Abstract
Insulin resistance is the rate-limiting step in the development of metabolic diseases, including type 2 diabetes. The gut microbiota has been implicated in host energy metabolism and metabolic diseases and is recognized as a quantitatively important organelle in host metabolism, as the human gut harbors 10 trillion bacterial cells. Gut microbiota break down various nutrients and produce metabolites that play fundamental roles in host metabolism and aid in the identification of possible therapeutic targets for metabolic diseases. Therefore, understanding the various effects of bacterial metabolites in the development of insulin resistance is critical. Here, we review the mechanisms linking gut microbial metabolites to insulin resistance in various insulin-responsive tissues.
Keywords: Insulin resistance, Skeletal muscle, Liver, Adipose tissue, Intestine, Gut bacterial metabolites
Core Tip: Since the gut microbiota has been implicated in host energy metabolism and metabolic diseases, understanding mechanisms linked to insulin resistance is a first step in discovery of new drugs and novel targets against metabolic diseases. Here, we review the mechanisms linking gut microbial metabolites to insulin resistance in major target tissues of insulin.
INTRODUCTION
Insulin resistance is a pathological state in which tissues do not respond normally to insulin in the process of glucose metabolism. Insulin is an endocrine hormone that binds to insulin receptors on the plasma membrane of target cells, which induces an anabolic response to nutrient availability[1]. Insulin directly regulates glucose homeostasis by acting on skeletal muscle, the liver, and adipose tissue. These tissues have different functions in metabolic homeostasis that are regulated via tissue-specific insulin signaling pathways. In skeletal muscle, insulin stimulates glucose uptake and storage by increasing the expression of glucose transporters (GLUTs) and glycogen synthesis[1,2]. In the liver, insulin activates glycogen synthesis and de novo lipogenesis and suppresses gluconeogenesis[1,2]. In adipose tissue, insulin suppresses lipolysis and increases both glucose and fatty acid uptake and lipogenesis[1,2]. In the insulin-resistant state, peripheral glucose disposal is impaired and hepatic gluconeogenesis and adipose lipolysis are not suppressed by insulin. Insulin resistance increases circulating glucose level, which results in increased insulin production in β cells as a compensatory response and hyperinsulinemia, leading to a vicious cycle that promotes further insulin resistance[1,3]. Non-treated and prolonged insulin resistance causes hyperglycemia and type 2 diabetes, and can lead to its complications including hyperlipidemia, metabolic syndrome, nonalcoholic fatty liver disease, and cardiovascular diseases[3,4].
Various factors have been implicated in the pathogenesis of insulin resistance, including genetic predisposition, aging, obesity, and a sedentary lifestyle. More recently, the gut microbiota has been considered to be a key factor leading to the insulin resistance[5]. The gut microbiota regulates host dietary intake, energy metabolism, and energy expenditure[6]. Changes in the composition of the intestinal bacteria might alter energy metabolism and exert various effects on the important metabolic organs, such as skeletal muscle, the liver, and adipose tissue[6]. In addition, the gut microbiota produces thousands of metabolites that accumulate in the gastrointestinal system and can be transferred to distant organs[7]. Lots of recent metabolomics studies examined the association of gut microbiota-derived metabolites with metabolic disease and their effects on host metabolism[8-10]. Therefore, understanding the various effects of bacterial metabolites in the development of insulin resistance becomes critical for discovering novel targets and developing new drugs against metabolic diseases. In this review, we review studies that provide evidence for a relationship between gut bacterial metabolites and insulin resistance, and summarize current mechanisms linking gut microbial metabolites to the development of insulin resistance in various metabolic organs, including skeletal muscle, liver, adipose tissue, and intestine.
EFFECTS OF GUT BACTERIAL METABOLITES ON THE PATHOGENESIS OF INSULIN RESISTANCE
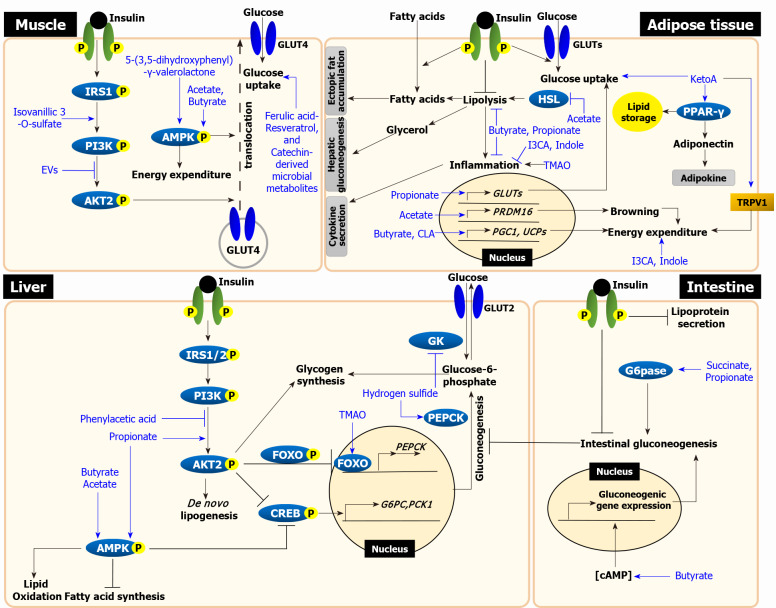
We split this section into four parts for each metabolic organ, and briefly describe the pathophysiology of insulin resistance first followed by further discussions on current mechanisms linking gut microbial metabolites to the development of insulin resistance. The mechanisms for each organ are graphically presented in Figure 1 and the studies for each metabolite are summarized in Table 1.
Figure 1.
