Abstract
Reproductive isolation is a prerequisite to form and maintain a new species. Multiple prezygotic and postzygotic reproductive isolation barriers have been reported in plants. In the model plant, Arabidopsis thaliana conspecific pollen tube precedence controlled by AtLURE1/PRK6-mediated signaling has been recently reported as a major prezygotic reproductive isolation barrier. By accelerating emergence of own pollen tubes from the transmitting tract, A. thaliana ovules promote self-fertilization and thus prevent fertilization by a different species. Taking advantage of a septuple atlure1null mutant, we now report on the role of AtLURE1/PRK6-mediated signaling for micropylar pollen tube guidance. Compared with wild-type (WT) ovules, atlure1null ovules displayed remarkably reduced micropylar pollen tube attraction efficiencies in modified semi-in vivo A. thaliana ovule targeting assays. However, when prk6 mutant pollen tubes were applied, atlure1null ovules showed micropylar attraction efficiencies comparable to that of WT ovules. These findings indicate that AtLURE1/PRK6-mediated signaling regulates micropylar pollen tube attraction in addition to promoting emergence of own pollen tubes from the transmitting tract. Moreover, semi-in vivo ovule targeting competition assays with the same amount of pollen grains from both A. thaliana and Arabidopsis lyrata showed that A. thaliana WT and xiuqiu mutant ovules are mainly targeted by own pollen tubes and that atlure1null mutant ovules are also entered to a large extent by A. lyrata pollen tubes. Taken together, we report that AtLURE1/PRK6-mediated signaling promotes conspecific micropylar pollen tube attraction representing an additional prezygotic isolation barrier.
A modified ovule targeting assay revealed that AtLURE1/PRK6-mediated signaling promotes micropylar guidance of Arabidopsis thaliana pollen tubes while discriminating tubes of related Arabidopsis lyrata.
Introduction
Reproductive isolation is an essential cause of speciation and diversity. Plant species are typically isolated by multiple prezygotic and postzygotic reproductive barriers (Howard, 1999; Widmer et al., 2009; Baack et al., 2015). During their life cycle, prezygotic barriers act earlier than the postzygotic barriers and contribute more significantly to reproductive isolation (Lowry et al., 2008). In flowering plants, the strength of prezygotic isolation is approximately twice than that of postzygotic isolation (Lowry et al., 2008).
In higher terrestrial plants, siphonogamy evolved as a new mechanism to promote fertilization independent of a moist environment. Sperm cells are enclosed by the vegetative pollen tube cell and transported as passive cargo via a long tube toward the female gametophyte (Zhang et al., 2017a; Glöckle et al., 2018). During their journey, pollen tubes intensively interact and communicate with different female tissues to secure successful germination, growth, guidance, and reception inside the ovule (Dresselhaus and Franklin-Tong, 2013; Qu et al., 2015; Johnson et al., 2019; Zhong and Qu, 2019a). Each germinated pollen tube competes with others to deliver and release its sperm cell cargo, while ovules simultaneously aim to promote own tubes and prevent those from other species. Conspecific pollen precedence was proposed to play an essential role in such competitions between closely related species by facilitating own pollen tubes to complete fertilization first (Howard, 1999; Baack et al., 2015; Meng et al., 2019). It seems very likely that conspecific pollen precedence occurs during many phases of the pollen tube journey.
Pollen tubes are guided toward receiving ovules. This process is divided into two major phases named as preovular and ovular guidance (Lausser et al., 2010; Higashiyama and Takeuchi, 2015). Preovular guidance starts when pollen tubes grow along stigmatic papilla cells toward the transmitting tract, is continued in the transmitting tract, and terminates when tubes emerge out of the septum to grow toward the funiculus, which connects the ovule with the placenta. The process in which pollen tubes eventually grow toward and inside the micropylar opening of the ovule is called ovular guidance, which terminates with the process of pollen tube reception and sperm cell release inside the receptive synergid cell. In the past few years, the synergid cell-secreted defensin-like peptides AtLURE1s were reported in the model plant Arabidopsis thaliana to participate in pollen tube guidance by interacting with their pollen tube receptor, the membrane-localized receptor-like kinase PRK6 (Takeuchi and Higashiyama, 2012, 2016; Liu et al., 2013; Zhang et al., 2017b). Recently, we adopted the CRIPSR/Cas9 technology to generate septuple mutants (named as atlure1null) with all seven AtLURE1 genes mutagenized and found that the efficiency of A. thaliana pollen tube emerging from the transmitting tract toward the funiculus was significantly lower in atlure1null pistils compared with wild-type (WT) pistils. Notably, this phenomenon occurred only for A. thaliana pollen tubes, thus own tubes, but not for pollen tubes of related species. It was concluded that AtLURE1/PRK6-mediated signaling plays an important role in promoting conspecific pollen precedence and thus contributes to prezygotic genetic isolation (Li et al., 2019; Zhong et al., 2019; Zhong and Qu, 2019b). AtLURE1-related defensin-like peptides XIUQIUs, which are also secreted from the synergid cells, were identified as general pollen tube attractants promoting pollen tube emergence in a weaker, nonspecies-specific manner in the genus Arabidopsis (Zhong et al., 2019; Zhong and Qu, 2019b).
