Abstract
Salmonella pullorum is a highly pathogenic bacteria in poultry industry. However, antibiotics were restricted in many countries because of the increasing risk of antibiotic resistance, Therefore, an environmental friendly and effective alternative strives to be developed. This study investigated the benefit of a probiotic-fermented herbal blend on the growth performance and gut microbiota of newborn broilers infected with S. pullorum. A total of 120 one-day-old dwarf male chicks were randomly allotted to 4 treatment groups, each including 5 replicates of 6 chicks: negative control (NC), positive control (PC), herbal blend (HB), and probiotic-fermented herbal blend (PF). All birds (n = 90), except for those in the NC, were infected with S. pullorum (1.69 × 108 CFU) on day 1. On day 11, body weight (BW), mortality, tissue pathology, cecal colony counts, immune organ indices, cecal mucosa secretory immunoglobulin A (sIgA) concentrations, and cecal cytokine mRNA expression levels were investigated. No mortality was observed after the PF treatment, and less pathological condition was in the ileum, cecum, and liver of HB and PF. BW, average daily gain and average daily feed intake were significant higher in the HB group compared to the PC and were the highest in the PF (P < 0.05). HB treatment significantly increased cecal populations of Lactobacilli, and decreased cecal populations of Escherichia coli and Salmonella, but results were more pronounced in the PF group (P < 0.05). Both HB and PF treatments increased cecal mucosa sIgA compared with the PC (P < 0.05). Tumor necrosis factor alpha and interferon gamma were lowest (P < 0.05) and interleukin 4 was the highest (P < 0.05) in PF, which exhibited similar levels to the NC group. PF treatment significantly improved the development of the thymus and bursa in S. pullorum-infected chicks. In conclusion, PF treatment prevented death, improved growth performance, regulated intestinal flora and enhanced immune ability of in S. pullorum-infected with chicks.
Key words: herb, chick, Salmonella, intestinal flora, immunity
Introduction
Pullorum disease (PD) is an avian-specific septicemic disease caused by Salmonella pullorum and leads to massive economic losses in the poultry industry. During the infection, S. pullorum were taken up and disseminated by macrophages to the intestinal mucosa, bursal follicles, and liver. The macrophages help the pathogen avoid humoral response-mediated clearance, resulting in prolonged colonization in chickens (Henderson et al., 1999; Wigley et al., 2002; Guo et al, 2019). Chickens infected with PD suffer from chronic or recessive disease, which leads to continuous horizontal and vertical transmission through transportation of eggs. Although many countries are reportedly free of PD, it is still a threat because of wild avian species and inefficient environmental control for the free-range reared chickens, especially in developing countries, such as Brazil, Argentina, and China (Barrow and Freitas Neto, 2011; Revolledo, 2018).
Antibiotics treatment is an effective method to prevent and treat PD. However, the widespread use of antibiotics has also raised concerns about increased antibiotic resistance in microorganisms. Many countries around the world have legislated limits on the use of antibiotics in poultry production (Gong et al., 2014; Lettini et al., 2016). Persistent infections in chickens and public concerns around antibiotic resistance necessitate the development of an environmentally friendly feed supplement that strengthens the defense mechanisms of chickens against PD, such as natural immunostimulants including probiotics and Chinese herbal medicines.
Herbs such as Astragalus, Panax notoginseng, licorice and chickpeas have been popular as traditional medicine and health food in China for over 1,000 years and are known to protect the intestinal mucosa, improve energy metabolism, reduce pathological damage to organs, promote gastrointestinal digestion and absorption and enhance growth and immunity (Qiu et al., 2007; Wu, 2018a; Rashidi et al., 2020). However, when added to feed, the cell wall of herbs interferes the release of the functional compounds, such as polysaccharides, saponins, flavonoids, isoflavones, coumarins, alkaloids and other ingredients, and lowers the efficacy of the herbs (Zhang et al., 2018; Wang et al., 2019a). Moreover, adding functional compounds directly to feed increases the costs and exceeds animal tolerance.
Fermenting herbs with probiotics increase organism tolerability and improves the activity and extraction yield of functional components (Liu et al., 2017). Probiotic-fermented herbal blend (PF), as prebiotics, can promote the growth and reproduction of probiotics and enhance their ability to colonize the intestine (Wang et al., 2017). Probiotics, particularly Lactobacillus species, have also been shown to be an effective way to inhibit colonization of invading pathogens, balance the intestinal microflora and stimulate the immune response (Rolfe, 2000; De Vrese and Schrezenmeir, 2008; Sharifi et al., 2012). Early intake of Lactobacillus-containing probiotics was shown to be effective in the prevention of PD. However, the effects were not great enough to protect chicks after infection (Chen et al., 2020). As an external antigen, PF activate the immune system by improving macrophage activity or antibody levels (Wang et al., 2019b). It was reported that PF improved ADG, immune organ indices and immunoglobulin secretion of Silkie chickens (Yang, 2015). There was also evidence that probiotic-fermented herbs reduce necrosis of liver cells, the activity of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and the level of total bilirubin in the liver (Tian et al., 2017). A study also showed that PF combined with Bacillus subtilis promoted the growth of anaerobic bacteria (such as Bifidobacterium, Lactobacillus, etc.), inhibited the growth of Escherichia coli and enhanced the humoral immune functioning (Xie et al., 2016). In past studies, PF have often been used in the aquaculture and livestock industries in the form of feed supplements to improve the production performance, but there are few reports their use in the treatment of avian diseases (Wang et al., 2017; Lei et al.,2018; Abarike et al., 2020).
This study investigated the effects of a traditional Chinese herb blend including Astragalus, P. notoginseng, licorice, chickpeas, and black beans fermented by Lactobacillus plantarum subsp. plantarum Zhang-LL and Lactobacillus paracasei KL1 on the growth performance, intestinal microflora and immune function of chicks infected with S. pullorum. The aim of this study is to provide an evidence for this PF as a new type of feed additive for the poultry production industry as well as a new strategy for treating PD.
