Abstract
Microbubbles are nanosized gas-filled bubbles. They are used in clinical diagnostics, in medical imaging, as contrast agents in ultrasound imaging, and as transporters for targeted drug delivery. They can also be used to treat thrombosis, neoplastic diseases, open arteries and vascular plaques and for localized transport of chemotherapies in cancer patients. Microbubbles can be filled with any type of therapeutics, cure agents, growth factors, extracellular vesicles, exosomes, miRNAs, and drugs. Microbubbles protect their cargo from immune attack because of their specialized encapsulated shell composed of lipid and protein. Filled with curative medicine, they could effectively circulate through the whole body safely and efficiently to reach the target area. The advanced bubble-based drug-delivery system, integrated with artificial intelligence for guidance, holds great promise for the targeted delivery of drugs and medicines.
Keywords: Artificial intelligence, Microbubbles, Nano-vesicles, Drug transportation, Targeted therapies
Introduction
Many drugs and medicines are susceptible to degradation, which makes it challenging to formulate them and deliver them to specific targets [1, 2]. The chemistry of these drugs makes the process even more challenging because it can lead to nonspecific side effects, interrupt normal physiology of intracellular receptors, damage healthy tissues, and result in unguided delivery [2–4]. Additionally, these drugs have reduced permeation across biological barriers, affinity towards unspecific sites, and a tendency to unload chemicals to multiple healthy targets [3]. To overcome these shortcomings, microbubbles deliver their cargo to molecular sites of disease while being tracked in real time by the latest simulation of artificial intelligence [3, 5, 6].
AI could potentially enhance the effectiveness of microbubble technology [6–8]. Bubbles guided by AI, and the medicine they encapsulate, have the power to improve the visualization of cardiac disease, which will give new life to the field of echocardiography or focused ultrasound imaging of the heart [9–12]. They are routinely used to evaluate myocardial perfusion and heart function and in kidney dialysis [11, 12]. Clinically, microbubbles are established for routine screening of a range of diseases, including cancer, cancerous lesions, inflammatory processes, cardiovascular pathologies, and diseases associated with aging, Table 1 [12–15].
Table 1.
Role of microbubbles in different diseases and in different clinical trials
| S. No | Name | Type | Disease type | Role | Technology | Clinical trials |
|---|---|---|---|---|---|---|
| 1 | The Role of Different Imaging Methods in the Diagnosis of Gallbladder Polyps | Interventional | Polyp of Gallbladder | Preoperative differential diagnostic accuracies of gallbladder polypoid lesions |
Device: Abdominal high-resolution CT Device: Contrast-enhanced ultrasonography Procedure: Cholecystectomy |
NCT02762227 |
| 2 | Novel MRI-Guided Ultrasound Stimulated Microbubble Radiation Treatment for Patients with Chest-wall and Locally-Advanced Breast Cancer | Interventional | Breast Cancer | Novel MRI-guided ultrasound stimulated microbubble treatment enhances radiation effects in humans receiving external beam radiotherapy delivered using radiation therapy device | Patients with locally advanced breast cancer and chest wall tumours will receive MRI-guided ultrasound-stimulated microbubble-treatment combined with radiotherapy | NCT04431674 |
| 3 | The Use of Focused Ultrasound and Microbubble Infusion for Altering Brain Perfusion and the Blood Brain Barrier | Interventional | Low Grade Glioma of Brain | The ability of focused ultrasound combined with microbubbles to open the blood brain barrier has the potential to revolutionize the delivery of therapeutic agents to the brain, allowing for more localized and efficient delivery | The ultrasound treatment will last either 1 h or 20 min total time for the DWL device. An infusion of Definity microbubbles will be infused intravenously over ten to thirty minutes as per routine approved application. Definity will be 1.3 mL added to 50 mL saline and infused no faster than 4 mL/minute | NCT04063514 |
| 4 | Microbubbles and Ultrasound in Stroke Trial | Interventional | Acute Ischemic Stroke | Transcranial 2-MHz ultrasound combined with intravenous administration of microbubbles improves early recanalization in patients with acute ischemic stroke caused by middle cerebral artery proximal occlusion treated with intravenous alteplase within 3 h of symptom onset |
Radiation: Ultrasound 2-MHz, low intensity transcranial ultrasound Drug: Levovist D-Galactose and palmitic palmitique intravenous 4 g |
NCT00222040 |
| 5 | Microbubble Cavitation for Improving Hepatocellular Carcinoma Radioembolization | Interventional |
Hepatocellular Carcinoma Liver Cancer |
Localized microbubble cavitation triggered by noninvasive ultrasound has been shown to sensitize malignant tissue to radiotherapy by inducing vascular endothelial cell apoptosis | Patients receive perflutren protein-type A microspheres IV over 10 min and undergo CEUS over 60 min at 1–6 h post radioembolization and at approximately 7 and 14 days after yttrium Y-90 radioembolization | NCT03199274 |
| 6 | Targeted Delivery of Chemotherapy with Ultrasound and Microbubbles (SONCHIMIO) | Interventional |
Colorectal Cancer Hepatic Metastases |
The oscillations of ultrasound (US) contrast agent microbubbles under their activation by US waves engender a modulation of the permeability of biological barriers amplifying hence the extravasation of drugs/fluorescent markers through a process known as sonoporation | Liver metastases randomized to receive sonoporation (US waves + gaseous microbubbles). The patient continues to receive the usual systemic chemotherapy | NCT03458975 |
Molecular imaging is an advanced way for decoding the biological processes to visualize and reveal the cellular events at molecular level [16, 17]. For example, quantum dots are photostable for longer duration and enhance the imaging of deep tissues [17]. Similarly, the fluorescence imaging with indocyanine green based system is valuable for monitoring of surgical procedures [17, 18]. Magnetic resonance imaging is clinically applied to visualize and expose the structural and pathological changes [19, 20]. Combination of different nanoparticles and contrast agents makes the field of molecular imaging more appealing for clinical applications [21–23]. The process of molecular imaging can be clinically improved by designing and engineering of new class of contrast agents, which are more sensitive, targeted, non-toxic and precise for molecular identification [24–26]. Such as monoclonal antibodies, nanoprobes, quantum dots, molecular dyes, and other targeting signatures are routinely attached to the surface of microbubble and extracellular vesicles for clinical monitoring of treatment process [5, 14, 21, 22, 26, 27]. Therapeutic drugs and medicines include growth factors, antibodies, peptides and recombinant proteins; microbubbles increase the effectiveness, specificity, and potency of these therapeutics [28–30].
