Abstract
International regulatory agencies such as the Food and Drug Administration have mandated that the scientific community develop humanized microphysiological systems (MPS) as an in vitro alternative to animal models in the near future. While the breast cancer research community has long appreciated the importance of three-dimensional growth dynamics in their experimental models, there are remaining obstacles preventing a full conversion to humanized MPS for drug discovery and pathophysiological studies. This perspective evaluates the current status of human tissue-derived cells and scaffolds as building blocks for an “idealized” breast cancer MPS based on bioengineering design principles. It considers the utility of adipose tissue as a potential source of endothelial, lymphohematopoietic, and stromal cells for the support of breast cancer epithelial cells. The relative merits of potential MPS scaffolds derived from adipose tissue, blood components, and synthetic biomaterials is evaluated relative to the current “gold standard” material, Matrigel, a murine chondrosarcoma-derived basement membrane-enriched hydrogel. The advantages and limitations of a humanized breast cancer MPS are discussed in the context of in-process and destructive read-out assays.
Impact statement
Regulatory authorities have highlighted microphysiological systems as an emerging tool in breast cancer research. This has been led by calls for more predictive human models and reduced animal experimentation. This perspective describes how human-derived cells, extracellular matrices, and hydrogels will provide the building blocks to create breast cancer models that accurately reflect diversity at multiple levels, that is, patient ethnicity, pathophysiology, and metabolic status.
Keywords: breast cancer, microphysiological system, three-dimensional cell culture, adipose-derived stromal/stem cells, Food and Drug Administration
Introduction
Over the past two decades, cell biologists exploring in vitro models of human disease have gained a greater appreciation for the importance of bioengineering principles.1 In the breast cancer field, these concepts have long been recognized in the literature. The originators of one of the most extensively studied estrogen receptor (ER)-dependent breast cancer cell lines, Michigan Cancer Foundation-7 (MCF-7), reported in 1976 that their cell model mimicked the tumor's original lobular architecture when cultured in a collagen sponge.2,3 Schmeichel and Bissell went on to articulate the contribution of the three-dimensional (3D) microenvironment to breast tumor pathophysiology.4 They recognized that two-dimensional (2D) tissue culture plastic growth of breast cancer cell lines as monotypic cultures failed to recapitulate the in vivo complexity of the actual tumor. To address this concern, they transformed the field by introducing a more sophisticated model, coculturing breast cancer cells together with supportive stromal cells and extracellular matrix (ECM) derived from laminin- and collagen-rich basement membrane tissues, thereby recapitulating the native 3D architecture.4 While this 3D culture approach was transformative, Griffith and Swartz recognized that the field required a cohesive set of design criteria and accepted standards before it could achieve widespread adoption.5 Prestwich elegantly and pragmatically laid out these challenges in his 2007 perspective on the development of ECM products for 3D models.6
Specifically, these materials would need to be available in multiple physical formats, remain consistent from batch to batch, be easy to use at physiological temperatures and pH, resist contractive and expansive events, be easy to see through with microscopy, suitable for high-throughput screening, have clinical translational potential, and, last but not least, be affordable.6 Prestwich argued persuasively that ECM meeting these goals would allow 3D assays to be used routinely in toxicology screening and drug discovery.6 Policy officials at the Food and Drug Administration (FDA), the Defense Advanced Research Project Agency (DARPA), and the National Center for Advancing Translational Science (NCATS) within NIH quickly recognized the importance of these concepts. Within a decade, program officers had introduced policies with goal-oriented roadmaps and associated funding to integrate humanized 3D models, termed inclusively as “microphysiological systems” (MPS), as a projected means to reduce, refine, and replace in vivo animal studies in human drug discovery research.7–9
Historically, human breast cancer studies have relied almost exclusively on immortalized human tumor cell lines that originated five or more decades ago.3,10 While there have been recent advances in the generation of patient-derived xenograft (PDX) models, these continue to require immunodeficient mice as in vivo hosts for both expansion and propagation-based studies.11–13 Studies have shown that both the ECM and murine host system impact the pathophysiology of human-derived breast cancer cells, thereby demonstrating a need for an alternative approach that is less dependent on the mouse.14,15 Based on the principles introduced by Bissell, Griffith, Prestwich, and their colleagues,4–6,16,17 an ideal human breast cancer MPS model would possess the above characteristics regarding performance consistency, ease of use, and amenability to high-throughput formats, in addition to the following attributes:
(a) Sourced materials entirely human in origin with respect to breast cancer cells, stromal cells, and ECM.
(b) Suitable for the direct generation of 3D culture using primary tumors for patient-derived MPS.
(c) Human stromal vascular fraction (SVF) cells that can provide not only fibroblastic features, but also adipocytes, endothelial cells, immune cells, and robust vascularization in vitro.
(d) ECM that can provide structural proteins as well as angiogenic and inflammatory factors, displays temperature- and temporal-dependent gelling properties, and will be “tunable” to allow for manipulation of biomechanical properties (viscoelasticity, porosity, stiffness) to mimic those of the pathophysiological microenvironment. Furthermore, the ECM will be transparent, thereby improving ease of visualization and in process microscopic monitoring.
(e) Validated pharmacodynamic/pharmacokinetic (PD/PK) features in vitro accurately reflecting known tumor pathophysiological behaviors in vivo, including invasion and metastasis to bone and other organs.18
(f) Predicted Absorption/Distribution/Metabolism/Excretion (ADME) toxicology suitable for human clinical testing.
(g) Compatible with economic, medium-throughput screening protocol (i) off-the-shelf, readily available components with long shelf life; (ii) low-complexity set-up and use; (iii) adaptable to automated morphological screening instruments.
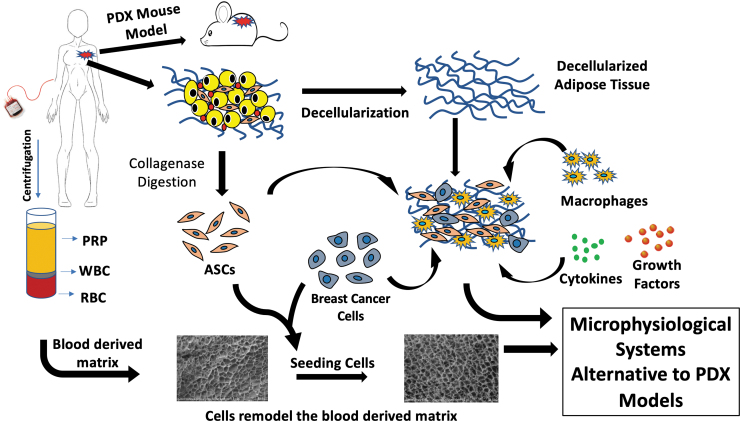
This perspective explores the current status of MPS as a breast cancer research discovery tool with a focus on the design criteria necessary to advance its broader adoption as a validated model (Fig. 1).
