Key summary points
Aim
Confusion was more prevalent in frail than in non-frail older patients at hospital admission.
Finding
COVID-19 and accelerated functional decline were associated among frail older hospitalised patients when compared to non-frail.
Message
Ninety-day all-cause mortality was 70% among frail hospitalised patients with COVID-19 and 15% among non-frail.
Keywords: COVID-19, Frailty, Aged, Cross-sectional study, Geriatrics
Abstract
Purpose
Older people are the most frequently hospital admitted patients with COVID-19. We aimed to describe the clinical presentation of COVID-19 among frail and nonfrail older hospitalised patients and to evaluate the potential association between frailty and clinical course, decision of treatment level with outcomes change in functional capacity and survival.
Methods
We performed a multi-center, retrospective cross-sectional cohort study examining data on clinical presentation and frailty-related domains for hospitalised people aged 75 + years with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test. Frailty was assessed at admission using record-based MPI (rMPI) and Clinical Frailty Scale (CFS). Decision on treatment level about invasive ventilation and cardiopulmonary resuscitation (CPR), change in CFS-score from admission to discharge, changed need of home care, and in-hospital, 30-day and 90-day mortality were registered.
Results
100 patients (median age 82 years (IQR 78–86), 56% female) with COVID-19 were included. 54 patients were assessed moderately or severely frail (rMPI-score = 2 or 3) and compared to non-frail (rMPI-score = 1). At admission, frail patients presented more frequently with confusion. At discharge, functional decline measured by change in CFS and increased home care was more prevalent among frail than the non-frail. Decisions about no invasive ventilation or CPR were more prevalent among frail older patients with COVID-19 than non-frail. Ninety-day mortality was 70% among frail patients versus 15% in non-frail.
Conclusion
Frailty seems to be associated with confusion, more frequent decisions about treatment level, larger functional decline at discharge and a higher mortality rate among older patients with COVID-19.
Background
Since the outbreak of COVID-19 caused by the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), older people have been the most frequently admitted patients with COVID-19 to hospitals worldwide [1]. High age and frailty are associated with mortality in older COVID-19 inpatients [1–6]. Patients who died from COVID-19 had a higher prevalence of coronary heart disease, hypertension, diabetes, malnutrition, multi-medication and functional disabilities [5]. Illness severity is an important marker of risk within the COVID-19 cohort and may be one of the most important prognostic factors [6]. In-hospital mortality rates are found between 27 and 31% in adults with COVID-19 [1, 4, 7]. Among patients aged 65 or older, the in-hospital mortality rate was 32% [6], and in frail older patients, up to 65% died within 30 days [8].
Frailty is defined as reduced physiological reserve and can be used to evaluate older patients in terms of risk of adverse outcomes [9, 10]. Findings of Hewitt et al. show the importance of frailty assessment, rather than age, in combination with other measures in the context of COVID-19 (1). There is no consensus on how to measure frailty. Until now, the validated tool Clinical Frailty Scale (CFS) has been preferred in studies identifying frailty in older patients with COVID-19. It is widely used across specialties and has short time-consumption [1–3, 5, 6, 8, 11, 12]. The CFS was in its origin developed to predict mortality. There is a building evidence worldwide that age, co-morbidity and frailty measured by CFS impact the course of the disease negatively in older patients who have been infected with COVID-19. In the COPE study, 49% of a cohort of European hospitalized COVID-19 positive adults were defined as frail corresponding to CFS score 5 or more [1]. In the older patients aged 65 years or older, 76–80% were assessed frail at hospital admission [6, 10]. In geriatric wards, the Multidimensional Prognostic Index (MPI) based on Comprehensive Geriatric Assessment (CGA) [13] is a validated and acknowledged tool [14, 15]. Since the MPI tool consists of components and several assessment tools, including illness severity, it makes identification of frailty rather time-consuming. However, due to the thorough assessment, the tool is valuable when making decisions about treatment level [15]. During the pandemic, lack of intensive care resources has been debated, highlighting the importance of front-line staff to address this important topic at admission in dialog with patients and relatives.
Frequent clinical manifestations in COVID-19 patients include cough, dyspnea, fever, sore throat, fatigue, myalgia and headache [16]. In addition, some patients may present with diarrhea, vomiting, taste and smell disturbances, and in few cases pleuritic chest pain [6, 16, 17]. Also, there are characteristic changes on chest X-ray, CT scan and in blood test. However, it is well known that older people often have atypical clinical presentation of infectious diseases [18]. So far, the clinical role of frailty among COVID-19-positive older patients is not fully clarified. Therefore, we aimed to describe the clinical presentations of COVID-19 in older inpatients with regard to frailty measured by the record-based MPI (rMPI) tool [19] and to explore possible associations between frailty level and decision of treatment level, change in physical functional abilities and survival. Subsequently, the predictive values of rMPI and CFS were compared.
Methods
Study population and setting
We undertook a multicenter, retrospective cross-sectional cohort study nested in the Central Denmark Region during the time period between March 1 and May 31, 2020 which equals to the first wave of the pandemic in Denmark. The region consists of 1,3 million inhabitants and approximately 8.5% of the population are 75 years or older [20]. In Denmark, the healthcare system is tax-funded and all acute admissions take place in public hospitals [21]. Due to a unique and universal identification number (Civil Registration Number, CRN) assigned to all inhabitants in Denmark at birth or immigration, and a region-wide use of electronic health records (EHR), it was possible to follow individual patients across hospital admissions and summarize data about home care needs collected in the municipalities of the region.
