Abstract
Naturally derived polymeric biomaterials, such as collagens, silks, elastins, alginates, and fibrins are utilized in tissue engineering due to their biocompatibility, bioactivity, and tunable mechanical and degradation kinetics. The use of these natural biopolymers in biomedical applications is advantageous because they do not release cytotoxic degradation products, are often processed using environmentally-friendly aqueous-based methods, and their degradation rates within biological systems can be manipulated by modifying the starting formulation or processing conditions. For these reasons, many recent in vivo investigations and FDA-approval of new biomaterials for clinical use have utilized natural biopolymers as matrices for cell delivery and as scaffolds for cell-free support of native tissues. This review highlights biopolymer-based scaffolds used in clinical applications for the regeneration and repair of native tissues, with a focus on bone, skeletal muscle, peripheral nerve, cardiac muscle, and cornea substitutes.
Keywords: Biopolymers, Scaffolds, Regenerative medicine
INTRODUCTION
Beginning in the mid 1970s, investigators explored the use of materials for biological and medical applications, leading to rapid growth in the field of biomaterials and their applications in tissue engineering.16,120,250 Successful tissue engineering strategies attempt to recreate or mimic the conditions present in healthy tissue in vivo. However, positive in vitro results have yielded limited translational success with in vivo models as well as in the clinic due to the simplified culture conditions, lack of cell retention in vivo, unknown biological factors, such as long-term immune response, which are difficult to predict or determine in vitro. A constant influx of new information from biological and medical research as well as improvements in in vitro experimental models will continue to guide methodologies and product development. Due to continued advancements in the fields of developmental biology, optics, and assay development, biomedical research continues to progress rapidly building from these improvements in the fundamental building blocks to generate better in vitro systems that more accurately recapitulate the in vivo environment (e.g., evaluation, utilization, and application of extracellular matrix composition, co-culture, and immunomodulation). Key components of this progress involve the careful selection and preparation of scaffold materials and use of physical, chemical, and/or electrical stimuli to direct delivered cell behavior to ultimately mimic the characteristic profile of the desired native tissue.
The Role of Biomaterials in Translational Medicine
Two traditional groups of biomaterials are available: synthetic polymers (Table 1) and natural biopolymers (Table 2). Since each group possesses distinct advantages and limitations, a wide variety of composite materials and interpenetrating networks have been utilized to achieve desired results. These include composites of natural materials, such as silk and collagen31,233 or chitosan and alginate,48,78,201,216,243,264,287 as well as blends of natural and synthetic systems, such as alginate, polyacrylamide, and poly(ε-caprolactone) interpenetrating networks.135 In addition, hybrid variants of these materials have emerged through synthetic designs and genetic engineering of peptide-based biopolymers.8,18,36,203
TABLE 1.
Common synthetic polymers used for tissue engineering and clinical applications.
| Synthetic polymer | Selective in vitro applications, clinical translation and commercialization | Refs. |
|---|---|---|
| Poly(l-lactic acid) (PLA or PLLA) | Composite materials, 2D gels, thin films Stent coatings, orthopedic applications, wound healing, soft tissue reconstruction |
15,48,62,130,144,162,185,215 |
| Poly(glycolic acid) (PGA) | 2D and 3D mesh and scaffolding Sutures, soft tissue reconstruction, nerve conduits |
12,46,104,111,127,148,274,283 |
| Poly(ethylene glycol) (PEG) | Composite materials, backbone for chemical modification, 2D gels and surfaces, easily modified, well-controlled structure Wound healing, soft tissue reconstruction |
27,28,97,116,134,136,179,180,184,188,205,251,286 |
| Poly(lactic-co-glycolic acid) (PLGA) | Nanoparticles, composites, electrospun scaffolds Drug delivery, cartilage tissue repair, bone scaffolds, vascular reconstruction, soft tissue engineering |
11,81,99,118,122,237 |
| Polyurethane | Wound healing, cardiac tissue engineering, skin grafting | 54,58,60,108,115,186,228,231,232 |
| Polytetrafluoroethylene (PTFE) | Cell free, non-modifiable structure with minimal degradation for 2D surfaces and implants Cardiac repair, vascular grafts |
103,145,202,221,259 |
| Poly(N-isopropylacrylamide) (poly(NIPAAm)) | Thermo-reversible; 2D cell sheets, thin tissue layers (≤600 μm), injectable gels Cartilage tissue engineering, cell sheet technology, soft tissue repair |
105,235,284 |
| Polycaprolactone (PCL) | Electrospun scaffolds, thin films, honeycomb architectures Cardiac tissue engineering, vascular grafts, cranial reconstruction |
37,47,49,51,163,196,272,286 |
| Poly(glycerol sebacate) (PGS) | 3D scaffolds and films; major focus is cardiac and bone Wound healing, nerve conduits, soft tissue reconstruction, bone scaffolding |
3,32,53,65,181,182,191,198,206,271,289 |
TABLE 2.
Common natural biopolymers used for tissue engineering and clinical applications.
| Biopolymer | Structure | Biological interactions | Applications and references |
|---|---|---|---|
| Alginate | 1 → 4 linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) residues G residues function with divalent cations to form ionic crosslinks |
Variations in local mechanical properties controlled by concentration of calcium ions Form soft gels, composite materials, interpenetrating networks Electrospun fibers with improved mechanical integrity |
Wound healing, drug delivery, soft tissue engineering, cell delivery, in vitro stem cell maintenance48,78,79,112,126,135,141,142,200,201,216,223,230,240,243,247,248,252,264,265,270,287,295 |
| Chitosan | Linear polysaccharide from deacetylation of chitin (main component in the exoskeletons of crustaceans) Randomly distributed β-(1–4)-linked d-glucosamine and N-acetyl-d-glucosamine |
Soluble, cationic in acidic conditions and insoluble in neutral and basic conditions | Wound healing, orthopedics, cardiac repair, neural tissue engineering, cornea repair, drug and gene delivery34,56,94,146,159,189,196,205,217,288,293 |
| Collagen | monomeric and crosslinked 3D triple helical structures Many types, type I consists of two identical α1 chains and one α2 chain Utilized in vitro in fibrous and hydrogel formats Content measured by quantifying hydroxyproline content Gelatin is a commonly used animal-based collagen product for gels |
Easily remodeled and degraded by cells Chemical crosslinking decreases degradation and improves long-term Mechanical properties Many types available, which are specific to different tissue types Collagen IV primarily found in the basal lamina, secreted by epithelial cells, collagen I is the main component in scar tissue, cartilage, bone, tendon, and other connective tissues and muscles Improper expression or mutation leads to disease |
Wound healing, skin grafts, muscle repair, nerve regeneration, anti-aging1,33,74,82–85,152–154,190 |
| Elastin | Elastic component in soft tissues that allows tissue to return to normal shape following stretch or pinch Crosslinking smaller tropoelastin polymers using lysyl oxidase to form mesh-like structures |
Small tropoelastin polymers can be used to form composite materials Can form films, gels, scaffolds, and electrospun fibers Easily remodeled by cells |
Cardiac stent coatings, soft tissue reconstruction, orthopedics5,10,14,86,110,172,273 |
| Fibrin | Formed when thrombin cleaves fibrinogen, generating soft gels Gelation kinetics controlled by ratio of thrombin to fibrinogen, calcium concentration, temperature |
Fibrinolytic inhibitors, like aprotinin or aminocaproic acid, reduce in vitro degradation rates | Wound healing, cardiac repair, in vivo cell delivery25,38,178,222,244,260,285,292 |
| Fibronectin | Glycoprotein secreted by fibroblasts Binds integrins, transmits mechanical cues from the environment to the cell binds other ECM proteins such as collagen and fibrin |
Improper regulation of expression leads to diseases such as cancer, fibrosis Fibronectin can be grafted onto 2D and 3D surfaces to improve biocompatibility Many sites for cell-based protein degradation |
Wound healing, stem cell differentiation, cardiac repair, bone regeneration158,193,227,275 |
| Keratin | Bundles form intermediate filaments that make up hair (α-keratins) and nails (β-keratins) Tough, insoluble polymer found in non-mineralized tissues Monomers form stable left-handed superhelical structures to form filaments |
Structure attributed to disulfide bridges; more bridges yields lower elasticity Classified as neutral-basic or acidic, dictating in vivo occurrence |
Cornea tissue engineering, wound healing, skin regeneration, cardiac repair, drug delivery, nerve repair23,210,213,238 |
| Silk | Extracted from the cocoons of Bombyx mon silkworm Foams, sponges, films and hydrogels are formed from silk solution |
In vivo degradation rate controlled by crystallinity (β-sheet content) Mechanics tailored by modifying concentration, crystallization, molecular weight, and scaffold format |
Muscle repair and regeneration, bone tissue engineering, cornea repair, drug delivery6,8,17,67,86,110,165,168,192,197,211,212,280 |
Synthetic Materials
Synthetic polymer-based materials are widely used as biomaterials for tissue engineering and regenerative medicine due to their well-defined chemical and structural characteristics, as well as the flexibility they afford in allowing the researcher to control and fine-tune the final properties of their scaffold. Thus, a diverse spectrum of synthetic polymers has been explored for use in virtually all tissue engineering applications. These polymers can be designed to be either non-degradable (e.g., polyethylene terephthalate/Dacron™) or degradable (e.g., poly(glycerol sebacate)). Non-biodegradable materials are advantageous in biological systems that experience high mechanical loading demands since the non-degradable materials can maintain their structural integrity over time. In contrast, fibrotic response to an inert, non-degradable scaffold due to inability to integrate within the native tissue or adverse inflammatory response can result in long-term implant failures or secondary complications. For example, the use of Dacron™ or PTFE for repair of congenital heart defects in children can lead to secondary complications such as aneurism or fatal arrhythmia.98,102,241 Biodegradable synthetic materials are advantageous because their chemical formulation can be tuned to obtain specific degradation properties (e.g., chemical hydrolysis or surface erosion). Unfortunately, cell-based degradation or natural deterioration may result in the release of cytotoxic or inflammatory small molecules, prompting the need to determine tissue-specific degradation rates, local dose–response sensitivity, and metabolic response of neighboring cells to these byproducts (See Refs.219,253 for a more detailed review). Commonly used synthetic biomaterials for tissue engineering are listed in Table 1. While Table 1 does not cover all possible synthetic materials, the polymers listed highlight novel structures and formulations utilized in current and emerging technologies as well as standard clinical products that represent areas in need of improvement.
Natural Materials
For the purpose of this review, we will define natural biomaterials as those found in nature, originating from a plant-based or tissue-based origin. Once properly purified for in vivo applications, these materials generally do not elicit an unwanted or unexpected immune response, leading to biocompatible and often bioactive matrices that can integrate with surrounding native tissue.132,204,214,267 In addition, the degradation products are generally more biocompatible, metabolically accessible, and less toxic when compared to their synthetic counterparts. A disadvantage of naturally derived biomaterials is their chemical heterogeneity and high dispersity, leading to variability in mechanical properties, structure, and performance, including variations in local degradation rates.132 Despite this variability, biopolymer scaffold materials have successfully advanced to the clinic for the repair of soft tissues, such as skin and muscle, and hard tissues, such as bone. Commonly investigated biopolymers and their benefits in vivo are listed in Table 2.
Natural materials can be further classified based on their origin and the processing methods used to obtain the material. Simple biopolymers are derived from a natural origin, such as rat-tail or bovine tendon for the isolation of collagen. These methods first require the removal of all cellular material and then the subsequent dissolution of the biopolymer in acidic medium to yield a semi-clear solution that has been purified to contain a specific protein (e.g., collagen type I).207 Alternatively, natural materials for scaffold fabrication can also be obtained from the decellularization of complex tissues. For example, collagen-based scaffolds can also arise from the decellularization of porcine dermis or pericardium. In some cases, such as in the use of demineralized (DMB) or decellularized (DCB) bone, the 3D architecture of the native tissue is maintained and utilized as the skeletal architecture of the scaffold. Both biopolymer-based scaffolds and decellularized matrices have achieved some clinical success, especially in the field of wound healing and skin regeneration. This review will discuss the use of biopolymer-based scaffolds for tissue repair and regeneration, with a focus on in vivo and clinical application (Table 3) to highlight the hurdles that still exist in the bench-to-bedside clinical transition.
TABLE 3.
Examples of commercially available biopolymer systems for various types of tissue repair.
| Manufacturer | Product | Application | Product description | Refs. |
|---|---|---|---|---|
| Baxter International, Inc. (Deerfield, IL) | TachoSil® | Cardiac Wound Sealant | Contains human fibrinogen and human thrombin to form a fibrin sealant Fibrinogen and thrombin formed on surface of an equine collagen patch Extensive literature on use for many applications other than FDA approved use |
40,66,90,149,157,171,195 |
| Collagen Matrix, Inc. (Franklin Lakes, NJ) sold by Stryker® (Kalamazoo, Ml) | NeuroFlex® | Nerve Repair and Regrowth | Type I Collagen mesh Flexible tube, kink-resistant |
4,52,93,119,166,167 |
| Collagen Matrix, Inc. (Franklin Lakes, NJ) sold by Stryker® (Kalamazoo, Ml) | NeuroMatrix® | Nerve repair and regrowth | Type I Collagen mesh Flexible tube |
4,52,93,119,166,167 |
| Collagen Matrix, Inc. (Franklin Lakes, NJ) sold by Stryker® (Kalamazoo, Ml) |
NeuroMend® | Nerve Repair and Regrowth | Type I Collagen mesh Designed to wrap injured nerves for a range of injuries |
4,52,93,119,166,167 |
| Cook Biotech, Inc. (West Lafayette, IN) | Dynamatrix® | Wound healing Soft Tissue Reconstruction | Acellular graft containing collagen (types I, III, VI), glycosaminoglycans (hyaluronic acid, chondroitin sulfate A and B, heparin, and heparan sulfate), proteoglycans, growth factors, and fibronectin |
19,121,183 |
| Cook Biotech, Inc. (West Lafayette, IN) | BioDesign® Grafts | Brain Surgery Abdominal Reconstruction Wound Healing Cardiac Surgery |
Acellular scaffold, non-cross-linked, non-dermis-based graft Forms water tight seal so fluid cannot leak from the closed wound |
64,124 |
| Japan Tissue Engineering Co., Ltd. (Gamagori City, Aichi, Japan) | LabCyte Cornea-Model |
Corneal epithelium | Trans-well permeable membrane seeded with primary human corneal epithelial cells Epidermal model also available |
113 |
| Living Cell Technologies™ (Manukau, Auckland, New Zealand) | DIABECELL® | Type 1 Diabetes | Alginate-based porcine-derived islet of Langerhans cell product | 79,112,141,252 |
| Living Cell Technologies™ (Manukau, Auckland, New Zealand) | NTCELL® | Parkinson’s Disease T reatment | Alginate-based choroid plexus cell product | 142,252,278 |
| MatTek Corporationa (Ashland, MA) | EpiOcular™ | Corneal epithelium | Fully biological scaffold containing human-derived epidermal keratinocytes | 161 |
| Medtronic Sofamor Danek (Minneapolis, MN) | INFUSE® | Bone Repair | Absorbable collagen sponge in a metal Delivers recombinant bone morphogenetic protein-2 |
96,234 |
| Mölnlyke Health Care (Norcross, GA) | Xelma | Ulcers Wound Healing | Extracellular matrix proteins mixed with propylene glycol and alginate | 22,164,268,269 |
| Musculoskeletal Transplant Foundation™ (Edison, NJ) | CASCADE® Autologous Platelet System | Soft Tissue Repair Bone Defects |
Autologous platelet delivery system using a fibrin gel Arthroscopic delivery, followed by suturing in place |
9,77 |
| Organogenesis, Inc. (Canton, MA) | Apligraft® | Skin Ulcers Wound Healing |
Cellular material comprised of foreskin-derived neonatal fibroblasts cultured in vitro, combined with bovine type I collagen coated in cultured neonatal keratinocytes First biomaterial with living cells to get FDA approval Achieves complete wound closure in chronic foot ulcers Initial production was not cost effective Shipping affected product reliability 10+ years to make profits |
29,44,63,70–72,76,92,109,117,123,208,224,246,249,256,291 |
| Organogenesis, Inc. (Canton, MA) | Dermagraft® | Skin Ulcers Wound Healing |
Cellular material comprised of human neonatal fibroblasts seeded on bioabsorbable polyglactin mesh scaffold Cells are cultured over time on the mesh to allow for ECM production on the mesh |
80,100,101,155,156,187,199 |
| Skin Ethic Laboratories (Lyon, France) | HCE | Corneal epithelium repair | Permeable polycarbonate substrate seeded with immortalized human corneal epithelial cells | 263 |
MatTek Corporation offers a variety of similar products for in vitro analysis.161
BIOPOLYMERS FOR BONE REPLACEMENT
The rising age of the population coupled with the increasing incidence of age-related conditions such as osteoarthritis and osteoporosis has produced an overwhelming market for bone replacement materials (Fig. 1).61 Despite modest success in the development of bone fillers, surgeons must still resort to autografts or allografts for critical sized defect repair. Over the past several decades, research has turned to tissue engineering as a strategy for functional bone repair. However, attempts to produce high-strength, porous scaffolds for bone regeneration have been limited by the intrinsic weakness associated with high porosity materials. Moreover, many formulations of natural or synthetic polymeric materials fail to promote adequate vascularization, innervation, and maturation of osteogenic cells.24,220 This section of the review addresses the critical role that biopolymers play in the repair of bone tissue, and highlights the clinical translation of these natural materials to the clinic.