The role of guided microbubbles in different diseases
Conventional medicine are known for their shortcomings such as toxicity to healthy tissues, not very specific to the targets, do not have the ability to cross the blood brain barrier, and have other side effects which restrict their applications [9, 31, 32]. New technologies have significantly improved the perspective of precision medicine [33–35]. The smart approach of using microbubbles loaded with degradable loaded drugs have the potential to deliver the medicine precisely to the targets where it is needed [36, 37]. Precision medicine (such as guided microbubbles) use the high throughput knowledge and artificial intelligence to enhance the process of clinical diagnosis and treatment [38, 39]. Moreover, the precision approach can also be used for preventive and surveillance measures of diseases. treatment efficiency and real time monitoring of drugs [39, 40]. Microbubbles loaded with drug are guided carriers that deliver medicine to targeted sites [2, 30, 41]. These bubbles increase the localized drug concentration at the site of disease, and mimicking the toxicity and unwanted delivery to healthy tissues and surrounding microenvironment [41, 42]. Ultrasound-guided microbubbles are routinely used in the treatment of many diseases’ such as cardiovascular disorders including thrombolysis, in the clinical imaging of tumor sites, and diagnosis of cancer and therapeutics [42–44]. Studies have shown that microbubbles loaded with drugs (such as growth factors, precision medicine, tissue plasminogen activator (tPA), regenerative molecules, and imaging probes) have improved the clinical outcome in different diseases [45, 46]. Microbubbles loaded with tPA successfully dissolved blood clots precisely at the tissue target sites, and bubbles loaded with regenerative cargo improved the healing effects in tissues repair process [42–44, 47]. Technology-guided microbubbles are well studied for targeted release of drugs at inflammatory and tumor sites Fig. 1 [3, 48]. Monoclonal antibodies, cytokines, tumor inhibitors, chimeric antigen receptor T cells therapies, and clinical chemotherapeutic drugs (for example, 5-fluorouracil and doxycycline) have successfully been loaded into microbubbles and applied in the treatment of neck, breast, pancreatic, ovarian, and hepatocellular carcinoma [49–51]. Ultrasound-targeted microbubbles have already been validated as an effective method for delivering microRNAs to tumor sites in clinical treatment of human malignancies [38, 49].
Fig. 1.
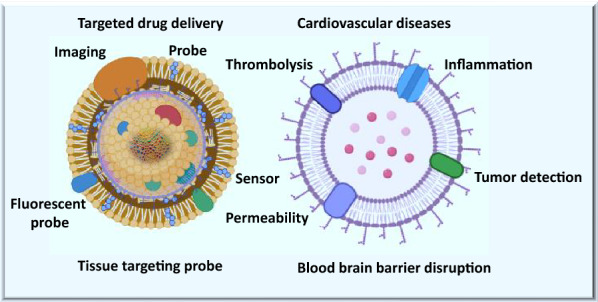
Role of guided microbubbles in drug delivery and imaging and in different diseases
AI could be used for medical imaging, patient monitoring, and for the targeted release of drugs at the damaged sites. It will enhance the therapeutic efficiency by increasing the localized drug concentration at diseased sites [8]. Microbubbles can cross the blood–brain barrier, meaning drugs can reach any brain cell in a targeted manner [8]. Microbubbles loaded with glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) have been shown to specifically accelerate the cell survival of dopaminergic neurons and protect neurons in treatment of many diseases, such as stroke, Alzheimer’s, Parkinson’s disease, seizure disorders, brain or spinal injuries, and other neurological disorders [8, 15, 44, 52–54]. Ultrasound has the potential for activation of drug release at targeted regions, and has the ability of precise-imaging to identify the diseased sites, thus enhancing the implications of microbubbles in treatment of different diseases [44, 55]. This technology includes the potential to monitor the drug and treatment response in real time, which increases the effectiveness of this approach Figs. 1, 2 and 3.
