Abstract
Tafazzin (TAZ) is a cardiolipin (CL) biosynthetic enzyme important for maintaining mitochondrial function. TAZ affects both the species and content of CL in the inner mitochondrial membrane, which are essential for normal cellular respiration. In pancreatic β cells, mitochondrial function is closely associated with insulin secretion. However, the role of TAZ and CL in the secretion of insulin from pancreatic islets remains unknown. Male 4-month-old doxycycline-inducible TAZ knock-down (KD) mice and wild-type littermate controls were used. Immunohistochemistry was used to assess β-cell morphology in whole pancreas sections, whereas ex vivo insulin secretion, CL content, RNA-sequencing analysis, and mitochondrial oxygen consumption were measured from isolated islet preparations. Ex vivo insulin secretion under nonstimulatory low-glucose concentrations was reduced ~52% from islets isolated from TAZ KD mice. Mitochondrial oxygen consumption under low-glucose conditions was also reduced ~58% in islets from TAZ KD animals. TAZ deficiency in pancreatic islets was associated with significant alteration in CL molecular species and elevated polyunsaturated fatty acid CL content. In addition, RNA-sequencing of isolated islets showed that TAZ KD increased expression of extracellular matrix genes, which are linked to pancreatic fibrosis, activated stellate cells, and impaired β-cell function. These data indicate a novel role for TAZ in regulating pancreatic islet function, particularly under low-glucose conditions.
Keywords: cardiolipin, tafazzin, pancreatic islet function, insulin secretion, mitochondrial respiration
The global prevalence of type 2 diabetes (T2D) exacts an enormous burden on health care systems as well as amplifies morbidity and premature mortality (1). One of the central components in the development and progression of T2D is the failure of pancreatic β cells to secrete sufficient insulin (2). In the presence of chronic fuel excess and obesity-associated insulin resistance, the β cell responds with increased mass, insulin synthesis, and secretory activity (2). Diabetes develops when the compensatory measures cannot be sustained in response to the metabolic stress. Although the specific molecular basis underlying the failure of pancreatic β cells remains unclear, the intimate relationship between insulin secretion and mitochondrial function has been widely established (1).
Tafazzin (TAZ) is a transacylase that alters the content and molecular structure of cardiolipin (CL) in the inner mitochondrial membrane (3). Specifically, TAZ promotes the enrichment of CL with linoleic acid, yielding the dominant species tetralinoleoyl CL (L4CL). It is well established that CL content and molecular structure are required to maintain proper mitochondrial cristae architecture, and multiple biological processes including fatty acid metabolism, protein transport, apoptosis, and mitochondrial fission and fusion (3). Moreover, the optimal activity of individual electron transport chain complexes as well as their assembly into respirasomes requires sufficient L4CL and total CL (3, 4). The importance of TAZ and CL content in maintaining mitochondrial function is underscored by a rare disorder called Barth syndrome in which patients lacking functional TAZ present with cardiomyopathy and skeletal dysfunction resulting from L4CL and total CL deficiency (5).
There are also reports that inadequate CL content may promote the development of mitochondrial dysfunction in pancreatic β cells (6). Mice lacking the phospholipid remodelling enzyme iPLA2β exhibit reduced insulin secretion from isolated islets as a result of increased oxidative damage to the mitochondrial membrane (6–8). Based on the enrichment of CL in the mitochondria and sensitivity of polyunsaturated fatty acids (PUFAs) to oxidative damage, the authors proposed that mitochondrial dysfunction could be a consequence of the inability to replace peroxidized fatty acid side chains (6, 7). Consistent with the idea that iPLA2β deficiency increases β-cell dysfunction and failure, db/db mice express only low levels of iPLA2β in isolated pancreatic islets (8). Nevertheless, the precise role of CL in maintaining β-cell function remains unclear because CL content nor degree of CL-specific oxidation was determined in iPLA2β-deficient islets. Furthermore, our previous work has indicated that the lack of TAZ is protective against the development of the metabolic syndrome, including hyperinsulinemia in mice aged to 10 months of age (9). Although overt insulin deficiency has not been reported in Barth syndrome patients, they do exhibit alterations in whole-body fatty acid, glucose, and amino acid metabolism (10). In this report, we investigated whether TAZ is critical for maintaining pancreatic islet mitochondrial function and insulin secretion in TAZ-deficient mice.
Materials and methods
Animal care
This study was performed with approval of the University of Manitoba Animal Policy and Welfare Committee, which adheres to the principles for biomedical research involving animals developed by the Canadian Council on Animal Care and the Council for International Organizations of Medical Sciences. This study is reported in accordance with Animal Research: Reporting of In Vivo Experiments guidelines. All animals were maintained in an environmentally controlled facility (22°C, 37% humidity, 12-hour light/dark cycle) with free access to food and water. Experimental animals were generated by mating male transgenic (Tg) mice (B6.Cg-Gt(ROSA)26Sortm1(H1/tetO-RNAi:Taz,CAG-tetR)Bsf/ZkhuJ, Jackson Laboratory, Bar Harbor, ME), which have a doxycycline (DOX)-inducible TAZ-specific short hairpin RNA (shRNA) with female C57BL/6J mice (Jackson Laboratory). The knock-down (KD) of TAZ was induced in utero by administering DOX (625 mg of DOX/kg of chow) as part of the standard lowfat 6% (w/w) fat rodent chow (Envigo Teklad, Rodent diet catalog #TD.01306) to female C57BL/6 mice both before mating and during pregnancy. TAZ deficiency is initiated in utero as previously described (11) to maximize the duration of TAZ deficiency. The dams with litters continued to receive the DOX diet (TD.01306) to maintain TAZ KD in the offspring for the entire suckling period. Male offspring were weaned at 3 weeks of age onto the lowfat 6% (w/w) (TD.01306) DOX-containing diet (625 mg/kg). In addition, some dams and corresponding male offspring (Tg and non-Tg) were fed a lowfat chow (6%, w/w) not treated with DOX that served to provide standard wild-type values. Male mice positive for the TAZ shRNA transgene were identified by PCR using primers (5′-CCATGGAATTCGAACGCTGACGTC-3′ forward, 5′-TATGGGCTATGAACTAATGACCC-3′ reverse) as described (9, 11).
Glucose tolerance test
Glucose tolerance tests (GTTs) were performed following IP administration of glucose as previously described (12). The circulating concentration of insulin was determined from plasma separated from tail vein blood samples by centrifugation (10 minutes at 4000 rpm, 4°C) followed by a colorimetric ELISA assay [RRID: AB_2792981 (13)] (ALPCO).
Pancreatic islet isolation and hormone assays
Mice were anesthetized with pentobarbital (110 mg/kg) and the pancreas perfused via the common bile duct with collagenase V as previously described (14). Islets were manually picked using a dissecting microscope (SZ61, Olympus) and incubated overnight at 37°C, 5% CO2 in 10% fetal bovine serum, 1% P/S RPMI 1640. The following day, islets from each animal were divided into 1.5-mL tubes (15 islets × 3 replicates). Insulin secretion experiments were performed by incubating each aliquot of islets at 37oC, 5% CO2 with Krebs-Ringer bicarbonate (KRB) buffer supplemented with 2.8 mM glucose for 1 hour, followed by KRB containing 2.8 mM for 30 minutes, 16.7 mM glucose for 30 minutes, and KRB containing 16.7 mM glucose plus 3 mM KCL for 30 minutes. The islets were then lysed (70% ethanol, 0.1 N HCl), dried down, and resuspended in water. Total pancreatic islet insulin content, secreted insulin [RRID:AB_2792981 (13)] (ALPCO), total pancreatic islet glucagon content, and secreted glucagon (radioimmunoassay, [RRID:AB_2757819 (15)], Millipore) were quantitated and normalized to DNA content.
