Abstract
Background
Ginseng, a traditional herbal medicine, has been used for thousands of years to treat various diseases including metabolic syndrome (MS). However, the underlying mechanism(s) of such beneficial actions of ginseng against MS is poorly understood. Emerging evidence indicates a close association of the host gut microbiota with MS. The present study was conducted to examine, whether the beneficial effects of Korean red ginseng (KRG) against MS could be influenced by gut microbial population and whether gut microbial profile could be considered a valuable biomarker for targeted treatment strategy for MS in compliance with the predictive, preventive, and personalized medicine (PPPM / 3PM).
Methods
This clinical study was a randomized, double-blind, placebo-controlled trial evaluating the effects of KRG treatment for 8 weeks on patients with MS. The anthropometric parameters, vital signs, metabolic biomarkers, and gut microbial composition through 16S rRNA gene sequencing were assessed at the baseline and endpoint. The impact of KRG was also evaluated after categorizing the subjects into responders and non-responders, as well as enterotypes 1 and 2 based on their gut microbial profile at the baseline.
Results
Fifty out of 60 subjects who meet the MS criteria completed the trial without showing adverse reactions. The KRG treatment caused a significant decrease in systolic blood pressure (SBP). Microbial analysis revealed a decrease in Firmicutes, Proteobacteria, and an increase in Bacteroidetes in response to KRG. In patient stratification analysis, the responders showing marked improvement in the serum levels of lipid metabolic biomarkers TC and LDL due to the KRG treatment exhibited higher population of both the family Lachnospiraceae and order Clostridiales compared to the non-responders. The homeostasis model assessment-insulin resistance (HOMA-IR) and insulin level were decreased in enterotype 1 (Bacteroides-abundant group) and increased in enterotype 2 (prevotella-abundant group) following the KRG treatment.
Conclusion
In this study, the effects of KRG on the glucose metabolism in MS patients were influenced by the relative abundances of gut microbial population and differed according to the individual enterotype. Therefore, the analysis of enterotype categories is considered to be helpful in predicting the effectiveness of KRG on glucose homeostasis of MS patients individually. This will further help to decide on the appropriate treatment strategy for MS, in compliance with the perspective of PPPM.
Keywords: Korean red ginseng, Herbal medicine, Metabolic syndrome, Patient stratification , Blood serum, Biomarker panel, Molecular pathways, Oxidative stress, Inflammation, ROS detoxification, Lipid metabolic biomarkers, Hyperlipidemia, Glucose homeostasis, Insulin level, Anthropometric parameters, Vital signs, Fat mass, BMI, Gender, Predictive preventive personalized medicine (PPPM / 3PM), Individual enterotype, Clinical trial, Gut microbiome profile, Drug response, Treatment strategy, Blood pressure, 16S rRNA gene sequencing, HOMA-IR
Introduction
Metabolic syndrome (MS) represents a group of clinical disorders, including obesity (central adiposity), insulin resistance, hyperglycemia, glucose intolerance, dyslipidemia, and hypertension [1–3]. Being a major risk factor for several diseases, such as type 2 diabetes (T2D), neurological disorders, cardiovascular disease (CVD), stroke, non-alcoholic steatohepatitis, polycystic ovary syndrome, and cancer [2], MS is now considered a serious global public health problem and it is becoming a major cause of morbidity and mortality in the world [4]. Therefore, MS appears to be a crucial challenge for the coming years [5]. According to the definition of MS from the International Diabetes Federation, the National Cholesterol Education Program, and the Adult Treatment Panel criteria, the prevalence of MS in the USA is estimated to be 22% to more than 30% [6]. Among adults, 12–37% of the Asian population, 12–63% of the African population, and 12–26% of the European population are affected by MS [7]. The WHO estimated that worldwide, more than 1.9 billion adults, 18 years and older were overweight in 2016. Among them, over 650 million were obese. More than 340 million children and adolescents aged 5–19 were overweight or obese in that year. Furthermore, 38 million children under the age of 5 were overweight or obese in 2019 [8]. A similar trend is also seen in the prevalence of diabetes. In 2013, 382 million people were found suffering from diabetes, and it has been predicted that the incidence of diabetes will increase abruptly to 600 million cases by 2045 [9, 10]. A nomogram prediction of the 3-year risk of diabetes based on analyses of the data of a clinical study on the healthy mainland China residents revealed 10.71% and 11.02% occurrences of this disease in 5557 participants (training cohorts) and 1870 participants (validation cohorts), respectively [11]. Furthermore, the WHO estimated that 17.9 million people died from cardiovascular diseases (CVDs) in 2016, representing 31% of all global deaths, and predicted that CVDs would persist over the next decade as the major cause of death [12].
Albeit, the detailed molecular mechanisms behind the onset and progression of MS are not completely elucidated, accumulating evidence indicates the involvement of oxidative stress (OS), a consequence of a decrease in the antioxidant systems and an increase in the production of reactive oxygen species (ROS), in this multifactorial disorder [13, 14]. Increased oxidative stress in accumulated fat is an early initiator of MS. The redox state in adipose tissue appears to be a potentially promising therapeutic target for obesity-associated MS [15]. Additionally, OS plays an important role in the onset and development of diabetes complications related to microvessels and coronary vessels. Diabetes mellitus triggers the production of excessive mitochondrial peroxide in vascular endothelial cells and the myocardium, leading to the activation of the main pathways involved in the pathogenesis of complications [16, 17]. Furthermore, a number of studies have revealed that MS is associated with a state of low-grade inflammation, characterized by abnormal proinflammatory cytokine production, augmented acute-phase reactants, and the activation of a network of inflammatory signaling pathways [13, 14, 18].
Accumulating evidence indicates that the microbiome, as well as probiotics and prebiotics, modulate the functions of distant organs, the mechanisms of which are well elucidated in the literature [19]. In adult humans, approximately 1014 bacterial cells are present in the intestine which are ten times more than the total body cells of the host [20, 21]. The gut microbes play an important role in metabolism and health [22] as evident by animal and human studies exhibiting a remarkable microbial influence on nutrient absorption, energy utilization and storage, immunologic system, and metabolic disease [23–28]. Accordingly, in the precision nutrition research field, the gut microbiome appears to be a potential tool for predicting and validating the personalized responses to diet and variability of human metabolism [29, 30]. A previous in vivo study reported that compared to lean mice, ob/ob animals had a 50% decrease in the abundance of Bacteroidetes and a proportional increase in Firmicutes indicating that obesity affects the gut microbial diversity [31]. In keeping with this, a clinical study showed that the relative proportion of Bacteroidetes decreased in obese participants compared to lean subjects. This proportion increased with weight loss driven by two types of low-calorie diet, further supporting that obesity has a microbial component that might have potential therapeutic applications [32].
An imbalance in the gut microbial communities (dysbiosis) might be associated with an elevation in the aberrant metabolite concentrations that disrupt the GLP-2–mediated tight junction integrity of the intestine, leading to an increase in the permeability of the gut epithelium. This allows the microbial lipopolysaccharide (LPS), trimethylamine (TMA), and other metabolites to enter the circulation, causing chronic inflammation of the liver and adipose tissue that triggers the onset and development of insulin resistance and other conditions associated with MS [33, 34]. Furthermore, gut microbial perturbations and related dysbiosis are associated with the progression and pathogenesis of CVD, including atherosclerosis, hypertension, and heart failure [33, 35]. Accordingly, various strategies, including diet, herbal medicines, probiotics, prebiotics, postbiotics, antibiotics, and fecal microbial transplantation (FMT), have been employed to maintain the balance in the gut microbial composition to improve type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), atherosclerosis, CVD, and cancer [36, 37]. Furthermore, emerging evidence indicates that the combined application of inorganic prebiotics and probiotics has great therapeutic potential to treat hypercholesterolemia [38]. It has been found that cholesterol metabolism is sensitive to the microbial profiles and the setup of appropriate probiotics, immunobiotics, and prebiotics is critical for a personalized clinical set [38]. Therefore, it is conceivable that the prebiotics-mediated modulation of the gut microbiota can be an important therapeutic strategy for predictive, preventive, and personalized medicine (PPPM) to combat the onset and development of obesity and MS.
Korean ginseng (Panax ginseng Meyer, Araliaceae), a traditional herbal medicine, has been used as a therapeutic agent to treat various diseases for thousands of years [17]. Korean red ginseng (KRG) is produced by steaming and drying fresh ginseng to potentiate its therapeutic activities [39]. Accumulating evidence suggests that ginseng, which is composed of multiple ingredients, possesses antioxidant, anti-inflammatory, antiapoptotic, and immunostimulant properties and exerts central nervous system effects, neuroprotective, immunomodulatory, and anticancer effects [40]. More specifically, as a potent natural antioxidant, ginseng effectively suppresses the inflammatory response in acute or chronic inflammation [41]. In parallel, growing evidence suggests that KRG is a potent therapeutic agent against MS disorders, including obesity, dyslipidemia, diabetes, and CVD [39]. For example, in vivo studies using rat and mouse models reported that ginseng or its components improved the glucose and lipid metabolism in MS [42–45]. Furthermore, our earlier clinical studies on South Korean obese women showed that a KRG treatment reduced the weight, BMI, waist-hip ratio, daily food intake, the Korean version of obesity-related quality of life scale [46, 47]. Another clinical study demonstrated the hypolipidemic action of a Panax ginseng extract on the lipid metabolism in humans, which was associated with a decrease in total cholesterol (TC), triglycerides (TG), and low-density lipoprotein (LDL) cholesterol, and an increase in high-density lipoprotein (HDL) cholesterol [48]. Furthermore, previous clinical trials in South Korea reported the anti-diabetic properties of KRG because it decreased the serum glucose and whole blood glucose levels, C-peptide, fasting insulin level, HOMA-IR, and current perception threshold of the lower extremities in type 2 diabetic patients [39]. Another clinical study reported that 12 weeks of supplementation with the selected KRG preparation maintained good glycemic control and improved the oral glucose tolerance test-plasma glucose, and plasma insulin regulation in well-controlled, type 2 diabetes [49]. Ginseng also exerted potent anti-stress effects in in vivo studies [41]. Moreover, accumulating clinical evidence indicates the beneficial impact of KRG on CVD. More specifically, the applications of KRG or its active constituents showed a marked decrease in central systolic and diastolic blood pressure (BP), end-systolic pressure, area under the systolic/diastolic BP curve, central and brachial mean arterial pressure, brachial systolic and diastolic BP [39]. Furthermore, ginseng suppresses the oxidative stress induced by ischemia, a major event causing stroke-induced tissue damage [41]. Previous in vivo studies reported that ginseng reduced the levels of ROS in myocardial tissue and improved blood circulation, facilitating the maintenance of cardiac function [41].
