Abstract
Although RNA helicases are essentially ubiquitous and perform roles in all stages of RNA metabolism, phylogenetic analysis of the DEAD (Asp-Glu-Ala-Asp)-box RNA helicase family in a single phylum has not been performed. Here, we performed a phylogenetic analysis on DEAD-box helicases from all currently available cyanobacterial genomes, comprising a total of 362 helicase protein sequences from 280 strains. DEAD-box helicases belonging to three distinct clades were observed. Two clades, the CsdA (cold shock DEAD-box A)-like and RhlE (RNA helicase E)-like helicases, cluster with the homologous proteins from Escherichia coli . The third clade, the CrhR (cyanobacterial RNA helicase Redox)-like helicases, is unique to cyanobacteria and characterized by a conserved sequence motif in the C-terminal extension. Restricted distribution is observed across cyanobacterial diversity with respect to both helicase type and strain. CrhR-like and CsdA-like helicases essentially never occur together, while RhlE always occurs with either a CrhR-like or CsdA-like helicase. CrhR-like and RhlE-like proteins occurred in filamentous cyanobacteria of the orders Nostocales , Oscillatoriales and Synechococcales . Similarly, CsdA- and RhlE-like proteins are restricted to unicellular cyanobacteria of the genera Cyanobium and Synechococcus . In addition, the unexpected occurrence of RhlE in two Synechococcus strains suggests recent acquisition and evolutionary divergence. This study, therefore, raises physiological and evolutionary questions as to why DEAD-box RNA helicases encoded in cyanobacterial lineages display restricted distributions, suggesting niches that require either CrhR or CsdA RNA helicase activity but not both. Extensive conservation of gene synteny surrounding the previously described rimO–crhR operon is also observed, indicating a role in the maintenance of photosynthesis. The analysis provides insights into the evolution, origin and dissemination of sequences within a single gene family to yield divergent functional roles.
Keywords: CrhR, cyanobacteria, DEAD-box RNA helicase, phylogenetic analysis
Data Summary
All of the protein sequences corresponding to the RNA helicases used in this study are publicly available in the GenBank database (https://www.ncbi.nlm.nih.gov). rpoC2 and most 16S rDNA sequences are also available in the GenBank database. Additional 16S rDNA sequences were obtained from the Joint Genome Institute Integrated Microbial Genomes and Microbiomes (JGI IMG/M) database (https://img.jgi.doe.gov/). Accession numbers are provided within the article and the supplementary data.
Impact Statement.
RNA helicases are enzymes that affect all aspects of RNA metabolism and, thus, are crucial for regulation of gene expression. Here, we applied a phylogenetic approach to analyse DEAD (Asp-Glu-Ala-Asp)-box RNA helicase distribution across all known cyanobacterial genomes. Cyanobacteria perform oxygenic photosynthesis, constituting an extremely diverse and ancient phylum that has dramatic impacts on primary productivity, water quality, and nitrogen, carbon and oxygen cycling worldwide. The analysis indicated that cyanobacteria only possess DEAD-box RNA helicases belonging to three clades, two of which correspond to groups commonly observed in other bacteria. Members of the third clade, the CrhR (cyanobacterial RNA helicase Redox)-like helicases, are only encoded in cyanobacteria, suggesting a unique role related to maintenance of photosynthesis in this phylum. Restricted distribution of the helicase classes is also observed across cyanobacterial diversity with respect to both helicase type and strain, suggesting niche-specific functionality. The phylum-specific clade and restricted distribution of DEAD-box RNA helicases in cyanobacteria provide unique insights into gene evolution and niche-specific functioning.
Introduction
Regulation of the expression of gene products occurs at many levels. At the post-transcriptional level, modulation of RNA structure can regulate gene expression by altering RNA stability or translation efficiency. In many cases, these RNA structural changes require interactions with proteins, such as chaperones [1, 2] or helicases [3–5]. Monomeric and multimeric helicases are classified into two large families: superfamily 1 (SF1) and superfamily 2 (SF2) [3–5]. RNA helicases generally belong to SF2 and function in all aspects of RNA metabolism, regulating a diverse range of cellular processes by unwinding and, more rarely, annealing RNA duplexes, performing local strand separation, and by mediating RNA-protein interactions [4, 6–8]. Generally, RNA helicases share a conserved core structure, with two RecA-like functional domains containing a series of conserved sequence motifs that participate in RNA binding, and ATP binding and hydrolysis (Fig. S1, available with the online version of this article) [9–11]. The largest family of RNA helicases, the DEAD (Asp-Glu-Ala-Asp)-box proteins, have 12 highly conserved sequence motifs [7], conferring ATP-dependent local RNA strand separation [12]. Some DEAD-box proteins can also displace proteins from RNA, or clamp to RNA and serve as a nucleation site for the recruitment of other proteins to form functional complexes [6, 7, 13]. In eukaryotes, DEAD-box helicases are essential for survival, as they are intimately involved in crucial cellular processes, including translation, splicing and RNA degradation [7]. Prokaryotic DEAD-box proteins also function in these processes; however, their function is generally only required under non-optimal conditions [14].
Classification of prokaryotic DEAD-box helicases was originally based on the RNA processes in which the protein participates [15, 16], but phylogenetic methods have also been used [14, 17]. Based on conserved sequence domains, López-Ramírez et al. [17] classified bacterial DEAD-box proteins into three major groups: (i) proteins that contain a DbpA (DEAD-box protein A) RNA binding domain, including CsdA (cold shock DEAD-box A) and DbpA from Escherichia coli , and DeaD/YxiN from Bacillus subtilis ; (ii) proteins lacking the DbpA domain, including SrmB and RhlB from E. coli , and CshA, CshB and YfmL from B. subtilis ; and (iii) RhlE (RNA helicase E)-like proteins. These classifications, based on sequence and structure, generally relate well to the cellular functions of the DEAD-box protein. For example, the DbpA RNA binding domain facilitates recognition and binding of hairpin 92 of the 23S rRNA by a DEAD-box RNA helicase [18–20]. All of the well-characterized DEAD-box proteins containing this domain, DbpA, CsdA and YxiN, participate in maturation of the 50S ribosomal subunit, and specifically the 23S rRNA [21–23]. These phylogenetic classifications aid in deducing putative functions of DEAD-box helicases from protein sequences as they are annotated in newly sequenced genomes.
Cyanobacteria is an ancient phylum of Gram-negative bacteria that evolved as early as 3.5 billion years ago [24]. These bacteria exhibit extreme genetic diversity and are distinguished by being the only known bacteria that perform oxygenic photosynthesis from which they obtain all of their energy [25, 26]. As a group, they have dramatic impacts on cycling of carbon dioxide, fixed carbon, oxygen and nitrogen, and primary productivity on a global scale [27]. In addition, their ability to sequester carbon dioxide makes them ideal platforms for carbon-neutral biotechnological applications [28]. Although phylogenetic analysis of bacterial DEAD-box RNA helicases has been performed [14, 17], extensive analysis within a specific phylum has not been conducted. An initial phylogenetic analysis of cyanobacterial DEAD-box helicases was performed [29]; however, there are a significantly larger number of cyanobacterial genomes now available that makes the analysis more robust. Therefore, it was of interest to evaluate how cyanobacterial DEAD-box RNA helicases relate to those encoded in other bacterial phyla.
Only four DEAD-box RNA helicases have previously been experimentally identified in cyanobacteria: CrhA from Anabaena variabilis UTCC 387 [30], CrhB and CrhC from Nostoc sp. PCC 7120 [31], and CrhR (cyanobacterial RNA helicase Redox) from Synechocystis sp. PCC 6803 (from here on Synechocystis ) [32], with two examples, CrhA and CrhB, receiving only limited study. CrhC is specifically induced at low temperature [31], and interacts with ribosomes and localizes to the cytoplasmic face of the plasma membrane near the cell poles [33]. Expression of CrhR is regulated in response to the redox poise of the plastoquinone pool of the photosynthetic electron transport chain [32, 34]. CrhR was shown to co-sediment with polysomes and RNA degradosome components, and localize to the thylakoid membrane [35]. Consistent with localization to the thylakoid membrane, crhR mutation results in disruption of photosynthesis, particularly at low temperatures [36, 37]. As the cyanobacterial DEAD-box proteins, and in particular CrhR, demonstrate localization and functions unique to cyanobacteria, further study of this protein family across the diversity of cyanobacteria was warranted.