We included all patients 75 years and older with a positive SARS-CoV-2 diagnostic test and an admission to one of the seven hospitals treating acute medical conditions in the Central Denmark Region during the study period. We included the positive SARS-CoV-2 diagnostic test results from all test modalities used during the study period verified by oropharyngeal swaps and lower respiratory tract aspirates, knowing that recommendations and test kits changed rapidly during the study period. During the first wave in Denmark, all nursing home residents with positive COVID-19 test were admitted to hospital. The Danish health care system never reached a point of where patients were denied admission due to age or frailty, rather the strategy was to admit everyone to isolate in-hospital rather than in, e.g., nursing homes.
Data source
Relevant CRNs were retrieved from the Central Denmark Region data warehouse that covers all hospitals in the region [22]. We were able to access the EHR and evaluate information about clinical presentation and measurements in the hospital. Also, from the EHR, we retrieved multidisciplinary assessments and information on preadmission cognitive and functional status and care needs generated in the local municipalities and automatically sent to the hospital at admission.
Data were stored in an approved REDCap database at Aarhus University [23].
Assessment
We used the record-based MPI (rMPI) that includes eight domains with weighted scores to assess and divide the patient’s frailty level at admission into low as the non-frail patient group, and moderate and severe as the frail group (“Appendix 1”) [19, 24]. Furthermore, we used the CFS both at admission and at discharge to describe any functional decline in relation to the hospitalization (“Appendix 2”).
The rMPI includes assessment of activities of daily living (ADL), mobility and instrumental ADL (IADL) by Functional Recovery Score, cognitive status by Short Portable Mental Status Questionnaire (SPMSQ), co-morbidity by Cumulated Illness Rating Scale—Geriatrics (CIRS-G), Mini-Nutritional Assessment – short form (MNA-SF), pressure sore risk by Exton Smith Scale (ESS), co-habitation status, and number of prescribed medications. We examined these frailty-related domains and disease characteristics for all included patients by review of the patients' EHR. When rMPI score was equal to one, patients were assessed to be non-frail and when equal to two or three, assessed to be frail.
CFS is a widely used global clinical measure of fitness and frailty that uses pictographs and clinical descriptions to help clinicians stratify older patients on a nine-point scale [25]. CFS scores 1–4 are regarded not frail, whereas scores 5–9 are increasingly frail. CFS was also assessed after thorough review of the EHRs.
Baseline covariates were collected from the EHR in the month leading up to admission and included in the rMPI and CFS, Also, CFS was estimated from descriptions in the EHR at discharge. Further, we identified registered symptoms noted in the EHR at admission, relevant vital measurements and blood tests, and information about decisions on treatment level and life support according to invasive ventilation and cardiopulmonary resuscitation (CPR). The EHR reviews were performed by three specialists in geriatric medicine, who have previously worked with the MPI in a clinical setting. Initially, four EHRs were reviewed by all three specialists to ensure inter-rater reliability. Inconsistencies were identified and debated before further individual assessments were performed.
Statistical methods
The study presents both descriptive data and associations. Descriptive baseline characteristics for categorical variables were calculated as percentages. Continuous variables were calculated using mean values with standard deviations for normally distributed data, and medians with interquartile range for non-normally distributed data.
Between frail and non-frail patients, continuous variables were compared and analyzed using Student’s t test for normally distributed variables and the Wilcoxon rank-sum test for non-normally distributed variables. Categorical variables were analyzed using the Pearson’s Chi squared test or Fisher’s exact test. Also, a binary linear regression model was used to compare the frail and non-frail patients’ clinical presentations. The models were adjusted for age and gender. The association between mortality of frailty was analyzed using a multivariable regression model adjusted for the clinical presentations at admission that were related to 30-day mortality (p value < 0.08). The regression models were checked by a receiver operating characteristic (ROC) curve.
To compare the discriminative abilities of CFS and rMPI measured at admission, the area under the ROC curve was used to assess the predictive value of 90-day mortality. The ROC curve plots the true-positive rate (sensitivity) against the false-positive rate (1 − specificity) at any given cut-off value. An area under the curve (AUC) of 0.5 indicates no discrimination above chance, whereas an AUC of 1.0 indicates perfect discrimination.
A p value < 0.05 was considered statistically significant. All statistical analyses were performed in STATA version 16 [26].
Results
The study population included 113 unique individuals with a positive SARS-CoV-2 test and hospital admission in the study period. Thirteen patients were excluded due to lack of relation between positive SARS-CoV-2 test and hospital admission.
At admission
In Table 1, baseline characteristics including clinical presentations are presented by frailty status. By rMPI rating, 54% of the patients were identified as frail. The frail patients were five years older than the non-frail (p = 0.001). Frail patients had similar symptoms and vital parameters at hospital arrival compared to the non-frail patients with exception of confusion, that was presented in 30% of the frail and only 13% of the non-frail patients (p = 0.05). Of all the patients with confusion, 63% were known with cognitive impairment. Mean hemoglobin level was 0.5 mmol/l lower in the frail patients compared to the non-frail. No other blood test values differed between groups.
