Abstract
Objectives:
Advances in genetic technologies provide opportunities for patient care and ethical challenges. Clinical care of patients with rare Mendelian disorders is often at the forefront of those developments. Whereas in classical polygenic inflammatory bowel disease (IBD), the predictive value of genetic variants is very low, predictive prenatal genetic diagnosis can inform families at high risk of severe genetic disorders. Patients with IL-10 signalling defects because of pathogenic variants in IL10RA, Il10RB, and IL10 develop severe infantile onset inflammatory bowel disease that is completely penetrant and has a high morbidity and substantial mortality despite treatment.
Methods:
We performed a survey among tertiary specialist paediatric centers of 10 countries on the utilization of predictive prenatal genetic diagnosis in IL-10 signalling defects. We retrospectively report prenatal genetics in a series of 8 families.
Results:
International variation in legislation, guidelines, expert opinion, as well as cultural and religious background of families and clinicians results in variable utilization of preimplantation and prenatal genetic testing for IL-10 signalling defects. Eleven referrals for prenatal diagnosis for IL-10 signalling defects were identified across 4 countries. We report on 8 families who underwent prenatal preimplantation monogenic testing after in vitro fertilization (n = 2) and/or by amniocentesis/chorion villus sampling (n = 6). A genetic diagnosis was established in 1 foetus and excluded in 7 foetuses (all IL10RA variants).
Conclusions:
Prenatal genetic testing for IL10R-defects is feasible, yet the legal and ethical considerations are complex and controversial. In some countries, predictive genetics for IL-10-related signalling defects is entering clinical practice.
Keywords: in vitro fertilization, preimplantation genetic testing, very-early-onset inflammatory bowel disease
Advances in genomics allow us to explain the genetic contribution in an increasing number of inherited disorders including polygenic and monogenic forms of inflammatory bowel disease (IBD) (1). Due to the combination of genetic and environmental factors at present it is largely impossible to predict the development of many immune-mediated disorders, such as IBD based on genetic markers. Among the increasing group of monogenic IBD gene defects, interleukin-10 (IL-10) signalling defects present with a particularly severe infantile-onset intestinal inflammation caused by mutations in IL-10 and the IL-10 receptor (encoded by IL10RA, IL10RB, and IL10) (2–4). Patients respond poorly to immunomodulatory treatments and biologics, such as anti-TNF therapies, and may require multiple surgical procedures (4). Allogeneic hematopoietic stem cell transplantation (HSCT) or umbilical cord stem cell transplantation has emerged as the standard of care approach for those disorders (5).
In particular, metabolic or developmental disorders with severe phenotype and complete penetrance because of chromosomal abnormalities or monogenic defects have been identified as candidate disorders for prenatal genetic diagnosis in a setting of multidisciplinary care involving pretest and post-test counselling, support from prenatal specialists, and adequate postnatal care (6). At the extreme early spectrum, preimplantation genetic testing for those monogenic diseases allows parents after in vitro fertilization to progress with implantation depending on the genotype (7). Preimplantation as well as intrauterine prenatal genetic testing, however, raise important ethical questions regarding the moral status of the embryo, genetic selection, resource allocation, and the emotional burden for women/couples (8,9). Several case series describe the use of prenatal genetic testing for fatal or severe monogenic disorders, including spinal muscular atrophy, myotonic dystrophy type 1, β-thalassemia, and Fragile X syndrome (10–13). A Japanese multicenter retrospective questionnaire survey on genetic counseling facilities showed that prenatal testing had been performed for 45 distinct genetic disorders (14). No report, however, has been issued on prenatal genetic testing of monogenic IBD.
We report the use of the prenatal genetic testing in couples who have previously had children with infantile IBD because of IL-10 signalling defects. The aim of this study was to evaluate the current international clinical practice of prenatal genetic testing for monogenic IBD because of IL-10 signalling defects and discuss ethical considerations.
MATERIALS AND METHODS
Audit on the Use of Prenatal Genetic Diagnosis in Different Countries
We surveyed the use of prenatal genetic diagnosis for infantile-onset IBD because of IL-10 signalling deficiency across 10 countries. Tertiary centres in China, United Kingdom, Germany, France, United States, Netherlands, Canada, South Korea, Japan, and Israel specialized in supporting patients with IL-10 signalling defects, and their families were approached. To avoid overinflation of similar opinion among clinicians in 1 center or collaborative care among centers in 1 country, we captured the clinical practice of the center/country. Using a structured e-mail survey we asked clinicians to provide their opinion whether there is medical need for prenatal diagnostics in families with known IL-10 signalling defects (YES/NO, comment) and whether families with known IL-10 signalling defects underwent prenatal diagnostics in their center (YES/NO; if yes how many families). As part of the survey, we asked open-ended questions to explore clinicians’ reasons to perform or not to perform prenatal diagnosis. The survey on current practice involves no patient identifiable data, and therefore, does not require ethical approval.
