Key Points
Question
Are chilblain-like lesions of the toes associated with SARS-CoV-2 infection or is the association merely temporal?
Findings
This case series of 17 adolescents found that chilblain-like lesions of the toes emerged during the COVID-19 pandemic in otherwise healthy adolescents without signs of SARS-CoV-2 infection or other inflammatory, autoimmune, or thrombophilic phenomena.
Meaning
These results suggest that chilblain-like lesions are not associated with systemic or localized SARS-CoV-2 infection.
Abstract
Importance
Chilblain-like lesions have been one of the most frequently described cutaneous manifestations during the COVID-19 pandemic. Their etiopathogenesis, including the role of SARS-CoV-2, remains elusive.
Objective
To examine the association of chilblain-like lesions with SARS-CoV-2 infection.
Design, Setting, and Participants
This prospective case series enrolled 17 adolescents who presented with chilblain-like lesions from April 1 to June 30, 2020, at a tertiary referral academic hospital in Italy.
Main Outcomes and Measures
Macroscopic (clinical and dermoscopic) and microscopic (histopathologic) analysis contributed to a thorough understanding of the lesions. Nasopharyngeal swab, serologic testing, and in situ hybridization of the skin biopsy specimens were performed to test for SARS-CoV-2 infection. Laboratory tests explored signs of systemic inflammation or thrombophilia. Structural changes in peripheral microcirculation were investigated by capillaroscopy.
Results
Of the 17 adolescents (9 [52.9%] male; median [interquartile range] age, 13.2 [12.5-14.3] years) enrolled during the first wave of the COVID-19 pandemic, 16 (94.1%) had bilaterally localized distal erythematous or cyanotic lesions. A triad of red dots (16 [100%]), white rosettes (11 [68.8%]), and white streaks (10 [62.5%]) characterized the dermoscopic picture. Histologic analysis revealed a remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate. SARS-CoV-2 infection was excluded by molecular and serologic testing. In situ hybridization did not highlight the viral genome in the lesions.
Conclusions and Relevance
This study delineated the clinical, histologic, and laboratory features of chilblain-like lesions that emerged during the COVID-19 pandemic, and its findings do not support their association with SARS-CoV-2 infection. The lesions occurred in otherwise healthy adolescents, had a long but benign course to self-resolution, and were characterized by a microvascular remodeling with perivascular lymphocytic infiltrate but no other signs of vasculitis. These results suggest that chilblain-like lesions do not imply a concomitant SARS-CoV-2 infection. Ongoing studies will help clarify the etiopathogenic mechanisms.
This case series examines the association of chilblain-like lesions with SARS-CoV-2 infection.
Introduction
SARS-CoV-2 was first identified in January 2020. Since then, COVID-19 disease has widely spread worldwide, becoming a pandemic emergency. Unlike adults, children are more mildly affected by SARS-CoV-2 for reasons that have not been fully elucidated.1 The most frequent symptoms in the pediatric population include fever (80%), cough (50%), rhinorrhea (27%), dyspnea (9%), sore throat (5%), and fatigue (2%),2 but diarrhea,2,3 dysgeusia, and anosmia (8%-13%) also occur. Numerous cutaneous manifestations have been reported during the pandemic, including extremely protean pictures, such as erythematous, varicelliform or morbilliform rash, and urticaria.4,5,6,7,8,9,10 However, a causative role of SARS-CoV-2 has not been confirmed, and the pathophysiologic mechanisms remain unknown.6
Cases of acral lesions resembling classic chilblains have been reported worldwide at a higher rate than before the pandemic,10,11 becoming one of the most commonly reported cutaneous manifestations during the pandemic. Also known as chilblain-like lesions or COVID toes, these acral lesions are described as erythematous to purple purpuric macules, papules, and/or vesicles that predominantly involve the feet and, to a lesser extent, the hands.12,13 Unlike other cutaneous findings, chilblain-like lesions tend to mostly affect patients without systemic or evident COVID-19 symptoms14; in fact, patients with such eruptions are less likely to have severe disease.15 SARS-CoV-2 infection remains unconfirmed in many patients with these lesions. Different pathogenic hypotheses have been advanced, including a delayed cytokine-mediated inflammatory response induced by SARS-CoV-2,16 viral-induced endothelial changes, obliterative microangiopathy, and coagulation abnormalities,17 although coagulation abnormalities have been related to acroischemia rather than COVID toes. However, none of these hypotheses have been convincingly confirmed, and a causal association with the virus has not been demonstrated. On the other hand, exposure to cold temperatures, typical of classic perniosis, has not been consistently reported, probably because of different geographic locations. This study examines the association of chilblain-like lesions with SARS-CoV-2 infection among pediatric patients
Methods
Study Design and Patients
In this cases series, we prospectively enrolled 17 pediatric patients referred by family pediatricians for chilblain-like lesions to our Pediatric Infectious Disease and Rheumatology Unit at the University of Naples Federico II, Naples, Italy, designated as the Regional COVID-19 Pediatric Hub. Exclusion criteria included other nonacral skin lesions. Seventeen children were enrolled from April 1 to June 30, 2020 (Table). At enrollment, all patients underwent a thorough clinical examination, laboratory workup, dermatologic evaluation (including dermoscopy),18 and a skin biopsy to investigate the histologic features and the presence of SARS-CoV-2. All patients underwent capillaroscopy to exclude systemic microvascular alterations.19,20 A follow-up appointment was set at 4 weeks after enrollment and included clinical, laboratory, and dermatologic evaluation. All patients underwent a nasopharyngeal swab for SARS-CoV-2 molecular testing at enrollement and quantitative IgM and IgG serologic testing against SARS-CoV-2 at enrollement and follow-up to investigate the association with SARS-CoV-2 infection. The study protocol was approved by the University of Naples Ethical Committee, and all enrolled patients provided written informed consent. All data were deidentified. The study followed the reporting guideline for case series.
