Abstract
Although genomic analyses predict many non-canonical open reading frames (ORFs) in the human genome, it is unclear whether they encode biologically active proteins. Here, we experimentally interrogated 553 candidates selected from non-canonical ORF datasets. Of these, 57 induced viability defects when knocked out in human cancer cell lines. Upon ectopic expression, 257 showed evidence of protein expression and 401 induced gene expression changes. CRISPR tiling and start codon mutagenesis indicated that their biological effects required translation as opposed to RNA-mediated effects. We found that one of these ORFs, G029442 — renamed GREP1 (Glycine-Rich Extracellular Protein-1) — encodes a secreted protein highly expressed in breast cancer, and its knock-out in 263 cancer cell lines showed preferential essentiality in breast cancer–derived lines. The secretome of GREP1-expressing cells has an increased abundance of the oncogenic cytokine GDF15, and GDF15 supplementation mitigated the growth inhibitory effect of GREP1 knock-out. Our experiments suggest that non-canonical ORFs can express biologically active proteins that are potential therapeutic targets.
Keywords: Cancer, gene dependency, translatome, CRISPR, unannotated genes
Early analyses of the human genome sequence suggested the existence of 100,000 or more protein-coding genes, but further scrutiny revealed that the majority of those candidate genes were more likely producing non-coding RNAs, fragmented cDNA clones, or RNAs expressed at inconsequential levels1–3. The current Human Proteome Project NeXtProt database recognizes ~17,600 proteins confirmed by mass spectrometry and ~2,100 unconfirmed proteins4. Nevertheless, a growing body of evidence utilizing high-throughput profiling of ribosome-associated RNAs suggests that additional, non-canonical translation exists in genes currently annotated as noncoding RNAs or pseudogenes, as well as 5’ and 3’ untranslated regions (UTRs) of protein-coding genes5–8. Yet, it is unclear whether such translation reflects proteins overlooked during the construction of reference genome databases9–12, leaky ribosome scanning, or confounded computational predictions13–15, since stringent conservation-based analyses have added only a small number of new proteins to the human genome13. Indeed, systematic experimental evidence interrogating whether such predicted proteins are in fact stably translated and biologically functional is lacking.
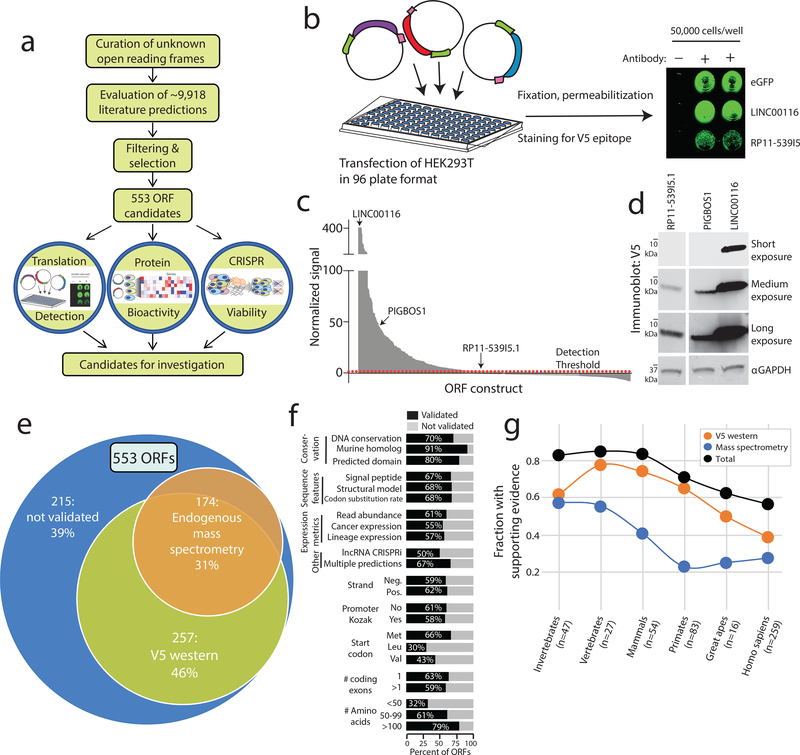
To address this, we curated a list of 553 high priority ORFs nominated in lncRNAs and regions upstream and downstream of known protein coding genes (uORFs and dORFs, respectively). These were selected based on integrative analyses of published predictions of ORF translation, with additional analyses to eliminate pseudogenes and ORFs representing variants of known protein coding regions5,6,14,16–33 (Supplementary Table 1, Supplementary Fig. 1 & 2, Methods). 227/553 (41%) were identified as translated by at least two independent studies. Overall, mass spectrometry and computational predictions contributed fewer candidates compared to ribosome profiling datasets (Supplementary Fig. 2). We annotated the 553 ORFs according to 12 metrics including evolutionary conservation, expression and structural features (Supplementary Tables 2–13, Supplementary Fig. 3, Methods). 450 of 553 selected ORFs (81%) scored highly for at least two metrics in support of relevance (Supplementary Fig. 1 and Supplementary Table 2).
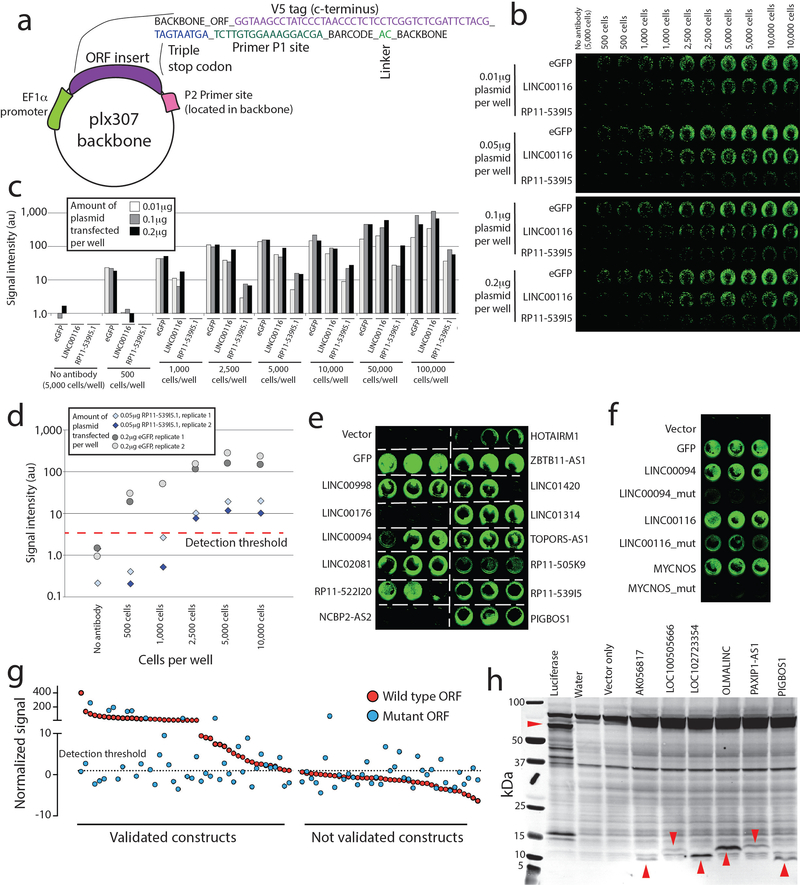
We next asked whether systematic functional studies could test the predicted translation of these ORFs (Fig. 1a). The capacity for the ORFs to produce a stably translated protein was assessed by three independent methods. First, we queried independent publicly-available mass spectrometry databases (Methods) and observed 707 distinct tryptic peptides supporting 174 of 553 ORFs (31%). Many tryptic peptides were reproducibly detected in numerous independent samples and datasets, for a total of 6,724 peptides identified (Supplementary Fig. 4, Supplementary Tables 14 & 15). Next, we designed an expression library of the 553 ORFs containing a V5 epitope tag and developed a scalable assay for individual protein evaluation by anti-V5 detection (Fig. 1b and Extended Data Figure 1a–d). 257 ORFs (46%) yielded a V5-tagged protein detectable by in-cell visualization (Fig. 1c–e, Extended Data Figure 1e–g and Supplementary Table 16). ORFs nominated through ribosome profiling, mass spectrometry, and bioinformatic approaches validated at similar rates (Supplementary Fig. 2). Lastly, we detected a protein for 10 of 30 ORFs tested by in vitro transcription and translation (Extended Data Figure 1h). Taken together, experimental evidence of protein translation was obtained for 334/553 (60%) of the ORFs. Translatability was associated with evolutionary conservation, with ancient ORFs being more likely to be translated compared to evolutionarily recent ORFs as determined by phylostratigraphy (p<0.001, two-way ANOVA, Fig. 1g, Supplementary Table 17). Pairwise analysis of combinations of ORF biological features highlighted conservation, size, and identification of a mass spectrometry peptide as the strongest predictors of V5-tagged ORF translation (Supplementary Fig. 5). ORFs predicted to encode proteins < 50 amino acids were less likely to yield a detectable protein, although this may be explained by the deleterious effect of fusing a 14-amino acid V5 tag to a very small protein. uORFs validated at a higher rate than lncRNA-derived ORFs, largely due to more frequent mass spectrometry evidence for small uORFs <50 amino acids, though this may be confounded by a small sample size of uORFs (n=18) (Supplementary Fig. 6).
Figure 1: Identification of translated unannotated or unstudied open reading frames.
a) A schematic overview of the research project. b) The experimental set-up for in vitro detection of protein translation by transfection of V5-tagged cDNAs into HEK293T cells followed by in-cell western blotting. c) In-cell western blot signal for each ORF. Values are the average of three replicates. d) Immunoblot correlates for three ORFs identified by in-cell western blotting, marked in panel c. Results were repeated in three independent experiments. e) An overview of biological support for translation of a subset of ORFs. f) Subgroup analyses of ORF biological features demonstrating fractions of ORFs supported by ectopic V5 translation assays, mass spectrometry or both. g) The fraction of ORFs supported by evidence of translation across major epochs in evolutionary time. Evidence of translation shown as the fraction of ORFs with V5 western blot signal, endogenous mass spectrometry peptides, and the summation of both.
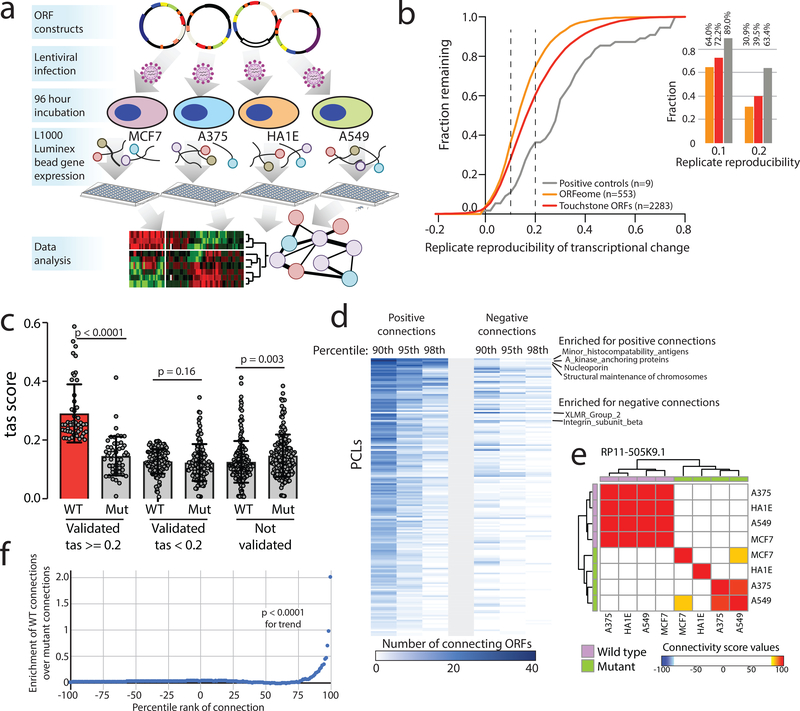
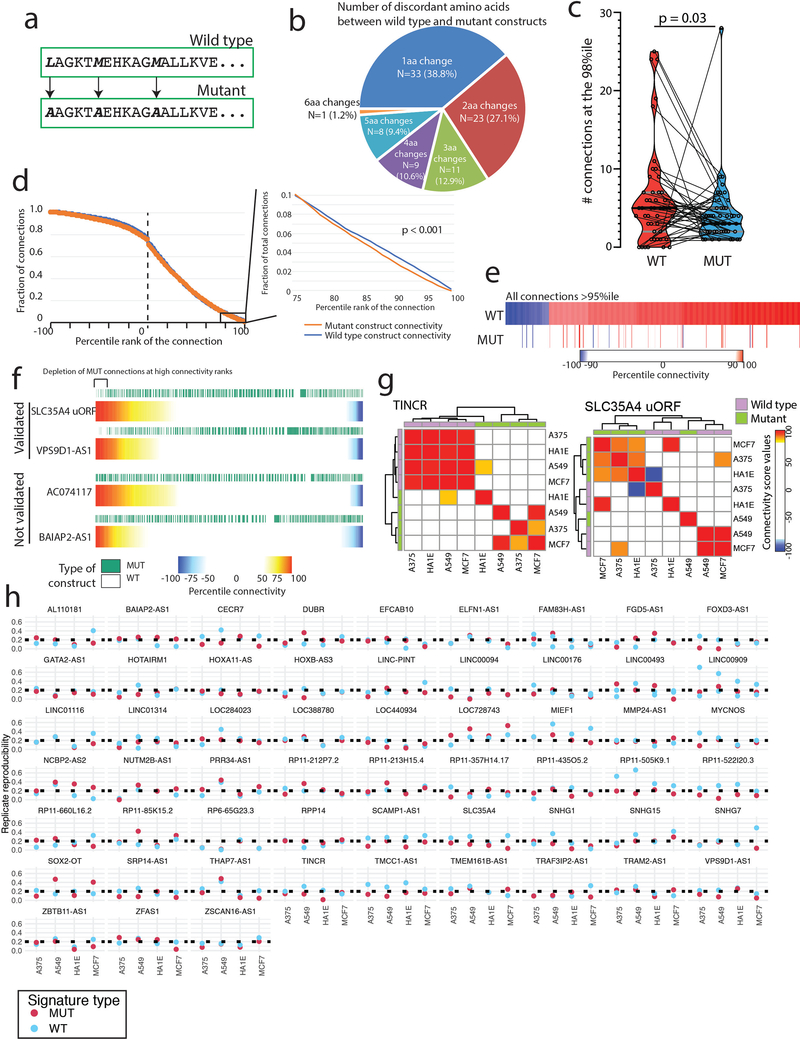
Since the majority of non-canonical ORFs have evidence of translatability, we next asked whether such translation was associated with biological activity. To address this, we expressed the 553 ORFs in each of four cell lines (MCF7, A549, A375, HA1E), and then performed RNA expression analysis using the L1000 platform34 (Fig. 2a), which monitors the expression of 978 mRNAs. Ectopic expression of 401 ORFs (73%) yielded a reproducible gene expression consequence, of which 237 ORFs induced a high transcriptional activation score (tas) indicating marked cellular changes34 (Fig. 2b, Supplementary Fig. 7 and Supplementary Table 18). In comparison, 81% of 2,283 canonical protein-coding genes yielded a gene expression consequence in this assay, indicating that the frequency of biological activity of known genes and unannotated ORFs is similar (Fig. 2b). To exclude the possibility that the observed transcriptional signature was due simply to overexpression of the RNA, we mutated translational start sites and repeated the L1000 profiling. In 48 of 51 (94%) cases, the perturbational response was lost when translation was prevented, indicating that the biological effect was indeed mediated by a protein, and not a non-coding RNA (Fig. 2c–f, Extended Data Figure 2, Supplementary Tables 19–20).
Figure 2: Defining bioactive ORFs through gene expression profiling.
a) A schematic showing the experimental set-up. Briefly, ORFs were individually transduced into 4 cell lines and expression was profiled 96 hours after infection using the L1000 platform. b) The fraction of ORFs resulting in transcriptional perturbation when overexpressed in 4 cell lines (A375, MCF7, HA1E, A549) compared to all profiled known genes and assay positive controls. Inset at the right, a barplot enumerating the percentage of ORFs in each group with a transcriptional signature above the indicated reproducibility threshold. c) A barplot showing the strength of transcriptional perturbation following expression of the indicated groups of wild-type or mutant ORF constructs. N for each pair of wild-type or mutant ORF data is indicated in the figure. P value by a two-sided Wilcoxon test. Error bars represent standard deviation. d) A heatmap showing the number of ORFs demonstrating positive or negative connections with individual Perturbational Classes (PCLs) at the indicated percentile rank. e) An example of RP11–505K9.1 showing the high concordance of connectivity signatures when the wild type ORF is expressed compared to the ORF with mutated translational start sites. f) Bland-Altman analysis demonstrating enrichment of high-ranking connectivity values following expression of wild type ORFs compared to mutant ORFs (N=19,012 for each). P value by a two-sided Wilcoxon test.