Case Series and Ethics
For all families that were included into a retrospective case series, written consent was obtained from each family and ethics approval obtained from the Ethics Committee for Human Subject research of the Obstetrics and Gynaecology Hospital, Fudan University [consent form 2010 (12)].
We recorded in families with genetically confirmed IL-10 signalling defect (pathogenic variants in IL10, IL10RA and IL10RB) of a previous child the family history, outcomes of previous pregnancies, prenatal genetic technologies (preimplantation genetic testing for a single gene defect, amniocentesis or chorionic villus sampling [CVS]), and genetic findings. Pregnancy outcomes and follow-up were identified from clinical records.
Procedures and genetic analysis was performed as standard of care in the local setting after clinical genetic counselling. In vitro fertilization, SNP-array for copy number analysis were performed as previously described (15) and Sanger Sequencing for variant analysis. For in vitro fertilization, fertilized oocytes were produced using intracytoplasmic sperm injection, and cultured for 5 to 6 days until the blastocyst stage. Three to 10 cells were removed from the trophectoderm on the fifth day of embryonic development. These cells were placed in polymerase chain reaction tubes with an alkaline denaturation buffer. Whole genomic amplification was performed by the multiple displacement amplification method. Isothermal DNA amplification was performed with phi 29 DNA polymerase (REPLI-g single cell kit, QIAGEN GmbH, Hilden, Germany). SNP genotypes were performed using Illumina Human Karyomap-12 V1.0 microarray as previously described (16). Each Karyomap-12 bead chip contains approximately 300,000 SNPs. The molecular karyotype analysis and the linkage analysis of haplotype were analysed with Blue-fuse1-Multi software v4.2 (Illumina, Inc. San Diego). The informative SNPs of 2 Mb around the mutation were selected to establish haplotypes of the regions covering the mutation.
Amniocentesis or CVS were performed according to standard protocol(17).FoetalDNAwasobtainedfromchorionicvillioramniotic fluid. Sanger sequencing was performed to confirm the mutations in IL10RA using specific primers as previously described (18).
RESULTS
Current Clinical Practice in Tertiary International Centres
Our survey from 12 centers in 10 different countries revealed a striking controversy with respect to the perceived clinical need, putative patient benefit, and ethical considerations for prenatal genetic testing in families with IL-10-related signalling defects (Fig. 1A and B). Opinion of clinicians ranged from opposing prenatal diagnostics (as with HSCT, an excellent treatment option is available and/or because of fundamental ethical concerns) to recognizing the benefits of predictive prenatal genetic diagnosis for infantile-onset IBD because of IL-10 signalling deficiency (because of severity of the disorder, difficulty to treat patients in the local setting, economic considerations for health care utilization where prenatal diagnosis costs is a fraction of the treatment costs of biologics, surgery, and stem cell transplantation in particular in a setting where health insurance is not available). Reasons for not performing prenatal diagnostics despite considering it as a potential option can be attributed to ethical or religious considerations of the clinicians, the social and religious background of the families. Some clinicians are open to consider prenatal diagnostics upon family request but there were no subsequent pregnancies in the affected families.
FIGURE 1.
