Abstract
Adipose tissue and arterial dysfunction are common in the obese state. Perivascular adipose tissue (PVAT) plays an important role in mediating arterial health, and with obesity the PVAT dysfunction negatively affects arterial health. Exercise training exerts direct and beneficial effects on PVAT providing an additional and novel pathway by which exercise can improve arterial health in diseased populations.
Keywords: Obesity, Exercise, Arterial Function, Perivascular Adipose Tissue, exercise training
Summary for table of contents:
Perivascular adipose tissue contributes to arterial dysfunction with obesity and exercise training can limit its role.
Introduction
Overweight and obesity affect 42.4% of the US population (1) which has significant cardiovascular (CV) consequences resulting in a reduced quality of life, and increased mortality rate (2). Arterial disease is an important clinical pathological consequence of obesity with significant macro- (aorta, femoral etc) and micro-vasculature (arterioles) changes that negatively affect the function of multiple organs. Obesity also results in an excessive accumulation of adipose tissue surrounding most of the arterial network. This perivascular adipose tissue (PVAT) was initially thought to provide structural support, however, in 2002 Lohn et al. (3) demonstrated that PVAT from healthy subjects contains perivascular relaxing factors (PVRF) that directly relaxed contracted arteries. Since then various labs, including our own, have shown a direct effect of PVAT on arterial health and that with obesity there is an excessive accumulation and a phenotypic change in PVAT (4, 5).
It is well known that regular exercise exerts beneficial physiological changes to the arterial system and adipose tissue morphology. However, recently my lab, and others, have suggested that exercise training exerts beneficial effects on PVAT with subsequent improved actions on arterial function/structure (6). As such, we hypothesized (Fig. 1) that obesity negatively alters PVAT phenotype which directly induces arterial dysfunction, and that exercise training can limit the obesity-PVAT arterial dysfunction. This review will summarize the effects of obesity on adipose tissue depots, arterial health, and their interaction, and how regular exercise improves arterial health with obesity.
Fig 1. The role of obesity and exercise on perivascular adipose tissue induced aortic (dys)function.
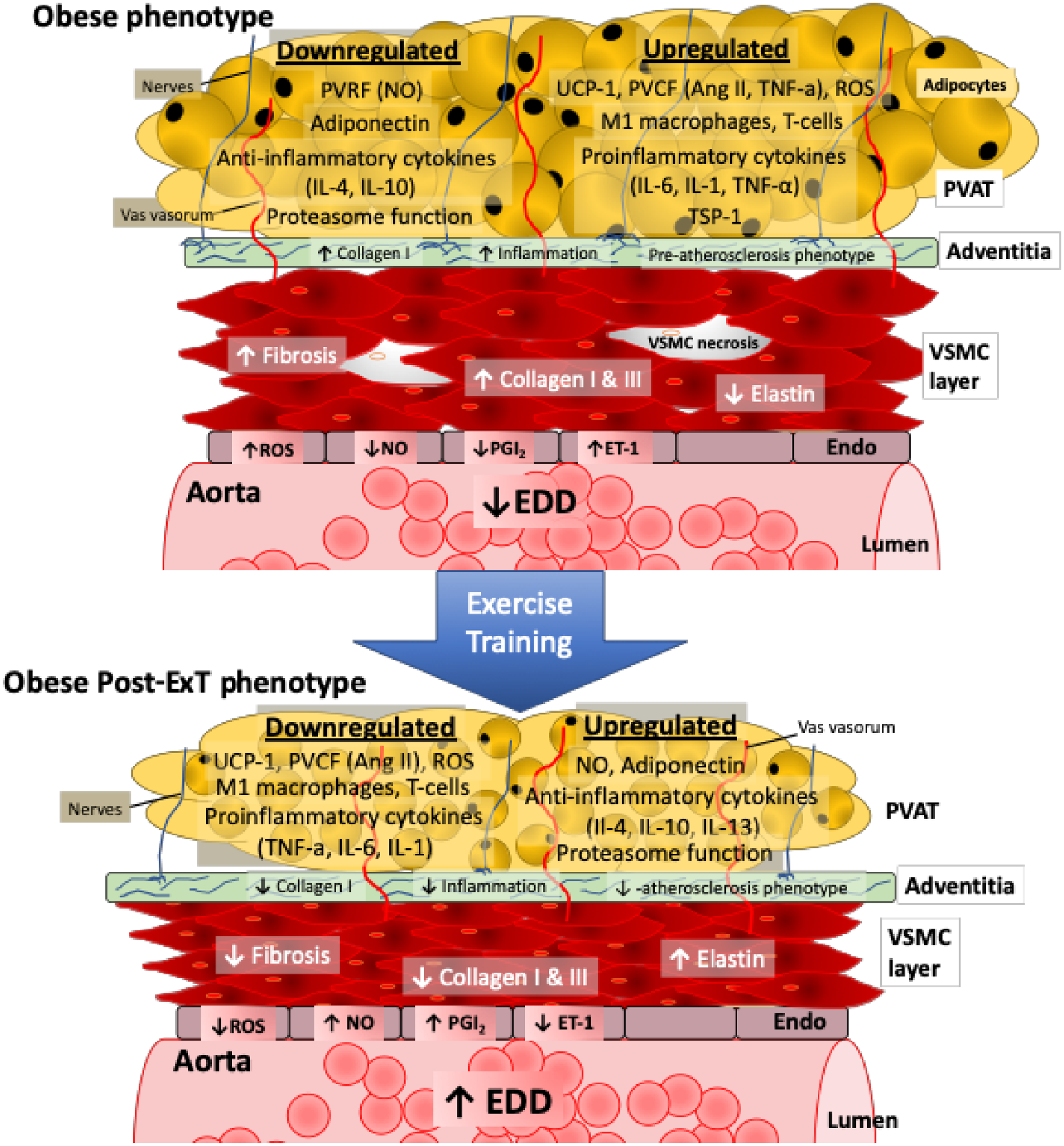
With obesity the PVAT becomes more white (decreased UCP-1) and dysfunctional, reflected by a greater infiltration of M1 macrophages and T-cells with a corresponding increase in pro-inflammatory cytokines, and ROS coupled with a downregulation in proteasome function and anti-inflammatory cytokines. This dysfunctional PVAT phenotype results in a decrease in PVRF (NO, adiponectin) and an increase in PVCF (ET-1, Ang II). As PVAT is closely positioned to the vascular adventitia without an anatomic barrier, it can directly communicate with vascular cells by transferring cytokines. Further, cytokines can be transferred through the vasa vasorum to VSMCs. As such, obesity increases collagen 1 content and inflammation to promote a pre-atherosclerotic phenotype in the adventitia. Significant changes also occur to the VSMC, reflected by an increase in fibrosis and collagen content, with a reduction in elastin content with more necrotic cells. In the endothelium, there is an impaired release of NO and PGI2 with an increase in ROS, and ET-1, which leads to reduced aortic EDD and increased stiffness. However, when obese animals under exercise training (blue arrow) the dysfunctional PVAT is partly reversed. That is obese-ExT PVAT reflects a more brown like phenotype, with reduced pro-inflammatory cytokines, ROS, and increased anti-inflammatory cytokines and proteasome function. Ultimately, exercise training improves PVRF and reduces PVCF so that aortic EDD and stiffness are improved.
