Abstract
Integrons confer a rapid adaptation capability to bacteria. Integron integrases are able to capture and shuffle novel functions embedded in cassettes. Here, we investigated cassette recruitment in the Vibrio cholerae chromosomal integron during horizontal transfer. We demonstrated that the endogenous integrase expression is sufficiently triggered, after SOS response induction mediated by the entry of cassettes during conjugation and natural transformation, to mediate significant cassette insertions. These insertions preferentially occur at the attIA site, despite the presence of about 180 attC sites in the integron array. Thanks to the presence of a promoter in the attIA site vicinity, all these newly inserted cassettes are expressed and prone to selection. We also showed that the RecA protein is critical for cassette recruitment in the V. cholerae chromosomal integron but not in mobile integrons. Moreover, unlike the mobile integron integrases, that of V. cholerae is not active in other bacteria. Mobile integrons might have evolved from the chromosomal ones by overcoming host factors, explaining their large dissemination in bacteria and their role in antibioresistance expansion.
INTRODUCTION
Mobile Genetic Elements (MGE) widely contribute to the evolution of bacterial genomes, notably by conveying adaptive traits such as the ability to resist to antibiotic treatments (1). This can have dramatic consequences, especially when occurring in pathogenic bacteria. Integrons are genetic structures that are considered as major contributors in the rise of multiple antibiotic resistance in Gram-negative bacteria. These genetic systems were discovered in the late 80s and described as platforms involved in the capture, stockpiling and expression of antibiotic resistance genes, embedded in structures termed ‘cassettes’ (2). These were later referred to as Mobile Integrons (MIs) because of their associations with transposable elements and conjugative plasmids. Larger integrons located on bacterial chromosomes were discovered later, the superintegron of Vibrio cholerae being the first identified (3). This superintegron is located on chromosome 2 of V. cholerae and contains about 180 gene cassettes coding mainly for proteins with no homologs in the databases or for proteins of unknown functions. In contrast to their mobile counterparts and to refer to their location, such structures are termed Sedentary Chromosomal Integrons (SCIs). They are common features of bacterial genomes from the Vibrio genus and are generally distributed in several genomes of β- and γ-proteobacteria (4,5). These large SCIs were proposed to be the ancestors of MIs (6) and the stockpiling capacity from both types of integrons suggests that they may have distinct but complementary roles (7). Indeed, large SCIs such as those found in genomes of Vibrio species, could constitute a reservoir of gene cassettes that can be captured and spread by MIs (7,8).
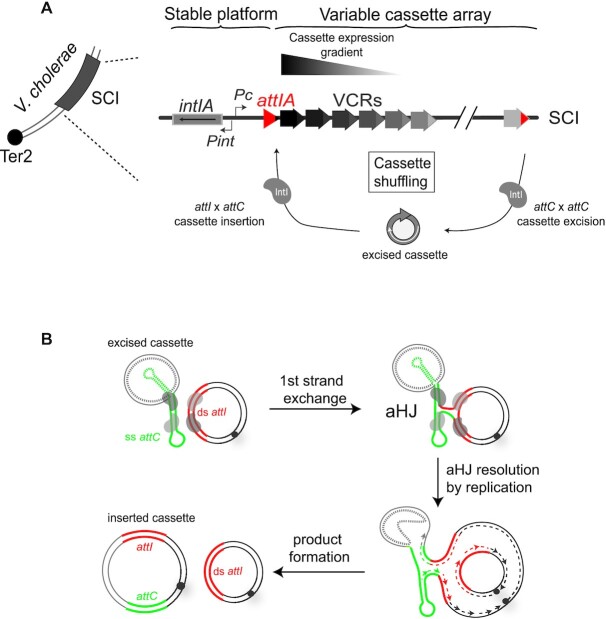
Both types of integrons are composed of a stable platform and a variable cassette array. The platform contains a gene coding for a tyrosine-recombinase, IntI, the attI recombination site and the resident promoter PC oriented towards the variable cassette array (Figure 1A). The cassette array contains a pool of gene cassettes generally composed of single promoter-less genes (coding sequence, CDS), so that their expression relies on the PC promoter. Starting from PC, their expression gradually decreases in the array (9) (Figure 1A). Then, the gene cassettes that are located close enough to the PC promoter are the only ones to be expressed, except for those that contain their own promoter (10–13). The integron system represents a low-cost and modular reservoir of adaptive functions for their host bacteria. Each gene cassette is flanked by an attC recombination site that is recognized and recombined by the integrase. The latter catalyzes the different reactions that lead to cassette mobilization. By recombining attI and attC sites, the integrase allows the recruitment of cassettes downstream of the PC promoter and express them (Figure 1A). By recombining two consecutive attC sites, the integrase ensures the excision of one cassette. Subsequent excision and integration of a circular cassette in the first position of the array, constitute a way for a previously silent gene to be expressed (Figure 1A).
Figure 1.
The integron. (A) The integron system in Vibrio cholerae. V. cholerae sedentary chromosomal integron is located on the second chromosome close to the termination site, Ter2. The four components of the integron stable platform are shown: the integrase expressing gene, intIA, the two promoters, PC and Pint and the attIA recombination site (red triangle). The variable cassette array contains a large number of cassettes, which are represented by small arrows. Their expression level is reflected by the colour intensity of each arrow. Only the first cassettes of the array are expressed, and the subsequent ones can be seen as a low-cost cassette reservoir. Upon expression of the integrase (grey forms) cassette shuffling can occur through cassette excision (attC × attC) and integration in the first position in the array (attIA × attC). (B) Integron cassette insertion in an attI site. Recombination between the double-stranded attI site (bold red lines) and a single-stranded bottom attC site (green lines) ending a cassette is shown. Since we do not exactly know the nature of the cassettes (ss or ds), the top strand of the attC site is represented as a dotted line. The synaptic complex comprises both att sites bound by four integrase monomers (grey ovals). One strand from each att site is cleaved and transferred to form an atypical Holliday junction (aHJ). aHJ resolution implies a replication step. The origin of replication is represented by a grey circle and the newly synthesized leading and lagging strands by dashed lines. Both products are represented: the initial substrate resulting from the top strand replication, and the reactional product containing the inserted cassette and resulting from the bottom strand replication.
A key feature of the integron is the ability of the integrase to recombine both single-stranded DNA (attC site) and double-stranded DNA (attI site) depending on their structure and sequence respectively (Figure 1B) (14,15). Indeed, each integrase recognizes the sequence of its cognate attI site (16,17). In contrast, the integrase does not recognize the sequence of attC sites, but rather the structure of the ss folded bottom strand (bs) (Figure 1B) (14,15,18–20). This specific recognition of the folded bottom strand of attC sites allows the insertion of cassettes in the proper orientation so that they can be expressed by the PC promoter (15,21). The recognition of this specific ssDNA substrate imposes some constraints for recombination reactions. Indeed, during the attI × attC reaction, an atypical Holliday junction (aHJ) is formed (Figure 1B), which cannot be resolved by the classical way but can be by a host-dependent replicative pathway (22). Several other host processes are implicated in cassette recombination, for instance, by influencing the proper folding of attC site (7,23). Host cells also control integrase and cassette expression (24–26). The most relevant regulatory network is the induction of integrase expression, for both Class 1 MI and V. cholerae SCI systems, in response to environmental stress through the SOS response (27,28). Such regulation allows the conditional reshuffling of cassettes, at a moment where the cells need to adapt to environmental changes. These examples show how integrons are intricate host-cell connected systems to maximize the potential benefit conveyed by this ‘adaptation on demand’ device (29).
Until now, the recombination processes occurring in integrons were mostly studied on MIs through assays developed and performed in Escherichia coli strains. Paradoxically, our knowledge of the SCI of the V. cholerae pathogenic strain remains predominantly descriptive more than 20 years after its discovery and despite its paradigmatic role in the field. Here, for the first time, we designed experimental assays to study cassette recruitment dynamics directly in the V. cholerae SCI. We delivered cassette substrates using conjugation, but also through natural transformation since V. cholerae is known to be naturally competent and to exchange DNA in this way. We measured significant cassette insertion rates mediated by the sole endogenous integrase. We confirmed that this integrase expression is due to the SOS activation probably triggered by the single-stranded cassette delivery during conjugation and transformation processes (24,30). Interestingly, cassettes are preferentially recruited into the attIA primary recombination site, directly downstream of the PC promoter, ensuring their expression and consecutive testing for selective advantages they can confer.
By performing in vivo recombination assays, we showed that the RecA protein (RecAVch) is critical for cassette recruitment at the attIA site of the V. cholerae SCI. The impact of RecAVch on cassette recombination is SOS-independent and seems specific to the attIA × attC reaction mediated by the integrase of V. cholerae, IntIA. Indeed, the RecAVch protein did not influence neither attC × attC recombination mediated by IntIA nor cassette recombination mediated by the MI Class 1 integrase, IntI1. Moreover, unlike that of IntI1, the V. cholerae SCI IntIA is not active in other bacterial hosts (e.g. in E. coli, even supplemented with RecAVch). Altogether, these results suggest that, in contrast to MIs, some specific host factors can regulate cassette recombination in SCIs. Therefore, MIs might have been selected to be independent of such host factors. In addition to their association with transposons and conjugative plasmids, this evolutionary trait may explain the large MI dissemination among bacteria and the antibioresistance expansion.
MATERIALS AND METHODS
Media
Escherichia coli and Vibrio cholerae strains were cultivated at 37°C in Luria Bertani (LB) media. V. cholerae and E. coli strains containing a plasmid with a thermo-sensible origin of replication were grown at 30°C. Thymidine (Thy) and diaminopimelic acid (DAP) were supplemented, when necessary, to a final concentration of 0.3 mM. Glucose (Glu), l-arabinose (Ara) and isopropyl-β-d-thiogalactopyranoside (IPTG) were added respectively at final concentrations of 10, 2 mg/ml and 0.8 mM. To induce the Ptet promoter, anhydrotetracycline (aTc) was supplemented into the media to a final concentration of 1 μg/ml. In the case of E. coli strains, antibiotics were added at the following concentrations: carbenicillin (Carb), 100 μg/ml, chloramphenicol (Cm), 25 μg/ml, kanamycin (Km), 25 μg/ml and spectinomycin (Sp), 50 μg/ml. V. cholerae strains were cultivated with the same antibiotic concentrations except in the case of Cm and Sp, that were supplemented at a final concentration of 5 μg/ml and 100 μg/ml respectively. When V. cholerae strains were cultivated in presence of glucose, the later concentration of Sp was increased 2-fold (200 μg/ml).