Table. Demographic and Clinical Data of the Study Patients.
| Patient No. | Patient sex | Patient age, y | Localization | Bilateral | Past chilblains | Prechilblains symptoms | COVID-19–related symptoms | SARS-CoV-2 nasopharyngeal swab result | Family history of SARS-CoV-2 | Relevant personal medical history | Family history of autoimmunity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 12.5 | Foot | Yes | No | URTI | None | Negative | Yes | None | No |
| 2 | M | 13.0 | Foot | No | No | URTI | None | Negative | No | None | Thromboangiitis obliterans |
| 3 | M | 11.0 | Foot | Yes | No | None | None | Negative | No | None | Hemolytic anemia during pregnancy |
| 4 | F | 12.5 | Foot | Yes | No | None | None | Negative | No | None | Hemolytic anemia during pregnancy |
| 5 | F | 12.8 | Foot | Yes | No | URTI | None | Negative | No | None | Autoimmune thyroiditis |
| 6 | F | 14.5 | Hand and foot | Yes | No | None | None | Negative | Not confirmed | None | No |
| 7 | F | 12.8 | Foot | Yes | No | None | None | Negative | Not confirmed | None | No |
| 8 | M | 14.5 | Foot | Yes | No | None | None | Negative | No | None | No |
| 9 | F | 10.5 | Hand and foot | Yes | No | None | None | Negative | No | None | No |
| 10 | M | 13.4 | Foot | Yes | No | None | None | Negative | No | Henoch-Schönlein purpura | No |
| 11 | M | 14.0 | Foot | Yes | No | Rhinitis | None | Negative | No | None | Autoimmune thyroiditis |
| 12 | M | 14.5 | Foot | Yes | No | None | None | Negative | No | None | Autoimmune thyroiditis and IBD |
| 13 | M | 14.3 | Foot | Yes | No | None | None | Negative | No | None | Autoimmune thyroiditis |
| 14 | M | 13.2 | Foot | Yes | No | None | None | Negative | No | None | No |
| 15 | M | 13.5 | Foot | Yes | No | None | None | Negative | No | None | No |
| 16 | F | 15.1 | Foot | Yes | No | None | None | Negative | No | None | No |
| 17 | F | 9.0 | Foot | Yes | No | URTI and GI symptoms | None | Negative | Yes | None | Autoimmune thyroiditis |
Abbreviations: GI, gastrointestinal; IBD, inflammatory bowel disease; URTI, upper respiratory tract infection.
Laboratory Workup
Laboratory tests were performed at both enrollement and follow-up. They included complete blood cell count; complete metabolic panel; coagulation; D-dimer; circulating immune complexes (circulating immune complex 1Q and circulating immune complex 3d); complement factors (C3 and C4); inflammatory markers, including procalcitonin, C-reactive protein, erythrocyte sedimentation rate, and ferritin; autoimmune panel, including antinuclear antibodies and extractable nuclear antigen profile; and total immunoglobulins (total IgA, IgM, and IgG).
SARS-CoV-2 Laboratory Tests
Real-time reverse transcriptase–polymerase chain reaction of nasopharyngeal swabs was performed at the centralized laboratory of the University of Naples Federico II. Quantitative SARS-CoV-2 serologic testing was performed by chemiluminescent immunoassay, which reported a sensitivity of 79% and a specificity of 97.5% for IgM (Snibe Diagnostics) and a sensitivity of 100% and a specificity of 99.6% for IgG (Abbott Laboratories).
Macroscopic (Clinical and Dermoscopic) and Microscopic (Histopathologic) Analysis
Patients were examined using video dermoscopy (DermaView) by 3 independent physicians (M.V., P.N., and G.F.) according to the International Dermoscopy Society’s expert consensus.18 In addition, histopathologic analysis was performed with a 3-mm skin punch biopsy taken at enrollement from a representative area of each patient. Then 4-mm-thick formalin-fixed, paraffin-embedded sections were stained with hematoxylin and eosin. Immunohistochemistry was performed as previously described21 with anti-CD3 and anti-CD31 antibodies to highlight the lymphocytic infiltrate and the endothelium, respectively.
In situ hybridization (RNAscope 2.5 HD Assay-BROWN; Advanced Cell Diagnostics) was used according to the manufacturer’s instructions to perform in situ hybridization on 5-μm-thick formalin-fixed, paraffin-embedded skin sections. POLR2A (OMIM 180660) messenger RNA labeling was used as a positive control. Slides were deparaffinized and air dried followed by hydrogen peroxide drying. Slides were submerged in fresh 1× target retrieval (Advanced Cell Diagnostics) at boiling temperature for 15 minutes, washed in deionized water followed by 100% ethanol, and air dried. Protease (Protease Plus; Advanced Cell Diagnostics) was applied for 20 minutes in a hybridization oven at 40 °C. Probes were incubated for 2 hours in a hybridization oven. Signal amplification reagents AMP1 to AMP6 were applied sequentially and incubated in a hybridization oven. 3,3′-Diaminobenzidine detection reagent was incubated for 10 minutes in a hybridization oven. Samples were counterstained with hematoxylin, rinsed with tap water, placed in 0.02% ammonia-water, rinsed with tap water, dehydrated in graded alcohols, treated with xylene, and coverslipped. Videocapillaroscopy19,20 of the fingers was also performed using a high-magnification lens (original magnification ×200) to allow for better visualization of the morphologic features of the capillaries.