FIGURE 1.
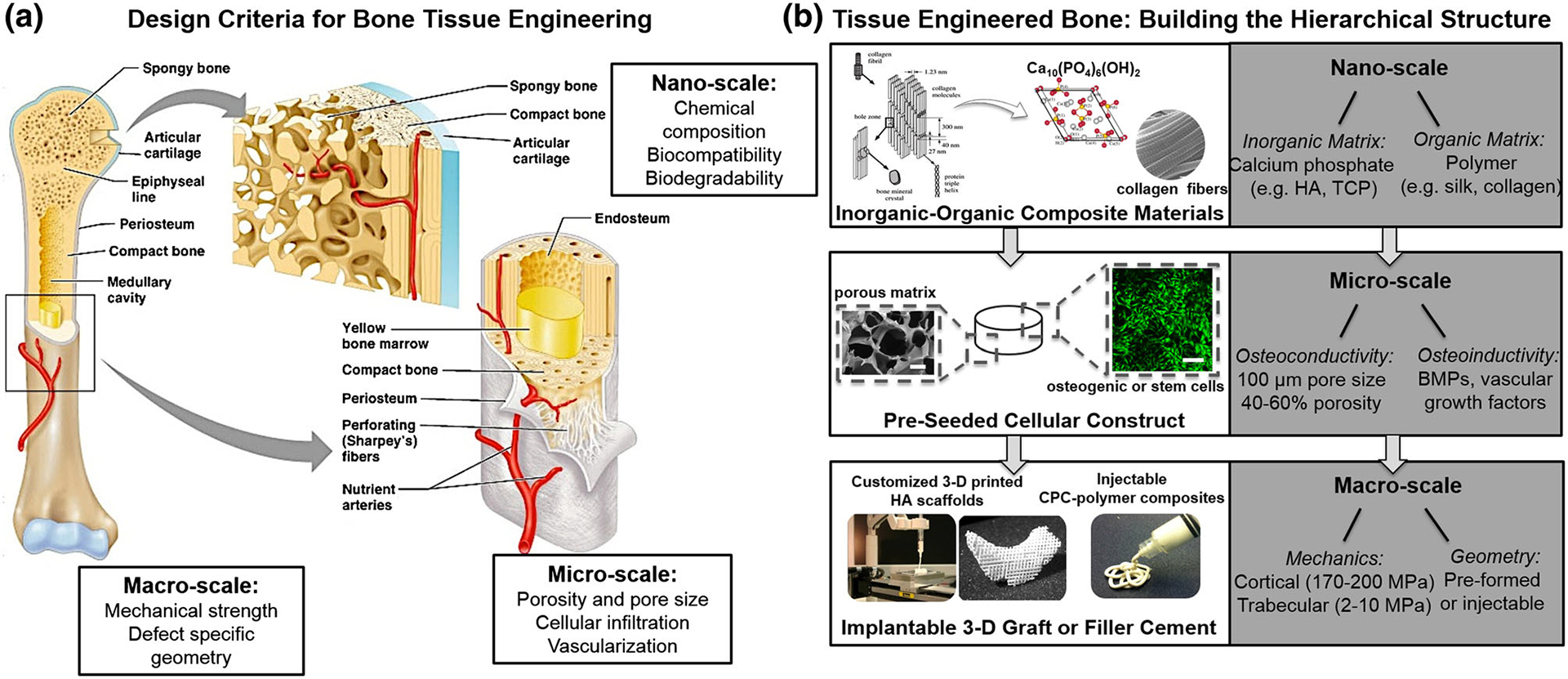
Bone tissue replacement. (a) Overview of the anatomical structure of human bone tissue and essential design criteria at the macro, micro, and nano-scales. (b) Building a functional bone graft or bone substitute material from a bottom-up approach involves careful selection of natural biocompatible and biodegradable materials to mimic the inorganic–organic structure of native bone. Calcium phosphates, such as hydroxyapatite (HA) and tricalcium phosphate (TCP), are frequently complexed with polymeric materials, such as collagen or silk, to form a composite structure. At a micro-scale, osteoconductivity of the composite scaffold is supported by porosity (40–60%) and pore size (>100 μm), and osteoinductivity to promote osteogenesis in vivo is accomplished by release of bone morphogenic proteins (BMPs) or vascular growth factors. On a macro-scale, scaffolds must withstand physiologic loading forces (2–10 MPa in trabecular bone and 170–200 MPa in cortical bone) and should be defect specific. This can be achieved with various methods including 3-D printing of patient-specific grafts or the use of an injectable, self-setting calcium phosphate cement (CPC)-polymeric fillers.
Design Criteria for Bone Replacement
From an engineering perspective, bone tissue is a composite, anisotropic material built to preferentially withstand mechanical forces in one direction (Fig. 1a).13 Due to the unique organic–inorganic composite structure of osseous tissue, design strategies in vitro aim to mimic natural mineralization processes. For example, mineralization can be induced via phosphorylation of collagen or the incorporation of bioactive agents (e.g., hydroxyapatite, bioactive glass particles).140,151–154 Additionally, mineralization can be achieved via the hybridization of collagenous biomaterial constructs with non-collagenous recombinant proteins, such as INFUSE®.234 Clinical success of peptide-based mineralization has been limited by the high cost of the peptides, as well as deleterious side effects (e.g., breathing difficulty, hematomas, swelling) caused by secondary inflammation near the site of implantation.96 The secondary inflammation can be caused by an unwanted immune response to the non-native peptides introduced for mineralization.234 For example, INFUSE® delivers recombinant bone morphogenic protein-2 (rhBMP-2) via a collagen sponge. Clinical results demonstrated that the desired inflammatory response at the treatment site may spread to adjacent critical structures, leading to increased postoperative morbidity.234
Despite the challenges tissue engineers face in achieving collagen scaffold mineralization in vivo, collagen-based biomaterials continue to be one of the most highly investigated natural materials for bone regeneration (Table 3). The implantation of pre-mineralized collagen matrices or pre-seeding with osteogenic cell lines remains the primary focus of in vivo collagen-based bone repair. The application of collagen hydrogels in particular has received much attention for bone repair. Implantation of a collagen hydrogel in a critical-sized rabbit segmental diaphyseal defect model resulted in supporting pre-seeded osteosarcoma cells,254 while collagen hydrogels with a collagen fibril density equivalent to native tissues (i.e., 10–15%) maintained structural stability up to 5 weeks post-implantation.177 These studies, among others, demonstrate the necessity of effective strategies for in vivo mineralization for successful graft integration and defect regeneration.
Silk
Silk protein has been widely used as a scaffolding material for tissue development and cell-based remodeling both in vitro and in vivo.20,57,150,165,192,261 Due to its robust mechanical profile, ability to be mineralized, controllable degradation, and excellent osteoconductive and osteoinductive properties, extensive research has been conducted on the use of silk for bone repair. The biocompatibility of silk has also been thoroughly investigated in the trabecular bone of sheep tibia and humerus defects.261 Despite the ability of silk scaffolds to deliver small molecules and growth factors while maintaining biocompatibility, silk exhibits a low compressive strength, thereby limiting its use in non-load-bearing bone regeneration. Within the past few years, however, several strategies have been implemented to overcome this mechanical limitation. Mandal et al. addressed the need for a stronger and stiffer natural polymeric matrix using a solvent-processed silk sponge scaffold reinforced with micron-sized silk fibers.150 Alternative strategies to improve the compressive strength of silk scaffolds utilize the addition of hydroxyapatite or bioglass particles, or in situ crystallization of calcium phosphate on the scaffold surface. Silk scaffolds have also been evaluated for the effect of pore size in bone repair in an in vivo model.165 Collectively, these studies demonstrate the potential of a natural silk material for bone tissue regeneration, and provide evidence for the utilization of silk or silk-based composites in clinical bone repair.
Alginate
The use of alginate-based materials for load bearing bone repair in critical-sized defects has been limited by the poor mechanical profile of alginate and the lack of cell-based degradation mechanisms, which can delay native tissue ingrowth into an alginate implant. However, despite these limitations, alginate-based materials and composites have been studied in orthopedic research for promoting osteogenesis, improving osteogenic differentiation, and delivering cells and growth factors to bone defect sites.265 One such study investigated the ability of an alginate/nanofiber mesh composites scaffold loaded with rhBMP-2 to enhance the repair of critically sized segmental bone defects in a rat model. Implantation with controlled release of rhBMP-2 resulted in consistent bony bridging within the defect, demonstrating the promise of these composites for growth factor delivery to repair of critically sized bone defects.126
Natural Materials as Bone Fillers and Cements
The rapidly rising demand for orthopedic tissue engineering products to repair spinal damage, musculoskeletal defects, or bone fractures has led to the development of several FDA-approved bone cements and filler materials. Calcium phosphates (CaP), particularly hydroxyapatite (HA), remain a focus for orthopedic applications due to their semblance to the mineral phase and crystalline structure of bone.59 A majority of bone filler materials are comprised of calcium phosphate cements (CPCs) (Table 4). In an aqueous environment, CPCs undergo rapid sedimentation and precipitation to apatite, hardening within minutes at body temperature. These reactive CPCs are optimal for on-site surgical filling of a bone gap, due to their moldability and rapid setting. Clinically, they are used in periodontal repair, cranio-maxillofacial surgery, and augmentation of an autograft or allograft. Once set, CPCs are very brittle and are therefore not applicable in the repair of load bearing critical-sized defects, where connection to the host vasculature, nervous, and muscular systems are paramount for clinical success.24,220 Overall, CPCs, especially when formulated using natural, non-toxic processing materials such as silk,165 represent a current clinical success story for research driven material design for clinical applications.
TABLE 4.
Commonly used synthetic bone filler materials on the US market.
| Product | Manufacturer | Composition | Forms |
|---|---|---|---|
| BonePlast | Interpore-Cross Intl. | Calcium sulfate | Injectable paste |
| PeoOsteon | Interpore-Cross Intl. | Coralline HA | Granules/blocks |
| Vitoss | Orthovita | β-tricalcium phosphate | Granules/blocks |
| α-BSM | Depuy Synthes | Calcium phosphate cement | Injectable paste |
| β-BSM | Depuy Synthes | Calcium phosphate cement | Injectable paste |
| γ-BSM | Depuy Synthes | Calcium phosphate cement | Moldable putty |
| Norian SRS | Depuy Synthes | Calcium phosphate cement | Injectable paste |
| Calceon6 | Depuy Synthes | Calcium sulfate | Pellets |
| CalStrux | Stryker Biotech | TCP with carboxymethycellulose | Moldable putty |
| BoneSource | Stryker Biotech | Calcium phosphate cement | Paste |
| HydroSet | Stryker Biotech | Calcium phosphate cement | Injectable paste |
| Collagraft | Zimmer/NeuColl | HA/TCP granules w/collagen gel | Strips |
| Cellplex | Wright Medical Inc. | Tricalcium phosphate | Granules |
| MIIG X3 | Wright Medical Inc. | Calcium sulfate | Pellets |
| OsteoSet | Wright Medical Inc. | Calcium sulfate | Pellets |
| Actifuse | Baxter International, Inc. | Silicate-substituted calcium phosphate ceramic | Injectable |
Most filler materials are calcium-based ceramics that can be mixed or cross-linked with polymeric materials to form composite scaffolds.
These bone fillers come in a variety of forms including injectable paste, powder/granules, pellets, and blocks.
HA, hydroxyapatite; TCP, β-tricalcium phosphate.
Clinical Translation and Commercialization
Cell-free bone cements and putties represent the state of the art in repair of small bone defects. However, secondary limitations, such as the lack of vascularization and organized structure of these cements, restrict their clinical potential. Given the recent progress in directing differentiation of human induced pluripotent stem (hiPS) cells towards osteoblast and osteoclast lineages50 in combination with improved material design, the development of successful cell-based therapies for repair of damaged or diseased bone is within reach.7 Progress towards clinical application of functional, critically sized bone grafts is underway, but current and future work must still address the combination of mineralized bone with other tissue structures such as bone marrow. In addition, connection of the new structure to the host system via vascularization/angiogenesis, innervation, and lymphangiogenesis has not been sufficiently addressed.7,24,43,106,114,129,220 Integration of a construct with the host tissue via these factors must be addressed in order to achieve long-term improvements in patient prognosis,.