Bacterial strains, plasmids and primers
The different strains and plasmids that were used in this study are described in Supplementary Tables S1 and S2. All sequences of primers that were used are available in Supplementary Table S3.
We performed allelic exchange to construct N16961 ΔrecA, N16961 ΔrecA ΔattIA, N16961 ΔrecA ΔattIA::attI1, N16961 hapR+ lexA(ind-) and N16961 hapR+ lexA(ind-) ΔrecA. To this purpose, we used different variants of the pMP7 vector, respectively pB203, pK590, pK584 and p6780 (28). We followed the same protocols as previously described (31,32). Briefly, the suicide vector pMP7 contains a R6K origin of replication and its replication is then dependent on the presence of the Π protein in the host cell. The Π3813 cell, a pir+ CcdB resistant E. coli strain (31), was used for cloning the different pMP7 plasmids. Once constructed, these vectors were transformed into the β3914 DAP auxotroph donor strain (31) in order to deliver by conjugation the pMP7 vector into the desired recipient strain. Homology regions corresponding to the genomic DNA from the recipient strain have been cloned in the different pMP7 vectors to allow the integration of the plasmid by homologous recombination. The only way for pMP7 vector to replicate within recipient strains is to then integrate into the host genome after a first crossover. After conjugation, integration of the entire pMP7 vector was then selected by plating cells on Cm plates lacking DAP. Next, cells were grown in presence of L-arabinose (0.2%) in order to express the CcdB toxin. The expression of this toxin allows the killing of cells in which the second crossover that leads to the excision of pMP7 backbone did not take place. This method allows us to obtain mutants of V. cholerae that are devoid of any antibiotic resistance marker. Note that for the deletion or replacement of the attIA site in N16961ΔrecA strains, we previously transformed this cell with pAM::recAEc vector (pCY579 (33)). The expression of the RecAEc protein allows allelic replacement to take place in N16961ΔrecA mutant. At the end of construction, strains were cultivated without Carb and IPTG and loss of the pAM::recAEc plasmid was assessed. For ectopic complementation of the recA mutation, we inserted a copy of the recA gene into the attTn7 site present on the chromosome of E. coli and V. cholerae. We used the same strategy as described in (34). The helper plasmid pMVM1 was transformed into both N16961 and MG1655 recA mutants. This vector has a thermo-sensitive origin of replication and carries a PBAD promoter that triggers the expression of TnsABCD transposases. These transposases catalyze insertion into attTn7 at high frequency. A second shuttle vector, pMP234, carries the IR sites that are recognized by the transposases and was modified for specific integration of the recA gene from E. coli or V. cholerae. The FRT-aph-FRT cassette was also added in between the IR, in order to select for transposition event. The pMP234 vector is a derivative of the pSW23T suicide vector, so its replication cannot take place into recipient cells that lack the Π protein. For integration, the pMP234 shuttle vector was delivered by conjugation into the recipient strains containing pMVM1. Transposition events were selected by plating conjugants on Km plates without DAP. These plates were incubated overnight at 42°C to get rid of the helper vector. The integration of PLAC-recA-FRT-aph-FRT fragment was assessed by testing UV sensitivity of the strains and by performing PCR and subsequent sequencing. After integration, the Flippase (Flp) expressing vector (pMP108, CarbR (34)) was delivered by conjugation into the V. cholerae strain in order to excise the Km resistance cassette. This plasmid is easily lost when culturing V. cholerae strains without Carb. In the case of E. coli, we transformed the strains with the pCP20 Flp expressing vector (CarbR (35)), which has a thermo-sensitive origin replication.
Suicide conjugation assay
This assay has been previously described (14) and implemented (16) for the delivery of one specific strand of a recombination site into recipient strains that express the integrase. In this study, we used the suicide conjugative vector pSW23T (pD060) that allows the delivery of the bottom strand of the attCaadA7 recombination site. An advantage of using this vector is its relatively small length (1817 bps) which enables to better mimicking the integron cassettes (7). This vector carries a RP4 origin of transfer and an oriVR6Kγ origin of replication. It was previously transformed into a pir+ donor strain, β2163, which contains the RP4 machinery of transfer. This later strain needs DAP to grow in rich medium, which allows its counter-selection after conjugation. The only possibility for pSW23T to replicate into the pir- recipient strains is thus to insert into the genome through a recombination reaction catalyzed by the IntI protein. Since the pSW23T vector contains a CmR cassette, recombination events can be selected with this marker. By plating in parallel conjugants on solid media with or without Cm, we are able to establish the frequency of a given recombination reaction. We adapted this protocol for the use of V. cholerae as the recipient strain, in which plasmids are more easily lost in absence of antibiotic selection than in E. coli. In this case, after an overnight culture, recipient cells were diluted (1:100) and grown in the presence of Sp and Ara (0.2%) respectively to maintain the pBAD43 vector and to allow the expression of the integrase. In the case of the recAVch and recAEc complemented strain, IPTG was also added in the media. The donor strain was grown in parallel in presence of DAP. When both donor and recipient cultures reach an OD600 nm of 0.7–0.8, recipient V. cholerae strains were washed by centrifugation at 3000 rcf for 6 min and resuspension of the pellet in 1 ml of LB. 1 ml of each donor and recipient cultures were then mixed and centrifuged at 3000 rcf for 6 min. The obtained pellet was re-suspended in a droplet of LB and spread on a 0.45 μm filter placed on MH DAP, Ara plates and incubated at 37°C for 3 h. After incubation, the filter was re-suspended in 5ml of LB and this suspension was used to spread appropriate dilutions on MH, Cm, Sp, Glu and MH, Sp, Glu plates. After 2 days of incubation at 37°C, the recombination frequency was calculated as the ratio of CmR clones over the total number of recipient colonies that grew on MH, Sp, Glu plates. Note that in the case where we did not detect recombination events for one replicate, we calculate the recombination rates as the ratio of the mean of recombinant clones over the mean of total recipient clones obtained for the different replicates. In this case no error bars are represented on our graphs.
Natural transformation assay
In this study, we used the same pSW23T vector (pD060) that was used in the suicide conjugation assay. V. cholerae natural transformation was previously described (36). Here, we adapted this protocol to assess integrase-mediated recombination frequency after natural transformation in competent strains of V. cholerae. As the natural V. cholerae N16961 strain contains a frameshift mutation in the hapR gene, which renders this strain non-transformable, we used the N16961 hapR+ strain, a genetically engineered N16961 variant with a repaired hapR gene. An overnight culture was used to inoculate 1:100 of bacteria in 5 ml of LB medium supplemented with Sp (50 μg/ml). The culture was grown at 30°C until they reached an OD600nm of 0.5. One milliliter of cells was then centrifuged (2200 rcf, 10 min) and resuspended in 1 ml of M9 minimal medium supplemented with MgSO4 (32 mM) and CaCl2 (5 mM) and Sp (100 μg/ml). Tubes containing 50–80 mg of chitin (C9213; Sigma) were inoculated with 0.5 ml of washed cells and 0.5 ml of fresh M9 medium supplemented with MgSO4, CaCl2 and Sp, vortexed, and grown 48 h at 30°C with shaking. Cultures were then washed (2200 rcf, 10 min) and resuspended in an equal volume of fresh M9 medium supplemented with MgSO4, CaCl2, Sp and anhydrotetracycline (aTc) to induce integrase expression. The cultures were incubated again for 30 min at 30°C with shaking. Then, 2 μg of plasmid DNA (the pD060 vector) were added to the cultures and incubated for 36 h at 30°C with shaking. After incubation, bacteria were detached from chitin by vortexing vigorously for 30 s. The obtained bacteria suspension was used to spread appropriate dilutions on MH, Cm, Sp and MH, Sp plates. After 2 days of incubation at 37°C, the recombination frequency was calculated as the ratio of CmR clones over the total number of recipient colonies that grew on MH, Sp plates in the same manner as for suicide conjugation assay.
Analysis of cassette insertion point localization
For each condition assay, at least 16 recombinant clones were isolated on appropriate plates and analyzed by PCR. For this, we performed different PCR reactions. In order to determine precisely if the pSW23T vector has been inserted into the attIA site of the SCI we used 5778 and SWend primers. These primers hybridize respectively in a sequence upstream of attIA in V. cholerae chromosome 2 or downstream of attCaadA7 in the pSW23T vector. For E. coli and V. cholerae strains transformed with the pSU38Δ or on pBAD43 vectors that harbor recombination sites, SWbeg and MFD or 1704 and SWend primers respectively were used to amplify one junction of the co-integrate. Finally, to detect insertion of pSW23T in the genome of V. cholerae, either at secondary sites or into the VCR sites of the SCI, we performed random PCR amplification. For these, we performed a first randomized PCR reaction using degenerate 1863 and 2405 primers. The 2405 primer hybridizes upstream of the attC sites on pSW23T plasmids. Due to the presence of degenerate nucleotides in the 1863 primer, low hybridization temperatures were used, first, 30°C during 5 cycles and after, 40°C during 30 cycles. The obtained amplified DNA fragments were subjected to a second PCR reaction in order to enrich for PCR products corresponding to cassette insertion. For this purpose, we used primers 1865 and 1388. These primers hybridize respectively to the fixed part of the degenerated 1863 primer and upstream (but closer than 2405) of the attC sites on pSW23T plasmids. Recombination points were precisely determined by sequencing PCR products using primer 1366. The 1366 primer hybridizes upstream (but closer than 1388) of the attC sites on pSW23T plasmids. For each condition assay, at least three PCR reactions were purified and sequenced to confirm the insertion point.
Recombination assay with unidirectional replicative substrate
This assay was previously described (23) and allows the determination of the recombination rates of a given recombination reaction when attC sites are carried by a replicative plasmid. The vectors that we used (p7523 or p7546, CmR) replicate unidirectionally and the attCaadA7 recombination sites they carry have been cloned so that the recombinogenic bottom strand is located either on leading or lagging strand template (‘lead’ or ‘lag’ orientation). V. cholerae strains were transformed with these unidirectional-replicative vectors and the IntIA expressing vector (p995, CarbR) or with the empty version of the plasmid (p979, CarbR). As p7523 and p7546 have both a thermo-sensitive origin of replication, the cells were cultivated overnight at 30°C. The next day, cultures were diluted (1:100) and incubated for 5h at 30°C in presence of Cm and Carb to maintain both pTSC29 and pBAD18 plasmid. In order for recombination to take place, L-arabinose was also added to the media at a concentration of 0.2% to allow the expression of the integrase. After 5h of incubation, appropriate dilutions of the cultures were plated in parallel on MH Cm, Carb, Glu and MH Carb, Glu plates and were incubated for two days at 42°C. Since pTSC29 vector cannot replicate at 42°C, the selection on Cm plates at this temperature allows the recovery of only the clones in which attCaadA7 site have been recombined by IntIA. Recombination rates were calculated as for suicide conjugation assays, by considering the ratio of CmR clones over the total number of recipient clones that grew on MH Carb, Glu plates. In order to determine if cassette insertion occurs into the attIA site of the SCI of V. cholerae, we performed PCR reactions on at least eight random isolated clones using 5778 and 573 or 5778 and MFD primers (respectively used for ‘lead’ and ‘lag’ orientation of the bs of attCaadA7 site on pTSC vector).
