Abstract
Background: Thyroid hormone (triiodothyronine [T3]) plays an important role in regulating vertebrate developmental, cellular, and metabolic processes via T3 receptor (TR). Liganded TR recruit coactivator complexes that include steroid receptor coactivators (SRC1, SRC2 or SRC3), which are histone acetyltransferases, to T3-responsive promoters. The functions of endogenous coactivators during T3-dependent mammalian adult organ development remain largely unclear, in part, due to the difficulty to access and manipulate late-stage embryos and neonates. We use Xenopus metamorphosis as a model for postembryonic development in vertebrates. This process is controlled by T3, involves drastic changes in every organ/tissue, and can be easily manipulated. We have previously found that SRC3 was upregulated in the intestine during amphibian metamorphosis.
Methods: To determine the function of endogenous SRC3 during intestinal remodeling, we have generated Xenopus tropicalis animals lacking a functional SRC3 gene and analyzed the resulting phenotype.
Results: Although removing SRC3 had no apparent effect on external development and animal gross morphology, the SRC3 (−/−) tadpoles displayed a reduction in the acetylation of histone H4 in the intestine compared with that in wild-type animals. Further, the expression of TR target genes was also reduced in SRC3 (−/−) tadpoles during intestinal remodeling. Importantly, SRC3 (−/−) tadpoles had inhibited/delayed intestinal remodeling during natural and T3-induced metamorphosis, including reduced adult intestinal stem cell proliferation and apoptosis of larval epithelial cells.
Conclusion: Our results, thus, demonstrate that SRC3 is a critical component of the TR-signaling pathway in vivo during intestinal remodeling.
Keywords: metamorphosis, Xenopus tropicalis, thyroid hormone receptor, SRC3, intestine, stem cells, apoptosis
Introduction
Thyroid hormone (triiodothyronine [T3]) is critical for development throughout vertebrates, including humans, amphibians, and fishes (1). In humans, high levels of T3 are present during the period of several months around birth (2), and T3 deficiency during this period causes severe developmental problems including improper maturation of the brain and intestine, and cretinism (3). T3 functions through T3 receptors (TRs). In the absence of T3, TRs form heterodimers with the receptors for 9-cis retinoic acid (the retinoid X receptors or RXRs) to recruit corepressors such as nuclear receptor corepressor N-CoR and the silencing mediator of retinoid and thyroid hormone receptors (SMRT) (4,5), which exist in large protein complexes containing histone deacetylases (HDAC) (6,7), to repress T3-inducible genes.
When T3 is present, TR/RXR heterodimers recruit coactivators to induce gene expression (8–10). Coactivators for TR include histone acetyltransferases (HATs) such as steroid receptor coactivators (SRCs) (11–15) and p300/CREB-binding protein (CBP) (16,17) and the histone methyltransferases such as PRMT1 and CARM1 (18,19). The SRC family contains three isoforms SRC-1, SRC-2, and SRC-3 (20). They bind to TR and other nuclear receptors in an agonist-dependent manner through LXXLL (L, Leucine; X, any amino acid) motifs, and they help to recruit other coactivators such as p300/CBP (16,21–23) into large complexes that can modify histones. These coactivators possess intrinsic HAT activity, and thus may regulate target gene expression in part through local histone acetylation. However, it remains unclear whether and how TR utilizes these coactivators in vivo, especially during vertebrate development, in part due to the difficulty to study this in mammals as the embryos and neonates depend on maternal supply for survival.
Because anuran amphibians develop externally in a biphasic process that is independent of maternal influence, we have utilized the T3-dependent metamorphosis in Xenopus tropicalis as a model to study the role of coactivators in TR function in development (24). During the second phase of anuran development, the tadpole undergoes metamorphosis to become a frog in a process that affects essentially all organs/tissues and can be easily manipulated by controlling the availability of T3 to tadpole rearing water (8,25,26). Notably, intestinal metamorphosis involves T3-dependent de novo formation of adult stem cells and mimics intestinal maturation during postembryonic mouse development (27). Thus, anuran metamorphosis is an advantageous and unique model to study not only how T3 regulates postembryonic vertebrate development but also how adult organ-specific stem cells are formed during vertebrate development, a critical problem that has been difficult to study in other species, including mammals.
Using the Xenopus model, we have previously reported that SRC3 expression in the intestine peaks at a climax of metamorphosis (stage 62) when larval epithelium is almost completely replaced by the newly formed, proliferating adult epithelial stem cells (28). Further, transgenic expression of a dominant negative SRC3 inhibits metamorphosis by blocking the activation of T3-responsive genes, demonstrating that coactivator recruitment is required for the developmental effects of T3 in vivo (29). However, the function of endogenous SRC3 during intestinal remodeling is still unclear. Here, we used the recently developed CRISPR-mediated gene knockout technology to test the hypothesis that endogenous SRC3 regulates intestinal remodeling, including the formation/proliferation of adult stem cells, by functioning as a TR coactivator.
Materials and Methods
All experiments involving X. tropicalis animals were carried out as approved by the National Institute of Child Health and Human Development Animal Use and Care Committee. The details are in the Supplementary Materials and Methods (Supplementary Data).
