Abstract
Introduction: Aidi injection (Aidi) is composed of cantharidin, astragaloside, ginsenoside, and elentheroside E. As an important adjuvant therapy, Aidi in combination with gemcitabine and cisplatin (GP) is often used in the treatment of non-small cell lung cancer (NSCLC).
Objectives: We performed a new evaluation to demonstrate the clinical efficacy and safety of the Aidi and GP combination and further explored an optimal strategy for achieving an ideal response and safety level in advanced NSCLC.
Methodology: We collected all the related trials from Chinese and English-language databases, analyzed their methodological bias risk using the Cochrane evaluation Handbook for Systematic Reviews of Interventions Version 5.1.0, extracted all the data using a predefined data extraction form, pooled the data using a series of meta-analyses, and finally summarized the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Results: We included 70 trials with 5,509 patients. Compared with GP alone, the Aidi and GP combination showed a significant improvement in the objective response rate (ORR) [1.82 (1.62–2.04)], disease control rate (DCR) [2.29 (1.97–2.67)], and quality of life (QOL) [3.03 (2.55–3.60)] and a low incidence of hematotoxicity and gastrointestinal and hepatorenal toxicity. Aidi might be more suitable for patients who are first-treated, elderly, or patients with a Karnofsky Performance Status (KPS) score ≥ 60 or anticipated survival time (AST) ≥3 months. An Aidi (50 ml/day, 7–14 days/cycle for one to two cycles), gemcitabine (1000 mg/m2), and cisplatin (20–30 mg/m2, 40–50 mg/m2, or 60–80 mg/m2) might be an optimal regimen for realizing an ideal response and safety level. Most results were robust and of moderate quality.
Conclusion: Current evidence indicates that Aidi's value in adjuvant chemotherapy may be broad-spectrum, not just for some regimens. The Aidi and GP combination may show a good short-term response, antitumor immunity, and safety level in patients with NSCLC. Aidi (50 ml/day, 7–14 days/cycle for one and two cycles) with GEM (1000 mg/m2) and DDP (20–30 mg/m2 or 40–50 mg/m2) may be an optimal regimen for realizing an ideal goal in patients who are first-treatment, elderly, or have a KPS score ≥ 60 or AST≥3 months.
Keywords: aidi injection, non-small cell lung cancer, gemcitabine and cisplatin, randomized controlled trial, optimal adjuvant strategy
Introduction
Lung cancer continues to be the most commonly diagnosed cancer and the leading cause of cancer death because of its poor prognosis (Chen et al., 2016; Torre et al., 2016; Siegel et al., 2017). Approximately 85% of lung cancers are non-small cell lung cancer. The combined use of cisplatin and gemcitabine is a standard regimen in the treatment of advanced NSCLC (Scagliotti et al., 2008; Scagliotti et al., 2012; Association et al., 2018). However, systemic chemotherapy often leads to multiple adverse drug reactions (ADRs), such as hematotoxicity, gastrointestinal toxicity, hepatorenal toxicity, and chemotherapy-induced immunosuppression (Pollera et al., 1987; Conroy et al., 2002; Waissbluth and Daniel, 2013; Shahid et al., 2018), which result in poor survival and quality of life.
In China, Chinese herb injections (CHIs) show important antitumor functions, upregulate antitumor immunity, and reduce chemotherapy-related ADRs in multiple malignant tumors (Cao et al., 2017; Duan et al., 2018a; Xiao et al., 2020a; Xiao et al., 2020b). As an important CHI, Aidi injection is composed of multiple active ingredients from Ginseng Radix Et Rhizoma, Astmgali Radix, Acanthopanacis Senticosi Radix Et Rhizoma Seu Caulis, and Mylabris (Supplementary Table S1; Xie et al., 2019). The active ingredients comprise the following main components: astragaloside (Re, Rb1, and Rg1), ginsenoside, cantharidin, elentheroside E, and syringin (Zhang et al., 2012; Zeng et al., 2016). These are purported to induce tumor cell apoptosis and to inhibit tumor cell proliferation and invasion (Duan et al., 2018b; Chen et al., 2019; Li et al., 2019), to reduce chemotherapy-related ADRs through anti-inflammation and antioxidative stress (Farag et al., 2019; Qu et al., 2019; Zhang et al., 2019), and to repair the host’s antitumor immunity though upregulating the levels of peripheral blood lymphocytes (PBLs) (Li et al., 2018; Zhou et al., 2018; Huang et al., 2019). In clinic, Aidi in combination with GP has been widely used in the treatment of NSCLC (Lv et al., 2018; Zhang, 2018; Zhou, 2018; Liu et al., 2019). According to the Cochrane systematic evaluation, three studies (Yang and Ding, 2012; Han et al., 2016; Xiao et al., 2017) including 36 trials evaluated the clinical efficacy and safety of Aidi injection with GP. However, there are many unacceptable methodological defects in previous systematic reviews (SRs) and meta-analyses. None of these evaluations ultimately demonstrate whether Aidi and GP combination shows a good clinical efficacy and safety levels. Moreover, no evaluation provides answers on the relationship between Aidi and GP, the optimal combination of Aidi and GP, optimal indication, treatment doses, or time and cycle. All these questions have become new obstacles to developing an optimal treatment strategy against advanced NSCLC and need to be confirmed by new evaluation.
Recently, new trials have been published (Geng et al., 2020; Guo, 2020; Tan et al., 2020; Xu, 2020; Xu and Li, 2020). Therefore, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we performed a new evaluation to demonstrate the clinical efficacy and safety of the Aidi and GP combination and to explore further its therapeutic threshold and optimal strategy for achieving an ideal response and safety level in advanced NSCLC.
Methods
Inclusion Criteria
According to the PICOS guidelines, all included trials met the following criteria. Patients with inoperable NSCLC (stages III–IV) were diagnosed using histopathological and cytological diagnostic criteria and the tumor node metastasis (TNM) staging system (Mountain, 1989). None of the restrictions were set on the Karnofsky Performance Status score, anticipated survival time, treatment process (primary treatment, PT/retreatment, and RT), age of patients, usages of Aidi and GP, or follow-up. The experimental group received the Aidi and GP combination and the control group received the GP alone; one month before therapy onset, no patients received chemotherapy, radiotherapy, targeted therapy, or traditional Chinese medicine (TCM). We analyzed the clinical efficacy using tumor responses, survival, QOL, and antitumor immunity, and the ADRs using hematotoxicity, gastrointestinal toxicity, hepatorenal toxicity, neurotoxicity, alopecia, and oral mucositis. The study design was a randomized controlled trial (RCT).
Exclusion Criteria
We excluded any study meeting the following criteria: duplicates; patients with non–NSCLC, non–Aidi, or Aidi alone; Aidi in combination with other chemotherapy, targeted therapy, radiotherapy, or other TCM; cohorts and case-control studies, and case series reports; meeting abstracts and reviews without available data; unrelated SRs/meta-analyses; and studies without data on tumor responses, survival, QOL, ADRs, or antitumor immunity.
Literature Search
Based on the principle of patients (P) and intervention (I), two reviewers (Cheng–Qiong Wang and Xiao-Tian Zheng) used standard medical subject headings and free-text words to build the search strategies and searched all records independently. The terms were “Lung Neoplasms” [Mesh], Pulmonary Neoplasms, Lung Neoplasm, Pulmonary Neoplasm, Lung Cancer, Lung Cancers, Pulmonary Cancer, Pulmonary Cancers, Lung carcinoma, Pulmonary carcinoma, NSCLC, Aidi, Aidi injection, Addie, and Compound Cantharis Injection. A systematic search of the literature published until November 2020 was conducted using the following databases: PubMed, Embase, Science Citation Index, the Cochrane Central Register of Controlled Trials (CENTRAL) database, China National Knowledge Infrastructure (CNKI) database, Chinese Scientific Journals Full-Text database (VIP), Wanfang database, and China Biological Medicine (CBM) database. In addition, the two reviewers read all the related SRs/meta-analyses about Aidi and GP combinations for NSCLC and collected eligible trials from their references.
Study Selection
Two independent reviewers (Shan-Shan Hu and Hong Jiang) selected eligible trials using predefined inclusion and exclusion criteria. Disputes of selections were resolved by discussion with each other or a third reviewer (Zheng Xiao).
Methodological Bias Risk
Two independent reviewers (Cheng–Qiong Wang and Xiao–Tian Zheng) critically assessed the methodological bias risk of all included trials using the Cochrane evaluation Handbook for SRs of Interventions version 5.1.0 (Higgins, 2011 JPT). The bias risk was appraised according to the following features: random sequence generation (selection bias), allocation concealment (selection bias), blinding of patients and researchers (performance bias), blinding of indicator measurement (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases, (e.g., whether the baseline was comparable). Each item was categorized into one of three levels—a low risk of bias, a high risk, or an unclear risk. If any domain was considered high risk, the trial was defined as poor quality. Disputes of assessments were resolved by discussion with each other or a third reviewer (Zheng Xiao).
Indicator Definition
We analyzed the clinical efficacy using tumor responses, survival, QOL, and levels of PBLs. In accordance with World Health Organization (WHO) criteria for solid tumor responses (Miller et al., 1981) or Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (Watanabe et al., 2003), the indicators used were complete response (CR), partial response (PR), no change (NC), and progressive disease (PD). We analyzed the tumor response using the objective response rate (ORR, ORR = CR + PR) and disease control rate (DCR, DCR = CR + PR + NC). We analyzed the survival using overall survival (OS), progression-free survival (PFS), OS, and PFS rates. In accordance with the Karnofsky Performance Status (KPS) Scale (Yates et al., 1980; Clancey, 1995), the scores increased by ≥ 10 points after treatment, and the QOL demonstrated an improvement. We analyzed antitumor immunity using the levels of CD3+ T cells, CD3+ CD4+ T cells, and CD3+ CD8+ T cells, and CD4+/CD8+ T cell ratios and natural killer cell (NK cell) activity, which were measured by using flow cytometry (FCM) or indirect immunofluorescence tests before and after treatment.
In accordance with WHO (Miller et al., 1981) or National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) (Trotti et al., 2003), we analyzed ADRs using hematotoxicity, gastrointestinal toxicity, liver or renal toxicity, neurotoxicity, alopecia, and oral mucositis. Hematotoxicity included myelosuppression, neutropenia (granulocytes <2×109/L), thrombocytopenia (platelets <100×109/L), and anemia (hemoglobin <110 g/L). Liver toxicity was detected using a level of serum aminotransferase or alkaline phosphatase >1.25 × N, and renal toxicity was detected using a level of serum urea nitrogen or creatinine >1.25 × N.
Data Extraction
Using a predefined data extraction form, two independent reviewers (Yuan Jiang and Xiao–Rong Huang) extracted the title, author, year, study design, and nationality; the KPS score, AST, PT/RT, and age of patients; the sample size; the usage of Aidi and GP combination; the measurement method of tumor response, ADRs, and PBLs; follow-up; and tumor response (ORR and DCR), OS, PFS, OS rate, PFS rate, QOL, ADRs, and PBLs. If articles provided the details, we directly extracted the data. Otherwise, we directly requested information from the author via email. If no author replied, we reconstructed the graphed data into analyzable data using a software graph digitizer scout (Guyot et al., 2012).
Statistical Analysis
We analyzed the ORR, DCR, OS, PFS, OS rate, PFS rate, QOL, and ADRs using odds ratios (ORs) and their 95% confidence intervals (CIs), the OS and PFS using hazard ratios (HRs) and their 95% Cis, and the levels of PBLs using standardized mean differences (SMDs) and their 95% CIs. If p < 0.05, the results were considered significant. We analyzed the potential statistical heterogeneity using Cochran’s χ2 test and I2 statistic, and I2 > 50% indicated statistical heterogeneity. Two independent reviewers (Cheng–Qiong Wang and Jun Huang) performed a series of meta-analyses using Review Manager 5.3 (as recommended by Cochrane Collaboration). If p > 0.1 and I2 ≤ 50%, we pooled the OR, HR, SMD, and their 95% CIs using a fixed-effects model (FEM); if I2 > 50% and without significant clinical heterogeneity, we pooled the data using a random-effects model (REM), and with significant clinical heterogeneity, we abandoned the pooling of data and described the data.
If the trials were greater than 10, we analyzed the potential publication bias using a funnel plot and Egger/Begg’s tests. Trials with poor quality, overestimated efficacy, and underestimated ADRs showed a negative influence on the outcome robustness. If the result was significantly different and beneficial to Aidi use, we defined it as an under- or overestimated trial. Then, we summarized the OR, HR, SMD, and their 95% CIs under extreme conditions, which rejected all poor trials, and trials with overestimated efficacy or underestimated ADRs (Xiao et al., 2018b; Xiao et al., 2019b). If the result before and after rejection had good consistency the result was robust; if not, the result was poorly robust.
According to variables such as KPS score, AST, treatment process (PT/RT), age of patients, and usage of Aidi and GP, we developed a subgroup analysis model to analyze the clinical heterogeneity and to reveal the effects of variables between trials on tumor response, ADRs, and PBL levels (Sun et al., 2010; Xiao et al., 2020a; Xiao et al., 2020b). In addition, we implemented a univariate random effects meta-regression to analyze the relationship between each variable and tumor response/ADRs and a post hoc multiple regression analysis to adjust for the OR of tumor response and ADRs of the variables.
Evidence Quality
According to the Grading of Recommendations Assessment, Development, and Evaluation guideline (Guyatt et al., 2008), two independent reviewers (Xiao-Fan Chen and Cheng–Qiong Wang) summarized the quality of evidence using the following five criteria (i) limitations in trial design (if most trials had unclear risk and no high risk, or if some trials had high risk and the result of sensitivity analysis was robust, we downgraded the quality by one level; if some trials had high risk and the result of sensitivity analysis was poorly robust, or all trials had high risk, we downgraded the quality by two levels. If neither of these applied, we downgraded them by one level) (ii) inconsistency (with heterogeneity, and the result was poorly robust) (iii) indirectness (the patients, interventions, outcomes, or controls did not meet the themes) (iv) imprecision (the sample size for outcome <300 cases); and (v) reporting bias (with reporting bias, and the result was poorly robust). Because of the (ii)–(v) domains, we downgraded the quality by one level. Finally, we summarized the quality into four grades: high, moderate, low, and very low.
Results
Search Results
We collected 2,436 records by searching. After scanning the titles, we collected 799 records. After scanning the abstracts and excluding the studies, such as those with non-NSCLC, non-Aidi injection, Aidi injection alone, and Aidi injection with other chemotherapy, we collected 91 original studies, three SRs/meta-analyses, and six related-SRs/meta-analyses. After evaluating original studies and excluding duplicates, cohort and case control studies, and case series reports, we included 70 eligible trials. In addition, we collected 36 eligible trials from the three SRs/meta-analyses (Yang and Ding, 2012; Han et al., 2016; Xiao et al., 2017) and 42 trials from the six related SRs/meta-analyses (Ma et al., 2009; Tian et al., 2014; Xiao et al., 2016; Wu et al., 2017a; Xiao et al., 2018b; Wang et al., 2018). Finally, we identified 70 eligible trials for this meta-analysis (Supplementary Tables S2–S5; Figure 1).
FIGURE 1.
PRISMA 2009 flow diagram for the eligible trials We identified 70 eligible trials for this analysis.