RESULTS
Horizontally transferred cassettes are efficiently inserted into the attIA site of the Vibrio cholerae SCI
Large SCIs such as the V. cholerae SCI constitute large reservoirs of functions for their host bacteria. In such massive integrons, several recombination sites can constitute potential targets for cassette insertion (attIA primary recombination site and/or the numerous VCR sites). In a previous study, recombination assays that were performed in V. cholerae aimed at evaluating cassette insertion in recombination sites carried on plasmids (16). Here, we attempted to visualize cassette insertion into the SCI of V. cholerae, using our classical conjugation assay (14) and developing a natural transformation assay. Interestingly, both assays reproduce the natural conditions in which the acquisition of cassettes could occur through horizontal gene transfer in V. cholerae (Figure 2A). The plasmids containing the donor attC site (pSW23T::attCaadA7) are delivered on a single-stranded form in a recipient V. cholerae strain containing a vector expressing the IntIA integrase. Once delivered, attC-containing plasmids cannot replicate and can therefore be assimilated to a non-replicative integron cassette. The unique way for these synthetic cassettes to be maintained is to be inserted in the V. cholerae host genome by a recombination between the cassette attC sites and the SCI attI or attC sites.
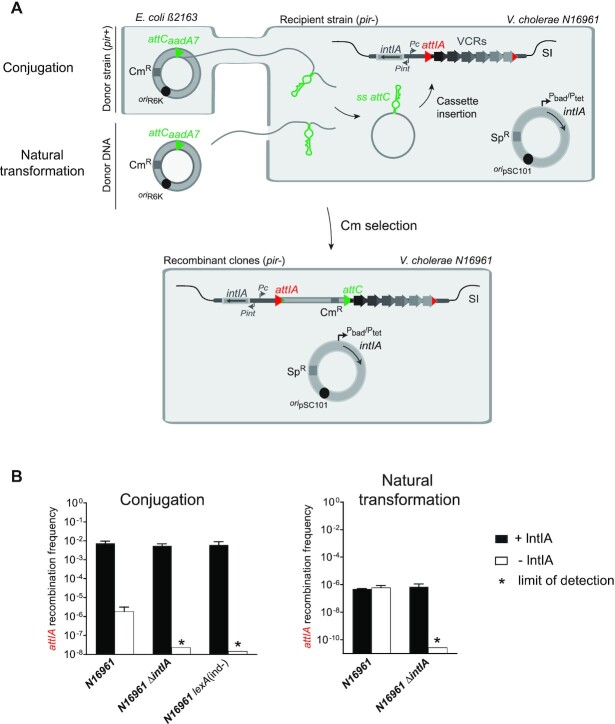
Figure 2.
Cassette recruitment in Vibrio cholerae SCI during horizontal gene transfer. (A) Experimental setup of the cassette insertion assay. The pSW23T::attCaadA7 suicide vector is delivered to N16961 V. cholerae recipient strains containing an integrase expressing vector or the sole endogenous integrase, and the SCI. The delivery occurs by two horizontal gene transfer processes: conjugation from the β2163 donor or natural transformation. As pSW23T cannot replicate in V. cholerae recipient strains, recombinant clones can be selected on appropriate Cm containing plates to evaluate the recombination frequency (see also Results and Materials and Methods). The attCaadA7 site carried by the suicide vector is represented by a green triangle and the attIA site on the V. cholerae SCI by a red triangle. (B) Frequency of insertion of the pSW23T::attCaadA7suicide vector into the attIA site. The recombination frequencies were calculated in N16961 V. cholerae wt, ΔintIA and lexA(ind-) strains. Results correspond to recombination frequencies that were normalized after analysis of PCR reactions (Materials and Methods). +IntIA: recipient strains transformed with the pBAD43 integrase expressing vector; -IntIA: control strains transformed with the empty pBAD43 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
When we carried out this test, by delivering cassette by conjugation, we detected a significant level of insertion (7.5 × 10–3) of the pSW23T::attCaadA7 vector using the N16961 wt V. cholerae strain (Figure 2B, +IntIA). We performed PCR reactions on some recombined clones (i.e. 24 clones) and demonstrated that, in all cases, the cassette insertion occurs at attIA sites from V. cholerae SCI platform (Supplementary Figure S1A). By sequencing some PCR products, we confirmed that insertion point was correctly localized in the 5′-AAC-3′ triplet.
We took advantage of V. cholerae’s natural competent state in the presence of chitin (37) to investigate cassette recombination in the context of another HGT mode. We adapted the natural transformation protocol to evaluate cassette insertion frequency in the V. cholerae SCI (Figure 2A). We obtained a recombination frequency of 4.8 × 10–7 when overexpressing the integrase (Figure 2B, +IntIA). We also performed PCR reactions on several recombined products (i.e. 32 clones) and demonstrated that cassette insertion occurs, for all tested clones, at attIA sites from V. cholerae SCI platform (Supplementary Figure S1B). These results show that, during conjugation or natural transformation, integron cassettes are efficiently released in the V. cholerae host cell and inserted in the SCI. Interestingly, all insertion events were detected in the integron platform attIA site in spite of the presence of about 180 attC sites.
The endogenous integrase efficiently inserts cassettes into the attIA site of the Vibrio cholerae SCI
We also performed both assays in the presence of the sole endogenous SCI IntIA integrase. Interestingly, in this case, we detected a significant level of insertion of the pSW23T::attCaadA7 vector using the N16961 wt V. cholerae strain (1.8 × 10–6 and 6.1 × 10–7 respectively for conjugation and transformation assays, Figure 2B, -IntIA). Here again, we performed PCR reactions on several recombined products and demonstrated that cassette insertion occurs, for all tested clones (i.e. respectively 16 and 48 clones), at attIA sites from the V. cholerae SCI platform (Supplementary Figure S1). This recombination activity is due to the expression of the endogenous intIA integrase gene since no recombination event was detected in the strain devoid of endogenous integrase (N16961 ΔintIA) for both assays (Figure 2B, -IntIA). We also demonstrated that the expression of endogenous integrase is dependant of the SOS system since no recombination event was detected below the detection limit of 1.5 × 10–8 in the N16961 lexA(ind-) strain in which the SOS response is not inducible. Indeed, in this strain, the SOS regulon genes are constitutively repressed because of the presence of the uncleavable LexAA91D version of the LexA repressor (28). As a supplementary control, we also overexpressed the IntIA integrase in both ΔintIA and lexA(ind-) mutant strains and as expected we obtained a very high recombination frequency (respectively 5.4 × 10–3 and 6.0 × 10–3, Figure 2B, +IntIA) corresponding to insertion events at the attIA site from the V. cholerae SCI (96/96 and 24/24 clones were inserted in attIA, Supplementary Figure S1).
Altogether, these results show that, during conjugation or natural transformation, integron cassettes are efficiently delivered in the V. cholerae host cell and inserted at the attIA site from the V. cholerae SCI even in the presence of the sole endogenous integrase. The level of endogenous integrase expression, triggered by SOS response induction initiated by the single-stranded cassette entry during conjugation and natural transformation (24,30) seems sufficient to insert cassettes at a significant level in the V. cholerae SCI. Note that, when performing both conjugation and natural transformation, we also detected some attIA insertion events associated with shuffling of internal remote cassettes in first position (1 event on the 160 performed PCR for conjugation and 12 events on the 152 performed PCR for natural transformation, Supplementary Figure S1).
RecAVch influences cassette insertion into the attIA site of the Vibrio cholerae SCI
Here, we precisely investigated the cassette recombination mechanism used by the SCI of V. cholerae. To define the network of intervening host factors, we tested the effect of the Vibrio cholerae RecA protein (RecAVch). The RecA protein is functionally conserved among bacterial species (38) and in eukaryotic organisms (39). RecA is a critical enzyme for homologous recombination process, during which it binds ssDNA catalyzing the pairing with complementary regions of dsDNA and strand exchange reactions (40–42). Because of the capacity of the RecA protein to bind ssDNA, we tested its impact on SCI cassette recombination catalyzed by IntIA in V. cholerae.
Among the different conditions previously developed, we chose to use the optimal one, i.e. the suicide conjugation assay with overexpression of integrase (Figure 3A). Here again, we obtained a very high recombination rate (2.0 × 10–2) when using the wt parental strain as recipient strain. We observed a decrease of more than two orders of magnitude in the recombination rates (7.7 × 10–5) in the corresponding N16961 ΔrecA mutant strain (Figure 3A). When performing PCR analysis for each reaction, we confirmed that insertions occur in the attIA site (222/222 and 108/111, respectively for the wt and ΔrecA strains). These results mean that the RecAVch protein favors insertion of cassettes in the attIA site of V. cholerae SCI. As expected, no recombination event was detected in the N16961 ΔrecA control strain that carries the empty pBAD43 vector (compare to the N16961 wt strain, Figure 3A), since the SOS response cannot be activated and induce the endogenous integrase expression in these cells. To confirm that the observed decrease in recombination rates is specifically due to the deletion of the recAVch gene and not to polar effects, we constructed a strain in which the recAVch deletion was ectopically complemented. For this, a copy of the recAVch gene was inserted into the attTn7 site located in the chromosome 1 of V. cholerae (34). This recAVch ectopic complementation allows recovery of a recombination rate similar to the one of the N16961 wt strain (Figure 3A), meaning that the effect on attIA × attCaadA7 recombination reaction, which we observed, is specific to the RecAVch protein. Furthermore, we tested the ability of the RecA protein of E. coli (RecAEc) to complement the recAVch deletion. The E. coli and V. cholerae RecA proteins are highly similar, but concentrate their variations in their C-terminal part (Supplementary Figure S2). This C-terminal region is implicated in the modulation of RecAEc activity notably by interacting with regulator proteins (43,44). We then tested if such variations could lead to host specific regulation that will impact cassette recombination. For this, we inserted a copy of the recA gene of E. coli into the attTn7 locus in V. cholerae. We observed that the complementation of the N16961 ΔrecA mutant with RecAEc was as efficient as with the native RecAVch of V. cholerae (Figure 3A). This is consistent with the high percentage of identity (almost 80%, Supplementary Figure S2) shared by both RecA proteins. Moreover, we observed that RecAEc was also mediating SOS induction, leading to endogenous integrase expression in the control strain harboring the empty pBAD43 plasmid (Figure 3A). Both RecA proteins seem then to share enough identity so that the RecAEc can replace RecAVch for several functions, including its apparent role in cassette recombination in V. cholerae.
