heterodimers then translocate into the nucleus, subsequently leading to the activation of noncanonical NF-kB target gene expression.283–285
CD146, a cell membrane protein, was knocked down to promote CAFs activation, which might have been caused by the modulation of NF-κB activity.286 In another study, Wu et al. found that gastric cancer-derived HTRA1 promoted CAFs generation from NFs through the activation of the NF-κB/bFGF/FGF2 signaling pathway.287 Furthermore, CXCR2 signaling in CAFs promoted the CAF acquisition of secretory phenotype by activating NF-κB,288 noting that NF-κB signaling pathway was involved in regulating CAF factor secretion. Similarly, in OSCC, CAFs were stimulated with IL-1β and exhibited increased CXCL1 secretion in an NF-κB-dependent manner.289 Cancer-derived IL-1α increased the expression of COX-2, CXCL8, CCL20, and IL-6 in CAFs to form an inflammatory environment in pancreatic cancer.290 In regard to the immune response, it was reported that decreased α-SMA in CAFs was observed after their incubation with the polysaccharide MPSSS and impaired the immunosuppressive effect of CAFs through TLR4/NF-κB signaling, but there was no obvious effect on CAF viability.291 Ligustilide had no effect on CAFs’ viability, but reversed the immunosuppressive function of CAFs through the TLR4/NF-κB signaling pathway.292 In sum, these evidences suggest that NF-κB signaling pathway is mainly implicated in the activation, secretory phenotype, and immunosuppressive functions of CAFs.
NF-κB signaling pathway-mediated crosstalk of CAFs with cancer cells and its targeted therapy
Fundamentally, CAF-driven NF-κB was reported as a pro-inflammatory gene signature critical for tumor progression (Fig. 5). For instance, CAF-derived CXCL1, IL-6, and COX-2, known as targets of the NF-κB transcription factor, were correlated with tumor-promoting inflammation and tumor invasiveness in human breast cancer.293 It was reported that oxidative stress triggered NF-κB activation and STAT3 in CAFs to upregulate CCL2, and inhibition of CCL2 could reduce tumor growth of oral cancer in a mouse model.294 IL-6 secreted by CAFs could trigger the induction of neoplastic OPN to promote the growth, migration, and invasion of cancer cells in HNSCC via the integrin αvβ3/NF-κB pathway.295 Notably, in SCC, the pro-inflammatory signaling driven by CAFs was NF-κB-dependent.36 In sum, these data suggest that CAF-driven NF-κB signaling plays a central role in mediating protumor inflammation. Interestingly, TLR4 expressed by tumor cells was significantly associated with decreased recurrence; however, its overexpression in CAFs was independently associated with increased recurrence in patients with colorectal cancer,296 indicating that TLR4 in CAFs might not be an independent prognostic factor for recurrence of colorectal cancer.
In addition, CAFs could mediate the EMT and tumor stemness through a pro-inflammatory signature strictly dependent on COX-2-, NF-κB-, and HIF-1α-related signaling cascades.297 In pancreatic cancer, phosphorylated NF-κB was positively correlated with CAV-1 expression, and knockdown of CAV-1 in CAFs could reduce the invasiveness and motility of cancer cells, but did not affect cell proliferation.298 Importantly, overexpression of Smad7 in IKKβ-deficient CAFs prevented HGF secretion, and pharmacological inhibition of Met in a CAC model supported that enhanced tumor promotion was dependent on HGF-Met signaling in the mucosa of IKKβ-mutant animals,299 suggesting that a tumor-suppressive function of IKKβ/NF-κB in CAFs might be related to HGF release. In our preliminary study, we found that topically applied Tat-Smad7 penetrated cells in both healthy oral mucosa and oral cancer, attenuating NF-κB signaling-related inflammation.300 In addition, the effect of antisense oligonucleotide-miR-221 transfection in CAFs caused the inhibition of migration/invasion by downregulating NF-κB in pancreatic cancer.301 In addition, ablation of Saa3, a member of the serum amyloid A apolipoprotein family, in pancreatic tumor cells rendered them insensitive to the inhibitory effect of Saa3-null CAFs,302 suggesting that saa3 in CAFs may provide potential therapeutic benefit to pancreatic ductal adenocarcinoma (PDAC) patients. Taken together, these results suggest that CAV-1, Smad7, saa3, and miRNAs might be candidates to target the CAF-mediated NF-κB signaling pathway in cancers.
Other signaling pathways in CAFs, crosstalk of CAFs with cancer cells, and its targeted therapy
CAF-induced signaling pathways not discussed above, including Notch,303–305 Hedgehog,306–308 p53,309–312 Rho/Rock,313,314 HIF-1α,44,315–317 and peroxisome proliferator-activated receptor (PPAR)318–320 signaling cascades, have been widely studied to understand CAFs crosstalk with cancer cells. Indeed, covering all aspects of all signaling pathways of CAFs in cancer progression is beyond the scope of this review. However, similar to the signaling pathways we summarized above, other signaling pathways exhibit unique characteristics. For instance, HIF-1α signal in CAFs promotes tumor progression mainly by regulating glycolytic metabolism. Radhakrishnan et al. demonstrated that LPA-induced glycolytic shift was the earliest, potentially priming event of the NF-CAF transition, and it was mediated through LPA/LPAR/HIF-1α signaling axis.44 In another example, CAF-specific RWE increased the expression of fructose bisphosphatase in cancer cells, leading to the modulation of HIF-1α function in non-small-cell lung cancer cells.321 Thus, all these signaling hubs in CAFs have great potential as targets for blocking CAFs crosstalk with cancer cells, and further investigations are warranted to identify the specific functions of these targets.
Immunotheray driven by CAFs
In addition to CAFs, the TME contains an array of other nonneoplastic cells, including resident and infiltrating immune cells. Particular emphasis has been placed on T lymphocytes, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and others. The immune cells in the TME play a dual role during cancer onset and progression, as they can mediate tumor clearance by killing immunogenic neoplastic cells and contribute to tumor escape by shaping tumor immunogenicity.322,323 Immunotherapies, harnessing the dual roles of the immune system in battling cancer, have emerged as new pillars within oncotherapy.324,325
To date, CAFs have been found mainly to regulate and rewire the TME to favor the malignant biological properties of tumors through the interaction of CAFs and T lymphocytes. In colorectal cancer, CD70 expression in CAFs and the CD70/CD27 axis affected the expansion and differentiation of T lymphocytes by activating the NF-κB signaling pathway.326 Chakravarthy et al. found that pan-cancer ECM dysregulation was linked to CAF-mediated TGF-β signaling, which was closely related to immunological activity and could predict failure of PD-1 blockade.327 Meanwhile, the combination of TGF-β blockers and anti-PD-L1 antibodies promoted T cell penetration into the tumor center, improving tumor responsiveness to anti-PD-L1 therapy.328 Activated CAFs can also produce TGF-β to form a positive-feedback loop for CAFs activation. Therefore, inhibition of TGF-β signaling, such as through treatment with galunisertib (LY2157299) or AVID200, is worthy of further exploration as a method to improve PD-L1 immunotherapy.329–332 In addition to TGF-β blockers, pirfenidone (PFD)-targeting CAFs possessed inhibitory effects on migration and decreased the expression of PD-L1 by targeting CCL17 and TNF-β. The regulation of CAFs through PFD treatment may reduce the acquisition of CAF-mediated immunosuppressive capacity in breast cancer cells, thereby leading to increased efficacy of chemotherapy.333 In addition, highly expressed presenilin 1 in CAFs also regulated tumor-infiltrating lymphocyte populations in the TME via the Wnt/β-catenin pathway. Inhibition of presenilin 1 by IL-1β promoted the proliferation and penetration of CD8+ T cells in multiple ovarian models and retrieved functional CD8+ T cells in the TME, which may improve the efficacy of current immunotherapies.334 The angiotensin receptor blocker losartan can drive myofibroblast CAFs into a quiescent state, attenuate immunosuppression, and increase T lymphocyte activity, thereby significantly improving the response of breast cancer cells to immune checkpoint blockers.335 Feig et al. found that pancreatic cancer cells coated with the CAF-derived CXCL12 caused the depletion of T cells and contributed to cancer unresponsiveness to α-PD-L1; administering a CXCR4 (a CXCL12 receptor) inhibitor, AMD3100, blocked CAF-directed immune evasion of the tumor to increase T cell infiltration in cancer cells and greatly diminish cancer volume when administered in combination with α-PD-L1.336 These studies demonstrate that the dysregulation of CAFs contributes to tumor-induced immunosuppression and that immunotherapy driven by CAFs sensitizes tumors to T cells, stimulating strong antitumor cellular immunity and tumor regression.
In addition, immunotherapies targeting TAMs have drawn significant attention, as they deplete and/or reprogram TAMs to block their protumor functions or restore their tumoricidal properties.337 Colony-stimulating factor-1 receptor (CSF1R) inhibitors targeting TAMs successfully in diffuse-type giant cell tumors failed to deliver an antitumor effect in other tumor models, as CSF1R inhibition altered CXCL1 and other chemokine secretion by CAFs and triggered a profound increase in protumor polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC) recruitment to tumors; combined inhibition of CSF1R and CXCR2 (a CXCL1 receptor on granulocytes) blocked CAF-mediated MDSC recruitment and reduced tumor growth.338 In OSCC, CAFs educated macrophages to suppress T cell proliferation, which was restored by the neutralization of TGF-β, IL-10, or arginase I.339 The CAF–TAM interaction was also regulated by pleiotropic glycoprotein stanniocalcin-1, which was secreted by CAFs and partially suppressed TAM differentiation.340 In colorectal cancer, CAFs could attract monocytes via IL-8/CXCR2 pathway to induce their polarization.341 Thus, CAFs play crucial roles in shaping the immunosuppressive TME by educating TAMs to induce a protumor phenotype, and methods for reversing CAF-mediated immunosuppression in TAM-targeted therapeutics need to be considered. In addition, CAFs can functionally sculpt other immune cells in the TME through their high secretory ability. CAF-derived cytokines, including IL-6 and CXCL12, induced the generation and activation of MDSCs to favor HCC progression.342 HCC CAFs recruited normal dendritic cells and educated them to acquire a tolerogenic phenotype through IL-6/STAT3 signaling.343 Taking these observations together, it can be concluded that crosstalk between CAFs and T cells, TAMs, MDSCs, etc. is involved in the formation of tumor immunosuppression (Fig. 5), and combination therapy driven by CAFs and immunotherapies might be an effective and promising strategy for treating insensitive tumors.
Meanwhile, the heterogeneity of intratumoral CAFs impels a paradigm shift in CAFs behaviors, especially immune-related function. Although the classification criteria for CAF subtype are diverse and are in a state of flux, some subpopulations of CAFs with an immunomodulating phenotype likely make more distinct contributions to tumor immunity. Based on the cellular source, Bartoschek et al. found that mCAFs from resident fibroblast can also regulate the immune response.57 Unlike this, from the perspective of function, in PDAC, iCAFs revealing a pro-inflammatory gene signature with IL-6 and CXCL12 expression promoted tumor growth, angiogenesis, and macrophage recruitment.344 Similarly, in breast cancer, CAF-S1 fibroblasts attracted or regulated the function and differentiation of T lymphocytes by secreting CXCL12 to format an immunosuppressive environment.58 Further, the CAF subtypes with a high level of IL-6 and FAP, which associated with a large cluster of pathways involved in immune regulation and the worse survival outcomes in ovarian cancer.345 These data suggest that the classification of different subpopulations based on CAFs’ function seems to be much more reliable in tumor immunity.
Therapeutic resistance caused by CAFs
At present, a number of CAF-mediated anticancer therapies have been reported, of which most are in phases of preclinical trials. Overall, CAF-mediated anticancer therapies mainly include the following five application approaches: inhibiting transformation from normal fibroblasts to CAF, promoting transformation from CAF to normal fibroblasts, inhibiting tumor development and progression, activating immunity system, and reversing tumor chemoresistance (Table 4). We found that breast cancer is the most widely studied for targeting CAFs in cancer treatment. Interestingly, no trials of pancreas cancer were found in the inhibition of tumor development and progression; however, a majority of studies in reversing tumor chemoresistance were related to pancreas cancer, suggesting that CAF-mediated chemoresistance might be tumor- or organ-specific. The interaction between tumor cells and CAFs or ECM blunts the response to cancer cell-directed therapies, including chemotherapy, small-molecule drug-based therapy, and radiotherapy (RT).346
Table 4.