FIG. 1.
Development of breast cancer microphysiological systems. The breast cancer microenvironment can be recapitulated in vitro by combinations of primary breast tumor cells, ASC, decellularized adipose tissue, blood-derived products, including PRP, RBC, and WBC, along with cytokines and growth factors. These can serve as alternatives to PDX models that require an immunodeficient murine host. ASC, adipose-derived stromal/stem cells; PDX, patient-derived xenograft; PRP, platelet-rich plasma; RBC, red blood cell; WBC, white blood cell.
Breast Cancer Cells and 3D Models
The field of cell biology has pursued in vitro breast cancer models for over 80 years.10,19 While initial attempts were impeded by the overgrowth of fibroblast/stromal cells in the cultures with the subsequent loss of the epithelial population, the advent of improved media formulations and culture techniques addressed these issues.10 Due to the coordinated efforts of surgeons, pathologists, and cell biologists, multiple breast cancer cell lines have been isolated and characterized during the intervening years.
Historically, the cell lines have been characterized based on their expression of receptors (ER, progesterone receptor [PR], human epidermal growth factor receptor 2 [HER2]) that in turn can be used to categorize the four major breast cancer pathologies: Luminal A (ER+, PR+/−, HER2−), Luminal B (ER+, PR+/−, HER2+), Her2 Amplified (ER−, PR−, HER2+), and Triple Negative (ER−, PR−, HER2−).20 More recently, breast cancer cells are being characterized by comprehensive genomic features such as mutation analyses (breast cancer gene BRCA, P53, and others), transcriptome, cistrome, and aneuploidy, as well as epithelial to mesenchymal transition (EMT) and other morphological features. Two of the most highly studied breast cancer cell lines are the MCF-7, an ER+ line, and MDA-MB-231 (M.D. Anderson-Metastasis Breast cancer-231), a triple-negative line; both were cloned by serial passage from the pleural effusion of metastatic adenocarcinomas.21,22
While the majority of the breast cancer cell lines can be cultured individually in 2D, the superiority of 3D coculture models with cancer-associated fibroblasts (CAF)/stromal cells has been recognized based on outcome measures, including cytokine secretion, aromatase expression, and vascularization.23–25 Cell spheroids created from primary tumor cells or cell lines and organoids created from embryonic or induced pluripotent stem cells have been established as 3D cancer models using multiple approaches.26–28 Mechanical methods can be used to create spheroids or organoids by continuous rotation in suspension culture devices, thereby mimicking microgravity conditions,29,30 by hanging drop cultures, by suspension on low adherent plastic surfaces, by use of spinner flasks, or by levitation using magnetic beads.31,32 More frequently, investigators choose to create spheroids or 3D constructs by encapsulating or suspending the cell(s) of interest in a biological scaffold mimicking the ECM.31
Breast cancer studies have recreated a 3D microenvironment by culturing the cells in scaffolds of collagen,2 Matrigel,33 hyaluronan,34 alginate,35 and polyethylene glycol (PEG)-modified fibrinogen,36 among others37 (see Section 4: [ECM: Biological and Synthetic Alternatives to Matrigel] for further details). The advancement of 3D bioprinting now allows for a more sophisticated approach by controlling the spatial dimensions and layered composition of the ECM scaffold relative to the cultured cells.18,38 These MPS models now provide a basis for screening breast cancer drugs as well as for studies on the influence of metabolic constraints within the tumor microenvironment, such as hypoxia.16,39–41
Recently, PDX models have emerged as an alternative to breast cancer cell line-based studies.42 These require close coordination between the operating room, pathological suite, and laboratory and are not strictly an in vitro experimental model. Fresh specimens from resected primary or metastatic breast cancers that are not required for diagnostic purposes must be rapidly transferred to the laboratory and implanted as small organoid fragments into immunodeficient murine hosts.11,12 Frequently, the tumor fragments are coimplanted with Matrigel or another ECM hydrogel to improve engraftment. The PDX breast cancer models are being deployed in studies evaluating tumor drug response at the physiological and transcriptomic levels.13,43–45 Nevertheless, the logistics of tissue processing and storage within a relatively short time frame are roadblocks to the widespread adoption of PDX as an avenue for individualized medical therapy and patient stratification in clinical trials.
This could be addressed, in part, by the ability to cryopreserve viable breast tumor tissues long term with appropriate storage. Preliminary studies have determined that intact human adipose tissue fragments retained ∼50% viability after cryopreservation in the presence of a cryoprotectant agent.46 It remains to be determined if a similar or improved cryopreservation outcome would be possible when applied to breast cancer primary or metastatic tissues. If successful, routine preservation of breast cancer specimens would permit the potential contribution of PDX studies in every patient's care. While the PDX model requires maintenance in a murine host, it nevertheless mimics the 3D microenvironment and cellular complexity of a human breast cancer.
Furthermore, the availability of an economic, reproducible PDX-based approach has the potential to advance personalized medicine in the context of breast cancer. If each primary and/or metastatic tumor could be retained in a format that accurately reflected the proteomic or transcriptomic profile as well as drug sensitivity of the parent tumor, this would have a substantial impact on individual breast cancer patient treatment outcomes and survival.47–49 Theoretically, the PDX model could be adapted from its current murine-based in vivo approach to an entirely MPS-based in vitro high-throughput assay using an appropriate combination of endothelial, immune, and stromal/stem cells and supportive hydrogels. For example, the successful construction of both an adipose and bone marrow MPS have been described recently in the literature.50–53 Using such MPS constructs, it would be possible to seed an adipose MPS with a primary breast cancer tumor and to monitor its ability to metastasize through a microperfusion circulatory system into a parallel bone marrow MPS. Alternatively, tissue from a bone marrow metastatic breast tumor could be seeded directly into a bone marrow microenvironment MPS. Such MPS systems could be used for drug screening as well as omics analyses. Although conceptual, it is likely that multiple investigators will pursue such personalized medical MPS approaches aggressively in the coming years.