Here, we report a phylogenetic analysis of cyanobacterial DEAD-box proteins indicating that they form three major clades, two with homology to the E. coli DEAD-box proteins CsdA and RhlE, and a third unique to cyanobacteria, the CrhR-like helicases. The CrhR-like proteins are found throughout cyanobacteria diversity and share a conserved sequence domain in the C-terminal extension that identifies them as a separate clade within the DEAD-box RNA helicase family. In addition, synteny surrounding the dicistronic rimO–crhR operon is extensively conserved, suggesting functional conservation and early acquisition of CrhR-like proteins in the cyanobacterial lineage. The three RNA helicase clades are also encoded in specifically restricted patterns throughout the phylum Cyanobacteria . CsdA-like helicases, which occur only in Synechococcales , have a DbpA RNA binding motif in the C-terminus and may have been acquired following a loss of the CrhR-like helicase in the ancestral lineage. Strains with RhlE-like helicases also encode at least one other DEAD-box protein in the genome. Based on similarities to characterized homologues, functions for CsdA, CrhR and RhlE in ribosome biogenesis, expression and maintenance of photosynthetic systems, and coordination of CsdA-CrhR function, respectively, are proposed.
Methods
Sequence data
Predicted and known amino acid sequences of cyanobacterial DEAD-box RNA helicases were obtained from the National Center for Biotechnology Information (NCBI) non-redundant (nr) protein database. The helicase core domain (amino acids F9–R338) of the sole DEAD-box RNA helicase in Synechocystis , CrhR, was retrieved from the NCBI database (accession no. WP_010873784.1) for use as the query sequence. A blastp search, restricted to cyanobacteria (taxid: 1117), yielded 461 unique DEAD-box RNA helicase sequences. Following alignment in mega7, these sequences were manually curated for full-length sequences that contained the 12 conserved motifs of the DEAD-box helicase protein family (Fig. S1). Curation was performed by initially removing sequences that lacked a start or stop codon, and subsequent deletion of sequences that did not span the full length of the helicase core region, extending from F9 to R338 of the Synechocystis sp. PCC 6803 slr0083-encoded helicase, CrhR. The remaining sequences were aligned with muscle [38] and those containing motifs in the helicase core characteristic of all SF1 subfamily members or other SF2 helicase clades, other than DEAD-box family members, were removed. Following curation, 362 DEAD-box RNA helicase sequences from 280 strains of cyanobacteria were identified. The sequences of the five DEAD-box proteins from E. coli were also retrieved from the NCBI to verify branching and clade assignment.
Sequence alignments and phylogenetic analysis
The helicase sequences were assembled in mega7 v7.0.21 [39], where multiple sequence alignments of the protein sequences were generated using the integrated muscle algorithm [38], using default parameters. This alignment was exported in fasta format and converted to relaxed phylip format using NCLconverter v2.1 [40], accessed via the CIPRES Science Gateway [41].
The maximum-likelihood tree was created using RAxML-HPC Blackbox v8.2.10 [42], accessed via CIPRES [41] using default settings, with the exception of the amino acid substitution matrix, which was set to LG [43]. Rapid bootstrapping ran for 252 replicates before auto-termination with MRE-based bootstrapping criteria [44]. The output tree was visualized in iTOL v4.3.2 [45]. Taxa colours correspond to the cyanobacterial order.
The Euler diagram representing the distribution of the helicase families across cyanobacterial strains was created as a three-set Euler diagram in EulerAPE v.3.0 [46], consisting of CrhR-like, CsdA-like and RhlE-like helicases. The fourth set, for the unclassified helicases, was added with approximate scaling in Adobe Illustrator to visually demonstrate the intersection of the unclassified helicases with the CrhR-like and RhlE-like proteins.
Gene context analysis
Gene synteny surrounding each DEAD-box protein in the respective cyanobacterial genome was identified using the NCBI Genome Viewer. Related genes were identified from annotations of conserved motifs.
C-terminal motif identification
Alignments of the C-terminal domains for sequences from each helicase subfamily were generated in muscle [38]. Alignments of a selection of sequences from the subfamily were used for visualization of the alignments. Alignments including all sequences in the subfamily were used to generate sequence logos in WebLogo 3 [47].
Pfam domain identification
Protein sequences were searched against the Pfam database [48] using the EMBL-EBI web portal with an E value cut-off of 1.0.
rpoC2 neighbour-joining tree
rpoC2 gene sequences were identified by searching the genome sequences with the RpoC2 protein of Synechocystis (BA000022.2 : 853497–857450). rpoC2 nucleotide sequences were used instead of 16S rDNA for tree reconstruction as the genome for Phormidesmis priestleyi Ana is incomplete, lacking sequences for the 16S rDNA and several core conserved genes often used in concatenated gene trees. rpoC2 nucleotide sequences were aligned by muscle [38] with default parameters. A neighbour-joining tree was generated in mega7 v7.0.21 [39] and a maximum-likelihood tree was generated using RAxML-HPC Blackbox v8.2.10 [42] using default settings, accessed via the CIPRES [41] Science Gateway.
Results
Cyanobacterial DEAD-box RNA helicases form three distinct clades
The amino acid sequence of the Synechocystis DEAD-box protein, CrhR, was used to generate a query sequence. The query sequence (F9–R338) was selected as it contained amino acids corresponding to the RNA helicase core, between the conserved phenylalanine upstream of the Q motif [49] and the final arginine of motif VI, HRIGR [50] (Fig. S1), which is highly conserved in all DEAD-box RNA helicases [7]. This sequence was used to search the NCBI non-redundant (nr) protein database, returning 461 unique cyanobacterial records, which were further curated to remove sequences from other SF1 and SF2 helicase families, as well as incomplete sequences. The resulting 362 cyanobacterial DEAD-box protein sequences (Table S1) were aligned, and the phylogenetic relationships were identified by reconstruction of an unrooted maximum-likelihood tree using RAxML [42].