Growing evidence suggests that KRG and its components have a reciprocal interrelationship with the host’s gut microbiota. Thus far, approximately 200 substances have been isolated from ginseng which include ginsenosides, polysaccharides, polyacetylenes, antioxidative aromatic compounds, amino acids, volatile oils, alkaloids, lignanes, and other nitrogen compounds (adenosine, acidic peptide, radiation protective protein, and immunocyte generative protein) [50, 51]. The major bioactive ingredients of P. ginseng are ginsenosides, which are triterpene saponins [51, 52]. In a rat model, it was found that the consumption of KRG powder increased the number of total ileal microbiota and Lactobacillus strains significantly compared to the control group [53]. Using the same animal model, it was shown that the ginseng extract affected the gut microbiota structure with a decreased abundance of TM7 and an increased population of Proteobacteria, Methylobacteriaceae, Parasutterella, and Sutterella [54]. A previous study reported that a treatment with ginseng saponins shaped the murine gut microbiome by enhancing the abundance of Akkermansia muciniphila and Parabacteroides distasonis [55]. In one of our clinical studies conducted on obese middle-aged Korean women showed that the order of dominance of the host’s gut microbial genera were changed from Blautia, Bifidobacterium, and Anaerostipes to Bifidobacterium, Blautia, and Faecalibacterium after KRG administration [47]. Furthermore, in NAFLD patients, KRG exerted beneficial effects that were associated with a decrease in the relative abundance of Firmicutes, whereas an increase was noted in the placebo group [56].
Overall, the abovementioned findings show that ginseng can modulate the gut microbial composition of the host, including those suffering from MS. Several lines of evidence indicate that gut microbes, in turn, enhance the therapeutic potential of ginseng. Our previous clinical study reported that treating of middle-aged obese Korean women with a ginseng extract affected weight loss and the gut microbiota, and that its anti-obesity effects differed according to the gut microbial composition prior to ginseng intake [47]. The gut microbes are enriched with enzymes that convert hydrophilic ginsenosides to hydrophobic compounds, making them easily absorbable. This enhances the bioavailability of ginsenosides and potentiates their efficacy [57, 58]. Taking account of the abovementioned beneficial effects of KRG, reciprocal relationship between KRG and gut microbiome, and the definition of prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [59], it is conceivable that KRG could be considered a potential prebiotic candidate [60].
PPPM, also considered “medicine of the future,” is an integrated approach addressing an individualized patient profile, predictive diagnosis, and targeted prevention. It is a new paradigm to provide both “health care” and “disease care,” combining the advantages of individual bio/medical fields and technologies and amalgamating a multi-professional collaboration [61]. In addition to personalized patient profiling, an analysis of the complex interactions between the host and gut microbes using integrated multiomics technology and patterning gut microbiota-based translational biomarkers would be vital in addressing an effective PPMM approach for MS [10, 62, 63]. Previous studies reported that the differences in the drug responses depend on the gut microbial composition at the baseline [47, 64]. Accordingly, the drugs could be administered to the “right” person and targeting the gut microbiota appears to be a potential option for improving the pharmacological effects of drugs. Analyzing the drug effects individually after stratifying the patients according to the gut microbial composition, such as “enterotypes,” is one of the key strategies to address PPPM [61, 65]. “Enterotypes” is a concept that categorizes the gut microbial population into three clusters determined by the composition of the predominant species, such as Bacteroides, Prevotella, and Ruminococcus [66]. Evaluating the pharmacological impact of KRG according to the gut microbial composition, such as enterotypes, will be the helpful in cost-effective use of KRG in the future.
This study evaluated the beneficial effects of KRG in improving the MS-related markers of subjects, as well as how these effects were influenced by gut microbial composition at the individual level. The outcome of this study could be used to evaluate the compatibility of the application of KRG as personalized medicine for MS based on the information on the response of individual patient’s enterotype, thereby establishing a targeted treatment strategy.
Materials and methods
Study design
The study was a single-center, randomized, double-blind, placebo-controlled clinical trial conducted on participants with MS. After 66 volunteers were screened, 60 participants who satisfied the criteria of this study were enrolled and assigned randomly to the KRG group (n = 30) or placebo group (n = 30) using a computerized procedure. The participants of the KRG and placebo groups received KRG and placebo capsules, respectively, for 8 weeks. All subjects visited the hospital for initial screening and at the 0th, 2nd, 4th, 6th, and 8th weeks of their KRG treatment for their assessment. At each visit, the subjects were scrutinized to determine if changes in their background medications had been made and the possible adverse effects of KRG.
Participants
Sixty subjects, both male and female, were registered. The inclusion criteria were as follows: (1) adults aged 19 to 65; (2) those who had 3 or more of the following MS features ((i) waist circumference (WC) male > 90 cm, female > 85 cm, (ii) TG ≥ 150 mg/dL or those taking medications for hyperlipidemia, (iii) HDL male < 40 mg/dL, female < 50 mg/dL, (iv) fasting glucose ≥ 100 mg/dL or those taking medications for diabetes, and (v) systolic blood pressure (SBP) ≥ 130 mm Hg or diastolic blood pressure (DBP) ≥ 85 mm Hg or those taking medication for hypertension)); (3) those who had been explained the outline of this clinical trial and signed a written consent form. The exclusion criteria were as follows: (1) those with endocrine diseases that could affect the body weight, such as hypothyroidism and Cushing’s syndrome; (2) those with heart diseases (heart failure, angina, and myocardial infarction) or a history of stroke or temporary ischemic heart attack; (3) those with a malignant tumor or lung disease; (4) those diagnosed with liver dysfunction [serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) > 2.5 times above the normal upper limit]; (5) those with cholelithiasis; (6) those showing serum creatinine level > 2.0 mg/dL; (7) those with narrow angle glaucoma; (8) those with a neurological or psychological disorder-related medical history or present illness; (9) those with a history of eating disorders, such as anorexia nervosa or bulimia nervosa; (10) those who had a history of taking drugs without prescription (especially, medicines that can affect the body weight, such as appetite suppressants, laxatives, and oral steroids), thyroid hormones, amphetamines, cyproheptadine, phenodiazin in the last 3 months; (11) those receiving weight-loss agents that might have an impact on central nervous system; (12) those receiving prohibited treatments (insulin, antidepressants, antiserotonins, barbiturates, antipsychotics, and drugs with a high potential for abuse); (13) those with anatomical changes, such as resection, and therefore, had difficulties in the measurement of body parameters; (14) those who were surgically operated for weight loss; (15) pregnant women, lactating women, women of childbearing potential who had a pregnancy plan or who did not agree to the selection of appropriate contraceptive methods (e.g. oral contraceptives, hormone transfer, intrauterine device (IUD), spermicide barrier method, condoms, abstinence); (16) those who participated in another clinical trial within the last month; (17) those who had lost more than 10% of their body weight within last 6 months; (18) those who were judged by the sub-investigator to be unable to follow the study’s protocol; and (19) those who had taken antibiotics or probiotics within the past month.
Intervention
The KRG and placebo groups received KRG capsules (Korean red ginseng powder 100%, 6000 mg/day) and placebo capsules (4200 mg/day), respectively, for 8 weeks. Each KRG capsule contained the ginsenosides Rg1 (2.90 mg/g), Re (1.48 mg/g), Rf (0.76 mg/g), Rg2s (0.19 mg/g), Rb1 (4.94 mg/g), Rc (1.98 mg/g), Rb2 (1.53 mg/g), Rd (0.31 mg/g), Rg3s (0.24 mg/g), Rg3r (0.14 mg/g), and Rh1 (0.23 mg/g). The placebo capsules, which were similar to the KRG capsules in shape and flavor, contained crystalline cellulose 70%, maltodextrin 28.37%, magnesium stearate 0.5%, silicon dioxide 1%, and food color 0.124%. The KRG and placebo capsules were provided by Korea Ginseng Corporation (Seoul, Republic of Korea).
Randomization and blinding strategies
Group allocation to either the KRG group or placebo group was based on randomization. Subjects were randomized in blocks of 4 using an online random code generation tool (http://www.jerrydallal.com/random/random_block_size_r.htm). Before being provisioned, KRG and placebo capsules were packed and labeled according to random code. All participants, pharmacists, and investigators remained blinded to the allocation until the end of the study. Subjects could not recognize the difference between the KRG and placebo capsules due to the similarity in their shape.
Assessment
The vital signs, body weight, total fat mass, fat percentage, body mass index (BMI), and WC of the participants were measured at each visit (0th, 2nd, 4th, 6th, and 8th week). The obesity-related quality of life of the subjects was evaluated at the 0th and 8th week [67], and fecal samples were collected. Blood samples were collected when volunteers were screened and at the 0th and 8th week of the KRG treatment. The sera were separated from the blood after clotting and centrifuging at 3000 rpm for 15 min at 4 °C.
Anthropometry
Vital signs, such as blood pressure and heart rate, were measured using an automatic digital sphygmomanometer (Easy X 800; SELVAS Healthcare, Daejeon, Republic of Korea). Body compositions, such as the fat mass and fat percentage, were determined using a bioelectrical impedance analysis method (Inbody 720; Biospace, Seoul, Republic of Korea).
Blood chemistry
At weeks 0 and 8 of the trial, biochemical tests were performed to determine the serum levels of insulin, glucose, TG, TC, and HDL using a Cobas 8000 modular analyzer (Roche, Brandford, CT, USA). Low-density lipoprotein (LDL) was estimated using the Friedewald equation [68]. The homeostasis model assessment-insulin resistance (HOMA-IR) was determined using the following equation
Sequencing of the 16S rRNA gene amplicon of fecal microbiota
Bacterial DNA was isolated from fecal samples using a QIAamp stool DNA mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The PCR of the V1-V3 region of the 16S rRNA gene was performed using a C1000 Touch thermal cycler equipped with a 96-deep-well reaction module (Bio-Rad, Hercules, CA, USA). The PCR products were purified using a LaboPass PCR purification kit (Cosmogenetech Inc., Seoul, Republic of Korea). The amplicons of each sample were pooled in equimolar amounts, and the resulting mixture was purified using AMPure XP beads (Agencourt Bioscience, Beverly, MA, USA). The genomic DNA in the samples was quantified using a PicoGreen dsDNA assay kit (Invitrogen, Carlsbad, CA, USA). The mixed amplicons were amplified on sequencing beads using emulsion PCR. The sequencing reactions were performed on a Roche/454 GS Junior system (454 Life Sciences, Branford, CT, USA) according to the manufacturer’s instructions.
Enterotype analysis
RStudio (RStudio Inc., Boston, MA, USA; version 3.6.2), a powerful, free, open-source programming language for statistical analyses and graphics, was used to categorize the enterotypes. For this, the samples were clustered using the partitioning around medoids (PAM) clustering algorithm [69], and an optimal number of clusters was validated using the Calinski-Harabasz index [70]. The sample distributions were calculated based on the relative gut microbial genus abundances using the Jensen-Shannon divergence (JSD) distance metric and visualized using a PCoA plot. All R codes used were obtained from the MetaHIT consortium [66].