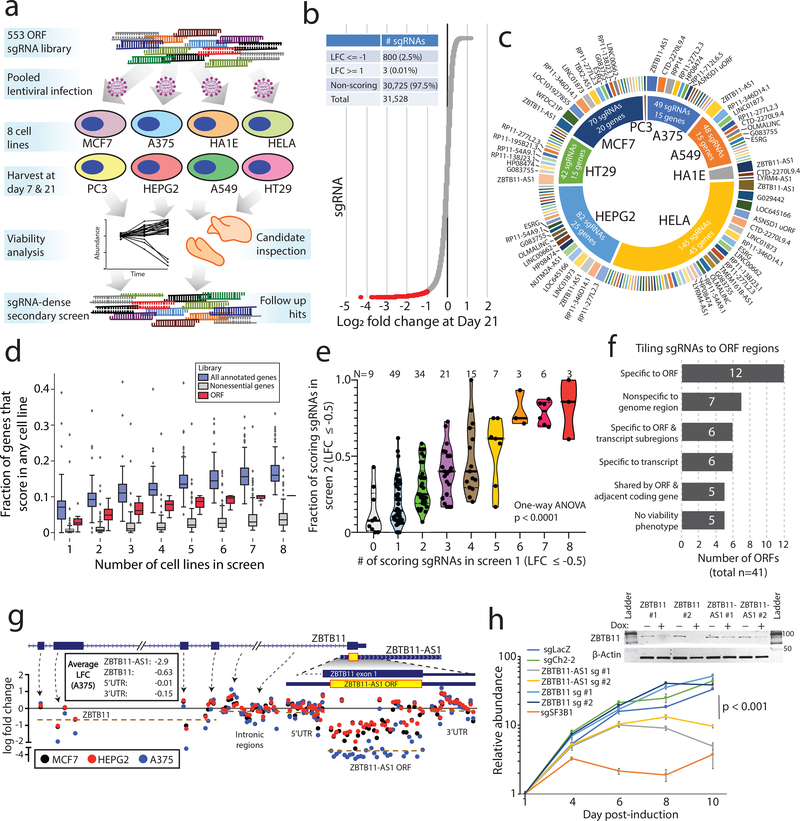
The transcriptional responses observed following ORF expression could conceivably be a consequence of overexpression of the transgene. To address the functional relevance of endogenous expression of these ORFs, we performed CRISPR/Cas9 loss-of-function viability screens in 8 cancer cell lines using a guide RNA library targeting the 553 ORFs (Fig. 3a, Supplementary Fig. 8a and Supplementary Table 21). Knock-out of 57 of the 553 ORFs (10%) demonstrated a growth inhibitory effect (Fig. 3b,c, Supplementary Tables 22–23). Of these, 31 (54%) impaired survival of all 8 cell lines, whereas 26 (46%) displayed selective dependency (Supplementary Fig. 8b–e).
Figure 3: CRISPR screening to identify unknown ORFs implicated in cancer cell viability.
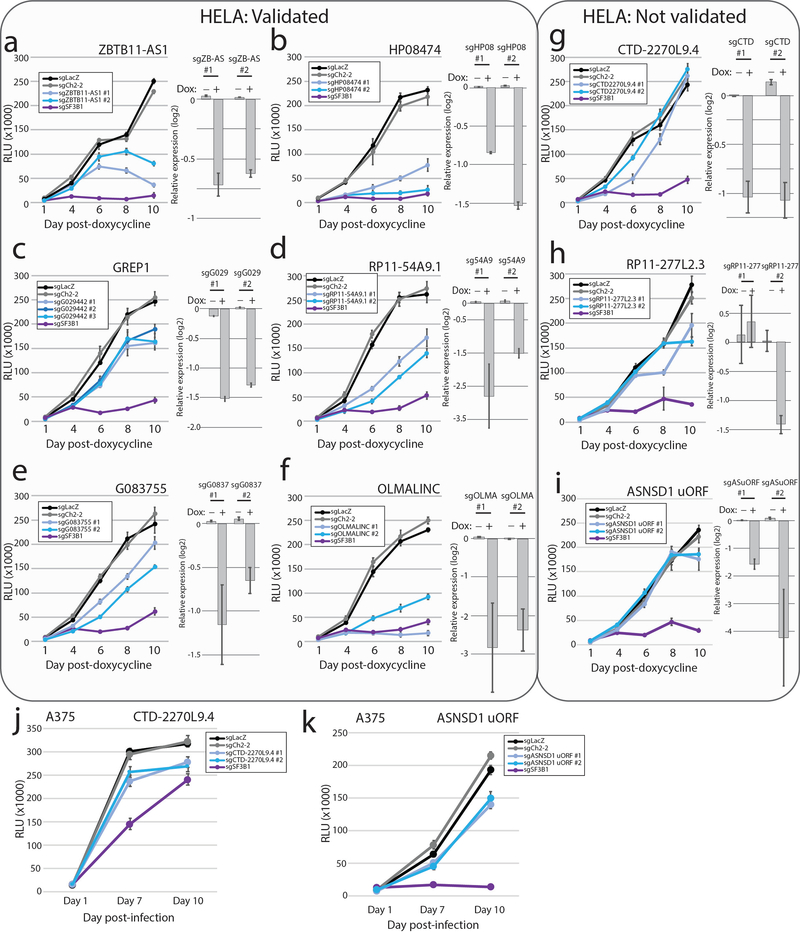
a) A schematic showing the experimental design, including a primary screen in 8 cancer cell lines and a secondary screen in 3 cancer cell lines. b) The distribution of sgRNA depletion at day +21 following lentiviral infection in the CRISPR screen across 8 cell lines. 2.5% of sgRNAs were identified as depleted in a particular cell line with a log2 fold change of <= −1. c) The distribution of nominated ORFs. For each cell line, the inner circle, the number of sgRNAs with a log2 fold change of <= −1, and the number of nominated genes are shown. The outer circle shows the ORFs nominated in that cell line, with the ORFs ranked by the number of supporting sgRNAs. The thickness of the outer circle boxes reflects the number of sgRNAs supporting that ORF’s nomination. Only ORFs nominated with >= 2 sgRNAs are shown. d) A boxplot showing the fraction of annotated genes, new ORF genes, and RNAi-defined nonessential genes that score as a vulnerability gene in the indicated number of cell lines. Each data point represents a unique cell line. The cell lines for ORF genes represent the cell lines used in this study. For annotated genes, the randomly selected cell lines from the Dependency Map were used. Box plots represent median with interquartile ranges (25% - 75%); the whiskers extend to the last data point up to 1.5x the interquartile distance from the box with individual data points shown beyond this range. e) The correlation between the number of sgRNAs producing a viability phenotype for a given ORF in the primary and the fraction of sgRNAs producing a viability phenotype in the secondary screen. The number of ORFs included in each group is indicated. P value by a one-way ANOVA. f) A barplot showing the number of ORFs with each category of viability phenotype in the tiling sgRNA CRISPR screens. g) An example of ZBTB11 and ZBTB11-AS1 for tiling CRISPR data, showing enhanced cell killing when the ZBTB11-AS1 ORF is knocked-out. Each data point represents a sgRNA. Data points are color-coded for the indicated cell lines. h) Individual CRISPR knockout experiments in a doxycycline-inducible Cas9 HeLa cell line using two sgRNAs targeting exclusively ZBTB11 or two sgRNAs targeting both the ZBTB11-AS1 ORF and ZBTB11. The line plot shows cell viability measured by cellular ATP following induction of Cas9 activity with 2ug/mL doxycycline. sgLacZ and sgCh2–2 are non-cutting and cutting negative controls, respectively, and sgSF3B1 is a pan-lethal positive control. N=6 technical replicates for each data point with two independent experiments performed. The inset western blot shows ZBTB11 protein abundance upon induction of Cas9. P value by a two-tailed Student’s t-test. Error bars represent standard deviation.
To compare these data to knock-out of canonical proteins, we analyzed the Cancer Dependency Map (www.depmap.org) for the viability effects of 553 randomly selected genes. Among canonical proteins, 17% demonstrated a viability effect in 8 randomly chosen cell lines, compared to approximately 10% for the non-canonical ORFs (Fig. 3d, Supplementary Fig. 8f–g), indicating that the frequency of dependencies between known genes and non-canonical ORFs is approximately on the same order of magnitude. These results were validated both in a secondary CRISPR screen of 147 ORFs (Fig. 3e, Supplementary Fig. 8h–i, Supplementary Tables 24–26), as well as individually-performed CRISPR assays for selected ORFs (Extended Data Figure 3 and Supplementary Table 29). Analyses for off-target effects of sgRNAs suggested that only 5 of the 57 CRISPR hits (RP11–138J23.1, RP11–346D14.1, LINC01873, LINC01184, RP11–277L2.3) were likely confounded by sgRNA cutting at unintended genomic loci (Supplementary Fig. 9, Supplementary Tables 30 & 31).
Because the viability effects from knock-out of non-canonical ORFs could be explained by loss of a regulatory region in the genome rather than the protein itself, we subjected 41 ORFs to dense tiling of sgRNAs across the genomic locus of each ORF. Only 7/41 (17%) genomic regions demonstrated non-specific viability loss suggestive of a regulatory region of the genome. For 18/41 ORFs (44%), the viability effect mapped exclusively to predicted coding exons or the coding region as well as adjacent nucleotides in the transcript, which may reflect sites of translational regulation or sgRNAs generating indels that also impact the ORF (Fig. 3f, Extended Data Figure 4, Supplementary Table 32). Further, there were 4/41 (10%) ORFs where the viability effect mapped exclusively to the predicted coding region, but a non-overlapping neighboring gene also demonstrated a viability effect upon knock-out (Extended Data Figure 4a,h).
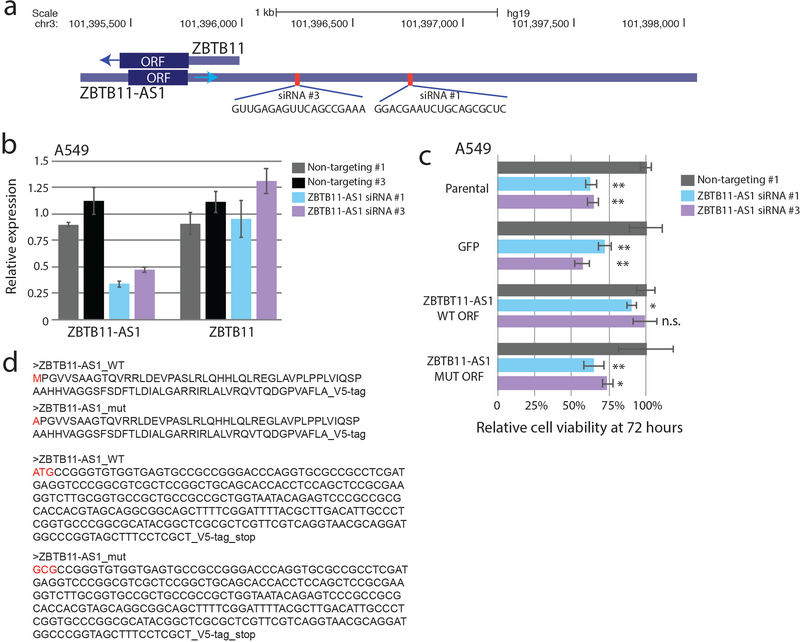
Interestingly, in several cases, a novel ORF overlapped with an annotated protein-coding gene, but it is the novel ORF that best explained the knock-out phenotype (Fig. 3g). As examples, we observed that ORFs arising from CTD-2270L9.4 and ZBTB11-AS1, which overlap coding exons of COG7 and ZBTB11, respectively, both demonstrated markedly more dramatic viability phenotypes using sgRNAs that target the novel ORF compared to adjacent sgRNAs that target only the known, parent ORF (Fig. 3g–h, Extended Data Figure 4b). These findings were supported by Cancer Dependency Map data in which sgRNAs targeting both the novel and the known ORFs had a more pronounced phenotype than sgRNAs targeting only the known ORF (Supplementary Fig. 10). For ZBTB11-AS1, we validated the specificity of this phenotype through exclusive siRNA knockdown of the ZBTB11-AS1 transcript, which was rescued by ectopic expression of a siRNA-resistant ZBTB11-AS1 ORF cDNA but not a mutant ZBTB11-AS1 cDNA removing the start codon (Extended Data Figure 5). Taken together, we conclude that a surprisingly high proportion of non-canonical ORFs exhibit a viability phenotype upon knock-out, and that prior CRISPR vulnerability screens may be confounded by cryptic, novel ORFs arising from the same genomic locus.
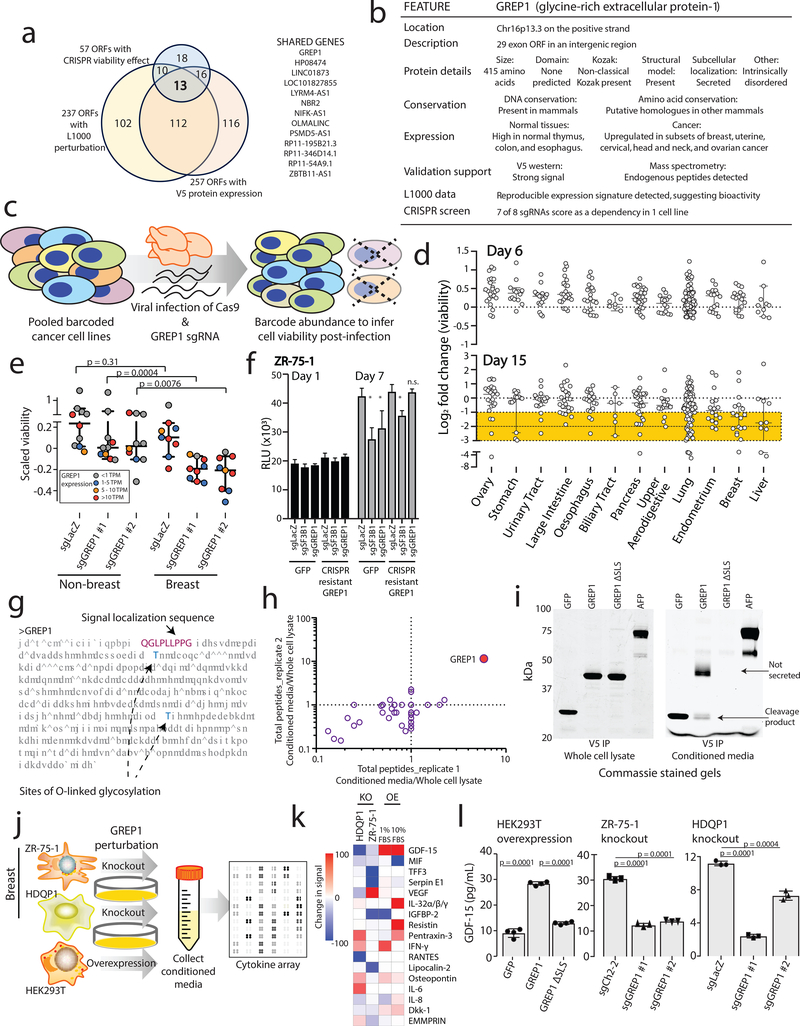
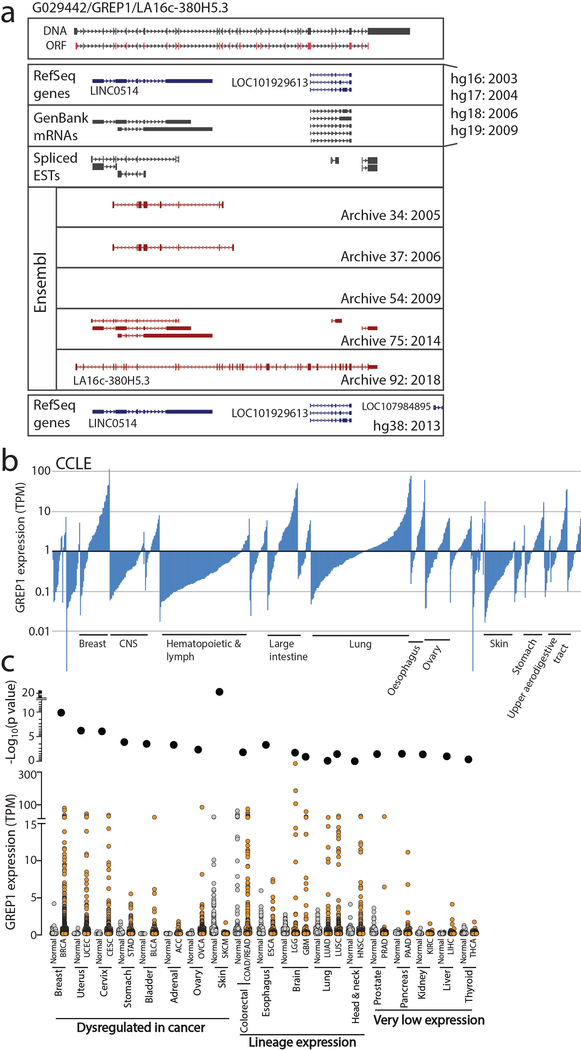
We next noted that 13 ORFs scored highly in all three high-throughput assays supporting translation, bioactivity and CRISPR vulnerability (Fig. 4a), suggesting that they may have particularly important biological roles. Among these, we especially focused on G029442 (LA16c-380H5.3 in GENCODE) because its knock-out resulted in selective cancer cell killing (1 of 8 cell lines killed), and it is highly expressed in several human cancer types (Fig. 4b and Extended Data Figure 6). We subsequently renamed this gene GREP1 (Glycine-Rich Extracellular Protein-1) for reasons elucidated below.
Figure 4: Characterizing GREP1 as a cancer dependency gene in breast cancer.