Statistical Analysis
For descriptive statistics, data are presented as mean (SD) for continuous values with gaussian distribution, as median and range for continuous values with non-Gaussian distribution, and as counts and percentages for categorical variables. Analyses were performed using GraphPad Prism, version 7.0 (GraphPad Software Inc).
Results
Clinical Features
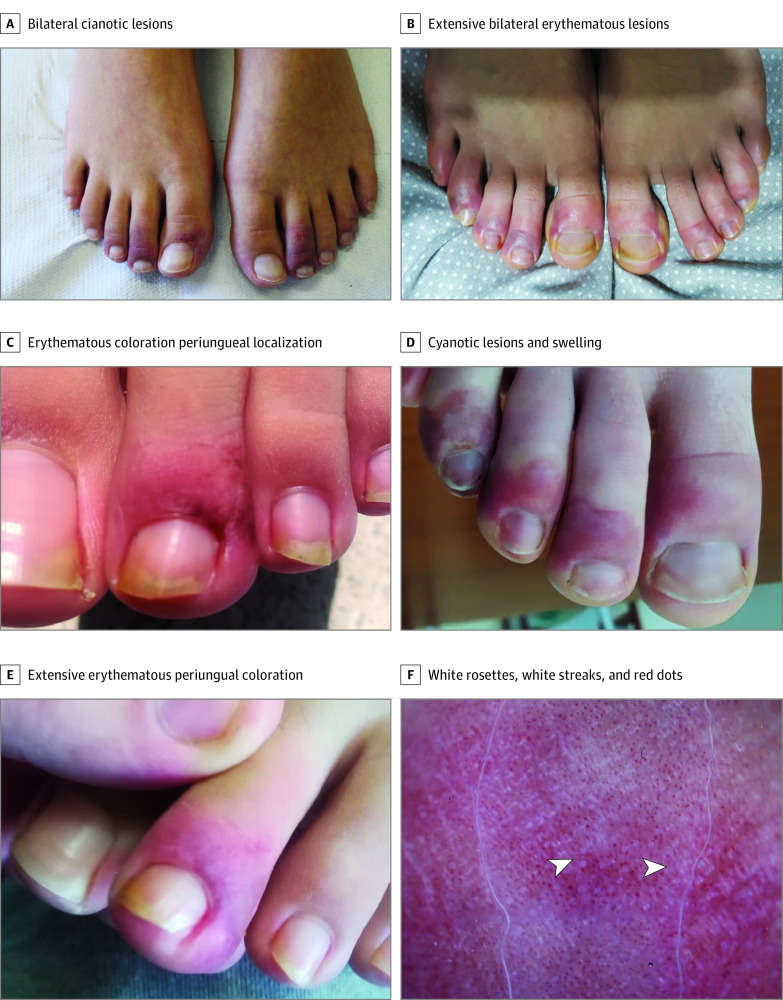
All 17 enrolled patients were healthy-appearing adolescents (9 [52.9%] male; median [interquartile range] age, 13.2 [12.5-14.3] years) who presented with erythematous or cyanotic acral lesions that involved the periungual area of the toes (Figure 1A-E). Lesions were bilaterally distributed (Figure 1A and B) in 16 patients (94.1%), and the skin area of the heels was involved in 7 patients (41.2%) (eFigure 1A-C in the Supplement). Ulceration complicated 1 case during the active phase of the disease (eFigure 2 in the Supplement), whereas desquamation developed over time in 3 cases (17.6%) (eFigure 2E in the Supplement). Concurrent involvement of the fingers was observed in only 2 patients (11.8%) (Table).
Figure 1. Bilateral Periungual Erythematous Lesions of the Toes in Adolescents During the COVID-19 Pandemic.
Representative pictures of the skin lesions highlighting the bilateral distribution (A-B), the periungual localization (A-E), and erythematous coloration (C-E). F, Dermoscopic image relative to the lesions pictured in panel D characterized by a peculiar triad, including white rosettes (left arrowhead), white streaks (right arrowhead), and red dots over an erythematous background.
Self-administered therapies included topical antibiotics and/or corticosteroids in 4 of 17 patients (23.5%), disinfectants in 4 of 17 patients (23.5%), and antifungal agents in 3 of 17 patients (17.6%), whereas systemic antibiotics or corticosteroids were used rarely (2 of 17 patients [11.8%]). None of the therapies substantially changed the course of the lesions, which was characterized initially by a gradual fading of the erythema and lastly by a gradual fading of the cyanosis, whereas the localization and distribution of the lesions remained unchanged over time (Figure 1; eFigure 2 in the Supplement). At onset, the lesions consisted of erythematous-purpuric papules and macules, whereas in the later stages they appeared as blurred, rosaceous, erythematous maculae with postinflammatory hyperpigmentation. In contrast to Raynaud phenomenon and perniosis, no discoloration of the extremities preceded the erythema and cyanosis, suggesting that vasospasm did not play a major role. The duration of the lesions was extremely variable, ranging from 49 to 145 days (eTable 1 in the Supplement); however, at follow-up, all patients had full resolution of the lesions.