Adipose-Derived Cells: SVF Cells and Adipose-Derived Stromal/Stem Cells
The breast microenvironment supporting the growth of mammary epithelial cells relies on an extensive network comprised of stromal/stem cells, adipocytes, fibroblasts, endothelial cells, and pericytes, as well as a heterogeneous population of immune cells (myeloid, lymphoid, mast cells). Indeed, the mammary gland is categorized as a unique adipose depot. Since subcutaneous white adipose depots display a comparable cell composition and functionality, it provides a logical tissue source for primary cells with which to create a functional breast cancer MPS. Over the past two decades, there has been considerable focus on the isolation, characterization, differentiation, culture expansion, and cryopreservation of adipose-derived cells by investigators from the fields of plastic surgery, endocrinology, and regenerative medicine.54–60
Adipose tissue can be reduced to a single cell suspension by mechanical disruption and subsequent digestion with collagenase and neutral peptidases.55 This initially releases a heterogeneous population known as SVF cells. These include endothelial progenitors, fibroblasts, lymphoid and myeloid immune cells, mature adipocytes, pericytes, and preadipocytes, which can be identified and sorted based on their expression of surface antigens using flow cytometry or antibody-linked magnetic beads.54 The SVF cells can be directly cryopreserved for later recovery and use in MPS culture models59,61 or transferred immediately to tissue culture flasks for expansion as an adherent population known as “Adipose-derived Stromal/Stem Cells” (ASCs).54,55
Compared with SVF cells, ASCs are relatively homogeneous, displaying a discrete set of mesenchymal stromal/stem cell-associated surface antigens, including CD29, CD44, CD73, and CD90.55 While ASCs were originally culture expanded in growth medium containing fetal bovine serum (FBS), recent studies have demonstrated that human serum and platelet lysate supplements can serve as effective replacements for FBS.62–65 Indeed, the use of human blood products for ASC expansion has already advanced to early phase clinical trials, including studies in breast reconstruction and augmentation.66,67 When exposed to lineage-specific inductive media formulations, ASCs are capable of differentiation along the adipocyte, chondrocyte, and osteoblast pathways.54,55 In addition to their multipotent differentiation potential, ASCs can release a complex secretome containing adipokines, cytokines, and microRNA-containing exosomes.68–70 Thus, ASCs are capable of immunomodulating their local microenvironment through paracrine mechanisms.71
Coculture models developing 3D adipose tissue models have evolved as from traditional 2D tissue plastic cultures. Several studies have employed murine models, most notably the immortalized 3T3-L1 preadipocyte line. In one study, investigators have cocultured 3T3-L1 adipocytes and RAW264.7 macrophages within an alginate scaffold to recreate a myeloid infiltrated adipose depot in vitro.72 The presence of the macrophages conferred insulin resistance upon this MPS construct and it proved to be suitable for screening of antidiabetic drugs with known peroxisome proliferator-activated receptor ligand mechanisms.72 Mass spectrometry proteomic analyses further determined that both macrophages and 3D structure modulated the expression of metabolically related proteins in the MPS constructs.73 In an independent study, investigators used magnetic nanomaterials to create 3D adipose constructs using either 3T3-L1 cells or murine fat-derived SVF cells.32 Compared with 2D cultures, the 3D MPS remained viable for extended periods of time and, in the case of the SVF cells, displayed evidence of vascular networks and lymphoid cells.32
Comparable MPS models have been created using human adipose-derived cells as recently reviewed.74 In one format, fragments of intact human adipose tissue are sandwiched between sheets of adherent ASC. These remained viable as 3D constructs for up to 8 weeks in vitro while retaining physiological function based on adipokine secretion and signaling response.75 Alternatively, human SVF cells suspended in a human blood-derived hydrogel exhibited robust adipogenic potential accompanied by the formation of vascular-like networks over a 4-week period.61 Furthermore, these human adipose MPS displayed glucose uptake responses to insulin and lipolytic responses to isoproterenol stimulation.61
Related studies have established cocultures combining isolated ASCs, endothelial cells, and macrophages obtained from a common human donor.76 While not strictly an MPS model, the authors found that the presence of the macrophages increased triglyceride accumulation and promoted a vascularized microenvironment suitable for analyses of paracrine proinflammatory factors.76 Consistent with these findings, independent studies cocultured human adipocytes with the U937 myeloid cell line in a perfusion bioreactor and observed immune cell-dependent insulin resistance.77
Recent studies have extended human adipose MPS constructs to include bioprinted matrices.78 Both human subcutaneous and visceral adipose-derived ASCs seeded into 3D constructs printed using polycaprolactone and gelatin displayed enhanced adipogenic hypertrophy and extended longevity relative to 2D cultures, remaining viable for up to 80 days in vitro. Furthermore, this MPS model could be conditioned to mimic both feeding and fasting with respect to physiological assay outputs, demonstrating its potential utility for drug screening and biomarker identification78
Presumably, an ideal human adipose MPS for breast cancer research would incorporate primary SVF cells and/or ASC isolated from the same donor as the source of the breast cancer cells under investigation. This would remove any potential for artifacts secondary to an immunogenic response resulting from an HLA mismatch between immune cells and cocultured adipocytes, breast cancer, endothelial, and stromal cells. Theoretically, it would be possible to isolate SVF cells from breast tissues resected along with a primary tumor specimen for a personalized medical application; however, a substantial level of future investigation and infrastructure would be required to insure the appropriate recovery and storage of such specimens in a consistent and scalable manner.