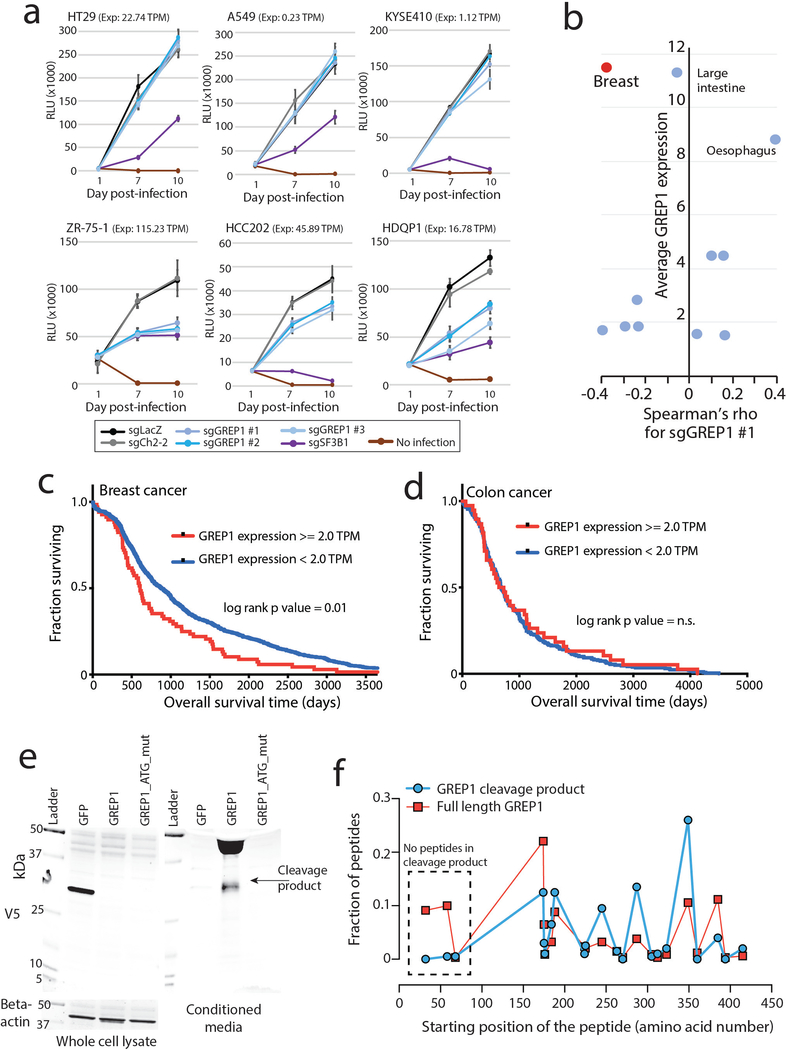
a) Nomination of candidate ORFs with evidence for protein translation, gene expression effect, and CRISPR phenotype. b) A table summarizing the characteristics of the GREP1 gene. c) A schematic showing the overview of pooled CRISPR screening. d) Log2 fold change abundance of cancer cell lines at Day 6 and Day 15 following pooled CRISPR screening. Cell lineages are ranked based on the median log2 fold change at Day 15. Each data point represents a unique cell line. e) Individual CRISPR validation experiments for GREP1 in a panel of non-breast (n=10) and breast (n=9) cell lines. Data are scaled so that 0 reflects the sgCh2–2 negative cutting control and −1 reflects the degree of viability loss from the sgSF3B1 positive control. Data were obtained 7 days after lentiviral infection. P value by a two-tailed Mann-Whitney test. f) Rescue of the CRISPR phenotype with overexpression of a CRISPR resistant GREP1 construct and not GFP. An asterisk (*) indicates a P value < 0.05. P values are as follows: for GFP cells, sgLacZ vs sgSF3B1 P = 0.0005, sgLacZ vs sgGREP1 P = 0.013; for GREP1 cells, sgLacZ vs sgSF3B1 P = 0.0005, sgLacZ vs sgGREP1 P = 0.08. P values by a two-tailed Student’s t-test. N=4 technical replicates per data point with two independent experiments performed. g) The GREP1 amino acid sequence with the signal localization sequence and the sites of glycosylation indicated. h) Immunoprecipitation followed by mass spectrometry of HEK293T conditioned media and whole cell lysate following ectopic expression of a pool of V5-tagged ORFs. The x and y axes represent the total number of MS peptides detected in two independent experiments. i) Expression of V5-tagged GREP1 or a truncated GREP1 lacking the N-terminal signal localization sequence in HEK293T cells. Cell lysates or conditioned media were subjected to V5 immunoprecipitation and then protein was visualized by Commassie stain. Two independent biological experiments performed. j) Experimental design for secreted cytokine profiling following GREP1 perturbation. k) A heatmap showing individual cell line changes in cytokine abundance following GREP1 perturbation. Cytokines are ranked according to the average of the absolute value of signal change for each cell line. l) Validation of GDF15 modulation upon GREP1 perturbation by ELISA in the indicated cell lines. N = 4 (HEK293T) or 3 (ZR-75–1, HDQP1) technical replicates per sample with either two (HDQP1) or three (ZR-75–1, HEK293T) independent experiments performed. P value by a two-tailed Student’s t-test. All error bars represent standard deviation.
To systematically explore the importance of GREP1 in cancer cell viability, we infected a pool of 486 barcoded human cancer cell lines with a single lentivirus harboring both Cas9 and a guide RNA targeting GREP1 (Fig. 4c, Methods). Because lentiviral infection rates vary across the cell lines, we focused our analysis on the 263 cell lines yielding highest quality data (Supplementary Fig. 11a–g, Supplementary Tables 33–34, Methods). GREP1 knockout resulted in preferential loss of viability in certain cell lineages, most notably breast cancer (Fig. 4d). We validated these pooled screening results with knock-out and rescue experiments for GREP1 in breast and non-breast cell lines, which confirmed a striking breast cancer viability phenotype that correlated with GREP1 mRNA expression (Fig. 4e–f, Extended Data Figure 7a,b, and Supplementary Fig. 11h). Sequencing of the GREP1 sgRNA genomic loci demonstrated an array of insertions, deletions, and substitutions at the expected genomic position, confirming sgRNA target specificity (Supplementary Fig. 12). Finally, GREP1 expression was higher in human breast cancers compared to normal breast tissue (p = 1.4 × 10−10) (Extended Data Figure 6c) and was associated with decreased patient survival in breast but not colon cancer patients (Extended Data Figure 7c,d). Together, these data implicate GREP1 as a previously unrecognized, prognostic breast cancer vulnerability gene.
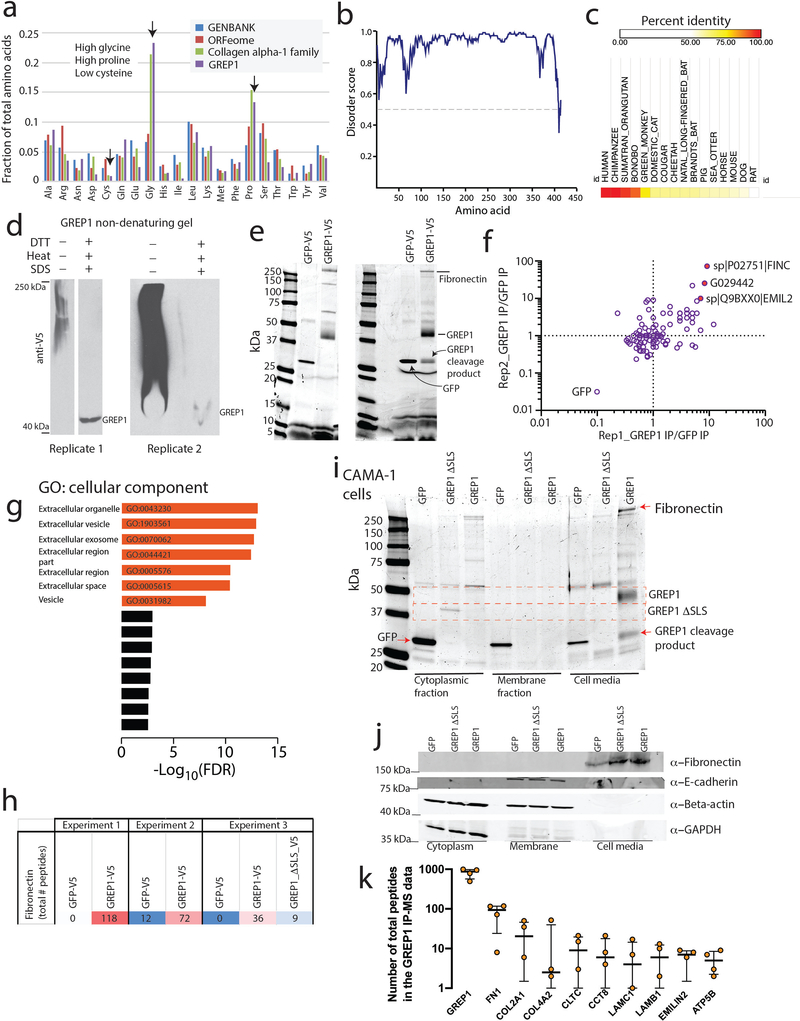
To explore the function of GREP1, we noted the presence of a signal localization sequence for extracellular secretion, as well as sites of glycosylation documented by mass spectrometry (Fig. 4g, Supplementary Table 35). We confirmed that ectopic expression of a GREP1 fusion protein with a C-terminus V5 epitope tag, but not an N-terminal truncation mutant lacking the signal localization sequence, was indeed secreted as well as cleaved into a smaller product (Fig. 4h–i, Extended Data Figure 7e–f, and Supplementary Table 36). Analyses of the GREP1 amino acid sequence revealed a conserved, glycine-rich, and intrinsically disordered protein (Extended Data Figure 8a–c), characteristics that resemble some members of the extracellular matrix35. As expected, immunoprecipitation of ectopically expressed GREP1 from cell culture media followed by mass spectrometry revealed a strong enrichment for extracellular matrix proteins, including fibronectin and collagen (Extended Data Figure 8d–k, Supplementary Table 37).
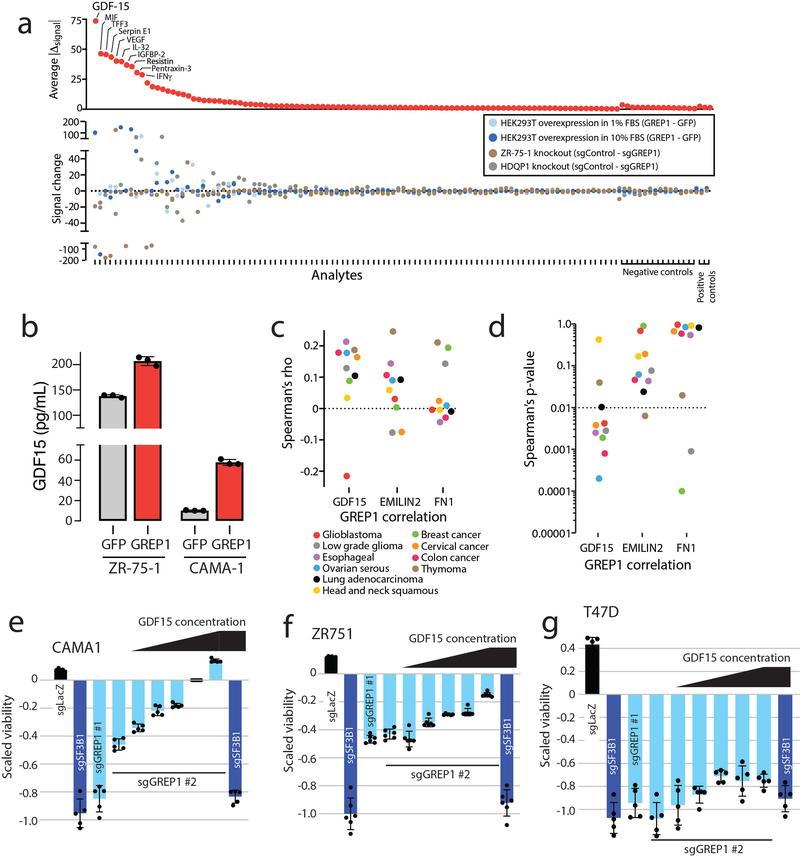
To establish the cellular consequence of GREP1 expression, we examined the impact of GREP1 knock-out and overexpression on other secreted proteins by testing a panel of 102 secreted proteins by antibody arrays across 3 cell lines (Fig. 4j). The metabolic cytokine GDF1536,37 demonstrated the most dramatic change, with GREP1 knockout resulting in decreased GDF15 levels and GREP1 overexpression resulting in increased GDF15 levels (Fig. 4k,l and Extended Data Figure 9a,b). Inducing non-specific cellular stress through pharmacologic treatment with toxic compounds did not increase GDF15 levels, indicating specificity (Supplementary Fig. 13a,b). In addition, impairment of GREP1 secretion through deletion of the signal localization sequence, but not mutation of the GREP1 glycosylation sites, prevented the increase in GDF15 secretion (Supplementary Fig. 13c,d). In human cancers, GREP1 and GDF15 expression were correlated across multiple tumor types in the TCGA database (Extended Data Figure 9c,d). To determine whether GDF15 was functionally important in cancer cells’ requirement of GREP1 for survival, we tested the effect of GREP1 knock-out in the presence and absence of recombinant GDF15. Remarkably, supplementation of recombinant human GDF15 rescued the loss of viability caused by GREP1 loss of function (Extended Data Figure 9e,f). The fact that GDF15 only partially rescues GREP1 knock-out in some cell lines suggests that there may be additional mechanisms downstream of GREP1 that regulate cell survival (Extended Data Figure 9g). While GDF15 has been previously implicated in a number of cancer phenotypes including chemoresistance38,39, immune evasion40, cellular survival and invasiveness41,42, its regulation by GREP1, which itself is a cancer dependency, is entirely new.
Despite the fact that the human genome was sequenced 18 years ago, the precise number of protein-coding genes in the genome remains a point of controversy. Our sampling of over 550 uncharacterized ORFs provides experimental evidence that a substantial proportion of such ORFs may be functional (Supplementary Fig. 14). Importantly, we establish that approximately 10% of the ORFs in our dataset were required for the survival of cancer cells, a rate only about half that observed for known, canonical proteins. Although our dataset represents a curated list of ORFs rather than a random sampling of all possible ORFs, these experiments suggest that further investigations of unannotated ORFs in cancer and other disease states will likely yield new insights. Since computational estimates of such ORFs now exceed 5,00043, our data suggest that a substantial number of those predicted ORFs may indeed encode functional proteins.
Consistent with this conclusion, a recent report by Chen et al. similarly suggests functional roles for a substantial fraction of non-canonical ORFs44. While a head-to-head comparison of the two datasets is difficult because they utilize different cell lines for functional analyses, the Chen et al. dataset identifies the existence of additional functional lincRNA-derived ORFs beyond those identified in our dataset (Supplementary Table 38). This result suggests that the functional ORFs discovered in our study do not represent the entirety of those encoded by the human genome; more functional ORFs likely remain to be discovered. Of note, whereas our study focused primarily on lncRNA-derived ORFs, Chen et al. also expand upon the possible functional importance of a subset of uORFs44. While the precise number of non-canonical ORFs encoded by the human genome remains to be determined, our work suggests that future systematic interrogation of non-canonical proteins is likely to yield a rich source of previously unrecognized proteins with key roles in development and disease.
Methods
Data statement:
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Cell lines and reagents:
All parental cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cas9-derived cell lines were obtained from the Broad Institute. Cell lines were maintained using standard media and conditions. In brief, cell lines derived from ZR-75–1, HCC1806, HCC1954, HCC202, T47D, HT29, HCC15, KYSE410, KYSE510, SNU503, SW837, HCT116, AU565, CAMA-1 and MDA-MB-231 were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA, Carlsbad, CA) supplemented with 10% FBS and 1% penicillin-streptomycin in a 5% CO2 cell culture incubator at 37°C. Cell lines derived from HDQP1, BT-474, JIMT1, A375, A549, MIAPACA2, MCF7, HEK293T, and MDA-MB-468 were maintained in DMEM supplemented with 10% FBS (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) in a 5% CO2 cell culture incubator. GFP-positive Cas9-derived cell lines were drug-selected using 2ug/mL blasticidin.
For stable knockout cell lines, ZR-75–1 Cas9 and HDQP1 Cas9 expressing cells were infected with lentivirus for the indicated sgRNAs which had been cloned into the LentiGuide-Puro plasmid (plasmid #52963, Addgene) with 4ug/mL of polybrene. 16 hours after transduction, cells were selected with cell culture media containing 2ug/mL of puromycin. Cells were maintained in puromycin-containing media for 72 hours before transitioned back to standard culture media. Stable GREP1-overexpressing cell lines were generated in ZR-75–1 and CAMA-1 cells by infecting with a sgRNA-resistant GREP1 cDNA construct and selecting with 350ug/mL of hygromycin for 96 hours, before transitioning back to standard culture media.