Because AtLURE1s are secreted from synergid cells and diffuse along the surface of the funiculus toward the septum (Takeuchi and Higashiyama, 2012), it is reasonable to hypothesize that AtLURE1/PRK6-mediated signaling may also regulate conspecific pollen tube precedence at the micropyle. This hypothesis, however, is rather difficult to prove in planta because defects in preovular guidance stages, that is growth of pollen tubes in the transmitting tract and/or emerging of pollen tubes from the septum, would mask defects occurring later at ovular guidance stages, that is micropylar guidance of pollen tubes, pollen tube reception, and fertilization (Guan et al., 2014). A semi-in vivo ovule targeting assay appeared as a very useful tool to overcome this difficulty, as it bypasses the stage of the pollen tube emergence from septum and allows pollen tubes emerging from style to directly interact with ovules so that ovular guidance can be studied independently from preovular guidance. Therefore, we adopted this assay to investigate whether AtLURE1/PRK6-mediated signaling is also involved in conspecific pollen tube precedence at the micropyle.
Results
AtLURE1s accelerate micropylar pollen tube attraction
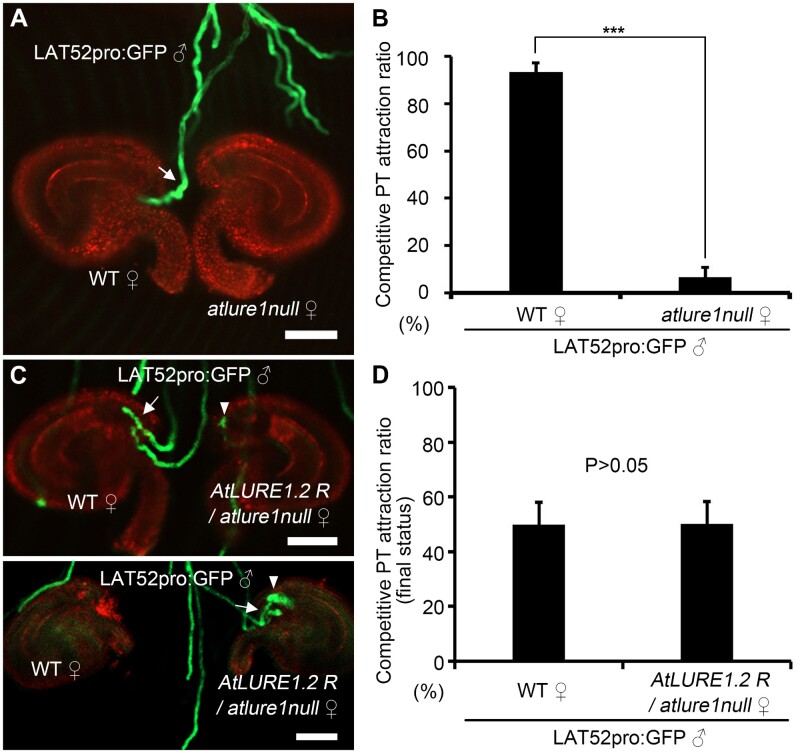
To elucidate the role of AtLURE1s in micropylar attraction of pollen tubes, we first employed a semi-in vivo ovule targeting assay (Palanivelu and Preuss, 2006) to compare the ovule targeting ratio of A. thaliana pollen tubes toward WT and atlure1null mutant ovules. Removal of the septum also eliminates the barrier that prevents multiple pollen tubes emerging from the transmitting tract and ultimately allows to evaluate whether the atlure1null mutant is capable of attracting pollen tubes or not. Only those pollen tubes that enter micropyles and thus are tightly associated with the ovules were considered and scored as successfully targeted pollen tubes in order to distinguish them from the pollen tubes that randomly target ovules. Green fluorescence protein (GFP)-labeled pollen tubes, that is LAT52pro:GFP transgenic pollen tubes, were used as A. thaliana WT tubes (Muschietti et al., 1994). We found that at 4 hours after pollination (HAP) ovule targeting efficiency of WT pollen tubes to WT ovules was similar to atlure1null mutant ovules (95.0 ± 3.3%, nr = 5, no = 110; 95.2 ± 3.5%, nr = 5, no = 112; P = 0.95; Supplemental Figure S1, A, B, and D), suggesting that atlure1null ovules are capable of attracting WT pollen tubes with a comparable efficiency as WT ovules. However, in ovule competition assays, when atlure1null ovules were placed head-to-head next to WT ovules, about 93.4% ± 3.9% of first arriving WT pollen tubes (nr = 5, no = 103) were attracted to enter micropyles of WT ovules (Figure 1, A and B). This pollen tube attraction preference was reversed when atlure1null was transformed with AtLURE1.2pro:AtLURE1.2 (Figure 1, C and D; Supplemental Figure S1, C and D). Altogether these findings demonstrate that AtLURE1s promote attraction competitiveness of pollen tubes at the micropyle. Notably, in most cases atlure1null ovules would eventually be targeted by later-arriving WT pollen tubes at 12 HAP (Supplemental Figure S2, A and B; 95.4 ± 3.4%, nr = 4, no = 102), consistent with a previously report showing that atlure1null mutants lack fertility defects (Zhong et al., 2019).