ECM: Biological and Synthetic Alternatives to Matrigel
The ECM components employed in the construction of breast cancer MPS is of equal importance as the cellular components. A number of protein hydrogels have been explored. Sponges created from lyophilized type 1 collagen were among the first to be examined in studies showing recapitulation of tumor architecture by MCF-7 breast cancer cell 3D cultures.2 Similar analyses showed that human platelet lysates improved the colony-forming unit activity of >50% of primary breast tumors examined in vitro.79 Nevertheless, Matrigel is employed most frequently as the hydrogel in 3D breast cancer modeling.4
Matrigel was isolated originally from the Engelbreth-Holm-Swarm (EHS) tumor, a chondrosarcoma, as a result of studies at the National Institute of Dental and Craniofacial Research focusing on the protein composition of basement membrane-rich structures.80–82 The investigators noted that the purified ECM proteins, which were enriched in type IV collagen, entactin, laminin, nidogen, and proteoglycans, spontaneously gelled when transferred from 4°C to 37°C.80–82 Breast cancer researchers soon discovered that Matrigel promoted the estrogen-dependent growth of the MCF-7 cell line in vitro and this was increased further by addition of fibroblast cells.83–85 Additionally, when the breast cancer cells were suspended in Matrigel and implanted as a xenograft in immunodeficient mice, the hydrogel enhanced the rate of tumor engraftment and growth rate in vivo.85 Consequently, subsequent studies of human breast cancer cell lines and PDX models routinely rely on the addition of Matrigel to improve engraftment outcomes in mice.4,11–13
The biophysical and mechanical properties of Matrigel can be manipulated during development, isolation, or postprocessing. The mice carrying the EHS tumor can be fed a diet containing the lathyrogen β aminopropionitrile, preventing collagen crosslinking and resulting in a tumor enriched in type IV collagen. Alternatively, the purified Matrigel can be precipitated with 20% NaCl following urea extraction to deplete the levels of growth factors in the final product.80 Additionally, Matrigel can be crosslinked with PEG and cyclodextrin-conjugated adhesion peptides to modify the rigidity of the scaffold.86 Increased stiffness of the Matrigel is correlated with reduced growth of normal and tumor breast cells, consistent with a relationship between mechanical properties and tumor invasion/metastatic properties.86
Currently, Matrigel commands a $600 million annual international market and this is predicted to double in size within 5 years. Nevertheless, because of its origins as a murine tumor-derived product, Matrigel has no future as a clinical product. Additionally, it exhibits batch-to-batch variability with respect to its gelling properties raising questions about its reliability and reproducibility at the manufacturing scale.4,6
Multiple groups have developed decellularized adipose tissue (DAT) as an alternative to Matrigel. The Flynn laboratory at Queens University in Canada was among the first to report on the isolation of DAT using a combination of chemical, enzymatic, and mechanical steps.87 Shortly thereafter, the Christman laboratory at the University of California-San Diego88 and the Elisseeff laboratory at Johns Hopkins University89 reported similar confirmatory findings. Over the next decade, the Flynn laboratory went on to demonstrate that DAT could be prepared in multiple formulations (hydrogel, microcarrier bead, powder) to regenerate soft tissue in vivo.90 In parallel, independent studies from multiple teams have demonstrated that DAT is biocompatible with fibroblasts and ASCs, can serve as a platform for the release of paracrine growth factors in MPS constructs, and supports the in vitro and in vivo adipogenic and osteogenic differentiation of ASCs (reviewed in Mohiuddin et al.91).
Indeed, the DAT hydrogels can be used as a bioink for 3D printing and this approach has been used successfully to generate a fully functional adipose MPS in vitro.92,93 As a result, preliminary clinical studies evaluating allogeneic DAT as a regenerative medical product in patients have confirmed its safety for use in reconstructive surgery.94 Like Matrigel, DAT can be chemically modified to alter its rigidity and mechanical properties. This can be accomplished by crosslinking with PEG thio-acrylate95 or with hexamethylene diisocyanate and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide.89 Additionally, the mechanical properties of DAT have been manipulated by combining it with silk fibroin scaffolds at a fixed ratio.96 In light of the emerging literature linking the biomechanical properties of the stromal ECM to cancer growth and metastasis, the tunability of DAT mechanical properties has added relevance as it is used in the development of breast cancer MPS.97
Mass spectrometry analyses of the DAT proteome have demonstrated that the product is enriched for ECM proteins, including nidogen, type I and type II collagens, while depleted for nuclear and cytoplasmic proteins relative to native adipose tissue.98 Furthermore, the proteome of DAT obtained from lean versus obese human donors, while distinct, does not show a significant difference in functionality based on in vitro assays of ASC biocompatibility and differentiation.99 Altogether, these findings document the feasibility and utility of DAT hydrogels as a component in MPS constructs suitable for screening assays with respect to pathophysiological features. The emerging body of literature relating to human DAT has been the subject of recent reviews.91,100
Further alternatives to Matrigel for breast cancer MPS construction have been generated from both biological and synthetic sources. Multiple groups have employed alginate as a relatively inert scaffold for the study of breast cancer cells as 3D spheroids.31,35,101 Prestwich and colleagues have modified hyaluronan and gelatin by crosslinking to create a synthetic biomaterial hydrogel known as Extracel™, which has proven suitable for the engraftment of breast cancer cell lines (MDA-MB-231, MDA-MB-468) into athymic mice.6,34 In contrast to the injection of cells alone, the Extracel suspended cells showed a higher frequency of engraftment, more uniform tumor size, reduced invasion of adjacent tissues by the tumor, and improved vascularity accompanied by reduced tumor necrosis.34 In similar studies, Ossipov and colleagues crosslinked hyaluronic acid (HA) with PEG, creating a tunable hydrogel biocompatible with the MCF-7 breast cancer cell line in vitro.102 Regardless of the stiffness, the HA hydrogels enhanced cell proliferation and promoted invasion, consistent with an EMT.102 Likewise, an international team used thiol–yne nucleotide addition reactions with PEG and selected peptides to create purely synthetic hydrogels supportive of the MCF-7, MDA-MB-231, and T47D cell lines.103 By incorporating peptides for adhesion sites (amino acid sequence Arginine, Glycine, and Aspartate; RGDs) or matrix metalloproteinase (MMP) cleavage sites at discretely spaced intervals, the investigators were able to manipulate the viscosity of the hydrogel as well as the growth characteristics of the tumor cell-based MPS.103
Using a similar approach, Shoichet and colleagues used photoactivatable crosslinking chemistries to modify HA with MMP cleavable crosslinkers.104,105 As a result, breast cancer lines suspended on the hydrogels displayed invasive (MDA-MB-231) or noninvasive (MCF-7, T47D) behaviors dependent on their expression of MMP.104,105 The authors postulate that the availability of such tunable hydrogels will enhance the potential of 3D MPS constructs for breast cancer drug discovery.104 Netti and colleagues have exploited such chemistry to deliver drugs (doxorubicin) to breast tumor models in vitro.106,107 By linking a polyglycolic-lactic acid (PGLA) PEG nanoparticle to an MMP cleavable synthetic peptide, the release of MMP by cocultured CAF and MCF-7 cells selectively and locally released the drug in a targeted manner.106,107
Finally, Obagel™ has recently been introduced commercially as a human blood product-derived hydrogel capable of supporting ASCs and SVF cell adipogenesis in MPS constructs.61,108 In contrast to Matrigel, the proteome of Obagel is enriched in coagulation-related proteins and robustly supports vascular network-like structures in vitro.61,108 Currently, studies are underway to explore the breast cancer-supportive ability of Obagel, alone or in combination with a DAT hydrogel (termed Adipogel™), in MPS constructs.