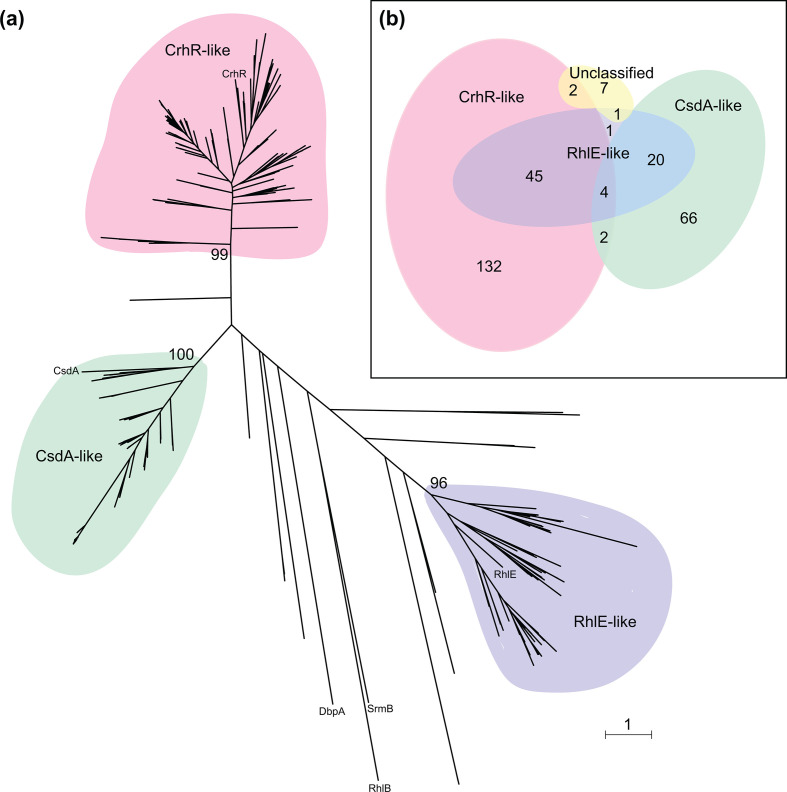
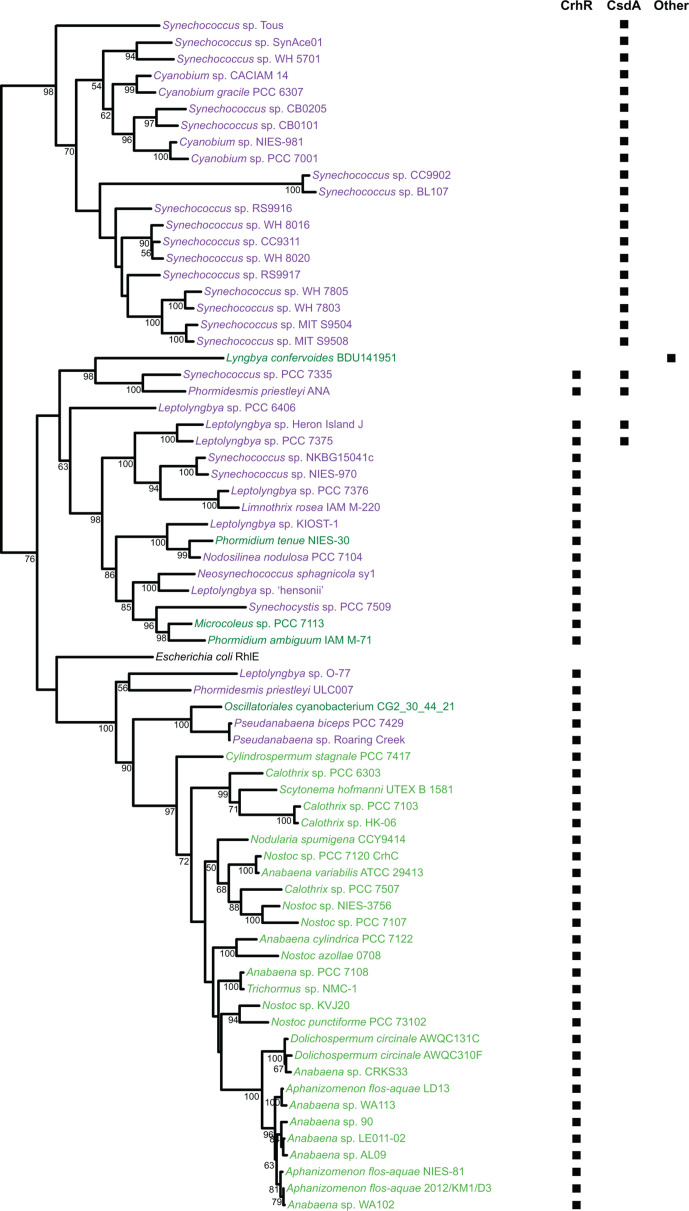
The majority of the cyanobacterial DEAD-box RNA helicase proteins cluster into three clades, each having a bootstrap value ≥95 % (Fig. 1a, b). Two of the E. coli helicase sequences, RhlE and CsdA, fall within these groups, while the remaining three, DbpA, RhlB and SrmB, do not group with the cyanobacterial helicase sequences. The three clades were designated as CsdA-like and RhlE-like, corresponding to the E. coli helicases that cluster with them, and CrhR-like, for the most-characterized protein sequence in this clade, CrhR, from Synechocystis [32]. Of the 362 cyanobacterial DEAD-box proteins in this analysis, 185 fall within the CrhR-like helicases, 92 in CsdA-like and 71 in RhlE-like (Fig. 1b). Fourteen of the cyanobacterial helicase proteins do not cluster with any of the three cyanobacterial clades nor with the three additional DEAD-box RNA helicase subfamilies present in E. coli (Fig. 1b).
Fig. 1.
Cyanobacterial DEAD-box proteins cluster into three clades. The phylogenetic tree (a) shows the 362 cyanobacterial DEAD-box proteins form three major clades with bootstrap values ≥95 %, corresponding to CsdA-like and RhlE-like proteins, and a clade unique to cyanobacteria, CrhR-like proteins, named for the sole DEAD-box RNA helicase encoded in the genome of Synechocystis sp. PCC 6803. Bootstrap values at the root of each clade, as well as the positions of the five DEAD-box proteins of E. coli and CrhR from Synechocystis sp. PCC 6803, are indicated. Branch lengths are proportional to the mean number of substitutions per amino acid site as indicated by the scale bar. The distribution of the DEAD-box protein clades identified in the 280 cyanobacterial strains used in the analysis is represented by the Euler diagram shown in the inset (b).
Examination of helicase distribution demonstrates that 199 cyanobacterial strains contain a single member of one of the DEAD-box clades, 67 contain two forms, while only 4 strains contain members of all three clades (Fig. 1a). Of the strains encoding ungrouped DEAD-box proteins, three strains also encode either a CrhR-like or RhlE-like protein, while the remaining seven encode solely DEAD-box proteins that do not cluster with the three protein subfamilies defined in this study. It is important to note that only a few examples of cyanobacterial strains that do not encode DEAD-box RNA helicases were identified. Interestingly, these include the model organism Synechococcus elongatus , of which there are several genome sequences available [51]. DEAD-box proteins were also not identified in some early branching cyanobacteria, including Synechococcus sp. JA3-3Ab, Synechococcus sp. JA2-3B′a, and the proposed order Gloeomargaritales , for which only a single genome exists [52]. Based on the currently available sequence data, it can be concluded that cyanobacterial species encode between zero and three DEAD-box proteins that group into separate clades.
CrhR-like proteins are conserved across cyanobacterial diversity
The maximum-likelihood tree of the cyanobacterial DEAD-box RNA helicases was also visualized, with the species, strain and order of the cyanobacteria encoding each protein indicated (Fig. S2). CrhR-like proteins were detected in every order of cyanobacteria that encode DEAD-box proteins except Gloeobacterales (Fig. S2), the oldest branching extant group of cyanobacteria [52]. This order represents a unique branch of cyanobacteria that lack thylakoid membranes, with photosynthetic light harvesting and electron transport located in the cytoplasmic membrane [53]. Interestingly, while Gloeobacter violaceus PCC 7421 and Gloeobacter kilaueensis JS1 encode DEAD-box proteins, only one of the Gloeobacter proteins clusters weakly with any of the three cyanobacterial DEAD-box helicase subfamilies defined in this study. In addition, CrhR-like helicases were not observed in the available sequences of the phylogenetically related non-photosynthetic proposed classes Melainabacteria and Sericytochromatia [26]. Conservation of the CrhR-like helicases throughout the diversity of cyanobacteria that evolved following branching from the Gloeobacterales and Gloeomargaritales , and absence of these proteins from organisms other than cyanobacteria, indicates that it is likely that the ancestral CrhR-like protein arose early in the radiation of the cyanobacterial crown group, after evolution of the non-photosynthetic Melainabacteria and the more basal Sericytochromatia .
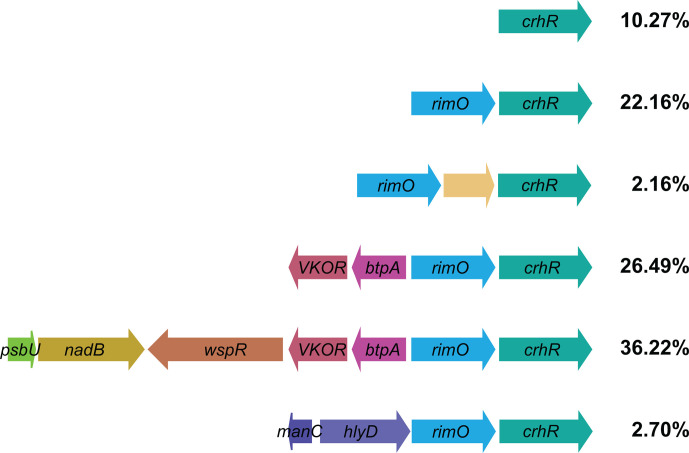
Conservation of the CrhR-like helicases in cyanobacteria was also investigated by examination of gene clustering surrounding the helicase. In Synechocystis , crhR is expressed as a dicistronic operon, downstream of the gene encoding the ribosomal protein S12 methylthiotransferase, rimO [54]. Of the 185 cyanobacterial strains that encode CrhR-like helicases in this study, only 19 (10.27 %) do not preserve the synteny of the rimO–crhR operon from Synechocystis (Fig. 2). Synteny is extensively maintained outside of the rimO–crhR operon with 62.71 % of strains encoding a vitamin K epoxide reductase (VKOR) and photosystem I (PSI) stabilizing protein (btpA) upstream of the rimO–crhR operon (Fig. 2). Of those, 57.7 6% also encode a diguanylate cyclase (wspR), l-aspartate oxidase (nadB) and photosystem II (PSII) complex extrinsic protein (psbU) further upstream. These proteins perform roles in photosynthesis, membrane protein stability, protein folding and cellular energetics. The strong conservation of the rimO–crhR gene pair, as well as upstream genes in a preponderance of strains, supports an early acquisition of the CrhR-like proteins in cyanobacteria. Maintenance of the syntenic gene organization throughout cyanobacterial diversity also intimates that the pathways in which the encoded proteins function, including RimO and CrhR, are likely related, as products of highly conserved gene pairs are typically functionally related and often physically interact [55].