Materials and Methods
Preparation of the Probiotic-Fermented Herbal Blend
The PF was prepared according to previous research (Li et al., 2018). Herbal blend powder (75% wt/wt) containing 40% (wt/wt) Astragalus, 30% (wt/wt) P. notoginseng, 10% (wt/wt) licorice and 20% (wt/wt) chickpeas (Suzhou Kemu Animal Medicine Co., Ltd., Suzhou, China) was mixed with 16.7% (wt/wt) black bean powder and 8.3% (wt/wt) glucose and dissolved in distilled water to a final concentration of 10% (wt/vol). The probiotics L. paracasei KL1 (CGMCC no.11533; 107 CFU/mL) and L. plantarum Zhang-LL (CGMCC no.6936; 107 CFU/mL) were cultured in de Man Rogosa and Sharpe (MRS) broth media at 37°C for 12 h and used as the inoculum for fermentation for 72 h at 34°C. Viable counts of L. paracasei KL1and L. plantarum Zhang-LL were determined on MRS agar plates (Liu et al., 2016).
Animals
A total of 120 one-day-old male Nongda No. 3 Dwarf chicks were obtained from the Animal Genetics and Breeding Laboratory, College of Animal Science and Technology (China Agricultural University, Beijing, China). All animal treatments, housing and feeding were in accordance with the General Rules for Animal Welfare Evaluation and the International Cooperation Committee of Animal Welfare (Beijing, China). All experimental procedures adhered to the institutional criteria for the care and use of laboratory animals. The chicks were raised in separate silos at a controlled temperature of 36°C under fluorescent light for 24 h, with light reduced by 1 hour per day from day 1 to day 10, and given ad libitum access to antibiotic-free feed and water throughout the experiment (Chen et al., 2020). The basal diet was formulated according to National Research Council (1994) standards (Table 1).
Table 1.
Ingredient and nutrient composition of basal diet (as fed).
| Item | Composition |
|---|---|
| Ingredient (%) | |
| Corn | 61.03 |
| Soybean meal | 30.80 |
| Soybean oil | 2.50 |
| Fish meal | 1.50 |
| CaHPO4 | 1.40 |
| Limestone | 1.40 |
| NaCl | 0.37 |
| Premix1 | 1.00 |
| Total | 100.00 |
| Calculated nutrient levels (%) | |
| Crude protein | 19.00 |
| Calcium | 0.90 |
| Available phosphorus | 0.35 |
| Lysine | 0.85 |
| Methionine | 0.40 |
| Methionine + cysteine | 0.65 |
| Metabolizable energy (MJ kg−1) | 12.40 |
The premix provided the following per kg of diet: vitamin A, 9500 IU; vitamin B1, 1.5 mg; vitamin B2, 9.0 mg; vitamin B6, 3.0 mg; vitamin B12, 0.02 mg; vitamin D3, 2,375 IU; vitamin E, 19 IU; vitamin K3, 1.40 mg; biotin, 0.95 mg; folic acid, 0.93 mg; D-pantothenic acid, 9.3 mg; Cu (as copper sulfate), 15 mg; Fe (as ferrous sulfate), 60 mg; Mn (as manganese sulfate), 100 mg, Zn (as zinc sulfate), 70 mg; I (as potassium iodide), 0.50 mg; Se (as sodium selenite), 0.59 mg.
Experimental Infection with S. pullorum
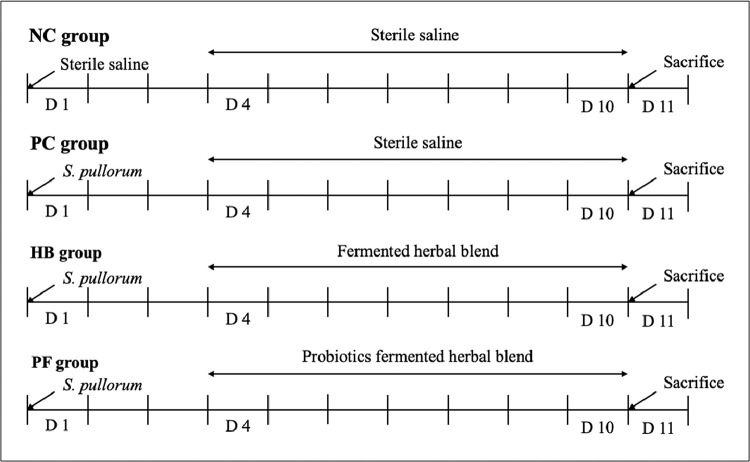
The S. pullorum (CVCC no. 533) strain was purchased from the China Veterinary Microbial Strain Preservation and Management Center (Beijing, China), activated in Luria-Bertani broth for 3 generations, centrifuged (3,500 × g, 15 min, 4°C), washed twice with PBS buffer (pH 7.2) and resuspended in sterile normal saline. A total of 120 male chicks were randomly divided into 4 treatment groups: negative control group (NC), positive control group (PC), herbal blend (HB) group and probiotic-fermented herbal blend (PF) group. Each treatment group included 5 replicates with 6 chicks in each replicate. On day 1, chicks in the PC, HB and PF groups were orally administered 0.2 mL per chick of a solution containing S. pullorum (8.45 × 108 CFU/mL; Geng et al., 2014). Chicks in the NC group were orally administered an equal amount of sterile normal saline. From days 4 to 10, chicks in the HB and PF groups were orally administered 0.2 mL per chick of a solution either containing the PF (1.20 × 109 CFU/mL) or HB, respectively (Figure 1).
Figure 1.
A total of 120 newborn Nongda No. 3 Dwarf chicks were randomly assigned to 4 treatments: negative control group (NC), positive control group (PC) herbal blend group (HB) or probiotic-fermented herbal blend group (PF). Each group included 5 replicates with 6 chicks in each replicate. Salmonella pullorum (CVCC no. 533; 8.45 × 108 CFU/mL) was orally administered to chicks in the PC, HB and PF groups at a total volume of 0.2 mL per chick. The NC group was orally administered an equal amount of sterile normal saline. From days 4 to 10, chicks in the HB and PF groups were orally administered 0.2 mL of a solution containing either the herbal blend or the probiotic-fermented herbal blend (1.20 × 109 CFU/mL), respectively.