Sequence analysis
The obtained sequence reads were filtered by eliminating the low-quality reads (average quality score < 20 or read length < 300 bp). The processed sequences and the operational taxonomic units (OTUs) were clustered using the open reference OTU picking method according to the Quantitative Insights into Microbial Ecology (QIIME) pipeline-version 1.9.1 [71]. For profiling taxa and finding differences in microbiota abundances between the KRG and placebo groups, a linear discriminant analysis effect size (LEfSe) assessment was performed using an online software tool (http://huttenhower.sph.harvard.edu/galaxy). For this evaluation, the threshold of the logarithmic linear discriminant analysis (LDA) score was set to > 2.0, and the alpha value of the factorial Kruskal–Wallis test among the classes was set to < 0.05.
Statistical analysis
The results are presented as mean ± standard deviation. A pre vs. post-treatment comparison of the variables within each study group (KRG or placebo) was performed using a paired t-test or Wilcoxon signed-rank test. An independent t-test or Wilcoxon’s rank-sum test was used to compare the KRG and placebo groups. All statistical analyses were performed using SPSS version 18.00 for Windows (SPSS Inc., Chicago, IL, USA). The strength of the relationship between the parameters was evaluated using the two-tailed Pearson’s correlation test; p-values < 0.05 were considered significant.
Results
Participants, anthropometric and biochemical parameters, vital signs, and adverse reactions
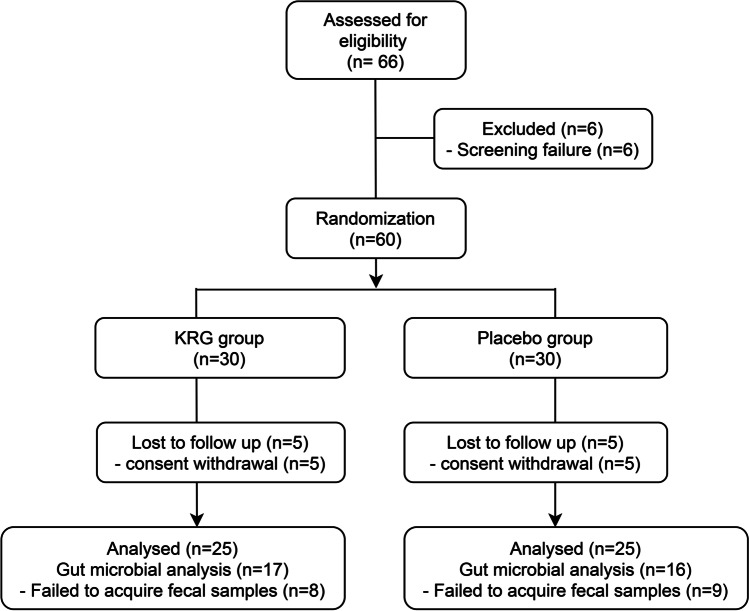
Among the 66 participants screened, 60 subjects fulfilled the study criteria and were registered for the trial. The selected volunteers were assigned randomly to the KRG group (n = 30) or the placebo group (n = 30). Fifty subjects (KRG, n = 25, placebo, n = 25) ultimately completed the study (Fig. 1). At the end of the trial, an analysis of the stool microbiota was accomplished for 17 and 16 subjects from the KRG and placebo groups, respectively. This analysis could not be performed for the rest of the participants due to the inadequate amount of fecal samples (Fig. 1). No significant inter-group differences in the anthropometric and biochemical parameters and vital signs were observed at the baseline (Table 1). Furthermore, no adverse effects of KRG on the subjects were observed throughout the entire trial period.
Fig. 1.
Study flow chart depicting the entire processes of the clinical trial
Table 1.
Baseline characteristics of the subjects
| Variables | KRG | Placebo | p-value ‡ |
|---|---|---|---|
| Gender (male/female) | 8/17 | 8/17 | 1.000† |
| Age (years) | 53.60 ± 8.45 | 49.84 ± 10.56 | 0.171 |
| Height (cm) | 162.48 ± 9.19 | 162.68 ± 7.22 | 0.932 |
| HOMA-IR | 3.01 ± 1.38 | 3.66 ± 3.38 | 0.930 |
| Insulin (µIU/mL) | 10.82 ± 4.73 | 12.04 ± 8.87 | 0.823 |
| Glucose (mg/dL) | 112.52 ± 21.16 | 117.72 ± 22.83 | 0.299 |
| TG (mg/dL) | 150.72 ± 57.51 | 166.36 ± 69.61 | 0.547 |
| TC (mg/dL) | 195.76 ± 39.71 | 186.72 ± 32.51 | 0.383 |
| HDL (mg/dL) | 52.52 ± 11.25 | 55.74 ± 9.75 | 0.107 |
| LDL (mg/dL) | 129.84 ± 40.20 | 116.48 ± 36.61 | 0.225 |
| Body weight (kg) | 74.30 ± 12.68 | 72.57 ± 16.31 | 0.676 |
| BMI (kg/m2) | 28.16 ± 4.47 | 27.22 ± 4.74 | 0.337 |
| WC (cm) | 95.90 ± 7.93 | 95.32 ± 9.29 | 0.503 |
| Fat mass (kg) | 26.22 ± 8.06 | 25.20 ± 10.12 | 0.273 |
| Fat percentage (%) | 35.10 ± 7.26 | 34.14 ± 7.48 | 0.646 |
| SBP (mmHg) | 132.36 ± 12.42 | 127.32 ± 15.62 | 0.213 |
| DBP (mmHg) | 77.40 ± 8.09 | 78.08 ± 13.57 | 0.831 |
| Pulse rate (/min) | 78.16 ± 11.42 | 79.32 ± 14.64 | 0.756 |
| CRP (mg/dL) | 0.13 ± 0.17 | 0.25 ± 0.45 | 0.189 |
| Number of MS factors | 3.60 ± 0.58 | 3.40 ± 0.65 | 0.243 |
| Obesity-related questionnaire | 29.64 ± 6.89 | 27.96 ± 9.82 | 0.487 |
Data are expressed as mean ± SD; †p-value obtained from a chi-square test; ‡p-value obtained from an independent t-test, Wilcoxon’s rank-sum test; *p < 0.05, **p < 0.01; HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rate; MS, metabolic syndrome
KRG administration improved SBP and led to shifts in gut microbiota
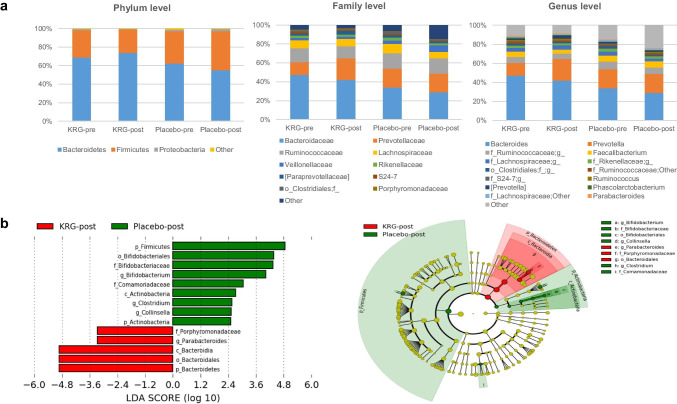
There were no significant differences in the body composition, anthropometric and biochemical parameters related to MS in the subjects between the pre and post-treatments of KRG (Table 2). In addition, these parameters were similar in the placebo and KRG groups at the end of the study. On the other hand, the SBP was observed significantly lower in the subjects exposed to KRG (p = 0.026). Furthermore, marked changes in the gut microbial communities were observed in the subjects at phylum, family, and genus levels in response to the KRG treatment. At the phylum level, although the changes were not significant, the relative abundance of the microbiota was increased from 68.87 ± 17.43% to 73.88 ± 21.00% (p = 0.156) in Bacteroidetes (B) and decreased from 29.47 ± 17.72% to 25.33 ± 20.84% (p = 0.332) in Firmicutes (F). In contrast, the population of phylum Proteobacteria had declined significantly from 1.27 ± 1.33 to 0.64 ± 0.72 (p = 0.023). Microbes representing more than 1% of the total population were considered for comparative analysis at the genus level. Among the dominant communities, although the changes were not significant, the relative abundance of Bacteroides decreased from 47.41 ± 27.09% to 41.93 ± 28.78% in response to the KRG treatment (p = 0.155). In contrast, the relative abundance of Prevotella increased from 13.29 ± 21.27% to 23.01 ± 30.19% (p = 0.286) (Fig. 2a). For a better understanding of the impact of KRG on the gut microbial communities, LEfSe analysis was performed to determine the differences in the distributional patterns of the gut microbes between the placebo and KRG groups. The results showed that after 8 weeks of KRG or placebo administration, Bacteroidetes, Bacteroidia, and Bacteroidales were the dominant gut microbial population at the phylum, class, and order levels, respectively, in the KRG group (Fig. 2b). In contrast, phylum Firmicutes, order Bifidobacteriales, family Bifidobacteriaceae, and genus Bifidobacterium were the dominant gut microbial communities in the placebo group.
Table 2.
