Version Changes
Revised. Amendments from Version 1
We would like to thank the three Reviewers for their insightful feedback. The authors have carefully considered all comments made by these Reviewers, and made necessary amendments to the article. Fully detailed responses to each of these comments can be found under the 'Reviewer Reports' section. In general, the manuscript has undergone several amendments including improvements in phrasing/terminology, clarity in potential limitations, and the addition of one more RCT in the systematic review portion of the article. Figures 1 and 7 have also been updated to reflect current data. Updated 'Extended data' can be found at: https://doi.org/10.6084/m9.figshare.12998549.v3
Abstract
Background: Exercise has been identified as an allied health strategy that can support the management of depression in older adults, yet the relative effectiveness for different exercise modalities is unknown. To meet this gap in knowledge, we present a systematic review and network meta-analysis of randomised controlled trials (RCTs) to examine the head-to-head effectiveness of aerobic, resistance, and mind-body exercise to mitigate depressive symptoms in adults aged ≥ 65 years.
Methods: A PRISMA-NMA compliant review was undertaken on RCTs from inception to September 12 th, 2019. PubMed, Web of Science, CINAHL, Health Source: Nursing/Academic Edition, PsycARTICLES, PsycINFO, and SPORTDiscus were systematically searched for eligible RCTs enrolling adults with a mean age ≥ 65 years, comparing one or more exercise intervention arms, and which used valid measures of depressive symptomology. Comparative effectiveness was evaluated using network meta-analysis to combine direct and indirect evidence, controlling for inherent variation in trial control groups.
Results: The systematic review included 82 RCTs, with 69 meeting eligibility for the network meta-analysis ( n = 5,379 participants). Pooled analysis found each exercise type to be effective compared with controls (Hedges’ g = -0.27 to -0.51). Relative head-to-head comparisons were statistically comparable between exercise types: resistance versus aerobic (Hedges’ g = -0.06, PrI = -0.91, 0.79), mind-body versus aerobic (Hedges’ g = -0.12, PrI = -0.95, 0.72), mind-body versus resistance (Hedges’ g = -0.06, PrI = -0.90, 0.79). High levels of compliance were demonstrated for each exercise treatment.
Conclusions: Aerobic, resistance, and mind-body exercise demonstrate equivalence to mitigate symptoms of depression in older adults aged ≥ 65 years, with comparably encouraging levels of compliance to exercise treatment. These findings coalesce with previous findings in clinically depressed older adults to encourage personal preference when prescribing exercise for depressive symptoms in older adults.
Registration: PROSPERO CRD42018115866 (23/11/2018).
Keywords: Older adults, elderly, seniors, exercise, physical activity, depression, randomised controlled trial, RCT
Introduction
At the close of this decade, the last remaining ‘baby boomers’ will transition to an expanding peer demographic aged ≥ 65 years projected to constitute more than one billion older adults, worldwide 1 . Physical exercise is proposed as a low-risk adjunctive mitigant of age-associated functional deterioration in mental health, including for dementia 2 and depression 3, 4 . In light of impending demographic shifts, and with the burden of age-associated depression estimated to affect ~20% of older adults 5– 7 , 2030 may confer a burden of 200 million adults aged ≥ 65 years presenting with clinical depression. In preparation for inevitable future demands on primary care systems 8 , there is prevailing opportunity for concerted efforts to support primary and allied health personnel with informed preventative strategies.
International public health consortia are in concert with the antidepressant effects of exercise as a low-risk adjunct for optimal mental health 9– 11 . While we do not yet have the answers for low uptake of exercise in older adults 12 , it may in some way be due to nuanced regimen design, which in turn, may similarly impact compliance in exercise prescription by primary and stakeholders in aged care. By exemplar, ‘Exercise is Medicine’ is a global initiative promulgated through 40 member countries by the American College of Sports Medicine with a platform to promote and encourage routine physical exercise for general health and a broad range of medical conditions. This manifesto encourages primary care physicians and health care providers to refer patients to qualified exercise personnel when prescribing treatment plans. However, despite conventional agreement for exercise as a prophylactic for geriatric depression, contemporary literature is yet to quantify the antidepressant treatment effectiveness for individual exercise types.
Perhaps a lesser appreciated obstacle to optimising exercise prescription for mental health in older adults lies with the ‘catch all’ characterisation of exercise. During the past four decades, widely different metabolic, social, and environmental demands between exercise modalities (i.e., running vs. weightlifting vs. Tai Chi) have been well-characterised. Given that there is variation between exercise regimens, and these variations are not merely semantics, one may be surprised to discover that only a few randomised controlled trials (RCTs) have deliberately compared the antidepressant effects of different exercise regimens in older adults 13, 14 . This begs a meaningful question, ‘are all exercise types equal?’.
In attempting to quantify the magnitude of potential antidepressant effects of exercise, researchers have deployed conventional pairwise meta-analysis 4, 15, 16 during recent years. In departure from the value offered by pooling conventional treatments during pairwise meta-analysis, this amalgamation is inherently prone to overgeneralisation and concomitant overestimation of treatment effectiveness 17 . More specifically in mental health literature, pooling individual trial effect comparisons during the pairwise meta-analytical process precludes any opportunity for head-to-head comparison between different exercise types. In the same vein, a further nuance of pairwise meta-analysis is that effect-sensitivity is compromised by foregoing potentially relevant characterisation of control arms (i.e., wait-list, usual care, attention-control) of included trials 18 . In this respect, one must acknowledge that pairwise meta-analysis has intrinsic restrictive boundaries in circumstances where head-to-head comparison of different exercise treatments are required.
In circumstances where relative effectiveness of multiple treatment comparisons are required, network meta-analysis offers a methodological solution to provide more precise pooled head-to-head effect estimates than may otherwise be achieved 19, 20 . Performed correctly, network meta-analysis can allow comparative effectiveness of exercise treatment regimens (aerobic, resistance, and mind-body), avoiding the assumption of treatment homogeneity, controlling for bias from small study effects, and likewise, controlling for characteristically different control arms (wait-list, usual care, and attention-control).
Exercise regimens are broadly categorized into either aerobic, resistance, or mind-body exercise types 21, 22 , and any individual exercise program may consist of multiple combinations of these activities. It is well established that independent regimens of either aerobic or resistance exercise elicit uniquely different metabolic and phenotypic adaptations, whereas mind-body exercise is unique as a low impact form of exercise. Therefore, it is reasonable to theorise comparative differences in the capacity to elicit any antidepressant effect. Until recently, investigation of head-to-head treatment effects of aerobic, resistance, and mind-body exercise had not been undertaken and their comparative ability to moderate symptoms of depression in older adults without pre-existing clinical depression is unknown. We direct the reader to the Extended data of this article for detailed description of the exercise classifications included in this review.
Recently, Miller and colleagues 23 quantitatively compared head-to-head effectiveness of aerobic, resistance, and mind-body exercise in older adults with pre-existing clinical depression. In realisation of their study hypothesis, Miller et al. 23 excluded studies of older adults which lacked participant-level diagnosis of clinical depression. In doing so, there is an assumption that older adults with diagnosis of clinical depression should not be pooled with similarly aged counterparts without diagnosis. These differences are not merely semantics, and while beyond the broad scope of the present study, are worthy of summary.
Present day phenotypic characterisation of clinically depression in older adults is supported by the coalition of more than six decades of cross-disciplinary research into major depressive disorders. Limbic brain regions, monoamine neurotransmitters, and the hypothalamic-pituitary-adrenal (HPA) axis contribute to the pathophysiology of depression 24 . In addition, hypothalamic oversight of pulsatile stress hormone perception from brain regions is central for homeostatic regulation of human stress response and maintenance of systemic feedback loop physiology in concert with the pituitary and adrenal cortex. Similarly, it is well-established that stress hormones have broad infiltration through large areas of the brain via neuronal supply originating from midbrain and brainstem regions. Indeed, these monoaminergic systems have been widely recognised by neuroscientists, pharmacologists, and clinicians alike as key determinants (thus targets) of a person’s mood, cognition, sleep quality, appetite, and reward systems; each of which is known to be affected by physical exercise and the cornerstone of many pharmacological treatments for major depressive disorders.
Long-term prospective data 25 has identified qualitatively distinct populations within patients with major depressive disorder, and a more recent systematic review 26 of 67,318 participants enrolled to longitudinal cohort studies identified that people with subthreshold depression had an elevated risk of developing major depressive disorder. However, the existence for a continuum of depressive symptomology is inconclusive 27 and subsyndromal symptoms are not yet classified on a continuum 28 . Taken together, it remains prudent to respect the binary threshold separating clinical diagnosis with subclinical depressive symptomology, and it stands to reason that clinical and subclinical categories should not be ‘merged’ into the same network in order to compare head-to-head effectiveness of different exercise treatments.
With these aspects in mind, the purpose of this systematic review and network meta-analysis was to quantitively assess the best evidence from RCTs to establish relative (head-to-head) effectiveness of resistance, aerobic, and mind-body exercise in adults aged ≥ 65 years, and below the clinical threshold for diagnosed depression. More specifically, we investigated whether (i) resistance, aerobic, and mind-body exercise training can induce substantive treatment effect on depressive symptoms in older adults, (ii) while considering relative treatment compliance to each exercise regimen, and further, (iii) to juxtapose the optimal exercise treatment for all adults aged ≥ 65 years irrespective of depressive symptomology.
Methods
This review was prospectively registered with PROSPERO (registration number: CRD42018115866, 23/11/2018). The network meta-analysis extension for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) guidelines 29 and the Cochrane Intervention Review that Compares Multiple Interventions 30 each provided further support in guiding this review. Guidelines specific for geriatric meta-analyses 31 were consulted to identify baseline characteristics, equity considerations, inclusion/exclusion criteria, known confounders, and potentially important effect modifiers. Extended data for this review can be found online 32 .
Eligibility criteria
Studies were eligible for inclusion if they (i) followed an RCT protocol, (ii) used a wait-list, usual care, or attention-control group, (iii) included one or more aerobic, resistance, or mind-body exercise intervention arms, (iv) reported depressive symptoms as an outcome at baseline and during follow-up, (v) used one or more psychometrically validated depression questionnaires, (vi) recruited participants with a minimum mean sample age of 65 years. Studies were excluded when (i) the intervention condition used a multicomponent treatment including non-exercise components with the exercise condition, or (ii) the participants were diagnosed with clinical depression, defined by DSM or ICD criteria, or a clinical threshold on a questionnaire validated against a structured diagnostic interview prior to study enrollment. Eligibility was not restricted to specific years, languages, or publication status.
Literature search
Studies were identified from computerised searches of the following databases: PubMed, Web of Science, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Health Source: Nursing/Academic Edition, PsycARTICLES, PsycINFO, and SPORTDiscus. Databases were searched for key terms pertaining to four main concepts: older adults, exercise, depressive symptoms, and RCTs. Search terms were identified using text mining procedures and an example of a search strategy is presented in detail as Extended data. Published studies, systematic reviews, and meta-analyses 4, 15, 16, 33– 36 were also screened for additional articles. Databases were searched up to and including September 12 th, 2019.
Study selection
All articles collated from the systematic search initially went through a title and abstract screening, followed by a full-text screening. Eligibility criteria were used to determine whether each article should be included or excluded. The screening process was performed concurrently for both the current review and a parallel review 23 . Once the screening process was complete, the remaining studies were included in the systematic review and descriptively reported. Studies were only included in the quantitative analysis if they contained sufficient outcome data.
Data extraction and coding
Detailed data extraction was undertaken independently by a minimum of two researchers (KJM, PA, and/or DH) in compliance with a data extraction form (see Extended data), with any inconsistencies being arbitrated by another researcher (CM). Study characteristics are presented in Table 1. Methodological characteristics of each study were used to evaluate the quality of evidence, which included publication status, intention-to-treat principle, use of a cluster design, and validated measure(s) of depressive symptoms used.
Table 1. Participant, intervention, and methodological characteristics of studies included in the systematic review.