Table 1.
Age, sex, social status, vital parameters, blood measurements, symptoms and examinations regarding to frailty status measured by record-based Multidimensional Prognostic Index (rMPI) in patients with COVID-19 and aged 75 + years
| Characteristics | All patients N = 100 | Non-frail patients (rMPI 1) n = 46 | Frail patients (rMPI 2 + 3) n = 54 | Relative Risk or coefficient* (95% CI) | p value |
|---|---|---|---|---|---|
| Median age, y (IQR) | 82 (78–86) | 79.5 (77–84) | 84.5 (79–89) | 3.57 (1.50; 5.64) | 0.001 |
| Female sex (%) | 56 | 30 (65) | 26 (48) | 1.49 (0.93; 2.39) | 0.10 |
| Social status (%) | |||||
| Living with spouse | 45 | 30 (65) | 15 (28) | 0.51 (0.32; 0.81) | 0.005 |
| Institution | 18 | 0 | 18 (33) | – | NA |
| Living alone | 37 | 16 (35) | 21 (39) | 0.97 (0.56; 1.66) | 0.91 |
| Co-morbidity (%) | |||||
| Low | 10 | 10 (22) | 0 | ||
| Moderate | 43 | 31 (67) | 12 (22) | ||
| Severe | 47 | 5 (11) | 42 (78) | 7.26 (3.12–16.9) | < 0.001 |
| COVID-19 as suspected referral diagnose (%) | 73 | 34 (77) | 39 (74) | 0.95 (0.76; 1.20) | 0.67 |
| Symptoms (%) | |||||
| Fever | 65 | 19 (54) | 16 (46) | 0.64 (0.36; 1,15) | 0.14 |
| Cough | 57 | 28 (61) | 29 (54) | 1.01 (0.75; 1.38) | 0.93 |
| Dyspnea | 50 | 21 (46) | 29 (54) | 1.24 (0.82; 1.88) | 0.31 |
| Chest pain | 9 | 3 (7) | 6 (11) | 1.86 (0.47; 7.31) | 0.37 |
| Myalgia | 18 | 12 (26) | 6 (11) | 0.60 (0.26; 1.41) | 0.24 |
| Abdominal pain | 38 | 14 (30) | 24 (44) | 1.27 (0.72; 2.23) | 0.4 |
| Headache | 9 | 6 (13) | 3 (6) | 0.53 (0.13; 2.05) | 0.35 |
| Weakness | 61 | 28 (61) | 33 (61) | 0.98 (0.70; 1.36) | 0.89 |
| Changed sense of smell/taste | 0 | 0 | 0 | – | NA |
| Confusion | 22 | 6 (13) | 16 (30) | 2.34 (1.00; 5.51) | 0.05 |
| Fall | 15 | 7 (15) | 8 (15) | 0.99 (0.59; 1.65) | 0.96 |
| Fatigue | 28 | 15 (33) | 13 (24) | 0.82 (0.52; 1.27) | 0.37 |
| Other | 30 | 14 (30) | 16 (30) | 0.98 (0.66–1.46) | 0.93 |
| Vital parameters, mean (SD) | |||||
| Respiratory frequency | 23.2 (6.4) | 22.0 (5.9) | 24.2 (6.7) | 1.28 (− 1.35; 3.90) | 0.34 |
| Body temperature | 38.1 (1.0) | 37.9 (1.0) | 38.1 (1.0) | 0.22 (− 0.19; 0.64) | 0.29 |
| Systolic blood pressure | 135 (23) | 139 (23) | 132 (23) | − 9.06 (− 18.7; 0.62) | 0.07 |
| Diastolic blood pressure | 73 (18) | 79 (21) | 69 (14) | − 9.75 (− 17.2; − 2.32) | 0.01 |
| Pulse | 87 (21) | 86 (22) | 88 (21) | 0.81 (− 8.14; 9.76) | 0.86 |
| Saturation without oxygen, n = 69 | 94 (4.1) | 94 (3.4) | 93 (4.8) | − 1.32 (− 3.54; 0.91) | 0.24 |
| Saturation with oxygen, n = 41 | 95 (3.4) | 95 (2.2) | 95 (3.9) | − 0.18 (− 2.78; 2.42) | 0.89 |
| Blood measurements, mean (SD) | |||||
| C-reactive protein [mg/l] | 79 (77.1) | 67 (61) | 90 (88) | 29 (− 4.07; 61.5) | 0.09 |
| Hemoglobin [mmol/l] | 7.7 (1.3) | 8.2 (1.2) | 7.4 (1.2) | − 0.69 (− 1.21; − 0.18) | 0.001 |
| Creatinine [µmol/l] | 105 (51) | 93 (36) | 114 (60) | 17.2 (− 4.32; 38.8) | 0.12 |
| LDH [U/l] n =89 | 301 (139) | 293 (102) | 308 (166) | 14.8 (− 49.0; 78.6) | 0.65 |
| Alkaline phosphatases [U/l] | 96 (65) | 87 (50.8) | 103 (74) | 14.3 (− 13.8; 42.3) | 0.32 |
| Albumin [g/l] n = 88 | 31 (4.4) | 32 (4.0) | 31 (4.7) | − 0.96 (− 2.94; 1.01) | 0.34 |
| Examinations (%) | |||||
| Chest X-ray | 86 | 38 (84) | 48 (89) | 1.10 (0.94; 1.28) | 0.22 |
| Chest CT-scan | 28 | 16 (36) | 12 (22) | 0.58 (0.29; 1.16) | 0.12 |
| Infiltrate verified by X-ray or CT-scan | 78 | 41 (89) | 37 (69) | 0.82 (0.66; 1.00) | 0.05 |
| Unilateral | 21 | 13 (28) | 8 (14) | 0.67 (0.31; 1.46) | 0.32 |