The mechanisms linking microbial metabolites to insulin resistance. In skeletal muscle, insulin stimulates glucose uptake by translocating glucose transporter (GLUT) 4 via insulin receptor substrate (IRS)-phosphoinositide-3-kinase (PI3K)-AKT signaling. Isovanillic 3-O-sulfate increases glucose uptake by activating PI3K-AKT pathway. Gut bacteria-derived extracellular vesicles (EVs) decrease glucose uptake by inhibiting AKT phosphorylation. Glucose uptake is also increased via AMP-activated protein kinase (AMPK) activation, insulin-independent. 5-(3,5-dihydroxyphenyl-γ-valerolactone activates AMPK phosphorylation, which enhances glucose uptake. Although mechanisms have been unknown, other ferulic acid, resveratrol, and catechin-derived microbial metabolites also enhance glucose uptake (left upper panel). In liver, insulin activates glycogen synthesis and de novo lipogenesis and suppresses gluconeogenesis via IRS-PI3K-AKT signaling. Propionate increases the phosphorylation of both AKT and AMPK, which suppresses gluconeogenesis. Hydrogen sulfide stimulates gluconeogenesis via phosphoenolpyruvate carboxykinase activation and reduces glycogen synthesis via the inhibition of glucokinase activity. Trimethylamine N-oxide (TMAO) increases gluconeogenesis via PKR-like ER kinase-FOXO1 pathway. Phenylacetic acid inhibits AKT phosphorylation. All short chain fatty acids (SCFAs), including acetate, propionate, and butyrate, activate AMPK phosphorylation, which lead to decrease lipid accumulation (left lower panel). In adipose tissue, insulin stimulates glucose and fatty acid uptake and suppress lipolysis. Failure to suppress lipolysis in insulin-resistant adipose tissue increases circulating free fatty acids and glycerol, which leads to an increase in ectopic fat accumulation in the liver and muscle and stimulates hepatic gluconeogenesis. 10-oxo-12(Z)-octadecenoic acid (KetoA) increases insulin-stimulated glucose uptake and energy expenditure via TRPV2 activation. KetoA also increases the production and secretion of adiponectin via peroxisome proliferator-activated receptor-γ activation. TMAO increases inflammation in adipocyte. Indole and I3CA have anti-inflammatory effects. Conjugated linoleic acid enhances energy expenditure by increasing the expression of uncoupling proteins (UCPs) genes. All SCFAs inhibit lipolysis. Acetate inhibits lipolysis by suppressing HSL and stimulates also browning by increasing the expression of browning-related genes. Butyrate and propionate attenuate inflammation. Propionate increases glucose uptake by increasing GLUT4 expression. Butyrate enhances energy expenditure by upregulating PPAR-γ coactivator 1 and UCPs genes (right upper panel). The intestine, as discussed in this review, is an organ that actively interacts with gut bacteria and accumulates microbial metabolites. Intestinal lipoprotein secretion and gluconeogenesis are suppressed by insulin. In intestine, propionate and succinate act as gluconeogenic substrate, which activate gluconeogenesis via G6Pase activation. Butyrate increases cyclic adenosine monophosphate levels, which upregulates the expression of gluconeogenic genes and increases gluconeogenesis. Through this mechanisms, increased intestinal gluconeogenesis suppresses hepatic gluconeogenesis (right lower panel). Black lines represent insulin resistance-related events and blue lines represent action of metabolites. Grey boxes represent the effects of adipose tissue on other tissues. EVs: Extracellular vesicles; PEPCK: Phosphoenolpyruvate carboxykinase; GK: Glucokinase; TMAO: Trimethylamine N-oxide; PERK: PKR-like ER kinase; FOXO1: Forkhead box protein O1; cAMP: Cyclic adenosine monophosphate; CREB: cAMP-response element binding protein; G6PC: Glucose 6-phosphatase catalytic subunit; PCK1: Phosphoenolpyruvate carboxykinase 1; TRPV1: Transient receptor potential vanilloid 1; PPAR: Peroxisome proliferator-activated receptor; I3CA: Indole-3-carboxylic acid; CLA: Conjugated linoleic acid; HSL: Hormone-sensitive lipase; PRDM16: PR domain containing 16; PGC1: Peroxisome proliferator-activated receptor-gamma coactivator 1; G6Pase: Glucose 6-phosphatase.
Table 1.
The effects of diet-derived gut bacterial metabolites on the pathogenesis of insulin resistance in various organs
|
Category
|
Metabolite
|
Target organ
|
Effects
|
Ref.
|
| Carbohydrate | ||||
| Fiber-derived | Acetate | Skeletal muscle | Increased lipid oxidation in vivo | Yamashita et al[75] |
| Liver | Decreased lipogenesis in vivo | den Besten et al[47] and Yamashita et al[51] | ||
| Increased lipid oxidation in vivo | den Besten et al[47], Yamashita et al[51], Kondo et al[52] and Sahuri-Arisoylu et al[53] | |||
| Adipose tissue | Stimulated adipogenesis in vitro | Ge et al[60] | ||
| Inhibited lipolysis in vitro and in vivo | Hong et al[59], Ge et al[60] and Jocken et al[61] | |||
| Increased browning in vitro and in vivo | Sahuri-Arisoylu et al[53] and Hanatani et al[73] | |||
| Whole body | Increased energy expenditure and fat oxidation in vivo and in humans | den Besten et al[47], Canfora et al[77] and van der Beek et al[78] | ||
| Propionate | Liver | Suppressed gluconeogenesis in vitro | Yoshida et al[29] | |
| Decreased lipogenesis in vivo | den Besten et al[47] | |||
| Increased lipid oxidation in vivo | den Besten et al[47] | |||
| Adipose tissue | Increased adipogenesis in vitro | Ge et al[60] | ||
| inhibit lipolysis in vitro and in vivo | Hong et al[59] and Ge et al[60] | |||
| Improved inflammation in ex vivo | Al-Lahham et al[66] | |||
| Intestine | Promoted gluconeogenesis in vivo | De Vadder et al[91] | ||
| Whole body | Increased energy expenditure and fat oxidation in vivo and in humans | den Besten et al[47], Canfora et al[77] and Chambers et al[79] | ||
| Butyrate | Skeletal muscle | Increased lipid oxidation in vitro and in vivo | Gao et al[48] | |
| Liver | Decreased lipogenesis in vivo | den Besten et al[47] | ||
| Increased lipid oxidation in vivo | den Besten et al[47], Gao et al[48] and Mollica et al[49] | |||
| Adipose tissue | decreased lipolysis in vitro | Ohira et al[67] | ||