Abbreviations: PVAT, perivascular adipose tissue; VSMCs, vascular smooth muscles cells; Endo, endothelium; EDD, endothelial dependent dilation; PVRF, perivascular derived relaxation factor; PVCF, perivascular derived contraction factors; NO, nitric oxide; IL-1, interlukin-1; IL-4, interlukin-4; IL-6, interlukin-6; IL-10, interlukin-10; IL-13, interlukin-13; UCP-1, uncoupling protein 1; Ang II, angiotensin II; TNF-a, Tumor necrosis factor-alpha; ROS, reactive oxygen species; TSP-1, thrombospondin-1; PGI2, prostacyclin; ET-1, endothelial-1
Adipose tissue morphology and pathology
In mammals, there are white (WAT), brown (BAT) and beige adipose tissue, which have distinct functions and different morphology, protein expression patterns, and developmental origins. Adipose tissue is composed of adipocytes and a stromal vascular fraction consisting of pericytes, endothelial cells, monocytes, macrophages, and stem cells. Adipose tissue has an endocrine role, secreting adipokines such as leptin, angiotensin, adiponectin, tumor-necrosis factor alpha (TNF-α), interleukin-6 (IL-6), etc. The function of WAT, which represents 10–30% of body weight, is to store energy in the form of lipids, and they have few mitochondria but have large single lipid droplets of triacylglycerol. There are two main depots of WAT, the subcutaneous (SAT) depot (adipose tissue located under the skin), and the visceral (VAT) depot. SAT, which makes up a majority of the total fat mass, usually consists of smaller adipocytes that produce low levels of pro-inflammatory adipokines (7) and high anti-inflammatory adipokines (8). SAT acts as an energy store, a thermal insulator, a cushion against mechanical stress, and a physical barrier to infection (9). VAT is composed of unilocular adipocytes tightly packed together and supported by loose connective tissue with a dense network of capillaries. In contrast, BAT has unique thermogenic properties and is a vital organ for maintaining body temperature in smaller mammals and human infants. BAT has a polygonal shape and contains multiple smaller lipid droplets, expressing uncoupling protein 1 (UCP1), Cidea and peroxisome proliferator-activated receptor-γ coactivator. The degree of thermogenesis in BAT can be influenced by a cold environment and BAT is highly vascularized and innervated. BAT was thought to only exist in children; however, BAT has been identified in adults (10). BAT have been found interspersed in WAT, however, they are not derived from the myf5 lineage (classical BAT) and thus are known as beige or brite cells. These cells have high UCP1 expression, thus beige fat can acquire a brown-like phenotype upon cold exposure or pharmacological stimulation. Both beige and brown adipocytes are important in regulating energy expenditure by reducing fat accumulation and improving metabolic health (11).
PVAT surrounds the adventitia of most arteries and represents around 3% of the total body adipose tissue mass. In addition to storing triglycerides, PVAT secretes a wide range of adipokines that have a direct impact on arterial health. It is suggested that PVAT release factors that reach the medial and endothelial layers via direct diffusion through the vasa vasorum (12), and/or via a dense reticular network of collagenous conduits connecting the medial layer with the underlying adventitia (13). In lean rodents, the mesenteric, carotid, and femoral arteries are surrounded by white PVAT, whereas the thoracic aorta is surrounded by brown PVAT and the abdominal aorta by beige PVAT (14). Epicardial adipose tissue (EAT) is located between the visceral pericardium and makes direct contact with the coronary vasculature, thus the EAT adipokines directly affect coronary function. Pericardial adipose tissue (PAT) on the other hand is located in and around the pericardial sac that surrounds the heart and should be considered an atypical PVAT depot. That is, although it does not make direct contact with the coronary vasculature, secreted adipokines reach the coronaries via the pericardial fluid (15). Figure 2 shows a simplified schematic of the different adipose tissue depots.
Fig 2. Typical and atypical perivascular adipose tissue distribution in humans.
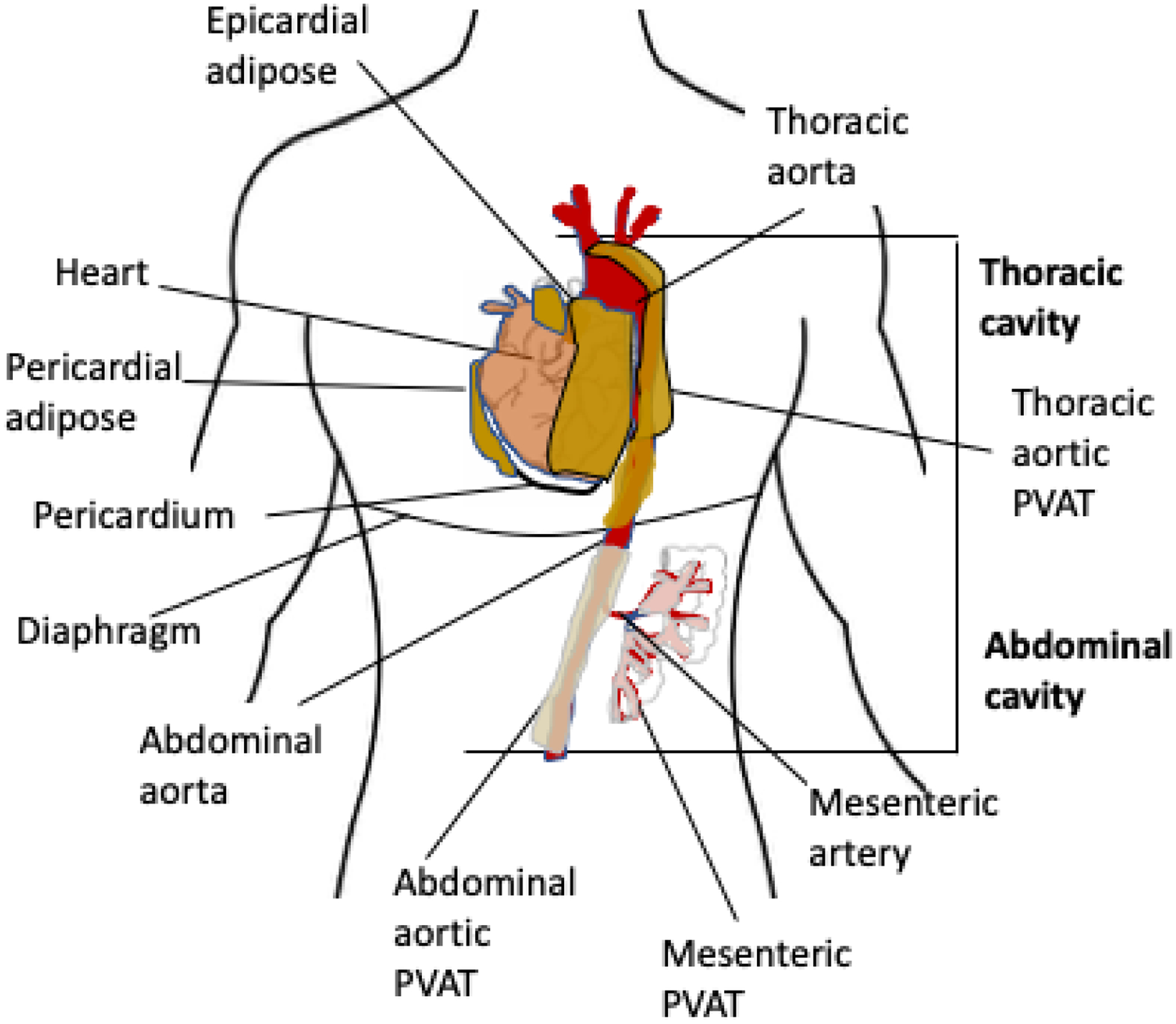
Diagram shows the location of typical and atypical perivascular adipose tissue depots in humans. The color differences reflect the different degrees of brown adipocytes in the adipose tissue. Further changes associated with obesity and exercise are described in Table 1.
