Abstract
Simple Summary
Although cognitive impairments have been complained about in patients with breast cancer who underwent chemotherapy, recent research has described possible neurocognitive decline prior to the start of chemotherapy and suggested that inflammatory cytokines may also have been involved. However, inconsistencies have been found in correlations of cognitive impairments with cancer, chemotherapy, and peridiagnostic cytokine levels. This cross-sectional study aimed to examine associations of cognitive functions and levels of cytokines in patients with newly- diagnosed breast cancer before chemotherapy, those that were 3 to 9 months after completing chemotherapy, and non-cancer controls, adjusting for baseline intelligence quotient, mood, and fatigue. We found that the performance in semantic association of verbal fluency in patients post chemotherapy might be affected by the status of cancer, IL-13, and anxiety. Our results indicated that verbal fluency and anxiety may be important when considering relevant psychosocial managements or prophylactic interventions for cognitive preservation associated with regulations in cytokines.
Abstract
Background: We aimed to investigate the associations of breast cancer (BC) and cancer-related chemotherapies with cytokine levels, and cognitive function. Methods: We evaluated subjective and objective cognitive function in BC patients before chemotherapy and 3~9 months after the completion of chemotherapy. Healthy volunteers without cancer were also compared as control group. Interleukins (IL) 2, 4, 5, 6, 10, 12p70, 13, 17A, 1β, IFNγ, and TNFα were measured. Associations of cancer status, chemotherapy and cytokine levels with subjective and objective cognitive impairments were analyzed using a regression model, adjusting for covariates, including IQ and psychological distress. Results: After adjustment, poorer performance in semantic verbal fluency was found in the post-chemotherapy subgroup compared to controls (p = 0.011, η2 = 0.070); whereas pre-chemotherapy patients scored higher in subjective cognitive perception. Higher IL-13 was associated with lower semantic verbal fluency in the post-chemotherapy subgroup. Higher IL-10 was associated with better perceived cognitive abilities in the pre-chemotherapy and control groups; while IL-5 and IL-13 were associated with lower perceived cognitive abilities in pre-chemotherapy and control groups. Our findings from mediation analysis further suggest that verbal fluency might be affected by cancer status, although mediated by anxiety. Conclusions: Our findings suggest that verbal fluency might be affected by cancer status, although mediated by anxiety. Different cytokines and their interactions may have different roles of neuroinflammation or neuroprotection that need further research.
Keywords: cancer, chemotherapy, subjective and objective cognitive functioning, neuropsychological testing, cytokines, inflammation
1. Introduction
Breast cancer (BC) is becoming the most prevalent cancer worldwide. The annual incidence of BC is over 45 per 100,000, and it is prevalent in more than 600,000 people per year [1]. With advances in treatments (e.g., chemotherapy, immunotherapies), BC survival rates have increased [2,3]; however, systemic chemotherapy can cause adverse reactions [3]. Although severe neurotoxic events are rare, risks of cognitive decline have varied in their estimates from 15~60% to the extent of interfering with the quality of everyday life [3,4,5,6,7]. Specifically, impairments in attention, concentration, learning, processing speed, and executive functioning have been reported [3]. Cognitive decline in BC may of course have multiple other underlying causes, including fatigue, sleep disturbance, psychological distress, or effects from other medication [8]. Although an up to 8-fold elevated risk of cognitive complaints was reported in BC patients who had undergone chemotherapy than those receiving other treatments [9], recent research has reported that 21–33% of BC patients experience neurocognitive decline prior to the start of chemotherapy [8]. Therefore, other factors might also be involved.
In this respect, there have been investigations of the relationship of cytotoxic agents, inflammatory cytokines, and cognitive impairments before [10], during [11,12,13], and after [14] BC chemotherapy [15]. Ganz et al. in a prospective study of BC patients after initial surgery, found a higher level of soluble tumor necrosis factor receptor type II (sTNFRII) among patients who received chemotherapy than in those who did not; this was correlated with initial subjective cognitive complaints. Declines in sTNFRII over years were associated with fewer complaints afterwards [14]. On the other hand, Patel et al. described reduced memory function in newly-diagnosed and pre-treatment BC compared to healthy controls, and this was associated with higher levels of pre-treatment sTNFRII [10]. Cheung et al. further reported a correlation between higher plasma IL-1β and poorer response speed; and higher IL-1β, IL-6, and more severe subjective cognitive complaints during chemotherapy receipt. Higher IL-4 was associated with better performances in response speed and fewer subjective complaints [11].