| Improved inflammation in vitro | Ohira et al[67] | |||
| Increased thermogenesis in vivo | Gao et al[48] and Li et al[74] | |||
| Intestine | Promoted gluconeogenesis in vitro and in vivo | De Vadder et al[91] | ||
| Whole body | Increased energy expenditure and fat oxidation in vivo and in humans | den Besten et al[47], Gao et al[48] and Canfora et al[77] | ||
| Succinate | Intestine | Promoted gluconeogenesis in vivo | De Vadder et al[92] | |
| Protein | ||||
| Protein-derived | Hydrogen sulfide | Liver | Increased gluconeogenesis in vitro | Zhang et al[32] |
| Decreased glycogen synthesis in vitro | Zhang et al[32] | |||
| Indole | Adipose tissue | Increased inflammation in vivo | Virtue et al[10] | |
| Indole-3-carboxylic acid | Adipose tissue | Increased inflammation in vivo | Virtue et al[10] | |
| Phenylacetic acid | Liver | Increased lipogenesis in ex vivo and in vivo | Hoyles et al[46] | |
| Lipid and others | ||||
| Linoleic acid-derived | 10-oxo-12(Z)-octadecenoic acid | Adipose tissue | Induced adipogenesis in vitro | Goto et al[55] |
| Increased thermogenesis in vivo | Kim et al[81] | |||
| Conjugated linoleic acid | Adipose tissue | Increased energy expenditure | Takahashi et al[82], Park et al[83] and Lee et al[84] | |
| Ferulic acid-derived | Ferulic acid 4-O-sulfate and Dihydroferulic acid 4-O-sulfate | Skeletal muscle | Increased glucose uptake in vitro | Houghton et al[19] |
| Resveratrol-derived | Trans-resveratrol 4’-O-glucuro-nide and Trans-resveratrol 3-O-sulfate | Skeletal muscle | Increased glucose uptake in vitro | Houghton et al[19] |
| Berries-derived | Isovanillic acid 3-O-sulfate | Skeletal muscle | Increased glucose uptake in vitro | Houghton et al[19] |
| Catecin-derived | 4-hydroxy-5-(3,4,5-trihydroxyphenyl) valeric acid, 5-(3,4,5-trihydroxyphenyl)-γ-valerolac-tone, and 5-(3-hydroxyphenyl) valeric acid | Skeletal muscle | Increased glucose uptake in vitro | Takagaki et al[22] |
| Catecin-derived | 5-(3,5-dihydroxyphenyl)-γ-valerolactone | Skeletal muscle | Increased glucose uptake in vitro and in vivo | Takagaki et al[22] |
| Bacteria-derived | Extracellular vesicles | Skeletal muscle | Decreased glucose uptake in vivo | Choi et al[20] |
| Choline-derived | Trimethylamine N-oxide | Liver | Increased gluconeogenesis in ex vivo and in vivo | Chen et al[33] and Gao et al[43] |
| Adipose tissue | Promoted inflammation in vivo | Gao et al[43] | ||
Skeletal muscle
Skeletal muscle is the primary organ for glucose disposal, accounting for up to 70% of glucose uptake in our body[11]. Insulin promotes glucose uptake in skeletal muscle by translocating the GLUT4 to the plasma membrane[12]. In insulin-sensitive skeletal muscle, the insulin receptor substrate 1-phosphoinositide-3-kinase (PI3K)-AKT arm of the insulin signaling cascade is activated, which increases glucose uptake and glycogen synthesis[1]. In insulin-resistant skeletal muscle, proximal insulin signaling events are impaired, which blocks the function of insulin to translocate GLUT4 to plasma membrane and to stimulate glycogen synthesis[1]. Furthermore, when calorie loads exceed the glucose uptake capacity of skeletal muscle, the circulating glucose mostly returns to the liver, triggering hepatic de novo lipogenesis[3], which causes ectopic fat deposition in the liver and other tissues, further exacerbating insulin resistance[13]. Therefore, impaired glucose uptake in skeletal muscle has been considered as a major culprit of type 2 diabetes[14-16] and targeted as a therapeutic strategy against insulin resistance[17,18].
A recent study suggests that microbial products derived from phenolic acids may increase glucose uptake in skeletal muscle under insulin-stimulated condition[19]. Microbiota-produced phenolic metabolites are derived from ferulic acid, resveratrol, and berries. The ferulic acid-derived metabolites, ferulic acid 4-O-sulfate and dihydroferulic acid 4-O-sulfate, and the resveratrol-derived metabolites, trans-resveratrol 4’-O-glucuronide and trans-resveratrol 3-O-sulfate, increased 2-deoxy-D-[1-14C(U)]-glucose uptake in LHCN-M2 human skeletal muscle cells[19]. Isovanillic acid 3-O-sulfate, which is primarily derived from berries, increased glucose uptake in myotubes through GLUT4-PI3K-AKT-dependent mechanisms and stimulated dose-dependent glucose uptake[19]. On the other hands, a study has been shown that bacterial-derived metabolite-complex can decrease glucose uptake, though the exact composition of the complex is not defined[20]. Gut bacteria-derived extracellular vesicles (EVs), which are phospholipid spherical bilayer, are ubiquitously produced by gram-negative bacteria[20]. Especially, Pseudomonas panacis-derived EVs increasing in a high-fat diet (HFD)-fed mice compared to regular chow-fed mice as well as gut microbe-derived EVs from HFD-fed mice stools induced insulin resistance, including impairment of insulin signaling both in vitro and in vivo, and impairment of glucose uptake by decreasing insulin-dependent GLUT4 translocation, both in myotubes and adipocytes [20].
Insulin-independent glucose uptake is also activated by microbial metabolites. Activation of AMP-activated protein kinase (AMPK) in response to exercise regulates the translocation of GLUT4 storage vesicles and promotes insulin-independent glucose uptake[21]. In particular, 5-(3,5-dihydroxyphenyl)-γ-valerolactone has been shown to increase GLUT4 translocation via activation of AMPK through an insulin-independent pathway in skeletal muscle both in vitro and in vivo[22]. The antidiabetic green tea catechin (-)-epigallocatechin gallate (EGCG) is further degraded by Flavonifractor plautii[23], and several gut bacteria-derived EGCG metabolites, including 5-(3,5-dihydroxyphenyl)-γ-valerolactone, 4-hydroxy-5-(3,4,5-trihydroxyphenyl)valeric acid, 5-(3,4,5-trihydroxyphenyl)-γ-valerolactone, and 5-(3-hydroxyphenyl) valeric acid, have been shown to promote 2-deoxy-glucose uptake in myotubes in vitro[22]. It has been reported that Flavonifractor plautii was decreased in fecal microbiota of subjects with mild fasting hyperglycemia[24].
