Abstract
In the early December 2019, a novel coronavirus named severe acute respiratory syndrome coronavirus 2 was first reported in Wuhan, China, followed by an outbreak that spread around the world. Numerous studies have shown that liver injury is common in patients with coronavirus disease 2019 (COVID-19), and may aggravate the severity of the disease. However, the exact cause and specific mechanism of COVID-associated liver injury needs to be elucidated further. In this review, we present an analysis of the clinical features, potential mechanisms, and treatment strategies for liver injury associated with COVID-19. We hope that this review would benefit clinicians in devising better strategies for management of such patients.
Keywords: COVID-19, Liver injury, Clinical features, Potential mechanism, Treatment
Core Tip: Coronavirus disease 2019 (COVID-19) has assumed pandemic proportions, and has resulted in several hundred thousand deaths globally. Although the lung is the main organ that is damaged in COVID-19, approximately 60% of the patients have been reported to develop various degrees of liver injury in several studies. Accumulating clinical data show that liver damage is related to the severity of COVID-19 and is a major cause of death from COVID-19, especially in the presence of hepatic failure. The exact cause of liver injury in patients with COVID-19 remains unclear and the specific underlying mechanism(s) need to be elucidated.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first broke out in Wuhan, China in December 2019, has become a great threat to public health worldwide. As of August 4, 2020, more than 1000000 deaths from COVID-19 have been confirmed[1]. Although the lung is the main organ that is damaged in COVID-19, approximately 60% of the patients were reported to develop various degrees of liver injury in previous studies[2-5]. Accumulating clinical data show that liver damage is related to the severity of COVID-19 and is also a major cause of death from COVID-19, especially in the presence of hepatic failure[6,7]. Thus, early detection, effective treatment, and elucidation of the mechanisms underlying the pathogenesis of liver damage are urgently needed for COVID-19 patients. In this review, we summarize the characteristics of COVID-19-associated liver injury from multiple perspectives, including clinical features (manifestation, laboratory examinations, liver biopsy, etc.), underlying pathogenesis (direct viral cytotoxicity, uncontrolled cytokine storm, drug-induced toxicity, etc.), special population of patients (those with cirrhosis, hepatitis B, liver transplantation, etc.), and clinical management (drugs, oxygen therapy, artificial liver blood purification, etc.). Based on the latest data, we hope to provide a feasible reference for follow-up clinical management of COVID-19.
CLINICAL FEATURES OF HEPATIC INJURY IN COVID-19
In patients with COVID-19, the most commonly used indicators of liver function impairment are liver transaminase, bilirubin, and albumin levels. Abnormal levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin were reported in 11%-56.3%, 15.0%-86.8%, and 2.7%-30.6% of patients with COVID-19, respectively, whereas 2%-11% of such patients had pre-existing liver disease[3-8]. In a recent study involving 228 patients with COVID-19, who did not have chronic liver disease (CLD), abnormal liver function was observed in 129 (56.3%) patients, which included elevations in the levels of ALT [84 (36.8%)], AST [58 (25.4%)], total bilirubin [59 (25.9%)], and gamma-glutamyl transferase [67 (29.5%)][9]. In a study on 99 patients, Chen et al[10] reported elevated levels of ALT and AST in 28 (28%) and 35 (35%) patients, respectively, and hypoalbuminemia and hyperbilirubinemia in 97 (98%) and 18 (18%) patients, respectively. In addition, several studies revealed that liver damage is more prevalent in severe cases of COVID-19 than in mild cases. In a large sample multicenter study[11], abnormally elevated levels of ALT were observed in 28.1% of critically ill patients and in 19.8% of non-critically ill patients, and of AST in 18.2% of non-critically ill patients and 39.4% of critically ill patients. Huang et al[4] also indicated that patients admitted to the intensive care unit (ICU) not only had higher plasma levels of the inflammatory indices [interleukin (IL)-2, IL-7, IL-10, tumor necrosis factor (TNF)-α, etc.], but also had abnormally high levels of AST [8 (62%) of 13 patients] compared with non-ICU patients [7 (25%) of 28 patients]. A recent descriptive study confirmed that the levels of ALT (35 vs 23, normal range 9-50 U/L, P = 0.007) and AST (52 vs 29, normal range 5-21 U/L, P < 0.001) were significantly higher in ICU patients[12]. In a Japanese cohort study concerning COVID-19, patients were classified into mild, moderate, and severe groups, respectively, based on gastrointestinal symptoms and severity of pneumonia; and the peak levels of AST (28 vs 48 vs 109, P < 0.001) and ALT (33 vs 47.5 vs 106, P = 0.0114) were significantly stratified according to these criteria[13].
Apart from the liver enzyme tests mentioned above, there are characteristic clinical manifestations of liver damage. In China, it has been reported that some patients recovering from severe COVID-19 exhibited darkening and pigmentation during the recovery process. Multiple organ damage, especially liver damage, is the main cause of darkening and hyperpigmentation[14]. Abnormal liver function may lead to pigmentation through the following three pathways: (1) Impaired liver function leads to hypofunction of the adrenal cortex. When the liver is unable to metabolize the melanin-stimulating hormone secreted by the anterior pituitary gland, the secretion of melanin increases[15]; (2) abnormal liver function hinders the inactivation of estrogen, leading to an increase in its level. The increase in estrogen levels in the body reduces the inhibition of tyrosinase by thiamine, thereby increasing the conversion of tyrosine to melanin[16]; and (3) liver damage increases the iron content in the blood. Iron delivered to the facial skin causes the darkening of the face.