Alternative 3D matrix platforms include electrospun fibers, which provide a 3D matrix that can provide for the recapitulation of tissue structure and mimic the tumor/stromal interface. The use of electrospun fibers and tumor models has recently been discussed.109 Emerging areas of interest include the role of aligned matrix fibers at the periphery of the tumor. Synthetic electrospun aligned fibers transform breast cancer cells to a more mesenchymal and elongated cell morphology. Aligned matrix fibers are commonly observed on the periphery of more aggressive tumors with a poor prognosis and allows for the study of cancer progression and cell motility.110
MPS Assay Approaches
Breast cancer MPS models can be quantitatively and qualitatively assessed based on multiple outcome metrics, including:
(a) Morphological features. The 3D constructs can be evaluated microscopically using either fixed or fresh frozen sections with phase-contrast, immunohistochemical, or confocal microscopy. Additionally, the use of live cell image-capturing instruments (such as IncuCyte™) have the potential for serial monitoring of MPS growth in real time over extended periods without destruction. By capturing images of the cells in culture, it is possible to recreate video displays monitoring the individual cell growth and interactive dynamics. These in vitro outcomes would reflect the migration, invasion, and EMT patterns of the primary tumor in vivo.
(b) Secretome features. The culture media conditioned by MPS constructs can be harvested nondestructively and serially at set time intervals and evaluated by enzyme-linked immunosorbent assay (ELISA), Antibody Array, or cytokine antibody-coupled fluorescent bead-based detection platforms (such as Luminex™) to assess the secretion of adipokine, cytokines, and related paracrine factors as a function of culture conditions, scaffold composition, and cell types.
(c) Transcriptomic features. The MPS constructs can be harvested for isolation of total RNA as a function of time and/or MPS composition. The RNA can be evaluated using commercially available, targeted PCR microarrays, transcriptomic microarrays, and unbiased NextGen sequencing. The resulting datasets can be evaluated further using bioinformatic algorithms. The transcriptomic outcomes could be used to define the genomic profile of the parent tumor and its metastases as well as to predict and monitor sensitivity to chemotherapeutic drugs and biological therapeutics.
(d) Proteomic features. The MPS constructs can be harvested for total protein and used in liquid chromatography/mass spectrometry and isobaric tag for relative and absolute quantitation (iTRAQ) to assess the global proteome in an unbiased fashion. These tools can be used in parallel with more targeted, traditional protein assays for enzyme activity or antibody detection of specific proteins (ELISA, western blot). The proteomic outcomes could be used in parallel with transcriptomics to monitor the profile of the tumor and its metastases. Additionally, this information could be used to identify novel biomarkers with prognostic or diagnostic value.
(e) Perfusion Bioreactors. Breast cancer MPS constructs can be adapted to perfusion bioreactors allowing for more robust evaluation of fluid dynamics and tumor pathophysiology. By linking the primary MPS circulation to that of parallel MPS reflective of tissue sites (bone, brain, liver, lung), it will be possible to evaluate metastases as a function of scaffold, cell composition, and drug intervention.
Conclusions and Future Directions
The majority of existing breast cancer MPS continue to rely on murine-derived scaffolds, cells, and/or physiological systems. In the case of PDX models, the reliance on the immunodeficient murine host complicates interpretation of outcomes and restricts the predictive value of the system for personalized medical and drug discovery applications. In response to mandates from international regulatory agencies, there remains a need to expand the available human-sourced components for construction of fully humanized breast cancer MPS with documented and validated predictive value with respect to drug discovery. While humanized MPS models are not immediately positioned to replace existing animal-based assays, they already have the potential to reduce and refine the need for in vivo studies in mice and other species. There has been considerable criticism of drug testing in animal models due to its inadequate recapitulation of the human condition. Indeed, the absence of efficacy and/or toxicity in animal models is no longer sufficient rationale for not advancing an investigational drug to human trials. This is especially relevant with respect to the immunogenicity of drugs identified in animals. Furthermore, the humanized MPS can be evaluated under conditions mimicking therapeutic drug interventions with omics-based read-out assays that can be correlated directly with outcomes in human subjects and clinical trials.
A final advantage of the humanized breast cancer MPS model is that it can be developed using cells and ECM scaffolds sourced from patients reflecting a diverse background with respect to adiposity as monitored by body mass index, ethnicity, age, and tumor histopathology. With increased access and availability of human-derived biomaterials, primary adipose and breast cancer cells, and related microfluidic perfusion devices, the bioengineering community is poised to elevate human breast cancer MPS discovery to a newt tier. In particular, MPS tumor models accurately reflecting the human immune composition and response would represent a paradigm shift for studies involving immunotherapeutics; such advances would extend well beyond current immunodeficient murine models.
Acknowledgments
The authors wish to thank Dr. Pamela Gehron Robey at the National Institute for Dental and Craniofacial Research for sharing her remembrances as a graduate student in the George Martin laboratory regarding the discovery of Matrigel, David Bode at Obatala Sciences for assistance with final article editing and submission.
Author Contributions
Discussion, Conception: T.F., M.H., E.R., X.W., and K.H., and J.M.G.; Outline: T.F. and J.M.G.; First Draft: T.F. and J.M.G.; Editing Drafts: C.W., M.H., T.D., E.R., X.W., K.H., E.C.M., O.M., S.S., R.D., B.R., and D.J.H.; Figure Preparation: S.S.; Finalizing Submission: T.F., M.H., E.R., K.H., and J.M.G.
Disclosure Statement
T.F., X.W., and J.M.G. are cofounders, coowners, and executives at Obatala Sciences Inc., whereas M.H., E.R., and K.H. are employees at the company.
Funding Information
No federal funding was used to support this research.