BIOPOLYMERS FOR SKELETAL MUSCLE AND NERVE TISSUE REGENERATION
Skeletal muscle tissue has the innate ability to repair and regenerate following acute injury, as evidenced by the successful increase in organized and functional muscle tissue following prolonged and repeated periods of exercise. Exercise induces small tears in the muscle fibers, which the body is able to not only repair, but also respond to, via increase in skeletal muscle volume. However, some of the most important parameters in dictating clinical success include the maintenance and growth of vascular and neural network architectures within the new muscle. In critically sized defects, such as those caused by volumetric muscle loss following of trauma,41,42,147 vasculature and neural networks are not maintained. Regeneration of these connections in vivo, specifically for the nerve (i.e., the neuromuscular junction (NMJ)), is paramount for functional muscle recovery resulting in controllable contractile function. Consequently, there is still an unmet need for the development of critically sized tissue replacements comprised of aligned, functional muscle containing nerve conduits with functional neuromuscular junctions.
Design Criteria for Critically Sized Skeletal Muscle Defects
Skeletal Muscle
Like bone tissue, the structure of skeletal muscle dictates its function. Muscle is composed of bundles of muscle fibers surrounded by connective tissue, with each muscle fiber representing a single, often multinucleated, muscle cell. Individual cells or fibers are bundled together into small groups called fascicle, which are surrounded by a thin layer of connective tissue. Multiple fascicle will make up a given skeletal muscle. The tubular structure formed by the fascicle is vascularized and innervated such that the vessels and nerve conduits run parallel to the bundled muscle fibers (Fig. 2).242
FIGURE 2.
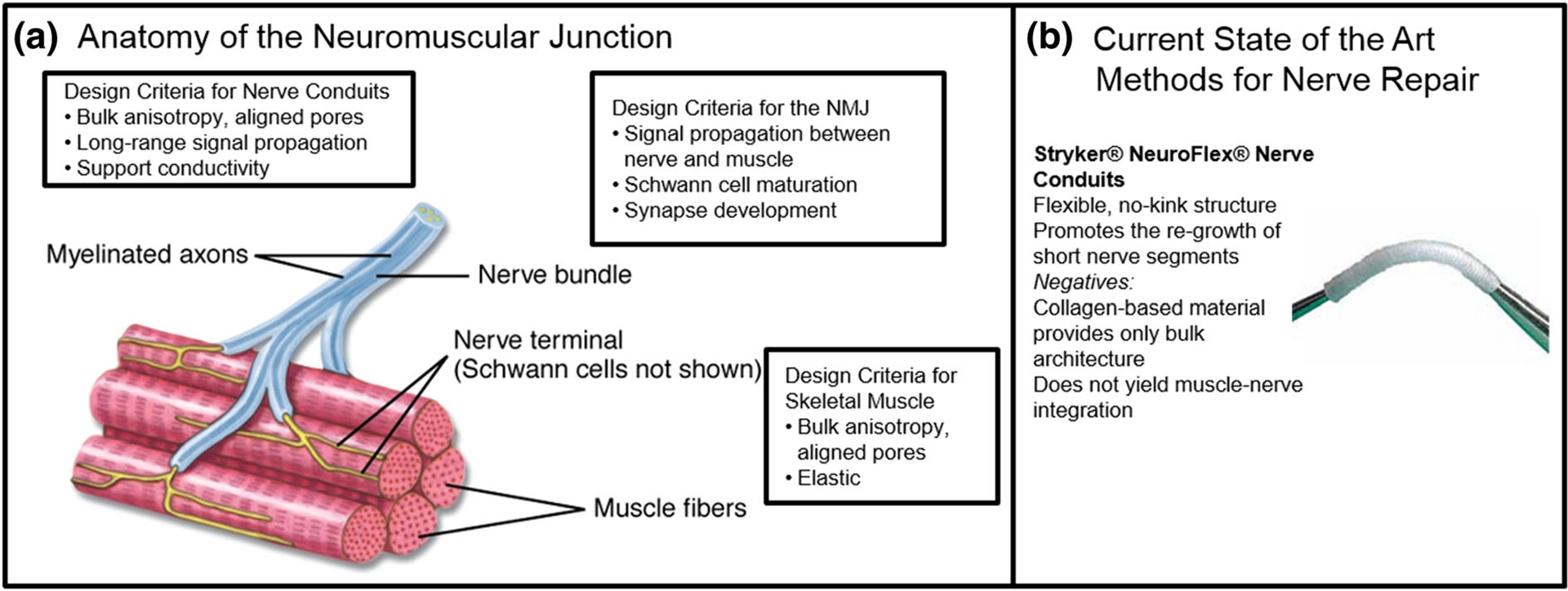
Engineering the neuromuscular junction (NMJ). (a) Anatomy of the neuromuscular junction showing myelinated axons of the nerve bundle interfacing with the striated muscle fibers at the nerve terminal. Design criteria for nerve conduits and skeletal muscle-NMJ interface include bulk alignment, elasticity, long-range signal propagation, and conductivity as well as efficient signal transmission to the sarcomere to elicit muscular response. (b) Commercially available scaffolds for nerve repair, such as Stryker NeuroFlex, are clinically available for implantation to promote the re-growth of short nerve segments. However, these conduits are only able to address nerve injuries or gaps of less than a critical size of 2.5 cm, nor can they provide effective muscle-nerve connectivity, which is essential for the regeneration of traumatic skeletal muscle and nerve injuries.
During development, changes in gene expression, structural alignment, and chemical cues facilitate the maturation of muscle progenitor cells into mature myoblasts or fibers, which are also key parameters in activating muscle progenitor or satellite cells in adult tissue.35,41,218,255,258,266,290 Many skeletal muscle engineering strategies have leveraged bulk alignment within the scaffold architecture, nanotopographical cues, and either passive tension or stretch applied by a bioreactor in order to mimic the native muscle structure.41,42,147,194
Engineering Skeletal Muscle with functional Neuromuscular Junctions (NMJs)
Apart from bulk alignment and cell–cell coupling, innervation of skeletal muscle is necessary for proper functioning of a critically sized muscle graft. Engineering the neuromuscular junction (NMJ) has proven extremely challenging due to the complexity required for proper cell-to-cell interactions that result in directed muscle movement.75,258 The neuromuscular junction develops in a complex multistep process involving both inter and extracellular signaling pathways, leading to the formation of synaptic contact between the terminal branches of the motor neuron and a specialized area of the muscle cell sarcolemma (i.e., plasma membrane) called the motor end-plate.281 To properly regenerate and build NMJs, interactions between motor neurons, skeletal muscle fibers, and glial cells must be tightly regulated through both time and space. As such, a thorough understanding how these cells interact and how to properly co-culture them is required before the development of critically sized constructs that achieve functional results will be successful.95,137,281 To further complicate the engineering problem, it is well known that improper cell-to-cell interaction and unorganized muscle-nerve interaction can lead to disease states such as amyotrophic lateral sclerosis (ALS), spinal muscular atrophy, and muscular dystrophy.245
In Vivo Accomplishments for Muscle and Nerve Tissue Regeneration
Many recent in vitro studies have demonstrated the potential use of natural biomaterial systems to guide skeletal and smooth muscle and nerve development, and further establish the functional connection between these two tissues.75,258 Current research aims to translate these successes to in vivo studies, further validating the use of natural materials for engineering critically sized muscle constructs. In vitro analyses suggest that constructs formed by the co-culture of two or more cell types may lead to better vascularization and innervation in vivo.75,258,281 By incorporating a mixed muscle progenitor cell population cultured on an aligned decellularized bladder matrix in the repair of volumetric muscle loss in rodents, the in vivo functional capacity of injured musculature improved 2.3-fold with the addition of a mixed cell population promoting the generation of functional skeletal muscle fibers. Additionally, a rodent model was used to demonstrate that innervation of rat skeletal muscle seeded fibrin gels prior to implantation significantly increased force generation and NMJ development in vivo.55 Addition of agrin, a large proteoglycan important for NMJ development, significantly increased the formation of acetylcholine receptor clusters on differentiated C2C12 mouse myoblasts in vitro.125 Pre-fabrication of acetylcholine receptor clusters on differentiated C2C12s in fibrin gels prior to implantation also accelerated innervation in vivo in a male nude rat model.125 These recent results demonstrate the potential for engineering the NMJ in vivo, while also elucidating current limitations in scaffold design, such as lack of control over cell-to-cell contacts, and physiologically inaccurate 3D geometries preventing proper NMJ development on aligned muscle fibers, all of which must be addressed in future designs.
Clinical Translation and Commercialization
While the clinical realization of innervated skeletal muscle constructs has yet to be achieved, clinical success in the repair of short nerve defects within muscle tissue has been demonstrated. Materials for nerve repair are commercially available and focus on guiding nerve growth using artificial conduits. Stryker® (Kalamazoo, MI) developed type I collagen-based biomaterials for peripheral nerve and soft tissue repair. Their products, NeuorMatrix™, NeuroFlex™, and NeuroMend™ all use type I collagen as a wrap or tube to repair peripheral nerve injuries (Fig. 2b). In addition, TissueMend™ (Stryker®, Kalamazoo, MI) acellular collagen scaffolds are designed to augment repair and provide mechanical reinforcement for damaged tissues. While these materials are FDA-approved, they do not meet many of the design criteria described above. For example, type I collagen-based materials do not provide bulk alignment, support NMJ formation, or promote differentiation of local progenitor cells into functional nerve or muscle tissue.
Similarly, both natural and synthetic materials have been utilized for form aligned skeletal muscle fibers using a variety of formats including scaffolds, films, and limited success has been demonstrated in animal models (see Ref. 279 for recent review).41,42 Overall, bridging the gap from promising in vivo animal studies to human trials and commercialization has proven difficult due to the multiple tissues that must integrate fully to provide functional recovery. However, as further progress is made using stem or progenitor cells, these remain a promising avenue for the repair of critically sized muscle defects.26
BIOPOLYMERS FOR CARDIAC TISSUE REGENERATION
Heart disease is the leading cause of death in both men and women, costing the United States $108.9 billion each year in health care services, medication, and loss of productivity.30 In adult patients, complications from heart disease often result in heart failure, but in children, heart failure is frequently caused by congenital malformations of the heart (e.g., birth defects), weakened heart muscle, or damaged tissue not specifically due to heart disease. Currently, the only clinically available treatments for congenital birth defects and end-stage heart failure include left ventricular assist devices (LVADs), total heart transplantation, and surgical reconstruction.225,282 Despite some success in heart repair and regeneration in small animal models, regeneration of electromechanically integrated human myocardium has yet to succeed, driving the need for new ideas and alternative engineering approaches for the repair and restoration of proper heart function.
Design Criteria for Cardiac Tissue Replacement
Scaffolds designed for cardiac tissue engineering must provide equivalent functionalities of physiological cardiac muscle, offering mechanical and electrical support for the native or encapsulated cardiomyocytes. Like skeletal muscle, cardiac muscle is striated. Therefore, alignment of scaffold architecture, whether achieved through passive stretch or tension, constant strain, or structural patterning, has shown to significantly improve cardiomyocyte function (Fig. 3a).21,170,174 Whether acellular or cell-seeded, scaffolds must demonstrate a high degree of elasticity, promote cellular remodeling, maintain cell viability, support stem cell differentiation, and eventually sustain cardiomyocyte hypertrophy. In a cell-delivery approach, the scaffold design must yield integration of the provided cells (e.g., stem cells) with the host tissue and electromechanically couple these cells with the native heart muscle through both host cell ingrowth and delivered cell outgrowth (Fig. 3a). Mechanical considerations depend on many factors, including the age of the patient and the presence of injury or disease, and therefore, the modulus of scaffolds for cardiac tissue engineering should range between 5 and 50 kPa with pore sizes ranging from 20 to 40 μm.204,225 Additionally, delivery of growth factors such as insulin-like growth factor-1 (IGF-1), human growth factor (HGF), vascular endothelial growth factor (VEGF), and stromal cell-derived factor-1 (SDF-1) have improved cardiac function and tissue integration following trauma.257,282 As designs for cardiac tissue engineering move forward, the use of time-dependent and slow-release delivery systems will be necessary to achieve the desired paracrine signaling profiles in vivo.
FIGURE 3.
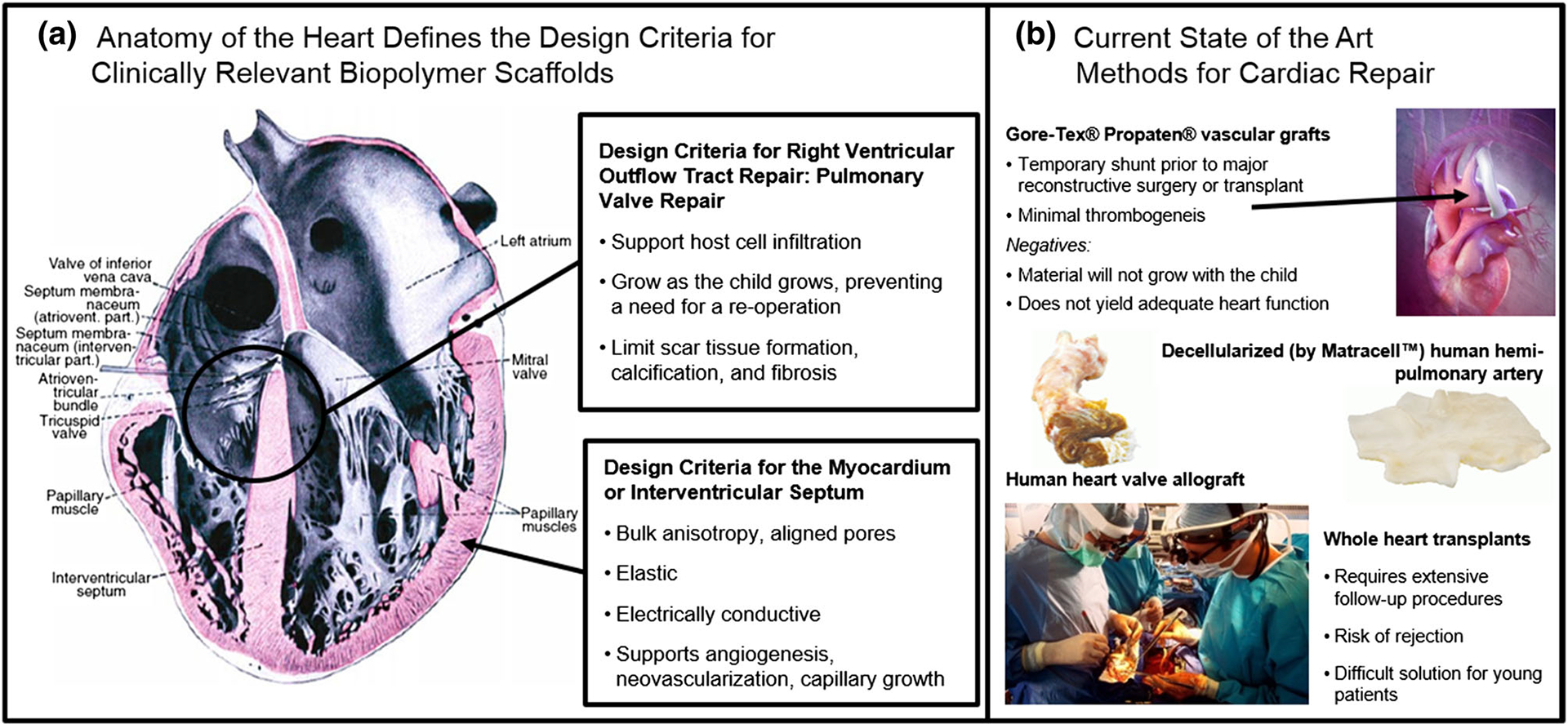
Cardiac tissue replacement. (a) Anatomy of the heart showing the striated alignment of cardiac muscle. Design criteria for cardiac tissue engineering include high elasticity, aligned porosity, support of vascular and capillary network growth, and methods for integration into the host tissue both mechanically and electrically. (b) A variety of materials are commercially available for temporary repair of cardiac defects, but most are made from non-degradable plastics, which do not grow with the patient or integrate with the tissue, leading to the risk of fatal arrhythmias. Some disadvantages of clinically available vascularized grafts or decellularized matrices are thrombosis or a lack of tissue integration and growth within the patient. Decellularized grafts represent an allogenic approach to repairing damaged tissue, but patients run the risk of rejection, infection, and fibrosis.