Clinical Symptoms and Growth Performance
During the experiment, the BW and feed intake of chicks in each group was determined on an empty stomach at 07:00 h, and clinical signs and mortality were observed every day. The mortality rate, ADG, ADFI, and feed conversion radio (FCR) were calculated.
Sample Collection
On day 11, 5 chicks from each treatment group (one chick from each replicate) were randomly selected and euthanized by cervical dislocation (Zhang et al., 2012; Wu et al., 2018b) The thymus, spleen and bursa were separated and weighed. The body of each chick was also weighed to determine relative organ weights. 1 g of cecal content was weighed and diluted with sterile saline. Approximately of 1 cm fresh cecal sample was taken, flash frozen with liquid nitrogen in a sterile centrifuge tube and stored at −80°C for analysis of cecal secretory immunoglobulin A (sIgA) concentrations and cytokine mRNA expression levels analysis.
Histological Analysis
Liver, ileum and cecum tissue samples were fixed in 10% neutral buffered formalin solution, routinely embedded in paraffin, cut into 5 mm-thick sections and processed for hematoxylin and eosin staining (Meng, 2004). Sections of the liver, ileum and cecum of the chicks were microscopically examined and photographed (CX21, Olympus Optical Co., Ltd., Tokyo, Japan).
Colony Counts of Lactobacillus, E. coli, and Salmonella
Cecal contents were serially diluted from 10−2 to 10−8, and each diluted sample was inoculated in eosin methylene blue agar, modified MRS agar and bismuth sulfite agar (Beijing Road Bridge Technology Co., Ltd., Beijing, China) at 37°C for 18 to 24 h to enumerate colonies of E. coli, Lactobacillus and Salmonella populations, respectively.
Measurement of Cecal sIgA Concentrations and Relative Cytokine mRNA Expression Levels
Cecal sIgA concentrations were determined using a Chicken sIgA ELISA Kit (JYM0012 Elisa Lab Co. Ltd., Wuhan, China) according to the manufacturer's instructions. Total RNA was extracted from cecal samples using an Ultrapure RNA Extraction Kit (CWbio Co., Ltd., Beijing, China) following the manufacturer's protocol. RNA quality was estimated by electrophoresis on 1% (wt/wt) agarose gels stained with ethidium bromide. The first cDNA synthesis reaction was performed with samples obtained by reverse transcription of total RNA using a HiFi-MMLVcDNA First Chain Synthesis Kit (CWbio Co., Ltd.). The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the amount of initial RNA in each sample. Real-time qPCR to measure cecal gene expression was carried out using the UltraSYBR Mixture (with ROX) real-time qPCR detection system (CWbio Co., Ltd) under the following conditions: 95°C for 10 min, 45 cycles of 95°C for 15 s, 60°C for 60 s. The primers for the target genes tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ) and interleukin 4 (IL4) and the internal gene (GAPDH) were designed by Genenode Co., Ltd. (Wuhan, China) and are shown in Table 2. The amplification efficiency of each gene was validated by constructing a standard curve with serial dilutions of cDNA. Relative quantitative analysis of the data was performed using the 2−△△CT method.
Table 2.
Sequences of primers used in this study.
| Gene | Forward sequence (5′–3′) | Reverse sequence (5′–3′) | Size/bp | Accession No. |
|---|---|---|---|---|
| GAPDH | AACTTTGGCATTGTGGAGGG | ACGCTGGGATGATGTTCTGG | 130 | NM_204305.1 |
| IFNγ | CCTCGCAACCTTCACCTCAC | CGCTGTAATCGTTGTCTTGGAG | 76 | FJ977575.1 |
| IL4 | GTGCCCACGCTGTGCTTAC | AGGAAACCTCTCCCTGGATGTC | 82 | GU119892.1 |
| TNFα | CTCAGGACAGCCTATGCCAACA | CCACCACACGACAGCCAAGT | 177 | XM_015294125.2 |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFNγ, interferon gamma; IL4, interleukin 4; TNFα, tumor necrosis factor alpha.
Statistical Analysis
Data were analyzed using one-way ANOVA with SPSS Statistics, Version 25.0 (IBM Corp., Armonk, NY, USA). Duncan's multiple comparison tests were performed to define the level of significance, and P < 0.05 was considered as a trend toward significance. Graphs were generated using Origin 9.0 (OriginLab, Northampton, MA, USA).
Results
Clinical Symptoms and Mortality in Chicks
After S. pullorum challenge, chicks exhibited lassitude, which was an inclination to huddle together with droopy wings and somnolescence. It was difficult for infected chicks to defecate due to adherence of a chalky white substance to the vent. Anatomic examination showed poor absorption of yolk fat, hepatomegaly and abnormal maculae, thickened pericardium, increased pericardial fluid and white nodes in the myocardium.
From days 1 to 3, the mortality rates of the PC, HB, and PF groups were 36.60%, 33.20%, and 33.40%, respectively. After treatment for 4 to 10 days, the mortality rate of the HB group was 70.37% lower than that of the PC group, and there were no deaths in the PF group. During the experiment, chicks in the NC group showed normal performance without adverse reactions (Table 3).
Table 3.
The mortality rate (%) of chicks in different treatment groups.
| Items | NC (n = 30) |
PC (n = 30) |
HB (n = 30) |
PF (n = 30) |
|---|---|---|---|---|
| On day 1-3 | 0.00 | 36.60 | 33.20 | 33.40 |
| On day 4-10 | 0.00 | 37.50 | 11.11 | 0.00 |
Abbreviations: HB, herbal blend group; n, number of birds; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Growth Performance
On day 1, no significant difference in BW was found among the groups (P > 0.05). On day 4, the BW was significantly lower (P < 0.05) in all groups compared with the NC group. At the end of the experiment, the BW of the HB group was significantly higher than that of the PC group by 18.94% but was not significantly different from that of the NC group. The BW of the PF group was also higher than that of the NC group by 17.23% (P < 0.05). The ADG of chicks in the PF group was significantly higher than that of chicks in the HB group by 36.90% (P < 0.05) and was also higher than that of chicks in the NC and PC groups (P < 0.05). Treatment of chicks with the HB and PF restored ADFI (P < 0.05); the ADFI of chicks in the PF group was comparable to that of chick in NC group. There was no significant difference in FCR between the groups (P > 0.05; Table 4).