Liver biopsy is also important in the etiological diagnosis of hepatic injury in COVID-19, particularly in cases where liver damage dominates the clinical manifestation, or where other alternative causes of damage need to be ruled out. Currently, most of the information on histological changes in the liver of patients with COVID-19 comes from autopsies. A case series by Bradley et al[17], based on ultrastructural findings and histopathology of samples from 14 fatal COVID-19 infections in the Washington State, showed centrilobular necrosis, consistent with hypoperfusion injury, in four patients; viral RNA was detected in the liver of these patients, as well. However, autopsies have several limitations. Death may occur long after the acute liver injury is noted; subsequently, histological changes may have been eliminated or obscured, and viral load would have diminished over time. Fiel et al[18] presented their findings from two liver biopsies performed on patients infected with SARS-CoV-2. Both patients had severe hepatic failure in the absence of obvious involvement of other organs. Detailed histological analysis, in situ hybridization, and electron microscopy revealed that apoptosis, abundant mitosis, mixed inflammatory infiltration in the portal area, severe bile duct injury, apparent viral particles, and viral RNA within hepatocytes are typical. These findings suggested hepatic involvement in infections with SARS-CoV-2. Another case report by Melquist et al[19] showed similar findings in a patient infected with SARS-CoV-2, manifesting as acute hepatitis without any respiratory symptoms, rapidly progressing to fulminant liver failure. Acute hepatitis (panacinar hepatitis, zone 3 necrosis, and focal hemophagocytosis) with viral-like changes was identified at the time of liver biopsy.
POTENTIAL MECHANISMS OF LIVER INJURY IN PATIENTS WITH COVID-19
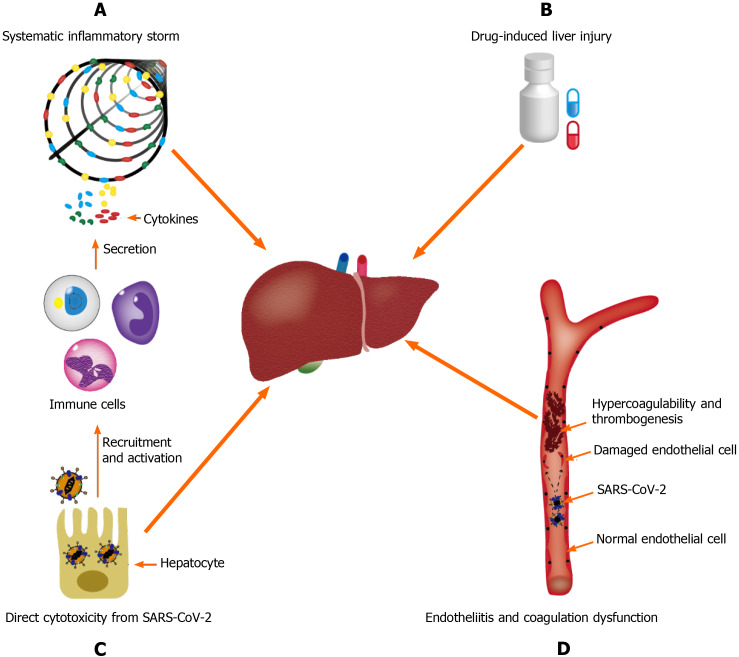
The available evidence supports that hepatic injury in SARS-CoV-2 infection is a consequence of a multifactorial attack. The potential mechanisms of pathogenesis may be broad spectrum, ranging from direct cytotoxicity from viral infection to indirect involvement of the inflammatory cytokine storm, hypoxic changes caused by respiratory failure, endotheliitis, and drug-induced liver injury (DILI)[20] (Figure 1).
Figure 1.
Underlying mechanisms of coronavirus disease-19-assocaited liver injury. A: Systematic inflammatory storm, which is generated by abnormal activation of the immune system; B: Drug-induced liver injury; C: Endotheliitis and coagulation dysfunction; D: Direct cytotoxicity from severe acute respiratory syndrome coronavirus 2. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Direct effect of viral infection on the liver
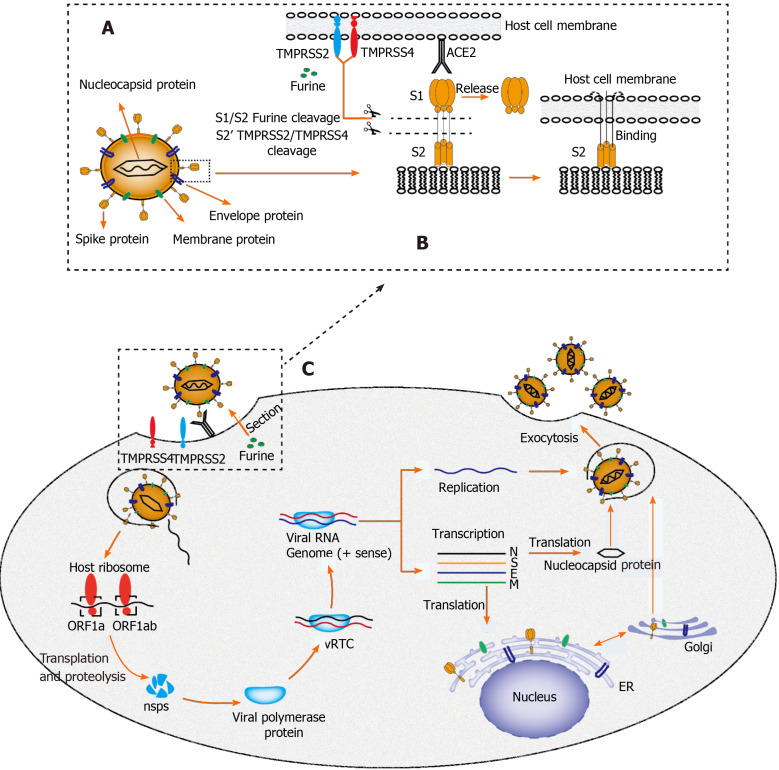
Recently, it was determined by quantitative reverse transcription-polymerase chain reaction that SARS-CoV-2 RNA is widely present in other organs outside the respiratory tract, such as the liver[21], although the exact cell location of replication has not been determined because of the isolation of nucleic acids by whole tissue homogenization. Until recently, a typical hepatitis picture is yet to be observed, and hepatic tropism and direct cytopathic effects of SARS-CoV-2 should be considered as the underlying mechanism of COVID-19 associated liver injury[22]. A major determinant of viral tropism is the availability of viral receptors on the surface of host cells in specific tissues. Cellular entry of SARS-CoV-2 is mediated by the spike (S) protein of the virus, which is cleaved by transmembrane serine protease 2/transmembrane serine protease 4 and specifically interacts with angiotensin converting enzyme 2 (ACE2) in the host[23] (Figure 2). According to Human Protein Atlas, ACE2 is highly expressed in the lung (type II alveolar cells), intestine, and gall bladder, but it seems to be almost absent in the liver. After in-depth research on ACE2 expression patterns, sinusoidal endothelial cells appear to be negative for ACE2, but this protein is expressed in the central hepatic vein and portal vein endothelial cells[24]. The expression level of ACE2 in the bile duct epithelium is comparable to that in alveolar epithelial cells, being almost 20-times higher than that in hepatocytes. Of note, Letko et al[25] revealed that compensatory differentiation and proliferation of liver parenchymal cells derived from bile duct cells leads to the upregulation of ACE2 expression in liver tissues, which might be the underlying mechanisms in COVID-19-associated liver injury.