| Author (year) | Treatment
M age (SD), females (%) |
Control
M age (SD), females (%) |
Source of
participants |
Inclusion
criteria |
Intention-
to-treat |
Cluster
design |
Treatment group | Control
group |
Length of
intervention |
Adherence
(%) |
Outcome
measure(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abd El-Kader
(2016) 37 |
68.9 (5.8)
70 |
69.1 (6.1)
75 |
N/R | Dementia | N/R | No |
n = 20
Aerobic (treadmill walking), supervised, individual, moderate intensity (60–70% HR max), 3 times/week, 10–30 mins 42 / session |
n = 20
N/R |
2 months | 100 | BDI |
| Adler (2007) 38 | 72.8 (5.4)
100 |
70.8 (8.0)
87.5 |
Community | Osteoarthritis | Yes | N/R |
n = 8
Mind-body (Tai Chi), supervised, group, low intensity, 1 time/week, 60 mins/session **Encouraged to practice at home at least 3 times per week for 15 minutes |
n = 6
Attention- control (bingo) |
10 weeks | 85 | CESD-20 |
| Angeles
(2016) 39 |
65.0 (3.3)
80 |
69.3 (7.4)
70 |
N/R | N/R | N/R | No |
n = 10
Mind-body (flexibility, toning, and balance - FlexToBa DVD), supervised, group, N/R, 3 times/week, 50 mins/session |
n = 10
Usual care |
12 weeks | N/R | POMS-D |
| Ansai (2015) 40 | 82.8 (2.8)
65.2 |
82.6 (2.6)
65.2 |
Community | Sedentary | Yes | No |
n = 23
Resistance (strength exercises with machines), supervised, group, N/R, 3 times/week, 60 mins/ session Adverse events: Mild muscle pain ( n = 9) |
n = 23
Usual care |
16 weeks | N/R | GDS-15 |
| Antunes
(2005) 41 |
68.1 (5.5)
0 |
65.9 (3.8)
0 |
Community | Sedentary | N/R | No |
n = 23
Aerobic (ergometric cycling), supervised, N/R, moderate intensity (50-60% VO 2max), 3 times/week, 20–60 mins/ session |
n = 23
Wait-list |
6 months | N/R | GDS-30 |
| Bernard
(2015) 42 |
65.5 (4.0)
100 |
65.5 (4.4)
100 |
Community | N/R | Yes | No |
n = 61
Aerobic (walking - Acti’March Program), supervised, individual, moderate intensity (40–75% HR max), 2 times/week, 40 mins/ session **One 40 minute unsupervised session |
n = 60
Wait-list |
6 months | 53.8 | BDI |
| Bethany
(2005) 43 |
83.1 (4.8)
92.9 |
Community | N/R | N/R | No |
n = 11
Aerobic (chair aerobics), supervised, group, N/R, 3 times/week, 30 mins/ session |
n = 10
Attention- control (social games) |
6 weeks | 71.7 | BDI-II | |
|
n = 10
Aerobic (walking), unsupervised, individual, N/R, 3 times/week, 30 mins/ session |
62.2 | ||||||||||
|
n = 11
Mind-body (chair yoga), supervised, group, N/R, 3 times/week, 30 mins/ session |
78.3 | ||||||||||
| Blumenthal
(1991) 44 |
66.5 (4.3)
48.4 |
66.8 (4.3)
52.9 |
Community | Sedentary | N/R | No |
n = 31
Aerobic (ergometric cycling, walking, jogging, and arm ergometry), supervised, group, moderate intensity (70% HR max), 3 times/week, 60 mins/session |
n = 32
Wait-list |
16 weeks | 95.8 | CESD-20 |
| 67.8 (5.9)
50 |
n = 34
Mind-body (yoga), supervised, group, low intensity, 2 times/week, 60 mins/session |
100 | |||||||||
| Bonura
(2014) 45 |
77.0 (7.3)
69.8 |
Community | N/R | N/R | No |
n = 33
Resistance (chair strength and balance), supervised, group, N/R, 1 time/week, 45 mins/session **Practice on their own for 15 minutes during non- class days |
n = 32
Wait-list |
6 weeks | 83.8 | GDS-30 | |
|
n = 33
Mind-body (chair yoga), supervised, group, N/R, 1 time/week, 45 mins/session **Practice on their own for 15 minutes during non- class days |
95 | ||||||||||
| Bostrom
(2016) 46 |
84.4 (6.2)
75.3 |
85.9 (7.8)
76.3 |
Residential | Dementia | N/R | Yes (36) |
n = 83
Resistance (functional weight-bearing and balance - High-Intensity Functional Exercise Program), supervised, group, moderate-high intensity, 2.5 times/week, 45 mins/session |
n = 80
Attention- control (seated social activities) |
4 months | 73 | GDS-15;
MADRS * |
| Bouaziz
(2019) 47 |
72.9 (2.5)
70 |
74.3 (3.4)
76.6 |
Community | Sedentary | N/R | No |
n = 27
Aerobic (cycle ergometry), supervised, individual, moderate intensity, 2 times/ week, 30 mins/session |
n = 29
Wait-list |
9.5 weeks | 94.7+ | GADS-D |
| Brown (2009) 48 | 81.5 (6.9)
91.6 |
78.1 (6.4)
86.8 |
Community | N/R | Yes | Yes (8) |
n = 26
Mind-body (flexibility and relaxation), supervised, group, low intensity, 2 times/week, 60 mins/ session |
n = 34
Usual care |
6 months | 44.8 | GDS-30 |
| Cancela
(2016) 49 |
80.6 (8.3)
43.8 |
82.9 (7.4)
81 |
Residential | Dementia | Yes | No |
n = 73
Aerobic (low resistance cycling), supervised, individual, N/R, 7 times/ week, 15+ mins/session |
n = 116
Attention- control (recreational activities) |
15 months | 88 | CSDD |
| Chen (2009) 50 | 65.8 (4.3)
83.9 |
72.4 (6.0)
62.1 |
Community | N/R | N/R | Yes (8) |
n = 62
Mind-body (silver yoga), supervised, group, N/R, 3 times/week, 70 mins/ session |
n = 66
Wait-list |
6 months | 87 | TDQ |
| Chen (2015) 51 | 79.2 (7.0)
49.1 |
Residential | Wheelchair-
bound |
N/R | Yes (10) |
n = 59
Aerobic (resistance bands, aerobic motion, and harmonic stretching - Wheelchair-bound Senior Elastic Band Program), supervised, group, N/R, 3 times/week, 40 mins/ session |
n = 55
Usual care |
6 months | 94.5 | TDQ | |
| Chen (2017) 52 | 80.7 (8.0)
58.5 |
81.6 (6.7)
54.8 |
Residential | Dementia;
wheelchair- bound |
N/R | Yes (8) |
n = 65
Aerobic (resistance bands, aerobic motion, and harmonic stretching - Wheelchair-bound Senior Elastic Band Program), supervised, group, N/R, 3 times/week, 40 mins/ session |
n = 62
Usual care |
15 months | 96.9 | CSDD |
| Chin A Paw
(2004) 53 |
81.0 (5.8)
73.2 |
81.3 (4.4)
82.9 |
Residential | N/R | Yes | No |
n = 41
Resistance (progressive resistance training), supervised, group, moderate intensity, 2 times/ week, 45–60 mins/session Adverse events: Program was too intensive ( n = 8) |
n = 35
Attention- control (educational programs) |
6 months | 78 | GDS-30 |
| Choi (2018) 54 | 77.6 (5.69)
90.9 |
78.8 (5.83)
96.7 |
Residential | N/R | N/R | Yes (6) |
n = 33
Mind-body (sitting yoga), supervised, group, moderate intensity (10– 14/20 RPE), 4 times/week, 30–40 mins/session |
n = 30
Wait-list |
12 weeks | N/R | GDS-15 |
| Clegg (2014) 55 | 79.4 (7.9)
73.3 |
78.0 (10.5)
69.2 |
Community | Frailty | Yes | No |
n = 40
Resistance (strengthening exercises - Home-based Older People’s Exercise Program), unsupervised, individual, N/R, 15 times/ week, 15 mins/session Adverse events: Fell at least once ( n = 7) |
n = 30
Usual care |
12 weeks | 67 | GDS-15 |
| Collier (1997) 56 | 71 (N/R)
63 |
69 (N/R)
46.2 |
Community | N/R | N/R | No |
n = 25
Resistance (strength exercises with machines), supervised, group, N/R, 3 times/week, 50 mins/ session |
n = 13
Usual care |
10 weeks | N/R | GDS-30 |
| Conradsson
(2010) 57 |
85.3 (6.1)
73.6 |
84.2 (6.8)
72 |
Residential | N/R | Yes | Yes (34) |
n = 75
Resistance (functional weight-bearing and balance - High-Intensity Functional Exercise Program), supervised, group, high intensity, 2.5 times/week, 45 mins/session |
n = 90
Attention- control (seated social activities) |
3 months | 72 | GDS-15 |
| Cramer
(2016) 58 |
68.7 (9.1)
37 |
67.8 (10.4)
40.7 |
Community | Colorectal
cancer |
Yes | No |
n = 27
Mind-body (hatha yoga), supervised, group, N/R, 1 time/week, 90 mins/session Adverse events: Muscle soreness ( n = 3), abdominal pain ( n = 1), neck pain ( n = 1), minor vertigo ( n = 1), hip pain ( n = 1) |
n = 27
Wait-list |
10 weeks | 53 | HADS-D |
| De Lima
(2019) 59 |
66.2 (5.5)
N/R |
67.2 (5.2)
N/R |
Community | Parkinson’s
diease |
N/R | No |
n = 17
Resistance (free weights and machines), supervised, group, N/R, 2 times/week, 30-40 mins/session |
n = 16
Usual care |
20 weeks | N/R | HRSD |
| Donesky-
Cuenco (2009) 60 |
72.2 (6.5)
73.3 |
67.7 (11.5)
71.4 |
Community | Chronic
obstructive pulmonary disease |
Yes | No |
n = 14
Mind-body (yoga), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 15
Usual care |
12 weeks | 83.3 | CESD-20 |
| Dong (2013) 61 | 68.5 (N/R)
100 |
Community | N/R | N/R | No |
n = 32
Mind-body (YiJinJing qigong), supervised, group, N/R, 3 times/week, 60 mins/ session |
n = 34
N/R |
12 weeks | N/R | GDS-15 | |
|
n = 36
Mind-body (LiuZiJue qigong), supervised, group, N/R, 3 times/week, 60 mins/ session |
N/R | ||||||||||
| Dorner (2007) 62 | 86.7 (6.1)
76.7 |
86.9 (5.7)
76.7 |
Residential | Frailty;
cognitive impairment |
N/R | No |
n = 15
Resistance (resistance bands, soft weights, and balance), supervised, group, N/R, 3 times/week, 50 mins/ session |
n = 15
N/R |
10 weeks | 91.8 | GDS-15 |
| Emery (1990) 63 | 72 (6)
83.3 |
Community | N/R | N/R | No |
n = 14
Aerobic (aerobic walking and rhythmic muscle strengthening), supervised, group, moderate intensity (70% HR max) 3 times/week, 60 mins/session |
n = 13
Wait-list |
12 weeks | 61-94 | CESD-20 | |
|
n = 11
Attention- control (social games) | |||||||||||
| Eyigor (2009) 64 | 73.5 (7.6)
100 |
71.2 (5.5)
100 |
Community | N/R | N/R | N/R |
n = 19
Aerobic (folkloric dance), supervised, group, N/R, 3 times/week, 60 mins/ session **Walk for 30 minutes at least twice a week |
n = 18
Usual care |
8 weeks | N/R | GDS-30 |
| Eyre (2017) 65 | 68.1 (8.7)
65.8 |
67.6 (8.0)
65.9 |
Community | Cognitive
impairment |
N/R | No |
n = 29
Mind-body (kundalini yoga), supervised, group, N/R, 1 time/week, 60 mins/session Adverse events: Dizziness ( n = 1) **Home meditation and finger movements for 12 minutes |
n = 32
Attention- control (memory enhancement training) |
12 weeks | 59.4 | GDS-30 |
| Fakhari
(2017) 66 |
69.2 (5.5)
48.1 |
69.3 (5.0)
58.6 |
Residential | Sedentary | N/R | No |
n = 27
Mind-body (Tai Chi), supervised, group, N/R, 3 times/week, 20-25 mins/ session |
n = 29
Usual care |
12 weeks | N/R | BDI-II |
| Fransen
(2007) 67 |
70.8 (6.3)
67.9 |
69.6 (6.1)
82.9 |
Community | Osteoarthritis | Yes | No |
n = 56
Mind-body (Tai Chi), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 41
Wait-list |
12 weeks | N/R | DASS-D |
| Frye (2007) 68 | 69.2 (9.3)
64.3 |
Community | Sedentary | N/R | No |
n = 23
Mind-body (Tai Chi), supervised, group, low- moderate intensity, 3 times/ week, 60 mins/session **Tai Chi video to assist with home practice, but was not monitored |
n = 21
Usual care |
12 weeks | 72.2-100 | CESD-20 | |
| Gary (2004) 69 | 67 (11)
100 |
69 (11)
100 |
Community | Diastolic
heart failure |
N/R | No |
n = 15
Aerobic (walking), supervised, individual, low- moderate intensity (40–60% HR max), 3 times/week, 20-30 mins/session |
n = 13
Attention- control (educational programs) |
12 weeks | N/R | GDS-15 |
| Gusi (2008) 70 | 71 (5)
100 |
74 (6)
100 |
Community | Moderate
depression or overweight |
N/R | No |
n = 55
Aerobic (walking), supervised, group, N/R, 3 times/week, 50 mins/ session |
n = 51
Usual care |
6 months | N/R | GDS-15 |
| Hars (2014) 71 | 75 (8)
97 |
76 (6)
95.6 |
Community | Risk of falling | Yes | No |
n = 66
Aerobic (aerobic training), supervised, group, N/R, 1 time/week, 60 mins/session |
n = 68
Wait-list |
6 months | 79 | HADS-D |
| Hsu (2016) 72 | 80.7 (9.7)
63.3 |
81.7 (6.3)
63.3 |
Residential | Wheelchair-
bound |
Yes | No |
n = 30
Mind-body (seated Tai Chi), supervised, group, N/R, 3 times/week, 40 mins/ session |
n = 30
Usual care |
26 weeks | 85.3 | GDS-15 |
| Irwin (2012) 73 | 70.7 (5.9)
69.6 |
71.4 (7.7)
51.4 |
Community | N/R | Yes | No |
n = 58
Mind-body (Tai Chi), supervised, group, N/R, 3 times/week, 40 mins/ session |
n = 53
Attention- control (health education) |
16 weeks | 83 | BDI |
| Irwin (2014) 74 | 66.3 (7.4)
64.6 |
66.4 (7.7)
72 |
Community | Insomnia | Yes | No |
n = 48
Mind-body (Tai Chi), supervised, group, N/R, 1 time/week, 120 mins/ session |
n = 25
Attention- control (sleep seminar education) |
4 months | 81 | IDS-C |
| Kekalainen
(2018) 75 |
68.9 (2.7)
53.8 |
68.3 (2.3)
47.8 |
Community | N/R | Yes | No |
n = 24
Resistance (progressive resistance training), supervised, group, N/R, 2 (month 1–3) and 1 (month 4–9) times/week, 60 mins/ session |
n = 19
Usual care |
9 months | N/R | BDI-II |
| 67.7 (2.8)
59.6 |
n = 25
Resistance (progressive resistance training), supervised, group, N/R, 2 (month 1–3) and 2 (month 4–9) times/week, 60 mins/ session |
N/R | |||||||||
| 69.0 (3.3)
57.1 |
n = 27
Resistance (progressive resistance training), supervised, group, N/R, 2 (month 1–3) and 3 (month 4–9) times/week, 60 mins/ session |
N/R | |||||||||
| Kim (2019) 76 | 76.1 (3.85)
100 |
76.40 (3.27)
100 |
Community | N/R | N/R | No |
n = 11
Resistance (strength exercises), N/R, N/R, moderate intensity (9-13/20 RPE), 3 times/week, 30–60 mins/session |
n = 10
Usual care |
24 weeks | N/R | GDS-15 |
| Kohut (2005) 77 | 73.1 (5.6)
50 |
70.3 (5.6)
53.8 |
Community | Sedentary | N/R | No |
n = 14
Aerobic (treadmills or ergometric cycling), supervised, group, high intensity (71% HR max), 3 times/week, 20–30 mins/ session |
n = 13
Usual care |
10 months | 80+ | GDS-30 |
| Krishnamurthy
(2007) 78 |
70.1 (8.3)
69.6 |
72.3 (7.4)
73.9 |
Residential | N/R | N/R | No |
n = 18
Mind-body (yoga), supervised, group, N/R, 6 times/week, 75 mins/ session |
n = 20
Wait-list |
24 weeks | N/R | GDS-15 |
| Kuo (2018) 79 | 68.93 (3.81)
80 |
70.38 (5.22)
85.7 |
Clinical | N/R | N/R | No |
n = 15
Aerobic (stepping exercises), supervised, N/R, moderate- high intensity (13-15/20 RPE; 60-80% HR max), 2 times/ week, 30 mins/session |