Fig. 2.
Conserved gene sequence surrounding CrhR-like DEAD-box RNA helicases. Gene synteny surrounding the DEAD-box RNA helicase in strains containing CrhR-like proteins. Percentages represent the relative proportion of the 185 CrhR-encoding cyanobacterial strains in this analysis possessing the indicated gene sequence surrounding CrhR. Functionally related genes have similar colours. The cut-off to be considered as a separate example of conserved gene context was conservation in 2 % of the strains.
Restricted distribution of DEAD-box proteins in cyanobacterial strains
Strict boundaries for DEAD-box RNA helicase distribution in cyanobacteria was evidenced by genomes that encode both CrhR- and RhlE-like proteins, which are restricted to filamentous cyanobacteria of the orders Nostocales , Oscillatoriales and Synechococcales. Similarly, co-occurrence of CsdA- and RhlE-like proteins is restricted to unicellular cyanobacteria of the genera Cyanobium and Synechococcus . The three exceptions are Synechocystis sp. PCC 7509, Synechococcus sp. NIES-970 and Synechococcus sp. NKBG15041c, which are unicellular strains that encode both a CrhR-like and RhlE-like helicase. Polyphyly has long been a known issue in the genera Synechococcus [56] and Synechocystis [57]. 16S rDNA and concatenated gene trees group Synechocystis sp. PCC 7509 near strains from the order Nostocales rather than other Synechococcales [58], suggesting this unicellular cyanobacterium encodes both a CrhR-like and RhlE-like helicase due to its common lineage with Nostocales and should be reclassified. In contrast, the other two unicellular strains that encode CrhR and RhlE, Synechococcus sp. NIES-970 and Synechococcus sp. NKBG15041c, are closely related to Synechococcus sp. PCC 7002 [59, 60], which encodes only a CrhR-like helicase. In a species evolution context, the presence of RhlE in only these two strains of unicellular Synechococcus is an important observation, since other closely related Synechococcus strains only contain CrhR-like helicases. This unpredicted occurrence implies that RhlE was acquired relatively recently in these two strains. Further study will be required to understand the physiological and evolutionary significance of RhlE helicase acquisition by these Synechococcus strains.
Furthermore, proteins belonging to the CsdA-like subfamily are only encoded in one cyanobacterial order, primarily in unicellular strains of the Synechococcales . This holds for all species examined except for the Synechococcales strains Leptolyngbya sp. Heron Island J, Leptolyngbya sp. PCC 7375 and Phormidesmis priestleyi Ana. These three filamentous strains, as well as the unicellular Synechococcus sp. PCC 7335, are the only strains that encode DEAD-box proteins from all three subfamilies and are discussed below. Other than the four strains encoding proteins from all three groups, CsdA-like proteins are only encoded in species of Acaryochloris , Cyanobium , Prochlorococcus and Synechococcus . The strains from the genera Cyanobium , Prochlorococcus and Synechococcus that encode CsdA are monophyletic, based on both 16S rRNA [60] and concatenated gene [58] phylogenies. The most closely related species of cyanobacteria to these strains is Synechococcus elongatus [58], which does not encode a DEAD-box protein.
Co-occurrence of CrhR-like and CsdA-like proteins, excepting the four strains that encode all three subfamilies, is restricted to the genus Acaryochloris (Fig. S2). Cyanobacteria belonging to Acaryochloris , like Gloeocapsa , have divergent photosynthetic pathways, as they are capable of photosynthesis using the far-red spectrum, with chlorophyll d as the primary photosynthetic pigment and an altered complement of phycobiliproteins that do not form phycobilisomes on the surface of the thylakoid membrane [61]. As Acaryochloris sp. and the four strains that encode all three subfamilies are more distantly related to the other Cyanobium , Prochlorococcus and Synechococcus strains that encode CsdA [58], further analysis will be required to discern whether the CsdA-like proteins were acquired independently by the different cyanobacterial lineages.
Conserved sequence motif in the C-terminus of CrhR-like proteins characterizes the CrhR-like clade
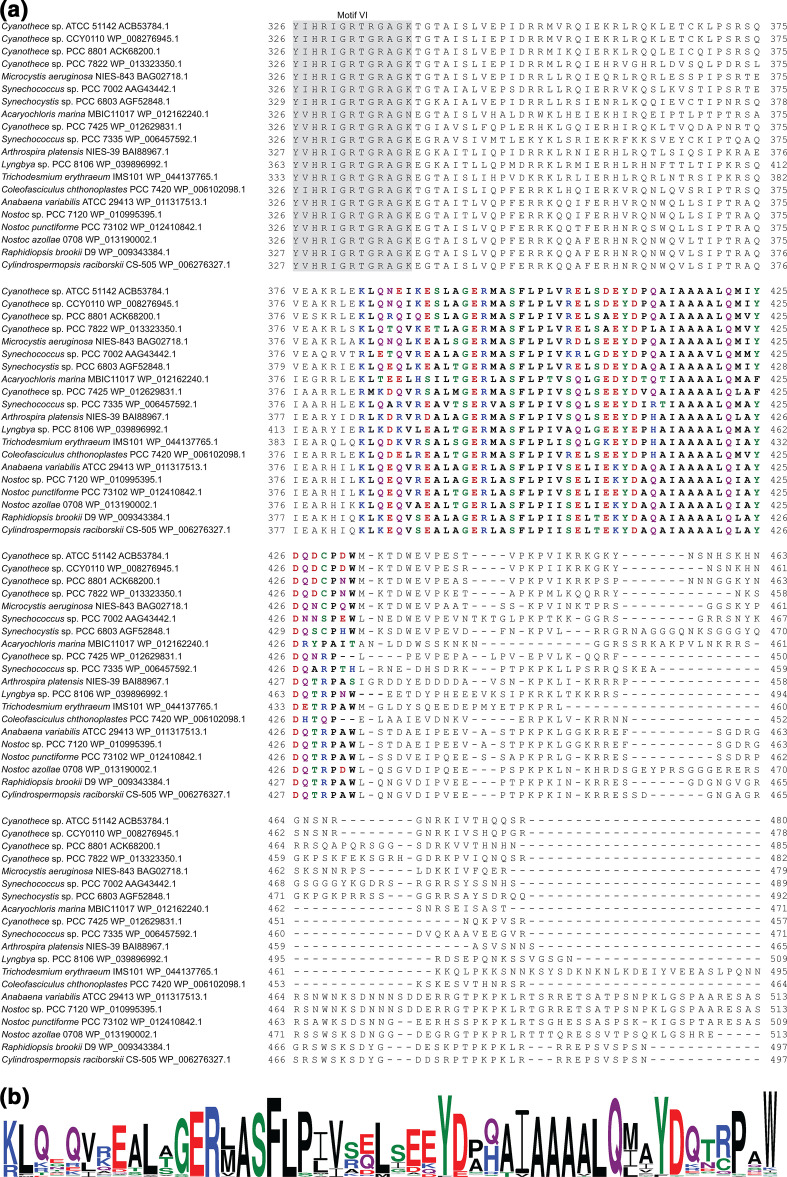
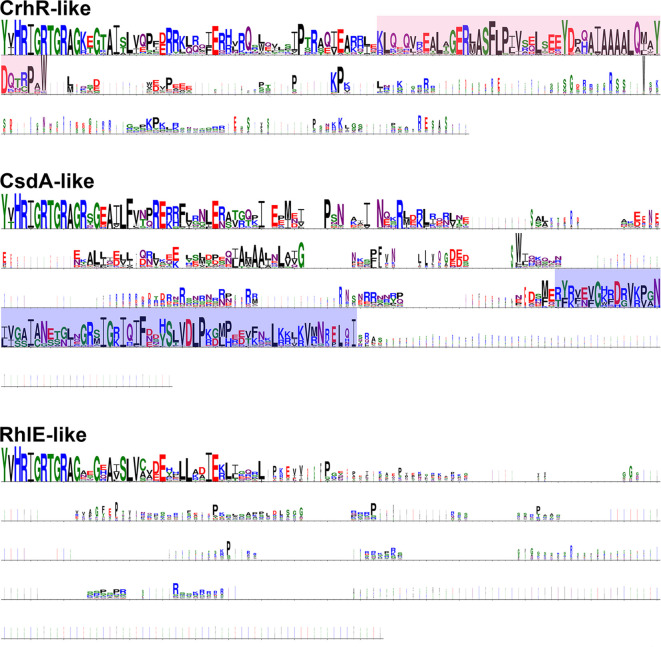
The C-terminal extension in CrhR-like helicases extends 150–200 amino acids downstream of the conserved helicase motif VI, HRIGR (Fig. S1). Within the C-terminal extension, a conserved sequence motif of approximately 50 amino acids is present in all CrhR-like helicases (Fig. 3). This motif is unique to and characterizes the CrhR-like RNA helicase clade as it is not found in the other two clades present in cyanobacteria (Fig. 4) and since other sequences containing this motif were not identified in either the Pfam protein families [48] or the NCBI protein databases. The DEAD-box protein from G. violaceus PCC 7421 (WP_011142503.1), the nearest branching protein to the CrhR-like group of DEAD-box proteins, also does not possess the conserved CrhR sequence motif in its C-terminus; however, it does have shorter regions of sequence conservation with the CrhR-like proteins in the C-terminal extension (Fig. S3). This was unexpected as, other than proteins containing the DbpA RNA binding domain, sequence conservation in the C-terminal extension of DEAD-box RNA helicases is generally minimal [17]. Therefore, it is likely that both the CrhR-like helicases and the DEAD-box protein from G. violaceus PCC 7421 share a common ancestral protein, with evolution of the CrhR-specific C-terminal motif occurring after the divergence of the order Gloeobacterales .