Figure 2.
Proposed structure diagram of severe acute respiratory syndrome coronavirus 2 and its life cycle in host cells. A: Structural sketch of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); B: Recognition and entry of SARS-CoV-2 into host cell. Transmembrane spike (S) glycoprotein on the surface of SARS-CoV-2 forms a homotrimer to recognize the human host angiotensin converting enzyme 2 (ACE2) receptor. The S protein is specifically cleaved by two mucose-specific serine proteases [recombinant transmembrane protease serine 2 (TMPRSS2) and TMPRSS4] and furine. The subunit of S protein (S1) is released, and another subunit (S2) is exposed and mediates the viral entry into host cells; C: Life cycle of SARS-CoV-2 in host cells. First, the S protein of SARS-CoV-2 binds to ACE2 to form an S protein-ACE2 complex, which directly mediates the cellular entry of virus and the process is facilitated by TMPRSS2, TMPRSS4, and furine. Second, viral RNA is released into host cytoplasm. Open reading frame (ORF) 1a and ORF1ab are translated into large polyproteins by host ribosome, which are further proteolytically cleaved into 16 non-structural proteins (nsps). Viral polymerase protein is assembled by nsps and viral replication/transcription complex (vRTC) is subsequnently formed by polymerase protein and genomic RNA. Third, a negative sense viral RNA is synthesized and used as a template to replicate progeny (+) sense viral genome and transcribes to form various mRNAs. The nucleocapsid protein is translated in the cytoplasm, whereas the S protein, membrane (M) protein, and envelope (E) protein are translated in the endoplasmic reticulum and transported to the Golgi apparatus for further packaging. Finally, a completely new viral particle is assembled by viral RNA-nucleocapsid complex and S, M, and E proteins in endoplasmic reticulum–Golgi intermediate compartment and is released from host cell via exocytosis. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; M: Membrane; E: Envelope; S: Spike; ER: Endoplasmic reticulum; vRTC: Viral replication/transcription complex; ORF: Open reading frame; TMPRSS: Transmembrane protease serine; ACE2: Angiotensin converting enzyme 2.
Notably, typical coronavirus particles (characterized by S structures) were identified in the cytoplasm of hepatocytes in an ultrastructural examination by Wang et al[26]. In this study, typical lesions of viral infection, including conspicuous mitochondrial swelling, decreased glycogen granules, and endoplasmic reticulum dilatation, were also observed in the SARS-CoV-2-infected hepatocytes, which indicated that hepatic impairment might be directly caused by SARS-CoV-2. Interestingly, massive hepatic apoptosis and some binuclear hepatocytes were also identified in this study. In addition, autopsy results in patients with SARS, in the study by Guo et al[27], showed a large number of hepatocyte balloons, central lobular necrosis, and obvious apoptosis. Similar histological findings were also observed in a study of liver biopsy from patients with SARS by Chau et al[28], which suggested that SARS-CoV may induce apoptosis of hepatocytes, thereby leading to liver damage. Furthermore, Tan et al[29] demonstrated that overexpression of p7a, a protein specifically expressed in SARS-CoV-infected cells, could induce apoptosis in cell lines derived from different organs (including lung, kidney, and liver) via a caspase-dependent pathway. This further confirmed the possibility that SARS-CoV can directly attack liver tissues and cause liver damage.
It is noteworthy that the expression level of ACE2 on hepatocytes is regulated by many factors. Several experimental studies, in both mice and humans, have confirmed increased expression of hepatic ACE2 under conditions of liver fibrosis/cirrhosis[30]. This may partially explain why pre-existing CLD increases the probability of liver damage in patients with COVID-19. Hypoxia, a typical feature in severe COVID-19 cases, has been proven to be a main regulator of ACE2 expression in hepatic cells[31]. This may explain why the dissemination of SARS-CoV-2 outside the lungs is mainly observed in patients with acute respiratory distress syndrome and other hypoxic conditions. Notably, the affinity of S protein in SARS-CoV-2 for its receptor can be increased when it is proteolytically activated by trypsin, a protein commonly expressed in liver epithelial cells[32]. A clinical drug trial by Fantini et al[33] indicated that ganglioside (GM1) might be another target that influences the S protein–ACE2 interaction, using a combination of structural and molecular modeling approaches. In the near future, new molecular and therapeutic insights concerning the S protein–ACE2 interactor are expected to be uncovered with the advancement of research.