Results
Disrupting SRC3 leads to reduction in histone acetylation without apparent effect on overall animal development
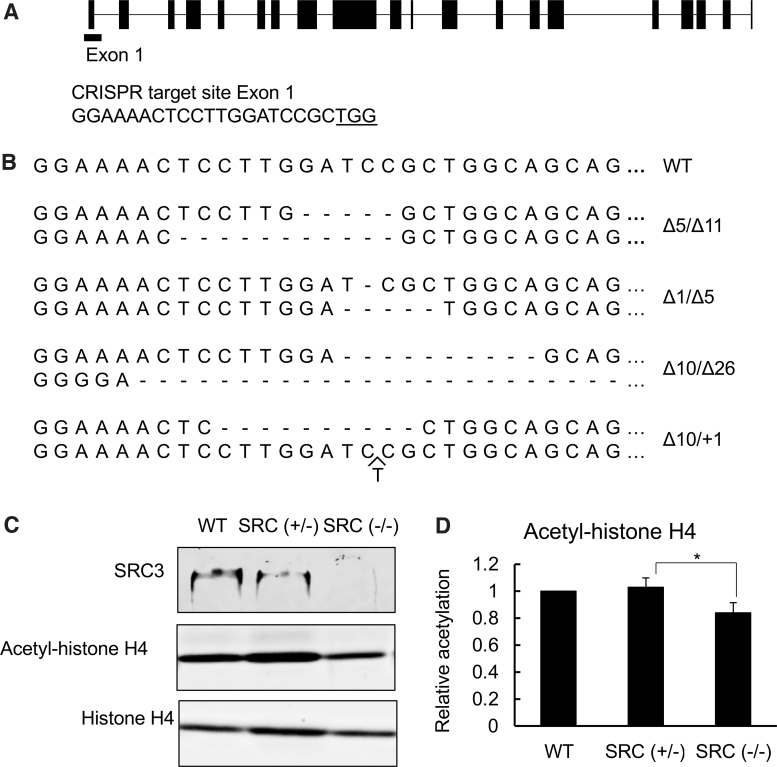
We designed an SRC3-specific gRNA targeting exon 1 of X. tropicalis SRC3 (Fig. 1A) and microinjected it together with Cas9 into fertilized egg to obtain mosaic mutant animals. After sex maturation, genomic DNA was extracted from skin to determine the out-of-frame mutation rate induced by the Cas9 RNA-guided endonuclease in the gRNA targeted region in SRC3 by using a color assay (30). Adult frogs with high rates of out-of-frame mutations (30% or more) were thus identified (data not shown), and they were crossed among each other to generate F1 animals. At stage 52 or other indicated stages, we extracted genomic DNA from tail clips and genotyped F1 tadpoles by sequencing to identify tadpoles with one wild type (WT) SRC3 allele and an out-of-frame mutation in the other SRC allele, that is, heterozygous mutants or SRC3 (+/−) tadpoles, and tadpoles with an out-of-frame mutation in each of the two SRC3 alleles, that is, homozygous mutants or SRC3 (−/−) tadpoles (Fig. 1B, C). To verify SRC3 knockout, Western blot analysis of the whole body protein samples from WT, SRC3 (+/−), and SRC3 (−/−) tadpoles at stage 46 was performed. The expected SRC3 protein of 152 kDa was detected in WT tadpoles, and consistent with the mutation in one SRC3 allele, the signal intensity in SRC3 (+/−) was weaker than that in WT (Fig. 1C). Importantly, SRC3 (−/−) tadpoles lack any detectable SRC3 protein (Fig. 1C). Further, when we analyzed acetylated histone H4 in whole animals, we observed that its level was reduced in homozygous SRC3 knockout tadpoles, although no significant difference was observed between WT and SRC3 (+/−) tadpoles (Fig. 1D), consistent with the fact that SRC3 is an HAT.
FIG. 1.
SRC3 knockout reduces histone H4 acetylation. (A) Schematic representation of Xenopus tropicalis SRC3 and the gRNA targeting exon 1. The gRNA complementary sequence is shown at bottom. The underline indicates the PAM sequence. (B) Sequences of the gRNA target site of the WT (top) and total knockout F1 animals. Deletions are indicated by dashes. Note that when each of the two alleles has out-of-frame deletion/insertion (i.e., with a net change in a length not divisible by 3), the resulting tadpole lacks any functional SRC3 gene and is thus considered a homozygous SRC3 knockout tadpole. (C, D) Western blots showing that homozygous SRC3 knockout tadpoles have no detectable SRC3 protein and reduced histone H4 acetylation. Total protein was isolated from the whole body at stage 46 and subjected to Western blotting with antibodies against SRC3, acetyl-histone H4, or histone H4 (C). The signal intensity of the bands was measured by image j and presented as the mean ± standard deviation of n = 3. Statistical analysis: analysis of one-way variance followed by Tukey analysis. *p < 0.05. SRC, steroid receptor coactivator; WT, wild type.
To determine the effect of SRC3 knockout, we measured the developmental rate of WT and SRC3 mutant tadpoles. We recorded the time for the animals to develop from fertilization to stage 54 (the onset of metamorphosis), or stage 58 (the beginning of metamorphic climax), and the time from stage 58 to stage 66 (the end of metamorphosis). We found that there was no difference among the three genotypes, WT, SRC3 (+/−), and SRC3 (−/−), for all periods measured (Supplementary Fig. S1), suggesting that SRC3 knockout does not affect gross animal development, at least based on external morphological criteria, possibly due to redundancy among three SRCs.
