Abstract
Autism spectrum disorder (ASD) is associated with atypical brain development. However, the phenotype of regionally specific increased cortical thickness observed in ASD may be driven by several independent biological processes that influence the gray/white matter boundary, such as synaptic pruning, myelination, or atypical migration. Here, we propose to use the boundary sharpness coefficient (BSC), a proxy for alterations in microstructure at the cortical gray/white matter boundary, to investigate brain differences in individuals with ASD, including factors that may influence ASD-related heterogeneity (age, sex, and intelligence quotient). Using a vertex-based meta-analysis and a large multicenter structural magnetic resonance imaging (MRI) dataset, with a total of 1136 individuals, 415 with ASD (112 female; 303 male), and 721 controls (283 female; 438 male), we observed that individuals with ASD had significantly greater BSC in the bilateral superior temporal gyrus and left inferior frontal gyrus indicating an abrupt transition (high contrast) between white matter and cortical intensities. Individuals with ASD under 18 had significantly greater BSC in the bilateral superior temporal gyrus and right postcentral gyrus; individuals with ASD over 18 had significantly increased BSC in the bilateral precuneus and superior temporal gyrus. Increases were observed in different brain regions in males and females, with larger effect sizes in females. BSC correlated with ADOS-2 Calibrated Severity Score in individuals with ASD in the right medial temporal pole. Importantly, there was a significant spatial overlap between maps of the effect of diagnosis on BSC when compared with cortical thickness. These results invite studies to use BSC as a possible new measure of cortical development in ASD and to further examine the microstructural underpinnings of BSC-related differences and their impact on measures of cortical morphology.
Keywords: autism spectrum disorder, cerebral cortex, microstructure, myelin, tissue contrast
Introduction
Neuroimaging studies of autism spectrum disorder (ASD) have repeatedly observed early increases in cortical thickness (Anagnostou and Taylor 2011; Courchesne et al. 2011; Hazlett et al. 2017; Park et al. 2018; Bedford et al. 2020) and altered structural and functional connectivity in individuals with ASD, particularly early in life (Just et al. 2012; Rudie et al. 2012; Hahamy et al. 2015). The early developmental period, which is coincident with ASD-onset, is a particularly sensitive time with respect to neuronal migration and cortical myelination. Indeed, neuronal migration disruptions from the ventricular and subventricular zones during early neocortex development have been observed in ASD (Huguet et al. 2013; Pinto et al. 2014; Reiner et al. 2016), potentially leading to the presence of supernumerary neurons proximal to the gray/white matter boundary. Additionally, maturational abnormalities of intracortical myelin, a key cortical maturational feature (Grydeland et al. 2013; Deoni et al. 2015), have been associated with ASD (Zikopoulos and Barbas 2010; Canali et al. 2018; Graciarena et al. 2018). This developmental variation may underlie measures seeking to capture so-called blurring at the interface of the cortex and the underlying superficial white matter, as measured using structural MRI (Andrews et al. 2017; Bezgin et al. 2018; Mann et al. 2018; Norbom et al. 2019). Despite advances in ASD research generated by studying the ratio of the tissue intensities between these two compartments, there are limitations that may complicate the interpretation of this measure as an index of cortical microstructure. Firstly, the ratio of intensities between the tissue classes is dependent on the placement of the boundary that separates them. However, the boundary placement may be influenced by signal ambiguity that arises from a less defined gray/white matter boundary, thereby potentially confounding the measurement of interest. Further, histological studies have shown that more convex regions such as gyral crowns are more myelinated in deeper cortical layers compared with concave regions, such as sulcal folds (Bok 1959; Sereno et al. 2013); thus measures of cortical microstructure are negatively correlated with cortical curvature, as T1-relaxation times are observed to be higher in gyral crowns and lower in sulcal folds (Sereno et al. 2013; Waehnert et al. 2016). In ASD, microstructural measurements obtained using the standard ratio of tissue intensities may be confounded by the well-characterized group differences in gyrification and curvature (Kohli et al. 2018; Libero et al. 2019).
Here, we propose to partly overcome these limitations using our newly developed metric, boundary sharpness coefficient (BSC), defined as the growth rate parameter of a sigmoid curve fit to the cortical intensity profile running perpendicular to the boundary surface (inspired by Avino and Hutsler 2010). Using this new measure that potentially reflects perturbations to neuronal migration and/or intracortical myelination, we performed a large-scale analysis using images collected from multiple acquisition sites and analyzed them with a meta-analytic technique (Bedford et al. 2020). In light of previous findings from our group (Bedford et al. 2020) and others (Anagnostou and Taylor 2011; Courchesne et al. 2011; Schuetze et al. 2016; Hazlett et al. 2017) of increased cortical thickness in ASD, we sought to further examine if previous studies of cortical thickness increases in ASD may have been influenced by cortical boundary abnormalities.
Given results from previous neuroimaging and histological studies (Avino and Hutsler 2010; Andrews et al. 2017; Bezgin et al. 2018; Mann et al. 2018), we expected to find a greater degree of cortical blurring in individuals with ASD relative to typically developing individuals, or a decrease in BSC. Since T1w intensity is thought to reflect underlying myeloarchitecture more so than cytoarchitecture (Eickhoff et al. 2005), blurring may arise from differences in intracortical myelination: an increase in myelin content (and of T1w signal) in the lower layers of the cortex would appear in T1w MRI as a more blurred transition in intensity moving from gray to white matter. An alternative process that may increase cortical blurring in ASD is neuronal migration. Neuronal migration defects are predicted to result in supernumerary neurons in the white matter compartment directly below the cortex (Chun and Shatz 1989; Avino and Hutsler 2010; Andrews et al. 2017), which would also result in a greater degree of blurring captured with BSC.
Another goal of this study was to examine the influence of clinical heterogeneity on BSC. Based on the previous studies of cortical anatomy, we expected age-related differences in BSC to be greatest in younger ASD individuals (Khundrakpam et al. 2017; Bedford et al. 2020), to vary by sex (Irimia et al. 2017; Zeestraten et al. 2017; Greenberg et al. 2018; Lai et al. 2015; Lai et al. 2017) and full-scale IQ (FIQ) (Lotspeich et al. 2004; Bedford et al. 2020).
Materials and Methods
Study Participants
Data included here were acquired from previous studies by the Hospital for Sick Children (Canada), the Cambridge Family Study of Autism (UK), and the UK Medical Research Council Autism Imaging Multicentre Study (UK MRC AIMS). We also included publicly available data from the Autism Brain Imaging Data Exchange (ABIDE) I and II datasets (di Martino et al. 2014, 2017) (Tables 1 and 2). All data used in this study were preprocessed by SB for analysis in Bedford et al. (2020), with an original sample size of 3145 participants (1415 individuals with ASD (1165 male/250 female) and 1730 controls (1172 male/558 female), aged 2–65 years. Sample characteristics (including image processing, and which subjects were excluded due to poor motion or segmentation quality) are equivalent between studies, with the exception of the NIMH site, which was not included in this study because the partial volume effects from its lower resolution would compromise the accuracy of BSC measurement. Quality control methodology is outlined in detail in a recent manuscript from our group (Bedford et al. 2020). Supplementary materials of that work (Supplementary Methods 2. Quality control [QC] and site elimination; 3. Image processing; 7. Quality control analysis) provide detailed accounts of our quality control choices for both the raw and processed data. In total, after segmentation quality control and motion quality control, there remained 1136 subjects: 415 with ASD (303 male/112 female) and 721 controls (438 male/283 female).