RNA isolation; cDNA synthesis; and qPCR experiments:
Total RNA was isolated using Qiazol and an miRNeasy Kit (Qiagen, Hilden, Germany) with DNase I digestion according to the manufacturer’s instructions. RNA integrity was verified on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA). cDNA was synthesized from total RNA using Superscript III (Invitrogen, Carlsbad, CA) and random primers (Invitrogen, Carlsbad, CA). Quantitative Real-time PCR (qPCR) was performed using Power SYBR Green Mastermix (Applied Biosystems, Foster City, CA) on a Thermo QStudio FLX Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). GAPDH was used the housekeeping control gene. The relative quantity of the target gene was completed for each sample using the ΔΔCt method by the comparing mean Ct of the gene to the average Ct of the geometric mean of the indicated housekeeping genes. The primer sequences are listed below:
GREP1 3’UTR-forward: AGCCTCCAAATGGCTATGGAC
GREP1 3’UTR-reverse: CTCGAGGCCACCATTAAAAC
GREP1 ORF-forward: CTGGATATCCGGCTGGAGATG
GREP1 ORF-reverse: ATTGCTGCCTCTCTTCACGTC
GAPDH-forward: TGCACCACCAACTGCTTAGC
GAPDH-reverse: GGCATGGACTGTGGTCATGAG
Beta-actin forward: AAGGCCAACCGCGAGAAG
Beta-actin reverse: ACAGCCTGGATAGCAACGTACA
Fibronectin forward: GAGAAAATGGCCAGATGATGA
Fibronectin reverse: AATGGCACCGAGATATTCCTT
Emilin2 forward: AACAAAGTGCTGGTGAACGAC
Emilin2 reverse: CTCTCCTGTACCCAGCGGTAT
ZBTB11-AS1 forward: CCGTTTTTACGTTTGAGACTCC
ZBTB11-AS1 reverse: ATGTAAATGGGCTGTCTCTGGT
ZBTB11 forward: GGAACGGGTGTGTGAAAAAT
ZBTB11 reverse: CAGCCCAAGCTACTCCACAT
HP08474 forward: GTGTAAAGAGGTCCTGGGACAG
HP08474 reverse: GCACTCCAGTCTAGACGACACA
RP11–54A9.1 forward: TTGGTGAGATGTTCCTTGAGC
RP11–54A9.1 reverse: CTCCACTTCACTGTCGGTCTC
G083755 forward: ATCCCATCTGAGTGCTTACCAA
G083755 reverse: CATGCATAATCTCCTTCCCTGC
OLMALINC forward: AGGAACATCTTGCCAATTTCA
OLMALINC reverse: TGTGGATCTTCAGTTGCTTCA
CTD-2270L9.4 forward: AGTCGTTGGCCGTTACCATA
CTD-2270L9.4 reverse: CTTCCCAGGCTCAAGCAAT
ASNSD1 uORF forward: ACAATTCGACCCCACACAAG
ASNSD1 uORF reverse: GGTTAGAAAGTTCATCCACCACA
RP11–277L2.3 forward: CTACGTGGGGCTGGAAATAC
RP11–277L2.3 reverse: CCCTTCCCAGTTCTCTGACC
GREP1_sgRNA1_amplicon_CRISPRSeq_Forward: GGCCTTAACCCTTTCTCTCCT
GREP1_sgRNA1_amplicon_CRISPRSeq_Reverse: ATCAAGGCGGGGTATGAATG
GREP1_sgRNA2_amplicon_CRISPRSeq_Forward: TTCTGGGGTGGATCTGAGTT
GREP1_sgRNA2_amplicon_CRISPRSeq_Reverse: CCCATTCCCATTCCCTAATC
Selection process for candidate ORFs:
Candidate ORFs were collated via manual curation from 25 published studies and one in-house analysis of ribosomal profiling data (Z. Ji, personal communication). Published studies are listed in Supplementary Table 1. Data types included were 14 studies with mass spectrometry data, 6 studies with ribosomal profiling data, 4 studies with computational ORF predictions, and 1 study with both mass spectrometry and ribosomal profiling data. In total, there were 9,918 candidate ORFs among which 4,433 unique Ensembl transcripts were represented.
We integrated the ORF nominations with mRNA expression data across the Cancer Cell Line Encyclopedia (CCLE). There were 6,305 ORFs arising from transcripts with expression >1 TPM (transcripts per million) with at least one cell line having >10 TPM. Because candidates nominated only from computational predictions were unlikely to have any ribosome profiling or mass spectrometry correlate (Supplementary Fig. 1 & 2), we only considered the 3,825 candidates that had either literature peptide support in mass spectrometry, ribosome profiling data, or both. Among this list, there were 917 annotated pseudogenes and 513 variants of known coding proteins (including N-terminal extension ORFs, ORFs of known proteins with new predicted exons, and alternative reading frame ORFs located entirely within the genomic nucleotides of an annotated protein); these were removed from consideration. For the remaining 2,395 ORFs arising from a putative noncoding RNA, we re-computed PhastCons scores, ribosome read abundance, PhyloCSF score, and protein domain scores as indicated below (Supplementary Tables 2 & 3). 553 high-priority ORFs were manually curated as candidates according to the criteria as described below. See Supplementary Figure 1 for an overview.
ORFeome library inclusion criteria
In order to be selected for the ORFeome library, an ORF had to exhibit at least one of the characteristics detailed below (Supplementary Tables 3 & 4). There were 669 of the 2,395 ORFs that exhibited two or more of the features. After manual inspection to eliminate overlapping candidates (e.g. isoforms or variants of the same ORF), we selected the longest ORF on each transcript for 353 of the 669 cases (53%). Of the 1,726 ORFs exhibiting only one feature, we eliminated overlapping candidates and manually inspected 1,018 cases to select 200 ORFs for inclusion in the ORFeome library. Below are details on the features: DNA conservation: An ORF was considered to have high DNA conservation if the average PhastCons score (version hg19_20110909) for 100 placental mammals was ≥0.20 for the entire ORF. 677 ORFs meeting this metric were manually inspected, filtered for overlapping predictions or multiple predictions on the same mRNA. 172 of the 677 ORFs (26%) were included in the ORFeome library.
Codon substitution rate:
ORFs were stratified is they had a codon PhyloCSF decibans score (29 mammal alignment) of ≥ 5.0 averaged across the whole ORF. 74 ORFs meeting this metric were manually inspected, filtered for overlapping predictions or multiple predictions on the same mRNA. 19 of 74 ORFs (26%) were included in the ORFeome library.
High read coverage:
Ribosome profiling read abundance data for ORFs identified by Ji et al.6 was used along with in-house analyses (Z. Ji, personal communication). ORFs were stratified if they had a read/length ratio of ≥1.0 in available ribosomal profiling data. 2,136 ORFs meeting this metric were manually inspected, filtered for overlapping predictions or multiple predictions on the same mRNA. 203 ORFs (9%) were included in the ORFeome library.
Protein domain structure:
We used the Pfam web server (http://pfam.xfam.org/search#tabview=tab1) to identify peptide sequences that harbored a putative Pfam domain (including both Pfam-A and Pfam-B), and used the default cutoff E-value <1. In addition, ORF amino acid sequences were also input into the NCBI Conserved Domain finder (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) with default settings to identify putative domains. ORFs with domain structures scoring at an e value confidence score of < 0.01 were considered. 195 ORFs meeting these criteria were then manually inspected and filtered for overlapping predictions. 88 of 195 ORFs (45%) were included in the ORFeome.
Multiple overlapping ORF predictions:
Published ORF predictions from 25 large datasets were integrated5,6,16,18–22,25–29,31,33 and queried for overlapping ORF predictions with at least 2 publications supporting their existence (Supplementary Tables 1–3). Of 643 candidates, we manually inspected and removed overlapping nominations or multiple isoforms of one gene. We included 227 of 643 ORFs (35%) in the ORFeome library.
Cancer expression:
We analyzed a dataset from Iyer et al.22 that identified 980 transcripts of unknown coding potential (TUCP) defined by a CPAT coding score of >0.5 and a statistical enrichment for human cancer tissue expression compared to benign tissue (n=707) or cancer lineage expression compared to other cancer types if no benign tissue was available (n=273). Of these, 437 (45%) exhibited an expression level of >= 1 TPM in one of the cell lines being used for CRISPR knock-out studies.
Lineage association:
ORFs were searched in the NIH Roadmap Epigenome Project data45, which transcriptionally profiled human embryonic stem cells before and after differentiation into mesenchymal stem cells, neural progenitor cells, trophoblast-like cells, or meso-endoderm. 243 transcripts were nominated, of which 123 (50%) harbored an ORF nomination and were included in the ORFeome.
Upstream and downstream ORFs:
We used candidates from Ji et al.6 and considered conserved upstream and downstream ORFs between mouse and human, as defined by an inter-species alignment with an E value of < 0.0001. We evaluated ORFs with all of the following attributes: a Ka/Ks conservation ratio of < 0.5, an ORF length of ≥25 amino acids, an ATG start site, a predicted higher translational efficiency compared to the annotated protein residing on the same mRNA, and the ORF was non-overlapping with the annotated ORF. 49 dORFs and 195 uORFs met these criteria. These were manually reviewed to select candidates included in the ORFeome.
Additional sub-analyses performed on the selected 553 ORF candidates were:
Murine homolog: Murine homologs were defined by the Slncky program46.
Cancer function association: ORFs were searched in the PubMed database for associations with the word “cancer” and screened for those studies implicating potential roles in cancer.
Predicted ORF CRISPR phenotype: Data from a CRISPR interference screen of lncRNAs were employed47. Of 492 lncRNA hits nominated in that study, there were 312 hits with GENCODE identifiers that could be further evaluated. Of those 312, there were 292 unique GENCODE identifiers, which were manually reviewed. 52 GENCODE identifiers overlapping ORFs in this ORFeome are indicated.
Signal peptide: All ORFeome ORFs were analyzed by SignalP version 4.1 using standard default settings48 and a D-score of ≥0.450 to nominate ORFs with a classical signal localization sequence.
Structural modeling: All ORFeome ORFs that are ≥40 amino acids were analyzed by the Phyre2 structural domain prediction software using default settings49. To distinguish ORFs enriched for structural models, we generated a random amino acid sequence library of 500 random 150-mer amino acid sequences with a methionine start codon. We derived a structural model score of (%ORF alignment to the structural model) * (%confidence of the model). A structural model score of 0.175 was used to maximally differentiate ORFeome ORFs from random amino acid sequences. 145 ORFs were classified as having a robust structural prediction score.
Overall ORF confidence score: Each criteria as above in addition to mass spectrometry peptide evidence (see below) was given a binary score of 1 if the criterion was met by the ORF or 0 if not met by the ORF. The ORF confidence score was the summation of these binary scores.
Identification of small open reading frames in proteomics datasets:
A fasta database containing the amino acid sequences of the 553 ORFs was appended to a reference protein database (UCSC RefSeq) and used to search peptide mass spectra of datasets acquired or analyzed in our laboratory. These datasets predominantly comprised of studies conducted by the Clinical Proteomics Tumor Analysis Consortium (CPTAC) (Supplementary Table 14). Raw mass spectrometry (MS) data were analyzed in Spectrum Mill MS Proteomics Workbench v6.0 (Agilent Technologies, Santa Clara, CA) employing search parameters specific for each project. Detailed descriptions of search parameters such as enzyme definition and specificity or the number of types variable modifications included in database search can be found in the corresponding publications (Supplementary Table 14). Peptide-spectrum matches (PSMs) to the ORF database were identified by automatically parsing through database search results generated by Spectrum Mill Software using an in-house developed R-script. Only PSMs validated by target-decoy based false-discovery (FDR) estimation were used for subsequent analysis. To further minimize the possibility of false positive identifications, we required a minimal Spectrum Mill PSM score of 8 which roughly translates to a minimum of eight identified fragment ions in the MS/MS spectrum. All PSMs meeting the criteria described above are listed in Supplementary Table 14.
Phylostratigraphy analysis:
All ORFs with an amino acid length of >= 40 amino acids was analyzed as described previously50,51, using TimeTree52 (http://www.timetree.org) to identify evolutionary strata. Using a BLASTP e-value threshold of 10−3 and a maximum number of 200,000 hits, we identified the phylostratum in which each ORF appeared. For clarity, we aggregated results into the following evolutionary eras: Invertebrates (phylostrata 1–9, including cellular organisms through Craniata, ~540 millions of years ago (Mya)), Vertebrates (phylostrata 10–17, including Vertebrata through Amniota (312 Mya)), Mammals (phylostrata 18 – 22, including Mammalia through Euarchontoglires (95 Mya)), Primates (phylostrata 23–27, including Primates through Hominoidae (20 Mya)), Great apes (phylostrata 28–30, including Hominidae through Homo), and Humans (phylostratum 31, including Homo sapiens).
Generation of the ORFeome library:
Initial prototype plasmids were generated in the pLX_TRC307 vector backbone designed for prior ORF studies53, obtained from the Broad Institute Genomic Perturbation Platform (Broad Institute, Cambridge, MA, USA), by PCR-amplification from cell line cDNA (HeLa, HEK293T, K562, or MCF7). PCR products were gel-purified (Qiagen, Hilden, Germany), cloned into the non-directional Gateway PCR8 vector (Invitrogen, Carlsbad, CA) as an entry vector, and shuttling to pLX_TRC307 using LR clonase II (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. pLX_307 is a Gateway-compatible expression vector where E1a is the promoter of the ORF and SV40 is the puromycin resistance gene (details available at https://portals.broadinstitute.org/gpp/public/resources/protocols). Following technical optimization of the insert sequence to include a barcode sequence following the V5 tag, the final ORF construct design is as follows: vector backbone -> ORF sequence lacking stop codon -> c-terminus V5 sequence (GGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACG) -> Triple stop codon (TAGTAATGA) -> P1 primer site (TCTTGTGGAAAGGACGA) -> Barcode sequence -> AC (linker sequence) -> vector backbone.
Following the ORF sequence, each construct therefore had the additional sequence:
GGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGTAGTAATGATCTTGTGGAAAGGACGA_BARCODE_AC
The ORFeome library was then generated via insert synthesis and cloning of unique plasmid inserts consisting unique barcodes (Supplementary Table 2) by a commercial vendor (GenScript, Piscataway, NJ) in arrayed barcoded tube format. Each plasmid therefore had a barcode sequence flanked by common PCR primer pair for amplification of a 233bp amplicon, where the sense primer was located in the ORF insert and the antisense primer was located in the plasmid backbone as follows:
P1 Sense primer: TCTTGTGGAAAGGACGA
P2 Antisense primer: TTAAAGCAGCGTATCCACATAGCGT
Generation of paired mutant ORFs:
The 85 mutant constructs employed and identical plasmid insert construct as detailed above with the following modifications: the putative ORF start codon was mutated to GCG (encoding alanine), and all internal in-frame ATG codons (encoding methionine) were mutated to GCG to reduce the chance of internal initiation of translation. Constructs were generated via commercial gene synthesis (GenScript, Piscataway, NJ).
In cell western blotting:
HEK293-T cells were plated at a density of 20,000 cells per well in a 96 well black plate format to minimize autofluorescence. 6 to 8 hours after plating, cells were transiently transfected with 0.1 ug of an individual plasmid with Fugene HD reagent (Promega, Madison, WI). 48 hours later, cell culture media was removed, and cells were fixed for 20 minutes with 150 uL of 3.7% formaldehyde solution in 1x phosphate-buffered saline at room temperature with no shaking. Fixing buffer was removed and cells were washed five times with 200 uL PBS containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for permeabilization. Following this, cells were blocked with 150uL of Odyssey Blocking Buffer (LI-COR, Lincoln, NE) for 90 minutes at room temperature on a plate shaker. Cells were then treated with anti-V5 antibody at 1:200 concentration in Odyssey Blocking Buffer or no-antibody control wells. Cells were incubated with the primary antibody overnight at 4°C. The next day, the primary antibody was removed and cells were washed five times with 200uL PBS containing 0.1% Triton X-100 as above. Then, 50uL secondary antibody was applied at 1:1000 dilution and samples were incubated for 1 hour with gentle shaking and protection from light. Afterwards, wells were washed five times with 200uL PBS containing 0.1% Tween20 (Sigma-Aldrich, St. Louis, MO). After the final wash, plates were blotted on tissue paper to remove excess wash buffer and immediately scanned on a LI-COR Odyssey system using the 800nm light channel to image and quantify anti-V5 abundance.
Analysis of in cell western data:
First, a preliminary dilution series was performed with decreasing amounts of transfected plasmid and decreasing numbers of HEK293T cells plated per well (Extended Data Figure 1). This was performed for two high-expressing plasmids that were verified by western blot (eGFP and LINC00116), and one low-expressing verified plasmid (RP11–539I5.1). Using eGFP and RP11–539I5.1 we defined a dynamic range for the assay (Extended Data Figure. 1) by normalizing V5 800nm light signal to the plate background. This defined a threshold above which signal was reproducibly detected even in low-expressing plasmids when transfected into 1,000 plated HEK293T cells.
Then, for the full ORFeome library, all plasmids were run in biological triplicate on 3 unique 96 well plates for in cell western analysis. Each plate was normalized by median-centering raw 800nm signals within each plate to minimize variance in plate background. Normalized 800nm signals were then averaged across replicates. Plasmids with averaged signal above the previously defined threshold based on RP11–539I5.1 expression were considered to generate a protein by V5 tag detection.
In vitro transcription/translation:
50 ORFs were selected at random from the ORFeome library for synthesis of the ORF insert lacking a V5 tag and containing a 5’ T7 promoter sequence. This tag-free insert was cloned into pUC57 plasmid. 1.0 mcg of linearized purified plasmid were subjected to wheat germ extract in vitro transcription/translation systems employing the non-radioactive Transcend tRNA system according to manufacturer’s instructions (Promega, Madison, WI). 10 of 50uL from the reaction volume was then heat-denatured in the presence of DTT and protein bands were detected by SDS-PAGE gel electrophoresis using a Tris-Glycine 10–20% gel (Thermo Fisher Scientific, Waltham, MA).