Liver
The liver is a central organ that coordinates whole-body metabolism, including carbohydrate, lipid, and protein metabolism. The liver is responsible for gluconeogenesis, glycogenolysis, glycogen synthesis, and de novo lipogenesis[1]. In contrast to skeletal muscle, hepatic glucose uptake is not regulated by insulin but blood glucose levels because GLUT2, a transporter with a high KM for glucose, is abundantly expressed in the liver and not translocated by insulin stimulation[25]. Rather than regulating the glucose uptake, in the liver, insulin suppresses hepatic glucose production by reducing the transcription of gluconeogenic enzymes[26,27] and induces a shift from net glucose production to net glucose storage by simultaneous regulation of glycogenolysis and glycogen synthesis[2]. However, in an insulin-resistant state, these regulations are not controlled by insulin, and the non-suppressed hepatic glucose production under insulin stimulated condition has been considered as a maker for hepatic insulin resistance[1,28]. It has been reported that propionate, a gut microbial product derived from carbohydrate fermentation, regulates hepatic gluconeogenesis under insulin stimulated condition[29]. Previously, stable isotope studies in both humans and animals have showed that propionate is used as a gluconeogenic substrate in the liver rather than being directly oxidized[30,31]. Recently, it was reported that propionate effectively suppresses hepatic glucose production in both presence and absence of long chain fatty acid by increasing the expression of gluconeogenesis-related genes, including G6PC and PCK1, via the G protein-coupled receptor (GPCR) 43-mediataed AMPK signaling pathway under insulin-stimulated condition as well as increases AKT phosphorylation in HepG2 hepatocyte[29]. In addition to insulin-stimulated condition, it has been reported that gut bacterial metabolites regulate hepatic glucose production under non-insulin stimulated basal condition [32,33]. Hydrogen sulfide, a product of protein fermentation, is not only generated in the body but also produced by sulfate-reducing bacteria, including Desulfovibrio, Desulfobacter, Desulfomonas, and Desulfobulbus, in the colon[32,34] and affects the basal hepatic glucose production. Under basal condition, this metabolite impairs glucose homeostasis by stimulating gluconeogenesis via increased phosphoenolpyruvate carboxykinase activity and decreased glucokinase by reducing glycogen synthesis in HepG2 human hepatoma cells[32]. In type 2 diabetes patients, it has been reported that the plasma hydrogen sulfide levels were reduced compared to healthy subjects[35,36], suggesting clinical association of microbial metabolites in hyperglycemia. Trimethylamine N-oxide (TMAO), which is known as a gut bacterial metabolite derived from choline, is converted by hepatic enzymes from trimethylamine, a choline-derived microbial metabolite, in liver[37]. The production of TMAO is completely suppressed in both antibiotics-treated humans and mice but the plasma of TMAO levels return to normal after the withdrawal of the antibiotics[38,39]. It has been reported that TMAO increases with insulin resistance in both humans and animals[40-42]. In mice, TMAO treatment promoted glucose intolerance, while a reduction of TMAO prevented glucose intolerance[33]. Under basal condition, the treatment of TMAO in mice activated PKR-like ER kinase and increased gluconeogenic gene expression, including G6pc and Pck1, via the forkhead box protein O1 transcription factor, which promoted hyperglycemia[33,43].
In addition to glucose metabolism, insulin also controls lipid metabolism in the liver. Since insulin normally promotes net hepatic de novo lipogenesis, one might expect decreased de novo lipogenesis in an insulin-resistant state; however, hepatic insulin resistance is highly associated with hepatic steatosis[1,3], and de novo lipogenesis is consistently elevated in insulin-resistant liver tissue[3]. This phenomenon has been termed “selective hepatic insulin resistance,” as glucose metabolism is affected by insulin resistance but lipid metabolism is not affected[44]. The increased de novo lipogenesis could be partly accounted by hyperinsulinemia, but still there are selective insulin resistance between glucose and lipid when considers the action of insulin per se[3,4]. Nevertheless, microbial metabolites can regulate the hepatic lipid metabolism. Phenylacetic acid is a microbial metabolite derived from aromatic compounds and produced by Bacteroides spp., which have aromatic amino acids fermentative activities[45]. Plasma phenylacetic acid positively correlates with the nonalcoholic fatty liver disease activity score in humans[9,46]. Phenylacetic acid induced the accumulation of hepatic triglycerides both in cellular and animal studies [46]. The metabolite also reduced insulin-induced AKT phosphorylation in human primary hepatocytes[46]. In contrast, short chain fatty acids (SCFAs), including acetate, propionate, and butyrate, decreased hepatic lipid accumulation. Administration of all three SCFAs in HFD-fed mice decreased not only total body fat content, without a change in food intake, but also the expression of genes related to hepatic lipogenesis and fatty acid synthase[47]. In addition, hepatic lipid oxidation capacity in SCFA-fed mice was increased via upregulation of mitochondrial uncoupling protein (UCP) 2 expression and activation of AMPK[47-49]. The SCFA acetate inhibited fatty acid synthesis in the liver via activation of AMPK. Oral administration of acetate stimulated the phosphorylation of AMPK, which inactivates carbohydrate-responsive element-binding protein[50], and in turn modulated the transcription of lipogenic genes in the liver[51]. Acetate also suppressed the increases in whole-body fat mass and hepatic lipid accumulation by increasing the expression of genes encoding peroxisome proliferator-activated receptor (PPAR) α and fatty acid oxidation-related proteins through AMPK α2 in the liver[52]. In mice, acetate treatment improved liver mitochondrial function by increasing the number of cristae, the location of the electron transport chain, per mitochondria, and the expression of complexes III, IV, and V[53]. Another SCFA, butyrate, increased mitochondrial mass and area and improved fatty acid oxidation in the liver of HFD-fed mice[49].
Adipose tissue
Adipose tissue is an energy storage organ[5]. In adipose tissue, insulin-stimulated glucose uptake also occurs via GLUT4 translocation, which is greatly reduced in insulin resistant condition, such as obesity and type 2 diabetes[54]. A linoleic acid-derived fatty acid generated by gut lactic acid bacteria, 10-oxo-12(Z)-octadecenoic acid (KetoA), induced adipocyte differentiation via activation of PPARγ, and increased the production of adiponectin and insulin-stimulated glucose uptake in 3T3-L1 murine adipocytes[55]. However, physiologically, adipose tissue is not quantitatively significant in insulin-stimulated glucose disposal because it accounts for < 5% of blood glucose uptake in our body[16]. Rather than glucose metabolism, insulin may have more critical roles in lipid metabolism of adipose tissues, thus the suppression of lipolysis is an important function of insulin in adipose tissue[4]. Failure to suppress lipolysis in insulin-resistant adipose tissue increases circulating free fatty acids and glycerol[15,56], and affects in hepatic glucose production[1]. These increased levels of circulating free fatty acids lead to an increase in ectopic fat accumulation in the liver and muscle, further exacerbating insulin resistance[15]. In addition, the glycerol released from adipose tissue serves as a gluconeogenic substrate and stimulates hepatic gluconeogenesis[1]. Suppression of lipolysis also reduces the levels of acetyl-CoA, an allosteric activator of pyruvate carboxylase, decreases pyruvate carboxylase activity[57]. As a result, gluconeogenic flux, involving glycerol and acetyl-CoA, is diminished, resulting in decreased hepatic gluconeogenesis[57]. Therefore, the regulation of lipolysis in adipose tissue is considered a therapeutic strategy against insulin resistance[58]. In the SCFAs, it has been reported both acetate and propionate stimulate adipogenesis and inhibit lipolysis via activation of GPCR43 but not GPCR41 [59,60]. Acetate might inhibit basal and beta-adrenergic receptor-mediated intracellular lipolysis via attenuation of hormone-sensitive lipase phosphorylation in human adipose tissue-derived adipocytes and lead to a reduction in non-esterified fatty acid release[61]. Injection of acetate into fasted mice led to decreased plasma free fatty acid levels via activation of GPCR43[60].
