Abstract
The global epidemic owing to COVID-19 has generated awareness to ensuring best practices for avoiding the microorganism spread. Indeed, because of the increase in infections caused by bacteria and viruses such as SARS-CoV-2, the global demand for antimicrobial materials is growing. New technologies by using polymeric systems are of great interest. Virus transmission by contaminated surfaces leads to the spread of infectious diseases, so antimicrobial coatings are significant in this regard. Moreover, antimicrobial food packaging is beneficial to prevent the spread of microorganisms during food processing and transportation. Furthermore, antimicrobial textiles show an effective role. We aim to provide a review of prepared antimicrobial polymeric materials for use in coating, food packaging, and textile during the COVID-19 pandemic and after pandemic.
Keywords: COVID-19, Microorganism, Coating, Textile industry, Food packaging
Graphical abstract
Introduction
COVID-19, a new disease caused by SARS-CoV-2, is a public concern, prevalent all over the world, and killing countless people [1, 2, 3, 4]. To date, various systems including antimicrobial metallic materials, metal oxide nanoparticles, and other antiviral materials were used to fight against microorganisms [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. For example, Randriantsilefisoa et al. [16] prepared hydrogel nanocomposites based on Au nanoparticles, sialic acid, polyethylene glycol, and polyglycerol cyclooctyne to catch the influenza virus. In another study, de Dicastillo et al. [17] organized an antimicrobial polymeric bilayer structure based on the insertion of zinc oxide nanotubes in the acrylic polymer for coating a polymeric substrate. In addition, a research team [18] prepared curcumin/chitosan nanocomposite as an antiviral agent for hepatitis C virus genotype 4a in human hepatoma cell lines. In another investigation, an antiviral polymeric system against the zika virus was prepared by encapsulating curcumin as a natural product into poly(lactic-co-glycolic acid) nanoparticles. Cytotoxicity and antiviral activity were evaluated in Vero cells. The outcomes showed promising candidates for the formulation of antiviral drugs [19∗]. Besides, to date, antimicrobial materials using metal and metal oxides were designed for diverse sectors such as coating [20,21].
Viruses including coronavirus can transmit through various surfaces. Because the stability of the virus varies on different surfaces and is possible for several days, therefore, the preparation of antimicrobial coatings is effective in eliminating pathogens and microorganisms [5,6,22]. Moreover, foods, fruits, and vegetables may become contaminated with microorganisms during processing and transportation [23]. Antimicrobial packaging using polymers and special polysaccharides to prevent plastic contamination can be useful in controlling the spread of microbes. So far, food packaging films with antimicrobial performance have been prepared and tested for microorganisms like viruses, bacteria, and fungi [24]. In addition, viruses can be transmitted through droplets and aerosols, so they can spread the disease. The spread of viruses has led to the widespread use of antimicrobial personal protective equipment or household fabric masks. The development of antimicrobial fabrics in the preparation of these materials can help to control the spread of microorganisms such as SARS-CoV-2.
Polymeric materials, including natural polymers, are a suitable option for the preparation of antimicrobial materials such as films, coatings, and so on. Polysaccharides, such as chitosan, alginate, gums, due to their natural nature, their biocompatibility, and biodegradability have received a lot of attention in this regard [25,26]. By introducing nanoparticles or using natural compounds with antimicrobial and antifungal properties into polysaccharides, composites with good performance are prepared that show effectiveness against microorganisms [27]. Hence, as per the latest information that has been reported in 2020 and 2021, this mini-review will be a good source for researchers by collecting useful information about antimicrobial polymeric materials for protective applications during the COVID-19 pandemic and after pandemic.
Antimicrobial coatings and membranes for protective applications
Human infections (almost 80%) can be transmitted through contaminated surfaces by bacteria, fungi, and viruses. Especially during the COVID-19 epidemic, it is necessary to provide antimicrobial coatings to control and prevent its spread [28]. On the other side, hospital infections are a threat to global health care. To counter this escalating threat, so far, by using polymeric materials and polymer hybrids, coating formulations have been proposed by researchers for pathogen inactivation. In fact, polymeric surfaces are widely used today and are one of the most ubiquitous substrates in life. In addition, metal nanoparticles and metal oxides can be used in the preparation of antimicrobial polymeric coatings. So far, polymeric systems for the preparation of coatings, membranes, and surfaces to fight against bacteria, viruses, and fungi have been widely reported. Here are some of the latest developments: Haldar et al. [28∗] reported an antimicrobial covalent coating to kill bacteria, fungi, and influenza viruses on clinical surfaces. For this aim, using UV irradiation, water-soluble quaternary benzophenone-based ester and amide were covalently immobilized on surfaces such as cotton sheets and polyurethane via dip-coating and drop-casting methods. The antibacterial (Staphylococcus aureus, Escherichia coli), antifungal, and antiviral (influenza virus) performances were tested, and the outcomes showed the killing of pathogens in a short time. This research team revealed a one-step curable coating that could covalently cover several surfaces and quickly kill influenza virus, bacteria, and fungi and was suitable for hospital coverage.
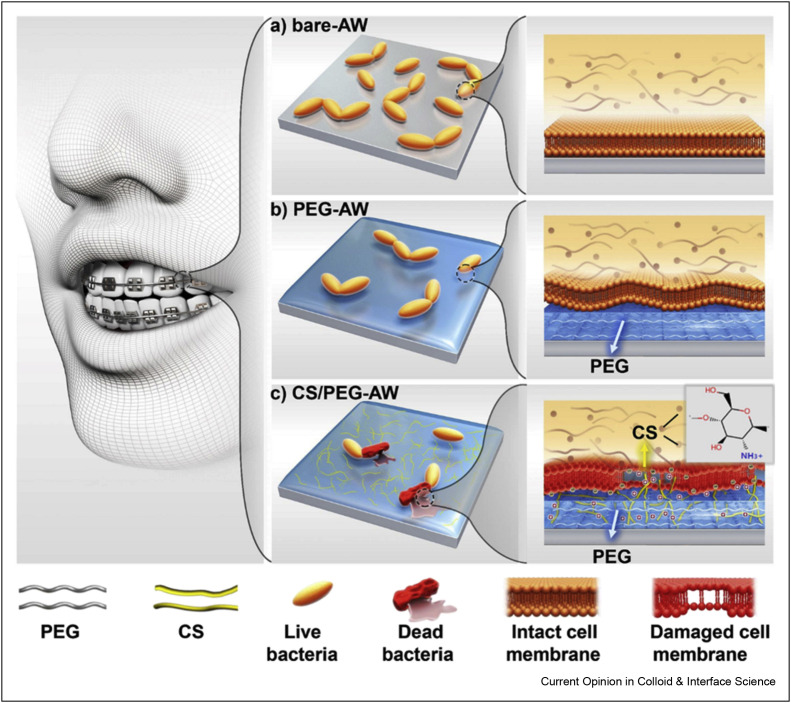
In addition, because of the spread of microbial infection in dental applications, an antimicrobial hydrogel coating was prepared for medical instruments using chitosan and poly(ethylene glycol) as low-volatile, low-toxic, and biocompatible materials to combat infections [29]. After coating the stainless-steel archwire with the fabricated coating, the prepared dental appliance showed adhesion-inhibiting and antibacterial capabilities, as can be observed in Figure 1 . The prepared bio-interface indicated great performance in early-stage adhesion inhibition (98.8%, 5 h) as well as long-lasting colony-suppression action (93.3%, 7 d). This nanomaterial containing many advantages such as excellent bio-compatibility, being safe and effective, dual-functional platform (antimicrobial and antifouling utilities) showed potential for coating in the biomedical device.
