Highlights
-
•
More patients with chronic hepatitis B (CHB) are surviving into old age.
-
•
The late-life health-related quality of life (HRQoL) they might experience remains unclear.
-
•
We repeatedly assessed HRQoL in a cohort of older adults with CHB over five years.
-
•
The HRQoL changes older CHB patients might experience were delineated.
-
•
Extrahepatic organ/system abnormalities were associated with changes in HRQoL.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIC, Bayesian information criterion; BMI, body mass index; BP, blood pressure; CES-D, Center for Epidemiological Studies Depression; CHB, chronic hepatitis B; CV, coefficient of variation; FIB-4, Fibrosis-4 Index for Liver Fibrosis; HALST, Healthy Aging Longitudinal Study in Taiwan; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HOMA-IR, Homeostasis Model Assessment of Insulin Resistance; HRQoL, health-related quality of life; hsCRP, high-sensitivity C-reactive protein; MCS, Mental Component Summary; MMSE, Mini-Mental State Examination; OR, odds ratio; PCS, Physical Component Summary; SF-12v2, the Short Form (12) Health Survey version 2; 95% CI, 95% confidence interval
Keywords: Health-related quality of life, Aging, Geriatric assessment, Group-based trajectory modeling, Healthy Aging Longitudinal Study in Taiwan (HALST)
Abstract
Despite the increasing health burden of chronic hepatitis B (CHB) in aging populations, little is known about the course of health-related quality of life (HRQoL) changes. We aimed to assess individual-level longitudinal HRQoL changes in elderly patients with CHB and to examine their correlates. A prospective 5.1 years-cohort study was conducted in community-dwelling adults aged 55 years with hepatitis B surface antigen-positive. Participants underwent serial measurement of HRQoL using the short-form (12) health survey version 2. Of 503 participants, 82.7% remained in good physical health throughout the study period, whereas 9.1% had declining physical health and 8.2% were in poor physical health. We likewise identified three trajectories of mental health changes (“good mental health” [86.9%], “declining mental health” [6.8%], and “poor mental health” [6.4%]). Three baseline characteristics were independently associated with a lower likelihood of remaining physically or mentally healthy: sarcopenic obesity (odds ratio [OR] with 95% confidence interval [95% CI] of 7.5 [2.8–20.5] for poor physical health, 3.1 [1.1–8.4] for declining physical health, 4.3 [1.4–13.0] for poor mental health), a higher number of metabolic abnormalities (OR [95% CI] of 3.6 [1.6–8.0] for poor physical health) and depressed mood (OR [95% CI] of 21.7 [5.8–81.0] for poor physical health, 5.3 [1.4–19.9] for declining physical health, 83.1 [19.7–350.2] for poor mental health, 13.6 [2.9–64.8] for declining mental health). In conclusion, in a cohort of elderly patients with CHB, we demonstrated the heterogeneity and nonlinearity of HRQoL changes and their associations with variations in specific extrahepatic organs/systems.
1. Introduction
Chronic hepatitis B (CHB) causes substantial health loss globally, and the burden of CHB is rising, driven in large part by an aging population (Stanaway et al., 2016). The risk of CHB complications increases with age, and health risks for elderly with CHB might be further compounded by the high prevalence of comorbid conditions, and frailty, as well as widely heterogeneous characteristics (Beard et al., 2016, Chen et al., 2010, Lai et al., 2018). As more patients with CHB survive into old age, the complexity and heterogeneity of their presentation pose challenges in meeting their health needs. An understanding of the course of health changes in later life in these patients is needed.
Increasing life’s quality, not merely its duration, is a major goal of health care and is of particular concern in elderly patients with low mortality diseases (Beard et al., 2016, Powers et al., 2016, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion, 2018). Health-related quality of life (HRQoL), defined as “the value assigned to duration of life as modified by the impairments, functional states, perceptions, and social opportunities that are influenced by disease, injury, treatment, or policy,” is a direct and multidimensional measure of health (Calvert et al., 2013, Maruish, 2012, Patrick and Erickson, 1993, Powers et al., 2016, U. S. Department of Health and Human Services F. D. A. Center for Drug Evaluation and Research et al., 2009, Younossi et al., 2016). Derived directly from a patient’s perspective, HRQoL provides the ideal means of assessing the effect of medical conditions on health and health needs (Maruish, 2012, Patrick and Erickson, 1993, U. S. Department of Health and Human Services F. D. A. Center for Drug Evaluation and Research et al., 2009, Younossi et al., 2016, Younossi et al., 2018). A change in HRQoL precedes and predicts multiple health outcomes in elderly people (Dorr et al., 2006, Rumsfeld et al., 1999). HRQoL is recognized as a crucial endpoint in clinical trials nowadays (Calvert et al., 2013, U. S. Department of Health and Human Services F. D. A. Center for Drug Evaluation and Research et al., 2009).
To date, it is unclear how health, as assessed by HRQoL, might change with time in older adults with CHB. Data derived from longitudinal studies with multiple repeated assessments are required to estimate the course of health change over time (health trajectory). Unfortunately, these data are scant, especially with regard to HRQoL in older patients with CHB. In this prospective study, we investigated the health trajectory in a cohort of older adults with CHB who have undergone repeated measurements of HRQoL. The primary aim was to delineate patterns of HRQoL changes in elderly patients with CHB. To further explore the likely determinants of HRQoL changes in older adults with CHB, we also examined whether these patterns are distinguishable in terms of diverse baseline characteristics, including sociodemographic features, organ/system abnormalities, geriatric conditions, and comorbidities.
2. Methods
2.1. Study design and participants
Healthy Aging Longitudinal Study in Taiwan (HALST) is a longitudinal, observational study launched to investigate the determinants of healthy aging. As such, it recruited adults aged 55 years and older and living in Taiwan and oversampled adults aged 65 years and older (see the supplement) (Hsu et al., 2017). We analyzed data of the HALST. The study was approved by the Institutional Review Board of the National Health Research Institutes and its participating hospitals.
At baseline assessment, 503 HALST participants were seropositive for hepatitis B surface antigen (HBsAg) (supplement) and were enrolled in the present study. Except in rare instances, most older residents in Taiwan who are seropositive for HBsAg acquired hepatitis B virus (HBV) perinatally before the age of 3 years and were chronically infected (Hsu et al., 1986).