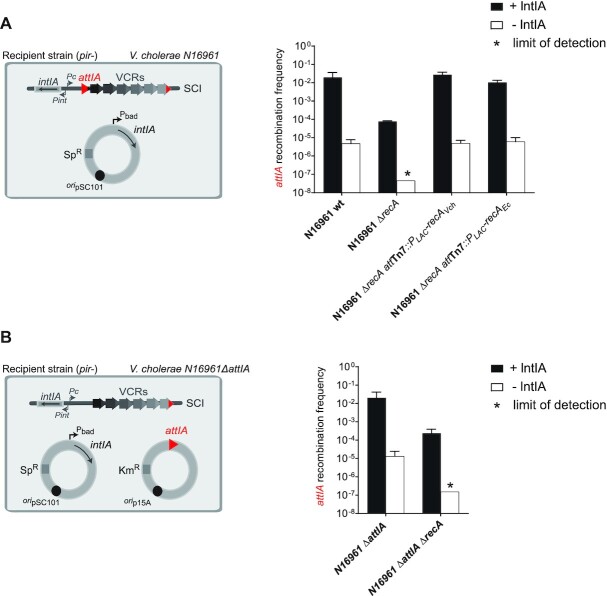
Figure 3.
Effect of the RecA protein on attIA × attC recombination in Vibrio cholerae SCI. (A) Experimental setup and frequency of insertion of the pSW23T::attCaadA7 suicide vector into the chromosomic attIA site. N16961 recipient strains transformed with the pBAD43 IntIA expressing vector were used (left panel). The recombination rates were calculated in N16961 V. cholerae wt and in the corresponding recA mutant (ΔrecA) and ectopic complemented (ΔrecA-atttn7::PLAC-recAVch and ΔrecA-atttn7::PLAC-recAEc) strains (right panel). (B) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the attIA site located on plasmid. N16961 recipient strains transformed with both pBAD43 IntIA expressing vector and pSU38Δ::attIA vector were used (left panel). The recombination rates were calculated in N16961 V. cholerae wt and in the corresponding recA mutant strains (ΔrecA, right panel). For both (A) and (B), results correspond to recombination frequencies that were normalized after analysis of PCR reactions (Materials and Methods). +IntIA: recipient strains transformed with the pBAD43 integrase expressing vector; -IntIA: control strains transformed with the empty pBAD43 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
RecA is involved in the induction of the SOS response, thus its effect on cassette recombination could be indirect and may involve one or more proteins of the SOS regulon. Using the lexA(ind-) mutant, in which the SOS response is non-inducible, we did not observe any effect on recombination efficiency compared to the wt strain (Figure 2B) and, using the lexA(ind-) ΔrecA mutant, we still observe a large decrease of more than two orders of magnitude in the recombination rates (Supplementary Figure S3). When performing PCR analysis for each reaction, we confirmed that insertions occur in the attIA site (24/24 and 48/48, respectively for the lexA(ind-) and lexA(ind-) ΔrecA strains). These results allow us to demonstrate that the RecAVch effect on IntIA mediated recombination is independent on the SOS regulon proteins. We also determined the role of the RecAVch protein during cassette recombination in attIA when this site is located on a plasmid (Figure 3B). For this, we constructed a V. cholerae strain deleted for the resident attIA site (V. cholerae N16961 ΔattIA) and transformed with an attIA-containing plasmid. Once again, we obtained a decrease of two orders of magnitude (from 2.0 × 10–2 to 2.4 × 10–4, Figure 3B).
Altogether these results show that the RecA protein of V. cholerae favors the attIA × attC recombination mediated by IntIA, in a SOS-independent manner and independently of where attIA is localized.
RecAVch does not influence attC × attC recombination in Vibrio cholerae
During both attC × attC and attI × attC reactions, the proper folding of attC site is essential for binding integrase monomers. Thus one could imagine that the effect seen for RecA here could be linked to its ssDNA binding function. In this case, we expect that it should impact both attI × attC and attC × attC reactions that are catalyzed by IntIA. To test this, we used different assays that allow assessment of cassette insertion frequencies directly into the VCR sites of the SCI or in attC sites carried on plasmids (Figure 4). To observe insertion events into the VCR sites of the SCI, we used the V. cholerae mutant strain deleted for the resident attIA site (V. cholerae N16961 ΔattIA, see just above). Here, we did not observe any differences in the recombination rates obtained with ΔattIA and ΔattIA ΔrecAVch mutant strains. To confirm the cassette insertion in VCRs, we performed random PCR reactions (Materials and methods). As the recombination rates into VCR sites is similar between both strains (Figure 4A), this means that the RecAVch protein does not affect the attC × attC recombination. Note that, in our experiment, the natural expression of the endogenous integrase is not high enough to mediate cassette insertion events in VCR sites at a detectable level. Interestingly, overexpressing the integrase enables to access to these rare recombination events and therefore to study the mechanism of integron recombination in more details.
Figure 4.
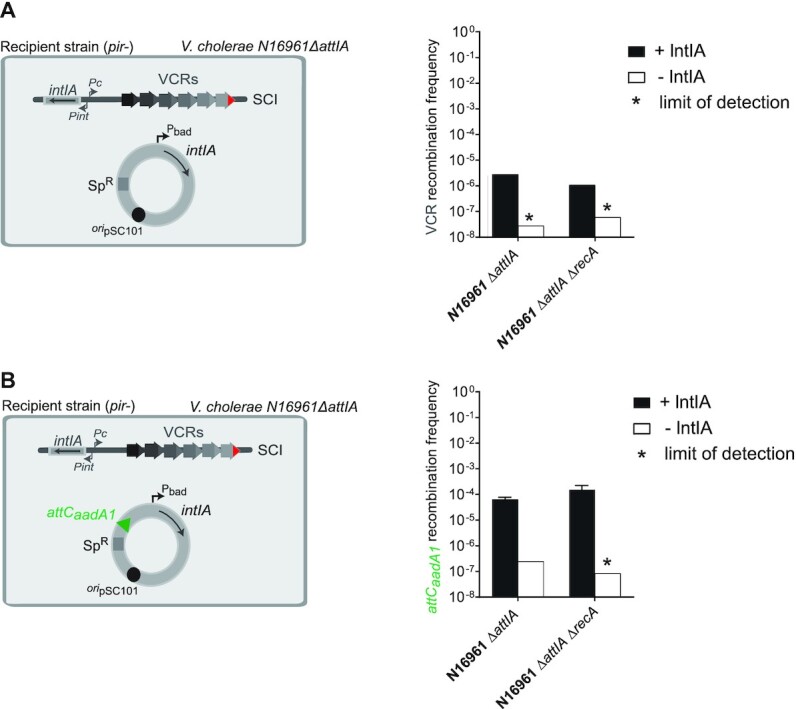
Effect of the RecA protein on attC × attC recombination in Vibrio cholerae. (A) Experimental setup and frequency of insertion of the pSW23T::attCaadA7 suicide vector into VCR sites of the SCI. N16961 recipient strains deleted for the attIA site (ΔattIA strains) and transformed with the pBAD43 IntIA expressing vector were used (left panel). The recombination rates were calculated in N16961 V. cholerae ΔattIA and in the corresponding recA mutant strain (ΔattIA ΔrecA, right panel). (B) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the attCaadA1 site located on the pBAD43 plasmid. N16961 recipient strains deleted for the attIA site (ΔattIA strains) and transformed with the IntIA expressing and attCaadA1 containing pBAD43 vector were used (left panel). The recombination rates were calculated in N16961 V. cholerae ΔattIA and the corresponding recA mutant strain (ΔattIA ΔrecA, right panel). For both (A) and (B), results correspond to recombination frequencies that were normalized after analysis of PCR reactions and sequencing (Materials and Methods). +IntIA: recipient strains transformed with the pBAD43 integrase expressing vector; -IntIA: control strains transformed with the empty pBAD43 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
To confirm this absence of RecAVch effect on attC × attC recombination, we perform a second conjugation assay in V. cholerae N16961 ΔattIA strains transformed with a plasmid carrying the attCaadA1 site (from the aadA1 gene cassette found in MIs). We obtained a significant rate of recombination for both ΔattIA and ΔattIA ΔrecAVch strains and we did not observe any impact of the RecAVch protein on attCaadA1 × attCaadA7 reaction catalyzed by IntIA in V. cholerae (Figure 4B). Together, these results, with those presented in the previous paragraph, indicate that the RecAVch protein seems to favor only attIA × attC reaction and has no influence on insertion reactions into attC sites, whether they are present on the chromosome (VCR) or on a plasmid (MI attC sites).