Fig. 3.
The C-terminal extension of CrhR-like helicases contains a unique, highly conserved sequence motif. (a) Alignment of the C-terminal extensions of selected CrhR-like DEAD-box proteins generated by muscle. The final conserved motif in the DEAD-box helicase core, motif VI, HRIGRXXR, is indicated with a grey background. The unique conserved sequence motif characteristic of CrhR-like helicases is indicated in bold, with amino acids coloured based on their chemical properties. (b) Sequence logo of the conserved CrhR-specific sequence motif, with the size of the amino acid indicating its conservation. The sequence logo was generated from an alignment of all 185 CrhR-like DEAD-box RNA helicase sequences using WebLogo 3 [47].
Fig. 4.
Conservation in the C-terminal extension of cyanobacterial DEAD-box proteins. Comparisons of the conservation in the C-terminal extensions of the three clades of cyanobacterial DEAD-box RNA helicases are shown using sequence logos, with the size of the amino acid indicating its conservation. The CrhR-specific sequence motif is indicated in pink and the DbpA RNA binding domain is marked in blue. Sequence logos were generated in WebLogo 3 from alignments of all cyanobacterial DEAD-box proteins in each clade.
CsdA-like proteins are characterized by a DbpA RNA binding domain in the C-terminal extension
The DbpA RNA binding motif is an arginine-rich protein motif that facilitates recognition and binding of RNA, specifically hairpin 92 of the 23S rRNA [18–20]. As the E. coli DEAD-box RNA helicase CsdA, which has a DbpA RNA binding motif in its C-terminal extension (Fig. 4), clustered within the cyanobacterial CsdA-like clade, the C-terminal extension of helicases in this study were also examined for a DbpA RNA binding motif. The C-terminal extension of the CsdA-like DEAD-box RNA helicases extends 200–235 amino acids past the final motif of the helicase core region, the HRIGR motif VI (Fig. S1). It was found that in CsdA-like cyanobacterial helicases, the C-terminal extension contains a DbpA RNA binding motif, a domain that is not observed in the CrhR-like helicases (Fig. 5). Two other cyanobacterial helicases also contain a DbpA RNA binding motif in their C-terminus: proteins from the unclassified cyanobacteria bacterium strain 13_1_20 CM_4_61_6 (OLE96526.1) and Hassallia byssoidea VB512170 (KIF31317.1). These strains cluster near the CsdA-like proteins and E. coli DbpA, respectively, with low bootstrapping support, suggesting a more distant relationship within the same larger group of DbpA-containing DEAD-box proteins.
Fig. 5.
The C-terminal extension of the CsdA-like helicases contains a DbpA RNA binding domain. (a) A schematic showing the Pfam domains present in the CsdA-like helicases: the DEAD/DEAH box helicase domain (DEAD, PF00270), the helicase conserved C-terminal domain (helicase C, PF00271) and the DbpA RNA binding domain (DbpA, PF03880). (b) Alignments generated with muscle of the C-terminal extensions of selected CsdA-like helicases. The final conserved DEAD-box helicase motif, HRIGRXXR, is marked with a grey background on the alignment. The conserved DbpA RNA binding domain is indicated in bold, with amino acids coloured based on their chemical properties. Note that this domain is not present in members of the CrhR-like clade.
RhlE-like helicases co-occur with another DEAD-box protein
RhlE-like proteins essentially only occur in strains of cyanobacteria with at least one other DEAD-box protein (Fig. 6). Leptolyngbya sp. PCC 6406 is the only cyanobacterium in this study that encodes an RhlE-like helicase as its only identified DEAD-box protein (Table S1); however, as the genome sequence of this organism is a high-quality draft sequence, we predict that an additional DEAD-box RNA helicase, either CrhR- or CsdA-like, is encoded in the remaining gapped regions.
Fig. 6.
Co-occurrence of RhlE-like helicases with CsdA- and CrhR-like proteins. The RhlE-like maximum-likelihood helicase tree was extracted from Fig. S2. Annotations indicate whether the second DEAD-box protein in each strain is from the CrhR-like or CsdA-like groups. Essentially, RhlE is only found in conjunction with either CrhR or CsdA. Bootstrap values ≥50 % are shown at the nodes. Taxa colours indicate the cyanobacterial taxonomic order: purple, Synechococcales ; light green, Nostocales ; dark green, Oscillatoriales . The position of E. coli RhlE is provided to support the classification.
The RhlE-like proteins cluster into two major related groups that, apart from the four strains that encode all three types of cyanobacterial DEAD-box proteins, correlate with the co-occurrence of CsdA- or CrhR-like helicases (Fig. 6). This suggests that RhlE-like helicases were acquired at least twice in cyanobacterial lineages, once in the lineage with CsdA-like helicases and at least once in strains with CrhR-like proteins.
The RhlE-like proteins have a C-terminal extension of approximately 75–100 amino acids that lacks sequence conservation (Fig. 4). It should also be noted that all RhlE-like proteins from the order Nostocales had a substitution of phenylalanine for serine in the conserved motif III [62], resulting in a sequence change of SAT→FAT.
Proteins from all three helicase clades are only encoded in four related strains of cyanobacteria
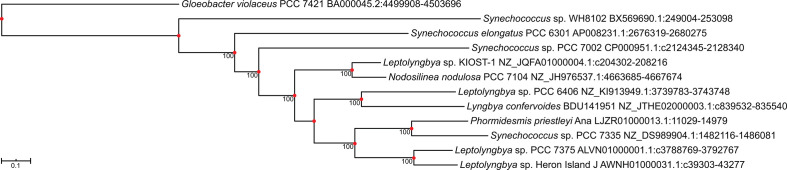
The only cyanobacterial strains that encode a member of all three clades, a CrhR-, CsdA- and RhlE-like helicase, within the same genome are Leptolyngbya sp. PCC 7375, Leptolyngbya sp. Heron Island J, Phormidesmis priestleyi Ana and Synechococcus sp. PCC 7335 (Fig. S2). Although these four strains are annotated as members of three separate genera, 16S rDNA, rpoC2 and/or concatenated gene phylogenies indicate they are more closely related than another closely related strain, Leptolyngbya sp. PCC 6406 [58, 63, 64]. As all four of these strains have not been included in the same phylogenetic analysis to date, an rpoC2 gene tree was reconstructed (Fig. 7) to confirm that the strains were closely related. As expected, Leptolyngbya sp. PCC 7375, Leptolyngbya sp. Heron Island J, Phormidesmis priestleyi Ana and Synechococcus sp. PCC 7335 cluster together (Fig. 7). This clustering was also supported by 16S rDNA analysis in which all nodes were duplicated, excepting the node nearest Phormidesmis priestleyi Ana (Fig. S4), for which a 16S rDNA sequence is not available. Clustering of these four strains confirms that, despite the differences in cellular morphology and current taxonomy, the strains of cyanobacteria that encode members of all three groups of cyanobacterial DEAD-box proteins are closely related and require reclassification.