Table 4.
The body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion radio (FCR) of chicks in the different treatment groups.1
| Item | NC | PC | HB | PF | SEM2 | P-value |
|---|---|---|---|---|---|---|
| BW day 1 (g) | 38.64 | 37.54 | 39.20 | 38.14 | 0.839 | 0.559 |
| BW day 4 (g) | 50.38a | 44.40b | 45.80b | 45.8b | 0.883 | 0.001 |
| BW day 10 (g) | 61.98b | 53.64c | 63.80b | 72.66a | 2.432 | 0.001 |
| ADG day 1-10 (g) | 3.86a | 1.61c | 2.52b | 3.45a | 0.245 | <0.001 |
| ADFI day 1-10 (g) | 4.85a | 2.12c | 3.15b | 4.14a | 0.319 | 0.001 |
| FCR 1-10 | 1.25 | 1.31 | 1.25 | 1.21 | 0.040 | 0.465 |
Abbreviations: HB, herbal blend group; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Data represent the mean value (n = 5) for each treatment group.
Pooled standard error of mean.
Different superscripts within a row indicate significantly different means (P < 0.05).
Histology of the Ileum, Cecum, and Liver
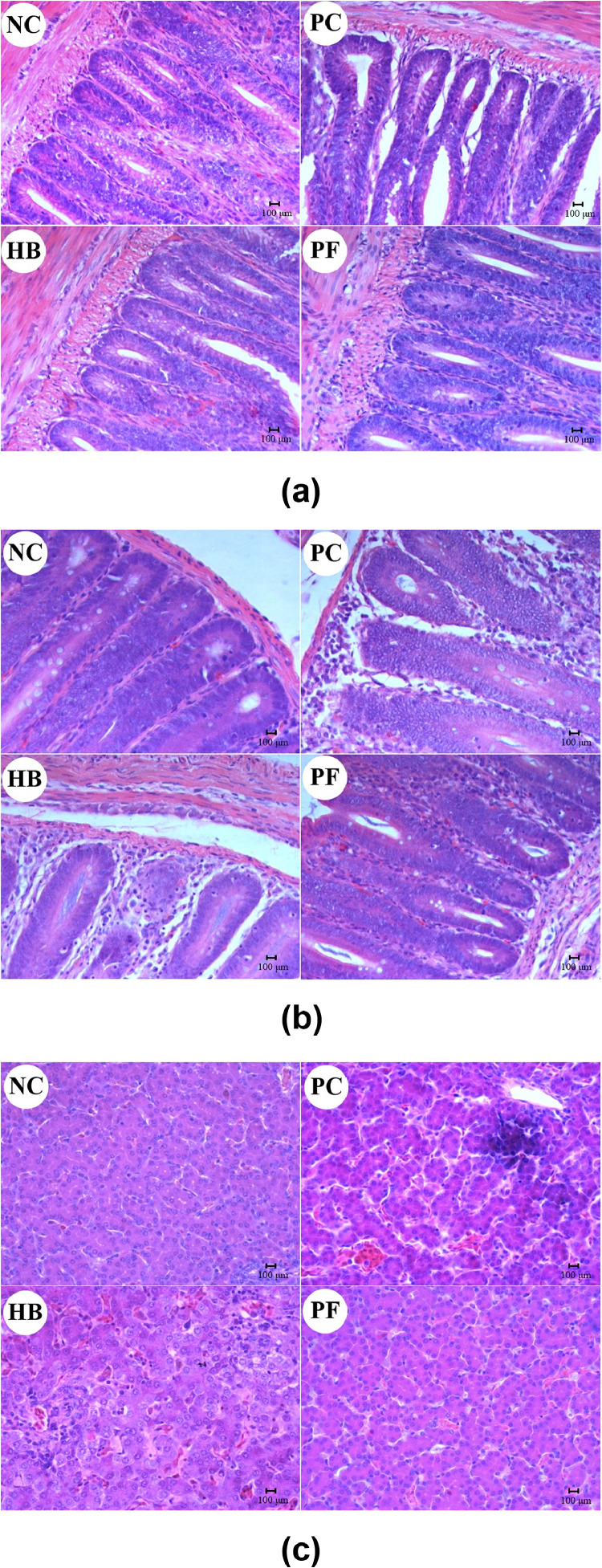
As shown in Figure 2a–2c, pathological changes were observed in histological sections of the ileum, cecum and liver of chicks in each group. Villi in the ileum and cecum of chicks in the NC group were arranged regularly, and the tissue was dense without obvious lesions, whereas the submucosal tissue in the ileum of chicks in the PC group was loosely organized with clots in the blood vessels. A large amount of lymphocyte infiltration covered the villi, and the intestinal villi became shorter and irregularly arranged along with shedding of the intestinal epithelial cells. Compared with that of the PC group, the structure of the cecal mucosa of chicks in the HB group was significantly improved, and there was lymphocyte infiltration in the mucosa propria of the ileum. There was a small amount of inflammatory cell infiltration in the ileal submucosa of chicks in the PF group only, and intact cecal structure was observed. The livers of chicks in the PC group showed focal necrosis, which was detached from surrounding cells and appeared as cytoplasmic voids of varying sizes. The livers also contained a large number of inflammatory cells. Chicks in the NC and PF groups showed no obvious lesion.
Figure 2.