Table 1.
Subject demographics of the cohort under study
| Subjects by site before QC (and after QC) | Age (years) [median] | Total (after QC) | Female ASD (after QC) | Male ASD (after QC) | Female control (after QC) | Male control (after QC) | FIQ [median] |
|---|---|---|---|---|---|---|---|
| ABIDE I—17 sites (4 sites) | 6–64 [14.7] (6–58 [16.9]) | 1101 (263) | 64 (14) | 466 (89) | 99 (38) | 472 (122) | 41–148 [109] (69–148 [110]) |
| ABIDE II—16 sites (4 sites) | 5–64 [11.7] (5–34 [11.1]) | 1044 (292) | 73 (25) | 414 (82) | 175 (69) | 382 (116) | 49–149 [112] (49–144 [110]) |
| SickKids Hospital, Toronto | 4–65 [14.0] (4–49 [14.8]) | 521 (321) | 25 (18) | 106 (62) | 194 (117) | 196 (124) | 69–149 [111] (69–149 [112]) |
| Cambridge Family Autism Study | 12–18 [14.7] (12–18 [15.0]) | 96 (52) | 17 (12) | 39 (15) | 20 (14) | 20 (11) | 73–146 [108] (82–139 [107]) |
| UK MRC Autism Imaging Multicentre Study (University of Cambridge, King’s College London) | 18–52 [25.7] (18–52 [27.7]) | 253 (208) | 54 (43) | 72 (55) | 54 (45) | 73 (65) | 73–137 [115] (73–137 [117]) |
| Total before QC | 2–65 [13.8] | 3015 | 233 | 1097 | 542 | 1143 | 41–149 [111] |
| Difference between sites (ANOVA/X 2 ; total sample) | F(29, 2913) = 101.9, P < 0.001 | X 2(87, N = 3015) = 544,35, P < 0.001 | F(29, 2893) = 7.985, P < 0.001 | ||||
| Total after QC | 2–65 [14.0] | 1136 | 112 | 303 | 283 | 438 | 49–149 [112] |
| Difference (ANOVA/X 2 ; QC’d sample) | F(9, 1126) = 118.1, P < 0.001 | X 2(27, N = 1136) = 148.2, P < 0.001 | F(9, 1126) = 0.94, P = 0.49 | ||||
Note: Group differences in age, sex distribution, and FIQ were also examined using t-tests (for continuous variables) and chi-squared tests (for categorical variables).
Table 2.
Subject demographics broken down by ABIDE I and II site
| Subjects by ABIDE site before QC (and after QC) | Age (years) [median] | Total (after QC) | F-ASD (after QC) | M-ASD (after QC) | F-Ctl (after QC) | M-Ctl (after QC) | FIQ [median] |
|---|---|---|---|---|---|---|---|
| Kennedy Krieger Institute (KKI; ABIDE I&II) | 8–13 [10.3] (8–13 [10.4]) | 256 (148) | 19 (11) | 58 (21) | 65 (45) | 123 (71) | 63–143 [113] (69–143 [114]) |
| Ludwig Maximilians University Munich (MAX MUN; ABIDE I) | 7–58 [26.0] (7–58 [29.5]) | 57 (38) | 3 (3) | 21 (14) | 4 (4) | 29 (17) | 79–133 [112] (93–133 [112]) |
| New York University (NYU; ABIDE I & II) | 5–39 [10.9] (5–39 [12.1]) | 289 (179) | 19 (10) | 135 (74) | 28 (19) | 107 (76) | 67–148 [109] (67–148 [109]) |
| Oregon Health and Science University (OHSU; ABIDE I & II) | 7–15 [11.0] (8–15 [11.0]) | 121 (97) | 7 (5) | 43 (33) | 29 (25) | 42 (34) | 69–140 [115] (69–136 [114]) |
| San Diego State University (SDSU; ABIDE I & II) | 7–18 [13.8] (8–18 [14.4]) | 94 (45) | 8 (6) | 39 (16) | 8 (3) | 39 (20) | 66–141 [105] (66–139 [104]) |
| University of Michigan (UM; ABIDE I) | 8–28 [14.0] (9–28 [15.8]) | 145 (55) | 10 (4) | 58 (17) | 18 (12) | 59 (22) | 76–147 [108] (78–147 [110]) |
Image Processing
Data were preprocessed using the minc-bpipe-library pipeline, including N4 bias field inhomogeneity correction (Tustison et al. 2010). Data were then processed through the CIVET 1.1.12 cortical segmentation and cortical thickness pipeline, which generated gray/white and pial surfaces and transformed subject brain volumes into standard MNI space (Ad-Dab’bagh et al. 2006). All intensity sampling and surface generation was performed in standard space.
BSC Calculation
For each of the 77 212 vertices (which exclude all vertices on the midline wall) on the cortical surface of each subject, signal intensity was measured at 10 surfaces that span the region around the gray/white boundary. Together, the 10 intensity samples derived from these new surfaces cover the bottom quarter of the cortex (the quarter that interfaces with the gray/white boundary) and a small portion of white matter below the gray/white boundary (Fig. 1).
Figure 1.

Intensity sampling and sigmoid fit method used to calculate BSC for each vertex. At each vertex, the T1w image intensity was measured at 10 cortical surfaces surrounding the gray/white boundary, including the mid-surface, gray/white boundary surface, and a total of eight newly generated gray and white surfaces equally spaced apart (A, B). A sigmoid curve (Equation 1) was fit to the 10 sample points (C) and parameter BSC, reflecting the sigmoid growth rate (D), was log-transformed to create the measure that is the BSC at that vertex. Higher BSC values reflect a quicker transition between gray and white matter and a less blurred cortical boundary, whereas lower BSC values reflect a slower transition between gray and white matter and a more blurred cortical boundary (D). The tissue contrast ratio was calculated by dividing the intensity sampled at the gray 25% surface by the intensity sampled at the white 25% surface.
Gray matter surfaces were created at increasing percentile fractions of the cortical thickness (0%, 6.25%, 12.5%, 18.75%, 25%, 50%), from the boundary to the pial surface (Fig. 1A,B). New white matter surfaces were generated at the same distance from the gray/white boundary surface as the gray matter surfaces, but in the direction of the white matter, with the exception of the white matter equivalent of the 50% gray matter surface, which was not included because it crossed into neighboring cortex in thin gyral crowns at certain vertices. See Supplementary Methods section S2 “Surface generation” for more details. A sigmoid curve was then fit to the 10 sample points using a nonlinear least squares estimator (Fig. 1C); see Supplementary Methods section S3 “Model fit” for more details.
Sampled intensities at each vertex and their distance along the axis perpendicular to the gray matter boundary surface were input to a nonlinear least-squares estimator to fit a sigmoid function:
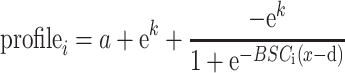 |
The d parameter reflects the translation of the sigmoid along the x-axis, where x is a vector of the displacement along the axis that runs orthogonal to the boundary, increasing toward the pial surface, parameter a translates the sigmoid along the T1 intensity, parameter k reflects the height of the sigmoid, and parameter BSCi reflects the growth rate (steepness) of the sigmoid.