Fig. 7.
rpoC2 maximum-likelihood tree showing that the four cyanobacterial strains that encode DEAD-box proteins from all three clades of cyanobacterial DEAD-box proteins are closely related. Nucleotide sequences for the RNA polymerase β′ subunit, rpoC2, were aligned by muscle and used to reconstruct a maximum-likelihood tree with G. violaceus PCC 7421 as an outgroup. Several Leptolyngbya species and closely related cyanobacteria, as well as several Synechococcus species, were included to confirm the clustering of the four strains that encode all three DEAD-box proteins. Bootstrap values ≥50 % are shown at the nodes. Branch lengths are proportional to the mean number of substitutions per nucleotide site as indicated by the scale bar. All nodes in the maximum-likelihood tree are duplicated in a neighbour-joining reconstruction, as indicated with a red circle.
Discussion
Cyanobacteria are unique among prokaryotes, as they are the only bacteria that perform oxygenic photosynthesis. In this study, it was shown that the complement of DEAD-box RNA helicases encoded by cyanobacteria is also unique, as proteins with homology to the helicases of E. coli , the CsdA-like and RhlE-like protein groups, are only encoded within the genomes of select cyanobacterial taxa. In contrast, the DEAD-box RNA helicase clade that can be found across cyanobacterial diversity, the CrhR-like proteins, is unique to cyanobacteria. The C-terminal extension domain of the DEAD-box RNA helicase is a defining characteristic of this unique clade.
A sequence motif of approximately 50 amino acids that is uniquely observed only in the CrhR-like clade of cyanobacterial DEAD-box RNA helicases is located within the C-terminal extension. This motif is not observed in other organisms, including higher plants and algae, but is widely distributed in cyanobacteria, suggesting that the CrhR-specific domain arose as a specialization of a DEAD-box protein in the ancestral cyanobacterial lineage. This implies that CrhR-like helicases perform a specific function that is unique to cyanobacteria. As CrhR-like helicases are often the sole DEAD-box protein in a cyanobacterial genome, it is likely that they function in multiple RNA processes. We propose that the unique function of CrhR-like helicases likely relates to oxygenic photosynthesis. This conjecture is supported by the co-precipitation of CrhR from Synechocystis with polysomes and RNA degradosome components [35], and the altered expression of ~10 % of the Synechocystis transcriptome, consisting of both mRNA and non-coding RNAs, in a crhR truncation mutant [65]. Physiologically, and consistent with the altered expression profiles, CrhR mutation results in significant disruption to photosynthesis, with cells exhibiting reduced pigment abundance, oxygen evolution rates and carbon dioxide fixation rates [36]. These physiological changes can also be associated with the regulation of crhR expression by a range of abiotic stresses [32, 34, 66, 67]. Taken together, these effects are consistent with CrhR RNA helicase localization to the thylakoid membrane [35], the site of light harvesting and ATP and NADPH formation in Synechocystis . Therefore, we propose that CrhR-like RNA helicases perform a unique function, coordinating expression of genes required to maintain oxygenic photosynthesis in response to abiotic stresses in cyanobacteria.
Support for CrhR functioning in maintenance of photosynthesis was also provided by the conserved gene synteny surrounding the CrhR-like proteins. The rimO–crhR operon of Synechocystis is conserved in 89.8 % of strains with CrhR-like helicases. Over 60 % of strains also have further conservation of synteny, with conserved genes relating to photosynthesis, energy transfer from the electron transport chain and membrane protein stability. In particular, the activities of many of these proteins could protect the photosynthetic electron transport chain from oxidative damage if an excess of reductant was to accumulate. For example, PsbU stabilizes the oxygen-evolving complex of photosystem II (PSII) and protects it from inactivation in response to stress, such as heat [68]. The vitamin K epoxide reductase (VKOR) and l-aspartate oxidase (nadB) act as electron sinks, as they both can use quinones from the electron transport chain as the source of energy for their biochemical activity [69, 70]. Expression of these proteins may be regulated under similar conditions as CrhR, which is induced in response to increasing reduction of the photosynthetic electron transport chain [34].
It is interesting that CrhR- and CsdA-like helicases are not observed in the same species, suggesting the requirement for divergent functionality. The CsdA-like helicases are characterized by a DbpA RNA binding domain in the C-terminus, similar to the canonical family member CsdA in E. coli [17]. The presence of the DbpA RNA binding domain, which assists DbpA in binding specifically to hairpin 92 of the 23S rRNA [18, 71], may indicate that the cyanobacterial CsdA-like helicases have a role in ribosome biogenesis, and more specifically maturation of the 23S rRNA, similar to DbpA [23, 72]. The domain may also provide more general RNA chaperone properties to the helicase, as some proteins with a DbpA RNA binding domain, including CsdA, do not bind to the 23S rRNA at hairpin 92 [73]. As these proteins have significant homology with CsdA, the cyanobacterial CsdA-like proteins may constitute a multifunctional helicase that can function in ribosome biogenesis, translation initiation and RNA degradation, as observed in E. coli [21, 23, 73–76]. The DbpA RNA binding domain is not encoded in CrhR-like helicases, suggesting CrhR is not involved in ribosome biogenesis.
Cyanobacterial RhlE-like helicases lack a conserved C-terminal domain. Interestingly, proteins from this group are essentially only encoded in genomes with at least one other DEAD-box helicase. This is consistent with the distribution of RhlE-like proteins generally in prokaryotes, with only a limited number of genomes encoding solely an RhlE-like DEAD-box helicase [17]. This also supports conservation of the proposed function of the RhlE helicase in E. coli as a helicase that functions cooperatively with other DEAD-box proteins during ribosome biogenesis [77].
Thus, distribution and sequence divergence of the CsdA and RhlE groups of DEAD-box RNA helicases in cyanobacteria suggest they were acquired independently several times. The CrhR-like proteins are widely distributed throughout cyanobacterial diversity and contain a sequence domain unique to the CrhR-like clade of DEAD-box RNA helicases, suggesting they perform a cyanobacterial specific function. Overall, the analysis suggests that the precursor of CrhR-like DEAD-box helicases arose early in cyanobacterial evolution. A common ancestor of the genera Synechococcus and Prochlorococcus likely lost the CrhR-like helicase. Early branching Synechococcus and Prochlorococcus , based on 16S rRNA sequence [78], contain CrhR-like helicases; however, CrhR-like proteins are absent from Synechococcus elongatus PCC 7942 and the strains with CsdA-like helicases. It is possible that the CsdA-like helicases were acquired by horizontal gene transfer in the ancestral lineage that lacked a CrhR-like helicase and were maintained as they conferred a selective advantage. Acquisition of RhlE-like helicases in cyanobacteria was likely also by horizontal gene transfer, possibly several events, as they are generally only found in proteobacteria [17].
This study raises questions as to why DEAD-box RNA helicases encoded in cyanobacterial genomes display distributions restricted on both the family member type and strain levels. This suggests certain lineages of cyanobacteria require the activity of specific DEAD-box RNA helicases. The specificity is potentially related to habitat niche for marine species, since CsdA-like helicases are principally encoded in Synechococcus and Prochlorococcus species, which are primarily marine; however, this does not apply to the CrhR-like helicases, which occur in cyanobacteria that occupy a diverse range of habitats. Cyanobacteria primarily encode helicases classified into three DEAD-box RNA helicase clades on the basis of conserved sequences in the C-terminal extension. Two of these clades, the CsdA-like and RhlE-like helicases, have significant similarity to the corresponding E. coli proteins. The third clade, the CrhR-like helicases, are unique to cyanobacteria and are characterized by a sequence motif in the C-terminal extension that is only found in CrhR-like helicases. Based on prior characterization of CrhR and the observed syntenic gene conservation, it is likely that CrhR-like proteins are multifunctional helicases, performing roles in RNA metabolism related to maintenance of photosynthesis.
Supplementary Data
Funding information
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant, grant number 171 319 to G.W.O.
Acknowledgements
We thank R. Glen Uhrig (University of Alberta, Canada) for his advice on molecular phylogenetics.
Author contributions
Conceptualization: D. S. W., B. T. W., G. W. O. Methodology: D. S. W., B. T. W., G. W. O. Validation: D. S. W. Formal analysis: D. S. W., G. W. O. Investigation: D. S. W. Resources: D. S. W., G. W. O. Data curation: D. S. W., G. W. O. Writing – original draft preparation: D. S. W. Writing – review and editing: D. S. W., G. W. O. Visualization: D. S. W., G. W. O. Supervision: G. W. O. Funding: G. W. O.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CrhR, cyanobacterial RNA helicase Redox; CsdA, cold shock DEAD-box A; DbpA, DEAD-box protein A; DEAD, Asp-Glu-Ala-Asp; NCBI, National Center for Biotechnology Information; RhlE, RNA helicase E; rimO, ribosomal protein S12 methylthiotransferase.