Pathology of the ileum (a), cecum (b) and liver (c) of chicks in the different treatment groups (hematoxylin eosin staining; scale bar = 100 μm). Abbreviations: HB, herbal blend group; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Colony Counts of Lactobacillus, E. coli, and Salmonella
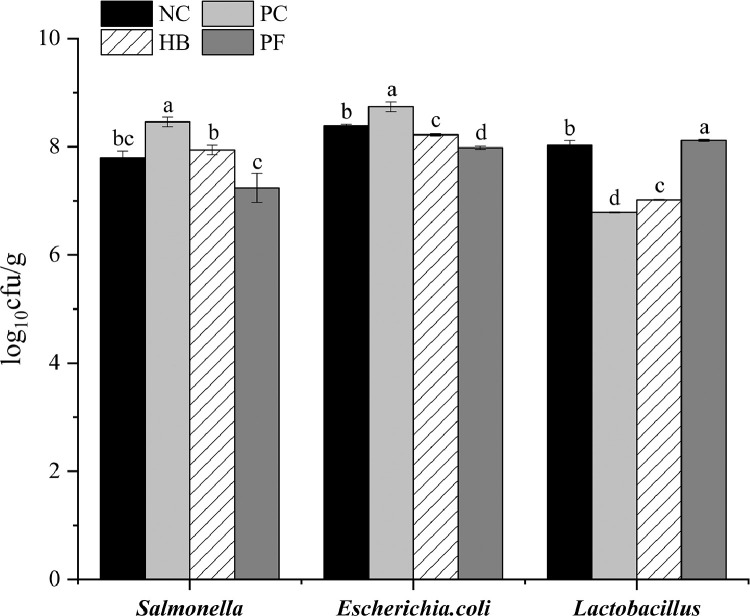
Compared with the PC group, the NC, HB and PF groups had significantly higher Lactobacillus counts in their cecum contents by 18.26%, 3.39%, and 19.59%, respectively (P < 0.05). In contrast, Salmonella counts of the NC, HB and PF groups were significantly lower by 7.92%, 6.14%, and 14.42%, respectively (P < 0.05). E. coli counts of the NC, HB and PF groups were also significantly lower by 4.00%, 5.95%, and 8.70%, respectively (P < 0.05). Compared with the HB group, the PF group had significantly lower Salmonella and E. coli counts by 8.81% and 2.92%, respectively (P < 0.05), but significantly higher Lactobacillus counts by 15.67% (P < 0.05; Figure 3).
Figure 3.
Logarithmic colony counts of Salmonella, Escherichia coli and Lactobacillus in the cecal contents of chicks in the different treatment groups. Significant differences between groups are indicated by different lowercase letters (a, b, c, d). The data are expressed as mean ± SD (n = 5). Abbreviations: HB, herbal blend group; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Immune Organ Indices
No significant difference was observed in spleen indices among the groups. There was no significant difference between the thymus indices of the PC and HB groups. Thymus indices of the PF group were significantly higher than those of the PC group (P < 0.05), but there was no significant difference from that of the NC group (P > 0.05). Compared with the PC and HB groups, bursa of Fabricius indices of chicks in the PF group were 38.10% and 31.82% higher (P < 0.05), respectively, and were not significantly difference from the NC group (P > 0.05; Table 5).
Table 5.
Immune organ indices of chicks in different treatment groups.1
| Item | NC | PC | HB | PF | SEM2 | P-value |
|---|---|---|---|---|---|---|
| thymus | 0.45a | 0.35b | 0.40ab | 0.46a | 0.020 | 0.038 |
| spleen | 0.11 | 0.09 | 0.13 | 0.12 | 0.014 | 0.434 |
| bursa of Fabricius | 0.26ab | 0.21b | 0.22b | 0.29a | 0.014 | 0.041 |
Abbreviations: HB, herbal blend group; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Data represent the mean value (n = 5) for each treatment group.
Pooled standard error of mean.
Different superscripts within a row indicate significantly different means (P < 0.05).
sIgA Concentrations and Relative Cytokine mRNA Expression Levels in the Cecum
Changes in sIgA concentrations and relative cytokine mRNA expression levels in the cecal mucosa are shown in Table 6. The sIgA concentrations in the cecal mucosa of chicks in the PF group was significantly higher than those in the PC group by 15.80% (P < 0.05), but the PF group was not significantly different from the NC or HB groups (P > 0.05). Compared with the PC group, TNFα and IFNγ mRNA expression levels were significantly lower in the PF group by 38.86% and 39.83%, respectively (P < 0.05). Compared with the HB group, TNFα and IFNγ mRNA expression levels were significantly lower in the PF group by 24.84% and 25.78%, respectively (P < 0.05). There was no significant difference in TNFα or IFNγ mRNA expression levels in the cecum between the NC and PF groups. There was no significant difference in IL4 mRNA expression level among NC, HB and PF group (P > 0.05). Compared with the PC group, IL4 mRNA expression level was significantly higher in the PF group by 81.01% (P < 0.05).
Table 6.
Cecal sIgA concentrations and relative cytokine mRNA expression levels of chicks in different treatment groups.1
| Item | NC | PC | HB | PF | SEM2 | P-value |
|---|---|---|---|---|---|---|
| TNFα | 1.13c | 1.93a | 1.57b | 1.18c | 0.069 | 0.002 |
| IFNγ | 1.76b | 3.59a | 2.87a | 2.13b | 0.174 | 0.002 |
| IL4 | 2.29ab | 1.79b | 3.08a | 3.24a | 0.255 | 0.031 |
| IgA/pg•mL−1 | 1156.55a | 1069.87b | 1203.10a | 1238.94a | 22.071 | 0.006 |
Abbreviations: HB, herbal blend group; NC, negative control group; PC, positive control group; PF, probiotic-fermented herbal blend group.
Data represent the mean value (n = 5) for each treatment group.
Pooled standard error of mean.
Different superscripts within a row indicate significantly different means (P < 0.05).