| Bilateral | 57 | 28 (61) | 29 (54) | 0.84 (0.58; 1.21) | 0.35 |
| SARS-CoV-2 verified by swab | 87 | 40 (87) | 47 (87) | 0.99 (0.86; 1.16) | 0.98 |
| SARS-CoV-2 verified by tracheal suction | 28 | 13 (28) | 15 (28) | 1.15 (0.61; 2.18) | 0.67 |
| CFS up to admission, median (IQR) | 5 (3–6) | 2.5 (2–3) | 6 (5–7) | – | < 0.001 |
*Relative risk ratio is used in comparison of dichotomous variables and coefficient is used in continuous and normal distributed variables
Adjustment of age and sex are applied in both analysis models. Not normal distributed variables are analyzed with Wilcoxon Rank-sum Test and presented with p values only
IQR Interquartile range, SD Standard Deviation, NA Not analysed, CFS Clinical Frailty Score
Outcomes
Table 2 shows that registered decisions about treatment level differed between the frail and the non-frail patients. Among frail patients, health care professionals had registered a decision about treatment level in 96% compared to 56% among the non-frail patients. Among the frail patients, 87% had a decision of “no critical care” such as invasive ventilation and 91% had a decision of “no attempt to resuscitation”, whereas among the non-frail it was decided in 17% and 22%, respectively. Median length of hospital stay was 8 days with no difference between groups.
Table 2.
Outcomes in 75 + -year-old patients with COVID-19 according to frailty status measured by record-based Multidimensional Prognostic Index (rMPI)
| Characteristics | All patients N = 100 | Non-frail patients (rMPI 1) n = 46 | Frail patients (rMPI 2 + 3) n = 54 | Relative risk or coefficient* (95% CI) | p value |
|---|---|---|---|---|---|
| Decision of treatment level | 77 | 25 (56) | 52 (96) | 1.82 (1.40; 2.36) | < 0.001 |
| Opting out of invasive ventilation | 55 | 8 (17) | 47 (87) | 5.09 (2.70; 9.62) | < 0.001 |
| Opting out of CPR | 58 | 10 (22) | 48 (91) | 4.22 (2.43; 7.33) | < 0.001 |
| Intensive care | |||||
| Intensive care unit | 14 | 8 (18) | 6 (11) | 0.63 (0.23; 1.72) | 0.37 |
| Non-invasive ventilation | 0 | 0 | 0 | – | NA |
| Invasive ventilation | 12 | 8 (17) | 4 (7) | 0.48 (0.15; 1.55) | 0.22 |
| Length of hospital stay, median days (IQR) | 8 (3–16) | 9 (2–17) | 7 (4–15) | – | 0.66 |
| Change* in CFS score, median (IQR) | 2 (1–4) | 1.5 (0–3) | 3 (1–4) | – | 0.02 |
| Need of increased home care after discharge (%) | |||||
| Domestic help | 35 | 19 (41) | 16 (30) | 0.70 (0.42; 1.21) | 0.21 |
| Personal help | 31 | 15 (33) | 16 (30) | 0.90 (0.50; 1.64) | 0.74 |
| Hospital readmission within 30 days (%) | 14 | 10 (25) | 4 (18) | 0.78 (0.25; 2.45) | 0.67 |
| Mortality (%) | |||||
| In-hospital | 37 | 6 (13) | 31 (57) | 4.68 (2.16; 10.1) | < 0.001 |
| 30-day | 38 | 3 (7) | 35 (65) | 10.3 (3.41; 31.2) | < 0.001 |
| 90-day | 45 | 7 (15) | 38 (70) | 4.85 (2.41; 9.76) | < 0.001 |
*Relative risk ratio is used in comparison of dichotomous variables and coefficient is used in continuous and normal distributed variables
Adjustment of age and gender are applied in both analysis models. Not normal distributed variables are analyzed with Wilcoxon Rank-sum Test
and presented with p values only
IQR Interquartile range, SD Standard Deviation, NA Not analysed, CFS Clinical Frailty Score, CPR Cardiopulmonary Resuscitation
The difference between the CFS score at admission and at discharge showed that frail patients had a more pronounced decline in functional capacity than non-frail patients. The loss of functional capacity was followed by an increased need for domestic and personal help after discharge.