CAF-mediated anticancer therapies
| Target | Cancer cell origin | Study type (sample size) | Anticancer therapies (NCT number) | Anticancer mechanisms | Refs. |
|---|---|---|---|---|---|
| Inhibiting transformation from normal fibroblasts to CAF | |||||
| NOX4 | Head, neck, colorectum | Preclinical | GKT137831 | Delays transdifferentiation of fibroblast to CAF | 530 |
| TGF-β | Breast, ovary | Preclinical | HA-PTX/MATT HNPs; LY2157299 | Blocks the fibroblast activation by downregulating the TGF-β expression | 531,532 |
| Promoting transformation from CAF to normal fibroblasts | |||||
| CAF | Solid tumors | Preclinical | sTRAIL LPD | Induces fibroblast reprogramming | 533 |
| TGF-β | Breast | Preclinical | Artemisinin derivatives | Inactivates CAF and inhibits metastasis | 534 |
| VDR | Pancreas | Preclinical | Calcipotriol | Reprises the quiescent state of CAF | 535 |
| Vitamin A | Pancreas | Preclinical | ATRA | Induces quiescence of stellate cells | 536 |
| Inhibiting tumor development and progression | |||||
| CAF | Esophagus, stomach | Preclinical | Bortezomib + panobinostat | Reduces the viability of CAF through inducing caspase-3-mediated apoptosis | 537 |
| CAF | Lung | Preclinical | 5-Fluorouracil | Eliminates CAF recruited by tumors | 538 |
| CAF | Stomach | Preclinical | Losartan + others | Reduces CAF activity and collagen fiber | 539 |
| CSF1R + CXCR2 | Lung, breast, colon, others | Preclinical | JNJ-40346527 + SB225002 | Blocks protumor PMN-MDSC recruitment and reduces tumor growth | 338 |
| FAP | Esophagus, breast, lung, colon, rectum | Preclinical; phase I (43) | PIT; 131I-mAbF19 PDT; Sibrotuzumab; αFAP-PE38 | Depletes CAF specifically or inhibits function of CAF to suppress tumor progression | 382,540–545 |
| FAP + DPPIV | Lung | Preclinical | FAP deletion; PT630 |
Increases collagen level, decreases CAF content and blood vessel density in tumor |
546 |
| Integrin αvβ3 | Breast | Preclinical | ProAgio | Reduces intratumoral collagen and decreases growth factors from CAF | 547 |
| MMP | Breast, solid tumors | Preclinical; phase I (32) | HA-PTX/MATT HNPs; S-3304 | Suppresses angiogenesis, degradation of extracellular matrix, and metastasis | 531,548 |
| MiR-214 + miR-301a | Stomach | Preclinical | Astragaloside IV | Reduces expression of oncogenic pluripotency factors SOX2 and NANOG | 549 |
| PDGFR signaling | Cervix uteri | Preclinical | Imatinib | Reduces epithelial cell growth factor and angiogenic factor by CAF | 550 |
| ROS/MAPK + ferroptosis pathways | Nasopharynx | Preclinical | Disulfiram/copper | Induces cytotoxic effects on CAF and tumor cells, promotes CAF apoptosis, and inactivates CAF | 179 |
| TEM-1 | Colorectum | Phase II (126) | MORAb-004 (NCT01507545) | Blocks specific TEM-1 receptor–ligand interactions | 551 |
| Tenascin | Brain | Phase II (43) | 131I-m81C6 | Delays tumors growth and metastasis | 552 |
| TGF-β | Breast, liver | Preclinical | Pirfenidone; LY2109761 | Induces CAF apoptosis and inhibits CAF proliferation | 90,553 |
| VEGF | Pleura | Phase III (448) | Bevacizumab + others | Inhibits tumor angiogenesis | 554 |
| VDR/1RARβ | Skin | Preclinical | EB1089; LE135 | Reduces cancer cell proliferation and/or increases apoptosis | 318 |
| Activating immunity system | |||||
| CAF | Liver | Preclinical | DC/CAF fusion | Activates cytotoxic T lymphocytes | 555 |
| CCR2 + IDO1 + NOX2 | Lung | Preclinical | CCR2i + epacadostat + GSK-2795039 | Reverses the interaction between CAF and monocytic myeloid cells | 556 |
| CXCR4 | Pancreas | Preclinical | AMD3100 + others | Reverses tumor immune evasion | 336 |
| FAP | Colon, breast | Preclinical | DNA vaccine; PIT | Enhances cytotoxic T cell infiltration | 336,545 |
| PD-L1 + TGF-β | Breast, colorectum | Preclinical | M7824 | Activates innate and adaptive immune to suppress tumor growth and metastasis | 557 |
| Reversing tumor chemoresistance | |||||
| AR | Pancreas | Phase II (49) | Losartan + others | Reduces collagen and hyaluronan level | 558 |
| ATRA | Pancreas | Preclinical | ATRA + gemcitabine | Targets multiple tumor–stromal pathways | 559 |
| Collagen I | Breast | Preclinical | Losartan-loaded C16-N | Inhibits CAF and collagen synthesis | 560 |
| CTGF | Pancreas | Preclinical; phase I (50) | FG-3019 gemcitabin (NCT01181245) | Decreases X-linked inhibitor of apoptosis protein to kill tumor cell | 561 |
| FAP-α | Prostate | Preclinical | Drug-loaded CAP-NPs | Disrupts stromal barrier to drug | 562 |
| GPR77 | Breast | Preclinical | Anti-GPR77 antibody | Reduces the infiltration of CAF | 563 |
| Hedgehog pathway | Pancreas | Preclinical | IPI-926 + gemcitabine | Depletes CAF to increase intratumoral concentration of gemcitabine | 395 |
| Hyaluronan | Pancreas | Phase II (279) | PEGPH20 + others | Increases drug delivery | 564 |
| IL-6 | Stomach | Preclinical | Tocilizumab | Renews chemotherapy-induced apoptosis | 388 |
| mTOR/4E-BP1 pathway | Pancreas | Preclinical | SOM230 + gemcitabine | Blocks insoluble or soluble proteins synthesis and secretion from CAF | 565 |
| Vimentin + SMA | Pancreas | Phase I (7) | PEGPH20 + Avelumab (NCT03481920) | Increases drug delivery and immune infiltration | 566 |
4E-BP1 4E-binding protein 1, AR angiotensin receptor, ATRA all-trans retinoic acid, CAF cancer-associated fibroblast, CAP-NP cleavable amphiphilic peptide nanoparticles, CSF1R colony-stimulating factor-1 receptor, CTGF connective tissue growth factor, CXCR2 chemokine receptor 2, DC dendritic cells, DPPIV dipeptidyl peptidase IV, ECM extracellular matrix, FAP fibroblast activation protein, GPR77 G-protein-coupled receptor 77, HA-PTX/MATT hyaluronic acid-paclitaxel/marimastat, HNP hybrid nanoparticles, IL interleukin, LPD lipid-coated protamine DNA complexes, MAPK mitogen-activated protein kinase, MMP matrix metalloproteinases, mTOR mammalian target of rapamycin, NOX4 NAD(P)H oxidase-4, PEGPH20 pegvorhyaluronidase alfa, PDGFR platelet-derived growth factor receptor, PDT photodynamic therapy, PIT photoimmunotherapy, PMN-MDSC polymorphonuclear myeloid-derived suppressor cells, RAR retinoic acid receptor, ROS reactive oxygen species, SMA smooth muscle actin, sTRAIL secretable TNF-related apoptosis-inducing ligand, TGF transforming growth factor, TEM-1 tumor endothelial marker-1, VEGF vascular endothelial growth factor, VDR vitamin D receptor.
Chemotherapy utilizing traditional cytotoxic antitumor drugs plays therapeutic roles in the lung, pancreatic, colorectal cancer, and other malignant tumors.347–350 First, CAFs generate physical obstacles around malignant elements by secreting a dense desmoplastic matrix into the TME. Laminin A1 secreted by CAFs, as well as tissue transglutaminase produced by PDAC cells, altered the response to gemcitabine,351 and breast CAFs increased MMP1 secretion synergistically with type IV collagen to promote Taxotere resistance via the TGF-β signaling, while GM6001 (an MMP1 inhibitor) recovered cancer cell chemosensitivity,352 perhaps by establishing physical barriers or inducing microvasculature compression through CAFs, and overcoming CAF-driven ECM hindrance of chemotherapeutic drugs delivery. Then, CAFs released regulatory molecules into tumor tissue in a paracrine-353–356 or exosome-driven357 manner to protect tumor cells from being eliminated. CAFs that were cocultured with cancer cells secreted IL-6 and upregulated the expression of CXCR7 through the STAT3/NF-κB pathway, which enhanced the proliferation and resistance of ESCC to cisplatin.218 In HNSCC, CAFs can upregulate autophagy by increasing the secretion of IL-6 and IL-8, thereby reducing cell sensitivity to cisplatin.358 In addition, CAFs can promote tumor stemness through the secretion of IL-17a and POSTN.190,359,360 Together, these data suggest that CAFs stimulated by agents in chemotherapy activate pro-survival signaling pathways, including those promoting the proliferation, stemness, and autophagy of cancer cells, to enhance treatment resistance (Fig. 6). In addition, activated CAFs can reprogram tumor cell metabolism to maintain tumor cell survival and protect them from apoptosis induced by treatment and/or stress. Augmented pyruvate in CAFs fed to tumor cells increased the activity of the tricarboxylic acid cycle to promote the survival of lymphoma cells.361 EMT mediated by CAFs decreased cellular adhesion, which was beneficial to tumor metastasis and resistance.362
Fig. 6.
CAF-mediated therapeutic resistance in anticancer treatment. a CAFs secreted dense desmoplastic matrix, including laminin A1, type IV collagen into the TME hindering the delivery of drugs, and increasing the resistance. b CAFs released regulatory molecules such as periostin, Nodal, EGF, IGF-2, and IL-6 or exosomes, which activated pro-survival signaling pathways, including proliferation, stemness, and apoptosis of cancer cells enhancing therapeutic resistance. c CAFs fed increased pyruvate, lactate, or GSSG to tumor cells by increasing energy supply and decreasing ROS. It also activated IGF-2 to increase autophagy via downstream pathways and the changes of metabolism in CAFs and tumor cells maintain the tumor survival in treatment. d CAFs released regulatory factors, including IGF-1, HGF, CXCL12, IL-6, and TGF-β1, to mediate EMT process through downstream ANAX2 and p38 pathways, subsequently increasing metastasis and resistance. TG2 tissue transglutaminase 2, LAMA1 laminin A1, Bcl-2 B cell lymphoma 2, Bax Bcl-2-associated X protein, Sox-2 SRY (sex-determining region Y)-box 2, TCA cycle tricarboxylic acid cycle, PP2A protein phosphatase 2A, CRM1 chromosomal region maintenance 1, GSSG glutathione disulfide, GSH glutathione, ANXA2 annexin A2
Small-molecule drugs targeting signaling pathways for tumor progression and growth have received attention and are widely used in the treatment of lung, breast, head and neck, and liver cancer.363–365 In comparing the potential mechanisms of CAF-mediated drug resistance that are substantially similar to those of chemical resistance, certain aspects of CAF-mediated drug resistance are unique (Fig. 6). To respond to drugs, the tumor–stroma or TME targets specific pathways and attenuates the effects of the drugs. For instance, excess CAF-secreted EGF can competitively bind to EGFR with cetuximab and activate MAPK to promote cetuximab resistance in colorectal cancer.253 Similarly, expressed PDGF-C in CAFs promotes angiogenesis and inhibits the effect of VEGF inhibitor.366
Similar to chemotherapy, CAF-induced pro-survival signaling, including proliferation, autophagy, and stemness, is able to induce resistance to RT (Fig. 6).367–369 In addition, the formation of a hypoxic microenvironment and the EMT driven by CAFs can also confer radioresistance onto cancer cells.370–373 In particular, we concluded that CSCs played an important role in the poor efficacy of RT and that CSCs survived more easily due to their potential innate radioresistance, which was partially induced by CAFs.374 CAFs induced a reduction response to antitumor treatment by maintaining activity via an autocrine periostin loop even during RT,371 and caused radiation-induced fibrosis, which was associated with retinoic acid or TGF-β,375 and with a severe adverse response to RT.376 These properties of CAFs make them potential targets for sensitizing tumors exposed to RT.
Challenges and future perspectives
In the present review, we concluded that several CAF-mediated signaling pathways exerted a supportive role in cancer progression. Key signaling pathway components, biomarkers in CAFs and CAF-derived factors, miRNAs, lncRNAs, etc., were predicted or found to have great potential for targeted therapy. Importantly, several clinical trials on CAFs have also been performed and shown that CAFs have a promising future in cancer therapy (Table 5). However, there are also multiple hurdles that need to be overcome before targeting CAFs in cancer treatment.
Table 5.
CAF-directed therapeutic resistance
| Therapy | Cancer type | Key molecule from CAFs | Molecular mechanism in cancer cell | Refs. |
|---|---|---|---|---|
| Chemotherapeutics | ||||
| 5-FU | GC | Paracrine low SPARC | Activates AKT/mTOR and MEK/ERK pathway | 148 |
| Adriamycin | PRAD | Paracrine IL-6 and exosomal miR-423 | Activates JAK/STAT and TGF-β pathway, and upregulates glutathione and GREM2 | 419,451,567 |
| Paclitaxel | ||||
| Adriamycin | BC | Paracrine CXCL12/HMGB1 | Downregulates H2AX phosphorylation | 568,569 |
| Adriamycin | SC | Paracrine Nodal | Activates Nodal/Samd/AKT pathway | 139 |
| Cisplatin | ESCA | Paracrine PAI-1/IL-6 | Activates AKT and ERK1/2 and CXCR7 pathway | 218,523,570 |
| Cisplatin | LC | Paracrine SDF-1/ANXA3/IL-6 | Activates NF-κB/Bcl-2 and JNK pathway, and upregulates p53 | 219,312,399,571 |
| Cisplatin | HNSCC | Exosomal miR-196a | Upregulates CDKN1B, ING5 LC3-II, and Beclin-1 | 435,572 |
| Cisplatin | OC | Paracrine POSTN/CXCL12 and exosomal miR-98 | Activates STAT3, PI3K/AKT, and /Wnt/β-catenin pathway and downregulates CDKN1A | 440,467,525,573 |
| Cisplatin | LUAD | Paracrine IL-11/COX-2 | Activates IL-11/STAT3 pathway and downregulates TNFSF4 | 461,529,574 |
| Cisplatin | HCC | Paracrine HGF | Activates c-Met and Mec-ERK1/2 pathway | 487 |
| Cisplatin | VSCC | Exosomal lncRNA UCA1 | Activates miR-103a/WEE1 pathway | 575 |
| Docetaxel | BC | Paracrine IL-8 | Upregulates CXCL2, MMP1, IL-8, RARRES1, FGF1, and CXCR7 | 362,576 |
| Ebimycin | BC | Paracrine pyruvate and lactate | Upregulates mitochondrial activity | 577 |
| Gemcitabine | PDAC | Paracrine LAMA1/Survivin/IL-6/SDF-1/MMP3/MMP9/PDGF/and CCL-7 and exosomal Snail/miR-146a/miR-106b | Activates protein kinase, AKT, and SDF-1/CXCR4/SATB-1 pathway, and upregulates Snail and TP53INP1 | 70,309,351,433,478,565,577 |
| Oxaliplatin | HNSCC | Paracrine IL-6 and IL-8 | Activates autophagy pathway | 358 |
| Oxaliplatin | CRC | Paracrine CM from CAFs | Activates STAT3 and p38 pathway | 578 |
| 5-FU | ||||
| Paclitaxel | BC | Paracrine MMP1 and collagen IV | Activates TGF-β pathway | 352 |
| Paclitaxel | LUAD | Paracrine HGF | Activates c-Met/PI3K/AKT pathway | 579 |
| Paclitaxel | OC | Paracrine CM from CAFs | Upregulates LPP | 580 |
| Targeted therapeutic | ||||
| Cetuximab | CRC | Paracrine EGF | Activates MAPK pathway | 253 |
| Cetuximab | HNSCC | Paracrine-soluble factors | Upregulates MMP1 | 319,581 |
| Lenzclutamiad | PRAD | Paracrine CM from CAFs | Activates PI3K/AKT pathway and upregulates E-cadherin and vimentin | 582 |
| EGFR-TKI | LUAD | Paracrine HGF | ND | 255 |
| Gefitinib | LC | Paracrine CM from CAFs | Activates AKT and ERK, ANXA2/EMT, and hedgehog pathway | 489,583,584 |
| Erlotinib | ||||
| Trastuzumab | BC | Paracrine IL-6/FGF-5 | Activates NF-κB, JAK/STAT3, AKT, and c-SRC/HER2 pathway | 585,586 |
| Trastuzumab | BC |
Paracrine pyruvate/lactate/ fibronectin |
Activates integrin-β1 pathway and promote mitochondrial activity | 577,586,587 |
| Tamoxifen | ||||
| Sorafenib | PRAD | Paracrine CM from CAFs | Activates autophagy pathway and upregulate AKT phosphorylation and Bcl-xL | 588 |
| Sorafenib | HCC | Paracrine HGF | Activates c-Met and Mec-ERK1/2 pathway | 487 |
| Radiotherapy therapy (RT) | ||||
| RT | LC | Paracrine FGF/IGF-2 | Activates autophagy pathway | 369,589 |
| RT | PDAC | Paracrine-soluble factors | Activates protein kinase and AKT pathways | 590 |
| RT | CESC | Paracrine IGF-2, EGF, FGF-4, IGFBPs, and GM-CSF | Activates p38 pathway | 591 |
| RT | LUAD | Paracrine CXCL12 | Activates CXCL12/CXCR4 pathway | 591 |
| RT | ESCA | Paracrine PDGFβ | Activates PDGFβ/PDGFβR/FOXO1 pathway and upregulates lncRNA DNM3OS | 592 |
| RT | CRC | Exosomal TGF-β/IGF-1 | Activates TGF-β and IGF-1/IGF1R pathway | 368,421,490 |
ANXA3 annexin A3, AKT protein kinase B, B-ALL B cell acute lymphoblastic leukemia. BC breast cancer, CESC cervical and endocervical cancer, CM conditioned medium, COX-2 cyclooxygenase, CRC colorectal cancer, EGF epidermal growth factor, ERK extracellular signal-related kinase, ESCA esophageal carcinoma, FGF fibroblast growth factor, GC gastric cancer, HCC hepatocellular carcinoma, HGF hepatocyte growth factor, HMGB1 high-mobility group box 1, HNSCC head and neck squamous cell carcinoma, IGF insulin-like growth factor, IL interleukin, JAK Janus kinase, LAMA1 laminin subunit alpha 1, LC lung cancer, LUAD lung adenocarcinoma, MMP matrix metalloproteinases, mTOR mammalian target of rapamycin, ND not determined, NF-κB nuclear factor kappa-B, OC ovarian cancer, PDAC pancreatic ductal adenocarcinoma, PAI-1 plasminogen activator inhibitor 1, PDGF platelet-derived growth factor, PI3K phosphatidylinositol-3-kinase, POSTN periostin, PRAD prostate adenocarcinoma, RCC renal cell carcinoma, SC stomach cancer, SPARC secreted protein acidic and rich in cysteine, STAT signal transducer and activator of transcription, TGF transforming growth factor, TSCC tongue squamous cell carcinoma.