n = 21
Usual care |
8 weeks | N/R | GDS-15 |
| Kupecz
(2001) 80 |
74.3 (6.2)
5.1 |
73.6 (5.9)
13 |
Community | N/R | N/R | No |
n = 30
Aerobic (walking), supervised, group, low- moderate intensity, 3 times /week, 45 mins/session Adverse events: Minor medical attention ( n =2) |
n = 35
Usual care |
12 weeks | 66 | CESD-20 |
| Li (2001) 81 | 73.2 (4.9)
76.5 |
Community | Sedentary | N/R | No |
n = 40
Mind-body (Tai Chi), supervised, group, N/R, 2 times/week, 60 mins/ session **Encouraged to practice at home |
n = 32
Wait-list |
24 weeks | 90 | CESD-20 | |
| Lin (2007) 82 | 77.1 (7.8)
51 |
76.2 (7.3)
51 |
Community | History of
falling |
N/R | No |
n = 39
Resistance (muscle strengthening and balance), supervised, individual, N/R, 0.5 times/week, 40–60 mins/ session **Instructed to practice exercises at least three times a week |
n = 40
Attention- control (educational programs) |
4 months | N/R | GDS-15 |
| Lincoln (2011) 83 | 66.0 (7.9)
69 |
66.6 (7.4)
58.6 |
Community | Type 2
diabetes |
N/R | No |
n = 29
Resistance (progressive resistance training), supervised, N/R, high intensity, 3 times/week, 45 mins/session |
n = 29
Attention- control (regular phone calls) |
16 weeks | N/R | GDS-30 |
| Ma (2018) 84 | 70.2 (10.3)
31.7 |
69.7 (10.8)
30.4 |
Community | Hypertension | Yes | No |
n = 79
Mind-body (Tai Chi), supervised, group, N/R, 4 times/week, 60 mins/ session |
n = 79
Usual care |
6 months | N/R | CESD-10 |
| Maki (2012) 85 | 71.9 (4.1)
69.3 |
72.0 (3.9)
72 |
Community | N/R | Yes | No |
n = 66
Aerobic (walking and group work), supervised, group, N/R, 1 time/week, 90 mins/ session |
n = 67
Attention- control (educational programs) |
3 months | 87.5 | GDS-30 |
| Martinez
(2015) 86 |
76.1 (8.1)
89.7 |
72.5 (8.0)
86.2 |
Clinical | N/R | Yes | No |
n = 29
Mind-body (qigong), supervised, group, N/R, 2 times/week, 90 mins/ session |
n = 25
Usual care |
4 weeks | 88.8 | GDS-5 |
| Martins
(2011) 13 |
75.9 (7.0)
66.7 |
77.7 (8.8)
58.1 |
Residential | Sedentary | N/R | No |
n = 24
Aerobic (rhythmic walking and stepping sequences), supervised, group, low-high intensity (40-85% HR max), 3 times/week, 45 mins/ session |
n = 31
Usual care |
16 weeks | N/R | POMS-SF-D |
| 73.4 (6.4)
56.5 |
n = 23
Resistance (resistance bands and calisthenics), supervised, group, moderate intensity, 3 times/ week, N/R |
N/R | |||||||||
| McMurdo
(1993) 87 |
82.3 (6.9)
80 |
79.3 (6.2)
80.7 |
Residential | N/R | N/R | N/R |
n = 10
Resistance (strengthening exercises), supervised, group, low intensity, 2 times/week, 45 mins/ session |
n = 23
Attention- control (reminiscence sessions) |
7 months | 91 | GDS-30 |
| Monga
(2005) 88 |
68 (4.2)
0 |
70.6 (5.3)
0 |
Community | Prostate
Cancer |
N/R | No |
n = 11
Aerobic (treadmill walking), supervised, group, moderate intensity (65% HR max), 3 times/week, 45–50 mins/session |
n = 10
Usual care |
8 weeks | N/R | BDI |
| Mortazavi
(2012) 89 |
71.7 (8.2)
64.1 |
71.7 (8.2)
61.3 |
Community | N/R | N/R | No |
n = 181
Aerobic (upper, lower, and whole body movements), supervised, group, low intensity, 2 times/week, 45 mins/session **Pamphlet explaining details of each session for exercising at home |
n = 191
Attention- control (physical activity pamphlets) |
2 months | N/R | GHQ-D |
| Netz (1994) 90 | 70.7 (6.8)
N/R |
74.1 (8.9)
N/R |
Clinical | Cognitive
or affective deterioration |
N/R | No |
n = 15
Aerobic (light callisthenic and rhythmic movements), supervised, group, low intensity, 3 times/week, 45 mins/session |
n = 15
Attention- control (social intellectual activity) |
8 weeks | N/R | GDS-30 |
| Ng (2017) 91 | 70.3 (5.3)
56.2 |
70.1 (5.0)
56 |
Community | Frailty | Yes | No |
n = 48
Resistance (functional strength and balance), supervised, group, moderate intensity, 2 times/ week, 90 mins/session **Weeks 13-24 were instructed to participate at home |
n = 50
Usual care |
24 weeks | 85 | GDS-15 |
| Oken (2006) 92 | 73.6 (5.1)
78.7 |
71.2 (4.4)
75 |
Community | Low activity
(aerobic exercise less than 210 minutes per week) |
N/R | No |
n = 38
Aerobic (walking), supervised, group, moderate intensity (6-7/10 RPE; 70% HR max), 1 time/ week, 60 mins/session Adverse events: Hip pain ( n = 1) **Exercise daily at least 5 times per week was strongly encouraged |
n = 42
Wait-list |
6 months | 68.7 | CESD-10 *; POMS-D |
| 71.5 (4.9)
70.5 |
n = 38
Mind-body (hatha yoga), supervised, group, N/R, 1 time/week, 90 mins/session Adverse events: Groin muscle strain ( n = 1) **Exercise daily at least 5 times per week was strongly encouraged |
77.6 | |||||||||
| Park (2016) 93 | 75.3 (7.5)
75 |
Community | Osteoarthritis | N/R | No |
n = 52
Mind-body (chair yoga), supervised, group, N/R, 2 times/week, 45 mins/ session |
n = 48
Attention- control (health education) |
8 weeks | 95 | PROMIS-
EDD SF-8a |
|
| Payne (2008) 94 | 64.7 (6.3)
100 |
Community | Breast cancer | N/R | N/R |
n = 9
Aerobic (walking), unsupervised, individual, moderate intensity, 4 times/ week, 20 mins/session |
n = 9
Usual care |
12 weeks | N/R | CESD-20 | |
| Pedersen
(2017) 95 |
79.2 (6.6)
52.6 |
81.3 (5.1)
50 |
Residential | N/R | N/R | N/R |
n = 19
Resistance (resistance training), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 12
N/R |
12 weeks | N/R | HADS-D |
| Penninx
(2002) 14 |
68.7 (5.6)
70.1 |
Community | Osteoarthritis | Yes | N/R |
n = 149
Aerobic (walking), supervised, N/R, moderate intensity (50–70% HR max), 3 times/week, 60 mins/ session **Months 4-18 were unsupervised |
n = 144
Attention- control (health education) |
18 months | N/R | CESD-6 | |
|
n = 146
Resistance (dumbbell and cuff weight exercises), supervised, N/R, N/R, 3 times/week, 60 mins/ session ** Months 4-18 were unsupervised |
N/R | ||||||||||
| Pinniger
(2013) 96 |
79.4 (N/R)
100 |
Community | Age-related
macular degeneration |
N/R | No |
n = 8
Aerobic (tango dance), supervised, group, N/R, 2 times/week, 90 mins/ session |
n = 9
Wait-list |
4 weeks | 96.9 | GDS-15 | |
| Ramanathan
(2017) 97 |
68.9 (7.6)
100 |
68.2 (8.8)
100 |
Residential | N/R | N/R | No |
n = 20
Mind-body (yoga), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 20
Wait-list |
12 weeks | N/R | HRSD |
| Roswiyani
(2019) 98 |
71.90 (8.57)
70.1 |
74.31 (9.57)
66.2 |
Residential | N/R | Yes | No |
n = 67
Mind-body (qigong), supervised, group, N/R, 2 times/week, 90 mins/ session |
n = 65
Usual care |
8 weeks | 61.2 | BDI-II |
| Sahin (2018) 99 | 84.5 (4.8)
N/R |
85.4 (4.7)
N/R |
Residential | Frailty | N/R | No |
n = 16
Resistance (dumbbell and cuff weight exercises), supervised, N/R, low intensity (40% 1R max), 3 times/week, 40 mins/ session |
n = 16
Usual care |
8 weeks | N/R | GDS-15 |
| 84.2 (6.9)
N/R |
n = 16
Resistance (dumbbell and cuff weight exercises), supervised, N/R, high intensity (70% 1R max), 3 times/week, 40 mins/ session |
N/R | |||||||||
| Sattin (2005) 100 | 80.4 (3.1)
95.4 |
80.5 (3.2)
93.6 |
Residential | Frailty; history
of falling |
Yes | Yes (20) |
n = 158
Mind-body (Tai Chi), supervised, group, N/R, 2 times/week, 60–90 mins/ session |
n = 153
Attention- control (health education) |
48 weeks | 76 | CESD-20 |
| Sola-Serrabou
(2019) 101 |
71.9 (5.0)
76.5 |
74.8 (6.1)
77.8 |
Residential | Sedentary | N/R | No |
n = 18
Resistance (strength exercises), supervised, N/R, moderate intensity (5-6/10 RPE), 2 times/week, 60 mins/session |
n = 17
Usual care |
24 weeks | 90 | GDS-15 |
| Song (2019) 102 | 76.22 (5.76)
80 |
75.33 (6.78)
70 |
Community | Cognitive
impairment; low activity (aerobic exercise less than 150 minutes per week) |
Yes | No |
n = 60
Aerobic (stepping exercises), supervised, group, moderate intensity (12- 14/20 RPE), 3 times/week, 60 mins/session |
n = 60
Attention- control (health education) |
16 weeks | 73.1 | GDS-30 |
| Sparrow
(2011) 103 |
70.3 (7.5)
32.7 |
71.7 (7.2)
29.4 |
Community | Low activity
(exercise less than 20 minutes per week) |
Yes | N/R |
n = 49
Resistance (resistance training - Telephone-Linked Computer-based Long- term Interactive Fitness Trainer), unsupervised, N/R, N/R, 3 times/week, 60 mins/session Adverse events: Musculoskeletal injury and discomfort ( n = 8) |
n = 51
Attention- control (health education) |
12 months | 54 | BDI-II |
| Swoap
(1994) 104 |
65.2 (4.2)
53.8 |
65.2 (4.2)
50 |
Community | Sedentary | N/R | No |
n = 24
Aerobic (walking), supervised, group, moderate intensity (65–70% HR max), 3 times/week, 30–65 mins/session |
n = 11
Wait-list |
26 weeks | N/R | GDS-30 |
| 65.2 (4.2)
58.3 |
n = 14
Aerobic (walking), supervised, group, high intensity (80–85% HR max), 3 times/week, 30–60 mins/ session |
N/R | |||||||||
| Tapps (2013) 105 | 76 (N/R)
N/R |
Residential | N/R | N/R | No |
n = 11
Resistance (resistance band and chair exercises), supervised, group, N/R, 3 times/week, 30 mins/ session |
n = 14
Usual care |
12 weeks | N/R | BDI-II | |
| Taylor-Piliae
(2014) 106 |
71.5 (10.3)
35.8 |
68.2 (10.3)
52.1 |
Community | History of
stroke |
Yes | No |
n = 53
Mind-body (Yang-style Tai Chi), supervised, group, N/R, 3 times/week, 60 mins/ session |
n = 48
Usual care |
12 weeks | 82 | CESD-20 |
| Tsang (2003) 107 | 72.9 (9.5)
62.5 |
76.3 (8.4)
37.5 |
Residential | Chronic
medical illness |
N/R | No |
n = 24
Mind-body (eight-section brocades qigong), supervised, group, N/R, 2 times/week, 60 mins/ session **Practice daily for at least 30 minutes |
n = 26
Attention- control (remedial rehabilitation activities) |
12 weeks | N/R | GDS-30 |
| Tsang (2013) 108 | 83.3 (6.3)
77 |
84.9 (6.0)
72.7 |
Clinical | Frailty | Yes | No |
n = 61
Mind-body (Yan Chai Yi Jin ten-section brocades qigong), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 55
Attention- control (newspaper reading) |
12 weeks | N/R | GDS-15 |
| Tsutsumi
(1998) 109 |
68.5 (6.1)
100 |
Community | Sedentary | N/R | N/R |
n = 12
Resistance (strength exercises with machines), supervised, group, moderate intensity (55–65% 1R max), 3 times/week, N/R |
n = 12
N/R |
12 weeks | N/R | POMS-D | |
|
n = 12
Resistance (strength exercises with machines), supervised, group, high intensity (75–85% 1R max), 3 times/week, N/R |
N/R | ||||||||||
| Vahlberg
(2017) 110 |
72.6 (5.5)
20.6 |
73.7 (5.3)
27.3 |
Community | History of
stroke |
Yes | No |
n = 34
Resistance (functional weight-bearing and balance - High-Intensity Functional Exercise Program), supervised, group, high intensity, 2 times/week, 55 mins/session |
n = 33
Usual care |
3 months | 91 | GDS-20 |
| Vankova
(2014) 111 |
83.4 (8.2)
91.6 |
82.9 (7.9)
92.4 |
Residential | N/R | N/R | No |
n = 79
Aerobic (ballroom dance - Exercise Dance for Seniors Program), supervised, group, N/R, 1 time/week, 60 mins/session |
n = 83
Wait-list |
3 months | 84.6 | GDS-15 |
| Wang (2009) 112 | 75.5 (9.2)
100 |
74.5 (8.1)
80 |
Community | N/R | N/R | No |
n = 7
Mind-body (yoga), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 10
Attention- control (social group) |
4 weeks | 78.1 | CESD-10 |
| Witham
(2005) 113 |
80 (6)
37 |
81 (4)
54 |
Community | Frailty;
chronic heart failure |
N/R | No |
n = 36
Aerobic (progressive aerobic seated exercise with small weights), supervised, group, N/R, 2 times/week, 20 mins/ session **Months 4–6 were home exercise 2–3 times per week with no face-to-face contact |
n = 32
Usual care |
6 months | 82.7 | HADS-D |
| Yang (2005) 114 | 72.6 (5.4)
68.4 |
72.7 (7.5)
90.5 |
Community | Chronic pain | N/R | No |
n = 19
Mind-body (Chun Soo Energy Healing qigong), supervised, group, N/R, 2 times/week, 20 mins/ session |
n = 21
Wait-list |
4 weeks | N/R | POMS-D |
| Yeh (2003) 115 | 65 (6)
40 |
66 (6)
40 |
Community | Chronic
obstructive pulmonary disease |
Yes | N/R |
n = 5
Mind-body (Tai Chi), supervised, group, N/R, 2 times/week, 60 mins/ session |
n = 5
Usual care |
12 weeks | 74.2 | CESD-20 |
| Zanuso
(2012) 116 |
74.3 (3.5)
50 |
67.1 (2.0)
50 |
Community | Sedentary | N/R | No |
n = 10
Resistance (resistance training), supervised, N/R, moderate intensity, 3 times/ week, 60 mins/session |
n = 10
Wait-list |
12 weeks | 90.7 | POMS-D |
Note. N/R = not reported; n = Total participants included in intention-to-treat or post-treatment data analysis; HR max = Heart rate maximum; VO 2max = Maximal oxygen uptake; 1R max = One-repetition maximum; RPE = Rating of perceived exertion; BDI = Beck Depression Inventory; CESD = Center for Epidemiological Studies Depression Scale; CSDD = Cornell Scale for Depression in Dementia; DASS-D = Depression, Anxiety, and Stress Scale (depression subscale); GADS-D = Goldberg Anxiety and Depression Scale (depression subscale); GDS = Geriatric Depression Scale; GHQ-D = General Health Questionnaire (depression subscale); HADS-D = Hospital Anxiety and Depression Scale (depression subscale); HRSD = Hamilton Rating Scale for Depression; IDS-C = Inventory of Depression Symptomatology; MADRS = Montgomery-Asberg Depression Rating Scale; POMS-D = Profile of Mood States (depression subscale); PROMIS-EDD SF-8a = Patient Reported Outcome Measurement Information System - Emotional Distress and Depression Short Form-8a; TDQ = Taiwanese Depression Questionnaire.