2.2. Assessment of HRQoL
HRQoL was assessed using the short-form (12) health survey version 2 (SF-12v2) scale at baseline and years 2, 3, and 5. SF-12 consists of 12 items (questions) that assess the effects of medical conditions on multiple health domains, including physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health (Fuh et al., 2000, Ju et al., 2002, Maruish, 2012, Tseng et al., 2002). As a generic measure of HRQoL, SF-12 allows capturing of the effects of diverse comorbid conditions in older adults with CHB (Maruish, 2012, Ware, 1995). The scales of SF-12 are summarized in two dimensions: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The PCS and MCS scores range from 0 to 100, with higher scores indicating better physical health and better mental health, respectively (Maruish, 2012).
2.3. Assessment of baseline characteristics
Sociodemographic, lifestyle, geriatric conditions, and comorbid diseases/conditions were comprehensively assessed during the baseline assessment (supplement) (Hsu et al., 2017). Hepatic complications of CHB (liver failure, ascites, esophageal varices, hepatic encephalopathy, hepatocellular carcinoma and liver fibrosis) and viral-load-lowering CHB treatment ever received were also assessed (supplement). The likelihood of concurrent advanced nonalcoholic steatohepatitis with fibrosis was also evaluated.
2.4. Statistical analysis
HRQoL was determined at the following time points: baseline and years 2, 3, and 5, and were analyzed in this study. We first modeled the individual-level trajectories of PCS and MCS score changes over time using group-based trajectory modeling (the PROC TRAJ procedure [SAS version 9.4 software; SAS Institute Inc., Cary, NC, USA]) (Nagin, 2005). In this procedure, individual-level heterogeneity in trajectories is modeled by fitting a semiparametric (discrete) mixture model to the longitudinal data of PCS and MCS scores by using the maximum likelihood method (Jones and Nagin, 2007, Nagin, 2005). The Bayesian information criterion (BIC) was applied to evaluate model fit and to determine the number of trajectories (Jones and Nagin, 2007, Nagin, 2005). Models that included a small (<5%) trajectory group were rejected. Adequacy of the model was assessed by examining the average posterior probabilities of membership of each trajectory (Jones and Nagin, 2007, Nagin, 2005).
We next assessed and compared the baseline characteristics of participants assigned to different HRQoL trajectories. Continuous data are expressed as the mean ± standard deviation, and categorical data are expressed as numbers and percentages. Categorical variables were compared using the chi-square test or Fisher’s exact test, and one-way analysis of variance was applied for continuous variables. Independent associations of baseline characteristics with HRQoL trajectories were examined using multinomial logistic regression analyses. The regression coefficient (e.g., B) for a variable in the logistic regression model represents change in the log odds associated with one-unit change in the variable. e raised to the power B represents the fold change in the odds associated with one-unit change in the variable (odds ratio). A two-sided p < .05 was considered significant. All the analyses were performed using SAS version 9.4, SPSS Version 25.0 (IBM Corp., Armonk, NY, USA) and R versions 3.6.0 (R Foundation for Statistical Computing).
3. Results
Participants characteristics at baseline are shown in Table 1. None of them had human immunodeficiency virus infection/acquired immune deficiency syndrome or viral hepatitis other than hepatitis B or C. The mean BMI was 24.4 ± 3.7 kg/m2. The mean PCS score was 47.42 ± 8.06, and the mean MCS score was 59.17 ± 7.84 at baseline.
Table 1.
Baseline characteristics of study participants by health-related quality of life trajectory groups. a
| PCS Trajectory Groups |
MCS Trajectory Groups |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Group 1 | Group 2 | Group 3 |
Pb |
Group 1 | Group 2 | Group 3 | Pb | ||||||||
| (Poor Physical Health) | (Good Physical Health) | (Declining Physical Health) | (Poor Mental Health) | (Good Mental Health) | (Declining Mental Health) | |||||||||||
| (n = 503) |
(n = 41) |
(n = 416) |
(n = 46) |
(n = 32) |
(n = 437) |
(n = 34) |
||||||||||
| Sociodemographic characteristics | ||||||||||||||||
| Age, y, mean (SD) | 67.3 | (7.8) | 71.4 | (9.3) | 66.7 | (7.5) | 68.7 | (7.8) | <0.001 | 68.4 | (7.8) | 67.1 | (7.8) | 68.7 | (8.4) | 0.340 |
| Sex | 0.005 | 0.001 | ||||||||||||||
| Female | 234 | (46.5) | 24 | (58.5) | 180 | (43.3) | 30 | (65.2) | 22 | (68.8) | 189 | (43.2) | 23 | (67.6) | ||