With obesity, the mature adipocytes undergo hyperplasia and hypertrophy. Hyperplasia of adipocytes is determined by the recruitment of pre-adipocytes within the tissue stroma, but the numbers of these are limited, suggesting that there is a limit to the degree to which adipocytes can expand by this means (16). Additionally, adipocyte hypertrophy is also limited by their capacity to store triglycerides and as such, results in excess triglycerides being stored in and around other tissues, including around the vasculature (17). Further, reaching this threshold of hyperplasia and hypertrophy causes stress in adipocytes and the activation of Jun N-terminal Kinase and Nuclear factor-Kß (Nf-Kß) signaling pathways which initiates an inflammatory response leading to local and systemic inflammation and impaired adipogenesis of precursor cells (18). In addition, there is a recruitment of monocytes to the adipose tissue, which differentiate to macrophages and amplify the inflammatory response (18). WAT is considered the most common fat depot responsible for the expansion of the adipose tissue, however, obesity also leads to maladaptive responses to BAT and PVAT. With obesity there is an expansion of BAT through hyperplasia, however, this may be associated with a loss of its protective role due to reduced glucose uptake and thermogenesis (19). Indeed, BAT seem to adopt a white-like phenotype, with increased lipid accumulation (20).
The actions of obesity on PVAT are multifactorial resulting in significant changes to its morphology, biochemistry and physiology (21). With obesity there is an increased release of adipokines (resistin, leptin and visfatin), pro-inflammatory cytokines (IL-6, IL-1, TNF-α etc), and chemokines (RANTES, CCL5, MCP-1, CCL2), with reduced anti-inflammatory cytokines (IL-10, IL-13, IL-4 etc) from the PVAT (4). With obesity, the adipose tissue inflammation is accompanied by increased infiltration (22) and phenotypic switching of macrophages to a proinflammatory activation profile (23). Further, CD8+ T cells produce monocyte chemoattractant proteins (MCPs) which modulate the infiltration of macrophages in PVAT (24). Further, cytotoxic CD8+ T cells secrete TNFα, IL-2, IFN-γ, whereas treatment of obese mice with CD8-specific antibodies was shown to attenuate M1 macrophage infiltration and adipose tissue inflammation (25). In this regard, inflammatory cells in PVAT are implicated in the recruitment and/or proliferation of adventitial myofibroblasts. Similarly, the EAT changes with obesity are characterized by hypertrophy, failure to store triglycerides, increased lipolysis and inflammation (26). As EAT expands it becomes hypoxic and dysfunctional and is invaded by increased numbers of macrophages and T lymphocytes, resulting in a shift in its metabolic profile (27). The result is increased secretion of pro-inflammatory cytokines that contribute to the inflammatory environment characteristic of atherogenesis (28), as well as reduced secretion of anti-atherosclerotic adipokines such as adiponectin (29). In this setting, the beneficial paracrine effects of EAT are lost, and such dysfunctional changes may play a causative role in local inflammation, with the potential to influence the development and progression of coronary artery plaque (30).
Unlike PVAT, our understanding as to how obesity impacts PAT phenotype is limited. Excessive adipose tissue around the heart is associated with obesity (31) leading to atrial fibrillation and calcification of the coronary vessels (32). Our most recent work in mice suggests that PAT has characteristics more similar to beige and BAT rather than WAT. Fatty acid binding protein 4, leptin, adiponectin mRNA levels were all significantly lower in PAT than in visceral WAT, whilst UCP1, Iodothyronine Deiodinase 2 (DIO2) and Cytochrome c oxidase subunit 8B were higher (33). This may suggest that PAT has increased flux through oxidative phosphorylation and the electron transport chain, although, UCP1 and DIO2 are more associated with increased thermogenesis (34). These data suggest that PAT has a beige or BAT phenotype which would usually be considered protective. However, despite PAT having more of a BAT gene signature, we also found some significant differences between the two depots, particularly with TNFα which was higher in PAT. High fat feeding also increased IL-6 only in PAT compared with VAT. These data suggest that PAT may be more prone to metabolic dysfunction as a result of pro-inflammatory macrophage infiltration following high fat feeding and obesity. Following obesity, PAT also showed the largest increase in cellularity, suggesting that this depot expands to a large degree through adipocyte hypertrophy (33). These data all indicate that many types of adipose depots at different locations (PAT, EAT, PVAT etc) can be negatively influenced by obesity with potentially severe implications on arterial health.
Obesity and Arterial Health
Arterial Function
One of the earliest manifestations of arterial disease with obesity is endothelial dysfunction. Indeed, an increase in BMI was an independent negative predictor of brachial endothelial dependent dilation (EDD; flow mediated dilation) (35). Even the resistance vessels isolated from a biopsy of abdominal SAT of patients with severe obesity showed a dramatic reduction in EDD compared to lean controls (36).
We have used the obese Zucker rat (OZR) model to show that aortic EDD to methacholine is significantly impaired (between 15–20%) in male and female OZR’s vs. lean controls (4, 37). However, endothelial independent dilation to sodium nitroprusside, which results in the direct stimulation of the vascular smooth muscle cells (VSMC) is not affected by obesity (4, 37). Similar findings have been noted in mouse models of obesity (high fat diet) with regard to EDD and EID (38). Further, we and others have consistently identified that the EDD of the muscular resistance arterioles are significantly impaired in obese vs. lean rats (39, 40). Similarly, the EDD of the middle cerebral artery (MCA) is decreased (-8 to -20%) in obese male and female rats/mice and that the degree of impairment accompanied the severity of obesity (37, 41). The dysfunctional resistance arterioles with obesity results in perfusion limitations (40) and greater injury from ischemic strokes (42).
What is inducing this obese arterial phenotype? Under normal physiological conditions, the secretion and release of endothelial-derived vasoactive factors are maintained and balanced. However, with obesity there is a reduction in arterial NO and prostacyclin, and an increase in ET-1 and thromboxane release (43). Inflammation and oxidative stress are proposed as the underlying factors that are inducing this imbalance. We have shown that circulating and tissue (aorta and MCA) levels of nitrotyrosine and TNF-α are elevated 1-to-3-fold in obese rats (4, 6, 37, 41). As such, the obesity-related decline in endothelial function is linked to the excessive oxidative stress (superoxide anion, hydroxyl racial, lipid radicals etc), however, the antioxidant defense pathway is also reduced with obesity (44). As such, the excessive production of superoxides leads to rapid reaction with NO to form peroxynitrite and thus reduces NO bioavailability and causes nitrosylation of proteins. Importantly, endothelial dysfunction in the aorta or resistance vessel returns to near normal healthy levels with the acute incubation of the vessels (ex-vivo) with the antioxidant Tempol (6, 45). Further, the daily delivery of Tempol in the drinking water of obese rats significantly lowered circulating nitrotyrosine and TNF-α, improved aortic NO bioavailability, and mostly restored EDD of the MCA in obese rats (45). Obesity is also associated with increased activation of the renin–angiotensin system, and increased concentrations of angiotensin II that can further promote oxidative stress in vessels and impair arterial function (46). Other factors influencing NO bioavailability, such as diminished sensitivity of the endothelium to insulin (47), or obesity-mediated alterations in matricellular proteins (such as thrombospondin-1, TSP-1) (48) that can directly impair NO synthesis pathway (49), are also likely to work in concert and/or further contribute to arterial dysfunction.