BSC at each vertex (BSC parameter of the sigmoid curve) was extracted from the estimated sigmoid curve (Fig. 1D), where a higher BSC reflects greater boundary “sharpness,” or a faster transition in intensity moving from gray matter to white matter (effectively a measure of contrast at the gray/white matter surface, with higher BSC reflecting high contrast which we take to mean a bigger difference between the microstructural composition of tissue compartments). See Supplementary Fig. S5 for representative sigmoid fits and associated BSC parameters and Supplementary Fig. S6 for a spatial distribution of model convergence failures prior to spatial smoothing. These values were then log-transformed and smoothed with a 20-mm full-width half-max (FWHM) smoothing kernel to reduce the effect of noise and simulate a Gaussian distribution. No smoothing was applied to the sampled intensity values prior to the model fit. Smoothing kernels at 10 mm (Mann et al. 2018; Norbom et al. 2019), 20 mm (Bezgin et al. 2018), and 30 mm (Salat et al. 2009; Uribe et al. 2018) FWHM have been used in prior studies investigating the tissue contrast ratio. Here, a 20-mm kernel was used with the a priori hypothesis that effects would be present at this intermediate kernel width. The analysis of main effect was repeated with a smoothing kernel of 10 mm FWHM. Values were then resampled to a common surface mesh to enable cross-subject comparisons (Lerch and Evans 2005).
By measuring T1 signal at multiple distances on either side of the gray/white boundary and fitting an adequately flexible sigmoid curve to the measurements, BSC is theoretically able to capture cortical blurring wherever it occurs relative to the segmented boundary and thus avoids the circular boundary placement problem mentioned in the Introduction. Finally, given that BSC maps also correlated with mean curvature (Supplementary Fig. S2B) in certain parts of the cortex, BSC values were further residualized for mean curvature at the vertex-wise level across all subjects within each site in standard space (Sereno et al. 2013).
Relationship between In-Scanner Motion and BSC
In order to assess the potential impact of motion on data that passed quality control, we obtained motion parameters from functional magnetic resonance imaging (fMRI) data in two sites (ABIDE I KKI; ABIDE II OHSU), which include subjects aged 7–15, a demographic for whom motion is traditionally higher overall. Though an indirect measure of T1 motion, fMRI realignment parameters acquired during the same session have been shown to correlate with anatomical scan motion (Alexander-Bloch et al. 2016). Linear regression was performed at each lobe (frontal, temporal, parietal, occipital, cingulate) to estimate the effect of in-scanner motion on BSC (Supplementary Methods S7).
Code Availability
Code for all processing steps, including surface generation and BSC calculation, is publicly available on the CoBrALab GitHub: https://github.com/CoBrALab/BSC.
Statistical Analysis of BSC
Data were amassed from multiple sites in order to be sufficiently powered to analyze sources of heterogeneity in ASD. However, analyzing multiple sites presents the challenge of site-specific confounds, such as scanner model, scan acquisition protocols, and sample demographics. These limitations were addressed by the prospective meta-analysis technique, where each site was treated as a separate study, and the results were pooled across sites to determine significance at each vertex (Thompson et al. 2014; van Erp et al. 2016; Bedford et al. 2020). Meta-analyses were performed at a vertex-wise level (i.e., performing regressions at each vertex across the brain). Specifically, within each site, for each vertex across the brain where BSC was estimated, a linear regression model was performed to derive per-site Cohen’s d effect sizes for the main effect of each variable of interest. Then, the final effect size representing the contribution of all sites was calculated in a random-effects meta-analysis (Borenstein et al. 2010) using the metafor package in R 3.4.0 (https://www.r-project.org/). See Supplementary Methods section S5 “Statistical Models” for more details. How models were chosen to study the impact of age, sex, and IQ are further detailed below. Vertexwise P-values obtained from the meta-analyses described in Figs 3-5 were adjusted for multiple comparisons (i.e., all vertices of all analyes performed) were corrected for multiple comparisons using the false discovery rate (FDR) correction, which controls the proportion of null hypotheses that are falsely rejected (Genovese et al. 2002).
Akaike Information Criterion Analysis of Variable Importance
The importance of age (linear term), age (quadratic term), sex, and FIQ were examined by using a vertex-wise Akaike Information Criterion (AIC) analysis. The AIC is a measure that assesses the relative quality of statistical models, where a model with the lowest AIC is considered the best fit for the data (Mazerolle 2006). AIC takes into account both accuracy and parsimony, because it carries a penalty for increasing the number of free parameters in the model. Within each site, the AIC was calculated at each vertex for the linear regression model without the variable of interest (e.g., diagnosis only), with the variable of interest (e.g., age + diagnosis) and with its interaction with diagnosis (e.g., age + diagnosis+age*diagnosis). The percentage of sites for which each of the above models was the best-fitting model, according to AICc (for small sample sizes), was calculated for each vertex as a weighted average based on site size (number of subjects scanned at each site after QC). Supplementary Fig. S7 displays these results for age, Supplementary Fig. S8 for sex, and Supplementary Fig. S9 for FIQ.
Based on the comparison of the models using AIC, it was determined that age (but not sex or FIQ) was an important explanatory variable at a substantial proportion of vertices across the brain for BSC, thus motivating our investigation into how this factor influences BSC in ASD. See Supplementary Methods section S5 “Statistical Models” for more details.
Age-Focused Analyses
The impact of age was examined by stratifying subjects by age, performing separate meta-analyses in subjects who were 18 years old and below, and in subjects who were above 18 years old, including sex and FIQ as covariates. Additionally, an age-centered analysis was performed to assess the trajectory of group differences by shifting the age at which group differences are assessed by 4-year intervals. This type of analysis allows for the interpretation of group differences at various 4-year cross-sections without splitting the dataset into age ranges, maximizing statistical power. The Cohen’s d effect size for the main effect of diagnosis was calculated for each site in each model (one for each age interval) and pooled in a random-effects meta-analysis in the same manner as the case–control comparisons.
Associations between BSC and ASD Symptoms/Characteristics
Since consistent measures of ASD symptoms or characteristics were not available across all sites, analyses were performed on a subset of individuals who had the same clinical measures. We chose the measure which had the largest number of individuals available, which was the ADOS-2 Calibrated Severity Scores (CSS) to examine overall symptom severity (N = 172; also conducted separately in males [N = 139] and females [N = 33]). The analysis of the relationship between BSC and severity measures (as measured by ADOS-2 CSS) was performed by conducting a multiple regression analysis, calculating the semipartial correlation of the ADOS-2 scores with BSC at each vertex, per site, with age, sex, and FIQ included in the model, in individuals with ASD only. The semipartial correlation was then pooled across sites in a random effects meta-analysis, in the same fashion as the Cohen’s d effect size in a typical meta-analysis (Bedford et al. 2020).
Assessing Spatial Overlap between BSC and Cortical Thickness Maps
The spatial correspondence between maps of the effect size of diagnosis on BSC and the effect size of diagnosis on cortical thickness was determined using a “spin test” (Alexander-Bloch et al. 2018), which generates null estimates of overlap by applying random rotations to spherical projections of a cortical surface. The correlation between the two original spatial maps is compared with the Pearson’s correlation coefficient measured between one original spatial map and the other map’s rotated permutations. Maps of cortical thickness were developed in (Bedford et al. 2020) from a near-identical dataset (excluding the NIMH dataset here).