Inconsistencies have been found in correlations of cognitive impairments with cancer status, chemotherapy, and peridiagnostic cytokine levels. No previous studies have adjusted for baseline intellectual functions [16]. We thus designed this cross-sectional study aimed to examine associations of self-perceived and objective cognitive functions, cancer status, and levels of cytokines in BC patients before receiving chemotherapy and 3 to 9 months after completing chemotherapy, incorporating comparisons with non-cancer controls. Baseline intelligence quotient, mood, and fatigue were also evaluated.
2. Methods
2.1. Participants
BC adult patients diagnosed with Stage I to Stage III invasive breast cancer without metastasis from the oncology clinic at a medical center located in Southern Taiwan were invited to participate. Our non-cancer controls were demographically 1:1 matched female volunteers within 5 years of age of each index patient. Participants were excluded if they were currently pregnant, unable to read/write, had histories of developmental delay, severe visual impairments, or major neurological diseases (e.g., stroke, movement disorders, epilepsy, multiple sclerosis, head injury with loss of consciousness or neurological sequela, or other lesions from central nervous system). Subjective and objective neuropsychological assessments were administered to controls, and to newly diagnosed cases before the start of their chemotherapy (categorized as the pre-chemotherapy (pre-C/T) group). Patients from the post-chemotherapy (post-C/T) group were those who had completed chemotherapy for 3 to 9 months (average: 4.2 months). A total of 70 and 36 participants were categorized in the pre-C/T and post-C/T group, respectively. The Research Ethics Committee at Chiayi Chang Gung Memorial Hospital approved this study (IRB number: 201700255B0) on 1 June 2017. Written informed consent was obtained from all participants before entering the study.
2.2. Measures
Subjective and objective neuropsychological measures were administered by research assistants supervised by a psychiatrist and clinical psychologists on weekly basis. Details of measures used are described elsewhere [17]. In short, Taiwanese versions of the assessments comprised: (i) the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) [18] to estimate intelligence; (ii) the Block Design subtests to test visuospatial functions; (iii) the Digit Symbol Substitution to measure processing speed; (iv) the Digit Span [18] and the first part of the Taiwanese Color Trails Test (CTT1) [19] to assess attention; (v) the second part of the Color Trails Test (CTT2), the Semantic Association of Verbal Fluency Test (SFT) [20], and the Orthographical Fluency Test (OFT) [20], to examine executive functioning; vi) the Word List subtest of the Wechsler Memory Scale, Third Edition [21] to evaluate memory function (the two single-trial tasks were used to test prospective memory) [22]. Higher scores on all above tests indicated better cognitive performance, apart from the CTT1 and CTT2, where higher completion times indicated poorer performance.
We used the Functional Assessment of Cancer Therapy Cognitive Function Version 3 (FACT-Cog) [23] to evaluate self-perceived cognitive function. The FACT-Cog comprises four subscales: Perceived Cognitive Impairment (FACT-PCI), Perceived Cognitive Abilities (FACT-PCA), Impact of Perceived Cognitive Impairment on Quality of Life (FACT-QoL), and Comments from Others on Cognitive Function (FACT-others). Higher FACT-Cog scores indicate better cognitive functioning and quality of life.
We also evaluated education years, BMI, levels of anxiety, depression, and fatigue by Taiwanese versions of the Anxiety subscale of the Hospital Anxiety and Depression Scale (HADS-A) [24], the Patient Health Questionnaire (PHQ-9) [25], and the Brief Fatigue Inventory (BFI) [26]. Interleukins (IL) from helper T cell type 1 (Th1, including IFNγ, IL-12p70, IL-1β, IL-2, and TNFα), type 2 (Th2, including IL-4, IL-5, IL-10, and IL-13), and type 17 (Th17, including IL-5 and IL-17A) were measured because past literature has described how these cytokines may be regarded as biomarkers related to cancer, chemotherapy, neurotoxicity, mental illnesses [27], or contribute to cognitive deficits among cancer patients [7,28].