The onset of lesions was not preceded by any prodromes. The most commonly associated signs and symptoms were pain, swelling (eFigure 1D in the Supplement), and pruritus (9 patients [52.9%]), whereas a burning sensation of the involved areas was reported by 4 patients (23.5%). Concomitant sore throat, cough, diarrhea, and dysgeusia were only sporadically reported. Subjective symptoms disappeared along with the clinical resolution.
A medical history of idiopathic perniosis and/or Raynaud phenomenon was not reported by any of the adolescents. Patients were interviewed in the presence of their parents, and either the patient or the parents could answer. Most often the parents answered. Similarly, a personal history of chronic autoimmune diseases was not reported by any of the patients. A positive family history of autoimmunity was noted in 6 patients (35.3%) (Table).
Laboratory Tests
All patients had normal laboratory test results, except for mild elevation of the complement C3 fraction (eTable 2 in the Supplement) found in all patients at enrollment (mean [SD], 123 [19] mg/dL; upper limit of the reference range, 95 mg/dL [to convert to grams per liter, multiply by 0.01]) and in 10 of the 17 tested patients at follow-up (mean [SD], 116 [17] mg/dL). The increase in C3 was mild and persisted at follow-up, despite the clinical resolution of the lesions, thus indicating a minor role in their onset. Of importance, the results of the laboratory tests performed (eTable 2 in the Supplement) allowed for exclusion of an ongoing systemic inflammatory process and ongoing autoimmune phenomena. Moreover, normal levels of D-dimer, fibrinogen, and platelets excluded a procoagulant state.
Dermoscopy
All but 1 of the enrolled patients (94.1%) underwent dermoscopic evaluation at enrollment, whereas only 4 (23.5%) received a second assessment. In line with previous reports,22 a dermoscopic triad almost invariably characterized the lesions, including red dots (16 of 16 patients [100%]), white rosettes (11 of 16 patients [68.8%]), and white streaks (10 of 16 patients [62.5%]) on an erythematous background (Figure 1F; eTable 1 in the Supplement). Red dots often appeared as dotted and comma-shaped congested vessels (9 of 16 patients [56.2%]), surrounding the rosettes in the early stage of the lesions. In later stages, red dots were still observed, whereas the rosettes disappeared. Although inconstantly found in inflammatory cutaneous conditions, these 3 signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process.