In Vivo Accomplishments for Cardiac Tissue Replacement
Recent in vitro and rodent-based in vivo data support the use of both cellular and acellular approaches for repairing and restoring heart tissue in both children and adults, yet clinical realization of these strategies has proven difficult.26,128 These therapies include the use of hydrogels or decellularized extracellular matrix solutions that are injected into or onto the heart,226 the implantation of scaffolds or matrices, or the implantation of films or cell sheets.160 Treatments are aimed at either restoring heart wall thickness and strengthening the muscle or minimizing scar tissue accumulation in the area and replacing it with electromechanically coupled tissue.
Alginate and Chitosan
Polysaccharides, such as alginate and chitosan, have been widely utilized for cardiac tissue engineering due to their biocompatibility and ease of use.239 Currently, liquid alginate solutions (Sodium Alginate and Calcium Gluconate, NCT01226563 and Algisyl-LVR™ intramyocardial injections of alginate hydrogel, NCT01311791) are undergoing thorough investigation in human clinical trials. However, a three-dimensional, pre-formed alginate-based cardiac patch has not yet reached the clinic despite promising in vitro results.239 The development of a cardiac patch from one or both of these materials is extremely challenging due to the limitations in electrical conductivity. Gold nanowires, gold nanoparticles, and carbon nanotubes have been incorporated into polysaccharide-based hydrogels to improve electrical coupling between adjacent cardiac cells in vitro. As with all critically sized constructs, vascularization is paramount to the success of a cardiac construct. One approach is to add pro-angiogenic growth factors to the matrix to improve host infiltration. Recent efforts using these polysaccharide based biopolymers have led to the successful delivery or growth factors and small molecules in vivo.133,262
Gelatin and Elastin
Use of collagen alone to repair heart defects is challenging since the protein itself causes stiffening of the heart tissue and induces reprogramming of cardiac fibroblasts. Instead, gelatin and elastin based scaffolds serve as sufficient alternatives for cardiac tissue engineering due to their rapid cellular degradation and bulk elasticity. Gelatin-based sheets for the fabrication of cardiac patches are currently under investigation in clinical trials (e.g., AutoLogous Human CArdiac-Derived stem cell to treat Ischemic cArdiomyopathy (ALCADIA), NCT00981006). Novel composites formed by methacrylating biopolymers, such as GelMa (methacrylated gelatin236) and MeTro (methacrylated tropoelastin10), have also been studied extensively in vitro. These biopolymers also lack electrical conductivity, which has prompted the in vivo investigation of composite materials containing single walled carbon nanotubes (SWNTs) for left ventricle repair following myocardial infarction. The implantation of these devices in vivo resulted in significantly increased fractional shortening and ejection fraction compared to untreated hearts, partially due to enhanced the expression of intercellular adhesive junctions and electrochemical junctions in rats who received SWCT-based implants.294 Overall, SWCT-based scaffolds integrated into infarct myocardium exerted beneficial effects on myocardial regeneration and remodeling in the infarct areas, resulting in the improvement of heart functions in rats.294 In vitro, the addition of the SWCTs enhanced spontaneous electrical activity in seeded cell constructs as well as cardiomyocyte connectivity, as demonstrated by the ability of SWCT-containing constructs to beat regionally after 2–3 days and contract synchronously at day 8, which was not observed in SWCT-free materials.294 SWNT-based materials supported the contractile properties of engineered cardiac tissue through enhancement of the formation of gap junctions and promotion of the excitation–contraction coupling of cardiomyocytes.294 However, the long-term biocompatibility of SWCTs in vivo raises concern for the translation of these scaffolds to human clinical trials.
Fibrin
Fibrin-based scaffolds show exciting promise for cardiac repair given the elastic nature of the scaffold and the rapid degradation and cellular remodeling potential afforded by the chemical structure.89 Initial work with this biopolymer demonstrated modulation of gelation rates by adjusting the fibrinogen to thrombin ratio to achieve an injectable material capable of delivering cells or growth factors to a damaged heart surface.39,178 Initial in vitro investigations of fibrin for cardiac repair established potential therapy to regenerate post-myocardial infarction cardiac tissue using 3D functional fibrin-based myocardial equivalent grafts formed in vitro. Results indicated that the aligned fibrin scaffolds demonstrated a significant increase in twitch force compared to the isotropic constructs.21 Parameters that could be modulated to tailor these construct properties prior to implantation include regulating the magnitude of stretch used to induce alignment, preconditioning the scaffold to stretch in a bioreactor, or pre-seeding the graft with cardiomyocytes prior to implantation.173–176,285,292
Clinical Translation and Future Directions
Current research on cardiac patch biomaterials is promising, yet full realization of the potential of these therapies remains elusive. Unless a patient is undergoing open-heart surgery, such as a valve replacement, surgical access to the outer left ventricle wall is limited. Regeneration strategies have instead focused on delivering cells or injectable gels at the damaged site by a catheter.226 Additionally, natural biopolymer-based cardiac patches may provide solutions to complex cardiac reconstruction in young patients with complex congenital heart defects. Natural-based cardiac patches have allowed engineers to overcome the limitations of current synthetic patches, which do not promote cellular remodeling, do not grow as the child grows, and often require reoperation (Fig. 3b).102 Furthermore, in young patients, both extracellular matrix composition and cardiomyocyte metabolism differ greatly from mature, adult tissue, necessitating a careful analysis of design criteria and biomaterial selection for cardiac graft fabrication.276,282 These results present two major implications for the future of cardiac tissue regeneration and remodeling: (1) repair in younger patients may be more successful due a greater percentage of proliferating cells and circulating progenitors and (2) the fetal environment (e.g., matrix composition, tissue density, tissue mechanics) may represent improved design criteria for repair and regeneration in the adult heart.
BIOPOLYMERS FOR CORNEAL TISSUE REPLACEMENT
The human cornea is a transparent, avascular, connective tissue that provides an optical interface with substantial refractive power, while protecting the eye from mechanical injury and potential infection. This organ is comprised of three distinct cellular layers: corneal epithelium, stroma, and endothelium, which are separated by two acellular collagenous interfaces known as the Bowman’s Layer and Descemet’s membrane (Fig. 4).214 Improper corneal development, damage to the cornea and limbal cells, or nerve injury resulting from infection or trauma can result in loss of corneal transparency, leading to either partial or complete loss of vision. Approximately 10 million people worldwide suffer from corneal vision loss, prompting the need for an effective corneal transplant therapy. These transplants can be categorized into two main repair options: allogenic and synthetic materials. Although allogenic materials from human donors are preferred, a shortage of quality donor graft material has limited the broad applicability of this clinical option. Alternatively, synthetic homologs to donor corneal grafts are primarily used as temporary replacements until suitable donor tissue becomes available. The use of a synthetic homolog for long-term repair is limited by intrinsic risks of corneal melting, bacterial endophthalmitis, and retinal detachment resulting in graft failure.
FIGURE 4.
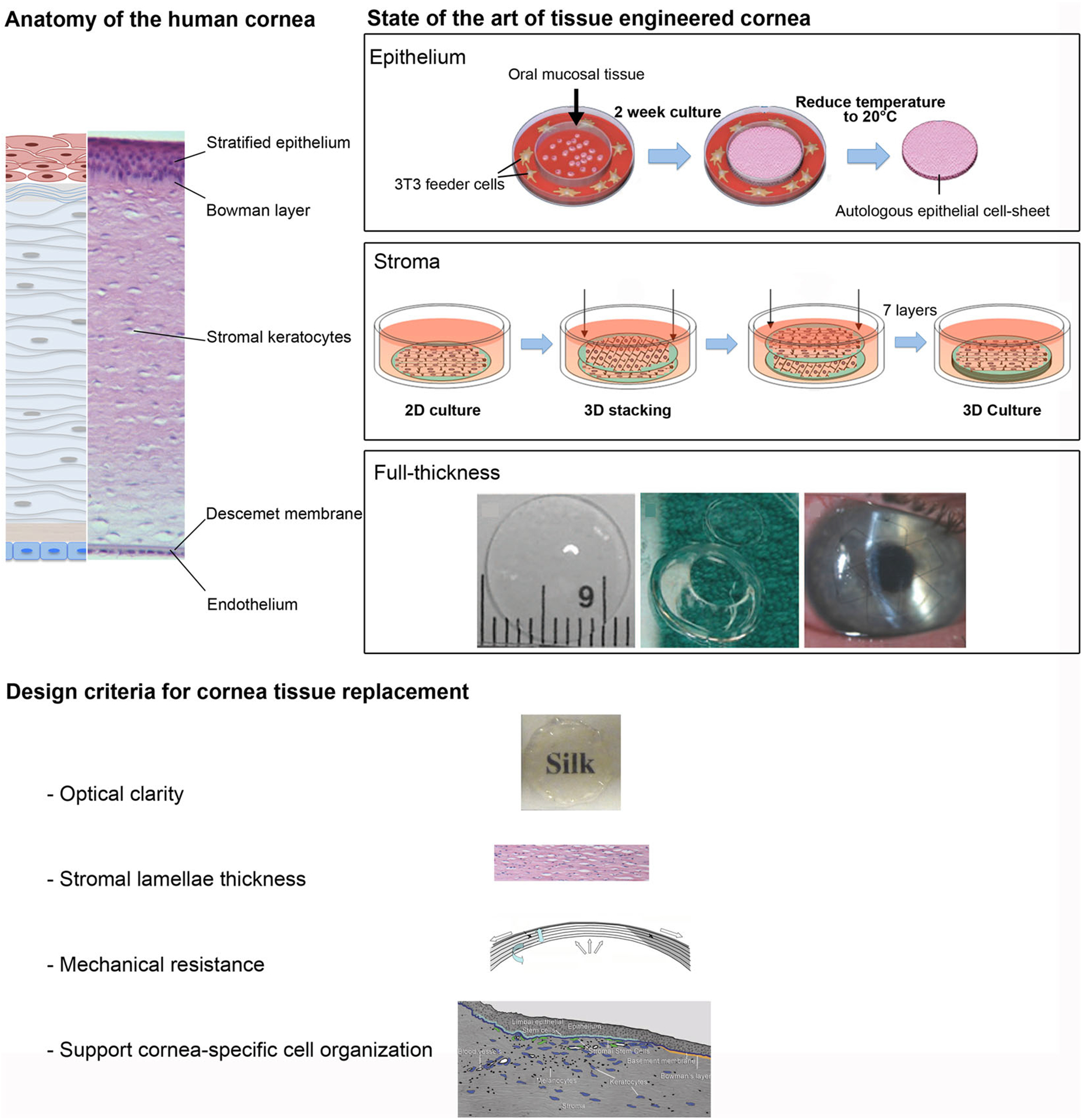
Cornea tissue replacement. (a) Anatomy of the human cornea: schematic and histological images of human cornea layers: the corneal stratified squamous epithelium with underlying the Bowman’s layer, the stroma with keratocytes for the maintenance and production of extracellular matrix, the Descemet’s membrane, and, the single-layer endothelium. State of the art of tissue engineered cornea: Epithelium Human epithelial cell sheet obtained from oral epithelial cells after removal of the cell sheet from the thermoresponsive surface; Stroma Assembly diagram for 3D silk film corneal constructs seeded with human corneal fibroblasts; Full-thickness Synthetically crosslinked collagen, molded into an implantable, full-thickness corneal substitute. Transparent samples were trephined to prepare a button for corneal implantation and then held in place by sutures in the patient eye.
Design Criteria for Cornea Tissue Replacement
The main functions of the cornea necessitate three major design requirements: protection, transparency, and an effective optical interface. In addition, a corneal replacement device must also be biocompatible in the human body and ideally bioactive such that the graft material can integrate within the surrounding host tissue (Fig. 4). Efforts to regenerate the cornea have focused on the in vitro regeneration of the epithelium, stroma, and endothelium strata, followed by the promotion of neural and vascular interfaces. Natural biopolymers have been used extensively to mimic the corneal epithelium layer. In particular, reconstituted and chemically cross-linked type I collagen or silk hydrogels have been used as substrates for human epithelial cell growth and functional tissue organization.138,169 Reconstruction of the corneal stroma has proven challenging due to the complex structure, mechanical strength requirements, and need for optical transparency. Therefore, corneal stroma engineering has focused on the development of functional corneal stroma substrates through chemical, morphological, and mechanical cues.88,131,277 In the particular context of the corneal stroma, type I collagen has been used extensively due to its dominant content in the native corneal tissue as well as its standardized processability.84 Alternatively, silk films have been optimized to support corneal stromal cell growth and organization in both 2D and 3D environments, in which topography, surface chemistry, porosity, degradation profiles, and transparency were controlled.87,229
In Vivo Accomplishments for Corneal Tissue Replacement
In order to study the integration of an implanted biomaterial within corneal native tissue, in vivo implantation of acellular corneal tissue equivalents has been investigated.139,205 Studies have focused on recapitulating the three-layer structure of the cornea (epithelium, stroma, and endothelium).2,91 Recent efforts have used decellularized biological material, such as amniotic membranes and animal-derived cornea. However, the results of an acellular porcine cornea in combination with amniotic epithelial cells in a rabbit lamellar keratoplasty resulted in degradation of the tissue-engineered cornea due to host rejection.143 Decellularized amniotic membrane was clinically evaluated in combination with human corneal endothelial cells in a lamellar keratoplasty model. The endothelium and part of the Descemet’s membrane were removed, and the construct was able to function as a corneal endothelium equivalent.73 In all cases, complications due to foreign material host response and material performance limitations such as lack in transparency, degradability, and mismatch in mechanical and permeability properties hindered success in the reported animal studies.