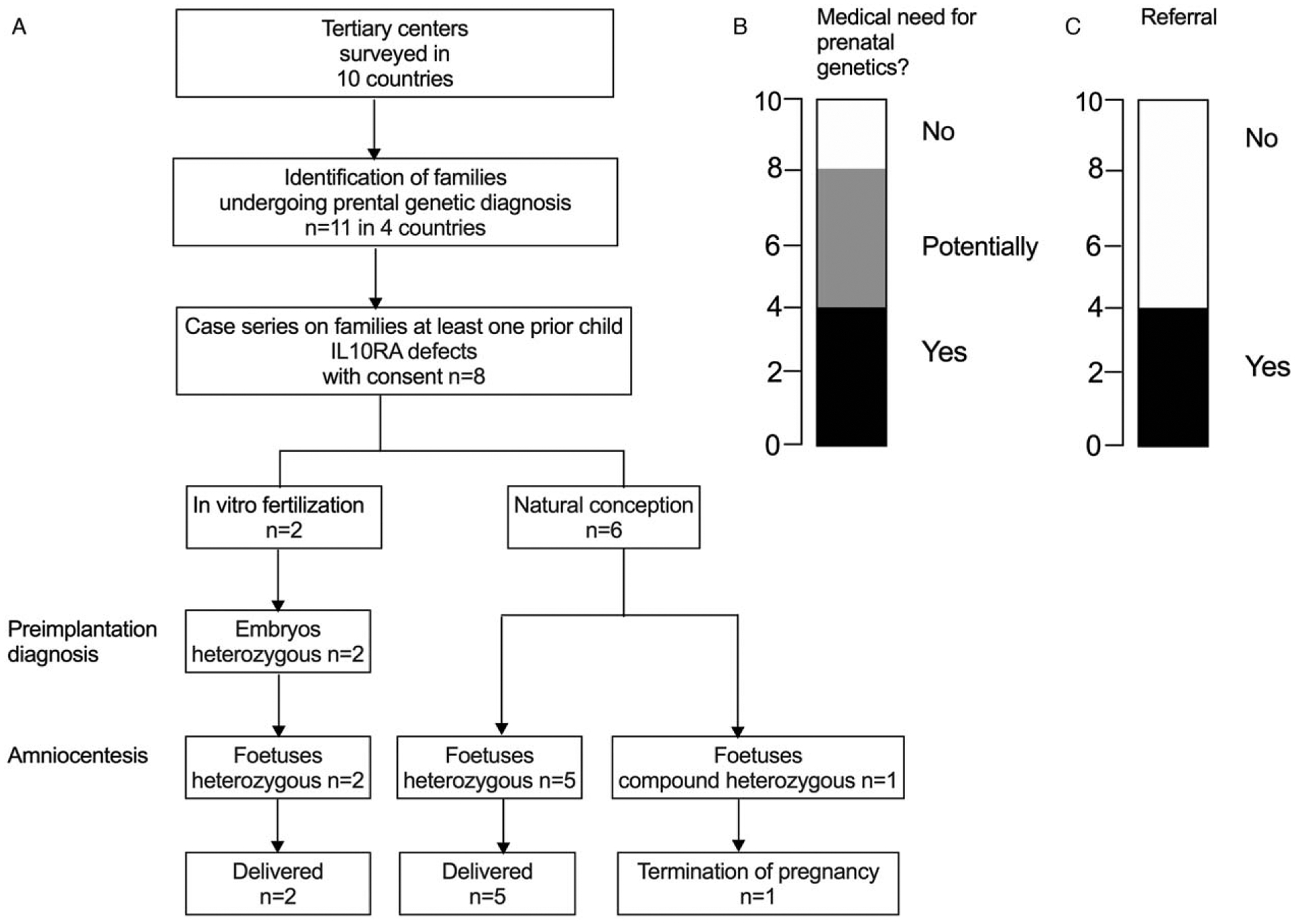
International survey on the use of prenatal genetic testing as part of clinical practice and case series. (A) Summary of responses from Prenatal genetics for monogenic IBD standardized survey on the use of prenatal genetic testing. Flow chart and pregnancy outcomes of families undergoing prenatal genetic testing. (B and C) Survey response regarding medical need and use of prenatal genetic diagnostics.
Centres from 4 out of 10 countries reported the use of prenatal genetic testing in clinical practice in families where a previous child was affected by infantile IBD because of IL-10 signalling defects (Fig. 1A and B).
Preimplantation and in-Utero Genetic Testing in Clinical Practice
Eight referrals for prenatal diagnosis for infantile-onset IBD with IL-10 signalling deficiency were identified as retrospective case series (Fig. 1). In all cases, state-of-the-art genetic counselling was provided. Of the 8 couples, 2 underwent in vitro fertilization and preimplantation genetic analysis. In families with natural conception (n = 6), genetic analysis was performed by amniocentesis or chorionic villus sampling.
Three foetuses were heterozygous for the maternal mutation, and 4 were heterozygous for the paternal mutation. These pregnancies continued and no children developed IBD over the mean follow-up period of 1.7 years (range: 9.6 months to 3.1 years; Fig. 1 and Table 1). One foetus was compound heterozygous for the pathogenic IL10RA mutations and the pregnancy was terminated.
TABLE 1.