First, how would decreasing the number of CAFs directly attenuate their tumor-promoting effects? Ample evidence suggests that FAP is an excellent target for CAFs, and antibodies against FAP and other FAP-targeting drugs are in development.377 T cell-mediated CAFs could be depleted by a DNA vaccine targeting FAP.378,379 Although FAP-specific CAR-T cells can selectively kill CAFs, they have been found to cause extensive lethal osteotoxicity and cachexia due to FAP expression in MSCs.338,380,381 In this regard, nanoparticles based on ZnF16pc loading and FAP-specific single-chain variable fragments or αFAP-Z@FRT can target FAP to effectively and safely deplete CAFs.382 In addition to CAF depletion, sibrolizumab (an anti-FAP monoclonal antibody) has been shown to target and prevent the activation of FAP+ CAFs to inhibit tumor growth.383,384 From another perspective, Noggin (a BMP signaling pathway inhibitor) can reverse the pericyte-CAF transition to decrease the number of CAFs.127 Notably, in addition to CAF depletion, the prevention of CAF generation and CAF normalization highly depend on their cellular phenotypes (Fig. 7a). Thus, there is an urgent need to enhance the understanding of the heterogeneity of CAFs and find more specific therapeutic targets.
Fig. 7.
CAF-driven targeted therapies and alternative targeting avenues, including epithelial–mesenchymal common target (EMCT), sequential target perturbation (STP), and crosstalk-directed signaling target (CDST) for CAF-directed or host cell-directed antitumor therapy. a Besides the inhibition of NFs to CAFs, there are still two major ways to decrease the CAFs number in TME: (i) targeting specific markers of CAFs by monoclonal antibody (mAb), recombinant virus, anti-precursor cell to deplete CAFs; (ii) revert the activated CAFs to quiescent phenotype by retinoic acid (RA), angiotensin receptor blockers (ARBs), gold nanoparticle (GNP), etc. b Both simultaneous overexpression of the same molecular protein in CAFs and cancer cells like TGF-βRIII and NRG1 has the potential to be targeted as EMCT. c In the regard to CAF-mediated signaling pathways in crosstalk with cancer cells, CDST is targeting simultaneously two different components of one signaling cascade in cancer cells and CAFs, respectively, in which the trigger locating in the upstream of CAFs (Target I), while effector locating in the downstream of cancer cells (Target II). d EGFR-TKI targets the mut-EGFR in cancer cells, while EGFR is up-expressed in CAFs. STP on cancer cells and CAFs, targeting the EGFR in cancer cells firstly (Step①), then in CAFs (Step②), may promote NK cell function to enhance antitumor efficacy and also avoid the IGF-2-mediated chemoresistance after long-term treatment. Since there are different functions in cancer cells and CAFs, STP also aims at targeting CAFs to block the protumor effect firstly (Step①: such as targeting ECM remodeling to move the barrier for CD8+ T cell infiltration) and then aiming at cancer cells (Step②: such as performing PD-1/PD-L1 to antitumor immunotherapy) might obtain well therapeutic efficacy
Second, since targeting cancer cells can unexpectedly render a therapeutic failure or even an acceleration of cancer, the option of EMCT targeting both in cancer cells and their adjacent stromal cells have become an attractive alternative. We have previously demonstrated that the common target perturbation of TGF-βRIII in oral cancerous epithelial cells and CAFs in conjunction with multiple antitumor effects that include depression of angiogenesis and metastasis and promotion of CAFs transition to an NF type, attenuating the CAF support of tumors.385 This finding suggests that the candidates for EMCTs are based on their simultaneous overexpression (Fig. 7b). Indeed, differentially expressed proteins in cancer cells and CAFs are common events in tumor progression. For these cases, we propose an alternative approach: sequential target perturbation (STP) in cancer cells and CAFs might lead to therapeutic efficacy, with targeting of CAFs to block the protumor effect first and then treating cancer cells to realize anticancer effects (Fig. 7d).
Third, to date, targeting two components of one signaling pathway related to specific tumor biology in cancer cells has been performed to treat malignancies.386,387 Since the crosstalk between cancer cells and CAFs is mediated by complexed signaling networks, we propose a novel therapeutic approach involving crosstalk-directed signaling targets (CDSTs) that simultaneously target two different components of one signaling cascade in cancer cells and CAFs, in which the trigger is located upstream of the CAFs and the effector is located in the downstream of the cancer cells (Fig. 7c). For instance, as CAF-derived IL-6 is beneficial for ECM remodeling218,219 and CAF generation220 to fuel tumor progression, and because IL-6 activates the IL-6/JAK/STAT3 signaling pathway in cancer cells,236,237 combining an IL-6-neutralizing antibody236 to treat the CAFs and the JAK inhibitor tofacitinib to treat the cancer cells388,389 might be valuable to study the targeted therapy. CAFs and their related signaling and/or downstream effectors in cancer cells are underexplored targets for cancer therapy.
Fourth, immunotherapy is considered an established pillar of cancer treatment; however, the current questions to answer include why does immunotherapy work well in some cancers but not at all in others? One of the major reasons that cancers fail to respond to anti-PD-1/PD-L immune checkpoint therapy is that CD8+ T cells cannot infiltrate into the TME of “Cold Tumor.”390 An important reason for this failure is CAF-mediated ECM forming a physical barrier to prevent CD8+ T cell infiltration and limits the delivery of drugs.391,392 Currently, there are three strategies for targeting CAF-mediated ECM remodeling: targeting the producer of ECM (activated CAFs),393 targeting the signaling pathways for ECM remodeling,394,395 and targeting ECM components.396,397 In this regard, with a method similar to STP, we propose that targeting CAF-mediated ECM to move the barrier first and then performing cancer immunotherapy might optimize therapeutic efficacy. In addition, since CAFs can recruit the PMN-MDSCs for protumor progression and resistance, attenuating this recruitment is a potential future cancer treatment. In sum, promising CAF-driven immunotherapy might focus on ECM normalization, prevention of disturbances from non-therapeutic immune cells, and combining other antitumor therapies with immunotherapy to maximize the efficacy of cancer immunotherapy.
In conclusion, since CAF-mediated signaling pathways have been implicated in the crosstalk with cancer cells that promote tumor progression and can affect the antitumor therapeutic efficacy, considering the evidence presented in this review, our group hypothesizes that EMCTs, STP, and CDSTs are alternative targeting avenues for CAF- or host cell-directed antitumor therapy.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 82071124, 82002884, and 81772898), Sichuan Science and Technology Program (Nos. 2021YFS0194, 2021YFH0143, and 2019YFS0361), and Science and Technology Program of Chengdu City (No. 2019YF0501151SN). Figures are created with BioRender.com.
Author contributions
H.Z. conceived and designed the study. F.W. and J.Y. drafted the manuscript. J.L., Y.W., J.M., Q.Z., S.D., F.W., and J.Y. searched and reviewed the literatures, and made the figures and tables. All the authors critically reviewed and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Fanglong Wu, Jin Yang
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 10.3322/caac.21660 (2021). [DOI] [PubMed]
- 3.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: the realities, challenges, and opportunities for new therapies. CA Cancer J. Clin. 2018;68:488–505. doi: 10.3322/caac.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts. https://seer.cancer.gov/statfacts (2021).
- 7.Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73:4965–4977. doi: 10.1158/0008-5472.CAN-13-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer. 2020;19:43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tommelein J, et al. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front. Oncol. 2015;5:63. doi: 10.3389/fonc.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knops AM, et al. Cancer-associated fibroblast density, prognostic characteristics, and recurrence in head and neck squamous cell carcinoma: a meta-analysis. Front. Oncol. 2020;10:565306. doi: 10.3389/fonc.2020.565306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanzaki R, Pietras K. Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 2020;111:2708–2717. doi: 10.1111/cas.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virchow, R. (eds). Die Cellularpathologie in Ihrer Begründung Auf Physiologische Und Pathologische Gewebelehre (A. Hirschwald, 1858).
- 14.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 15.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe PT. Neural crest and tooth morphogenesis. Adv. Dent. Res. 2001;15:4–7. doi: 10.1177/08959374010150011001. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. S100a4 is secreted by alternatively activated alveolar macrophages and promotes activation of lung fibroblasts in pulmonary fibrosis. Front. Immunol. 2018;9:1216. doi: 10.3389/fimmu.2018.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dave JM, Bayless KJ. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation. 2014;21:333–344. doi: 10.1111/micc.12111. [DOI] [PubMed] [Google Scholar]
- 20.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi N, et al. Nuclear expression of S100A4 is associated with aggressive behavior of epithelial ovarian carcinoma: an important autocrine/paracrine factor in tumor progression. Cancer Sci. 2006;97:1061–1069. doi: 10.1111/j.1349-7006.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, et al. Separation, cultivation and biological characteristics of oral carcinoma-associated fibroblasts. Oral Dis. 2006;12:375–380. doi: 10.1111/j.1601-0825.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 23.Österreicher CH, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl Acad. Sci. USA. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rognoni E, et al. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 2018;14:e8174. doi: 10.15252/msb.20178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fintha A, Gasparics Á, Rosivall L, Sebe A. Therapeutic targeting of fibrotic epithelial-mesenchymal transition-an outstanding challenge. Front. Pharmacol. 2019;10:388. doi: 10.3389/fphar.2019.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J. Mol. Cell. Cardiol. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Guen L, Marchal S, Faure S, de Santa Barbara P. Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cell. Mol. Life Sci. 2015;72:3883–3896. doi: 10.1007/s00018-015-1975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 31.Sakai N, et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017;7:5392. doi: 10.1038/s41598-017-05624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr MJ, Li Y, Rezakhanlou AM, Ghahary A. Keratinocyte-releasable factors stimulate the expression of granulocyte colony-stimulating factor in human dermal fibroblasts. J. Cell. Biochem. 2017;118:308–317. doi: 10.1002/jcb.25638. [DOI] [PubMed] [Google Scholar]
- 33.Brown FD, Turley SJ. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J. Immunol. 2015;194:1389–1394. doi: 10.4049/jimmunol.1402520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 35.Kuzet SE, Gaggioli C. Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res. 2016;365:607–619. doi: 10.1007/s00441-016-2467-x. [DOI] [PubMed] [Google Scholar]
- 36.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, et al. MiR-21/Smad 7 signaling determines TGF-β1-induced CAF formation. Sci. Rep. 2013;3:2038. doi: 10.1038/srep02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ringuette Goulet C, et al. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Mol. Cancer Res. 2018;16:1196–1204. doi: 10.1158/1541-7786.MCR-17-0784. [DOI] [PubMed] [Google Scholar]
- 39.Sharon Y, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–973. doi: 10.1158/0008-5472.CAN-14-1990. [DOI] [PubMed] [Google Scholar]
- 40.Dror S, et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016;18:1006–1017. doi: 10.1038/ncb3399. [DOI] [PubMed] [Google Scholar]
- 41.Hu T, Hu J. Melanoma-derived exosomes induce reprogramming fibroblasts into cancer-associated fibroblasts via Gm26809 delivery. Cell Cycle. 2019;18:3085–3094. doi: 10.1080/15384101.2019.1669380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelton M, et al. The role of CAF derived exosomal microRNAs in the tumour microenvironment of melanoma. Biochim. Biophys. Acta. 2021;1875:188456. doi: 10.1016/j.bbcan.2020.188456. [DOI] [PubMed] [Google Scholar]
- 43.Orang AV, Petersen J, McKinnon RA, Michael MZ. Micromanaging aerobic respiration and glycolysis in cancer cells. Mol. Metab. 2019;23:98–126. doi: 10.1016/j.molmet.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radhakrishnan R, et al. Ovarian cancer cell-derived lysophosphatidic acid induces glycolytic shift and cancer-associated fibroblast-phenotype in normal and peritumoral fibroblasts. Cancer Lett. 2019;442:464–474. doi: 10.1016/j.canlet.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, et al. Metabolic reprogramming of normal oral fibroblasts correlated with increased glycolytic metabolism of oral squamous cell carcinoma and precedes their activation into carcinoma associated fibroblasts. Cell. Mol. Life Sci. 2020;77:1115–1133. doi: 10.1007/s00018-019-03209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen T, et al. YAP1 plays a key role of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to prostate cancer progression. J. Exp. Clin. Cancer Res. 2020;39:36. doi: 10.1186/s13046-020-1542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mhaidly R, Mechta-Grigoriou F. Fibroblast heterogeneity in tumor micro-environment: role in immunosuppression and new therapies. Semin. Immunol. 2020;48:101417. doi: 10.1016/j.smim.2020.101417. [DOI] [PubMed] [Google Scholar]
- 49.LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020;34:3519–3536. doi: 10.1096/fj.201903188R. [DOI] [PubMed] [Google Scholar]
- 50.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Pavlides S, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, et al. Glycolysis reprogramming in cancer-associated fibroblasts promotes the growth of oral cancer through the lncRNA H19/miR-675-5p/PFKFB3 signaling pathway. Int. J. Oral Sci. 2021;13:12. doi: 10.1038/s41368-021-00115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu T, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo JJ, Young CD, Zhou HM, Wang XJ. Mouse models for studying oral cancer: impact in the era of cancer immunotherapy. J. Dent. Res. 2018;97:683–690. doi: 10.1177/0022034518767635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartoschek M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–479. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Macosko EZ, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costea DE, et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 2013;73:3888–3901. doi: 10.1158/0008-5472.CAN-12-4150. [DOI] [PubMed] [Google Scholar]
- 61.Biffi G, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bliss LA, et al. Use of postmortem human dura mater and scalp for deriving human fibroblast cultures. PLoS ONE. 2012;7:e45282. doi: 10.1371/journal.pone.0045282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. 2010;5:2033. doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambrechts D, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 66.Li H, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017;49:708–718. doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 67.Puram SV, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171:1611–1624. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sebastian, A. et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers12, 1307 (2020). [DOI] [PMC free article] [PubMed]
- 69.Li S, et al. The fibroblast TIAM2 promotes lung cancer cell invasion and metastasis. J. Cancer. 2019;10:1879–1889. doi: 10.7150/jca.30477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richards KE, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng R, et al. Reduced CX3CL1 secretion contributes to the susceptibility of oral leukoplakia-associated fibroblasts to Candida albicans. Front. Cell. Infect. Microbiol. 2016;6:150. doi: 10.3389/fcimb.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Grunsven LA. 3D in vitro models of liver fibrosis. Adv. Drug Deliv. Rev. 2017;121:133–146. doi: 10.1016/j.addr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Balachander GM, Balaji SA, Rangarajan A, Chatterjee K. Enhanced metastatic potential in a 3D tissue scaffold toward a comprehensive in vitro model for breast cancer metastasis. ACS Appl. Mater. Interfaces. 2015;7:27810–27822. doi: 10.1021/acsami.5b09064. [DOI] [PubMed] [Google Scholar]
- 74.Croce S, Peloso A, Zoro T, Avanzini MA, Cobianchi L. A hepatic scaffold from decellularized liver tissue: food for thought. Biomolecules. 2019;9:813. doi: 10.3390/biom9120813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Rel. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanoni M, et al. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020;13:97. doi: 10.1186/s13045-020-00931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J. Hematol. Oncol. 2019;12:142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim E, et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature. 2020;588:664–669. doi: 10.1038/s41586-020-3034-x. [DOI] [PubMed] [Google Scholar]