SRC3 is important for intestinal remodeling during natural metamorphosis
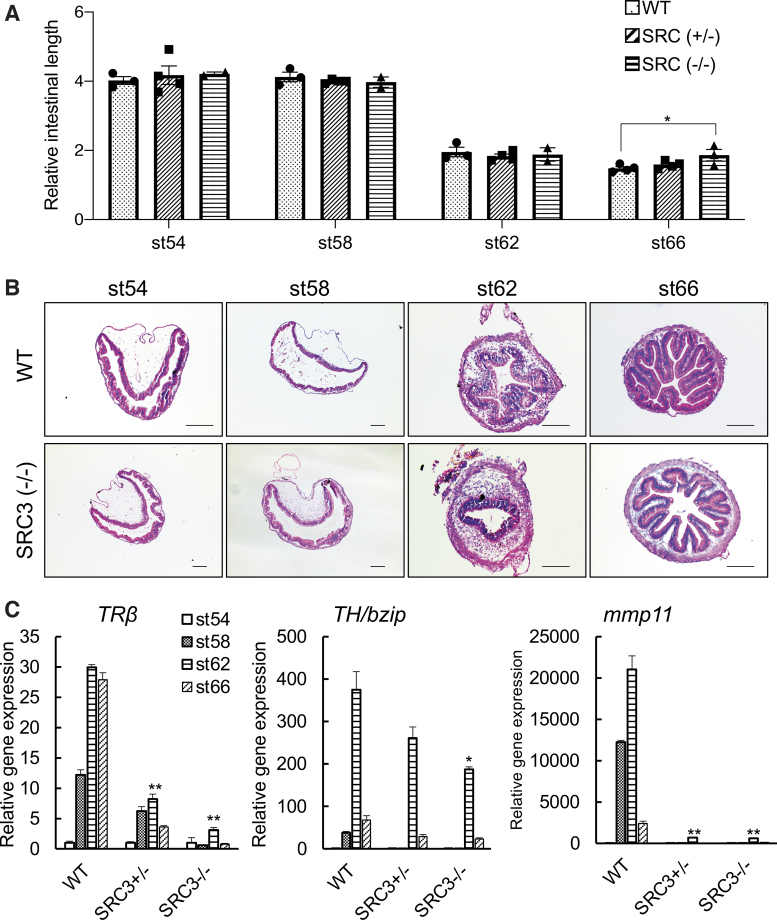
We next investigated whether SRC3 knockout had any effect on the intestine during metamorphosis. Intestinal remodeling involves near complete degeneration of the larval epithelium with a single epithelial fold and de novo development of a multiply folded adult epithelium via the formation of adult epithelial stem cells and their subsequent proliferation and differentiation, accompanied by drastic shortening of the length of the intestine. Thus, we first measured intestine length during metamorphosis. Since individual animals of the same age and/or stage can vary in size, we normalized the length of the intestine against the length of the body (from tip of the head to the end of the belly). As expected, we observed that the length of the WT intestine was shortened by more than two-fold by the climax of metamorphosis (stage 62) and a little further by the end of metamorphosis (stage 66) (Fig. 2A). Heterozygous SRC3 knockout animals behaved similarly. However, the homozygous SRC3 knockout animals had a longer intestine compared with the other two genotypes at the end of metamorphosis, suggesting that SRC3 knockout delayed or inhibited intestinal remodeling during natural metamorphosis.
FIG. 2.
SRC3 knockout reduces TR target gene expression in the intestine and delays intestinal remodeling during metamorphosis. (A) Relative intestinal length is longer in SRC3 (−/−) tadpoles at the end of metamorphosis (stage 66). Sibling tadpoles of the three genotypes at stage 54, 58, 62, and 66 were euthanized for measurement of the body length (from tip of the head to the end of the belly) and the length of the intestine. The relative intestine length normalized against the body length was presented as the mean ± standard deviation of. Note that the relative intestine length in the homozygous knockout animals was longer compared with the other two genotypes at the end of metamorphosis (*p < 0.05). (B) SRC3 knockout delays intestinal remodeling at the climax of the metamorphosis (stage 62). Cross-sections of the intestine from WT and SRC3 (−/−) tadpoles at indicated stages during natural metamorphosis were stained with methyl green-pyronin Y (with methyl green staining the DNA blue and pyronin-Y staining the RNA red). Note that at stage 62, numerous adult intestinal epithelial folds were formed in the WT but not SRC3 (−/−) tadpoles, indicating a delay in adult intestinal development. Scale bar indicates 20 μm. (C) The expression of known TR target genes TRβ, TH/bzip, and mmp11 is reduced in the intestine of SRC3 knockout tadpoles during natural metamorphosis. Intestinal RNA was isolated from stage-matched sibling animals of the three genotypes and analyzed by qRT-PCR. The expression levels, after normalization against the control gene rpl8, from at least three samples per genotype were presented as the mean fold induction ± SEM relative to that at stage 54. *p < 0.05; **p < 0.01 vs. WT at stage 62. qRT-PCR, quantitative real-time-polymerase chain reaction; T3, triiodothyronine; TR, T3 receptor.
To investigate whether SRC3 knockout affected cellular transformation, we stained cross-sections of the intestine from WT and SRC3 (−/−) tadpoles at different stages. The result showed that at stage 62, numerous adult intestinal epithelial folds were formed in the WT but not SRC3 (−/−) tadpoles (Fig. 2B), indicating a delay/inhibition in adult intestinal development by the SRC3 knockout. Further, both epithelial cell death and proliferation were easily detected at the climax of metamorphosis (stage 62) (Supplementary Fig. S2) when drastic intestinal remodeling took place in the WT intestine. The signals were much less in SRC3 (−/−) tadpoles, supporting an important role of SRC3 during intestinal remodeling.
To determine whether SRC3 affected intestinal metamorphosis via functioning as a TR coactivator, we analyzed the expression of TR target genes during intestinal remodeling. We chose three well-known, direct, and ubiquitous T3-responsive genes, TRβ (31), TH/bzip (32), and mmp11 (33). As shown in Figure 2C, SRC3 knockout reduced the upregulation of all three genes in the intestine during natural metamorphosis, with stronger effect by the homozygous knockout compared with heterozygous knockout, suggesting that SRC3 functions as a TR coactivator during intestinal remodeling.