Genetic sequencing results as part of prenatal genetic diagnosis
| Case | Gene | Paternal mutation | Maternal mutation | Conception | Prenatal result |
|---|---|---|---|---|---|
| 1 | IL10RA | c.689–1G>T | c.301C>T | In vitro fertilization | Heterozygous c.301C>T |
| 2 | IL10RA | c.537G>A | c.301C>T | In vitro fertilization | Heterozygous c.301C>T |
| 3 | IL10RA | c.301C>T | c.569C>T | Natural | Heterozygous c.301C>T |
| 4 | IL10RA | c.301C>T | c.537G>A | Natural | Heterozygous c.537G>A |
| 5 | IL10RA | c.299T>G | c.301C>T | Natural | Heterozygous c.299T>G |
| 6 | IL10RA | c.301C>T | c.537G>A | Natural | Heterozygous c.301C>T |
| 7 | IL10RA | c.349C>T | c.301G>A | Natural | Heterozygous c.349C>T |
| 8 | IL10RA | c.301C>T | c.537G>A | Natural | Compound heterozygous c.301C>T/c.537G>A |
Two representative examples illustrate the family history and clinical utilization of prenatal genetics (Fig. 2). In family I, the parents had a child (1-II.1) with infantile IBD because of confirmed IL10RA defects. This child had severe disease with onset in the neonatal period requiring multiple surgical procedures. Partial remission was obtained with azathioprine and thalidomide and the parents did not opt for allogeneic hematopoietic stem cell transplantation or umbilical cord stem cell transplantation. The couple underwent in vitro fertilization, preimplantation genetic testing, and an embryo heterozygous for a pathogenic variant was implanted. The genotype was subsequently confirmed by CVS. 1-II.2 now is a healthy 16 months old female.
FIGURE 2.
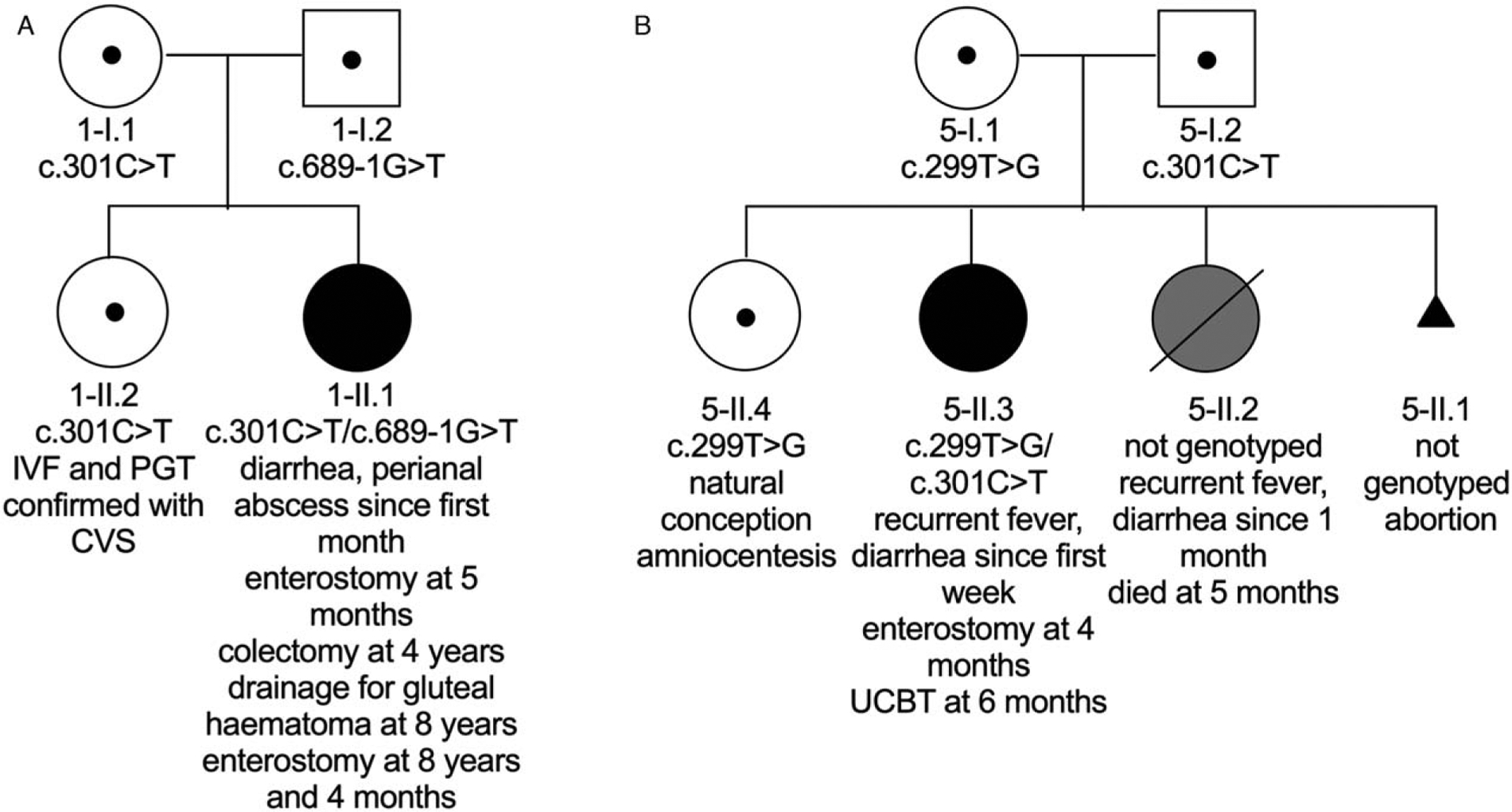
Examples of prenatal genetic testing. (A) Pedigree of family I. (B) Pedigree of family V. Black symbols represent affected individuals with biallelic pathogenic IL10RA variants, grey symbols represent a child with infantile IBD where no genotype is available. Dots represent individuals with heterozygous pathogenic variants. CVS = chorionic villus sampling, UCBT = umbilical cord blood transplantation.
Family V had 2 previous children who developed infantile IBD. Child 5-II.2 died from severe infantile IBD (not genotyped). In the subsequent child, 5-II.3 whole exome sequencing identified an IL10RA defect after the onset of infantile IBD. Allogeneic HSCT using umbilical cord blood stem cells was performed. After 4 years of follow-up, child 5-II.3 is in complete clinical remission. In the subsequent pregnancy, the couple underwent prenatal diagnosis with amniocentesis, which demonstrated a heterozygous variant (patient 5-II.4). This child developed into a healthy 3-year-old with no evidence of IBD.
DISCUSSION