Inflammatory storm in COVID-19-associated hepatic injury
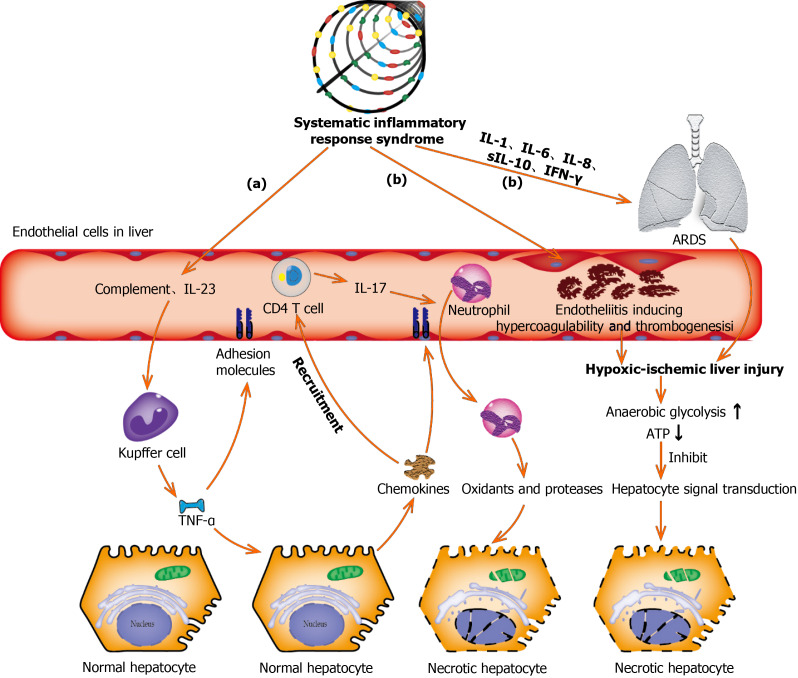
Inflammatory cytokine storm generated by the excessive immune response induced by coronavirus infection might also be one of the key factors in hepatic injury[34,35]. Higher plasma levels of inflammatory cytokines (IL-2, IL-7, IL-10, GSCF, IP10, MCP1, MIP1A, and TNF-α) and lower lymphocyte counts (both helper T cells and suppressor T cells) were commonly observed in patients with COVID-19, especially in the critically ill ones[4,36]. Qin et al[37] showed that COVID-19 is a systemic inflammatory viral response, first during the viral infection period and subsequently during the inflammatory period. This may explain why the conditions of illness in some patients with COVID-19 are not serious in the early stage, but if they do not receive timely medical treatment, the disease deteriorates rapidly in a short time and enters a state of multiple organ failure[38]. A cohort study of 192 patients revealed that an increase in IL-6 and IL-10 and a decrease in CD4+ T cells were independent risk factors related to severe liver damage[39]. In another study recently published in World J Gastroenterol[40], lymphopenia and C-reactive protein levels were found to be independently associated with hepatic injury (Figure 3).
Figure 3.
Underlying molecular mechanisms of coronavirus disease-19-associated liver injury caused by systematic inflammatory response syndrome and hypoxic ischemia. (a): Complement and interleukin-23 are released into the blood during the systemic inflammation, which subsequently activate Kupffer cells and induce their production of tumor necrosis factor α (TNF-α). As a pro-inflammatory cytokine, TNF-α aggravates the inflammation responses by up-regulating the expression of endothelial cell adhesion molecules and inducing hepatocytes to secrete chemokines. Under the induction of chemokines, CD4 T cells and neutrophils are rapidly recruited to the liver, in which CD4 T cells assist mucosal molecules to promote neutrophils into the liver parenchyma. Finally, neutrophils directly damage liver cells by releasing oxidants and proteases, leading to necrotic cell death; (b): Acute respiratory distress syndrome and endotheliitis are the two main causes leading to hypoxic-ischemic liver injury in the period of systematic inflammatory response syndrome. Increased anaerobic glycolysis leads to a decrease in ATP production, which ultimately leads to the death of hepatocytes by inhibiting hepatocyte signal transduction. ARDS: Acute respiratory distress syndrome; TNF-α: Tumor necrosis factor α; IL: Interleukin; IFN-γ: Interferon-γ.
Burra[14] confirmed that the incidence of liver damage in patients with COVID-19 having elevated ferritin levels was significantly higher (52.3% vs 20.0%) than that of patients with normal ferritin levels. This suggests that ferritin could be employed as an easy-to-use tool to ascertain liver injury. A possible reason for this is that ferritin, acting as an inflammatory cytokine like IL-6, participates in acute liver damage[41]. Inflammasome activation and apoptosis/pyrolysis in SARS-CoV-2 induced inflammatory cells may cause multiorgan dysfunction[42]. Interestingly, pathological changes, such as spleen atrophy and lymph node necrosis, were observed in severe cases of SARS infection, which indicated the presence of immune-mediated injury[43].
Endotheliitis in COVID-19-associated hepatic injury
COVID-19 is considered to be a thrombo-inflammatory disease that affects the lungs and, beyond that, endothelium, which is one of the largest organs in the human body. A variety of viruses, such as the HIV, dengue fever virus, and Ebola virus, have been previously reported to affect the coagulation system[44]. SARS-CoV-2 enters the endothelial cells by endocytosis via binding to the ACE2 receptor as well[45]. A recent study from Switzerland[42] showed the presence of viral inclusion structures within endothelial cells and diffuse endothelial inflammation. The vascular endothelium is indispensable in regulating the vascular tone and in maintaining vascular homeostasis, and intact endothelial cells provide potent anti-coagulant properties[46]. When the vascular endothelium is destroyed, either directly by viral infection or through immune-mediated inflammation, vasoconstriction and procoagulant behavior can occur rapidly. Spiezia et al[47] found that plasma levels of fibrinogen and D-dimer in severe cases of COVID-19 were significantly higher than those in healthy controls. In this study, markedly hypercoagulable thromboelastometry profiles, as reflected by shorter clot formation time and higher maximum clot firmness, were also observed in patients with COVID-19. In a recent study, multiple areas of microthrombi were revealed in a patient with COVID-19 by contrast-enhanced ultrasound of the lung[48], which confirmed the involvement of microvessels during the disease process. In addition, the frequency of acute pulmonary embolus in patients with COVID-19 was 30%[49] higher than that usually occurring in critically ill (1.3%)[50] or emergency (3% to 10%)[51] patients without COVID-19 (Figure 3).