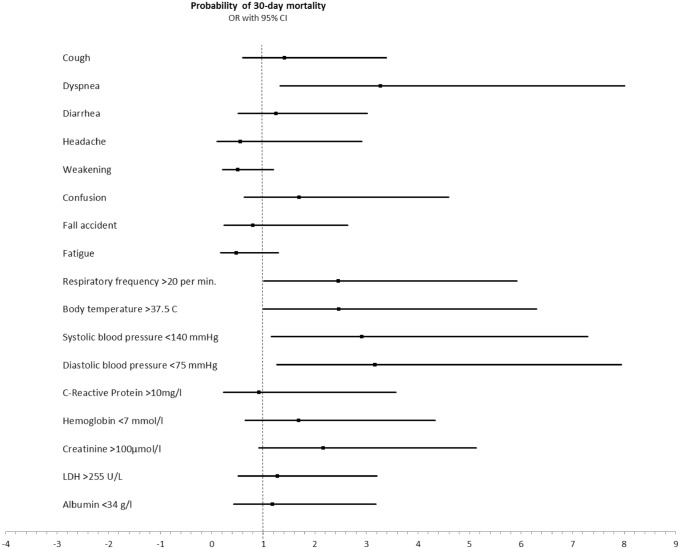
In-hospital mortality was significantly higher in the frail patients (57% vs. 13%), p < 0.001. All-cause 30-day mortality rate was 65% in the frail group versus 7% in the non-frail groups, and 90-day mortality was 70% among the frail patients and 15% in the non-frail group (p < 0.001). At admission, data showed that temperature above 37.5 °C, tachypnea (> 20 breaths per minute) and systolic blood pressure below 140 mmHg were associated with 30-day mortality; and creatinine more than 100 µmol/l was non-significantly associated with 30-day mortality (Fig. 1). The relative risk of 90-day mortality in the frail patients was 4.85 (95% CI: 2.41–9.76), p < 0.001). None of the mortality-related clinical presentations added higher or lower risk of death to the model. Adjustment for confusion did not alter the risk of 90-day mortality (RR = 0.97 (95% CI: 0.67–1.42), p = 0.89). No difference was found in unplanned 30-day readmission rate according to frailty.
Fig. 1.
Prediction of 30-day mortality according to clinical presentations at admission
rMPI versus CFS
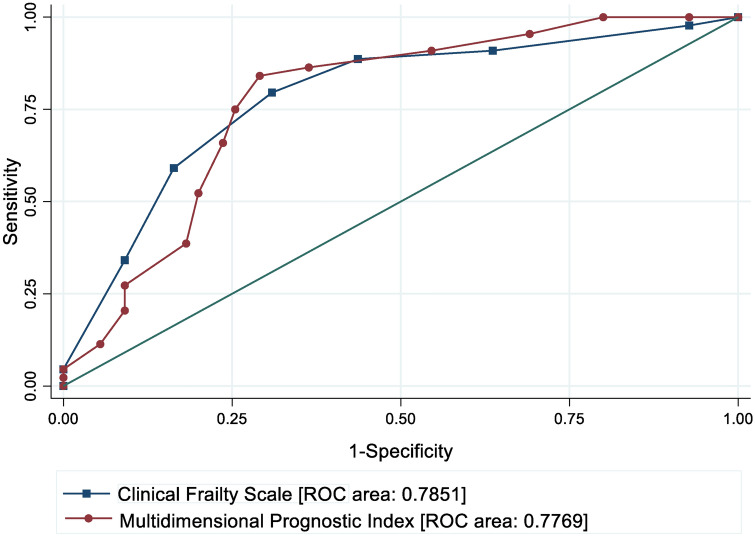
Figure 2 illustrates equality of the predictive values of rMPI and CFS measured at admission. Both of the frailty tools were capable of predicting 90-day mortality.
Fig. 2.
Receiver Operating Characteristic (ROC) curves illustrate the prediction of 90-day mortality using classification thresholds of Clinical Frailty Scale or Multidimensional Prognostic Index. Area under the ROC curves illustrates accuracy of the predictions
Discussion
Clinical presentation
The most frequent clinical presentations of COVID-19 in the 75 + -year-old patients were fever, weakness, coughs and dyspnea. Frailty among these patients seems to have a minor impact on the clinical presentation at admission. However, confusion was more prevalent in the frail compared to the non-frail, and short-term mortality was significantly more present in the frail patients.
In an American study, 28% of older patients admitted to an emergency department (ED) with COVID-19 had delirium at presentation [27]. Marengoni et al. found that 27.5% of patients admitted with COVID-19 to an acute geriatric ward in Italy had delirium at hospital admittance [28] which corresponds to the incidence in a Danish study of 80 + -year-old patients with COVID-19 [29]. However, Vrillon et al. found an even higher incidence of confusion/delirium by 71% in patients with COVID-19 aged 85 years or more [30]. Also, the Italian group found that patients with delirium compared to patients with no delirium were four times more likely to die during their hospital stay than those without. The seriousness of delirium during admission for COVID-19 has been investigated in a Brazilian study, where delirium was independently associated with in-hospital death, increased length of stay, and admission to intensive care in older adults [31]. Patients with both frailty and confusion often have a history of known cognitive impairment [32] and we found such an association.
Similar to our findings, Karlsson et al. found that most patients displayed classical symptoms of COVID-19: fever, cough, respiratory distress, and fatigue [29]. Lui et al. found fever to be less frequent (78%) among older patients aged 60 years or more compared to younger patients [33], which is consistent with our study where only 65% had fever at admission. We found a tendency towards a lower incidence of fever in the frail patients compared to the non-frail.