Figure 1.
Schematic illustration of the design of a dental appliance with both adhesion-inhibiting and antibacterial capabilities. (a) Significant aggregation of bacteria on bare stainless-steel AW. (b) PEGylation of stainless-steel AW can significantly reduce bacterial adhesion owing to the existence of a thin water layer caused by PEG. (c) Stainless-steel AW with CS/PEG hydrogel coating exhibits both adhesion-inhibiting and antibacterial capabilities (PEG: polyethylene glycol, CS: chitosan, AW: stainless-steel archwire). This was reprinted with permission from Ref. [29].
Outbreaks of viral diseases, including COVID-19, are possible through the air and have recently become a major problem for human health. Even if air conditioning and heating systems restrict virus transmission, their filters are contaminated. The virus can also be transmitted through aerosol, and it is essential to design a system to absorb the virus-containing aerosol. In this way, a research team [30] prepared an antiviral composite coating including Ag nanoclusters/silica for deposition on cotton, glass, and metallic filters through the co-sputtering method. These coatings were tested for several respiratory viruses including influenza A and human rhinovirus. After placing the coating on the filter, its function did not change. The outcomes of antiviral studies showed that the composite coating had a strong antiviral performance. These coatings can be used to provide filters and masks in most areas, including hospitals, gyms, and other areas to control the risk of viruses.
In a recent study, an antibacterial polymeric composite coating on the Ti (titanium) surface was prepared by using Ag nanoparticles (20–30 nm), sodium alginate, poly-l-lysine, and dopamine for infection prevention. The fabricated coating showed the release of Ag+ for >27 days and successfully killed and inhibited S. aureus and Streptococcus. mutans bacteria [31]. In addition, in a recent study by Ballarre et al. [32], antibacterial surface coating systems based on chitosan, gentamicin, and silica on titanium substrates were developed via spray and electrophoretic deposition as a simple and versatile method. The prepared coating showed great antibacterial performance against S. aureus and E. coli. In addition, Fabra et al. [33] prepared antiviral edible coatings using gelatin and Persian gum in a 50:50 ratio. Diverse percentages of the polymer blend were used. The mechanical and barrier features of Persian gum were developed owing to the interactions (electrostatic) among polymers, which enhance the cohesive system. The prepared coating showed good antiviral performance.
He et al. [34] proposed an eco-friendly approach, based on superior antimicrobial composites and based on immobilization of Ag/ZnO nanoparticles on sericin/agarose film via the adhesion feature of polydopamine. For the preparation of the film, sericin (as a natural hydrophilic protein) was blended with agarose (as a neutral polysaccharide). Then, polydopamine was coated on it to capture ZnO/Ag. The outcomes displayed good mechanical and excellent antimicrobial performance against both Gram-positive and Gram-negative bacteria. It showed good potential in the fabrication of antimicrobial coatings. In a similar investigation, the antimicrobial coating was prepared by in situ fabrication of Ag nanoparticles in dopamine-containing hydrophilic glycopolymers. The prepared coating showed superior antimicrobial action toward E. coli and S. aureus microorganisms [35]. These polymeric materials with antimicrobial properties are well used to cover different surfaces to kill microorganisms.
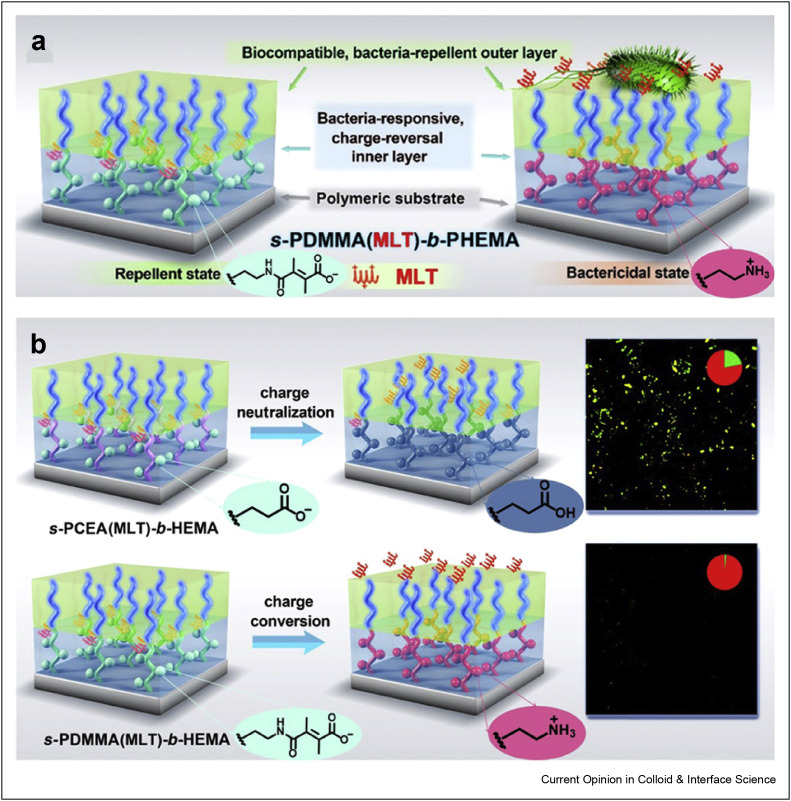
Surface-tethered hierarchical polymer brushes can be used to fabricate surfaces against microorganisms. In a recent study, an antibacterial surface with the construction of a hierarchical polymer brush on the polymer substrate was prepared via light-induced living surface grafting polymerization (Figure 2 ), which can be used in the medical field for the protection of surface against microorganisms. The micrometer-thick polymer brush system has a hierarchical structure consisting of an outer polymer layer poly(hydroxyethyl methacrylate) and an anionic inner layer loaded with antimicrobial peptides. It binds to bacteria on the surface and multiplies, causing acidification and the cleavage of unstable amide bonds. With the release of melittin, the bacteria that adhere to the surface are killed. This polymeric system with antimicrobial properties has excellent potential for use in preventing infection [36].