Clinical Translation and Commercialization
Anterior partial keratoplasty performed in humans using biosynthetic corneas made from cross-linking recombinant human collagen type III showed that these naturally derived collagen scaffolds provide effective tissue regeneration by promoting endogenous tissue growth and innervation without signs of vascularization for up to two years. However, a delay in epithelial closure and a fibrotic response were observed, likely caused by surgical sutures.68 A 4-year follow up showed a stably integrated implant, although a more robust material with better shape retention may improve visual acuity.69 Partial or full-thickness engineered corneal tissues have been developed for in vitro preclinical cornea tissue repair models to reduce animal testing for commercial products for eye irritancy tests.45 A multi-layer collagen hydrogel scaffold was developed and evaluated using primary corneal endothelial, stromal, and epithelial cells.209 The commercially available products mainly referred to engineered epithelium based on trans-well permeable membrane architecture, as in the case of Clonetics™ Human Corneal Epithelial Culture Model (Lonza, Hopkinton, MA), LabCyte Cornea-Model (Japan Tissue Engineering Co., Ltd., Gamagori City, Aichi, Japan), and EpiOcular™ (MatTek Corporation, Ashland, MA).
THE FUTURE OF BIOPOLYMERS: A FOCUS ON CLINICAL TRANSLATION
Given the wide range of products available on the market today, there still exists a pressing need for further product development to repair full thickness wounds. The path to clinical translation and commercialization of wound healing products presents a case where academics, start-up companies, and venture capitalists can reflect on both the successes and failures of product implementation in the medical field. A major lesson learned is that there is no “one-size-fits-all” solution such that a single product or biomaterial will not meet the design criteria and patient need for every clinical application. For instance, wound healing is more complex and takes longer in diabetic patients compared to healthy patients, as these patients often suffer from ischemia and neuropathy in their extremities. Thus, challenges with cost-to-patient-benefit analysis, proper doctor recommendation and utilization, long times between initial development and FDA approval, and other unforeseen challenges have impeded the clinical translation of many promising biopolymer-based products.107
A fundamental understanding of native tissue structure and organization, as well as physical, and biochemical properties is essential for the successful design of biomaterial scaffold systems to produce effective tissue replacement therapies. The goal of this review was to provide an overview of recent efforts based on natural polymers to mimic in vivo structures for tissue types including bone, muscle, peripheral nerve, cardiac, and cornea, and to highlight the progression of these systems from animal models into clinical settings. Despite major recent advancements in the tissue engineering field, significant challenges such as lack of vascularization, fine control of biomaterial degradation rate and byproducts, and construct re-innervation in critical-size grafts are limiting factors in the translation of tissue engineered products to clinical scenarios. Thus, there remains a significant opportunity to develop new methods and techniques for the generation of pre-vascularized and re-innervated tissues to promote angiogenesis and neovascularization, lymphangiogenesis, and innervation in order to create fully functioning tissues.
ACKNOWLEDGMENTS
We thank the NIH for support (P41 EB002520, R01 EB011620, R01 EY020856, R01 DE017207). W.L.S acknowledges funding from the Tufts University Training in Education and Critical Research Skills NIH IRACDA program (K12 GM074869).
REFERENCES
- 1.Abou Neel EA, Bozec L, Knowles JC, Syed O, Mudera V, Day R, and Hyun JK. Collagen—emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev 65:429–456, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Alaminos M, Sánchez-Quevedo MDC, Muñoz-Ávila JI, Serrano D, Medialdea S, Carreras I, and Campos A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Investig. Ophthalmol. Vis. Sci 47:3311–3317, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Allen RA, Wu W, Yao M, Dutta D, Duan X, Bachman TN, Champion HC, Stolz DB, Robertson AM, and Kim K. Nerve regeneration and elastin formation within poly (glycerol sebacate)-based synthetic arterial grafts one-year post-implantation in a rat model. Biomaterials 35:165–173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alluin O, Wittmann C, Marqueste T, Chabas J-F, Garcia S, Lavaut M-N, Guinard D, Feron F, and Decherchi P. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30:363–373, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Almine JF, Bax DV, Mithieux SM, Nivison-Smith L, Rnjak J, Waterhouse A, Wise SG, and Weiss AS. Elastin-based materials. Chem. Soc. Rev 39:3371–3379, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, and Kaplan DL. Silk-based biomaterials. Biomaterials 24:401–416, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Amini AR, Laurencin CT, and Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng 40:363–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An B, DesRochers TM, Qin GK, Xia XX, Thiagarajan G, Brodsky B, and Kaplan DL. The influence of specific binding of collagen-silk chimeras to silk biomaterials on hMSC behavior. Biomaterials 34:402–412, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anitua E, Andia I, Ardanza B, Nurden P, and Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost 91:4–15, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, and Weiss AS. Highly elastic micropatterned hydrogel for engineering functional cardiac tissue. Adv. Funct. Mater 23:4950–4959, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astete CE, and Sabliov CM. Synthesis and characterization of plga nanoparticles. J. Biomater. Sci. Polym. Ed 17:247–289, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Athanasiou KA, Niederauer GG, and Agrawal C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 17:93–102, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Athanasiou KA, Zhu CF, Lanctot DR, Agrawal CM, and Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 6:361–381, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Baldock C, Oberhauser AF, Ma L, Lammie D, Siegler V, Mithieux SM, Tu Y, Chow JY, Suleman F, Malfois M, Rogers S, Guo L, Irving TC, Wess TJ, and Weiss AS. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proc. Natl. Acad. Sci. USA 108:4322–4327, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartus C, William Hanke C, and Daro-Kaftan E. A decade of experience with injectable poly-l-lactic acid: a focus on safety. Dermatol. Surg 39:698–705, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Bell E, Ivarsson B, and Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci 76:1274–1278, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellas E, Panilaitis BJB, Glettig DL, Kirker-Head CA, Yoo JJ, Marra KG, Rubin JP, and Kaplan DL. Sustained volume retention in vivo with adipocyte and lipoaspirate seeded silk scaffolds. Biomaterials 34:2960–2968, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beun LH, Storm IM, Werten MW, de Wolf FA, Cohen Stuart MA, and de Vries R. From micelles to fibers: balancing self-assembling and random coiling domains in ph-responsive silk-collagen-like protein-based polymers. Biomacromolecules 15:3349–3357, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhola M, Sanchez S, and Kolhatkar S. Use of an extracellular matrix membrane for root coverage: case series and review of the literature. Clin. Adv. Periodontics 3:16–21, 2013. [Google Scholar]
- 20.Bhumiratana S, Grayson WL, Castaneda A, Rockwood DN, Gil ES, Kaplan DL, and Vunjak-Novakovic G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 32:2812–2820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black LD III, Meyers JD, Weinbaum JS, Shvelidze YA, and Tranquillo RT. Cell-induced alignment augments twitch force in fibrin gel–based engineered myocardium via gap junction modification. Tissue Eng. Part A 15:3099–3108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond E, Barrett S, Pragnell J, and Victoria R. Successful treatment of nonhealing wounds with xelma®. Br. J. Nursing 18:1404–1409, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli M, Reichl S, Feng Y, Schargus M, Schrader S, and Geerling G. In vitro characterization and ex vivo surgical evaluation of human hair keratin films in ocular surface reconstruction after sterilization processing. J. Mater. Sci. Mater. Med 24:221–230, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Bose S, Roy M, and Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 30:546–554, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, and Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 11:1122–1132, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Burdick JA, Mauck RL, Gorman JH, and Gorman RC. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci. Transl. Med 5:176ps4, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buskens E, Meijboom MJ, Kooijman H, and Van Hout BA. The use of a surgical sealant (coseal) in cardiac and vascular reconstructive surgery: an economic analysis. J. Cardiovasc. Surg 47:161–170, 2006. [PubMed] [Google Scholar]
- 28.Cannata A, Taglieri C, Russo CF, Bruschi G, and Martinelli L. Use of coseal in a patient with a left ventricular assist device. Ann. Thorac. Surg 87:1956–1958, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Carlson M, Faria K, Shamis Y, Leman J, Ronfard V, and Garlick J. Epidermal stem cells are preserved during commercial-scale manufacture of a bilayered, living cellular construct (apligraf®). Tissue Eng. Part A 17:487–493, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Heart disease fact sheet. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 31.Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, and Ouyang HW. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 31:9438–9451, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q-Z, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, Chrzanowski W, Boccaccini AR, Ali NN, Knowles JC, and Harding SE. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials 31:3885–3893, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Chicatun F, Pedraza CE, Ghezzi CE, Marelli B, Kaartinen MT, McKee MD, and Nazhat SN. Osteoid-mimicking dense collagen/chitosan hybrid gels. Biomacromolecules 12:2946–2956, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Chicatun F, Pedraza CE, Muja N, Ghezzi CE, McKee MD, and Nazhat SN. Effect of chitosan incorporation and scaffold geometry on chondrocyte function in dense collagen type I hydrogels. Tissue Eng. Part A 19:2553–2564, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho OH, Mallappa C, Hernandez-Hernandez JM, Rivera-Perez JA, and Imbalzano AN. Contrasting roles for myod in organizing myogenic promoter structures during embryonic skeletal muscle development. Dev. Dyn 2014. doi: 10.1002/dvdy.24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow D, Nunalee ML, Lim DW, Simnick AJ, and Chilkoti A. Peptide-based biopolymers in biomedicine and biotechnology. Mater. Sci. Eng. R 62:125–155, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choy DKS, Nga VDW, Lim J, Lu J, Chou N, Yeo TT, and Teoh S-H. Brain tissue interaction with three-dimensional, honeycomb polycaprolactone-based scaffolds designed for cranial reconstruction following traumatic brain injury. Tissue Eng. Part A 19:2382–2389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christman KL, Fok HH, Sievers RE, Fang Q, and Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 10:403–409, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, and Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J. Am. Coll. Cardiol 44:654–660, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Cormio L, Perrone A, Di Fino G, Ruocco N, De Siati M, de la Rosette J, and Carrieri G. Tachosil® sealed tubeless percutaneous nephrolithotomy to reduce urine leakage and bleeding: outcome of a randomized controlled study. J. Urol 188:145–150, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, Zhao W, and Christ GJ. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. Part A 18:1213–1228, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corona BT, Ward CL, Baker HB, Walters TJ, and Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A 20:705–715, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corpas Ldos S, Lambrichts I, Quirynen M, Collaert B, Politis C, Vrielinck L, Martens W, Struys T, and Jacobs R. Peri-implant bone innervation: histological findings in humans. Eur. J. Oral Implantol 7:283–292, 2014. [PubMed] [Google Scholar]
- 44.Curran MP, and Plosker GL. Bilayered bioengineered skin substitute (apligraf®). BioDrugs 16:439–455, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Curren RD, and Harbell JW. Ocular safety: a silent (in vitro) success story. Altern. Lab. Anim 30(Suppl 2):69–74, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, and Niklason LE. Readily available tissue-engineered vascular grafts. Sci. Transl. Med 3:68ra9, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Dan H, Vaquette C, Fisher AG, Hamlet SM, Xiao Y, Hutmacher DW, and Ivanovski S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 35:113–122, 2014. [DOI] [PubMed] [Google Scholar]
- 48.De la Riva B, Nowak C, Sanchez E, Hernandez A, Schulz-Siegmund M, Pec MK, Delgado A, and Evora C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm 73:50–58, 2009. [DOI] [PubMed] [Google Scholar]
- 49.De Luca AC, Stevens JS, Schroeder SLM, Guilbaud JB, Saiani A, Downes S, and Terenghi G. Immobilization of cell-binding peptides on poly-ε-caprolactone film surface to biomimic the peripheral nervous system. J. Biomed. Mater. Res. Part A 101:491–501, 2013. [DOI] [PubMed] [Google Scholar]
- 50.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, and Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 110:8680–8685, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Valence S, Tille J-C, Mugnai D, Mrowczynski W, Gurny R, Möller M, and Walpoth BH. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 33:38–47, 2012. [DOI] [PubMed] [Google Scholar]
- 52.Deal DN, Griffin JW, and Hogan MV. Nerve conduits for nerve repair or reconstruction. J. Am. Acad. Orthop. Surg 20:63–68, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Deng Y, Bi X, Zhou H, You Z, Wang Y, Gu P, and Fan X. Repair of critical-sized bone defects with anti-mir-31-expressing bone marrow stromal stem cells and poly (glycerol sebacate) scaffolds. Eur. Cells Mater 27:13, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Dhanraj P A clinical study comparing helicoll with scarlet red and opsite in the treatment of split thickness skin graft donor sites—a randomized controlled trial. Indian J. Surg 2013. doi: 10.1007/s12262-013-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dhawan V, Lytle IF, Dow DE, Huang Y-C, and Brown DL. Neurotization improves contractile forces of tissue-engineered skeletal muscle. Tissue Eng. 13:2813–2821, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Di Martino A, Sittinger M, and Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26:5983–5990, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Diab T, Pritchard EM, Uhrig BA, Boerckel JD, Kaplan DL, and Guldberg RE. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J. Mech. Behav. Biomed. Mater 11:123–131, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dornseifer U, Lonic D, Gerstung TI, Herter F, Fichter AM, Holm C, Schuster T, and Ninkovic M. The ideal split-thickness skin graft donor-site dressing: a clinical comparative trial of a modified polyurethane dressing and aquacel. Plast. Reconstr. Surg 128:918–924, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Dorozhkin SV Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci 44:2343–2387, 2009. [Google Scholar]
- 60.Downie F, and Gannon R. Opsite flexifix gentle: preventing skin breakdown in vulnerable skin. Br. J. Nurs 22:698–700, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Driscoll P Tissue engineering, cell therapy, and transplantation: products, technologies, and market opportunities worldwide: 2009–2018. Tissue Eng. Cell Ther 2010. http://www.mediligence.com/rpt/rpt-s520.htm. [Google Scholar]