Basic Features of the Included Trials
In this meta-analysis, we identified 70 trials from China, which involved 5,509 NSCLC patients including 3,278 males and 1,995 females with ages ranging from 21–86 years old (Table 1). The intervention was Aidi injection which was intravenously injected with 30–100 ml/day, 7–28 days per cycle for one to four cycles. The experimental group with 2,783 cases received Aidi and GP combination, and the control with 2,726 cases received GP alone. GEM (1000 mg/m2) was used in combinations with DDP (20–100 mg/m2). The efficacy and safety were evaluated after follow-up of six weeks to two years. Sixty-three trials with 4,851 patients reported the tumor response rate (ORR and DCR) according to WHO (Miller et al., 1981) or RECIST guidelines (Watanabe et al., 2003); four trials with 320 patients reported the survival (Sun et al., 2008; Cheng et al., 2014; Li and Yang, 2014; Guo, 2020) and no trials reported the PFS0 .31 trials with 2,485 patients reported the QOL; 18 trials with 1,688 patients reported the antitumor immunity before and after therapy which were detected using a FCM (Feng et al., 2008; Jiang et al., 2011; Xu et al., 2013; Zhang, 2014; Han et al., 2015; Li, 2015; Zhao et al., 2015; Li et al., 2016; Wu et al., 2017b; Huang et al., 2017; Ma, 2017; Su, 2017; Lv et al., 2018; Liu et al., 2019; Zhao and Li, 2019; Guo, 2020; Tan et al., 2020; Xu, 2020). 58 trials with 4,596 patients reported ADRs according to WHO (Miller et al., 1981) or Common Terminology Criteria for Adverse Events (CTCAE) (Trotti et al., 2003).
TABLE 1.
Basic features of the included trials.
| First author. Year | Non-small cell lung cancer (NSCLC) | Interventions | Fellow up | Criteria | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNM | KPS | AST | TP | E/C | M/F | Age | Aidi injection (usages) | GP (dosages) | ||||
| Zou et al. (2006) | IIIb–IV | ≥60 | >3 m | Un | 42/39 | 56/25 | 35–73 | 80 ml, 14 days, 1cycle | G:1 g/m2; P:30 mg/m2 | 6–12 w | WHO,WHO | O1,2,4 |
| Feng et al. (2008) | III–IV | ≥70 | Un | PT | 68/62 | 88/42 | 38–74 | 50 ml, 15 days, 2cycles | G:1 g/m2; P:75 mg/m2 | 6 w | WHO,WHO | O1,2,4,5 |
| Sun et al. (2008) | IIIb–IV | ≥60 | >3 m | PT/RT | 33/30 | 54/9 | 34–73 | 100 ml,14 days,2cycles | G:1 g/m2; P:30 mg/m2 | 1 year | WHO, Un | O1,3 |
| Yang et al. (2008) | III–IV | ≥60 | ≥3 m | Un | 30/27 | 39/18 | 34–82 | 80 ml,8 days,2cycles | G:1 g/m2; P:75 mg/m2 | 6 w | WHO, No | O1,2 |
| Zhao et al. (2008) | III–IV | Un | >3 m | Un | 30/20 | 31/19 | 29–73 | 30 ml,21 days,3cycles | G:1 g/m2;P:60–80 mg/m2 | 9 w | WHO,WHO | O1,2,4 |
| Lv et al. (2009) | IIIb–IV | ≥60 | ≥3 m | Un | 30/30 | 42/18 | 45–70 | 80 ml,10 days,2cycles | G:1 g/m2; P:60–80 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Song et al. (2009) | III–IV | >60 | >3 m | PT | 30/30 | 36/24 | 53–76 | 50 ml,14 days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Wang. (2009) | IIIa–IV | ≥60 | >3 m | Un | 32/27 | 48/11 | Un | Un,10 days,2cycles | G:1 g/m2; P:20 mg/m2 | 6 w | WHO,WHO | O1,O4 |
| Wen et al. (2009) | IIIa–IV | Un | >3 m | PT/RT | 38/38 | 52/24 | 32–77 | 50 ml,8–10 days,2cycles | G:1 g/m2; P:75 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Zhang. (2009) | IIIb–IV | ≥60 | >3 m | PT/RT | 32/31 | 44/19 | 31–79 | 80 ml,14 days,2cycles | G:1 g/m2; P:80 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Hong et al. (2010) | IIIb–IV | ≥60 | ≥3 m | PT | 90/70 | 82/78 | 38–70 | 60 ml,14 days,2cycles | G:1 g/m2; P:25 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Hou and Zhang. (2010) | III–IV | ≥60 | ≥3 m | Un | 40/38 | 49/29 | 32–79 | 50 ml,14 days,2cycles | G:1 g/m2; P:25 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Li et al. (2010) | III–IV | >60 | >3 m | Un | 36/36 | 39/33 | 29–75 | 50–100 ml,15 days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Liu et al. (2010) | III–IV | >60 | >3 m | Un | 32/32 | 37/27 | 45–75 | 50 ml,14days,4cycles | G:1 g/m2; P:30 mg/m2 | 12 w | WHO, No | O1,4 |
| Shi et al. (2010) | IIIa–IV | ≥60 | ≥3 m | PT | 28/28 | 47/9 | 48–72 | 50 ml,14days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Ding et al. (2011) | III–IV | >50 | >3 m | Un | 18/22 | 27/13 | Un | 50 ml,10days,2cycles | G:1.4 g/m2; P:40 mg/m2 | 8 w | Un, WHO | O1,2,4 |
| Fan et al. (2011) | IIIb–IV | ≥60 | >3 m | Un | 41/38 | 54/25 | 39–73 | 50 ml,21 days,2-4cycles | G:1 g/m2; P:30 mg/m2 | 6–12 w | WHO, No | O1,2 |
| He et al. (2011) | IIIb–IV | ≥60 | >3 m | Un | 29/23 | 29/23 | 21–74 | 50–100 ml,15 days,2-3cycles | G:1 g/m2; P:75 mg/m2 | 8–11 w | WHO,WHO | O1,2,4 |
| Jiang et al. (2011) | IIIb–IV | ≥60 | >3 m | PT | 32/30 | 39/23 | 60–75 | 100 ml,14days,2cycles | G:1 g/m2; P:80 mg/m2 | 6 w | WHO, No | O1,2,5 |
| Lu et al. (2011) | IIIb–IV | ≥60 | >6 m | Un | 34/34 | 39/29 | 40–76 | 100 ml,14 days,2cycles | G:1 g/m2; P:80 mg/m2 | 6 w | WHO, Un | O1,2,4 |
| Wu and He. (2011) | III–IV | Un | Un | Un | 30/30 | 41/19 | 45–77 | 100 ml,16 days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | WHO, No | O1 |
| Fu. (2012) | IIIb–IV | Un | >3 m | Un | 35/35 | Un | 61–84 | 50 ml,14 days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | WHO, Un | O1,4 |
| Pei. (2012) | IIIb–IV | Un | Un | PT | 40/40 | 47/33 | 39–72 | 50 ml,8 days,2cycles | G:1 g/m2; P:20 mg/m2 | 6 w | RECIST | O1 |
| Sun et al. (2012) | IIIb–IV | Un | >3 m | Un | 34/34 | 42/26 | 60–86 | 50 ml,10 days,2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | RECIST, CTCAE | O1,2,4 |
| Wang. (2012) | III–IV | ≥60 | >3 m | Un | 25/24 | 35/14 | 56.8 ± 9.1/57.8 ± 10.2 | 60 ml,14 days,3cycles | G:1 g/m2; P:25 mg/m2 | 9 w | WHO, Un | O1,4 |
| Wang and Peng. (2012) | IIIb–IV | ≥70 | ≥3 m | Un | 36/36 | 46/26 | 32–74 | 80 ml,10 days,2-4cycles | G:1 g/m2; P:40 mg/m2 | 6–12 w | RECIST, WHO | O1,2,4 |
| Xu et al. (2012) | IIIb–IV | ≥70 | Un | Un | 33/33 | 36/30 | Un | 80 mg,10 days,4cycles | G:1.25 g/m2;P:100 mg/m2 | 13 w | RECIST, WHO | O1,4 |
| Zhang. (2012) | IIIb–IV | ≥60 | >3 m | Un | 41/42 | 63/20 | 57.2 ± 9.4/58.2 ± 10.3 | 60 ml,14 days,3cycles | G:1 g/m2; P:25 mg/m2 | 9 w | WHO, Un | O1,4 |
| Cai et al. (2013) | IIIa–IV | ≥60 | Un | Un | 19/19 | 21/17 | 36–68 | 50–100 ml,15 days,2cycles | G:1 g/m2; P:30mg/m2 | 6 w | Un | O4 |
| Ju et al. (2013) | IIIb–IV | Un | >3 m | Un | 34/34 | 36/32 | 61–81 | 50 ml,14 days,2cycles | G:1 g/m2; P:50 mg/m2 | 6 w | WHO, Un | O1,2,4 |
| Lai. (2013) | IIIb–IV | Un | Un | Un | 70/70 | 73/67 | 45–79 | 50 ml,14 days,>2cycles | G:1 g/m2; P:30 mg/m2 | Un | WHO,WHO | O1,2,4 |
| Xu et al. (2013) | IIIb–IV | Un | Un | Un | 38/42 | 55/25 | 39–81 | 50 ml/14 days/3cycles | G:1 g/m2; P:30 mg/m2 | 9 w | WHO,WHO | O1,2,4,5 |
| Li and Yang. (2014) | IIIb–IV | Un | >3 m | PT | 27/27 | 32/22 | 34–68 | 50ml/8–10 days/4cycles | G:1 g/m2; P:75 mg/m2 | 12 w | RECIST, CTCAE | O1,3,4 |
| Liu and Zhao. (2014) | IIIb–IV | ≥60 | ≥3 m | Un | 43/43 | 53/33 | 39–73 | 50ml/8–10 days/2cycles | G:1 g/m2; P:50 mg/m2 | 6 w | WHO,WHO | O1,4 |
| Liu and Zhang. (2014) | IIIb–IV | Un | Un | Un | 24/24 | 30/18 | 35–80 | 60 ml/21 days/2cycles | G:0.2 g/m2; P:25 mg/m2 | 6 w | Un, Un | O2,4 |
| Wen. (2014) | IIIb–IV | ≥60 | ≥3 m | Un | 45/45 | 64/26 | 61–81 | 50 ml/21 days/2cycles | G:1 g/m2; P:50 mg/m2 | 6 w | RECIST, CTCAE | O1,2,4 |
| Cheng et al. (2014) | IIIb–IV | ≥70 | ≥3 m | PT | 49/52 | 78/23 | 27–74 | 50–100 ml/10 days/2cycles | G:1–1.25 g/m2;P:25 mg/m2 | 2 years | RECIST, WHO | O1-4 |
| Li et al. (2014) | IIIb–IV | >60 | >3 m | Un | 30/30 | 28/32 | 40–81 | 50 ml/10 days/2cycles | G:1 g/m2; P:50 mg/m2 | Un | WHO, Un | O1,2,4 |
| Zhang. (2014) | III–IV | Un | Un | Un | 64/64 | 68/60 | 57–79 | 50 ml/10days/Un | G:1 g/m2; P:30 mg/m2 | 8 w | ELISA,FCM | O5 |
| Han et al. (2015) | IIIb–IV | Un | Un | Un | 36/36 | 39/33 | 48–67 | 50 ml,Un,3cycles | G:1 g/m2; P:Un | 12 w | WHO, Un, FCM | O1,4,5 |
| Li. (2015) | IIIb–IV | ≥60 | ≥3 m | Un | 20/20 | 24/16 | 45–74 | 50 ml/10 days/2cycles | G:1 g/m2; P:80 mg/m2 | 6 w | WHO, Un | O2,4,5 |
| Ning et al. (2015) | III–IV | Un | Un | RT | 31/31 | 49/13 | 45–75 | 50 ml/14 days/3cycles | G:1 g/m2; P:75 mg/m2 | 1 year | WHO,WHO | O1,3,4 |
| Zhang. (2015) | III–IV | Un | Un | Un | 39/32 | 37/34 | 60–83 | 50 ml/10 days/2cycles | G:1 g/m2; P:30 mg/m2 | 8 w | RECIST, CTCAE | O1,4 |
| Zhao et al. (2015) | III–IV | ≥70 | >3 m | Un | 43/43 | 58/28 | 43–79 | 100 ml/10 days/Un | G:1 g/m2; P:25 mg/m2 | Un | WHO,WHO,FCM | O1,2,4,5 |
| Zhu. (2015) | IIIb–IV | ≥60 | >3 m | Un | 21/21 | 22/20 | 60–75 | 100 ml/14 days/Un | G:1 g/m2; P:25 mg/m2 | Un | WHO,WHO | O1,4 |
| Chen. (2016) | III–IV | >60 | >3 m | Yes | 30/30 | 36/24 | 42–76 | 80 ml/8 days/2cycles | G:1 g/m2; P:25 mg/m2 | 6 w | WHO | O1 |
| Fang. (2016) | III–IV | ≥70 | Un | Un | 45/45 | Un/Un | 40–70 | 50 ml/10 days/2cycles | G:1 g/m2; P:75 mg/m2 | 6 w | WHO,CTCAE | O1,4 |
| Li et al. (2016) | IIIb–IV | ≥60 | >3 m | Un | 47/47 | 52/42 | 40–70 | 50–100 ml/28 days/1cycles | G:1 g/m2; P:30 mg/m2 | 12 w | WHO, Un, FCM | O1,2,4,5 |
| Li. (2016) | III–IV | >60 | >6 m | Un | 35/35 | 43/27 | 44–82 | 100 ml/14 days/1cycles | G:1 g/m2; P:80 mg/m2 | 6 w | WHO,WHO | O1,2,4 |
| Ma and Jiang. (2016) | III–IV | >60 | >3 m | Un | 33/35 | 39/29 | Un | 60 ml/14 days/1cycles | G:1 g/m2; P:25 mg/m2 | Un | WHO, Un | O1,4 |
| Zhang. (2016a) | III–IV | Un | Un | Un | 19/19 | 21/17 | 45–76 | 50 ml/28 days/1cycles | G:1 g/m2; P:25 mg/m2 | 6 w | WHO, Un | O1,2,4 |
| Zhang. (2016b) | IV | Un | >3 m | PT | 25/25 | Un/Un | 32–70 | 50 ml/10 days/4cycles | G:1 g/m2; P:75 mg/m2 | 12 w | RECIST, WHO | O1,2,4 |
| Huang et al. (2017) | IIIb–IV | ≥60 | ≥6 m | Un | 39/40 | 46/33 | 49–70 | 60 ml/21 days/3cycles | G:1 g/m2; P:25 mg/m2 | 9 w | RECIST,WHO,FCM | O1,4,5 |
| Ma. (2017) | IIIb–IV | ≥60 | ≥6 m | Un | 42/42 | 55/29 | 44–75 | 50ml/Un/4cycles | G:1 g/m2; P:75 mg/m2 | 16 w | WHO, Un, FCM | O1,4,5 |
| Su. (2017) | IIIb–IV | Un | ≥3 m | Un | 40/39 | 45/34 | 40–70 | 50 ml/21 days/2cycles | G:1 g/m2; P:30 mg/m2 | 6 w | RECIST, Un, FCM | O1,4,5 |
| Wu and Chen. (2017) | III–IV | Un | Un | Un | 67/68 | 83/52 | 43–71 | 100 ml/10 days/4cycles | G:1 g/m2; P:20 mg/m2 | 12 w | WHO, Un | O1,4 |
| Wu et al. (2017b) | III–IV | Un | Un | Un | 109/109 | 137/79 | 32–72 | 50 ml/14 days/3cycles | G:1 g/m2; P:80 mg/m2 | 9 w | Un, FCM | O2,4,5 |
| Zhang et al. (2017) | III–IV | Un | Un | Un | 54/54 | 64/40 | 46–79 | 60–100 ml/10 days/4cycles | G:1 g/m2; P:30 mg/m2 | 12 w | WHO, Un | O1,2,4 |
| Lv et al. (2018) | III–IV | >70 | >3 m | Un | 30/30 | 35/25 | 56–75 | 50 ml/14 days/Un | G:1 g/m2; P:25 mg/m2 | Un | WHO,FCM | O1,5 |
| Zhang. (2018) | III–IV | Un | ≥5m | Un | 40/40 | 43/27 | 38–72 | 60 ml/10 days/2cycles | G:1 g/m2; P:25 mg/m2 | 6 w | Un | O4 |
| Zhou. (2018) | III–IV | Un | ≥3 m | Un | 58/58 | 63/53 | 41–70 | 50 ml/20 days/Un | G:1 g/m2; P:25 mg/m2 | Un | RECIST, Un | O1,4 |
| Su and Zhang. (2019) | III–IV | ≥60 | ≥3 m | Un | 41/41 | 54/28 | 42–78 | Un/21 days/4-6cycles | G:1–1.25 g/m2; P:50 mg/m2 | 12–18 w | RECIST, Un | O1,2,4 |
| Liu et al. (2019) | IIIb–IV | Un | Un | Un | 44/44 | 54/34 | 42–76 | 50ml/Un/2cycles | G:1 g/m2; P:20 mg/m2 | Un | Un | O1,5 |
| Zhao and Li. (2010) | III–IV | Un | ≥3 m | PT | 43/43 | 55/31 | 64.0 ± 2.3/63.5 ± 2.6 | 50 ml/21dayays/2cycles | G:1.0 g/m2; P:80 mg/m2 | 6 w | WHO, Un | O1,3,5 |
| Chen. (2020) | III–IV | Un | Un | Un | 49/49 | 51/47 | 61–86 | 50–100 ml/10 days/Un | G:1–1.25 g/m2; P:25 mg/m2 | Un | RECIST, Un | O1,2,4 |
| Geng et al. (2020) | III–IV | ≥70 | ≥3 m | Un | 45/45 | 61/29 | 44–79 | 50 ml/14 days/4cycles | G:1.0 g/m2; P:30 mg/m2 | 8 w | WHO, Un | O1,4 |
| Tan et al. (2020) | IIIb–IV | >60 | ≥3 m | Un | 60/60 | 78/42 | 60–80 | 50 ml/10 days/2cycles | G:1.0 g/m2; P:30 mg/m2 | 6 w | RECIST,WHO,FCM | O1,2,4,5 |
| Guo. (2020) | IIIb–IV | Un | ≥3 m | Un | 51/51 | 58/44 | 43–75 | 60 ml/14 days/4cycles | G:1.0 g/m2; P:25 mg/m2 | 12 w-1 year | WHO,FCM | O3,4,5 |
| Xu and Li. (2020) | IIIb–IV | Un | Un | Un | 51/45 | 53/37 | 42–82 | 60 ml/21 days/3cycles | G:1.0 g/m2; P:25 mg/m2 | 9 w | WHO | O1,2 |
| Xu. (2020) | III–IV | Un | >6 m | Un | 40/40 | 43/27 | 49–72 | 50–100 ml/21 days/2cycles | G:1.0 g/m2; P:20 mg/m2 | 6 w | RECIST,Un,FCM | O1,4,5 |
Note: GP: Gemcitabine and cisplatin; E: Experimental group (Aidi plus GP); C: Control group (GP alone); KPS score: Karnofsky Performance Status score; TP: treatment process; PT: primary treatment; RT: retreatment; AST: anticipated survival time; M: male; F: female; WHO: World Health Organization guidelines for solid tumor responses; RECIST: Response Evaluation Criteria in Solid Tumors; FCM: flow cytometry; O1: clinical efficacy included ORR and DCR; O2: quality of life (QOL); O3: patient survival; O4: adverse drug reactions (ADRs); and O5: antitumor immunity.