Figure 2.
Schematic diagram of the antibacterial mechanism of the hierarchical platform. (a) The hierarchical platform provides a biocompatible and nonadhesive surface under normal physiological conditions. In the early stage of surface colonization by bacteria, the bacterially induced acidification triggers MLT releasing, turning the surface from nonadhesive to bactericidal when needed to attack the adhering bacteria. (b) Compared with the prevailing release model based on charge neutralization, the negative-to-positive charge-conversion mechanism renders the surface highly responsive to bacterial acidification and eliminates infections at early stages of the bacterial colonization of biomaterials. This was reprinted with permission from Ref. [36].
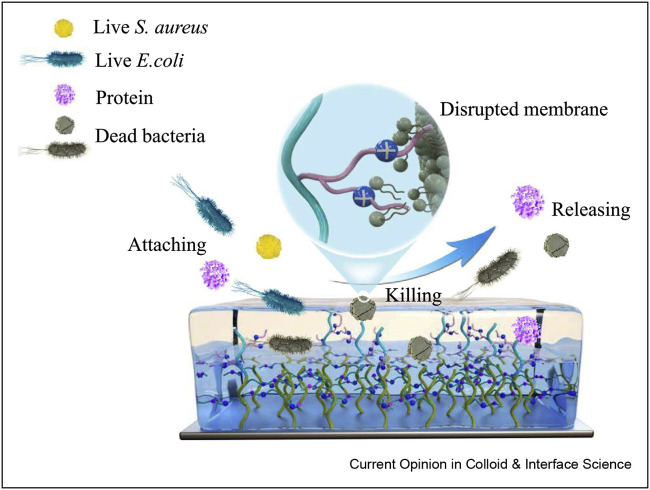
In a similar study, a hierarchical polymer brush surface with super-antibacterial and self-cleaning features toward microorganisms was prepared. The antibacterial upper layer (geminized cationic amphiphilic) and zwitterionic antifouling sublayer were applied for this aim. As can be observed in Figure 3 , this polymeric system kills microorganisms, releases dead bacteria, and resists the adhesion of protein [37∗].
Figure 3.
Schematic illustration of the antibacterial mechanisms for the hierarchical surface. This was reprinted with permission from Ref. [37].
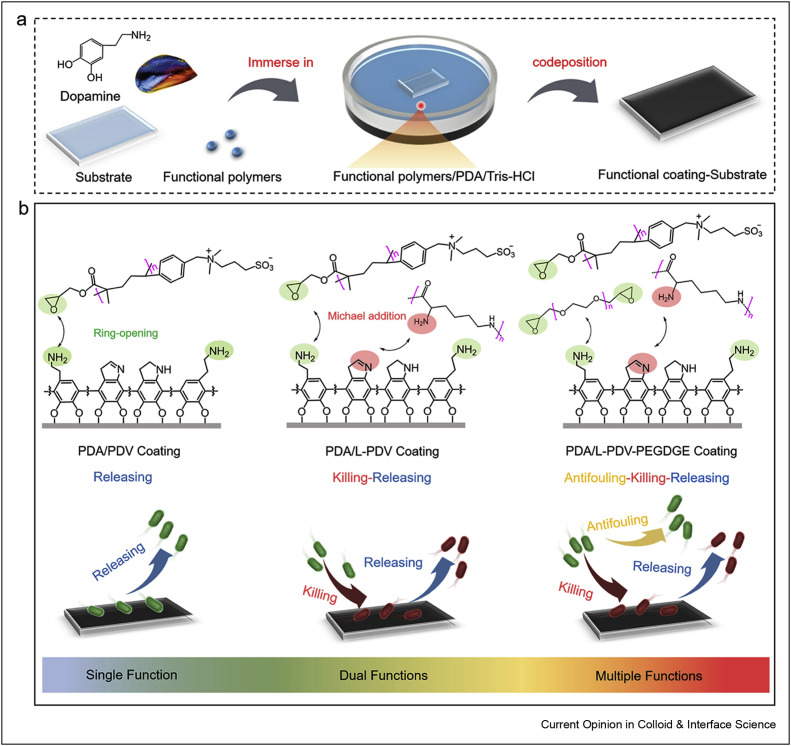
Zhang et al. [38] designed and prepared a series of mussel-inspired polymeric coatings with different antimicrobial functions (single function [release], dual functions [touch-killing/release], as well as triple functions [antifouling/touch-killing/release]) in a mussel-inspired co-deposition method. The fabrication process of coatings can be observed in Figure 4 , polydopamine selected as an adhesive layer for the preparation of multifunctional antimicrobial surfaces. Owing to Michael's addition/ring–opening reaction in the preparation process, coatings could strongly anchor onto several substrates such as polypropylene, polyethylene terephthalate, and glass. The fabricated coatings showed high performance against microorganisms.
Figure 4.
Mussel-inspired polymeric coatings realizing functions from single to multiple antimicrobial mechanisms. (a) Schematic illustration of one-step fabrication for functional polymeric coatings by PDA-assisted techniques. (b) Chemical structures and corresponding practical behaviors of as-designed polymeric coatings from a single function (release) to triple functions (antifouling–killing–release). Three functional polymers, that is, polylysine, diethylene glycol diglycidyl ether (PEGDGE), and poly[glycidylmethacrylate-co-3-(dimethyl(4-vinylbenzyl)ammonium)propyl sulfonate] (poly(GMA-co-DVBAPS)), were selected to demonstrate our strategy (PDA: polydopamine). This was reprinted with permission from Ref. [38].
An antimicrobial strategy in medicine is to use nano-gel antimicrobial coatings to prevent and combat infection. These coatings create a hydrated surface layer for promoting the antifouling features successfully. The antimicrobial activity of these nano-gels can be increased with the help of quaternary ammonium compounds because of positive charges and the alkyl chain. In a study, nano-gel coatings with high antimicrobial performance by using tertiary amine were designed. The nano-gels were quaternized by N-alkylation, which induces antibacterial activity through membrane binding. The quaternized nano-gels caused intraparticle hydrophobic domains. Triclosan-incorporated nano-gels killed 99.99% of pathogens on the surface of clinical coatings [39].