RecAVch does not influence attI1 × attC recombination mediated by IntI1 in Vibrio cholerae
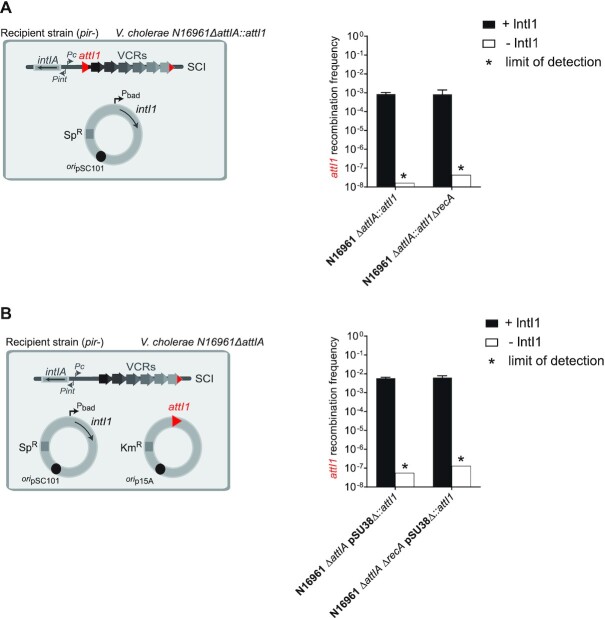
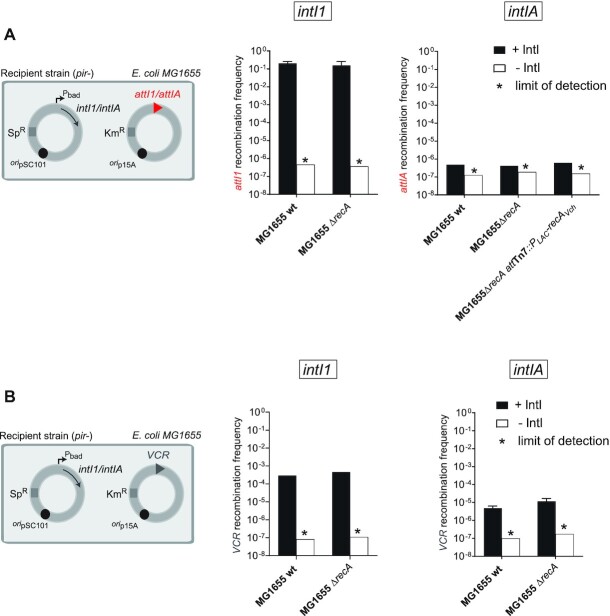
The previously demonstrated influence of the RecAVch protein on cassette recombination was striking since it has been previously shown that the RecAEc protein does not influence the recombination reactions catalyzed by IntI1 in E. coli (45). To determine if the role of the RecAVch protein is specifically linked to the V. cholerae SCI system, we used two different recombination tests to establish, in V. cholerae, the activity of the integrase IntI1. First, we performed the suicide conjugation assays in a V. cholerae strain where the attIA site from the SCI platform was replaced by the attI1 site (the attI site of the IntI1 integrase). Secondly, we performed this assay in V. cholerae strains deleted for the attIA site (V. cholerae N16961 ΔattIA) and transformed with an attI1-carrying plasmid. We observed, using these two assays, that the frequencies of attI1 × attC reaction catalyzed by IntI1 were similar for both ΔattIA and ΔattIA ΔrecA recipient strains (Figure 5A and B). Therefore, we demonstrated that, in V. cholerae, the attI1 × attC reaction catalyzed by IntI1 is not influenced by RecAVch. This suggests that the role of RecAVch on the attI × attC reaction is specific to IntIA.
Figure 5.
Effect of the RecA protein on attI1 × attC recombination mediated by IntI1 in Vibrio cholerae. (A) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the attI1 site located in the SCI platform. N16961 recipient strains with an attI1 site in place of the attIA site (ΔattIA::attI1 strains) were transformed with the pBAD43 IntI1 expressing vector (left panel). The recombination rates were calculated in N16961 V. cholerae ΔattIA::attI1 and the corresponding recA mutant strain (ΔattIA::attI1 ΔrecA, right panel). (B) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the attI1 site located on a plasmid. N16961 recipient strains deleted for the attIA site (ΔattIA::attI1 strain) and transformed with both pBAD43 IntI1 expressing vector and pSU38Δ::attI1 vector were used (left panel). The recombination rates were calculated in V. cholerae ΔattIA::attI1 and the corresponding recA mutant strain (ΔattIA::attI1 ΔrecA, right panel). For both (A) and (B), results correspond to recombination frequencies that were normalized after analysis of PCR reactions (Materials and Methods). +IntIA: recipient strains transformed with the pBAD43 integrase expressing vector; -IntIA: control strains transformed with the empty pBAD43 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
Effect of RecA on cassette recombination in Escherichia coli
As already mentioned, the impact of the RecA protein on cassette recombination had already been tested in E. coli (45). In this previous study, a setup based on the reconstitution of a functional dapA gene after cassette excision through an intramolecular reaction was used. In this case, only the efficiency of reactions catalyzed by the IntI1 integrase was studied (45). Here, we extended this by assessing the efficiency of either attI × attC or attC × attC intermolecular reactions and tested the effect of recA deletion on reactions catalyzed by both IntI1 and IntIA integrases in E. coli. We also used another attC site, VCRVCA0441 (45). We still observed the RecA-independence of IntI1 for both attI1 × attCaadA7 and VCR × attCaadA7 reactions (Figure 6A and B, left panels). Indeed, respective recombination frequencies were identical in MG1655 wt and ΔrecA recipient strains. For the reaction catalyzed by IntIA, we observed that, in E. coli, the VCR × attCaadA7 reaction was not affected by the deletion of the recA gene (Figure 6B, right panel). In accordance with the study of Biskri and coll. (16), we found that IntIAVch was not able to efficiently catalyze the attIA × attCaadA7 reaction in E. coli in the wt strain (Figure 6A, right panel). As proposed earlier, if IntIA recombines much less efficiently in E. coli this may reflect the absence, or a too large divergence, of at least one host factor required for this reaction. Thus, in order to determine if RecA could correspond to one of these divergent factors, we tested if RecAVch could rescue the lack of attIA × attC recombination in E. coli. As performed previously in V. cholerae, we inserted the recAVch gene into the unique attTn7 site of E. coli. However, we found that the expression of RecAVch do not restore the recombination capacity of IntIA for the attIA × attCaadA7 reaction in E. coli. Thus, it shows that the RecA protein is not the unique factor whose divergence hampers the attIA × attCaadA7 reaction catalyzed by IntIA to take place in E. coli.
Figure 6.
Effect of the RecA protein on recombination reactions catalysed by IntI1 and IntIA in Escherichia coli. (A) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the att sites (attI1 or attIA) located on a plasmid. MG1655 recipient strains transformed with both pBAD43 IntI expressing vector (IntI1 or IntIA) and the pSU38Δ::attI vector (attI1 or attIA) were used (left panel). The recombination rates were calculated in MG1655 and in the corresponding recA mutant (ΔrecA) and ectopic complemented (ΔrecA-atttn7::PLAC-recAVch) strains (right panels). (B) Experimental setup and frequency of insertion of the pSW23T::attCaadA7suicide vector into the VCRVCA0441 site located on a plasmid. MG1655 recipient strains transformed with both pBAD43 IntI expressing vector (IntI1 or IntIA) and the pSU38Δ::VCRVCA0441 vector were used (left panel). The recombination rates were calculated in MG1655 and in the corresponding recA mutant strain (ΔrecA, right panels). For both (A) and (B), results correspond to recombination frequencies that were normalized after analysis of PCR reactions (Materials and Methods). +IntI: recipient strains transformed with the pBAD43 integrase expressing vector; -IntI: control strains transformed with the empty pBAD43 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
RecAVch influences attIA cassette insertion no matter how the attC site is delivered
In all previously presented assays, we used horizontal gene transfer mechanisms as a means for delivering the single-stranded recombinogenic bottom strand of the attC site on non-replicative substrate mimicking integron cassettes. To determine if RecA still favors cassette insertion in the case where attC sites are folded from double-stranded molecules, we performed a recombination assay that relies on the use of replicative vectors (pTSC29 derivatives). These vectors replicate unidirectionally and the attCaadA7 site that they carry was cloned in two orientations, so that the bottom strand of the attC site is either present on the lagging or leading strand template (Figure 7A). When the bs of the attC site is located on the lagging strand template, in which large region of ssDNA are available during replication (i.e., between Okazaki fragments), bs attC site folding from ssDNA is favored (Figure 7B). When the bs of the attC site is located on the leading strand template, bs attC site folding can only occur from dsDNA by cruciform structure extrusion (23) (Figure 7B). Since the pTSC29 vectors have a thermo-sensitive origin of replication, the selection of recombinant CmR clones at 42°C allows us to evaluate the efficiency of recombination events. When we performed this replicative assay in the V. cholerae N16961 wt strain, we observed a decrease of about one order of magnitude when the attCaadA7 bs is present on the leading strand template (Figure 7C). Such differences in the recombination frequency between the two orientations of the attCaadA7 site were previously observed when the same recombination assays were carried out in E. coli with the integrase IntI1 (23). In the N16961 ΔrecA mutant, we still observed a large decrease in recombination rates for both attCaadA7 site orientations (Figure 7C). These results demonstrate that RecAVch is involved in the mechanism of recombination during attIA × attC reaction whatever the manner that the attC site is delivered (i.e. from ssDNA or dsDNA).
Figure 7.
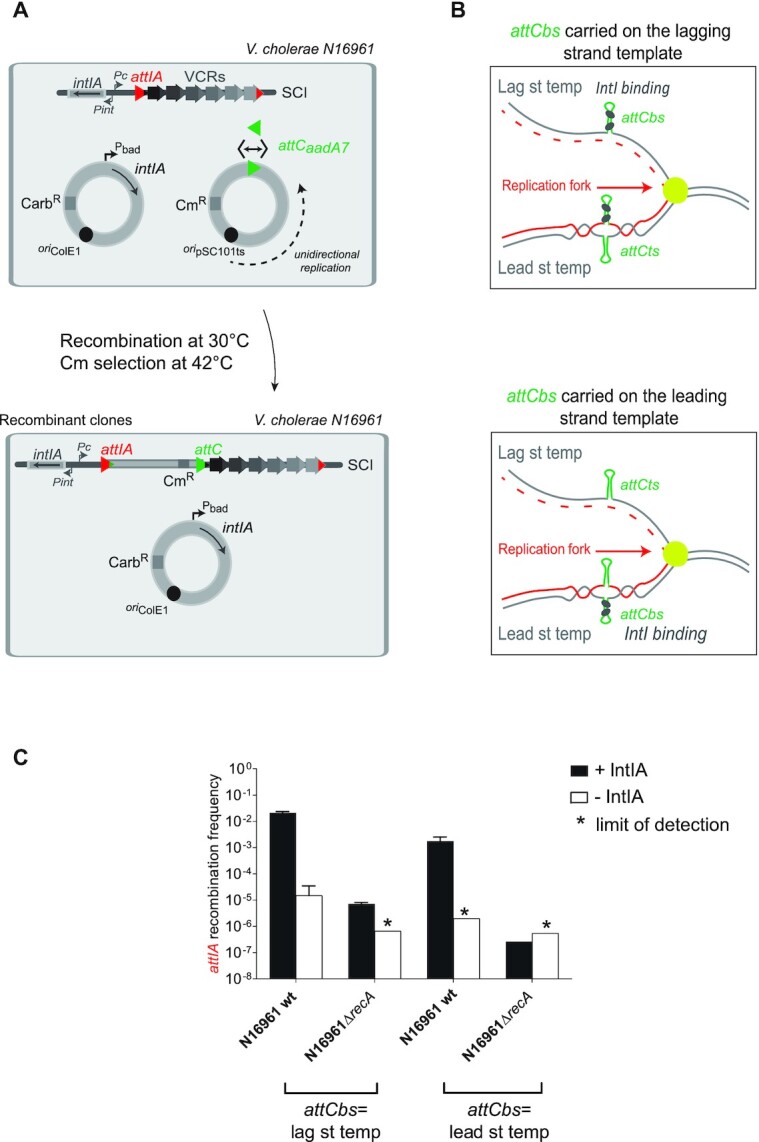
Effect of the RecA protein on attC × attI reaction when attC sites are carried by a replicative vector in Vibrio cholerae. (A) Experimental setup of the replicative assay. V. cholerae N16961 strains transformed with both pBAD18 IntIA expressing vector and the unidirectional-replicative vector, pTSC29::attCaadA7 were used. attCaadA7 sites (green triangles) were cloned in both orientations (double arrow) in the pTSC29 vector. Since pTSC29 has a thermosensitive origin of replication, the recombination reaction is performed at 30°C and, to evaluate the recombination frequency, recombinant clones selection was performed on Cm containing plates at 42°C (see also Results and Materials and methods). The attIA site on the V. cholerae SCI is represented by a red triangle. (B) Folding of attCaadA7 site on a replicative vector. Replicated and template strands are coloured in red and grey respectively and grey circles represent IntIA monomers. bs: bottom strand; ts: top strand; Lag st temp: Lagging strand template; Lead st temp: Leading strand template. (C) Frequency of insertion of the pTSC29::attCaadA7unidirectional-replicative vector into the attIA site. The recombination rates were calculated in N16961 V. cholerae wt and in the corresponding recA mutant strains (ΔrecA). Under plots, the orientation of attCaadA7 bs on lagging or leading strand template (lag st temp or lead st temp) are indicated. Results correspond to recombination frequencies that were normalized after analysis of PCR reactions (Materials and Methods). +IntI: recipient strains transformed with the pBAD18 integrase expressing vector; -IntI: control strains transformed with the empty pBAD18 vector. * correspond to the limits of detection. Values represent the mean of at least three independent experiments and error bars correspond to average deviations from the mean.