Prenatal predictive genetics is an emerging field in medicine with opportunities but also specific legal implications and challenging ethical considerations. In this article, we report the medical perspective and current clinical practice of prenatal testing in tertiary referral centres of 10 different countries that are specialized to support families with children affected by IL-10 signalling defects. In this context, it is important to discuss the indication, family considerations for decision-making, health care provider considerations, the legal framework, and ethical considerations.
Criteria, such as penetrance of the disease, age of onset, disease severity, phenotypic expression, and treatability are key for health care professionals to determine indications of clinical prenatal genetics (9,19). Infantile-onset IBD because of IL-10 signalling defects meets many of those criteria. Patients with IL-10 signalling defects, including mutations in IL10, IL10RA, and IL10RB, develop a particularly severe phenotype with neonatal or infantile onset of disease (5,20). Biallelic loss-of-function IL-10 signalling defects are characterized by complete penetrance of intestinal inflammation. Patients with IL-10 signalling defects do not achieve long-term sustained remission with standard therapeutics for IBD, including 5-ASA, immunomodulators, and biologics (21), and develop complications of B cell lymphoma at a young age (20,22). Allogeneic HSCT in particular bone marrow stem cell, and less commonly used umbilical cord blood stem cell tansplantation has been successfuly applied to treat patients with IL-10-related signalling defects (3,23,24). Indeed, the majority of patients with IL-10 signalling defects are in complete clinical remission 2 years after HSCT (4,25). This therapeutic modality has emerged as the standard of care but requires strong expertise, interdisciplinary collaboration, and is thus only available at selected transplant centers. The outcome might vary among centers. Allogeneic HSCT is associated with complications, such as acute or chronic graft-versus-host disease, graft failure/graft rejection, and life-threatening infections, causing morbidity and mortality in a proportion of patients (20,21,23). In a cohort of 40 patients with IL-10 signaling defects, an overall mortality of 17.5% was observed after HSCT (20). Furthermore, allogeneic HSCT is associated with very high costs, preventing many patients with insufficient health insurance and financial opportunities from access (26). More targeted approaches, such as hematopoietic stem cell gene therapy, may offer alternative therapies for definitive cures in the future.