Adipose tissue also functions as an endocrine organ and releases adipokines, lipids, and cytokines, which regulates whole-body metabolism[62]. Adipose tissue can secrete molecules associated with improved insulin sensitivity, including adiponectin and branched fatty acid esters of hydroxyl fatty acids[63]. Chronic low-grade inflammation occurs in obese individuals with insulin resistance, which is mainly induced by adipose tissue inflammation[64]. Inflammation of adipose tissue is caused by macrophage infiltration, which impairs the insulin sensitivity of insulin target organs, resulting in insulin resistance[65]. TMAO, a microbial metabolite derived from choline, promoted adipose tissue inflammation in HFD-fed mice by increasing mRNA and serum levels of monocyte chemoattractant protein-1 (MCP-1), the proinflammatory cytokine, and decreasing mRNA and serum levels of interleukin (IL)-10, the anti-inflammatory cytokine, in adipose tissue[43]. Conversely, the SCFAs propionate and butyrate improved adipose tissue inflammation. Propionate may have a directly beneficial effect on adipose tissue in overweight subjects, as it reduced the mRNA expression and secretion of inflammatory cytokines and increased the mRNA expression of genes involved in lipogenesis (e.g., LPL, SREBP1c) and glucose uptake (e.g., GLUT4)[66]. Butyrate suppressed lipolysis and inflammatory responses, including the upregulation of tumor necrosis factor-α, MCP-1, and IL-6, which are generated by the interaction of adipocytes and macrophages [67]. It has been reported that gut bacterial metabolites derived from protein fermentation have anti-inflammatory effects in adipose tissue. Indole-3-carboxylic acid and indole, a tryptophan-derived microbial metabolites, are decreased in the cecal contents of HFD-fed mice compared to regular chow-fed mice[10]. These metabolites increased energy expenditure and improved insulin sensitivity by decreasing the expression of the microRNA miR-181, which is upregulated in HFD feeding and increases white adipose tissue (WAT) inflammation[10].
Brown adipose tissue and whole body energy expenditure
Unlike WAT, because brown adipose tissue (BAT) is responsible for energy expenditure by burning fatty acids to produce heat, it is an important organ that effects on whole body energy metabolism[68]. Similarly increasing beige adipocytes in WAT, a process termed “browning,” results in increased heat production and energy expenditure[69]. Therefore, enhanced BAT activity and browning of WAT are important for energy expenditure and are thought to influence insulin sensitivity[68,69]. It has been reported that gut bacterial metabolites derived from carbohydrates and fatty acids increase energy expenditure via browning of WAT and/or enhancing the function of BAT. SCFAs, including acetate, propionate, and butyrate, which are products of dietary fiber fermentation by gut bacteria. Acetate is mainly produced by Bifidobacteria and Lactobacillus and propionate is largely produced by Bacteroides and Veillonella, such as Bacteroides eggerthii, Bacteroides fragilis, and Veillonella parvula[70,71]. Butyrate is mostly produced by anaerobic bacteria, including Faecalibacterium prausnitzii, and Eubacterium rectale[72]. Acetate enhanced beige fat differentiation of white adipocytes in vitro[73]. Acetate and butyrate promoted browning in adipocytes[53,74]. In obese diabetic mice, acetate also induced browning of adipocytes by increasing thermogenesis-related gene expression, altered adipocyte morphology, and increased the thermogenic capacity of adipose tissue, independent of BAT, in HFD-fed mice[53,73]. Butyrate exerted an anti-obesity effect in animal models by strengthening the function of BAT. This anti-obesity effect occurs by increasing in energy expenditure and fat oxidation through upregulation of the expression of thermogenesis-related genes in BAT, such as PPAR-γ coactivator 1-α (PGC1α) and UCP1[48,74]. All SCFAs stimulated lipid oxidation by activating AMPK in the liver and adipose tissue[47]. Similarly, in skeletal muscle, acetate improved oxygen consumption by increasing the expression of lipid oxidation-related genes and AMPK activity in animal models[75]. Butyrate increased oxygen consumption and energy expenditure both in vitro and in vivo. These effects are caused by activation of AMPK and inhibition of histone deacetylases, which activate PGC1α and subsequently increase the expression of PPARδ, thus promoting fatty acid oxidation and increasing the proportion of type I muscle fibers, which are characterized by their high oxidative capacity[48]. In contrast to animal models, treatment of both lean and metabolic syndrome subjects with butyrate had no effect on BAT function[76], and open to debate over the single treatment. However, infusion of SCFA mixtures of acetate, propionate, and butyrate increased fat oxidation and whole-body energy expenditure in overweight/obese men[77,78]. These effects were observed following treatment with each SCFA alone as well as mixtures of the SCFAs. Propionate increased whole-body energy expenditure and fat oxidation in healthy and overweight/obese humans [79]. These findings suggested that SCFAs affect whole-body energy expenditure through a combination of mechanisms in different tissues, including skeletal muscle, the liver, and adipose tissue.
There are several studies show that fatty acid-derived microbial metabolites effect on energy expenditure. KetoA, a linoleic acid-derived microbial metabolite, has been suggested as the regulator of host energy metabolism. The anti-obesity effect of KetoA is shown via the activation of transient receptor potential vanilloid 1 (TRPV1), a member of the TRPV channel family, which has been reported to be important for the regulation of energy metabolism in adipocytes[80,81]. KetoA-induced TRPV1 activation enhanced energy expenditure by increasing the function of both BAT and WAT in diabetic mice[81]. Conjugated linoleic acid (CLA) is also mainly produced from linoleic acid by lactic acid bacteria, and enhances energy expenditure via increase in the expression of UCPs genes in adipose tissue[82,83]. Mice fed CLA-producing bacteria, Lactobacillus rhamnosus PL60, are prevented from diet-induced obesity and hepatic steatosis[84].