| Male | 269 | (53.5) | 17 | (41.5) | 236 | (56.7) | 16 | (34.8) | 10 | (31.3) | 248 | (56.8) | 11 | (32.4) | ||
| Marital status | 0.001 | <0.001 | ||||||||||||||
| Not married | 102 | (20.3) | 15 | (36.6) | 72 | (17.3) | 15 | (32.6) | 15 | (46.9) | 78 | (17.8) | 9 | (26.5) | ||
| Married | 401 | (79.7) | 26 | (63.4) | 344 | (82.7) | 31 | (67.4) | 17 | (53.1) | 359 | (82.2) | 25 | (73.5) | ||
| Lifestyle characteristics | ||||||||||||||||
| Smoking | 0.221 | 0.580 | ||||||||||||||
| Never | 340 | (67.6) | 28 | (68.3) | 275 | (66.1) | 37 | (80.4) | 23 | (71.9) | 292 | (66.8) | 25 | (73.5) | ||
| Ever | 88 | (17.5) | 9 | (22.0) | 73 | (17.5) | 6 | (13.0) | 7 | (21.9) | 76 | (17.4) | 5 | (14.7) | ||
| Current | 75 | (14.9) | 4 | (9.8) | 68 | (16.3) | 3 | (6.5) | 2 | (6.3) | 69 | (15.8) | 4 | (11.8) | ||
| Alcohol drinking | 0.030 | 0.783 | ||||||||||||||
| Never | 301 | (59.8) | 28 | (68.3) | 242 | (58.2) | 31 | (67.4) | 22 | (68.8) | 259 | (59.3) | 20 | (58.8) | ||
| Ever | 68 | (13.5) | 9 | (22.0) | 52 | (12.5) | 7 | (15.2) | 3 | (9.4) | 59 | (13.5) | 6 | (17.6) | ||
| Current | 134 | (26.6) | 4 | (9.8) | 122 | (29.3) | 8 | (17.4) | 7 | (21.9) | 119 | (27.2) | 8 | (23.5) | ||
| Diseases | ||||||||||||||||
| Diabetes mellitus | 135 | (26.8) | 21 | (51.2) | 99 | (23.8) | 15 | (32.6) | 0.001 | 11 | (34.4) | 112 | (25.6) | 12 | (35.3) | 0.288 |
| Cardiovascular disease | 88 | (17.5) | 15 | (36.6) | 62 | (14.9) | 11 | (23.9) | 0.001 | 8 | (25.0) | 74 | (16.9) | 6 | (17.6) | 0.511 |
| Arthritis | 73 | (14.5) | 16 | (39.0) | 48 | (11.5) | 9 | (19.6) | <0.001 | 3 | (9.4) | 60 | (13.7) | 10 | (29.4) | 0.031 |
| Hypertension | 235 | (46.7) | 26 | (63.4) | 189 | (45.4) | 20 | (43.5) | 0.080 | 14 | (43.8) | 205 | (46.9) | 16 | (47.1) | 0.941 |
| Stroke | 21 | (4.2) | 5 | (12.2) | 15 | (3.6) | 1 | (2.2) | 0.025 | 0 | (0.0) | 17 | (3.9) | 4 | (11.8) | 0.041 |
| Cancer | 36 | (7.2) | 2 | (4.9) | 32 | (7.7) | 2 | (4.3) | 0.593 | 3 | (9.4) | 31 | (7.1) | 2 | (5.9) | 0.851 |
| Lung diseases | 18 | (3.6) | 0 | (0.0) | 17 | (4.1) | 1 | (2.2) | 0.351 | 2 | (6.3) | 13 | (3.0) | 3 | (8.8) | 0.147 |
| Chronic kidney disease | 62 | (12.3) | 10 | (24.4) | 47 | (11.3) | 5 | (10.9) | 0.049 | 3 | (9.4) | 53 | (12.1) | 6 | (17.6) | 0.559 |
| Dementia | 1 | (0.2) | 0 | (0.0) | 1 | (0.2) | 0 | (0.0) | 0.901 | 0 | (0.0) | 1 | (0.2) | 0 | (0.0) | 0.927 |
| Hepatitis C | 14 | (2.8) | 0 | (0.0) | 10 | (2.4) | 4 | (8.7) | 0.026 | 0 | (0.0) | 13 | (3.0) | 1 | (2.9) | 0.612 |
| Chronic hepatitis B treatment | 26 | (5.2) | 4 | (9.8) | 20 | (4.8) | 2 | (4.4) | 0.382 | 3 | (9.4) | 22 | (5.0) | 1 | (2.9) | 0.470 |
| Hepatic complications | ||||||||||||||||
| Liver failure | 3 | (0.6) | 1 | (2.4) | 2 | (0.5) | 0 | (0.0) | 0.258 | 0 | (0.0) | 3 | (0.7) | 0 | (0.0) | 0.796 |
| Ascites | 6 | (1.2) | 1 | (2.4) | 5 | (1.2) | 0 | (0.0) | 0.579 | 1 | (3.1) | 4 | (0.9) | 1 | (2.9) | 0.338 |
| Varices/varices bleeding | 5 | (1.0) | 0 | (0.0) | 5 | (1.2) | 0 | (0.0) | 0.589 | 1 | (3.1) | 4 | (0.9) | 0 | (0.0) | 0.398 |
| Hepatic encephalopathy | 2 | (0.4) | 0 | (0.0) | 2 | (0.5) | 0 | (0.0) | 0.810 | 1 | (3.1) | 1 | (0.2) | 0 | (0.0) | 0.040 |
| Hepatocellular carcinoma | 12 | (2.4) | 2 | (4.9) | 8 | (1.9) | 2 | (4.3) | 0.329 | 1 | (3.1) | 9 | (2.1) | 2 | (5.9) | 0.359 |
| FIB-4, mean (SD) | 2.3 | (2.2) | 2.8 | (2.3) | 2.3 | (2.2) | 2.3 | (1.1) | 0.289 | 2.4 | (1.8) | 2.3 | (2.2) | 1.9 | (0.8) | 0.578 |
| Cirrhosis (FIB-4 greater than 0.7) | 5 | (1.0) | 2 | (4.9) | 3 | (0.7) | 0 | (0.0) | 0.030 | 0 | (0.0) | 4 | (0.9) | 1 | (2.9) | 0.436 |
| Metabolic health | ||||||||||||||||
| Number of metabolic abnormalities | 0.001 | 0.020 | ||||||||||||||
| Low | 337 | (67.0) | 17 | (41.5) | 292 | (70.2) | 28 | (60.9) | 19 | (59.4) | 302 | (69.1) | 16 | (47.1) | ||
| High | 166 | (33.0) | 24 | (58.5) | 124 | (29.8) | 18 | (39.1) | 13 | (40.6) | 135 | (30.9) | 18 | (52.9) | ||
| Geriatric conditions | ||||||||||||||||
| Sarcopenic obesity | 37 | (7.4) | 11 | (26.8) | 19 | (4.6) | 7 | (15.2) | <0.001 | 7 | (21.9) | 26 | (6.0) | 4 | (11.8) | 0.002 |
| Cognitive impairment | 86 | (17.1) | 16 | (39.0) | 57 | (13.7) | 13 | (28.3) | <0.001 | 9 | (28.1) | 65 | (14.9) | 12 | (35.3) | 0.002 |
| Depressed mood | 19 | (3.8) | 7 | (17.1) | 8 | (1.9) | 4 | (8.7) | <0.001 | 11 | (34.4) | 4 | (0.9) | 4 | (11.8) | <0.001 |
Data are n (%) unless otherwise specified. PCS indicates Physical Component Summary score of the Short Form (12) Health Survey version 2; MCS, Mental Component Summary score of the Short Form (12) Health Survey version 2; and FIB-4, Fibrosis-4 Index for Liver Fibrosis.
Continuous variables were analyzed using a one-way analysis of variance, whereas categorical variables (proportions) were analyzed using the chi-square test or Fisher’s exact test.