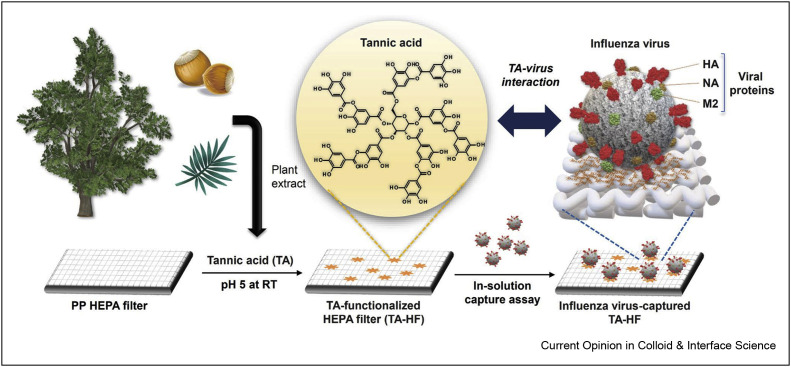
Tannic acid is a phenolic acid that has unique antiviral and antibacterial properties, and there have been many reports of its action against a variety of viruses (influenza, papilloma, HIV, herpes, noroviruses) and bacteria (Gram-positive and -negative). Modifying coating and filters with these natural molecules is a promising way to binding and capture viruses and preparative antiviral materials [40]. In this regard, polypropylene filter fabrics were functionalized with tannic acid for the manufacture of antiviral filters in preparation of protective equipment against the influenza A virus (Figure 5 ). Through a dipping/washing procedure, polypropylene filters were covered with tannic acid. As per the results, fast and efficient performance was achieved, and also, no cytotoxicity was observed. The functionalized filter displayed a high virus capture proficiency (2723 pfu/mm2 in 10 min), which was very higher than the nonmodified one [41∗].
Figure 5.
Preparation of TA-HF for influenza virus capture. Schematic illustration of TA-HF preparation and influenza virus capture based on interaction of TA with viral proteins (HA hemagglutinin, NA neuraminidase, M2 matrix-2) (TA: tannic acid, HF: high-efficiency particulate air filter). This was reprinted with permission from Ref. [41].
For the preparation of antivirals and bacteria personal protective equipment or medical devices, in a study, antibacterial and antiviral nanofibrous membranes including vitamin K compounds and poly(vinyl alcohol-co-ethylene) were produced. The prepared membranes displayed strong photoactivity in producing reactive oxygen species in daylight and UVA. In a short time, great antimicrobial and antiviral proficiency (>99.9%) was obtained. These membranes showed great potential for use as antibacterial and antiviral materials in the production of personal protective equipment such as face masks [42∗].
Antimicrobial textiles and fabrics for protective applications
Today, the demand for environmentally friendly antimicrobial fabrics and textiles is growing. To reduce contamination caused by nondegradable plastics, in a study, an antimicrobial cellulose fabric containing Ag nanoparticles was prepared. Cellulose fabrics are used in many industries, including medical textiles, and are also widely used in the preparation of personal protective equipment during the COVID-19 period. In this study, fabric-metal nanocomposites were prepared via ultrasonic waves in a simple, green, and one-step process. Cellulose mechanoradicals were generated via ultrasonication of cotton solution and breakage of 1,4-glycosidic bonds. Then, by generated mechanoradicals, the metal ions (Au3+ and Ag+ ions) in the solution were reduced to metal nanoparticles. Finally, metal nanoparticles were decorated on fabrics. The antimicrobial performance of the prepared fabric toward E. coli (Gram-negative bacteria) and Bacillus subtilis (Gram-positive bacteria) was tested, and the outcomes showed the toxicity of nanocomposite toward bacteria [43∗].
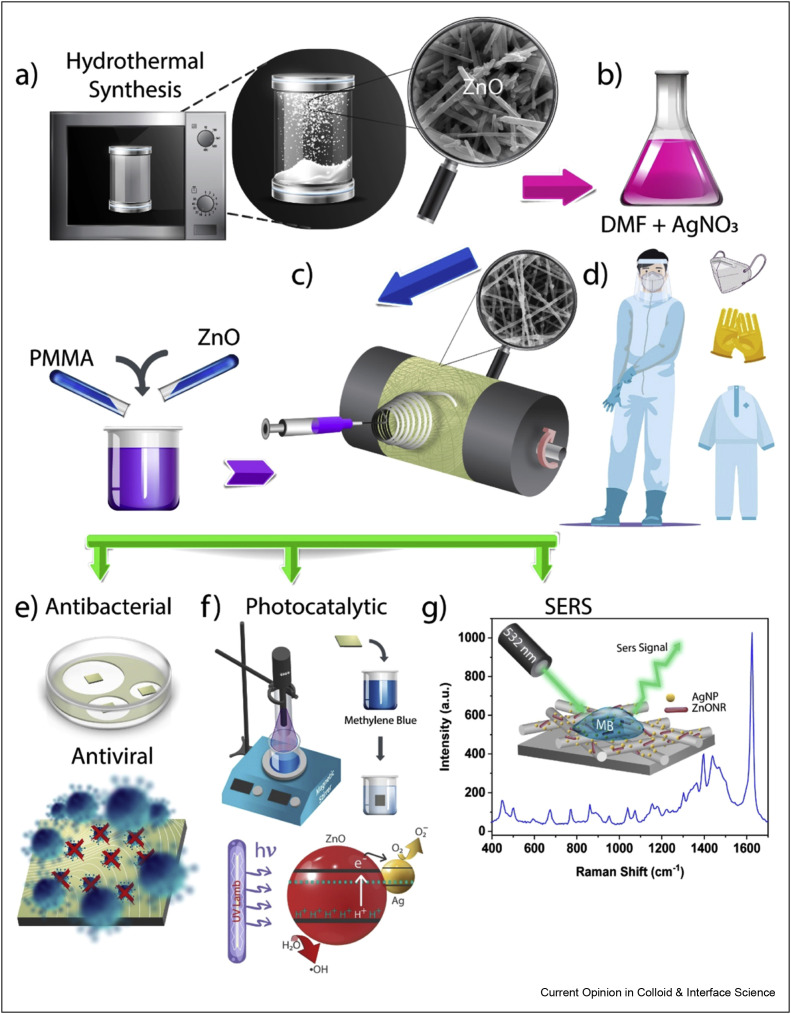
A research team prepared antimicrobial, antiviral, and self-cleaning nanofibers with the decoration of poly(methyl methacrylate) with ZnO nanorods and nano-Ag [44]. By the direct electrospinning method, multifunctional mats for modification of protective clothing were designed (Figure 6 ). For this aim, first, ZnO was manufactured by a hydrothermal technique, and Ag was prepared via reduction of AgNO3. A solution containing polymer, ZnO, Ag nanoparticles was prepared, and then, this solution was electrospun on a mat and placed on the inner side of the fabric. The prepared nanofibers with 450 nm size showed great presentation with 4 functionalities: Antiviral performance toward influenza and coronaviruses, antibacterial behavior against Gram-negative and -positive bacteria, photocatalyst to eliminate organic contaminants, and reusable surface-enhanced Raman scattering substrate to quantitative analysis of impurities on the fabric.