Arterial Remodeling
The transition from being healthy to having obesity/metabolic diseases coincides with an increase in carotid intima medial thickness (0.011 vs. 0.005 mm/year), and lumen diameter (0.055 vs. 0.023 mm/year) (50). The remodeling of the large arteries in response to obesity is largely due to an increase in circumferential wall stress and flow-mediated shear stress (51). Thus, the arteries undergo either changes in lumen size and/or arterial wall thickness to maintain tensile wall stress within ideal limits. Animal models also demonstrate that the conduit vessels undergo remodeling similar to that noted in obese humans (52). Clinical data also suggests that the aorta undergoes some form of remodeling with obesity. A large population of subjects, free from CV risk factors, showed that increasing BMI was associated with increasing aortic size (53). In addition to large artery remodeling, small resistance arteries dissected from the abdominal SAT of obese individuals had increased wall thickness and media-to-lumen ratio compared to healthy controls, indicating hypertrophic remodeling (54). Studies in the OZR showed atrophic remodeling (reduced lumen size and thinner vascular walls) of the microvasculature (55). There is also a profound reduction in microvessel density, which negatively impacts mass transport and exchange with the surrounding parenchymal tissues within skeletal muscle (56). Further, conduit and resistance vessels become stiffer with obesity. Results from a prospective cohort study showed that central obesity predicted the development of arterial stiffness over a period of 16 years (57).
Structural changes to the extracellular matrix directly impacts arterial stiffening. Mouse models of obesity are known to induce arterial stiffness (58). An accumulation of arterial collagen content (which are 100–1000 times stiffer than elastin) is noted with obesity which is linked to increased transforming growth factor (TGF)-ß signaling and mineralocorticoid receptor activation (59), and that mineralocorticoid receptor blockade can prevent the diet-induced arterial stiffness (59). In addition to the collagen accumulation, a high-fat-high-refined carbohydrate western diet resulted in considerable cross-linking in the arterial wall mediated by the increased activation of transglutaminase 2 (TG2; a collagen cross-linking enzyme), lysyl oxidase and/or advanced glycation end products (60). This cross-linking of collagen further compounds arterial stiffness. Of note, a decrease in NO promotes TG2 activation (61) and increases TGF-ß activation, which in turn increases TG2 expression and activation (60). Elastin breakdown is also a feature of arterial stiffness with obesity (62). Several matricellular proteins have been found to be important. Obesity increases TSP-1 expression in adipose tissue (48) and blood vessels (63). TSP-1 is multifunctional anti-angiogenic protein whose actions include, potent activator of TGF-β, inhibition of NO synthesis via its CD36 and CD47 receptors, and inhibition of matrix metalloproteinases (MMPs). MMPs are implicated in the elastin breakdown and therefore play a crucial role in arterial remodeling by degrading components of the extracellular matrix. Increased oxidative stress can trigger MMP activity and changes in collagen and elastin deposition/resorption (64). Thus, obesity can lead to arterial stiffening via a variety of pathways, which can negatively affect the ability of the vessels to vasodilate.
PVAT induced arterial dysfunction
A current hypothesis is that PVAT plays a key role in arterial function, and that with obesity PVAT undergoes pathological adaptations that induce arterial structural and functional changes. An exception to this is the cerebral network, which does not have any adipose tissue contained within the brain. However, the cerebrovasculature is negatively affected by obesity. It is thought that the excess WAT elicits a chronic low-grade inflammatory and oxidative state, which can have autocrine, paracrine, and endocrine effects thus exerting their actions on the local and systemic arterial system, including the cerebrovasculature. Indeed, obesity enhances pro-inflammatory markers in the brain (65). However, more recently, research has focused on the adipose tissue that is in direct contact with the arterial system, specifically PVAT.
Various labs, including our own, have explored the role of PVAT mediated arterial function. Using a wire-myography chamber, we have shown that the EDD of the thoracic aorta (from lean healthy rat) cleaned of the surrounding thoracic PVAT (tPVAT) is improved when the healthy lean tPVAT conditioned media is administered into the myography chamber (4). However, maladaptive responses of tPVAT during obesity impair aortic EDD and induce arterial stiffness (4). For example, with obesity the release of PVRF from PVAT are diminished, there is an increase in pro-inflammatory cytokines and this toxic PVAT environment contributes to the arterial dysfunction (5). Indeed, the anticontractile properties of lean healthy PVAT are lost with obesity (4). Xia et al (66) showed that endothelial nitric oxide synthase (eNOS) is expressed in PVAT which aids in the production of NO, and that obesity leads to PVAT eNOS uncoupling. We have shown that the blunted aortic EDD in obese rats was further reduced in the presence of obese tPVAT due to reduce NO bioavailability (4). Of note, exposing a lean healthy aorta to the obese tPVAT significantly impaired EDD by ~ 25%, suggesting that factors in the obese tPVAT exert significant vasoactive actions. Conversely, exposing the unhealthy obese aorta with healthy lean tPVAT lowered ROS and improved NO production, which improved EDD in the obese aorta by ~ 20% (4). Our data suggests that the aortic endothelial dysfunction with tPVAT was due to obese-tPVAT derived TNFα inducing aortic oxidative stress (4). As such, we acutely inhibited TNFα by exposing the tPVAT to a TNFα-neutralizing antibody which restored aortic EDD in the presence of the tPVAT. We have also shown that inhibiting NADPH oxidase (NOX) 2 in obese tPVAT had the same impact as TNFα neutralization antibody, suggesting tPVAT impairment of the aorta is dependent on oxidative stress. Additional pathways by which obese tPVAT may inhibit aortic EDD NO production is through increased expression of caveolin-1, which negatively regulates eNOS via the interruption of calcium/calmodulin signaling (67). Another potential reason for the reduction in tPVAT-derived NO is due to a deficiency in PVAT adiponectin (4) that normally stimulates eNOS activity in PVAT adipocytes (68). Increased TNFα inhibits adiponectin removing its protective effects on tPVAT and aortic NO (68).
In addition to the aorta PVAT, during the early phase of diet-induced obesity there is an adaptive overproduction of NO from mesenteric PVAT (69), whereas chronic exposure to diet-induced obesity leads to a reduction in mesenteric PVAT NO production and impaired EDD (70). Again, the increased oxidative environment within the PVAT seems to play a significant role, whereby incubation of the obese PVAT with antioxidant enzymes (superoxide dismutase and catalase) improved PVAT-intact mesenteric arterial function (70).