Methodological Bias Risk
For the method of generating random sequences, only 24 trials used the random number table (Lai, 2013; Liu and Zhao, 2014; Wen, 2014; Zhang, 2014; Han et al., 2015; Li, 2015; Ning et al., 2015; Zhang, 2015; Huang et al., 2017; Ma, 2017; Su, 2017; Zhang et al., 2017; Zhang, 2018; Liu et al., 2019; Zhao and Li, 2019; Geng et al., 2020; Guo, 2020; Tan et al., 2020; Xu, 2020; Xu and Li, 2020), draw (Liu and Zhang, 2014), computer random (Wu et al., 2017b), or odd–even random (Fu, 2012; Sun et al., 2012). For the allocation, only two trials (Fu, 2012; Sun et al., 2012) reported the exposure of allocation. None of the trials reported blinding, and all trials had complete follow-up. Two trials selectively reported the tumor response (Ding et al., 2011; Xu and Li, 2020) and survival (Li and Yang, 2014; Zhao and Li, 2019). Seven trials selectively reported QOL (Ding et al., 2011; Lu et al., 2011; Xu et al., 2012; Li et al., 2014; Li et al., 2016; Zhang et al., 2017; Xu and Li, 2020), 27 trials selectively reported ADRs, and four trials (Feng et al., 2008; Xu et al., 2013; Su, 2017; Liu et al., 2019) selectively reported the levels of PBLs. In addition, 66 trials had baseline comparability, and four trials (Lv et al., 2009; Wang, 2009; Cai et al., 2013; Fang, 2016) had unclear comparability. We summarize the risk features of methodological bias in Figure 2.
FIGURE 2.
Risk of methodological bias Only 24 trials reported the random number table, draw, computer random, or odd–even random. Two trials reported the exposure of allocation. None of the trials reported the blinding and all trials had complete follow-up.
Tumor Responses
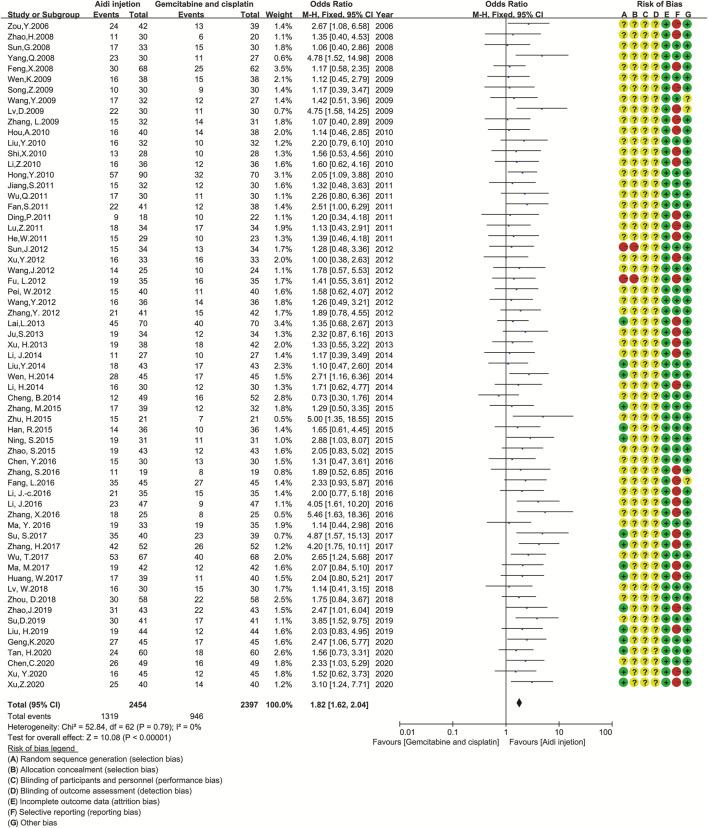
Sixty-three trials involving 4,851 patients compared the ORR and DCR (Figures 3A,B). Cochran’s χ2 test and I 2 statistic showed no statistical heterogeneity in ORR and DCR (I 2 = 0%). Therefore, we pooled the OR of ORR and DCR using a FEM. The pooled result showed a significant improvement in tumor responses (ORR and DCR) in the Aidi and GP combination group compared to that in the control group (OR = 1.82, 95% CI [1.62 to 2.04], p < 0.00001; OR = 2.29, 95% CI [1.97 to 2.67], p < 0.00001).
FIGURE 3.
The analysis of tumor response between two groups 3a.The analysis of ORR between two groups. The pooled result showed a significant improvement in ORR in the Aidi and GP combination group compared to that in the control group (OR = 1.82, 95% CI [1.62 to 2.04], p < 0.00001). Note: GP, Gemcitabine, and cisplatin; ORR, objective response rate. 3b. The analysis of DCR between two groups. The pooled result showed a significant improvement in DCR in the Aidi and GP combination group compared to that in the control group (OR = 2.29, 95% CI [1.97 to 2.67], p < 0.00001). Note: GP, Gemcitabine, and cisplatin; DCR, disease control rate.
Quality of Life
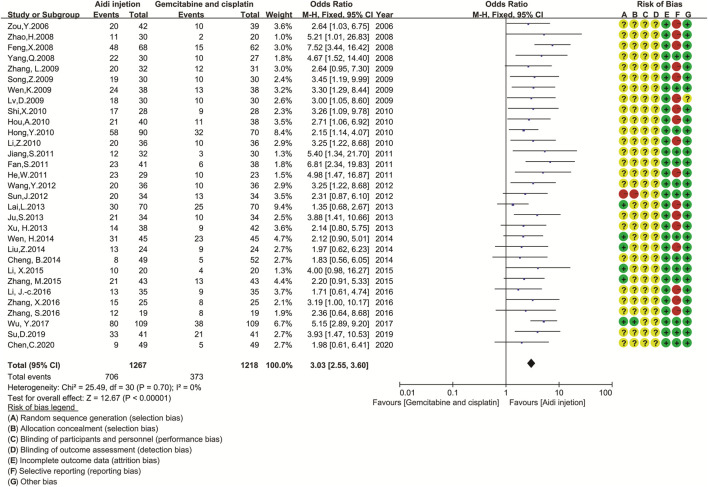
Thirty-one trials involving 2,485 patients completely compared the QOL in the two groups (Figure 4). Cochran’s χ2 test and I 2 statistic showed no significant heterogeneity in QOL (I 2 = 0%). Therefore, we pooled the OR of QOL using an FEM. The pooled result showed a significant improvement in QOL in the Aidi and GP combination compared to that in the control (OR = 3.03, 95% CI [2.55 to 3.60], p < 0.00001).
FIGURE 4.
The analysis of QOL between two groups. The pooled result showed a significant improvement in QOL in the Aidi and GP combination compared to that in the control group (OR = 3.03, 95% CI [2.55 to 3.60], p < 0.00001). Note: GP, Gemcitabine and cisplatin; QOL, quality of life.
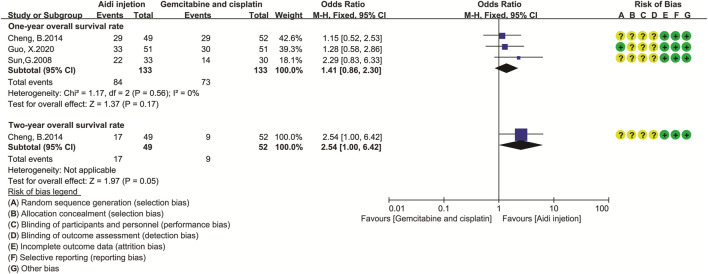
Overall Survival
Only three trials (Sun et al., 2008; Cheng et al., 2014; Guo, 2020) compared the OS rate in the two groups (Figure 5). Cochran’s χ2 test and I 2 statistic showed no significant heterogeneity in the one-year OS rate (I 2 = 0%). Therefore, we pooled the ORs of the one-year OS rate using a FEM. The results showed no significant difference in the one-year OS rate and two-year OS rate between the two groups (OR = 1.41, 95% CI [0.86 to 2.30], p = 0.17; and OR = 2.54, 95% CI [1.00 to 6.42], p = 0.05).
FIGURE 5.
The analysis of overall survival rate. The result showed no difference in one- and two-year OS rate between the two groups (OR = 1.41, 95% CI [0.86 to 2.30], p = 0.17; and OR = 2.54, 95% CI [1.00 to 6.42], p = 0.05). Note: GP, Gemcitabine and cisplatin.
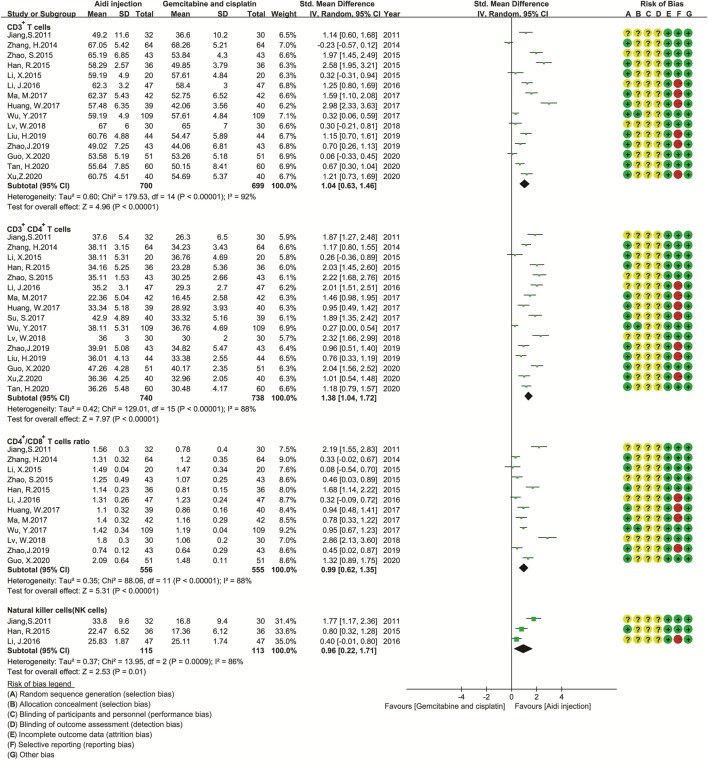
Levels of Antitumor Immunity
Eighteen trials involving 1,688 patients (Feng et al., 2008; Jiang et al., 2011; Xu et al., 2013; Zhang, 2014; Han et al., 2015; Li, 2015; Zhao et al., 2015; Li et al., 2016; Wu et al., 2017b; Huang et al., 2017; Ma, 2017; Su, 2017; Lv et al., 2018; Liu et al., 2019; Zhao and Li, 2019; Guo, 2020; Tan et al., 2020; Xu, 2020) compared the antitumor immunity in the two groups (Figure 6). Cochran’s χ2 test and I2 statistic showed significant heterogeneity in CD3+ T cells (I 2 = 92%), CD3+ CD4+ T cells (I 2 = 88%), CD4+/CD8+ T cell ratios (I 2 = 88%), and NK cells (I 2 = 86%) and significant clinical heterogeneity in CD3+ CD8+ T cells. Then, we pooled only the SMD of CD3+ T cells, CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and NK cell activity using REM. The pooled result showed a significant upregulation in CD3+ T cells, CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and NK cell activity (SMD = 1.04, 95% CI [0.63 to 1.46], p < 0.00001); SMD = 1.38, 95% CI [1.04 to 1.72], p < 0.00001; SMD = 0.99, 95% CI [0.62 to 1.35], p < 0.00001; SMD = 0.96, 95% CI [0.22 to 1.71], p = 0.01).