Fig. 2.
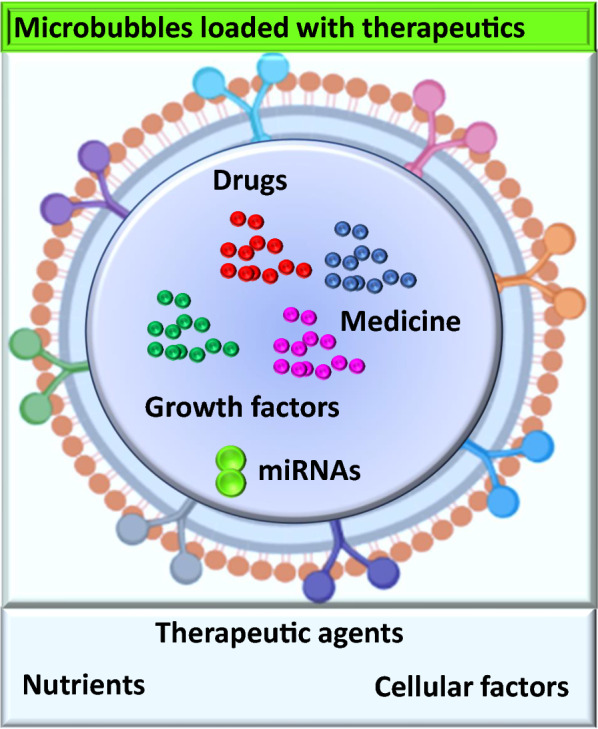
Microbubbles and loaded therapeutic cargo
Fig. 3.
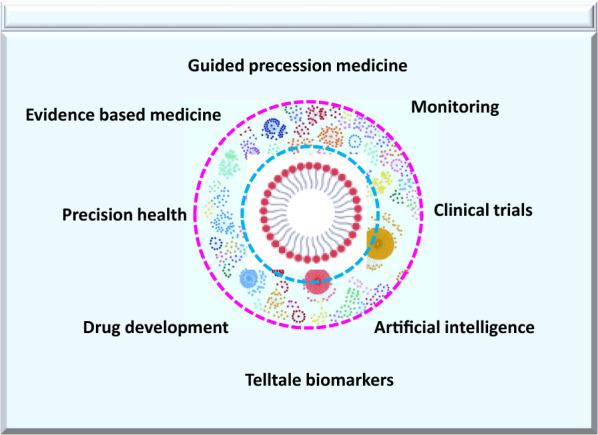
Guided microbubbles and technology
Technology and microbubbles
The technology behind the smart design of microbubbles has attracted great attention due to its wide application in many fields of science and technology [21, 56]. The nano-sized microbubbles are negatively charged [56, 57]. Particles with positive charges, known as free radicals, engulf electrons from healthy cells to neutralize their own charge, causing cellular damage [15, 58, 59]. In contrast, negatively charged bubbles fight free radicals to improve the health of damaged cells and detoxify the inflammatory fluids in diseased tissue [15, 60].
Microbubbles newly designed through biomedical engineering and nanomaterials approaches are crucial for intracellular delivery of proteins, drugs, growth factors, and peptides, Fig. 2 [9, 30, 42]. They may revolutionize the whole biopharmaceutical drug industry [14, 58]. Although technological breakthroughs have been made in the development, monitoring, and tracking of drugs by artificial intelligence and in the delivery of biopharmaceutical drugs, challenges and unanswered questions remain [61]. The medicine in the bubbles can target both extracellular and intracellular targets and guide the localized drug delivery to specific sites [61].
Medicine and the machine
Artificial intelligence (AI)–based technologies have the potential to transform the healthcare industry by deriving innovative approaches to the discovery of drugs, Fig. 3 [5, 61]. Examples of innovation through AI range from self-driving cars to pattern- and image-recognition tools to clinical diagnostics that allow expedited drug discovery, earlier detection of disease, more precise diagnosis, identification of new biomarkers, and development of personalized diagnostics and therapeutics [6, 7, 10, 62]. AI has the power to treat, diagnose, cure, mitigate, or prevent disease or other critical or serious conditions [29, 61, 63]. Recent studies have shown that AI can expedite diabetic retinopathy and eye scan [29, 61, 63, 64]. AI has incredible pattern-recognizing abilities within big data and thus holds the potential to solve many key clinical challenges [64]. Leveraging AI with microbubble technology may expedite and enhance early detection of disease and patient care [5, 62].