Figure 1.
atlure1null ovules exhibit weak micropylar pollen tubes attracting activity compared with WT ovules. A, Semi-in vivo pollen tube competition assay with WT and atlure1null ovules targeted by A. thaliana LAT52pro:GFP (WT) pollen tubes (indicated by white arrows). B, Statistical analysis of (A). Five repeats of semi-in vivo ovule targeting assays with more than 20 ovules each were conducted. Data are mean values ± sd. ***P < 0.01 (Student’s t test). C, Two representative examples showing the semi-in vivo pollen tube competition assay with WT and AtLURE1.2 rescued atlure1null ovules (AtLURE1.2R/atlure1null; indicated by white arrowheads) targeted by A. thaliana LAT52pro:GFP (WT) pollen tubes (indicated by white arrows). D, Statistical analyses of (C). Three repeats of semi-in vivo ovule targeting assays with 30–40 ovules each were conducted. Data are mean values ± sd. P > 0.05 means no significant difference (Student’s t test). Scale bars, 50 μm.
The “dragging” method is useful and efficient in pollen tube targeting as well as pollen tube and ovule competition assays
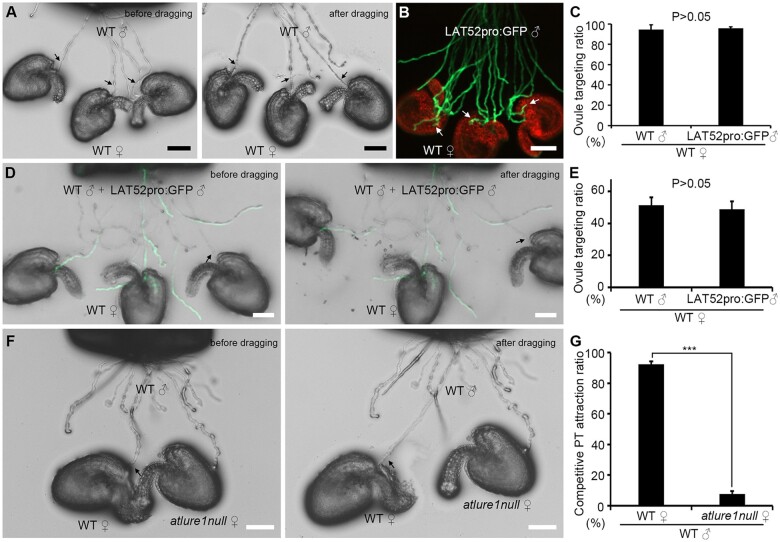
Compared with GFP-labeled WT pollen tubes, it is usually difficult to judge whether a pollen tube lacking fluorescence targets an ovule or not (Supplemental Figure S3, A and B). Therefore, we first established a modified semi-in vivo ovule targeting method by using a glass needle to drag ovules. This allowed to better visualize whether a pollen tube lacking fluorescence has successfully targeted and penetrated an ovule. The rationale is as follows: if a pollen tube has successfully targeted an ovule, the pollen tube would be tightly associated with the ovule and would not be separated from the ovule by the dragging force. We then tested and validated the feasibility of this method in different experiments, that is pollen tube targeting as well as pollen tube and ovule competition assays. First, we tested WT pollen tube targeting efficiency on WT ovules. Using this method, we found that 94.6% ± 4.5% WT pollen tubes lacking fluorescence and 95.6% ± 1.4% LAT52pro:GFP WT pollen tubes (with green fluorescence) were attracted by WT ovules. We did not observe significant differences (nr = 4, no = 107 and nr = 4, no = 113, respectively; P = 0.67; Figure 2, A–C). This finding demonstrates that the dragging method is well suited to measure efficiency of successful pollen tube targeting. Next, we analyzed whether this method could also be successfully used to compare the targeting efficiency of two different pollen tube types in ovule targeting assays. When a pistil was pollinated with a mixture of the same amount of WT and LAT52pro:GFP WT pollen grains, we found that these two types of WT pollen tubes targeted WT ovules at a comparable efficiency (51.2% ± 4.9% versus 48.8% ± 4.9%, nr = 4, no = 134, P = 0.5; Figure 2, D and E). These numbers indicate that the method is reliable in pollen tube competition assays. We also tested whether the method is suitable to compare the attraction ability of two different types of ovules (i.e. ovule competition assays). We found that first arriving WT pollen tubes were almost exclusively attracted by WT ovules compared with atlure1null ovules (92.4% ± 1.8% versus 7.6% ± 1.8%, nr = 3, no = 143, P < 0.01; Figure 2, F and G). This is consistent with the data shown in Figure 1A, suggesting that the dragging method is also reliable in ovule competition assays. Altogether, the above results demonstrate that the dragging method is well suited for ovule targeting as well as pollen tube and ovule competition assays.
Figure 2.
The “dragging” method can be used in semi-in vivo pollen tube targeting as well as pollen and ovule competition assays in Arabidopsis. A–C, Semi-in vivo pollen tube targeting with WT (indicated by black arrows) and LAT52pro:GFP transgenic pollen tubes (indicated by white arrows), respectively. A, Bright field images showing WT pollen tubes targeting ovules. Before dragging (left) and after dragging (right). B, Merged images showing LAT52pro:GFP transgenic pollen tubes (green fluorescence-labeled, indicated by white arrows) targeting ovules (red autofluorescence) in a semi-in vivo assay. C, Statistical analysis of (A) and (B). Four repeats with more than 25 ovules each were conducted. D and E, Semi-in vivo pollen tube competition assay of WT (indicated by black arrows) and LAT52pro:GFP transgenic pollen tubes targeting WT ovules. Bright field and GFP channel-merged images are shown before and after dragging, as indicated. E, Statistical analysis of (D). Four repeats of semi-in vivo ovule targeting assays with 30–40 ovules each were conducted. F and G, Semi-in vivo ovule competition assay of WT pollen tube (indicated by black arrows) targeting WT and atlure1null ovules shown before and after dragging, as indicated. G, Statistical analysis of (F). Three repeats of semi-in vivo ovule targeting assays with 30–60 ovules were each conducted. Data are mean values ± sd. P > 0.05 means no significant difference (Student’s t test). Scale bars, 50 μm.