Figure 6.
Fabrication steps of PMMA/ZnO–Ag NFs (a–d): (a) synthesis of ZnO nanorods by the hydrothermal method and the SEM image of ZnO nanorods, (b) preparation of the electrospinning solution by mixing PMMA and ZnO nanorods with the solution of Ag NPs synthesized by in situ reduction of AgNO3 in the presence of DMF, (c) fabrication of PMMA/ZnO–Ag NFs on a mat by electrospinning and integration of NF mats to use protective clothes, (d) schematic illustration of a protective clothing containing PMMA/ZnO–Ag NF mats, and (e–g) multifunctional properties of the fabricated PMMA/ZnO–Ag NF mats (PMMA: Polymethyl methacrylate). This was reprinted with permission from Ref. [44].
The antibacterial fabric was prepared for preventing wound infection in the hospital. For this aim, polydopamine and polyethyleneimine were deposited on the cotton fabric. Gallic acid/Ag nanoparticles were cross-linked with them by H- bonding and Michael addition. The modified fabric showed an anionic surface because of gallic acid/Ag nanoparticles. This low-cytotoxicity modified fabric was resistant to bacteria owing to electrostatic repulsion. In addition, with releasing Ag+, it had strong antimicrobial performances [45].
Because viruses such as influenza and SARS-CoV-2 can be transmitted through fluid droplets and aerosols, they can spread the disease. Fabric face masks are a barrier to their prevalence. On the other hand, these viruses remain on the surface for several days and are stable. Metal nanoparticles, including zinc, can inactivate these viruses. In one study, zinc was embedded in polyamide 6.6 fibers to inactivate viruses in preparation of effective masks. The results showed that these viruses were easily absorbed by the fabric and inactivated by zinc ions [46]. In a study by Tremiliosi et al. [47], by pad–dry–cure technique, poly-cotton fabrics were functionalized with Ag nanoparticles. Ag nanoparticles have an excellent ability to inactivate pathogens and are widely used in the textile industry. In this process, the results exposed that this polymeric composite effectively inhibits the SARS-CoV-2 virus (around 100% in 2 min) as well as other pathogens (S. aureus, E. coli, and Candida albicans) and does not cause any allergies.
El-Naggar et al. [48] fabricated self-cleanable cotton fibers with an antimicrobial performance by using Ag nanoparticles for decorating on them in a simplistic and cheap in situ pad–dry–cure process to prepare UV protection and brilliant colors. Via thermal reduction (at 130 °C) of Ag ions on the surface of fibrous cotton, Ag nanoparticles were prepared. Through plasma activation, Ag particles were better immobilized on it. The obtained cotton fibers showed antimicrobial performance toward diverse pathogens (S. aureus, E. coli, and C. albicans). Another research team [49] prepared metal oxide–coated textiles using a coating of ZnO nanoparticles on starched cotton fibers via ultrasonication, and the antimicrobial activity was examined. Corn starch was applied to enhance the cotton adhesion to ZnO and for better stabilization of nanoparticles. Deposition of ZnO was improved (53%) after using 3 wt.% starch for 10 washing cycles. With the functionalization of ZnO-coated cotton by curcumin and Ag nanoparticles, the antimicrobial performance of the fabric was enhanced because of the synergistic behavior of ZnO, Ag, and curcumin. In addition, in a conventional dyeing technique, CuO nanoparticles were in situ grown onto cotton textiles for the fabrication of antimicrobial textile samples. For functionalization of textiles, diverse percentages of Cu(CH3COO)2.H2O were used. The functionalized textile with antimicrobial agents displayed a clear inhibition zone toward Gram-positive and -negative microorganisms [50]. Noorian et al. [51] produced multifunctional fabrics by in situ manufacture of ZnO nanoparticles on modified cotton. At first, for enhancement of the active sites in the fabric, the OH group was oxidized to aldehyde. Then, it was treated with 4-aminobenzoic acid. In the last stage, the nano-ZnO was in situ manufactured on the treated fabric. The fabric showed good antibacterial features and UV protection after 100 cycles of abrasion and 20 cycles of washing.
Antimicrobial packaging for protective applications
The world is currently facing a new challenge from the infectious disease of COVID-19. Food products can carry the virus during various stages of processing and transportation and reach the customer and so cause the spread of disease. The SARS-CoV-2 can remain on surfaces for several days. Antimicrobial food packaging may be useful in this case. For example, Hunt et al. [52] used hydrochloric acid and TiO2 nanoparticles for the fabrication of photocatalytic coatings with good antimicrobial performance and mechanical robustness for food contact surfaces to enhance food safety during food processing. The produced coating in combination with water, oxygen, and light clears contaminants by releasing reactive oxygen species (.OH, 1O2, and H2O2). The fabricated coating showed the potential for preventing cross-contamination in the processing of foods.
Via blending antimicrobial and antiviral components such as curcumin into poly(vinyl acetate) matrixes, a new protecting coating with antimicrobial photodynamic performance toward Salmonella typhimurium and S. aureus was prepared for food decontamination. The killing efficiency of microorganisms was depended on the curcumin concentration. Poly(vinyl acetate)/curcumin10 coating reached 93% at an energy density of 72 J/cm2 [53]. Antimicrobial active food packaging was developed by carboxymethyl cellulose, montmorillonite (MMT) clay, as well as ε-poly-(L-lysine) through solution casting technique. Fillers enhanced the antimicrobial, mechanical, and UV barrier properties. Carboxymethyl cellulose/MMT/ε-PL nanocomposite films demonstrated respectable antimicrobial performance toward fungi (Botrytis cinerea and Rhizopus oligosporus), S. aureus, and E. coli [54].
In another study, by the incorporation of green tea extract into chitosan edible films, antiviral and antibacterial coatings for food safety were produced [55]. The fabricated nanocomposite coating effectively inactivated and reduced the virus (murine norovirus) and bacteria (such as E. coli). Moreover, nanocomposite films with antimicrobial behavior by using chitosan, cellulose nanofiber, and curcumin were designed for food packaging applications. Curcumin is known as an antioxidant and a natural antiviral and antibacterial agent. Smooth and uniform films with suitable mechanical properties were prepared to prevent pathogens. Excellent antibacterial activity was reported in this study. In another study, a natural antiviral agent (Larrea nitida extract) was inserted into agar, alginate, or agar/alginate film for the preparation of antiviral edible coatings for food packaging. The prepared coatings exhibited good antiviral and antibacterial performance [56].