*Outcome measure used in network meta-analysis.
**Additional unsupervised exercise component.
Control groups within each individual study were further categorised as either wait-list, usual care, or attention-control. Participants undergoing wait-list conditions received the exercise intervention following trial completion. Participants randomised to usual care were those in sole receipt of conventional treatment during the trial. Participants randomised to attention-control conditions (also known as attention placebo control or active control) received activities completely unrelated to physical activity and/or exercise during their respective trials (e.g., social activities, educational programs, etc.).
Important participant and intervention characteristics were used to verify whether potential effect modifiers were similarly distributed across comparisons within the network. Participant characteristics were coded according to sample size, age (mean and standard deviation), percentage of females, and place of residence (community-dwelling, residential care, clinical setting). Relevant inclusion criteria (e.g., sedentary, dementia, etc.) were further used to assess the risk of bias from equity considerations 117 .
Intervention characteristics were appropriately coded. Exercise types were categorised as aerobic, resistance, or mind-body exercise. Length of interventions were coded in weeks or months. Exercise intensities were coded according to ratings of perceived exertion 118 , heart rate maximum (HR max; low = <50%, moderate = 50-70%, high = >70%), maximal oxygen uptake (VO 2max; low = <40%, moderate = 40-60%, high = >60%) or one-repetition maximum (1R max; low = <50%, moderate = 50-74%, high = >74%) 119 , or where unavailable, this was estimated according to the assessment of the original author(s). Frequency was coded as total sessions per week. Duration was coded as the average number of minutes engaging in exercise per session. Format of program was dichotomously coded as exercise in a group or individual setting. Format of supervision was dichotomously coded as supervised or unsupervised exercise. Agreement between the three researchers was 91.3%.
Risk of bias and quality assessment
Risk of bias of the included RCTs was assessed using the Cochrane Collaboration’s Tool for Assessing Risk of Bias 120 , which were independently conducted by a minimum of two researchers (KJM, PA, and/or DH). Discrepancies were arbitrated by another co-author (CM). Appraisal for ‘other sources of bias’ were evaluated with consideration for small sample size ( n < 15), low adherence (less than 80%), cluster randomisation, and inequity in the selection of the sample.
Summary of outcomes
Outcome statistics including means ( M), standard deviations ( SD), and sample sizes ( n) for depressive symptoms were used to calculate the mean change in the primary outcome. Test statistics (i.e., t-, F-, and p-values) were used to estimate effect sizes when descriptive statistics were unavailable. Pairwise relative (head-to-head) treatment effects for depressive symptoms were estimated using Hedges’ g 121 , which corrects for overestimation biases due to small sample sizes 122 . Hedges’ g coefficients were interpreted according to Cohen’s conversions 123 , whereby effects were considered small (0.2), medium (0.5), and large (0.8). Independent subgroups (e.g., males vs. females) within studies were treated as independent effect size estimates 124 . When individual studies reported more than one post-treatment depression score for the same group of participants, only the initial post-treatment time-point was used. When studies reported depression scores on multiple outcome measures, the included depression measure was selected in compliance with clinical applicability 125, 126 .
Secondary outcome data were also extracted for study attrition, treatment adherence, and adverse events. Pairwise relative (head-to-head) treatment effects for study attrition were reported as odds ratios based on the pre-treatment sample size versus post-treatment dropout in the treatment and comparison conditions. Treatment adherence were reported as a percentage of total attendance in, or compliance to, the treatment condition. Adverse events were qualitatively reported according to the descriptive information provided in the transcript.
Data synthesis
One notable assumption of network meta-analysis relates to comparison group estimates, which are derived from pooling studies with homogenous between-study effect modifiers 127 . If the distribution of an effect modifier is heterogeneous across studies within a specific comparison group, the assumption of transitivity can be violated. In the context of this network meta-analysis, we separate exercise conditions (i.e., aerobic, resistance, mind-body) and control conditions (i.e., wait-list, usual care, attention-control) to account for the between-study variation of these effect modifiers. Given the intricacies of depression severity, a forest plot was generated to depict the juxtaposition of treatment effects between (i) participants in the present network meta-analysis with depressive symptomology but not clinically diagnosed and (ii) participants in a previous network meta-analysis with clinical depression 23 .
Risk of bias assessment identified the potential for unit-of-analysis error within studies using a cluster design for treatment allocation. Thus, sample sizes were recalculated to account for error in cluster RCTs by determining a design effect 128 with a conservative intracluster correlation coefficient of 0.05, in accordance with past studies 46, 129 . The certainty of evidence contributing to network estimates was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach 130 .
Data were analysed and figures were generated using STATA/SE 15.1 131 . Comparison-adjusted funnel plots were used to evaluate publication bias and small-study effects. Random-effects meta-regression was used to investigate modifying effects of age, gender, source of participants, length of intervention, exercise intensity, frequency, duration, and format of program, which have been identified as potential risks to transitivity in geriatric meta-analyses 4, 35, 132 .
A multivariate random-effects meta-analysis was conducted using the ‘mvmeta’ command 133 . A random-effects model assumes variance both within and between studies, explaining the heterogeneity of treatment effects 134 . A common heterogeneity parameter was assumed across comparisons. Heterogeneity was evaluated using tau-squared (τ 2), which estimates the deviation in effect sizes across the population of studies 124 . The 95% prediction interval ( PrI) was also used to estimate the true dispersion of effect within two standard deviations of the mean effect size 124 . The significance level was p < 0.05 for all analyses.
Results
Study selection
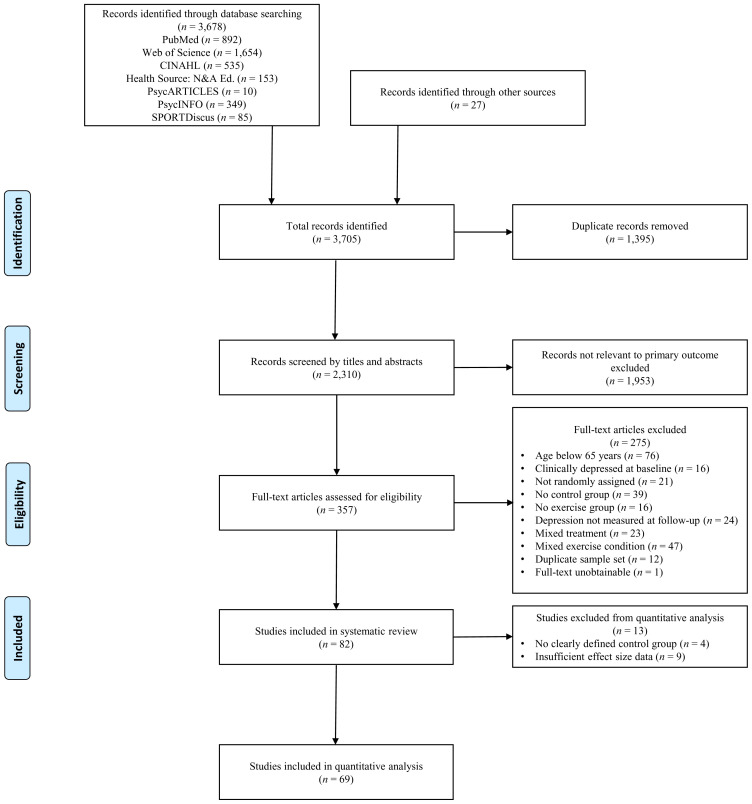
The initial systematic search yielded a total of 3,705 citations. When duplicate studies were removed ( n = 1,395), 2,310 eligible records were identified. Titles and abstracts were screened by two independent researchers (KJM and DCGB) with an agreement of 91.9%. Subsequently, 357 full-text articles were assessed for compliance with inclusion criteria and outcome data. Full-text screening was performed independently by two researchers (KJM and PA) with an agreement of 81.8%, where discrepancies were arbitrated and resolved by consensus with a third researcher (CM). Studies using duplicate sample sets ( n = 12) were identified, where the most informative publication with complete data being included in the quantitative analysis. Finally, 82 studies fulfilled all inclusion criteria to be included in the systematic review. In cases of missing data, authors were emailed by the first author (KJM). If authors failed to respond following two contact efforts, and there were insufficient data to calculate effect size estimates, the study was excluded from the quantitative analysis ( n = 9). Additionally, four studies 37, 61– 63 fulfilled all criteria but necessitated exclusion from the quantitative analysis due to control conditions being insufficiently defined, and the authors being unobtainable. Figure 1 outlines study selection and additional exclusion criteria.
Figure 1. Flowchart of screening process.
Characteristics of the studies
Data from a total of 5,379 participants (2,815 treatment and 2,564 control) across 69 studies were pooled in the quantitative analysis. Each study reported pre- and post-treatment measures of depression symptoms, and data from 60 of the 69 studies were obtained for post-treatment attrition. Figure 2 illustrates the network of pairwise comparisons across all studies. Aerobic, resistance, and mind-body conditions had one or more direct comparison with each of the five comparison conditions. Direct comparisons for depressive symptoms were robustly characterised between intervention and corresponding control conditions (aerobic = 28, resistance = 22, mind-body = 32), with proportions being similar for study attrition (aerobic = 24, resistance = 21, mind-body = 25).
Figure 2. Network plot of comparisons for all studies included in the network meta-analysis.
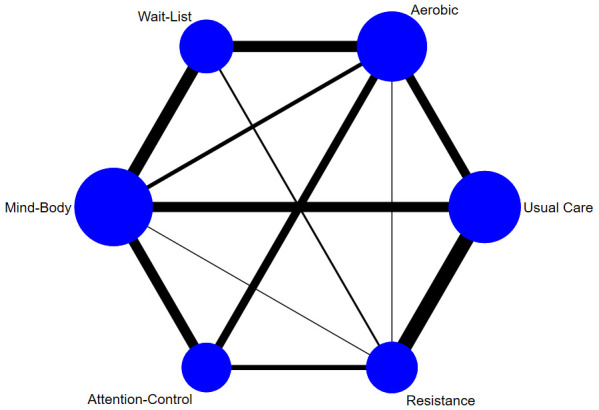
Line width is proportional to the number of pairwise effect size estimates and node size is proportional to the number of participants.
From the 69 included studies, 43 studies enrolled young-old samples (65–75 years) and 26 studies enrolled old-old samples (>75 years). Participants were identified as either community-dwelling ( n = 46), living in residential care ( n = 18), clinical ( n = 4), or uncategorised ( n = 1). Twelve studies enrolled participants with sedentary lifestyles, while the remainder enrolled participants with a range of active and sedentary lifestyles. Some studies recruited older adults that were either frail ( n = 5), wheelchair bound ( n = 3), or identified as presenting with a risk of falling ( n = 2). Participants with age-associated comorbidities were also recruited, including cardiovascular problems ( n = 6), dementia ( n = 3), cognitive impairment ( n = 3), cancer ( n = 2), and other chronic illness ( n = 6).
From 79 independent exercise interventions, the majority were supervised in group exercise classes ( n = 64). The remainder were either individually supervised ( n = 5), unsupervised in an individual format ( n = 2), supervised in an undisclosed format ( n = 1), or entirely undisclosed ( n = 1). The average exercise program length (weeks) was shorter for mind-body ( M = 13.67, SD = 6.77) than aerobic ( M = 19.76, SD = 14.44) or resistance ( M = 18.00, SD = 9.07). Average weekly exercise minutes (calculated as frequency multiplied by duration) were calculated for aerobic ( M = 107.78, SD = 40.88), resistance ( M = 126.90, SD = 50.28), and mind-body exercise ( M = 132.75, SD = 76.07). Treatment adherence ( n = 44) was comparable between aerobic ( M = 81.01, SD = 13.72), resistance ( M = 81.17, SD = 9.00), and mind-body exercise ( M = 79.34, SD = 14.73). Adverse events were observed during several of the included trials, which are presented in Table 2.
Table 2. Adverse events from included studies.
| Exercise group | Study | Adverse event(s) |
|---|---|---|
| Aerobic | Kupecz (2001) 80 | Minor medical attention ( n = 2) |
| Oken (2006) 92 | Hip pain ( n = 1) | |
| Resistance | Ansai (2015) 40 | Mild muscle pain ( n = 9) |
| Chin A Paw (2004) 53 | Program was too intensive ( n = 8) | |
| Clegg (2014) 55 | Fell at least once ( n = 7) | |
| Mind-body | Cramer (2016) 58 | Muscle soreness (
n = 3), abdominal pain (
n = 1), neck
pain ( n = 1), minor vertigo ( n = 1), hip pain ( n = 1) |
| Eyre (2017) 65 | Dizziness ( n = 1) | |
| Oken (2006) 92 | Groin muscle strain ( n = 1) |
Risk of bias and quality assessment
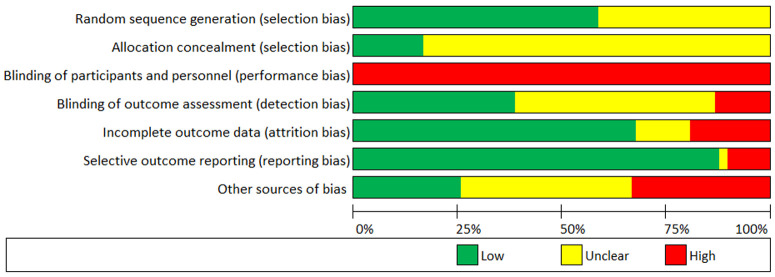
Risk of bias for each study is presented comprehensively as Extended data (see Figure S1). Both blinding of participants and personnel to treatment was implausible due to the implicit nature of exercise training interventions, and thus, the remaining six criteria were used to assess the overall risk of bias within each study. Methodological quality of included studies can be considered low-to-moderate ( M = 4.08/6, where low = 1, unclear, = 0.5, high = 0). Assessment of random sequence generation (selection bias), incomplete outcome data (attrition bias), and selective outcome reporting (reporting bias) may be considered adequate for most studies, whereas allocation concealment (selection bias) and blinding of outcome assessment (detection bias) were diverse. High ‘other sources of bias’ was due to low study adherence ( n = 14), small sample sizes ( n = 8), or both ( n = 1). See Figure 3 for a summary of the risk of bias in the included studies.