- 79.Frolik CA, Dart LL, Meyers CA, Smith DM, Sporn MB. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc. Natl Acad. Sci. USA. 1983;80:3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu T, Feng XH. Regulation of TGF-beta signalling by protein phosphatases. Biochem. J. 2010;430:191–198. doi: 10.1042/BJ20100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 82.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karagiannis GS, et al. Collective migration of cancer-associated fibroblasts is enhanced by overexpression of tight junction-associated proteins claudin-11 and occludin. Mol. Oncol. 2014;8:178–195. doi: 10.1016/j.molonc.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shangguan L, et al. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells. 2012;30:2810–2819. doi: 10.1002/stem.1251. [DOI] [PubMed] [Google Scholar]
- 85.Hawinkels LJ, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 86.Untergasser G, et al. Profiling molecular targets of TGF-beta1 in prostate fibroblast-to-myofibroblast transdifferentiation. Mech. Ageing Dev. 2005;126:59–69. doi: 10.1016/j.mad.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 87.Lamprecht S, et al. Teaming up for trouble: cancer cells, transforming growth factor-β1 signaling and the epigenetic corruption of stromal naïve fibroblasts. Cancers. 2018;10:61. doi: 10.3390/cancers10030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barcellos-de-Souza P, et al. Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-β1. Stem Cells. 2016;34:2536–2547. doi: 10.1002/stem.2412. [DOI] [PubMed] [Google Scholar]
- 89.Tan HX, et al. TGFβ1 is essential for MSCs-CAFs differentiation and promotes HCT116 cells migration and invasion via JAK/STAT3 signaling. OncoTargets Ther. 2019;12:5323–5334. doi: 10.2147/OTT.S178618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazzocca A, et al. Down-regulation of connective tissue growth factor by inhibition of transforming growth factor beta blocks the tumor-stroma cross-talk and tumor progression in hepatocellular carcinoma. Hepatology. 2010;51:523–534. doi: 10.1002/hep.23285. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Outschoorn UE, et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int. J. Biochem. Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guido C, et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang D, et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep. 2015;10:1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Power surge: supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012;15:4–5. doi: 10.1016/j.cmet.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Shi X, Young CD, Zhou H, Wang X. Transforming growth factor-β signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules. 2020;10:1666. doi: 10.3390/biom10121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasegawa T, et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-β signaling. Int. J. Cancer. 2014;134:1785–1795. doi: 10.1002/ijc.28520. [DOI] [PubMed] [Google Scholar]
- 97.Fuyuhiro Y, et al. Cancer-associated orthotopic myofibroblasts stimulates the motility of gastric carcinoma cells. Cancer Sci. 2012;103:797–805. doi: 10.1111/j.1349-7006.2012.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carloni V, Luong TV, Rombouts K. Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever. Liver Int. 2014;34:834–843. doi: 10.1111/liv.12465. [DOI] [PubMed] [Google Scholar]
- 99.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshida GJ, Azuma A, Miura Y, Orimo A. Activated fibroblast program orchestrates tumor initiation and progression; molecular mechanisms and the associated therapeutic strategies. Int. J. Mol. Sci. 2019;20:2256. doi: 10.3390/ijms20092256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen T, et al. Novel inactivating mutations of transforming growth factor-beta type I receptor gene in head-and-neck cancer metastases. Int. J. Cancer. 2001;93:653–661. doi: 10.1002/ijc.1381. [DOI] [PubMed] [Google Scholar]
- 104.Lu SL, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meng W, et al. Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer. 2011;11:88. doi: 10.1186/1471-2407-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshida GJ. Emerging role of epithelial-mesenchymal transition in hepatic cancer. J. Exp. Clin. Cancer Res. 2016;35:141. doi: 10.1186/s13046-016-0419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kojima Y, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl Acad. Sci. USA. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pavlides S, et al. The autophagic tumor stroma model of cancer: Role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho JA, et al. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol. Oncol. 2011;123:379–386. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 111.Jeon ES, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 112.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Model. Mech. 2018;11:dmm029447. doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Motohara T, et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. 2019;38:2885–2898. doi: 10.1038/s41388-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ko SY, et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J. Clin. Invest. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang J, et al. Vascular mimicry formation is promoted by paracrine TGF-β and SDF1 of cancer-associated fibroblasts and inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett. 2016;383:18–27. doi: 10.1016/j.canlet.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 116.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 118.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Update. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weichhart T, Säemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 2008;67:iii70–iii74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- 120.Knowles MA, Platt FM, Ross RL, Hurst CD. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009;28:305–316. doi: 10.1007/s10555-009-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loewith R, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/S1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 122.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int. J. Mol. Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 125.Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr. Opin. Cell Biol. 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 127.Ning X, Zhang H, Wang C, Song X. Exosomes released by gastric cancer cells induce transition of pericytes into cancer-associated fibroblasts. Med. Sci. Monit. 2018;24:2350–2359. doi: 10.12659/MSM.906641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang YM, Wang W, Qiu ED. Osteosarcoma cells induce differentiation of mesenchymal stem cells into cancer associated fibroblasts through Notch and Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2017;10:8479–8486. [PMC free article] [PubMed] [Google Scholar]
- 129.Chapoval AI, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 130.Zhang S, Zhou C, Zhang D, Huang Z, Zhang G. The anti-apoptotic effect on cancer-associated fibroblasts of B7-H3 molecule enhancing the cell invasion and metastasis in renal cancer. OncoTargets Ther. 2019;12:4119–4127. doi: 10.2147/OTT.S201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pham LK, et al. Contextual effect of repression of bone morphogenetic protein activity in prostate cancer. Endocr. Relat. Cancer. 2013;20:861–874. doi: 10.1530/ERC-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barone I, et al. Activation of farnesoid X receptor impairs the tumor-promoting function of breast cancer-associated fibroblasts. Cancer Lett. 2018;437:89–99. doi: 10.1016/j.canlet.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 133.Catalano S, et al. Inhibition of Leydig tumor growth by farnesoid X receptor activation: the in vitro and in vivo basis for a novel therapeutic strategy. Int. J. Cancer. 2013;132:2237–2247. doi: 10.1002/ijc.27915. [DOI] [PubMed] [Google Scholar]
- 134.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 135.Yang F, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 136.Li YY, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–220. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H, et al. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling pathway. Am. J. Transl. Res. 2019;11:4077–4088. [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Z, et al. VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 2020;473:62–73. doi: 10.1016/j.canlet.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 139.Pang T, et al. Cancer-associated fibroblasts promote malignancy of gastric cancer cells via Nodal signalling. Cell Biochem. Funct. 2020;38:4–11. doi: 10.1002/cbf.3446. [DOI] [PubMed] [Google Scholar]
- 140.Ma J, et al. Fibroblast-derived CXCL12 regulates PTEN expression and is associated with the proliferation and invasion of colon cancer cells via PI3k/Akt signaling. Cell Commun. Signal. 2019;17:119. doi: 10.1186/s12964-019-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Subramaniam KS, et al. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS ONE. 2013;8:e68923. doi: 10.1371/journal.pone.0068923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cacheux W, et al. Interaction between IGF2-PI3K axis and cancer-associated-fibroblasts promotes anal squamous carcinogenesis. Int. J. Cancer. 2019;145:1852–1859. doi: 10.1002/ijc.32178. [DOI] [PubMed] [Google Scholar]
- 143.Zhang R, Qi F, Shao S, Li G, Feng Y. Human colorectal cancer-derived carcinoma associated fibroblasts promote CD44-mediated adhesion of colorectal cancer cells to endothelial cells by secretion of HGF. Cancer Cell Int. 2019;19:192. doi: 10.1186/s12935-019-0914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Williamson SC, et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiang T, et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat. Commun. 2018;9:5009. doi: 10.1038/s41467-018-07308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim HS, et al. Role of EphA2-PI3K signaling in vasculogenic mimicry induced by cancer-associated fibroblasts in gastric cancer cells. Oncol. Lett. 2019;18:3031–3038. doi: 10.3892/ol.2019.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding X, et al. HGF derived from cancer‑associated fibroblasts promotes vascularization in gastric cancer via PI3K/AKT and ERK1/2 signaling. Oncol. Rep. 2018;40:1185–1195. doi: 10.3892/or.2018.6500. [DOI] [PubMed] [Google Scholar]
- 148.Ma Y, et al. Low expression of SPARC in gastric cancer-associated fibroblasts leads to stemness transformation and 5-fluorouracil resistance in gastric cancer. Cancer Cell Int. 2019;19:137. doi: 10.1186/s12935-019-0844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benetatos L, Voulgaris E, Vartholomatos G. The crosstalk between long non-coding RNAs and PI3K in cancer. Med. Oncol. 2017;34:39. doi: 10.1007/s12032-017-0897-2. [DOI] [PubMed] [Google Scholar]
- 150.Dong P, et al. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 2014;12:231. doi: 10.1186/s12967-014-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klingenberg M, Matsuda A, Diederichs S, Patel T. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J. Hepatol. 2017;67:603–618. doi: 10.1016/j.jhep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 152.Jahangiri B, Khalaj-Kondori M, Asadollahi E, Sadeghizadeh M. Cancer-associated fibroblasts enhance cell proliferation and metastasis of colorectal cancer SW480 cells by provoking long noncoding RNA UCA1. J. Cell Commun. Signal. 2019;13:53–64. doi: 10.1007/s12079-018-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ogier C, et al. Targeting the NRG1/HER3 pathway in tumor cells and cancer-associated fibroblasts with an anti-neuregulin 1 antibody inhibits tumor growth in pre-clinical models of pancreatic cancer. Cancer Lett. 2018;432:227–236. doi: 10.1016/j.canlet.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 154.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Imajo M, Tsuchiya Y, Nishida E. Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life. 2006;58:312–317. doi: 10.1080/15216540600746393. [DOI] [PubMed] [Google Scholar]
- 156.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 157.Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khokhlatchev AV, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/S0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 159.Hossen MN, et al. Gold nanoparticle transforms activated cancer-associated fibroblasts to quiescence. ACS Appl. Mater. Interfaces. 2019;11:26060–26068. doi: 10.1021/acsami.9b03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adam O. Dietary fatty acids and immune reactions in synovial tissue. Eur. J. Med. Res. 2003;8:381–387. [PubMed] [Google Scholar]
- 161.Ando N, et al. Eicosapentaenoic acid suppresses angiogenesis via reducing secretion of IL‑6 and VEGF from colon cancer‑associated fibroblasts. Oncol. Rep. 2019;42:339–349. doi: 10.3892/or.2019.7141. [DOI] [PubMed] [Google Scholar]
- 162.Pappalardo G, Almeida A, Ravasco P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015;31:549–555. doi: 10.1016/j.nut.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 163.de Carvalho C, Caramujo MJ. The various roles of fatty acids. Molecules. 2018;23:2583. doi: 10.3390/molecules23102583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 165.Menendez JA, Oza BP, Colomer R, Lupu R. The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival. Int. J. Oncol. 2005;26:1507–1515. [PubMed] [Google Scholar]
- 166.Santolla MF, et al. G protein-coupled estrogen receptor mediates the up-regulation of fatty acid synthase induced by 17β-estradiol in cancer cells and cancer-associated fibroblasts. J. Biol. Chem. 2012;287:43234–43245. doi: 10.1074/jbc.M112.417303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ishii G, et al. Fibroblasts associated with cancer cells keep enhanced migration activity after separation from cancer cells: a novel character of tumor educated fibroblasts. Int. J. Oncol. 2010;37:317–325. doi: 10.3892/ijo_00000680. [DOI] [PubMed] [Google Scholar]
- 168.Eck SM, Côté AL, Winkelman WD, Brinckerhoff CE. CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol. Cancer Res. 2009;7:1033–1044. doi: 10.1158/1541-7786.MCR-09-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong Y, et al. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS ONE. 2013;8:e77366. doi: 10.1371/journal.pone.0077366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang K, et al. Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts. J. Cell Sci. 2016;129:1989–2002. doi: 10.1242/jcs.186437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neufert C, et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Invest. 2013;123:1428–1443. doi: 10.1172/JCI63748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu X, et al. Dual role of twist1 in cancer-associated fibroblasts and tumor cells promoted epithelial-mesenchymal transition of esophageal cancer. Exp. Cell Res. 2019;375:41–50. doi: 10.1016/j.yexcr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 174.Wang X, et al. Cancer-associated fibroblasts-stimulated interleukin-11 promotes metastasis of gastric cancer cells mediated by upregulation of MUC1. Exp. Cell Res. 2018;368:184–193. doi: 10.1016/j.yexcr.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 175.Tian B, et al. Urokinase plasminogen activator secreted by cancer-associated fibroblasts induces tumor progression via PI3K/AKT and ERK signaling in esophageal squamous cell carcinoma. Oncotarget. 2017;8:42300–42313. doi: 10.18632/oncotarget.15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Borriello L, et al. Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Cancer Res. 2017;77:5142–5157. doi: 10.1158/0008-5472.CAN-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kikuchi Y, et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 2014;184:859–870. doi: 10.1016/j.ajpath.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 178.Wen S, et al. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–332. doi: 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 179.Li Y, et al. Disulfiram/copper induces antitumor activity against both nasopharyngeal cancer cells and cancer-associated fibroblasts through ROS/MAPK and ferroptosis pathways. Cancers. 2020;12:138. doi: 10.3390/cancers12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int. J. Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Latres E, Chiaur DS, Pagano M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 182.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Metcalfe C, Mendoza-Topaz C, Mieszczanek J, Bienz M. Stability elements in the LRP6 cytoplasmic tail confer efficient signalling upon DIX-dependent polymerization. J. Cell Sci. 2010;123:1588–1599. doi: 10.1242/jcs.067546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell. Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 185.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 2011;291:21–71. doi: 10.1016/B978-0-12-386035-4.00002-1. [DOI] [PubMed] [Google Scholar]
- 186.Zhou L, Yang K, Randall Wickett R, Zhang Y. Dermal fibroblasts induce cell cycle arrest and block epithelial-mesenchymal transition to inhibit the early stage melanoma development. Cancer Med. 2016;5:1566–1579. doi: 10.1002/cam4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mosa MH, et al. A Wnt-induced phenotypic switch in cancer-associated fibroblasts inhibits EMT in colorectal cancer. Cancer Res. 2020;80:5569–5582. doi: 10.1158/0008-5472.CAN-20-0263. [DOI] [PubMed] [Google Scholar]
- 188.Zhou L, Yang K, Wickett RR, Kadekaro AL, Zhang Y. Targeted deactivation of cancer-associated fibroblasts by β-catenin ablation suppresses melanoma growth. Tumor Biol. 2016;37:14235–14248. doi: 10.1007/s13277-016-5293-6. [DOI] [PubMed] [Google Scholar]