Intestine
The small intestine takes up glucose from the intestinal lumen mainly through sodium-glucose cotransporter 1 and transports glucose from enterocytes to the blood via GLUT2[85]. Although it has been reported that insulin inhibits the translocation of GLUT2 from the basolateral surface to the apical epithelial membrane, the role of insulin in intestinal glucose uptake is unclear[85,86]. Insulin signaling has been implicated in both increased and decreased glucose uptake from the intestinal lumen to the enterocytes in both humans and in vitro studies[87]. Beside the glucose uptake, insulin’s action on lipid metabolism and gluconeogenesis seems to be well established in intestine. Insulin modulates lipoprotein metabolism in the intestine and suppresses lipoprotein secretion[86]. Production of apolipoproteinB48-containing chylomicrons by the small intestine increases insulin resistance[86,88]. Like liver, the intestine shows gluconeogenic capacity. Intestinal glucose production is suppressed by insulin and increased in insulinopenic states, such as a 48-h fasting and type 1 diabetes[89,90]. In a postprandial state, intestinal gluconeogenesis (IGN) accounts for about 5%-7% of the total endogenous glucose production[91-93]. A protein-rich diet increases the expression of genes involved in IGN in the intestine and the regulatory enzymes in gluconeogenesis and glutaminase[94]. In intestine, the peptides digested from protein-rich diet act on μ-opioide receptors presenting in the portal vein nerves[94], which signals to the brain and releases of the neuromediator vasoactive intestinal peptide from brain during the postprandial period[95]. This neuromediator activates adenylate cyclase and increases cAMP levels, which induces the expression of IGN-related genes[95]. The IGN-related enzymes are progressively induced during the postprandial period, and the amount of enzyme is maintained during the post-absorptive period[94,96]. In addition, because protein-rich diets provide major IGN substrates, including glutamine and glutamate, these substrates can be utilized by IGN induced during the post-absorptive period[97]. It was recently reported that IGN protects against diabetes and obesity by suppressing hepatic gluconeogenesis and positively regulating glucose homeostasis[91,92]. Gastric bypass surgery also has been reported to increase IGN and suppress hepatic gluconeogenesis in diabetic animal model[93,98].
It has been reported that IGN is induced by the SCFAs and succinate, the gut microbial metabolites derived from carbohydrate fermentation[91,92]. The SCFA propionate, as a gluconeogenic substrate, activated G6Pase activity and increased IGN gene expression via vasoactive intestinal peptide released from brain through a gut-brain neural circuit involving the free fatty acid receptor 3-dependent stimulation [91,95]. Propionate showed the strongest capacity to induce intestinal glucose production among SCFAs[91]. Another SCFA, butyrate increased the levels of ATP, a substrate of adenylate cyclase, which promoted the production of cyclic AMP[91]. Cyclic AMP functions as an intracellular messenger that stimulates the expression of genes involved in gluconeogenesis[99]. Through this mechanism, butyrate promoted gluconeogenesis in enterocytes[91]. Microbial-derived succinate not only showed an anti-obesity effect but also improved glucose tolerance and insulin sensitivity. Succinate functioned as a gluconeogenic substrate such as propionate, and it was shown to promote activation of gluconeogenesis in the intestine of high-fat and high-sucrose diet-fed mice[92]. In addition, succinate-fed wild-type mice showed a decreased capacity for hepatic glucose production, and this suppression of hepatic gluconeogenesis was absent in succinate-fed G6pc intestinal-specific knockout mice[92]. In humans, it has been reported that succinate-producing bacteria, including Bacteroidaceae and Prevotella, were found to be increased in fecal samples of patients with non-alcoholic steatohepatitis[92,100].
CONCLUSION
Since the association between microbiome and metabolic diseases in the last 20 years has been increasingly revealed, microbial metabolites are considered to be the link between microbiome and metabolic diseases. This review summarized the role of microbial metabolites in the major mechanisms representing insulin resistance in each tissue. Through this, in addition to SCFAs, which has been studied a lot in the past, recent studies have found some candidates that protein-derived (e.g., hydrogen sulfide, Indole-3-carboxylic acid, and phenylacetic acid) and lipid-derived microbial metabolites (e.g., KetoA and CLA) can play a role in the pathogenesis of insulin resistance. However, metabolites by gut bacteria are highly diverse depending on intestinal environments (e.g., dietary substrates, host enzyme, acidity, temperature, and antibiotics), yet only limited number of metabolites have been identified and functionally studied in metabolic diseases. Indeed, according to Human Microbiome Database, over 100 thousand of metabolites are exited in our body, but only hundreds are counted as bacterial-specific (https://hmdb.ca/statistics).
What makes this even more challengeable is the complex etiology of insulin resistance. In glucose and lipid metabolism, each organ is highly interrelated. Muscle insulin resistance can divert ingested glucose into the liver, and increase hepatic de novo lipogenesis and gluconeogenesis. Adipose tissue insulin resistance can release lipogenic and gluconeogenic substrates to liver as well intestinal IGN reversely controls the hepatic gluconeogenesis. In order to develop bacterial metabolites as a therapeutic agent for insulin resistance in humans, not only clarifying the exact mechanism of action for which stage of insulin resistance, but also understanding metabolic complexities between multiple organs should be conducted in parallel. Nevertheless, insulin resistance is a common prerequisite for various metabolic diseases, the discovery of metabolites that specifically act on insulin resistance is a strategy to overcome metabolic diseases in terms of more fundamental etiology and early prevention, and more research should be conducted.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 10, 2021
First decision: March 8, 2021
Article in press: May 20, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng JT, Peng XC S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ
Contributor Information
Hye Rim Jang, Laboratory of Mitochondrial and Metabolic Diseases, Department of Health Sciences and Technology, GAIHST, Gachon University, Incheon 21999, South Korea.
Hui-Young Lee, Laboratory of Mitochondrial and Metabolic Diseases, Department of Health Sciences and Technology, GAIHST, Gachon University, Incheon 21999, South Korea; Korea Mouse Metabolic Phenotyping Center, Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon 21999, South Korea; Division of Molecular Medicine, Department of Medicine, Gachon University College of Medicine, Incheon 21936, South Korea. hylee@gachon.ac.kr.