Histologic Analysis
A punch biopsy of the involved skin was taken in 12 of 17 cases (70.6%). Histologic analysis showed a slight remodeling and increased number of the dermal blood vessels with a tendency to a lobular arrangement (Figure 2; eTable 3 in the Supplement). Immunohistochemical analysis revealed a slight wall thickening with endothelial hyperplasia (anti-CD31 staining) (Figure 3A-C) associated with mild lymphocytic perivascular infiltrate (anti-CD3 staining) (Figure 3D-F). Interestingly, the histologic features evolved from a low-grade lymphocytic infiltration (earlier lesions) to a reorganization of the vascular structures, which appeared lobular and hyperplastic (advanced lesions). In contrast with idiopathic perniosis (eFigure 3A in the Supplement), none of the patients had edema of the papillary derma or fluffy edema of the vessels. Moreover, despite several biopsy specimens having been taken at the onset of the lesions, none of them revealed the eosinophilic or neutrophilic infiltrate typical of early stages of perniosis. In line with a milder histologic picture than classic perniosis, no vacuolar changes of the basal layer or signs of leukocytoclastic vasculitis (eFigure 3B in the Supplement) were found.
Figure 2. Microvascular Remodeling Characterized by Lobular Organization of the Vessels and Wall Thickening.
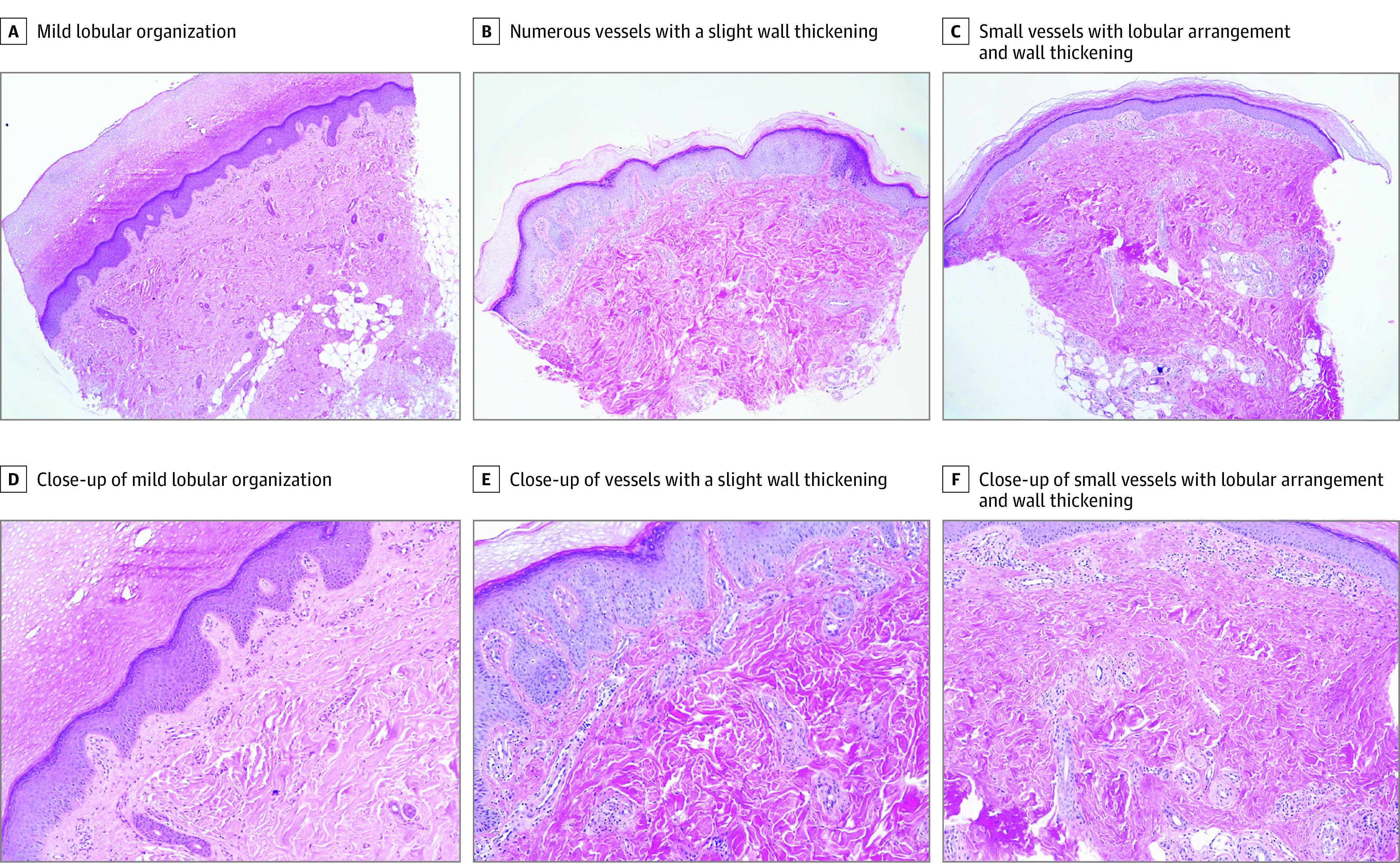
Hematoxylin-eosin immunohistochemistry images from 3 representative patients (each column includes images from the same case). Original magnification ×4 (A-C); original magnification ×10 (D-F).
Figure 3. Microvascular Remodeling Featuring Endothelial Hyperplasia and a Mild Perivascular Lymphocytic Infiltrate.
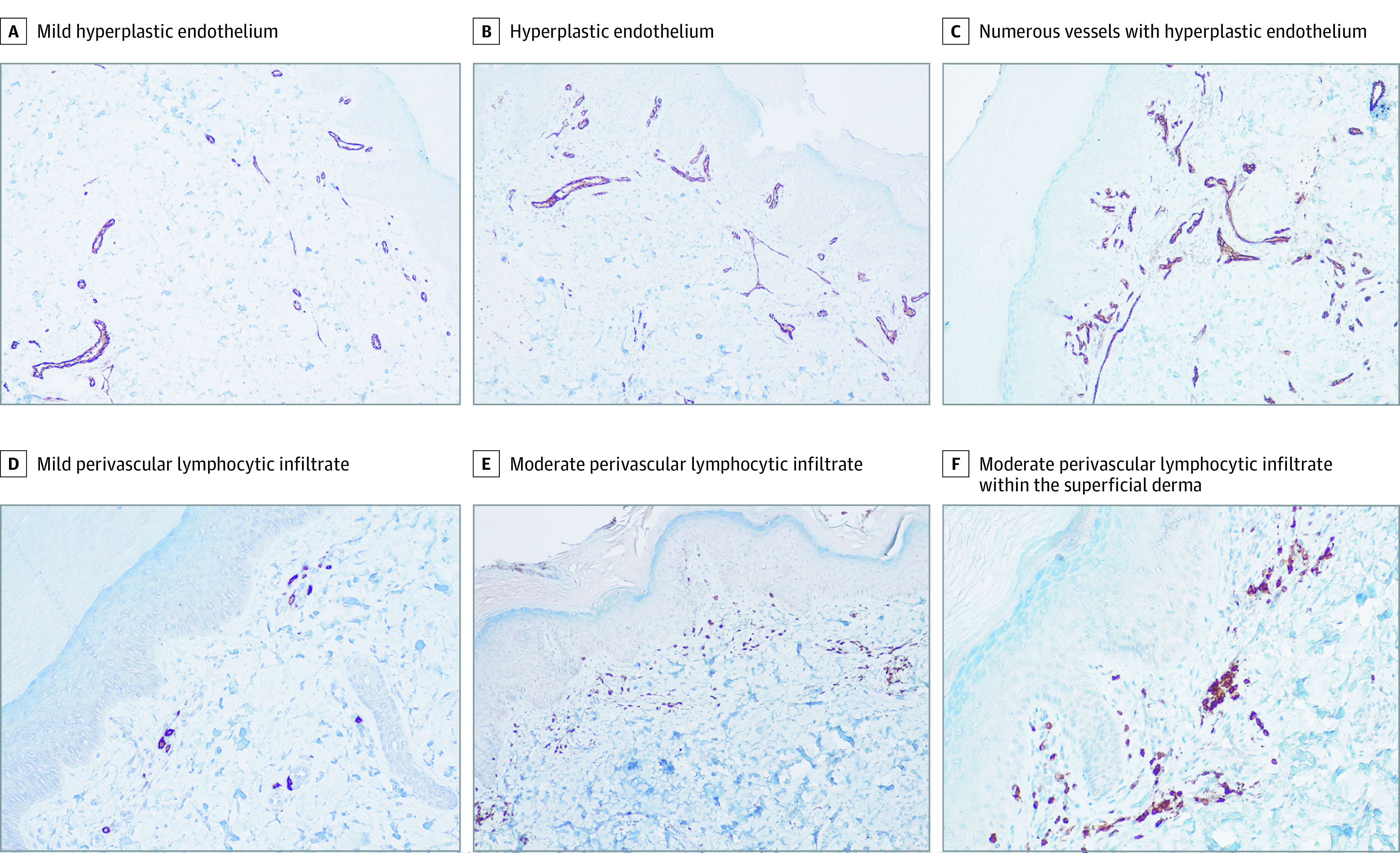
Immunohistochemistry images from 3 representative patients (original magnification ×10). As in Figure 2, each column includes images from the same case. A-C, Endothelial cells are highlighted by anti-CD31 staining. D-F, Lymphocytes are highlighted by anti-CD3 staining.