Figure 3. Risk of bias chart of studies included in the quantitative analysis.
Assessment of inconsistency
Inconsistency network models were used to test the global consistency of direct and indirect effects of pairwise and multi-arm comparisons. Assumption of consistency was satisfied for each treatment ( p > 0.05). Tests for inconsistency between direct and indirect estimates were not significant ( p > 0.05), thus indirect and direct estimates were not different to direct evidence. Inconsistency tables can be found as Extended data (see Tables S1 and S2).
Loop-specific heterogeneity was explored using inconsistency plots (see Extended data, Figure S7 and S8). Within the depressive symptoms network, inconsistency factors ( IF) did not indicate high inconsistency ( IF = 0.00 to 0.65) or loop-specific heterogeneity (τ 2 = 0.05 to 0.28). The AER-MB-UC loop departed from the minimum lower-bound confidence interval ( CI), yet fell short of presenting risk for heterogeneity ( IF = 0.54, τ 2 = 0.05, CI = 0.04, 1.03). Within the attrition network, the ratio of two odds ratios ( RoR) indicated a high degree of inconsistency for the AER-RES-UC ( RoR = 1.87) and MB-RES-UC ( RoR = 1.77) loops. Each were interpreted as presenting elevated risk for heterogeneity, which were subsequently downgraded during GRADE assessment. All remaining loops satisfied assumption of consistency.
Publication bias and sensitivity analyses
Comparison-adjusted funnel plots were used to detect publication bias and small-study effects. Funnel plots were roughly symmetrical for both depressive symptoms and attrition, indicative of low risk of publication bias and no presence of small-study effects. See Figure 4 for the depressive symptoms network and Extended data for the attrition network (see Figure S9).
Figure 4. Comparison-adjusted funnel plot for the depressive symptoms network.
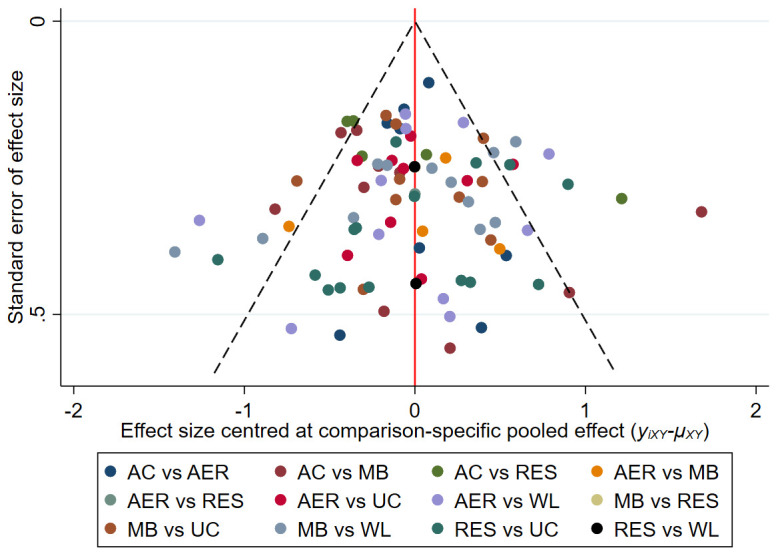
The red vertical line represents the null hypothesis that independent effect size estimates are not different to comparison-specific pooled estimates. AC = attention-control; AER = aerobic; MB = mind-body; RES = resistance; UC = usual care; WL = wait-list.
In order to test transitivity across networks, potential effect modifiers were tested with meta-regression sensitivity analyses for the entire pool of studies and separately for each exercise comparison (aerobic vs. resistance vs. mind-body). No significant modifying effects were observed for the pool of studies or any separate exercise comparison for age, gender, source of participants, length of intervention, format of program, exercise intensity, frequency, duration, adherence, year of study, risk of bias, publication status, intention-to-treat analysis, nor cluster design, indicating that the assumption of transitivity was upheld. Full analyses are presented as Extended data (see Tables S6– S8).
Results of the network meta-analysis
Data pooled from 69 eligible studies provided a total of 88 individual comparisons for depressive symptoms and 76 individual comparisons for study attrition. Table 3 presents the network meta-analysis of depressive symptoms and attrition. Network estimates were calculated to establish relative effectiveness between pairs of comparisons.
Table 3. League table for head-to-head comparisons.
| Wait-list | -0.11
(-0.40, 0.17) |
-0.45
(-0.77, -0.13) |
-0.51
(-0.74, -0.27) |
-0.39
(-0.63, -0.14) |
-0.12
(-0.41, 0.18) |
| 1.10
(0.48, 2.48) |
Usual care | -0.34
(-0.57, -0.10) |
-0.39
(-0.62, -0.16) |
-0.27
(-0.51, -0.04) |
-0.00
(-0.27, 0.27) |
| 0.74
(0.30, 1.83) |
0.68
(0.37, 1.26) |
Resistance | -0.06
(-0.33, 0.22) |
0.06
(-0.22, 0.35) |
0.33
(0.05, 0.61) |
| 1.00
(0.50, 2.01) |
0.92
(0.47, 1.77) |
1.35
(0.61, 2.99) |
Mind-body | 0.12
(-0.12, 0.35) |
0.39
(0.15, 0.63) |
| 1.03
(0.50, 2.14) |
0.94
(0.48, 1.84) |
1.39
(0.62, 3.10) |
1.03
(0.51, 2.07) |
Aerobic | 0.27
(0.02, 0.52) |
| 1.03
(0.41, 2.57) |
0.94
(0.41, 2.12) |
1.38
(0.60, 3.19) |
1.02
(0.47, 2.23) |
1.00
(0.46, 2.15) |
Attention-control |
Note. Depressive symptoms (upper) are reported as Hedges’ g and 95% confidence intervals. Negative scores indicate a greater decrease in depressive symptoms for the column group. Attrition (lower) is reported as odds ratios and 95% confidence intervals. An odds ratio lower than 1 indicate a greater attrition for the column-defining comparison.
Each exercise type effectively reduced depressive symptoms compared with control conditions (see Figure 5). Ranking of treatments for depressive symptoms are presented on surface under the cumulative ranking curve (SUCRA) plots of ranked mean values, which can be found as Extended data (see Figure S11 and Table S3). Ranked order of quantitative values determined mind-body exercise to be the most effective type of exercise to mitigate depressive symptoms, followed closely by resistance and aerobic exercise, respectively. The magnitude of study effect did not reach statistical threshold to favour any individual exercise treatment.
Figure 5. Predictive interval plot for the depressive symptoms network.
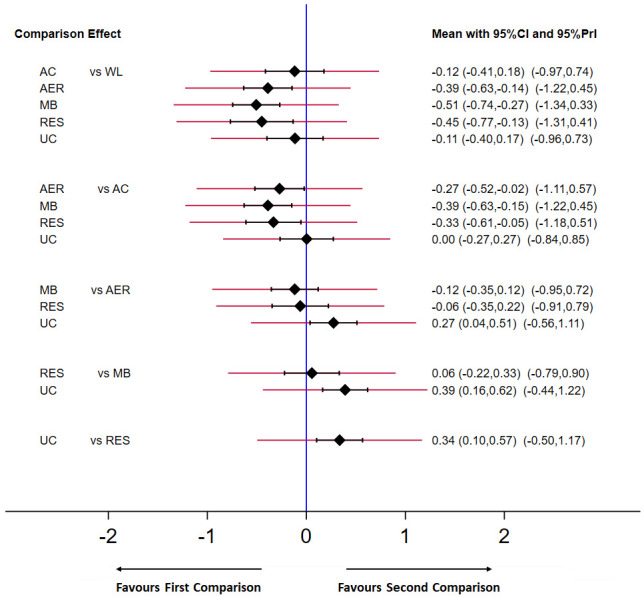
Black diamonds represent the difference in the effect size estimate (Hedges’ g). Narrow horizontal lines represent the confidence intervals (95% CI) and the wider horizontal lines represent the prediction intervals (95% PrI). The blue vertical line represents the null hypothesis (Hedges’ g = 0). Negative scores indicate a greater decrease in depressive symptoms for the comparison (left) group. AC = attention-control; AER = aerobic; MB = mind-body; RES = resistance; UC = usual care; WL = wait-list.
Resistance exercise demonstrated the highest study compliance compared with each of the other comparison groups, but the dispersion of effect estimates presented a level of heterogeneity that confounded any substantive differences. Comprehensive reporting of study attrition can be found as Extended data (see Figure S10) in addition to SUCRA plots and ranked order (see Figure S12 and Table S4).
Effectiveness vs. attrition
A two-dimensional clustered ranking plot was employed to illustrate the average reduction in depressive symptoms for each comparison, relative to average attrition rate. Figure 6 presents the ranking of the exercise conditions with respective control conditions in conjunction with SUCRA values for depressive symptoms (effectiveness) and attrition. The three exercise conditions amalgamated a single cluster and were more effective than control conditions.
Figure 6. Clustered ranking plot of the network presenting clustered analysis of SUCRA values for depressive symptoms (effectiveness) and attrition outcomes.
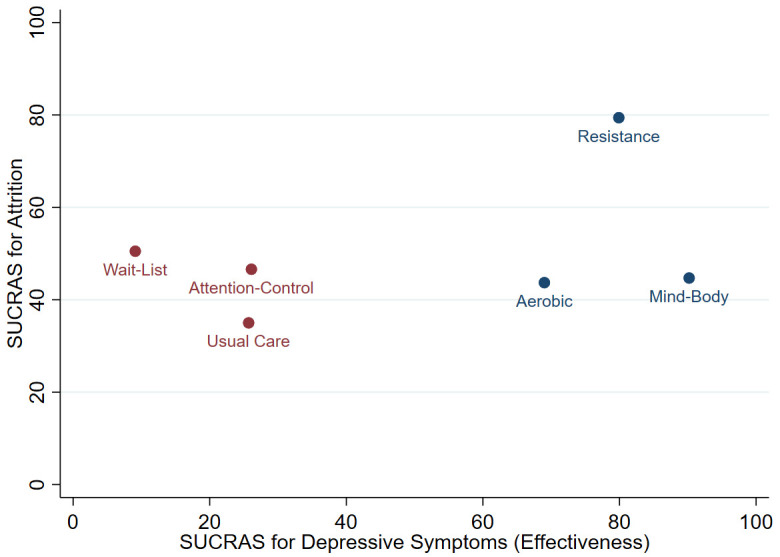
Each colour represents a group of treatments that belong to the same cluster. Treatments in the upper right corner represent effective treatments with low attrition.
GRADE assessment
The certainty of evidence was assessed with the GRADE approach 130 . All control comparisons in the depressive symptoms network were rated as high or moderate certainty, which were downgraded due to small sample size or potential risks of bias (i.e., attrition bias, detection bias, or bias resulting from low adherence). Comparisons between exercise interventions were moderate-to-low certainty, resulting from imprecision in confidence intervals and small sample sizes. Detailed summary of the depressive symptoms network is presented in Table 4. Estimates of the attrition network were downgraded due to inconsistency and imprecision, which reflect moderate to very low confidence this outcome (see Extended data, Table S5).
Table 4. Summary of GRADE assessment for the certainty in depressive symptoms estimates.
| Comparison Effect | Number of
participants |
Number of direct
comparisons |
Nature of
evidence |
Certainty | Reason for
downgrading |
|---|---|---|---|---|---|
| Aerobic vs. wait-list | 371 vs. 357 | 11 | Mixed | High | None |
| Aerobic vs. usual care | 309 vs. 310 | 9 | Mixed | Moderate | Risk of bias a |
| Aerobic vs. attention-control | 431 vs. 472 | 8 | Mixed | High | None |
| Resistance vs. wait-list | 43 vs. 42 | 2 | Mixed | Moderate | Imprecision b |
| Resistance vs. usual care | 358 vs. 272 | 15 | Mixed | High | None |
| Resistance vs. attention-control | 267 vs. 274 | 5 | Mixed | Moderate | Risk of bias c |
| Mind-body vs. wait-list | 380 vs. 363 | 12 | Mixed | High | None |
| Mind-body vs. usual care | 358 vs. 356 | 10 | Mixed | Moderate | Risk of bias d |
| Mind-body vs. attention-control | 298 vs. 265 | 10 | Mixed | High | None |
| Aerobic vs. resistance | 24 vs. 23 | 1 | Mixed | Low | Imprecision b e |
| Aerobic vs. mind-body | 90 vs. 83 | 4 | Mixed | Moderate | Imprecision e |
| Resistance vs. mind-body | 33 vs. 33 | 1 | Mixed | Low | Imprecision b e |
aPotential attrition bias due to high number of studies with incomplete outcome data. bSmall sample size. cPotential risk of bias due to high number of studies with low adherence. dPotential detection bias due to high number of studies without blinding of outcome assessment. eConfidence intervals include values favouring either treatment.
Generalisation for all adults aged ≥ 65 years
Figure 7 presents collective representation for all adults aged ≥ 65 years. This comparison employs scaled distribution (Hedges’ g) for the present data and that of clinically depressed older adults 23 .
Figure 7. Forest plot depicting juxtaposed collective RCT evidence of older adults ( n = 5,975) in the depressive symptoms network.