DISCUSSION
Incoming integron cassettes are efficiently inserted in the attIA site of the Vibrio cholerae integron
Exchange of integron gene cassettes between bacterial species (46) and gene cassette shuffling in the V. cholerae SCI (30) have been detected previously but at very low frequencies and have only been linked to homologous recombination events. Here, for the first time, we experimentally succeeded to visualize cassette recruitment events in the SCI of the Vibrio cholerae pathogenic strain, catalyzed by the sole endogenous integron integrase. We used both conjugation and natural transformation processes to deliver the integron cassettes through horizontal gene transfer in V. cholerae strains. Up to now, we always used our classical conjugation assay to deliver cassettes in recipient strains and study integron recombination. However, this assay does not perfectly mimic the integron system since cassettes are not themselves conjugative elements and have to be excised from the conjugative plasmid before their insertions. This is why we attempted here to develop for the first time another assay involving natural transformation, which is thought to be an important road for cassette recruitment in V. cholerae.
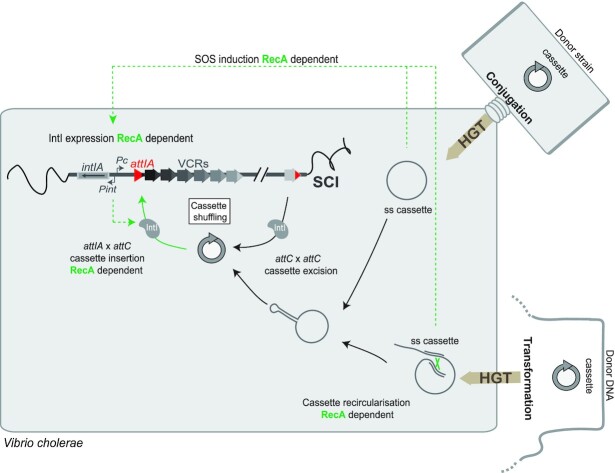
When delivering cassettes by conjugation assay, we detected a high rate of cassette insertion (∼10–2) in conditions where IntIA is overexpressed. However, we were also able to detect a significant level of cassette insertion (>10–6) catalyzed by expression of the sole endogenous integrase. We also detected cassette insertion events catalyzed by the endogenous integrase by delivering cassettes during natural transformation. The entry of single-stranded cassettes by natural gene transfer processes activates the SOS response sufficiently to express the endogenous integrase at levels allowing the proper insertion of these incoming cassettes. We therefore reproduce the whole natural integron pathway in which single-stranded integron cassettes entry, mediated by HGT processes, activates their proper insertion in the integron platform (Figure 8).
Figure 8.
Snap shot of SCI recombination during horizontal gene transfer in Vibrio cholerae. The SCI activity is represented and its connections with bacterial physiology. The steps, which involve the RecA protein, are indicated in green. HGT: horizontal gene transfer; ss: single-stranded DNA; VCR: Vibrio cholerae Repeat sequences; SCI: sedentary chromosomal integron.
Interestingly, by analyzing the insertion events, we demonstrated that despite the presence of about 180 VCR sites in the large array of cassettes, the attIA site constitutes the major recruitment site for cassette insertion in the V. cholerae SCI. It has been demonstrated that expression levels are maximal for these first cassettes in the array, and gradually decrease for those following (47,48). Nonetheless, in the large cassette arrays such as those of SCIs, the cassette expression level can vary along of the array depending on the nature of the cassettes (49,50). First, even though these are a minority, cassettes can carry their own promoters and therefore be expressed whatever their position in the array. Second, cassettes can have a RBS site ensuring a better translation (51). Third, for cassettes devoid of RBS, the presence of translated ORF just upstream increases their translation rates favoring the destabilization of the attC site structure on the mRNA transcript and ensuring the ribosome progression (9). Nevertheless, these observed preferential insertions of incoming cassettes in the first position, i.e. in the PC vicinity, ensure their efficient expression whatever their nature. This means that, even if the frequency of cassette insertion seems low when mediated by the endogenous integrase (∼10–6), these events can be selected and evolutionarily fixed if cassette expression leads to an advantageous new function. Thus, the knowledge of the precise cassette functions in SCI would allow us to trace back the environmental conditions of cell growth and the evolutionary history of SCI-containing cells.
RecAVch is critical for cassette insertion in the attIA site of the Vibrio cholerae SCI
Previous studies have shown how integrons are intimately connected with their hosts. For instance, the folding of single-stranded attC substrates depends on several cellular processes including conjugation, replication or DNA topology (7,23). attC site folding can also be modulated by the binding of the SSB host protein, which, by hampering attC hairpin folding in vivo in the absence of IntI, would play an important role in maintaining attC integrity and thus its recombinogenic functionality (45). It has also been shown that the pathway that allows the resolution of aHJ formed during the attI × attC reaction is linked to a host-replicative process (22). Moreover, integrase expression is also extensively regulated by environmental stresses, thus connecting cassette recombination to the host-cell environment. Both integrase and cassette expressions, ensured respectively by Pint and PC, are regulated by catabolite repression in V. cholerae SCI (24,26). Expression of the MI class 1 integrase is in part regulated by the stringent response in biofilms (25). The most relevant example among these regulatory pathways is undoubtedly the induction of integrase expression (of class 1 MI and V. cholerae SCI) during the SOS response. Such regulation is ensured by the binding of the LexA repressor in the promoter region of Pint (27,28). Integrase expression, similar to all genes of the SOS regulon (more than 40 SOS genes) (50), is triggered by the autocatalytic cleavage of LexA, a process induced by the binding of RecA on single-stranded DNA, constituting a nucleofilament. The SOS response is a global response to DNA damage ensuring a state of high-activity DNA repair.
In this study, we investigated the role of the RecA protein at another regulatory level of the integron system, cassette recombination. We choose to study this protein for two reasons. First, because of its ssDNA binding properties, since we know that cassette recombination involves one of the recombination sites, the attC site, in a ssDNA form. Secondly, because efficient cassette recombination could be dependent on one or several proteins belonging to the SOS regulon and therefore whose expression is induced in presence of RecA. We found that the RecAVch protein is critical for the attIA × attC reaction in the SCI of V. cholerae and that the RecA effect is independent on SOS regulon proteins. On the contrary, when we tested the effect of RecAVch on cassette insertions in VCR sites of the SCI, we found that these attC × VCR reactions were independent of the RecAVch protein. We also confirmed the absence of the RecA effect on attC × attC recombination by testing cassette insertion in an attCaadA1 site-carrying plasmid. Interestingly, these results show that the RecA effect depends on the nature of att sites (attI or attC), which are recombined. Altogether, our results indicate that the RecA protein is a host factor specifically involved in the cassette recruitment in the attIA site of the V. cholerae SCI during environmental stress conditions. Finally, the RecA protein acts at several steps during the integron recombination process (Figure 8). First, as described above, in the presence of antibiotics or when single-stranded cassettes are released in the cell by HGT, RecA favors the expression of the SOS-dependent integrase gene (28,52). Secondly, RecA ensures the cassette recruitment in the SCI of V. cholerae. Since the recA gene itself also belongs to the V. cholerae SOS regulon (50), its expression is probably up-regulated as in E. coli (ten-fold within minutes (53,54)). This release of the RecA protein would allow RecA to be available to trigger its biological functions, among which its effect on cassette recruitment at attIA SCI (Figure 8).
Model of RecAVch mechanism of action
RecA nucleofilament indirectly activates expression of a large number of genes during the SOS response (50). The mechanism of action of RecAVch on integron recombination could thus be the consequence of an indirect effect. Using a V. cholerae mutant strain lexA(ind-) in which the SOS response is constitutively repressed, we showed that SOS response abrogation did not affect recombination rates and therefore that the observed RecAVch effect on attIA × attC recombination does not involve any other protein belonging to the SOS regulon. Besides, we know that the E. coli RecA protein differs from the V. cholerae RecA protein in the extreme C-terminal part and that this part is known to modulate interactions with regulatory proteins (Supplementary Figure S2) (Cox, 2007b). We decided to study the effect of the RecAEc protein in attIA × attC recombination mediated by IntIA. We performed complementation of the V. cholerae ΔrecA mutant by the recAEc gene. We observed that the RecAEc protein fully restores the recombination activity, meaning that both RecA proteins may ensure cassette insertion in the attIA site.