Histology and immunohistochemistry
The 4% paraformaldehyde-fixed pancreas was cryopreserved, sectioned completely (12 µm) and mounted onto superfrost plus slides (Fisher). To assess islet morphology, some sections were stained with cresyl violet. For single immunofluorescence analysis, sections were preincubated in blocking solution (PBS, goat serum 5% serum, 0.2% Triton X-100, 0.02% sodium azide, 0.1% BSA fraction V) for 2 hours at room temperature (RT). Slides were then incubated with the corresponding primary IgG: rabbit glucagon (1:200) [RRID:AB_659831 (16)], rabbit insulin (1:100) [RRID:AB_659820 (17)], and rabbit activated caspase 3 (1:200) [RRID:AB_2341188 (18)] (Cell Signaling Technologies) overnight at 4°C. The next day, after several washes in 1X PBS/Triton (PBS-T), slides were incubated in goat anti-rabbit Alexa-Fluor 488 or 594 conjugate [RRID:AB_10583036 (19); RRID:AB_10578938 (20)] (LifeTechnologies) in blocking serum buffer for 2 hours at RT. After several washes in PBS-T, slides were then mounted with Vectashield/DAPI (Vector Laboratories). Signal for primary antibody 4-hydroxynonenal (4-HNE) (1:100) [RRID:AB_2016554 (21)] (Kamiya Biomedical) was amplified. Primary antibody for 4-HNE was incubated as described previously. Biotinylated goat anti-rabbit IgG secondary antibody [RRID:AB_2313606 (22)] (Vector Laboratories) was added for 2 hours at RT. After PBS-T washes, streptavidin Alexa-Fluor conjugate 488 was added for an additional 2 hours at RT. Negative controls for insulin, glucagon, activated caspase-3, and 4-HNE staining were obtained in the absence of primary antibody. Colocalization of insulin and glucagon were obtained by performing double immunofluorescence. For double immunofluorescence analysis, slides were blocked in donkey serum blocking solution (PBS, donkey serum 5% serum, 0.2% Triton X-100, 0.02% sodium azide, 0.1% BSA fraction V). Slides were then incubated in goat polyclonal insulin (1:50) [RRID:AB_2280400 (23)] (Santa Cruz) and rabbit polyclonal glucagon (1:50) [RRID:AB_659831 (16)] (Cell Signalling Technology) overnight at 4°C. The next day, slides were washed with PBS-T, and incubated with donkey anti-goat Alexa-fluor 594 and goat anti-rabbit Alexa-fluor 488 secondaries [RRID:AB_2338046 (24)] (Jackson ImmunoResearch) diluted in donkey blocking solution for 2 hours at RT. For assessment of fibrosis, the pancreas was formalin-fixed and sectioned (5 µm) before trichrome or picro Sirius red staining. Images were obtained using an Olympus IX70 inverted microscope and quantified using Image J software as previously described (14).
Mitochondrial analysis
Oxygen consumption rate (OCR) was measured in islets from 4-month-old animals using a Seahorse Bioscience Extracellular Flux Analyzer (model XF24, Agilent Technologies). Mouse islets (50 islets per well) were assayed in Seahorse XF media containing 2.8 mM glucose. Islets were then treated with 16.7 mM glucose followed by 10 µM oligomycin and finally 5 µM antimycin A + 5 µM rotenone. Rotenone is an inhibitor of complex 1 that results in blocking electron flow through the electron transport chain and thus ATP synthesis. Basal oxygen consumption was considered to be the respiration sensitive to inhibition by 5 μM antimycin A + 5 μM rotenone. ATP-sensitive oxygen consumption was inhibited by 10 μM oligomycin, and ATP-insensitive respiration (heat) was the remaining proportion of basal oxygen consumption. Maximal oxygen consumption was achieved with 16.7 mM glucose. The addition of antimycin A inhibits all mitochondrial respiration; therefore, any respiration in the presence of antimycin A was subtracted from all OCR values before calculating the average. The glucose-stimulated respiration was calculated by subtracting basal respiration from maximal respiration.
Quantitation of cardiolipin species
The molecular species of CL, oxidized CL (Ox-CL) and peroxidized CL (Per-CL) were quantitated from 4-month-old mouse islets using liquid chromatography coupled to electrospray ionization mass spectrometry in an API 4000 mass spectrometer (Sciex, Framingham, MA), as described previously (25). Briefly, ~300 islets per sample (pooled from ~10 mice of the same genotype) lysed in PBS (200 µg protein) and lipids were extracted according to previously published methods (26, 27). A total of 1000 nmoles of tetramyristyl CL was used as an internal standard (Avanti Polar Lipids, Alabaster, AL). CL species were quantified per milligram of protein based on a protein assay of homogenates (BCA, Pierce). Analysis was performed using the Analyst software with Ox-CL and Per-CL species determined by the M+16 and M+32 species, respectively. Although CL species had a retention time of approximately 8 minutes, Ox-CL and Per-CL had a retention time of 9 to 10 minutes using normal phase solvents. Ox-CL and Per-CL species underwent tandem mass spectometry to verify acyl composition. The total CL was calculated from the sum of the 8 most prominent CL species (1442, 1424, 1448, 1450, 1472, 1474, 1496, 1498) that were previously defined by fatty acid side chain composition (28).
RNA-sequencing library preparation and analysis
RNA was extracted from mouse islets of 4-month-old animals (~300 islets per sample, pooled from ~10 mice of the same genotype) using RNAeasy Micro Kit (Qiagen, Valencia, CA) as per the manufacturer’s instructions. Ribosomal RNA was removed using Ribo-Zero rRNA removal kit (Illumina) and the purity and integrity assessed using Bioanalyzer 2000 (Agilent Technologies). The library was prepared using NEBNExt Ultra II Directional RNA Library Prep for Ilumina (Ipswich, MA). Paired-end RNA sequencing was performed using Illumina HiSeq 4000 PE100 in genome Quebec. The details of the sequencing reads are provided in Supplementary Table 1 (29). The raw and processed data were submitted to the GEO database with accession ID GSE136242. For the alignment of the reads, default parameters of STAR version 020201 (PMID: 23104886) was used and the mouse reference genome version used was mm10. The differentially regulated genes were obtained by using HOMER v4.10 (PMID:20513432) script getDiffExpression.pl. The algorithm used for differentially regulated genes was R package DESeq2(PMID: 25516281).
RNA isolation and quantitative RT-PCR analysis
Total RNA was isolated from pancreatic islets of 4-month-old animals using a RNeasy micro kit (Qiagen) and first-strand cDNA synthesis performed (0.5 µg total RNA) with SuperScript II (Invitrogen). PCR was performed using Eppendorf Realplex2 instrument with the gene specific primers as indicated (Supplementary Table 2) (29). All mRNA levels were quantitated using a standard curve followed by normalization to TfIIb.
Statistical analysis
Data are expressed as means ± SEM. Comparisons between animal treatment groups was performed by unpaired 2-tailed Student t test or analysis of variance using Tukey post hoc analysis where appropriate. Student t test was used for comparisons between low glucose and high glucose conditions within the same genotype. A probability value of <0.05 was considered significant. For RNA-sequencing analysis, statistical analysis was performed using R packages DESEQ2 using the Bonferroni Hochberg method as previously described (13).