3.1. Trajectories of physical health and mental health changes
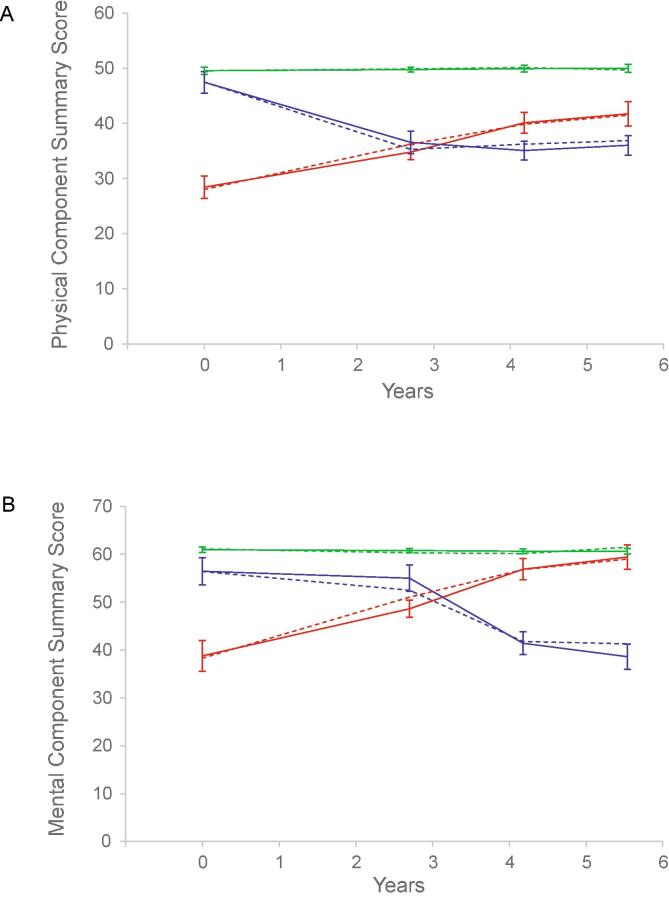
Group-based trajectory modeling analysis yielded three distinct trajectories of PCS score changes over a mean follow-up time of 5.1 years: “poor physical health” (group 1); “good physical health” (group 2); and “declining physical health” (group 3) (Fig. 1A; Supplementary Table 1). For the MCS score, we likewise identified three distinct trajectories of changes: “poor mental health” (group 1); “good mental health” (group 2); and “declining mental health” (group 3) (Fig. 1B; Supplementary Table 1).
Fig. 1.
Trajectories of health-related quality of life changes over time. Changes of the Physical Component Summary (PCS) (A) and Mental Component Summary (MCS) (B) scores of the Short Form (12) Health Survey version 2 over time since baseline assessment were modeled using a group-based trajectory modeling procedure. For the PCS score, 416 individuals (82.7%; group 2) were assigned to a trajectory featuring a persistently normal PCS score that did not change much over time (good physical health) (green lines), 46 (9.1%; group 3) to a trajectory featuring a rapid decline from an initially near-normal PCS score (declining physical health) (blue lines), and 41 (8.2%; group 1) to a trajectory that began with a very low PCS score (poor physical health) (red lines). For the MCS score, 437 individuals (86.9%; group 2) were assigned to a trajectory featuring a persistently high MCS score that did not change much over time (good mental health) (green lines), 34 (6.8%; group 3) to a trajectory featuring a gradual decline from an initially high-normal MCS score (declining mental health) (blue lines), and 32 (6.4%; group 1) to a trajectory that began with a very low MCS score (poor mental health) (red lines). Dashed lines represent identified trajectories; solid lines, predicted trajectories. The error bars represent 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Trajectory groups differed in diverse baseline characteristics
Participants who experienced different trajectories of PCS changes and different trajectories of MCS changes differed significantly in sociodemographic characteristics and prevalence of metabolic abnormalities, sarcopenic obesity, cognitive impairment, depressed mood, and diseases (Table 1). Also, compared with other groups, a larger proportion of participants with poor physical health were former alcohol drinkers. There were no marked differences in proportion of participants receiving CHB treatment or having hepatic complications.
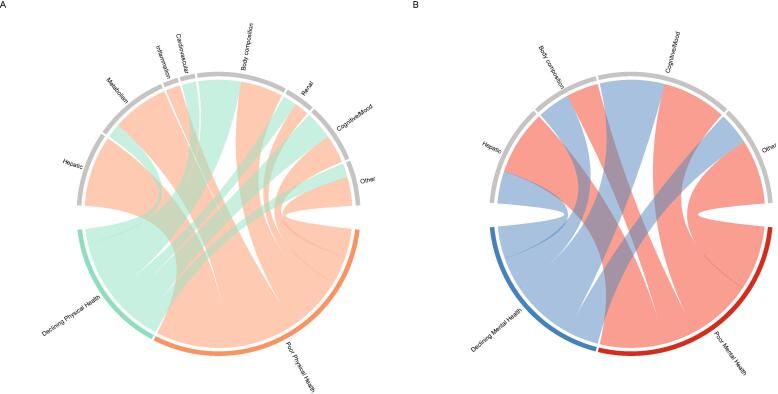
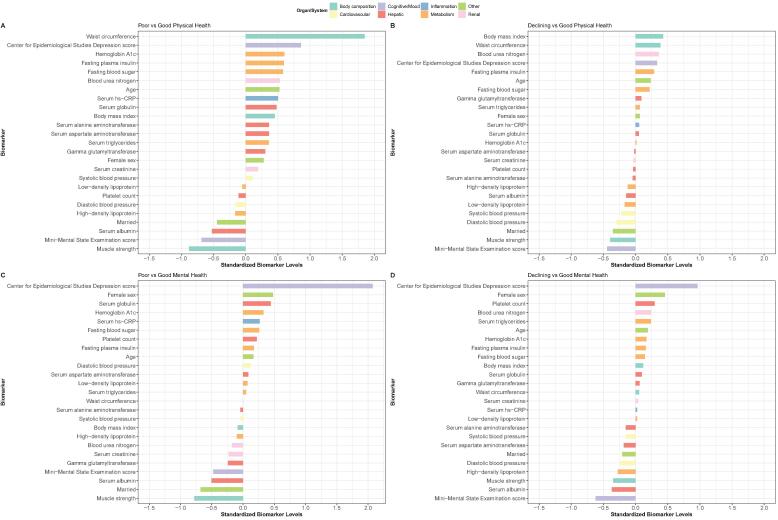
The patterns of organ/system abnormalities differed between physical health trajectory groups (Fig. 2A). Marked deviations in the levels of body composition and metabolic markers, in addition to cognition and mood, were observed in participants who already had a very low PCS score at the beginning (Fig. 3A). A trend of similar deviations, although less in degree, was also noted in those who began with a near-normal PCS score but experienced a rapid decline thereafter (Fig. 3B).
Fig. 2.
Organ/system abnormalities by health-related quality of life trajectory groups. Patterns of organ/system abnormalities by Physical Component Summary score trajectory groups (A) and Mental Component Summary score trajectory groups (B) are shown. Where there was a difference in the levels of a biomarker of an organ/system between a trajectory group and the cohort consisting of that trajectory group and the reference group (group 2 [good physical health] in A; group 2 [good mental health] in B, a ribbon is connected between the organ/system and the trajectory group. The more biomarkers of an organ/system showing differences between a trajectory group and the cohort consisting of that trajectory group and the reference group, the broader the ribbon.
Fig. 3.