Association With SARS-CoV-2 Infection
Despite most chilblain-like lesions having been described in asymptomatic or minimally symptomatic SARS-CoV-2 cases,14 because all of the patients were enrolled during the first wave of the COVID-19 pandemic, a thorough interview exploring the occurrence of SARS-CoV-2–suggestive symptoms was conducted. Those symptoms were only episodically reported, with sore throat reported in 2 of the 17 patients (11.8%) and cough, dysgeusia, and diarrhea reported in 1 of the 17 patients (5.9%). Three weeks before the onset of the skin lesions, upper respiratory tract symptoms were reported by 3 patients (17.6%) and diarrhea by 2 patients (11.8%). At enrollment, 2 patients reported previous contact with a person with a confirmed positive SARS-CoV-2 case, whereas 2 patients had contact with a suspected but not a confirmed case (Table). Nevertheless, molecular test results were negative for SARS-CoV-2 in all patients. Similarly, quantitative determination of SARS-CoV-2–specific IgM and IgG antibodies was negative in all patients at enrollment and follow-up.
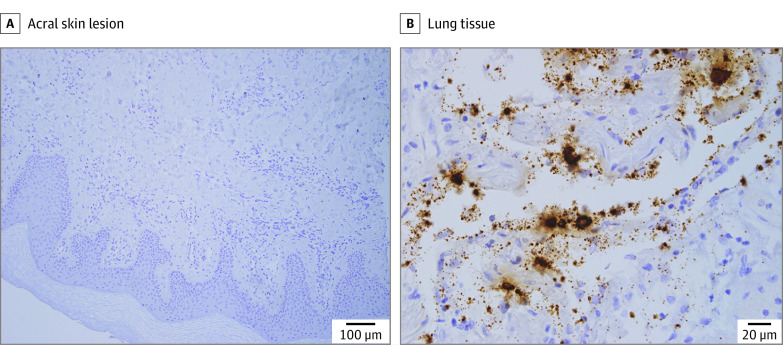
Because some authors23 ascribed chilblain-like lesions to a local SARS-CoV-2 infection, to further explore its potential role, we investigated the presence of SARS-CoV-2 RNA in the skin lesions by in situ hybridization, and in line with the laboratory tests, we did not find the viral genome in the lesions of any of the enrolled adolescents (Figure 4). Altogether these data do not support the association of the lesions with SARS-CoV-2 infection.
Figure 4. Absence of SARS-CoV-2 Viral Genome in the Skin Lesions.
In situ hybridization was performed on skin biopsies of the acral lesions (1 representative image, scale bar = 100 μm) (A) and lung tissue as a positive control (B) from a SARS-CoV-2–positive case (scale bar = 20 μm).
Capillaroscopy
To exclude the presence of signs suggestive of a systemic inflammatory process of the acral microvascular districts, we performed capillaroscopy at enrollment in 15 of the 17 patients (88.2%) (eTable 4 and eFigure 4 in the Supplement). In 80% of the patients, capillaroscopic results were either completely normal (6 of 15 [40.0%]) or showed rare ectasias (6 of 15 [40.0%]), supporting the lack of a systemic inflammatory process. A winding organization of vessels (3 of 15 patients [20.0%]) or more frequent ectasias (3 of 15 patients [20.0%]) were rarely described.
Discussion
This case series of otherwise healthy adolescents delineated a clinical picture that emerged during the COVID-19 pandemic of chilblain-like lesions of the toes associated with microvascular remodeling with a long but benign course to self-resolution not associated with signs of SARS-CoV-2 infection. These skin lesions were characterized by erythematous or cyanotic acral lesions typically bilaterally localized at the distal extremities, with invariable involvement of the periungual area of the toes. The onset was often associated with pain, swelling, and pruritus. A dermoscopic triad, including red dots, white rosettes, and white streaks, was invariably reported, in line with previous findings.22
Classic erythema pernio is an inflammatory vascular response of the superficial dermis of the proximal extremities, typically occurring in middle-aged women after exposure to cold or after a sudden temperature change.8 Together with their occurrence in childhood and distal localization, another unique feature of the lesions observed in this case series was the involvement of the heels, which is absent in classic chilblains. Whether walking barefoot at home, which has occurred more frequently during the COVID-19 lockdown, contributed to the development of lesions remains a hypothesis.24
Histopathologic analysis revealed signs of dermal vascular remodeling, including a tendency toward lobular arrangement, slight wall thickening, and variable endothelial hyperplasia. In contrast to idiopathic perniosis,25 none of the patients had dermal edema or eosinophilic infiltrate, as found in the early stages of perniosis and in previously reported cases of COVID toes.26 In line with this adult case series, the perivascular lymphocytic infiltrate and endothelial cell swelling were 2 of the most commonly reported features, although parakeratosis or vacuolar changes of the basal layer, which are commonly described in autoimmune chilblains, were not observed. The milder histologic findings in this case series might have been associated with the younger age of the patients and suggest a chronic rather than an acute inflammatory process, resulting in tissue remodeling that resembles vascular stasis more than vasculitis. Accordingly, capillaroscopy did not reveal signs of impaired microcirculation in the upper limbs, in contrast to systemic vasculitis.