Results
CL species are altered in pancreatic islets from TAZ-deficient mice
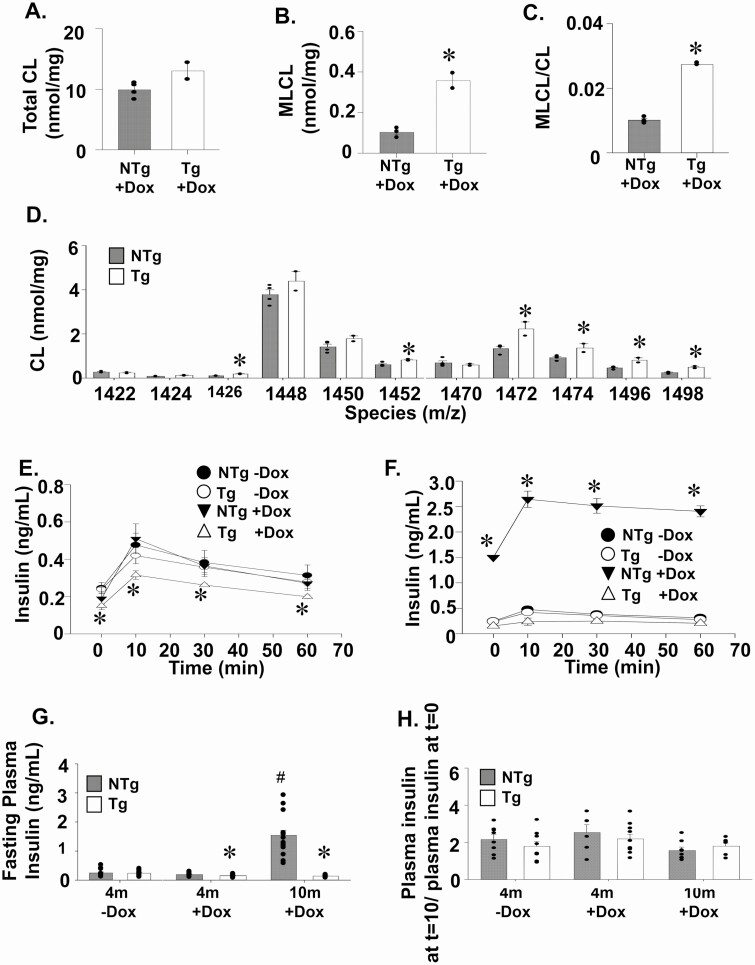
The total CL content of isolated islets remained similar between the 4-month-old TAZ KD (Tg+DOX) and wild-type animals (non-TG [NTg]+DOX, Fig. 1A). Mass spectrometry analysis indicated that several CL species were elevated in the TAZ KD mice, including those (m/z 1472, 1474, 1496, 1498) that contain 1 or more very long chain PUFAs (arachidonic acid, eicosapentaenoic acid) (Fig. 1D). We confirmed that DOX feeding reduced TAZ in the pancreatic islets of TAZ shRNA Tg animals using the ratio of monolysocardiolipin (MLCL) to CL. Significant elevations in the MLCL/CL ratio are used to diagnose Barth syndrome patients because of 100% sensitivity and specificity for the loss of adequate TAZ function (30). We determined that the level of MLCL (359%) and the ratio of MLCL/CL (170%) were significantly elevated in the islets of TAZ KD mice compared with the NTg+DOX littermate controls (Fig. 1B-C).
Figure 1.
Tafazzin deficiency reduces plasma insulin levels. Islets isolated from Tg and NTg mice were used to quantitate (A) total CL, (B) MLCL, and (C) the ratio of MLCL/CL by tandem mass spectrometry (n = 2-4). (D) Quantitation of individual CL species from isolated islets (n = 2-4). Plasma insulin levels following intraperitoneal glucose administration (2 g/mL) of Tg and NTg animals at (E) 4 months and (F) 10 months of age (n = 8-10). (G) Fasting plasma insulin levels. (H) The plasma insulin level at t = 10 minutes after glucose injection during GTT/plasma insulin level after 16 hours of fasting at t = 0 minutes (before glucose injection). Values are means ± SEMs. *P < 0.05 compared with NTg mice fed the same diet. #P < 0.05 compared with all other groups. CL, cardiolipin; MLCL, monolysocardiolipin; NTg, nontransgenic; Tg, transgenic.
TAZ deficiency reduces plasma insulin levels and ex vivo insulin secretion
Our previous work indicated that TAZ deficiency (Tg+DOX) was protective against the development of metabolic syndrome, which occurred in aged littermate controls (10-month-old NTg+DOX) (9). Specifically, we previously determined lower plasma insulin levels in fasted 10-month-old TAZ KD mice (9). Thus, it was expected that TAZ KD (Tg+DOX) mice in the current study would also have reduced plasma insulin levels in part because of the development of hyperinsulinemia in control animals (10-month-old NTg+DOX), which accompanies the elevated body weight and impaired glucose tolerance (Supplementary Fig. 1A, C) (9, 29). At 4 months of age, the TAZ KD (Tg+DOX) mice had significantly reduced plasma insulin levels (~25%) at all time points during GTT compared with littermate controls (NTg+DOX, Fig. 1E). This was complemented with small but significant increases (~13%) of plasma glucose levels (Supplementary Fig. 1B) (29). At 10 months, plasma insulin levels were also reduced during GTT in TAZ-deficient (Tg+DOX) mice compared with littermate controls (NTg +DOX, Fig. 1F). The downward shift in plasma insulin throughout the GTT for both 4- and 10-month-old TAZ KD mice was due to a significant reduction in baseline fasting plasma insulin levels (Fig. 1G) and not a reduction in the fold increase in insulin secretion stimulated by glucose (Fig. 1H).
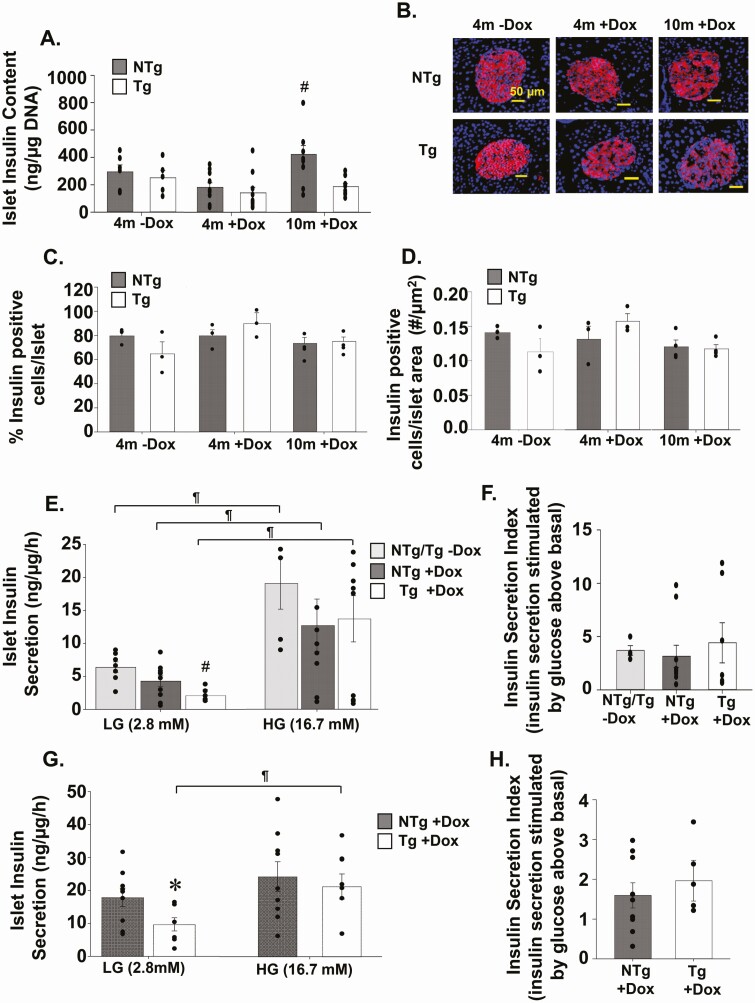
To investigate whether the significantly lower plasma insulin levels observed in TAZ-deficient (Tg+DOX) mice were due to an inherent low insulin secretion defect, we looked at insulin synthesis and secretion. We determined that the islet insulin content was maintained at a constant level when TAZ KD (Tg+DOX) mice were aged from 4 to 10 months (Fig. 2A). In contrast, insulin content increased (~132%) in islets isolated from littermate controls as they aged from 4 to 10 months (NTg+DOX, Fig. 2A). Both the percent of insulin-positive cells per islet and number of insulin-positive cells per islet area were comparable between animal groups at both 4 and 10 months of age (Fig. 2B-D). Similarly, islet number and size were unaltered by TAZ deficiency (Supplementary Fig. 2A-D) (29). Ex vivo secretion of insulin under nonstimulatory low-glucose conditions (basal) was significantly reduced (~48%) in the 4- and 10-month TAZ KD mice compared to age matched wild-type controls (NTg+DOX, Fig. 2E and G, respectively). However, there was no change in insulin secretion under high-glucose conditions or the insulin secretion index between genotypes for either age group (Fig. 2E-H). We also determined that the TAZ KD mice did not have dysfunctional insulin secretion machinery because membrane depolarization with KCL produced similar rates of insulin secretion between experimental groups (Supplementary Fig. 2E, F) (29).