Results
The Tissue Intensity Ratio Correlates with Mean Curvature
To determine the extent to which tissue intensity ratio may be influenced by cortical curvature, we assessed the correlation between mean curvature and the tissue intensity ratio (see Supplementary Methods section S4 “Tissue intensity ratio” for methods). In a vertex-wise single-subject analysis, we found that the tissue intensity ratio was lower in gyri compared with sulci (Supplementary Fig. S1). Furthermore, in a cross-subject analysis to determine in which areas of the brain this relationship is present, we found a negative correlation between the tissue intensity ratio and mean curvature for most vertices in the brain (Supplementary Fig. S2A,C,D), including vast areas of the frontal, parietal, and occipital lobes. These results are consistent with the hypothesis that the impact of curvature on cortical T1w intensity extends to the tissue intensity ratio and calls for a measure of cortical contrast between the gray and white matter that is less susceptible to bias by other cortical features. When assessed for its relationship to curvature, we found that BSC correlated with mean curvature in regions of the anterior frontal, parietal, and temporal lobes (Supplementary Fig. S2B) and did not correlate in any regions after regressing the values against mean curvature at the vertex level.
Average BSC Map Recapitulates T1/T2 Ratio Map
We observed regional differences in mean BSC across healthy individuals ages 22–35 within the Sick Kids (Toronto) cohort (Fig. 2A), with the lowest mean BSC in areas such as the motor-somatosensory strip in the central sulcus, the visual cortex in the occipital lobe, and in early auditory areas in the Sylvian fissure, matching areas that were reported to contain the heaviest intracortical myelination as measured by the T1/T2 ratio in Glasser et al. (2011).
Figure 2.

Diagnostic group comparisons of BSC. (A) Average BSC map versus average T1/T2 ratio myelin map from Glasser et al. (2011). Olafson et al. BSC map depicts the average BSC values across control subjects aged 22–35 for a single site (Sick Kids) for correspondence with the HCP dataset used by Glasser et al. (2011) to derive T1/T2 ratio maps. The top row displays lateral views (left hemisphere on the left, right hemisphere on the right), and the bottom row displays medial views (with midline vertices masked out), for each map. (B) Individuals with ASD had significantly higher BSC measures (<5% FDR, peak Cohen’s d = 0.38) in the bilateral superior temporal gyrus, inferior temporal gyrus, and left inferior frontal gyrus. (C) Forest plot displaying site-specific effect sizes at a peak vertex in the left frontal gyrus represented by an asterisk in (B).
Normative Effect of Age and Sex on BSC in Control Subjects
In order to contextualize diagnostic group differences in BSC, we performed a vertexwise analysis of the main effects of age, age squared, and sex on BSC across all control individuals in the Sick Kids (Toronto) cohort (165F/149M; aged 4–65) (Supplementary methods section S6). Main effects of age on BSC were significant across the cortex with a negative relationship in association cortices and positive relationship in sensory areas. Likewise, age-squared was negatively related to BSC in association cortices (an inverted U-shaped relationship), and positively related in primary sensory areas (U-shaped relationship) (Supplementary Fig. S3A,B). Significant sex differences were only observed in the bilateral medial temporal cortices where males have higher BSC than females (Supplementary Fig. S3C).
Motion Does Not Correlate with BSC
Multiple regression between average BSC and in-scanner motion (as estimated by Framewise Displacement (Power et al. 2012); see Supplementary Methods S7) did not provide evidence for a relationship between BSC and in-scanner motion (Supplementary Fig. S4; Supplementary Table S1).
Greater BSC in Individuals with ASD
We observed regions of significantly greater BSC in individuals with ASD compared with controls in the bilateral superior temporal gyrus, inferior temporal gyrus, and left inferior frontal gyrus (<5% FDR, peak Cohen’s d = 0.36) (Fig. 2B), corresponding to a faster transition from cortical gray matter to white matter relative to controls. Effect sizes varied moderately between sites but were mostly positive (Fig. 2C, Supplementary Fig. S10). The analysis of the main effect of ASD was repeated with a smoothing kernel of 10 mm FWHM, where the pattern of group differences is similar, and more extensive (Supplementary Fig. S11).
Age-Specific Patterns of Boundary Alterations
In the age-stratified analysis, individuals with ASD above and below 18 both showed significantly greater BSC than their typically developing counterparts (Fig. 3A), though these group differences showed age-specific patterning and effect sizes, and the main effect of diagnosis was stronger in the age group over 18. Individuals with ASD under 18 had significantly greater BSC in the bilateral superior temporal gyrus and right postcentral gyrus, with a peak Cohen’s d of 0.41 (Fig. 3A). Individuals with ASD over 18 had significantly increased BSC in the bilateral precuneus and superior temporal gyrus, with a peak Cohen’s d of 0.62 (Fig. 3A). In the age-centered analysis, which examined group differences at specific ages, group differences were greatest between the ages of 12 and 20 in the right superior temporal gyrus and left inferior temporal gyrus (Fig. 3B,C,D). Threshold effects were not responsible for the pattern of differences between the two age groups (Supplementary Fig. S12A).
Figure 3.

Age-stratified and age-centered analyses. Individuals under 18 with ASD had significantly higher BSC measures in the bilateral superior temporal gyrus as well as the left precentral gyrus (<5% FDR, peak Cohen’s d = 0.41) (A). Individuals over 18 with ASD showed significantly increased BSC in the bilateral precuneus and superior temporal gyrus (<5% FDR, peak Cohen’s d = 0.62). (B) For the age-centered analysis, group differences in BSC were greatest between the ages of 12 and 20 in the right superior temporal gyrus and left inferior temporal gyrus. (C) Zoom of the left superior temporal lobe (top) and right inferior temporal lobe (bottom) displaying qvalues between 1% and 5% FDR (same colormap as in B). (D) Plot of BSC across age in a single site (Toronto) at vertex highlighted with a white asterisk in A.
Sex-Specific Boundary Alterations
A sex-focused analysis on group differences in BSC was performed (Supplementary Methods S8). In both the male and female subgroups, individuals with ASD had significant increases in BSC, but these increases were observed in different brain regions in males and females and with larger effect sizes in the female group (Supplementary Fig. S14A,B). Females with ASD had significantly greater BSC in the bilateral superior parietal gyrus and superior temporal gyrus, with a peak Cohen’s d of 0.63 (Supplementary Fig. S14A). Males with ASD displayed significantly greater BSC in the bilateral inferior temporal gyrus and left inferior frontal lobe, with a peak Cohen’s d of 0.32 (Supplementary Fig. S14B). Threshold effects were not responsible for the pattern of differences between the sexes (Supplementary Fig. S12B).
Minimal Correlation between BSC and ASD Severity Measures
We observed a significant positive correlation between BSC and ADOS-2 CSS in individuals with ASD in the right medial temporal pole (Fig. 4A). Given our findings of sex-specific regions of BSC differences in subjects with ASD, we explored the relationship between BSC and ASD severity separately in males and females. In the female group, we found a significant positive association between BSC and ADOS-2 CSS in the left parietal lobe (Fig. 4B). No correlations between severity score and BSC were observed in the male-only subset.
Figure 4.

Relationship between BSC and ADOS-CSS. Across all subjects with ASD with severity scores, ADOS-CSS was positively correlated with BSC, shown for a peak vertex in the right medial temporal gyrus (A). Correlations between ADOS-CSS and BSC were also observed in the female-only group in the left parietal lobe (B).