Since falls are an important predictor of negative health outcome among older patients one could have expected these findings in our study. However, 15% of the patients had a fall leading up to admission with COVID-19. Falls in this acute setting are most likely an acute symptom of severe disease rather than an expression of chronic falling among frail individuals. In the Danish study by Karlsson et al., fall was registered in 8% of the 80 + -year-old patients [29]. Vrillon et al. found a higher fall incidence of 25% prior to COVID-19-related hospitalisations of 85 + years [30]. Looking into larger study populations in the future may provide more details about presence and importance of fall in older COVID-19 patients.
The significance of frailty
Our data indicate that front-line staff do pay more attention to decision on treatment level among frail older patients. Ideally, we believe that all patients should be involved in the decision of treatment level and it should be noted in the patient’s health record when admitted with an acute condition. Continuous attention should be paid to this topic both in pandemics and beyond.
Not surprisingly, frail older patients with COVID-19 were found to have a more pronounced decline in functional level and a higher need of home care than the non-frail patients at discharge. More detailed knowledge about declining functional level and potential benefits of early in-hospital rehabilitation efforts is needed, as it has important socio-economic perspectives due to increased expenses. Concordant with our findings, a large multi-centre study found that frailty in COVID-19 patients has been associated with increased care needs at discharge in a large cohort study [37].
In a recent prospective study by Pilotto et al. using bedside MPI, they also found that older patients with COVID-19 in MPI level 3 had a significant higher mortality rate than the patients in MPI level 1 [38]. Besides being a strong predictor of mortality in a Brazil cohort during the first COVID-19 wave, frailty assessment was found to provide extra prognostic information by capturing risk factors apart from known risk factors associated with age, co-morbidity and acute disease [39]. As in the study of Karlsson et al. [29] we found no association between confusion and death.
In the blood tests measured at admission, we only found one significant difference, where frail patients presented a lower hemoglobin than the non-frail. Few patients presented bleedings so our finding might be due to a more severe load of co-morbidity in the frail group which can lead to chronical anemia [34]. By analyzing the vital parameters at admission, we can only assume that a systolic blood pressure less than 140 mmHg and a diastolic blood pressure less than 75 mmHg were associated with 30-day mortality due to our small sample size. Nevertheless, emerging studies have demonstrated a high prevalence of hypertension among patients with COVID-19 [35]. At present, there is no clear epidemiological evidence supporting that hypertension itself is an independent risk factor for developing severe disease in patients with COVID-19 [36]. Therefore, these results must be examined in larger studies on frail older patients with COVID-19.
In our data, rMPI and CFS showed a fair and equal efficiency to predict 90-day mortality. Our rMPI accuracy in predicting 90-day mortality was as good as the bedside MPI in predicting in-hospital mortality in Pilotto's study [38]. Different settings may impact choice of frailty assessment tool as CFS takes a short time to complete, whereas bedside MPI is more time-consuming. Therefore, CFS might be more appropriately used in the ED screening patients for frailty [10], whereas completing a bedside MPI is valuable as a systematic application of CGA. MPI can be recommended to detangle complex physical and mental health needs among elderly and for early discharge planning in the geriatric wards.
Strengths and limitations
To our knowledge, this is the first study to evaluate change in functional status during hospitalization based on detailed data from EHRs and home care data from municipalities. We realize that since data were collected in the beginning of the pandemic with limited test resources, some frail older inpatients were not diagnosed with COVID-19 and therefore not included in the database. Due to an established public data warehouse, all admitted patients with known COVID-19 disease are enrolled in the cohort. During the study period, efforts were made to bring patients with COVID-19 from nursing home (NH) to hospitals to avoid contamination of cohabitants. The same preventive actions were applied among patients receiving home care. The hospitals in Central Denmark Region and in Denmark as a total did not reach a critical number of patients during the study period and it is not likely that any patients were kept out of hospital due to lack of capacity.
Our study has some important limitations that needs to be considered when interpreting the data. The record-based reviews were only performed by one reviewer per patient. However, in a recent study by Hansen et al., rMPI was found to have an acceptable inter-rater reliability and high agreement when compared to bedside-rated MPI [19]. In our study, none of the included hospitals have used standardized screening tools for delirium of whether the confusion was an expression of delirium. We based our assessment on EHR descriptions from receiving nurses and physicians notes and often the description was in agreement with clinical presentation of delirium. Also, despite lack of validation of the tool, we used changes in pre- and post-CFS scores and increased home care as a proxy intending to evaluate the potential functional decline during the hospital stay.
We did not account for differences in treatment across hospitals and departments, and also did not account for competing risk of death from other acute or chronical diseases with high mortality that might had led to admission in the first place. This might have introduced bias in our results although we have adjusted for gender and age.
Due to restriction on test kits in the beginning of the pandemic, only patients with relevant travel activity to pandemic areas and later only patients with classical symptoms like coughing and dyspnea were tested. Also, one can speculate if people living in a nursing home facility may more often have expressed a will about admission avoidance or restrictions to end-of-life care and therefore has not been admitted, but rather have received palliative care at home. This might have led to underdiagnoses of COVID-19 and therefore potentially limited entrance to this study.