Figure 2.
Mice lacking TAZ secrete less insulin during low glucose conditions. (A) Islets isolated from Tg and NTg mice were used to quantitate total insulin content (n = 5-11). (B) Representative immunofluorescent images (40×) of insulin-stained pancreas sections. The % of insulin-positive cells (C) per islet and number of insulin-positive cells (D) per islet area (n = 3-4). Ex vivo insulin secretion by isolated islets from Tg and NTg mice at (E) 4 months and (G) 10 months of age in low-glucose (LG) and high-glucose (HG) conditions (n = 5-10). The insulin secretion index (insulin secretion rate at HG conditions/insulin secretion rate at LG conditions) from islets isolated from (F) 4- and (H) 10-month-old mice. Values are means ± SEMs. *P < 0.05 compared with NTg mice fed the same diet. #P < 0.05 compared with all other groups. ¶ P < 0.05 compared with LG conditions from the same animal group. NTg, nontransgenic; TAZ, tafazzin; Tg, transgenic.
Glucagon content is reduced but α-cells preserved in TAZ-deficient islets
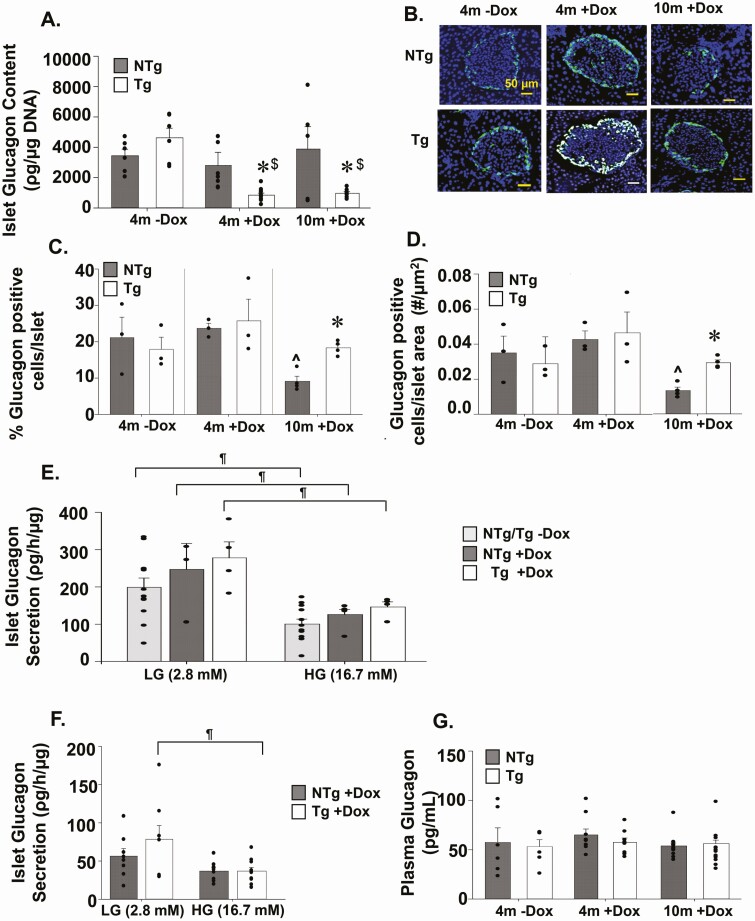
Analysis of islet glucagon content revealed significant reduction in glucagon content in TAZ KD (Tg+DOX) animals at both 4 (~56%) and 10months (~68%) of age compared with age matched wild-type controls (NTg+DOX, Fig. 3A). However, the reduction in glucagon content in TAZ KD mice did not correlate with reductions in either percent glucagon-positive cells per islet or number of α-cells per islet area (Fig. 3B-D). Alternatively, TAZ deficiency protected against the loss of α-cells, which occurred in 10-month-old wild-type controls (Fig. 3B-D). This was not due to enhanced dedifferentiation of β cells into intermediate cells capable of secreting glucagon because similar levels of insulin and glucagon colocalization were observed in islets from TAZ KD and wild-type controls (NTG+DOX) (Supplementary Fig. 3A) (29). Ex vivo secretion of glucagon from islets under low-glucose conditions (basal) and high-glucose conditions were similar between genotypes in both age groups (Fig. 3E, F). Fasting plasma glucagon levels were also constant between experimental groups (Fig. 3G).
Figure 3.
Analysis of pancreatic glucagon levels and secretion. (A) Islets isolated from Tg and NTg mice were used to quantitate total glucagon content (n = 5-10). (B) Representative immunofluorescent images (40×) of glucagon-stained pancreas sections. The % of glucagon positive cells (C) per islet and number of glucagon positive cells (D) per islet area (n = 3-4). Ex vivo glucagon secretion by isolated islets from Tg and NTg mice at (E) 4 and (F) 10 months of age in low-glucose (LG) and high-glucose (HG) conditions (n = 4-12). (G) Fasting plasma glucagon levels (n = 6-14). Values are means ± SEMs. *P < 0.05 compared with NTg mice fed the same diet. $P < 0.05 compared with 4m-DOX of the same genotype. ^P < 0.05 compared to 4m+DOX of the same genotype. ¶P < 0.05 compared with LG conditions from the same animal group. 4m, 4 months; DOX, doxycycline; NTg, nontransgenic; Tg, transgenic.
TAZ deficiency reduces basal respiration
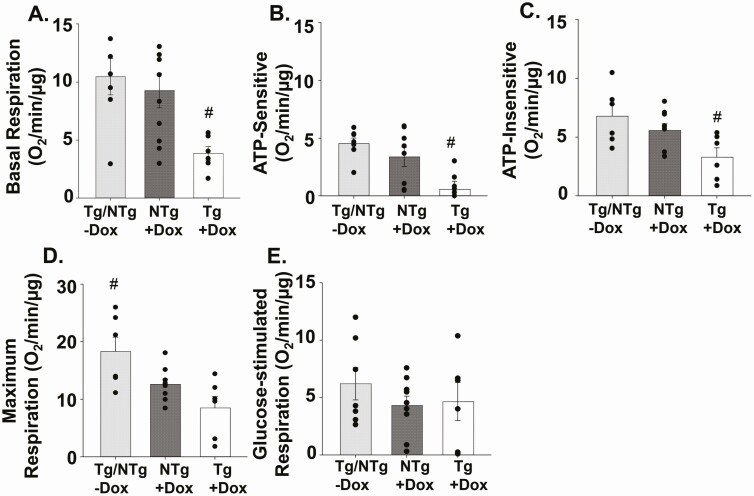
We sought to determine whether the reduction of insulin secretion from islets isolated from TAZ KD (Tg+DOX) mice was associated with impaired mitochondrial respiratory function. Therefore, we measured OCR in islets from 4-month-old animals in the presence of nonstimulatory low-glucose conditions. We determined that the level of basal OCR was significantly reduced (~58%) in islets isolated from TAZ KD mice compared with the wild-type controls (NTg+DOX, Fig. 4A). This reduction was due to decreases in both the ATP-sensitive (~83%) (oligomycin-sensitive) and ATP-insensitive (~41%) oxygen consumption (heat) in the TAZ KD mice (Fig. 4B, C). Maximal respiratory capacity, which was measured in the presence of high glucose, was comparable between TAZ KD mice and wild-type controls (NTg+DOX, Fig. 4D). Similarly, the glucose-stimulated respiration (maximal OCR – basal OCR) was not altered by TAZ deficiency (Fig. 4E). These data indicate that, in TAZ KD mice, the attenuation of insulin secretion from islets incubated with low-glucose concentrations is associated with mitochondrial dysfunction.