Immunoblot Analysis:
Cells were lysed in RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO) and allowed to homogenize on ice for 30 minutes after lysis. Cell debris was removed by centrifugation for 15 minutes at 13,200 RPM and the debris pellet was discarded. 1x HALT protease inhibitor (Thermo Fisher Scientific, Waltham, MA) was added to lysate supernatants. Protein abundance was quantified by the bicinchoninic acid (BCA) method using and bovine-specific albumin standard curve for normalization of protein abundance. Aliquots of each protein extract were boiled in LDS sample buffer, size fractionated by SDS-PAGE at 4°C by Tris-Glycine 10–20% gels, and transferred onto nitrocellulose membranes with pre-cast gels via the iBlot-2 system (Thermo Fisher Scientific, Waltham, MA). The membrane was then incubated at room temperature for 1–2 hours in LICOR Odyssey blocking buffer and incubated at 4°C with the appropriate antibody overnight. Following incubation, the blot was washed 4 times with 1x TBS with 0.1% Tween20 and incubated with fluorophore-specific IRDye secondary antibodies (LI-COR, Lincoln, NE) and imaged on a LI-COR Odyssey machine.
For conditioned media western blots, conditioned media of GFP- or GREP1-expressing HEK293T cells was concentrated using 3kDa size exclusion filter tubes (Millipore, Burlington, MA) by a factor of 5-fold. Then, 1x HALT protease inhibitor was added to the sample. Samples were kept at 4°C and not frozen to preserve protein fidelity. Western blots were then performed as detailed above. Uncropped and unprocessed scans of relevant western blots are included in the Supplementary Materials as Immunoblot Figures 1–6.
Antibodies used:
| Antibody | Species | Monoclonal/Polyclonal | Dilution | Catalogue Number | Vendor | Conditions |
|---|---|---|---|---|---|---|
| V5 (D3H8Q) | Rabbit | Monoclonal | 1:2000 | 13202S | Cell Signaling Technology | 4C overnight |
| ZBTB11 | Rabbit | Polyclonal | 1:1000 | A303–240A-M | Bethyl Laboratories | 4C overnight |
| Beta-Actin | Mouse | Monoclonal | 1:4000 | A5316 | Sigma-Aldrich | 4C overnight |
| Goat anti-mouse secondary | Goat | N/A | 1:5000 | 926–32210 | LI-COR | 20C for 1 hour |
| Goat anti-rabbit secondary | Goat | N/A | 1:5000 | 926–68021 | LI-COR | 20C for 1 hour |
| Goat anti-rabbit HRP | Goat | N/A | 1:10000 | 7074S | Cell Signaling Technology | 20C for 1 hour |
Non-denaturing western blot:
Non-denaturing western analysis was performed using the NativePage system (Thermo Fisher Scientific, Waltham, MA). In brief, HEK293T cells were transfected with plasmid encoding GREP1. 72 hours after transfection, conditioned media was collected and cellular debris was removed via centrifugation and filtering of the media. Protease inhibitor was added to the conditioned media for preservation. Conditioned media was then prepared with 4x NativePAGE sample buffer without heat, detergents, or reducing agents. For comparison, conditioned media was also prepared using 4x NativePAGE sample buffer and also 1% SDS and 10% NuPAGE sample reducing agent (Thermo Fisher Scientific, Waltham, MA) followed by boiling at 95°C for 5 minutes. Samples were then run on a NativePAGE Novex Bis-Tris gel using NativePAGE running buffer and NativePAGE 20x Cathode Buffer according to manufacturer’s instructions. Proteins were transferred to a PVDF membrane after membrane activation with isopropanol using a semi-dry system of 7V for 30 minutes at room temperature. After blocking for 1 hour at room temperature in Odyssey Blocking Buffer, membranes were treated with rabbit anti-V5 antibody at a 1:2000 dilution (Clone D3H8Q, #13202S, Cell Signaling Technology, Danvers, MA) overnight at 4°C, then washed 4 times in 1x TBS-Tween, and treated with goat anti-rabbit HRP secondary antibody at a 1:10000 dilution (Cell Signaling, #7074S). Chemilluminence was achieved with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, MA), and images were developed with CareStream Kodak BioMax light film (Kodak, Rochester, NY).
Lentivirus production for L1000 experiments:
Complete details of standard virus production pipelines can be found at the Broad Institute Genetic Perturbation Platform website https://portals.broadinstitute.org/gpp/public/.
Virus was produced in arrayed 96 well plates via triple transfection of HEK293T cells with each packaging vector (100 ng), envelope plasmid (10 ng), and each respective pLX317 plasmid (100 ng). Lentiviral-containing supernatants were harvested at 32–56 hours post-transfection and stored in polypropylene plates at −80°C until use.
Cell lines and lentiviral transduction for L1000 expression profiling:
A549 and A375 cells were cultured in RPM1 media supplemented with 10% FBS and 1% penicillin/streptomycin. MCF7 and HA1E cells were cultured in DMEM media supplemented with 10% FBS and 1% penicillin/streptomycin. To perform L1000 HT gene expression profiling, cells were robotically seeded (40uL per well) into 384 well plates. Optimized seeding densities were 250 cells per well (MCF7), 400 cells per well (A549, A375 and HA1E). Twenty-four hours post-seeding cells were spin-infected in the presence of polybrene (4 ug/mL for A549 and HA1E and 8 ug/mL for MCF7 and A375 cells). The plates were then centrifuged for 30 minutes at 1,178 g at 37°C. The supernatant was robotically removed and replaced with fresh media 3 hours (A549) or 24 hours post-infection (A375, MCF7, HA1E) and cells cultured for an additional 72 hours till assay.
Infections were carried out in 5 replicates, 3 of which were used for L1000 assay and 2 used for assessing the infection efficiency. To assess infection efficiency, cells were treated with or without puromycin selection (1.5 ug/mL) 24 hours post-infection, and cell viability was determined using CellTiterGlo (Promega, Madison, WI) after 72 hours of selection. For the remaining plates, media was removed 96 hours post-infection, and the cells were lysed with the addition of TCL buffer (Qiagen, Hilden, Germany). Plates were then sealed and stored at −80 °C until gene expression profiling.
L1000 experimental design:
Two 384 well plates of perturbational ORFs were designed for cell treatment prior to L1000 profiling, each containing 352 unique ORFs, negative control ORFs, internal technical controls, and untreated wells. Plate format can be found in Supplementary Fig. 7. In each plate, 346 wells were devoted to treatment ORFs, and ten to ORFs targeting L1000 landmark genes were included for positive control purposes. These positive control wells would later be assessed for targeted gene z-score (≥ 2) and targeted gene rank (computed relative to the expression levels of that same gene across the assay plate). Control genes included were ACAA1, ACD, AURKB, BMP4, CBR1, CCDC90A, CDK6, CSNK1A1, ETV1, and SOX2. Genes were selected for overall for high baseline expression levels in the lines profiled and previous reproducibility in the L1000 assay. Additionally, 16 wells of negative control ORFs targeting BFP, EGFP, or HCRED were added. Each plate also contained 12 untreated wells.
Cell lines MCF7, HA1E, A549, and A375 were chosen to represent a diversity of tissue types and also to match CMap cell lines that had been profiled extensively in the past and were constituents of the CMap reference database Touchstone34.
L1000 data processing:
Detailed protocols for the L1000 assay are provided at https://clue.io/sop-L1000.pdf. Each plate was profiled 96 hours after infection. Antibiotic selection was not employed, and each plate was processed using the standard L1000 data processing pipeline which has been described elsewhere34. Briefly, mRNA was initially captured using 384-well oligo dT-coated Turbocapture plates; after removing lysate and adding a reverse-transcription mix containing MLLV, the plate was washed and a mixture of both upstream and downstream probes (each containing a gene-specific sequence and a universal primer site) for each of the 978 (“Landmark”) genes measured was added. The probes were first annealed to cDNA over a six hour period, and then ligated together to form a PCR template. After ligation, Hot Start Taq and universal primers were added to the plate, the upstream primer was biotinylated to allow for later staining with streptavidin-pycoerythrin, and the PCR amplicon was hybridized to Luminex microbeads using the complementary and probe-specific barcode on each bead. After overnight hybridization, the beads were washed and stained with streptavidin-pycoerythrin and Luminex FlexMap 3D scanners were used to measure each bead independently, reporting bead colour, identity, and fluorescence intensity of the stain. Fluorescence intensity of the stain values were then converted into median intensity values for each of the 978 measured genes using a deconvolution algorithm (resulting in “GEX” level data). These GEX data were then normalized relative to a set of invariant genes, and subsequently quantile normalized (resulting in “QNORM”) level data. An inference model was applied to the QNORM data to infer gene expression changes for a total of 10,174 genes, which corresponds to the “BING” (Best INferred Genes) genes we report below. Next, expression values of each individual well was converted to robust z-scores by z-scoring gene expression relative to corresponding expression across the entire plate population; these z-scored differential expression gene signatures were lastly replicate collapsed to a single differential expression vector per treatment, which we term a signature (and “MODZ” level data).
L1000 quality control:
All samples profiled passed internal technical L1000 assay quality control measures described elsewhere34. Additionally, all samples included passed an internal fingerprinting algorithm that verifies the identity of cell lines on L1000 plates by comparing quantile-normalized gene expression data in each will to a ranked reference library of over 1000 cancer cell lines; samples are defined as passing if their Spearman correlation to their respective reference profile is higher than equivalent correlation values to all other reference cancer profiles. Additionally, 67% of positive control ORFs included had a replicate correlation of 0.25 or greater and induced a z-score of 2 or greater in their target gene. Notably, ORFs targeting CNSK1A1 represented the majority of poorly performing positive control ORFs. Positive control ORFs that showed high transcriptional activity (TAS) also clustered together (Supplementary Fig. 7c).
Measures of L1000 signature bioactivity:
Each perturbagen’s transcriptional activity was represented using a Transcriptional Activity Score (TAS), which has been described in depth elsewhere34. Briefly, TAS is computed as a geometric mean of signature strength (SS; or, the number of landmark (n=978) genes in a signature with absolute z-score greater than or equal to 2) and replicate correlation (RC; or, the 75th quantile of all pairwise Spearman correlations between replicate level z-score profiles): )). Signatures were considered to be bioactive if they had a TAS score of 0.2 or higher, which represents the value at which 95% of negative control wells fall below34.
L1000 signature queries:
Each MODZ-level signature profiled was queried both against the other L1000 signatures in the dataset and against the Connectivity Map dataset that has been published and described elsewhere34. Similarity values between these signatures was assessed using a percentile score derived from a normalized weighted connectivity score (WTCS). Briefly, WTCS is a similarity measure based on the weighted Kolmogorov-Smirnov enrichment statistic (ES) described previously54 and is computed as follows for a given query gene set pair (q_up, q_down) and a reference signature r:
Where ES_up is the enrichment of q_up in r and ES_down is the enrichment of q_down in r. WTCS ranges between −1 and 1, and is positive for signatures that are positively related, negative for the converse, and near zero for unrelated signatures.
WTCS is then normalized to allow for comparison of connectivity scores across cell and perturbagen types; this normalization is similar to that used in Gene Set Enrichment Analysis and accounts for differences in connectivity that may occur across such covariates. Given a vector of WTCS values from a query, normalization occurs as follows:
Where NCSc,t, wc,t, U+c,t, U−c,t are the normalized connectivity scores, raw WTCS, and signed means (the mean of the positive and negative values evaluated separately) of the WTCS values within the subset of signatures corresponding to cell line c and perturbagen type t, respectively.
Lastly, NCS scores are converted to percentile scores accounting for whether the connectivity between the queried (“q”) and reference signature (“r”) are significantly different from that observed between r and other queries. This is done by comparing each observed NCS value ncsq,r between the query q and a reference signature r to a distribution of NCS values representing the similarities between a reference compendium of queries (Qref) and r. This procedure results in a standardized measure we refer to as Tau (τ) that ranges from −100 to +100 and represents the percentage of queries in Qref with a lower |NCS| than |ncsq,r|, adjusted to retain the sign of ncsq,r and relies on the following formula:
Where ncsq,r is the normalized connectivity score for signature r w.r.t query q, ncsi,r is the normalized connectivity score for signature r relative to the i-th query in Qref (a set of query signatures obtained from exemplar signatures of perturbagens matching the cell line and perturbagen type of signature r) and N is the number of queries in Qref.
L1000 software packages used:
L1000 data were analysed using the ‘tidyverse’ suite55 of R packages (v1.2.1) and the ‘cmapR’ package56 (v1.0.1) in R v3.5.0 (R Core Team 2018).
CRISPR sgRNA design:
sgRNAs for the ORFs in this study were designed using the Broad Institute GPP sgRNA designer for S. Pyogenes Cas9 against genome coordinates for the GRCh38 assembly of the human genome (https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design). Only exonic coding regions for the ORFs were used. A maximum of 8 unique sgRNAs were employed per gene. If fewer than 8 were nominated due to small gene size and lack of available PAM sites, then all nominated sgRNAs were used. If more than 8 sgRNAs were nominated, then the top 8 ranked sgRNAs were used according the Broad Institute GPP sgRNA designer pick analysis. For the secondary CRISPR screen, 147 ORFs were tested. These were chosen to include all ORFs with a viability phenotype in the primary screen in the appropriate cell lines (A375, MCF7, HEPG2), as well as additional ORFs that did not have viability phenotype.
For tiling sgRNA analyses, additional nominated sgRNAs for each ORF were selected. Also, we selected sgRNAs to putative 3’UTR, 5’UTR, and promoter regions (defined as within 1000 basepairs of the transcript start site). A maximum of 16 sgRNAs were designed for each region. If there were multiple UTR exons, then a maximum of 16 sgRNAs were designed for each UTR exon. Intronic sgRNAs were used were available and limited to 6 sgRNAs per intron. sgRNAs for adjacent protein coding genes were also employed as indicated, and designed in an identical manner. The number of sgRNAs for adjacent coding genes and various genome regions is detailed in Supplementary Tables 25 and 26.
Determination of infection conditions for CRISPR pooled screens:
Optimal infection conditions were determined in each cell line in order to achieve 30–50% infection efficiency, corresponding to a multiplicity of infection (MOI) of ~0.5 – 1. Spin-infections were performed in 12-well plate format with 3 × 106 cells each well. Optimal conditions were determined by infecting cells with different virus volumes with a final concentration of 4 ug/mL polybrene. Cells were spun for 2 hours at 1000 g at 30°C. Approximately 24 hours after infection, cells were trypsinized and 2×10e5 for A375, HT-29, and PC-3 cells; 1.5×10e5 for A549 and HeLa cells; 3×10e5 for HepG2 cells; 5×10e5 for MCF-7 cells from each infection were seeded 2 wells of a 6-well plate, each with complete medium, one supplemented with the appropriate concentration of puromycin (1.5 ug/mL for A375; 2 ug/mL for A549, MCF7 and PC-3; 1 ug/mL for HeLa, HA1E, HepG2, and HT-29). For the secondary screen, only HepG2, MCF7 and A375 were used. Cells were counted 4–5 days post selection to determine the infection efficiency, comparing survival with and without puromycin selection. Volumes of virus that yielded ~30 – 50% infection efficiency were used for screening.
Primary and secondary CRISPR pooled proliferation screens
The lentiviral barcoded library used in the primary screen contains 5235 sgRNAs, which includes an average of 8 guides per gene and 500 non –targeting control guides. The validation library contains 6996 sgRNAs targeting selected regions of the smORFs. Genome-scale infections were performed in three replicates with the pre-determined volume of virus in the same 12-well format as the viral titration described above, and pooled 24 h post-centrifugation. Infections were performed with enough cells per replicate, in order to achieve a representation of at least 1000 cells per sgRNA following puromycin selection (~1.5×10e7 surviving cells). Approximately 24 hours after infection, all wells within a replicate were pooled and were split into T225 flasks. 24 hours after infection, cells were selected with puromycin for 7 days to remove uninfected cells. After selection was complete, 1.5–2×10e7 of cells were harvested for assessing the initial abundance of the library. Cells were passaged every 3–4 days and harvested ~21 days after infection. For all genome-wide screens, genomic DNA (gDNA) was isolated using Midi or Maxi kits for the validation screens gDNA was isolated using and Midi kits according to the manufacturer’s protocol (Qiagen, Hilden, Germany). PCR and sequencing were performed as previously described57,58. Samples were sequenced on a HiSeq2000 (Illumina, San Diego, CA). For analysis, the read counts were normalized to reads per million and then log2 transformed. The log2 fold-change of each sgRNA was determined relative to the initial time point for each biological replicate.
Analysis of CRISPR screening data:
CRISPR data was analyzed as log2 fold change values computed between the day 21 timepoint and the input plasmid DNA. A log2 fold change of <= −1 was defined as a scoring sgRNA which was depleted in the analysis. In the primary screen, a gene with at least 2 sgRNAs with a log2 fold change of <= −1 in at least 1 cell line was defined a putative vulnerability hit. Because the vast majority of genes in the primary screen had 8 sgRNAs per cell line, genes could be compared against each other with this metric. In the secondary screen, because the number of sgRNAs for each gene varied, a scoring candidate was defined as a gene in which at least 10% of the sgRNAs had a log2 fold change of <= −1, and there were at least 2 sgRNAs with a log2 fold change of <= −1 in at least 1 cell line. sgRNAs were also analyzed via STARS v1.3 and CERES scores as previously described57,59.
Analysis of CRISPR tiling screen:
Log2 fold change values for each sgRNA at day 21 of the screen were considered as above. sgRNAs were then grouped into their respective genomic region (e.g. UTR, ORF exon, adjacent gene exon, intron). The mean log2 fold change for each region was computed. A mean log2 fold change of <= −1 was considered to be a scoring hit. Genes were then classified in the following manner according to the viability affect of the sgRNAs: “specific to ORF” if only the ORF region sgRNAs scored; “specific to ORF and transcript subregion” if the ORF sgRNAs and sgRNAs to only one other region of the RNA transcript scored; “specific to transcript” if sgRNAs to any part of the ORF or RNA transcript scored, but not sgRNAs to introns or genomic regions; “shared with adjacent gene” if the ORF and an annotated adjacent protein coding gene both scored; “nonspecific to the genome” if sgRNAs to any part of the genomic region, intron, RNA transcript or ORF all demonstrated depletion.