Despite the confined number of patients included might restrict the conclusions that can be made based on our results, we believe that the focus on multidimensional frailty assessment is an important piece in the puzzle of COVID-19.
Implications
The included patients presented with a wide range of symptoms when admitted to the hospital. We only found minor differences in the clinical presentation of the disease when comparing frail older patients with non-frail, which is important knowledge when working with geriatric patients in the ED.
Our results suggest that COVID-19 in frail patients accelerate functional decline during hospitalization. This calls for further evaluation of impact of pre- and post-discharge rehabilitation. A large excess mortality among frail elderly with COVID-19 calls for both disease preventive actions like vaccinations and application of known hygienic precautions, and continuous improvement of treatment and care of frail older patients with COVID-19 both in-hospital and at home. A systematic use of frailty measurements will shed light on COVID-19 interaction with frailty domains and potentially improve patient-centered approaches.
Bedsides, MPI and standardized assessment of delirium among patients with COVID-19 infection are wanted to further investigate possible impacts of frailty on COVID-19 infection. Detailed knowledge about frailty status and frailty interactions with, e.g., co-morbidity enable the interdisciplinary team to closely monitor frailty domains like, e.g., functional status to identify specific interventions aimed to reduce negative health outcomes.
Conclusion
In older patients with COVID-19, it seems that frailty was associated with confusion at admission to hospital. Decision of treatment level about invasive ventilation and cardiopulmonary resuscitation was more prevalent among frail older patients than non-frail. Frail patients experienced more often a decline in physical functional abilities and had a higher risk of short-term mortality than non-frail patients.
Appendix 1
Record-based multidimensional prognostic index (see Table 3).
Table 3.
MPI items and applied data collection methods in the bedside- and record-based ratings
| MPI-item | Bedside rating data sources | Solution applied in the record-based rating |
|---|---|---|
| C0-habitation |
Interviewing patients, relatives and/or home care staff Medical audit |
Medical record audit |
| No. of drugs |
Drug chart review Interviewing patients, relatives and/or home care staff Medical record audit |
Medical record audit Drug chart review Clinical pharmacist notes |
| FRS-ADL |
Physical examination Clinical observation Interviewing patients, relatives and/or home care staff Medical record audit |
Medical record audit Rated as self-sufficient if no information was available |
| FRS-IADL |
Interviewing patients, relatives and/or home care staff Clinical observation Physical examination Medical record audit |
Medical record audit Rated as self-sufficient if no information was available |
| SPMSQ | Structured interview (10-item questionnaire) |
Medical record audit Rated as: “Low” (3 points, score = 0) if no cognitive impairment was described “moderate” (5 points, score = 0.5) if described as delirious “High” (10 points, score = 1) if diagnosed with dementia |
| ESS |
Physical examination Clinical observation Interviewing patients, relatives and/or home care staff Medical record audit |
Medical record audit Rated as self-sufficient if no information was available |
| CIRS-G | Medical record audit | Medical record audit |
| MINA-SF |
Interviewing patients, relatives and/or home care staff Physical examination Clinical observation Medical record audit |
Medical record audit Neuropsychological prblems were rated: 0 points if the patients was diagnosed with dementia, moderate or severe depression, or treated with anti-dementia drugs or antidepressants 1 point if described mild cogniti ve impairment or mild depression 2 points if no cogniti ve or depressive symptoms were described If no BMI available, BMI was rated: 0 points if described as underweight, malnouri shed, cachectic, suffering from sarcopenia or atrophy of the muscles 1 point if nutritional status was not described 2 points if described as of normal weight or well-nourished 3 points if described as overweight or obese |
MPI-item: The Multidimensional Prognostic Index was an aggregate score based on the eight items
Co-habitation co-habitation status, No. of drugs number of drugs used at admission, FRS-ADL Functional Recovery Score Activities of Daily Living, FRS-IADL Functional Recovery Score Instrumentalized Activities of Daily Living, SPMSQ Short Portable Mental Status Questionnaire, ESS Exton Smith Scale, CIRS-G Cumulative Illness Rating Scale-Geriatrics, MNA-SF Mini Nutritional Assessment-Short Form; BMI: Body Mass Index (kg/m2)
Appendix 2
See Fig. 3.
Fig. 3.