Medicine in microbubbles and the blood–brain barrier
The blood–brain barrier (BBB) is responsible for protection against circulating toxins, preventing harmful pathogens from entering the brain [44, 65]. The defensive wall of the BBB prevents brain infections, but it also blocks medicines that could treat brain diseases, neurological disorders, and neurodegenerative diseases [44, 65, 66]. This protective wall presents an obstacle for most of the available drugs in the market [66, 67]. Medicine in microbubbles, in contrast, can reach and open the BBB to target the disease site effectively instead of circulating randomly in the system [67, 68]. The brain is the only organ known to have its own security system; however, medicine in the bubbles breaks the defensive wall of the BBB and allows lifesaving drugs to reach their targets to repair the injured or diseased brain [68]. Usually drugs are chemicals, and the brain senses these harsh molecules and blocks its defensive door using the BBB; however, medicines in bubbles are difficult to interpret as chemicals or dangerous enemies as they are encapsulated in a shell, Figs. 1, 2 and 3 [15, 53, 66, 68].
Conclusion
Microbubbles have the potential to protect their cargo from degradation, restrict the drug release to disease sites, and prevent nonspecific drug delivery to healthy tissues [69, 70]. Medicine in bubbles enhances targeted drug delivery, tumor targeting, ultrasound imaging, and intracellular drug release [7, 71]. These microbubbles can be used for the delivery of oxygen in stroke patients, and delivery of immune cells in those patients who has weak immune system [54, 72, 73]. AI-powered capabilities, including data integration and interpretation, are fundamental for clinical transformation of microbubbles to enhance treatment efficacy [7, 38]. Leveraging technology will enhance the ability of microbubbles and extracellular vesicles for oxygen release to energizes cells and stimulates the immune system against different diseases [74, 75]. Ultrasound guided microbubbles can be used for opening blocked arteries, for increasing the permeability of blood brain barrier and drug delivery to those tissues which are otherwise difficult target for conventional drug delivery [76–78].
The delivery of medicine in bubbles has some limitations, such as undesired shell cracking due to acoustic pressure, limited capacity for drug loading and cavitation in the ultrasound field [30, 61]. The biostability of microbubbles is poor in some organs, less biocompatible, structurally unstable, and limited circulation time in certain tissues. Sometimes these bubbles have difficulty reaching deep and hard ossified tissues [30, 38]. The safety, ethics, effectiveness, and functionality of the process should be considered to improve the development of next generation of microbubbles with innovative engineering approaches to enhance the drug loading capacity of bubbles [21, 79–81]. Designing of bubbles for precise imaging should revolutionize the field of molecular imaging and precision medicine for treatment of cancer, aging, cardiovascular and neurological diseases [13, 30, 82].
Microbubbles are inert, nonreactive vesicles which makes them ideal for molecular imaging, bypassing microcirculation, and ideal cargo for conventionally challenged targets [83–85]. Although there are still some challenges in the clinical translation, but scientists are expecting that newly designed microbubbles can be leveraged to AI methods and techniques [86, 87]. These emerging bubbles can be used to train neural networks and other tissues and to monitor drugs for real time imaging and precise treatment [87–89]. Microbubbles with integrated molecular sensor-probes have the ability to distinguish contrast agents and differentiate healthy versus diseased sites [90, 91]. The technological platform of microbubbles applications should be upgraded with AI integration for more safer, and real-time tracking of drugs in clinical translation [92]. Further research of guided microbubbles is needed to explore the field drug delivery. The field of bioengineering for designing smart microbubbles will revolutionize this technology further, which has already been shown its role in real time molecular imaging and precise treatment of different diseases such as cardiovascular and neurological disorders [93, 94]. The integration of AI and other technologies in the field of microbubbles will accelerate the development of strategies for detection, prevention, diagnosis, treatment, and cure.
Acknowledgements
The schematic representations were designed in the Adobe Illustrator. We also used the support of ChemDraw (PerkinElmer Informatics) and Biorender (©BioRender—biorender.com) for combination figures.
Abbreviations
- EVs
Extracellular vesicles
- BDNF
Brain-derived neurotrophic factor
- GDNF
Glial cell line-derived neurotrophic factor
- BBB
Blood–brain barrier
Authors’ contributions