FIGURE 6.
The analysis of antitumor immunity. The pooled result showed a significant up-regulation in CD3+ T cells, CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and NK cell activity in the Aidi and GP combination group (SMD = 1.04, 95% CI [0.63 to 1.46], p < 0.00001); SMD = 1.38, 95% CI [1.04 to 1.72], p < 0.00001; SMD = 0.99, 95% CI [0.62 to1.35], p < 0.00001; SMD = 0.96, 95% CI [0.22 to 1.71], p = 0.01).
In addition, we developed a subgroup analysis model to analyze the causes of heterogeneity in the levels of PBLs (Supplementary Table S6; Supplementary Figures S1–S24). The results of the subgroup analysis showed that the AST, treatment time, and dosage of DDP might be the causes of heterogeneity in CD3+ T cells (Supplementary Table S6; Supplementary Figures S4, S16, S22); the KPS score, AST, dosage of Aidi and DDP, and treatment cycles might be the causes in CD3+ CD4+ T cells (Supplementary Table S6; Supplementary Figures S2, S5, S14, S20, S23). Theage, dosage of Aidi and DDP, treatment time, and treatment cycle might be the causes in CD4+/CD8+ T cells (Supplementary Table S6; Supplementary Figures S12, S15, S18, S21, S24).
Adverse Drug Reactions
Fifty-eight trials involving 4,596 patients compared the ADRs in the two groups (Table 2; Supplementary Figures S25–S34). Cochran’s χ2 test and the I 2 statistic showed a significant heterogeneity in alopecia (I 2 = 70%), minimal heterogeneity in myelosuppression (I 2 = 31%), and no heterogeneity (I 2 = 0%) in other ADRs. Therefore, we pooled the ORs of alopecia using an REM, and the ORs of other ADRs using an FEM. The pooled result showed a significant decrease in myelosuppression, neutropenia, thrombocytopenia, anemia, gastrointestinal toxicity, liver toxicity, and renal toxicity (OR = 0.36, 95% CI [0.28 to 0.47], p < 0.00001; OR = 0.41, 95% CI [0.35 to 0.49], p < 0.00001); OR = 0.48, 95% CI [0.39 to 0.59], p < 0.00001; OR = 0.59, 95% CI [0.43 to 0.80], p = 0.0009; OR = 0.45, 95% CI [0.39 to 0.51], p < 0.00001; OR = 0.58, 95% CI [0.47 to 0.72], p < 0.00001; and OR = 0.62, 95% CI [0.48 to 0.79], p = 0.0001) and no significant differences in alopecia, neurotoxicity, and oral mucositis.
TABLE 2.
Meta-analysis results of adverse drug reactions (Supplementary Figures S25–S34).
| Indicators | Trials | Aidi injection with GP (Events/Total) | GP alone (Events/Total) | SM | Odds ratio, 95% CI | I2 | P |
|---|---|---|---|---|---|---|---|
| Myelosuppression (Supplementary Figure S25) | 17 | 218/642 | 342/632 | FEM | 0.36 [0.28, 0.47] | 31% | p < 0.00001 |
| Neutropenia (Supplementary Figure S26) | 40 | 626/1701 | 862/1670 | FEM | 0.41 [0.35, 0.49] | 0% | p < 0.00001 |
| Thrombocytopenia (Supplementary Figure S27) | 28 | 286/1181 | 409/1156 | FEM | 0.48 [0.39, 0.59] | 0% | p < 0.00001 |
| Anemia (Supplementary Figure S28) | 11 | 156/524 | 200/523 | FEM | 0.59 [0.43, 0.80] | 5% | p = 0.0009 |
| Gastrointestinal toxicity (Supplementary Figure S29) | 49 | 756/2043 | 1059/2002 | FEM | 0.45 [0.39, 0.51] | 0% | p < 0.00001 |
| Liver toxicity (Supplementary Figure S30) | 29 | 192/1284 | 285/1257 | FEM | 0.58 [0.47, 0.72] | 0% | p < 0.00001 |
| Renal toxicity (Supplementary Figure S31) | 24 | 132/1070 | 192/1044 | FEM | 0.62 [0.48, 0.79] | 0% | p = 0.0001 |
| Alopecia (Supplementary Figure S32) | 3 | 41/94 | 57/89 | REM | 0.27 [0.05, 1.37] | 70% | p = 0.11 |
| Neurotoxicity (Supplementary Figure S33) | 5 | 26/208 | 37/208 | FEM | 0.63 [0.35, 1.12] | 0% | p = 0.11 |
| Oral mucositis (Supplementary Figure S34) | 3 | 10/106 | 18/106 | FEM | 0.50 [0.22, 1.16] | 0% | p = 0.11 |
Note: GP: gemcitabine and cisplatin; SM: statistical method; REM: random-effects model; and FEM: fixed-effects model.
Subgroup and Meta-Regression Analysis
This study included patients with KPS scores (≥60 or ≥70) or AST (≥3 months or ≥5 months). For patients with a KPS score ≥60 or AST ≥3 months, the results of subgroup analyses showed that the Aidi and GP combination achieved a significant improvement in the ORR and DCR and a low incidence rate of neutropenia and gastrointestinal toxicity (Tables 3A,B; ; Supplementary Figures S35–S50). We included patients with treatment processes (PT, RT, or PT/RT) and with age (≥60 or others). For patients with PT or age (≥60), the pooled results showed that the Aidi and GP combination also achieved the same effects (Tables 3C,D; Supplementary Figures S51–S66). Univariate random effects meta-regression manifested significant differences in the relationship between age and neutropenia (Tables 3D; Supplementary Figures S63–S64).
TABLE 3.
Subgroup and meta-regression analysis.
| Subgroups | Objective response rate (ORR) | Disease control rate (DCR) | Neutropenia | Gastrointestinal toxicity | ||||||||
| OR (95% CI) | UM | MM | OR (95% CI) | UM | MM | OR (95% CI) | UM | MM | OR (95% CI) | UM | MM | |
| Table 3A.Subgroups analysis according to KPS score (Supplementary Figures S35–S42) | ||||||||||||
| KPS score (≥60) | 1.87 [1.58, 2.22] | 0.22 | 0.27 | 2.31 [1.84, 2.89] | 0.57 | 0.47 | 0.42 [0.33, 0.54] | 0.82 | 0.90 | 0.41 [0.33, 0.50] | 0.32 | 0.64 |
| KPS score (≥70) | 1.40 [1.03, 1.90] | 2.01 [1.36, 2.98] | 0.33 [0.20, 0.54] | 0.45 [0.31, 0.66] | ||||||||
| KPS score (others) | 1.94 [1.61, 2.33] | 2.39 [1.87, 3.06] | 0.43 [0.33, 0.55] | 0.50 [0.40, 0.62] | ||||||||
| Table 3B.Subgroups analysis according to AST (Supplementary Figures S35–S50) | ||||||||||||
| AST (≥3m) | 1.81 [1.56, 2.09] | 0.28 | 0.99 | 2.20 [1.82, 2.66] | 0.72 | 0.98 | 0.42 [0.34, 0.53] | 0.78 | 0.90 | 0.47 [0.39, 0.56] | 0.32 | 0.42 |
| AST (≥5m) | 1.98 [1.31, 3.00] | 2.90 [1.69, 4.99] | 0.40 [0.26, 0.61] | 0.26 [0.15, 0.46] | ||||||||
| AST (unclear) | 1.80 [1.45, 2.24] | 2.35 [1.74, 3.18] | 0.40 [0.29, 0.55] | 0.46 [0.36, 0.59] | ||||||||
| Table 3C. Subgroups analysis via treatment process (Supplementary Figures S51–S58) | ||||||||||||
| Primary treatment (PT) | 1.46 [1.10, 1.94] | 0.08 | 0.06 | 2.04 [1.41, 2.94] | 0.27 | 0.20 | 0.46 [0.28, 0.76] | 0.42 | 0.52 | 0.54 [0.36, 0.82] | 0.81 | 0.41 |
| Retreatment (RT) | 2.88 [1.03, 8.07] | 2.13 [0.62, 7.29] | 1.00 [0.37, 2.71] | 0.59 [0.22, 1.62] | ||||||||
| PT and RT | 1.08 [0.62, 1.89] | 1.43 [0.65, 3.14] | 0.29 [0.13, 0.63] | 0.32 [0.16, 0.65] | ||||||||
| Unclear | 1.95 [1.71, 2.23] | 2.41 [2.02, 2.87] | 0.40 [0.33, 0.48] | 0.44 [0.37, 0.51] | ||||||||
| Table 3D. Subgroups analysis via age (Supplementary Figures S59–S66) | ||||||||||||
| Age (≥60) | 1.85 [1.36, 2.51] | 0.91 | 0.88 | 2.22 [1.49, 3.30] | 0.86 | 0.56 | 0.25 [0.15, 0.42] | 0.04 | 0.09 | 0.46 [0.31, 0.69] | 0.80 | 0.99 |
| Age (others) | 1.81 [1.60, 2.06] | 2.30 [1.95, 2.72] | 0.44 [0.37, 0.53] | 0.44 [0.38, 0.52] | ||||||||
| Table 3E. Subgroups analysis according to dosage (Supplementary Figures S67–S74) | ||||||||||||
| 50–60 ml | 1.72 [1.49, 2.00] | 0.75 | 0.88 | 2.21 [1.82, 2.68] | 0.35 | 0.21 | 0.45 [0.37, 0.56] | 0.32 | 0.58 | 0.47 [0.39, 0.56] | 0.09 | 0.21 |
| 70–80 ml | 1.85 [1.28, 2.68] | 2.80 [1.73, 4.52] | 0.24 [0.12, 0.48] | 0.39 [0.25, 0.62] | ||||||||
| 90–100 ml | 1.89 [1.35, 2.64] | 2.55 [1.57, 4.14] | 0.34 [0.21, 0.53] | 0.30 [0.19, 0.48] | ||||||||
| Others | 2.16 [1.61, 2.89] | 2.18 [1.48, 3.21] | 0.41 [0.27, 0.63] | 0.48 [0.35, 0.67] | ||||||||
| Table 3F. Subgroups analysis according to time per cycle (Supplementary Figures S75–S82) | ||||||||||||
| 7–10days | 1.75 [1.43, 2.13] | 0.18 | 0.18 | 2.19 [1.70, 2.82] | 0.48 | 0.53 | 0.35 [0.27, 0.46] | 0.32 | 0.61 | 0.44 [0.35, 0.57] | 0.97 | 0.41 |
| 14–15days | 1.62 [1.35, 1.94] | 1.87 [1.46, 2.38] | 0.40 [0.31, 0.53] | 0.45 [0.37, 0.55] | ||||||||
| 21–28days | 2.48 [1.90, 3.25] | 3.59 [2.46, 5.24] | 0.62 [0.39, 1.00] | 0.42 [0.29, 0.63] | ||||||||
| Others | 1.92 [1.13, 3.28] | 3.23 [1.64, 6.37] | 0.56 [0.28, 1.14] | No | ||||||||
| Table 3G. Subgroups analysis according to treatment cycle (Supplementary Figures S83–S90) | ||||||||||||
| One cycle | 2.22 [1.44, 3.43] | 0.70 | 0.40 | 2.54 [1.46, 4.41] | 1.00 | 0.74 | 0.37 [0.21, 0.63] | 0.35 | 0.78 | 0.41 [0.26, 0.66] | 0.21 | 0.08 |
| Two cycles | 1.65 [1.40, 1.94] | 2.08 [1.67, 2.58] | 0.35 [0.27, 0.45] | 0.46 [0.37, 0.57] | ||||||||
| Three cycles | 1.75 [1.24, 2.47] | 2.48 [1.55, 3.96] | 0.61 [0.41, 0.90] | 0.45 [0.31, 0.65] | ||||||||
| Four cycles | 2.32 [1.68, 3.22] | 2.59 [1.69, 3.95] | 0.40 [0.27, 0.61] | 0.35 [0.24, 0.51] | ||||||||
| Others | 1.91 [1.44, 2.52] | 2.53 [1.75, 3.64] | 0.45 [0.28, 0.70] | 0.51 [0.37, 0.72] | ||||||||
| Table 3H. Subgroups analysis according to DDP dosage (Supplementary Figures S91–S98) | ||||||||||||
| 20–30 mg/m2 | 1.83 [1.58, 2.12] | 0.77 | 0.34 | 2.26 [1.87, 2.72] | 0.98 | 0.75 | 0.43 [0.35, 0.53] | 0.55 | 0.37 | 0.49 [0.41, 0.59] | 0.28 | 0.27 |
| 40–50 mg/m2 | 1.88 [1.32, 2.69] | 2.56 [1.52, 4.31] | 0.38 [0.19, 0.76] | 0.39 [0.26, 0.59] | ||||||||
| 60–80 mg/m2 | 1.83 [1.43, 2.32] | 2.25 [1.61, 3.15] | 0.39 [0.28, 0.55] | 0.39 [0.29, 0.52] | ||||||||
| Others | 1.28 [0.64, 2.55] | 2.68 [1.02, 7.01] | 0.27 [0.11, 0.68] | 0.22 [0.08, 0.66] | ||||||||
| Table 3I. Subgroups analysis according to DDP dosage (Supplementary Figures S35–S50) | ||||||||||||
| WHO | 1.81 [1.58, 2.08] | 0.85 | 0.87 | 2.17 [1.80, 2.62] | 0.63 | 0.43 | 0.40 [0.32, 0.51] | 0.66 | 0.91 | 0.45 [0.36, 0.55] | 0.69 | 0.49 |
| RECIST/NCI-CTCAE | 1.85 [1.47, 2.31] | 2.50 [1.90, 3.30] | 0.36 [0.20, 0.66] | 0.38 [0.22, 0.66] | ||||||||
| Other | 1.70 [0.82, 3.50] | 3.64 [1.18, 11.23] | 0.43 [0.34, 0.56] | 0.46 [0.37, 0.56] | ||||||||
Note: AST: anticipated survival time; PT: primary treatment; RT: retreatment; Others: unclear or ungroupable; WHO: World Health Organization for solid tumor responses; OR: odds ratio; RECIST: Response Evaluation Criteria in Solid Tumors guideline; NCI-CTCAE: National Cancer Institute-Common Terminology Criteria for Adverse Events; UM: univariate meta-regression; and MM: multiple meta-regression.
Aidi was injected intravenously at 30–100 ml/day and 7–28 days/cycle for one to four cycles. In subgroups with Aidi usage (50–100 ml/day, 7–15 days/cycle for one to four cycles), the Aidi and GP combination showed a significant improvement in the tumor response and a low incidence rate of neutropenia and gastrointestinal toxicity (Tables 3E–G; Supplementary Figures S67–S90). Univariate meta-regression analysis also manifested any statistical difference in the relationship between Aidi usage and tumor response/ADRs (Tables 3E–G; Supplementary Figures S67–S90).