2.3. Statistical Analysis
Analysis of variance (ANOVA) was used to examine differences in patients’ characteristics and cognitive performance measures of three comparison groups: (i) pre-C/T, (ii) post-C/T, (iii) controls. Bonferroni correction was performed for post hoc tests. Since the distributions of cytokine levels were skewed to the right, these were transformed into log values for normality [10]. Analysis of covariance (ANCOVA) was performed to examine differences in log values of cytokines of the three subgroups while adjusting for significant covariates and cytokines.
Associations of cognitive performance and cytokine levels in the three subgroups while controlling for demographic (age, IQ, BMI, education years) and psychological (anxiety, depression, fatigue) covariates were modelled in stepwise multivariate linear regression analyses. A p-value of less than or equal to 0.05 was defined as a cut-off for further investigation since this study was planned to be exploratory.
When statistically significant correlations with neurocognitive function were found in the multivariate regression analysis, individual effects were examined in the post-hoc analysis after controlling for all other covariates. Further secondary multivariate regression analyses included comparing subgroups of breast cancer (pre-C/T and post-C/T subgroups) and controls as the ‘cancer vs. non-cancer’ comparison; post-C/T and control subgroups as ‘chemotherapy vs. non-cancer’ comparison; and pre-C/T and post C/T subgroups in the cancer group as ‘chemotherapy vs. non-chemotherapy’. Mediation analyses were performed to investigate whether mood symptoms or cytokines may mediate cognitive impairment differences between comparison groups.
3. Results
A total of 136 participants were included in this study: 106 BC patients, with 70 in the pre-C/T subgroup and 36 in the post-C/T subgroup, and 30 controls. Table 1 shows the demographic and clinical characteristics of our participants. The mean age of the combined sample was 50.5 (SD = 11.0) years. The pre-C/T subgroup was older, and had lower mean years of education and mean IQ scores than controls. The pre-C/T group also scored significantly higher on PHQ-9, HADS-A, and BFI-fatigue interference than controls or the post-C/T group. No significant differences were found in BMI between the three groups. Most of the BC patients were in Stage II. All patients in the post-C/T group had menopause (n = 36, 100%). Half of patients in the post-C/T subgroup received cyclophosphamide, 5-fluorouracil, epirubicin and doxorubicin, and docetaxel as the main chemotherapy regimen. More than 90% of post-C/T BC patients received a mastectomy. The range of intervals between surgery and recruitment was 93 to 288 days (Table 1).
Table 1.
Summary of subject characteristics.
| Characteristics, Cognitive Status, and Cytokine Levels | Pre-C/T | Post-C/T | Non-Cancer | ANOVA | ||
|---|---|---|---|---|---|---|
| Cancer Patients | Cancer Patients | Controls | ||||
| (N = 70) | (N = 36) | (N = 30) | ||||
| Basic Information | Mean (SD) | Mean (SD) | Mean (SD) | p | η 2 | Post Hoc Tests ‡ |
| Age (years) | 51.74 (11.39) | 49.97 (10.04) | 48.13 (11.30) | 0.312 | 0.017 | |
| BMI | 24.14 (3.73) | 24.42 (4.15) | 24.77 (3.00) | 0.738 | 0.005 | |