Variation in biomarker levels across the health-related quality of life trajectory groups. Variation in the levels of organ/system biomarkers across Physical Component Summary score trajectory groups (A and B) and Mental Component Summary score trajectory groups (C and D) are shown. The biomarker levels (x-axis) are standardized. A value of 1 indicates that biomarker levels in participants of the trajectory group (group 1 [poor physical health] in A; group 3 [declining physical health] in B; group 1 [poor mental health] in C; group 3 [declining mental health] in D) were, on average, 1 standard deviation higher than the mean level across all participants in that trajectory group and the reference group (group 2 [good physical health] in A and B; group 2 [good mental health] in C and D). We ranked the biomarkers (y-axis) according to the standardized mean difference (from most positive to most negative). The biomarker levels of different organs/systems are shown in different colors.
For mental health trajectory groups, patterns of organ/system abnormalities different from those associated with physical health trajectories were noted (Fig. 2B). We observed deviations predominantly in cognition and mood measures in participants who already had a very low MCS score at the beginning of the study period (Fig. 3C). Deviations in muscle strength levels were also observed in this group of older adults with CHB. Participants who began with a high MCS score but experienced a gradual decline over time had generally similar, although less pronounced, alterations in biomarkers (Fig. 3D).
3.3. Associations of baseline characteristics with trajectory groups
In multinomial logistic regression analyses after adjustment for multiple variables (Table 2), participants with a higher number of metabolic abnormalities had a 3.6-fold (95% confidence interval [95% CI], 1.6–8.0; p = .002) greater odds of already having a very low PCS score at the beginning of the study (poor physical health) than those without. Sarcopenic obesity was associated with as high as 7.5-fold (95% CI, 2.8–20.5; p < .001) greater odds of being in the poor physical health trajectory group and 3.1-fold (95% CI, 1.1–8.4; p = .028) greater odds of being in the declining physical health trajectory group. We observed similar results when insulin resistance was defined on the basis of an HOMA-IR greater than 1 (Supplementary Table 2). Independent of other characteristics, depressed mood was associated with greater odds of being in the poor physical health trajectory group and of being in the declining physical health trajectory group (Table 2 and Supplementary Table 2).
Table 2.
Multivariate-adjusted odds ratios of physical health trajectory groups associated with baseline characteristics.a
| Group 1 | Group 3 | |||||
|---|---|---|---|---|---|---|
| (Poor Physical Health) |
(Declining Physical Health) |
|||||
| Characteristic | Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P |
| Number of metabolic abnormalities | ||||||
| Low | 1.0 | (Reference) | 1.0 | (Reference) | ||
| High | 3.6 | 1.6–8.0 | 0.002 | 1.3 | 0.7–2.6 | 0.419 |
| Sarcopenic obesity | 7.5 | 2.8–20.5 | <0.001 | 3.1 | 1.1–8.4 | 0.028 |
| Age per 1-y increase | 1.0 | 1.0–1.1 | 0.082 | 1.0 | 1.0–1.1 | 0.286 |
| Male sex | 1.1 | 0.4–3.5 | 0.857 | 0.6 | 0.2–1.5 | 0.298 |
| Married status | 0.5 | 0.2–1.1 | 0.080 | 0.6 | 0.3–1.2 | 0.131 |
| Arthritis | 7.2 | 3.0–17.2 | <0.001 | 1.9 | 0.8–4.3 | 0.147 |
| Depressed mood | 21.7 | 5.8–81.0 | <0.001 | 5.3 | 1.4–19.9 | 0.013 |
| Smoking | ||||||
| Never | 1.0 | (Reference) | 1.0 | (Reference) | ||
| Ever | 0.7 | 0.2–3.2 | 0.687 | 0.7 | 0.2–2.3 | 0.585 |
| Current | 1.3 | 0.3–4.7 | 0.715 | 0.4 | 0.1–1.8 | 0.246 |
| Alcohol drinking | ||||||
| Never | 1.0 | (Reference) | 1.0 | (Reference) | ||
| Ever | 2.2 | 0.7–7.0 | 0.165 | 2.1 | 0.7–6.2 | 0.164 |
| Current | 0.5 | 0.1–1.9 | 0.295 | 1.1 | 0.4–2.7 | 0.892 |
| FIB-4 | 1.1 | 1.0–1.3 | 0.017 | 1.0 | 0.8–1.2 | 0.968 |
Relative to group 2 (good physical health). FIB-4 indicates Fibrosis-4 Index for Liver Fibrosis.
After adjustment for multiple variables, participants with depressed mood at baseline had an 83.1-fold (95% CI, 19.7–350.2; p < .001) greater odds of being in the poor mental health trajectory group and 13.6-fold (95% CI, 2.9–64.8; p = .001) greater odds of being in the declining mental health trajectory group (Table 3). Interestingly, participants with sarcopenic obesity also had greater odds of being in the poor mental health trajectory group (Table 3). Similar results were observed in the sensitivity analysis in which insulin resistance was defined on the basis of an HOMA-IR greater than 1 (Supplementary Table 3).
Table 3.
Multivariate-adjusted odds ratios of mental health trajectory groups associated with baseline characteristics.a
| Group 1 | Group 3 | |||||
|---|---|---|---|---|---|---|
| (Poor Mental Health) |
(Declining Mental Health) |
|||||
| Characteristic | Odds Ratio | 95% CI | P | Odds Ratio | 95% CI | P |
| Number of metabolic abnormalities | ||||||
| Low | 1.0 | (Reference) | 1.0 | (Reference) | ||
| High | 1.3 | 0.5–3.2 | 0.581 | 2.3 | 1.1–4.8 | 0.034 |
| Sarcopenic obesity | 4.3 | 1.4–13.0 | 0.010 | 1.3 | 0.4–4.4 | 0.666 |
| Age per 1-y increase | 1.0 | 0.9–1.1 | 0.873 | 1.0 | 1.0–1.1 | 0.170 |
| Male sex | 0.4 | 0.1–1.7 | 0.206 | 0.2 | 0.1–0.7 | 0.013 |
| Married status | 0.3 | 0.1–0.8 | 0.021 | 0.8 | 0.3–2.0 | 0.643 |
| Depressed mood | 83.1 | 19.7–350.2 | <0.001 | 13.6 | 2.9–64.8 | 0.001 |
| Smoking | ||||||
| Never | 1.0 | (Reference) | 1.0 | (Reference) | ||
| Ever | 3.2 | 0.6–16.3 | 0.163 | 1.6 | 0.4–7.4 | 0.524 |
| Current | 0.4 | 0.1–3.5 | 0.447 | 1.2 | 0.3–5.7 | 0.815 |
| Alcohol drinking | ||||||
| Never | 1.0 | (Reference) | 1.0 | (Reference) | ||
| Ever | 1.2 | 0.2–5.8 | 0.836 | 3.3 | 1.0–11.3 | 0.058 |
| Current | 1.5 | 0.5–5.1 | 0.479 | 2.3 | 0.8–6.4 | 0.128 |
| FIB-4 | 1.1 | 0.9–1.3 | 0.307 | 0.8 | 0.5–1.2 | 0.195 |
Relative to group 2 (good mental health). FIB-4 indicates Fibrosis-4 Index for Liver Fibrosis.