PVAT proteasome dysfunction also seems to play a role in the arterial dysfunction with obesity. Proteasomes are large protein complexes responsible for the proper regulation of proteins that control cell-cycle progression and apoptosis. The proteasome consists of catalytic and regulatory subunits. The basic particle of the proteasome is the 20S core, which forms a gated channel through which a limited number of peptides and proteins enter (71). To alter the gate conformation and allow the degradation of a wider range of proteins (ubiquitinated, damaged and misfolded proteins), 19S regulatory complexes bind onto the 20S proteasome. The proteasome is able to recognize and degrade peptides and proteins to maintain equilibrium between normal protein production and degradation, or to eliminate the damaged, misfolded/unfolded or pathogenic proteins. Upon substrate recognition, 19S subunits are activated to unfold the substrate and facilitate its entrance through the gate and, finally, its route to the catalytic subunits (72). Oxidative stress is known to damage and misfold proteins, and the 20S complex of the proteasomes possess a major role in recognizing and removing the damaged proteins. However, we have shown that obesity results in proteasome dysfunction in tPVAT resulting in an accumulation of ubiquitinated proteins in the tPVAT (6). The increase accumulation of damaged and misfolded proteins can lead to further cellular and oxidative stress. Specifically, buildup of oxidized and ubiquitin products through activation of endoplasmic reticulum stress induced production of inflammatory cytokines (73). Suggesting, proteasome dysfunction may contribute to the increased pro-inflammatory cytokine production in tPVAT. The importance of the proteasome is highlighted by its inhibition with MG132 in lean healthy tPVAT blunted the beneficial actions of healthy tPVAT on aortic EDD (6).
In addition to functional changes, PVAT also seems to play a critical role in the development of arterial stiffness and remodeling. The Framingham Offspring and Third Generation cohorts showed that PVAT volume was associated with higher thoracic and abdominal aortic dimensions (74). Fleenor et al.(75) showed that oxidative stress within PVAT due to the aging process contributed to aortic stiffness in old mice. Aortic wall hypertrophy and adventitial collagen I accumulation was associated with greater superoxide production in PVAT, and that inhibiting superoxide production with tempol reversed arterial wall hypertrophy and stiffness. In obese and aged mice, tPVAT was shown to increase arterial stiffness through alterations of oxidative status, leading to elastin fragmentation (76). To explore the role of tPVAT in aortic stiffness with obesity we perform a co-culture experiment in which a healthy aorta from a lean rat was exposed to either tPVAT from a lean or obese rat. The elastic modulus (a measure of arterial stiffness) of the lean rat aorta was not altered with the lean tPVAT, however, the lean aorta cultured with obese-tPVAT showed an increased elastic modulus (4). tPVAT production of TNFα may play an important role in the aortic stiffening. Indeed, the aortic stiffening in a lean rat in the presence of obese tPVAT was completely inhibited with a TNFα neutralizing antibody (4). TNFα is known to stimulate the production of MMP9 and we found that obese tPVAT had increased MMP9 activity, which was associated with increased arterial stiffness. Inhibition of TNFα by TNFα neutralizing antibody treatment also decreased MMP9 activity. Together, these data strongly suggest that dysfunctional PVAT plays a key role in the arterial dysfunction and remodeling associated with obesity.
Exercise training, arterial function, and PVAT.
Regularly performed exercise induces structural and functional adaptations to large and small vessels. A meta-analysis of 17 studies revealed that exercise interventions significantly improved brachial flow mediated dilation in overweight and obese adults (77). The repeated episodes of elevated blood flow and shear stress (mechanical stimulus) during exercise represent the primary physiological signal (integrins, ion channels, G protein-coupled receptors, and receptor tyrosine kinases) for endothelial structural and functional adaptations (78). The mechanisms of improved endothelial vasodilation after exercise training have been attributed to an increase in release of prostaglandin and NO bioavailability via an increase in eNOS expression and production of NO (79). Exercise has also been shown to reduce pro-inflammatory cytokines and oxidative stress molecules and improve anti-inflammatory cytokines (IL-4, IL-10, IL-13 etc) (80). Indeed, we have shown that exercise training in obese rats leads to the classical arterial adaptive response whereby aortic oxidative stress was less and NO abundance was higher with improved aortic EDD (4). In addition to the aorta, we have also shown that other arterial regions (mesenteric and MCA) are improved with exercise training in obese rats (41). Further, we have shown that aerobic exercise training in obese rats blunts the severity of the microcirculation rarefaction via an improved inflammatory profile (81). Despite the important of exercise induced shear stress on improving arterial function, arteries with a similar structure and exposed to similar hemodynamic forces display distinctly different arterial phenotypes. This would suggest that other additional local factors influence the regulation of arterial function, and that tPVAT may be involved.
It is noted that PVAT phenotype is modified by continued weight lost, low calorie diets and after bariatric surgery which is linked to the beneficial arterial effects observed under these conditions (70). In addition, bariatric surgery reduced PVAT inflammation and restored the normal anticontractile PVAT phenotype in human small arteries (70). We have shown that 8 weeks of treadmill running in OZRs increased tPVAT UCP-1 expression suggesting a browner like phenotype (6). Similarly, exercise training increased UCP-1 content and reduced the size of the adipocyte in the mesenteric PVAT (82) suggesting a restoration of PVAT morphology. In addition, the improved mesenteric PVAT phenotype limited the hypercontractile response of the mesenteric artery to serotonin (82).
We have shown that exercise training also lowered the tPVAT expression of immuno-attractant cytokines (MCP-1, CXCL1), and immune cell specific markers (TNFα, CD68, CD8) while limiting the reduction in anti-inflammatory cytokines (adiponectin, IL-10, IL-13 and IL-4) in OZR (6). These data suggest that exercise training reduced tPVAT inflammation, in part, by preventing the infiltration of T-cells and macrophages. Similarly, exercise training for 16 weeks in mice feed a high fat diet, reduced CD11c+ inflammatory macrophages and CD8+ T cells in epididymal adipose tissue, which was accompanied by a lower mRNA expression of TNFα (83). Exercise also increases the secretion of adiponectin in obese tPVAT which may also contribute to improved aortic EDD mediated eNOS phosphorylation and production of NO, in a caveolin-1 dependent manner. The reduced production of TNFα and immune cells in the exercise trained obese tPVAT likely exert a couple of important actions on the autocrine signaling of tPVAT. First, lower TNFα levels removes autocrine activation of oxidative stress from tPVAT, which can subsequently: a) decrease the oxidative dissociation of the 19S from the proteasome thereby improving proteasome function; b) decrease the sequestration of NO; and c) decrease inflammation-controlled gene expression. Indeed, proteasome function in the tPVAT was improved in the obese exercise trained rat, which likely improved the recognition and breakdown of ubiquitinated proteins. Second, flipping the TNFα-adiponectin balance towards adiponectin would promote the autocrine activation of enhanced NO abundance and phenotype maintenance. We also note an increase in GTP cyclohydrolase 1 expression, which is involved in the production of tetrahydrobiopterin, an essential cofactor for NO generation via eNOS (6) after exercise training in obese rat tPVAT. Further, exercise training mitigated the expression of NOX2 in obese tPVAT, which was accompanied by a significant reduction in oxidative stress. The reduction in oxidative stress and limited eNOS uncoupling permitted improved NO bioavailability in the aorta and tPVAT and as such a corresponding improvement in aortic EDD with obesity (6).
We have also noted significant improvements in aortic stiffness after exercise training in OZR, which we believe are partly mediated through the improved tPVAT phenotype (reduced oxidative stress and MMP-9, improved NO, etc) (6). Another study showed that exercise training for 17 weeks prevented coronary PVAT advanced glycation end products (AGE) expression and secretion, as well as the increased arterial stiffness with reduction of elastin content and AGE accumulation in the presence of the PVAT conditioned medium from an heart failure swine model (84). In support of these data, inhibition of AGE with aminoguanidine prevented the detrimental impact of PVAT conditioned medium from the swine group on mouse aortic stiffness and wall remodeling (84). These results provide mechanistic evidence that chronic exercise training exerts its protective effect on pressure overload-induced coronary arterial stiffness mediated by a reduction in PVAT-related AGE secretion and associated oxidative stress and inflammation. The same study showed that exercise training attenuated the increases in coronary PVAT nitrotyrosine abundance, NF-κB p65 subunit expression, and IL-6/IL-8 secretion, indicating a potential integrative mechanism by which exercise prevents increased coronary arterial stiffness.