Discussion
Chicks with PD showed somnolescence, weakness, depressed appetite, poor growth, high mortality, and the adherence of a chalky white material to the vent region, which was consistent with the results of a previous study (Shivaprasad, 2000). The mortality rate of PC group was 74.10% on day 11. The infected chicks treated with the PF exhibited improved mental state with no death. However, the results of treatment with the unfermented herbal blend were not as pronounced, confirming that the fermentation of the blend was crucial for its therapeutic use. Jung et al. (2010) reported that the mortality of Salmonella-infected chicks decreased from 85% to 55% seventeen days after treatment with a probiotic-fermented 4-herb combination via potent stimulation of non-specific immune responses, which was similar to our results. Inherently complex PF may be considered medicines owing to their ability to regulate the intestinal flora and modulate biological immune responses, which may contribute to the control of disease progression and development in infected chicks. S. pullorum invading also induce high consumption of nutrients which could show impaired performance in chickens subsequently (Wang et al., 2019b). Previous evidence has shown that both functional compounds of traditional Chinese herb and probiotics can increase animal growth performance by enhancing digestibility (Yin et al., 2009; Symonds et al., 2012). In present study, PF treatment significantly increased the BW, ADG, and ADFI. Similar to the positive effects on growth performance that we observed, a previous study showed that daily feeding with the fermented herbal blend for 6 weeks improved the growth performance of broilers (Hinz et al., 2019). Lei et al., (2018) also reported the promotion effect of 16 weeks feed fermented herbs supplementation on production performance of growing-finishing pigs.
As shown in a previous study, S. pullorum can multiply rapidly and destroy the intestinal flora of young chicks owing to their underdeveloped gut flora (Barrow and Freitas Neto, 2011). In the present study, the HB and the PF reduced colony counts of pathogenic bacteria (Salmonella) and harmful bacteria (E. coli), but increased colony counts of beneficial bacteria (Lactobacillus) in the cecal contents of chicks infected with S. pullorum. Interestingly, the PF group showed the best performance in this regard. Furthermore, in the PF group, only a few inflammatory cells infiltrated the ileal submucosa, and the tissues were dense and intact, similar to those of chicks in the NC group. These results indicated that the PF had the positive effect of regulating the intestinal flora and protecting intestinal tissue. In previous research, phytogenic feed additives have been shown to suppress harmful bacteria, select beneficial bacteria by controlling the intestinal microbial ecosystem, and favoring healthier microbial groups (Guo et al., 2004; Tiihonen et al., 2010; Mao, 2016; Lin et al., 2021). Moreover, introduction of probiotics in the PF had the positive effect of reconstructing the intestinal flora of infected chicks. At the same time, colonization of probiotics increases the resistance of chicks to pathogens and maintains the long-term stability of the intestinal flora (Li et al., 2009; Revolledo et al., 2009; Mookiah et al., 2012). As mentioned in previous studies, probiotics suppress the proliferation and virulence of bacterial pathogens via local production of bacteriocins within the enteric microenvironment or competitive exclusion of pathogens (Pascual et al., 1999; Higgins et al., 2008). Similarly, Xie et al. (2016) found that herbal medicine fermented by lactic acid bacteria increased Lactobacillus counts and reduced E. coli counts in the intestinal tract of broilers. Qu et al. (2021) have also demonstrated that the gut microbiota of mice with antibiotic-associated diarrhea mice was restored by treatment with a fermented herb.
After invading the digestive tract of chicks, S. pullorum preferentially targets the cloacal bursa and spleen prior to eliciting inflammation in the intestine and causing systemic infection (Shivaprasad and Barrow, 2007; Ding et al., 2021). The immunity of young chicks is extremely immature and, therefore, not able to trigger a cellular immune response robust enough to avoid systemic infection. In the present study, the PF promoted the development of immune organs, the secretion of sIgA, and IL4 expression level. At the same time, the mRNA expression levels of TNFα and IFNγ significantly decreased. Liver lesions were also improved by treatment with the PF, which were in accordance with decreased activity of serum ALT and AST (Table S1). Although, many studies have suggested that humoral immunity has little effect on Salmonella elimination, the decrease of mortality in the HB and PF groups was accompanied by the up-regulation of humoral-related immune cytokines. This result may be explained by the fact that the PF regulated the balance of immune response. The functional components that are released by enzymolysis during microbial fermentation of PF, such as saponins, flavonoids and polysaccharides, have been demonstrated to globally downregulate the expression of inflammatory cytokines, affect mitogen-activated protein kinase and nuclear factor-kappa B and protect against diseases such as Newcastle and infectious bursal disease (Wei et al., 2017; De Camrgo et al., 2019; Mohanad et al., 2019). A variety of Lactobacillus-containing probiotics have been found to regulate the expression of IL4, contribute to the development of B cells, promote the secretion of sIgA and inhibit the expression of many pro-inflammatory factors (Galdeano and Perdigon, 2006; Haghighi et al., 2008). It was shown that synergism between probiotics and Astragalus polysaccharides enhanced the development of the immune organs of Hy-Line chicks (Li et al., 2009). Zhao et al. (2018) demonstrated that fermented herb residue enhanced host immunity and inflammatory responses and improved the immunity by promoting the diversity of beneficial bacteria.
In conclusion, the PF examined in this study improved clinical symptoms, increased growth performance, and enhanced the immune function of S. pullorum-infected newborn chicks. Remarkably, no deaths were observed in infected chicks treated with the PF. In light of the limitations associated with antibiotic use in the poultry industry, the PF examined here provides an environmentally-friendly feed additive to treat chicks infected with S. pullorum, and our results provide a basis for future feed additive research and development. Further studies should be performed to investigate the long-term effects of the PF on the production performance and health status of infected and healthy chickens.
Acknowledgments
This research was part of the “Collaborative Innovation Team Project of Green Layer Breeding and Food Safety” funded by the Beijing University of Agriculture, Beijing, China.
Disclosures
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101196.