- 189.Bielefeld KA, et al. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 2011;286:27687–27697. doi: 10.1074/jbc.M111.261677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu B, et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:1082. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Mao B, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 192.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 193.Ferrari N, et al. Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat. Commun. 2019;10:130. doi: 10.1038/s41467-018-07987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scherz-Shouval R, et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158:564–578. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kudo J, et al. Aberrant nuclear localization of beta-catenin without genetic alterations in beta-catenin or Axin genes in esophageal cancer. World J. Surg. Oncol. 2007;5:21. doi: 10.1186/1477-7819-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Soulieres D, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 197.Clements WM, et al. Beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 198.Laurent-Puig P, et al. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 199.Pan T, Xu J, Zhu Y. Self-renewal molecular mechanisms of colorectal cancer stem cells. Int. J. Mol. Med. 2017;39:9–20. doi: 10.3892/ijmm.2016.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Novellasdemunt L, et al. USP7 is a tumor-specific WNT activator for APC-mutated colorectal cancer by mediating β-catenin deubiquitination. Cell Rep. 2017;21:612–627. doi: 10.1016/j.celrep.2017.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Giannakis M, et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014;46:1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zucman-Rossi J, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 204.Nanki K, et al. Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis. Cell. 2018;174:856–869. doi: 10.1016/j.cell.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 205.Aizawa T, et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019;8:6370–6382. doi: 10.1002/cam4.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Unterleuthner D, et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159–177. doi: 10.1007/s10456-019-09688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang M, et al. Cancer- Med. Sci. Monit. 2017;23:3904–3912. doi: 10.12659/MSM.902870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maeda M, et al. Cancer cell niche factors secreted from cancer-associated fibroblast by loss of H3K27me3. Gut. 2020;69:243–251. doi: 10.1136/gutjnl-2018-317645. [DOI] [PubMed] [Google Scholar]
- 209.Xiang L, Song Z, Rong G. Taxotere-induced WNT16 expression in carcinoma-associated fibroblasts might associate with progression and chemoresistance of breast cancer. Ann. Clin. Lab. Sci. 2020;50:205–212. [PubMed] [Google Scholar]
- 210.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/S0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 211.Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 212.Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 213.Quintás-Cardama A, Verstovsek S. Molecular pathways: Jak/STAT pathway: mutations, inhibitors, and resistance. Clin. Cancer Res. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stroud RM, Wells JA. Mechanistic diversity of cytokine receptor signaling across cell membranes. Sci. STKE. 2004;2004:re7. doi: 10.1126/stke.2312004re7. [DOI] [PubMed] [Google Scholar]
- 215.Delgoffe GM, Murray PJ, Vignali DA. Interpreting mixed signals: the cell’s cytokine conundrum. Curr. Opin. Immunol. 2011;23:632–638. doi: 10.1016/j.coi.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ina K, et al. Intestinal fibroblast-derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J. Immunol. 2005;175:2000–2009. doi: 10.4049/jimmunol.175.3.2000. [DOI] [PubMed] [Google Scholar]
- 217.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 218.Qiao Y, et al. IL6 derived from cancer-associated fibroblasts promotes chemoresistance via CXCR7 in esophageal squamous cell carcinoma. Oncogene. 2018;37:873–883. doi: 10.1038/onc.2017.387. [DOI] [PubMed] [Google Scholar]
- 219.Shintani Y, et al. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J. Thorac. Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 220.Nightingale J, et al. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J. Am. Soc. Nephrol. 2004;15:21–32. doi: 10.1097/01.ASN.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- 221.Albrengues J, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sengupta TK, Schmitt EM, Ivashkiv LB. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc. Natl Acad. Sci. USA. 1996;93:9499–9504. doi: 10.1073/pnas.93.18.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu M, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat. Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 224.Mishra R, Haldar S, Suchanti S, Bhowmick NA. Epigenetic changes in fibroblasts drive cancer metabolism and differentiation. Endocr. Relat. Cancer. 2019;26:R673–R688. doi: 10.1530/ERC-19-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fan J, Xu G, Chang Z, Zhu L, Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin. Sci. 2020;134:807–825. doi: 10.1042/CS20200039. [DOI] [PubMed] [Google Scholar]
- 226.Teramoto K, et al. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer. 2019;137:56–63. doi: 10.1016/j.lungcan.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 227.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu. Rev. Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 228.Giannoni E, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 229.Vicent S, et al. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Res. 2012;72:5744–5756. doi: 10.1158/0008-5472.CAN-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sun X, et al. Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer. BMC Cancer. 2019;19:1180. doi: 10.1186/s12885-019-6333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Heichler C, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69:1269–1282. doi: 10.1136/gutjnl-2019-319200. [DOI] [PubMed] [Google Scholar]
- 232.Zhang J, et al. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann. Transl. Med. 2020;8:877. doi: 10.21037/atm-20-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang X, Sun X, Mu L, Chen W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition in endometrial cancer cells by regulating pituitary tumor transforming gene. Cancer Invest. 2019;37:134–143. doi: 10.1080/07357907.2019.1575969. [DOI] [PubMed] [Google Scholar]
- 234.Jia C, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma. Int. J. Biol. Sci. 2020;16:2542–2558. doi: 10.7150/ijbs.45446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang Y, et al. Epiregulin reprograms cancer-associated fibroblasts and facilitates oral squamous cell carcinoma invasion via JAK2-STAT3 pathway. J. Exp. Clin. Cancer Res. 2019;38:274. doi: 10.1186/s13046-019-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sung PJ, et al. Cancer-associated fibroblasts produce Netrin-1 to control cancer cell plasticity. Cancer Res. 2019;79:3651–3661. doi: 10.1158/0008-5472.CAN-18-2952. [DOI] [PubMed] [Google Scholar]
- 237.Sanz-Moreno V, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 238.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 240.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 241.Ferguson KM, et al. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell. 2003;11:507–517. doi: 10.1016/S1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 242.Ogiso H, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–787. doi: 10.1016/S0092-8674(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 243.Siwak DR, et al. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J. Oncol. 2010;2010:568938. doi: 10.1155/2010/568938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tzahar E, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996;16:5276–5287. doi: 10.1128/MCB.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Normanno N, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 246.Shin N, et al. Cancer-associated fibroblasts and desmoplastic reactions related to cancer invasiveness in patients with colorectal cancer. Ann. Coloproctol. 2019;35:36–46. doi: 10.3393/ac.2018.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 248.Luo H, et al. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr. Relat. Cancer. 2014;21:355–369. doi: 10.1530/ERC-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pisano A, et al. GPER, IGF-IR, and EGFR transduction signaling are involved in stimulatory effects of zinc in breast cancer cells and cancer-associated fibroblasts. Mol. Carcinogen. 2017;56:580–593. doi: 10.1002/mc.22518. [DOI] [PubMed] [Google Scholar]
- 250.Aguiar DP, et al. New strategy to control cell migration and metastasis regulated by CCN2/CTGF. Cancer Cell Int. 2014;14:61. doi: 10.1186/1475-2867-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li F, et al. α1,6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC) Am. J. Cancer Res. 2020;10:816–837. [PMC free article] [PubMed] [Google Scholar]
- 252.Costa D, et al. Targeting the epidermal growth factor receptor can counteract the inhibition of natural killer cell function exerted by colorectal tumor-associated fibroblasts. Front. Immunol. 2018;9:1150. doi: 10.3389/fimmu.2018.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Garvey CM, et al. Anti-EGFR therapy induces EGF secretion by cancer-associated fibroblasts to confer colorectal cancer chemoresistance. Cancers. 2020;12:1393. doi: 10.3390/cancers12061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mink SR, et al. Cancer-associated fibroblasts derived from EGFR-TKI-resistant tumors reverse EGFR pathway inhibition by EGFR-TKIs. Mol. Cancer Res. 2010;8:809–820. doi: 10.1158/1541-7786.MCR-09-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Suzuki E, et al. Secretion of high amounts of hepatocyte growth factor is a characteristic feature of cancer-associated fibroblasts with EGFR-TKI resistance-promoting phenotype: a study of 18 cases of cancer-associated fibroblasts. Pathol. Int. 2019;69:472–480. doi: 10.1111/pin.12838. [DOI] [PubMed] [Google Scholar]
- 256.Vaquero J, et al. The IGF2/IR/IGF1R pathway in tumor cells and myofibroblasts mediates resistance to egfr inhibition in cholangiocarcinoma. Clin. Cancer Res. 2018;24:4282–4296. doi: 10.1158/1078-0432.CCR-17-3725. [DOI] [PubMed] [Google Scholar]
- 257.Álvarez-Teijeiro S, et al. Factors secreted by cancer-associated fibroblasts that sustain cancer stem properties in head and neck squamous carcinoma cells as potential therapeutic targets. Cancers. 2018;10:334. doi: 10.3390/cancers10090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Grasset EM, et al. Matrix stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res. 2018;78:5229–5242. doi: 10.1158/0008-5472.CAN-18-0601. [DOI] [PubMed] [Google Scholar]
- 259.Sieg DJ, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 260.Gao Q, et al. Heterotypic CAF-tumor spheroids promote early peritoneal metastatis of ovarian cancer. J. Exp. Med. 2019;216:688–703. doi: 10.1084/jem.20180765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur. J. Cancer. 2001;37:S9–S15. doi: 10.1016/S0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 262.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 263.Zheng Y, Pan D. The Hippo signaling pathway in development and disease. Dev. Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nishio, M. et al. In Innovative Medicine: Basic Research and Development (eds Nakao, K., Minato, N. & Uemoto, S.) 79–94 (Springer, 2015). [PubMed]
- 265.Yang CC, et al. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc. Natl Acad. Sci. USA. 2015;112:1785–1790. doi: 10.1073/pnas.1420850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bao M, Xie J, Piruska A, Huck WTS. 3D microniches reveal the importance of cell size and shape. Nat. Commun. 2017;8:1962. doi: 10.1038/s41467-017-02163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu. Rev. Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 268.Rognoni E, Walko G. The roles of YAP/TAZ and the Hippo pathway in healthy and diseased skin. Cells. 2019;8:411. doi: 10.3390/cells8050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr. Opin. Pharmacol. 2016;29:26–33. doi: 10.1016/j.coph.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ge L, et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS ONE. 2011;6:e27529. doi: 10.1371/journal.pone.0027529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Santolla MF, et al. miR-221 stimulates breast cancer cells and cancer-associated fibroblasts (CAFs) through selective interference with the A20/c-Rel/CTGF signaling. J. Exp. Clin. Cancer Res. 2018;37:94. doi: 10.1186/s13046-018-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Eissa S, Matboli M, Sharawy A, El-Sharkawi F. Prognostic and biological significance of microRNA-221 in breast cancer. Gene. 2015;574:163–167. doi: 10.1016/j.gene.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 274.Gallo Cantafio ME, et al. Pharmacokinetics and pharmacodynamics of a 13-mer LNA-inhibitor-miR-221 in mice and non-human primates. Mol. Ther. 2016;5:e336. doi: 10.1038/mtna.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Gullà A, et al. A 13 mer LNA-i-miR-221 inhibitor restores drug sensitivity in melphalan-refractory multiple myeloma cells. Clin. Cancer Res. 2016;22:1222–1233. doi: 10.1158/1078-0432.CCR-15-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 277.Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors. 2010;28:232–242. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Huang DB, Vu D, Ghosh G. NF-kappaB RelB forms an intertwined homodimer. Structure. 2005;13:1365–1373. doi: 10.1016/j.str.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 281.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 282.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 283.Cildir G, Low KC, Tergaonkar V. Noncanonical NF-κB signaling in health and disease. Trends Mol. Med. 2016;22:414–429. doi: 10.1016/j.molmed.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 284.Sun, S. C. Non-canonical NF-κB signaling pathway. Cell Res. 21, 71–85 (2011). [DOI] [PMC free article] [PubMed]
- 285.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 2001;7:401–409. doi: 10.1016/S1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 286.Zheng B, et al. CD146 attenuation in cancer-associated fibroblasts promotes pancreatic cancer progression. Mol. Carcinogen. 2016;55:1560–1572. doi: 10.1002/mc.22409. [DOI] [PubMed] [Google Scholar]
- 287.Wu H, Ma S, Xiang M, Tong S. HTRA1 promotes transdifferentiation of normal fibroblasts to cancer-associated fibroblasts through activation of the NF-κB/bFGF signaling pathway in gastric cancer. Biochem. Biophys. Res. Commun. 2019;514:933–939. doi: 10.1016/j.bbrc.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 288.Awaji M, et al. CXCR2 signaling promotes secretory cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. FASEB J. 2020;34:9405–9418. doi: 10.1096/fj.201902990R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wei LY, et al. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019;88:115–123. doi: 10.1016/j.oraloncology.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 290.Tjomsland V, et al. Interleukin 1α sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13:664–675. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Xu Y, et al. MPSSS impairs the immunosuppressive function of cancer-associated fibroblasts via the TLR4-NF-κB pathway. Biosci. Rep. 2019;39:BSR20182171. doi: 10.1042/BSR20182171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ma J, et al. Ligustilide inhibits the activation of cancer-associated fibroblasts. Life Sci. 2019;218:58–64. doi: 10.1016/j.lfs.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 293.Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013;437:397–402. doi: 10.1016/j.bbrc.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 294.Li X, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. 2014;35:1362–1370. doi: 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- 295.Qin X, et al. Cancer-associated fibroblast-derived IL-6 promotes head and neck cancer progression via the osteopontin-NF-kappa B signaling pathway. Theranostics. 2018;8:921–940. doi: 10.7150/thno.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Eiró N, et al. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J. Immunother. 2013;36:342–349. doi: 10.1097/CJI.0b013e31829d85e6. [DOI] [PubMed] [Google Scholar]
- 297.Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid. Redox Signal. 2011;14:2361–2371. doi: 10.1089/ars.2010.3727. [DOI] [PubMed] [Google Scholar]
- 298.Yamao T, et al. Cellular senescence, represented by expression of caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann. Surg. Oncol. 2019;26:1552–1559. doi: 10.1245/s10434-019-07266-2. [DOI] [PubMed] [Google Scholar]
- 299.Pallangyo CK, Ziegler PK, Greten FR. IKKβ acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J. Exp. Med. 2015;212:2253–2266. doi: 10.1084/jem.20150576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Luo J, et al. Smad7 promotes healing of radiotherapy-induced oral mucositis without compromising oral cancer therapy in a xenograft mouse model. Clin. Cancer Res. 2019;25:808–818. doi: 10.1158/1078-0432.CCR-18-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ali S, et al. Contribution of microRNAs in understanding the pancreatic tumor microenvironment involving cancer associated stellate and fibroblast cells. Am. J. Cancer Res. 2015;5:1251–1264. [PMC free article] [PubMed] [Google Scholar]