Decision-making factors for families, and attitudes towards prenatal testing in general include motivation for a healthy child, ability (psychological and socioeconomical) to take care of a sick child, costs, and ethical or religious considerations (27,28). Similarly to the families reported in this report, in other disease areas many affected patients and families are open to consider prenatal genetic diagnosis. The majority of patients who are affected by severe autosomal dominant disease (ie, patients are affected by the disease and likely to transmit the disorder with 50% probability) would consider prenatal testing (29). The motivation of families with infantile monogenic IBD and IL-10 signalling defects has not been investigated.
Our survey describing prenatal testing for IL-10 signalling defects illustrate that there is no consensus in regards to clinical practice and clinicians attitudes. Again, this likely reflects a broader lack of consensus in the field of prenatal medicine. Clinicians are faced with ethical dilemmas, including concerns about adequate pretest counselling, issues revolving around the selection of the embryo as well as conflicts in regards to their own ethical convictions and religious believes (30,31). In a worldwide web-based survey, most health care providers supported preimplantation genetic testing over testing in utero (32).
Regulation of prenatal genetic testing varies across the world (33,34). For example, the use of preimplantation genetic testing is controlled by legislation in some countries and professional guidelines in others (7,9). For instance, professional guidelines largely govern the use of preimplantation genetic testing in the United States, China, and Japan (7,35). Preimplantation diagnostics is regulated by legislation in many European countries, such as Germany without specific reference to individual immune-mediated disorders. In the South Korea Enforcement Decree of Bioethics and Biosafety act, 153 disorders including a small number of immune dysregulation defects are specified but not IL-10 signalling defects (36). In the UK, preimplantation genetic testing can only occur once a condition has been approved by the Human Fertilization and Embryology Authority and IL-10-related signalling disorders are such specified conditions.
Prenatal genetic diagnosis poses highly complex ethical and societal issues (8,37). From a parents’ or healthcare system’s perspective, reasons to perform prenatal genetic testing for an increasing number of conditions include the aim to improve ‘health’, reduce psychosocial burden of affected families, and to optimize costs. With regard to the moral and ethical implications of prenatal testing, there is controversy regarding parental decision and responsibility to undergo testing in order to select the child based on available genetic information (38,39). An ethical position prioritizing the respect of the dignity of every human being over utilitarian considerations to maximise the ‘good’, in this context health, is often in contrast to this.
Ethical controversies include questions concerning the moral status of the embryo, the risk of genetic selection, the definition of a ‘good’ life, potential discrimination of people living with a particular condition, short- and long-term safety of preimplantation and intrauterine sampling and testing, costs, and psychological burden (8). The potential harms to the embryo, the child, and the family need to be carefully balanced with harms that may occur when no testing is undertaken and a child is born with severe infantile IBD requiring treatment associated with high economic and emotional costs. Genetic testing allows women or parents to make informed reproductive decisions whether it is about preimplantation genetic testing, termination of pregnancy, or preparation for a child with a severe condition. Whatever decision a woman or parents will make, it is important that professionals provide adequate genetic counselling allowing for an informed balancing of the harms and benefits involved, and ultimately for an informed decision (8).
In summary, our data suggest that in some tertiary centers, prenatal genetic testing is currently adapted in clinical practice. Families at high risk of developing infantile IBD because of known genetic pathogenic variants in the IL-10 receptor complex have chosen to undergo prenatal testing. This highlights the speed how genetic technologies find their way into clinical practice. A number of further developments, such as fetal exome sequencing (40), noninvasive prenatal diagnosis with cell-free fetal DNA (41–43) might accelerate this development. Our brief study not only illustrates the technical advances but also the challenging ethical considerations for families and health care professionals and the need for careful counselling.
CONCLUSIONS
We show that prenatal genetic diagnostics for couples with known genetic risk for infantile IBD because of IL-10 signalling defects is technically feasible, is about to enter clinical practice, and poses ethical concerns that must be addressed by health care professionals in interdisciplinary studies.
What Is Known
Patients with interleukin-10 signalling defects develop infantile-onset inflammatory bowel disease refractory to conventional immunomodulatory and immunosuppressive treatments. There is a high degree of morbidity and mortality.
Genomic technologies are increasingly used to explain the immunopathology in patients with monogenic causes of intestinal inflammation.
What Is New
In families with high recurrence risk of infantile onset inflammatory bowel disease caused by interleukin-10 signalling defects, prenatal diagnosis has been performed.
Predictive genetic testing is about to enter clinical practice in some countries.