Contrary to the attC × attIA reaction, we did not observe any influence of RecAVch on attC × attC reaction mediated by IntIA in V. cholerae, while both reactions require a ss attC site on a double-stranded DNA molecule, we still observed an important effect of the RecAVch protein. Then, in whatever way attC sites are delivered, i.e. single-stranded (conjugation and replicative assays) or double-stranded (replicative assay), we observed an important effect of the RecAVch protein in attIA × attC recombination. Altogether, these results show that the observed RecAVch effect is not linked to the regulation of attC site folding. We therefore suppose that the attIA × attC synaptic complex formed during the recombination could impede the aHJ resolution by blocking replication fork progression. The effect of RecA would be to bind the ss attC sites and help to maintain the attC site in a non-folded single-stranded form destabilizing the synaptic complex and favoring the replicative resolution step. Note that we did not observe this RecAVch effect on attC × attC recombination suggesting a differential configuration between both attC × attC and attIA × attC complexes. Indeed, synapses formed during both reactions are known to be different since involving respectively two flexible bs attC sites versus a flexible bs attC and a stiffer ds attI (55). Such differences would explain that the synapse architecture for the attIA × attC reaction versus attC × attC may potentially require the assistance of additional accessory proteins. Furthermore, the attC × attC reaction can be catalyzed by IntIA in E. coli while, productive attIA × attC reactions are almost undetectable in this host (16).
Host factor recruitment differs between mobile and sedentary chromosomal Integrons
Biskri et al. previously observed the integrase of the SCI of V. cholerae recombines 2000-fold less efficiently during an attIA × attC reaction when expressed in a heterologous host such as E. coli (16). This observation suggests that IntIA requires host factors that are absent or too divergent in E. coli to carry out this reaction (16). Here, we confirmed this result and failed at recovering attIA × attC recombination by expressing RecAVch in E. coli, suggesting that the RecAVch protein is not the missing factor (or not the single one) in E. coli impeding this reaction to efficiently occur. This is not surprising since, even though they show some slight variations in the C terminal part, RecAEc and RecAVch present a very high level of identity (80%, Supplementary Figure S2). In addition, this is in accordance with the fact that RecAEc was able to functionally complement the absence of RecAVch to achieve this reaction in V. cholerae (Figure 3A). Therefore, we hypothesize that other specific V. cholerae host factors are missing in E. coli.
In contrast, RecA is not involved in any reactions mediated by IntI1 (neither in E. coli or V. cholerae) and all these reactions are efficient in V. cholerae. Therefore, while SCI cassette recombination activity seems restricted to a given host, MI recombination seems efficient in different species suggesting a host factor recruitment more stringent for SCIs compared to MIs. This is consistent with the observed widespread MIs dissemination among bacterial species. The evolutionary success of class 1 MIs clearly reflects the fact that they are functional in a wide range of bacterial hosts. This could result from a co-evolution between the integrase IntI1 and its attI1 site to ensure that cassette recombination takes place in absence of specific accessory proteins. Both IntI and attI level of divergence, 45% between IntIA and IntI1 (55) and the lack of identity between attIA and attI1 sites, likely reflect their functional differences. Indeed, the attI1 site harbors supplementary IntI1 binding sites called ‘direct repeats’ (DRs, Supplementary Figure S4), that constitute accessory sequences to which the integrase is able to bind (56). These DRs favor attI1 × attC reactions (57). However, such sequences are absent from the majority of attI sites (58) and, for instance, the attIA site of the V. cholerae SCI does not harbor any DRs (Supplementary Figure S4). Upon mobilization of integrons, attI sites may have evolved and selected such motifs (e.g. DRs), to replace trans-acting host factors necessary for the attI1 × attC reactions. For instance, DRs of attI1 site acting as topological filters (57) could therefore replace host factors that regulate supercoiling.
Natural transformation represents an efficient pathway for SCI cassette recruitment
HGT contributes to the emergence of pathogens and the spread of virulence factors, and also enables many disease-causing bacteria to rapidly evolve in response to environmental pressures such as antibiotic use (59–65). Among HGT processes, natural transformation is able to mediate the absorption and exchange of free DNA when sufficient homology is present between the incoming DNA and the bacterial genome (66,67). Here, we demonstrated that acquisition of material during natural transformation can be also mediated by homology independent DNA mechanisms, notably by exchanging and recruiting gene cassettes inside integrons in an integrase dependent way. We used the causative agent of the diarrheal disease cholera, Vibrio cholerae, responsible for seven major pandemics since 1817, the latter of which being still ongoing (68). Cassette recruitments occur directly in the attIA site of the SCI contained in V. cholerae where they are expressed and selected if providing an advantage to the V. cholerae strain. The natural bacterial competence has already been demonstrated for at least 80 species of bacteria but remains little explored and is probably underestimated (69,70). Notably, natural transformation is largely widespread among Vibrionaceae, where SCIs are mainly found and constitute very plastic regions (71,72). Here, we validated a new pathway for integron cassette recruitment in SCIs triggered by natural transformation probably involved in adaptation and making integrons important motors of evolution in Vibrionaceae species.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Evelyne Krin for providing the N16961 recA mutant and Sandra Arroyo-Beck and Marcos Manero for their experimental help. We also thank Jason Bland for helpful reading of the manuscript and all the lab members for helpful discussion.
Author contributions: C.L., D.M. and V.P. designed the research. C.L., C.V., E.R., F.F., C.W., X.E. and D.L. performed the experiments. C.L. and C.V. wrote the draft of the manuscript. All authors read, amended the manuscript, and approved its final version.
Contributor Information
Claire Vit, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France; Sorbonne Université, Collège doctoral, F-75005 Paris, France.
Egill Richard, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France; Sorbonne Université, Collège doctoral, F-75005 Paris, France.
Florian Fournes, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France.
Clémence Whiteway, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France.
Xavier Eyer, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France.
Delphine Lapaillerie, CNRS, UMR5234, Fundamental Microbiology and Pathogenicity laboratory, University of Bordeaux. Département de Sciences Biologiques et Médicales, Bordeaux, France; Viral DNA Integration and Chromatin Dynamics Network (DyNAVir), France.
Vincent Parissi, CNRS, UMR5234, Fundamental Microbiology and Pathogenicity laboratory, University of Bordeaux. Département de Sciences Biologiques et Médicales, Bordeaux, France; Viral DNA Integration and Chromatin Dynamics Network (DyNAVir), France.
Didier Mazel, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France.
Céline Loot, Institut Pasteur, Unité Plasticité du Génome Bactérien, CNRS UMR3525, Paris, France.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Institut Pasteur, Centre National de la Recherche Scientifique, the French Government's Investissement d’Avenir program Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases [ANR-10-LABX-62- IBEID]; Fondation pour la Recherche Médicale and Ecole Doctorale Complexité du Vivant. Funding for open access charge: Institut Pasteur.
Conflict of interest statement. None declared.
REFERENCES
- 1. Partridge S.R., Kwong S.M., Firth N., Jensen S.O.. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018; 31:e00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stokes H.W., Hall R.M.. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 1989; 3:1669–1683. [DOI] [PubMed] [Google Scholar]
- 3. Mazel D., Dychinco B., Webb V.A., Davies J.. A distinctive class of integron in the Vibrio cholerae genome. Science. 1998; 280:605–608. [DOI] [PubMed] [Google Scholar]
- 4. Cambray G., Guerout A.M., Mazel D. Integrons. 2010; 44:141–166. [DOI] [PubMed] [Google Scholar]
- 5. Cury J., Jove T., Touchon M., Neron B., Rocha E.P.. Identification and analysis of integrons and cassette arrays in bacterial genomes. Nucleic. Acids. Res. 2016; 44:4539–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowe-Magnus D.A., Guerout A.M., Ploncard P., Dychinco B., Davies J., Mazel D.. The evolutionary history of chromosomal super-integrons provides an ancestry for multiresistant integrons. Proc. Natl. Acad. Sci. U.S.A. 2001; 98:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loot C., Nivina A., Cury J., Escudero J.A., Ducos-Galand M., Bikard D., Rocha E.P., Mazel D.. Differences in integron cassette excision dynamics shape a trade-off between evolvability and genetic capacitance. mBio. 2017; 8:e02296-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowe-Magnus D.A., Guerout A.M., Mazel D.. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol. Microbiol. 2002; 43:1657–1669. [DOI] [PubMed] [Google Scholar]
- 9. Jacquier H., Zaoui C., Sanson-le Pors M.J., Mazel D., Bercot B.. Translation regulation of integrons gene cassette expression by the attC sites. Mol. Microbiol. 2009; 72:1475–1486. [DOI] [PubMed] [Google Scholar]
- 10. Biskri L., Mazel D.. Erythromycin esterase gene ere(A) is located in a functional gene cassette in an unusual class 2 integron. Antimicrob. Agents Chemother. 2003; 47:3326–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Fonseca E.L., Vicente A.C.. Functional characterization of a Cassette-specific promoter in the class 1 integron-associated qnrVC1 gene. Antimicrob. Agents Chemother. 2012; 56:3392–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stokes H.W., Hall R.M.. Sequence analysis of the inducible chloramphenicol resistance determinant in the Tn1696 integron suggests regulation by translational attenuation. Plasmid. 1991; 26:10–19. [DOI] [PubMed] [Google Scholar]
- 13. Szekeres S., Dauti M., Wilde C., Mazel D., Rowe-Magnus D.A.. Chromosomal toxin-antitoxin loci can diminish large-scale genome reductions in the absence of selection. Mol. Microbiol. 2007; 63:1588–1605. [DOI] [PubMed] [Google Scholar]