Comparison of the changes in the anthropometric and biochemical parameters as well as the vital signs between the KRG and placebo groups
| KRG (n = 25) | Placebo (n = 25) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Initial | Final | Within† | Initial | Final | Within† | p-value‡ |
| HOMA-IR | 3.01 ± 1.38 | 3.09 ± 1.90 | 0.882 | 3.66 ± 3.38 | 3.93 ± 3.40 | 0.510 | 0.528 |
| Insulin (µIU/mL) | 10.82 ± 4.73 | 10.75 ± 5.69 | 0.840 | 12.04 ± 8.87 | 12.49 ± 8.15 | 0.412 | 0.467 |
| Glucose (mg/dL) | 112.52 ± 21.16 | 114.28 ± 25.34 | 0.210 | 117.72 ± 22.83 | 118.72 ± 30.06 | 0.688 | 0.503 |
| TG (mg/dL) | 150.72 ± 57.51 | 157.44 ± 58.95 | 0.563 | 166.36 ± 69.61 | 146.64 ± 59.07 | 0.115 | 0.479 |
| TC (mg/dL) | 195.76 ± 39.71 | 191.40 ± 37.07 | 0.380 | 186.72 ± 32.51 | 176.36 ± 32.71 | 0.010 | 0.135 |
| HDL (mg/dL) | 52.52 ± 11.25 | 51.80 ± 13.91 | 0.537 | 55.74 ± 9.75 | 53.68 ± 9.23 | 0.132 | 0.145 |
| LDL (mg/dL) | 129.84 ± 40.20 | 124.96 ± 39.33 | 0.241 | 116.48 ± 36.61 | 109.00 ± 36.77 | 0.066 | 0.145 |
| Body weight (kg) | 74.30 ± 12.68 | 74.58 ± 13.01 | 0.384 | 72.57 ± 16.31 | 72.37 ± 16.39 | 0.367 | 0.600 |
| BMI (kg/m2) | 28.16 ± 4.47 | 28.46 ± 4.47 | 0.014* | 27.22 ± 4.74 | 27.13 ± 4.71 | 0.292 | 0.240 |
| WC (cm) | 95.90 ± 7.93 | 95.35 ± 7.94 | 0.167 | 95.32 ± 9.29 | 94.04 ± 8.79 | 0.005* | 0.331 |
| Fat mass (kg) | 26.22 ± 8.06 | 26.24 ± 8.15 | 0.864 | 25.20 ± 10.12 | 25.29 ± 10.06 | 0.891 | 0.318 |
| Fat percentage (%) | 35.10 ± 7.26 | 35.06 ± 7.54 | 0.896 | 34.14 ± 7.48 | 34.40 ± 7.43 | 0.328 | 0.755 |
| SBP (mmHg) | 132.36 ± 12.42 | 126.80 ± 12.87 | 0.026* | 127.32 ± 15.62 | 124.88 ± 17.27 | 0.249 | 0.658 |
| DBP (mmHg) | 77.40 ± 8.09 | 75.84 ± 7.51 | 0.415 | 78.08 ± 13.57 | 75.72 ± 11.41 | 0.159 | 0.965 |
| Pulse rate (/min) | 78.16 ± 11.42 | 78.28 ± 9.84 | 0.657 | 79.32 ± 14.64 | 77.32 ± 12.82 | 0.257 | 0.977 |
| CRP (mg/dL) | 0.13 ± 0.17 | 0.21 ± 0.31 | 0.315 | 0.25 ± 0.45 | 0.22 ± 0.31 | 0.988 | 0.869 |
| Number of MS factors | 3.60 ± 0.58 | 3.32 ± 0.80 | 0.106 | 3.40 ± 0.65 | 3.08 ± 0.95 | 0.033* | 0.395 |
| Obesity-related questionnaire | 29.64 ± 6.89 | 29.44 ± 7.12 | 0.869 | 27.96 ± 9.82 | 27.60 ± 7.71 | 0.500 | 0.186 |
Data are expressed as mean ± SD; †p-value obtained from a paired t-test, Wilcoxon signed-rank test; ‡p-value obtained from an independent t-test, Wilcoxon’s rank-sum test; *p < 0.05, **p < 0.01; HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; MS, metabolic syndrome; CRP, C-reactive protein
Fig. 2.
Effects of KRG or placebo treatments on the gut microbiota. a Comparison of the relative abundances of the gut microbiota at phylum, family, and genus levels between before and after KRG, placebo administration; b comparison of the dominant gut microbial taxa between the KRG and placebo groups at the termination of the study, as shown by LEfSe
Impact of KRG on the serum glucose and lipid parameters differed depending on the gut microbial composition at the baseline
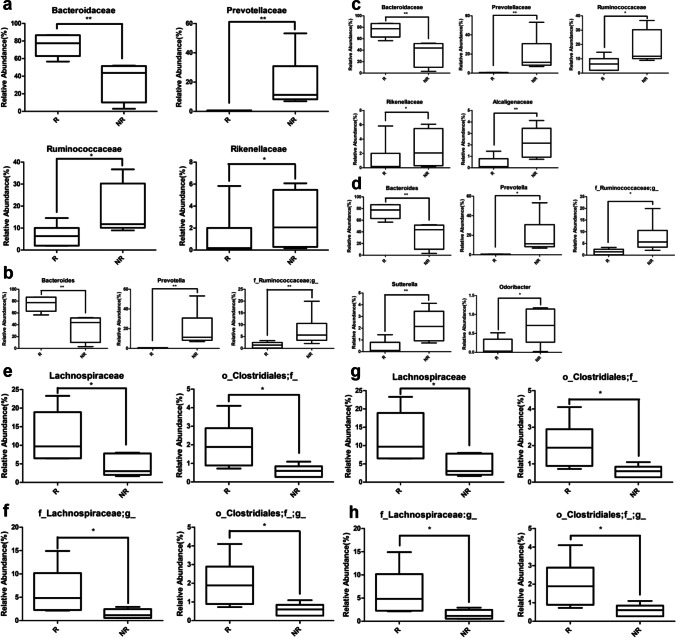
Next, we evaluated the influence of gut microbial population at baseline on the subjects’ response to the KRG treatment. For this, the participants were sorted into responder and non-responder groups based on the degree of improvement of the metabolic parameters HOMA-IR and serum levels of insulin, TC, and LDL in response to KRG treatment. Subjects who ranked among the top and bottom 6 responders, were categorized as the responder and non-responder groups, respectively (Table 3). The gut microbial profile of the responders exhibiting marked improvement in HOMA-IR upon exposure to KRG showed that at the family level, the abundance of Bacteroidaceae (p = 0.002) was significantly higher than that of the non-responders while the populations of Prevotellaceae (p = 0.004), Ruminococcaceae (p = 0.025), and Rikenellaceae (p = 0.016) were significantly lower (Fig. 3a). Among the genera, Bacteroides (p = 0.002) was significantly higher in the responders than the non-responders, whereas Prevotella (p = 0.004) and the family Ruminococcaceae (p = 0.010) were significantly lower (Fig. 3b). On the other hand, the gut microbial communities of the responders demonstrating noticeable improvement in the serum insulin level after the KRG treatment showed that at the family level, the population of Bacteroidaceae (p = 0.002) was significantly higher in the responders than the non-responders, while that of Prevotellaceae (p = 0.004), Ruminococcaceae (p = 0.025), Riknellaceae (p = 0.016), and Alcaligenaceae (p = 0.010) were significantly lower (Fig. 3c). Furthermore, among the genera, Bacteroides (p = 0.002) was significantly higher in the responders than the non-responders, whereas Prevotella (p = 0.027) and the family Ruminococcaceae (p = 0.048), Sutterella (p = 0.010), and Odoribacter (p = 0.024) were significantly lower (Fig. 3d). The pattern of the gut microbial distribution of the responders, who exhibited marked improvement in the serum levels of TC and LDL due to the KRG treatment, showed that the population of both the family Lachnospiraceae and order Clostridiales were significantly higher than the non-responders (all p < 0.05, Fig. 3e–h).
Table 3.
Alignment of the subjects according to the improvement of the HOMA-IR, insulin, TC, and TG after KRG administration
| Participants | ΔHOMA-IR | Participants | ΔInsulin | Participants | ΔTC | Participants | ΔLDL |
|---|---|---|---|---|---|---|---|
| S-15* | − 2.78 | S-15* | − 10.85 | S-64* | − 53 | S-44 | − 43 |
| S-44 | − 1.51 | S-44 | − 5.44 | S-44 | − 49 | S-64* | − 39 |
| S-37 | − 1 | S-20 | − 3.92 | S-32 | − 42 | S-51 | − 39 |
| S-18 | − 1 | S-37 | − 3.73 | S-15* | − 39 | S-32 | − 33 |
| S-20 | − 0.91 | S-18 | − 3.62 | S-28 | -28 | S-15* | − 23 |
| S-01* | − 0.86 | S-53 | − 3.29 | S-51 | − 26 | S-28 | − 23 |
| S-53 | − 0.82 | S-45* | − 3.22 | S-54* | − 22 | S-54* | − 14 |
| S-46* | − 0.78 | S-01* | − 3 | S-02 | − 12 | S-02 | − 14 |
| S-45* | − 0.59 | S-46* | − 2.43 | S-25 | − 9 | S-25 | − 14 |
| S-23 | − 0.52 | S-23 | − 1.77 | S-23 | − 7 | S-12* | − 11 |
| S-25 | − 0.4 | S-25 | − 1.46 | S-45* | − 6 | S-22* | − 10 |
| S-36 | − 0.22 | S-36 | − 0.5 | S-46* | − 6 | S-23 | − 6 |
| S-52 | 0.02 | S-12* | − 0.04 | S-22* | − 1 | S-18 | − 2 |
| S-12* | 0.04 | S-52 | 0.26 | S-18 | 3 | S-45* | − 1 |
| S-51 | 0.25 | S-02 | 0.73 | S-12* | 3 | S-39 | − 1 |
| S-02 | 0.39 | S-22* | 1.67 | S-63 | 8 | S-46* | 2 |
| S-22* | 0.46 | S-51 | 1.73 | S-39 | 10 | S-63 | 11 |
| S-63 | 0.64 | S-63 | 2.31 | S-53 | 11 | S-53 | 12 |
| S-54* | 0.88 | S-54* | 2.37 | S-61 | 18 | S-20 | 13 |
| S-64* | 0.96 | S-39 | 2.68 | S-36 | 19 | S-52 | 16 |
| S-39 | 1.29 | S-64* | 3.51 | S-52 | 19 | S-08 | 16 |
| S-61 | 1.74 | S-28 | 4.04 | S-20 | 21 | S-61 | 18 |
| S-08 | 1.96 | S-08 | 5.82 | S-01* | 22 | S-01* | 19 |
| S-32 | 2.21 | S-61 | 6.98 | S-37 | 25 | S-37 | 21 |
| S-28 | 2.56 | S-32 | 9.44 | S-08 | 32 | S-36 | 23 |
The degree of improvement was evaluated according to the decrease in size of each index value, and it was considered that smaller the delta value, greater the improvement. Participants who failed to isolate bacterial DNA sample were marked with “*” and excluded from the microbiological analysis. Δ means delta value of each item. HOMA-IR, homeostatic model assessment-insulin resistance; TC, total cholesterol; LDL, low-density lipoprotein cholesterol
Fig. 3.