Comparison of CRISPR screen data with Project Achilles:
For each gene of ORF in each of the eight cell lines used in the primary ORF CRISPR screen, knockout was determined to produce depletion if at least two guides produced at least 50% depletion from initial abundance after RPM normalization. The file “Achilles_logfold_change” in DepMap_public_19Q4 was used for Achilles screens (available at https://depmap.org/portal/download). To determine the expected number of genes or ORFs that deplete in any cell line given N cell lines, all possible subsets of N lines were selected and the number of genes with at least one depleted line were counted. For a negative control, this process was repeated in Achilles screens using only genes proposed as non-essential by previously published RNA interference data60, to generate a null distribution.
Off-target sgRNA effect prediction
For the 57 putative hits in the primary CRISPR screen, we analyzed scoring sgRNAs for off-target genomic homology sites Using the Cas-OFFinder v1.0 algorithm61. Homology sites were computed using default program settings with a mismatch tolerance of 0, DNA bulge of 0, and RNA bulge of 0. Predicted off-target sites are listed in Supplementary Tables 30 & 31. In addition, all ORF-targeting sgRNAs in the primary screen were analyzed for specificity or off-target sites using BLAT through the UCSC Genome Browser.
GREP1 annotation analysis and expression data:
GREP1 annotation status was evaluated using the indicated historical versions of the GENCODE database with graphic visualization of the locus. In cell lines, GREP1 expression was evaluated through Cancer Cell Line Encyclopedia data for LINC00514 (NR_033861.1), a RefSeq annotation which incorporates the first portion of GREP1. CCLE data was downloaded from https://portals.broadinstitute.org/ccle.
Pooled GREP1 knockout:
For the pooled GREP1 CRISPR knockout assay, we used a pool of 486 barcoded, adherent human cancer cell lines developed at the Broad Institute62. The cell line pool was grown in RPMI1640 media supplemented with 10% FBS. sgRNAs used for this experiment were non-cutting control sgLacZ (AACGGCGGATTGACCGTAAT), cutting control sgChr2 (GGTGTGCGTATGAAGCAGTGG), sgGREP1 #1 (ACTCAAAATGGCTATAGACC), and sgGREP1 #2 (AGGCTTTAGAGGGGACATGA). On Day 0, the cell line pool was plated in 6 well plates at 400,000 cells per well in 3mL of cell culture media. 24 hours later, using an all-in-one Cas9/sgRNA plasmid, the cell line pool was infected with each lentivirus at an MOI of 10; lentivirus was concentrated prior to use to obtain a concentration of >1e7 particle/ml. Cells were also treated with 4ug/mL polybrene in 2mL/well for the lentiviral infection, and spun at 2250rpm for 1 hour at 37°C. 24 hours after transduction, cells were split from 1 well in a 6 well plate into two T25 flasks; at this time the baseline cell DNA lysate was harvested as a “no infection” control. 72 hours after infection, cell culture media was changed and puromycin selection was started at a concentration of 1ug/mL puromycin. Thereafter, cell culture media was changed every 72 hours and cells were expanded as needed into T75 and T175 flasks. Pooled cell line DNA was collected from the input plasmid pool, on day +6 as an early timepoint, and day +15 as a late timepoint to assess for dropout of cell line. At each sample timepoint, cells were counted and 2e6 cells were removed for lysis for DNA. For lysis, cells were pelleted, washed in PBS, and genomic DNA was extracted with the DNA Blood and Tissue Kit according to manufacturer’s instructions (Qiagen, Hilden, Germany). The remainder to the cells not taken for lysis were re-seeded into T75 and T175 flasks for continuing cell growth.
For sequencing, timepoint DNA was subjected to PCR using universal barcode primers. PCR products were run on a 2% agarose gel to confirm amplicon size. Then 10uL from each PCR product was pooled, purified with AMPur beads (Beckman Coulter, Brea, CA). DNA concentration was measured via Qubit fluorometric quantification (Thermo Fisher Scientific, Waltham, MA) and DNA was sequenced on a NovaSeq (Illumina, San Diego, CA) at the Genomics Platform at the Broad Institute.
Analysis of pooled GREP1 knockout sequencing data:
Cell line abundance was calculated based on cell line barcode detection by next generation sequencing as previously described62. To analyze the pooled GREP1 CRISPR knockout data, we first calculated the theoretical number of cells in each well at each timepoint based on the experimental measurements of the total number of cells and the number of cells removed for sequencing. We accounted for these removed cells by scaling the measured number of cells at a given timepoint by the ratio of the total number of cells at the previous timepoint to the number of reseeded, or continued, cells from the previous timepoint.
Next, for quality control, we computed the purity of each sample as the percentage of the read counts mapping to cell lines not in the pool. We removed samples with lower than 95% purity. We also filtered out cell lines with fewer than 12 reads in more than one replicate of either of the two negative control conditions, LacZ and Chr2. The conservative threshold of 12 was determined from the minimum number of counts at which we are able to distinguish between that number of counts and half that number, at a confidence level of 0.05, under a Poisson distribution.
Then, we added a pseudocount of 1 to each of the read counts and normalized the updated read counts by the library size and the theoretical total cell count. We define the log fold-change of a cell line in a sample as the log2-transform of the ratio of the normalized read count of the cell line in the sample to the normalized read count of the cell line at day 0. Finally, we define the viability as the difference between the log fold-change in the cell line and treatment of interest and the average of the log fold-changes in the cell line and the two negative controls.
Next, we developed a series of data processing steps to empirically improve the quality of the dataset (see Supplementary Fig. 11). First, we excluded cell lines believed to be puromycin resistant based on the criterion of positive viability in the puromycin, no-virus condition. These filters resulted in a viability dataset of 400 out of 486 cell lines. Then, we removed cell lines that exhibited excessive lentiviral toxicity given the high MOI used for this experiment. This left 320 cell lines. Next, we eliminated cancer type cohorts with less than or equal to 5 cell lines, due to insufficient numbers for analysis, leaving 294 cell lines. Lastly, we calculated the number of cell lines per cancer cohort that expressed GREP1 above a minimal threshold, and excluded cohorts with insufficient expression as any change in those cohorts may be spurious due to population shifts in the cell line pool or off-target effects.
CRISPR-seq
ZR-75–1 cells infected with lentivirus for sgCh2–2 negative control, sgGREP1 #1 or sgGREP1 #2, and antibiotic-resistant cells were selected with 2ug/mL puromycin for 48 hours as described previously. 96 hours after infection, genomic DNA from cells was isolated using the Qiagen DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructors. 100ng of DNA was amplified by PCR with the following thermocycler conditions: 94C for 2 minutes, followed by 30 cycles of 94C for 30 seconds, 52C for 30 seconds, and 68C for 1 minute; final elongation was 68C for 7 minutes. PCR products were confirmed for specificity with a 1% agarose gel and then gel-purified with a Qiagen Gel Extraction kit according to manufacturer’s instructions. DNA was diluted to a concentration of 25ng/uL and submitted to the Massachusetts General Hospital Center for Computational and Integrative Biology (CCIB) DNA Core for sequencing. FASTQ sequencing files were analyzed using CRISPResso63 v2 (http://crispresso.pinellolab.partners.org) according to default parameters.
Patient outcomes analysis for GREP1:
Expression data for GREP1 in the TCGA samples was acquired from the MiPanda publicly available tool using the LA16c-H380H5.3 gene annotation as a query64. Data for the GDC TCGA Breast Cancer and GDC TCGA Colon Cancer datasets were used. LINC00514 expression was extracted as a proxy for GREP1 given that LINC00514 is a fragment of the longer gene. Overall survival was also extracted for these datasets. Kaplan-Meier curves and statistical significance via Log-rank P value were generated using GraphPad Prism8 software, with a p value of < 0.05 being considered statistically significant.
GREP1 copy number analysis:
CCLE copy number data from the 2013–12-03 segmentation was downloaded from https://depmap.org/portal/download. Data for LINC00514 (283875) was used as a proxy for GREP1 given overlapping genomic regions. Copy number data was then aggregated by cell line lineage.
CRISPR validation experiments:
Cells were plated in 96-well plates and allow to grow for 4–8 hours prior to infection with the indicated sgRNA or treatment condition. 1,000 – 5,000 cells per well were plated depending on the cell line. sgRNAs were obtained from the Broad Institute Genomic Perturbation Platform (Broad Institute, Cambridge, MA, USA) or from direct synthesis into the BRDN0003 backbone via commercial vendor (GenScript, Piscataway, NJ). sgRNA sequences are listed below:
| Gene | sgRNA # | sgRNA sequence |
|---|---|---|
| ASNSD1 | 1 | GCTCACGTCCTACACTTGAG |
| ASNSD1 | 2 | TTTGGGTGCCAACTGAAGAG |
| ASNSD1 uORF | 1 | GCTTAGATCCTCCTTGTGTG |
| ASNSD1 uORF | 2 | TAAAGAACAAAAAATTGTGG |
| chr2–2 | N/A | GGTGTGCGTATGAAGCAGTGG |
| COG7 | 2 | TGTTGAAGCCCTAAAACAGG |
| COG7 | 1 | CTACTACTACAAGTGTCACA |
| GREP1 | 1 | ACTCAAAATGGCTATAGACC |
| GREP1 | 2 | AGGCTTTAGAGGGGACATGA |
| GREP1 | 3 | GCTCAAAATGGCTTTGGACC |
| HP08474 | 1 | TGTGTTTGAGCCAGGCATGG |
| HP08474 | 2 | AGTCCCAGCAGCTACTCCGG |
| RP11–277L2.3 | 1 | CGCCTCCTGGGTTCCAGCAG |
| RP11–277L2.3 | 2 | GGGACTAGATGGAGCCGAAG |
| RP11–54A9.1 | 1 | TGGGTCTCCTCACAGAGTGA |
| RP11–54A9.1 | 2 | TCCTCAGACCAACCAGCTCA |
| LacZ | N/A | AACGGCGGATTGACCGTAAT |
| ZBTB11-AS1 | 1 | GCGGGACTCTGTATTACCAG |
| ZBTB11-AS1 | 2 | GCGACGCCGGGACCTCATCG |
| CTD-2270L9.4 | 1 | CGTGAAGGAGTGGATCAATG |
| CTD-2270L9.4 | 2 | GAACTTGGAGAAGTCCATGG |
| G083755 | 1 | CCAACAGGTGACCTCAGCAA |
| G083755 | 2 | GGACCTCTTACATCATGGAA |
| SF3B1 | N/A | AAGGGTATCCGCCAACACAG |
| ZBTB11 | 1 | ACAGGTTGACACCAAAGGAG |
| ZBTB11 | 2 | GCATATATTCGACTACACAA |
| OLMALINC | 1 | ACAGGGCACTGGTCTCCCAA |
| OLMALINC | 2 | CAAGGCTGTATATTTCACCT |
All sgRNAs were sequenced and verified. After sequence verification, constructs were transfected with packaging vectors into HEK-293T with Fugene HD (Sigma-Aldrich, St. Louis, MO). After plating, cells were then infected with sgRNA lentivirus to achieve maximal knockout but without viral toxicity. 16 hours after infection, cells were selected with 2ug/uL puromycin (Invitrogen, Carlsbad, CA) for 48 hours. Cell viability was measured CellTiter-Glo reagent (Promega, Madison, WI) was measured at 16 hours post-transfection for a baseline assessment, and additional timepoints as needed. For stable knockout cell lines, cells were plated at equal densities and cell viability was measured by CellTiter-Glo every 24 hours as indicated.
GREP1 overexpression rescue experiments:
For CRISPR rescue experiments, Cas9-derivatized cell lines were infected with lentivirus GFP or GREP1 coding plasmids cloned into the pLX_TRC313 vector, which has EF1a promoter and hygromycin resistance (see https://portals.broadinstitute.org/gpp/public/vector for details). Cells were selected in 350ug/mL of hygromycin for 72 hours prior to transitioning back to standard culture media.
In 96 well plates, 5000 ZR-75–1 derived cells were plated and infected with the indicated sgRNA lentivirus 4–6 hours after plating. 16 hours after infection, cells were selected with 2ug/mL puromycin for 48 hours and grown for 7 days prior to cell viability analysis using CellTiter-Glo reagent.
Conditioned media rescue experiments:
First, on day −2 HEK293-T cells were plated and transiently transfected with GFP and GREP1 with Fugene HD reagent. On day −2, 5000 ZR-75–1 derived cells or 2500 AU565-derived cells were plated in wells of a 96-well plate. On day −1, ZR-75–1 and AU565 cells were switched to serum-free media. On day 0, conditioned media from GFP- or GREP1-expressing HEK293-T cells was cleared of cellular debris via centrifugation and then 100uL of conditioned media was applied to each well. Conditioned media was then refreshed daily and cell viability was determined with the CellTiter-Glo reagent at the indicated time points.
Immunoprecipitation:
HEK293-T cells were transiently transfected with GFP-V5 or GREP1-V5 fusion proteins using OptiMem and Fugene HD (Sigma-Aldrich, St. Louis, MO). 72 hours after transfection, cell culture media was harvested and cell debris was sedimented by centrifugation at 1500 rpm x 5 minutes twice. Resulting cell culture media was concentrated in a 10:1 manner using 3kDa size-exclusion filter (Millipore, Burlington, MA). Concentrated culture media was treated with HALT protease inhibitor. Next, all immunoprecipitation steps were performed on ice or in a 4°C cold room. First, culture media was cleared with Pierce magnetic A/G beads (Thermo Fisher Scientific, Waltham, MA) for 1 hour while rotating at 18–20 rpm. Beads were then discarded and 10% of the media was removed as an input sample and kept at 4°C without freezing. The remained of the culture media was then treated with 50uL of magnetic anti-V5 beads (MBL International, Woburn, MA) and rotated at 18–20 rpm overnight at 4°C. The next day, the supernatant was discarded and beads were washed four times in IP wash buffer (50nM TricHCl, pH 8.0, 150nM NaCl, 2mM EDTA, pH 8.0, 0.2% NP-40, and 1ug/mL PMSF protease inhibitor) with rotation for 10 minutes per wash. After the final wash, beads were gently centrifuged and residual wash buffer was removed. Then, proteins were eluted twice with 2 ug/uL V5 peptide in water (Sigma-Aldrich, St. Louis, MO) at 37°C for 15 minutes with shaking at 1000rpm. The two elution fractions were pooled and samples were prepared with 4x LDS sample buffer and 10x Sample Reducing Agent (Thermo Fisher Scientific), followed by boiling at 95°C for 5 minutes. One-third of the eluate was then run on a 10–20% Tris-Glycine SDS Page gel and stained with SimplyBlue Commassie stain (Thermo Fisher Scientific, Waltham, MA) for 2 hours. Gels were destained with a minimum of 3 washes in water for at least 2 hours per wash. Bands were visualized using Commassie autofluorescence on the LI-COR Odyssey in the 800nM channel. Gel lanes were then cut into 6 equal-sized pieces using a sterile razor in sterile conditions, and stored in 1mL of DEPC-treated water prior to mass spectrometry analysis.
Methods for Protein Sequence Analysis by LC-MS/MS:
LC-MS/MS was performed in the Taplin Biological Mass Spectrometry Facility at the Harvard Medical School. Briefly, excised gel bands were cut into approximately 1 mm3 pieces. Gel pieces were then subjected to a modified in-gel trypsin digestion procedure65. Gel pieces were washed and dehydrated with acetonitrile for 10 min. followed by removal of acetonitrile. Pieces were then completely dried in a speed-vac. Rehydration of the gel pieces was with 50 mM ammonium bicarbonate solution containing 12.5 ng/μl modified sequencing-grade trypsin (Promega, Madison, WI) at 4ºC. After 45 min., the excess trypsin solution was removed and replaced with 50 mM ammonium bicarbonate solution to just cover the gel pieces. Samples were then placed in a 37ºC room overnight. Peptides were later extracted by removing the ammonium bicarbonate solution, followed by one wash with a solution containing 50% acetonitrile and 1% formic acid. The extracts were then dried in a speed-vac (~1 hr). The samples were then stored at 4ºC until analysis.
On the day of analysis the samples were reconstituted in 5 – 10 μl of HPLC solvent A (2.5% acetonitrile, 0.1% formic acid). A nanoscale reverse-phase HPLC capillary column was created by packing 2.6 μm C18 spherical silica beads into a fused silica capillary (100 μm inner diameter x ~30 cm length) with a flame-drawn tip66. After equilibrating the column each sample was loaded via a Famos auto sampler (LC Packings, San Francisco CA) onto the column. A gradient was formed and peptides were eluted with increasing concentrations of solvent B (97.5% acetonitrile, 0.1% formic acid).
As peptides eluted they were subjected to electrospray ionization and then entered into an LTQ Orbitrap Velos Pro ion-trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Peptides were detected, isolated, and fragmented to produce a tandem mass spectrum of specific fragment ions for each peptide. Peptide sequences (and hence protein identity) were determined by matching protein databases with the acquired fragmentation pattern by the software program, Sequest67 (Thermo Fisher Scientific, Waltham, MA). All databases include a reversed version of all the sequences and the data was filtered to between a one and two percent peptide false discovery rate. Glycosylated peptides were defined using the A score method as described68.