SRC3 knockout inhibits gene activation and intestinal remodeling during T3-induced metamorphosis
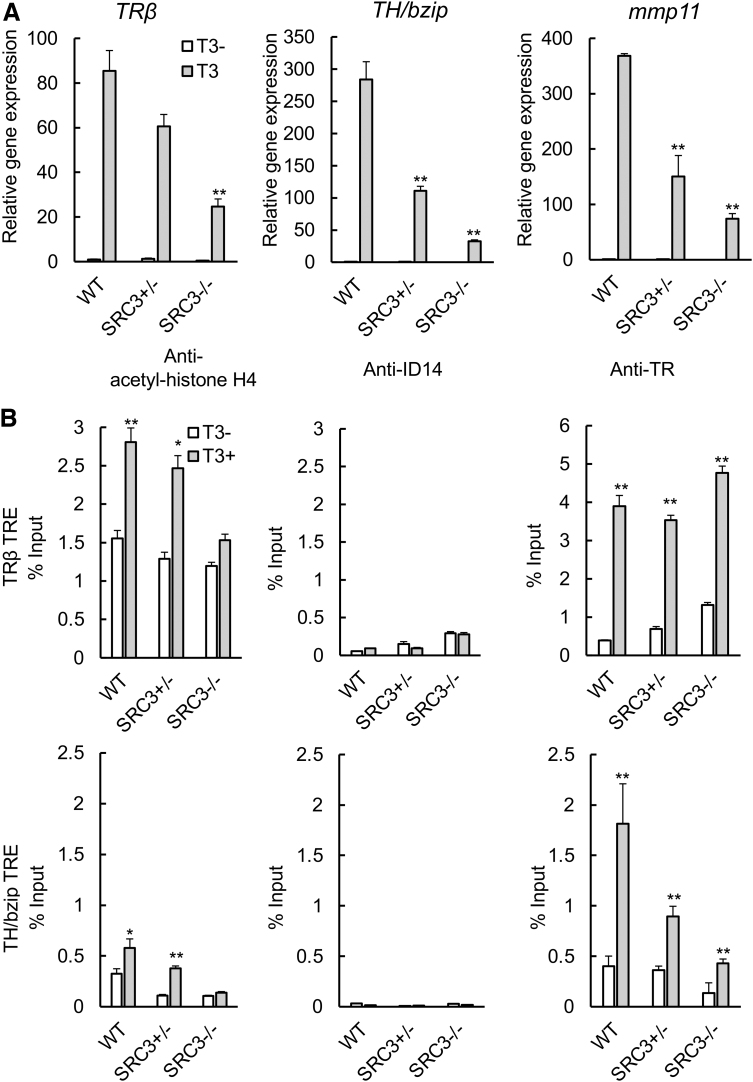
Natural metamorphosis is a lengthy process, requiring four weeks or longer for the tadpole to develop from stage 54 (onset of metamorphosis) to stage 66 (end of metamorphosis). This may enable various endogenous compensatory mechanisms to reduce the potential effects of SRC3 knockout, consequently leading to relatively small effects due to the knockout. In addition, there is also considerable heterogeneity among sibling animals during such a lengthy developmental process, making it difficult to measure knockout effect. To overcome these issues, we next took advantage of the fact that many processes during anuran metamorphosis, including intestinal remodeling, can be induced by treating premetamorphic tadpoles with physiological concentrations of T3 for as little as three days. We first treated premetamorphic WT and SRC3 knockout tadpoles at stage 54 with 10 nM T3 for 18 hours and isolated total RNA from the intestine for analysis of the three known TR target genes, as studied earlier. All three were strongly induced by T3 in WT animals (Fig. 3A). Their induction was reduced in SRC3 (+/−) and SRC3 (−/−) animals, with a much bigger effect seen in homozygous knockout animals (Fig. 3A).
FIG. 3.
SRC3 knockout reduces T3-induced target gene expression, accompanied by reduced histone H4 acetylation but without affecting TR binding. (A) SRC3 knockout reduces gene activation by T3 in the intestine. The intestine was isolated from WT, SRC3+/−, and SRC3−/− tadpoles at stage 54 with or without T3 treatment for 18 hours for RNA isolation and qRT-PCR analysis of the indicated TR target genes. **p < 0.01 for T3 treated vs. WT intestine. (B) Histone acetylation but not TR binding is decreased at TR target genes in the intestine of SRC3−/− tadpoles at stage 54 treated with T3. ChIP assay was performed on intestine samples as in (A) with antibodies against acetyl-histone H4 and TR or the control ID14, an extracellular protein. The presence of the genomic DNA of the TRβ TRE region (top) or TH/bzip TRE (bottom) was analyzed by qPCR. Error bars indicate SEM. *p < 0.05 and ** p < 0.01 vs. T3−. qRT-PCR, quantitative real-time-polymerase chain reaction.
To investigate how SRC3 knockout affected TR target genes, we carried out ChIP assays to measure the binding of TR to and association of histone H4 acetylation with the TREs of two well-known target genes, TRβ and TH/bzip, in the animals. As shown in Figure 3B, H4 acetylation was specifically increased by T3 treatment at the TRE regions of both genes but not the negative control region, the exon 5 of TRβ gene that lacks any TRE (Supplementary Fig. S3), in the WT intestine. This increase was reduced in the SRC3 (+/−) intestine and essentially abolished in the SRC3 (−/−) intestine (Fig. 3B). As a control, we also carried out ChIP assay by using an antibody against an extracellular protein encoded by X. laevis gene ID14 and observed only low background signals (Fig. 3B and Supplementary Fig. S3). We further analyzed the binding of TR to the TREs and found that expectedly, TR was specifically bound to the TREs of both genes but not the exon 5 of the TRβ gene in the intestine and this binding was not affected by SRC3 knockout (Fig. 3B and Supplementary Fig. S3). These results suggest that SRC3 is an important TR coactivator in the intestine.