Comparison of the relative abundances of gut microbial strains between the responder and non-responder groups at the baseline. a Strains that showed a significant difference in the HOMA-IR (at family level); b strains that showed a significant difference in the HOMA-IR (at genus level); c strains that showed a significant difference in the serum insulin level (at family level); d strains that showed a significant difference in the serum insulin level (at genus level); e strains that showed a significant difference in the serum TC level (at family level); f strains that showed a significant difference in the serum TC level (at genus level); g strains that showed a significant difference in the serum LDL level (at family level); h strains that showed a significant difference in the LDL (at genus level); HOMA-IR, homeostatic model assessment-insulin resistance; TC, total cholesterol; LDL, low-density lipoprotein cholesterol; R, responder; NR, non-responder; *p < 0.05, **p < 0.01
Effects of KRG on the blood glucose parameters differed depending on the enterotype
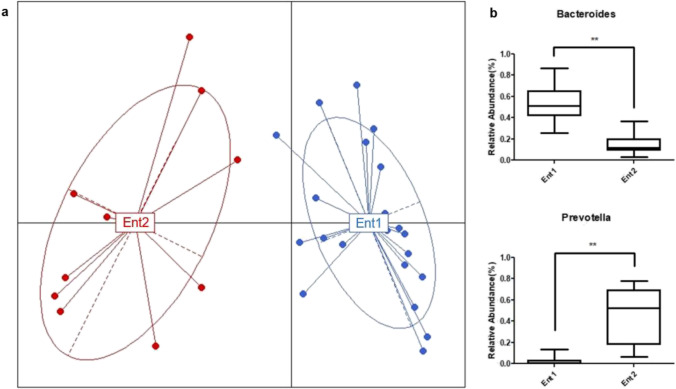
The participants of the KRG group were categorized into two enterotypes, enterotypes 1 and 2, according to the abundance of Bacteroides and Prevotella at the baseline to understand the influence of the gut microbial composition on the mode of action of KRG further. The relative abundance of Bacteroides was significantly higher in enterotype 1 than enterotype 2 (p < 0.001), whereas that of Prevotella was significantly higher in enterotype 2 than enterotype 1 (p < 0.001) (Fig. 4). In contrast, the anthropometric parameters, such as BMI, WC, and fat mass, were significantly lower in enterotype 1 than enterotype 2 at the baseline (p < 0.05, Table 4). When the delta values (differences between post- and pre-treatment of KRG) of the anthropometric, body composition, and biochemical parameters were compared between the enterotypes, a decreasing trend in HOMA-IR was observed in enterotype 1 compared to enterotype 2 (p = 0.070, Table 5). Furthermore, the delta value of the serum insulin level was significantly lower in enterotype 1 than enterotype 2. (p = 0.026).
Fig. 4.
Analysis of enterotypes. a Profiling of the enterotypes. The red and blue colors represent the participants belonging to enterotypes 2 and 1, respectively. Ent1, enterotype 1; Ent2, enterotype 2. b Enterotype-specific dominant strains
Table 4.
Baseline characteristics of the subjects according to the enterotype in the KRG group
| Variables | Enterotype 1 (n = 12) | Enterotype 2 (n = 5) | p-value† |
|---|---|---|---|
| HOMA-IR | 2.80 ± 1.53 | 2.66 ± 1.08 | 0.861 |
| Insulin (µIU/mL) | 9.68 ± 4.42 | 9.29 ± 2.87 | 0.860 |
| Glucose (mg/dL) | 114.58 ± 24.34 | 113.40 ± 22.91 | 0.879 |
| TG (mg/dL) | 143.16 ± 33.39 | 150.20 ± 64.11 | 0.767 |
| TC (mg/dL) | 202.91 ± 47.36 | 188.60 ± 46.18 | 0.576 |
| HDL (mg/dL) | 53.41 ± 12.27 | 47.50 ± 7.99 | 0.341 |
| LDL (mg/dL) | 137.58 ± 47.39 | 126.40 ± 41.06 | 0.653 |
| Body weight (kg) | 69.35 ± 10.93 | 79.30 ± 13.86 | 0.134 |
| BMI (kg/m2) | 26.59 ± 3.13 | 31.46 ± 3.12 | 0.011* |
| WC (cm) | 93.08 ± 7.87 | 102.4 ± 8.33 | 0.045* |
| Fat mass (kg) | 23.07 ± 5.92 | 31.16 ± 4.03 | 0.020* |
| Fat percentage (%) | 33.27 ± 6.42 | 39.70 ± 4.73 | 0.063 |
| SBP (mmHg) | 129.58 ± 10.57 | 132.40 ± 15.58 | 0.669 |
| DBP (mmHg) | 76.50 ± 6.69 | 76.20 ± 7.19 | 0.935 |
| Pulse rate (/min) | 74.33 ± 10.39 | 78.00 ± 5.91 | 0.476 |
| CRP (mg/dL) | 0.095 ± 0.100 | 0.128 ± 0.048 | 0.130 |
Data are expressed as the mean ± SD; †p-value obtained from an independent t-test, Mann Whitney U-test; *p < 0.05, **p < 0.01; HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein
Table 5.
Comparison of the delta values of the anthropometric and biochemical parameters as well as the vital signs between enterotypes 1 and 2
| Variables | Enterotype 1 (n = 12) | Enterotype 2 (n = 5) | p-value† |
|---|---|---|---|
| HOMA-IR | − 0.08 ± 1.25 | 1.13 ± 0.92 | 0.070 |
| Insulin (µIU/mL) | − 0.82 ± 3.55 | 4.02 ± 4.03 | 0.026* |
| Glucose (mg/dL) | 0.00 ± 7.30 | 3.80 ± 5.17 | 0.310 |
| TG (mg/dL) | 17.42 ± 54.54 | 10.60 ± 66.70 | 0.828 |
| TC (mg/dL) | 0.00 ± 24.56 | − 1.40 ± 25.90 | 0.921 |
| HDL (mg/dL) | − 0.50 ± 8.01 | − 0.30 ± 2.61 | 0.958 |
| LDL (mg/dL) | − 2.58 ± 22.79 | − 2.80 ± 21.37 | 0.986 |
| Body weight (kg) | 0.40 ± 1.63 | − 0.66 ± 1.70 | 0.273 |
| BMI (kg/m2) | 0.27 ± 0.43 | 0.02 ± 0.83 | 0.423 |
| WC (cm) | − 0.33 ± 1.85 | − 2.10 ± 2.33 | 0.116 |
| Fat mass (kg) | 0.47 ± 1.67 | − 1.22 ± 2.68 | 0.133 |
| Fat percentage (%) | 0.37 ± 1.59 | − 0.96 ± 2.62 | 0.213 |
| SBP (mmHg) | − 4.00 ± 11.48 | − 6.60 ± 10.06 | 0.667 |
| DBP (mmHg) | − 3.00 ± 8.71 | − 2.60 ± 3.85 | 0.721 |
| Pulse rate (/min) | 1.83 ± 9.63 | − 1.60 ± 8.91 | 0.499 |
| CRP (mg/dL) | 0.18 ± 0.34 | 0.00 ± 0.08 | 0.130 |
Data are expressed as the mean ± SD; †p-value obtained from an independent t-test, Mann Whitney U-test; *p < 0.05, **p < 0.01; HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; CRP, C-reactive protein
Certain gut microbial strains have strong links to MS markers
Fifty-nine gut microbial strains correlated with several biomarkers of MS (p < 0.05) (Fig. 5). Among them, Lachnospiraceae showed a positive correlation with HOMA-IR, BMI, and the serum levels of insulin and glucose. Acidaminococcus and Holdemania were positively correlated with the HOMA-IR and serum insulin concentration. Actinomyces demonstrated a positive correlation with the HOMA-IR, serum glucose level, and BMI. In contrast, Anaerostipes was negatively correlated with the HOMA-IR and serum insulin concentration.
Fig. 5.
Correlation between the gut microbiota and metabolic markers. Heatmap showing the correlation between the changes in the gut microbiota at the genus level and alterations in metabolic markers. The red and blue colors represent the positive and negative relationships, respectively. *p < 0.05; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rate; HOMA-IR, homeostatic model assessment-insulin resistance; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol
Discussion
This study was a single-center, randomized, double-blind, placebo-controlled clinical trial conducted to evaluate the beneficial impact of KRG on MS and determine if the gut microbial composition and enterotypes could influence these effects.
Influences of KRG administration on MS-related markers
Although the molecular mechanisms underlying MS are not fully understood, OS and inflammatory insults are actively involved in MS [13]. OS, which is imposed due to an imbalance between ROS production and biological systems’ ability to detoxify the reactive intermediates or to repair the resulting damages. This condition plays an important role in MS and MS-related diseases [13, 72]. Specifically, enhanced oxidative damage reflected by reduced α-tocopherol and vitamin C levels, decreased antioxidant protection and superoxide dismutase (SOD) activity, as well as increased malondialdehyde concentration, lipid peroxidation, protein carbonyls, and xanthine oxidase activity are strongly correlated with MS occurrence [13, 73]. Fat accumulation correlates with systemic oxidative stress in both humans and mice. The generation of ROS increases selectively in adipose tissue of obese mice, accompanied by upregulated expression of NADPH oxidase and downregulated expression of antioxidative enzymes. In contrast, in cultured adipocytes, increased levels of fatty acids trigger oxidative stress via NADPH oxidase activation, and oxidative stress causes dysregulated production of adipocytokines (fat-derived hormones), including adiponectin. The contribution of OS to MS is supported further by the fact that in obese mice, treatment with a NADPH oxidase inhibitor, which reduces ROS production in the adipose tissue, attenuated the dysregulation of adipocytokines, and improved diabetes, hyperlipidemia, and hepatic steatosis [15]. Accumulating evidence also indicates that obesity and MS are associated with a low-grade, chronic (smoldering) state of inflammation characterized by augmented circulating levels of free fatty acids, increased chemoattraction of immune cells (such as macrophages), as well as elevated concentrations of various proinflammatory cytokines including interleukin 1β (IL-1β), IL-8, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor α (TNFα), and prothrombotic mediator plasminogen activator inhibitor-1 (PAI-1), and biomarkers of inflammation (e.g. C-reactive protein) [13, 18]. Furthermore, dysregulated inflammation with impaired wound healing is a characteristic feature of diabetes [74].
Several animal and human studies have reported that ginseng and its major active components have antioxidant effects, which are mediated mainly through the activation of antioxidant enzymes, such as SOD, catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferases (GSTs), as well as the attenuation of lipid peroxidation and MDA production [17]. Accordingly, ginseng has therapeutic potential in the prevention and treatment of a wide range of diseases that are associated with oxidative stress such as types 1 and 2 diabetes, cancer, rheumatoid arthritis, heart disease, schizophrenia, Parkinson’s disease, and Alzheimer’s disease [17]. Furthermore, growing evidence suggests that the anti-obesity effects of KRG and ginsenosides are exerted via the regulation of lipid synthesis and adipocyte differentiation, which are driven by several mechanisms: downregulated expression of lipogenesis and adipogenesis factors (peroxisome proliferator-activated receptor (PPARγ), CCAAT enhancer-binding protein alpha (C/EBPα), fatty acid-binding protein 4 (FABP4), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), sterol regulatory element-binding protein 1 (SREBP-1C), stearoyl-coenzyme A desaturase 1 (SCD1), and perilipin) in adipose tissue; upregulated expression of glucose transporter type 4 (GLUT4) and PPARγ; increased phosphorylation of AMP-activated protein kinase (AMPK) in the skeletal muscle [39, 75]. On the other hand, the effects of KRG and ginsenosides against diabetes and insulin resistance were found to be associated with the following: increased phosphorylation of Akt and AMPK; decreased phosphorylation of signal transducer and activator of transcription 5 (STAT5); and increased expressions of insulin receptor (IR), glucose transporter type 1 (GLUT1), PPARγ, and lipoprotein lipase (LPL) [39, 75]. Furthermore, as stated before, MS is associated with a state of low-grade inflammation, and ginseng has been found to exert strong inhibitory effects on the key players in the inflammatory cascade, such as the p38 mitogen-activated protein kinase (p38 MAPK) pathway, nuclear factor κβ (NF-κβ), inducible NOS (iNOS), and proinflammatory cytokines such as TNFα, interleukin (IL)-1β (IL-1β), IL-6, IL-12, IL-18, and interferon-gamma (IFN-γ) as well as the inflammatory markers CRP and cyclooxygenase-2 (COX-2) [39, 76, 77].