- 62.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, Jin H, Parker KK, Langer R, and Kohane DS. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol 6:720–725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eaglstein WH, and Falanga V. Tissue engineering and the development of apligraf®, a human skin equivalent. Clin. Ther 19:894–905, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Ellis CN Outcomes with the use of bioprosthetic grafts to reinforce the ligation of the intersphincteric fistula tract (biolift procedure) for the management of complex anal fistulas. Dis. Colon Rectum 53:1361–1364, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Engelmayr Jr., Cheng GC,M, Bettinger CJ, Borenstein JT, Langer R, and Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater 7:1003–1010, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erb MA, Claus T, Hartrumpf M, Bachmann S, and Albes JM. The use of tachosil® surgical patch or fibrin glue in coronary artery surgery does not affect quality of anastomosis or provoke postoperative adhesions in pigs. Eur. J. Cardiothorac. Surg 36:703–707, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Etienne O, Schneider A, Kluge JA, Bellemin-Laponnaz C, Polidori C, Leisk GG, Kaplan DL, Garlick JA, and Egles C. Soft tissue augmentation using silk gels: an in vitro and in vivo study. J. Periodontol 80:1852–1858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Soderqvist M, and Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med 2:46ra61, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, Suuronen EJ, Liu Y, Brunette I, and Griffith M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 35:2420–2427, 2014. [DOI] [PubMed] [Google Scholar]
- 70.Falabella AF, Schachner LA, Valencia IC, and Eaglstein WH. The use of tissue-engineered skin (apligraf) to treat a newborn with epidermolysis bullosa. Arch. Dermatol 135:1219–1222, 1999. [DOI] [PubMed] [Google Scholar]
- 71.Falabella AF, Valencia IC, Eaglstein WH, and Schachner LA. Tissue-engineered skin (apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch. Dermatol 136:1225–1230, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Falanga V, and Sabolinski M. A bilayered living skin construct (apligraf®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 7:201–207, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Fan T, Ma X, Zhao J, Wen Q, Hu X, Yu H, and Shi W. Transplantation of tissue-engineered human corneal endothelium in cat models. Mol. Vis 19:400–407, 2013. [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira AM, Gentile P, Chiono V, and Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 8:3191–3200, 2012. [DOI] [PubMed] [Google Scholar]
- 75.Fishman JM, Tyraskis A, Maghsoudlou P, Urbani L, Totonelli G, Birchall MA, and De Coppi P. Skeletal muscle tissue engineering: which cell to use? Tissue Eng. Part B 19:503–515, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Fivenson D, and Scherschun L. Clinical and economic impact of apligraf® for the treatment of nonhealing venous leg ulcers. Int. J. Dermatol 42:960–965, 2003. [DOI] [PubMed] [Google Scholar]
- 77.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, and Rodeo SA. Platelet-rich plasma from basic science to clinical applications. Am. J. Sports Med 37:2259–2272, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Francis NL, Hunger PM, Donius AE, Riblett BW, Zavaliangos A, Wegst UGK, and Wheatley MA. An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. J. Biomed. Mater. Res. Part A 101:3493–3503, 2013. [DOI] [PubMed] [Google Scholar]
- 79.Garkavenko O, Wynyard S, Nathu D, Quane T, Durbin K, Denner J, and Elliott R. The first clinical xenotransplantation trial in new zealand: efficacy and safety. Xenotransplantation 19:6, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, and Lipkin S. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care 19:350–354, 1996. [DOI] [PubMed] [Google Scholar]
- 81.Geuze RE, Theyse LF, Kempen DH, Hazewinkel HA, Kraak HY, Oner FC, Dhert WJ, and Alblas J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng. Part A 18:2052–2062, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghezzi CE, Marelli B, Muja N, Hirota N, Martin JG, Barralet JE, Alessandrino A, Freddi G, and Nazhat SN. Mesenchymal stem cell-seeded multilayered dense collagen-silk fibroin hybrid for tissue engineering applications. Biotechnol. J 6:1198–1207, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Ghezzi CE, Marelli B, Muja N, and Nazhat SN. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 8:1813–1825, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Ghezzi CE, Muja N, Marelli B, and Nazhat SN. Real time responses of fibroblasts to plastically compressed fibrillar collagen hydrogels. Biomaterials 32:4761–4772, 2011. [DOI] [PubMed] [Google Scholar]
- 85.Ghezzi CE, Risse PA, Marelli B, Muja N, Barralet JE, Martin JG, and Nazhat SN. An airway smooth muscle cell niche under physiological pulsatile flow culture using a tubular dense collagen construct. Biomaterials 34:1954–1966, 2013. [DOI] [PubMed] [Google Scholar]
- 86.Ghezzi CE, Rnjak-Kovacina J, Weiss AS, and Kaplan DL. Multifunctional silk-tropoelastin biomaterial systems. Isr. J. Chem 53:777–786, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gil ES, Mandal BB, Park SH, Marchant JK, Omenetto FG, and Kaplan DL. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 31:8953–8963, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gil ES, Park SH, Marchant J, Omenetto F, and Kaplan DL. Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci 10:664–673, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grassl ED, Oegema TR, and Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J. Biomed. Mater. Res 60:607–612, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Grau AE, and Durán JA. Treatment of a large corneal perforation with a multilayer of amniotic membrane and tachosil. Cornea 31:98–100, 2012. [DOI] [PubMed] [Google Scholar]
- 91.Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, Laycock NL, Hakim M, Song Y, and Watsky MA. Functional human corneal equivalents constructed from cell lines. Science 286:2169–2172, 1999. [DOI] [PubMed] [Google Scholar]
- 92.Griffiths M, Ojeh N, Livingstone R, Price R, and Navsaria H. Survival of apligraf in acute human wounds. Tissue Eng. 10:1180–1195, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Gu X, Ding F, and Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35:6143–6156, 2014. [DOI] [PubMed] [Google Scholar]
- 94.Guan L, Ge H, Tang X, Su S, Tian P, Xiao N, Zhang H, Zhang L, and Liu P. Use of a silk fibroin-chitosan scaffold to construct a tissue-engineered corneal stroma. Cells Tissues Organs 198:190–197, 2013. [DOI] [PubMed] [Google Scholar]
- 95.Guo X, Das M, Rumsey J, Gonzalez M, Stancescu M, and Hickman J. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng. Part C 16:1347–1355, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guyer RD, Tromanhauser SG, and Regan JJ. An economic model of one-level lumbar arthroplasty versus fusion. Spine J. Off. J. N. Am. Spine Soc 7:558–562, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Hall KK, Gattás-Asfura KM, and Stabler CL. Microencapsulation of islets within alginate/poly(ethylene glycol) gels cross-linked via staudinger ligation. Acta Biomater 7:614–624, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hallab NJ Hypersensitivity to implant debris. Degrad. Implant Mater 2012:329–345, 2012. [Google Scholar]
- 99.Han J, Lazarovici P, Pomerantz C, Chen X, Wei Y, and Lelkes PI. Co-electrospun blends of plga, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 12:399–408, 2010. [DOI] [PubMed] [Google Scholar]
- 100.Hansbrough JF, Mozingo DW, Kealey GP, Davis M, Gidner A, and Gentzkow GD. Clinical trials of a biosynthetic temporary skin replacement, dermagraft-transitional covering, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J. Burn Care Res 18:43–51, 1997. [DOI] [PubMed] [Google Scholar]
- 101.Hart CE, Loewen-Rodriguez A, and Lessem J. Dermagraft: use in the treatment of chronic wounds. Adv. Wound Care 1:138–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hauser M, Eicken A, Kuehn A, Hess J, Fratz S, Ewert P, and Kaemmerer H. Managing the right ventricular outflow tract for pulmonary regurgitation after tetralogy of fallot repair. Heart Asia 5:106–111, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayabuchi Y, Mori K, Kitagawa T, Sakata M, and Kagami S. Polytetrafluoroethylene graft calcification in patients with surgically repaired congenital heart disease: evaluation using multidetector-row computed tomography. Am. Heart J 153:806.e1–806.e8, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Herrmann JB, Kelly RJ, and Higgins GA. Polyglycolic acid sutures: laboratory and clinical evaluation of a new absorbable suture material. Arch. Surg 100:486–490, 1970. [DOI] [PubMed] [Google Scholar]
- 105.Hoffman AS Stimuli-responsive polymers: biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev 65:10–16, 2013. [DOI] [PubMed] [Google Scholar]
- 106.Hofmann S, Hilbe M, Fajardo RJ, Hagenmueller H, Nuss K, Arras M, Mueller R, von Rechenberg B, Kaplan DL, Merkle HP, and Meinel L. Remodeling of tissue-engineered bone structures in vivo. Eur. J. Pharm. Biopharm 85:119–129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Noeth U, Jakob F, Rudert M, Groll J, and Hutmacher DW. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev 65:581–603, 2013. [DOI] [PubMed] [Google Scholar]
- 108.Hong Y, Huber A, Takanari K, Amoroso NJ, Hashizume R, Badylak SF, and Wagner WR. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials 32:3387–3394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu S, Kirsner RS, Falanga V, Phillips T, and Eaglstein WH. Evaluation of apligraf® persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 14:427–433, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Hu X, Wang X, Rnjak J, Weiss AS, and Kaplan DL. Biomaterials derived from silk-tropoelastin protein systems. Biomaterials 31:8121–8131, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang AH, and Niklason LE. Engineering biological-based vascular grafts using a pulsatile bioreactor. J. Vis. Exp 2011. doi: 10.3791/2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ilic D Industry update: latest developments in the field of stem cell research and regenerative medicine compiled from publicly available information and press releases from nonacademic institutions from 1 November 2013 until 31 December 2013. Regen. Med 9:137–143, 2014. [Google Scholar]
- 113.Japan Tissue Engineering Company. Labcyte cornea model product page. 2014. http://www.jpte.co.jp/english/business/LabCyte/CORNEAmodel.html.
- 114.Jell G, Kerjaschki D, Revell P, and Al-Saffar N. Lymphangiogenesis in the bone-implant interface of orthopedic implants: importance and consequence. J. Biomed. Mater. Res. A 77:119–127, 2006. [DOI] [PubMed] [Google Scholar]
- 115.Kaiser D, Hafner J, Mayer D, French LE, and Lauchli S. Alginate dressing and polyurethane film versus paraffin gauze in the treatment of split-thickness skin graft donor sites: a randomized controlled pilot study. Adv. Skin Wound Care 26:67–73, 2013. [DOI] [PubMed] [Google Scholar]
- 116.Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, and Donovan D. Effects of sterilization on poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. Part A 87A:608–617, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Karr JC Retrospective comparison of diabetic foot ulcer and venous stasis ulcer healing outcome between a dermal repair scaffold (primatrix) and a bilayered living cell therapy (apligraf). Adv. Skin Wound Care 24:119–125, 2011. [DOI] [PubMed] [Google Scholar]
- 118.Kawazoe N, Inoue C, Tateishi T, and Chen G. A cell leakproof PLGA-collagen hybrid scaffold for cartilage tissue engineering. Biotechnol. Prog 26:819–826, 2010. [DOI] [PubMed] [Google Scholar]
- 119.Kehoe S, Zhang XF, and Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43:553–572, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Kierstan M, and Bucke C. The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels. Biotechnol. Bioeng 19:387–397, 1977. [DOI] [PubMed] [Google Scholar]
- 121.Kim DM, Nevins M, Camelo M, Schupbach P, Kim S-W, Camelo JM, Al Hezaimi K, and Nevins ML. The feasibility of demineralized bone matrix and cancellous bone chips in conjunction with an extracellular matrix membrane for alveolar ridge preservation: a case series. Int. J. Periodontics Restor. Dent 31:39–47, 2011. [PubMed] [Google Scholar]
- 122.Kim J, McBride S, Tellis B, Alvarez-Urena P, Song Y-H, Dean DD, Sylvia VL, Elgendy H, Ong J, and Hollinger JO. Rapid-prototyped PLGA/beta-TCP/hydroxyapatite nanocomposite scaffolds in a rabbit femoral defect model. Biofabrication 4:025003, 2012. [DOI] [PubMed] [Google Scholar]
- 123.Kirsner RS The use of apligraf in acute wounds. J. Dermatol 25:805–811, 1998. [PubMed] [Google Scholar]
- 124.Knoll LD Use of porcine small intestinal submucosal graft in the surgical management of peyronie’s disease. Urology 57:753–757, 2001. [DOI] [PubMed] [Google Scholar]
- 125.Ko IK, Lee B-K, Lee SJ, Andersson K-E, Atala A, and Yoo JJ. The effect of in vitro formation of acetylcholine receptor (ACHR) clusters in engineered muscle fibers on subsequent innervation of constructs in vivo. Biomaterials 34:3246–3255, 2013. [DOI] [PubMed] [Google Scholar]
- 126.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, and Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 32:65–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komura M, Komura H, Kanamori Y, Tanaka Y, Suzuki K, Sugiyama M, Nakahara S, Kawashima H, Hatanaka A, Hoshi K, Ikada Y, Tabata Y, and Iwanaka T. An animal model study for tissue-engineered trachea fabricated from a biodegradable scaffold using chondrocytes to augment repair of tracheal stenosis. J. Pediatr. Surg 43:2141–2146, 2008. [DOI] [PubMed] [Google Scholar]
- 128.Kreutziger KL, and Murry CE. Engineered human cardiac tissue. Pediatr. Cardiol 32:334–341, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Krishnan L, Willett N, and Guldberg R. Vascularization strategies for bone regeneration. Ann. Biomed. Eng 42:432–444, 2014. [DOI] [PubMed] [Google Scholar]
- 130.Kwon H, Sun L, Cairns DM, Rainbow RS, Preda RC, Kaplan DL, and Zeng L. The influence of scaffold material on chondrocytes under inflammatory conditions. Acta Biomater. 9:6563–6575, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, and Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials 30:1299–1308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee E, Kasper FK, and Mikos A. Biomaterials for tissue engineering. Ann. Biomed. Eng 42:323–337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee KY, and Mooney DJ. Alginate: properties and biomedical applications. Prog. Polym. Sci 37:106–126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leslie-Barbick JE, Saik JE, Gould DJ, Dickinson ME, and West JL. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials 32:5782–5789, 2011. [DOI] [PubMed] [Google Scholar]
- 135.Liao IC, Moutos FT, Estes BT, Zhao X, and Guilak F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater 23:5833–5839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin CC, and Anseth KS. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules 10:2460–2467, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, and Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410:1057–1064, 2001. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Lawrence BD, Liu A, Schwab IR, Oliveira LA, and Rosenblatt MI. Silk fibroin as a biomaterial substrate for corneal epithelial cell sheet generation. Investig. Ophthalmol. Vis. Sci 53:4130–4138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu W, Merrett K, Griffith M, Fagerholm P, Dravida S, Heyne B, Scaiano JC, Watsky MA, Shinozaki N, Lagali N, Munger R, and Li F. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 29:1147–1158, 2008. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, Li N, Qi Y-P, Dai L, Bryan TE, Mao J, Pashley DH, and Tay FR. Intrafibrillar collagen mineralization produced by biomimetic hierarchical nanoapatite assembly. Adv. Mater 23:975–980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Living Cell Technologies. Diabecell product page. 2014. http://www.lctglobal.com/products/diabecell.
- 142.Living Cell Technologies. Ntcell product page. 2014. http://www.lctglobal.com/products/ntcell.