Conclusion
Obesity continues to be an epidemic with approximately 42.4% of Americans (2017–2018) presenting with obesity and reports suggest that by 2030 nearly 1 in 2 adults (49%) will have obesity and 1 in 4 adults are projected to have severe obesity (1). Obesity results in various alterations to the macro- and micro-vascular structure and function that accelerates the risk for future CV events. Adipose tissues, through the release of adipokines, chemo/cytokines, hormones, and other currently unknown factors, impact, depending on the type of adipose tissue and its pathology, CV structure and function. The dysfunctional adipose tissues, due to obesity, is one of the major risk factors for CV disease. As PVAT is adjacent and integrated within the vessels it is a major regulator of arterial physiology and pathophysiology. Indeed, PVAT may be considered a predictor of arterial disease and a therapeutic target. Obesity results in a phenotypic change to the PVAT manifested by reduced UCP-1 expression (reflecting a whiter-like adipose tissue composition) coupled with increased infiltration of T-cell and monocyte/macrophage resulting in a pro-oxidative, pro-inflammatory, and reduced anti-inflammatory tPVAT-environment. This obese PVAT phenotype results in arterial dysfunction, wall remodeling, and arterial stiffness (Fig 1). Importantly, the obese PVAT phenotype is modifiable as evident with the beneficial changes noted with exercise training. This is reflected by an improved oxidative and inflammatory tPVAT environment with increased PVRF (including improved NO) and proteasome function. This improved tPVAT environment following exercise training results in improved aortic function and structure (Fig 1). Therapeutically targeting tPVAT with exercise training or other therapeutics might accelerate the beneficial arterial adaptation and may provide a vital approach to reduce CV burden. However, more extensive and mechanistic research on the PVAT signaling pathways and its cross-talk with the various arterial components (EC, VSMC, fibroblasts etc) is needed to further understand the physiological and pathological role of PVAT.
Table 1.
Typical and atypical perivascular adipose tissue phenotype in lean, obesity and following exercise.
| Adipose tissue type | Normal/lean | Obese | Exercise | References |
|---|---|---|---|---|
| Mesenteric PVAT | Low UCP-1/thermogenesis | Thermogenesis ↓ Leptin ↑ Adipocyte hypertrophy |
Tissue volume ↓ Thermogenesis ↑ |
14, 17, 69, 70, 82, 85 |
| Abdominal PVAT | medium UCP-1 thermogenesis | Thermogenesis ↓ Leptin ↑ Adipocyte hypertrophy |
Tissue volume ↓ Thermogenesis ↑ |
14, 86–88 |
| Thoracic PVAT | High UCP1/thermogenesis Protective |
Thermogenesis ↓ Leptin ↑ Adipocyte hypertrophy |
Tissue volume ↓ Thermogenesis ↑ Inflammation ↓ |
4, 6, 14, 18, 86, 88 |
| Pericardial adipose tissue | High UCP-1/thermogenesis Protective low inflammation |
Thermogenesis High inflammation Leptin ↑ Adipocyte hypertrophy/hyperplasia? |
Tissue volume ↓ Thermogenesis? Inflammation? |
29, 32, 33, 89 |
| Epicardial adipose tissue | UCP-1 expression High thermogenesis Protective low inflammation |
Thermogenesis ↓ Function: lipid uptake/ less protective Leptin ↑ Adipose hyperplasia |
Tissue volume ↓ Thermogenesis ↑ Inflammation ↓ |
28, 30, 31, 84, 90–93 |
Key Points.
Obesity leads to a toxic perivascular adipose tissue (PVAT).
PVAT mediates aortic function via paracrine and autocrine pathways.
Exercise training improves PVAT phenotype and directly improves arterial function and structure with obesity.
Funding:
This study was in part supported by the National Institute of General Medical Sciences of the National Institutes of Health (U54GM104942, and 5P20GM109098).
Footnotes
Disclosure of conflicts of interest: I have no conflicts of interest
References
- 1.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N Engl J Med. 2019;381(25):2440–50. Epub 2019/12/19. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 2.Wade KH, Chiesa ST, Hughes AD, Chaturvedi N, Charakida M, Rapala A, et al. Assessing the causal role of body mass index on cardiovascular health in young adults: Mendelian randomization and recall-by-genotype analyses. Circulation. 2018;138(20):2187–201. Epub 2018/12/14. doi: 10.1161/CIRCULATIONAHA.117.033278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16(9):1057–63. Epub 2002/06/28. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 4.DeVallance E, Branyan KW, Lemaster K, Olfert IM, Smith DM, Pistilli EE, et al. Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFalpha- and NOX2-dependent pathway. Exp Physiol. 2018;103(4):590–603. Epub 2018/01/20. doi: 10.1113/EP086818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Garcia-Barrio MT, Chen YE. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2020;40(5):1094–109. Epub 2020/03/20. doi: 10.1161/ATVBAHA.120.312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVallance E, Branyan KW, Lemaster KC, Anderson R, Marshall KL, Olfert IM, et al. Exercise training prevents the perivascular adipose tissue-induced aortic dysfunction with metabolic syndrome. Redox Biol. 2019;26:101285. Epub 2019/08/03. doi: 10.1016/j.redox.2019.101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero-Diaz B, Rodriguez-Flores M, Sanchez-Munoz V, Monraz-Preciado F, Ordonez-Ortega S, Becerril-Elias V, et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front Physiol. 2018;9:1781. Epub 2019/01/09. doi: 10.3389/fphys.2018.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaud A, Lacroix-Pepin N, Pelletier M, Daris M, Biertho L, Fortier MA, et al. Expression of genes related to prostaglandin synthesis or signaling in human subcutaneous and omental adipose tissue: depot differences and modulation by adipogenesis. Mediators Inflamm. 2014;2014:451620. Epub 2014/12/06. doi: 10.1155/2014/451620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. Epub 2016/03/12. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. Epub 2009/04/10. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thyagarajan B, Foster MT. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig. 2017;31(2). Epub 2017/07/05. doi: 10.1515/hmbci-2017-0016. [DOI] [PubMed] [Google Scholar]
- 12.Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernandez-Alfonso MS. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab. 2015;26(7):367–75. Epub 2015/05/27. doi: 10.1016/j.tem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012;110(6):889–900. Epub 2012/03/20. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34(8):1621–30. Epub 2014/05/17. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57(17):1745–51. Epub 2011/04/23. doi: 10.1016/j.jacc.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 16.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–7. Epub 2008/05/06. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 17.Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2018;19(3):406–20. Epub 2017/12/16. doi: 10.1111/obr.12646. [DOI] [PubMed] [Google Scholar]