Chilblains might also occur secondary to underlying conditions, such as connective tissue diseases, cryopathies, neoplastic diseases, blood hyperviscosity, genetic diseases (ie, familial chilblains lupus or Aicardi-Goutières syndrome), anorexia, and malnutrition.25 However, the patients in this case series did not report a personal history of autoimmunity and did not have any laboratory signs of underlying autoimmune, inflammatory, or proliferative conditions.
Of interest, vascular deposits of C3, together with IgA and IgM, were previously reported in 5 patients who presented with chilblain-like lesions.26 In contrast to a depletion of circulating complement, as expected in association with vascular deposits, a mild elevation of the C3 fraction was seen in all patients and persisted during follow-up. Despite this finding seeming nonspecific, the role of the complement in the genesis of the lesions remains to be elucidated. Hypercoagulability, which might be associated with acroischemia more than chilblain-like lesions, was also excluded.
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions, to the point that the media now refers to it as COVID toes, suggests that this condition could be a cutaneous sign of SARS-CoV-2 disease.25,27,28 However, data on the association with SARS-CoV-2 are controversial.29,30 The first case series failed to perform SARS-CoV-2 testing in all patients.8,12,31 In line with several studies,32,33 in this case series, molecular, serologic, and tissue data did not show signs of current or past SARS-CoV-2 infection in any of the patients. A French study27 reported similar skin lesions in adults enrolled during April 2020. In contrast to the findings of this case series, the French investigators observed a more frequent bullous and necrotic evolution and an increase in antinuclear antibodies in approximately 20% of patients and in D-dimer levels in 60% of patients. In the previous study,27 histopathologic analysis revealed a more intense lymphocytic infiltration, probably because only the most severe cases underwent skin biopsy. Whether these differences might be attributable to patient age or to a distinct pathogenic mechanism remains elusive. The authors did not identify any patients with SARS-CoV-2, yet they hypothesized, in line with other authors,30 that the lesions might have been the result of a delayed immune response to SARS-CoV-2 exposure. In contrast with this hypothesis, all adolescents included in the present case series also had negative IgM- and IgG-specific serologic test results at 4 weeks of follow-up. Moreover, to rule out a direct role of the virus in the development of the lesions, the current study performed in situ hybridization of the skin biopsy specimens and systematically failed to detect the viral genome in the skin lesions of the patients, further demolishing the causative role of SARS-CoV-2. This finding is in contrast to the hypothesis of a local endothelial or cutaneous infection proposed by some authors23,26,34 but in line with previous studies22,35 that failed to detect SARS-CoV-2 in chilblain-like lesions by in situ hybridization or immunohistochemical analysis.
Despite the increasing incidence of COVID-19 cases in Campania in Southern Italy, from 10 to 20 per 100 000 population during the first wave of the pandemic to 75 per 100 000 population during the second wave,36 only 3 new chilblain-like cases were reported after June 2020. That only 3 new cases were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection; in addition, none of these patients tested positive for SARS-CoV-2. The fact that all 3 cases during the second wave occurred in winter months (November 2020, January 2021, and March 2021) suggests that exposure to the cold might, at least in some cases, trigger the skin lesions. In line with this hypothesis, 7 of the enrolled adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
Limitations
This study has limitations, the main limitation being the small sample size. In addition, the study design did not allow risks to be calculated or cause-effect relationships to be established.
Conclusions
This case series included 17 adolescents who presented with chilblain-like lesions of the toes associated with microvascular remodeling but no evidence of current, past, or local SARS-CoV-2 infection. Lifestyle changes, including reduced physical activity and avoidance of footwear at home (related to the lockdown restrictive measures imposed by the government), might have contributed to the onset of the lesions. The increased emphasis by the media on the pandemic and the associated emerging clinical manifestations might have contributed to the increase in attention on milder cases that would have not undergone clinical examination in the prepandemic era. Whether genetic predisposing factors contribute to the onset of these lesions in selected adolescents remains to be explored. Larger ongoing studies will help delineate the genetic, immune, and metabolic changes of this recently emerging clinical entity.