- 302.Djurec M, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc. Natl Acad. Sci. USA. 2018;115:E1147–E1156. doi: 10.1073/pnas.1717802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Du Y, et al. Intracellular Notch1 signaling in cancer-associated fibroblasts dictates the plasticity and stemness of melanoma stem/initiating cells. Stem Cells. 2019;37:865–875. doi: 10.1002/stem.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Pazolli E, et al. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res. 2012;72:2251–2261. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pelon F, et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020;11:404. doi: 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chong Y, et al. Galectin-1 from cancer-associated fibroblasts induces epithelial-mesenchymal transition through β1 integrin-mediated upregulation of Gli1 in gastric cancer. J. Exp. Clin. Cancer Res. 2016;35:175. doi: 10.1186/s13046-016-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Valenti G, et al. Cancer stem cells regulate cancer-associated fibroblasts via activation of hedgehog signaling in mammary gland tumors. Cancer Res. 2017;77:2134–2147. doi: 10.1158/0008-5472.CAN-15-3490. [DOI] [PubMed] [Google Scholar]
- 308.Xu G, et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int. J. Biol. Sci. 2019;15:2320–2329. doi: 10.7150/ijbs.33750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Fang Y, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2019;383:111543. doi: 10.1016/j.yexcr.2019.111543. [DOI] [PubMed] [Google Scholar]
- 310.Mertens JC, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Procopio MG, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat. Cell Biol. 2015;17:1193–1204. doi: 10.1038/ncb3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Schmid JO, et al. Cancer cells cue the p53 response of cancer-associated fibroblasts to cisplatin. Cancer Res. 2012;72:5824–5832. doi: 10.1158/0008-5472.CAN-12-1201. [DOI] [PubMed] [Google Scholar]
- 313.Erdogan B, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017;216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Stadler S, et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca(2+) signalling. Cell. Mol. Life Sci. 2017;74:1907–1921. doi: 10.1007/s00018-016-2441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Becker LM, et al. Epigenetic reprogramming of cancer-associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 2020;31:107701. doi: 10.1016/j.celrep.2020.107701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.De Francesco EM, et al. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Murata T, et al. HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells with cancer-associated fibroblasts to support progression of uterine cervical cancers. Cancer Res. 2011;71:6633–6642. doi: 10.1158/0008-5472.CAN-11-0034. [DOI] [PubMed] [Google Scholar]
- 318.Chan JSK, et al. Targeting nuclear receptors in cancer-associated fibroblasts as concurrent therapy to inhibit development of chemoresistant tumors. Oncogene. 2018;37:160–173. doi: 10.1038/onc.2017.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Johansson AC, et al. Cancer-associated fibroblasts induce matrix metalloproteinase-mediated cetuximab resistance in head and neck squamous cell carcinoma cells. Mol. Cancer Res. 2012;10:1158–1168. doi: 10.1158/1541-7786.MCR-12-0030. [DOI] [PubMed] [Google Scholar]
- 320.Li D, et al. SERS analysis of carcinoma-associated fibroblasts in a tumor microenvironment based on targeted 2D nanosheets. Nanoscale. 2020;12:2133–2141. doi: 10.1039/C9NR08754K. [DOI] [PubMed] [Google Scholar]
- 321.Duda P, Janczara J, McCubrey JA, Gizak A, Rakus D. The reverse Warburg effect is associated with Fbp2-dependent Hif1α regulation in cancer cells stimulated by fibroblasts. Cells. 2020;9:205. doi: 10.3390/cells9010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lei X, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 324.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 325.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 2015;125:3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Inoue S, et al. CD70 expression in tumor-associated fibroblasts predicts worse survival in colorectal cancer patients. Virchows Arch. 2019;475:425–434. doi: 10.1007/s00428-019-02565-1. [DOI] [PubMed] [Google Scholar]
- 327.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018;9:4692. doi: 10.1038/s41467-018-06654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Holmgaard RB, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer. 2018;6:47. doi: 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Joyce CE, et al. TGFβ signaling underlies hematopoietic dysfunction and bone marrow failure in Shwachman-Diamond Syndrome. J. Clin. Invest. 2019;129:3821–3826. doi: 10.1172/JCI125375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 333.Aboulkheyr Es,H, Zhand S, Thiery JP, Warkiani ME. Pirfenidone reduces immune-suppressive capacity of cancer-associated fibroblasts through targeting CCL17 and TNF-beta. Integr. Biol. 2020;12:188–197. doi: 10.1093/intbio/zyaa014. [DOI] [PubMed] [Google Scholar]
- 334.Zhang H, et al. CTL attenuation regulated by PS1 in cancer-associated fibroblast. Front. Immunol. 2020;11:999. doi: 10.3389/fimmu.2020.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chauhan VP, et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl Acad. Sci. USA. 2019;116:10674–10680. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Feig C, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kumar V, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. 2017;32:654–668.e655. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Takahashi H, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–8647. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kamata T, et al. Fibroblast-derived STC-1 modulates tumor-associated macrophages and lung adenocarcinoma development. Cell Rep. 2020;31:107802. doi: 10.1016/j.celrep.2020.107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhang R, et al. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019;10:273. doi: 10.1038/s41419-019-1435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Deng Y, et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36:1090–1101. doi: 10.1038/onc.2016.273. [DOI] [PubMed] [Google Scholar]
- 343.Cheng JT, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020;17:487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Hussain A, et al. Distinct fibroblast functional states drive clinical outcomes in ovarian cancer and are regulated by TCF21. J. Exp. Med. 2020;217:e20191094. doi: 10.1084/jem.20191094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yan Y, et al. The effects and the mechanisms of autophagy on the cancer-associated fibroblasts in cancer. J. Exp. Clin. Cancer Res. 2019;38:171. doi: 10.1186/s13046-019-1172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Houthuijzen JM, Jonkers J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 2018;37:577–597. doi: 10.1007/s10555-018-9768-3. [DOI] [PubMed] [Google Scholar]
- 348.Kobayashi H, et al. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 349.Sun Y, et al. Cancer associated fibroblasts tailored tumor microenvironment of therapy resistance in gastrointestinal cancers. J. Cell. Physiol. 2018;233:6359–6369. doi: 10.1002/jcp.26433. [DOI] [PubMed] [Google Scholar]
- 350.von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017;10:76. doi: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lee J, et al. Tissue transglutaminase activates cancer-associated fibroblasts and contributes to gemcitabine resistance in pancreatic cancer. Neoplasia. 2016;18:689–698. doi: 10.1016/j.neo.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cui Q, et al. Upregulating MMP-1 in carcinoma-associated fibroblasts reduces the efficacy of Taxotere on breast cancer synergized by collagen IV. Oncol. Lett. 2018;16:3537–3544. doi: 10.3892/ol.2018.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Izar B, et al. Bidirectional cross talk between patient-derived melanoma and cancer-associated fibroblasts promotes invasion and proliferation. Pigment Cell Melanoma Res. 2016;29:656–668. doi: 10.1111/pcmr.12513. [DOI] [PubMed] [Google Scholar]
- 354.Liu Y, Zhang J, Sun X, Su Q, You C. Down-regulation of miR-29b in carcinoma associated fibroblasts promotes cell growth and metastasis of breast cancer. Oncotarget. 2017;8:39559–39570. doi: 10.18632/oncotarget.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Long X, et al. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERβ/Bcl-2 signalling. Cell Death Dis. 2019;10:375. doi: 10.1038/s41419-019-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Xu Y, Zhang Z, Zhang L, Zhang C. Novel module and hub genes of distinctive breast cancer associated fibroblasts identified by weighted gene co-expression network analysis. Breast Cancer. 2020;27:1017–1028. doi: 10.1007/s12282-020-01101-3. [DOI] [PubMed] [Google Scholar]
- 357.Wei JR, Dong J, Li L. Cancer-associated fibroblasts-derived gamma-glutamyltransferase 5 promotes tumor growth and drug resistance in lung adenocarcinoma. Aging. 2020;12:13220–13233. doi: 10.18632/aging.103429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.New J, et al. Secretory autophagy in cancer-associated fibroblasts promotes head and neck cancer progression and offers a novel therapeutic target. Cancer Res. 2017;77:6679–6691. doi: 10.1158/0008-5472.CAN-17-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lotti F, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ren J, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sakamoto A, et al. Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. 2019;110:269–278. doi: 10.1111/cas.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rong G, Kang H, Wang Y, Hai T, Sun H. Candidate markers that associate with chemotherapy resistance in breast cancer through the study on Taxotere-induced damage to tumor microenvironment and gene expression profiling of carcinoma-associated fibroblasts (CAFs) PLoS ONE. 2013;8:e70960. doi: 10.1371/journal.pone.0070960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Chen TH, Chang PM, Yang MH. Novel immune-modulating drugs for advanced head and neck cancer. Head Neck. 2019;41:46–56. doi: 10.1002/hed.25929. [DOI] [PubMed] [Google Scholar]
- 364.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435–451. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 365.Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J. Hematol. Oncol. 2019;12:47. doi: 10.1186/s13045-019-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Francia G, Emmenegger U, Kerbel RS. Tumor-associated fibroblasts as “Trojan Horse” mediators of resistance to anti-VEGF therapy. Cancer Cell. 2009;15:3–5. doi: 10.1016/j.ccr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 367.Chen X, et al. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J. Exp. Clin. Cancer Res. 2020;39:65. doi: 10.1186/s13046-019-1507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Liu L, et al. Cancer associated fibroblasts-derived exosomes contribute to radioresistance through promoting colorectal cancer stem cells phenotype. Exp. Cell Res. 2020;391:111956. doi: 10.1016/j.yexcr.2020.111956. [DOI] [PubMed] [Google Scholar]
- 369.Wang Y, et al. Cancer-associated fibroblasts promote irradiated cancer cell recovery through autophagy. EBioMedicine. 2017;17:45–56. doi: 10.1016/j.ebiom.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Al-Assar O, et al. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014;111:243–251. doi: 10.1016/j.radonc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 371.Erkan M, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 372.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016;57:i90–i98. doi: 10.1093/jrr/rrw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhang H, et al. Cancer-associated fibroblast-promoted LncRNA DNM3OS confers radioresistance by regulating DNA damage response in esophageal squamous cell carcinoma. Clin. Cancer Res. 2019;25:1989–2000. doi: 10.1158/1078-0432.CCR-18-0773. [DOI] [PubMed] [Google Scholar]
- 374.Liu Y, Yang M, Luo J, Zhou H. Radiotherapy targeting cancer stem cells “awakens” them to induce tumour relapse and metastasis in oral cancer. Int. J. Oral Sci. 2020;12:19. doi: 10.1038/s41368-020-00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Forrester HB, Li J, Leong T, McKay MJ, Sprung CN. Identification of a radiation sensitivity gene expression profile in primary fibroblasts derived from patients who developed radiotherapy-induced fibrosis. Radiother. Oncol. 2014;111:186–193. doi: 10.1016/j.radonc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 376.Straub JM, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39:783–803. doi: 10.1007/s10555-020-09909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lappano R, Rigiracciolo DC, Belfiore A, Maggiolini M, De Francesco EM. Cancer associated fibroblasts: role in breast cancer and potential as therapeutic targets. Expert Opin. Ther. Targets. 2020;24:559–572. doi: 10.1080/14728222.2020.1751819. [DOI] [PubMed] [Google Scholar]
- 379.Miao L, Guo S, Lin CM, Liu Q, Huang L. Nanoformulations for combination or cascade anticancer therapy. Adv. Drug Deliv. Rev. 2017;115:3–22. doi: 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.de Sostoa J, et al. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Freedman JD, et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 382.Zhou S, et al. FAP-targeted photodynamic therapy mediated by ferritin nanoparticles elicits an immune response against cancer cells and cancer associated fibroblasts. Adv. Funct. Mater. 2021;31:2007017. doi: 10.1002/adfm.202007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Hofheinz RD, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 384.Siska PJ, Singer K, Evert K, Renner K, Kreutz M. The immunological Warburg effect: Can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy? Immunol. Rev. 2020;295:187–202. doi: 10.1111/imr.12846. [DOI] [PubMed] [Google Scholar]
- 385.Meng W, et al. A systems biology approach identifies effective tumor-stroma common targets for oral squamous cell carcinoma. Cancer Res. 2014;74:2306–2315. doi: 10.1158/0008-5472.CAN-13-2275. [DOI] [PubMed] [Google Scholar]
- 386.Faratian D, et al. Trastuzumab and pertuzumab produce changes in morphology and estrogen receptor signaling in ovarian cancer xenografts revealing new treatment strategies. Clin. Cancer Res. 2011;17:4451–4461. doi: 10.1158/1078-0432.CCR-10-2461. [DOI] [PubMed] [Google Scholar]
- 387.Weiss A, et al. Identification of a synergistic multi-drug combination active in cancer cells via the prevention of spindle pole clustering. Cancers. 2019;11:1612. doi: 10.3390/cancers11101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ham IH, et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer. 2019;18:68. doi: 10.1186/s12943-019-0972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hosseini A, et al. Janus kinase inhibitors: a therapeutic strategy for cancer and autoimmune diseases. J. Cell. Physiol. 2020;235:5903–5924. doi: 10.1002/jcp.29593. [DOI] [PubMed] [Google Scholar]
- 390.Noman MZ, et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci. Adv. 2020;6:eaax7881. doi: 10.1126/sciadv.aax7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Puré E, Lo A. Can targeting stroma pave the way to enhanced antitumor immunity and immunotherapy of solid tumors? Cancer Immunol. Res. 2016;4:269–278. doi: 10.1158/2326-6066.CIR-16-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.van Elsas MJ, van Hall T, van der Burg SH. Future challenges in cancer resistance to immunotherapy. Cancers. 2020;12:935. doi: 10.3390/cancers12040935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Chronopoulos A, et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016;7:12630. doi: 10.1038/ncomms12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Jiang H, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Hauge A, Rofstad EK. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020;18:207. doi: 10.1186/s12967-020-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Provenzano PP, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Yin Z, et al. Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma. J. Hematol. Oncol. 2019;12:101. doi: 10.1186/s13045-019-0782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Li J, Guan J, Long X, Wang Y, Xiang X. mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol. Rep. 2016;35:3523–3531. doi: 10.3892/or.2016.4714. [DOI] [PubMed] [Google Scholar]
- 400.Zhang J, et al. miR-101 represses lung cancer by inhibiting interaction of fibroblasts and cancer cells by down-regulating CXCL12. Biomed. Pharmacother. 2015;74:215–221. doi: 10.1016/j.biopha.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 401.Yang F, et al. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell Signal. 2020;73:109675. doi: 10.1016/j.cellsig.2020.109675. [DOI] [PubMed] [Google Scholar]
- 402.Shen H, et al. Reprogramming of normal fibroblasts into cancer-associated fibroblasts by miRNAs-mediated CCL2/VEGFA signaling. PLoS Genet. 2016;12:e1006244. doi: 10.1371/journal.pgen.1006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Tao, S. et al. Elevating microRNA-1-3p shuttled by cancer-associated fibroblasts-derived extracellular vesicles suppresses breast cancer progression and metastasis by inhibiting GLIS1. Cancer Gene Ther. 10.1038/s41417-020-00244-x (2020). [DOI] [PubMed]