Pectin-based antimicrobial coatings were developed by Ghorbani et al. [57]. The pomegranate has high antioxidant and antimicrobial properties. In this study, pomegranate peel extract containing flavonoids and polyphenols including gallic acid and caffeic acid was used. The extract was added to the pectin-containing solution, and a coating with high antimicrobial properties was prepared for food packaging. Pomegranate peel extract showed good inhibitory and antibacterial effects against Gram-positive and Gram-negative bacteria (S. aureus and E. coli). Pectin-based coatings showed good antimicrobial properties against pathogenic microorganisms and also increased food shelf life. In addition, Simona et al. [58] prepared antimicrobial food packaging films with UV protector behavior by incorporation of orange essential oil into the carrageenan matrix. The prepared films were resistant to S. aureus bacteria. In addition, antimicrobial electrospun zein fibers were developed to apply in the food packaging sector. It showed great capability for inactivation of wide spectrum of pathogens [59].
Conclusions
Demand for antimicrobials is growing during the COVID-19 pandemic and will be even more important in the postpandemic time to make the world safer. In this regard, polymeric composites or other polymeric materials that are resistant to microorganisms have received much attention. Antimicrobial coatings are very important for preventing the spearing of microbes and viruses. On the other side, antimicrobial packaging may be beneficial to disinfect microorganisms. Moreover, for the fabrication of antimicrobial fabrics in manufacturing household masks or personal protective equipment, polymeric materials can play an effective role. At last but not least, these kinds of material will be developed and would be needed to fight against COVID-19 pandemic and after pandemic.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are immensely grateful for financial support from the Research Affairs Division of Isfahan University of Technology (IUT), Isfahan, I. R. Iran and Iran Nanotechnology Initiative Council (INIC) Tehran, I. R. Iran. The authors would also like to show their gratitude to the National Elite Foundation (NEF), Tehran, I. R. Iran and Center of Excellence in Sensors and Green Chemistry IUT.
This review comes from a themed issue on Hot Topic: COVID-19
Edited by Reinhard Miller and Libero Liggieri
References
- 1.Suthar S., Das S., Nagpure A., Madhurantakam C., Tiwari S.B., Gahlot P., et al. Epidemiology and diagnosis, environmental resources quality and socio-economic perspectives for COVID-19 pandemic. J Environ Manag. 2021;280:111700. doi: 10.1016/j.jenvman.2020.111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz A., Asif M., Ashraf G., Farooq U., Yang Q., Wang S. Trends in biosensing platforms for SARS-CoV-2 detection: a critical appraisal against standard detection tools. Curr Opin Colloid Interface Sci. 2021;52:101418. doi: 10.1016/j.cocis.2021.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao M., Liu H., Wang X., Hu X., Huang Y., Liu X., et al. A technical review of face mask wearing in preventing respiratory COVID-19 transmission. Curr Opin Colloid Interface Sci. 2021;52:101417. doi: 10.1016/j.cocis.2021.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emani V.R., Goswami S., Nandanoor D., Emani S.R., Reddy N.K., Reddy R. Randomised controlled trials for COVID-19: evaluation of optimal randomisation methodologies—need for data validation of the completed trials and to improve ongoing and future randomised trial designs. Int J Antimicrob Agents. 2021;57:106222. doi: 10.1016/j.ijantimicag.2020.106222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14:12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramaniam B., Prateek, Ranjan S., Saraf M., Kar P., Singh S.P., et al. Antibacterial and antiviral functional materials: chemistry and biological activity toward tackling COVID-19-like pandemics. ACS Pharmacol Transl Sci. 2021;4:8–54. doi: 10.1021/acsptsci.0c00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koduru J.R., Kailasa S.K., Bhamore J.R., Kim K.H., Dutta T., Vellingiri K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: a review. Adv Colloid Interface Sci. 2018;256:326–339. doi: 10.1016/j.cis.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Mallakpour S., Azadi E., Hussain C. M Environmentally benign production of cupric oxide nanoparticles and various utilizations of their polymeric hybrids in different technologies. Coord Chem Rev. 2020:213378. doi: 10.1016/j.ccr.2020.213378. [DOI] [Google Scholar]
- 9.Mallakpour S., Azadi E., Hussain C.M. The latest strategies in the fight against the COVID-19 pandemic: the role of metal and metal oxide nanoparticles. New J Chem. 2021;45:6167. doi: 10.1039/d1nj00047k. [DOI] [Google Scholar]
- 10.Mallakpour S., Azadi E., Hussain C.M. Fight against COVID-19 pandemic with the help of carbon-based nanomaterials. New J Chem. 2021;45:8832–8846. doi: 10.1039/D1NJ01333E. [DOI] [Google Scholar]
- 11.Mallakpour S., Ramezanzade V. Green fabrication of chitosan/tragacanth gum bionanocomposite films having TiO2@Ag hybrid for bioactivity and antibacterial applications. Int J Biol Macromol. 2020;162:512–522. doi: 10.1016/j.ijbiomac.2020.06.163. [DOI] [PubMed] [Google Scholar]
- 12.Mallakpour S., Abbasi M. Hydroxyapatite mineralization on chitosan-tragacanth gum/silica@silver nanocomposites and their antibacterial activity evaluation. Int J Biol Macromol. 2020;151:909–923. doi: 10.1016/j.ijbiomac.2020.02.167. [DOI] [PubMed] [Google Scholar]
- 13.Pang W.Y., Ahmad A.L., Zaulkiflee N.D. Antifouling and antibacterial evaluation of ZnO/MWCNT dual nanofiller polyethersulfone mixed matrix membrane. J Environ Manag. 2019;249:109358. doi: 10.1016/j.jenvman.2019.109358. [DOI] [PubMed] [Google Scholar]
- 14.Duval J.F.L., Leeuwen HP van, Norde W., Town R.M. Chemodynamic features of nanoparticles: application to understanding the dynamic life cycle of SARS-CoV-2 in aerosols and aqueous biointerfacial zones. Adv Colloid Interface Sci. 2021:144034. doi: 10.1016/j.cis.2021.102400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Mu C., Lin W., Xiao H. Functional-modified polyurethanes for rendering surfaces antimicrobial: an overview. Adv Colloid Interface Sci. 2020;283:102235. doi: 10.1016/j.cis.2020.102235. [DOI] [PubMed] [Google Scholar]
- 16.Randriantsilefisoa R., Nie C., Parshad B., Pan Y., Bhatia S., Haag R. Double trouble for viruses: a hydrogel nanocomposite catches the influenza virus while shrinking and changing color. Chem Commun. 2020;56:3547–3550. doi: 10.1039/c9cc09069j. [DOI] [PubMed] [Google Scholar]
- 17.de Dicastillo C.L., Vidal C.P., Falcó I., Sánchez G., Márquez P., Escrig J. Antimicrobial bilayer nanocomposites based on the incorporation of as-synthetized hollow zinc oxide nanotubes. Nanomaterials. 2020;10:1–14. doi: 10.3390/nano10030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loutfy S.A., Elberry M.H., Farroh K.Y., Mohamed H.T., Mohamed A.A., Mohamed E.B., et al. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int J Nanomed. 2020;15:2699–2715. doi: 10.2147/IJN.S241702. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pacho M.N., Pugni E.N., Díaz Sierra J.B., Morell M.L., Sepúlveda C.S., Damonte E.B., et al. Antiviral activity against Zika virus of a new formulation of curcumin in poly lactic- co-glycolic acid nanoparticles. J Pharm Pharmacol. 2021;73:357–365. doi: 10.1093/jpp/rgaa045. [DOI] [PubMed] [Google Scholar]; In this study, for the first time, effective antiviral formulation by natural product curcumin and the green copolymer is presented for Zika virus infection.