Although the previous randomized clinical trials (RCTs) showed improvement of the glucose and lipid parameters, such as HbA1c, fasting blood glucose, and serum LDL levels in type 2 diabetic patients in response to the ginseng treatment [78, 79], the administration of the subjects with MS to KRG did not grossly elicit significant improvement in the glucose and lipid profiles in the present study. On the other hand, a significant decrease in SBP (− 5.56 ± 11.71, p = 0.026) and a slight change in DBP were noted in the subjects exposed to KRG at a dose of 6000 mg/day for 8 weeks. This agrees with a previous RCT showing a reduction in SBP in pre-hypertensive subjects in response to treatment with KRG at a dose of 5000 mg/day for 12 weeks [80]. Previous studies reported that compared to DBP, SBP is related more closely to the risk of cardiovascular disease [81] and that a 2 mm Hg decrease in SBP among middle-aged people would result in a 10% and 7% decrease in the risks of mortality from stroke and ischemic heart disease, respectively, as well as a decrease in other cardiovascular complications [82]. Based on this, it is conceivable that in the present study, the decrease in SBP following KRG administration is a beneficial outcome with clinical significance. Indeed, many ginsenosides have vasodilating effects and appeared to be potential candidates for the treatment of hypertension [83].
Reciprocal interaction of KRG with gut microbiota: potential use of KRG as prebiotics
The gut microbiota plays crucial roles in the host’s metabolic functions, such as vitamin production, amino acid biosynthesis, bile acid transformation, lipid metabolism, fermentation of non-digestible substrates, and production of short chain fatty acids (SCFAs) [84]. An imbalance in the gut microbial communities (dysbiosis) represented by lower-diversity and higher abundance of pathogenic species is associated with MS and related diseases, including obesity, insulin resistance, type 2 diabetes, hypertension, and cardiovascular disease [33–35, 85–89]. Accumulating evidence suggests the therapeutic potential of ginseng and its active constituents to improve MS, which is mediated by restoration of the gut microbial composition, amelioration of the gut barrier function, and regulation of metabolites of gut flora [90]. In the present study, the 16S rRNA sequencing data of the gut microbial analyses revealed changes in the relative abundances of several bacterial strains in response to the KRG treatment. In particular, an increase in Bacteroidetes and a decrease in Firmicutes were observed at the phylum level, but these changes were statistically insignificant. Consistent with this, LEfSe also revealed a remarkable difference in the distributional pattern of the dominant microbial species between the KRG and placebo group. Accumulating evidence suggests that a higher F/B ratio in the gut is related to the increased storage of energy by the host’s adipose tissue, which is mediated by promoting energy extraction [91]. In an earlier study, an increased gut F/B ratio in HFD-fed or ob/ob mice was reversed in response to the treatment with ginseng saponins [55]. Furthermore, in the present study, the abundance of Proteobacteria decreased significantly after KRG administration. An increase in Proteobacteria is associated with gut microbial dysbiosis and diseases with an inflammatory phenotype [92, 93]. Therefore, it is conceivable that the reduction in F/B ratio and Proteobacteria population caused by KRG would have a beneficial impact on energy and gut microbial homeostasis. In a previous clinical study, silymarin-treated NAFLD patients showed an increased abundance of Proteobacteria upon exposure to KRG, but they exhibited a declining trend in the F/B ratio [56]. The differences in the pathophysiological conditions of the subjects and treatment regimens between these two studies might account for these discrepancies. Furthermore, the outcomes of correlation analysis elucidating the relationship between the gut microbiomes and MS-related biomarkers are in parallel with previous studies. For example, high abundances of Lachnospiraceae were positively correlated with metabolic disturbances [94]. Acidaminococcus was positively linked to the parameters for glucose and insulin, while Holdemania was correlated with the clinical indicators of an impaired lipid and glucose metabolism [95]. Actinomyces was abundant in obese adolescent subjects [96]. A negative correlation was noted between Anaerostipes and the metabolic markers, such as the fasting glucose and insulin resistance index levels [97].
The gut microbes do not only exhibit changes in their abundance in response to ginseng treatment, rather, in turn, they enhance the therapeutic potential of this herb. One of our earlier clinical studies showed that the anti-obesity effects of ginseng extract on middle-aged obese Korean women differed according to the gut microbiota composition prior to ginseng intake [47]. In the host, the bioavailability of ginsenosides is low because they are hydrophilic compounds and difficult to absorb. On the other hand, the metabolism of ginsenosides by the gut microbial enzymes make these compounds more polar, which facilitates their absorption [57, 58]. In vitro models showed that various biotransformations, such as deglycosylation, hydration, esterification are the major metabolic pathways of ginsenoside driven by the gut microbiota [98, 99]. In humans, compound K (CK) is one of the leading products of the gut microbial-mediated metabolism of orally administered ginsenosides, such as Rb1, Rb2, Rc, and Rd [100]. This metabolite has more potent pharmacological effects, such as anti-inflammatory and anti-diabetic activities, compared to the parent ginsenosides [58]. Taken together, it is conceivable that the reciprocal interaction between KRG and gut microbiota plays a vital role in the beneficial effects exerted by KRG.
The term “prebiotic” was first defined in 1995 as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health” [101]. In 2016, the definition of prebiotic was modified as “a substrate that is selectively utilized by host microorganisms conferring a health benefit.” Several indigestible dietary carbohydrates, such as oligofructose, galactooligosaccharides, lactulose, and inulin (long chain β-fructan), are shown to be prebiotics because they selectively promote the growth of several beneficial gut bacteria (probiotics) and simultaneously maintain the homeostasis of the gut microbial community along with host health [102, 103]. Accordingly, it has been found that among the ginseng constituents, the polysaccharide fractions especially, sulfated acidic polysaccharides, have prebiotic-like properties, such as sustaining the balance in the gut microbial population, stimulating the growth of beneficial probiotic flora, including Lactobacillus and Bacteroides species, and enhancing the absorption of metabolites [104–107]. The treatment of hyperlipidemic rats with red ginseng acidic polysaccharide (RGAP) reduced the serum and hepatic levels of TG and increased the activity of LPL in the serum, a key hydrolytic enzyme of lipid molecules in lipoprotein [108]. Taking account of the abovementioned beneficial impact of KRG and its reciprocal interaction with the host’s gut microbiome, KRG is considered a potentially promising prebiotic candidate [60].
Patient stratification strategy for KRG use: enterotypes as potential biomarkers for MS
The differences in the intestinal microbial communities between the responder and non-responder groups were analyzed at the baseline to understand the influence of bacterial strains on the beneficial action of KRG further. The responders who had a higher abundance of Bacteroides and Ruminococaceae and a lower population of Prevotella compared to the non-responders showed marked improvement in HOMA-IR and serum insulin level after the KRG treatment. The responders who had a higher abundance of Lachnospiraceae and Clostridiales than the non-responders demonstrated decreases in the serum TC and LDL levels after the KRG treatment. These findings are in keeping with a comparative analysis of the gut microbiota in people with different degrees of ginsenoside metabolism activities. The study showed that Bacteroides, Ruminoccus, and Clostridiales were higher in the groups with fecal activity metabolizing ginsenoside Rb1 to CK [109].
The recent advances in PPPM have led to a paradigm shift from the uniform treatment strategy for a particular disease to personalized health care by considering the characteristics of each patient [61]. Over the last few years, predictive medicine has attracted considerable attention as a promising therapeutic approach to meet the need for increasing the crisis of diseases, including MS using technologies, such as personalized profiling, biomarker patterning, multiomics, big data, and machine learning [10, 11, 74, 110–112]. Emerging evidence indicates that the use of personalized data, including the gut microbial profile, is helpful to predict individual glycemic responses and to lower the post-meal blood glucose level [113]. Drugs affect the human gut microbiota, and the gut microbiota facilitates the effects of many drugs [114]. Enterotype, a concept that categorizes individuals according to their gut microbial profile, helps analyze the correlation between the gut microbiota and the overall functioning of the body. This facilitates the strategic application of precision medicine for many diseases, including MS [64, 65, 115]. The enterotypes are independent of age, gender, cultural background and geography [114]. Thus far, three enterotypes of bacterial patterns have been traditionally reported: Bacteroides-rich (enterotype 1), Prevotella-rich (enterotype 2), and Ruminococcus-rich (enterotype 3) [66]. Functional compositions and properties including coenzyme, lipid, amino acid, and carbohydrate metabolism show differences among the enterotypes owing to their different microbial profile [116]. Furthermore, in MS patients, there are several associations between species abundance of gut microbiota and metabolic parameters that are enterotype-specific [115]. Because the microbial enterotypes have different digestion capacities and substrate preferences, individuals with different enterotypes would respond differently to specific diets and medications [114]. Indeed, many clinical studies confirmed differential metabolic or pharmacological responses of the subjects to diets, dietary products or dietary supplements, probiotics or drugs correlating with specific gut enterotypes [65, 114, 117].
In enterotype analyses, the anthropometric parameters, such as BMI, WC, and fat mass, in the KRG group were significantly lower in enterotype 1 (Bacteroides-rich) than enterotype 2 (Prevotella-rich) at the baseline, which is in agreement with a previous study showing a significantly lower basal body weight and BMI in the low Prevotella-to Bacteroides (P/B) group compared to the high P/B group [118]. Furthermore, in the present study, enterotype 1 responded more effectively in improving the serum insulin level to KRG than enterotype 2. Bacteroides metabolizes polysaccharides and oligosaccharides and provides nutrition and vitamins to the host and other intestinal microbial communities; hence, they positively impact human health [119]. Accumulating evidence suggests that Bacteroides is negatively associated with type 2 diabetes. It has been found that this bacterial genus plays a beneficial role in the glucose metabolism in humans and experimental animals by improving glucose intolerance and insulin resistance [120]. Emerging evidence also suggests that Bacteroides is involved in the metabolism of ginsenosides through deglycosylation, oxygenation, and hydrolysis [57, 121]. Rc, Rg3, Re, and Rb1 are potentially transformed into CK, and protopanaxadiol, Rh1, Rh2, and F1 by the action of enzymes of Bacteroides [109, 122–124]. Therefore, the greater effectiveness of KRG in the glucose metabolism in enterotype 1 than enterotype 2 is due to the improved pharmacokinetic properties of ginsenosides contributed mainly by Bacteroides.