Overlap between Maps of Diagnostic Effect on Cortical Thickness and BSC
We observed a significant overlap between the maps of Cohen’s d effect size of diagnosis on BSC and the map of Cohen’s d effect size of diagnosis on CT (P < 0.01) (Fig. 5A,B), as well as between the maps of FDR-corrected q-values (P < 0.01) (Fig. 5C,D, Supplementary Fig. S13).
Figure 5.

Cohen’s d effect size maps of BSC (A) and cortical thickness (B, used with permission from Bedford et al. 2020). Spatial correspondence assessed by the Pearson correlation coefficient in a permutation-based “spin-test” analysis between BSC and cortical thickness is demarcated in red (C) was significant (P = 0.00; P < 0.001 as per the software output) with 1000 null spatial permutations. FDR-thresholded q-value maps of significant increases in BSC and increases in cortical thickness in individuals with ASD (D).
Discussion
In this study, we employed a vertex-wise meta-analysis on a large multisite dataset to investigate BSC in ASD, finding significant group-level increases in BSC in lateral frontal and temporal regions as well as sex-specific and age-specific patterns of BSC increases in individuals with ASD. As BSC is parameterized to capture cortical blurring which may be a product of differences in neural migration and intracortical myelination, there are several considerations for interpreting our observation of increased BSC in ASD. Neuronal migration defects are predicted to result in supernumerary neurons in the white matter compartment directly below the cortex (Chun and Shatz 1989; Avino and Hutsler 2010; Andrews et al. 2017). In previous neuroimaging studies examining cortical blurring at the gray/white boundary using the tissue intensity ratio, the presence of ectopic neurons is thought to be captured by an intensity differential that is lower in individuals with ASD (Andrews et al. 2017). Thus, our finding of a greater intensity difference (as indexed by greater BSC) between cortex and white matter is unlikely to reflect differences in neuronal migration.
Since T1w intensity is thought to reflect underlying myeloarchitecture more so than cytoarchitecture (Eickhoff et al. 2005), BSC may also reflect intracortical myelination: a reduction of myelin content (and presumably, of T1w signal) in the lower layers of the cortex would appear in T1w MRI as a sharper transition in intensity moving from gray to white matter. This assumption is supported by the general agreement between the group-average BSC map (Supplementary Fig. S8) and the intracortical myelin map derived using the T1w/T2w ratio by Glasser et al. (2011) and is further supported by evidence from studies suggesting altered intracortical myelination in ASD, including genetic (Richetto et al. 2017) and molecular (Canali et al. 2018; Lee et al. 2019) studies, preclinical mouse models (Graciarena et al. 2018; Shen et al. 2018), and postmortem histology (Zikopoulos and Barbas 2010). Furthermore, age-related effects of BSC in healthy controls (Supplementary Fig. S3) recapitulate normative developmental patterns of intracortical myelination. The results suggest that BSC follows an inverted U shape trajectory across most of the cortex, which is consistent with studies of intracortical myelin, which describe cortical development over the life span as occurring in phases: an early maturation phase that lasts into adulthood, followed by a stable period, and then a decline of intracortical myelin (Rowley et al. 2017; Grydeland et al. 2019). Here, across most of the cortex, BSC trajectories follow a similar pattern—an early decrease in BSC (increase in myelin) during adolescence, and then a plateau, and then a moderate increase in BSC (decrease in myelin).
Mounting evidence from diffusion imaging and resting state fMRI studies support the characterization of ASD as a connectopathy, or a brain network disorder. Since our proposed measure is parameterized to capture myelination in the lower layers of the cortex (a region through which fibers that connect distant brain regions pass), the higher BSC observed in ASD is supported by studies that find reduced long-range cortical connectivity (Kikuchi et al. 2015; O’Reilly et al. 2017) and thalamo-cortical connectivity (Nair et al. 2013; Tomasi and Volkow 2019) in ASD. As such, the contribution of myelination is increasingly relevant to understanding the neurodevelopmental mechanisms of ASD.
Two neuroimaging studies to date investigated boundary microstructure in ASD using a different metric called the tissue intensity ratio, reporting lower tissue intensity ratio in ASD which may indicate greater intracortical myelination or the presence of supernumerary neurons in the superficial white matter compartment directly beneath the gray/white boundary (Andrews et al. 2017; Mann et al. 2018; Norbom et al. 2019). This discrepancy to the present findings could be due, in part, to differences in preprocessing and analysis methods (particularly the use of the tissue intensity ratio). The tissue intensity ratio suffers from the ambiguity arising from the blurring around the cortical boundary which, in turn, alters placement of the gray/white boundary that serves as a reference point for the component gray and white measurements. Additionally, the present study uses a sample size that is considerably larger than previous studies and is more suitably powered to detect group differences in high-variability populations. Examining the directionality in each of the forest plots reveals how different studies may, indeed, yield different findings and how they may be dependent on age range and demographic composition. The data in the present study were rigorously filtered for motion and image processing artifacts (Bedford et al. 2020), whereas quality control procedures were not described in detail in previous studies, making it difficult to assess the effect of motion or inaccurate segmentation on reported results. It is possible that measures of cortical blurring using the tissue intensity ratio in previous ASD studies have been obscured by motion-induced blurring of the gray-white matter boundary (Alexander-Bloch et al., 2016; Reuter et al. 2015), especially considering individuals with ASD are more likely to move during the scan than controls (Pardoe et al. 2016; Bedford et al. 2020). It is also possible that factors such as age, sex, FIQ, and symptom severity are influencing case–control differences in previous studies of cortical blurring in autism (Lombardo et al. 2019). Therefore, we attempted to determine the degree to which diagnostic differences in BSC are modulated by these factors.
The male bias in ASD prevalence (3:1 males: females diagnosed (Baxter et al. 2015) as well as sex differences in behavior and key ASD-related phenotypes such as restrictive and repetitive behaviors (Mandy et al. 2012; Knutsen et al. 2019) have spurred the investigation of neuroanatomical sex differences in ASD (Lai et al. 2017). Given their scarcity, females with ASD are a difficult population to recruit, and, as such, our understanding of the modulating effect of sex on neuroanatomy in individuals with ASD is relatively rudimentary. However, this knowledge gap is beginning to narrow as a result of efforts to curate large-scale publicly-available neuroimaging datasets (Di Martino et al. 2014) and an increased awareness of the importance of female representation in ASD studies (Lai et al. 2015). By combining publicly-available datasets with several multicentre consortium datasets, we were able to incorporate data from 117 females with ASD in this study. We observed sex-specific patterns of BSC, where females with ASD displayed higher BSC in the superior temporal and parietal lobes, whereas males with ASD displayed a greater BSC in the inferior temporal and frontal lobes. Sex-specific patterns of cortical neuroanatomy in autism have recently been reported, in both gross volumetric measures (Retico et al. 2016; Bedford et al. 2020) and altered connectivity (Irimia et al. 2017; Zeestraten et al. 2017). The maximum effect size of diagnosis in the female sample was almost twice as high as the effect size of diagnosis observed in the male sample. BSC also correlated with ADOS-CSS in the female-only sample. Taken together, these findings support a differential neuroanatomical presentation of autism in males and females.