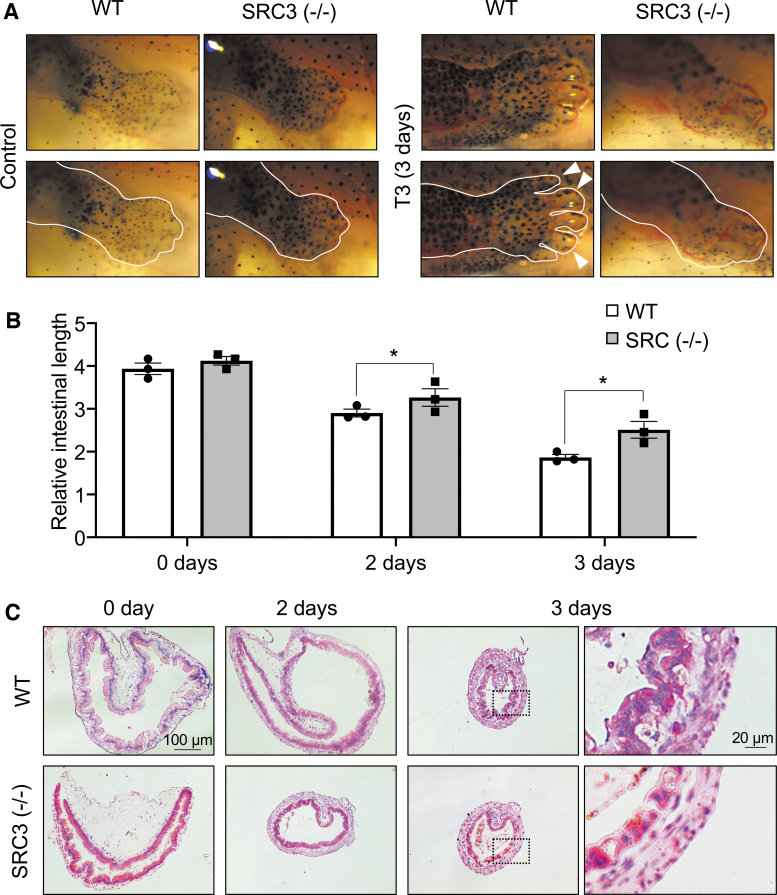
To study the effect of SRC3 (−/−) on T3-induced metamorphosis, WT and knockout siblings at premetamorphic stage 54 were treated with 10 nM T3 for up to three days. As shown in Figure 4A, three day of T3 treatment led to dramatic metamorphic changes in the WT animals, most noticeably limb development (digit formation) and reshaping of the head structure, which is, in part, due to resorption of the gills (Fig. 4A and Supplementary Fig. S4). In SRC3 knockout animals, T3-induced limb development was inhibited (Fig. 4A). In addition, structural changes in the head region, including gill resorption, was also reduced compared with the WT ones (Fig. 4A and Supplementary Fig. S4). Thus, SRC3 is important for T3-induced external metamorphic changes.
FIG. 4.
SRC3 knockout inhibits T3-induced limb development and intestinal remodeling. (A) SRC knockout inhibits T3-induced limb metamorphosis. WT and SRC3 knockout (SRC3−/−) tadpoles at stage 54 were treated with 10 nM T3 for three days at 25°C, and the hindlimb region was photographed. The lower panels were the same as the top panels, except with the added white line marking the approximate boundary of the outer limb epithelium and/or the boundaries of the digits (white arrowheads indicate some of the digits of the WT animal treated with T3). Note that limb development (digit formation) is the earliest and most dramatic early event in the WT animals treated with T3. Quantitative analysis revealed that the T3-induced gill resorption was also inhibited in the knockout tadpoles (Supplementary Fig. S4). (B) T3-induced intestinal shortening is reduced in the knockout tadpoles. The length of the intestine in WT and SRC3−/− tadpoles as in (A) was measured and normalized against body length. Error bars indicate SEM. *p < 0.05. (C) Adult epithelium development is inhibited by SRC3 knockout. Cross-sections of the intestine in WT and SRC3−/− tadpoles as in (A) were stained with methyl green-pyronin Y (staining DNA blue and RNA red). Note that the adult epithelium folds were formed after three days of T3 treatment only in WT but not SRC3−/− tadpoles.
We next first measured intestine length. As expected, T3 induced intestine shortening in WT animals within two days of treatment (Fig. 4B). Importantly, homozygous SRC3 knockout reduced this shortening after two or three days of T3 treatment (Fig. 4B). We further stained intestinal cross-sections and found that after three days of T3 treatment, numerous adult epithelial folds were formed in WT but SRC3 (−/−) tadpoles (Fig. 4C), suggesting that SRC3 is important for T3-induced intestinal remodeling.