Limitations
This study had some limitations. First, the factors that can modulate the intestinal floral communities, such as diet [125], sleep [126], and exercise [127], were not controlled completely. Second, because MS is a cluster of diseases, including hyperlipidemia, hypertension, and type 2 diabetes, various classes of drugs have already been taken by a large number of participants of the KRG (15 out of 30) and placebo groups (16 out of 30) to combat those conditions. This might mask the effects of KRG because many of the metabolic parameters are improved by these background medications in both groups. Furthermore, these background medications might also interact with KRG. Third, both enterotypes 1 and 2 encompassed only a small number of participants. Therefore, the statistical power might not be sufficient to validate the outcomes of enterotype analyses. Fourth, the ginsenoside metabolites and short chain fatty acids were not measured to analyze the effects of the gut microbiota, and the phenotype biomarkers, such as visceral fat, were not estimated. It has been found that the excessive visceral fat accumulation is associated with insulin resistance and multiple risk factors for MS [128, 129]. Earlier studies reported that visceral fat area (VFA) was superior to BMI and waist circumference to predict MS and consequently, various cut-off values of VFA have been developed according to race, gender, and age [130–133]. Furthermore, it has been shown that VFA could be an independent phenotype marker of MS in type 2 diabetic patients [134]. Based on the abovementioned points, we opined that after the KRG treatment, the trial should be continued with routine analyses of the parameters and markers until the gut microbial profile is restored to its initial condition. Therefore, a further follow-up trial without the abovementioned limitations should be carried out in the future.
Conclusion and expert recommendations
To the best of our knowledge, this is the first randomized, double-blind, placebo-controlled clinical trial evaluating the effects of KRG on patients with MS, especially from the context of PPPM. The results showed that the administration of KRG capsules at a dose of 6000 mg/day for 8 weeks elicited clinically meaningful improvement in SBP. Gut microbial analysis revealed a decrease in Firmicutes and Proteobacteria, and an increase in Bacteroidetes due to the abovementioned treatment. Moreover, the patients’ response to KRG was influenced by the gut microbial composition. In patient stratification analysis, the responders who had a higher abundance of Lachnospiraceae and Clostridiales than non-responders showed decreases in serum TC and LDL levels. Further differentiation of the subjects into two enterotypes showed that Bacteroides might play a role in the beneficial impacts of KRG on the parameters related to the glucose metabolism, particularly the HOMA-IR and serum insulin level.
In this study, a reciprocal interaction between the gut microbiota and KRG was observed. Taking account into the abovementioned interactions and the beneficial impact of KRG on the host, ginseng is thought to be a potentially promising prebiotics candidate. Additionally, the findings on the impact of KRG on blood glucose parameters of the participants with MS can be interpreted from the perspective of personalized medicine that the effects of KRG on the glucose metabolism vary depending on the enterotypes. Therefore, it is conceivable that having information on the enterotype in advance will be helpful in predicting the effectiveness of KRG on glucose homeostasis of MS patients at an individual level. This will also help to establish a targeted treatment strategy for MS, in compliance with the concept of PPPM.
Author contribution
E Seong contributed to manuscript writing and data analysis; S Bose, SY Han, and M Lee contributed to the manuscript review; E Song and Y Nam contributed to the microbial analysis; and H Kim contributed the overall research design and progress.
Funding
This study was supported by the Korea Ginseng Corporation (Seoul, Republic of Korea) and by the Main Research Program (E0170601-04) of the Korea Food Research Institute funded by the Ministry of Science and ICT. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and material
Data can be provided upon request from the journal.
Code availability
Related information was provided in the manuscript.
Declarations
Ethics approval and consent to participate
This trial was performed at Dongguk University-Ilsan Oriental Medical Hospital (Ilsan, Gyeonggi-do, Republic of Korea) from February 27, 2019, to February 21, 2020. The Institutional Review Board of the Hospital approved this study (approval number: DUIOH 2018–12-004). The study protocol was also registered in the Clinical Research Information Service online registration system (registration number: KCT0004823). It was conducted according to the principles of the Declaration of Helsinki. Voluntary written consent was obtained after providing a detailed explanation regarding the research to the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young-Do Nam, Email: youngdo98@kfri.re.kr.
Hojun Kim, Email: kimklar@dongguk.ac.kr.
References
- 1.Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Mendrick DL, Diehl AM, Topor LS, Dietert RR, Will Y, La Merrill MA, et al. Metabolic syndrome and associated diseases: from the bench to the clinic. Toxicol Sci. 2018;162(1):36–42. doi: 10.1093/toxsci/kfx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce KD, Byrne CD. The metabolic syndrome: common origins of a multifactorial disorder. Postgrad Med J. 2009;85(1009):614–621. doi: 10.1136/pgmj.2008.078014. [DOI] [PubMed] [Google Scholar]
- 4.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engin A. The definition and prevalence of obesity and metabolic syndrome. In: Engin A, Engin A, editors. Obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:1–17. 10.1007/978-3-319-48382-5_1. [DOI] [PubMed]
- 6.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health. 2017;17(1):101. doi: 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity and overweight. In: Fact sheet. WHO. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 18 Mar 2021.
- 9.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9(2):125–131. doi: 10.1007/s13167-018-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Gong M, Xie S, Zhang M, Zheng H, Zhao X, et al. Nomogram prediction for the 3-year risk of type 2 diabetes in healthy mainland China residents. EPMA J. 2019;10(3):227–237. doi: 10.1007/s13167-019-00181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Cardiovascular diseases (CVDs). In: Fact sheet. WHO. 2017. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 18 Mar 2021.
- 13.Alicka M, Marycz K. The effect of chronic inflammation and oxidative and endoplasmic reticulum stress in the course of metabolic syndrome and its therapy. Stem Cells Int. 2018;2018:4274361. doi: 10.1155/2018/4274361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-García JC, Cardona F, Tinahones FJ. Inflammation, oxidative stress and metabolic syndrome: dietary modulation. Curr Vasc Pharmacol. 2013;11(6):906–919. doi: 10.2174/15701611113116660175. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SK, Hyun SH, In G, Park C-K, Kwak Y-S, Jang Y-J, et al. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: a systemic review through in vivo and clinical trials. J Ginseng Res. 2020 doi: 10.1016/j.jgr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271(1):82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benef Microbes. 2017;8(4):521–533. doi: 10.3920/BM2016.0222. [DOI] [PubMed] [Google Scholar]
- 20.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 22.Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 24.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012;7(1):91–109. doi: 10.2217/fmb.11.142. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 27.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587(Pt 17):4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535(7610):65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 29.Hughes RL, Marco ML, Hughes JP, Keim NL, Kable ME. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models-part I: overview of current methods. Adv Nutr. 2019;10(6):953–978. doi: 10.1093/advances/nmz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes RL, Kable ME, Marco M, Keim NL. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models. Part II results. Adv Nutr. 2019;10(6):979–998. doi: 10.1093/advances/nmz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JH, Bose S, Kim HG, Han KS, Kim H. Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci Rep. 2015;5:8391. doi: 10.1038/srep08391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad AF, Dwivedi G, O'Gara F, Caparros-Martin J, Ward NC. The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiol Heart Circ Physiol. 2019;317(5):H923–H938. doi: 10.1152/ajpheart.00376.2019. [DOI] [PubMed] [Google Scholar]
- 36.Lyu M, Wang YF, Fan GW, Wang XY, Xu SY, Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microbiol. 2017;8:2146. doi: 10.3389/fmicb.2017.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39(26):4925–4943. doi: 10.1038/s41388-020-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bubnov R, Babenko L, Lazarenko L, Kryvtsova M, Shcherbakov O, Zholobak N, et al. Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J. 2019;10(4):317–335. doi: 10.1007/s13167-019-00190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon SJ, Kim SK, Lee NY, Choi YR, Kim HS, Gupta H, et al. Effect of Korean red ginseng on metabolic syndrome. J Ginseng Res. 2020 doi: 10.1016/j.jgr.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22(7):851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Rhee DK. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res. 2017;41(4):589–594. doi: 10.1016/j.jgr.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun SN, Ko SK, Lee KH, Chung SH. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch Pharm Res. 2007;30(5):587. doi: 10.1007/BF02977653. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Luo J, Anandh Babu PV, Zhang W, Gilbert E, Cline M, et al. Dietary supplementation of Chinese ginseng prevents obesity and metabolic syndrome in high-fat diet-fed mice. J Med Food. 2014;17(12):1287–1297. doi: 10.1089/jmf.2014.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kho MC, Lee YJ, Park JH, Kim HY, Yoon JJ, Ahn YM, et al. Fermented red ginseng potentiates improvement of metabolic dysfunction in metabolic syndrome rat models. Nutrients. 2016;8(6):369. doi: 10.3390/nu8060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh RK, Lui E, Wright D, Taylor A, Bakovic M. Alcohol extract of North American ginseng (Panax quinquefolius) reduces fatty liver, dyslipidemia, and other complications of metabolic syndrome in a mouse model. Can J Physiol Pharmacol. 2017;95(9):1046–1057. doi: 10.1139/cjpp-2016-0510. [DOI] [PubMed] [Google Scholar]
- 46.Kwon DH, Bose S, Song MY, Lee MJ, Lim CY, Kwon BS, et al. Efficacy of Korean red ginseng by single nucleotide polymorphism in obese women: randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2012;36(2):176–189. doi: 10.5142/jgr.2012.36.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song M-Y, Kim B-S, Kim H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J Ginseng Res. 2014;38(2):106–115. doi: 10.1016/j.jgr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim S-H, Park K-S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48(5):511–513. doi: 10.1016/S1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 49.Vuksan V, Sung M-K, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18(1):46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res. 2020;161:105263. doi: 10.1016/j.phrs.2020.105263. [DOI] [PubMed] [Google Scholar]
- 51.Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res. 1998;10(1):37–43. doi: 10.1038/sj.ijir.3900300. [DOI] [PubMed] [Google Scholar]
- 52.Kim JH. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han KS, Balan P, Hong HD, Choi WI, Cho CW, Lee YC, et al. Korean ginseng modulates the ileal microbiota and mucin gene expression in the growing rat. Food Funct. 2014;5(7):1506–1512. doi: 10.1039/c4fo00087k. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Chen S, Wei R, Xie X, Wang C, Fan S, et al. Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 2018;9(6):3547–3556. doi: 10.1039/C8FO00025E. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Wang N, Tan HY, Li S, Zhang C, Zhang Z, et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–11323. doi: 10.7150/thno.47746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong JT, Lee M-J, Yoon SJ, Shin SP, Bang CS, Baik GH, et al. Effect of Korea red ginseng on nonalcoholic fatty liver disease: an association of gut microbiota with liver function. J Ginseng Res. 2020 doi: 10.1016/j.jgr.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Zou H, Gao Y, Luo J, Xie X, Meng W, et al. Insights into gastrointestinal microbiota-generated ginsenoside metabolites and their bioactivities. Drug Metab Rev. 2020;52(1):125–138. doi: 10.1080/03602532.2020.1714645. [DOI] [PubMed] [Google Scholar]
- 58.Kim D-H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42(3):255–263. doi: 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev. 2017;14(8):491. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 60.Xiao-Meng W, Xiao-Bo L, Ying P. Impact of Qi-invigorating traditional Chinese medicines on intestinal flora: a basis for rational choice of prebiotics. Chin J Nat Med. 2017;15(4):241–254. doi: 10.1016/S1875-5364(17)30041-9. [DOI] [PubMed] [Google Scholar]
- 61.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation-EPMA position paper 2016. EPMA J. 2016;7(1):1–13. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao S, Zhao L. Gut microbiota-based translational biomarkers to prevent metabolic syndrome via nutritional modulation. FEMS Microbiol Ecol. 2014;87(2):303–314. doi: 10.1111/1574-6941.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daliri EB-M, Ofosu FK, Chelliah R, Lee BH, Oh D-H. Challenges and perspective in integrated multi-omics in gut microbiota studies. Biomolecules. 2021;11(2):300. doi: 10.3390/biom11020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song E-J, Han K, Lim T-J, Lim S, Chung M-J, Nam MH, et al. Effect of probiotics on obesity-related markers per enterotype: a double-blind, placebo-controlled, randomized clinical trial. EPMA J. 2020;11(1):31–51. doi: 10.1007/s13167-020-00198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng M, Ning K. Stereotypes about enterotype: the old and new ideas. Genom Proteomics Bioinform. 2019;17(1):4–12. doi: 10.1016/j.gpb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HS, Sung SW, Ou SW, Lee KY, Kim BS, Han JH, et al. Development of Korean version of obesity-related quality of life scale. Korean J Obes. 2003;12(4):280–293. [Google Scholar]
- 68.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 69.Kaufman L, Rousseeuw PJ. Clustering by means of medoids. Statistical Data Analysis based on the L1 Norm. Y Dodge, Editor 1987:405–16.