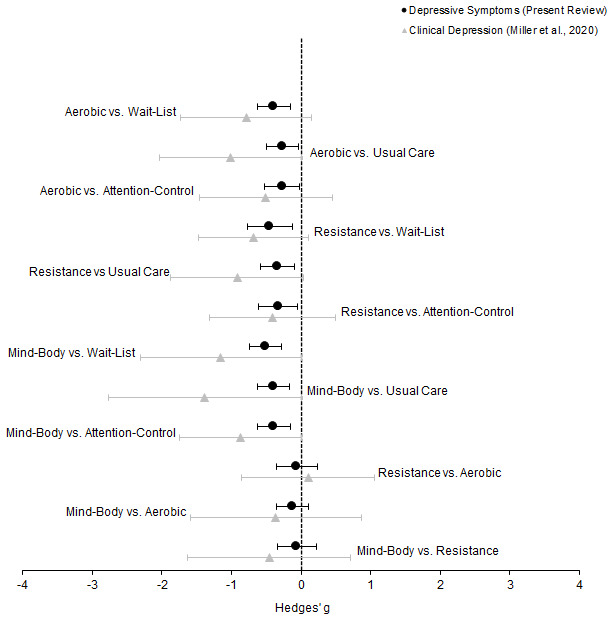
Circles represent the difference in the effect size estimate (Hedges’ g) for those with depressive symptomology but not diagnosed with clinical depression ( n = 5,379). Triangles represent the difference in the effect size estimate (Hedges’ g) for those with clinical depression ( n = 596; effect sizes adapted from Miller et al., 2020). Horizontal lines represent the confidence intervals (95% CI). The dotted vertical line represents the null hypothesis (Hedges’ g = 0). Negative scores indicate a greater decrease in depressive symptoms for the left group, and vice versa.
Discussion
The present review offers new information for general exercise prescription to support mental health outcomes for adults aged ≥ 65 years. Specifically, (i) aerobic, resistance, and mind-body each demonstrate equivalent benefit to mitigate symptoms of depression in adults aged ≥ 65 years, (ii) compliance to exercise treatment is notably encouraging for each exercise types, and (iii) when combined with the pool of data from clinically depressed older adults 23 , the effectiveness of aerobic, resistance, and mind-body exercise is comparably consistent for all adults aged ≥ 65 years. These findings should provide reassurance for personnel and stakeholders in healthy ageing to encourage exercise prescription from a point of pragmatism and in collaboration with patient preference.
Theoretical implications for the current findings
Behavioural and physiological research 24, 25, 27, 28 coalesce to provide ample reasoning to separate participant cohorts with existing clinical depression from those without diagnosis. This study is no different. In agreement with findings from previous research 13, 14, 35 , aerobic and resistance exercise demonstrated similar treatment effectiveness in older samples aged ≥ 65 years (Hedges’ g = -0.06, PrI = -0.91, 0.79). Exercise characteristics (i.e., intensity, frequency, duration, etc.) are often similar between aerobic and resistance exercise, representing two sides of the same coin. Meta-analytical data 15 on older adults with existing clinical depression observed that exercise programs incorporating a combination of aerobic and resistance training were most beneficial. Although the synergistic effects of combined exercise types were beyond the scope of the current review, it is conceivable that aerobic and resistance exercise may complement one another in an exercise intervention.
Pooled direct and indirect estimates marginally favoured treatment with mind-body exercise over either aerobic (Hedges’ g = -0.12, PrI = -0.95, 0.72) or resistance exercise (Hedges’ g = -0.06, PrI = -0.90, 0.79). However, it must be noted that the magnitude of effect falls short of being statistically different between groups and should be considered equivalent. Certainty of evidence is moderate, due to the dispersion in effect size estimates resulting in imprecision. Direct comparisons from multi-arm RCTs have offered mind-body exercise to be more effective than aerobic 92 or resistance 45 exercise. Moreover, subgroup analyses 16 have indicated that clinically depressed older adults respond more favourably to mind-body exercise, but this hypothesis has not be substantiated 4 .
Since mind-body exercise engages low intensity muscular activity (i.e., yoga, Tai Chi, qigong), the novel evidence in the current systematic analysis challenges the perception that exercise intensity is the primary mechanism for the antidepressive effect of exercise. Rather, mind-body exercise combines the mental and physical aspects of exercise, which may result in similar antidepressive effects to higher intensity exercise 135, 136 . Critical to these mental aspects is interoceptive sensations such as an internally directed focus on breathing and proprioception, which have previously been linked to the resilience of depressive states 137, 138 . Thus, it is plausible that mind-body exercise allows older adults to regulate negative mood states, though the relative contribution of mindfulness per se remains to be elucidated 139 .
Other important determinants of successful programming include study attrition, adherence rate, and adverse events. Here, we anticipated that each exercise condition would demonstrate lower compliance to treatment than wait-list, usual care, and attention-control comparisons. Contrary to expectations, pooled direct and indirect estimates indicated that study attrition was comparable for all comparisons apart from resistance exercise, which offered a higher degree of compliance. However, on deeper scrutiny of absolute sample size, any differences observed in attrition become abrogated due to relatively smaller participant numbers within the resistance exercise studies ( n = 705) compared with aerobic ( n = 1,143) or mind-body ( n = 1,005). Thus, in consideration of the wide dispersion in effect size estimates and a moderate risk of bias in individual studies, there are limitations in the certainty to confidently conclude substantive difference in study attrition between any comparisons.
Each of the three types of exercise had similar adherence rates and time spent per week (calculated as frequency multiplied by duration). Interestingly, mind-body exercise had relatively shorter intervention length than either aerobic or resistance exercise ( M diff = 6.09 and 4.33 weeks, respectively) despite having a greater reduction in depressive symptoms. This could be explained by (i) mind-body exercise having the same antidepressive effects in a shorter time than aerobic and resistance exercise, or (ii) a potential plateau effect whereby the antidepressive effects reach a maximum threshold during the first 10–15 weeks of an exercise program and are then maintained during the remaining weeks. Either way, it seems plausible that mind-body exercise provides a slightly more effective intervention against depressive symptoms in older populations without clinical depression, but that these treatment effects are not substantive enough to constitute statistical difference (we direct the reader to the Extended data for further discussion relating to potential mechanisms for the antidepressant effects of exercise in older adults).
Practical considerations
With consideration to projected population estimates over the next decade and the consequential demand on healthcare services 1, 8 , the findings from this network meta-analysis offer a message of support for exercise prescription to promote mentally healthy ageing. When considering the collective findings of the present review in conjunction with the recent network meta-analysis in clinically depressed adults aged ≥ 65 years 23 , stakeholders in healthy ageing and exercise prescription have encouraging pooled RCT evidence for the antidepressant effects of either aerobic, resistance, or mind-body exercise for older adults across the mental health continuum.
Treatment safety is a matter of ongoing importance in gerontological health, and exercise treatment programs are no different. Systematic scrutiny of the included RCTs found that study participants reported no major adverse events and only a few minor somatic complaints ( n = 28). Taken together, this provides encouraging support for personnel wanting to safely prescribe exercise-based intervention programs in older populations. Of course, there is always a possibility of underreporting adverse events in clinical trials, and the present review was no exception. Therefore, the importance of reporting event outcomes, adverse or otherwise, cannot be understated. In fact, there is a known phenomenon in geriatric exercise research whereby adverse events are often underreported because authors do not consider minor adverse events to be noteworthy and/or essential to the primary purpose of the trial 140 , giving rise to an ongoing issue that will not be corrected until all studies routinely report event outcomes.
Nevertheless, participants engaging in aerobic exercise reported the least adverse events ( n = 3), including minor medical attention and hip pain. Amongst studies included in this meta-analysis, aerobic exercise predominantly involved walking and stationary cycling, which may reflect a safe and natural form of exercise for older adults. Resistance exercise was typically associated with participants experiencing mild muscular pain and falls ( n = 16), which may be explained by the progressive overloading of resistance-based training. Notably, incidents of falling were reported in an unsupervised exercise program. Finally, mind-body exercise was typically associated with different types of muscular pain and body strain ( n = 9). It is speculated that the higher rates of injury in mind-body exercise are predominantly because it incorporates flexibility, balance, and stability movements, which may be unique to older bodies. In general, exercise seems to be a relatively safe intervention for older adults living in both the local community and residential aged care, although intensity and supervision, particularly for resistance training, should be monitored to ensure falls and injury do not occur.
The present review has some notable advantages above a traditional pairwise meta-analysis. RCTs with considerable non-exercising components, such as those using a multicomponent exercise intervention, were excluded because they may have overestimated the magnitude of the true effect in past reviews. Specifically, it is likely that multicomponent exercise interventions such as laughter therapy 141 , depression awareness training 129 , or self-efficacy training 142 may have introduced a risk of bias by inflating the observed effectiveness of the exercise program on depressive symptoms through a secondary, complementary treatment effect. Pairwise meta-analysis also assumes that all control groups are the same, which is not always the case 18 . To manage heterogeneity from this assumption, control groups were separated into individual network comparisons. Taken together, the current findings provide a more accurate estimate of the true effects of exercise on depressive symptoms in adults aged ≥ 65 years.
Limitations and future directions
The present network meta-analysis is not without limitations. As study participants and personnel cannot be successfully blinded, there is an inherent risk of performance bias. It is also believed that many exercise-based interventions have a small number of participants, shorter follow-up, and do not adequately conceal randomisation 143 , which are all likely to reduce the quality of RCTs and increase the risk of bias. However, we mitigated the impact of this by comparing relative effects with multiple control groups in order to increase reliability and specificity. This, combined with the relatively low risk of bias in individual RCTs, were extremely important in minimising overall risk of bias and achieving accurate effect comparisons in the present review.
Since most RCTs did not explicitly describe the exclusion of participants with ongoing diagnosis of clinical depression, there was potential contamination with data from participants with existing clinical diagnosis and medical treatment that may have been underreported. Here, this was managed by explicitly separating (i) participants without clinically diagnosed depression from (ii) participants with clinical depression as described in another recent network meta-analysis 23 . Within this review, we further mitigated this effect modifier by only including RCTs, where this risk would be counter balanced with respective control participants. Similarly, in the absence of evidence to the contrary, and confirmed by separate analysis (see Extended data), we selected to include a small number of trials where participants met existing criteria in the presence of co-morbidity (i.e., cognitive decline, Parkinson’s disease, dementia). Despite a lack of impact here, we offer this as a cautionary note should evidence of differential antidepressant response to exercise become available for these subgroups, and encourage researchers to report all ongoing pharmacological regimens and co-morbidities in RCTs recruiting older participants.
Although modifying effects were explored using meta-regression, potential compounding effects from exercise modifiers (e.g., fitness improvements, length of program, session frequency and duration, exercise intensity, supervision, group format) were outside the scope of our network meta-analysis. There has been a modicum of such exploration in subgroup and meta-regression analyses of previous reviews 4, 16, 35 , providing researchers with an encouraging opportunity in their planning of future similar work. Future meta-analyses with extensive subgroup analyses should explain the heterogeneity of effect sizes between similar exercise intervention studies in older persons.
Conclusions
Pooled RCT evidence highlights that each individual exercise mode (aerobic, resistance, and mind-body) demonstrate equivalence to mitigate symptoms of depression in older adults. As each exercise treatment demonstrated encouraging levels of treatment compliance, we endorse personnel and stakeholders in healthy ageing to encourage individual/patient preference when prescribing exercise to older adults ≥ 65 years presenting with depressive symptomology.
Data availability
Underlying data
Figshare: Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: A systematic review and network meta-analysis of randomised controlled trials (extended data). https://doi.org/10.6084/m9.figshare.12998549.v3 32 .
This project contains the following underlying data:
-
-
Data File D1 (PDF file containing raw outcome data)
-
-
STATA/SE 15.1 files (DTA files containing raw data)
Extended data
Figshare: Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: A systematic review and network meta-analysis of randomised controlled trials (extended data). https://doi.org/10.6084/m9.figshare.12998549.v3 32 .
This project contains the following extended data:
-
-
Supplementary File S1 (PDF file containing additional information, tables, and figures not in the main manuscript)
Reporting guidelines
Figshare: PRISMA-NMA checklist for “Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: A systematic review and network meta-analysis of randomised controlled trials (extended data). https://doi.org/10.6084/m9.figshare.12998549.v3 32
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgments
The authors graciously acknowledge the skilled contributions of Dr. Beyon Miloyan for his technical guidance, thoughtful discussions, and expert critique of the present work.