Age is another factor that is known to modulate diagnostic group differences. Though most brain differences in ASD have been investigated in a child or adolescent population, there is evidence that differences in brain anatomy related to ASD are present even in adulthood (Ecker et al. 2013, 2012; Lazar et al. 2014). Intracortical myelination has shown to be ongoing even past adolescence, with accelerated myelination until ~30 years of age, followed by a period of stability, and then a decrease in myelination from the late 50s (Grydeland et al. 2013; Tullo et al. 2019). Though our use of cross-sectional data limits our interpretation of the age-centered results as being reflective of developmental processes, the peak diagnostic group differences observed in adolescence and adulthood may be the result of reduced or protracted myelination in ASD relative to the rate of myelination in typical development, as observed in postmortem histology (Zikopoulos and Barbas 2010) and mouse models of ASD (Ellegood et al. 2015; Graciarena et al. 2018).
Intracortical myelination has been shown to drive MRI-based measures of cortical thickness in the visual cortex across development (Natu et al. 2018), potentially by pushing the gray-white matter boundary deeper into the cortex. In our analysis, we found a significant spatial overlap between maps of the effect size of diagnosis on BSC and the effect size of diagnosis on cortical thickness (Bedford et al. 2020). These results suggest that the neuro-phenotype of increased cortical thickness in ASD, as observed in Bedford et al. (2020) and many other studies, may be partially driven by lower levels of intracortical myelination in ASD relative to controls. Moving forward, a longitudinal design would allow us to determine in the same individual, the evolution of the pattern of BSC across development, and would allow for relating patterns of BSC over time with respect to behaviors and autism symptomatology. Additionally, since age may substantially moderate the pattern of sex differences, a longitudinal framework would allow for a more precise investigation into this time-sensitive relationship.
However, while cortical thickness differences observed in our previous work (Bedford et al. 2020) correlate with autism severity scores in many regions of the brain, we found minimal correlations between BSC and ASD severity. This disparity suggests that intracortical myelination may inflate MRI-based measures of cortical thickness in ASD for several areas of the brain, but the cortical overgrowth phenotype itself is driven by separate biological processes (such as reduced synaptic pruning) that are more clinically relevant to ASD severity. On the other hand, deficits in intracortical myelination may be a neurobiological hallmark of the disorder that does not alter ASD behavioral severity beyond a certain point and thus does not correlate with the ADOS-CSS score.
There are several limitations to this study. First, although our BSC maps correspond well with established patterns of intracortical myelination, the T1w intensity also reflects other biological properties including water content, iron, and dendrite density and therefore may reflect more than the degree of myelination in the cortex (Stüber et al. 2014). Second, abnormalities in the superficial white matter may also drive some of the changes to BSC. Though a vertex-wise analysis of the intracortical or superficial white matter intensities independently is precluded by between-scanner intensity profile differences and by the distorting effect of inhomogeneity gradients across images, it would be possible to evaluate differences in normalized intracortical intensities that have been scaled to the intensity of a predetermined sample of white matter within each subjects’ brain. Finally, since the distance sampled in the white matter is based on a percentage of the cortical thickness, the depth at which white matter is measured varies between regions with different cortical thicknesses.
Given these findings of increased BSC in individuals with ASD, which may reflect the degree of myelination in the lower layers of the cortex, it will be pertinent to investigate how subcortical volume changes in ASD relate to BSC in cortical sensory regions with high thalamic input (Uddin 2015; Schuetze et al. 2016). Considering the importance of intracortical myelination on the fidelity of neural connections and the maintenance of networks, these results may help us better understand the cognitive and behavioral atypicalities seen in ASD. Finally, the significant spatial correspondence observed between maps of cortical thickness and of BSC calls for a reconsideration of what biological phenomena may underlie the MRI-derived measures of cortical thickness increases in autism.
Supplementary Material
Contributor Information
Emily Olafson, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Department of Neuroscience, Weill Cornell Graduate School of Medical Sciences, New York City, NY 10021, USA.
Saashi A Bedford, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Integrated Program in Neuroscience, McGill University, Montreal H3A 2B4, Canada; Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
Gabriel A Devenyi, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Department of Psychiatry, McGill University, Montreal H3A 2B4, Canada.
Raihaan Patel, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Department of Biological and Biomedical Engineering, McGill University, Montreal H3A 2B4, Canada.
Stephanie Tullo, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Integrated Program in Neuroscience, McGill University, Montreal H3A 2B4, Canada.
Min Tae M Park, Department of Psychiatry, Schulich School of Medicine and Dentistry, Western University, London N6A 3K7, ON, Canada.
Olivier Parent, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Departement de Psychologie, Universite de Montreal, Montreal, QC, Canada.
Evdokia Anagnostou, Holland Bloorview Kids Rehabilitation Hospital, Toronto M4G 1R8, Canada; Department of Pediatrics, University of Toronto, Toronto, ON, Canada.
Simon Baron-Cohen, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
Edward T Bullmore, Brain Mapping Unit, Department of Psychiatry, University of Cambridge, Cambridge CB2 0SZ, UK.
Lindsay R Chura, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
Michael C Craig, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK; National Autism Unit, Bethlem Royal Hospital, London BR3 3BX, UK.
Christine Ecker, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, University Hospital of the Goethe University, Frankfurt am Main 60528, Germany.
Dorothea L Floris, Donders Center for Brain, Cognition and Behavior, Radboud University Nijmegen, Nijmegen 6525 HR, The Netherlands; Department for Cognitive Neuroscience, Radboud University Medical Center Nijmegen, Nijmegen 02.275, The Netherlands.
Rosemary J Holt, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
Rhoshel Lenroot, Department of Psychiatry, University of New South Wales, Sydney, NSW 2052, Australia.
Jason P Lerch, Department of Medical Biophysics, The University of Toronto, Toronto, ON M5G 1L7, Canada; Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford OX3 9DU, UK.
Michael V Lombardo, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK; Laboratory for Autism and Neurodevelopmental Disorders, Center for Neuroscience and Cognitive Systems, @UniTn, Istituto Italiano di Tecnologia, 38068 Rovereto, Italy.
Declan G M Murphy, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Armin Raznahan, Section on Developmental Neurogenomics, Human Genetics Branch, National Institute of Mental Health, Bethesda, MD 20892-9663, USA.
Amber N V Ruigrok, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
Michael D Spencer, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK.
John Suckling, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK; Brain Mapping Unit, Department of Psychiatry, University of Cambridge, Cambridge CB2 0SZ, UK.
Margot J Taylor, Diagnostic Imaging, The Hospital for Sick Children, Toronto M5G 1X8, Canada; Program in Neurosciences and Mental Health, The Hospital for Sick Children, Toronto M5G 1X8, Canada; Department of Medical Imaging, University of Toronto, Toronto M5G 1X8, Canada.
Meng-Chuan Lai, Autism Research Center, Department of Psychiatry, University of Cambridge, Cambridge CB2 8AH, UK; The Margaret and Wallace McCain Centre for Child, Youth & Family Mental Health and Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto M6J 1H4, Canada; Department of Psychiatry, University of Toronto, Toronto M5T 1R8, Canada; Department of Psychiatry, National Taiwan University Hospital and College of Medicine, Taipei 100229, Taiwan; Department of Psychiatry, The Hospital for Sick Children, Toronto M5G 1X8, Canada.
M Mallar Chakravarty, Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal H4H 1R3, Canada; Integrated Program in Neuroscience, McGill University, Montreal H3A 2B4, Canada; Department of Psychiatry, McGill University, Montreal H3A 2B4, Canada; Department of Biological and Biomedical Engineering, McGill University, Montreal H3A 2B4, Canada.