- 404.Gao Y, et al. CD63(+) cancer-associated fibroblasts confer tamoxifen resistance to breast cancer cells through exosomal miR-22. Adv. Sci. 2020;7:2002518. doi: 10.1002/advs.202002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Verghese ET, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J. Pathol. 2013;231:388–399. doi: 10.1002/path.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Dou D, et al. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front. Immunol. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 407.Sun Y, et al. Primed atypical ductal hyperplasia-associated fibroblasts promote cell growth and polarity changes of transformed epithelium-like breast cancer MCF-7 cells via miR-200b/c-IKKβ signaling. Cell Death Dis. 2018;9:122. doi: 10.1038/s41419-017-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tang X, et al. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23:132–145. doi: 10.1038/cdd.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Du YE, et al. MiR-205/YAP1 in activated fibroblasts of breast tumor promotes VEGF-independent angiogenesis through STAT3 signaling. Theranostics. 2017;7:3972–3988. doi: 10.7150/thno.18990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bronisz A, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Wang H, et al. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. 2020;19:654–667. doi: 10.1016/j.omtn.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Liu Y, Yang Y, Du J, Lin D, Li F. MiR-3613-3p from carcinoma-associated fibroblasts exosomes promoted breast cancer cell proliferation and metastasis by regulating SOCS2 expression. IUBMB Life. 2020;72:1705–1714. doi: 10.1002/iub.2292. [DOI] [PubMed] [Google Scholar]
- 413.Kim JE, et al. The stromal loss of miR-4516 promotes the FOSL1-dependent proliferation and malignancy of triple negative breast cancer. Cancer Lett. 2020;469:256–265. doi: 10.1016/j.canlet.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 414.Wu HJ, Hao M, Yeo SK, Guan JL. FAK signaling in cancer-associated fibroblasts promotes breast cancer cell migration and metastasis by exosomal miRNAs-mediated intercellular communication. Oncogene. 2020;39:2539–2549. doi: 10.1038/s41388-020-1162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Tang X, et al. Autocrine TGF-β1/miR-200s/miR-221/DNMT3B regulatory loop maintains CAF status to fuel breast cancer cell proliferation. Cancer Lett. 2019;452:79–89. doi: 10.1016/j.canlet.2019.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Musumeci M, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30:4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]
- 417.Zhang Y, et al. Loss of exosomal miR-146a-5p from cancer-associated fibroblasts after androgen deprivation therapy contributes to prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2020;39:282. doi: 10.1186/s13046-020-01761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Josson S, et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin. Cancer Res. 2014;20:4636–4646. doi: 10.1158/1078-0432.CCR-14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Shan G, et al. Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-β signaling pathway. Exp. Mol. Med. 2020;52:1809–1822. doi: 10.1038/s12276-020-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Bullock MD, et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013;4:e684. doi: 10.1038/cddis.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yang X, et al. miR-31 affects colorectal cancer cells by inhibiting autophagy in cancer-associated fibroblasts. Oncotarget. 2016;7:79617–79628. doi: 10.18632/oncotarget.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Hu JL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer. 2019;18:91. doi: 10.1186/s12943-019-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Ast V, et al. MiR-192, miR-200c and miR-17 are fibroblast-mediated inhibitors of colorectal cancer invasion. Oncotarget. 2018;9:35559–35580. doi: 10.18632/oncotarget.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Shi L, Wang Z, Geng X, Zhang Y, Xue Z. Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging. 2020;12:8549–8564. doi: 10.18632/aging.103157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 425.Yang TS, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162–2169. doi: 10.1016/j.febslet.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 426.Li P, et al. Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res. 2015;25:588–603. doi: 10.1038/cr.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wang R, et al. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J. Exp. Clin. Cancer Res. 2019;38:20. doi: 10.1186/s13046-018-0995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Liu X, et al. Exosomal miR-29b from cancer-associated fibroblasts inhibits the migration and invasion of hepatocellular carcinoma cells. Transl. Cancer Res. 2020;9:2576–2587. doi: 10.21037/tcr.2020.02.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Zhang Z, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 430.Fang T, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Utaijaratrasmi P, et al. The microRNA-15a-PAI-2 axis in cholangiocarcinoma-associated fibroblasts promotes migration of cancer cells. Mol. Cancer. 2018;17:10. doi: 10.1186/s12943-018-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Zhang, X. et al. Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway. Cancer Gene Ther. 10.1038/s41417-020-00238-9 (2020). [DOI] [PubMed]
- 433.Zhang L, Yao J, Li W, Zhang C. Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol. Res. 2018;26:827–835. doi: 10.3727/096504017X14934840662335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Shen Z, et al. Cancer-associated fibroblasts promote cancer cell growth through a miR-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget. 2017;8:1290–1303. doi: 10.18632/oncotarget.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Qin X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20:12. doi: 10.1186/s13059-018-1604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wang X, et al. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. J. Exp. Clin. Cancer Res. 2019;38:151. doi: 10.1186/s13046-019-1144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zhou X, et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J. Exp. Clin. Cancer Res. 2018;37:242. doi: 10.1186/s13046-018-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang JW, Wu XF, Gu XJ, Jiang XH. Exosomal miR-1228 from cancer-associated fibroblasts promotes cell migration and invasion of osteosarcoma by directly targeting SCAI. Oncol. Res. 2019;27:979–986. doi: 10.3727/096504018X15336368805108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Au Yeung CL, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Guo H, et al. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347. doi: 10.1186/s12935-019-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Mitra AK, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2:1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Aprelikova O, et al. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle. 2010;9:4387–4398. doi: 10.4161/cc.9.21.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Aprelikova O, et al. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene. 2013;32:3246–3253. doi: 10.1038/onc.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Li BL, et al. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell. Physiol. 2019;234:2943–2953. doi: 10.1002/jcp.27111. [DOI] [PubMed] [Google Scholar]
- 445.Huang YH, et al. Cancer-associated fibroblast-derived interleukin-1β activates protumor C-C motif chemokine ligand 22 signaling in head and neck cancer. Cancer Sci. 2019;110:2783–2793. doi: 10.1111/cas.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.De Marco P, et al. GPER signalling in both cancer-associated fibroblasts and breast cancer cells mediates a feedforward IL1β/IL1R1 response. Sci. Rep. 2016;6:24354. doi: 10.1038/srep24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Zhang X, Hwang YS. Cancer-associated fibroblast stimulates cancer cell invasion in an interleukin-1 receptor (IL-1R)-dependent manner. Oncol. Lett. 2019;18:4645–4650. doi: 10.3892/ol.2019.10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Subramaniam KS, et al. Cancer-associated fibroblasts promote endometrial cancer growth via activation of interleukin-6/STAT-3/c-Myc pathway. Am. J. Cancer Res. 2016;6:200–213. [PMC free article] [PubMed] [Google Scholar]
- 449.Wang L, et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8:76116–76128. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Li Y, et al. Cancer-associated fibroblasts promote the stemness of CD24(+) liver cells via paracrine signaling. J. Mol. Med. 2019;97:243–255. doi: 10.1007/s00109-018-1731-9. [DOI] [PubMed] [Google Scholar]
- 451.Cheteh EH, et al. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Dis. 2020;6:42. doi: 10.1038/s41420-020-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Ding X, et al. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9:867. doi: 10.1038/s41419-018-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Goulet CR, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer. 2019;19:137. doi: 10.1186/s12885-019-5353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Pan MS, et al. Gallbladder cancer-associated fibroblasts promote vasculogenic mimicry formation and tumor growth in gallbladder cancer via upregulating the expression of NOX4, a poor prognosis factor, through IL-6-JAK-STAT3 signal pathway. J. Exp. Clin. Cancer Res. 2020;39:234. doi: 10.1186/s13046-020-01742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wu X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741–20750. doi: 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Zhang Y, Cong X, Li Z, Xue Y. Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int. Immunopharmacol. 2020;78:105937. doi: 10.1016/j.intimp.2019.105937. [DOI] [PubMed] [Google Scholar]
- 457.Xiong S, et al. Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. Am. J. Cancer Res. 2018;8:302–316. [PMC free article] [PubMed] [Google Scholar]
- 458.Cheng Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Zhai J, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 460.Ma J, Song X, Xu X, Mou Y. Cancer-associated fibroblasts promote the chemo-resistance in gastric cancer through secreting IL-11 targeting JAK/STAT3/Bcl2 pathway. Cancer Res. Treat. 2019;51:194–210. doi: 10.4143/crt.2018.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Tao L, et al. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016;6:38408. doi: 10.1038/srep38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Fukui H, et al. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br. J. Cancer. 2014;111:763–771. doi: 10.1038/bjc.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Yin SY, et al. Induction of IL-25 secretion from tumour-associated fibroblasts suppresses mammary tumour metastasis. Nat. Commun. 2016;7:11311. doi: 10.1038/ncomms11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Chen SF, et al. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J. Pathol. 2013;231:180–189. doi: 10.1002/path.4226. [DOI] [PubMed] [Google Scholar]
- 465.Li Z, et al. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer. 2019;145:1946–1957. doi: 10.1002/ijc.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Bian L, Sun X, Jin K, He Y. Oral cancer-associated fibroblasts inhibit heat-induced apoptosis in Tca8113 cells through upregulated expression of Bcl-2 through the Mig/CXCR3 axis. Oncol. Rep. 2012;28:2063–2068. doi: 10.3892/or.2012.2019. [DOI] [PubMed] [Google Scholar]
- 467.Zhang F, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer via CXCL12/CXCR4 axis. Fut. Oncol. 2020;16:2619–2633. doi: 10.2217/fon-2020-0095. [DOI] [PubMed] [Google Scholar]
- 468.Izumi D, et al. CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer. Int. J. Cancer. 2016;138:1207–1219. doi: 10.1002/ijc.29864. [DOI] [PubMed] [Google Scholar]
- 469.Li D, et al. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am. J. Cancer Res. 2016;6:2192–2206. [PMC free article] [PubMed] [Google Scholar]
- 470.Sugihara H, et al. Cancer-associated fibroblast-derived CXCL12 causes tumor progression in adenocarcinoma of the esophagogastric junction. Med. Oncol. 2015;32:618. doi: 10.1007/s12032-015-0618-7. [DOI] [PubMed] [Google Scholar]
- 471.Wald O, et al. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J. Thorac. Cardiovasc. Surg. 2011;141:1503–1512. doi: 10.1016/j.jtcvs.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 472.Wang M, et al. The primary growth of laryngeal squamous cell carcinoma cells in vitro is effectively supported by paired cancer-associated fibroblasts alone. Tumor Biol. 2017;39:1–12. doi: 10.1177/1010428317705512. [DOI] [PubMed] [Google Scholar]
- 473.Noh KH, et al. Crosstalk between prostate cancer cells and tumor-associated fibroblasts enhances the malignancy by inhibiting the tumor suppressor PLZF. Cancers. 2020;12:1083. doi: 10.3390/cancers12051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Yang T, et al. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol. Ther. 2017;18:806–815. doi: 10.1080/15384047.2017.1373219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Inoue C, et al. PD-L1 induction by cancer-associated fibroblast-derived factors in lung adenocarcinoma cells. Cancers. 2019;11:1257. doi: 10.3390/cancers11091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Allaoui R, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 2016;7:13050. doi: 10.1038/ncomms13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Peng C, et al. Integrin αvβ6 plays a bi-directional regulation role between colon cancer cells and cancer-associated fibroblasts. Biosci. Rep. 2018;38:BSR20180243. doi: 10.1042/BSR20180243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Wei L, et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018;9:1065. doi: 10.1038/s41419-018-1104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Teng F, et al. Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis. J. Hematol. Oncol. 2016;9:8. doi: 10.1186/s13045-015-0231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Sun Y, et al. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling. Tumor Biol. 2017;39:712592. doi: 10.1177/1010428317712592. [DOI] [PubMed] [Google Scholar]
- 481.Hegab AE, et al. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J. Pathol. 2019;249:193–205. doi: 10.1002/path.5290. [DOI] [PubMed] [Google Scholar]
- 482.Knuchel S, Anderle P, Werfelli P, Diamantis E, Rüegg C. Fibroblast surface-associated FGF-2 promotes contact-dependent colorectal cancer cell migration and invasion through FGFR-SRC signaling and integrin αvβ5-mediated adhesion. Oncotarget. 2015;6:14300–14317. doi: 10.18632/oncotarget.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Suh J, Kim DH, Lee YH, Jang JH, Surh YJ. Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling. Mol. Carcinogen. 2020;59:1028–1040. doi: 10.1002/mc.23233. [DOI] [PubMed] [Google Scholar]
- 484.Sun C, et al. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Cancer. 2015;15:333. doi: 10.1186/s12885-015-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Bruzzese F, et al. Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15. Cancer Res. 2014;74:3408–3417. doi: 10.1158/0008-5472.CAN-13-2259. [DOI] [PubMed] [Google Scholar]
- 486.Kumar D, et al. Mitigation of tumor-associated fibroblast-facilitated head and neck cancer progression with anti-hepatocyte growth factor antibody ficlatuzumab. JAMA Otolaryngol. Head Neck Surg. 2015;141:1133–1139. doi: 10.1001/jamaoto.2015.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Peng H, et al. Cancer-associated fibroblasts enhance the chemoresistance of CD73(+) hepatocellular carcinoma cancer cells via HGF-Met-ERK1/2 pathway. Ann. Transl. Med. 2020;8:856. doi: 10.21037/atm-20-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Lau EY, et al. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep. 2016;15:1175–1189. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 489.Yi Y, et al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim. Biophys. Acta. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 490.Tommelein J, et al. Radiotherapy-activated cancer-associated fibroblasts promote tumor progression through paracrine IGF1R activation. Cancer Res. 2018;78:659–670. doi: 10.1158/0008-5472.CAN-17-0524. [DOI] [PubMed] [Google Scholar]
- 491.Zhang Q, Yang J, Bai J, Ren J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018;109:944–955. doi: 10.1111/cas.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Sun DY, Wu JQ, He ZH, He MF, Sun HB. Cancer-associated fibroblast regulate proliferation and migration of prostate cancer cells through TGF-β signaling pathway. Life Sci. 2019;235:116791. doi: 10.1016/j.lfs.2019.116791. [DOI] [PubMed] [Google Scholar]
- 493.Yu Y, et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Shen J, et al. Cancer-associated fibroblasts-derived VCAM1 induced by H. pylori infection facilitates tumor invasion in gastric cancer. Oncogene. 2020;39:2961–2974. doi: 10.1038/s41388-020-1197-4. [DOI] [PubMed] [Google Scholar]
- 495.Gao MQ, et al. Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-α cleavage by ADAM17. Cancer Lett. 2013;336:240–246. doi: 10.1016/j.canlet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 496.Bauer J, et al. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci. Rep. 2020;10:50. doi: 10.1038/s41598-019-55687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Wang L, et al. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019;110:1609–1620. doi: 10.1111/cas.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Satoyoshi R, Kuriyama S, Aiba N, Yashiro M, Tanaka M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene. 2015;34:650–660. doi: 10.1038/onc.2013.584. [DOI] [PubMed] [Google Scholar]
- 499.Bae CA, et al. Inhibiting the GAS6/AXL axis suppresses tumor progression by blocking the interaction between cancer-associated fibroblasts and cancer cells in gastric carcinoma. Gastric Cancer. 2020;23:824–836. doi: 10.1007/s10120-020-01066-4. [DOI] [PubMed] [Google Scholar]