References
- 1. Guilak, F., Cohen, D.M., Estes, B.T., Gimble, J.M., Liedtke, W., and Chen, C.S.. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russo, J., Soule, H.D., McGrath, C., and Rich, M.A.. Reexpression of the original tumor pattern by a human breast carcinoma cell line (MCF-7) in sponge culture. J Natl Cancer Inst 56, 279, 1976 [DOI] [PubMed] [Google Scholar]
- 3. Soule, H.D., Vazguez, J., Long, A., Albert, S., and Brennan, M.. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 51, 1409, 1973 [DOI] [PubMed] [Google Scholar]
- 4. Schmeichel, K.L., and Bissell, M.J.. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci 116, 2377, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffith, L.G., and Swartz, M.A.. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7, 211, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Prestwich, G.D. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem 101, 1370, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Sutherland, M.L., Fabre, K.M., and Tagle, D.A.. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res Ther 4 Suppl 1, I1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson, D.E., Hunziker, R., and Wikswo, J.P.. Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology. Exp Biol Med (Maywood) 242, 1559, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marx, U., Akabane, T., Andersson, T.B., et al. . Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 37, 365, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lasfargues, E.Y., and Ozzello, L.. Cultivation of human breast carcinomas. J Natl Cancer Inst 21, 1131, 1958 [PubMed] [Google Scholar]
- 11. DeRose, Y.S., Gligorich, K.M., Wang, G., et al. . Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol Chapter 14, Unit14 23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeRose, Y.S., Wang, G., Lin, Y.C., et al. . Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17, 1514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matossian, M.D., Burks, H.E., Bowles, A.C., et al. . A novel patient-derived xenograft model for claudin-low triple-negative breast cancer. Breast Cancer Res Treat 169, 381, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shuhendler, A.J., Prasad, P., Cai, P., et al. . Matrigel alters the pathophysiology of orthotopic human breast adenocarcinoma xenografts with implications for nanomedicine evaluation. Nanomedicine 9, 795, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Reyal, F., Guyader, C., Decraene, C., et al. . Molecular profiling of patient-derived breast cancer xenografts. Breast Cancer Res 14, R11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prestwich, G.D. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res 41, 139, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Prestwich, G.D., Liu, Y., Yu, B., Shu, X.Z., and Scott, A.. 3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul 47, 196, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Belgodere, J.A., King, C.T., Bursavich, J.B., Burow, M.E., Martin, E.C., and Jung, J.P.. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol 6, 66, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cameron, G., Chambers, R.. Neoplasm studies; organization of cells of human tumors in tissue culture. Am J Cancer 30, 115, 1937 [Google Scholar]
- 20. Collection, A.T.C. Breast Cancer Research Book. Manassas, VA: ATCC, 2015. https://www.atcc.org/~/media/PDFs/Culture%20Guides/breast_cancer_resource.ashx
- 21. Brooks, S.C., Locke, E.R., and Soule, H.D.. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem 248, 6251, 1973 [PubMed] [Google Scholar]
- 22. Cailleau, R., Young, R., Olive, M., and Reeves, W.J.Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst 53, 661, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miki, Y., Ono, K., Hata, S., Suzuki, T., Kumamoto, H., and Sasano, H.. The advantages of co-culture over mono cell culture in simulating in vivo environment. J Steroid Biochem Mol Biol 131, 68, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Regier, M.C., Alarid, E.T., and Beebe, D.J.. Progress towards understanding heterotypic interactions in multi-culture models of breast cancer. Integr Biol (Camb) 8, 684, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holliday, D.L., Brouilette, K.T., Markert, A., Gordon, L.A., and Jones, J.L.. Novel multicellular organotypic models of normal and malignant breast: tools for dissecting the role of the microenvironment in breast cancer progression. Breast Cancer Res 11, R3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang, C., Yang, Z., Dong, D.L., et al. . 3D culture technologies of cancer stem cells: promising ex vivo tumor models. J Tissue Eng 11, 2041731420933407, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lv, D., Hu, Z., Lu, L., Lu, H., and Xu, X.. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncol Lett 14, 6999, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zanoni, M., Cortesi, M., Zamagni, A., Arienti, C., Pignatta, S., and Tesei, A.. Modeling neoplastic disease with spheroids and organoids. J Hematol Oncol 13, 97, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaur, P., Ward, B., Saha, B., et al. . Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue. J Histochem Cytochem 59, 1087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nassef, M.Z., Melnik, D., Kopp, S., et al. . Breast cancer cells in microgravity: new aspects for cancer research. Int J Mol Sci 21, 7345, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamatar, A., Gunay, G., and Acar, H.. Natural and synthetic biomaterials for engineering multicellular tumor spheroids. Polymers (Basel) 12, 2506, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daquinag, A.C., Souza, G.R., and Kolonin, M.G.. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods 19, 336, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albini, A., Graf, J., Kitten, G.T., et al. . 17 beta-estradiol regulates and v-Ha-ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci U S A 83, 8182, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu, Y., Shu, X.Z., and Prestwich, G.D.. Tumor engineering: orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan-derived hydrogels. Tissue Eng 13, 1091, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Estrada, M.F., Rebelo, S.P., Davies, E.J., et al. . Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression. Biomaterials 78, 50, 2016 [DOI] [PubMed] [Google Scholar]
- 36. Pradhan, S., Clary, J.M., Seliktar, D., and Lipke, E.A.. A three-dimensional spheroidal cancer model based on PEG-fibrinogen hydrogel microspheres. Biomaterials 115, 141, 2017 [DOI] [PubMed] [Google Scholar]
- 37. Gill, B.J., and West, J.L.. Modeling the tumor extracellular matrix: tissue engineering tools repurposed towards new frontiers in cancer biology. J Biomech 47, 1969, 2014 [DOI] [PubMed] [Google Scholar]
- 38. Datta, P., Dey, M., Ataie, Z., Unutmaz, D., and Ozbolat, I.T.. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis Oncol 4, 18, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nii, T., Kuwahara, T., Makino, K., and Tabata, Y.. A co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng Part A 26, 1272, 2020 [DOI] [PubMed] [Google Scholar]