eFigure 1. Heel Involvement and Swelling of the Lesions
eFigure 2. Ulceration
eFigure 3. Histology of Disease Controls Including Perniosis and Leukocytoclastic Vasculitis
eFigure 4. Capillaroscopy
eTable 1. Dermoscopy Findings in Patients
eTable 2. Laboratory Tests in Patients
eTable 3. Histology Findings
eTable 4. Capillaroscopy Findings
References
- 1.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702 [DOI] [Google Scholar]
- 2.Garazzino S, Montagnani C, Donà D, et al; Italian SITIP-SIP Pediatric Infection Study Group. Multicentre Italian study of SARS-CoV02 infection in children and adolescents, preliminary data as at 10 April 2020. Eur Surveill. 2020;25(18):34. doi: 10.2807/1560-7917.ES.2020.25.18.2000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663-1665. doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183(3):431-442. doi: 10.1111/bjd.19264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34(8):e346-e347. doi: 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matar S, Oulès B, Sohier P, et al. Cutaneous manifestations in SARS-CoV-2 infection (COVID-19): a French experience and a systematic review of the literature. J Eur Acad Dermatol Venereol. 2020;34(11):e686-e689. doi: 10.1111/jdv.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouaziz JD, Duong TA, Jachiet M, et al. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol. 2020;34(9):e451-e452. doi: 10.1111/jdv.16544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EE, McMahon DE, Lipoff JB, et al. ; American Academy of Dermatology Ad Hoc Task Force on COVID-19 . Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486-492. doi: 10.1016/j.jaad.2020.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280-285. doi: 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Comment on “Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak.” J Am Acad Dermatol. 2020;83(3):e241. doi: 10.1016/j.jaad.2020.05.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45(6):746-748. doi: 10.1111/ced.14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonna C, Genovese G, Monzani NA, et al. Outbreak of chilblain-like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83(3):965-969. doi: 10.1016/j.jaad.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccolo V, Neri I, Filippeschi C, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34(7):e291-e293. doi: 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeck M, Herman A. COVID toes: where do we stand with the current evidence? Int J Infect Dis. 2021;102:53-55. doi: 10.1016/j.ijid.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DS, Mirmirani P, McCleskey PE, Mehrpouya M, Gorouhi F. Cutaneous manifestations of COVID-19: a systematic review and analysis of individual patient-level data. Dermatol Online J. 2020;26(12):13030/qt7s34p8rw. [PubMed] [Google Scholar]
- 16.Zhou Z, Ren L, Zhang L, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883-890.e2. doi: 10.1016/j.chom.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology (Basel). 2020;7(1):3-16. doi: 10.3390/dermatopathology7010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Errichetti E, Zalaudek I, Kittler H, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182(2):454-467. doi: 10.1111/bjd.18125 [DOI] [PubMed] [Google Scholar]
- 19.Latuskiewicz-Potemska J, Chmura-Skirlinska A, Gurbiel RJ, Smolewska E. Nailfold capillaroscopy assessment of microcirculation abnormalities and endothelial dysfunction in children with primary or secondary Raynaud syndrome. Clin Rheumatol. 2016;35(8):1993-2001. doi: 10.1007/s10067-016-3340-8 [DOI] [PubMed] [Google Scholar]
- 20.Kayser C, Bredemeier M, Caleiro MT, et al. Position article and guidelines 2018 recommendations of the Brazilian Society of Rheumatology for the indication, interpretation and performance of nailfold capillaroscopy. Adv Rheumatol. 2019;59(1):5. doi: 10.1186/s42358-018-0046-4 [DOI] [PubMed] [Google Scholar]
- 21.Mascolo M, Romano MF, Ilardi G, et al. Expression of FK506-binding protein 51 (FKBP51) in Mycosis fungoides. J Eur Acad Dermatol Venereol. 2018;32(5):735-744. doi: 10.1111/jdv.14614 [DOI] [PubMed] [Google Scholar]
- 22.Fabbrocini G, Vastarella M, Nappa P, et al. A new dermoscopic pattern for chilblain-COVID-19-like skin lesions in adolescents. JAAD Case Rep. 2020;6(12):1271-1274. doi: 10.1016/j.jdcr.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729-737. doi: 10.1111/bjd.19327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Hachem M, Diociaiuti A, Concato C, et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620-2629. doi: 10.1111/jdv.16682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406-411. doi: 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870-875. doi: 10.1016/j.jaad.2020.05.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157(2):202-206. doi: 10.1001/jamadermatol.2020.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bristow IR, Borthwick AM. The mystery of the COVID toes—turning evidence-based medicine on its head. J Foot Ankle Res. 2020;13(1):38. doi: 10.1186/s13047-020-00408-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology. 2021;237(1):1-12. doi: 10.1159/000512932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinkai K, Bruckner AL. Dermatology and COVID-19. JAMA. 2020;324(12):1133-1134. doi: 10.1001/jama.2020.15276 [DOI] [PubMed] [Google Scholar]
- 31.de Masson A, Bouaziz JD, Sulimovic L, et al. ; SNDV (French National Union of Dermatologists-Venereologists) . Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667-670. doi: 10.1016/j.jaad.2020.04.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caselli D, Chironna M, Loconsole D, et al. No evidence of SARS-CoV-2 infection by polymerase chain reaction or serology in children with pseudo-chilblain. Br J Dermatol. 2020;183(4):784-785. doi: 10.1111/bjd.19349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156(9):998-1003. doi: 10.1001/jamadermatol.2020.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko CJ, Harigopal M, Gehlhausen JR, Bosenberg M, McNiff JM, Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2020;48(1):47-52. doi: 10.1111/cup.13866 [DOI] [PubMed] [Google Scholar]
- 35.Magro C, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19 associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2020. doi: 10.1111/bjd.19415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GIMBE Evidence for Health. Coronavirus epidemic monitoring in Italian regions and provinces. Accessed December 2020. https://coronavirus.gimbe.org/regioni.it-IT.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Heel Involvement and Swelling of the Lesions
eFigure 2. Ulceration
eFigure 3. Histology of Disease Controls Including Perniosis and Leukocytoclastic Vasculitis
eFigure 4. Capillaroscopy
eTable 1. Dermoscopy Findings in Patients
eTable 2. Laboratory Tests in Patients
eTable 3. Histology Findings
eTable 4. Capillaroscopy Findings