Funding
This work was supported by a Clinician Scientist Fellowship from the Medical Research Council (G0701919 to MDS); the Medical Research Council UK to (GO 400061 to DM, EB, SBC) as the Autism Imaging Multicentre Study (AIMS); the Canadian Institutes of Health Research (MOP-106582, MOP119541 and MOP-142379 to MT and EA); Brain Canada, in partnership with Health Canada, for the Canadian Open Neuroscience Platform initiative to GAD; a European Research Council Starting Grant (ERC-2017-STG; AUTISMS; no. 755816 to MVL); the National Institutes of Mental Health (Clinical trial NCT00001246, clinicaltrials.gov; NIH Annual Report Number, 1ZIAMH002949-03 to AR); the Academic Scholars Award from the Department of Psychiatry, University of Toronto, the Canadian Institutes of Health Research (CIHR) Sex and Gender Science Chair (GSB 171373), and the CAMH Foundation to M.-C.L.; the Canadian Institutes of Health Research, Natural Science and Engineering Research Council of Canada, Fonds de la recherches en santé du Québec, the Weston Brain Institute, and the Healthy Brains for Healthy Lives Initiative (Canada First Research Excellence Fund-McGill University) to MMC.
Notes
This study was performed by E.O. while at the Cerebral Imaging Centre, Douglas Mental Health University Institute, Montreal, Canada. E.O. is supported by the Natural Science and Engineering Research Council of Canada (NSERC) and the Fonds de la recherches en santé du Québec (FRQS). Conflict of Interest: None declared.
References
- Ad-Dab’bagh Y., Lyttelton, O., Muehlboeck, J.S., Lepage, C., Einarson, D., Mok, K., Ivanov, O., Vincent, R.D., Lerch, L., Fombonne, E., et al. 2006. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: Corbetta M., editor. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Neuroimage. http://www.bic.mni.mcgill.ca/users/yaddab/Yasser-HBM2006-Poster.pdf (last accessed 5 July 2020).
- Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A. 2016. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp. 37(7):2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Shou H, Liu S, Satterthwaite TD, Glahn DC, Shinohara RT, Vandekar SN, Raznahan A. 2018. On testing for spatial correspondence between maps of human brain structure and function. Neuroimage. 178:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E, Taylor MJ. 2011. Review of neuroimaging in autism Spectrum disorders: what have we learned and where we go from here. Mol Autism. 2:4. doi: 10.1186/2040-2392-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DS, Avino TA, Gudbrandsen M, Daly E, Marquand A, Murphy CM, Lai MC, Lombardo MV, Ruigrok ANV, Williams SCet al. 2017. In vivo evidence of reduced integrity of the Gray-white matter boundary in autism spectrum disorder. Cereb Cortex. 27(2):877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino TA, Hutsler JJ. 2010. Abnormal cell patterning at the cortical gray-white matter boundary in autism spectrum disorders. Brain Res. 1360:138–146. [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. 2015. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 45(3):601–613. [DOI] [PubMed] [Google Scholar]
- Bedford, Saashi A, Min Tae M, Park, Gabriel A. Devenyi, Stephanie Tullo, Jurgen Germann, Raihaan Patel, Evdokia Anagnostouet al. 2020. Large-Scale Analyses of the Relationship between Sex, Age and Intelligence Quotient Heterogeneity and Cortical Morphometry in Autism Spectrum Disorder. Molecular Psychiatry. 25(3):614–628. [DOI] [PubMed] [Google Scholar]
- Bezgin G, Lewis JD, Evans AC. 2018. Developmental changes of cortical white–gray contrast as predictors of autism diagnosis and severity. Transl Psychiatry. 8(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok ST. 1959. Histonomy of the Cerebral Cortex. Amsterdam: Elsevier. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. 2010. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 1(2):97–111. [DOI] [PubMed] [Google Scholar]
- Canali G, Garcia M, Hivert B, Pinatel D, Goullancourt A, Oguievetskaia K, Saint-Martin M, Girault J-A, Faivre-Sarrailh C, Goutebroze L. 2018. Genetic variants in autism-related CNTNAP2 impair axonal growth of cortical neurons. Hum Mol Genet. 27(11):1941–1954. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. 1989. Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol. 282(4):555–569. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. 2011. Neuron number and size in prefrontal cortex of children with autism. JAMA. 306(18):2001–2010. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, Dean DC 3rd, Remer J, Dirks H, O’Muircheartaigh J. 2015. Cortical maturation and myelination in healthy toddlers and young children. Neuroimage. 115:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, Balsters JH, Baxter L, Beggiato A, Bernaerts Set al. 2017. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Scientific Data. 4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto Met al. 2014. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 19(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Ronan L, Feng Y, Daly E, Murphy C, Ginestet CE, Brammer M, Fletcher PC, Bullmore ET, Suckling Jet al. 2013. Intrinsic gray-matter connectivity of the brain in adults with autism spectrum disorder. Proc Natl Acad Sci U S A. 110(32):13222–13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJet al. 2012. Brain anatomy and its relationship to behavior in adults with autism Spectrum disorder: a Multicenter Magnetic Resonance Imaging Study. Arch Gen Psychiatry. 69(2):195–209. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JDG, Amunts K. 2005. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp. 24(3):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau BA, Crawley JN, Lin L, Genestine M, DiCicco-Bloom E, Lai JKY, Foster JA, Peñagarikano Oet al. 2015. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry. 20(1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UKet al. 2016. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA Consortium. Mol Psychiatry. 21(4):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15(4):870–878. [DOI] [PubMed] [Google Scholar]
- Glasser MF, David C, Essen V. 2011. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 31(32):11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graciarena M, Seiffe A, Nait-Oumesmar B, Depino AM. 2018. Hypomyelination and oligodendroglial alterations in a mouse model of autism spectrum disorder. Front Cell Neurosci. 12:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DM, Warrier V, Allison C, Baron-Cohen S. 2018. Testing the empathizing-systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc Natl Acad Sci U S A. 115(48):12152–12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Vértes PE, Váša F, Romero-Garcia R, Whitaker K, Alexander-Bloch AF, Bjørnerud A, Patel AX, Sederevicius D, Tamnes CKet al. 2019. Waves of maturation and senescence in micro-structural MRI markers of human cortical myelination over the lifespan. Cereb Cortex. 29(3):1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. 2013. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci: Off J Soc Neurosci. 33(47):18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R. 2015. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Neurosci. 18(2):302–309. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KNet al. 2017. Early brain development in infants at high risk for autism spectrum disorder. Nature. 542(7641):348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet G, Ey E, Bourgeron T. 2013. The genetic landscapes of autism spectrum disorders. Annu Rev Genomics Hum Genet. 14:191–213. doi: 10.1146/annurev-genom-091212-153431. [DOI] [PubMed] [Google Scholar]