SARS-CoV-2 infection causes inflammation of the vascular endothelium, which in turn leads to vascular dysfunction, especially in capillaries. Subsequently, microvascular dysfunction leads to a hypercoagulable state, tissue edema, and organ ischemia[44,52]. Liver ischemia reperfusion injury, a pathophysiological process commonly occurring after rapid recovery of blood circulation, may be the underlying mechanism of COVID-19-associated hepatic injury. Liver ischemia-reperfusion can activate neutrophils, Kupffer cells, and platelets, inducing a series of destructive cellular reactions, such as reactive oxygen species and calcium overload, which ultimately lead to an inflammatory response and cell damage. It has also been reported that hepatic sinusoidal endothelial cell damage causes microcirculation disorders and further aggravates liver ischemia and hypoxia. Wang et al[9] observed different degrees of hypoxemia by blood gas analysis in more than 40% of patients with COVID-19. Patients receiving oxygen therapy have a faster recovery of liver function, and the average length of hospital stay is considerably shortened. In addition, lymphatic vessels are also reported to be involved in the pathological process of acute liver injury, prevent the occurrence of acute liver damage, and delay the progression of COVID-19[53]. Lymphatic vessels participate in the clearance of virus through absorption and transportation of inflammatory exudates, inflammatory cytokines, dead cell debris, and immune cells[54].
Drug-induced hepatic injury during treatment
DILI is defined as liver damage caused by the drug and/or its metabolites, or by hypersensitivity or reduced tolerance of the drug owing to special physique during the use of drugs[55]. Based on the available data, a variety of drugs widely used to treat COVID-19, such as antibiotics (macrolides and quinolones), antiviral drugs (ribavirin and lopinavir/ritonavir), and non-steroidal anti-inflammatory drugs, have been reported to cause liver damage[56]. A meta-analysis by Kulkarni et al[57] showed a pooled incidence of DILI of 25.4% in patients with confirmed SARS-CoV-2 infection. Interestingly, Fan et al[58] found that the proportion of patients with abnormal liver function who received lopinavir/ritonavir after admission (57.8%) was significantly higher than that of patients with normal liver function (31.3%). Moreover, the average hospital stay of patients with abnormal liver function was significantly longer than that of patients with normal liver function (15.09 ± 4.79 d vs 12.76 ± 4.14 d, P = 0.021). Previous studies have indicated that patients with severe COVID-19 disease are prone to hepatic injury[4,5]. The reason for this might be that severe and critically ill patients require long-course and/or more metered administration of antiviral drugs, antibiotics, or other potentially hepatotoxic drugs during hospitalization. Similarly, Qin et al[37] reported that up to 70% of critically ill patients received systemic corticosteroid therapy. Furthermore, a case report from Italy suggested that tocilizumab, a drug used to reduce inflammation by blocking the IL-6 signal transduction pathway, played a beneficial role in the management of severe COVID-19 disease[59]. In the case of poor efficacy of azithromycin, hydroxychloroquine, and lopinavir, the administration of tocilizumab rapidly improved the clinical condition. However, mild to moderate elevations in transaminases have been observed in patients with COVID-19 treated with tocilizumab. A possible reason is that the use of immunosuppressive drugs, such as tocilizumab, tofacitinib, and dexamethasone, can reactivate hepatitis B virus (HBV) in patients with occult infection and induce liver damage[57]. However, randomized controlled clinical trials evaluating the safety of remdesvir and tocilizumab in the treatment of COVID-19 have not yet revealed any significant difference in the incidence of liver injury between the treatment and placebo groups[60,61].
The Novel Coronavirus Pneumonia Diagnosis and Treatment Scheme (Trial Version 8) issued by the National Health Commission of the People's Republic of China on August 19, 2020[62], provides a brief summary of the antiviral drugs under trial. Some drugs have been shown to have certain therapeutic effects in clinical observation studies, but no antiviral drugs have been determined to be effective in strict “randomized, double-blind, placebo-controlled studies.” It is recommended that drugs with potential antiviral effects should be used early in the course of the disease and should be applied to patients with high-risk factors for severe illness and severe illness tendencies. It is not recommended to use lopinavir/ritonavir and ribavirin alone, as well as hydroxychloroquine, in combination with azithromycin. The trial of alpha-interferon, ribavirin (recommended to be used in combination with interferon or lopinavir/ritonavir), chloroquine phosphate, and arbidol can be continued, and their efficacy, adverse reactions, contraindications, and interactions with other drugs should be evaluated in further clinical applications. It is not recommended to use more than three antiviral drugs at the same time.
COVID-19-ASSOCIATED LIVER INJURY IN SPECIAL POPULATIONS
As of date, there are approximately 400 million patients with CLD in China, including those with chronic viral hepatitis, fatty liver, alcoholic liver disease, cirrhosis, or other liver diseases. Therefore, in patients with SARS-CoV-2 infection, the effect of pre-existing liver disease on the liver injury status cannot be ignored.