Appendix. Supplementary materials
References
- Abarike E.D., Jiang J.C., Tang J.F., Cai J., Sakyi E.M., Kuebutornye F.K. A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquacult. Rep. 2020;18 [Google Scholar]
- Barrow P.A., Freitas Neto O.C. Pullorum disease and fowl typhoid-new thoughts on old diseases: a review. Avian. Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Li J.Y., Zhang H.X., Xie Y.H., Xiong L.X., Liu H., Wang F. Effects of a probiotic on the growth performance, intestinal flora, and immune function of chicks infected with Salmonella pullorum. Poult. Sci. 2020;99:5316–5323. doi: 10.1016/j.psj.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camrgo A.C., Favero B.T., Morzelle M.C., Franchin M., Parrilla E.A., Rosa L.A.D.L., Geraldi M.V., Junior M.R.M., Shahidi F., Schwember A. Is chickpea a potential substitute for soybean? Phenolic Bioactives and potential health benefits. Int. J. Mol. Sci. 2019;20:1–42. doi: 10.3390/ijms20112644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrese M., Schrezenmeir J. Probiotics, prebiotics and synbiotics. Adv. Biochem. Engin. Biot. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- Ding J.M., Zhou H., Luo L.X., Xiao L., Yang K.X., Zheng Y.M., Xu K., He C., Han C.X., Luo H.X., Qin C., Akinyemi F.T., Gu C., Zhou Z.X., Huang Q.Z., Meng H. Heritable gut microbiome associated with Salmonella enterica Serovar pullorum infection in chickens. mSystems. 2021;6:1–12. doi: 10.1128/mSystems.01192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdeano C.M., Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine. Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S.Z., Jiao X.N., Barrow P., Pan Z.M., Chen X. Virulence determinants of Salmonella Gallinarum biovar Pullorum identified by PCR signature-tagged mutagenesis and the spiC mutant as a candidate live attenuated vaccine. Vet. Microbiol. 2014;168:388–394. doi: 10.1016/j.vetmic.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Gong J., Yin F., Hou Y., Yin Y. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: Potential and challenges in application. Can. J. Anim. Sci. 2014;94:223–241. [Google Scholar]
- Guo F.C., William B.A., Kwakkel R.P., Li H.S., Li X.P., Luo J.Y., Li W.K., Verstegen W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004;83:175–182. doi: 10.1093/ps/83.2.175. [DOI] [PubMed] [Google Scholar]
- Guo R.X., Li Z.Y., Zhou X.H., Huang C.Y., Hu Y.C., Geng S.Z., Chen X., Li Q.C., Pan Z.M., Jiao X.N. Induction of arthritis in chickens by infection with novel virulent Salmonella Pullorum strains. Vet. Microbiol. 2019;228:165–172. doi: 10.1016/j.vetmic.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Haghighi H.R., Careem M.F.A., Dara R.A., Chambers J.R., Sharif S. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 2008;126:225–233. doi: 10.1016/j.vetmic.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Henderson S.C., Bounous D.I., Lee M.D. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 1999;67:3580–3586. doi: 10.1128/iai.67.7.3580-3586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S.E., Higgins J.P., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Tellez G., Hargis B. Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella Enteritidis in neonatal broiler chicks. Poult. Sci. 2008;87:27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- Hinz K, Stracke J., Schattler J., K., Spindler B., Kemper N. Foot pad health and growth performance in broiler chickens as affected by supplemental charcoal and fermented herb extract (FKE): an on-farm study. Europ. Poult. Sci. 2019;83:1–13. [Google Scholar]
- Jung B.G., Ko J.H., Lee B.J. Dietary supplementation with a probiotic fermented four-herb combination enhances immune activity in broiler chicks and increases survivability against Salmonella Gallinarum in experimentally infected broiler chicks. J. Vet. Med. Sci. 2010;72:1565–1573. doi: 10.1292/jvms.10-0152. [DOI] [PubMed] [Google Scholar]
- Lei J.X., Yun H.M., Kim I.H. Effects of dietary supplementation of natural and fermented herbs on growth performance, nutrient digestibility, blood parameters, meat quality and fatty acid composition in growing-finishing pigs. Ital. J. Anim. Sci. 2018;17:984–993. [Google Scholar]
- Lettini A.A., Vo Than T., Marafin E., Longo A., Antonello K., Zavagnin P., Barco L., Mancin M., Cibin V., Morini M., Dang Thi Sao M., Nguyen Thi T., Pham Trung H., Le L., Nguyen Duc T., Ricci A. Distribution of Salmonella serovars and antimicrobial susceptibility from poultry and swine farms in central Vietnam. Zoonoses Public Hlth. 2016;63:569–576. doi: 10.1111/zph.12265. [DOI] [PubMed] [Google Scholar]
- Li J.Y., Zhang H.X., Xie Y.H., Xiong L.X., Gao X.Z., Liu H., Lian Z.X. Optimization of fermentation for producing polysaccharides from Chinese herbal by combined probiotics. J. Beijing Univ. Agric. 2018;33:83–87. (in Chinese) [Google Scholar]
- Li S.P., Zhao X J, Wang J.Y. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult. Sci. 2009;88:519–525. doi: 10.3382/ps.2008-00365. [DOI] [PubMed] [Google Scholar]
- Liu H., Zhang H.X., Gao X.Z. China Light Industry Press; China: 2016. Modern Experimental Technology of Food Microbiology. 2nd rev. [Google Scholar]
- Lin T.L., Lu C.C., Lai W.F., Wu T., Lu J.J., Chen Y.M., Tzeng C.M., Liu H.T., Wei H., Lai H.C. Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell. 2021;12:394–410. doi: 10.1007/s13238-020-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jin S.Y., Chang J., Wang P., Chao C.Q., Yin Q.Q., Gao T.Z., Zhu Q., Lu F.S. Changes of active ingredients before and after compound probiotic fermented Chinese Herbs. Anhui. Agric. Sci. 2017;45:123–125. (in Chinese) [Google Scholar]
- Mao Y.L. Zhejiang University; 2016. The Immunomodulatory Effects of Glycyrrhizin and its Mechanisms Against Salmonella Infection. PhD Diss. (in Chinese) [Google Scholar]
- Meng Y.L. 1st rev. ed. Wuhan Univ. Press; Wuhan, China: 2004. Pages 8–27 in Modern Histology and Cytology. [Google Scholar]