The AtLURE1 receptor PRK6 is required to accelerate micropylar pollen tube attraction
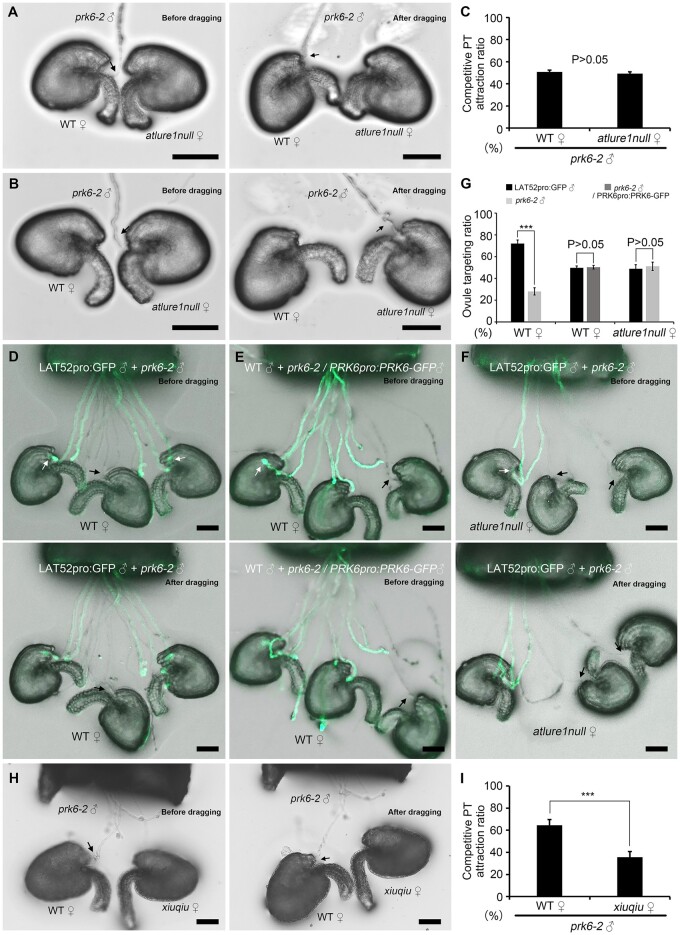
We next tested whether PRK6, the receptor of AtLURE1s, is also involved in accelerating micropylar pollen tube attraction. Using the dragging method, we found that prk6-2 mutant pollen tubes were successfully attracted by both WT and atlure1null ovules (97.0% ± 2.4%, nr = 4, no=102 vs 95.3% ± 0.6%, nr = 4, no = 107, respectively; P = 0.23; Supplemental Figure S4, A–C). Loss of PRK6 function did not affect micropylar pollen tube attraction, consistent with the targeting ratio of pollen tubes attracted to atlure1null ovules (Supplemental Figure S1). In ovule competition assays, we found that prk6-2 pollen tubes exhibited a comparable ovule targeting efficiency toward both WT and atlure1null ovules (50.8% ± 1.7% versus 49.2% ± 1.7%, respectively; nr=4, no = 105, P = 0.21; Figure 3, A–C). Ultimately, almost all ovules were penetrated by prk6-2 pollen tubes (Supplemental Figure S5, A and B). When equal amounts of LAT52pro:GFP (WT) and prk6-2 pollen grains were mixed to pollinate WT pistils, the majority of WT ovules (71.9% ± 3.3%, nr = 4, no = 110) were targeted by LAT52pro:GFP (WT) pollen tubes. This efficiency was almost three times higher compared with ovules targeted by prk6-2 mutant pollen tubes (28.1% ± 3.3%, nr = 4, no = 110; Figure 3, D and G). When the same amount of WT and PRK6pro:PRK6-GFP complemented prk6-2 pollen grains was used, a comparable targeting efficiency was observed (49.7% ± 1.7% versus 50.3% ± 1.7%, respectively; nr = 3, no = 185, P = 0.68) indicating full complementation of the mutant (Figure 3, E and G). This indicates that LAT52pro:GFP (WT) pollen tubes outcompeted prk6-2 pollen tubes and that this prepotency was indeed caused by PRK6. Notably, when atlure1null mutant ovules were tested, the targeting ratio of LAT52pro:GFP (WT) pollen tubes was comparable with that of prk6-2 mutant pollen tubes (51.0% ± 3.8% vs 49.0% ± 3.8%, respectively; nr = 4, no = 112, P = 0.46; Figure 3, F and G). Altogether, these results further confirm that loss of either AtLURE1 or PRK6 function reduces attraction competitiveness of pollen tubes at the micropyle.
Figure 3.