Previous studies have shown that patients having cirrhosis with relatively lower immunity are vulnerable to liver decompensation or acute chronic liver failure after influenza virus infection[63]. Notably, similar results were also reported in a large, multicenter, international cohort study; cirrhosis in patients with COVID-19 was closely associated with a poor model for end-stage liver disease score and decompensated events[64]. The authors indicated that mortality was strongly correlated with hepatic decompensation following SARS-CoV-2 infection; 63.2% of patients with new decompensated events died, whereas the proportion of death in those without new decompensation events was 26.2%. According to a study by Sarin et al[65], decompensating one-fifth of cirrhosis was observed, in which 57% of patients had progression of liver damage and the mortality rate was 43%. Notably, among patients with liver cirrhosis, the mortality rate of COVID-19 was significantly higher than that in patients hospitalized for bacterial infection[66]. However, in a contemporaneously enrolled study conducted in the United States[67], Bajaj et al[67] determined that the mortality rate in patients with cirrhosis + COVID-19 was similar to that in patients with cirrhosis alone (30% vs 20%, P = 0.16), but was higher than that in patients with COVID-19 alone (30% vs 13%, P = 0.03) after matching for age/sex. In this study, the Charlson Comorbidity Index, a prognostic comorbidity score, was identified as the only independent variable predictive of mortality in the entire matched cohort [odds ratio 1.23, 95% confidence interval (CI): 1.11-1.37; P < 0.001]. Thus, whether the mortality rate in patients with cirrhosis infected with SARS-CoV-2 is higher than in those infected with other viruses or bacteria is yet to be determined.
Based on previous studies[68], patients with chronic hepatitis B co-infected with severe acute respiratory syndrome (SARS) virus are more likely to develop severe hepatitis. A possible reason is that the SARS virus triggers HBV reactivation and massive replication, and chronic hepatitis B patients co-infected with SARS virus may require a longer time to fully clear the SARS virus from their bodies. Very recently, an observation from China[69] revealed that two patients with HBV infection had a slower clearance of SARS-CoV-2 (mean difference 10.6 d; 95%CI: 6.2-15.1 d). The mechanism may involve the dysfunction of T cells in HBV-infected patients, which causes the body's immune response to other viruses to weaken, but whether there is an exact connection between the two remains to be elucidated. However, Chen et al[70] reviewed the clinical characteristics of patients with SARS-CoV-2/HBV coinfection and found no significant difference in the hepatic function index between 20 patients with HBV infection (6.1%) and 306 patients without HBV infection (93.9%). Moreover, there is no evidence that SARS-CoV-2/HBV coinfection would reduce the discharge rate and lengthen the hospital stay. A similar phenomenon was observed in another study[71] showing that patients with COVID-19 co-infected with hepatitis B were not significantly associated with worse outcomes compared to those without hepatitis B.
Patients with non-alcoholic fatty liver disease (NAFLD) were previously reported to be more prone to liver damage when infected with SARS-CoV-2; many of these were cases of mild to moderate liver damage, and severe disease was rare[72,73]. Ji et al[73] showed that patients with pre-existing NAFLD infected with SARS-CoV-2 had a higher possibility of abnormal hepatic function during hospitalization, higher risk of disease progression, and longer duration of virus shedding compared to those without NAFLD. In this study, the pattern of liver injury was mainly hepatocellular rather than cholestatic, which was contrary to the existing finding that SARS-CoV-2 has a high affinity for the ACE2 receptor highly expressed in biliary tract cells. Metabolic diseases, such as obesity, hypertension, diabetes, and cardiovascular disease, are common in patients with NAFLD. Studies have shown that coexisting metabolic risk factors in patients with NAFLD are independent risk factors for severe COVID-19 disease, and the risk of severity increases with the number of metabolic risk factors present[74]. To further verify whether NAFLD itself affects liver function in patients with COVID-19, Hashemi et al[75] adjusted for potential confounding factors (age, sex, hypertension, diabetes, obesity, hyperlipidemia, heart diseases, and pulmonary disorders), and determined that NAFLD was still independently associated with ICU admission (49.3% vs 35.0%, P = 0.028) and mechanical ventilation (47.8% vs 30.3%, P = 0.0055), but was not associated with mortality. Patients with NAFLD who were infected with SARS-CoV-2 had a higher prevalence of elevated transaminases on admission. Coincidently, a very recent study[76] revealed a similar result that NAFLD is an independent predictor of liver injury in COVID-19, but not a predictor of death and disease severity (presentation or progression). However, the debate concerning whether NAFLD increases the risk of death in patients with COVID-19 continues.
Liver transplant recipients are also a special population affected by the global spread of COVID-19. Long-term immunosuppressive treatment may increase the risk of contracting respiratory viruses, especially in patients with preoperative organ decompensation and chronic disease[77]. A prospective cohort study of 111 cases[75] showed that liver transplant patients had an increased risk of contracting SARS-CoV-2 owing to chronic immunosuppression, but the mortality rate was lower than that in the matched general population. Mycophenolate, a baseline immunosuppressive drug, was identified as an independent predictor of severe COVID-19, but no such deterioration was observed with calcineurin inhibitors or everolimus. In a series of cases in Brazil[78], a negative effect of COVID-19 on liver transplantation was reported, especially in elderly patients with comorbidities. One case was a 69-year-old patient with severe cardiovascular disease who showed a rapid deterioration after being diagnosed with COVID-19, and another case was a patient with NAFLD complicated with kidney failure, who eventually died of a secondary bacterial infection. However, different opinions have been forwarded in other studies. A large international observational study conducted by Webb et al[79] indicated that liver transplantation did not significantly increase the proportion of ICU admission and the risk of death. Similarly, D'Antiga et al[80] also showed that patients with COVID-19 receiving liver transplantation were not at an increased risk of severe pulmonary disease, despite their immunosuppressed status. Moreover, three COVID-19-related deaths observed at an Italian transplant center were of patients undergoing long-term treatment with a minimal immunosuppressive regimen, rather than of fully immunosuppressed patients who recently received transplants[81].