4. Discussion
Through group-based trajectory analysis of longitudinal data gathered from serial examinations, we identified patterns of HRQoL changes among older adults with CHB. Moreover, we showed that the degree of extrahepatic organ/system abnormality (in particular, sarcopenic obesity, metabolic abnormalities, and depressed mood) was independently associated with differential changes in physical and mental health in older adults with CHB.
Health is featured by its dimensionality and encompasses two distinct major constructs: physical health and mental health (Maruish, 2012, Salomon et al., 2003). As such, it is argued that attempts to measure health should include measurements of both physical and mental functioning and well-being (Maruish, 2012, Salomon et al., 2003). This is particularly true for patients with chronic diseases (Stewart et al., 1989). In response to the need for assessing the health impacts of diseases, the concept of HRQoL was developed, and its measures were constructed (Maruish, 2012).
Examination of the effects of chronic hepatitis on health as assessed by HRQoL represents a major focus of research. Using the Short Form Health Survey scale, multiple studies showed that chronic hepatitis C virus infection is significantly associated with both poor physical health and poor mental health (Boscarino et al., 2015, Younossi et al., 2016). However, much less is known about HRQoL in adults, particularly older adults, with CHB.
This is the first study, to our knowledge, to identify distinct HRQoL trajectories that might occur in older adults affected by CHB. Traditional approaches of estimating a trajectory rely on substantial a priori assumptions about the trajectory itself, including ad hoc classification rules (Nagin, 2005). Group-based trajectory modeling, on the other hand, takes a data-driven approach and enables discovery of distinct courses of change that would otherwise be difficult to detect using traditional methods (Nagin, 2005). Applying this data-driven analytic approach, we showed that not all older adults with CHB experienced health decline. Those with normal initial physical health were most likely to stay healthy physically, whereas those with initial physical health that was near-normal (with marginally low PCS scores) were likely to experience a rapid decline in the following years. Those with very poor physical health at the beginning tended to remain unhealthy physically throughout the following years, although with a trend of increasing the PCS score over time. For mental health, we likewise identified three distinct trajectories. Those with good initial mental health were most likely to stay mentally healthy, and those with normal initial mental health (with high-normal MCS scores) were likely to experience a decline in the following years. Participants with poor mental health at the beginning tended to remain mentally unhealthy, with a trend of increasing MCS score over time.
This study further provides an exploratory examination of diverse baseline characteristics in relation to the HRQoL trajectories in older adulthood, which may reveal some mechanistic hints. Obesity and related cardiometabolic risks are increasingly prevalent globally, especially in Asia (Ng et al., 2014, Yoon et al., 2006). Notably, an increasing amount of research indicates contribution of these risks to hepatic complications in patients with CHB (Huang et al., 2013, Stepanova et al., 2010, Wong et al., 2009, Yu et al., 2017, Yu et al., 2008). Recent research suggests that, owing to their complex interrelationship, multiple metabolic abnormalities in aggregate may additionally increase the risk of hepatic complications of CHB (Yu et al., 2017). Our study showed that among older adults with CHB, those with multiple metabolic abnormalities are likely to have overall poor physical functioning and well-being. Interestingly, we noticed that even among those with physical health still within normal limits, those simply having sarcopenic obesity were at a high risk of physical health decline in the near future. These associations are in general consistent with the emerging roles of skeletal muscle in the early pathogenic cascades of multiple obesity-related metabolic disorders (Lawan et al., 2018). In this study, we also observed an association of sarcopenic obesity with a mental health trajectory characterized by poor baseline mental health, which agrees with recent studies (Hamer et al., 2015).
We found that depressed mood at baseline was independently associated with greater odds of being in poor mental health and declining mental health trajectory groups as well as greater odds of being in poor physical health and declining physical health trajectory groups. Although previous studies have demonstrated the high risk of depressed mood and its deleterious effects on HRQoL in patients with chronic viral hepatitis C (Boscarino et al., 2015, Gallegos-Orozco et al., 2003, Golden et al., 2005, Younossi et al., 2016), much less is known about mood disorders in patients with CHB. In this study, only 19 (3.8%) of 503 older adults with CHB had a depressed mood. In contrast to those with chronic hepatitis C, in whom a depression prevalence of 24.5% has been reported (Younossi et al., 2016), a much lower prevalence was observed in patients with CHB (Carta et al., 2007). Despite its low prevalence, our study demonstrated that depressed mood in older adults with CHB might accompany mental health in a poor state or even precede its decline. Notably, we revealed an association between depressed mood and physical health that was either in decline or in a poor state. Cross-sectional studies have showed that patients with chronic hepatitis C who had depressive mood had poorer physical health in addition to poorer mental health (Gallegos-Orozco et al., 2003, Hauser et al., 2004). Interestingly, a recent observation in patients with chronic hepatitis C suggested that depression and poor physical health might share common risk factors (Boscarino et al., 2015).
In contrast to other markers, fewer variations in hepatic marker levels existed among participants following different physical health and mental health trajectories. Also, we failed to observe significant differences among these participants in the prevalence of either hepatic complications or viral-load-lowering antiviral therapies. In multinomial logistic regression analyses after adjustment for multiple variables, a higher FIB-4 index, indicating a greater likelihood of HBV-related liver fibrosis, was independently associated greater odds of being allocated to the poor physical health trajectory. However, no association was found with the declining physical health trajectory. After adjustment for other variables, the FIB-4 index was not associated with any mental health trajectories. The relationships of cirrhosis and its stages with physical health and mental health may be partly due to (or confounded by) sarcopenic obesity, depressed mood, and other comorbid conditions. Our findings are consistent with the results of previous works. Previous studies of patients with chronic hepatitis C found an association of cirrhosis with physical health only (Younossi et al., 2016). Other studies showed that after taking into account psychiatric disorders and other comorbid diseases, the severity of liver disease was no longer associated with physical health and mental health in patients with chronic liver disease (Hauser et al., 2004). Complex relationships between infection severity and HRQoL in CHB patients were illustrated by recent studies. In a group of 242 CHB patients, Younossi el al. showed that a decrease in HBV DNA by antiviral therapies was associated with an improvement in mental health but not in physical health (Younossi et al., 2018). HBV viral suppression was neither associated with improvements in physical health nor with improvements in mental health (Younossi et al., 2018).