- Irimia A, Torgerson CM, Jacokes ZJ, Van Horn JD. 2017. The connectomes of males and females with autism spectrum disorder have significantly different white matter connectivity densities. Sci Rep. 7(April):46401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. 2012. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 36(4):1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Lewis JD, Kostopoulos P, Carbonell F, Evans AC. 2017. Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: a Large-Scale MRI Study. Cereb Cortex. 27(3):1721–1731. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Yoshimura Y, Hiraishi H, Munesue T, Hashimoto T, Tsubokawa T, Takahashi T, Suzuki M, Higashida H, Minabe Y. 2015. Reduced long-range functional connectivity in young children with autism spectrum disorder. Soc Cogn Affect Neurosci. 10(2):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen J, Crossman M, Perrin J, Shui A, Kuhlthau K. 2019. Sex differences in restricted repetitive behaviors and interests in children with autism spectrum disorder: an Autism Treatment Network Study. Autism: Int J Res Practice. 23(4):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli JS, Kinnear MK, Fong CH, Fishman I, Carper RA, Müller R-A. 2018. Local cortical Gyrification is increased in children with autism spectrum disorders, but decreases rapidly in adolescents. Cereb Cortex. 29(6):2412–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lerch JP, Floris DL, Ruigrok ANV, Pohl A, Lombardo MV, Baron-Cohen S. 2017. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res. 95:380–397. doi: 10.1002/jnr.23948. [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. 2015. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. 54(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M, Miles LM, Babb JS, Donaldson JB. 2014. Axonal deficits in young adults with high functioning autism and their impact on processing speed. NeuroImage Clinical. 4(February):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Thacker S, Sarn N, Dutta R, Eng C. 2019. Constitutional mislocalization of Pten drives precocious maturation in oligodendrocytes and aberrant myelination in model of autism spectrum disorder. Transl Psychiatry. 9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. 2005. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 24(1):163–173. [DOI] [PubMed] [Google Scholar]
- Libero LE, Schaer M, Li DD, Amaral DG, Nordahl CW. 2019. A longitudinal study of local Gyrification index in young boys with autism spectrum disorder. 29(6):2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Lai M-C, Baron-Cohen S. 2019. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry January. 24:1435–1450. doi: 10.1038/s41380-018-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL, Buonocore MH, Lammers CR, Amaral DG, Reiss AL. 2004. Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry. 61(3):291–298. [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. 2012. Sex differences in autism Spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 42(7):1304–1313. [DOI] [PubMed] [Google Scholar]
- Mann C, Bletsch A, Andrews D, Daly E, Murphy C, MRC AIMS Consortium, Murphy D, Ecker C. 2018. The effect of age on vertex-based measures of the Grey-white matter tissue contrast in autism spectrum disorder. Mol Autism. 9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle M. 2006. Improving data analysis in herpetology: using Akaike’s information criterion (AIC) to assess the strength of biological hypotheses. Amphibia-Reptilia: Publication of the Societas Europaea Herpetologica. 27(2):169–180. [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. 2013. Impaired Thalamocortical connectivity in autism Spectrum disorder: a study of functional and anatomical connectivity. Brain: A Journal of Neurology. 136(Pt 6):1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu VS, Gomez J, Barnett M, Jeska B, Kirilina E, Jaeger C, Zhen Zet al. 2018. Apparent thinning of visual cortex during childhood is associated with myelination, not pruning. PNAS. 116(41):20750–20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbom LB, Doan NT, Alnæs D, Kaufmann T, Moberget T, Rokicki J, Andreassen OA, Westlye LT, Tamnes CK. 2019. Probing brain developmental patterns of myelination and associations with psychopathology in youths using Gray/white matter contrast. Biol Psychiatry. 85(5):389–398. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, Lewis JD, Elsabbagh M. 2017. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PloS One. 12(5):e0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Hiess RK, Kuzniecky R. 2016. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 135(July):177–185. [DOI] [PubMed] [Google Scholar]
- Park MTM, Raznahan A, Shaw P, Gogtay N, Lerch JP, Mallar Chakravarty M. 2018. Neuroanatomical phenotypes in mental illness: identifying convergent and divergent cortical phenotypes across autism, ADHD and schizophrenia. J Psych Neurosci. 43(3):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, Thiruvahindrapuram Bet al. 2014. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 94(5):677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Karzbrun E, Kshirsagar A, Kaibuchi K. 2016. Regulation of neuronal migration, an emerging topic in autism spectrum disorders. J Neurochem. 136(3):440–456. [DOI] [PubMed] [Google Scholar]
- Retico A, Giuliano A, Tancredi R, Cosenza A, Apicella F, Narzisi A, Biagi L, Tosetti M, Muratori F, Calderoni S. 2016. The effect of gender on the neuroanatomy of children with autism spectrum disorders: a support vector machine case-control study. Mol Autism. (7):5. doi: 10.1186/s13229-015-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Dylan Tisdall M, Qureshi A, Buckner RL, Kouwe AJW, Fischl B. 2015. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 15;107:107–115. doi: 10.1016/j.neuroimage.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Chesters R, Cattaneo A, Labouesse MA, Gutierrez AMC, Wood TC, Luoni A, Meyer U, Vernon A, Riva MA. 2017. Genome-wide transcriptional profiling and structural magnetic resonance imaging in the maternal immune activation model of neurodevelopmental disorders. 27(6):3397–3413. [DOI] [PubMed] [Google Scholar]
- Rowley CD, Sehmbi M, Bazin P-L, Tardif CL, Minuzzi L, Frey BN, Bock NA. 2017. Age-related mapping of Intracortical myelin from late adolescence to middle adulthood using T1-weighted MRI. Hum Brain Mapp. 38(7):3691–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, Bookheimer SY, Dapretto M. 2012. Altered functional and structural brain network organization in autism. NeuroImage Clinical. 2(November):79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Lee SY, Kouwe AJ, Greve DN, Fischl B, Rosas HD. 2009. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage. 48(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze M, Park MTM, Cho IY, MacMaster FP, Mallar Chakravarty M, Bray SL. 2016. Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology. 41(11):2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Lutti A, Weiskopf N, Dick F. 2013. Mapping the human cortical surface by combining quantitative T1 with retinotopy. Cereb Cortex. 23(9):2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-Y, Huang N, Reemmer J, Xiao L. 2018. Adenosine actions on oligodendroglia and myelination in autism spectrum disorder. Front Cell Neurosci. 12:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C, Streicher Met al. 2014. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage. 93(Pt 1 June):95–106. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro Ret al. 2014. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8(2):153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2019. Reduced local and increased long-range functional connectivity of the thalamus in autism spectrum disorder. Cereb Cortex. 29(2):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo S, Patel R, Devenyi GA, Salaciak A, Bedford SA, Farzin S, Wlodarski N, Tardif C, Breitner J, Chakravarty MM. 2019. MR-based age-related effects on the striatum, globus pallidus, and thalamus in healthy individuals across the adult lifespan. Hum Brain Mapp August. 40(18):5269–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. 2015. Idiosyncratic connectivity in autism: developmental and anatomical considerations. Trends Neurosci. 38(5):261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe C, Segura B, Baggio HC, Abos A, Garcia-Diaz AI, Campabadal A, Marti MJet al. 2018. Gray/white matter contrast in Parkinson’s disease. Front Aging Neurosci. 10(MAR):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehnert MD, Dinse J, Schäfer A, Geyer S, Bazin PL, Turner R, Tardif CL. 2016. A subject-specific framework for in vivo myeloarchitectonic analysis using high resolution quantitative MRI. Neuroimage. doi: 10.1016/j.neuroimage.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Zeestraten EA, Gudbrandsen MC, Daly E, Schotten MT, Catani M, Dell’Acqua F, Lai M-Cet al. 2017. Sex differences in frontal lobe connectivity in adults with autism spectrum conditions. Transl Psychiatry. 7(4):e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. 2010. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. doi: 10.1523/jneurosci.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