Funding Statement
Kyle J. Miller was supported by an Australian Government Research Training Program (RTP) Fee-Offset Scholarship through Federation University Australia.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved]
References
- 1. United Nations DoEaSA, Population Division: World Population Prospects 2019. Edition. Rev. 1.2019. Reference Source [Google Scholar]
- 2. Tari AR, Nauman J, Zisko N, et al. : Temporal changes in cardiorespiratory fitness and risk of dementia incidence and mortality: a population-based prospective cohort study. Lancet Public Health. 2019;4(11):e565–e74. 10.1016/S2468-2667(19)30183-5 [DOI] [PubMed] [Google Scholar]
- 3. Murri MB, Ekkekakis P, Menchetti M, et al. : Physical exercise for late-life depression: Effects on symptom dimensions and time course. J Affect Disord. 2018;230:65–70. 10.1016/j.jad.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 4. Rhyner KT, Watts A: Exercise and Depressive Symptoms in Older Adults: A Systematic Meta-Analytic Review. J Aging Phys Act. 2016;24(2):234–46. 10.1123/japa.2015-0146 [DOI] [PubMed] [Google Scholar]
- 5. Barua A, Ghosh MK, Kar N, et al. : Prevalence of depressive disorders in the elderly. Ann Saudi Med. 2011;31(6):620–4. 10.4103/0256-4947.87100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luppa M, Sikorski C, Luck T, et al. : Age- and gender-specific prevalence of depression in latest-life--systematic review and meta-analysis. J Affect Disord. 2012;136(3):212–21. 10.1016/j.jad.2010.11.033 [DOI] [PubMed] [Google Scholar]
- 7. Volkert J, Schulz H, Härter M, et al. : The prevalence of mental disorders in older people in Western countries - A meta-analysis. Ageing Res Rev. 2013;12(1):339–53. 10.1016/j.arr.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 8. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Psychiatric Association: Practice guideline for the treatment of patients with Major Depressive Disorder. 3 ed. Washington, DC: American Psychiatric Association;2010. Reference Source [Google Scholar]
- 10. Stubbs B, Vancampfort D, Hallgren M, et al. : EPA guidance on physical activity as a treatment for severe mental illness: A meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry. 2018;54:124–44. 10.1016/j.eurpsy.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization: Global recommendations on physical activity for health. Geneva, Switzerland: World Health Organization;2010. Reference Source [PubMed] [Google Scholar]
- 12. Knowles AM, Herbert P, Easton C, et al. : Impact of low-volume, high-intensity interval training on maximal aerobic capacity, health-related quality of life and motivation to exercise in ageing men. Age (Dordr). 2015;37(2):25. 10.1007/s11357-015-9763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martins R, Silva MCE, Pindus D, et al. : Effects of strength and aerobic-based training on functional fitness, mood and the relationship between fatness and mood in older adults. J Sports Med Phys Fitness. 2011;51(3):489–96. [PubMed] [Google Scholar]
- 14. Penninx BWJH, Rejeski WJ, Pandya J, et al. : Exercise and depressive symptoms: A comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P124–32. 10.1093/geronb/57.2.p124 [DOI] [PubMed] [Google Scholar]
- 15. Bridle C, Spanjers K, Patel S, et al. : Effect of exercise on depression severity in older people: Systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201(3):180–5. 10.1192/bjp.bp.111.095174 [DOI] [PubMed] [Google Scholar]
- 16. Heinzel S, Lawrence JB, Kallies G, et al. : Using exercise to fight depression in older adults: A systematic review and meta-analysis. GeroPsych. 2015;28(4):149–62. 10.1024/1662-9647/a000133 [DOI] [Google Scholar]
- 17. Bucher HC, Guyatt GH, Griffith LE, et al. : The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91. 10.1016/s0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 18. Pagoto SL, McDermott MM, Reed G, et al. : Can attention control conditions have detrimental effects on behavioral medicine randomized trials? Psychosom Med. 2013;75(2):137–43. 10.1097/PSY.0b013e3182765dd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caldwell DM, Ades AE, Higgins JPT: Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. 10.1136/bmj.331.7521.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salanti G, Higgins JPT, Ades AE, et al. : Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17(3):279–301. 10.1177/0962280207080643 [DOI] [PubMed] [Google Scholar]
- 21. National Health Lung and Blood Institute: Your guide to physical activity and your heart. (NIH Publication No. 06–5714). Washington, DC: U.S. Department of Health and Human Services;2006. Reference Source [Google Scholar]
- 22. US Department of Health and Human Services: Physical activity guidelines for Americans. 2 ed. Washington, DC: U.S. Department of Health and Human Services;2018. Reference Source [Google Scholar]
- 23. Miller KJ, Gonçalves-Bradley DC, Areerob P, et al. : Comparative effectiveness of three exercise types to treat clinical depression in older adults: A systematic review and network meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;58:100999. 10.1016/j.arr.2019.100999 [DOI] [PubMed] [Google Scholar]
- 24. Jesulola E, Micalos P, Baguley IJ: Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 2018;341:79–90. 10.1016/j.bbr.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 25. Klein DN, Kotov R: Course of depression in a 10–year prospective study: Evidence for qualitatively distinct subgroups. J Abnorm Psychol. 2016;125(3):337–48. 10.1037/abn0000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YY, Stockings EA, Harris MG, et al. : The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol Med. 2019;49(1):92–102. 10.1017/S0033291718000557 [DOI] [PubMed] [Google Scholar]
- 27. Solomon A, Haaga DA, Arnow BA: Is clinical depression distinct from subthreshold depressive symptoms? A review of the continuity issue in depression research. J Nerv Ment Dis. 2001;189(8):498–506. 10.1097/00005053-200108000-00002 [DOI] [PubMed] [Google Scholar]
- 28. Ruscio AM: Normal Versus Pathological Mood: Implications for Diagnosis. Annu Rev Clin Psychol. 2019;15:179–205. 10.1146/annurev-clinpsy-050718-095644 [DOI] [PubMed] [Google Scholar]
- 29. Hutton B, Salanti G, Caldwell DM, et al. : The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 30. Cochrane Methods: Cochrane intervention review that compares multiple interventions.2014. Reference Source [Google Scholar]
- 31. Shenkin SD, Harrison JK, Wilkinson T, et al. : Systematic reviews: Guidance relevant for studies of older people. Age Ageing. 2017;46(5):722–8. 10.1093/ageing/afx105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller K, Areerob P, Hennessy D, et al. : Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: A systematic review and network meta-analysis of randomised controlled trials (extended data). 2020. 10.6084/m9.figshare.12998549.v3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blake H, Mo P, Malik S, et al. : How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;23(10):873–87. 10.1177/0269215509337449 [DOI] [PubMed] [Google Scholar]
- 34. Mura G, Carta MG: Physical activity in depressed elderly. A systematic review. Clin Pract Epidemiol Ment Health. 2013;9:125–35. 10.2174/1745017901309010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schuch FB, Vancampfort D, Rosenbaum S, et al. : Exercise for depression in older adults: A meta-analysis of randomized controlled trials adjusting for publication bias. Braz J Psychiatry. 2016;38(3):247–54. 10.1590/1516-4446-2016-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sjosten N, Kivela SL: The effects of physical exercise on depressive symptoms among the aged: A systematic review. Int J Geriatr Psychiatry. 2006;21(5):410–8. 10.1002/gps.1494 [DOI] [PubMed] [Google Scholar]
- 37. Abd El-Kader SM, Al-Jiffri OH: Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr Health Sci. 2016;16(4):1045–55. 10.4314/ahs.v16i4.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adler PA: The effects of Tai Chi on pain and function in older adults with osteoarthritis. Case Western Reserve University;2007. Reference Source [Google Scholar]
- 39. Angeles MAV, Jimenez JM, Sanchez JJG, et al. : Effects of a Pilates-based exercise program on mood states in older adults in Mexico. Retos-Nuevas Tendencias En Educacion Fisica Deporte Y Recreacion. 2016; (30):106–9. Reference Source [Google Scholar]
- 40. Ansai JH, Rebelatto JR: Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: A randomized controlled trial. Geriatr Gerontol Int. 2015;15(9):1127–34. 10.1111/ggi.12411 [DOI] [PubMed] [Google Scholar]
- 41. Antunes HKM, Stella SG, Santos RF, et al. : Depression, anxiety and quality of life scores in seniors after an endurance exercise program. Braz J Psychiatry. 2005;27(4):266–71. 10.1590/s1516-44462005000400003 [DOI] [PubMed] [Google Scholar]
- 42. Bernard P, Ninot G, Bernard PL, et al. : Effects of a six-month walking intervention on depression in inactive post-menopausal women: a randomized controlled trial. Aging Ment Health. 2015;19(6):485–92. 10.1080/13607863.2014.948806 [DOI] [PubMed] [Google Scholar]
- 43. Bethany KH: The effects of selected exercise modalities on stress, anxiety, and depression responses in the elderly [Thesis].2005. Reference Source [Google Scholar]
- 44. Blumenthal JA, Emery CF, Madden DJ, et al. : Long-term effects of exercise on psychological functioning in older men and women. J Gerontol. 1991;46(6):P352–61. 10.1093/geronj/46.6.p352 [DOI] [PubMed] [Google Scholar]
- 45. Bonura KB, Tenenbaum G: Effects of yoga on psychological health in older adults. J Phys Act Health. 2014;11(7):1334–41. 10.1123/jpah.2012-0365 [DOI] [PubMed] [Google Scholar]
- 46. Bostrom G, Conradsson M, Hornsten C, et al. : Effects of a high-intensity functional exercise program on depressive symptoms among people with dementia in residential care: A randomized controlled trial. Int J Geriatr Psychiatry. 2016;31(8):868–78. 10.1002/gps.4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bouaziz W, Schmitt E, Vogel T, et al. : Effects of a short-term Interval Aerobic Training Programme with active Recovery bouts (IATP-R) on cognitive and mental health, functional performance and quality of life: A randomised controlled trial in sedentary seniors. Int J Clin Pract. 2019;73(1):e13219. 10.1111/ijcp.13219 [DOI] [PubMed] [Google Scholar]
- 48. Brown AK, Liu-Ambrose T, Tate R, et al. : The effect of group-based exercise on cognitive performance and mood in seniors residing in intermediate care and self-care retirement facilities: a randomised controlled trial. Br J Sports Med. 2009;43(8):608–14. 10.1136/bjsm.2008.049882 [DOI] [PubMed] [Google Scholar]
- 49. Cancela JM, Ayán C, Varela S, et al. : Effects of a long-term aerobic exercise intervention on institutionalized patients with dementia. J Sci Med Sport. 2016;19(4):293–8. 10.1016/j.jsams.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 50. Chen KM, Chen MH, Chao HC, et al. : Sleep quality, depression state, and health status of older adults after silver yoga exercises: cluster randomized trial. Int J Nurs Stud. 2009;46(2):154–63. 10.1016/j.ijnurstu.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 51. Chen KM, Huang HT, Cheng YY, et al. : Sleep quality and depression of nursing home older adults in wheelchairs after exercises. Nurs Outlook. 2015;63(3):357–65. 10.1016/j.outlook.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 52. Chen KM, Kuo CC, Chang YH, et al. : Resistance Band Exercises Reduce Depression and Behavioral Problems of Wheelchair-Bound Older Adults with Dementia: A Cluster-Randomized Controlled Trial. J Am Geriatr Soc. 2017;65(2):356–63. 10.1111/jgs.14526 [DOI] [PubMed] [Google Scholar]
- 53. Paw MJMCA, van Poppel MNM, Twisk JWR, et al. : Effects of resistance and all-round, functional training on quality of life, vitality and depression of older adults living in long-term care facilities: a 'randomized' controlled trial [ISRCTN87177281]. BMC Geriatr. 2004;4:5. 10.1186/1471-2318-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Choi MJ, Sohng KY: The Effects of Floor-seated Exercise Program on Physical Fitness, Depression, and Sleep in Older Adults: A Cluster Randomized Controlled Trial. Int J Gerontol. 2018;12(2):116–21. 10.1016/j.ijge.2017.06.003 [DOI] [Google Scholar]
- 55. Clegg A, Barber S, Young J, et al. : The Home-based Older People's Exercise (HOPE) trial: a pilot randomised controlled trial of a home-based exercise intervention for older people with frailty. Age Ageing. 2014;43(5):687–95. 10.1093/ageing/afu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Collier CD: Isotonic resistance training related to functional fitness, physical self-efficacy, and depression in adults ages 65–85. US: ProQuest Information & Learning;1998. Reference Source [Google Scholar]
- 57. Conradsson M, Littbrand H, Lindelof N, et al. : Effects of a high-intensity functional exercise programme on depressive symptoms and psychological well-being among older people living in residential care facilities: A cluster-randomized controlled trial. Aging Ment Health. 2010;14(5):565–76. 10.1080/13607860903483078 [DOI] [PubMed] [Google Scholar]
- 58. Cramer H, Pokhrel B, Fester C, et al. : A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psychooncology. 2016;25(4):412–20. 10.1002/pon.3927 [DOI] [PubMed] [Google Scholar]
- 59. de Lima TA, Ferreira-Moraes R, Alves W, et al. : Resistance training reduces depressive symptoms in elderly people with Parkinson disease: A controlled randomized study. Scand J Med Sci Sports. 2019;29(12):1957–1957. 10.1111/sms.13528 [DOI] [PubMed] [Google Scholar]
- 60. Donesky-Cuenco D, Nguyen HQ, Paul S, et al. : Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med. 2009;15(3):225–34. 10.1089/acm.2008.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dong L, Lee JB, Kim YK, et al. : The effects of health Qigong training of elderly single women on pain consciousness and depression. Int J Appl Sports Sci. 2013;25(2):118–26. 10.24985/IJASS.2013.25.2.118 [DOI] [Google Scholar]
- 62. Dorner T, Kranz A, Zettl-Wiedner K, et al. : The effect of structured strength and balance training on cognitive function in frail, cognitive impaired elderly long-term care residents. Aging Clin Exp Res. 2007;19(5):400–5. 10.1007/BF03324721 [DOI] [PubMed] [Google Scholar]
- 63. Emery CF, Gatz M: Psychological and cognitive effects of an exercise program for community-residing older adults. Gerontologist. 1990;30(2):184–8. 10.1093/geront/30.2.184 [DOI] [PubMed] [Google Scholar]
- 64. Eyigor S, Karapolat H, Durmaz B, et al. : A randomized controlled trial of Turkish folklore dance on the physical performance, balance, depression and quality of life in older women. Arch Gerontol Geriatr. 2009;48(1):84–8. 10.1016/j.archger.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 65. Eyre HA, Siddarth P, Acevedo B, et al. : A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr. 2017;29(4):557–67. 10.1017/S1041610216002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fakhari M: Effects of Tai Chi exercise on depression in older adults: A randomized controlled trial. Bali Medical Journal. 2017;6(3):679–83. 10.15562/bmj.v6i3.706 [DOI] [Google Scholar]
- 67. Fransen M, Nairn L, Winstanley J, et al. : Physical activity for osteoarthritis management: A randomized controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum. 2007;57(3):407–14. 10.1002/art.22621 [DOI] [PubMed] [Google Scholar]
- 68. Frye B, Scheinthal S, Kemarskaya T, et al. : Tai chi and low impact exercise: Effects on the physical functioning and psychological well-being of older people. J Appl Gerontol. 2007;26(5):433–53. 10.1177/0733464807306915 [DOI] [Google Scholar]
- 69. Gary RA, Sueta CA, Dougherty M, et al. : Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33(4):210–8. 10.1016/j.hrtlng.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 70. Gusi N, Reyes MC, Gonzalez-Guerrero JL, et al. : Cost-utility of a walking programme for moderately depressed, obese, or overweight elderly women in primary care: A randomised controlled trial. BMC Public Health. 2008;8:231. 10.1186/1471-2458-8-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hars M, Herrmann FR, Gold G, et al. : Effect of music-based multitask training on cognition and mood in older adults. Age Ageing. 2014;43(2):196–200. 10.1093/ageing/aft163 [DOI] [PubMed] [Google Scholar]