Our study has several limitations. First, this is an observational study. The possibility of residual confounding could not be excluded. Second, study participants consisted mainly of community-dwelling older adults with CHB, with very few having hepatic complications of CHB. Findings may not be generalizable to patient populations with greater severity of the disease. Third, to capture the effects of diverse comorbid conditions that are prevalent in older adults, we assessed HRQoL by using a generic measure rather than disease-specific measures. In addition to comparisons across diseases or conditions, as enabled by the Short Form Health Survey, future studies examining changes in disease-specific measures of HRQoL are likely to provide additional information about late-life health in patients with CHB. Another limitation is that sociodemographics, lifestyles, metabolic health, geriatric conditions, and comorbid diseases/conditions were only assessed at baseline. We lacked information on the status of these during the follow-up period, and were unable to estimate the effects of these changes on the observed physical and mental health trajectories. Finally, we evaluated the hidden nonalcoholic steatohepatitis using non-invasive serum biomarkers. The standard liver biopsy should be included in future researches to assess the potential effects of nonalcoholic fatty liver disease across its spectrum of severity.
In conclusion, this longitudinal observational study of older adults with CHB demonstrated that HRQoL changes in later life, although quite varied, followed distinct patterns. Three nonlinear trajectories of physical health and mental health changes were revealed. Also, this study documented that differential changes in HRQoL in older adults with HBV were associated with variations in specific extrahepatic organs/systems, namely degree of sarcopenic obesity, metabolic abnormalities, and depressed mood. The hidden complexity indicates the potential value of comprehensive assessment and management in this geriatric population and underscores the need for further research.
Funding
This work was supported by the National Health Research Institutes in Taiwan. (Project nos. BS-097-SP-04, PH-098-SP-02, PH-099-SP-01, PH-100-SP-01, PH-101-SP-01, PH-102-SP-01, PH-103-SP-01, PH-104-SP-01, PH-105-SP-01, PH-106-SP-01, PH-107-SP-01, PH-107-PP-22, PH-108-SP-01, PH-108-PP-22). The sponsors had no roles in the design, methods, subject recruitment, data collections, analysis, or preparation of the paper.
CRediT authorship contribution statement
Chang-Hua Chen: Conceptualization, Methodology, Investigation, Writing - original draft. Ming-Shiang Wu: Data curation, Formal analysis, Project administration, Visualization, Writing - original draft. Yu-Wen Yang: Data curation, Formal analysis, Project administration, Visualization, Investigation, Writing - review & editing. Yen-Tze Liu: Investigation, Writing - review & editing. Yen-Feng Chiu: Investigation, Writing - review & editing. Chih-Cheng Hsu: Investigation, Writing - review & editing. Shu-Chun Chuang: Investigation, Writing - review & editing. Tieh-Chi Chung: Investigation, Writing - review & editing. Tsung-Lung Tsai: Investigation, Writing - review & editing. Wen-Hao Huang: Investigation, Writing - review & editing. Wei-Lin Huang: Investigation, Writing - review & editing. Chung-Chou Juan: Investigation, Writing - review & editing. Li-Ming Lien: Investigation, Writing - review & editing. Chao A. Hsiung: Investigation. I-Chien Wu: Conceptualization, Methodology, Investigation, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank all members of the HALST study group. The members of the HALST study group are as follows: Drs. Chao-Agnes Hsiung, Chih-Cheng Hsu, I-Chien Wu, Hsing-Yi Chang, Chu-Chih Chen, Yen-Feng Chiu, Hui-Ju Tsai, and Shu-Chun Chuang of the National Heath Research Institutes; Dr. Ching-Yu Chen of National Taiwan University; Dr. Kiang Liu of Northwestern University Medical School; Dr. Marion Lee of University of California at San Francisco; Dr. Ida Chen of University of California at Los Angeles; Dr. Li-Ming Lien of Shin Kong Wu Ho-su Memorial Hospital; Dr. Wen-Jin Liaw of Yee Zen General Hospital; Dr. Tieh-Chi Chung of Hope Doctors Hospital; Dr. I-Ching Lin of Changhua Christian Hospital; Dr. Tsung-Lung Tsai of Puzi Hospital; Dr. Chung-Chou Juan of Yuan's General Hospital; and Dr. Chi-Chung Wang of Mennonite Christian Hospital. We also acknowledge the significant contributions of the HALST staff.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101432.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Beard J.R., Officer A., de Carvalho I.A., Sadana R., Pot A.M., Michel J.P., Lloyd-Sherlock P., Epping-Jordan J.E., Peeters G.M. The World report on ageing and health: a policy framework for healthy ageing. Lancet (London, England) 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J.A., Lu M., Moorman A.C., Gordon S.C., Rupp L.B., Spradling P.R., Teshale E.H., Schmidt M.A., Vijayadeva V. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: the Chronic Hepatitis Cohort Study (CHeCS) Hepatology (Baltimore Md.) 2015;61:802–811. doi: 10.1002/hep.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert M., Blazeby J., Altman D.G., Revicki D.A., Moher D., Brundage M.D. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309:814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- Carta M.G., Hardoy M.C., Garofalo A., Pisano E., Nonnoi V., Intilla G., Serra G., Balestrieri C., Chessa L. Association of chronic hepatitis C with major depressive disorders: irrespective of interferon-alpha therapy. Clinical practice and epidemiology in mental health. CP & EMH. 2007;3:22. doi: 10.1186/1745-0179-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.D., Yang H.I., Iloeje U.H., You S.L., Lu S.N., Wang L.Y., Su J., Sun C.A., Liaw Y.F. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747–1754. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- Dorr D.A., Jones S.S., Burns L., Donnelly S.M., Brunker C.P., Wilcox A., Clayton P.D. Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. J. Am. Geriatr. Soc. 2006;54:667–673. doi: 10.1111/j.1532-5415.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- Fuh J.L., Wang S.J., Lu S.R., Juang K.D., Lee S.J. Psychometric evaluation of a Chinese (Taiwanese) version of the SF-36 health survey amongst middle-aged women from a rural community. Qual. Life Res. 2000;9:675–683. doi: 10.1023/a:1008993821633. [DOI] [PubMed] [Google Scholar]