Clinical Frailty Scale, CFS
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Due to national guidelines for quality improvement projects, no ethical approval was required from the local ethical committee (No: 1-10-72-1-20). EHR reviews were approved by all the hospital boards on the involved hospitals in Central Denmark Region.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Smet R, Mellaerts B, Vandewinckele H, Lybeert P, Frans E, Ombelet S, et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aw D, Woodrow L, Ogliari G, Harwood R. Association of frailty with mortality in older inpatients with Covid-19: a cohort study. Age Ageing. 2020;49(6):915–922. doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter B, Collins JT, Barlow-Pay F, Rickard F, Bruce E, Verduri A, et al. Nosocomial COVID-19 infection: examining the risk of mortality The COPE-Nosocomial Study (COVID in Older PEople) J Hosp Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellelli G, Rebora P, Valsecchi MG, Bonfanti P, Citerio G. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020;46(8):1634–1636. doi: 10.1007/s00134-020-06087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendes A, Serratrice C, Herrmann FR, Genton L, Périvier S, Scheffler M, et al. Predictors of in-hospital mortality in older patients with covid-19: the COVID Age study. J Am Med Dir Assoc. 2020;21(11):1546–54.e3. doi: 10.1016/j.jamda.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino M, Pentakota SR, Mosenthal AC, Glass NE. The palliative performance scale predicts mortality in hospitalized patients with COVID-19. Palliat Med. 2020;34(9):1228–1234. doi: 10.1177/0269216320940566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owen RK, Conroy SP, Taub N, Jones W, Bryden D, Pareek M, et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2020 doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dent E, Morley JE, Cruz-Jentoft AJ, Woodhouse L, Rodríguez-Mañas L, Fried LP, et al. Physical frailty: icfsr international clinical practice guidelines for identification and management. J Nutr Health Aging. 2019;23(9):771–787. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns GP, Lane ND, Tedd HM, Deutsch E, Douglas F, West SD, et al. Improved survival following ward-based non-invasive pressure support for severe hypoxia in a cohort of frail patients with COVID-19: Retrospective analysis from a UK teaching hospital. BMJ Open Respiratory Res. 2020;7(1):621. doi: 10.1136/bmjresp-2020-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins JT, Short R, Carter B, Verduri A, Myint PK, Quinn TJ, et al. The clinical frailty scale: estimating the prevalence of frailty in older patients hospitalised with COVID-19 the cope study. Geriatrics (Basel) 2020;5(3):58. doi: 10.3390/geriatrics5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017 doi: 10.1002/14651858.CD006211.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilotto A, Ferrucci L, Franceschi M, D'Ambrosio LP, Scarcelli C, Cascavilla L, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregersen M, Hansen TK, Jørgensen BB, Damsgaard EM. Frailty is associated with hospital readmission in geriatric patients: a prognostic study. Eur Geriatr Med. 2020 doi: 10.1007/s41999-020-00335-w. [DOI] [PubMed] [Google Scholar]
- 16.Landi F, Barillaro C, Bellieni A, Brandi V, Carfì A, D’Angelo M, et al. The new challenge of geriatrics: saving frail older people from the SARS-COV-2 pandemic infection. J Nutr Health Aging. 2020;24(5):466–470. doi: 10.1007/s12603-020-1356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen TK, Damsgaard EM, Shahla S, Bruun JM, Gregersen M. A reliable and record-based frailty assessment method for older medical inpatients. Eur Geriatr Med. 2020;11(5):803–812. doi: 10.1007/s41999-020-00345-8. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Denmark (2020) https://www.dst.dk/en/OmDS. (Accessed 19 Jan 2020).
- 21.Schmidt M, Pedersen L, Sørensen HT. The danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 22.Region BICD. Central Denmark Region. (2021) https://www.rm.dk/om-os/organisation/it/business-intelligence (Accessed 19 Jan 2021).
- 23.Harris PA. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen TK, Shahla S, Damsgaard EM, Bossen SRL, Bruun JM, Gregersen M. Mortality and readmission risk can be predicted by the record-based multidimensional prognostic index: a cohort study of medical inpatients older than 75 years. Eur Geriat Med. 2021 doi: 10.1007/s41999-021-00453-z. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.StataCorp . Stata statistical software release 16. StataCorp LCC; 2019. [Google Scholar]
- 27.Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia JM, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3(11):e2029540. doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020;49(6):923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson LK, Jakobsen LH, Hollensberg L, Ryg J, Midttun M, Frederiksen H, et al. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19-A retrospective cohort study. Arch Gerontol Geriatr. 2020;94:104335. doi: 10.1016/j.archger.2020.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrillon A, Hourregue C, Azuar J, Grosset L, Boutelier A, Tan S, et al. COVID-19 in older adults: a series of 76 patients aged 85 years and older with COVID-19. J Am Geriatr Soc. 2020;68(12):2735–2743. doi: 10.1111/jgs.16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcez FB, Aliberti MJR, Poco PCE, Hiratsuka M, Takahashi SF, Coelho VA, et al. Delirium and adverse outcomes in hospitalized patients with COVID-19. J Am Geriatr Soc. 2020;68(11):2440–2446. doi: 10.1111/jgs.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis DHJ, Muniz-Terrera G, Keage HAD, Stephan BCM, Fleming J, Ince PG, et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population-based cohort studies. JAMA Psychiat. 2017;74(3):244–251. doi: 10.1001/jamapsychiatry.2016.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarychanski R, Houston DS. Clinical paradigms - Anemia of chronic disease: a harmful disorder or an adaptive, beneficial response? CMAJ. 2008;179(4):333–337. doi: 10.1503/cmaj.071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hyper Res. 2020;43(10):1028–1046. doi: 10.1038/s41440-020-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021 doi: 10.1093/ageing/afab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilotto A, Azzini M, Cella A, Cenderello G, Castagna A, Pilotto A, et al. The multidimensional prognostic index (MPI) for the prognostic stratification of older inpatients with COVID-19: a multicenter prospective observational cohort study. Arch Gerontol Geriatr. 2021;95:104415. doi: 10.1016/j.archger.2021.104415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliberti MJR, Szlejf C, Avelino-Silva V, Suemoto CK, Apolinario D, Dias MB, et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriat Soc. 2021 doi: 10.1111/jgs.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]