All supporting data and protocols have been provided within the article or through supplementary data files. One supplementary table and four supplementary figures are available with the online version of this article.
References
- 1.Herschlag D. RNA chaperones and the RNA folding problem. J Biol Chem. 1995;270:20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- 2.Woodson SA, Panja S, Santiago-Frangos A. Proteins that chaperone RNA regulation. In: Storz G, Papenfort K, editors. Regulating with RNA in Bacteria and Archaea. Washington, DC: American Society for Microbiology; 2019. pp. 385–397. [Google Scholar]
- 3.Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Jankowsky E. RNA helicases at work: binding and rearranging. Trends Biochem Sci. 2011;36:19–29. doi: 10.1016/j.tibs.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linder P, Fuller-Pace F. Happy birthday: 25 years of DEAD-box proteins. Methods Mol Biol. 2015;1259:17–33. doi: 10.1007/978-1-4939-2214-7_2. [DOI] [PubMed] [Google Scholar]
- 6.Jarmoskaite I, Russell R. RNA helicase proteins as chaperones and remodelers. Annu Rev Biochem. 2014;83:697–725. doi: 10.1146/annurev-biochem-060713-035546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder P, Jankowsky E. From unwinding to clamping – the DEAD box RNA helicase family. Nat Rev Mol Cell Biol. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- 8.Tanner NK, Linder P. DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell. 2001;8:251–262. doi: 10.1016/s1097-2765(01)00329-x. [DOI] [PubMed] [Google Scholar]
- 9.Schmid SR, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 10.Schütz P, Karlberg T, van den Berg S, Collins R, Lehtiö L, et al. Comparative structural analysis of human DEAD-box RNA helicases. PLoS One. 2010;5:e12791. doi: 10.1371/journal.pone.0012791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Jankowsky E. The DEAD-box protein Ded1 unwinds RNA duplexes by a mode distinct from translocating helicases. Nat Struct Mol Biol. 2006;13:981–986. doi: 10.1038/nsmb1165. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Putnam AA, Jankowsky E. DEAD-box helicases form nucleotide-dependent, long-lived complexes with RNA. Biochemistry. 2014;53:423–433. doi: 10.1021/bi401540q. [DOI] [PubMed] [Google Scholar]
- 14.Redder P, Hausmann S, Khemici V, Yasrebi H, Linder P. Bacterial versatility requires DEAD-box RNA helicases. FEMS Microbiol Rev. 2015;39:392–412. doi: 10.1093/femsre/fuv011. [DOI] [PubMed] [Google Scholar]
- 15.Khemici V, Linder P. RNA helicases in bacteria. Curr Opin Microbiol. 2016;30:58–66. doi: 10.1016/j.mib.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Owttrim GW. RNA helicases: diverse roles in prokaryotic response to abiotic stress. RNA Biol. 2013;10:96–110. doi: 10.4161/rna.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Ramírez V, Alcaraz LD, Moreno-Hagelsieb G, Olmedo-Álvarez G. Phylogenetic distribution and evolutionary history of bacterial DEAD-box proteins. J Mol Evol. 2011;72:413–431. doi: 10.1007/s00239-011-9441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diges CM, Uhlenbeck OC. Escherichia coli DbpA is an RNA helicase that requires hairpin 92 of 23S rRNA. EMBO J. 2001;20:5503–5512. doi: 10.1093/emboj/20.19.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kossen K, Uhlenbeck OC. Cloning and biochemical characterization of Bacillus subtilis YxiN, a DEAD protein specifically activated by 23S rRNA: delineation of a novel sub-family of bacterial DEAD proteins. Nucleic Acids Res. 1999;27:3811–3820. doi: 10.1093/nar/27.19.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicol SM, Fuller-Pace FV. The "DEAD box" protein DbpA interacts specifically with the peptidyltransferase center in 23S rRNA. Proc Natl Acad Sci USA. 1995;92:11681–11685. doi: 10.1073/pnas.92.25.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charollais J, Dreyfus M, Iost I. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 2004;32:2751–2759. doi: 10.1093/nar/gkh603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehnik-Habrink M, Rempeters L, Kovács Ákos T, Wrede C, Baierlein C, et al. DEAD-box RNA helicases in Bacillus subtilis have multiple functions and act independently from each other. J Bacteriol. 2013;195:534–544. doi: 10.1128/JB.01475-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peil L, Virumäe K, Remme J. Ribosome assembly in Escherichia coli strains lacking the RNA helicase DeaD/CsdA or DbpA. FEBS J. 2008;275:3772–3782. doi: 10.1111/j.1742-4658.2008.06523.x. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner RJ, Van Kranendonk MJ, Wacey D, Fiorentini ML, Saunders M, et al. Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life. Geology. 2019;47:1039–1043. doi: 10.1130/G46365.1. [DOI] [Google Scholar]
- 25.Beck C, Knoop H, Axmann IM, Steuer R. The diversity of cyanobacterial metabolism: genome analysis of multiple phototrophic microorganisms. BMC Genomics. 2012;13:56. doi: 10.1186/1471-2164-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soo RM, Hemp J, Parks DH, Fischer WW, Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science. 2017;355:1436–1440. doi: 10.1126/science.aal3794. [DOI] [PubMed] [Google Scholar]
- 27.Fuchsman CA, Palevsky HI, Widner B, Duffy M, Carlson MCG, et al. Cyanobacteria and cyanophage contributions to carbon and nitrogen cycling in an oligotrophic oxygen-deficient zone. ISME J. 2019;13:2714–2726. doi: 10.1038/s41396-019-0452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmour DJ. Microalgae for biofuel production. Adv Appl Microbiol. 2019;109:1–30. doi: 10.1016/bs.aambs.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Georg J. PhD thesis. Universitat Freiburg; Breisgau, Germany: 2010. A hidden layer of genetic information – regulatory non-protein-coding RNAs in Synechocystis PCC6803. [Google Scholar]
- 30.Magee WC. MSc thesis. University of Alberta; Edmonton, AB, Canada: 1997. Characterization of a cyanobacterial RNA helicase gene. [Google Scholar]
- 31.Chamot D, Magee WC, Yu E, Owttrim GW. A cold shock-induced cyanobacterial RNA helicase. J Bacteriol. 1999;181:1728–1732. doi: 10.1128/JB.181.6.1728-1732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kujat SL, Owttrim GW. Redox-regulated RNA helicase expression. Plant Physiol. 2000;124:703–714. doi: 10.1104/pp.124.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Fahmawi B, Owttrim GW. Polar-biased localization of the cold stress-induced RNA helicase, CrhC, in the cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol. 2003;50:1439–1448. doi: 10.1046/j.1365-2958.2003.03783.x. [DOI] [PubMed] [Google Scholar]
- 34.Ritter SPA, Lewis AC, Vincent SL, Lo LL, Cunha APA, et al. Evidence for convergent sensing of multiple abiotic stresses in cyanobacteria. Biochim Biophys Acta. 2020;1864:129462. doi: 10.1016/j.bbagen.2019.129462. [DOI] [PubMed] [Google Scholar]
- 35.Rosana ARR, Whitford DS, Fahlman RP, Owttrim GW. Cyanobacterial RNA helicase CrhR localizes to the thylakoid membrane region and cosediments with degradosome and polysome complexes in Synechocystis sp. strain PCC 6803. J Bacteriol. 2016;198:2089–2099. doi: 10.1128/JB.00267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosana ARR, Ventakesh M, Chamot D, Patterson-Fortin LM, Tarassova O, et al. Inactivation of a low temperature-induced RNA helicase in Synechocystis sp. PCC 6803: physiological and morphological consequences. Plant Cell Physiol. 2012;53:646–658. doi: 10.1093/pcp/pcs020. [DOI] [PubMed] [Google Scholar]
- 37.Sireesha K, Radharani B, Krishna PS, Sreedhar N, Subramanyam R, et al. RNA helicase, CrhR is indispensable for the energy redistribution and the regulation of photosystem stoichiometry at low temperature in Synechocystis sp. PCC6803. Biochim Biophys Acta. 2012;1817:1525–1536. doi: 10.1016/j.bbabio.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Stecher G, Tamura K. mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis PO. NCL: a C++ class library for interpreting data files in NEXUS format. Bioinformatics. 2003;19:2330–2331. doi: 10.1093/bioinformatics/btg319. [DOI] [PubMed] [Google Scholar]
- 41.Miller MA, Pfeiffer W, Schwartz T. Proceedings of the 2010 Gateway Computing Environments Workshop (GCE 2010) New Orleans, LA, USA: ACM; 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. [Google Scholar]