- Gallegos-Orozco J.F., Fuentes A.P., Gerardo Argueta J., Perez-Pruna C., Hinojosa-Becerril C., Sixtos-Alonso M.S., Cruz-Castellanos S., Gutierrez-Reyes G., Olivera-Martinez M.A. Health-related quality of life and depression in patients with chronic hepatitis C. Arch. Med. Res. 2003;34:124–129. doi: 10.1016/s0188-4409(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Golden J., O'Dwyer A.M., Conroy R.M. Depression and anxiety in patients with hepatitis C: prevalence, detection rates and risk factors. Gen. Hosp. Psychiatry. 2005;27:431–438. doi: 10.1016/j.genhosppsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hamer M., Batty G.D., Kivimaki M. Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. Int. J. Obesity. 2015;2005(39):1717–1720. doi: 10.1038/ijo.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W., Holtmann G., Grandt D. Determinants of health-related quality of life in patients with chronic liver diseases. Clin. Gastroenterol. Hepatol. 2004;2:157–163. doi: 10.1016/s1542-3565(03)00315-x. [DOI] [PubMed] [Google Scholar]
- Hsu C.C., Chang H.Y., Wu I.C., Chen C.C., Tsai H.J., Chiu Y.F., Chuang S.C., Hsiung W.C., Tsai T.L. Cohort Profile: The Healthy Aging Longitudinal Study in Taiwan (HALST) Int. J. Epidemiol. 2017;46:1106–1206. doi: 10.1093/ije/dyw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.Y., Chang M.H., Chen D.S., Lee C.Y., Sung J.L. Baseline seroepidemiology of hepatitis B virus infection in children in Taipei, 1984: a study just before mass hepatitis B vaccination program in Taiwan. J. Med. Virol. 1986;18:301–307. doi: 10.1002/jmv.1890180402. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Wang T.C., Lin S.C., Chang H.Y., Chen D.S., Hu J.T., Yang S.S., Kao J.H. Increased risk of cirrhosis and its decompensation in chronic hepatitis B patients with newly diagnosed diabetes: a nationwide cohort study. Clin. Infect. Dis. 2013;57:1695–1702. doi: 10.1093/cid/cit603. [DOI] [PubMed] [Google Scholar]
- Jones B.L., Nagin D.S. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol. Methods Res. 2007;35:542–571. [Google Scholar]
- Ju J.F.R., Tseng H.M., Tsai Y.J. Assessment of health-related quality of life in Taiwan (I): development and psychometric testing of SF-36 Taiwan version. Taiwan J. Publ. Health. 2002;22:501–511. [Google Scholar]
- Lai J.C., Covinsky K.E., McCulloch C.E., Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am. J. Gastroenterol. 2018;113:235–242. doi: 10.1038/ajg.2017.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawan A., Min K., Zhang L., Canfran-Duque A., Jurczak M.J., Camporez J.P.G., Nie Y., Gavin T.P., Shulman G.I. Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes. 2018;67:624–635. doi: 10.2337/db17-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruish M.E. thrid ed. QualityMetric Incorporated; Lincoln, RI: 2012. User’s Manual for the SF-12v2 Health Survey. [Google Scholar]
- Nagin D. Harvard University Press; Cambridge, Massachusetts: 2005. Group-based Modeling of Development. [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D.L., Erickson P. Oxford University Press; New York: 1993. Health Status and Health Policy: Quality of Life in Health Care Evaluation and Resource Allocation. [Google Scholar]
- Powers J.H., 3rd, Howard K., Saretsky T., Clifford S., Hoffmann S., Llorens L., Talbot G. Patient-reported outcome assessments as endpoints in studies in infectious diseases. Clin. Infect. Dis. 2016;63(Suppl 2):S52–S56. doi: 10.1093/cid/ciw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsfeld J.S., MaWhinney S., McCarthy M., Jr., Shroyer A.L., VillaNueva C.B., O'Brien M., Moritz T.E., Henderson W.G., Grover F.L. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281:1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- Salomon J.A., Mathers C.D., Chatterji S., Sadana R., Ustun T.B., Murray C.J.L. Quantifying individual levels of health: definitions, concepts and measurement issues. In: Murray C.J.L., Evans D.B., editors. Health Systems Performance Assessment: Debates, Measures and Empiricism. World Health Organization; Geneva: 2003. pp. 301–318. [Google Scholar]
- Stanaway J.D., Flaxman A.D., Naghavi M., Fitzmaurice C., Vos T., Abubakar I., Abu-Raddad L.J., Assadi R., Bhala N. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet (London, England) 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova M., Rafiq N., Younossi Z.M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59:1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- Stewart A.L., Greenfield S., Hays R.D., Wells K., Rogers W.H., Berry S.D., McGlynn E.A., Ware J.E., Jr. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- Tseng H.M., Lu J.F.R., Tsai Y.J. Assessment of health-related quality of life in Taiwan (Ⅱ): norming and validation of SF-36 Taiwan version. Taiwan J Publ. Health. 2002;22:512–518. [Google Scholar]
- U. S. Department of Health and Human Services F. D. A. Center for Drug Evaluation and Research, U. S. Department of Health and Human Services F. D. A. Center for Biologics Evaluation and Research, U. S. Department of Health and Human Services F. D. A. Center for Devices and Radiological Health, 2009. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims.
- U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion Healthy People 2020 2018 Washington DC. [PubMed]
- Ware J.E., Jr. The status of health assessment 1994. Annu. Rev. Public Health. 1995;16:327–354. doi: 10.1146/annurev.pu.16.050195.001551. [DOI] [PubMed] [Google Scholar]
- Wong G.L., Wong V.W., Choi P.C., Chan A.W., Chim A.M., Yiu K.K., Chan H.Y., Chan F.K., Sung J.J. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58:111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H., Zimmet P., Son H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet (London, England) 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- Younossi Z., Park H., Henry L., Adeyemi A., Stepanova M. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology. 2016;150:1599–1608. doi: 10.1053/j.gastro.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Stepanova M., Janssen H.L.A., Agarwal K., Nguyen M.H., Gane E., Tsai N., Younossi I., Racila A. Effects of Treatment of Chronic Hepatitis B Virus Infection on Patient-Reported Outcomes. Clin. Gastroenterol. Hepatol. 2018;16 doi: 10.1016/j.cgh.2018.02.037. 1641-49.e6. [DOI] [PubMed] [Google Scholar]
- Yu M.W., Lin C.L., Liu C.J., Yang S.H., Tseng Y.L., Wu C.F. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: a large cohort study. Gastroenterology. 2017;153 doi: 10.1053/j.gastro.2017.07.001. 1006-17.e5. [DOI] [PubMed] [Google Scholar]
- Yu M.W., Shih W.L., Lin C.L., Liu C.J., Jian J.W., Tsai K.S., Chen C.J. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J. Clin. Oncol. 2008;26:5576–5582. doi: 10.1200/JCO.2008.16.1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.