References
- 1.Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erion DM, Park HJ, Lee HY. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016;49:139–148. doi: 10.5483/BMBRep.2016.49.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Lee HY. Revisiting the Bacterial Phylum Composition in Metabolic Diseases Focused on Host Energy Metabolism. Diabetes Metab J. 2020;44:658–667. doi: 10.4093/dmj.2019.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 8.Chen MX, Wang SY, Kuo CH, Tsai IL. Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc. 2019;118 Suppl 1:S10–S22. doi: 10.1016/j.jfma.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, Joannas L, Basavappa MG, Spencer SP, Clark ML, Eisennagel SH, Williams A, Levy M, Manne S, Henrickson SE, Wherry EJ, Thaiss CA, Elinav E, Henao-Mejia J. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aav1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 12.Zorzano A, Muñoz P, Camps M, Mora C, Testar X, Palacín M. Insulin-induced redistribution of GLUT4 glucose carriers in the muscle fiber. In search of GLUT4 trafficking pathways. Diabetes. 1996;45 Suppl 1:S70–S81. doi: 10.2337/diab.45.1.s70. [DOI] [PubMed] [Google Scholar]
- 13.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab. 2014;307:E859–E871. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 17.Kalinovich A, Dehvari N, Åslund A, van Beek S, Halleskog C, Olsen J, Forsberg E, Zacharewicz E, Schaart G, Rinde M, Sandström A, Berlin R, Östenson CG, Hoeks J, Bengtsson T. Treatment with a β-2-adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet-induced obesity. Diabetologia. 2020;63:1603–1615. doi: 10.1007/s00125-020-05171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira R, Souto SB, Sánchez-López E, Machado AL, Severino P, Jose S, Santini A, Fortuna A, García ML, Silva AM, Souto EB. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Review of Classical and New Compounds: Part-I. Pharmaceuticals (Basel) 2019;12 doi: 10.3390/ph12040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houghton MJ, Kerimi A, Mouly V, Tumova S, Williamson G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2019;33:1887–1898. doi: 10.1096/fj.201801209R. [DOI] [PubMed] [Google Scholar]
- 20.Choi Y, Kwon Y, Kim DK, Jeon J, Jang SC, Wang T, Ban M, Kim MH, Jeon SG, Kim MS, Choi CS, Jee YK, Gho YS, Ryu SH, Kim YK. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. 2015;5:15878. doi: 10.1038/srep15878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 22.Takagaki A, Yoshioka Y, Yamashita Y, Nagano T, Ikeda M, Hara-Terawaki A, Seto R, Ashida H. Effects of Microbial Metabolites of (-)-Epigallocatechin Gallate on Glucose Uptake in L6 Skeletal Muscle Cell and Glucose Tolerance in ICR Mice. Biol Pharm Bull. 2019;42:212–221. doi: 10.1248/bpb.b18-00612. [DOI] [PubMed] [Google Scholar]
- 23.Takagaki A, Kato Y, Nanjo F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin. Arch Microbiol. 2014;196:681–695. doi: 10.1007/s00203-014-1006-y. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Gummesson A, Perkins R, Bergström G, Bäckhed F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab 2020; 32: 379-390. :e3. doi: 10.1016/j.cmet.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Thorens B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia. 2015;58:221–232. doi: 10.1007/s00125-014-3451-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J 3rd, Olefsky J, Guarente L, Montminy M. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, Birkenfeld AL, Jornayvaz FR, Jurczak MJ, Kanda S, Popov V, Frederick DW, Zhang D, Guigni B, Bharadwaj KG, Choi CS, Goldberg IJ, Park JH, Petersen KF, Samuel VT, Shulman GI. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54:1650–1660. doi: 10.1002/hep.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida H, Ishii M, Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys. 2019;672:108057. doi: 10.1016/j.abb.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 30.den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, Reijngoud DJ. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305:G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 31.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Yang G, Untereiner A, Ju Y, Wu L, Wang R. Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology. 2013;154:114–126. doi: 10.1210/en.2012-1658. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, Schell M, Sandoval-Espinola WJ, Tao J, Sha B, Graham M, Crooke R, Kleinridders A, Balskus EP, Rey FE, Morris AJ, Biddinger SB. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab 2019; 30: 1141-1151. :e5. doi: 10.1016/j.cmet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Dordević D, Jančíková S, Vítězová M, Kushkevych I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J Adv Res. 2021;27:55–69. doi: 10.1016/j.jare.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K, Sagara M, Aoki C, Tanaka S, Aso Y. Clinical Implication of Plasma Hydrogen Sulfide Levels in Japanese Patients with Type 2 Diabetes. Intern Med. 2017;56:17–21. doi: 10.2169/internalmedicine.56.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rath S, Rud T, Pieper DH, Vital M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front Microbiol. 2019;10:2966. doi: 10.3389/fmicb.2019.02966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svingen GF, Schartum-Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njølstad PR, Seifert R, Strand E, Karlsson T, Nygård O. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin Chem. 2016;62:755–765. doi: 10.1373/clinchem.2015.250761. [DOI] [PubMed] [Google Scholar]
- 41.Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp Clin Endocrinol Diabetes. 2016;124:251–256. doi: 10.1055/s-0035-1569330. [DOI] [PubMed] [Google Scholar]
- 42.Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB Morbid Obesity Study Group. Vicent D, Biddinger SB. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–481. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Brown MS, Goldstein JL. Selective vs total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57:523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- 46.Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, Chilloux J, Myridakis A, Martinez-Gili L, Moreno-Navarrete JM, Benhamed F, Azalbert V, Blasco-Baque V, Puig J, Xifra G, Ricart W, Tomlinson C, Woodbridge M, Cardellini M, Davato F, Cardolini I, Porzio O, Gentileschi P, Lopez F, Foufelle F, Butcher SA, Holmes E, Nicholson JK, Postic C, Burcelin R, Dumas ME. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24:1070–1080. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, Bakker BM. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 48.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mollica MP, Mattace Raso G, Cavaliere G, Trinchese G, De Filippo C, Aceto S, Prisco M, Pirozzi C, Di Guida F, Lama A, Crispino M, Tronino D, Di Vaio P, Berni Canani R, Calignano A, Meli R. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes. 2017;66:1405–1418. doi: 10.2337/db16-0924. [DOI] [PubMed] [Google Scholar]
- 50.Sato S, Jung H, Nakagawa T, Pawlosky R, Takeshima T, Lee WR, Sakiyama H, Laxman S, Wynn RM, Tu BP, MacMillan JB, De Brabander JK, Veech RL, Uyeda K. Metabolite Regulation of Nuclear Localization of Carbohydrate-response Element-binding Protein (ChREBP): ROLE OF AMP AS AN ALLOSTERIC INHIBITOR. J Biol Chem. 2016;291:10515–10527. doi: 10.1074/jbc.M115.708982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, Hiemori M, Tsuji H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007;71:1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- 52.Kondo T, Kishi M, Fushimi T, Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 53.Sahuri-Arisoylu M, Brody LP, Parkinson JR, Parkes H, Navaratnam N, Miller AD, Thomas EL, Frost G, Bell JD. Reprogramming of hepatic fat accumulation and 'browning' of adipose tissue by the short-chain fatty acid acetate. Int J Obes (Lond) 2016;40:955–963. doi: 10.1038/ijo.2016.23. [DOI] [PubMed] [Google Scholar]