Figure 4.
TAZ deficiency reduced basal mitochondrial respiration in isolated islets. (A) Basal mitochondrial respiration was measured in isolated islets from 4-month-old animals in low-glucose (2.8 Mm). The (B) ATP-sensitive (oligomycin-sensitive) and (C) ATP-insensitive (ie, heat) oxygen consumption rates. (D) Maximum respiration in response to high glucose (16.7 mM). (E) Glucose-stimulated respiration ([glucose-stimulated – basal]) (n = 6-10). All measurements were normalized to µg of DNA. Values are means ± SEMs. #P < 0.05 compared with all other groups. TAZ, tafazzin.
CL oxidation and peroxidation in TAZ-deficient islets
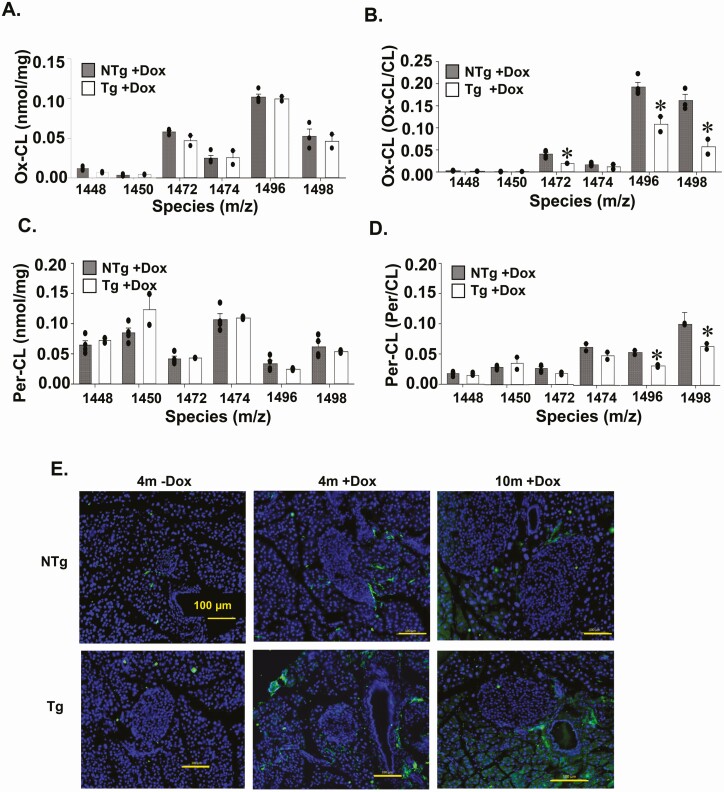
There are previous reports that lack of CL remodelling in pancreatic islets may promote mitochondrial dysfunction because of impaired replacement of peroxidized/oxidized fatty acid side chains (6, 7). Therefore, we quantitated the level of oxidative damage to CL species using mass spectrometry. We determined that the amount of oxidized and peroxidized CL species in pancreatic islets were not different between 4-month-old TAZ KD (Tg+DOX) and wild-type control animals (NTg+DOX, Fig. 5A, C). Furthermore, significant decreases in oxidized (~54%) (m/z 1472, 1496, 1498) and peroxidized (~38%) CL species (m/z 1496, 1498) were identified in TAZ-deficient pancreatic islets when normalized to the corresponding unoxidized species (Fig. 5B, D).
Figure 5.
Quantitation of oxidized CL species in isolated pancreatic islets. Islets isolated from 4-month-old Tg and NTg mice were used to quantitate oxidized CL species by (A) tandem mass spectrometry and (B) expressed as a fraction of each individual CL species. Peroxidized CL species in islets were quantitated by (C) tandem mass spectrometry and (D) expressed as a fraction of each individual CL species. Values are means ± SEMs (n = 2-4). (E) Representative immunofluorescent (40×) images of 4-HNE–stained pancreas sections (n = 3-4). *P < 0.05 compared with NTg mice fed the same diet. 4-HNE, 4-hydroxynonenal; CL, cardiolipin; NTg, nontransgenic; Tg, transgenic.
We also assessed the pancreatic level of 4-HNE, which is one of the dominant end-products of lipid peroxidation and mediators of oxidative stress-induced apoptosis (31). Overall, minimal 4-HNE was detected within the islet and was similar across all experimental groups (Fig. 5E) (Supplementary Fig. 3B) (29). The amount of activated caspase-3, a final step in the apoptotic cascade, was also not different between islets from TAZ KD and wild-type littermate controls (Supplementary Fig. 4A, B) (29). Together, these data indicate that the mitochondrial dysfunction of islets from TAZ KD mice was not from elevated lipid peroxidation, induction of apoptosis, or an accumulation of oxidated CL species.
TAZ deficiency increases fibrosis in pancreatic islets
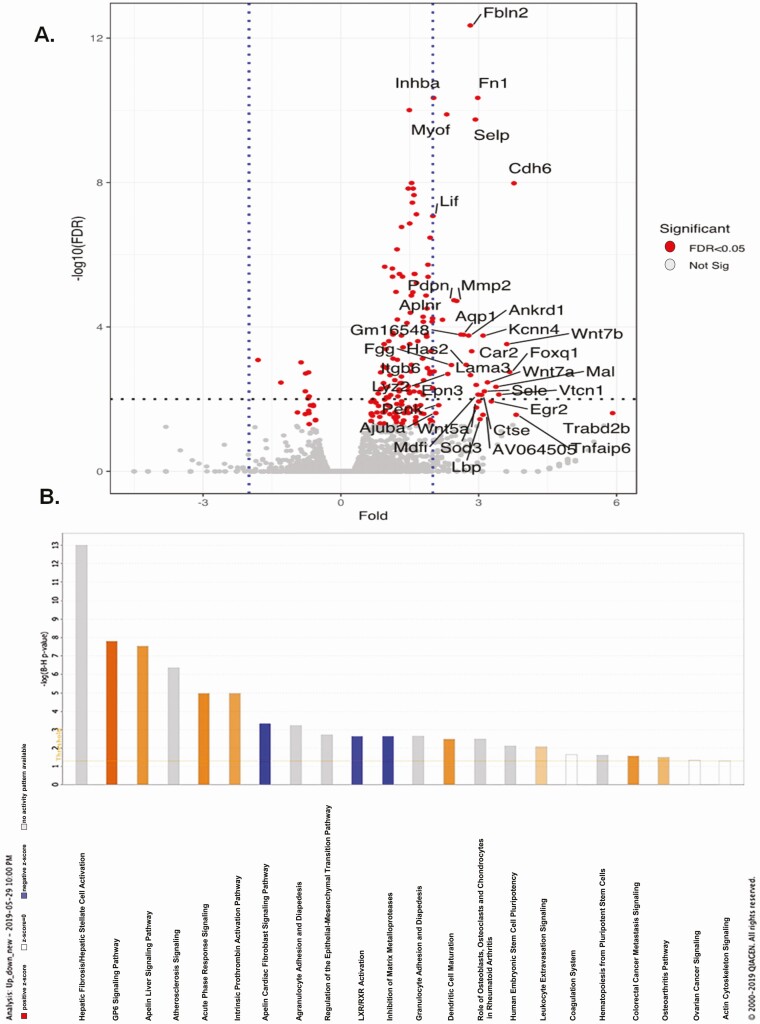
We performed RNA sequencing on isolated pancreatic islets in the TAZ KD mice. Next-generation sequencing of mRNA from islets isolated from 4-month-old TAZ KD (Tg+DOX) and TAZ wild-type controls (NTg+DOX) detected a total of 141 different genes (139 upregulated, 2 downregulated). Among the upregulated set of genes, Fbln2, Fn1, Selp, and Cdh6 showed the largest changes in expression (Fig. 6A, Supplementary Table 3) (29). Novel pathways disrupted by TAZ KD were detected by functionally annotating differentially expressed genes using Ingenuity Pathway Analysis (Fig. 6B) The genes that were most highly represented clustered in the stellate cell activation and fibrosis pathways, which included various types of collagen and fibronectin (eg, Col1a1, Col5a1, Fn1) (Supplementary Table 4) (29). Enrichment of genes also occurred in the pathways associated with the biosynthesis of extracellular matrix components (epithelial mesenchymal transition pathway) and regulation of lipid metabolism (LXR/RXR activation) (Supplementary Tables 5 and 6) (29).