AtLURE1/PRK6-mediated signaling enhances micropylar pollen tube attraction. A and B, Bright field images of semi-in vivo ovule competition assays using prk6-2 pollen tubes targeting WT (A) and atlure1null (B) ovules before and after dragging, as indicated. C, Statistical analysis of (A and B). Four repeats each with >25 ovules were conducted. Data are mean values ± SD. P > 0.05 means no significant difference (Student’s t test). Scale bars, 50 μm. D–F, Semi-in vivo pollen tube competition assays using mixed WT and prk6-2 as well as mixed WT and PRK6pro:PRK6-GFP rescued prk6-2 pollen tubes targeting WT ovules and atlure1null ovules, as indicated. Images are shown before and after dragging. G, Statistical analysis of (D–F). Three or four repeats each with 20–70 ovules were conducted. White arrows indicate A. thaliana LAT52pro:GFP transgenic pollen tubes (WT, green fluorescence-labeled) and black arrows indicate prk6-2 mutant pollen tubes. H, Semi-in vivo ovule competition assays using prk6-2 pollen tubes targeting WT and xiuqiu ovules before and after dragging, as indicated. I, Statistical analysis of (H). Data are mean values ± sd. P < 0.01 means significant difference (Student’s t test). Scale bars, 50 μm.
We next tested whether nonspecies-specific pollen tube attractants XIUQIUs require PRK6 for micropylar attraction of pollen tubes. In the ovule competition assays with WT and xiuqiu ovules, the majority of prk6-2 pollen tubes were preferentially attracted by WT ovules instead of xiuqiu ovules (64.5% ± 5.2% versus 35.5% ± 5.2%, respectively; nr = 4, no = 98, P < 0.01; Figure 3, H and I). This result indicates that, when lacking AtLURE1/PRK6-mediated signaling, WT ovules have stronger pollen tube-attracting activities at the micropyle than xiuqiu ovules. This finding suggests that the function of XIUQIU peptides in micropyle attraction of pollen tubes is independent of PRK6.
AtLURE1/PRK6-mediated signaling promotes conspecific micropylar pollen tube precedence
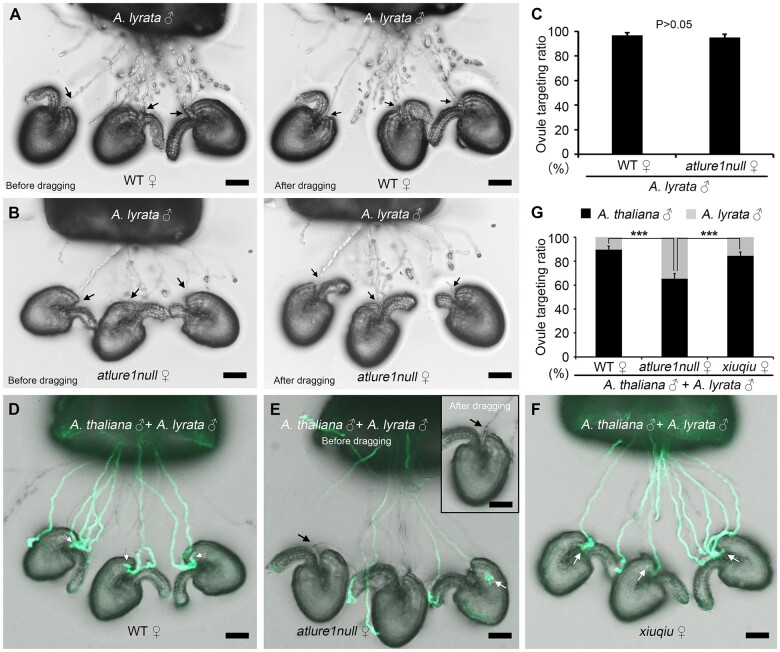
It has been reported previously that AtLURE1/PRK6-mediated signaling promotes pollen tube emergence from the septum in a species-specific manner, therefore, serving as a conspecific pollen tube precedence factor during preovular guidance (Zhong et al., 2019; Zhong and Qu, 2019b). Thus, we next investigated whether AtLURE1/PRK6-mediated signaling may also act as a conspecific pollen tube precedence factor for ovular guidance. To address this question, we exposed WT and atlure1null mutant ovules to both A. thaliana LAT52pro:GFP (WT) and Arabidopsis lyrata pollen tubes. We found that A. thaliana WT and atlure1null ovules were successfully targeted by A. lyrata pollen tubes at high efficiencies (96.7% ± 2.3%, nr = 4, no=109% versus 95.0% ± 2.5%, nr = 4, no = 111, P = 0.39; Figure 4, A–C). This observation indicated that A. thaliana ovules are capable of attracting A. lyrata pollen tubes at the micropyle in an AtLURE1-independent manner. However, when the same amount of pollen grains of the two species was applied simultaneously in a mixed pollen competition assay, A. thaliana WT ovules mainly attracted own pollen tubes and discriminated A. lyrata pollen tubes (89.5% ± 3.0% versus 10.5% ± 3.0%, respectively, nr = 4, no = 105; Figure 4, D and G). This observation indicated that A. thaliana pollen tubes outcompeted A. lyrata pollen tubes at the micropyle of WT ovules. When atlure1null ovules were used for the mixed pollination competition assay (Figure 4E), 34.6% ± 4.1% A. lyrata pollen tubes (nr = 4, no = 101) successfully targeted atlure1null ovules (Figure 4, E and G). This number was more than three times higher than that of A. lyrata pollen tubes targeting A. thaliana WT ovules. In conclusion, these findings demonstrate that AtLURE1s also promote conspecific pollen tube precedence during micropylar pollen tube guidance.