- 20.Valenzuela L., Iglesias A., Faraldos M., Bahamonde A., Rosal R. Antimicrobial surfaces with self-cleaning properties functionalized by photocatalytic ZnO electrosprayed coatings. J Hazard Mater. 2019;369:665–673. doi: 10.1016/j.jhazmat.2019.02.073. [DOI] [PubMed] [Google Scholar]
- 21.Jalvo B., Faraldos M., Bahamonde A., Rosal R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J Hazard Mater. 2017;340:160–170. doi: 10.1016/j.jhazmat.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Pemmada R., Zhu X., Dash M., Zhou Y., Ramakrishna S., Peng X., et al. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials. 2020;13:4041. doi: 10.3390/ma13184041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yekta R., Vahid-Dastjerdi L., Norouzbeigi S., Mortazavian A.M. Food products as potential carriers of SARS-CoV-2. Food Contr. 2021:107754. doi: 10.1016/j.foodcont.2020.107754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Tayyar N.A., Youssef A.M., Al-hindi R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: a review. Food Chem. 2020;310:125915. doi: 10.1016/j.foodchem.2019.125915. [DOI] [PubMed] [Google Scholar]
- 25.Cabañas-Romero L.V., Valls C., Valenzuela S.V., Roncero M.B., Pastor F.I.J., Diaz P., et al. Bacterial cellulose-chitosan paper with antimicrobial and antioxidant activities. Biomacromolecules. 2020;21:1568–1577. doi: 10.1021/acs.biomac.0c00127. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud Hamdy A.E.-A., Mohamed Salah K. Antiviral and antinematodal potentials of chitosan: review. J Plant Sci Phytopathol. 2020;4 doi: 10.29328/journal.jpsp.1001051. 055–059. [DOI] [Google Scholar]
- 27.Porter G.C., Schwass D.R., Tompkins G.R., Bobbala S.K.R., Medlicott N.J., Meledandri C.J. AgNP/Alginate Nanocomposite hydrogel for antimicrobial and antibiofilm applications. Carbohydr Polym. 2021;251:117017. doi: 10.1016/j.carbpol.2020.117017. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Mukherjee R., Basak D., Haldar J. One-step curable, covalently immobilized coating for clinically relevant surfaces that can kill bacteria, fungi, and influenza virus. ACS Appl Mater Interfaces. 2020;12:27853–27865. doi: 10.1021/acsami.9b22610. [DOI] [PubMed] [Google Scholar]; This is the first study of an antimicrobial coating which can kill all of bacteria, fungi, and influenza virus with 100% killing for application in healthcare settings.
- 29.Peng L., Chang L., Si M., Lin J., Wei Y., Wang S., et al. Hydrogel-coated dental device with adhesion-inhibiting and colony-suppressing properties. ACS Appl Mater Interfaces. 2020;12:9718–9725. doi: 10.1021/acsami.9b19873. [DOI] [PubMed] [Google Scholar]
- 30.Balagna C., Francese R., Perero S., Lembo D., Ferraris M. Nanostructured composite coating endowed with antiviral activity against human respiratory viruses deposited on fibre-based air filters. Surf Coating Technol. 2021;409:126873. doi: 10.1016/j.surfcoat.2021.126873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo C., Cui W., Wang X., Lu X., Zhang L., Li X., et al. Poly-l-lysine/Sodium alginate coating loading nanosilver for improving the antibacterial effect and inducing mineralization of dental implants. ACS Omega. 2020;5:10562–10571. doi: 10.1021/acsomega.0c00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballarre J., Aydemir T., Liverani L., Roether J.A., Goldmann W.H., Boccaccini A.R. Versatile bioactive and antibacterial coating system based on silica, gentamicin, and chitosan: improving early stage performance of titanium implants. Surf Coating Technol. 2020;381:125138. doi: 10.1016/j.surfcoat.2019.125138. [DOI] [Google Scholar]
- 33.Sharif N., Falcó I., Martínez-Abad A., Sánchez G., López-Rubio A., Fabra M.J. On the use of Persian gum for the development of antiviral edible coatings against murine norovirus of interest in blueberries. Polymers. 2021;13:1–12. doi: 10.3390/polym13020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Huang Z., Cai R., Yang W., He H., Wang Y. Rational design of Ag/ZnO hybrid nanoparticles on sericin/agarose composite film for enhanced antimicrobial applications. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng K., Peng L., Yu L., Zheng Y., Chen R., Zhang W., et al. Universal antifogging and antimicrobial thin coating based on dopamine-containing glycopolymers. ACS Appl Mater Interfaces. 2020;12:27632–27639. doi: 10.1021/acsami.0c07949. [DOI] [PubMed] [Google Scholar]
- 36.Liu T., Yan S., Zhou R., Zhang X., Yang H., Yan Q., et al. Self-adaptive antibacterial coating for universal polymeric substrates based on a micrometer-scale hierarchical polymer brush system. ACS Appl Mater Interfaces. 2020;12:42576–42585. doi: 10.1021/acsami.0c13413. [DOI] [PubMed] [Google Scholar]
- Chen T., Zhao L., Wang Z., Zhao J., Li Y., Long H., et al. Hierarchical surface inspired by geminized cationic amphiphilic polymer brushes for super-antibacterial and self-cleaning properties. Biomacromolecules. 2020;21:5213–5221. doi: 10.1021/acs.biomac.0c01295. [DOI] [PubMed] [Google Scholar]; For biomedical applications, in a novel study, super-antibacterial and ultrahigh self-cleaning surface is prepared using geminized cationic amphiphilic polymer brushes.