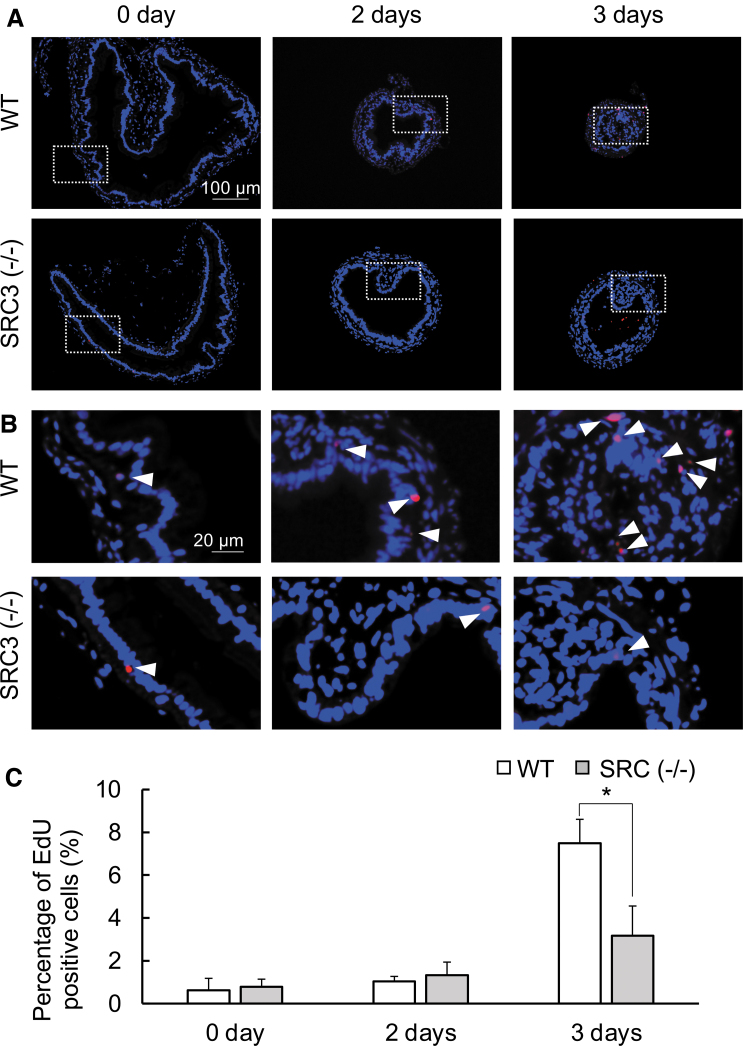
Intestinal metamorphosis involves near total degeneration of larval epithelium via apoptosis and development of adult epithelium via de novo formation of adult epithelial stem cells followed by their proliferation and differentiation. Thus, we next analyzed cell proliferation with EdU labeling. The results showed that expectedly, intestinal epithelial cell proliferation as detected by EdU labeling was induced during T3-induced metamorphosis in WT animals (Fig. 5). In SRC3 knockout animals, in contrast, T3-induced cell proliferation was drastically reduced (Fig. 5A, B). This was further confirmed by quantification of the percentage of EdU-positive cells (Fig. 5C).
FIG. 5.
Adult stem cell proliferation is reduced in T3-treated SRC3 knockout tadpoles. (A) EdU labeling for proliferating cells in WT and SRC3−/− intestine during T3-induced metamorphosis. The intestinal cross-sections from stage 54 tadpoles treated with 10 nM T3 for up to three days were double stained with EdU for cell proliferation (red) and DAPI for DNA (blue). Scale bar indicates 100 μm. (B) The boxed region in (A) is shown at a higher magnification. White arrowheads denote EdU-positive cells. Scale bar indicates 20 μm. (C) Quantification of cell proliferation as detected in (A, B). The EdU-positive areas (red) in the epithelium were quantified with image j software and normalized against the total cellular area in epithelium as determined by DAPI staining. The experiment was repeated twice with similar results. Each bar represents the mean ± SE. *p < 0.05.
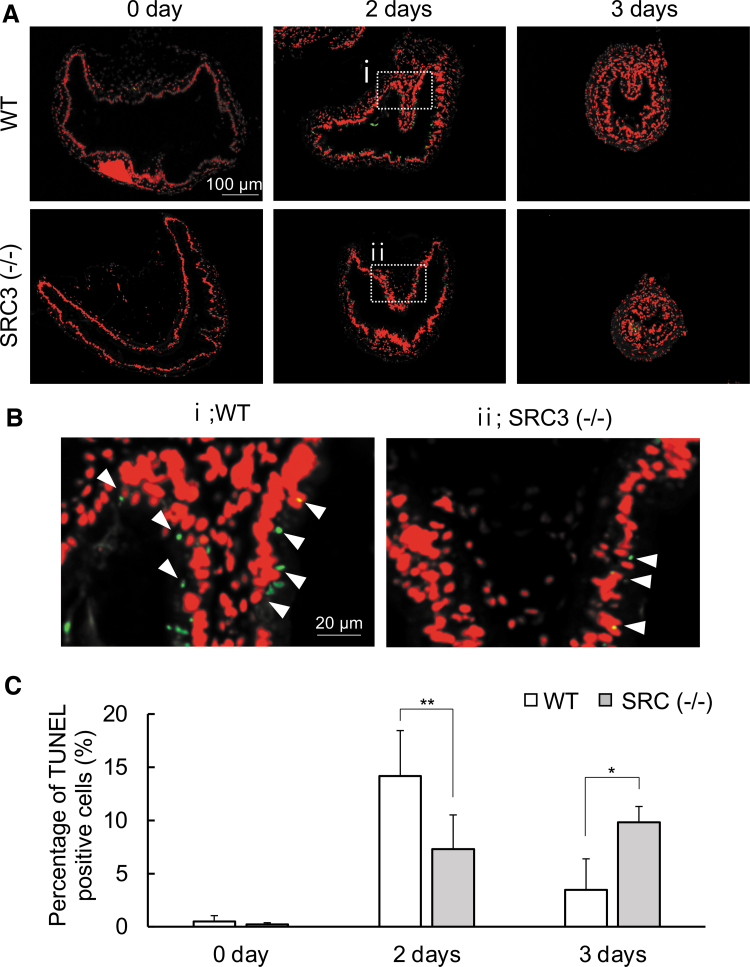
We also investigated apoptosis in the intestine by using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). We found that after two days of T3 treatment, high levels of apoptotic signals were present in the WT intestinal epithelium (Fig. 6A, B) and that the signals decreased in the WT intestine after three days of treatment (Fig. 6C) when adult cell proliferation took place (Fig. 5). In SRC3 (−/−) animals, T3-induced apoptosis in the intestinal epithelium was much lower than that in WT animals after two days of T3 treatment (Fig. 6A, B). This was again confirmed by quantifying the TUNEL signals (Fig. 6C). Apoptosis was higher in SRC3 (−/−) animals compared with WT animals after three days of T3 treatment (Fig. 6C), suggesting that T3-induced apoptosis was delayed in SRC3 (−/−) tadpoles.