- 143.Luo H, Lu Y, Wu T, Zhang M, Zhang Y, and Jin Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 34:6748–6759, 2013. [DOI] [PubMed] [Google Scholar]
- 144.Luvizuto ER, Tangl S, Zanoni G, Okamoto T, Sonoda CK, Gruber R, and Okamoto R. The effect of bmp-2 on the osteoconductive properties of [beta]-tricalcium phosphate in rat calvaria defects. Biomaterials 32:3855–3861, 2011. [DOI] [PubMed] [Google Scholar]
- 145.Ma HY, Hu JA, and Ma PX. Polymer scaffolds for small-diameter vascular tissue engineering. Adv. Funct. Mater 20:2833–2841, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, and Han C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 24:4833–4841, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, and Christ GJ. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng. Part A 17:2291–2303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mackinnon SE, and Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast. Reconstr. Surg 85:419–424, 1990. [DOI] [PubMed] [Google Scholar]
- 149.Maisano F, Kjærgård HK, Bauernschmitt R, Pavie A, Rábago G, Laskar M, Marstein JP, and Falk V. Tachosil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: a randomised controlled trial. Eur. J. Cardiothorac. Surg 36:708–714, 2009. [DOI] [PubMed] [Google Scholar]
- 150.Mandal BB, Grinberg A, Gil ES, Panilaitis B, and Kaplan DL. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 109:7699–7704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marelli B, Ghezzi CE, Alessandrino A, Barralet JE, Freddi G, and Nazhat SN. Silk fibroin derived polypeptide-induced biomineralization of collagen. Biomaterials 33:102–108, 2012. [DOI] [PubMed] [Google Scholar]
- 152.Marelli B, Ghezzi CE, Barralet JE, Boccaccini AR, and Nazhat SN. Three-dimensional mineralization of dense nanofibrillar collagen-bioglass hybrid scaffolds. Biomacromolecules 11:1470–1479, 2010. [DOI] [PubMed] [Google Scholar]
- 153.Marelli B, Ghezzi CE, Barralet JE, and Nazhat SN. Collagen gel fibrillar density dictates the extent of mineralization in vitro. Soft Matter 7:9898–9907, 2011. [Google Scholar]
- 154.Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, and Nazhat SN. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 32:8915–8926, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Marston WA Dermagraft®, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev. Med. Devices 1:21–31, 2004. [DOI] [PubMed] [Google Scholar]
- 156.Marston WA, Hanft J, Norwood P, and Pollak R. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers results of a prospective randomized trial. Diabetes Care 26:1701–1705, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Marta GM, Facciolo F, Ladegaard L, Dienemann H, Csekeo A, Rea F, Dango S, Spaggiari L, Tetens V, and Klepetko W. Efficacy and safety of tachosil® versus standard treatment of air leakage after pulmonary lobectomy. Eur. J. Cardiothorac. Surg 38:683–689, 2010. [DOI] [PubMed] [Google Scholar]
- 158.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Müller R, Livne E, Eming SA, and Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med 3:100ra89, 2011. [DOI] [PubMed] [Google Scholar]
- 159.Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, and Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 15:635–643, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matsuura K, Haraguchi Y, Shimizu T, and Okano T. Cell sheet transplantation for heart tissue repair. J. Controlled Release 169:336–340, 2013. [DOI] [PubMed] [Google Scholar]
- 161.MatTekCorporation. Mattek corporation: In vitro tissue models, Ashland, MA. 2014. http://www.mattek.com/.
- 162.McCarty LP, Buss DD, Datta MW, Freehill MQ, and Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-l-lactic acid implants. J. Bone Jt. Surg 95:507–511, 2013. [DOI] [PubMed] [Google Scholar]
- 163.McHugh KJ, Tao SL, and Saint-Geniez M. Porous poly (ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Investig. Ophthalmol. Vis. Sci 55:1754–1762, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McInnes A Consensus statement on the use of xelma in diabetic foot ulcers. Diabetic Foot 13:148–151, 2010. [Google Scholar]
- 165.McNamara SL, Rnjak-Kovacina J, Schmidt DF, Lo TJ, and Kaplan DL. Silk as a biocohesive sacrificial binder in the fabrication of hydroxyapatite load bearing scaffolds. Biomaterials 35:6941–6953, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meek MF, and Coert JH. US food and drug administration/conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann. Plast. Surg 60:110–116, 2008. [DOI] [PubMed] [Google Scholar]
- 167.Meek MF, and Coert JH. Recovery of two-point discrimination function after digital nerve repair in the hand using resorbable FDA- and CE-approved nerve conduits. J. Plast. Reconstr. Aesthet. Surg 66:1307–1315, 2013. [DOI] [PubMed] [Google Scholar]
- 168.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, and Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 26:147–155, 2005. [DOI] [PubMed] [Google Scholar]
- 169.Mi SL, Chen B, Wright B, and Connon CJ. Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng. Part A 16:2091–2100, 2010. [DOI] [PubMed] [Google Scholar]
- 170.Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, and Radisic M. Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 6:024113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mirza D, Millar AJW, Sharif K, Vilca-Melendez H, Rela M, and Heaton N. The use of tachosil in children undergoing liver resection with or without segmental liver transplantation. Eur. J. Pediatr. Surg 21:111–115, 2011. [DOI] [PubMed] [Google Scholar]
- 172.Mithieux SM, Wise SG, and Weiss AS. Tropoelastin—a multifaceted naturally smart material. Adv. Drug Deliv. Rev 65:421–428, 2013. [DOI] [PubMed] [Google Scholar]
- 173.Morgan KY, and Black LD III. It’s all in the timing: modeling isovolumic contraction through development and disease with a dynamic dual electromechanical bioreactor system. Organogenesis 10:1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morgan KY, and Black LD III. Mimicking isovolumic contraction with combined electromechanical stimulation improves the development of engineered cardiac constructs. Tissue Eng. Part A 20:1654–1667, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morgan KY, and Black LD III. Creation of a bioreactor for the application of variable amplitude mechanical stimulation of fibrin gel-based engineered cardiac tissue. Methods Mol. Biol 1181:177–187, 2014. [DOI] [PubMed] [Google Scholar]
- 176.Morgan KY, and Black LD III. Investigation into the effects of varying frequency of mechanical stimulation in a cycle-by-cycle manner on engineered cardiac construct function. J. Tissue Eng. Regen. Med 2014. doi: 10.1002/term.1915. [DOI] [PubMed] [Google Scholar]
- 177.Mudera V, Morgan M, Cheema U, Nazhat SN, and Brown RA. Ultra-rapid engineered collagen constructs tested in an in vivo nursery site. J. Tissue Eng. Regen. Med 1:192–198, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Nakamuta JS, Danoviz ME, Marques FLN, Dos Santos L, Becker C, Gonçalves GA, Vassallo PF, Schettert IT, Tucci PJF, and Krieger JE. Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS ONE 4:e6005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Napoleone CP, Oppido G, Angeli E, and Gargiulo G. Resternotomy in pediatric cardiac surgery: Coseal® initial experience. Interact. Cardiovasc. Thorac. Surg 6:21–23, 2007. [DOI] [PubMed] [Google Scholar]
- 180.Napoleone CP, Valori A, Crupi G, Ocello S, Santoro F, Vouhé P, Weerasena N, and Gargiulo G. An observational study of coseal® for the prevention of adhesions in pediatric cardiac surgery. Interact. Cardiovasc. Thorac. Surg 9:978–982, 2009. [DOI] [PubMed] [Google Scholar]
- 181.Neal RA, Jean A, Park H, Wu PB, Hsiao J, Engelmayr GC Jr., Langer R, and Freed LE. Three-dimensional elastomeric scaffolds designed with cardiac-mimetic structural and mechanical features. Tissue Eng. Part A 19:793–807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Neuenschwander P Scaffolds for artificial heart valves and vascular structures, Eidgenössische Technische Hochschule Zürich. 2007.
- 183.Nevins M, Nevins ML, Camelo M, Camelo JM, Schupbach P, and Kim DM. The clinical efficacy of dynamatrix extracellular membrane in augmenting keratinized tissue. Int. J. Periodontics Restor. Dent 30:151–161, 2010. [PubMed] [Google Scholar]
- 184.Nguyen LH, Kudva AK, Guckert NL, Linse KD, and Roy K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 32:1327–1338, 2011. [DOI] [PubMed] [Google Scholar]
- 185.Nishio S, Kosuga K, Igaki K, Okada M, Kyo E, Tsuji T, Takeuchi E, Inuzuka Y, Takeda S, and Hata T. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents Igaki-Tamai stents. Circulation 125:2343–2353, 2012. [DOI] [PubMed] [Google Scholar]
- 186.O’Brien G, Buckley K, Vanwalleghem G, Vanrenterghem D, Dharma H, Winter RL, and Douglass J. A multi-centre, prospective, clinical in-market evaluation to assess the performance of opsite™ post-op visible dressings. Int. Wound J 7:329–337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Omar AA, Mavor AID, Jones AM, and Homer-Vanniasinkam S. Treatment of venous leg ulcers with dermagraft®. Eur. J. Vasc. Endovasc. Surg 27:666–672, 2004. [DOI] [PubMed] [Google Scholar]
- 188.Ouasti S, Donno R, Cellesi F, Sherratt MJ, Terenghi G, and Tirelli N. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 32:6456–6470, 2011. [DOI] [PubMed] [Google Scholar]
- 189.Ozcelik B, Brown KD, Blencowe A, Daniell M, Stevens GW, and Qiao GG. Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 9:6594–6605, 2013. [DOI] [PubMed] [Google Scholar]
- 190.Parenteau-Bareil R, Gauvin R, and Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials 3:1863–1887, 2010. [Google Scholar]
- 191.Park H, Larson BL, Kolewe ME, Vunjak-Novakovic G, and Freed LE. Biomimetic scaffold combined with electrical stimulation and growth factor promotes tissue engineered cardiac development. Exp. Cell Res 321:297–306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, Marelli B, Mitropoulos AN, Omenetto FG, and Kaplan DL. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater 24:4615–4624, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pellenc D, Berry H, and Gallet O. Adsorption-induced fibronectin aggregation and fibrillogenesis. J. Colloid Interface Sci 298:132–144, 2006. [DOI] [PubMed] [Google Scholar]
- 194.Pennisi CP, Olesen CG, De Zee M, Rasmussen J, and Zachar V. Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng. Part A 17:2543–2550, 2011. [DOI] [PubMed] [Google Scholar]
- 195.Pocar M, Passolunghi D, Bregasi A, and Donatelli F. Tachosil® for postinfarction ventricular free wall rupture. Inter. Cardiovasc. Thorac. Surg 14:866–867, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pok S, Benavides OM, Hallal PA, and Jacot J. Use of myocardial matrix in a chitosan-based full thickness heart patch. Tissue Eng. 20:1877–1887, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Preda RC, Leisk G, Omenetto F, and Kaplan DL. Bioengineered silk proteins to control cell and tissue functions. Methods Mol. Biol. (Clifton, N.J.) 996:19–41, 2013. [DOI] [PubMed] [Google Scholar]
- 198.Pryor HI II, O’Doherty E, Hart A, Owens G, Hoganson D, Vacanti JP, Masiakos PT, and Sundback CA. Poly(glycerol sebacate) films prevent postoperative adhesions and allow laparoscopic placement. Surgery 146:490–497, 2009. [DOI] [PubMed] [Google Scholar]
- 199.Purdue GF, Hunt JL, Still JM Jr., Law EJ, Herndon DN, Goldfarb IW, Schiller WR, Hansbrough JF, Hickerson WL, and Himel HN. A multicenter clinical trial of a biosynthetic skin replacement, dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J. Burn Care Res 18:52–57, 1997. [DOI] [PubMed] [Google Scholar]
- 200.Qi M, Strand BL, Morch Y, Lacik I, Wang Y, Salehi P, Barbaro B, Gangemi A, Kuechle J, Romagnoli T, Hansen MA, Rodriguez LA, Benedetti E, Hunkeler D, Skjak-Braek G, and Oberholzer J. Encapsulation of human islets in novel inhomogeneous alginate-Ca2+/Ba2+ microbeads: in vitro and in vivo function. Artif. Cells Blood Substit. Biotechnol 36:403–420, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qiao P, Wang J, Xie Q, Li F, Dong L, and Xu T. Injectable calcium phosphate-alginate-chitosan microencapsulated mc3t3-e1 cell paste for bone tissue engineering in vivo. Mater. Sci. Eng. C 33:4633–4639, 2013. [DOI] [PubMed] [Google Scholar]
- 202.Quintessenza J 2011, Bicuspid vascular valve and methods for making and implanting same, Google Patents.