MU: Conceptualization, data mining, coordinated the project and wrote the paper, MU, NP, AA, SJ: Data analysis and writing of review manuscript. MU, NP, SJ, and AA: Conceptualization, manuscript review, editing suggestions, and final approval. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The data is original for this article and the sources used have been cited both within the article and within the Figures.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 2.Blessy M, Patel RD, Prajapati PN, Agrawal YK. Development of forced degradation and stability indicating studies of drugs—a review. J Pharm Anal. 2014;4(3):159–165. doi: 10.1016/j.jpha.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choonara BF, Choonara YE, Kumar P, Bijukumar D, du Toit LC, Pillay V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol Adv. 2014;32(7):1269–1282. doi: 10.1016/j.biotechadv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Ullah M. The Pandemic of Novel Coronavirus Disease 2019 (COVID-19): need for an immediate action. Open Access J Biomed Sci. 2020 doi: 10.38125/OAJBS.000168. [DOI] [Google Scholar]
- 5.Dilsizian SE, Siegel EL. Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep. 2014;16(1):441. doi: 10.1007/s11886-013-0441-8. [DOI] [PubMed] [Google Scholar]
- 6.Ullah M, Akbar A, Yannarelli G. Applications of artificial intelligence in, early detection of cancer, clinical diagnosis and personalized medicine. Artificial Intelligence Cancer. 2020;1(2):39–44. [Google Scholar]
- 7.Jin X, Liu C, Xu T, Su L, Zhang X. Artificial intelligence biosensors: Challenges and prospects. Biosens Bioelectron. 2020;165:112412. doi: 10.1016/j.bios.2020.112412. [DOI] [PubMed] [Google Scholar]
- 8.Toccaceli G, Barbagallo G, Peschillo S. Low-intensity focused ultrasound for the treatment of brain diseases: safety and feasibility. Theranostics. 2019;9(2):537–539. doi: 10.7150/thno.31765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Rodríguez J, Sevilla A, Martínez-Bazán C, Gordillo JM. Generation of microbubbles with applications to industry and medicine. Annu Rev Fluid Mech. 2015;47:405–429. doi: 10.1146/annurev-fluid-010814-014658. [DOI] [Google Scholar]
- 10.Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Lindner JR. Therapeutic contrast echocardiography: bubbles become medicine. In: American College of Cardiology Foundation Washington, DC; 2019. [DOI] [PubMed]
- 12.Ling ZY, Shu SY, Zhong SG, Luo J, Su L, Liu ZZ, Lan XB, Yuan GB, Zheng YY, Ran HT, et al. Ultrasound targeted microbubble destruction promotes angiogenesis and heart function by inducing myocardial microenvironment change. Ultrasound Med Biol. 2013;39(11):2001–2010. doi: 10.1016/j.ultrasmedbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Postema M, Schmitz G. Ultrasonic bubbles in medicine: influence of the shell. Ultrason Sonochem. 2007;14(4):438–444. doi: 10.1016/j.ultsonch.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, Lutz AM, Chen IY, Chen X, Gambhir SS. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246(2):508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung YS, Marquet F, Teichert T, Ferrera V, Konofagou EE. Feasibility of noninvasive cavitation-guided blood-brain barrier opening using focused ultrasound and microbubbles in nonhuman primates. Appl Phys Letters. 2011;98(16):163704. doi: 10.1063/1.3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullah M, Kodam SP, Mu Q, Akbar A. Microbubbles versus extracellular vesicles as therapeutic cargo for targeting drug delivery. ACS Nano. 2021;15(3):3612–3620. doi: 10.1021/acsnano.0c10689. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Guo B, Rao Z, Zhang B, Gong JR. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013;13(6):2436–2441. doi: 10.1021/nl400368v. [DOI] [PubMed] [Google Scholar]
- 18.Terasawa M, Ishizawa T, Mise Y, Inoue Y, Ito H, Takahashi Y, Saiura A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surv Methodol. 2017;31(12):5111–5118. doi: 10.1007/s00464-017-5576-z. [DOI] [PubMed] [Google Scholar]
- 19.Alhadlaq HA, Xia Y, Moody JB, Matyas JR. Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarised light microscopy. Ann Rheum Dis. 2004;63(6):709–717. doi: 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris MF, Verma DR, Sheikh H, Su W, Pershad A. Outcomes after magnetic resonance imaging in patients with pacemakers and defibrillators and abandoned leads. Cardiovasc Revasc Med. 2018;19(6):685–688. doi: 10.1016/j.carrev.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Hettiarachchi K, Talu E, Longo ML, Dayton PA, Lee AP. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip. 2007;7(4):463–468. doi: 10.1039/b701481n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology. 2010;257(1):24–39. doi: 10.1148/radiol.10091210. [DOI] [PubMed] [Google Scholar]
- 23.Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal Bioanal Chem. 2011;399(1):3–27. doi: 10.1007/s00216-010-4207-5. [DOI] [PubMed] [Google Scholar]
- 24.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92(2):897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 25.Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, Cunningham VJ, Grasby PM. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33(7):1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissleder R. Molecular imaging: exploring the next frontier. Radiology. 1999;212(3):609–614. doi: 10.1148/radiology.212.3.r99se18609. [DOI] [PubMed] [Google Scholar]
- 27.Wong FC, Kim EE. A review of molecular imaging studies reaching the clinical stage. Eur J Radiol. 2009;70(2):205–211. doi: 10.1016/j.ejrad.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Ullah M, Qiao Y, Concepcion W, Thakor AS. Stem cell-derived extracellular vesicles: role in oncogenic processes, bioengineering potential, and technical challenges. Stem Cell Res Ther. 2019;10(1):347. doi: 10.1186/s13287-019-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng R, Yu F, Xu J, Hu X. Knowledge gaps in immune response and immunotherapy involving nanomaterials: databases and artificial intelligence for material design. Biomaterials. 2021;266:120469. doi: 10.1016/j.biomaterials.2020.120469. [DOI] [PubMed] [Google Scholar]