IP-MS and gene ontology analysis:
We analyzed IP-MS data from two independent experiments for V5 immunoprecipitation for GFP-V5 and GREP1-V5 conditioned media in HEK293T cells and one biological replicate for GFP-V5 and GREP1-V5 in CAMA-1 and ZR-75–1 cells each. IP-MS data was merged for the two experiments and all proteins with < 2 total peptides were removed to exclude technical artifacts. To the remaining proteins, a pseudocount of 1 was added to ensure a non-zero denominator. Next, fold change of (GREP1+1)/(GFP+1) peptide count was calculated and log10-transformed. Enriched peptides with a (GREP1+1)/(GFP+1) ratio of >= 2 were further analyzed using the Gene Ontology database (http://geneontology.org) for cellular component analysis and corrected false discovery rates were plotted as shown.
GREP1 disorder analysis:
The GREP1 primary amino acid sequence was analyzed via the DISOPRED3 package69 on the PsiPred server (http://bioinf.cs.ucl.ac.uk/psipred/) using default settings. Disorder scores were plotted as indicated.
GREP1 evolutionary analysis:
The GREP1 amino acid sequence (ENST00000573315.2_prot) was aligned to non-redundant protein sequences using the NCBI BlastP suite as well as manually aligned to the genomes of the common rat (RGSC 6.0/rn6, July 2014 assembly) and domestic dog (Broad CanFam3.1/canFam3 assembly). Resulting protein hits were then ranked by E-value value and the most significant result was used for each organism. Predicted proteins and low-quality protein assemblies were included in this analysis. Resultant species-specific amino acid sequences were then aligned by the Clustal Omega sequence aligner (https://www.ebi.ac.uk/Tools/msa/clustalo/) and percent similarity to human GREP1 was plotted.
GREP1 codon usage analysis:
We calculated the triplet codon frequency for all triplet codons for the GREP1 amino acid sequence, the whole ORFeome in total, and GENBANK genes by collating all mRNA sequences within these respective groups and calculating the codon usage per group. Each codon usage was normalized to a standard rate of codon usage per 1000 codons. Triplet codons were then collapsed into single amino acids by scaling the codon usage rate to the relative frequency of usage for each codon per amino acid. Aggregate frequency of amino acid representation was then calculated and compared across groups.
Cytokine profiling array:
Cytokine profiling was performed simultaneously using the Human XL Cytokine Array (R&D Systems, ARY022, Minneapolis, MN). Briefly, cell culture media were cleared of cellular debris and Halt protease inhibitor was added as above. Then, cytokine arrays were blocked in 2mL of Array Buffer 6 (blocking buffer) each for 1 hour on a shaker at room temperature. Samples were prepared with 300uL of culture media and diluted with 1200uL of Array Buffer 6. Cytokine arrays were then removed from blocking buffer and incubated with samples overnight at 4°C on a rocker. The next morning array membranes were washed in 20mL 1x Wash Buffer for a total of 3 washes. Then, arrays were placed in 1.5mL of 1x Array Buffer 4/6 (a 1:2 mixture of Array Buffer 4 and Array Buffer 6), and 30uL of reconstituted detection antibody cocktail was added. Samples were incubated for 1 hour at room temperature on a shaker. Subsequently, membranes were washed in 20mL 1x Wash Buffer for a total of 3 washes, and then transferred to 2.0mL of 1x streptavidin-HRP for 30 minutes at room temperature on a shaker, followed by three more washes in 20mL of 1x Wash Buffer. Afterwards, the membranes were blotted on tissue paper to remove excess buffer, and signal was developed with chemiluminescent reagent mix. Images were developed with CareStream Kodak BioMax light film (Kodak, Rochester, NY).
Cytokine profiling analysis:
Immunoblot images of the cytokine arrays were scanned and the signal intensity of all array antibody spots was determined using ImageJ v2.0.0 (https://imagej.nih.gov/ij/index.html). Raw data values were then inverted using the formula y = 255 - x, where x is the raw signal intensity. Inverted values were then normalized according to knockout or overexpression experiments. For knockout experiments, signal was analyzed as sgControl - sgGREP1. For overexpression experiments, signal was analyzed as GREP1 - GFP. Then, the absolute value of signal change was averaged across experiments and rank-listed according to the magnitude of average change.
GDF15 ELISA:
The GDF15 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) was used. In brief, cell culture media samples were diluted 1:3 by volume in Diluent RD5–20. To prepare microplate wells, 100uL of Assay Diluent RD1–9 was added to each well. Then, 50uL of standards, controls, or diluted samples were added to a given well. The plates were incubated at 2 hours at room temperature on a horizontal orbital microplate shaker at 500rpm. Wells were then washed four times with 400uL of 1x Wash Buffer for five minutes per wash; after the final wash, plates were inverted and blotted on tissue paper to remove excess. Then, 200uL of Human GDF-15 conjugate was added to each well and the plate was incubated for 1 hour at room temperature on an orbital shaker. Following this, wells were then washed four times with 400uL of 1x Wash Buffer for five minutes per wash; after the final wash, plates were inverted and blotted on tissue paper to remove excess. Then, 200uL of Substrate Solution was added per well, and plates were incubated for 30 minutes at room temperature without shaking and protected from light. Then, 50uL of Stop Solution was added per well and samples were mixed with gentle tapping. The optical density of samples at 450nM and 570nM was determined on a microplate reader within 15 minutes of completion of the protocol. For analysis, background signal from 570nM was subtracted per well from the 450nM signal. Samples were then calculated based on a standard curve to obtain GDF-15 concentration values. For pharmacologic treatments preceding GDF15 measurements, HEK293T cells were treated with 10uM of vorinostat, idarubicin, GSK265, bortezomib, GSK132, or luminespib for 24 hours. Cells with transient transfection of GFP or GREP1 cDNAs were treated with DMSO as controls. After 24 hours, GDF15 abundance was measured in conditioned by ELISA.
Correlation of GREP1 and GDF15 expression:
GREP1, GDF15, FN1, and EMIL2 expression was downloaded via the MiPanda portal64 as TPM values. GTex and TCGA samples were used. Spearman rho correlation coefficients and Spearman p values were calculated using GraphPad Prism8 and plotted as shown.
Recombinant GDF15 experiments:
Recombinant human GDF15 (R&D Systems, Minneapolis, MN, catalogue number 957-GD-025) was resuspended in water at 10ug/uL. Knockout with sgGREP1 #2 or controls in ZR-75–1 was performed as described above. 24 hours after infection with lentiviral sgRNA, cell culture media was refreshed to contain puromycin as above for antibiotic selection, and GDF15 or vehicle control was supplemented at the following concentrations: 0.01 pg/mL, 0.1 pg/mL, 1pg/mL, 10pg/mL, 100pg/mL. Thereafter cell culture media and recombinant GDF15 was refreshed every 24 hours. Cell viability was measured 7 days after lentiviral infection using the CellTiter-Glo reagent (Promega, Madison, WI).
Generation of GREP1 glycosylation mutants
V5-tagged GREP1 T63V, T265V, and T63V/T265V double mutant cDNA constructs were generated through a commercial service with GenScript in the plx307 vector. Briefly, for each respective construct, threonine at position 63 was mutated to valine with the following two mutations, A187G and C188T to change the codon ACC to GTC; the threonine at position 265 was mutated to valine with the following two mutations, A748G and C749T to change the codon ACC to GTC. The GREP1 T63V/T265V double mutant construct harbored all four basepair changes. For GDF15 analyses, the indicated constructs were transiently transfected into HEK293T cells as described previously and GDF15 was measured in conditioned media 48 hours later, as previously detailed.
ZBTB11-AS1 knockdown experiments
A549 cells with transduced with lentivirus encoding GFP, ZBTB11-AS1 ORF or mutant ZBTB11-AS1 ORF with mutated ATG and antibiotic-resistant cells were isolated with 2ug/mL puromycin treatment for 72 hours. 500,000 cells of the given cell line were plated in 6 well plates in serum-free media. Four hours after plating, wells were individually transfected with 20uM of the indicated siRNA oligonucleotide or non-targeting controls mixed in 135uL of OptiMem with 10uL Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA). Twelve hours later, serum-containing media was added and cells were grown for 48 hours. Then, cells were trypsinized and plated in 96 well plates at a density of 5,000 cells per well in 6 replicates. Cell viability was measured 72 hours later using the CellTiter-Glo reagent (Promega, Madison, WI). siRNA sequences: Lincode ZBTB11-AS1 #1, 5’-GGACGAAUCUGCAGCGCUC-3’ (Cat # N-188908–01-0002, Dharmacon, Horizon Discovery, UK), Lincode ZBTB11-AS1 #3, 5’-GUUGAGAGUUCAGCCGAAA-3’ (Cat # N-188908–03-0002, Dharmacon, Horizon Discovery, UK), ON-TARGET plus Non-targeting siRNA #1, 5’-UGGUUUACAUGUCGACUAA-3’ (Cat # D-001810–01-20, Dharmacon, Horizon Discovery, UK), ON-TARGET plue Non-targeting siRNA #3, 5’-UGGUUUACAUGUUUUCUGA-3’ (Cat # D-001810–03-20, Dharmacon, Horizon Discovery, UK). Knockout efficiency was monitored by quantitative RT-PCR (qPCR).
Statistical analyses for experimental studies
All data are expressed as means ± standard deviation. All experimental assays were performed in duplicate or triplicate. Statistical analysis was performed by a two-tailed Student’s t-test, one-way or two-way analysis of variance (ANOVA), Kolmogorov-Smirnov test, log-rank P value, or other tests as indicated. A p value <0.05 was considered statistically significant.
Data availability statement
Processed data for CRISPR screens (in Figure 3 and Figure 4d) are available in Supplementary Tables 22 and 27. Raw data are available in the Source Data files accompanying this manuscript as well as through the NCBI Sequence Read Archive (SRA) at: SRR13126801, SRR13128583, SRR13132373, SRR13142215 and SRR13142421. Mass spectrometry data relating to Figure 1 are available in Supplementary Table 14. Raw MS spectra are available through the original datasets at: https://cptac-data-portal.georgetown.edu/study-summary/S060 (CPTAC2_BRCA_prosp), https://cptac-data-portal.georgetown.edu/study-summary/S045 (CPTAC2_COAD_prosp), https://cptac-data-portal.georgetown.edu/study-summary/S050 (CPTAC3_ccRCC), https://cptac-data-portal.georgetown.edu/study-summary/S056 (CPTAC3_LUAD), https://cptac-data-portal.georgetown.edu/study-summary/S051 (CPTAC3_PTRC_DP1), https://cptac-data-portal.georgetown.edu/study-summary/S053 (CPTAC3_UCEC), ftp://massive.ucsd.edu/MSV000080527 (HLA_Abelin), ftp://massive.ucsd.edu/MSV000084787 (HLA_Ouspenskaia), ftp://massive.ucsd.edu/MSV000084172/; ftp://massive.ucsd.edu/MSV000080527; ftp://massive.ucsd.edu/MSV000084442/ (HLA_Sarkizova), ftp://massive.ucsd.edu/MSV000082644 (CPTAC Medulloblastoma), http://www.peptideatlas.org (PeptideAtlas database). L1000 data relating to Figure 2 and Supplementary Figures 8 & 9 is available through the NIH LINCS program and at https://clue.io/data. The website lincsproject.org provides information about the LINCS consortium, including data standards. Please see the Life Sciences Reporting Summary accompanying this manuscript for more details.
Code availability statement
L1000 data analysis code and pre-processed data are available via GitHub https://github.com/cmap/cmapM. There is additional information about this database and tools at http://clue.io/connectopedia. L1000 data were analyzed via: the ‘tidyverse’ suite36 of R packages (v1.2.1), the ‘cmapR’ package37 (v1.0.1) in R v3.5.0 (R Core Team 2018), in-house code available through github (https://github.com/johnprensner/smORF_analyses). Mass spectrometry peptides were processed via Spectrum Mill MS Proteomics Workbench v6.0. Additional code for computational tools used in this study are listed here: PhyloCSF (https://github.com/mlin/PhyloCSF/wiki) for 29 mammal alignment, Slncky (https://slncky.github.io), STARS v1.3 (http://www.broadinstitute.org/rnai/public/software/index) and CERES v1.0 (https://github.com/cancerdatasci/ceres). Please see the Life Sciences Reporting Summary accompanying this manuscript for more details.
Extended Data
Extended Data Fig. 1. Generation and validation of a noncanonical ORF cDNA library.
a) Vector design and sequence details for the ORF library. The vector used is a modified version of the plx307 vector developed by the Genomic Perturbation Platform at the Broad Institute. b) Titration analyses of in cell western experiments. Three ORFs were chosen: eGFP (positive control), LINC00116 (high-expressing ORF), and RP11–539I5 (low expressing ORF). Increasing amounts of plasmid were transfected into increasing numbers of HEK293T cells as shown. c) Quantification the in cell western titration shown in b, demonstrating signal detection over noise and signal plateau. Signal was quantified using pixel density in the 800nM green color channel. d) Replicate experiments assessing signal-to-noise thresholds for a low-expressing ORF transfected into HEK293T cells with a low DNA plasmid concentration, as well as a high-expressing ORF (eGFP) transfected into HEK293T cells at a high DNA plasmid concentration. e) Example in cell western data in triplicate experiments for selected ORFs. f) Abrogation of protein translation via mutation of the ORF for selected examples. g) A systematic evaluation of in cell western signal for wild type and mutant ORFs for all pairs. ORFs are separated into those with signal above the baseline threshold, and those without reproducible signal. h) An immunoblot showing in vitro transcription/translation of selected tag-free ORFs using a wheat germ lysate system. Red arrows indicate the translated ORFs. Results were repeated in two independent experiments.
Extended Data Fig. 2. Analysis of paired wild-type and mutant constructs in L1000 data.
a) A strategy for ORF mutagenesis strategy in which the start codon and downstream methionines were mutated to alanine. The shown amino acid sequence is a fictional sequence. b) A pie chart showing the number and percentage of amino acids changed per ORF from the mutagenesis. c) A violin plot showing the number of Perturbational Class (PCL) connections made at the 98th percentile for matched mutant and wild type constructs (n=47 for each, all data points are biologically independent experiments). P value by a two-tailed Wilcoxon matched pairs rank test. d) Left, the overall distribution of PCL connections across all ranks in wild type and mutant constructs (n=19,012 independent comparisons for each). Right, an inset image of distribution of PCL connections at high connectivity, showing a bias in connections made with wild type compared to mutant constructs (n=1,920 independent comparisons each). P value by a two-tailed Wilcoxon matched pairs rank test. e) All PCL connections in wild type constructs at either the >=95th percentile or <= −95th percentile, with the matched percentile connectivity in the mutant constructs. f) The distribution of percentile connectivity results in wild type or mutant constructs for the indicated genes. In brief, all ORF L1000 signatures were queried against all PCL classes and a percentile connectivity was generated for each individual cell line and for both wild type and mutant constructs. Cell line and construct data was then aggregated and ranked from highest to lowest connectivity. The rank positions of wild type and mutant ORFs were then plotted to reveal a depletion of mutant constructs at high connectivity scores. g) Two example heatmaps for the TINCR and SLC35A4 uORF plasmids showing clustering of PCL connectivity among wild type constructs that is not shared with mutant constructs. Purple bars denote wild type ORF experiments and green bars denote mutant ORF experiments. h) L1000 signature replicate reproducibility for all wild type and mutant pairs across all cell lines. All ORF signatures with at least one reproducible wild type signature are shown.
Extended Data Fig. 3. Validation of CRISPR hits via manual assays.
a-i) CRISPR assays using doxycycline-inducible Cas9 in HeLa cells. Targets are divided in ones that validated and ones that did not. For each experiment, the right-set panel is qPCR data of expression 96 hours after induction of Cas9 with doxycycline. a) ZBTB11-AS1 b) HP08474 c) GREP1 d) RP11–54A9.1 e) G083755 f) OLMALINC g) CTD-2270L9.4 h) RP11–277L2.3 i) ASNSD1 uORF. j-k) CRISPR assays using stably-expressing A375 Cas9 cells. j) CTD-2270L9.4 k) ASNSD1 uORF. For all data in this figure, n=6 technical replicates for each data point. Error bars represent standard deviation. Data was also acquired a 3 independent biological replicates based on doxycycline dose level (0.2 ug/mL, 1.0 ug/mL and 2.0 ug/mL doxycycline, as well as 0 ug/mL doxycycline). The data shown are the 1.0 ug/mL dosing level, with similar results observed for the 0.2 ug/mL and 2.0 ug/mL doxycycline dosing levels.
Extended Data Fig. 4. Tiling CRISPR assays to elucidate functional non-canonical ORFs.
a) A heatmap showing log fold change viability loss at Day +21 in the secondary CRISPR screen for the indicated non-canonical ORFs tested by multiple tiling sgRNA regions. b-e) Examples of non-canonical ORFs with a CRISPR tiling phenotype. b-e) Graphical representation of tiling CRISPR assays in which each dot represents an individual sgRNA. sgRNAs are mapped to their genomic loci and the genomic region of the tiling assay is shown. The location of the putative non-canonical ORF is shown in the gene annotation above. b) CTD-2270L9.4 c) OLMALINC d) RP11–54A9.1 e) RPP14 dORF / HTD2. f - k) Representative sgRNA log fold change data for the indicated transcripts. Each tiling experiment is classified as indicated. f) LINC00662 g) RP11–195B21.3 h) LYRM4-AS1 i) ESRG j) TCONS_I2_00007040 k) LINC01184.