FIG. 6.
Apoptosis is reduced in SRC3 knockout tadpoles. (A) TUNEL labeling for apoptotic cells in WT and SRC3−/− intestine during T3-induced metamorphosis. The cross-sections of the intestine from stage 54 tadpoles treated with 10 nM T3 for 0–3 days were double stained with TUNEL for apoptotic cells (green) and with PI for DNA (red). Scale bar indicates 100 μm. (B) The boxed region in (A) is shown at a higher magnification. White arrowheads denote TUNEL-positive cells. Scale bar indicates 20 μm. (C) Quantification of apoptosis as detected in (A, B). The TUNEL-positive cells (green) in the epithelium were counted with image j software and normalized against the total cells in epithelium, as determined by PI staining. *p < 0.05 and **p < 0.01. The experiment was repeated three times, with similar results. Each bar represents the mean ± SE. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
The T3-dependent Xenopus intestinal remodeling during metamorphosis as a model of mammalian postembryonic development has provided new insights on the de novo formation of adult organ-specific stem cells in vertebrates (34). Importantly, this process is similar to the development of mammalian adult intestine around the neonatal period (27). Earlier studies of Xenopus intestinal metamorphosis have identified many T3 response genes that are likely involved during Xenopus intestinal remodeling. Among them is the TR-coactivator SRC3 (28,29,35). There are many known TR-coactivators, which often function as coactivators for other nuclear hormone receptors as well. Gene knockout studies in mouse have shown that removing coactivators causes various developmental defects (36). However, it is difficult to determine the molecular mechanisms since the coactivators are often involved in gene regulation by many nuclear hormone receptors and other transcription factors. This is further compounded by the difficulty to manipulate mammalian postembryonic development. Taking advantage of many unique properties of anuran metamorphosis, our results here thus provide for the first time in vivo evidence to support an important role of an endogenous coactivator, the HAT SRC3, in intestinal metamorphosis by functioning as a TR coactivator and increasing local histone acetylation either directly through its own acetyltransferase activity or indirectly through recruitments of other acetyltransferases such as CBP/P300 as a TR-coactivator complex.
Intestinal remodeling during Xenopus metamorphosis involves near-complete degeneration of the larval epithelial through apoptosis and de novo formation of adult epithelial stem cells in a process totally regulated by T3 (37–40). Earlier studies have shown that the HAT SRC3 is upregulated during Xenopus intestinal metamorphosis and can function as a TR coactivator, suggesting that SRC3 is upregulated by T3 and, in turn, feeds back positively by functioning as a TR coactivator to further enhance T3 signaling during intestinal metamorphosis. Consistently, we have shown here that SRC3 knockout leads to a delay in intestinal remodeling with a reduction of intestinal stem cell proliferation and apoptosis of larval epithelial cells during both natural and T3-induced metamorphosis. Mechanistically, we found that the expression of TR response genes is strongly reduced in SRC3 knockout tadpoles and that SRC3 knockout results in reduced histone acetylation at a TR response gene without affecting TR binding to the TRE. Thus, endogenous SRC3 appears to function as a TR coactivator to increase local histone acetylation to enhance the promoter activity, thereby ensuring proper intestinal remodeling during metamorphosis. It is worth noting that SRC3 is likely recruited to TR target genes during metamorphosis as a large complex containing P300/CBP (29,35), which have stronger HAT activities. Thus, the effects of SRC3 knockout on local histone acetylation might be due to the failure to recruit P300/CBP via the SRC3-P300/CBP complex.
Unliganded TR binding to the TRE region recruits corepressors such as N-CoR and SMRT to a target promoter, whereas ligand-bound TR recruits co-activators such as SRC1, SRC2, and SRC3. Among the three members of the SRC family of coactivators, SRC3 expression has been shown to be drastically increased during intestinal metamorphosis (28). Thus, somewhat surprisingly, SRC3 knockout has little effect on overall tadpole development, at least based on external morphology. Even for the intestine, the effects of SRC3 knockout appear to be fairly modest during natural metamorphosis, with a slightly longer intestine at the end of metamorphosis, suggesting a slight delay/inhibition of the remodeling process. Such mild effects of the knockout likely reflect potential redundant functions among the three SRC family members, as also suggested by genetic studies in mouse. Total knockout of mouse SRC3 also has a relatively mild effect, with the resulting animals having retarded growth, delayed puberty, decreased reproductive function, and blunted mammary gland development (36). In addition, the mice lacking SRC1 have even less defect, although having blunted mammary gland development (41). On the other hand, double knockout of SRC1 and SRC3 leads to embryonic lethality (42), supporting compensatory effects of the two members. Thus, it would be interesting to investigate the role of other SRC family members during anuran metamorphosis.
By using T3-induced metamorphosis under physiological levels of T3, we were able to observe much bigger effects of the SRC3 knockout on metamorphosis, based on both external morphology (limb development and gill resorption) and intestinal remodeling. This was likely due to the fact that T3-induced metamorphosis occurs in a much shorter time frame and more synchronous manner, thus reducing the variations among animals and the likelihood of compensatory events that knockout animals may have in a much longer natural metamorphic process. Our findings here, therefore, not only reveal, for the first time, an important role of an endogenous TR coactivator in T3 signaling and the formation/proliferation of adult organ-specific stem cell, but also further highlight the advantages of the anuran metamorphosis for studying adult organ development/maturation and gene function during postembryonic development.
Supplementary Material
Acknowledgment
The authors thank Nga Luu for experimental help and laboratory management.