TREATMENT STRATEGIES FOR LIVER INJURY IN PATIENTS WITH COVID-19
Although liver damage is a common complication of COVID-19, most cases of COVID-19 show mildly abnormal liver function, which is usually temporary and can return to normal without any special treatment[7]. According to the Chinese Pharmaceutical Association, the “Four-Anti and Two-Balance” strategy is recommended, which includes antivirus, anti-shock, anti-hyoxemia, anti-secondary infection therapy, and maintenance of water, electrolyte, acid–base, and microecological balance. Patients with COVID-19 exhibiting obvious liver damage could be treated with hepatoprotective, anti-jaundice, or anti-inflammatory drugs, such as polyene phosphatidylcholine, glycyrrhizic acid, ursodeoxycholic acid, and adenosylmethionine. For liver injury in critically ill patients infected with SARS-CoV-2, one or two kinds of drugs can be chosen to avoid drug abuse and aggravation of liver burden, and reduce drug interactions. A recent study by Hoever et al[82] revealed that glycyrrhizic acid derivatives, which are preferred anti-hepatitis drugs, may also have antiviral activity against SARS-CoV-2. Glycyrrhizic acid has a strong affinity for liver steroid metabolism enzymes, and hinders the inactivation of cortisol and aldosterone; it also shows obvious corticosteroid-like effects, such as anti-inflammatory, anti-allergic, and protective film structures, without obvious cortical hormone-like side effects.
Hu et al[34] retrospectively analyzed the clinical characteristics, susceptible population, and treatment strategies for patients with new coronavirus infection and showed that the mainstay to manage COVID-19-associated liver injury was to suppress inflammatory response, correct hypoxemia, and provide symptomatic support. Conservative oxygen therapy is preferred, and ventilator-associated pneumonia should be strictly supervised in patients receiving mechanical ventilation[83]. Xu et al[83] showed that an artificial liver blood purification system could improve the treatment effect in critically ill patients by rapidly removing inflammatory mediators, blocking cytokine storms, and favoring the water–electrolyte balance. Similar findings were reported by Liu et al[84] who showed a significant declining trend in the levels of cytokines and inflammatory factors (IL-6 and C-reactive protein) in patients with COVID-19 after a course of artificial liver blood purification. For patients with COVID-19 who are suspected of having liver damage caused by drugs, consideration should be given to stop or reduce the drug dose. Prevention is better than management. Monitoring the liver function and avoiding liver damage play key roles in the treatment of COVID-19-associated liver injury.
CONCLUSION
In this review, we summarize the latest advances in research on the clinical features, potential mechanisms, exacerbation of underlying hepatic dysfunction in patients with CLD, and treatment strategies for patients with COVID-19 as of January 2021. Patients with COVID-19 showing liver injury may experience darkening of the skin and hyperpigmentation. To ascertain the presence of liver injury, liver enzymes are the most commonly used markers. Liver biopsy is strongly recommended for those with unexplained acute liver failure. Apparent viral particles have been observed in hepatocytes. However, to date, studies involving liver biopsy in COVID-19 are still limited to case reports. The potential mechanisms of COVID-19-associated liver injury may include the direct effects of viral infection, inflammatory storm, hypoxemia, endotheliitis, and drugs. The S protein-ACE2 interactor may be the main tunnel for the entry of virus, the activity of which can be regulated by multiple factors, such as hypoxia, fibrosis/cirrhosis, and GM1. Among patients with CLD, NAFLD was indicated as an independent factor associated with ICU admission and mechanical ventilation after adjusting for comorbidities, such as hypertension, diabetes, and obesity. Patients with cirrhosis, co-infected with SRAS-CoV-2, experience high mortality, whereas whether hepatitis B and liver transplantation increase the severity of COVID-19 disease remains an open question. The “Four-Anti and Two-Balance” strategy, in which it is necessary to avoid drug abuse that aggravates liver burden and prevention is preferred to management, is recommended to manage COVID-19-associated liver injury.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest related to this manuscript.
Manuscript source: Invited manuscript
Peer-review started: January 24, 2021
First decision: March 7, 2021
Article in press: April 21, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Campani C, Casanova Rituerto D, Elshaarawy O, Skrypnyk I S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Yue Cai, Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China; Department of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China.
Li-Ping Ye, Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China; Department of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China; School of Medicine, Zhejiang University, Hangzhou 310000, Zhejiang Province, China.
Ya-Qi Song, School of Medicine, Zhejiang University, Hangzhou 310000, Zhejiang Province, China.
Xin-Li Mao, Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China; Department of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China.
Li Wang, College of Basic Medicine, Inner Mongolia Medical University, Hohhot 010000, Inner Mongolia Autonomous Region, China.
Yan-Zhi Jiang, Department of Gastroenterology and Hepatology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200000, China.
Wei-Tao Que, Department of Surgery, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200000, China.
Shao-Wei Li, Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China; Department of Gastroenterology, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Linhai 317000, Zhejiang Province, China. li_shaowei81@hotmail.com.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic, 2020. [cited 10 January 2021]. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_1 .