- 203.Rabotyagova OS, Cebe P, and Kaplan DL. Protein-based block copolymers. Biomacromolecules 12:269–289, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Radisic M, and Christman KL. Materials science and tissue engineering: repairing the heart. Mayo Clin. Proc 88:884–898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rafat M, Li F, Fagerholm P, Lagali NS, Watsky MA, Munger R, Matsuura T, and Griffith M. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 29:3960–3972, 2008. [DOI] [PubMed] [Google Scholar]
- 206.Rai R, Tallawi M, Grigore A, and Boccaccini AR. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): a review. Prog. Polym. Sci 37:1051–1078, 2012. [Google Scholar]
- 207.Rajan N, Habermehl J, Coté MF, Doillon CJ, and Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc 1:2753–2758, 2007. [DOI] [PubMed] [Google Scholar]
- 208.Redekop WK, McDonnell J, Verboom P, Lovas K, and Kalo Z. The cost effectiveness of apligraf® treatment of diabetic foot ulcers. Pharmacoeconomics 21:1171–1183, 2003. [DOI] [PubMed] [Google Scholar]
- 209.Reichl S, Bednarz J, and Muller-Goymann CC. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br. J. Ophthalmol 88:560–565, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Richter JR, de Guzman RC, and Van Dyke ME. Mechanisms of hepatocyte attachment to keratin biomaterials. Biomaterials 32:7555–7561, 2011. [DOI] [PubMed] [Google Scholar]
- 211.Rnjak-Kovacina J, Wray LS, Golinski JM, and Kaplan DL. Arrayed hollow channels in silk-based scaffolds provide functional outcomes for engineering critically sized tissue constructs. Adv. Funct. Mater 24:2188–2196, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, and Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc 6:1612–1631, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rouse JG, and Van Dyke ME. A review of keratin-based biomaterials for biomedical applications. Materials 3:999–1014, 2010. [Google Scholar]
- 214.Ruberti JW, Roy AS, and Roberts CJ. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng 13:269–295, 2011. [DOI] [PubMed] [Google Scholar]
- 215.Saffer EM, Tew GN, and Bhatia SR. Poly(lactic acid)-poly(ethylene oxide) block copolymers: new directions in self-assembly and biomedical applications. Curr. Med. Chem 18:5676–5686, 2011. [DOI] [PubMed] [Google Scholar]
- 216.Sajesh KM, Jayakumar R, Nair SV, and Chennazhi KP. Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol 62:465–471, 2013. [DOI] [PubMed] [Google Scholar]
- 217.Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, Moroni L, and Dubruel P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev 41:7147–7194, 2012. [DOI] [PubMed] [Google Scholar]
- 218.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, and Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138:3647–3656, 2011. [DOI] [PubMed] [Google Scholar]
- 219.Santerre JP, Woodhouse K, Laroche G, and Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials 26:7457–7470, 2005. [DOI] [PubMed] [Google Scholar]
- 220.Santos MI, and Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol. Biosci 10:12–27, 2010. [DOI] [PubMed] [Google Scholar]
- 221.Sasson L, Houri S, Raucher Sternfeld A, Cohen I, Lenczner O, Bove EL, Kapusta L, and Tamir A. Right ventricular outflow tract strategies for repair of tetralogy of fallot: effect of monocusp valve reconstruction. Eur. J. Cardiothorac. Surg 43:743–751, 2013. [DOI] [PubMed] [Google Scholar]
- 222.Schaaf S, Shibamiya A, Mewe M, Eder A, Stoehr A, Hirt MN, Rau T, Zimmermann W-H, Conradi L, Eschenhagen T, and Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. Plos One 6:e26397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schneider S, Feilen PJ, Brunnenmeier F, Minnemann T, Zimmermann H, Zimmermann U, and Weber MM. Long-term graft function of adult rat and human islets encapsulated in novel alginate-based microcapsules after transplantation in immunocompetent diabetic mice. Diabetes 54:687–693, 2005. [DOI] [PubMed] [Google Scholar]
- 224.Schonfeld WH, Villa KF, Fastenau JM, Mazonson PD, and Falanga V. An economic assessment of apligraf® (graftskin) for the treatment of hard-to-heal venous leg ulcers. Wound Repair Regen. 8:251–257, 2000. [DOI] [PubMed] [Google Scholar]
- 225.Seif-Naraghi SB, and Christman KL. Tissue engineering and the role of biomaterial scaffolds: the evolution of cardiac tissue engineering. In: Resident Stem Cells and Regenerative Therapy, edited by Goldenberg RCS and de Carvalho ACC. Elsevier, 2013, pp. 43–67. http://www.sciencedirect.com/science/book/9780124160125. [Google Scholar]
- 226.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, and Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med 5:10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shachar M, Tsur-Gang O, Dvir T, Leor J, and Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 7:152–162, 2011. [DOI] [PubMed] [Google Scholar]
- 228.Shafei S, Sharifudin MA, Ismail MS, Ab Rahman S, and Sadagatullah AN. A comparative study of Tualang honey spray versus film spray (opsite®) as post-long bone fracture fixation wound dressing. In: Kelantan Research Day 2013. Rural Transformation Centre (RTC), Terminal Agribisnes Negara (TEMAN), Kota Bharu. 2013. http://irep.iium.edu.my/32605/. [Google Scholar]
- 229.Shang K, Rnjak-Kovacina J, Lin Y, Hayden R, Tao H, and Kaplan D. Accelerated in vitro degradation of optically clear low beta-sheet silk films by enzyme mediated pretreatment. Trans. Vis. Sci. Technol 2:2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson P, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, and Lakey JRT. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med 355:1318–1330, 2006. [DOI] [PubMed] [Google Scholar]
- 231.Sharifpoor S, Simmons CA, Labow RS, and Santerre JP. A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomater. 6:4218–4228, 2010. [DOI] [PubMed] [Google Scholar]
- 232.Sharifpoor S, Simmons CA, Labow RS, and Santerre JP. Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials 32:4816–4829, 2011. [DOI] [PubMed] [Google Scholar]
- 233.Shen W, Chen X, Hu Y, Yin Z, Zhu T, Hu J, Chen J, Zheng Z, Zhang W, Ran J, Heng BC, Ji J, Chen W, and Ouyang HW. Long-term effects of knitted silk-collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials 35:8154–8163, 2014. [DOI] [PubMed] [Google Scholar]
- 234.Shields LBE, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, and Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 31:542–547, 2006. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 235.Shimizu T, Yamato M, Kikuchi A, and Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 24:2309–2316, 2003. [DOI] [PubMed] [Google Scholar]
- 236.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim Sb, Nikkhah M, Khabiry M, Azize M, Kong J, Wan K-t, Palacios T, Dokmeci MR, Bae H, Tang X, and Khademhosseini A. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 7:2369–2380, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sicchieri LG, Crippa GE, de Oliveira PT, Beloti MM, and Rosa AL. Pore size regulates cell and tissue interactions with PLGA-cap scaffolds used for bone engineering. J. Tissue Eng. Regen. Med 6:155–162, 2012. [DOI] [PubMed] [Google Scholar]
- 238.Sierpinski P, Garrett J, Ma J, Apel P, Klorig D, Smith T, Koman LA, Atala A, and Van Dyke M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29:118–128, 2008. [DOI] [PubMed] [Google Scholar]
- 239.Silva AKA, Juenet M, Meddahi-Pellé A, and Letourneur D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr. Polym 116:267–277, 2014. [DOI] [PubMed] [Google Scholar]
- 240.Siti-Ismail N, Bishop AE, Polak JM, and Mantalaris A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 29:3946–3952, 2008. [DOI] [PubMed] [Google Scholar]
- 241.Skulstad H, Erikssen G, Estensen ME, and Lindberg HL. Insufficient long term follow up and risk for aneurism in patients operated with dacron patch for coarctatio aortae. Eur. Heart J 34:P2117, 2013. [Google Scholar]
- 242.Sosa H, Popp D, Ouyang G, and Huxley HE. Ultrastructure of skeletal muscle fibers studied by a plunge quick freezing method: myofilament lengths. Biophys. J 67:283–292, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sowjanya JA, Singh J, Mohita T, Sarvanan S, Moorthi A, Srinivasan N, and Selvamurugan N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf. B 109:294–300, 2013. [DOI] [PubMed] [Google Scholar]
- 244.Spotnitz WD Fibrin sealant: past, present, and future: a brief review. World J. Surg 34:632–634, 2010. [DOI] [PubMed] [Google Scholar]
- 245.Stavarachi M, Apostol P, Toma M, Cimponeriu D, and Gavrila L. Spinal muscular atrophy disease: a literature review for therapeutic strategies. J. Med. Life 3:3, 2010. [PMC free article] [PubMed] [Google Scholar]
- 246.Steinberg JS, Edmonds M, Hurley DP Jr., and King WN. Confirmatory data from eu study supports apligraf for the treatment of neuropathic diabetic foot ulcers. J. Am. Podiatr. Med. Assoc 100:73–77, 2010. [DOI] [PubMed] [Google Scholar]
- 247.Stoppel WL, White JC, Horava SD, Bhatia SR, and Roberts SC. Transport of biological molecules in surfactant-alginate composite hydrogels. Acta Biomater. 7:3988–3998, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Stoppel WL, White JC, Horava SD, Henry AC, Roberts SC, and Bhatia SR. Terminal sterilization of alginate hydrogels: efficacy and impact on mechanical properties. J. Biomed. Mater. Res. Part B 102:877–884, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Streit M, and Braathen LR. Apligraf–a living human skin equivalent for the treatment of chronic wounds. Int. J. Artif. Organs 23:831–833, 2000. [PubMed] [Google Scholar]
- 250.Takata I, Tosa T, and Chibata I. Screening of matrix suitable for immobilization of microbial cells. J. Solid-Phase Biochem 2:225–236, 1977. [Google Scholar]
- 251.Tan C, Utley M, Paschalides C, Pilling J, Robb JD, Harrison-Phipps KM, Lang-Lazdunski L, and Treasure T. A prospective randomized controlled study to assess the effectiveness of coseal® to seal air leaks in lung surgery. Eur. J. Cardiothorac. Surg 40:304–308, 2011. [DOI] [PubMed] [Google Scholar]
- 252.Tan PLJ Company profile: tissue regeneration for diabetes and neurological diseases at living cell technologies. Regen. Med 5:181–187, 2010. [DOI] [PubMed] [Google Scholar]
- 253.Taylor MS, Daniels AU, Andriano KP, and Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J. Appl. Biomater 5:151–157, 1994. [DOI] [PubMed] [Google Scholar]
- 254.Themistocleous GS, Katopodis HA, Khaldi L, Papalois A, Doillon C, Sourla A, Soucacos PN, and Koutsilieris M. Implants of type I collagen gel containing mg-63 osteoblast-like cells can act as stable scaffolds stimulating the bone healing process at the sites of the surgically-produced segmental diaphyseal defects in male rabbits. In Vivo 21:69–76, 2007. [PubMed] [Google Scholar]
- 255.Tonami K, Hata S, Ojima K, Ono Y, Kurihara Y, Amano T, Sato T, Kawamura Y, Kurihara H, and Sorimachi H. Calpain-6 deficiency promotes skeletal muscle development and regeneration. PLoS Genet. 9:e1003668, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Trent JF, and Kirsner RS. Tissue engineered skin: apligraf, a bi-layered living skin equivalent. Int. J. Clin. Pract 52:408–413, 1998. [PubMed] [Google Scholar]
- 257.Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, and Lavandero S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab 25:128–137, 2014. [DOI] [PubMed] [Google Scholar]
- 258.Turner NJ, Keane TJ, and Badylak SF. Lessons from developmental biology for regenerative medicine. Birth Defects Res. Part C 99:149–159, 2013. [DOI] [PubMed] [Google Scholar]
- 259.Turrentine MW, McCarthy RP, Vijay P, McConnell KW, and Brown JW. PTFE monocusp valve reconstruction of the right ventricular outflow tract. Ann. Thorac. Surg 73:871–880, 2002. [DOI] [PubMed] [Google Scholar]
- 260.Twardowski R, and Black LD III. Cardiac fibroblasts support endothelial cell proliferation and sprout formation but not the development of multicellular sprouts in a fibrin gel co-culture model. Ann. Biomed. Eng 42:1074–1084, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Uebersax L, Apfel T, Nuss KMR, Vogt R, Kim HY, Meinel L, Kaplan DL, Auer JA, Merkle HP, and von Rechenberg B. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur. J. Pharm. Biopharm 85:107–118, 2013. [DOI] [PubMed] [Google Scholar]
- 262.Ungerleider JL, and Christman KL. Concise review: Injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl. Med 3:1090–1099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Van Goethem F, Adriaens E, Alepee N, Straube F, De Wever B, Cappadoro M, Catoire S, Hansen E, Wolf A, and Vanparys P. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicol. In Vitro 20:1–17, 2006. [DOI] [PubMed] [Google Scholar]
- 264.Venkatesan J, Bhatnagar I, and Kim S-K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 12:300–316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Venkatesan J, Bhatnagar I, Manivasagan P, Kang K-H, and Kim S-K. Alginate composites for bone tissue engineering: a review. Int. J. Biol. Macromol 72C:269–281, 2014. [DOI] [PubMed] [Google Scholar]
- 266.von Maltzahn J, Jones AE, Parks RJ, and Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 110:16474–16479, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vorndran C Recent us patents on extracellular matrix in tissue engineering and regenerative medicine. Recent Patents Regen. Med 4:34–39, 2014. [Google Scholar]
- 268.Vowden K, McGowan J, Pilcher M, D’Arcy A, Renton C, Warner V, Megson J, and Vowden P. Experience with the use of an amelogenin-based extracellular matrix substitute (xelma®) in the management of a variety of complex hard-to-heal chronic wounds. In: European Wound Management Association Conference Abstracts, May 2007, Glasgow, Scotland. Oral presentation #91. [Google Scholar]
- 269.Vowden P, Romanelli M, Peter R,Boström Å, Josefsson A, and Stege H. The effect of amelogenins (xelma™) on hard-to-heal venous leg ulcers. Wound Repair Regen. 14:240–246, 2006. [DOI] [PubMed] [Google Scholar]
- 270.Wang X, Wang W, Ma J, Guo X, Yu X, and Ma X. Proliferation and differentiation of mouse embryonic stem cells in apa microcapsule: a model for studying the interaction between stem cells and their niche. Biotechnol. Prog 22:791–800, 2006. [DOI] [PubMed] [Google Scholar]
- 271.Wang Y, Kim YM, and Langer R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 66A:192–197, 2003. [DOI] [PubMed] [Google Scholar]
- 272.Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng X-L, Zhu Y, Kong D, and Zhao Q. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macro-phage polarization and arterial regeneration. Biomaterials 35:5700–5710, 2014. [DOI] [PubMed] [Google Scholar]
- 273.Waterhouse A, Wise SG, Ng MKC, and Weiss AS. Elastin as a nonthrombogenic biomaterial. Tissue Eng. Part B 17:93–99, 2011. [DOI] [PubMed] [Google Scholar]
- 274.Weber RA, Breidenbach WC, Brown RE, Jabaley ME, and Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast. Reconstr. Surg 106:1036–1045, 2000. [DOI] [PubMed] [Google Scholar]
- 275.White ES, and Muro AF. Fibronectin splice variants: understanding their multiple roles in health and disease using engineered mouse models. IUBMB Life 63:538–546, 2011. [DOI] [PubMed] [Google Scholar]
- 276.Williams C, Quinn KP, Georgakoudi I, and Black III LD. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomater. 10:194–204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wilson SL, Wimpenny I, Ahearne M, Rauz S, El Haj AJ, and Yang Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Funct. Mater 22:3641–3649, 2012. [Google Scholar]
- 278.Wise AK, Fallon JB, Neil AJ, Pettingill LN, Geaney MS, Skinner SJ, and Shepherd RK. Combining cell-based therapies and neural prostheses to promote neural survival. Neurotherapeutics 8:774–787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wolf MT, Dearth CL, Sonnenberg SB, Loboa EG, and Badylak SF. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev 2014. doi: 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, and Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials 33:9214–9224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wu H, Xiong WC, and Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137:1017–1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Xin M, Olson EN, and Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol 14:529–541, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou GD, Liu W, and Cao Y. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials 29:1464–1472, 2008. [DOI] [PubMed] [Google Scholar]
- 284.Yamato M, and Okano T. Cell sheet engineering. Mater. Today 7:42–47, 2004. [Google Scholar]
- 285.Ye KY, Sullivan KE, and Black LD III. Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. J. Vis. Exp 2011. doi: 10.3791/3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yin H, Gong C, Shi S, Liu X, Wei Y, and Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J. Biomed. Mater. Res. B 92B:129–137, 2010. [DOI] [PubMed] [Google Scholar]
- 287.Yu C-C, Chang J-J, Lee Y-H, Lin Y-C, Wu M-H, Yang M-C, and Chien C-T. Electrospun scaffolds composing of alginate, chitosan, collagen and hydroxyapatite for applying in bone tissue engineering. Mater. Lett 93:133–136, 2013. [Google Scholar]
- 288.Yuvarani I, Kumar SS, Venkatesan J, Kim S-K, and Sudha PN. Preparation and characterization of curcumin coated chitosan-alginate blend for wound dressing application. J. Biomater. Tissue Eng 2:54–60, 2012. [Google Scholar]
- 289.Zaky SH, Lee K-W, Gao J, Jensen A, Close J, Wang Y, Almarza AJ, and Sfeir C. Poly (glycerol sebacate) elastomer: a novel material for mechanically loaded bone regeneration. Tissue Eng. Part A 20:45–53, 2013. [DOI] [PubMed] [Google Scholar]
- 290.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, and Beauchamp JR. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci 119:1824–1832, 2006. [DOI] [PubMed] [Google Scholar]
- 291.Zaulyanov L, and Kirsner RS. A review of a bi-layered living cell treatment (apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2:93, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhang D, Shadrin IY, Lam J, Xian H-Q, Snodgrass HR, and Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human esc-derived cardiomyocytes. Biomaterials 34:5813–5820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhang X, Zhu L, Lv H, Cao Y, Liu Y, Xu Y, Ye W, and Wang J. Repair of rabbit femoral condyle bone defects with injectable nanohydroxyapatite/chitosan composites. J. Mater. Sci. Mater. Med 23:1941–1949, 2012. [DOI] [PubMed] [Google Scholar]
- 294.Zhou J, Chen J, Sun H, Qiu X, Mou Y, Liu Z, Zhao Y, Li X, Han Y, Duan C, Tang R, Wang C, Zhong W, Liu J, Luo Y, Xing MM, and Wang C. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep 4:1–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zimmermann H, Shirley S, and Zimmermann U. Alginate-based encapsulation of cells: past, present, and future. Curr. Diab.Rep 7:314–320, 2007. [DOI] [PubMed] [Google Scholar]