Author Contributions
Y.T. and Y.B.-S. designed the research plan, L.B. generated the F0 mutant animals, and Y.T. carried out the research and data analyses. All authors participated in the article preparation and approved the final version of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the intramural Research Program of NICHD, NIH. Y.T. was supported in part by a Japan Society for the Promotion of Science Research Fellowship for Japanese Biomedical and Behavioral Researchers at the National Institutes of Health (No. 29-71715).
Supplementary Material
References
- 1. Yen PM, Heart B. 2001. Physiological and molecular basis of Thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 2. Fisher DA 2002. Developmental Endoccrinology. Humana Press, New Jersey [Google Scholar]
- 3. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. 2018. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 14:301–316 [DOI] [PubMed] [Google Scholar]
- 4. Chen JD, Evans RM. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- 5. Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK, Rosenfeld MG. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- 6. Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev 14:1048–1057 [PMC free article] [PubMed] [Google Scholar]
- 7. Jones PL, Shi Y-B 2003 N-CoR-HDAC corepressor complexes: roles in transcriptional regulation by nuclear hormone receptors. In: Workman JL (ed) Protein Complexes that Modify Chromatin. Springer-Verlag Berlin Heidelberg, New York, pp 237–268 [DOI] [PubMed] [Google Scholar]
- 8. Buchholz DR, Paul BD, Fu L, Shi Y-B. 2006. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- 9. Cheng S-Y, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y-B, Matsuura K, Fujimoto K, Wen L, Fu L. 2012. Thyroid hormone receptor actions on transcription in amphibia: the roles of histone modification and chromatin disruption. Cell Biosci 2:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oñate SA, Tsai SY, Tsai M-J, O'Malley BW. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science (80-) 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- 12. Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667–3675 [PMC free article] [PubMed] [Google Scholar]
- 13. Anzick SL 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science (80-) 277:965–968 [DOI] [PubMed] [Google Scholar]
- 14. Suen C-S, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE. 1998. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem 273:27645–27653 [DOI] [PubMed] [Google Scholar]
- 15. Takeshita A, Cardona GR, Koibuchi N, Suen C-S, Chin WW. 1997. TRAM-1, A Novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem 272:27629–27634 [DOI] [PubMed] [Google Scholar]
- 16. Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580 [DOI] [PubMed] [Google Scholar]
- 17. Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677–684 [DOI] [PubMed] [Google Scholar]
- 18. Chen D 1999. Regulation of transcription by a protein methyltransferase. Science (80-) 284:2174–2177 [DOI] [PubMed] [Google Scholar]
- 19. Koh SS, Chen D, Lee Y-H, Stallcup MR. 2001. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem 276:1089–1098 [DOI] [PubMed] [Google Scholar]
- 20. Leo C, Chen JD. 2000. The SRC family of nuclear receptor coactivators. Gene 245:1–11 [DOI] [PubMed] [Google Scholar]
- 21. Yao TP, Ku G, Zhou N, Scully R, Livingston DM. 1996. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci U S A 93:10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voegel JJ 1998. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J 17:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, Evans RM, Wright PE. 2002. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415:549–553 [DOI] [PubMed] [Google Scholar]
- 24. Shi Y-B 2013 Unliganded thyroid hormone receptor regulates metamorphic timing via the recruitment of histone deacetylase complexes. In: Wassarman PM (ed) Current Topics in Developmental Biology. Elsevier Inc., USA, pp 275–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tata JR 1993. Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 [DOI] [PubMed] [Google Scholar]
- 26. Shi Y-B 1999. Amphibian Metamorphosis: From Morphology to Molecular Biology. John Wiley and Sons Ltd, New York [Google Scholar]
- 27. Buchholz DR 2015. More similar than you think: frog metamorphosis as a model of human perinatal endocrinology. Dev Biol 408:188–195 [DOI] [PubMed] [Google Scholar]
- 28. Paul BD, Shi Y-B. 2003. Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis. Cell Res 13:459–464 [DOI] [PubMed] [Google Scholar]
- 29. Paul BD, Fu L, Buchholz DR, Shi Y-B. 2005. Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol 25:5712–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fu L, Wen L, Luu N, Shi Y-B. 2016. A simple and efficient method to visualize and quantify the efficiency of chromosomal mutations from genome editing. Sci Rep 6:35488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ranjan M, Wong J, Shi YB. 1994. Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem 269:24699–24705 [PubMed] [Google Scholar]
- 32. Furlow JD, Brown DD. 1999. In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol Endocrinol 13:2076–2089 [DOI] [PubMed] [Google Scholar]
- 33. Fu L, Tomita A, Wang H, Buchholz DR, Shi Y-B. 2006. Transcriptional regulation of the Xenopus laevis stromelysin-3 gene by thyroid hormone is mediated by a DNA element in the first intron. J Biol Chem 281:16870–16878 [DOI] [PubMed] [Google Scholar]
- 34. Fu L, Yin J, Shi Y-BB. 2019. Involvement of epigenetic modifications in thyroid hormone-dependent formation of adult intestinal stem cells during amphibian metamorphosis. Gen Comp Endocrinol 271:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paul BD, Buchholz DR, Fu L, Shi YB. 2007. SRC-p300 coactivator complex is required for thyroid hormone-induced amphibian metamorphosis. J Biol Chem 282:7472–7481 [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A 97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi YB, Ishizuya-Oka A. 2001. Thyroid hormone regulation of apoptotic tissue remodeling: implications from molecular analysis of amphibian metamorphosis. Prog Nucleic Acid Res Mol Biol 65:53–1100 [DOI] [PubMed] [Google Scholar]
- 38. Sun G, Fu L, Shi YB. 2014. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci 4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi Y-BB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. 2011. The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishizuya-Oka A, Shimozawa A. 1992. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux Arch Dev Biol 201:322–329 [DOI] [PubMed] [Google Scholar]
- 41. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science (80-) 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Liu Z, Xu J. 2010. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol Endocrinol 1:1917–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.