- 500.Miki Y, et al. CD9-positive exosomes from cancer-associated fibroblasts stimulate the migration ability of scirrhous-type gastric cancer cells. Br. J. Cancer. 2018;118:867–877. doi: 10.1038/bjc.2017.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Bonneau C, et al. A subset of activated fibroblasts is associated with distant relapse in early luminal breast cancer. Breast Cancer Res. 2020;22:76. doi: 10.1186/s13058-020-01311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Zhu HF, et al. Cancer-associated fibroblasts promote colorectal cancer progression by secreting CLEC3B. Cancer Biol. Ther. 2019;20:967–978. doi: 10.1080/15384047.2019.1591122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Li X, Li Q, Yu X, Li H, Huang G. Reverse of microtubule-directed chemotherapeutic drugs resistance induced by cancer-associated fibroblasts in breast cancer. OncoTargets Ther. 2019;12:7963–7973. doi: 10.2147/OTT.S211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Begum A, et al. Direct interactions with cancer-associated fibroblasts lead to enhanced pancreatic cancer stem cell function. Pancreas. 2019;48:329–334. doi: 10.1097/MPA.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Gong J, et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020;11:267. doi: 10.1038/s41419-020-2434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.He XJ, et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin β1. Cancer Sci. 2014;105:1402–1410. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Tang D, et al. Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumor Biol. 2016;37:1889–1899. doi: 10.1007/s13277-015-3942-9. [DOI] [PubMed] [Google Scholar]
- 508.Ren J, et al. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. 2019;21:109. doi: 10.1186/s13058-019-1194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Kugeratski FG, et al. Hypoxic cancer-associated fibroblasts increase NCBP2-AS2/HIAR to promote endothelial sprouting through enhanced VEGF signaling. Sci. Signal. 2019;12:eaan8247. doi: 10.1126/scisignal.aan8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Du X, et al. HIC-5 in cancer-associated fibroblasts contributes to esophageal squamous cell carcinoma progression. Cell Death Dis. 2019;10:873. doi: 10.1038/s41419-019-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zhao XL, et al. High-mobility group box 1 released by autophagic cancer-associated fibroblasts maintains the stemness of luminal breast cancer cells. J. Pathol. 2017;243:376–389. doi: 10.1002/path.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Sun K, et al. Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling. EBioMedicine. 2019;41:370–383. doi: 10.1016/j.ebiom.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Li Q, et al. Lysyl oxidase promotes liver metastasis of gastric cancer via facilitating the reciprocal interactions between tumor cells and cancer associated fibroblasts. EBioMedicine. 2019;49:157–171. doi: 10.1016/j.ebiom.2019.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Xuefeng X, et al. Epithelial-mesenchymal transition and metastasis of colon cancer cells induced by the FAK pathway in cancer-associated fibroblasts. J. Int. Med. Res. 2020;48:300060520931242. doi: 10.1177/0300060520931242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Wang X, et al. Cancer-associated fibroblast-derived Lumican promotes gastric cancer progression via the integrin β1-FAK signaling pathway. Int. J. Cancer. 2017;141:998–1010. doi: 10.1002/ijc.30801. [DOI] [PubMed] [Google Scholar]
- 516.Zhang A, et al. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 2017;6:463–470. doi: 10.1002/cam4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Principe S, et al. Proteomic analysis of cancer-associated fibroblasts reveals a paracrine role for MFAP5 in human oral tongue squamous cell carcinoma. J. Proteome Res. 2018;17:2045–2059. doi: 10.1021/acs.jproteome.7b00925. [DOI] [PubMed] [Google Scholar]
- 518.Zhang D, et al. Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci. Rep. 2017;7:16231. doi: 10.1038/s41598-017-13431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Fullár A, et al. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer. 2015;15:256. doi: 10.1186/s12885-015-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br. J. Cancer. 2014;111:1230–1237. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Choi SY, et al. NAB 2-expressing cancer-associated fibroblast promotes HNSCC progression. Cancers. 2019;11:388. doi: 10.3390/cancers11030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Liu C, et al. LSD1 stimulates cancer-associated fibroblasts to drive Notch3-dependent self-renewal of liver cancer stem-like cells. Cancer Res. 2018;78:938–949. doi: 10.1158/0008-5472.CAN-17-1236. [DOI] [PubMed] [Google Scholar]
- 523.Che Y, et al. Cisplatin-activated PAI-1 secretion in the cancer-associated fibroblasts with paracrine effects promoting esophageal squamous cell carcinoma progression and causing chemoresistance. Cell Death Dis. 2018;9:759. doi: 10.1038/s41419-018-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Wei WF, et al. Periostin(+) cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol. Oncol. 2021;15:210–227. doi: 10.1002/1878-0261.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Chu L, et al. Periostin secreted by carcinoma-associated fibroblasts promotes ovarian cancer cell platinum resistance through the PI3K/Akt signaling pathway. Technol. Cancer Res. Treat. 2020;19:153303382097753. doi: 10.1177/1533033820977535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Underwood TJ, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J. Pathol. 2015;235:466–477. doi: 10.1002/path.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Elmusrati AA, Pilborough AE, Khurram SA, Lambert DW. Cancer-associated fibroblasts promote bone invasion in oral squamous cell carcinoma. Br. J. Cancer. 2017;117:867–875. doi: 10.1038/bjc.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.You J, et al. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM. 2019;112:581–590. doi: 10.1093/qjmed/hcz093. [DOI] [PubMed] [Google Scholar]
- 529.Li Y, et al. Stress-induced upregulation of TNFSF4 in cancer-associated fibroblast facilitates chemoresistance of lung adenocarcinoma through inhibiting apoptosis of tumor cells. Cancer Lett. 2021;497:212–220. doi: 10.1016/j.canlet.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 530.Hanley CJ, et al. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J. Natl Cancer Inst. 2018;110:109–120. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Lv Y, et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano. 2018;12:1519–1536. doi: 10.1021/acsnano.7b08051. [DOI] [PubMed] [Google Scholar]
- 532.Zhang Q, et al. LY2157299 monohydrate, a TGF-βR1 inhibitor, suppresses tumor growth and ascites development in ovarian cancer. Cancers. 2018;10:260. doi: 10.3390/cancers10080260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Miao L, et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 2017;77:719–731. doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Yao Y, et al. Artemisinin derivatives inactivate cancer-associated fibroblasts through suppressing TGF-β signaling in breast cancer. J. Exp. Clin. Cancer Res. 2018;37:282. doi: 10.1186/s13046-018-0960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Sherman MH, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Froeling FEM, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–1497. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 537.Lee H-M, et al. Drug repurposing screening identifies bortezomib and panobinostat as drugs targeting cancer associated fibroblasts (CAFs) by synergistic induction of apoptosis. Invest. N. Drugs. 2018;36:545–560. doi: 10.1007/s10637-017-0547-8. [DOI] [PubMed] [Google Scholar]
- 538.Ma Y, et al. Extreme low dose of 5-fluorouracil reverses MDR in cancer by sensitizing cancer associated fibroblasts and down-regulating P-gp. PLoS ONE. 2017;12:e0180023. doi: 10.1371/journal.pone.0180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Yao H, Xu K, Zhou J, Zhou L, Wei S. A tumor microenvironment destroyer for efficient cancer suppression. ACS Biomater. Sci. Eng. 2020;6:450–462. doi: 10.1021/acsbiomaterials.9b01544. [DOI] [PubMed] [Google Scholar]
- 540.Fang J, et al. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int. J. Cancer. 2016;138:1013–1023. doi: 10.1002/ijc.29831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Li L, et al. Photosensitizer-encapsulated ferritins mediate photodynamic therapy against cancer-associated fibroblasts and improve tumor accumulation of nanoparticles. Mol. Pharm. 2018;15:3595–3599. doi: 10.1021/acs.molpharmaceut.8b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Scott AM, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 543.Watanabe S, et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 2019;20:1234–1248. doi: 10.1080/15384047.2019.1617566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Welt S, et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 545.Zhen Z, et al. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic t cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 546.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Sharma, M. et al. Simultaneously targeting cancer-associated fibroblasts and angiogenic vessel as a treatment for TNBC. J. Exp. Med. 218, e20200712 (2021). [DOI] [PMC free article] [PubMed]
- 548.Chiappori AA, et al. A phase I pharmacokinetic and pharmacodynamic study of s-3304, a novel matrix metalloproteinase inhibitor, in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2007;13:2091–2099. doi: 10.1158/1078-0432.CCR-06-1586. [DOI] [PubMed] [Google Scholar]
- 549.Wang Z-F, et al. Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts. World J. Gastroenterol. 2017;23:8512–8525. doi: 10.3748/wjg.v23.i48.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.US National Library of Medicine. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT01507545 (2021).
- 552.Reardon DA, et al. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. J. Clin. Oncol. 2006;24:115–122. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 553.Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7:82889–82901. doi: 10.18632/oncotarget.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Zalcman G, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 555.Qian L, et al. Fusion of dendritic cells and cancer-associated fibroblasts for activation of anti-tumor cytotoxic T lymphocytes. J. Biomed. Nanotechnol. 2018;14:1826–1835. doi: 10.1166/jbn.2018.2616. [DOI] [PubMed] [Google Scholar]
- 556.Xiang H, et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol. Res. 2020;8:436–450. doi: 10.1158/2326-6066.CIR-19-0507. [DOI] [PubMed] [Google Scholar]
- 557.Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 10, eaan5488 (2018). [DOI] [PubMed]
- 558.Murphy JE, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Carapuça EF, et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J. Pathol. 2016;239:286–296. doi: 10.1002/path.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Hu C, et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60–72. doi: 10.1016/j.biomaterials.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 561.Neesse A, et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc. Natl Acad. Sci. USA. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ji T, et al. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew. Chem. Int. Ed. Engl. 2016;55:1050–1055. doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Su S, et al. CD10GPR77 cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 564.Hingorani SR, et al. HALO 202: randomized phase II study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 565.Duluc C, et al. Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol. Med. 2015;7:735–753. doi: 10.15252/emmm.201404346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.US National Library of Medicine. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT03481920 (2021).
- 567.Cheteh EH, et al. Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death. Cell Death Dis. 2017;8:e2848–e2848. doi: 10.1038/cddis.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Amornsupak K, et al. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer. 2014;14:955. doi: 10.1186/1471-2407-14-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Soon PSH, et al. Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr. Relat. Cancer. 2013;20:1–12. doi: 10.1530/ERC-12-0227. [DOI] [PubMed] [Google Scholar]
- 570.Tanaka K, et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis. 2015;36:894–903. doi: 10.1093/carcin/bgv067. [DOI] [PubMed] [Google Scholar]
- 571.Wang L, et al. Cancer‐associated fibroblasts contribute to cisplatin resistance by modulating ANXA 3 in lung cancer cells. Cancer Sci. 2019;110:1609–1620. doi: 10.1111/cas.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Liao JK, et al. Cancer-associated fibroblasts confer cisplatin resistance of tongue cancer via autophagy activation. Biomed. Pharmacother. 2018;97:1341–1348. doi: 10.1016/j.biopha.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 573.Yan H, Guo B-Y, Zhang S. Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis in ovarian cancer cells by promoting STAT3 signaling. Biochem. Biophys. Res. Commun. 2016;470:947–954. doi: 10.1016/j.bbrc.2016.01.131. [DOI] [PubMed] [Google Scholar]
- 574.Broekgaarden M, et al. Modulation of redox metabolism negates cancer-associated fibroblasts-induced treatment resistance in a heterotypic 3D culture platform of pancreatic cancer. Biomaterials. 2019;222:119421. doi: 10.1016/j.biomaterials.2019.119421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Gao Q, Fang X, Chen Y, Li Z, Wang M. Exosomal lncRNA UCA1 from cancer-associated fibroblasts enhances chemoresistance in vulvar squamous cell carcinoma cells. J. Obstet. Gynaecol. Res. 2021;47:73–87. doi: 10.1111/jog.14418. [DOI] [PubMed] [Google Scholar]
- 576.Li Y, Rong G, Kang H. Taxotere-induced elevated expression of IL8 in carcinoma-associated fibroblasts of breast invasive ductal cancer. Oncol. Lett. 2017;13:1856–1860. doi: 10.3892/ol.2017.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Yu T, et al. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene. 2017;36:2131–2145. doi: 10.1038/onc.2016.370. [DOI] [PubMed] [Google Scholar]
- 578.Gonçalves-Ribeiro S, et al. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget. 2016;7:59766–59780. doi: 10.18632/oncotarget.11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Ying L, et al. Cancer associated fibroblast-derived hepatocyte growth factor inhibits the paclitaxel-induced apoptosis of lung cancer A549 cells by up-regulating the PI3K/Akt and GRP78 signaling on a microfluidic platform. PLoS ONE. 2015;10:e0129593. doi: 10.1371/journal.pone.0129593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Leung CS, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J. Clin. Invest. 2018;128:589–606. doi: 10.1172/JCI95200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Yegodayev KM, et al. TGF-beta-activated cancer-associated fibroblasts limit cetuximab efficacy in preclinical models of head and neck cancer. Cancers. 2020;12:339. doi: 10.3390/cancers12020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Eder T, et al. Cancer-associated fibroblasts modify the response of prostate cancer cells to androgen and anti-androgens in three-dimensional spheroid culture. Int. J. Mol. Sci. 2016;17:1458. doi: 10.3390/ijms17091458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Choe C, et al. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. OncoTargets Ther. 2015;8:3665–3678. doi: 10.2147/OTT.S89659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Yoshida T, et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin. Cancer Res. 2015;21:642–651. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 585.Mao Y, et al. Cancer-associated fibroblasts induce trastuzumab resistance in HER2 positive breast cancer cells. Mol. Biosyst. 2015;11:1029–1040. doi: 10.1039/C4MB00710G. [DOI] [PubMed] [Google Scholar]
- 586.Fernández-Nogueira P, et al. Tumor-associated fibroblasts promote HER2-targeted therapy resistance through FGFR2 activation. Clin. Cancer Res. 2020;26:1432–1448. doi: 10.1158/1078-0432.CCR-19-0353. [DOI] [PubMed] [Google Scholar]
- 587.Yuan J, et al. Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: a new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and β1-integrin signaling pathway in tumor cells. Breast Cancer Res. 2015;17:69. doi: 10.1186/s13058-015-0579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Kharaziha P, et al. Targeting of distinct signaling cascades and cancer-associated fibroblasts define the efficacy of Sorafenib against prostate cancer cells. Cell Death Dis. 2012;3:e262–e262. doi: 10.1038/cddis.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Hellevik T, et al. Changes in the secretory profile of NSCLC-associated fibroblasts after ablative radiotherapy: potential impact on angiogenesis and tumor growth. Transl. Oncol. 2013;6:66–74. doi: 10.1593/tlo.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Hwang RF, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Chu T-Y, Yang J-T, Huang T-H, Liu H-W. Crosstalk with cancer-associated fibroblasts increases the growth and radiation survival of cervical cancer cells. Radiat. Res. 2014;181:540–547. doi: 10.1667/RR13583.1. [DOI] [PubMed] [Google Scholar]
- 592.Zhang H, et al. Cancer-associated fibroblast–promoted LncRNADNM3OS confers radioresistance by regulating DNA damage response in esophageal squamous cell carcinoma. Clin. Cancer Res. 2019;25:1989–2000. doi: 10.1158/1078-0432.CCR-18-0773. [DOI] [PubMed] [Google Scholar]