- 18.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–43. Epub 2017/08/12. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 19.Thoonen R, Hindle AG, Scherrer-Crosbie M. Brown adipose tissue: The heat is on the heart. Am J Physiol Heart Circ Physiol. 2016;310(11):H1592–605. Epub 2016/04/17. doi: 10.1152/ajpheart.00698.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124(5):2099–112. Epub 2014/04/10. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa RM, Neves KB, Tostes RC, Lobato NS. Perivascular Adipose Tissue as a Relevant Fat Depot for Cardiovascular Risk in Obesity. Front Physiol. 2018;9:253. Epub 2018/04/06. doi: 10.3389/fphys.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–7. Epub 2006/02/24. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. Epub 2007/01/04. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–9. Epub 2008/10/09. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. Epub 2009/07/28. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3(2):e000582. Epub 2014/03/07. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119(12):1661–70. Epub 2009/03/18. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 28.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108(20):2460–6. Epub 2003/10/29. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 29.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(5):781–6. Epub 2009/02/21. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 30.Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol. 2017;595(12):3907–17. Epub 2017/02/14. doi: 10.1113/JP273049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99(9):1242–5. Epub 2007/05/05. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 32.Al-Rawahi M, Proietti R, Thanassoulis G. Pericardial fat and atrial fibrillation: Epidemiology, mechanisms and interventions. Int J Cardiol. 2015;195:98–103. Epub 2015/05/31. doi: 10.1016/j.ijcard.2015.05.129. [DOI] [PubMed] [Google Scholar]
- 33.Al-Dibouni A, Gaspar R, Ige S, Boateng S, Cagampang FR, Gibbins J, et al. Unique Genetic and Histological Signatures of Mouse Pericardial Adipose Tissue. Nutrients. 2020;12(6). Epub 2020/06/26. doi: 10.3390/nu12061855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz MB, Herzig S, Vegiopoulos A. Thermogenic adipocytes: from cells to physiology and medicine. Metabolism. 2014;63(10):1238–49. Epub 2014/08/12. doi: 10.1016/j.metabol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Corrigan FE 3rd, Kelli HM, Dhindsa DS, Heinl RE, Al Mheid I, Hammadah M, et al. Changes in truncal obesity and fat distribution predict arterial health. J Clin Lipidol. 2017;11(6):1354–60 e3. Epub 2017/09/25. doi: 10.1016/j.jacl.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grassi G, Seravalle G, Scopelliti F, Dell’Oro R, Fattori L, Quarti-Trevano F, et al. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring). 2010;18(1):92–8. Epub 2009/06/13. doi: 10.1038/oby.2009.195. [DOI] [PubMed] [Google Scholar]
- 37.Brooks SD, Hileman SM, Chantler PD, Milde SA, Lemaster KA, Frisbee SJ, et al. Protection from chronic stress- and depressive symptom-induced vascular endothelial dysfunction in female rats is abolished by preexisting metabolic disease. Am J Physiol Heart Circ Physiol. 2018;314(5):H1085–H97. doi: 10.1152/ajpheart.00648.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayasi R, Akamine EH, Davel AP, Rodrigues MA, Carvalho CR, Rossoni LV. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J Hypertens. 2010;28(10):2111–9. Epub 2010/07/10. doi: 10.1097/HJH.0b013e32833ca68c. [DOI] [PubMed] [Google Scholar]
- 39.Branyan KW, Devallance ER, Lemaster KA, Skinner RC, Bryner RW, Olfert IM, et al. Role of Chronic Stress and Exercise on Microvascular Function in Metabolic Syndrome. Med Sci Sports Exerc. 2018;50(5):957–66. doi: 10.1249/MSS.0000000000001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1124–34. Epub 2003/07/12. doi: 10.1152/ajpregu.00239.2003. [DOI] [PubMed] [Google Scholar]
- 41.Brooks S, Branyan KW, DeVallance E, Skinner R, Lemaster K, Sheets JW, et al. Psychological stress-induced cerebrovascular dysfunction: the role of metabolic syndrome and exercise. Exp Physiol. 2018;103(5):761–76. Epub 2018/02/14. doi: 10.1113/EP086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity Increases Blood Pressure, Cerebral Vascular Remodeling, and Severity of Stroke in the Zucker Rat. Hypertension. 2009;53(2):381–6. doi: 10.1161/hypertensionaha.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Heart Circ Physiol. 2011;301(3):H689–95. Epub 2011/06/15. doi: 10.1152/ajpheart.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas I, Papademetriou L, Economou M, et al. The implication of obesity on total antioxidant capacity in apparently healthy men and women: the ATTICA study. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2007;17(8):590–7. Epub 2006/08/12. doi: 10.1016/j.numecd.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Brooks SD, DeVallance E, d’Audiffret AC, Frisbee SJ, Tabone LE, Shrader CD, et al. Metabolic Syndrome Impairs Reactivity and Wall Mechanics of Cerebral Resistance Arteries in Obese Zucker Rats. Am J Physiol Heart Circ Physiol. 2015;309(11):H1846–59. doi: 10.1152/ajpheart.00691.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egan BM, Greene EL, Goodfriend TL. Insulin resistance and cardiovascular disease. Am J Hypertens. 2001;14(6 Pt 2):116S–25S. Epub 2001/06/20. doi: 10.1016/s0895-7061(01)02078-7. [DOI] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854–8. Epub 1994/05/24. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong P, Cavalera M, Frangogiannis NG. The role of thrombospondin (TSP)-1 in obesity and diabetes. Adipocyte. 2014;3(1):81–4. Epub 2014/02/28. doi: 10.4161/adip.26990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31(3):162–9. Epub 2012/01/24. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira I, Beijers HJ, Schouten F, Smulders YM, Twisk JW, Stehouwer CD. Clustering of Metabolic Syndrome Traits Is Associated With Maladaptive Carotid Remodeling and Stiffening: A 6-Year Longitudinal Study. Hypertension. 2012;60(2):542–9. doi: 10.1161/hypertensionaha.112.194738. [DOI] [PubMed] [Google Scholar]
- 51.Glagov S, Vito R, Giddens DP, Zarins CK. Micro-architecture and composition of artery walls: Relationship to location, diameter and the distribution of mechanical stress. Journal of Hypertension. 1992;10(SUPPL. 6):S101–S4. [PubMed] [Google Scholar]
- 52.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial Nitric Oxide Synthase Uncoupling and Perivascular Adipose Oxidative Stress and Inflammation Contribute to Vascular Dysfunction in a Rodent Model of Metabolic Syndrome. Hypertension. 2009;54(6):1384–92. doi: 10.1161/hypertensionaha.109.138305. [DOI] [PubMed] [Google Scholar]
- 53.Davis AE, Lewandowski AJ, Holloway CJ, Ntusi NA, Banerjee R, Nethononda R, et al. Observational study of regional aortic size referenced to body size: production of a cardiovascular magnetic resonance nomogram. J Cardiovasc Magn Reson. 2014;16:9. Epub 2014/01/23. doi: 10.1186/1532-429X-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grassi G, Seravalle G, Brambilla G, Facchetti R, Bolla G, Mozzi E, et al. Impact of the metabolic syndrome on subcutaneous microcirculation in obese patients. J Hypertens. 2010;28(8):1708–14. Epub 2010/06/04. doi: 10.1097/HJH.0b013e32833af3c9. [DOI] [PubMed] [Google Scholar]
- 55.Stepp DW, Pollock DM, Frisbee JC. Low-flow vascular remodeling in the metabolic syndrome X. Am J Physiol Heart Circ Physiol. 2004;286(3):H964–70. Epub 2003/12/03. doi: 10.1152/ajpheart.00836.2003 00836.2003 [pii]. [DOI] [PubMed] [Google Scholar]
- 56.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12(5):383–92. [DOI] [PubMed] [Google Scholar]