- 38.Mao S., Zhang D., He X., Yang Y., Protsak I., Li Y., et al. Mussel-inspired polymeric coatings to realize functions from single and dual to multiple antimicrobial mechanisms. ACS Appl Mater Interfaces. 2021;13:3089–3097. doi: 10.1021/acsami.0c16510. [DOI] [PubMed] [Google Scholar]
- 39.Keskin D., Tromp L., Mergel O., Zu G., Warszawik E., Van Der Mei H.C., et al. Highly efficient antimicrobial and antifouling surface coatings with triclosan-loaded nanogels. ACS Appl Mater Interfaces. 2020;12:57721–57731. doi: 10.1021/acsami.0c18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaczmarek B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials-A minireview. Materials. 2020;13:3224. doi: 10.3390/ma13143224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Chung J., Lee S.H., Yoon J.H., Kweon D.H., Chung W.J. Tannic acid-functionalized HEPA filter materials for influenza virus capture. Sci Rep. 2021;11:1–7. doi: 10.1038/s41598-020-78929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this interesting and successful study, the authors report a simple, cost-effective, and robust strategy for the development anti-influenza filter using tannic acid as an antiviral compound.
- Sun G., Zhang Z., El-Moghazy A.Y., Wisuthiphaet N., Nitin N., Castillo D., et al. Daylight-induced antibacterial and antiviral nanofibrous membranes containing Vitamin K derivatives for personal protective equipment. ACS Appl Mater Interfaces. 2020;12:49416–49430. doi: 10.1021/acsami.0c14883. [DOI] [PubMed] [Google Scholar]; For the development of personal protective equipment during the COVID-19 pandemic, the authors using daylight active functional polymeric materials containing vitamin K, prepare antibacterial and antiviral membranes with great antimicrobial and antiviral efficiency (>99.9%).
- Kwiczak-Yiǧitbaşl J., Demir M., Ahan R.E., Canll S., Şafak Şeker U.Ö., Baytekin B. Ultrasonication for environmentally friendly preparation of antimicrobial and catalytically active nanocomposites of cellulosic textiles. ACS Sustainable Chem Eng. 2020;8:18879–18888. doi: 10.1021/acssuschemeng.0c05493. [DOI] [Google Scholar]; In this study, the authors report the first-time preparation of cotton and cotton fabric/Au and Ag nanoparticle composites via sonication in green, simple, and one-pot method.
- 44.Karagoz S., Kiremitler N.B., Sarp G., Pekdemir S., Salem S., Goksu G., et al. Antibacterial , antiviral , and self-cleaning mats with sensing capabilities based on electrospun nano fi bers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl Mater Interfaces. 2021;13:5678–5690. doi: 10.1021/acsami.0c15606. [DOI] [PubMed] [Google Scholar]
- 45.Liu G., Xiang J., Xia Q., Li K., Yan H., Yu L. Fabrication of durably antibacterial cotton fabrics by robust and uniform immobilization of silver nanoparticles via mussel-inspired polydopamine/polyethyleneimine coating. Ind Eng Chem Res. 2020;59:9666–9678. doi: 10.1021/acs.iecr.9b07076. [DOI] [Google Scholar]
- 46.Gopal V., Nilsson-Payant B.E., French H., Siegers J.Y., tenOever B.R., Yung W.S., et al. Zinc-embedded fabrics inactivate SARS-CoV-2 and influenza a virus. BioRxiv. 2020 doi: 10.1101/2020.11.02.365833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremiliosi G.C., Simoes L.G.P., Minozzi D.T., Santos R.I., Vilela D.C.B., Durigon E.L., et al. Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.06.26.152520. [DOI] [Google Scholar]
- 48.El-Naggar M.E., Khattab T.A., Abdelrahman M.S., Aldalbahi A., Hatshan M.R. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose. 2021;28:1105–1121. doi: 10.1007/s10570-020-03537-4. [DOI] [Google Scholar]
- 49.El-Nahhal I.M., Salem J., Anbar R., Kodeh F.S., Elmanama A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci Rep. 2020;10:1–10. doi: 10.1038/s41598-020-61306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Román L.E., Amézquita M.J., Uribe C.L., Maurtua D.J., Costa S.A., Costa S.M., et al. In situ growth of CuO nanoparticles onto cotton textiles. Adv Nat Sci Nanosci Nanotechnol. 2020;11 doi: 10.1088/2043-6254/ab8a2f. [DOI] [Google Scholar]
- 51.Noorian S.A., Hemmatinejad N., Navarro J.A.R. Ligand modified cellulose fabrics as support of zinc oxide nanoparticles for UV protection and antimicrobial activities. Int J Biol Macromol. 2020;154:1215–1226. doi: 10.1016/j.ijbiomac.2019.10.276. [DOI] [PubMed] [Google Scholar]
- 52.Torres Dominguez E., Nguyen P., Hylen A., Maschmann M.R., Mustapha A., Hunt H.K. Design and characterization of mechanically stable, nanoporous TiO2 thin film antimicrobial coatings for food contact surfaces. Mater Chem Phys. 2020;251:123001. doi: 10.1016/j.matchemphys.2020.123001. [DOI] [Google Scholar]
- 53.Chen L., Song Z., Zhi X., Du B. Photoinduced antimicrobial activity of curcumin-containing coatings: molecular interaction, stability and potential application in food decontamination. ACS Omega. 2020;5:31044–31054. doi: 10.1021/acsomega.0c04065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Y., Fei X., Li H. Carboxymethyl cellulose-based nanocomposites reinforced with montmorillonite and ε-poly-l-lysine for antimicrobial active food packaging. J Appl Polym Sci. 2020;137:1–12. doi: 10.1002/app.48782. [DOI] [Google Scholar]
- 55.Amankwaah C., Li J., Lee J., Pascall M.A. Antimicrobial activity of chitosan-based films enriched with green tea extracts on murine norovirus, Escherichia coli, and Listeria innocua. Int J Food Sci. 2020:3941924. doi: 10.1155/2020/3941924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreno M.A., Bojorges H., Falcó I., Sánchez G., López-Carballo G., López-Rubio A., et al. Active properties of edible marine polysaccharide-based coatings containing Larrea nitida polyphenols enriched extract. Food Hydrocolloids. 2020;102:105595. doi: 10.1016/j.foodhyd.2019.105595. [DOI] [Google Scholar]
- 57.Ghorbani E., Moghaddam A.D., Sharifan A., Kiani H. Emergency food product packaging by pectin-based antimicrobial coatings functionalized by pomegranate peel extracts. J Food Qual. 2021:6631021. [Google Scholar]
- 58.Simona J., Dani D., Petr S., Marcela N., Jakub T., Bohuslava T. Edible films from carrageenan/orange essential oil/trehalose—structure, optical properties, and antimicrobial activity. Polymers. 2021;13:1–19. doi: 10.3390/polym13030332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aytac Z., Huang R., Vaze N., Xu T., Eitzer B.D., Krol W., et al. Development of biodegradable and antimicrobial electrospun zein fibers for food packaging. ACS Sustainable Chem Eng. 2020;8:15354–15365. doi: 10.1021/acssuschemeng.0c05917. [DOI] [Google Scholar]