GEM (1000 mg/m2) is often used in combination with DDP (20–100 mg/m2). In subgroups with DDP usage (20–30 mg/m2, 40–50 mg/m2, or 60–80 mg/m2), the Aidi and GP combination also showed a significant improvement in the tumor response and a low incidence rate of neutropenia and gastrointestinal toxicity (Table 3H; Supplementary Figures S91–S98). There was no significance in the relationship between the dosage of DDP and tumor response/ADRs (Table 3H; Supplementary Figures S91–S98). The tumor responses (ORR and DCR) were evaluated using WHO (Miller et al., 1981) or RECIST guidelines (Watanabe et al., 2003), and the ADRs were evaluated using WHO (Miller et al., 1981) or CTCAE criteria (Trotti et al., 2003). Different criteria showed no positive effect on tumor responses and ADRs (Table 3I; Supplementary Figures S99–S106). Post hoc multiple regression analysis manifested no positive relationship between all variables and indicators (Table 3).
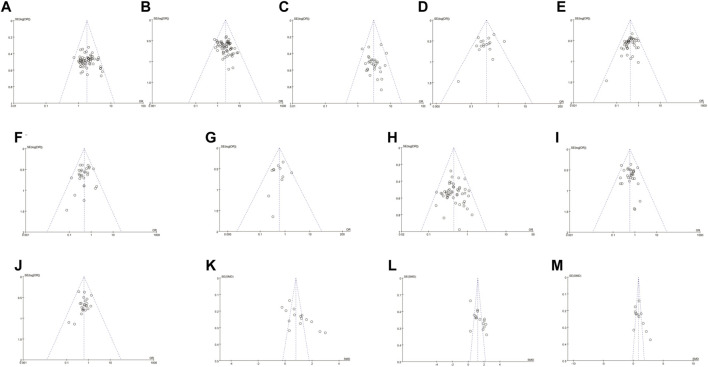
Publication Bias Analysis
We analyzed the potential publication bias using a funnel plot and Egger/Begg’s tests (Figure 7). The results showed that no publication bias was found for ORR, QOL, myelosuppression, thrombocytopenia, anemia, gastrointestinal toxicity, liver toxicity, renal toxicity, or CD4+/CD8+ T cell ratios (p = 0.32, 95% CI −0.81–2.41; p = 0.68, 95% CI −1.20–1.81; p = 0.15, 95% CI −3.84–0.62; p = 0.34, 95% CI −1.84–0.64; p = 0.49, 95% CI −2.64–1.36; p = 0.73, 95% CI −0.96–1.37; p = 0.44, 95% CI −0.72–1.60; p = 0.52, 95% CI −1.43–0.75; and p = 0.11, 95% CI −1.43–12.04, and the trials objectively reported them. However, significant publication bias was found for DCR, neutropenia, CD3+ T cells, and CD3+ CD4+ T cells (p = 0.02, 95% CI 0.21 to 2.28; p = 0.03, 95% CI −2.24–−0.13; p = 0.01, 95% CI 3.94 to 16.11; and p = 0.001, 95% CI 4.04–13.58). The trials overestimated DCR, CD3+ T cells, and CD3+ CD4+ T cells and underestimated neutropenia.
FIGURE 7.
The analysis of publication bias. There was a significant publication bias for DCR, neutropenia, CD3+ T cells, and CD3+ CD4+ T cells (p = 0.02, 95% CI 0.21 to 2.28; p = 0.03, 95% CI –2.24 to –0.13; p = 0.01, 95% CI 3.94 to 16.11; and p = 0.001, 95% CI 4.04–13.58). Note: ORR, objective response rate; DCR, disease control rate.
Sensitivity Analysis
Poor trials were found for ORR, DCR, QOL, myelosuppression, neutropenia, thrombocytopenia, anemia, gastrointestinal toxicity, liver toxicity, renal toxicity, neurotoxicity, CD3+ T cells, CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and natural killer cells. Before and after rejecting the poor trials, the pooled results were robust. The ORs for anemia and alopecia were poorly robust (Table 4A). Overestimated trials were found for ORR, DCR, QOL, CD3+ T cells, CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and natural killer cells. Underestimated trials were found for myelosuppression, neutropenia, thrombocytopenia, anemia, gastrointestinal toxicity, liver toxicity, renal toxicity, and alopecia. Before and after rejecting the trials with overestimated efficacy or underestimated ADRs, the pooled results were robust (Table 4B).
TABLE 4.
Sensitivity analysis.
Note: PBL: Peripheral blood lymphocyte; SM: statistical method; FEM: fixed-effects model; OR: odds ratio; SMD: standardized mean difference; CI: confidence interval; Poor trial (Poor*) that had at least one domain being considered as high risk of bias; and Over* or Under*: over or underestimated trial which the result was significant difference and beneficial to Aidi injection use.
Quality of Evidence
In methodology, this meta-analysis included 36 poor trials. The ORs of anemia and alopecia were poorly robust, and then we downgraded the quality by two levels. The ORs of other indicators were robust, and then we downgraded the quality by only one level. Cochran’s χ2 test and the I 2 statistic found significant heterogeneity for alopecia and levels of PBLs, and all indicators were robust, and not downgraded. The OS rates of patients with alopecia, oral mucositis, and natural killer cells were less than 300. Therefore, we downgraded their quality by one level. There was significant publication bias in DCR, neutropenia, CD3+ T cells, and CD3+ CD4+ T cells, and the pooled results were robust; therefore, their quality was not downgraded. Upgrade was unsuitable for any indicators. Finally, we summarized the quality of ORR, DCR, QOL, myelosuppression, neutropenia, thrombocytopenia, gastrointestinal toxicity, hepatorenal toxicity, neurotoxicity, CD3+ T cells, CD3+ CD4+ T cells, and CD4+/CD8+ T cell ratios as “moderate” and other indicators as “low to very low” (Table 5).
TABLE 5.
GRADE evidence profile.
| Table 5A. The clinical efficacy and safety | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indicators (Trials) | Quality assessment | NSCLC | Clinical efficacy and safety | Quality | ||||||
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Aidi injection | GP | Odds ratios (95% CI) | Absolute effects | ||
| Objective response rate (63) | Serious a | No | No | No | None | 1319/2454 (53.7%) | 946/2397 (39.5%) | 1.82 (1.62–2.04) | 148 more per 1000 (from 119 more to 176 more) | ⊕⊕⊕Ο Moderate |
| Disease control rate (61) | Serious a | No | No | No | None b | 2095/2406 (87.1%) | 1766/2355 (75%) | 2.29 (1.97–2.67) | 123 more per 1000 (from 105 more to 139 more) | ⊕⊕⊕Ο Moderate |
| Quality of life (31) | Serious a | No | No | No | None | 706/1267 (55.7%) | 373/1218 (30.6%) | 3.03 (2.55–3.6) | 266 more per 1000 (from 223 more to 308 more) | ⊕⊕⊕Ο Moderate |
| 1-year OS rate (3) | Serious c | No | No | Serious d | None | 84/133 (63.2%) | 73/133 (54.9%) | 1.41 (0.86–2.3) | 83 more per 1000 (from 38 fewer to 188 more) | ⊕⊕ΟΟ Low |
| 2-years OS rate (1) | Serious c | No | No | Serious d | None | 17/49 (34.7%) | 9/52 (17.3%) | 2.54 (1–6.42) | 174 more per 1000 (from 0 more to 400 more) | ⊕⊕ΟΟ Low |
| Myelosuppression (17) | Serious a | No | No | No | None | 218/642 (34%) | 342/632 (54.1%) | 0.36 (0.28–0.47) | 243 fewer per 1000 (from 185 fewer to 293 fewer) | ⊕⊕⊕Ο Moderate |
| Neutropenia (40) | Serious a | No | No | No | None e | 626/1701 (36.8%) | 862/1670 (51.6%) | 0.41 (0.35–0.49) | 212 fewer per 1000 (from 173 fewer to 244 fewer) | ⊕⊕⊕Ο Moderate |
| Thrombocytopenia (28) | Serious a | No | No | No | None | 286/1181 (24.2%) | 409/1156 (35.4%) | 0.48 (0.39–0.59) | 146 fewer per 1000 (from 110 fewer to 178 fewer) | ⊕⊕⊕Ο Moderate |
| Anemia (11) | Very serious f | No | No | No | None | 156/524 (29.8%) | 200/523 (38.2%) | 0.59 (0.43–0.8) | 115 fewer per 1000 (from 51 fewer to 172 fewer) | ⊕⊕ΟΟ Low |
| Gastrointestinal toxicity (49) | Serious a | No | No | No | None | 756/2043 (37%) | 1059/2002 (52.9%) | 0.45 (0.39–0.51) | 193 fewer per 1000 (from 165 fewer to 224 fewer) | ⊕⊕⊕Ο Moderate |
| Liver toxicity (29) | Serious a | No | No | No | None b | 192/1284 (15%) | 285/1257 (22.7%) | 0.58 (0.47–0.72) | 81 fewer per 1000 (from 52 fewer to 106 fewer) | ⊕⊕⊕Ο Moderate |
| Renal toxicity (24) | Serious a | No | No | No | None | 132/1070 (12.3%) | 192/1044 (18.4%) | 0.62 (0.48–0.79) | 61 fewer per 1000 (from 33 fewer to 86 fewer) | ⊕⊕⊕Ο Moderate |
| Alopecia (3) | Very serious f | No g | No | Serious d | None | 41/94 (43.6%) | 57/89 (64%) | 0.27 (0.05–1.37) | 316 fewer per 1000 (from 559 fewer to 69 more) | ⊕ΟΟΟ Very low |
| Neurotoxicity (5) | Serious a | No | No | Serious d | None | 26/208 (12.5%) | 37/208 (17.8%) | 0.63 (0.35–1.12) | 58 fewer per 1000 (from 107 fewer to 17 more) | ⊕⊕⊕Ο Moderate |
| Oral mucositis (3) | Serious a | No | No | Serious d | None | 10/106 (9.4%) | 18/106 (17%) | 0.5 (0.22–1.16) | 77 fewer per 1000 (from 127 fewer to 22 more) | ⊕⊕ΟΟ Low |
| Table 5B. The levels of peripheral blood lymphocytes | ||||||||||
| Indicators (Trials) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Aidi injection | GP | Odds ratios (95% CI) | SMD (95% CI) | Quality |
| CD3+ T cell (15) | Serious a | No g | No | No | None b | 700 | 699 | No | 1.04 higher (0.63–1.46 higher) | ⊕⊕⊕Ο Moderate |
| CD3+ CD4+ T cell (16) | Serious a | No g | No | No | None b | 740 | 738 | No | 1.38 higher (1.04–1.72 higher) | ⊕⊕⊕Ο Moderate |
| CD4+/CD8+ T cell ratios (12) | Serious a | No g | No | No | None | 556 | 555 | No | 0.99 higher (0.62–1.35 higher) | ⊕⊕⊕Ο Moderate |
| Natural killer cell (3) | Serious a | No g | No | Serious d | None | 115 | 113 | No | 0.96 higher (0.22–1.71 higher) | ⊕⊕ΟΟ Low |
Note: NSCLC: non-small cell lung cancer; GP: gemcitabine and cisplatin; OS: overall survival; SMD: standardized mean difference; and CI: confidence interval.
Most trials had unclear risk and with high risk, the result of sensitivity analysis was robust, and the evidence was downgraded by only one level
with publication bias, the result was overestimated and robust, and not downgraded
most trials had unclear risk and without high risk, the evidence was downgraded by only one level
the sample size for result <300 cases, and the evidence was downgraded by one level
with publication bias, the result was underestimated and robust, and not downgraded
most trials had unclear risk and with high risk, the result of sensitivity analysis was poorly robust, and the evidence was downgraded by two levels
with heterogeneity, the results was robust, and not downgraded
Discussion
Based on three previous SRs/meta-analyses (Yang and Ding, 2012; Han et al., 2016; Xiao et al., 2017) and six related SRs/meta-analyses (Ma et al., 2009; Tian et al., 2014; Xiao et al., 2016; Wu et al., 2017a; Xiao et al., 2018b; Wang et al., 2018), we included 70 trials for this meta-analysis, which involved 5,509 NSCLC patients including 3,278 males and 1,995 females with ages ranging from 21–86 years old. The experimental group with 2,783 cases received the Aidi and GP combination, and the control group with 2,726 cases received the GP alone. Aidi was intravenously injected at 30–100 ml/day, 7–28 days per cycle for one to four cycles. GEM (1000 mg/m2) was mainly used in combination with DDP (20–100 mg/m2). After six weeks to two years of follow-up, the trials evaluated tumor response, survival, QOL, antitumor immunity, and ADRs.
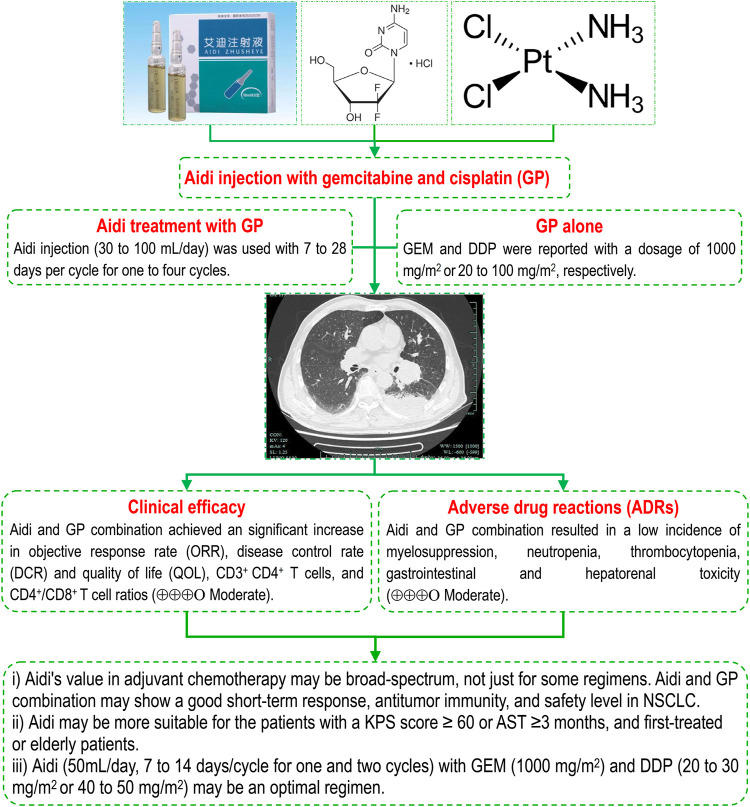
Gemcitabine and cisplatin is one of the standard regimens in the treatment of advanced NSCLC. As a cantharidin-based CHI and important adjuvant drug, Aidi is often used in combination with GP to treat NSCLC (Yang and Ding, 2012; Han et al., 2016; Xiao et al., 2017). Three years ago, we reported that the Aidi and GP combination might improve the tumor response and QOL with a low risk of hematotoxicity and gastrointestinal toxicity in patients (Xiao et al., 2017). However, the methodology had many shortcomings, and new trials have been published (Chen, 2020; Guo, 2020; Tan et al., 2020; Xu, 2020). Therefore, we further improved the methodology, integrated all previous three SRs/meta-analyses (Yang and Ding, 2012; Han et al., 2016; Xiao et al., 2017), and supplemented 34 trials with 2,927 cases for this meta-analysis. The pooled results demonstrated that the Aidi and GP combination significantly improved the ORR, DCR, and QOL, reduced the incidences of hematotoxicity and gastrointestinal and hepatorenal toxicity, and upregulated the levels of CD3+ T cells and CD3+ CD4+ T cells, CD4+/CD8+ T cell ratios, and NK cell activity. However, the pooled results of PBLs showed significant heterogeneity, and further subgroup analysis revealed the patient features, DDP, and Aidi usage might be important causes of heterogeneity. Moreover, four trials with 320 patients reported survival (Sun et al., 2008; Cheng et al., 2014; Li and Yang, 2014; Guo, 2020), and no trials reported PFS. However, current evidence does not support whether the Aidi and GP combination improves survival. Most trials had unclear risk, some had high risk, and some indicators were overestimated or underestimated. Nevertheless, most results were robust, and their quality was “moderate” (Figure 8).