Figure 6.
Transcriptomic profiling of TAZ KD islets revealed increased expression of extracellular matrix protein. Islet genes plotted as fold change against -log10(FDR) in a volcano plot. (A) Significantly regulated genes (>1.5-fold) are marked in red. (B) Highly enriched transcriptional pathways from the Ingenuity Pathway Analysis of mouse islet mRNA. Orange bars represent positive z-score, open bars represent z-score = 0, blue bars represent negative z-score, gray bars represent no activity pattern available. FDR, false discovery rate; KD, knock-down, TAZ, tafazzin.
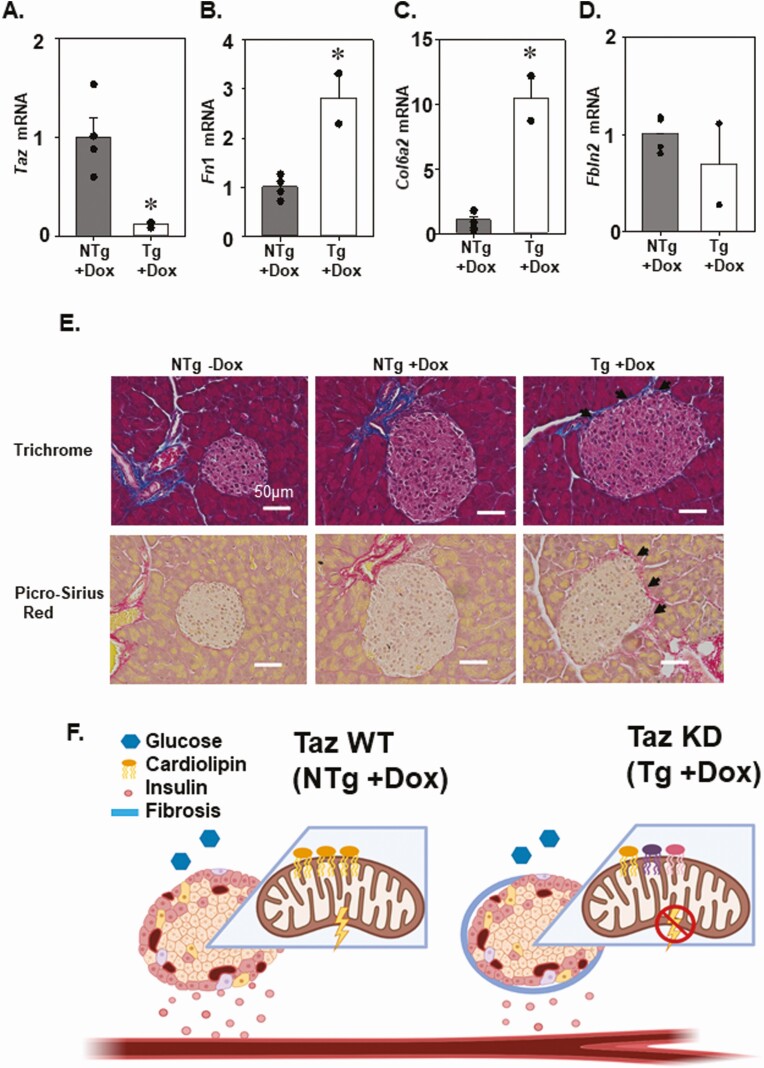
We performed a targeted assessment of TAZ and three genes with known roles in pancreatic fibrosis by quantitative PCR analysis (Fn1, Col6a2, Fbln2) (Fig. 7B-D). As expected, we confirmed that taz gene expression was significantly reduced (~89%) in islets isolated from 4-month-old TAZ KD mice (Fig. 7A). Two of the genes (Fn1, Col6a2) associated with fibrosis were differentially expressed with significant increases (2.8- to 10-fold) in the TAZ KD animals, which is consistent with our RNA-sequencing analysis (Fig. 7B-D). The extent of fibrosis occurring in the pancreas was further assessed by histological visualization (Fig. 7E). Whereas in the controls, picrosirius red and trichrome staining of collagen was limited to the blood vessels, islets derived from the TAZ KD mice displayed collagen along the periphery (Fig. 7E).
Figure 7.
TAZ KD islets revealed increased expression of extracellular matrix protein. (A) Quantitation of TAZ (Taz), (B) fibronectin 1 (Fn1), (C) collagen 6A2 (Col6A2), and (D) fibulin 2 (Fbln2) mRNA by quantitative PCR (n = 2-4). (E) Representative trichrome and picro-Sirius red staining (40×) of pancreas sections with black arrowheads indicating the fibrotic changes (n = 4). Schematic summary: in NTg + DOX (TAZ WT) animals, low-glucose conditions are associated with a corresponding level of mitochondrial respiration and insulin secretion. (F) In Tg + DOX (TAZ KD) animals, alteration in CL species (as indicated by different colors), increased fibrosis, and reduced mitochondrial respiration are associated with a reduction of insulin secretion in low-glucose conditions. *P < 0.05 compared with NTg mice. DOX, doxycycline; KD, knock-down; NTg, nontransgenic; TAZ, tafazzin; Tg, transgenic; WT, wild-type.
Discussion
The present study has defined a role for TAZ deficiency in regulating insulin release from pancreatic islets. We demonstrated that TAZ deficiency reduced ex vivo insulin secretion and mitochondrial oxygen consumption from isolated islets under nonstimulatory low-glucose concentrations. These effects were associated with significant increase in CL species containing very-long-chain PUFAs. Interestingly, our results also revealed that TAZ KD increased expression of extracellular matrix (ECM) genes, which are directly linked to pancreatic fibrosis, activated stellate cells, and impaired β-cell function (Fig. 7F).
CL is almost exclusively localized to the inner mitochondrial membrane, where it functions to maintain normal mitochondrial respiratory function. In the pancreatic islet, mitochondrial oxidative phosphorylation is tightly linked to insulin release, yet very little is known about the precise role of TAZ in this process. Previously, it was demonstrated that inadequate CL remodeling in iPLA2β KO promotes pancreatic β-cell failure and reduces ex vivo insulin secretion (6–8). We similarly determined that deficiency of the CL remodelling enzyme TAZ reduced insulin secretion from isolated pancreatic islets.
We demonstrated for the first time that a reduction in insulin secretion occurred in islets isolated from TAZ KD mice under basal conditions (low glucose levels). This result contrasts with the reduction in insulin secretion stimulated by high-glucose conditions identified in iPLA2β KO mice (7, 8). A potential explanation for the difference between animal models is the divergence in underlying mechanisms. In the iPLA2β KO mice, the mechanism is characterized by oxidation of mitochondrial phospholipids and induction of apoptosis that reduced β-cell mass (8). In addition, the inability to remove oxidized CL side chains has been proposed to increase production of 4-HNE and subsequently damage mitochondria in the iPLA2β KO mice (32). Our data clearly indicate that pancreatic islets isolated from TAZ KD mice exhibit normal β-cell mass, 4-HNE levels, and caspase-3 activation as well as reduced levels of CL oxidation (Fig. 2A-C, Fig. 5A-E, Supplementary Fig 4A, B) (29). This indicates that a separate mechanism is responsible for the reduction in insulin secretion from islets isolated from TAZ KD animals.