- 30.Chang S, Si T, Zhang S, Merrick MA, Cohn DE, Xu RX. Ultrasound mediated destruction of multifunctional microbubbles for image guided delivery of oxygen and drugs. Ultrason Sonochem. 2016;28:31–38. doi: 10.1016/j.ultsonch.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;2(2):123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- 32.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol Imag Biol. 2003;5(6):376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Ghasemi M, Nabipour I, Omrani A, Alipour Z, Assadi M. Precision medicine and molecular imaging: new targeted approaches toward cancer therapeutic and diagnosis. Am J Nucl Med Mol Imaging. 2016;6(6):310–327. [PMC free article] [PubMed] [Google Scholar]
- 34.Penet MF, Krishnamachary B, Chen Z, Jin J, Bhujwalla ZM. Molecular imaging of the tumor microenvironment for precision medicine and theranostics. Adv Cancer Res. 2014;124:235–256. doi: 10.1016/B978-0-12-411638-2.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullah M, Akbar A. Clinical relevance of RNA editing to early detection of cancer in human. Int J Stem Cell Res Ther. 2020;7(1):66. doi: 10.23937/2469-570x/1410066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiordelisi MF, Auletta L, Meomartino L, Basso L, Fatone G, Salvatore M, Mancini M, Greco A. Preclinical molecular imaging for precision medicine in breast cancer mouse models. Contrast Media Mol Imaging. 2019;2019:8946729. doi: 10.1155/2019/8946729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48(4):260–270. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo S, Takagi K, Nishida M, Iwai T, Kudo Y, Ogawa K, Kamiyama T, Shibuya H, Kahata K, Shimizu C. Computer-aided diagnosis of focal liver lesions using contrast-enhanced ultrasonography with perflubutane microbubbles. IEEE Trans Med Imaging. 2017;36(7):1427–1437. doi: 10.1109/TMI.2017.2659734. [DOI] [PubMed] [Google Scholar]
- 39.Zhu JQ, Rowland EM, Harput S, Riemer K, Leow CH, Clark B, Cox K, Lim A, Christensen-Jeffries K, Zhang G, et al. 3D Super-resolution US imaging of rabbit lymph node vasculature in vivo by using microbubbles. Radiology. 2019;291(3):642–650. doi: 10.1148/radiol.2019182593. [DOI] [PubMed] [Google Scholar]
- 40.de Senneville BD, Frulio N, Laumonier H, Salut C, Lafitte L, Trillaud H. Liver contrast-enhanced sonography: computer-assisted differentiation between focal nodular hyperplasia and inflammatory hepatocellular adenoma by reference to microbubble transport patterns. Eur Radiol. 2020;30(5):2995–3003. doi: 10.1007/s00330-019-06566-1. [DOI] [PubMed] [Google Scholar]
- 41.Sirsi SR, Hernandez SL, Zielinski L, Blomback H, Koubaa A, Synder M, Homma S, Kandel JJ, Yamashiro DJ, Borden MA. Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors. J Control Release. 2012;157(2):224–234. doi: 10.1016/j.jconrel.2011.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Saint VM, Crake C, Coussios CC, Stride E. Properties, characteristics and applications of microbubbles for sonothrombolysis. Expert Opin Drug Deliv. 2014;11(2):187–209. doi: 10.1517/17425247.2014.868434. [DOI] [PubMed] [Google Scholar]
- 43.Tsivgoulis G, Culp WC, Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics. 2008;48(4):303–311. doi: 10.1016/j.ultras.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Hynynen K, Clement G. Clinical applications of focused ultrasound-the brain. Int J Hyperthermia. 2007;23(2):193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 45.Yan WC, Chua QW, Ong XJ, Sharma VK, Tong YW, Wang CH. Fabrication of ultrasound-responsive microbubbles via coaxial electrohydrodynamic atomization for triggered release of tPA. J Colloid Interface Sci. 2017;501:282–293. doi: 10.1016/j.jcis.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Xie F, Lof J, Sayyed S, Porter TR. Utilization of modified diagnostic ultrasound and microbubbles to reduce myocardial infarct size. Heart. 2015;101(18):1468–1474. doi: 10.1136/heartjnl-2015-307625. [DOI] [PubMed] [Google Scholar]
- 47.Hua X, Zhou L, Liu P, He Y, Tan K, Chen Q, Gao Y, Gao Y. In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J Thromb Thrombolysis. 2014;38(1):57–64. doi: 10.1007/s11239-014-1071-8. [DOI] [PubMed] [Google Scholar]
- 48.Escoffre J-M, Deckers R, Bos C, Moonen C. Bubble-assisted ultrasound: application in immunotherapy and vaccination. In: Therapeutic Ultrasound. Springer; 2016, p. 243–61. [DOI] [PubMed]
- 49.Abed Z, Khoei S, Ghalandari B, Beik J, Shakeri-Zadeh A, Ghaznavi H, Shiran MB. The measurement and mathematical analysis of 5-Fu release from magnetic polymeric nanocapsules, following the application of ultrasound. Anticancer Agents Med Chem. 2018;18(3):438–449. doi: 10.2174/1871520617666170921124951. [DOI] [PubMed] [Google Scholar]