FIGURE 8.
Aidi injection and GP combination in NSCLC, Aidi's value in adjuvant chemotherapy may be broad-spectrum, not just for some regimens. The Aidi and GP combination may show a good short-term response, antitumor immunity, and safety level in NSCLC. Aidi may be more suitable for patients with a KPS score ≥60 or AST ≥3 months, and first-treated or elderly patients. Aidi (50 ml/day, 7–14 days/cycle for one and two cycles) with GEM (1000 mg/m2) and DDP (20–30 mg/m2 or 40–50 mg/m2) may be an optimal regimen. Note: AST, anticipated survival time; DCR, disease control rate; DDP, cisplatin; GEM, Gemcitabine; KPS: KPS, Karnofsky Performance Status; NSCLC, non-small cell lung cancer; ORR, objective response rate.
Aidi injection is composed of cantharidin, astragaloside, ginsenoside, elentheroside E, and syringin (Zhang et al., 2012; Zeng et al., 2016). The results from clinical and basic studies revealed that Aidi has an important antitumor, immunoregulatory, anti-inflammatory and antioxidative stress function (Duan et al., 2018b; Li et al., 2018; Zhou et al., 2018; Xiao et al., 2019a; Chen et al., 2019; Farag et al., 2019; Huang et al., 2019; Li et al., 2019; Qu et al., 2019; Zhang et al., 2019). The results of previous SRs/meta-analyses (Ma et al., 2009; Xiao et al., 2016; Wu et al., 2017a; Xiao et al., 2018a; Xiao et al., 2018b; Wang et al., 2018; Xiao et al., 2019b) demonstrated that Aidi in combination with chemotherapy showed significant improvements in tumor response, QOL, and OS rate, and decreases in ADRs. Recently, we found that Aidi in combination with NP might improve the tumor response and QOL, upregulate antitumor immunity, and reduce the incidence of hematotoxicity and gastrointestinal and liver toxicity in patients with NSCLC (Xiao et al., 2020b). After this study, we further found that Aidi in combination with chemotherapy resulted in a low incidence of hepatorenal toxicity in patients with lung cancer (Xiao et al., 2020a). In addition, another meta-analysis (Xiao et al., 2016) revealed that Aidi might significantly upregulate the antitumor immunity in NSCLC patients undergoing platinum-based chemotherapy. This meta-analysis further demonstrated that the Aidi and GP combination also significantly improved the tumor response and QOL, upregulated the antitumor immunity, and decreased the incidences of ADRs, especially GP-induced hepatorenal toxicity (Figure 8). More interestingly, the results further demonstrated that Aidi may show an important protective function for the liver and kidney in NSCLC patients undergoing chemotherapy. In addition, current evidence does not support that the Aidi and GP combination improves survival. All these findings demonstrated that Aidi had an important clinical value in improving short-term responses and antitumor immunity and reducing ADRs. Aidi's value in adjuvant chemotherapy may be broad-spectrum, not just for some regimens. Therefore, the strategy of Aidi use should focus on reducing toxicity and improving tumor responses and QOL.
In previous studies (Xiao et al., 2020a; Xiao et al., 2020b), we found that patient features and Aidi and DDP usage might be important influencing factors in obtaining an ideal tumor response and safety level for NSCLC. For patients with a KPS score ≥60, the Aidi and NP combination may produce an ideal tumor response and achieve a good safety level (Xiao et al., 2020b). Aidi decreased the risk of hepatorenal toxicity for patients with KPS scores ≥60, PT and who are elderly (Xiao et al., 2020a). The results indicate that patients who are first-treated, elderly, patients with a KPS score ≥60, or AST ≥3 months may be appropriate populations for Aidi. In this meta-analysis, series subgroup analyses further revealed that the Aidi and GP combination also achieved an ideal response and safety level in patients with KPS scores ≥60, AST ≥3 months, PT or age ≥60. The patients with a KPS score ≥ 60 or AST ≥3 months and patients who were first-treated or elderly might be appropriate populations for Aidi and GP combinations. These populations may have a treatment threshold for Aidi and GP combinations, which is of important clinical significance for standardizing the Aidi treatment (Figure 8). Unfortunately, no evidence supports that Aidi achieves the same effects in patients with retreatment or drug resistance, which needs to be confirmed by new trials.
In the GP regimen, GEM and DDP are recommended at dosages of 1000 mg/m2 or 75 mg/m2, respectively, according to the guidelines (Association et al., 2018). Wang Z. et al. (Wang and Zhu, 2015) reported that Aidi treatment with a 60% dose of GP or NP might achieve the same tumor response as the conventional dose. Ruan F. et al. (Ruan and Lu, 2012) reported that Aidi treatment with low-dose capecitabine might improve tumor responses and survival in advanced gastric cancer. Our previous studies (Xiao et al., 2020a; Xiao et al., 2020b) also reported that Aidi in combination with vinorelbine and cisplatin (low- or high-dose) might achieve the same tumor response and good safety level (Xiao et al., 2020b). In this meta-analysis, we further confirmed a similar relationship between Aidi and GP. The results of subgroup analyses further revealed that Aidi (50 ml/day, 7–14 days/cycle for one and two cycles) in combination with GEM (1000 mg/m2) and DDP (20–30 mg/m2, 40–50 mg/m2) might obtain the above effects (Figure 8). The results indicate that Aidi has a similar dosage, treatment time, and cycle in combination with different regimens, which is beneficial to further standardized/rational drug use. In addition, Aidi treatment with low- or high-dose DDP might both obtain satisfactory effects, and Aidi might decrease the dosage of DDP and show a synergistic effect with different chemotherapy regimens, which will be of important clinical significance in improving the prognosis of patients by innovating chemotherapy strategies based on synergistic effects. However, a post hoc univariate and multiple regression analysis found only one positive correlation. These conclusions were drawn from the subgroup analysis and need to be further confirmed by new studies. Based on the optimization of efficacy and safety, Aidi treatment (50 ml/day, 7–14 days/cycle for one and two cycles) with GEM (1000 mg/m2) and DDP (20–30 mg/m2 or 40–50 mg/m2) may be an optimal therapy for realizing an ideal goal. If confirmed, these findings will be of importance for developing a standardized and rational drug use strategy against advanced NSCLC.
Some limitations exist in this meta-analysis. First, we collected the trials only from Chinese and English-language databases, which might have resulted in retrieval bias. Second, methodologically, the bias risk of most trials was unclear, and 36 trials selectively reported the clinical efficacy, ADRs, and PBLs; the quality was “moderate” to “very low.” Third, only some trials provided the baseline information such as age, KPS score, AST, retreatment, and drug resistance. Limited trials and patients were available to analyze the OS, phlebitis, and antitumor immunity, and none of the trials reported the PFS. Fourth, this meta-analysis did not support that the Aidi and GP combination is suitable for patients with retreatment or drug resistance or improves survival. We have not further explored the optimal conditions to achieve ideal antitumor immunity. Fifth, the univariate and multiple meta-regression analyses found only one positive correlation, and these conclusions from the subgroup analysis belonged to indirect evidence. All of these questions need further high-quality study to determine.
Conclusion
Current evidence indicates that Aidi’s value in adjuvant chemotherapy may be broad-spectrum, not just for some regimens. The Aidi and GP combination may show a good short-term response, antitumor immunity, and a safety level in patients with NSCLC. Aidi may be more suitable for patients with a KPS score ≥60 or AST ≥3 months, patients first-treated, and elderly patients. Aidi treatment (50 ml/day, 7–14 days/cycle for one and two cycles) with GEM (1000 mg/m2) and DDP (20–30 mg/m2 or 40–50 mg/m2) may be an optimal therapy for realizing an ideal goal. Moreover, Aidi may decrease the dosage of DDP use and show a synergistic effect with different regimens. Finally, we hope that this meta-analysis will provide valuable evidence by which to develop an optimal CHI treatment strategy against advanced NSCLC.
Author Contributions
Conception and design by ZX, XX, and X-FL; development of methodology by ZX, C-QW, and X-FC; trials retrieval by C-QW and X-TZ; study selection by S-SH and HJ; Evaluation of methodological bias risk by C-QW and X-TZ; data extraction by YJ and X-RH; statistical analysis by C-QW and JH; rating quality of evidence by X-FC and C-QW; preparing the manuscript draft by C-QW, ZX, S-YL, XX, and X-FL; review and revision of the manuscript by XX, X-FC, Q-HG, and J-HF; and study supervision by ZX, XX, and X-FL. All authors read and approved the final version of the manuscript.
Funding
This work was funded by special funds for academic seedlings training and innovation at Zunyi Medical College (Qian Kehe Pingtai Rencai No (2017) 5733–034), special funds for science and technology research into traditional Chinese and national medicine in Guizhou (No QZYY 2017–084), innovation talent team of Guizhou science and Technology Department (Qian Kehe Platform Talents (2020) 5007), and a high-level innovative talent program in Guizhou (No. fzc 120,171,001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.582447/full#supplementary-material
Glossary
- ADRs
Adverse drug reactions
- Aidi
Aidi injection
- AST
anticipated survival time
- CBM
China Biological Medicine database
- CENTRAL
Cochrane Central Register of Controlled Trials database;
- CHIs
Chinese herbs injections
- CIs
Confidence intervals
- CNKI
China National Knowledge Infrastructure (CNKI) database
- CR
Complete response
- CTCAE
Common terminology criteria for adverse events
- DCR
disease control rate
- DDP
cisplatin
- FCM
flow cytometry
- FEM
Fixed-effects model
- GP
Gemcitabine and cisplatin
- GRADE
Grading of Recommendations Assessment Development and Evaluation approach
- GEM
Gemcitabine
- HR
Hazard ratio
- KPS
Karnofsky Performance Status score
- MM
Multiple meta-regression
- NC
No change
- NK cell
Natural killer cell
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- ORR
Objective response rate
- OS
overall survival
- PBLs
Peripheral blood lymphocytes
- PD
Progressive disease
- PFS
Progression-free survival
- PR
Partial response
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines
- PT
Primary treatment
- QOL
Quality of life
- RCT
Randomized controlled trial
- RECIST
Response Evaluation Criteria in Solid Tumors
- REM
Random-effects model
- RT
Retreatment
- SM
Statistical method
- SMD
Standardized mean difference
- SRs
Systematic reviews
- TCM
Traditional Chinese medicine
- TNM staging system
Tumor node metastasis staging system
- TP
Treatment process
- UM
Univariate meta-regression
- VIP
Chinese Scientific Journals Full-Text database
- WHO
World Health Organization
References
- Association C. M., Association O. So. C. M., House C. M. A. P. (2018). Chinese Medical Association Guidelines for Clinical Diagnosis and Treatment of Lung Cancer ( Edition 2018). Chin. J. Oncol. 40, 935–964. 10.3760/cma.j.issn.0253-3766.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Cai H., Zou H., Chai Y., Zhu H. (2013). Clinical Study of Aidi Injection and GP Chemotherapy to Improve the Quality of Life in Patients with Non-small Cell Lung Cancer. Med. Pharm. Yunnan 34, 365. [Google Scholar]
- Cao A., He H., Jing M., Yu B., Zhou X. (2017). Shenfu Injection Adjunct with Platinum-Based Chemotherapy for the Treatment of Advanced Non-small-cell Lung Cancer: A Meta-Analysis and Systematic Review. Evid. Based Complement. Alternat Med. 2017, 1068751. 10.1155/2017/1068751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. (2020). Clinical Efficacy of Aidi Injection Combined with GP Chemotherapy in the Treatment of Advanced Non-small Cell Lung Cancer. Capital Med. 27. [DOI] [PubMed] [Google Scholar]
- Chen W., Zheng R., Baade P. D., Zhang S., Zeng H., Bray F., et al. (2016). Cancer Statistics in China, 2015. CA Cancer J. Clin. 66, 115–132. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Chen X., Gao J., Yang H., Duan Y., Feng Y., He X., et al. (2019). Astragaloside III Enhances Anti-tumor Response of NK Cells by Elevating NKG2D and IFN-Gamma. Front. Pharmacol. 10, 898. 10.3389/fphar.2019.00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. (2016). Efficacy of Aidi Injection Combined with Chemotherapy on Expression of VEGF-C and CYFR21 -1 in Peripheral Blood in Patients with Advanced Non-small Cell Lung Cancer. Chin. J. Clin. Rational Drug Use 9, 5–6. 10.15887/j.cnki.13-1389/r.2016.17.003 [DOI] [Google Scholar]
- Cheng B., Wang Z., Zhou L., Lou G., Yang G., Weng L. (2014). Clinical Effects of Aidi Injection Combined with Chemotherapy of GP Regimen in Treating Patients with Advanced Non-small Cell Lung Cancer. Chin. Arch. TCM 32, 1666–1668. 10.13193/j.issn.1673-7717.2014.07.042 [DOI] [Google Scholar]
- Clancey J. K. (1995). Karnofsky Performance Scale. J. Neurosci. Nurs. 27, 220. 10.1145/212094.212110 [DOI] [PubMed] [Google Scholar]