- 70.Caliński T, Harabasz J. A dendrite method for cluster analysis. Commun Stat Theory Methods. 1974;3(1):1–27. doi: 10.1080/03610927408827101. [DOI] [Google Scholar]
- 71.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. 2014;2014:908539. doi: 10.1155/2014/908539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. J Nutr Biochem. 2008;19(8):491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Maturo MG, Soligo M, Gibson G, Manni L, Nardini C. The greater inflammatory pathway—high clinical potential by innovative predictive, preventive, and personalized medical approach. EPMA J. 2020;11(1):1–16. doi: 10.1007/s13167-019-00195-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H, Kong G, Tran Q, Kim C, Park J, Park J. Relationship between ginsenoside Rg3 and metabolic syndrome. Front Pharmacol. 2020;11:130. doi: 10.3389/fphar.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 Suppl):183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 77.Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16(4):2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vuksan V, Xu ZZ, Jovanovski E, Jenkins AL, Beljan-Zdravkovic U, Sievenpiper JL, et al. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial. Eur J Nutr. 2019;58(3):1237–1245. doi: 10.1007/s00394-018-1642-0. [DOI] [PubMed] [Google Scholar]
- 79.Jovanovski E, Lea-Duvnjak-Smircic KA, Au-Yeung F, Zurbau A, Jenkins AL, et al. Vascular effects of combined enriched Korean Red ginseng (Panax Ginseng) and American ginseng (Panax Quinquefolius) administration in individuals with hypertension and type 2 diabetes: a randomized controlled trial. Complement Ther Med. 2020;49:102338. doi: 10.1016/j.ctim.2020.102338. [DOI] [PubMed] [Google Scholar]
- 80.Cha TW, Kim M, Kim M, Chae JS, Lee JH. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA 2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens Res. 2016;39(6):449–456. doi: 10.1038/hr.2016.7. [DOI] [PubMed] [Google Scholar]
- 81.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med. 1993;153(5):598–615. doi: 10.1001/archinte.1993.00410050036006. [DOI] [PubMed] [Google Scholar]
- 82.Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/S0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 83.Karmazyn M, Gan XT. Chemical components of ginseng, their biotransformation products and their potential as treatment of hypertension. Mol Cell Biochem. 2020:1–15. 10.1007/s11010-020-03910-8. [DOI] [PubMed]
- 84.Pascale A, Marchesi N, Marelli C, Coppola A, Luzi L, Govoni S, et al. Microbiota and metabolic diseases. Endocrine. 2018;61(3):357–371. doi: 10.1007/s12020-018-1605-5. [DOI] [PubMed] [Google Scholar]
- 85.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26(1):26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):1–19. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. J Am Heart Assoc. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Watanabe K, Kimura I. Gut microbiota dysbiosis drives and implies novel therapeutic strategies for diabetes mellitus and related metabolic diseases. Front Immunol. 2017;8:1882. doi: 10.3389/fimmu.2017.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo Z, Xu W, Zhang Y, Di L, Shan J. A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota. Pharmacol Res. 2020:105088. 10.1016/j.phrs.2020.105088. [DOI] [PubMed]
- 91.Kim B, Choi H-N, Yim J-E. Effect of diet on the gut microbiota associated with obesity. J Obes Metab Syndr. 2019;28(4):216. doi: 10.7570/jomes.2019.28.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin N-R, Whon TW, Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33(9):496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8(4):573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes. 2017;8(4):545–556. doi: 10.3920/BM2016.0184. [DOI] [PubMed] [Google Scholar]
- 96.Del Chierico F, Abbatini F, Russo A, Quagliariello A, Reddel S, Capoccia D, et al. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front Microbiol. 2018;9:1210. doi: 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ren S, Mei L, Huang H, Cao S, Zhao R, Zheng P. Correlation analysis of gut microbiota and biochemical indexes in patients with non-alcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2019;27(5):369–375. doi: 10.3760/cma.j.issn.1007-3418.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Kong H, Wang M, Venema K, Maathuis A, van der Heijden R, van der Greef J, et al. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid chromatography–high resolution Fourier transform ion cyclotron resonance mass spectrometry. J Chromatogr A. 2009;1216(11):2195–2203. doi: 10.1016/j.chroma.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 99.Wang H-Y, Hua H-Y, Liu X-Y, Liu J-H, Yu B-Y. In vitro biotransformation of red ginseng extract by human intestinal microflora: metabolites identification and metabolic profile elucidation using LC–Q-TOF/MS. J Pharm Biomed Anal. 2014;98:296–306. doi: 10.1016/j.jpba.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 100.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31(8):1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 101.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 102.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 104.Zhou S-S, Xu J, Zhu H, Wu J, Xu J-D, Yan R, et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep. 2016;6(1):1–13. doi: 10.1038/srep22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen H, Gao X-J, Li T, Jing W-H, Han B-L, Jia Y-M, et al. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J Ethnopharmacol. 2018;216:47–56. doi: 10.1016/j.jep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Qi Y, Chen L, Qu D, Li Z, Gao K, et al. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol Macromol. 2019;124:931–937. doi: 10.1016/j.ijbiomac.2018.11.271. [DOI] [PubMed] [Google Scholar]
- 107.Shalaby ASG, Ragab TIM, Mehany ABM, Helal MMI, Helmy WA. Antitumor and prebiotic activities of novel sulfated acidic polysaccharide from ginseng. Biocatal Agric Biotechnol. 2018;14:402–409. doi: 10.1016/j.bcab.2018.04.008. [DOI] [Google Scholar]
- 108.Kwak YS, Kyung JS, Kim JS, Cho JY, Rhee MH. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33(3):468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 109.Kim KA, Jung IH, Park SH, Ahn YT, Huh CS, Kim DH. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE. 2013;8(4):e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Worachartcheewan A, Shoombuatong W, Pidetcha P, Nopnithipat W, Prachayasittikul V, Nantasenamat C. Predicting metabolic syndrome using the random forest method. Sci World J. 2015;2015:581501. doi: 10.1155/2015/581501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crigna AT, Samec M, Koklesova L, Liskova A, Giordano FA, Kubatka P, et al. Cell-free nucleic acid patterns in disease prediction and monitoring—hype or hope? EPMA J. 2020:1–25. 10.1007/s13167-020-00226-x. [DOI] [PMC free article] [PubMed]
- 112.Koklesova L, Liskova A, Samec M, Qaradakhi T, Zulli A, Smejkal K, et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020;11:261–287. doi: 10.1007/s13167-020-00210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Christensen L, Roager HM, Astrup A, Hjorth MF. Microbial enterotypes in personalized nutrition and obesity management. Am J Clin Nutr. 2018;108(4):645–651. doi: 10.1093/ajcn/nqy175. [DOI] [PubMed] [Google Scholar]
- 115.Wutthi-In M, Cheevadhanarak S, Yasom S, Kerdphoo S, Thiennimitr P, Phrommintikul A, et al. Gut microbiota profiles of treated metabolic syndrome patients and their relationship with metabolic health. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-67078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3(1):8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, et al. Healthy Subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab. 2016;101(12):4681–4689. doi: 10.1210/jc.2016-2786. [DOI] [PubMed] [Google Scholar]
- 118.Hjorth MF, Blædel T, Bendtsen LQ, Lorenzen JK, Holm JB, Kiilerich P, et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int J Obes (Lond) 2019;43(1):149–157. doi: 10.1038/s41366-018-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zafar H, Saier MH., Jr Gut Bacteroides species in health and disease. Gut Microbes. 2021;13(1):1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santangelo R, Silvestrini A, Mancuso C. Ginsenosides, catechins, quercetin and gut microbiota: current evidence of challenging interactions. Food Chem Toxicol. 2019;123:42–49. doi: 10.1016/j.fct.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 122.Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25(6):743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 123.Bae EA, Han MJ, Choo MK, Park SY, Kim DH. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25(1):58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 124.Bae EA, Shin JE, Kim DH. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28(10):1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 125.Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- 127.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 128.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113(11):1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629–639. doi: 10.5551/jat.7922. [DOI] [PubMed] [Google Scholar]
- 130.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36(1):54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 131.Kanai H, Matsuzawa Y, Kotani K, Keno Y, Kobatake T, Nagai Y, et al. Close correlation of intra-abdominal fat accumulation to hypertension in obese women. Hypertension. 1990;16(5):484–490. doi: 10.1161/01.hyp.16.5.484. [DOI] [PubMed] [Google Scholar]
- 132.Després JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38(3):304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 133.Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, et al. Insulin resistance and body fat distribution. Diabetes Care. 1996;19(3):287–291. doi: 10.2337/diacare.19.3.287. [DOI] [PubMed] [Google Scholar]
- 134.Yang X, Lin Y, Xu GD, Chen YS, Zhou Y, Sun J, et al. Optimal cut-off values of visceral fat area for predicting metabolic syndrome among type 2 diabetes patients in Ningbo, China. Diabetes Metab Syndr Obes. 2021;14:1375–1383. doi: 10.2147/DMSO.S304164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be provided upon request from the journal.
Related information was provided in the manuscript.