Previously, we determined that TAZ deficiency was protective against the development of obesity and hyperinsulinemia in aged 10-month-old animals (9). The TAZ KD mice had a ~70% reduction in fasting plasma insulin levels compared with the age-matched control animals (9). Our current data support a role for β-cell function in the protective effect of TAZ deficiency. We have demonstrated for the first time that TAZ KD significantly reduces fasting plasma insulin levels because of reductions in islet insulin secretion under basal unstimulated conditions. Elevated basal insulin secretion rates are closely associated with hyperinsulinemia and the progression of T2D because they account for as much as half of the total daily insulin delivered (33).
Little is currently known about the mechanisms that control the rate of basal insulin secretion. Most processes identified focus on organization of the exocytotic machinery and regulation of ion channel activity. For example, there is evidence supporting the involvement of plasma membrane rafts, the organization of molecular scaffolding, and gap junctions (34–36). Alternatively, we have determined that the reduction in insulin secretion under low-glucose conditions correlated with a reduction in mitochondrial oxidative consumption rate. This is consistent with the connection between mitochondrial function and insulin secretion as well as the well-established role of TAZ in maintaining normal mitochondrial performance. For example, β-cell mitochondria are key determinants in glucose-stimulated insulin secretion. The uptake and metabolism of glucose into pyruvate activates the mitochondrial respiratory chain. The subsequent ATP production couples mitochondrial metabolism to insulin secretion because cytosolic ATP levels are also increased, resulting in the closure of Katp channels and opening of Ca2+ channels. The ATP-mediated influx of extracellular Ca2+ completes the chain of events by promoting insulin exocytosis. Furthermore, increased mitochondrial ATP production provides energy for insulin exocytosis, replenishing of insulin granules, and establishment of a new steady state of ion gradients in the β cell (37). The prevailing model is that both CL content and acyl chain composition are critical for maintaining optimal mitochondrial function. Both reductions in CL content (>60%) and altered CL remodeling correlate with mitochondrial respiratory dysfunction in cardiac tissue from TAZ KD mice (38, 39), barth syndrome (BTHS) induced plueripotent stem cell (iPSC)-derived cardiomyocytes (40, 41) and TAZ-deficient lymphocytes (42). However, there is evidence that CL acyl chain remodeling may not be sufficient to regulate mitochondrial respiration. Mitochondrial respiratory dysfunction was not induced in yeast as a result of increased saturation of CL acyl chains (43), and in mice, high-fat-diet-induced CL acyl chain remodelling was linked to diminished mitochondrial respiration in the liver, but not the heart (44, 45).
The specific acyl chain composition of CL has additionally been linked to the initiation of cellular effects through signaling functions (46). For example, in response to acute injury, the initiation of apoptotic death signals was linked to specific CL oxidation products (linoleic, arachidonic acids, and monolyso-CLs) across several tissues (brain, liver, heart, small intestine) (47). Additional processes linked to CL signaling include mitophagy and immune responses (inflammasome activation, phagocytosis), although the specific acyl chains involved remain unknown (46). Potentially, numerous cellular processes may be regulated by CL mediated signaling based on the vast number of species, oxidative metabolites, and lipid mediators (>107) (46). However, there is a paucity of information on signaling pathways within the mitochondria, including those that regulate the posttranslational modification of respiratory complexes (48). Nevertheless, our results indicate that in isolated mouse pancreatic islets, increased levels of very long PUFA-containing CL species were sufficient to impair mitochondrial oxidative phosphorylation in the presence of normal CL content. This finding underscores the sensitivity of pancreatic islets to CL molecular species and mitochondrial dysfunction.
Additionally, our data indicate that pancreatic islets isolated from TAZ-deficient animals may have elevated stimulation of stellate cells. Stellate cells have been extensively studied in the injured liver where they are the major ECM protein-producing cell (49). There is accumulating evidence for the role of stellate cells in the development of pancreatic fibrosis (50, 51) and in directly altering β-cell function (52). Interestingly, pancreatic stellate cells were identified within the islets of healthy mice, particularly within the peripheral capsule (52) where fibrosis was localized in the TAZ KD mice.
Islet fibrosis has been observed in patients (53) and rodent models of T2D (50, 54, 55). Increased deposition of ECM components including collagen and fibronectin is consistent with the elevations observed in TAZ KD mice. However, the TAZ KD animals are actually protected against the development of metabolic syndrome (9). This apparent contradiction may be explained by the relatively small increase in fibrosis and ECM gene expression in the TAZ KD mice (~1.1- to 3-fold) compared with animal diabetic models (2- to 30-fold) (50, 54, 55). Although the effect of ECM manipulations, particularly within the peripheral capsule, remains unclear (56), it is established that the relationship between the ECM and cellular function is dynamic. Therefore, the elevated ECM production in pancreatic islets from the TAZ KD mice may reflect the changes in cellular function (mitochondrial dysfunction) and/or promote changes in cellular function through surface receptors (57, 58).
In conclusion, we have found that pancreatic islets isolated from TAZ KD mice have reduced ex vivo insulin secretion and mitochondrial oxygen consumption under nonstimulatory low-glucose concentrations. Our findings also demonstrate that TAZ deficiency in pancreatic islets was associated with significant alteration in CL molecular species, with elevated PUFA CL content and increased expression of ECM genes. Together, our data reveal a novel role for TAZ in regulating pancreatic islet mitochondrial function and insulin secretion.
Acknowledgments
Financial Support : P. A. is the recipient of a postdoctoral fellowship from Research Manitoba. L. K. C. was the recipient of a CIHR/HSFC IMPACT Fellowship. G. M. H. is a Canada Research Chair in Molecular Cardiolipin Metabolism. C. A. D. is the Dr. J.A. Moorhouse Fellow of the Diabetes Foundation of Manitoba. V. W. D. is the Allen Rouse-Manitoba Medical Services Foundation Basic Scientist. This research was supported by an Environments, Genes and Chronic Disease Canadian Institutes for Health Research (CIHR) Team Grant #144626, the Heart and Stroke Foundation of Canada, the Natural Sciences and Engineering Research Council, and Children’s Hospital Research Institute of Manitoba. We thank McGill University and Genome Quebec Innovation Centre for performing the sequencing of the RNA libraries.
Author Contributions: L. K. C., P. A., M. F., B. Z., G. C. S., N. S., and M. V. performed experiments. L. K. C., C. A. D., V. W. D., and G. M. H. were responsible for conceptual design. L. K. C., C. A. D., V. W. D., and G. M. H. wrote and edited the manuscript. All authors read and approved the manuscript.