- Conroy T., Etienne P. L., Adenis A., Ducreux M., Paillot B., Oliveira J., et al. (2002). Vinorelbine and Cisplatin in Metastatic Squamous Cell Carcinoma of the Oesophagus: Response, Toxicity, Quality of Life and Survival. Ann. Oncol. 13, 721–729. 10.1093/annonc/mdf063 [DOI] [PubMed] [Google Scholar]
- Ding P., Ge H., Li M., Zhu M. (2011). Aidi Injection with Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. J. Clin. Pulm. Med. 16, 1802–1803. 10.3969/j.issn.1009-6663.2011.11.086 [DOI] [Google Scholar]
- Duan B., Xie J., Rui Q., Zhang W., Xi Z. (2018a). Effects of Shengmai Injection Add-On Therapy to Chemotherapy in Patients with Non-small Cell Lung Cancer: a Meta-Analysis. Support Care Cancer. 10.1007/s00520-018-4167-4 [DOI] [PubMed] [Google Scholar]
- Duan Z., Wei B., Deng J., Mi Y., Dong Y., Zhu C., et al. (2018b). The Anti-tumor Effect of Ginsenoside Rh4 in MCF-7 Breast Cancer Cells In Vitro and In Vivo. Biochem. Biophys. Res. Commun. 499, 482–487. 10.1016/j.bbrc.2018.03.174 [DOI] [PubMed] [Google Scholar]
- Fan S., Peng L., Qi X. (2011). Aidi Injection with Chemotherapy for Advanced Non-small Cell Lung Cancer: a Clinical Study. Zhejiang J. ITCWM 21, 232–233. 10.3969/j.issn.1005-4561.2011.04.007 [DOI] [Google Scholar]
- Fang L. (2016). Clinical Study of Addie Injection Combined Cavity Perfusion Hyperthermic Chemotherapy in Treatment of Patients with Advanced Non Small Cell Lung Cancer. J. Med. Forum 37, 74–75. [Google Scholar]
- Farag M. R., Elhady W. M., Ahmed S. Y. A., Taha H. S. A., Alagawany M. (2019). Astragalus Polysaccharides Alleviate Tilmicosin-Induced Toxicity in Rats by Inhibiting Oxidative Damage and Modulating the Expressions of HSP70, NF-kB and Nrf2/HO-1 Pathway. Res. Vet. Sci. 124, 137–148. 10.1016/j.rvsc.2019.03.010 [DOI] [PubMed] [Google Scholar]
- Feng X., Li X., Cui E. (2008). Aidi Injection Combined with GP Regimen in the Treatment for 68 Cases with Advanced Non-small Cell Lung Cancer. J. Oncol. 14, 892–893. [Google Scholar]
- Fu L. (2012). Aidi Injection with GP in the Treatment of Elderly Patients with Advanced Non-small Cell Lung Cancer: a Clinical Study. Guide China Med. 10, 466. 10.3969/j.issn.1671-8194.2012.26.360 [DOI] [Google Scholar]
- Geng K., Dong J., Su H., Dai C., Chen J., Yue X. (2020). Efficacy and Safety of Aidi Injection Combined with Gemcitabine and Cisplatin in the Treatment of Advanced Non-small Cell Lung Cancer. Henan Med. Res. 29, 2231–2232. 10.3969/j.issn.1004-437X.2020.12.059 [DOI] [Google Scholar]
- Guo X. (2020). Effect Analysis of Aidi Injection Combined with GP Regimen in the Treatment of Advanced NSCLC. Pract. Clin. J. ITCWM 20, 104–105. 10.13638/j.issn.1671-4040.2020.04.053 [DOI] [Google Scholar]
- Guyatt G. H., Oxman A. D., Vist G. E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 336, 924–926. 10.1136/bmj.39489.470347.ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot P., Ades A. E., Ouwens M. J., Welton N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R., Wang A., Yang G., Yang Y., Liu B., Cao X., et al. (2015). Effect of Aidi Injection on Chemotherapeutic Efficacy and Immune Function of Advanced Non-small Cell Lung Cancer. Chin. J. Gerontol. 35, 4878–4879. 10.3969/j.issn.1005-9202.2015.17.067 [DOI] [Google Scholar]
- Han Y., Jie K., Zhang H. (2016). Systematic Review of Aidi Injection Auxiliary GP Regimen for Non-small Cell Lung Cancer. Chin. J. Exp. Tradit Med. Formulae 22, 188–193. 10.13422/j.cnki.syfjx.2016100188 [DOI] [Google Scholar]
- He W., Wu L., Yu C., Song Y. (2011). Observation on the Clinical Effect of Combined Therapy of GP Regimen Plus Aidi Injection on Advanced Stage Non-small-cell Lung Cancer. Acta Acad. Med. CPAR 20, 296–297. 10.3969/j.issn.1008-5041.2011.04.015 [DOI] [Google Scholar]
- Higgins J. P. T. G. S. (2011). Cochrane Handbook for Systematic Reviewsof Interventions. [updated March 2011]. Available at:http://wwwhandbookcochraneorg/.Version 5.1.0 (Accessed January 2017). [Google Scholar]
- Hong Y., Wang J., Jiao Z. (2010). Clinical Observation on Aidiinjection Combined with GP Chemotherapy in Treating Advanced Non Small Cell Lung Cancer. Chin. J. Clin. Oncol. Rehab 17, 247–249. 10.1017/s000748531000012x [DOI] [Google Scholar]
- Hou A., Zhang L. (2010). Aidi Injection with Gemcitabine and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study of 78 Cases. World Health Dig. 07, 285–286. 10.3969/j.issn.1672-5085.2010.31.303 [DOI] [Google Scholar]
- Huang H., Luo S. H., Huang D. C., Cheng S. J., Cao C. J., Chen G. T. (2019). Immunomodulatory Activities of Proteins from Astragalus Membranaceus Waste. J. Sci. Food Agric. 99, 4174–4181. 10.1002/jsfa.9650 [DOI] [PubMed] [Google Scholar]
- Huang W., Zheng J., Guo B. (2017). Clinical Efficacy and Immune Function of Aidi Injection Combined with GP Regimen in the Treatment of Advanced Non-small Cell Lung Cancer. J. Med. Theor. Prac 30, 995–997. 10.19381/j.issn.1001-7585.2017.07.029 [DOI] [Google Scholar]
- Jiang S., Mao X., Hong J. (2011). Aidi Injection with Chemotherapy for Advanced Non Small Cell Lung Cancer: a Clinical Study of 32 Cases. Zhejiang J. ITCWM 21, 24–26. 10.3969/j.issn.1005-4561.2011.01.012 [DOI] [Google Scholar]
- Ju S., Li C., Xu Y. (2013). Aidi Injection Combined with GP in the Treatment of Elderly Patients with Advanced Non-small Cell Lung Cancer: a Clinical Study. Strait Pharma J. 25, 206–207. 10.3969/j.issn.1006-3765.2013.03.137 [DOI] [Google Scholar]
- Lai L. (2013). Aidi Injection with GP in the Treatment of Elderly Patients with Advanced Non-small Cell Lung Cancer: a Clinical Study. Mod. J. ITCWM 22, 257–258. 10.3969/j.issn.1008-8849.2013.03.013 [DOI] [Google Scholar]
- Li H., Deng C., Yu R., An N., He Y. (2014). Effects of Kang'ai and Aidi Injection Combined with Chemotherapy on Non-small Cell Lung Cancer. Guiding J. TCM Pharm. 20, 27–28. 10.13862/j.cnki.cn43-1446/r.2014.12.010 [DOI] [Google Scholar]
- Li J. (2016). Effect of Aidi Injection on Advanced Non-small Cell Lung Cancer. Clin. J. Chin. Med. 8, 82–84. 10.3969/j.issn.1674-7860.2016.08.044 [DOI] [Google Scholar]
- Li J., Xu L., Sang R., Yu Y., Ge B., Zhang X. (2018). Immunomodulatory and Anti-inflammatory Effects of Total Flavonoids of Astragalus by Regulating NF-KappaB and MAPK Signalling Pathways in RAW 264.7 Macrophages. Pharmazie 73, 589–593. 10.1691/ph.2018.8633 [DOI] [PubMed] [Google Scholar]
- Li J., Yang G. (2014). Clinical Evaluation of Aidi Injection Combined with GP for Advanced Non-small Cell Lung Cancer. Heilongjiang Med. J. 38, 447–448. 10.3969/j.issn.1004-5775.2014.04.049 [DOI] [Google Scholar]
- Li J., Yang Z., Song X., Jian Q., Sun X., Zhang H., et al. (2016). Study on Short-Term Efficacy and Safety of Aidi Injection Combined with GP Regimen in the Treatment of Advanced Non-small Cell Lung Cancer. Hebei Med. J. 38, 1345–1347. 10.3969/j.issn.1002-7386.2016.09.020 [DOI] [Google Scholar]
- Li W., Hu X., Wang S., Jiao Z., Sun T., Liu T., et al. (2019). Characterization and Anti-tumor Bioactivity of astragalus Polysaccharides by Immunomodulation. Int. J. Biol. Macromol, 189. 10.1016/j.ijbiomac.2019.09 [DOI] [PubMed] [Google Scholar]
- Li X. (2015). A Comparative Study of Aidi Injection and Kang Ai Injection Combined with Chemotherapy in Middle-Aged and Elderly Patients with Advanced Non-small Cell Lung Cancer (Qi-Yin Deficiency Syndrome) [Master]. Fuzhou: Fujian University of Traditional Chinese Medicine. [Google Scholar]
- Li Z., Liu X., Yuan L. (2010). Aidi Injection with Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. J. Commun. Med. 08, 23–24. [Google Scholar]
- Liu H., Nie Q., Lu Y., Liu X. (2019). Effect of Aidi Injection Combined with GP Regimen on the Expression of Serum Vascular Endothelial Growth Factor and Immune Function in Patients with Advanced Non-small Cell Lung Cancer. Jiangxi J. TCM 50, 42–44. [Google Scholar]
- Liu Y., Liu Y., Zhang T. (2010). Aidi Injection and Chemotherapy for Advanced Non-small Cell Lung Cancer:a Short-Term Curative Effect Study. Natl. Med Front China 05, 46. 10.3969/j.issn.1673-5552.2010.19.0033 [DOI] [Google Scholar]
- Liu Y., Zhao Y. (2014). Efect of Addie Injection Combined with GP Regimen on Advanced Non·small Cell Lung Cancer. China Pract. J. Med. 41, 67–69. 10.3760/cma.j.issn.1674-4756.2014.24.028 [DOI] [Google Scholar]
- Liu Z., Zhang C. (2014). Advanced Small Cell Lung Parallel Randomized Controlled Study of Non-aidi Injection Combined with GP Regimen. J. Pract. Tradit Chin. Intern. Med. 28, 65–67. 10.13729/j.issn.1671-7813.2014.12.32 [DOI] [Google Scholar]
- Lu Z., Xu L., Yu J., Wang D. (2011). Aidi Injection with Chemotherapy for Advanced Non-small Cell Lung Cancer: a Clinical Study. China Mod. Med. 18, 72–73. 10.3969/j.issn.1674-4721.2011.17.044 [DOI] [Google Scholar]
- Lv D., Guo Q., Qiu Y. (2009). Aidi Injection and Gemcitabine and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study of 30 Cases. Jiangxi J. TCM 40, 47–48. 10.3969/j.issn.0411-9584.2009.10.036 [DOI] [Google Scholar]
- Lv W., Hu S., Xiong G., Xiong H. (2018). Effect of Integrated Traditional Chinese and Western Medicine Chemotherapy on Clinical Efficacy and Immune Function of Patients with Non-small Cell Lung Cancer. Anti-infect Pharm. 15, 544–547. 10.13493/j.issn.1672-7878.2018.03-070 [DOI] [Google Scholar]
- Ma M. (2017). Clinical Effect of Aidi Injection Combined with GP Chemotherapy on Advanced NSCLC Patients with Deficiency of Both Qi and Yin and its Influence on Immune Function and Coagulation Function. Asia-pacific Tradit Med. 13, 108–110. 10.11954/ytctyy.201721046 [DOI] [Google Scholar]
- Ma W., Duan K., Feng M., She B., Chen Y., Zhang R. (2009). Aidi Injection as an Adjunct Therapy for Non-small Cell Lung Cancer: a Systematic Review. J. Integr. Med. 7, 315–324. 10.3736/jcim20090404 [DOI] [PubMed] [Google Scholar]
- Ma Y., Jiang X. (2016). Influence of Aidi Injection Combined with GP Chemotherapy Regimen in Treating Patients with Non - Small Cell Lung Cancer. PJCCPVD 24, 89–91. 10.3969/j.issn.1008-5971.2016.06.023 [DOI] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. (1981). Reporting Results of Cancer Treatment. Cancer 47, 207–214. [DOI] [PubMed] [Google Scholar]
- Mountain C. F. (1989). Value of the New TNM Staging System for Lung Cancer. Chest 96, 47S–49S. 10.1378/chest.96.1_supplement.47s [DOI] [PubMed] [Google Scholar]
- Ning S., Zhan B., Xu H. (2015). Clinical Observation of Aidi Injection Combined with GP Schemes in Treatment of Advanced Non-small Cell Lung Cancer with Acquired Drug-Resistance to EGFR-TKI. Drugs & Clinic 30, 691–695. 10.7501/j.issn.1674-5515.2015.06.019 [DOI] [Google Scholar]
- Pei W. (2012). Efficacy of Aidi Injection Combined with GP Regimen in Patients with Advanced Non-small Cell Lung Cancer and the Impact on Expression of Serum VEGF. China Med. Herald 09, 99–100. 10.3969/j.issn.1673-7210.2012.16.041 [DOI] [Google Scholar]
- Pollera C. F., Ameglio F., Nardi M., Vitelli G., Marolla P. (1987). Cisplatin-induced Hepatic Toxicity. J. Clin. Oncol. 5, 318–319. 10.1200/JCO.1987.5.2.318 [DOI] [PubMed] [Google Scholar]
- Qu X., Gao H., Tao L., Zhang Y., Zhai J., Sun J., et al. (2019). Astragaloside IV Protects against Cisplatin-Induced Liver and Kidney Injury via Autophagy-Mediated Inhibition of NLRP3 in Rats. J. Toxicol. Sci. 44, 167–175. 10.2131/jts.44.167 [DOI] [PubMed] [Google Scholar]
- Ruan F., Lu B. (2012). Therapeutic Effect of Low-Dose Capecitabine Combined with Aidi Injection on Senile Advanced Gastric Cancer. J. North. Pharm. 9, 13. [Google Scholar]
- Scagliotti G. V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., et al. (2008). Phase III Study Comparing Cisplatin Plus Gemcitabine with Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients with Advanced-Stage Non-small-cell Lung Cancer. J. Clin. Oncol. 26, 3543–3551. 10.1200/jco.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- Scagliotti G. V., Pastorino U., Vansteenkiste J. F., Spaggiari L., Facciolo F., Orlowski T. M., et al. (2012). Randomized Phase III Study of Surgery Alone or Surgery Plus Preoperative Cisplatin and Gemcitabine in Stages IB to IIIA Non-small-cell Lung Cancer. J. Clin. Oncol. 30, 172–178. 10.1200/jco.2010.33.7089 [DOI] [PubMed] [Google Scholar]
- Shahid F., Farooqui Z., Khan F. (2018). Cisplatin-induced Gastrointestinal Toxicity: An Update on Possible Mechanisms and on Available Gastroprotective Strategies. Eur. J. Pharmacol. 827, 49–57. 10.1016/j.ejphar.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Shi X., Yu A., Yang Y., Jin X., Dai L., Zhang T., et al. (2010). Aidi Injection with Chemotherapy for Advanced Non-small Cell Lung Cancer: a Clinical Study. Zhejiang J. ITCWM 20, 747–748. 10.3969/j.issn.1005-4561.2010.12.011 [DOI] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2017). Cancer Statistics. CA Cancer J. Clin. 67, 7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- Song Z., Liu X., Zhang H., Shang Y., Xu Y. (2009). Aidi Injection and Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. Med. Res. Edu 26, 36–37. 10.3969/j.issn.1674-490X.2009.05.014 [DOI] [Google Scholar]
- Su D., Zhang X. (2019). Clinical Observation of Aidi Injection Combined with GP Regimen Chemotherapy in the Treatment of Non-small Cell Lung Cancer . J. Basic Clin. Oncol. 32, 31–34. 10.3969/j.issn.1673-5412.2019.01.010 [DOI] [Google Scholar]
- Su S. (2017). Aidi Injection Combined with Gemcitabine and Cisplatin in the Chemotherapy Treatment of Advanced Non-small Cell Lung Cancer. Chin. J. Pract. Med. 44, 112–115. 10.3760/cma.j.issn.1674-4756.2017.24.037 [DOI] [Google Scholar]