Figure 4.
Although A. thaliana WT and xiuqiu ovules are mainly targeted by own pollen tubes, atlure1null mutant ovules are also entered by A. lyrata pollen tubes. A and B, Bright field images showing semi-in vivo pollen tube targeting of A. lyrata pollen tubes toward A. thaliana WT and atlure1null ovules, respectively, before and after dragging, as indicated. C, Statistical analysis of (A) and (B). Four repeats each with more than 25 ovules were conducted. Data are mean values ± sd. P > 0.05 (Student’s t test). D–F, Semi-in vivo pollen tube competition assays with WT, atlure1null, and xiuqiu ovules targeted by mixed pollen tubes from LAT52pro:GFP (A. thaliana) and A. lyrata, as indicated. G, Statistical analysis of (D–F). Four repeats each with more than 20 ovules were conducted. White arrows indicate A. thaliana LAT52pro:GFP transgenic pollen tubes (WT, green fluorescence-labeled) and black arrows show A. lyrata pollen tubes. Data are mean values ± sd. P < 0.01 means significant difference (Student’s t test). Scale bars, 50 μm.
Finally, we also conducted ovule targeting assays with xiuqiu ovules. We first determined that the targeting ratios of A. thaliana and A. lyrata pollen tubes to xiuqiu ovules were 93.3% ± 1.5% (nr = 3, no = 179) and 93.8% ± 2.5% (nr = 3, no = 117), respectively, being almost identical (Supplemental Figure S6, A–C). This result shows that both A. thaliana and A. lyrata pollen tubes exhibited a high, while compatible, targeting efficiency to xiuqiu ovules, suggesting that there exist other nonspecies-specific pollen tube attractants in addition to XIUQIUs. In the subsequent pollen tube competition assays, 84.4% ± 3.0% of the A. thaliana pollen tubes were targeted to xiuqiu ovules (nr = 4, no = 101; Figure 4, F and G). This targeting ratio was slightly lower than that of WT ovules (89.5% ± 3.0%, nr = 4, no = 105, Figure 4, D and G), suggesting that the lack of XIUQIUs did not change much of the attraction precedence of the A. thaliana pollen tubes at the micropyle and that XIUQIUs function as nonspecies-specific pollen tube attractants. Meanwhile, this targeting ratio was significantly higher than that of atlure1null ovules (65.4% ± 4.1%, nr = 4, no = 101; Figure 4, E and G), suggesting that AtLURE1s contribute primarily to the attraction precedence of A. thaliana pollen tubes at the micropyle. This result supports the role of AtLURE1s as species-specific pollen tube attractants at the micropyle.
Discussion
Prezygotic reproductive isolation in plants is mainly associated with arrested and defective pollen tube growth and guidance, respectively. It is believed that each process along the pollen tube journey could potentially become a genetic barrier contributing to reproduction isolation (Baack et al., 2015). This includes pollen–stigma recognition, pollen tube penetration into the stigma and style, growth in the transmitting tissue, pollen tube emergence out of the septum, further growth toward ovules where pollen tubes penetrate the micropylar region of the ovule, and ultimately pollen tube reception culminating in sperm cell release and gamete fusion (Dresselhaus and Franklin-Tong, 2013). Reproductive isolation barriers associated with self-incompatibility during pollen stigma interaction and initial tube growth are well investigated in the Brassicaceae and Papaveraceae (Nasrallah, 2019; Wang et al., 2019). But our molecular understanding of how the other processes may contribute to reproductive isolation is still scarce. In contrast to its relatives like A. lyrata, a self-incompatible species that only allows foreign pollen of the own species to grow inside the pistil and toward ovules, A. thaliana is a self-compatible species lacking early mechanisms to discriminate between own and foreign pollen of related species (Nasrallah, 2019). Recently, AtLURE1/PRK6-mediated signaling was reported to contribute to reproductive isolation by promoting emergence of own pollen tubes from the septum to grow toward the ovule for fertilization (Zhong et al., 2019). The goal of this study was to clarify whether AtLURE1s and their receptors are also involved in formation of a prezygotic reproductive isolation barrier at the micropyle.
To answer this question, we adopted the semi-in vivo ovule targeting assay (Palanivelu and Preuss 2006), a very useful method to study solely micropylar effects as it bypasses the earlier stages described above. Furthermore, the semi-in vivo ovule targeting assay was used in three different ways: (1) to test general ovule targeting efficiencies of pollen tubes, (2) in pollen tube competition assays to test competitiveness of two different types of pollen tubes that target the same ovule type, and (3) in ovule competition assays to test competitiveness of two different types of ovules to attract pollen tubes. In order to confirm that a pollen tube has indeed successfully targeted an ovule, we further developed the “dragging” method, which we described to be well suited to discriminate between loosely associated pollen tubes, pollen tubes that successfully entered the micropyle, as well as fluorescent and nonfluorescent tubes. By using this modified method, we demonstrate that AtLURE1s and their receptor PRK6 are involved in promoting attraction of own pollen tubes at the micropyle. Together with our previous report (Zhong et al., 2019), it is now clear that the AtLURE1/PRK6 signaling pathway promotes conspecific pollen precedence at two stages, that is pollen tube emergence from the septum to the funiculus and pollen tube attraction at the micropyle, thus playing a major role during the whole process of ovular pollen tube guidance (Hater et al., 2020). The AtLURE1/PRK6-mediated signaling pathway thus guarantees a strong and biased precedence for own pollen tubes and contributes to prezygotic reproductive isolation in the genus Arabidopsis. Notably, previously reported XIUQIUs, nonspecies-specific pollen tube attractant peptides of the Brassicaceae were shown in this study to attract pollen tubes independent of PRK6 and to not be involved in discriminating pollen tubes of A. thaliana from those of A. lyrata at the micropyle.