- 40. Nii, T., Makino, K., and Tabata, Y.. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers (Basel) 12, 2754, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aggarwal, V., Miranda, O., Johnston, P.A., and Sant, S.. Three dimensional engineered models to study hypoxia biology in breast cancer. Cancer Lett 490, 124, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dobrolecki, L.E., Airhart, S.D., Alferez, D.G., et al. . Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev 35, 547, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sikora, M.J., Cooper, K.L., Bahreini, A., et al. . Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res 74, 1463, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matossian, M.D., Burks, H.E., Elliott, S., et al. . Panobinostat suppresses the mesenchymal phenotype in a novel claudin-low triple negative patient-derived breast cancer model. Oncoscience 5, 99, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matossian, M.D., Burks, H.E., Elliott, S., et al. . Drug resistance profiling of a new triple negative breast cancer patient-derived xenograft model. BMC Cancer 19, 205, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zanata, F., Bowles, A., Frazier, T., et al. . Effect of cryopreservation on human adipose tissue and isolated stromal vascular fraction cells: in vitro and in vivo analyses. Plast Reconstr Surg 141, 232e, 2018 [DOI] [PubMed] [Google Scholar]
- 47. Hidalgo, M., Amant, F., Biankin, A.V., et al. . Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4, 998, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Whittle, J.R., Lewis, M.T., Lindeman, G.J., and Visvader, J.E.. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res 17, 17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parsons, J., and Francavilla, C.. ‘Omics approaches to explore the breast cancer landscape. Front Cell Dev Biol 7, 395, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin, H., Lozito, T.P., Alexander, P.G., Gottardi, R., and Tuan, R.S.. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1beta. Mol Pharm 11, 2203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Donnell, B.T., Al-Ghadban, S., Ives, C.J., et al. . Adipose tissue-derived stem cells retain their adipocyte differentiation potential in three-dimensional hydrogels and bioreactors (dagger). Biomolecules 10, 1070, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romero-Lopez, M., Li, Z., Rhee, C., et al. . Macrophage effects on mesenchymal stem cell osteogenesis in a three-dimensional in vitro bone model. Tissue Eng Part A 26, 1099, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Makarczyk, M.J., Gao, Q., He, Y., et al. . Current models for development of disease-modifying osteoarthritis drugs. Tissue Eng Part C Methods 2, 138, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bourin, P., Bunnell, B.A., Casteilla, L., et al. . Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gimble, J.M., Katz, A.J., and Bunnell, B.A.. Adipose-derived stem cells for regenerative medicine. Circ Res 100, 1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gimble, J.M., Bunnell, B.A., Chiu, E.S., and Guilak, F.. Concise review: adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells 29, 749, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Zuk, P.A., Zhu, M., Mizuno, H., et al. . Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Shaik, S., Wu, X., Gimble, J., and Devireddy, R.. Effects of decade long freezing storage on adipose derived stem cells functionality. Sci Rep 8, 8162, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thirumala, S., Gimble, J.M., and Devireddy, R.V.. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med 4, 224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goh, B.C., Thirumala, S., Kilroy, G., Devireddy, R.V., and Gimble, J.M.. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1, 322, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Bender R, M.M., Brown T, Bukowska J, et al. . Human adipose derived cells in two- and three-dimensional cultures: functional validation of an in vitro fat construct stem cells. International 2020, 4242130, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cowper, M., Frazier, T., Wu, X., et al. . Human platelet lysate as a functional substitute for fetal bovine serum in the culture of human adipose derived stromal/stem cells. Cells 8, 724, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patrikoski, M., Lee, M.H.C., Makinen, L., et al. . Effects of macromolecular crowding on human adipose stem cell culture in fetal bovine serum, human serum, and defined xeno-free/serum-free conditions. Stem Cells Int 2017, 6909163, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patrikoski, M., Sivula, J., Huhtala, H., et al. . Different culture conditions modulate the immunological properties of adipose stem cells. Stem Cells Transl Med 3, 1220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kyllonen, L., Haimi, S., Mannerstrom, B., et al. . Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res Ther 4, 17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sandor, G.K., Numminen, J., Wolff, J., et al. . Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl Med 3, 530, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kolle, S.T., Duscher, D., Taudorf, M., et al. . Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial. Stem Cells Transl Med 9, 1277, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kilroy, G.E., Foster, S.J., Wu, X., et al. . Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 212, 702, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Zvonic, S., Lefevre, M., Kilroy, G., et al. . Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics 6, 18, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Martin, E.C., Qureshi, A.T., Dasa, V., Freitas, M.A., Gimble, J.M., and Davis, T.A.. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: perspectives on miRNA biogenesis and cellular transcriptome. Biochimie 124, 98, 2016 [DOI] [PubMed] [Google Scholar]
- 71. McIntosh, K.R., Frazier, T., Rowan, B.G., and Gimble, J.M.. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol 9, 175, 2013 [DOI] [PubMed] [Google Scholar]
- 72. Park, S.B., Lee, S.Y., Jung, W.H., et al. . Development of in vitro three-dimensional co-culture system for metabolic syndrome therapeutic agents. Diabetes Obes Metab 21, 1146, 2019 [DOI] [PubMed] [Google Scholar]
- 73. Lee, S.Y., Park, S.B., Kim, Y.E., et al. . iTRAQ-based quantitative proteomic comparison of 2D and 3D adipocyte cell models co-cultured with macrophages using online 2D-nanoLC-ESI-MS/MS. Sci Rep 9, 16746, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McCarthy, M., Brown, T., Alarcon, A., et al. . Fat-on-a-Chip models for research and discovery in obesity and its metabolic co-morbidities. Tissue Eng Part B Rev 6, 586, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lau, F.H., Vogel, K., Luckett, J.P., et al. . Sandwiched white adipose tissue: a microphysiological system of primary human adipose tissue. Tissue Eng Part C Methods 24, 135, 2018 [DOI] [PubMed] [Google Scholar]