- Sun G., Liu J., Wang T., Zhu J. (2008). Aidi Injection Plus Gemcitabine and Cisplatin for Advanced Non Small Cell Lung Cancer:a Clinical Study of 33 Cases. Fujian Med. J. 30, 134. 10.3969/j.issn.1002-2600.2008.06.089 [DOI] [Google Scholar]
- Sun J., Xu P., Shi D., Cao B. (2012). Evaluation on Clinical Efficacy of Aidi Injection Combined with GP Chemotherapy in Treatment of Advanced Non-small Cell Lung Cancer in Elder Patients. J. Jilin Univ. (Med Edit) 38, 151–154. [Google Scholar]
- Sun X., Briel M., Walter S. D., Guyatt G. H. (2010). Is a Subgroup Effect Believable? Updating Criteria to Evaluate the Credibility of Subgroup Analyses. BMJ 340, c117. 10.1136/bmj.c117 [DOI] [PubMed] [Google Scholar]
- Tan H., Tan C., Feng X., Pan B. (2020). Efficacy of GP Chemotherapy Combined with Aidi Injection on Elderly Advanced Non-small Cell Lung Cancer and its Effects on Immune Function and Quality of Life of Patients. Pract. J. Cancer 35, 1327–1330+1346. 10.3969/j.issn.1001-5930.2020.08.028 [DOI] [Google Scholar]
- Tian J., Zhao Y., Li J., Ge L., Yang K. (2014). Network Meta-Analysis of 12 Chinese Herb Injections Combined with Gemcitabine and Cisplatin for Non-small Cell Lung Cancer. Chin. J. Drug Eval. 31, 350–355. 10.3969/j.issn.2095-3593.2014.06.010 [DOI] [Google Scholar]
- Torre L. A., Siegel R. L., Jemal A. (2016). Lung Cancer Statistics. Adv. Exp. Med. Biol. 893, 1–19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- Trotti A., Colevas A. D., Setser A., Rusch V., Jaques D., Budach V., et al. (2003). CTCAE v3.0: Development of a Comprehensive Grading System for the Adverse Effects of Cancer Treatment. Semin. Radiat. Oncol. 13, 176–181. 10.1016/s1053-4296(03)00031-6 [DOI] [PubMed] [Google Scholar]
- Waissbluth S., Daniel S. J. (2013). Cisplatin-induced Ototoxicity: Transporters Playing a Role in Cisplatin Toxicity. Hear. Res. 299, 37–45. 10.1016/j.heares.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Wang J. (2012). Clinical Observation of Aidi Injection Combined with GP Chemotherapy on Treatment of Non-small Cell Lung Cancer. J. Clin. Pulm. Med. 17, 312–313. 10.3969/j.issn.1009-6663.2012.02.060 [DOI] [Google Scholar]
- Wang J., Li G., Yu L., Mo T., Wu Q., Zhou Z. (2018). Aidi Injection Plus Platinum-Based Chemotherapy for Stage IIIB/IV Non-small Cell Lung Cancer: A Meta-Analysis of 42 RCTs Following the PRISMA Guidelines. J. Ethnopharmacol 221, 137–150. 10.1016/j.jep.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Wang Y. (2009). Aidi Injection and Chemotherapy in the Treatment of Elderly Patients with Advanced Non-small Cell Lung Cancer: a Clinical Study. Chin. Prim. Health Care 23, 101–102. 10.3969/j.issn.1001-568X.2009.11.058 [DOI] [Google Scholar]
- Wang Y., Peng L. (2012). Clinical Study on Aidi Injection Combined GP for Treating Advanced Non-small Cell Lung Cancer. Chin. Commun. Doctors 14, 231–232. 10.3969/j.issn.1007-614x.2012.18.209 [DOI] [Google Scholar]
- Wang Z., Zhu L. (2015). Low-dose Chemotherapy Combined with Aidi Injection in the Treatment of Non-small Cell Lung Cancer. Mod. Instrum Med. Treat. 21, 92–94. 10.11876/mimt201505035 [DOI] [Google Scholar]
- Watanabe H., Yamamoto S., Kunitoh H., Sekine I., Yamamoto N., Ohe Y., et al. (2003). Tumor Response to Chemotherapy: the Validity and Reproducibility of RECIST Guidelines in NSCLC Patients. Cancer Sci. 94, 1015–1020. 10.1111/j.1349-7006.2003.tb01394.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H. (2014). Clinical Evaluation of TCM Combined with Western Medicine for Elderly Patients with Advanced Non-small Cell Lung Cancer. Mod. J. ITCWM 23, 2325–2326. 10.3969/j.issn.1008-8849.2014.21.018 [DOI] [Google Scholar]
- Wen K., Li J., Peng D. (2009). Aidi Injection with Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. Chin. J. Basic Med. TCM 15, 716–717. 10.1111/j.1365-294X.2010.04789.x [DOI] [Google Scholar]
- Wu J., Wang Q., Lin L., Sun L. (2017a). Clinical Efficacy of Aidi Injection Combined with First-Line Chemotherapy in the Treatment of Non-small Cell Lung Cancer:a Meta-Analysis. Chin. Tradit Patent Med. 39, 1323–1328. 10.3969/j.issn.1001-1528.2017.06.051 [DOI] [Google Scholar]
- Wu Q., He X. (2011). Aidi Injection with Chemotherapy for Advanced Non-small Cell Lung Cancer: a Clinical Study. China J. Chin. Mater. Med. 36, 3364–3365. [Google Scholar]
- Wu T., Chen S. (2017). Research on Feasibility of Aidi Injection and Chemotherapy in the Non-small Cell Lung Cancer. China Foreign Med. Treat. 36, 126–127. 10.16662/j.cnki.1674-0742.2017.15.126 [DOI] [Google Scholar]
- Wu Y., Qu Z., Tao H. (2017b). Effect of Aidi Injection Combined with Chemotherapy on Advanced Non-small Cell Lung Cancer. Shaanxi J. TCM 38, 883–884. 10.3969/j.issn.1000-7369.2017.07.035 [DOI] [Google Scholar]
- Xiao Z., Jiang Y., Chen X. F., Wang C. Q., Xu W. H., Liu Y., et al. (2020a). The Hepatorenal Toxicity and Tumor Response of Chemotherapy with or without Aidi Injection in Advanced Lung Cancer: A Meta-Analysis of 80 Randomized Controlled Trials. Clin. Ther. 42, 515–543 e531. 10.1016/j.clinthera.2020.01.011 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Jiang Y., Wang C. Q., Hu S. S., Huang X. R., Chen X. F., et al. (2020b). Clinical Efficacy and Safety of Aidi Injection Combination with Vinorelbine and Cisplatin for Advanced Non-small-cell Lung Carcinoma: A Systematic Review and Meta-Analysis of 54 Randomized Controlled Trials. Pharmacol. Res. 153, 104637. 10.1016/j.phrs.2020.104637 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Chen L., Tang X., Li L., Li N., et al. (2017). Has Aidi Injection the Attenuation and Synergistic Efficacy to Gemcitabine and Cisplatin in Non-small Cell Lung Cancer? A Meta-Analysis of 36 Randomized Controlled Trials. Oncotarget 8, 1329–1342. 10.18632/oncotarget.13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Li L., Tang X., Li N., Li J., et al. (2018a). Clinical Efficacy and Safety of Aidi Injection Plus Docetaxel-Based Chemotherapy in Advanced Nonsmall Cell Lung Cancer: A Meta-Analysis of 36 Randomized Controlled Trials. Evid. Based Complement. Alternat Med. 2018, 7918258. 10.1155/2018/7918258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Sun Y., Li N., Li J., Chen L., et al. (2016). Can Aidi Injection Restore Cellular Immunity and Improve Clinical Efficacy in Non-small-cell Lung Cancer Patients Treated with Platinum-Based Chemotherapy? A Meta-Analysis of 17 Randomized Controlled Trials Following the PRISMA Guidelines. Medicine (Baltimore) 95, e5210. 10.1097/MD.0000000000005210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Tan Z., Hu S., Chen Y., Zhou M., et al. (2019a). Clinical Efficacy and Safety of Sodium Cantharidinate Plus Chemotherapy in Non-small-cell Lung Cancer: A Systematic Review and Meta-Analysis of 38 Randomized Controlled Trials. J. Clin. Pharm. Ther. 44, 23–38. 10.1111/jcpt.12761 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Zhou M., Hu S., Jiang Y., Huang X., et al. (2019b). Clinical Efficacy and Safety of Aidi Injection Plus Paclitaxel-Based Chemotherapy for Advanced Non-small Cell Lung Cancer: A Meta-Analysis of 31 Randomized Controlled Trials Following the PRISMA Guidelines. J. Ethnopharmacol 228, 110–122. 10.1016/j.jep.2018.09.024 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Wang C., Zhou R., Hu S., Yi N., Feng J., et al. (2018b). Can Aidi Injection Improve Overall Survival in Patients with Non-small Cell Lung Cancer? A Systematic Review and Meta-Analysis of 25 Randomized Controlled Trials. Complement. Ther. Med. 37, 50–60. 10.1016/j.ctim.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Xie G., Cui Z., Peng K., Zhou X., Xia Q., Xu D. (2019). Aidi Injection, a Traditional Chinese Medicine Injection, Could Be Used as an Adjuvant Drug to Improve Quality of Life of Cancer Patients Receiving Chemotherapy: A Propensity Score Matching Analysis. Integr. Cancer Ther. 18, 1534735418810799. 10.1177/1534735418810799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Yang T., Zhou M. (2013). Clinical Evaluation of Aidi Injection Combined with Chemotherapy for Advanced Non-small Cell Lung Cancer. Inter. J. Resp 33, 765–767. 10.3760/cma.j.issn.1673-436X.2013.010.011 [DOI] [Google Scholar]
- Xu Y., Li W. (2020). Clinical Observation of Aidi Injection Combined with GP Regimen in the Treatment of Non-small Cell Lung Cancer. Capital Med. 27, 58 [Google Scholar]
- Xu Y., Zhang Y., Lu B. (2012). Clinical Study on Aidi Injection Combined Chemotherapy for Treating Middle and Late Stage Lung Cancer. China Pharma 21, 56–57. 10.3969/j.issn.1006-4931.2012.23.038 [DOI] [Google Scholar]
- Xu Z. (2020). Effect of Aidi Injection Combined with GP Chemotherapy in the Treatment of Advanced Non-small Cell Lung Cancer. Henan Med. Res. 29, 5133–5135. 10.3969/j.issn.1004-437X.2020.27.060 [DOI] [Google Scholar]
- Yang J., Ding M. (2012). Eddie Combined with Gemcitabine and Cisplatin for Advanced Non-small Cell Lung Cancer: Meta-Analysis. Chin. Gen. Pract. 15, 2794–2798. 10.3969/j.issn.1007-9572.2012.08.100 [DOI] [Google Scholar]
- Yang Q., Chen W., Huang J., Chen X. (2008). Aidi Injection and Chemotherapy in the Treatment of Advanced Lung Cancer. Guangming J. Chin. Med. 23, 1760–1761. 10.3969/j.issn.1003-8914.2008.11.072 [DOI] [Google Scholar]
- Yates J. W., Chalmer B., McKegney F. P. (1980). Evaluation of Patients with Advanced Cancer Using the Karnofsky Performance Status. Cancer 45, 2220–2224. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Liu X., Li C., Zhou X., Zhang X., Yang Y., et al. (2016). Chemical Constituents from Mylabris Phalerata and Their Cytotoxic Activity In Vitro. J. Chin. Mater. Med. 41, 859–863. 10.4268/cjcmm20160516 [DOI] [PubMed] [Google Scholar]
- Zhang H., Cai S., Chen J., Chen F. (2017). Feasibility Study on Aidi Injection Combined with Chemotherapy for Non-small Cell Lung Cancer. China Contin. Med. Edu 9, 195–196. 10.3969/j.issn.1674-9308.2017.23.104 [DOI] [Google Scholar]
- Zhang H. (2014). Effect of Aidi Injection Combined with GP Chemotherapy on Non-small Cell Lung Cancer Patients' Serum VEGF Levels and Immune Function Indicators. China J. Mod. Med. 24, 40–43. [Google Scholar]
- Zhang H. (2018). Effect of Aidi Injection on Toxic and Side Reactions in Patients with Advanced NSCLC Chemotherapy. Jilin Med. J. 39, 134–136. 10.3969/j.issn.1004-0412.2018.01.052 [DOI] [Google Scholar]
- Zhang L. (2009). Aidi Injection with Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. J. Pract. Med. 25, 2929–2930. 10.3969/j.issn.1006-5725.2009.17.054 [DOI] [Google Scholar]
- Zhang M., Liu Y., Chen C., Li X., Xu Q., Yang S. (2012). Studies on Chemical Constituents from Aidi Injection. Chin. Tradit Herb Drugs 43, 1462–1470. [Google Scholar]
- Zhang M. (2015). Clinical Observation of Chemotherapy for Senile Advanced Non-small Cell Lung Cancer. J. Today Health 14, 48. [Google Scholar]
- Zhang S. (2016a). Observation of Short-Term Efficacy of Aidi Injection in Adjuvant Treatment of Advanced Lung Cancer. J. Today Health 15, 46. [Google Scholar]
- Zhang X. (2016b). Clinical Observation of Addie Injection Combined with GP Regimen in the Treatment of Non Small Cell Lung Cancer. J. Liaoning Univ. TCM 18, 173–175. 10.13194/j.issn.1673-842x.2016.02.060 [DOI] [Google Scholar]
- Zhang Y. (2012). Aidi Injection with GP for Non Small Cell Lung Cancer: a Clinical Study. Mod. J. ITCWM 21, 2095–2096. 10.3969/j.issn.1008-8849.2012.19.022 [DOI] [Google Scholar]
- Zhang Y. M., Liu Y. Q., Liu D., Zhang L., Qin J., Zhang Z., et al. (2019). The Effects of Astragalus Polysaccharide on Bone Marrow-Derived Mesenchymal Stem Cell Proliferation and Morphology Induced by A549 Lung Cancer Cells. Med. Sci. Monit. 25, 4110–4121. 10.12659/msm.914219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhao Y., Su H., Wang C. (2008). Aidi Injection and Gemcitabine Hydrochloride and Cisplatin for Advanced Non Small Cell Lung Cancer: a Clinical Study. Chin. J. Misdiag 8, 5873–5874. 10.3969/j.issn.1009-6647.2008.24.068 [DOI] [Google Scholar]
- Zhao J., Li J. (2019). Application Effect of Aidi Injection Combined with GP Scheme in Advanced Non-small Cell Lung Cancer. Clin. Res. Pract. 4, 117–119. 10.19347/j.cnki.2096-1413.201925049 [DOI] [Google Scholar]
- Zhao S., Zhang Q., Wang D. (2015). Efficacy Analysis of Aidi Injection in Combination with Chemotherapy on Immunity in Patients with Lung Cancer. Med. J. West. China 27, 1663–1666. 10.3969/j.issn.1672-3511.2015.11.018 [DOI] [Google Scholar]
- Zhou D. (2018). Clinical Efficacy of Aidi Injection Combined with Gemcitabine and Cisplatin (GP) Regimen in the Treatment of Advanced Non-small Cell Lung Cancer. J. Clin. Med. Lit. (Electron Edit) 5, 47–48. 10.3877/j.issn.2095-8242.2018.A1.026 [DOI] [Google Scholar]
- Zhou X., Liu Z., Long T., Zhou L., Bao Y. (2018). Immunomodulatory Effects of Herbal Formula of astragalus Polysaccharide (APS) and Polysaccharopeptide (PSP) in Mice with Lung Cancer. Int. J. Biol. Macromol 106, 596–601. 10.1016/j.ijbiomac.2017.08.054 [DOI] [PubMed] [Google Scholar]
- Zhu H. (2015). Effect of GP Regimen Combined with Aidi Injection on Elderly Patients with Advanced Non-small Cell Lung Cancer. Contemp. Med. Forum 13, 135–136. [Google Scholar]
- Zou Y., Li J., Luo X. (2006). Aidi Injection with GP Chemotherapy for Advanced Lung Cancer: a Clinical Study. Hubei J. TCM 28, 33–34. 10.3969/j.issn.1000-0704.2006.03.017 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.