- 50.Ullah M, Akbar A, Ng NN, Concepcion W, Thakor AS. Mesenchymal stem cells confer chemoresistance in breast cancer via a CD9 dependent mechanism. Oncotarget. 2019;10(37):3435–3450. doi: 10.18632/oncotarget.26952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ullah M, Akbar A, Thakor AS. An emerging role of CD9 in stemness and chemoresistance. Oncotarget. 2019;10(40):4000–4001. doi: 10.18632/oncotarget.27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess MT, Apostolakis I, Konofagou EE. Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles. Phys Med Biol. 2018;63(6):065009. doi: 10.1088/1361-6560/aab05c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipsman N, Meng Y, Bethune AJ, Huang YX, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1):1–8. doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Frutos B, Otero-Ortega L, Ramos-Cejudo J, Martinez-Sanchez P, Barahona-Sanz I, Navarro-Hernanz T, Gomez-de FrutosMdel C, Diez-Tejedor E, Gutierrez-Fernandez M. Enhanced brain-derived neurotrophic factor delivery by ultrasound and microbubbles promotes white matter repair after stroke. Biomaterials. 2016;100:41–52. doi: 10.1016/j.biomaterials.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 55.Huynh E, Lovell JF, Helfield BL, Jeon M, Kim C, Goertz DE, Wilson BC, Zheng G. Porphyrin shell microbubbles with intrinsic ultrasound and photoacoustic properties. J Am Chem Soc. 2012;134(40):16464–16467. doi: 10.1021/ja305988f. [DOI] [PubMed] [Google Scholar]
- 56.Lin H, Chen J, Chen C. A novel technology: microfluidic devices for microbubble ultrasound contrast agent generation. Med Biol Eng Comput. 2016;54(9):1317–1330. doi: 10.1007/s11517-016-1475-z. [DOI] [PubMed] [Google Scholar]
- 57.Delalande A, Postema M, Mignet N, Midoux P, Pichon C. Ultrasound and microbubble-assisted gene delivery: recent advances and ongoing challenges. Ther Deliv. 2012;3(10):1199–1215. doi: 10.4155/tde.12.100. [DOI] [PubMed] [Google Scholar]
- 58.Abdalkader R, Kawakami S, Unga J, Suzuki R, Maruyama K, Yamashita F, Hashida M. Evaluation of the potential of doxorubicin loaded microbubbles as a theranostic modality using a murine tumor model. Acta Biomater. 2015;19:112–118. doi: 10.1016/j.actbio.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Chertok B, Langer R. Circulating magnetic microbubbles for localized real-time control of drug delivery by ultrasonography-guided magnetic targeting and ultrasound. Theranostics. 2018;8(2):341–357. doi: 10.7150/thno.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullah M. Novel Coronavirus (COVID-19) treatment options. Biomed J Sci Tech Res. 2020;27(3):20872–20874. [Google Scholar]
- 61.Fleming N. How artificial intelligence is changing drug discovery. Nature. 2018;557(7706):S55–S55. doi: 10.1038/d41586-018-05267-x. [DOI] [PubMed] [Google Scholar]
- 62.Zhu H. Big data and artificial intelligence modeling for drug discovery. Annu Rev Pharmacol Toxicol. 2020;60:573–589. doi: 10.1146/annurev-pharmtox-010919-023324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassanzadeh P, Atyabi F, Dinarvand R. The significance of artificial intelligence in drug delivery system design. Adv Drug Deliv Rev. 2019;151–152:169–190. doi: 10.1016/j.addr.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Winkler-Schwartz A, Bissonnette V, Mirchi N, Ponnudurai N, Yilmaz R, Ledwos N, Siyar S, Azarnoush H, Karlik B, Del Maestro RF. Artificial intelligence in medical education: best practices using machine learning to assess surgical expertise in virtual reality simulation. J Surg Educ. 2019;76(6):1681–1690. doi: 10.1016/j.jsurg.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB) J Microencapsul. 2013;30(1):49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- 67.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Can Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tung YS, Vlachos F, Feshitan JA, Borden MA, Konofagou EE. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am. 2011;130(5):3059–3067. doi: 10.1121/1.3646905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pillalamarri N, Ren G, Khan L, Ullah A, Jonnakuti S, Ullah M. Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage. Transl Oncol. 2021;14(7):101095. doi: 10.1016/j.tranon.2021.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Y, Zhang B, Chen Y, Jin Q, Wu J, Yan F, Zheng H. Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl Mater Interfaces. 2017;9(25):21190–21199. doi: 10.1021/acsami.7b05346. [DOI] [PubMed] [Google Scholar]
- 71.Park JI, Jagadeesan D, Williams R, Oakden W, Chung S, Stanisz GJ, Kumacheva E. Microbubbles loaded with nanoparticles: a route to multiple imaging modalities. ACS Nano. 2010;4(11):6579–6586. doi: 10.1021/nn102248g. [DOI] [PubMed] [Google Scholar]
- 72.Unger E, Porter T, Lindner J, Grayburn P. Cardiovascular drug delivery with ultrasound and microbubbles. Adv Drug Deliv Rev. 2014;72:110–126. doi: 10.1016/j.addr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Feshitan JA, Legband ND, Borden MA, Terry BS. Systemic oxygen delivery by peritoneal perfusion of oxygen microbubbles. Biomaterials. 2014;35(9):2600–2606. doi: 10.1016/j.biomaterials.2013.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meairs S, Alonso A. Ultrasound, microbubbles and the blood-brain barrier. Prog Biophys Mol Biol. 2007;93(1–3):354–362. doi: 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 75.Kwan JJ, Kaya M, Borden MA, Dayton PA. Theranostic oxygen delivery using ultrasound and microbubbles. Theranostics. 2012;2(12):1174–1184. doi: 10.7150/thno.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, Coussios CC, Borden M, Nomikou N, McHale AP, et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Control Release. 2015;203:51–56. doi: 10.1016/j.jconrel.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal K, Chatterjee D. The role of encapsulated microbubbles in the diagnosis of stenosis in arteries. In: Journal of Physics: Conference Series: 2015. IOP Publishing: 012002.