In conclusion, by extending studies on AtLURE1/PRK6-mediated signaling using modified semi-in vivo ovule targeting assays, we demonstrated in this study that conspecific micropylar pollen tube guidance represents another mechanism to promote own pollen tubes in A. thaliana. In addition, our previous and this study have collectively demonstrated that AtLURE1/PRK6-mediated signaling promotes conspecific pollen tube precedence for A. thaliana both at the septum emergence point and at the micropyle. This adds another layer to our understanding of how signaling mechanisms contribute to the complexity of the formation of reproductive isolation barriers in plants.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as WT. The prk6-2 (Salk_076923C) mutant was obtained from the Arabidopsis Biological Resource Center (ABRC). Arabidopsis lyrata ssp. lyrata was provided by Dr Ya-Long Guo’s laboratory (Institute of Botany, Chinese Academy of Sciences). All seeds were sown on half-strength Murashige and Skoog medium (Murashige and Skoog, 1962). Plates with seeds were placed at 4°C for 2 d and then transferred toward a growth incubator with 16-h light/8-h dark cycles for 10 d at 22°C. Seedlings were transferred to soil and grown in the greenhouse with LED lights (GPL production modules DR/W and DR/B/FR, Philips) under long-day conditions (16-h light/8-h dark) at 22°C ± 2°C.
Semi-in vivo ovule targeting assay
The semi-in vivo ovule targeting assay was conducted as described previously (Willemse et al., 1995; Palanivelu and Preuss, 2006). We used male sterile 1 (ms1; CS301367, Col-0 genetic background) pistils as acceptors of pollen grains (Yang et al., 2007). In brief, ms1 stigmata were handcut from the pistil and placed onto solid pollen germination medium (SPGM: 14% w/v sucrose, 0.001% w/v boric acid, 1.27 mM Ca(NO3)2, 0.4 mM MgSO4, pH adjusted to 7.0 with KOH in 1.5% w/v low gelling temperature agarose) in a small culture dish with a 2-mm-thick, 14-mm-diametral cover glass in the bottom center. After detached stigmata were pollinated, ovules were placed at appropriate positions below cut stigmata and then, culture dishes were placed into a 22°C incubator allowing pollen germination, growth, and attraction. To investigate whether pollen tubes without fluorescence entered the micropyle, each of the corresponding ovules was gently dragged with a glass needle (with the diameter of the needle tip about 40 μm) by hand under a ×10/0.25 objective in a NIKON ECLIPSE TS100 inverted microscope. If a pollen tube was dragged into a straight line, and could not be separated from the ovule, it was considered as a pollen tube that had successfully entered the ovule.
Imaging
Images of the ovule targeting assays were acquired with a Zeiss Axio Imager D2 microscope equipped with a Yokogawa CSU-X1 spinning disk. The Plan-Apochromat ×10/0.45 and Plan-Apochromat ×20/0.8 objectives were used under 488 nm (intensity 60%, collection bandwidth 500–550 nm, gains 800) and 561 nm (intensity 35%, collection bandwidth 598–660 nm, gains 400) for LAT52pro:GFP transgenic pollen tubes (green fluorescence-labeled) and ovules with autofluorescence observation, respectively, and under bright field (5.10 V for intensity, gains 89) for nonfluorescence-labeled pollen tube targeting assays.
Supplemental data
The following supplemental materials are available.
Supplemental Figure S1. LAT52pro:GFP pollen tube targeting efficiencies to WT and atlure1null mutant and AtLURE1.2 rescued atlure1null mutant ovules, respectively.
Supplemental Figure S2. Final status of WT and LAT52pro:GFP pollen tubes in the semi-in vivo ovule competition assay.
Supplemental Figure S3. Estimation of the targeting status in GFP channel and bright field.
Supplemental Figure S4. prk6-2 mutant pollen tube targeting efficiencies to WT and atlure1null mutant ovules, respectively.
Supplemental Figure S5. Confirmation of final status of prk6-2 pollen tubes in the semi-in vivo ovule competition assay by the “dragging” method.
Supplemental Figure S6. Pollen tube targeting efficiencies of LAT52pro:GFP pollen tube and A. lyrata pollen tube to xiuqiu mutant ovules, respectively.
Funding
This work was supported by grants from National Natural Science Foundation of China (Grant no. 31991202, 31830004, 31620103903 and 31621001 to L.-J.Q., and 32070854 to S.Z., and 91331201 to H.G.), the Peking-Tsinghua Joint Center for Life Sciences in-house grant to L.-J.Q. and the German Research Council DFG via the Collaborative Research Center (SFB924 to T.D.).
Conflict of interest statement. None declared.