- Mohanad K.U., Saleem M.R.I., Kadhim M.J. Effect of using natural apple vinegar, garlic powder (Alsin) and black bean seed on the immune system and some of characteristics of the blood broilers Ross 308. J. Phys. Conf. Ser. 2019;9:1294. [Google Scholar]
- Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2012;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Pascual M., M.Hugas J.Badiola, Monfort J., Garriga M. Lactobacillus salivarus CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microb. 1999;65:4981–4986. doi: 10.1128/aem.65.11.4981-4986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Hu Y.L., Cui B.A., Zhang H.Y., Kong X.F., Wang D.Y., Wang Y.G. Immunopotentiating effects of four Chinese herbal polysaccharides administered at vaccination in chickens. Poult. Sci. 2007;86:2530–2535. doi: 10.3382/ps.2007-00076. [DOI] [PubMed] [Google Scholar]
- Qu Q.S., Yang F., Zhao C.Y., Liu X., Yang P.S., Li Z.X., Han L., Shi X.Y. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 2021;267 doi: 10.1016/j.jep.2020.113594. [DOI] [PubMed] [Google Scholar]
- Rashidi N., Ali K., Kamran T., Mohammad A.G., Hassan S. Effects of licorice extract, probiotic, toxin binder and poultry litter biochar on performance, immune function, blood indices and liver histopathology of broilers exposed to aflatoxin-B1. Poult. Sci. 2020;99:5896–5906. doi: 10.1016/j.psj.2020.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revolledo L., Ferreira C.S.A., Ferreira A.J.P. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009;88:734–743. doi: 10.3382/ps.2008-00410. [DOI] [PubMed] [Google Scholar]
- Revolledo L. Vaccines and vaccination against fowl typhoid and pullorum disease: An overview and approaches in developing countries. Appl. Poult. Res. 2018;27:279–291. [Google Scholar]
- Rolfe R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- Sharifi S.D., Dibamehr A., Lotfollahian H., Baurhoo B. Effects of flavomycin and probiotic supplementation to diets containing different sources of fat on growth performance, intestinal morphology, apparent metabolizable energy, and fat digestibility in broiler chickens. Poult. Sci. 2012;91:918–927. doi: 10.3382/ps.2011-01844. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L., Barrow P.A. Pullorum disease and fowl typhoid. Dis. Poult. 2007;16:678–693. [Google Scholar]
- Symonds E.L., O'Mahony C., Lapthorne S., O'Mahony D., Sharry J.M., O' Mahony L., Shanahan F. Bifidobacterium infantis 35624 protects against Salmonella-induced reductions in digestive enzyme activity in mice by attenuation of the host inflammatory response. Clin. Transl. Gastroen. 2012;3:1–10. doi: 10.1038/ctg.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., He Y.X., Hou Y.X., Guo J.Y., Qian X.Q., Wang L.C. Effects of composite herbal-probiotics agents substitute for antibiotic on growth performance, immune function and organs & tissue development of Yellow Broilers. Chin. J. Anim. Sci. 2017;53:90–95. (in Chinese) [Google Scholar]
- Tiihonen K., Kettunen H., Bento M.H.L., Saarinen M., Lahti- nen S., Ouwehand A.C., Schulze H., Rautonen N. The effect of feeding essential oils on broiler performance and gut microbiota. Brit. Poult. Sci. 2010;51:381–392. doi: 10.1080/00071668.2010.496446. [DOI] [PubMed] [Google Scholar]
- Wang X., Xie H.J., Liu F., Wang Y.H. Production performance, immunity, and heat stress resistance in Jersey cattle fed a concentrate fermented with probiotics in the presence of a Chinese herbal combination. J. Anim. Feed. Sci. 2017;228:59–65. [Google Scholar]
- Wang R., Cai W.T., Wang X.L., Gao J., Huang M. Progress in Chinese medicine-probiotics compound microecological preparations for livestock and poultry. Chin. J. Biotech. 2019;35:972–987. doi: 10.13345/j.cjb.180443. (in Chinese) [DOI] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., G. H. Qi. Supplemental plant extracts from Flos lonicerae in combination with Baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J.R., Wen X.F., Bible P.W., Li Z.Y., Nussenblatt R.B., Wei L. Panax notoginseng saponin controls IL-17 expression in helper T cells. J. Ocul. Pharmacol. Th. 2017;33:285–289. doi: 10.1089/jop.2016.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley P., Jones M.A., Barrow P.A. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian Pathol. 2002;31:501–506. doi: 10.1080/0307945021000005879. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3439–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Wu Q.J., Zheng X.C., Wang T., Zhang T.Y. Effect of dietary oridonin supplementation on growth performance, gut health, and immune response of broilers infected with Salmonella pullorum. Irish Vet. J. 2018;71:1–6. doi: 10.1186/s13620-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q.X., Zhang J.M., Qi X.Y., Xu H., Xu H.Y., Gui W. Research Lactobacillus rhamnosus fermented herbal and Bacillus subtilis complex on immune performance, E. coli infection of broiler. Chin. Anim. Husbandry. Vet. Med. 2016;43:1523–1529. (in Chinese) [Google Scholar]
- Yang R.L. Henan University of Science and Technology; 2015. Effects of Bacillus from gut of silkie combined with chinese herbal medicine on the growth and immunity function of silkie. PhD Diss. (in Chinese) [Google Scholar]
- Yin F.G., Liu Y.L., Yin Y.L., Kong X.F., Huang R.L., Li T.J., Wu G.Y., Hou Y.Q. Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids. 2009;37:263–270. doi: 10.1007/s00726-008-0142-6. [DOI] [PubMed] [Google Scholar]
- Zhang D.X., Li R., Li J.C. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res. Vet. Sci. 2012;93:366–373. doi: 10.1016/j.rvsc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liu Y.C., Liu Y.P., Li J.Z., Li L.C. Characteristics and application of synergistic effect of probiotics and Chinese herbal medicine in livestock and poultry breeding. Mod. Anim. Husbandry. 2018;2:30–34. (in Chinese) [Google Scholar]
- Zhao X.X., Chen T.T., Meng F.J., Wang H., Tian P.Y., Tang X.Y., Wang X., Wang X.L., Xin H.G., Wei H. Therapeutic effect of herb residue fermentation supernatant on spleen‑deficient mice. Mol. Med. Rep. 2018;17:2764–2770. doi: 10.3892/mmr.2017.8150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.