- 14. Bouvier M., Demarre G., Mazel D.. Integron cassette insertion: a recombination process involving a folded single strand substrate. EMBO J. 2005; 24:4356–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouvier M., Ducos-Galand M., Loot C., Bikard D., Mazel D.. Structural features of single-stranded integron cassette attC sites and their role in strand selection. PLos Genet. 2009; 5:e1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biskri L., Bouvier M., Guerout A.M., Boisnard S., Mazel D.. Comparative study of class 1 integron and Vibrio cholerae superintegron integrase activities. J. Bacteriol. 2005; 187:1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collis C.M., Kim M.J., Stokes H.W., Hall R.M.. Integron-encoded IntI integrases preferentially recognize the adjacent cognate attI site in recombination with a 59-be site. Mol. Microbiol. 2002; 46:1415–1427. [DOI] [PubMed] [Google Scholar]
- 18. Francia M.V., Zabala J.C., de la Cruz F., Garcia-Lobo J.M.. The IntI1 integron integrase preferentially binds single-stranded DNA of the attC site. J. Bacteriol. 1999; 181:6844–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johansson C., Kamali-Moghaddam M., Sundstrom L.. Integron integrase binds to bulged hairpin DNA. Nucleic. Acids. Res. 2004; 32:4033–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacDonald D., Demarre G., Bouvier M., Mazel D., Gopaul D.N.. Structural basis for broad DNA specificity in integron recombination. Nature. 2006; 440:1157–1162. [DOI] [PubMed] [Google Scholar]
- 21. Nivina A., Escudero J.A., Vit C., Mazel D., Loot C.. Efficiency of integron cassette insertion in correct orientation is ensured by the interplay of the three unpaired features of attC recombination sites. Nucleic. Acids. Res. 2016; 44:7792–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loot C., Ducos-Galand M., Escudero J.A., Bouvier M., Mazel D.. Replicative resolution of integron cassette insertion. Nucleic Acids Res. 2012; 40:8361–8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loot C., Bikard D., Rachlin A., Mazel D.. Cellular pathways controlling integron cassette site folding. EMBO J. 2010; 29:2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baharoglu Z., Krin E., Mazel D.. Connecting environment and genome plasticity in the characterization of transformation-induced SOS regulation and carbon catabolite control of the Vibrio cholerae integron integrase. J. Bacteriol. 2012; 194:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strugeon E., Tilloy V., Ploy M.C., Da Re S.. The stringent response promotes antibiotic resistance dissemination by regulating integron integrase expression in biofilms. mBio. 2016; 7:e00868-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krin E., Cambray G., Mazel D.. The superintegron integrase and the cassette promoters are co-regulated in Vibrio cholerae. PLoS One. 2014; 9:e91194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cambray G., Sanchez-Alberola N., Campoy S., Guerin E., Da Re S., Gonzalez-Zorn B., Ploy M.C., Barbe J., Mazel D., Erill I.. Prevalence of SOS-mediated control of integron integrase expression as an adaptive trait of chromosomal and mobile integrons. Mobile DNA. 2011; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guerin E., Cambray G., Sanchez-Alberola N., Campoy S., Erill I., Da Re S., Gonzalez-Zorn B., Barbe J., Ploy M.C., Mazel D.. The SOS response controls integron recombination. Science. 2009; 324:1034. [DOI] [PubMed] [Google Scholar]
- 29. Escudero J.A., Loot C., Nivina A., Mazel D. The integron: adaptation on demand. Microbiology Spectrum. 2015; 3:MDNA3–0019–2014. [DOI] [PubMed] [Google Scholar]
- 30. Baharoglu Z., Bikard D., Mazel D.. Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 2010; 6:e1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le Roux F., Binesse J., Saulnier D., Mazel D.. Construction of a Vibrio splendidus mutant lacking the metalloprotease gene vsm by use of a novel counterselectable suicide vector. Appl. Environ. Microbiol. 2007; 73:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Val M.E., Skovgaard O., Ducos-Galand M., Bland M.J., Mazel D.. Genome engineering in Vibrio cholerae: a feasible approach to address biological issues. PLoS Genet. 2012; 8:e1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cronan J.E. Cosmid-based system for transient expression and absolute off-to-on transcriptional control of Escherichia coli genes. J. Bacteriol. 2003; 185:6522–6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Lemos Martins F., Fournes F., Mazzuoli M.V., Mazel D., Val M.E.. Vibrio cholerae chromosome 2 copy number is controlled by the methylation-independent binding of its monomeric initiator to the chromosome 1 crtS site. Nucleic Acids Res. 2018; 46:10145–10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cherepanov P.P., Wackernagel W.. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995; 158:9–14. [DOI] [PubMed] [Google Scholar]
- 36. Marvig R.L., Blokesch M.. Natural transformation of Vibrio cholerae as a tool–optimizing the procedure. BMC Microbiol. 2010; 10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meibom K.L., Blokesch M., Dolganov N.A., Wu C.Y., Schoolnik G.K.. Chitin induces natural competence in Vibrio cholerae. Science. 2005; 310:1824–1827. [DOI] [PubMed] [Google Scholar]
- 38. Goldberg I., Mekalanos J.J.. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J. Bacteriol. 1986; 165:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shinohara A., Ogawa T.. Rad51/RecA protein families and the associated proteins in eukaryotes. Mutat. Res. 1999; 435:13–21. [DOI] [PubMed] [Google Scholar]
- 40. Cox M.M. Motoring along with the bacterial RecA protein. Nat. Rev. Mol. Cell Biol. 2007; 8:127–138. [DOI] [PubMed] [Google Scholar]
- 41. Kowalczykowski S.C. Initiation of genetic recombination and recombination-dependent replication. Trends Biochem. Sci. 2000; 25:156–165. [DOI] [PubMed] [Google Scholar]
- 42. Lusetti S.L., Cox M.M.. The bacterial RecA protein and the recombinational DNA repair of stalled replication forks. Annu. Rev. Biochem. 2002; 71:71–100. [DOI] [PubMed] [Google Scholar]
- 43. Lusetti S.L., Voloshin O.N., Inman R.B., Camerini-Otero R.D., Cox M.M.. The DinI protein stabilizes RecA protein filaments. J. Biol. Chem. 2004; 279:30037–30046. [DOI] [PubMed] [Google Scholar]
- 44. Cox M.M. Regulation of bacterial RecA protein function. Crit. Rev. Biochem. Mol. Biol. 2007; 42:41–63. [DOI] [PubMed] [Google Scholar]
- 45. Loot C., Parissi V., Escudero J.A., Amarir-Bouhram J., Bikard D., Mazel D.. The integron integrase efficiently prevents the melting effect of Escherichia coli single-stranded DNA-binding protein on folded attC sites. J. Bacteriol. 2014; 196:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Domingues S., Harms K., Fricke W.F., Johnsen P.J., da Silva G.J., Nielsen K.M.. Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog. 2012; 8:e1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Collis C.M., Hall R.M.. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob. Agents Chemother. 1995; 39:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Souque C., Escudero J.A., MacLean R.C.. Integron activity accelerates the evolution of antibiotic resistance. Elife. 2021; 10:e62474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Michael C.A., Labbate M.. Gene cassette transcription in a large integron-associated array. BMC Genet. 2010; 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krin E., Pierle S.A., Sismeiro O., Jagla B., Dillies M.A., Varet H., Irazoki O., Campoy S., Rouy Z., Cruveiller S.et al.. Expansion of the SOS regulon of Vibrio cholerae through extensive transcriptome analysis and experimental validation. BMC Genomics. 2018; 19:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hanau-Bercot B., Podglajen I., Casin I., Collatz E.. An intrinsic control element for translational initiation in class 1 integrons. Mol. Microbiol. 2002; 44:119–130. [DOI] [PubMed] [Google Scholar]
- 52. Baharoglu Z., Mazel D.. Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: a route towards multiresistance. Antimicrob. Agents Chemother. 2011; 55:2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Courcelle J., Khodursky A., Peter B., Brown P.O., Hanawalt P.C.. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001; 158:41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Renzette N., Gumlaw N., Nordman J.T., Krieger M., Yeh S.P., Long E., Centore R., Boonsombat R., Sandler S.J.. Localization of RecA in Escherichia coli K-12 using RecA-GFP. Mol. Microbiol. 2005; 57:1074–1085. [DOI] [PubMed] [Google Scholar]
- 55. Demarre G., Frumerie C., Gopaul D.N., Mazel D. Identification of key structural determinants of the IntI1 integron integrase that influence attC x attI1 recombination efficiency. Nucleic Acids Res. 2007; 35:6475–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gravel A., Fournier B., Roy P.H.. DNA complexes obtained with the integron integrase IntI1 at the attI1 site. Nucleic Acids Res. 1998; 26:4347–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Partridge S.R., Recchia G.D., Scaramuzzi C., Collis C.M., Stokes H.W., Hall R.M.. Definition of the attI1 site of class 1 integrons. Microbiology. 2000; 146:2855–2864. [DOI] [PubMed] [Google Scholar]
- 58. Nield B.S., Holmes A.J., Gillings M.R., Recchia G.D., Mabbutt B.C., Nevalainen K.M., Stokes H.W.. Recovery of new integron classes from environmental DNA. FEMS Microbiol. Lett. 2001; 195:59–65. [DOI] [PubMed] [Google Scholar]
- 59. de la Cruz F., Davies J.. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 2000; 8:128–133. [DOI] [PubMed] [Google Scholar]
- 60. Gogarten J.P., Doolittle W.F., Lawrence J.G.. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 2002; 19:2226–2238. [DOI] [PubMed] [Google Scholar]
- 61. Ochman H., Lerat E., Daubin V.. Examining bacterial species under the specter of gene transfer and exchange. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:6595–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blokesch M. Natural competence for transformation. Curr. Biol. 2016; 26:3255. [DOI] [PubMed] [Google Scholar]
- 63. Waldor M.K., Mekalanos J.J.. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996; 272:1910–1914. [DOI] [PubMed] [Google Scholar]
- 64. Frost L.S., Leplae R., Summers A.O., Toussaint A.. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 2005; 3:722–732. [DOI] [PubMed] [Google Scholar]
- 65. Prudhomme M., Attaiech L., Sanchez G., Martin B., Claverys J.P.. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006; 313:89–92. [DOI] [PubMed] [Google Scholar]
- 66. Griffith F. The significance of Pneumococcal types. J. Hyg. (Lond). 1928; 27:113–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen I., Dubnau D.. DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2004; 2:241–249. [DOI] [PubMed] [Google Scholar]
- 68. Lippi D., Gotuzzo E., Caini S.. Cholera. Microbiol. Spectr. 2016; 4:doi:10.1128/microbiolspec.PoH-0012-2015. [DOI] [PubMed] [Google Scholar]
- 69. Johnsborg O., Eldholm V., Havarstein L.S.. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 2007; 158:767–778. [DOI] [PubMed] [Google Scholar]
- 70. Johnston C., Campo N., Berge M.J., Polard P., Claverys J.P.. Streptococcus pneumoniae, le transformiste. Trends Microbiol. 2014; 22:113–119. [DOI] [PubMed] [Google Scholar]
- 71. Le Roux F., Blokesch M.. Eco-evolutionary dynamics linked to horizontal gene transfer in Vibrios. Annu. Rev. Microbiol. 2018; 72:89–110. [DOI] [PubMed] [Google Scholar]
- 72. Matthey N., Stutzmann S., Stoudmann C., Guex N., Iseli C., Blokesch M.. Neighbor predation linked to natural competence fosters the transfer of large genomic regions in Vibrio cholerae. Elife. 2019; 8:e48212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.