Acknowledgments
Z.Y. is supported by the H.M. LUI Memorial Fund. H.H.U. is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. Y.H. is supported by the National Children’s Medical Center, Haiju International Joint Lab Fund. A.M., S.S., C.K., and H.H.U. are supported by the Leona M. and Harry B. Helmsley Charitable Trust. R.H. is supported by the Wellcome Centre Grant (203132/Z/16/Z).
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Uhlig HH, Powrie F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu Rev Immunol 2018;36:755–81. [DOI] [PubMed] [Google Scholar]
- 2.Uhlig HH, Schwerd T, Koletzko S, et al. , COLORS in IBD Study Group and NEOPICS. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147:990.e3–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glocker EO, Frede N, Perro M, et al. Infant colitis–it’s in the genes. Lancet 2010;376:1272. [DOI] [PubMed] [Google Scholar]
- 4.Kotlarz D, Beier R, Murugan D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology 2012;143:347–55. [DOI] [PubMed] [Google Scholar]
- 5.Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009;361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chitty LS. Advances in the prenatal diagnosis of monogenic disorders. Prenat Diagn 2018;38:3–5. [DOI] [PubMed] [Google Scholar]
- 7.Ginoza MEC, Isasi R. Regulating preimplantation genetic testing across the world: a comparison of international policy and ethical perspectives. Cold Spring Harb Perspect Med 2020;10:a036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn R, Parker M. Opening Pandora’s box?: ethical issues in prenatal whole genome and exome sequencing. Prenat Diagn 2018;38:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayefsky MJ. Comparative preimplantation genetic diagnosis policy in Europe and the USA and its implications for reproductive tourism. Reprod Biomed Soc Online 2016;3:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez RM, Pecina A, Lozano-Arana MD, et al. Clinical and technical overview of preimplantation genetic diagnosis for Fragile X syndrome: experience at the University Hospital Virgen del Rocio in Spain. Biomed Res Int 2015;2015:965839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian M, Lee CG, Chong SS. Robust preimplantation genetic testing strategy for myotonic dystrophy type 1 by bidirectional triplet-primed polymerase chain reaction combined with multi-microsatellite haplotyping following whole-genome amplification. Front Genet 2019;10:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao M, Lian M, Cheah FSH, et al. Identification of novel microsatellite markers flanking the SMN1 and SMN2 duplicated region and inclusion into a single-tube tridecaplex panel for haplotype-based preimplantation genetic testing of spinal muscular atrophy. Front Genet 2019;10:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Shen X, Chen D, et al. Multiple displacement amplification as the first step can increase the diagnostic efficiency of preimplantation genetic testing for monogenic disease for beta-thalassemia. J Obstet Gynaecol Res 2019;45:1515–21. [DOI] [PubMed] [Google Scholar]
- 14.Nobuzane T, Yamada T, Miura K, et al. Survey of prenatal testing for genetic disorders in Japan: recent report. J Obstet Gynaecol Res 2016;42:375–9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Lei C, Wu J, et al. The establishment and application of preimplantation genetic haplotyping in embryo diagnosis for reciprocal and Robertsonian translocation carriers. BMC Med Genomics 2017;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handyside AH, Harton GL, Mariani B, et al. Karyomapping: a universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J Med Genet 2010;47:651–8. [DOI] [PubMed] [Google Scholar]
- 17.Ghi T, Sotiriadis A, Calda P, et al. , International Society of Ultrasound in Obstetrics and Gynecology (ISUOG). ISUOG Practice Guidelines: invasive procedures for prenatal diagnosis. Ultrasound Obstet Gynecol 2016;48:256–68. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z, Peng K, Li X, et al. Mutations in interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: a Chinese VEO-IBD Collaboration Group Survey. Inflamm Bowel Dis 2017;23:578–90. [DOI] [PubMed] [Google Scholar]
- 19.Klitzman R. Challenges, dilemmas and factors involved in PGD decision-making: providers’ and patients’ views, experiences and decisions. J Genet Couns 2018;27:909–19. [DOI] [PubMed] [Google Scholar]