- 54.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto T, Kim YI, Furuzono T, Takahashi N, Yamakuni K, Yang HE, Li Y, Ohue R, Nomura W, Sugawara T, Yu R, Kitamura N, Park SB, Kishino S, Ogawa J, Kawada T. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, potently activates PPARγ and stimulates adipogenesis. Biochem Biophys Res Commun. 2015;459:597–603. doi: 10.1016/j.bbrc.2015.02.154. [DOI] [PubMed] [Google Scholar]
- 56.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perry RJ, Camporez JG, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. doi: 10.1016/j.biochi.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- 60.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 61.Jocken JWE, González Hernández MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, Canfora EE. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front Endocrinol (Lausanne) 2017;8:372. doi: 10.3389/fendo.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 63.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, Venema K. Propionic acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest. 2012;42:357–364. doi: 10.1111/j.1365-2362.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 67.Ohira H, Fujioka Y, Katagiri C, Mamoto R, Aoyama-Ishikawa M, Amako K, Izumi Y, Nishiumi S, Yoshida M, Usami M, Ikeda M. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. 2013;20:425–442. doi: 10.5551/jat.15065. [DOI] [PubMed] [Google Scholar]
- 68.Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014;25:168–177. doi: 10.1016/j.tem.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lizcano F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Callaghan A, van Sinderen D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front Microbiol. 2016;7:925. doi: 10.3389/fmicb.2016.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N, Murayama MA, Ueda Y, Wakisaka S, Suzuki T, Suzuki N. Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS One. 2018;13:e0203657. doi: 10.1371/journal.pone.0203657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11:1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanatani S, Motoshima H, Takaki Y, Kawasaki S, Igata M, Matsumura T, Kondo T, Senokuchi T, Ishii N, Kawashima J, Kukidome D, Shimoda S, Nishikawa T, Araki E. Acetate alters expression of genes involved in beige adipogenesis in 3T3-L1 cells and obese KK-Ay mice. J Clin Biochem Nutr. 2016;59:207–214. doi: 10.3164/jcbn.16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, Gao Y, van den Heuvel JK, Meijer OC, Berbée JFP, Heijink M, Giera M, Willems van Dijk K, Groen AK, Rensen PCN, Wang Y. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 75.Yamashita H, Maruta H, Jozuka M, Kimura R, Iwabuchi H, Yamato M, Saito T, Fujisawa K, Takahashi Y, Kimoto M, Hiemori M, Tsuji H. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2009;73:570–576. doi: 10.1271/bbb.80634. [DOI] [PubMed] [Google Scholar]
- 76.Bouter K, Bakker GJ, Levin E, Hartstra AV, Kootte RS, Udayappan SD, Katiraei S, Bahler L, Gilijamse PW, Tremaroli V, Stahlman M, Holleman F, van Riel NAW, Verberne HJ, Romijn JA, Dallinga-Thie GM, Serlie MJ, Ackermans MT, Kemper EM, Willems van Dijk K, Backhed F, Groen AK, Nieuwdorp M. Differential metabolic effects of oral butyrate treatment in lean vs metabolic syndrome subjects. Clin Transl Gastroenterol. 2018;9:155. doi: 10.1038/s41424-018-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, Lenaerts K, Dejong CHC, Blaak EE. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7:2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, Masclee AAM, Dejong CHC, Blaak EE. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) 2016;130:2073–2082. doi: 10.1042/CS20160263. [DOI] [PubMed] [Google Scholar]
- 79.Chambers ES, Byrne CS, Aspey K, Chen Y, Khan S, Morrison DJ, Frost G. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes Obes Metab. 2018;20:1034–1039. doi: 10.1111/dom.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christie S, Wittert GA, Li H, Page AJ. Involvement of TRPV1 Channels in Energy Homeostasis. Front Endocrinol (Lausanne) 2018;9:420. doi: 10.3389/fendo.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim M, Furuzono T, Yamakuni K, Li Y, Kim YI, Takahashi H, Ohue-Kitano R, Jheng HF, Takahashi N, Kano Y, Yu R, Kishino S, Ogawa J, Uchida K, Yamazaki J, Tominaga M, Kawada T, Goto T. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017;31:5036–5048. doi: 10.1096/fj.201700151R. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi Y, Kushiro M, Shinohara K, Ide T. Dietary conjugated linoleic acid reduces body fat mass and affects gene expression of proteins regulating energy metabolism in mice. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:395–404. doi: 10.1016/s1096-4959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 83.Park Y, Park Y. Conjugated fatty acids increase energy expenditure in part by increasing voluntary movement in mice. Food Chem. 2012;133:400–409. doi: 10.1016/j.foodchem.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 84.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, Paek KS, Lee Y. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. 2020;472:1207–1248. doi: 10.1007/s00424-020-02439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes. 2010;59:580–587. doi: 10.2337/db09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ussar S, Haering MF, Fujisaka S, Lutter D, Lee KY, Li N, Gerber GK, Bry L, Kahn CR. Regulation of Glucose Uptake and Enteroendocrine Function by the Intestinal Epithelial Insulin Receptor. Diabetes. 2017;66:886–896. doi: 10.2337/db15-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Federico LM, Naples M, Taylor D, Adeli K. Intestinal insulin resistance and aberrant production of apolipoprotein B48 Lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia: evidence for activation of protein tyrosine phosphatase-1B, extracellular signal-related kinase, and sterol regulatory element-binding protein-1c in the fructose-fed hamster intestine. Diabetes. 2006;55:1316–1326. doi: 10.2337/db04-1084. [DOI] [PubMed] [Google Scholar]
- 89.Croset M, Rajas F, Zitoun C, Hurot JM, Montano S, Mithieux G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes. 2001;50:740–746. doi: 10.2337/diabetes.50.4.740. [DOI] [PubMed] [Google Scholar]
- 90.Rajas F, Croset M, Zitoun C, Montano S, Mithieux G. Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes. 2000;49:1165–1168. doi: 10.2337/diabetes.49.7.1165. [DOI] [PubMed] [Google Scholar]
- 91.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 92.De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 93.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Duraffourd C, De Vadder F, Goncalves D, Delaere F, Penhoat A, Brusset B, Rajas F, Chassard D, Duchampt A, Stefanutti A, Gautier-Stein A, Mithieux G. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell. 2012;150:377–388. doi: 10.1016/j.cell.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 95.De Vadder F, Plessier F, Gautier-Stein A, Mithieux G. Vasoactive intestinal peptide is a local mediator in a gut-brain neural axis activating intestinal gluconeogenesis. Neurogastroenterol Motil. 2015;27:443–448. doi: 10.1111/nmo.12508. [DOI] [PubMed] [Google Scholar]
- 96.Mithieux G, Misery P, Magnan C, Pillot B, Gautier-Stein A, Bernard C, Rajas F, Zitoun C. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–329. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Soty M, Gautier-Stein A, Rajas F, Mithieux G. Gut-Brain Glucose Signaling in Energy Homeostasis. Cell Metab. 2017;25:1231–1242. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 98.Yan Y, Zhou Z, Kong F, Feng S, Li X, Sha Y, Zhang G, Liu H, Zhang H, Wang S, Hu C, Zhang X. Roux-en-Y Gastric Bypass Surgery Suppresses Hepatic Gluconeogenesis and Increases Intestinal Gluconeogenesis in a T2DM Rat Model. Obes Surg. 2016;26:2683–2690. doi: 10.1007/s11695-016-2157-5. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Yang S, Chen J, Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front Endocrinol (Lausanne) 2018;9:802. doi: 10.3389/fendo.2018.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]