Glossary
Abbreviations
- 4-HNE
4-hydroxynonenal
- CL
cardiolipin
- DOX
doxycycline
- ECM
extracellular matrix
- GTT
glucose tolerance test
- KD
knock-down
- KRB
Krebs-Ringer bicarbonate
- L4CL
tetralinoleoyl cardiolipin
- MLCL
monolysocardiolipin
- NTg
nontransgenic
- OCR
oxygen consumption rate
- Ox-CL
oxidized cardiolipin
- PBS-T
PBS-Triton
- Per-CL
peroxidized cardiolipin
- PLA
phospholipase A
- PUFA
polyunsaturated fatty acid
- RT
room temperature
- shRNA
short hairpin RNA
- TAZ
tafazzin
- Tg
transgenic
- T2D
type 2 diabetes
Additional Information
Disclosures: The authors of this study have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Las G, Oliveira MF, Shirihai OS. Emerging roles of β-cell mitochondria in type-2-diabetes. Mol Aspects Med. 2020;71:100843. [DOI] [PubMed] [Google Scholar]
- 2. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dudek J, Hartmann M, Rehling P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim Biophys Acta Mol Basis Dis. 2019;1865(4):810-821. [DOI] [PubMed] [Google Scholar]
- 4. Dolinsky VW, Cole LK, Sparagna GC, Hatch GM. Cardiac mitochondrial energy metabolism in heart failure: role of cardiolipin and sirtuins. Biochim Biophys Acta. 2016;1861(10):1544-1554. [DOI] [PubMed] [Google Scholar]
- 5. Finsterer J. Barth syndrome: mechanisms and management. Appl Clin Genet. 2019;12:95-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bao S, Song H, Wohltmann M, et al. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis. J Biol Chem. 2006;281(30):20958-20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Z, Zhang X, Zhao C, et al. Protection of pancreatic beta-cells by group VIA phospholipase A(2)-mediated repair of mitochondrial membrane peroxidation. Endocrinology. 2010;151(7):3038-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole LK, Mejia EM, Vandel M, et al. Impaired cardiolipin biosynthesis prevents hepatic steatosis and diet-induced obesity. Diabetes. 2016;65(11):3289-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cade WT, Spencer CT, Reeds DN, et al. Substrate metabolism during basal and hyperinsulinemic conditions in adolescents and young-adults with Barth syndrome. J Inherit Metab Dis. 2013;36(1):91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Acehan D, Vaz F, Houtkooper RH, et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem. 2011;286(2):899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira TJ, Fonseca MA, Campbell KE, et al. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol. 2015;593(14):3181-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. RRID: AB_2792981. https://antibodyregistry.org/search.php?q=AB_2792981. [Google Scholar]
- 14. Agarwal P, Brar N, Morriseau TS, et al. Gestational diabetes adversely affects pancreatic islet architecture and function in the male rat offspring. Endocrinology. 2019;160(8):1907-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RRID:AB_2757819. https://antibodyregistry.org/search.php?q=AB_2757819. [Google Scholar]
- 16. RRID:AB_659831. https://antibodyregistry.org/search.php?q=AB_659831. [Google Scholar]
- 17. RRID:AB_659820. https://antibodyregistry.org/search.php?q=AB_659831. [Google Scholar]
- 18. RRID:AB_2341188. https://antibodyregistry.org/search.php?q=AB_2341188. [Google Scholar]
- 19. RRID:AB_10583036. https://antibodyregistry.org/search.php?q=AB_10583036. [Google Scholar]
- 20. RRID:AB_10578938. https://antibodyregistry.org/search.php?q=AB_10578938. [Google Scholar]
- 21. RRID:AB_2016554. https://antibodyregistry.org/search.php?q=AB_2016554. [Google Scholar]
- 22. RRID:AB_2313606. https://antibodyregistry.org/search.php?q=AB_2313606. [Google Scholar]
- 23. RRID:AB_2280400. https://antibodyregistry.org/search.php?q=AB_2280400. [Google Scholar]
- 24. RRID:AB_2338046. https://antibodyregistry.org/search.php?q=AB_2338046. [Google Scholar]
- 25. Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292(1):C33-C44. [DOI] [PubMed] [Google Scholar]
- 26. Sparagna GC, Chicco AJ, Murphy RC, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48(7):1559-1570. [DOI] [PubMed] [Google Scholar]
- 27. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911-917. [DOI] [PubMed] [Google Scholar]
- 28. Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46(6):1196-1204. [DOI] [PubMed] [Google Scholar]
- 29. Cole LK, Agarwal P, Doucette CA, et al. Data from: Tafazzin Deficiency Reduces Basal Insulin Secretion and Mitochondrial Function in Pancreatic Islets from Male Mice. Mendeley Data 2021. Deposited May 7, 2021. doi: 10.17632/78p2rds4c6.1. [DOI] [PMC free article] [PubMed]
- 30. Houtkooper RH, Rodenburg RJ, Thiels C, et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal Biochem. 2009;387(2):230-237. [DOI] [PubMed] [Google Scholar]
- 31. Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, Awasthi S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol. 2015;289(3):361-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu W, Porter NA, Schneider C, Brash AR, Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radic Biol Med. 2011;50(1):166-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162-185. [DOI] [PubMed] [Google Scholar]
- 34. Carvalho CP, Oliveira RB, Britan A, et al. Impaired β-cell-β-cell coupling mediated by Cx36 gap junctions in prediabetic mice. Am J Physiol Endocrinol Metab. 2012;303(1):E144-E151. [DOI] [PubMed] [Google Scholar]
- 35. Tsang SW, Shao D, Cheah KS, Okuse K, Leung PS, Yao KM. Increased basal insulin secretion in Pdzd2-deficient mice. Mol Cell Endocrinol. 2010;315(1-2):263-270. [DOI] [PubMed] [Google Scholar]
- 36. Jezek P, Jaburek M, Holendova B, Plecita-Hlavata L. Fatty acid stimulated insulin secretion vs. Lipotoxicity. Molecules. 2018; 23(6):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagaraj V, Kazim AS, Helgeson J, et al. Elevated basal insulin secretion in type 2 diabetes caused by reduced plasma membrane cholesterol. Mol Endocrinol. 2016;30(10):1059-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cole LK, Mejia EM, Sparagna GC, et al. Cardiolipin deficiency elevates susceptibility to a lipotoxic hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2020;144:24-34. [DOI] [PubMed] [Google Scholar]
- 39. Kiebish MA, Yang K, Liu X, et al. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res. 2013;54(5):1312-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dudek J, Cheng IF, Balleininger M, et al. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 2013;11(2):806-819. [DOI] [PubMed] [Google Scholar]
- 41. Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20(6):616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mejia EM, Zegallai H, Bouchard ED, Banerji V, Ravandi A, Hatch GM. Expression of human monolysocardiolipin acyltransferase-1 improves mitochondrial function in Barth syndrome lymphoblasts. J Biol Chem. 2018;293(20):7564-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baile MG, Sathappa M, Lu YW, et al. Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J Biol Chem. 2014;289(3):1768-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le CH, Mulligan CM, Routh MA, et al. Delta-6-desaturase links polyunsaturated fatty acid metabolism with phospholipid remodeling and disease progression in heart failure. Circ Heart Fail. 2014;7(1):172-183. [DOI] [PubMed] [Google Scholar]
- 45. Sullivan EM, Fix A, Crouch MJ, et al. Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J Nutr Biochem. 2017;45:94-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maguire JJ, Tyurina YY, Mohammadyani D, et al. Known unknowns of cardiolipin signaling: the best is yet to come. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(1):8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tyurina YY, Poloyac SM, Tyurin VA, et al. A mitochondrial pathway for biosynthesis of lipid mediators. Nat Chem. 2014;6(6):542-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stram AR, Payne RM. Post-translational modifications in mitochondria: protein signaling in the powerhouse. Cell Mol Life Sci. 2016;73(21):4063-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hoffmann C, Djerir NEH, Danckaert A, et al. Hepatic stellate cell hypertrophy is associated with metabolic liver fibrosis. Sci Rep. 2020;10(1):3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee E, Ryu GR, Ko SH, Ahn YB, Song KH. A role of pancreatic stellate cells in islet fibrosis and β-cell dysfunction in type 2 diabetes mellitus. Biochem Biophys Res Commun. 2017;485(2):328-334. [DOI] [PubMed] [Google Scholar]
- 51. Xue R, Jia K, Wang J, et al. A rising star in pancreatic diseases: pancreatic stellate cells. Front Physiol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zang G, Sandberg M, Carlsson PO, Welsh N, Jansson L, Barbu A. Activated pancreatic stellate cells can impair pancreatic islet function in mice. Ups J Med Sci. 2015;120(3):169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao HL, Lai FM, Tong PC, et al. Prevalence and clinicopathological characteristics of islet amyloid in chinese patients with type 2 diabetes. Diabetes. 2003;52(11):2759-2766. [DOI] [PubMed] [Google Scholar]
- 54. Pick A, Clark J, Kubstrup C, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47(3):358-364. [DOI] [PubMed] [Google Scholar]
- 55. Homo-Delarche F, Calderari S, Irminger JC, et al. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55(6):1625-1633. [DOI] [PubMed] [Google Scholar]
- 56. Almaça J, Caicedo A, Landsman L. Beta cell dysfunction in diabetes: the islet microenvironment as an unusual suspect. Diabetologia. 2020;63(10):2076-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61(6):1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147(6):2643-2649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.