- 42.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25:1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 44.Pattengale ND, Alipour M, Bininda-Emonds ORP, Moret BME, Stamatakis A. How many bootstrap replicates are necessary? J Comput Biol. 2010;17:337–354. doi: 10.1089/cmb.2009.0179. [DOI] [PubMed] [Google Scholar]
- 45.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crooks GE, Hon G, Chandonia J-M, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn RD, Mistry J, Tate J, Coggill P, Heger A, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordin O, Tanner NK, Doère M, Linder P, Banroques J. The newly discovered Q motif of DEAD-box RNA helicases regulates RNA-binding and helicase activity. EMBO J. 2004;23:2478–2487. doi: 10.1038/sj.emboj.7600272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pause A, Méthot N, Sonenberg N. The HRIGRXXR region of the DEAD box RNA helicase eukaryotic translation initiation factor 4A is required for RNA binding and ATP hydrolysis. Mol Cell Biol. 1993;13:6789–6798. doi: 10.1128/MCB.13.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugita C, Ogata K, Shikata M, Jikuya H, Takano J, et al. Complete nucleotide sequence of the freshwater unicellular cyanobacterium Synechococcus elongatus PCC 6301 chromosome: gene content and organization. Photosynth Res. 2007;93:55–67. doi: 10.1007/s11120-006-9122-4. [DOI] [PubMed] [Google Scholar]
- 52.Moreira D, Tavera R, Benzerara K, Skouri-Panet F, Couradeau E, et al. Description of Gloeomargarita lithophora gen. nov., sp. nov., a thylakoid-bearing, basal-branching cyanobacterium with intracellular carbonates, and proposal for Gloeomargaritales ord. nov. Int J Syst Evol Microbiol. 2017;67:653–658. doi: 10.1099/ijsem.0.001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rippka R, Waterbury J, Cohen-Bazire GA. A cyanobacterium which lacks thylakoids. Arch Microbiol. 1974;100:419–436. doi: 10.1007/BF00446333. [DOI] [Google Scholar]
- 54.Rosana ARR, Whitford DS, Migur A, Steglich C, Kujat-Choy SL, et al. RNA helicase-regulated processing of the Synechocystis rimO-crhR operon results in differential cistron expression and accumulation of two sRNAs. J Biol Chem. 2020;295:6372–6386. doi: 10.1074/jbc.RA120.013148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dandekar T, Snel B, Huynen M, Bork P. Conservation of gene order: a fingerprint of proteins that physically interact. Trends Biochem Sci. 1998;23:324–328. doi: 10.1016/S0968-0004(98)01274-2. [DOI] [PubMed] [Google Scholar]
- 56.Robertson BR, Tezuka N, Watanabe MM. Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. Int J Syst Evol Microbiol. 2001;51:861–871. doi: 10.1099/00207713-51-3-861. [DOI] [PubMed] [Google Scholar]
- 57.Juteršek M, Klemenčič M, Dolinar M. Discrimination between Synechocystis members (cyanobacteria) based on heterogeneity of their 16S rRNA and ITS regions. Acta Chim Slov. 2017;64:804–817. doi: 10.17344/acsi.2017.3262. [DOI] [PubMed] [Google Scholar]
- 58.Shih PM, Wu D, Latifi A, Axen SD, Fewer DP, et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci USA. 2013;110:1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimura Y, Hirose Y, Misawa N, Wakazuki S, Fujisawa T, et al. Complete genome sequence of a coastal cyanobacterium, Synechococcus sp. strain NIES-970. Genome Announc. 2017;5:e00139–17. doi: 10.1128/genomeA.00139-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshino T, Honda T, Tanaka M, Tanaka T. Draft genome sequence of marine cyanobacterium Synechococcus sp. strain NKBG15041c. Genome Announc. 2013;1:e00954-13. doi: 10.1128/genomeA.00954-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swingley WD, Chen M, Cheung PC, Conrad AL, Dejesa LC, et al. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina . Proc Natl Acad Sci USA. 2008;105:2005–2010. doi: 10.1073/pnas.0709772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banroques J, Doère M, Dreyfus M, Linder P, Tanner NK. Motif III in superfamily 2 "helicases" helps convert the binding energy of ATP into a high-affinity RNA binding site in the yeast DEAD-box protein Ded1. J Mol Biol. 2010;396:949–966. doi: 10.1016/j.jmb.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Nelson WC, Maezato Y, Wu Y-W, Romine MF, Lindemann SR. Identification and resolution of microdiversity through metagenomic sequencing of parallel consortia. Appl Environ Microbiol. 2015;82:255–267. doi: 10.1128/AEM.02274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul R. PhD thesis. Arizona State University; Tempe, AZ, USA: 2014. Genome sequencing of Leptolyngbya Heron Island, 2a crystal structure of phycoerythrin and spectroscopic investigation of chromatic acclimation. [Google Scholar]
- 65.Georg J, Rosana ARR, Chamot D, Migur A, Hess WR, et al. Inactivation of the RNA helicase CrhR impacts a specific subset of the transcriptome in the cyanobacterium Synechocystis sp. PCC 6803. RNA Biol. 2019;16:1205–1214. doi: 10.1080/15476286.2019.1621622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosana AR, Chamot D, Owttrim GW. Autoregulation of RNA helicase expression in response to temperature stress in Synechocystis sp. PCC 6803. PLoS One. 2012;7:e48683. doi: 10.1371/journal.pone.0048683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarassova OS, Chamot D, Owttrim GW. Conditional, temperature-induced proteolytic regulation of cyanobacterial RNA helicase expression. J Bacteriol. 2014;196:1560–1568. doi: 10.1128/JB.01362-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishiyama Y, Los DA, Murata N. PsbU, a protein associated with photosystem II, is required for the acquisition of cellular thermotolerance in Synechococcus species PCC 7002. Plant Physiol. 1999;120:301–308. doi: 10.1104/pp.120.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerdes SY, Kurnasov OV, Shatalin K, Polanuyer B, Sloutsky R, et al. Comparative genomics of NAD biosynthesis in cyanobacteria. J Bacteriol. 2006;188:3012–3023. doi: 10.1128/JB.188.8.3012-3023.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li W, Schulman S, Dutton RJ, Boyd D, Beckwith J, et al. Structure of a bacterial homologue of vitamin K epoxide reductase. Nature. 2010;463:507–512. doi: 10.1038/nature08720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S, Hu Y, Overgaard MT, Karginov FV, Uhlenbeck OC, et al. The domain of the Bacillus subtilis DEAD-box helicase YxiN that is responsible for specific binding of 23S rRNA has an RNA recognition motif fold. RNA. 2006;12:959–967. doi: 10.1261/rna.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharpe Elles LM, Sykes MT, Williamson JR, Uhlenbeck OC. A dominant negative mutant of the E. coli RNA helicase DbpA blocks assembly of the 50S ribosomal subunit. Nucleic Acids Res. 2009;37:6503–6514. doi: 10.1093/nar/gkp711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones PG, Mitta M, Kim Y, Jiang W, Inouye M. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli . Proc Natl Acad Sci USA. 1996;93:76–80. doi: 10.1073/pnas.93.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butland G, Krogan NJ, Xu J, Yang W-H, Aoki H, et al. Investigating the in vivo activity of the DeaD protein using protein-protein interactions and the translational activity of structured chloramphenicol acetyltransferase mRNAs. J Cell Biochem. 2007;100:642–652. doi: 10.1002/jcb.21016. [DOI] [PubMed] [Google Scholar]
- 75.Prud'homme-Généreux A, Beran RK, Iost I, Ramey CS, Mackie GA, et al. Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a 'cold shock degradosome'. Mol Microbiol. 2004;54:1409–1421. doi: 10.1111/j.1365-2958.2004.04360.x. [DOI] [PubMed] [Google Scholar]
- 76.Vakulskas CA, Pannuri A, Cortés-Selva D, Zere TR, Ahmer BM, et al. Global effects of the DEAD-box RNA helicase DeaD (CsdA) on gene expression over a broad range of temperatures. Mol Microbiol. 2014;92:945–958. doi: 10.1111/mmi.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain C. The E. coli RhlE RNA helicase regulates the function of related RNA helicases during ribosome assembly. RNA. 2008;14:381–389. doi: 10.1261/rna.800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner S, Pryer KM, Miao VPW, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol. 1999;46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.