- 57.Johansen NB, Vistisen D, Brunner EJ, Tabak AG, Shipley MJ, Wilkinson IB, et al. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS One. 2012;7(5):e37165. Epub 2012/05/26. doi: 10.1371/journal.pone.0037165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foote CA, Castorena-Gonzalez JA, Ramirez-Perez FI, Jia G, Hill MA, Reyes-Aldasoro CC, et al. Arterial Stiffening in Western Diet-Fed Mice Is Associated with Increased Vascular Elastin, Transforming Growth Factor-beta, and Plasma Neuraminidase. Front Physiol. 2016;7:285. Epub 2016/07/28. doi: 10.3389/fphys.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, et al. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66(1):99–107. Epub 2015/05/28. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Akker J, VanBavel E, van Geel R, Matlung HL, Guvenc Tuna B, Janssen GM, et al. The redox state of transglutaminase 2 controls arterial remodeling. PLoS One. 2011;6(8):e23067. Epub 2011/09/09. doi: 10.1371/journal.pone.0023067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eringa EC, Bakker W, van Hinsbergh VW. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascular pharmacology. 2012;56(5–6):204–9. Epub 2012/03/01. doi: 10.1016/j.vph.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, et al. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118(6):935–43. Epub 2016/02/18. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, et al. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107(25):3209–15. Epub 2003/06/18. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 64.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503. Epub 1994/12/01. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andre C, Dinel AL, Ferreira G, Laye S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav Immun. 2014;41:10–21. Epub 2014/04/01. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler Thromb Vasc Biol. 2016;36(1):78–85. Epub 2015/11/21. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 67.Lee MH, Chen SJ, Tsao CM, Wu CC. Perivascular adipose tissue inhibits endothelial function of rat aortas via caveolin-1. PLoS One. 2014;9(6):e99947. Epub 2014/06/14. doi: 10.1371/journal.pone.0099947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51(1):8–14. Epub 2007/11/14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 69.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151(7):3299–306. Epub 2010/04/23. doi: 10.1210/en.2009-1464. [DOI] [PubMed] [Google Scholar]
- 70.Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J Vasc Res. 2015;52(5):299–305. Epub 2016/02/26. doi: 10.1159/000443885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groll M, Bajorek M, Kohler A, Moroder L, Rubin DM, Huber R, et al. A gated channel into the proteasome core particle. Nat Struct Biol. 2000;7(11):1062–7. Epub 2000/11/04. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- 72.Ciechanover A, Stanhill A. The complexity of recognition of ubiquitinated substrates by the 26S proteasome. Biochim Biophys Acta. 2014;1843(1):86–96. Epub 2013/07/23. doi: 10.1016/j.bbamcr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh AK, Garg SK, Mau T, O’Brien M, Liu J, Yung R. Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci. 2015;70(11):1320–9. Epub 2014/10/18. doi: 10.1093/gerona/glu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, et al. Periaortic adipose tissue and aortic dimensions in the Framingham Heart Study. J Am Heart Assoc. 2012;1(6):e000885. Epub 2013/01/15. doi: 10.1161/JAHA.112.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD, Seals DR. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell. 2014;13(3):576–8. Epub 2013/12/18. doi: 10.1111/acel.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, et al. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol. 2013;33(4):839–46. Epub 2013/02/16. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 77.Son Y, Kim K, Jeon S, Kang M, Lee S, Park Y. Effect of Exercise Intervention on Flow-Mediated Dilation in Overweight and Obese Adults: Meta-Analysis. Int J Vasc Med. 2017;2017:7532702. Epub 2017/11/04. doi: 10.1155/2017/7532702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol. 2008;59 Suppl 7:71–88. Epub 2009/03/11. [PMC free article] [PubMed] [Google Scholar]
- 79.Stapleton PA, Goodwill AG, James ME, Frisbee JC. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1110–9. doi: 10.1152/ajpregu.00410.2007. [DOI] [PubMed] [Google Scholar]
- 80.Bai Y, Sigala W, Adams GR, Vaziri ND. Effect of exercise on cardiac tissue oxidative and inflammatory mediators in chronic kidney disease. American journal of nephrology. 2009;29(3):213–21. doi: 10.1159/000156715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291(5):H2483–92. doi: 10.1152/ajpheart.00566.2006. [DOI] [PubMed] [Google Scholar]
- 82.Liao J, Yin H, Huang J, Hu M. Dysfunction of perivascular adipose tissue in mesenteric artery is restored by aerobic exercise in high-fat diet induced obesity. Clinical and experimental pharmacology & physiology. 2020. Epub 2020/09/08. doi: 10.1111/1440-1681.13404. [DOI] [PubMed] [Google Scholar]
- 83.Kawanishi N, Mizokami T, Yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc. 2013;45(9):1684–93. Epub 2013/08/21. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 84.Ouyang A, Olver TD, Emter CA, Fleenor BS. Chronic exercise training prevents coronary artery stiffening in aortic-banded miniswine: role of perivascular adipose-derived advanced glycation end products. J Appl Physiol (1985). 2019;127(3):816–27. Epub 2019/07/12. doi: 10.1152/japplphysiol.00146.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, et al. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008;19(6):371–5. Epub 2007/07/07. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Victorio JA, Fontes MT, Rossoni LV, Davel AP. Different Anti-Contractile Function and Nitric Oxide Production of Thoracic and Abdominal Perivascular Adipose Tissues. Front Physiol. 2016;7:295. Epub 2016/07/28. doi: 10.3389/fphys.2016.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li RM, Chen SQ, Zeng NX, Zheng SH, Guan L, Liu HM, et al. Browning of Abdominal Aorta Perivascular Adipose Tissue Inhibits Adipose Tissue Inflammation. Metabolic syndrome and related disorders. 2017;15(9):450–7. Epub 2017/09/22. doi: 10.1089/met.2017.0074. [DOI] [PubMed] [Google Scholar]
- 88.Cheng CK, Bakar HA, Gollasch M, Huang Y. Perivascular Adipose Tissue: the Sixth Man of the Cardiovascular System. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2018;32(5):481–502. Epub 2018/09/02. doi: 10.1007/s10557-018-6820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christensen RH, Wedell-Neergaard AS, Lehrskov LL, Legaard GE, Dorph E, Larsen MK, et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019;4(8):778–87. Epub 2019/07/04. doi: 10.1001/jamacardio.2019.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Company JM, Booth FW, Laughlin MH, Arce-Esquivel AA, Sacks HS, Bahouth SW, et al. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J Appl Physiol (1985). 2010;109(6):1904–12. Epub 2010/10/16. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16(5):406–15. Epub 2015/03/11. doi: 10.1111/obr.12270. [DOI] [PubMed] [Google Scholar]
- 92.Packer M Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: the potential mediating influence of epicardial adipose tissue. Cardiovascular diabetology. 2019;18(1):121. Epub 2019/09/26. doi: 10.1186/s12933-019-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bairapareddy KC, Maiya AG, Kumar P, Nayak K, Guddattu V, Nayak V. Effect of aerobic exercise on echocardiographic epicardial adipose tissue thickness in overweight individuals. Diabetes Metab Syndr Obes. 2018;11:303–12. Epub 2018/06/29. doi: 10.2147/DMSO.S145862. [DOI] [PMC free article] [PubMed] [Google Scholar]