Supplementary Material
M.L., Z.W., and S.Z. formulated the research plan and designed the experiments. M.L. and Z.W. performed experiments and interpreted results. S.H. and L.W. helped with genotyping and microscopic imaging. Q.H. helped to generate atlure1null quintuple mutant plants. H.G. and L.-J.Q. supervised the experiments. M.L., Z.W., S.Z., and L.-J.Q. wrote the article. M.L., Z.W., H.G., T.D., S.Z., and L.-J.Q. discussed results and commented on the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Li-Jia Qu (qulj@pku.edu.cn)
References
- Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D (2015) The origins of reproductive isolation in plants. New Phytol 207: 968–984 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Franklin-Tong N (2013) Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 6: 1018–1036 [DOI] [PubMed] [Google Scholar]
- Glöckle B, Urban WJ, Nagahara S, Andersen ED, Higashiyama T, Grini PE, Schnittger A (2018) Pollen differentiation as well as pollen tube guidance and discharge are independent of the presence of gametes. Development 145: dev152645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Lu J, Xu J, Mcclure B, Zhang S (2014) Two mitogen-activated protein kinases, MPK3 and MPK6, are required for funicular guidance of pollen tubes in Arabidopsis. Plant Physiol 165: 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hater F, Nakel T, Groß-Hardt R (2020) Reproductive multitasking: the female gametophyte. Annu Rev Plant Biol 71: 517–546 [DOI] [PubMed] [Google Scholar]
- Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66: 393–413 [DOI] [PubMed] [Google Scholar]
- Howard DJ (1999) Conspecific sperm and pollen precedence and speciation. Annu Rev Ecol Syst 30: 109–132 [Google Scholar]
- Johnson MA, Harper JF, Palanivelu R (2019) A fruitful journey: pollen tube navigation from germination to fertilization. Annu Rev Plant Biol 70: 809–837 [DOI] [PubMed] [Google Scholar]
- Lausser A, Kliwer I, Srilunchang K-O, Dresselhaus T (2010) Sporophytic control of pollen tube growth and guidance in maize. J Exp Bot 61: 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Pei JQ, Tang WH (2019) What took you so long? Peptide-receptor kinase signaling mediates reproductive isolation in plants. Sci Bullet 64: 1390–1392 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhong S, Guo X, Hao L, Wei X, Huang Q, Hou Y, Shi J, Wang C, Gu H, et al. (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23: 993–998 [DOI] [PubMed] [Google Scholar]
- Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH (2008) Review. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philos Trans R Soc Lond B Biol Sci 363: 3009–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JG, Zhang MX, Yang WC, Li HJ (2019). TICKET attracts pollen tubes and mediates reproductive isolation between relative species in Brassicaceae. Sci China Life Sci 62: 1413–1419 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantar 15: 473–497 [Google Scholar]
- Muschietti J, Dircks L, Vancanneyt G, McCormick S (1994) LAT52 protein is essential for tomato pollen development: Pollen expressing antisense LAT52 RNA hydrates and germinatesabnormally and cannot achieve fertilization. Plant J 6: 321–338 [DOI] [PubMed] [Google Scholar]
- Nasrallah JB (2019). Self-incompatibility in the Brassicaceae: regulation and mechanism of self-recognition. Curr Top Dev Biol 131: 435–452 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L-J, Li L, Lan Z, Dresselhaus T (2015) Peptide signalling during the pollen tube journey and double fertilization. J Exp Bot 66: 5139–5150 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2012) A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 10: e1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Wang L, Lin Z, Trivino M, Nowack MK, Franklin-Tong VE, Bosch M (2019) Self-incompatibility in Papaver pollen: programmed cell death in an acidic environment. J Exp Bot 70: 2113–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer A, Lexer C, Cozzolino S (2009). Evolution of reproductive isolation in plants. Heredity (Edinb) 102: 31–38 [DOI] [PubMed] [Google Scholar]
- Willemse MTM, Plyushch TA, Reinders MC (1995) In vitro micropylar penetration of the pollen tube in the ovule of Gasteria verrucosa (Mill.) H. Duval and Lilium longiflorum Thunb.: conditions attraction and application. Plant Sci 108: 201–208 [Google Scholar]
- Yang C, Vizcay-Barrena G, Conner K, Wilson ZA (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19: 3530–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Huang Q, Zhong S, Bleckmann A, Huang J, Guo X, Lin Q, Gu H, Dong J, Dresselhaus T, et al. (2017a) Sperm cells are passive cargo of the pollen tube in plant fertilization. Nat Plants 3: 17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu W, Nagae TT, Takeuchi H, Zhang H, Han Z, Higashiyama T, Chai J. (2017b) Structural basis for receptor recognition of pollen tube attraction peptides. Nat Commun 8: 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Qu L-J (2019a) Peptide/receptor-like kinase-mediated signaling involved in male-female interactions. Curr Opin Plant Biol 51: 7–14 [DOI] [PubMed] [Google Scholar]
- Zhong S, Qu L-J (2019b) Cysteine-rich peptides: signals for pollen tube guidance, species isolation and beyond. Sci China Life Sci 62: 1243–1245 [DOI] [PubMed] [Google Scholar]
- Zhong S, Liu M, Wang Z, Huang Q, Hou S, Xu YC, Ge Z, Song Z, Huang J, Qiu X, et al. (2019). Cysteine-rich peptides promote interspecific genetic isolation in Arabidopsis. Science 364: eauu3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.