- 72. Hsu CY, Moyle W, Cooke M, et al. : Seated Tai Chi versus usual activities in older people using wheelchairs: A randomized controlled trial. Complement Ther Med. 2016;24:1–6. 10.1016/j.ctim.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 73. Irwin MR, Olmstead R: Mitigating cellular inflammation in older adults: A randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20(9):764–72. 10.1097/JGP.0b013e3182330fd3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Irwin MR, Olmstead R, Carrillo C, et al. : Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: A randomized controlled comparative efficacy trial. Sleep. 2014;37(9):1543–52. 10.5665/sleep.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kekalainen T, Kokko K, Sipila S, et al. : Effects of a 9-month resistance training intervention on quality of life, sense of coherence, and depressive symptoms in older adults: Randomized controlled trial. Qual Life Res. 2018;27(2):455–65. 10.1007/s11136-017-1733-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim YS, O'Sullivan DM, Shin SK: Can 24 weeks strength training reduce feelings of depression and increase neurotransmitter in elderly females? Exp Gerontol. 2019;115:62–8. 10.1016/j.exger.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 77. Kohut ML, Lee W, Martin A, et al. : The exercise-induced enhancement of influenza immunity is mediated in part by improvements in psychosocial factors in older adults. Brain Behav Immun. 2005;19(4):357–66. 10.1016/j.bbi.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 78. Krishnamurthy MN, Telles S: Assessing depression following two ancient Indian interventions: Effects of yoga and ayurveda on older adults in a residential home. J Gerontol Nurs. 2007;33(2):17–23. 10.3928/00989134-20070201-05 [DOI] [PubMed] [Google Scholar]
- 79. Kuo MC, Chen CM, Jeng C: A Randomized Controlled Trial of the Prescribed Stepper Walking Program in Preventing Frailty Among the Dwelling Elderly Application of Comprehensive Geriatric Assessment. Top Geriatr Rehabil. 2018;34(3):223–33. 10.1097/TGR.0000000000000198 [DOI] [Google Scholar]
- 80. Kupecz DB: Effects of a structured exercise program in older veteran patients.University of Northern Colorado;2001. Reference Source [Google Scholar]
- 81. Li F, Duncan TE, Duncan SC, et al. : Enhancing the psychological well-being of elderly individuals through Tai Chi exercise: A latent growth curve analysis. Struct Equ Modeling. 2001;8(1):53–83. 10.1207/S15328007SEM0801_4 [DOI] [Google Scholar]
- 82. Lin MR, Wolf SL, Hwang HF, et al. : A randomized, controlled trial of fall prevention programs and quality of life in older fallers. J Am Geriatr Soc. 2007;55(4):499–506. 10.1111/j.1532-5415.2007.01146.x [DOI] [PubMed] [Google Scholar]
- 83. Lincoln AK, Shepherd A, Johnson PL, et al. : The impact of resistance exercise training on the mental health of older Puerto Rican adults with type 2 diabetes. J Gerontol B Psychol Sci Soc Sci. 2011;66(5):567–70. 10.1093/geronb/gbr034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma C, Zhou W, Tang Q, et al. : The impact of group-based Tai chi on health-status outcomes among community-dwelling older adults with hypertension. Heart Lung. 2018;47(4):337–44. 10.1016/j.hrtlng.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 85. Maki Y, Ura C, Yamaguchi T, et al. : Effects of intervention using a community-based walking program for prevention of mental decline: A randomized controlled trial. J Am Geriatr Soc. 2012;60(3):505–10. 10.1111/j.1532-5415.2011.03838.x [DOI] [PubMed] [Google Scholar]
- 86. Martinez N, Martorell C, Espinosa L, et al. : Impact of Qigong on quality of life, pain and depressive symptoms in older adults admitted to an intermediate care rehabilitation unit: A randomized controlled trial. Aging Clin Exp Res. 2015;27(2):125–30. 10.1007/s40520-014-0250-y [DOI] [PubMed] [Google Scholar]
- 87. McMurdo ME, Rennie L: A controlled trial of exercise by residents of old people's homes. Age Ageing. 1993;22(1):11–5. 10.1093/ageing/22.1.11 [DOI] [PubMed] [Google Scholar]
- 88. Monga U, Garber SL, Thornby J, et al. : Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88(11):1416–22. 10.1016/j.apmr.2007.08.110 [DOI] [PubMed] [Google Scholar]
- 89. Mortazavi SS, Mohammad K, Ardebili HE, et al. : Mental disorder prevention and physical activity in Iranian elderly. Int J Prev Med. 2012;3(Suppl 1):S64–72. [PMC free article] [PubMed] [Google Scholar]
- 90. Netz Y, Yaretzki A, Salganik I, et al. : The effect of supervised physical activity on cognitive and affective state of geriatric and psychogeriatric in-patients. Clin Gerontol. 1994;15(1):47–56. 10.1300/J018v15n01_06 [DOI] [Google Scholar]
- 91. Ng TP, Nyunt MSZ, Feng L, et al. : Multi-domains lifestyle interventions reduces depressive symptoms among frail and pre-frail older persons: Randomized controlled trial. J Nutr Health Aging. 2017;21(8):918–26. 10.1007/s12603-016-0867-y [DOI] [PubMed] [Google Scholar]
- 92. Oken BS, Zajdel D, Kishiyama S, et al. : Randomized, controlled, six-month trial of yoga in healthy seniors: Effects on cognition and quality of life. Altern Ther Health Med. 2006;12(1):40–7. [PMC free article] [PubMed] [Google Scholar]
- 93. Park J, Newman D, McCaffrey R, et al. : The effect of chair Yoga on biopsychosocial changes in English- and Spanish-speaking community-dwelling older adults with lower-extremity osteoarthritis. J Gerontol Soc Work. 2016;59(7–8):604–26. 10.1080/01634372.2016.1239234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Payne JK, Held J, Thorpe J, et al. : Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35(4):635–42. 10.1188/08.ONF.635-642 [DOI] [PubMed] [Google Scholar]
- 95. Pedersen MT, Vorup J, Nistrup A, et al. : Effect of team sports and resistance training on physical function, quality of life, and motivation in older adults. Scand J Med Sci Sports. 2017;27(8):852–64. 10.1111/sms.12823 [DOI] [PubMed] [Google Scholar]
- 96. Pinniger R, Brown RF, Thorsteinsson EB, et al. : Tango programme for individuals with age-related macular degeneration. Br J Vis Impair. 2013;31(1):47–59. 10.1177/0264619612470651 [DOI] [Google Scholar]
- 97. Ramanathan M, Bhavanani AB, Trakroo M: Effect of a 12-week yoga therapy program on mental health status in elderly women inmates of a hospice. Int J Yoga. 2017;10(1):24–8. 10.4103/0973-6131.186156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roswiyani R, Hiew CH, Witteman CLM, et al. : Art activities and qigong exercise for the well-being of older adults in nursing homes in indonesia: A randomized controlled trial. Aging Ment Health. 2020;24(10):1569–1578. 10.1080/13607863.2019.1617239 [DOI] [PubMed] [Google Scholar]
- 99. Sahin UK, Kirdi N, Bozoglu E, et al. : Effect of low-intensity versus high-intensity resistance training on the functioning of the institutionalized frail elderly. Int J Rehabil Res. 2018;41(3):211–7. 10.1097/MRR.0000000000000285 [DOI] [PubMed] [Google Scholar]
- 100. Sattin RW, Easley KA, Wolf SL, et al. : Reduction in fear of falling through intense tai chi exercise training in older, transitionally frail adults. J Am Geriatr Soc. 2005;53(7):1168–78. 10.1111/j.1532-5415.2005.53375.x [DOI] [PubMed] [Google Scholar]
- 101. Sola-Serrabou M, Lopez JL, Valero O: Effectiveness of Training in the Elderly and its Impact on Health-related Quality of Life. Apunts Educacion Fisica Y Deportes. 2019; (137):30–42. 10.5672/apunts.2014-0983.cat [DOI] [Google Scholar]
- 102. Song D, Yu DSF: Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: A randomised controlled trial. Int J Nurs Stud. 2019;93:97–105. 10.1016/j.ijnurstu.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 103. Sparrow D, Gottlieb DJ, Demolles D, et al. : Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(11):1251–7. 10.1093/gerona/glr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Swoap RA, Norvell N, Graves JE, et al. : High versus moderate intensity aerobic exercise in older adults: Psychological and physiological effects. J Aging Phys Act. 1994;2(4):293–303. 10.1123/japa.2.4.293 [DOI] [Google Scholar]
- 105. Tapps TP: An Investigation into the Effects of Resistance Based Physical Activity Participation on Depression of Older Adults in a Long-term Care Facility. Annual in Therapeutic Recreation. 2013;21:63–72. Reference Source [Google Scholar]
- 106. Taylor-Piliae RE, Hoke TM, Hepworth JT, et al. : Effect of Tai Chi on physical function, fall rates and quality of life among older stroke survivors. Arch Phys Med Rehabil. 2014;95(5):816–24. 10.1016/j.apmr.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 107. Tsang HWH, Mok CK, Au Yeung YT, et al. : The effect of Qigong on general and psychosocial health of elderly with chronic physical illnesses: A randomized clinical trial. Int J Geriatr Psychiatry. 2003;18(5):441–9. 10.1002/gps.861 [DOI] [PubMed] [Google Scholar]
- 108. Tsang HWH, Lee JLC, Au DWH, et al. : Developing and testing the effectiveness of a novel health qigong for frail elders in Hong Kong: A preliminary study. Evid Based Complement Alternat Med. 2013;2013:827392. 10.1155/2013/827392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tsutsumi T, Don BM, Zaichkowsky LD, et al. : Comparison of high and moderate intensity of strength training on mood and anxiety in older adults. Percept Mot Skills. 1998;87(3 Pt 1):1003–11. 10.2466/pms.1998.87.3.1003 [DOI] [PubMed] [Google Scholar]
- 110. Vahlberg B, Cederholm T, Lindmark B, et al. : Short-term and long-term effects of a progressive resistance and balance exercise program in individuals with chronic stroke: A randomized controlled trial. Disabil Rehabil. 2017;39(16):1615–22. 10.1080/09638288.2016.1206631 [DOI] [PubMed] [Google Scholar]
- 111. Vankova H, Holmerova I, Machacova K, et al. : The effect of dance on depressive symptoms in nursing home residents. J Am Med Dir Assoc. 2014;15(8):582–7. 10.1016/j.jamda.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 112. Wang DS: The impact of a yoga intervention on the mental well-being and physical functioning in older adults living in the community.US: ProQuest Information & Learning; 2009. Reference Source [Google Scholar]
- 113. Witham MD, Gray JM, Argo IS, et al. : Effect of a seated exercise program to improve physical function and health status in frail patients > or = 70 years of age with heart failure. Am J Cardiol. 2005;95(9):1120–4. 10.1016/j.amjcard.2005.01.031 [DOI] [PubMed] [Google Scholar]
- 114. Yang KH, Kim YH, Lee MS: Efficacy of Qi-therapy (external Qigong) for elderly people with chronic pain. Int J Neurosci. 2005;115(7):949–63. 10.1080/00207450590901378 [DOI] [PubMed] [Google Scholar]
- 115. Yeh GY, Roberts DH, Wayne PM, et al. : Tai chi exercise for patients with chronic obstructive pulmonary disease: A pilot study. Respir Care. 2010;55(11):1475–82. [PMC free article] [PubMed] [Google Scholar]
- 116. Zanuso S, Sieverdes JC, Smith N, et al. : The effect of a strength training program on affect, mood, anxiety, and strength performance in older individuals. Int J Sport Psychol. 2012;43(1):53–66. Reference Source [Google Scholar]
- 117. O'Neill J, Tabish H, Welch V, et al. : Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. 10.1016/j.jclinepi.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 118. Borg GA: Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 119. Sigal RJ, Kenny GP, Wasserman DH, et al. : Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–39. 10.2337/diacare.27.10.2518 [DOI] [PubMed] [Google Scholar]
- 120. Higgins JP, Altman DG, Gotzsche PC, et al. : The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hedges LV: Distribution Theory for Glass's Estimator of Effect Size and Related Estimators. J Educ Stat. 1981;6(2):107–28. 10.3102/10769986006002107 [DOI] [Google Scholar]
- 122. Hedges LV, Olkin I: Statistical methods for meta-analysis.New York, NY: Academic Press;1985. 10.2307/1164953 [DOI] [Google Scholar]
- 123. Cohen J: Statistical power analysis for the behavioral sciences.2 ed. Hillsdale, NJ: Lawrence Erlbaum Associates;1988. Reference Source [Google Scholar]
- 124. Borenstein M, Hedges LV, Higgins JPT, et al. : Introduction to meta-analysis.Chichester, UK: John Wiley & Sons;2009. 10.1002/9780470743386 [DOI] [Google Scholar]
- 125. Costa MV, Diniz MF, Nascimento KK, et al. : Accuracy of three depression screening scales to diagnose major depressive episodes in older adults without neurocognitive disorders. Braz J Psychiatry. 2016;38(2):154–6. 10.1590/1516-4446-2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Smarr KL, Keefer AL: Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S454–66. 10.1002/acr.20556 [DOI] [PubMed] [Google Scholar]
- 127. Jansen JP, Naci H: Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11:159. 10.1186/1741-7015-11-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Higgins JP, Deeks JJ, Altman DG: Special topics in statistics. Cochrane handbook for systematic reviews of interventions. England: John Wiley & Sons;2008;481–529. 10.1002/9780470712184.ch16 [DOI] [Google Scholar]
- 129. Underwood M, Lamb SE, Eldridge S, et al. : Exercise for depression in elderly residents of care homes: A cluster-randomised controlled trial. Lancet. 2013;382(9886):41–9. 10.1016/S0140-6736(13)60649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Salanti G, Del Giovane C, Chaimani A, et al. : Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. StataCorp: Stata Statistical Software: Release 15.College Station, TX: StataCorp LLC;2017. [Google Scholar]
- 132. Netz Y, Wu MJ, Becker BJ, et al. : Physical activity and psychological well-being in advanced age: A meta-analysis of intervention studies. Psychol Aging. 2005;20(2):272–84. 10.1037/0882-7974.20.2.272 [DOI] [PubMed] [Google Scholar]
- 133. White IR: Multivariate random-effects meta-analysis. The Stata Journal. 2009;9(1):40–56. 10.1177/1536867X0900900103 [DOI] [Google Scholar]
- 134. Borenstein M, Hedges LV, Higgins JP, et al. : A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 135. La Forge R: Mind-body fitness: encouraging prospects for primary and secondary prevention. J Cardiovasc Nurs. 1997;11(3):53–65. 10.1097/00005082-199704000-00006 [DOI] [PubMed] [Google Scholar]
- 136. Prakhinkit S, Suppapitiporn S, Tanaka H, et al. : Effects of Buddhism walking meditation on depression, functional fitness, and endothelium-dependent vasodilation in depressed elderly. J Altern Complement Med. 2014;20(5):411–6. 10.1089/acm.2013.0205 [DOI] [PubMed] [Google Scholar]
- 137. Avery JA, Drevets WC, Moseman SE, et al. : Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76(3):258–66. 10.1016/j.biopsych.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Paulus MP, Stein MB: Interoception in anxiety and depression. Brain Struct Funct. 2010;214(5–6):451–63. 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li SYH, Bressington K: The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: A systematic review and meta-analysis. Int J Ment Health Nurs. 2019;28(3):635–56. 10.1111/inm.12568 [DOI] [PubMed] [Google Scholar]
- 140. Liu CJ, Latham N: Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch Phys Med Rehabil. 2010;91(9):1471–3. 10.1016/j.apmr.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 141. Hirosaki M, Ohira T, Kajiura M, et al. : Effects of a laughter and exercise program on physiological and psychological health among community-dwelling elderly in Japan: randomized controlled trial. Geriatr Gerontol Int. 2013;13(1):152–60. 10.1111/j.1447-0594.2012.00877.x [DOI] [PubMed] [Google Scholar]
- 142. Resnick B, Luisi D, Vogel A: Testing the Senior Exercise Self-efficacy Project (SESEP) for use with urban dwelling minority older adults. Public Health Nurs. 2008;25(3):221–34. 10.1111/j.1525-1446.2008.00699.x [DOI] [PubMed] [Google Scholar]
- 143. Daley A, Jolly K: Exercise to treat depression. BMJ. 2012;344:e3181. 10.1136/bmj.e3181 [DOI] [PubMed] [Google Scholar]