- 2.Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: Etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286–2293. doi: 10.3748/wjg.v26.i19.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Zhu L, Xue L, Liu L, Yan X, Huang S, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Shao H, Chen K, Zhao XA, Sang D, Zhao H, Guan X, Chen X, Chen Y, Liu J, Huang R, Zhu C, Wu C. Risk factors of liver injury in patients with coronavirus disease 2019 in Jiangsu, China: A retrospective, multi-center study. J Med Virol. 2020 doi: 10.1002/jmv.26663. [DOI] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko S, Kurosaki M, Nagata K, Taki R, Ueda K, Hanada S, Takayama K, Suzaki S, Harada N, Sugiyama T, Nagasawa M, Izumi N. Liver injury with COVID-19 based on gastrointestinal symptoms and pneumonia severity. PLoS One. 2020;15:e0241663. doi: 10.1371/journal.pone.0241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burra P. Liver abnormalities and endocrine diseases. Best Pract Res Clin Gastroenterol. 2013;27:553–563. doi: 10.1016/j.bpg.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jee SH, Lee SY, Chiu HC, Chang CC, Chen TJ. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun. 1994;199:1407–1412. doi: 10.1006/bbrc.1994.1387. [DOI] [PubMed] [Google Scholar]
- 17.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763–770. doi: 10.1016/j.jcmgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore) 2020;99:e22818. doi: 10.1097/MD.0000000000022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol. 2020;26:2323–2332. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan YJ, Fielding BC, Goh PY, Shen S, Tan TH, Lim SG, Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78:14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Q, Xie Q, Shi CC, Xiang XG, Lin LY, Gong BD, Zhao GD, Wang H, Jia NN. Expression of angiotensin-converting enzyme 2 in CCL4-induced rat liver fibrosis. Int J Mol Med. 2009;23:717–723. doi: 10.3892/ijmm_00000185. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed FF, Khokha R. Thinking outside the cell: proteases regulate hepatocyte division. Trends Cell Biol. 2005;15:555–563. doi: 10.1016/j.tcb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy] Zhonghua Gan Zang Bing Za Zhi. 2020;28:97–99. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggi E, Canonica GW, Moretta L. COVID-19: Unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol. 2020;146:18–22. doi: 10.1016/j.jaci.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan K, Liao S, Li J, Bai Y, Lv L, Yu K, Qiu L, Li C, Yuan G, Zhang A, Mei Z. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut. 2021;70:628–629. doi: 10.1136/gutjnl-2020-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Liao YS, Gong J, Liu J, Zhang H. Clinical characteristics and risk factors for liver injury in COVID-19 patients in Wuhan. World J Gastroenterol. 2020;26:4694–4702. doi: 10.3748/wjg.v26.i31.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao JM, Zhou GD, Sun YL, Wang SS, Yang JF, Meng EH, Pan D, Li WS, Zhou XS, Wang YD, Lu JY, Li N, Wang DW, Zhou BC, Zhang TH. [Clinical pathology and pathogenesis of severe acute respiratory syndrome] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:217–221. [PubMed] [Google Scholar]
- 44.Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605–2613. doi: 10.1182/blood-2013-09-526277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020; 181: 894-904. :e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, Navalesi P, Simioni P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tee A, Wong A, Yusuf GT, Rao D, Sidhu PS. Contrast-enhanced ultrasound (CEUS) of the lung reveals multiple areas of microthrombi in a COVID-19 patient. Intensive Care Med. 2020;46:1660–1662. doi: 10.1007/s00134-020-06085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Molière S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, Dodek P, McIntyre L, Hall R, Heels-Ansdell D, Fowler R, Pai M, Guyatt G, Crowther MA, Warkentin TE, Devereaux PJ, Walter SD, Muscedere J, Herridge M, Turgeon AF, Geerts W, Finfer S, Jacka M, Berwanger O, Ostermann M, Qushmaq I, Friedrich JO, Cook DJ PROphylaxis for ThromboEmbolism in Critical Care Trial Investigators. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med. 2015;43:401–410. doi: 10.1097/CCM.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 51.Corrigan D, Prucnal C, Kabrhel C. Pulmonary embolism: the diagnosis, risk-stratification, treatment and disposition of emergency department patients. Clin Exp Emerg Med. 2016;3:117–125. doi: 10.15441/ceem.16.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antoniak S. The coagulation system in host defense. Res Pract Thromb Haemost. 2018;2:549–557. doi: 10.1002/rth2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witte MH, Daley SK. SARS-CoV-2/COVID-19, Lymphatic vessels, lymph, and lymphology. Lymphology. 2020;53:97–98. [PubMed] [Google Scholar]
- 54.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 55.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60–63. [PubMed] [Google Scholar]
- 57.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. doi: 10.1177/1756284820959183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medical Administration Board. China National Health Commission (2020) Diagnosis and treatment of 2019-nCoV pneumonia in China (version 8) (in Chinese). [cited 10 January 2021]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml . Accessed 19 August 2020.
- 63.Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M APASL COVID Task Force. APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zha L, Li S, Pan L, Tefsen B, Li Y, French N, Chen L, Yang G, Villanueva EV. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu , Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597–603. doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, Huang S, Li Y, Zhang B, Xu T, Li C, Ji F, Ming F, Zhao Y, Cheng J, Wang Y, Zhao H, Hong S, Chen K, Zhao XA, Zou L, Sang D, Shao H, Guan X, Chen X, Chen Y, Wei J, Zhu C, Wu C. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020 doi: 10.1002/hep4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, Iqbal P, Elfert K, Balaraju G, Almaslamani M, Al-Ejji K, AlKaabi S, Kamel YM. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482–484. doi: 10.1016/j.jhep.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sessa A, Mazzola A, Lim C, Atif M, Pappatella J, Pourcher V, Scatton O, Conti F. COVID-19 in a liver transplant recipient: Could iatrogenic immunosuppression have prevented severe pneumonia? World J Gastroenterol. 2020;26:7076–7084. doi: 10.3748/wjg.v26.i44.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 81.Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoever G, Baltina L, Michaelis M, Kondratenko R, Tolstikov GA, Doerr HW, Cinatl J Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 83.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. [Management of COVID-19: the Zhejiang experience] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Dong YQ, Yin J, He G, Wu X, Li J, Qiu Y, He X. Critically ill patients with COVID-19 with ECMO and artificial liver plasma exchange: A retrospective study. Medicine (Baltimore) 2020;99:e21012. doi: 10.1097/MD.0000000000021012. [DOI] [PMC free article] [PubMed] [Google Scholar]