- 20.Zheng C, Huang Y, Hu W, et al. Phenotypic characterization of very early-onset inflammatory bowel disease with interleukin-10 signaling deficiency: based on a large cohort study. Inflamm Bowel Dis 2019;25:756–66. [DOI] [PubMed] [Google Scholar]
- 21.Ouahed J, Spencer E, Kotlarz D, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflamm Bowel Dis 2020;26:820–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neven B, Mamessier E, Bruneau J, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood 2013;122:3713–22. [DOI] [PubMed] [Google Scholar]
- 23.Peng K, Qian X, Huang Z, et al. Umbilical cord blood transplantation corrects very early-onset inflammatory bowel disease in Chinese patients with IL10RA-associated immune deficiency. Inflamm Bowel Dis 2018;24:1416–27. [DOI] [PubMed] [Google Scholar]
- 24.Ye Z, Huang Y, Zheng C, et al. Clinical and genetic spectrum of children with congenital diarrhea and enteropathy in China. Genet Med 2019;21:2224–30. [DOI] [PubMed] [Google Scholar]
- 25.Pigneur B, Escher J, Elawad M, et al. Phenotypic characterization of very early-onset IBD due to mutations in the IL10, IL10 receptor alpha or beta gene: a survey of the Genius Working Group. Inflamm Bowel Dis 2013;19:2820–8. [DOI] [PubMed] [Google Scholar]
- 26.Poudyal B, Tuladhar S, Neupane S, et al. Socio-economic determinants of hematopoietic stem cell transplantation (HSCT) in Nepal. Biol Blood Marrow Transplant 2020;26:S298. [Google Scholar]
- 27.Genoff Garzon MC, Rubin LR, Lobel M, et al. Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet 2018;94:22–42. [DOI] [PubMed] [Google Scholar]
- 28.Brezina PR, Kutteh WH. Clinical applications of preimplantation genetic testing. BMJ 2015;350:g7611. [DOI] [PubMed] [Google Scholar]
- 29.Swift O, Vilar E, Rahman B, et al. Attitudes in patients with autosomal dominant polycystic kidney disease toward prenatal diagnosis and preimplantation genetic diagnosis. Genet Test Mol Biomarkers 2016;20:741–6. [DOI] [PubMed] [Google Scholar]
- 30.Hens K, Dondorp W, Handyside AH, et al. Dynamics and ethics of comprehensive preimplantation genetic testing: a review of the challenges. Hum Reprod Update 2013;19:366–75. [DOI] [PubMed] [Google Scholar]
- 31.Harper J, Geraedts J, Borry P, et al. , ESHG, ESHRE and EuroGentest2. Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Hum Reprod 2014;29:1603–9. [DOI] [PubMed] [Google Scholar]
- 32.Weissman A, Shoham G, Shoham Z, et al. Preimplantation genetic screening: results of a worldwide web-based survey. Reprod Biomed Online 2017;35:693–700. [DOI] [PubMed] [Google Scholar]
- 33.Knoppers BM, Isasi RM. Regulatory approaches to reproductive genetic testing. Hum Reprod 2004;19:2695–701. [DOI] [PubMed] [Google Scholar]
- 34.Skirton H, Goldsmith L, Jackson L, et al. Offering prenatal diagnostic tests: European guidelines for clinical practice [corrected]. Eur J Hum Genet 2014;22:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayefsky M Who should regulate preimplantation genetic diagnosis in the United States? AMA J Ethics 2018;20:E1160–7. [DOI] [PubMed] [Google Scholar]
- 36.Kim NK. Legislation on genetic diagnosis: comparison of South Korea and Germany: with focus on the application and communication structure. Dev Reprod 2015;19:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botkin JR, Belmont JW, Berg JS, et al. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet 2015;97:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savulescu J Procreative beneficence: why we should select the best children. Bioethics 2001;15:413–26. [DOI] [PubMed] [Google Scholar]
- 39.Parker M The best possible child. J Med Ethics 2007;33:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monaghan KG, Leach NT, Pekarek D, et al. , ACMG Professional Practice and Guidelines Committee. The use of fetal exome sequencing in prenatal diagnosis: a points to consider document of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2020;22:675–80. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins LA, Deans ZC, Lewis C, et al. Delivering an accredited noninvasive prenatal diagnosis service for monogenic disorders and recommendations for best practice. Prenat Diagn 2018;38:44–51. [DOI] [PubMed] [Google Scholar]
- 42.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics, Committee on Genetics; Society for Maternal–Fetal Medicine. Practice Bulletin No. 162 Summary: Prenatal Diagnostic Testing for Genetic Disorders. Obstet Gynecol 2016;127:e108–22. [Google Scholar]
- 43.Fan HC, Gu W, Wang J, et al. Non-invasive prenatal measurement of the fetal genome. Nature 2012;487:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