- 78.Zhou XB, Qin H, Li J, Wang B, Wang CB, Liu YM, Jia XD, Shi N. Platelet-targeted microbubbles inhibit re-occlusion after thrombolysis with transcutaneous ultrasound and microbubbles. Ultrasonics. 2011;51(3):270–274. doi: 10.1016/j.ultras.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Li MD, Bussonniere A, Bronson M, Xu ZH, Liu QX. Study of Venturi tube geometry on the hydrodynamic cavitation for the generation of microbubbles. Miner Eng. 2019;132:268–274. doi: 10.1016/j.mineng.2018.11.001. [DOI] [Google Scholar]
- 80.Das D, Sivasubramanian K, Yang C, Pramanik M. On-chip generation of microbubbles in photoacoustic contrast agents for dual modal ultrasound/photoacoustic in vivo animal imaging. Sci Rep. 2018;8(1):6401. doi: 10.1038/s41598-018-24713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zandi A, Khayamian MA, Saghafi M, Shalileh S, Katebi P, Assadi S, Gilani A, SalemizadehParizi M, Vanaei S, Esmailinejad MR, et al. Microneedle-based generation of microbubbles in cancer tumors to improve ultrasound-assisted drug delivery. Adv Healthc Mater. 2019;8(17):e1900613. doi: 10.1002/adhm.201900613. [DOI] [PubMed] [Google Scholar]
- 82.Feng R, Ullah M, Chen K, Ali Q, Lin Y, Lei H, Sun ZJ. Inducible pluripotent stem cell-derived extracellular vesicles mitigates aging-associated arterial stiffness and hypertension. Circulation. 2017;136(suppl_1):A17570–A17570. doi: 10.1080/20013078.2020.1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang LW, Hou ML, Hung SH, Lin LC, Tsai TH. Pharmacokinetics of quercetin-loaded nanodroplets with ultrasound activation and their use for bioimaging. Int J Nanomed. 2015;10:3031–3042. doi: 10.2147/IJN.S78983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.López-Marín LM, Rivera AL, Fernández F, Loske AM. Shock wave-induced permeabilization of mammalian cells. Phys Life Rev. 2018;26:1–38. doi: 10.1016/j.plrev.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Thakur A, Foged C. Nanoparticles for mucosal vaccine delivery. Nanoeng Biomater Adv Drug Deliv. 2020 doi: 10.1016/B978-0-08-102985-5.00025-5. [DOI] [Google Scholar]
- 86.Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4(4):432–444. doi: 10.7150/thno.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi JJ, Selert K, Vlachos F, Wong A, Konofagou EE. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci USA. 2011;108(40):16539–16544. doi: 10.1073/pnas.1105116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui Z, Li D, Feng Y, Xu T, Wu S, Li Y, Bouakaz A, Wan M, Zhang S. Enhanced neuronal activity in mouse motor cortex with microbubbles' oscillations by transcranial focused ultrasound stimulation. Ultrason Sonochem. 2019;59:104745. doi: 10.1016/j.ultsonch.2019.104745. [DOI] [PubMed] [Google Scholar]
- 89.Scarcelli T, Jordao JF, O'Reilly MA, Ellens N, Hynynen K, Aubert I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7(2):304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li F, Li DY, Yan F. Improvement of detection sensitivity of microbubbles as sensors to detect ambient pressure. Sensors. 2018;18(12):4083. doi: 10.3390/s18124083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Y, Ward J, Chormaic SN. Quasi-droplet microbubbles for high resolution sensing applications. Opt Express. 2014;22(6):6881–6898. doi: 10.1364/OE.22.006881. [DOI] [PubMed] [Google Scholar]
- 92.Kogan P, Gessner RC, Dayton PA. Microbubbles in imaging: applications beyond ultrasound. Bubble Sci Eng Technol. 2010;2(1):3–8. doi: 10.1179/175889610X12730566149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ullah M, Akbar A, Yannarelli G. Clinical applications of RNA editing technology for the early detection of cancer and future directions. Technol Cancer Res Treat. 2020;19:1533033820964194. doi: 10.1177/1533033820964194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullah M, Qian NPM, Yannarelli G. Advances in innovative exosome-technology for real time monitoring of viable drugs in clinical translation prognosis and treatment response. Oncotarget. 2021;12(11):1029–1031. doi: 10.18632/oncotarget.27927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