Extended Data Fig. 5. Specific siRNA knockdown of ZBTB11-AS1 mRNA transcript causes a viability phenotype which is specifically rescued by the wild type ZBTB11-AS1 ORF.
a) A schematic showing the genomic location and sequences for the two siRNAs used for ZBTB11-AS1. b) mRNA expression levels for ZBTB11-AS1 or ZBTB11 transcripts 48 hours after siRNA knockdown of ZBTB11-AS1 in A549 cells. N=3 independent replicates for all conditions. Barplots represent mean +/− standard deviation. c) Relative cell viability of A549 cells treated with ZBTB11-AS1 siRNAs at 72 hours. Parental A549 cells were used along with A549 cells expressing cDNAs for GFP, wild type ZBTB11-AS1 ORF sequence, or mutant ZBTB11-AS1 ORF lacking translational start sites. Only the wild-type ZBTB11-AS1 ORF sequence rescues the viability phenotype. N=6 independent replicates for all conditions. Barplots represent mean +/− standard deviation. d) DNA and amino acid sequences of the wild type and mutant ZBTB11-AS1 ORF cDNAs. * p < 0.05, ** p < 0.01. n.s., non-significant. For P values: Parental, non-targeting vs siRNA #1 P < 0.0001, non-targeting vs siRNA #2 P < 0.0001; GFP, non-targeting vs siRNA #1 P = 0.0008, non-targeting vs siRNA #2, P < 0.0001; WT ORF, non-targeting vs siRNA #1 P = 0.04, non-targeting vs siRNA #2 P = 0.83; MUT ORF, non-targeting vs siRNA #1 P = 0.001, non-targeting vs siRNA #2 P = 0.02. P values by a two-tailed Student’s T test.
Extended Data Fig. 6. The GREP1 locus and expression.
a) A schematic representation of the GREP1 gene structure and the annotation of this locus in the indicated databases. The year of release for each database is indicated. b) mRNA expression level of GREP1 across tumor lineages in the Cancer Cell Line Encyclopedia. The Y axis is in a log10 scale. c) mRNA expression of GREP1 across tumor types using TCGA and GTex data. A two-tailed Student’s t-test was used to calculate significance of change between normal and cancer tissues. Cell lineages are grouped according to whether GREP1 expression is specifically modulated in cancer, universally expressed as a lineage gene, or not robustly expressed in the indicated lineage.
Extended Data Fig. 7. GREP1 is implicated in cell proliferation and breast cancer patient outcomes.
a) Cell viability curves following GREP1 knockout in three sensitive and three insensitive cell lines. GREP1 expression in the Cancer Cell Line Encyclopedia is indicated in transcripts per million (TPM) b) A scatter plot showing lineage-specific correlation between cell viability and GREP1 mRNA expression on the X axis with the average GREP1 expression level on the Y axis. c) Overall survival for breast cancer patients in the TCGA database stratified by GREP1 expression. N=1,036 individual patients. N=969 GREP1-high and N=67 GREP1-low patients. Significance by a one-sided log-rank P value. d) Overall survival for colon cancer patients in the TCGA database stratified by GREP1 expression. N=296 individual patients. N=38 GREP1-high and N=258 GREP1-low patients. Significance by a one-sided log-rank P value. e) Immunoblot of V5-tagged GREP1 or GFP in HEK293T cells in both whole cell lysate and conditioned media. A mutant GREP1, in which translational start sites were mutated to alanine, lacks protein translation initiation ability. Results were repeated in three independent experiments. i) Abundance of mass spec peptides detected in the full length GREP1 or cleavage product GREP1 proteins. Peptide abundance is represented as a fraction of total peptides detected. All error bars represent standard deviation.
Extended Data Fig. 8. GREP1 is associated with the extracellular matrix.
a) Total fraction of amino acid usage in the ORFeome, GENBANK, GREP1, and the Collagen alpha-1 family. Sequence similarities between GREP1 and the collagen family are indicated. b) Predicted disorder score for the GREP1 amino acid sequence. c) Amino acid conservation for detected homologs of GREP1 in the indicated species. d) Non-denaturing native western blot of GREP1 in conditioned media from HEK293T cells expressing V5-tagged GREP1. e) Representative Commassie-stained gels for immunoprecipitation of GREP1 from the conditioned media of HEK293T cells. Two representative biological replicates are shown. f) Enrichment of extracellular matrix proteins in the IP-MS data for GREP1 compared to IP-MS data for GFP. g) Gene Ontology Cellular Component analysis of proteins >= 2 fold enriched in GREP1 immunoprecipitation compared to GFP immunoprecipitations. h) IP MS total peptide count for fibronectin shown for three separate experiments. i) Commassie stain of V5 immunoprecapitation of V5-tagged GFP, GREP1 del_SLS or GREP1 constructs expressed in CAMA-1 cells following fractionation of cell lysate into cytoplasmic, membrane and cell media components. Results were repeated in 2 independent experiments. j) Western blot of endogenous fibronectin, E-cadherin, beta-actin and GAPDH in cell lysate or cell culture media for CAMA-1 cells expressing GFP, GREP1 del_SLS or GREP1 constructs as in panel i. Results were repeated in two independent experiments. k) IP mass spectrometry data showing the total peptide count for GREP1 and other top-scoring proteins following IP of V5-tagged GREP1 in HEK293T, ZR-75–1, and CAMA-1 cells. N=4 independent IP MS experiments. Lines represent median +/− interquartile (25–75%) range.
Extended Data Fig. 9. GREP1 regulates GDF15 in vitro and correlates with GDF15 expression in patient tumor tissues.
a) Cytokine profiling in HEK293T cells with transient ectopic GREP1 or GFP overexpression, ZR-75–1 cells with stable GREP1 knockout, or HDQP1 cells with stable GREP1 knockout. The change in signal abundance was calculated for each control/GREP1 pair. To rank cytokines, the average of the absolute values for the individual signal changes was plotted. b) GDF15 abundance by ELISA in ZR-75–1 and CAMA-1 cells overexpressing a GREP1 or GFP cDNA plasmid. N=3 technical replicates. N=2 independent experiments performed, with representative results shown. c) Spearman’s rho for GREP1 expression correlation with GDF15, EMILIN2, or FN1 in the indicated TCGA datasets. d) Spearman’s p value for the GREP1 correlation coefficient for GREP1 correlation with GDF15, EMILIN2, or FN1 in the indicated TCGA datasets. e-g) Recombinant GDF15 partially rescues GREP1 knockout. CAMA-1, ZR-75–1 or T47D Cas9 cells were infected with the indicated sgRNAs. 24 hours after infection, cells were treated with vehicle control or increasing concentration of recombinant human GDF15 as shown. Relative abundance was measured 7 days after infection. N=5 for all conditions in panel e. N=6 for all conditions in panel f. N=5 for all conditions in panel g. All error bars represent standard deviation. Two independent experiments were performed for panels e-g.
Supplementary Material
Acknowledgements
We thank D. Bondeson, P. Tsvetkov, S. Corsello, U. Ben-David and T. Ouspenskaia for helpful discussions and critical reading of the manuscript. We thank M. Zhong for technical assistance with cloning and Z. Demere for assistance with CRISPR-seq. We thank D. Nusinow and S. Gygi for insights in identifying small peptides in proteomics datasets. We thank R. Tomaino for assistance with mass spectrometry at the Talpin Biological Mass Spectrometry Facility at the Harvard Medical School. We thank J. Chen for assistance with the Slncky algorithm. We thank J. Gould for assistance with gene datasets. We thank I. Cheeseman for providing doxycycline-inducible HeLa Cas9 cells. J.R.P. was supported by the Harvard K-12 in Central Nervous System tumors (5K12 CA 90354–18). V.L and M.W.K. were supported by the National Institutes of Health (R01 HD073104 and RO1 HD091846 to M.W.K.).
Footnotes
Competing Interests Statement
The authors declare no relevant competing interests.
References
- 1.Ewing B & Green P Analysis of expressed sequence tags indicates 35,000 human genes. Nat. Genet 25, 232–234 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Fields C, Adams MD, White O & Venter JC How many genes in the human genome? Nat. Genet 7, 345–346 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Liang F et al. Gene index analysis of the human genome estimates approximately 120,000 genes. Nat. Genet 25, 239–240 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Omenn GS et al. Progress on Identifying and Characterizing the Human Proteome: 2018 Metrics from the HUPO Human Proteome Project. J. Proteome Res. 17, 4031–4041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingolia NT et al. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep. 8, 1365–1379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Z, Song R, Regev A & Struhl K Many lncRNAs, 5’UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 4, e08890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pertea M et al. CHESS: a new human gene catalog curated from thousands of large-scale RNA sequencing experiments reveals extensive transcriptional noise. Genome Biol. 19, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Heesch S et al. The Translational Landscape of the Human Heart. Cell 178, 242–260 e229 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Burge C & Karlin S Prediction of complete gene structures in human genomic DNA. J. Mol. Biol 268, 78–94 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Dinger ME, Pang KC, Mercer TR & Mattick JS Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput. Biol. 4, e1000176 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lander ES et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Mouse Genome Sequencing C et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Mudge JM et al. Discovery of high-confidence human protein-coding genes and exons by whole-genome PhyloCSF helps elucidate 118 GWAS loci. Genome Res. 29, 2073–2087 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banfai B et al. Long noncoding RNAs are rarely translated in two human cell lines. Genome Res. 22, 1646–1657 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jungreis I, Tress ML, Mudge J, Sisu C, Hunt T, Johnson R, Uszcysnka-Ratajczak B, Lagarde J, Wright J, Muir P, Gerstein M, Guigo R, Kellis M, Frankish A, Flicek P, The GENCODE Consortium Nearly all new protein-coding predictions in the CHESS database are not protein-coding. Preprint at 10.1101/360602v1 (2018). [DOI]
- 16.Bazzini AA et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 33, 981–993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branca RM et al. HiRIEF LC-MS enables deep proteome coverage and unbiased proteogenomics. Nat. Methods 11, 59–62 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Cabili MN et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 25, 1915–1927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calviello L et al. Detecting actively translated open reading frames in ribosome profiling data. Nat. Methods 13, 165–170 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Gao X et al. Quantitative profiling of initiating ribosomes in vivo. Nat. Methods 12, 147–153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gascoigne DK et al. Pinstripe: a suite of programs for integrating transcriptomic and proteomic datasets identifies novel proteins and improves differentiation of protein-coding and non-coding genes. Bioinformatics 28, 3042–3050 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Iyer MK et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 47, 199–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS et al. A draft map of the human proteome. Nature 509, 575–581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch A et al. A proteogenomics approach integrating proteomics and ribosome profiling increases the efficiency of protein identification and enables the discovery of alternative translation start sites. Proteomics 14, 2688–2698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J et al. Discovery of human sORF-encoded polypeptides (SEPs) in cell lines and tissue. J. Proteome Res. 13, 1757–1765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackowiak SD et al. Extensive identification and analysis of conserved small ORFs in animals. Genome Biol. 16, 179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Orera J, Messeguer X, Subirana JA & Alba MM Long non-coding RNAs as a source of new peptides. Elife 3, e03523 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaid AG et al. Chemoproteomic discovery of cysteine-containing human short open reading frames. J. Am. Chem. Soc. 135, 16750–16753 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavoff SA et al. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat. Chem. Biol. 9, 59–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H et al. Integration of mass spectrometry and RNA-Seq data to confirm human ab initio predicted genes and lncRNAs. Proteomics 14, 2760–2768 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Zhang C et al. Systematic analysis of missing proteins provides clues to help define all of the protein-coding genes on human chromosome 1. J. Proteome Res. 13, 114–125 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Vanderperre B et al. Direct detection of alternative open reading frames translation products in human significantly expands the proteome. PLoS One 8, e70698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilhelm M et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Subramanian A et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 171, 1437–1452 e1417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nassa M et al. Analysis of human collagen sequences. Bioinformation 8, 26–33 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breit SN, Tsai VW & Brown DA Targeting Obesity and Cachexia: Identification of the GFRAL Receptor-MIC-1/GDF15 Pathway. Trends Mol. Med. 23, 1065–1067 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Mullican SE & Rangwala SM Uniting GDF15 and GFRAL: Therapeutic Opportunities in Obesity and Beyond. Trends Endocrinol. Metab. 29, 560–570 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Baroni M et al. Distinct response to GDF15 knockdown in pediatric and adult glioblastoma cell lines. J. Neurooncol. 139, 51–60 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Huang CY et al. Molecular alterations in prostate carcinomas that associate with in vivo exposure to chemotherapy: identification of a cytoprotective mechanism involving growth differentiation factor 15. Clin. Cancer Res. 13, 5825–5833 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Ratnam NM et al. NF-kappaB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J. Clin. Invest. 127, 3796–3809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corre J et al. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 72, 1395–1406 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Peake BF, Eze SM, Yang L, Castellino RC & Nahta R Growth differentiation factor 15 mediates epithelial mesenchymal transition and invasion of breast cancers through IGF-1R-FoxM1 signaling. Oncotarget 8, 94393–94406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez TF et al. Accurate annotation of human protein-coding small open reading frames. Nat. Chem. Biol. 16, 458–468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J et al. Pervasive functional translation of noncanonical human open reading frames. Science 367, 1140–1146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-Only References
- 45.Xie W et al. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 153, 1134–1148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J et al. Evolutionary analysis across mammals reveals distinct classes of long non-coding RNAs. Genome Biol. 17, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu SJ et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen TN, Brunak S, von Heijne G & Nielsen H SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Kelley LA, Mezulis S, Yates CM, Wass MN & Sternberg MJ The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domazet-Loso T, Brajkovic J & Tautz D A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 23, 533–539 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Domazet-Loso T et al. No Evidence for Phylostratigraphic Bias Impacting Inferences on Patterns of Gene Emergence and Evolution. Mol. Biol. Evol 34, 843–856 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S, Stecher G, Suleski M & Hedges SB TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 34, 1812–1819 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Yang X et al. A public genome-scale lentiviral expression library of human ORFs. Nat. Methods 8, 659–661 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian A et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross Z, Wickham H, Robinson D Declutter your R workflow with tidy tools. Preprint at https://peerj.com/preprints/3180.pdf (2017).
- 56.Enache OM et al. The GCTx format and cmap{Py, R, M, J} packages: resources for optimized storage and integrated traversal of annotated dense matrices. Bioinformatics 35, 1427–1429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doench JG et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piccioni F, Younger ST & Root DE Pooled Lentiviral-Delivery Genetic Screens. Curr. Protoc. Mol. Biol 121, 32 31 31–32 31 21 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Meyers RM et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet 49, 1779–1784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart T, Brown KR, Sircoulomb F, Rottapel R & Moffat J Measuring error rates in genomic perturbation screens: gold standards for human functional genomics. Mol. Syst. Biol 10, 733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae S, Park J & Kim JS Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu C et al. High-throughput identification of genotype-specific cancer vulnerabilities in mixtures of barcoded tumor cell lines. Nat. Biotechnol. 34, 419–423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinello L et al. Analyzing CRISPR genome-editing experiments with CRISPResso. Nat. Biotechnol. 34, 695–697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niknafs YS et al. MiPanda: A Resource for Analyzing and Visualizing Next-Generation Sequencing Transcriptomics Data. Neoplasia 20, 1144–1149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shevchenko A, Wilm M, Vorm O & Mann M Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem 68, 850–858 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Peng J & Gygi SP Proteomics: the move to mixtures. J. Mass Spectrom. 36, 1083–1091 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Eng JK, McCormack AL & Yates JR An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom 5, 976–989 (1994). [DOI] [PubMed] [Google Scholar]
- 68.Beausoleil SA, Villen J, Gerber SA, Rush J & Gygi SP A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol 24, 1285–1292 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Jones DT & Cozzetto D DISOPRED3: precise disordered region predictions with annotated protein-binding activity. Bioinformatics 31, 857–863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Processed data for CRISPR screens (in Figure 3 and Figure 4d) are available in Supplementary Tables 22 and 27. Raw data are available in the Source Data files accompanying this manuscript as well as through the NCBI Sequence Read Archive (SRA) at: SRR13126801, SRR13128583, SRR13132373, SRR13142215 and SRR13142421. Mass spectrometry data relating to Figure 1 are available in Supplementary Table 14. Raw MS spectra are available through the original datasets at: https://cptac-data-portal.georgetown.edu/study-summary/S060 (CPTAC2_BRCA_prosp), https://cptac-data-portal.georgetown.edu/study-summary/S045 (CPTAC2_COAD_prosp), https://cptac-data-portal.georgetown.edu/study-summary/S050 (CPTAC3_ccRCC), https://cptac-data-portal.georgetown.edu/study-summary/S056 (CPTAC3_LUAD), https://cptac-data-portal.georgetown.edu/study-summary/S051 (CPTAC3_PTRC_DP1), https://cptac-data-portal.georgetown.edu/study-summary/S053 (CPTAC3_UCEC), ftp://massive.ucsd.edu/MSV000080527 (HLA_Abelin), ftp://massive.ucsd.edu/MSV000084787 (HLA_Ouspenskaia), ftp://massive.ucsd.edu/MSV000084172/; ftp://massive.ucsd.edu/MSV000080527; ftp://massive.ucsd.edu/MSV000084442/ (HLA_Sarkizova), ftp://massive.ucsd.edu/MSV000082644 (CPTAC Medulloblastoma), http://www.peptideatlas.org (PeptideAtlas database). L1000 data relating to Figure 2 and Supplementary Figures 8 & 9 is available through the NIH LINCS program and at https://clue.io/data. The website lincsproject.org provides information about the LINCS consortium, including data standards. Please see the Life Sciences Reporting Summary accompanying this manuscript for more details.