- 76. Huttala, O., Sarkanen, J.R., Mannerstrom, M., Toimela, T., Heinonen, T., and Ylikomi, T.. Development of novel human in vitro vascularized adipose tissue model with functional macrophages. Cytotechnology 72, 665, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu, Y., Kongsuphol, P., Chiam, S.Y., et al. . Adipose-on-a-chip: a dynamic microphysiological in vitro model of the human adipose for immune-metabolic analysis in type II diabetes. Lab Chip 19, 241, 2019 [DOI] [PubMed] [Google Scholar]
- 78. Pope, B.D., Warren, C.R., Dahl, M.O., et al. . Fattening chips: hypertrophy, feeding, and fasting of human white adipocytes in vitro. Lab Chip 22, 4152, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cowan, D.H., and Graham, J.. Stimulation of human tumor colony formation by platelet lysate. J Lab Clin Med 102, 973, 1983 [PubMed] [Google Scholar]
- 80. Kleinman, H.K., and Martin, G.R.. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15, 378, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Kleinman, H.K., McGarvey, M.L., Hassell, J.R., et al. . Basement membrane complexes with biological activity. Biochemistry 25, 312, 1986 [DOI] [PubMed] [Google Scholar]
- 82. Kleinman, H.K., McGarvey, M.L., Liotta, L.A., Robey, P.G., Tryggvason, K., and Martin, G.R.. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188, 1982 [DOI] [PubMed] [Google Scholar]
- 83. Noel, A., Nusgens, B., Lapiere, C.H., and Foidart, J.M.. Interactions between tumoral MCF7 cells and fibroblasts on matrigel and purified laminin. Matrix 13, 267, 1993 [PubMed] [Google Scholar]
- 84. Noel, A., Simon, N., Raus, J., and Foidart, J.M.. Basement membrane components (matrigel) promote the tumorigenicity of human breast adenocarcinoma MCF7 cells and provide an in vivo model to assess the responsiveness of cells to estrogen. Biochem Pharmacol 43, 1263, 1992 [DOI] [PubMed] [Google Scholar]
- 85. Noel, A., De Pauw-Gillet, M.C., Purnell, G., Nusgens, B., Lapiere, C.M., and Foidart, J.M.. Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer 68, 909, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Beck, J.N., Singh, A., Rothenberg, A.R., Elisseeff, J.H., and Ewald, A.J.. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 34, 9486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Flynn, L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 31, 4715, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Young, D.A., Ibrahim, D.O., Hu, D., and Christman, K.L.. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater 7, 1040, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu, I., Nahas, Z., Kimmerling, K.A., Rosson, G.D., and Elisseeff, J.H.. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg 129, 1247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Morissette Martin, P., Shridhar, A., Yu, C., Brown, C., and Flynn, L.E.. Decellularized adipose tissue scaffolds for soft tissue regeneration and adipose-derived stem/stromal cell delivery. Methods Mol Biol 1773, 53, 2018 [DOI] [PubMed] [Google Scholar]
- 91. Mohiuddin, O.A., Campbell, B., Poche, J.N., et al. . Decellularized adipose tissue: biochemical composition, in vivo analysis and potential clinical applications. Adv Exp Med Biol 1212, 57, 2020 [DOI] [PubMed] [Google Scholar]
- 92. Pati, F., and Cho, D.W.. Bioprinting of 3D tissue models using decellularized extracellular matrix bioink. Methods Mol Biol 1612, 381, 2017 [DOI] [PubMed] [Google Scholar]
- 93. Pati, F., Jang, J., Ha, D.H., et al. . Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5, 3935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kokai, L.E., Sivak, W.N., Schilling, B.K., et al. . Clinical evaluation of an off-the-shelf allogeneic adipose matrix for soft tissue reconstruction. Plast Reconstr Surg Glob Open 8, e2574, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li, S., Poche, J.N., Liu, Y., et al. . Hybrid synthetic-biological hydrogel system for adipose tissue regeneration. Macromol Biosci 18, e1800122, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kayabolen, A., Keskin, D., Aykan, A., Karslioglu, Y., Zor, F., and Tezcaner, A.. Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization. Biomed Mater 12, 035007, 2017 [DOI] [PubMed] [Google Scholar]
- 97. Lu, P., Weaver, V.M., and Werb, Z.. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196, 395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thomas-Porch, C., Li, J., Zanata, F., et al. . Comparative proteomic analyses of human adipose extracellular matrices decellularized using alternative procedures. J Biomed Mater Res A 106, 2481, 2018. 29693792 [Google Scholar]
- 99. Mohiuddin, O.A., Motherwell, J.M., Rogers, E., et al. . Characterization and proteomic analysis of decellularized adipose tissue hydrogels derived from lean and overweight/obese human donors. Adv Biosyst 4, e2000124, 2020 [DOI] [PubMed] [Google Scholar]
- 100. Sharath, S.S., Ramu, J., Nair, S.V., Iyer, S., Mony, U., and Rangasamy, J.. Human adipose tissue derivatives as a potent native biomaterial for tissue regenerative therapies. Tissue Eng Regen Med 17, 123, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lan, S.F., and Starly, B.. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol Appl Pharmacol 256, 62, 2011 [DOI] [PubMed] [Google Scholar]
- 102. Ranga, A., Lutolf, M.P., Hilborn, J., and Ossipov, D.A.. Hyaluronic acid hydrogels formed in situ by transglutaminase-catalyzed reaction. Biomacromolecules 17, 1553, 2016 [DOI] [PubMed] [Google Scholar]
- 103. Macdougall, L.J., Wiley, K.L., Kloxin, A.M., and Dove, A.P.. Design of synthetic extracellular matrices for probing breast cancer cell growth using robust cyctocompatible nucleophilic thiol-yne addition chemistry. Biomaterials 178, 435, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tam, R.Y., Smith, L.J., and Shoichet, M.S.. Engineering cellular microenvironments with photo- and enzymatically responsive hydrogels: toward biomimetic 3D cell culture models. Acc Chem Res 50, 703, 2017 [DOI] [PubMed] [Google Scholar]
- 105. Baker, A.E.G., Tam, R.Y., and Shoichet, M.S.. Independently tuning the biochemical and mechanical properties of 3D hyaluronan-based hydrogels with oxime and diels-alder chemistry to culture breast cancer spheroids. Biomacromolecules 18, 4373, 2017 [DOI] [PubMed] [Google Scholar]
- 106. Brancato, V., Garziano, A., Gioiella, F., et al. . 3D is not enough: building up a cell instructive microenvironment for tumoral stroma microtissues. Acta Biomater 47, 1, 2017 [DOI] [PubMed] [Google Scholar]
- 107. Brancato, V., Gioiella, F., Imparato, G., Guarnieri, D., Urciuolo, F., and Netti, P.A.. 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro. Acta Biomater 75, 200, 2018 [DOI] [PubMed] [Google Scholar]
- 108. Frazier, T., Alarcon, A., Wu, X., et al. . Clinical translational potential in skin wound regeneration for adipose-derived, blood-derived, and cellulose materials: cells, exosomes, and hydrogels. Biomolecules 10, 1373, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chen, S., Boda, S.K., Batra, S.K., Li, X., and Xie, J.. Emerging roles of electrospun nanofibers in cancer research. Adv Healthc Mater 7, e1701024, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Conklin, M.W., Eickhoff, J.C., Riching, K.M., et al. . Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178, 1221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]