| Education (years) | 11.01 (4.46) | 11.97 (3.82) | 12.50 (3.97) | 0.220 | 0.023 | |
| IQ † | 100.00 (11.37) | 101.14 (13.66) | 106.60 (10.43) | 0.039 * | 0.049 | control > pre-C/T |
| PHQ-9 | 5.14 (4.42) | 4.06 (3.91) | 2.07 (2.56) | 0.002 * | 0.088 | pre-C/T > control |
| HADS-A | 4.21 (3.71) | 2.44 (2.56) | 1.97 (2.68) | 0.002 * | 0.090 | pre-C/T > control |
| BFI—fatigue severity score | 1.43 (2.29) | 2.26 (2.14) | 2.29 (2.62) | 0.108 | 0.033 | |
| BFI—fatigue interference score | 4.04 (6.23) | 4.94 (8.04) | 0.68 (1.36) | 0.014 * | 0.063 | pre-C/T > control post-C/T > control |
| N (%) | N (%) | N (%) | ||||
| Menopausal status (No) | 37 (52.9) | 36 (100) | 14 (46.7) | <0.001 | ||
| Stage | 0.823 | |||||
| Stage I | 21 (30.00) | 12 (30.00) | - | |||
| Stage II | 36 (51.43) | 16 (44.44) | - | |||
| Stage III | 12 (17.14) | 7 (19.44) | - | |||
| Treatment | ||||||
| Mastectomy | 17 (24.64) | 33 (94.29) | - | |||
| Time from Mastectomy to recruitment, days, Mean (SD) | 93.53 (181.55) | 288.43 (56.84) | ||||
| Radiotherapy | 1 (1.43) | 30 (83.33) | - | |||
| Hormonal therapy | 3 (4.29) | 28 (77.78) | - | |||
| Targeted therapy | 0 | 0 | - | |||
| Chemotherapy regimen | ||||||
| Cyclophosphamide, 5-fluorouracil, epirubicin and doxorubicin, and docetaxel | - | 15 (50.00) | - | |||
| Cyclophosphamide, 5-fluorouracil, epirubicin and doxorubicin, docetaxel, and cisplatin | - | 4 (13.33) | - | |||
| Cyclophosphamide, 5-fluorouracil, epirubicin and doxorubicin | - | 4 (13.33) | - | |||
| Cyclophosphamide, methotrexate, and 5-fluorouracil | - | 3 (1.00) | - | |||
| Cyclophosphamide, methotrexate, epirubicin and doxorubicin | - | 1 (0.03) | - | |||
| Functional Assessment of Cancer Therapy Cognitive Scale (FACT-Cog) | 119.26 (10.54) | 118.58 (10.11) | 116.13 (16.25) | 0.485 | 0.011 | |
| Perceived Cognitive Impairment | 66.03 (6.41) | 65.83 (6.25) | 64.50 (9.70) | 0.615 | 0.007 | |
| Comments from Others | 15.71 (0.89) | 15.56 (0.81) | 15.27 (1.82) | 0.201 | 0.024 | |
| Perceived Cognitive Abilities | 22.13 (3.73) | 21.81 (3.48) | 21.30 (4.79) | 0.624 | 0.007 | |
| Impact of Perceived Cognitive Impairments on Quality of Life | 15.39 (1.96) | 15.39 (1.42) | 15.07 (2.16) | 0.714 | 0.005 | |
| Cytokines | ||||||
| Th1 | ||||||
| IFNγ | 6.35 (5.68) | 6.72 (4.62) | 12.45 (7.32) | <0.001 * | 0.156 | control > pre-C/T control > post-C/T |
| IL-12p70 | 1.90 (1.56) | 1.93 (1.42) | 3.25 (2.00) | 0.001 | 0.016 | control > pre-C/T control > post-C/T |
| IL-1β | 0.74 (0.81) | 0.64 (0.49) | 0.95 (0.50) | 0.175 | 0.026 | |
| IL-2 | 1.29 (1.10) | 0.96 (0.80) | 1.66 (1.29) | 0.036 * | 0.049 | control > post-C/T |
| TNF | 4.38 (2.55) | 4.99 (2.58) | 5.61 (2.83) | 0.093 | 0.035 | |
| Th2 | ||||||
| IL-4 | 10.09 (11.11) | 14.75 (22.05) | 18.62 (18.01) | 0.047 | 0.045 | |
| IL-5 | 1.53 (1.22) | 1.16 (0.95) | 1.70 (1.18) | 0.134 | 0.030 | |
| IL-10 | 4.64 (3.83) | 3.45 (3.34) | 6.28 (4.83) | 0.016 * | 0.060 | control > post-C/T |
| IL-13 | 2.56 (2.71) | 2.84 (4.03) | 4.68 (2.72) | 0.008 * | 0.071 | control > pre-C/T |
| Th17 | ||||||
| IL-6 | 1.43 (1.68) | 1.63 (1.88) | 2.33 (1.69) | 0.060 | 0.041 | |
| IL-17A | 5.40 (3.97) | 4.87 (2.65) | 7.70 (3.93) | 0.004 * | 0.078 | control > pre-C/T control > post-C/T |
† Intelligence Quotient (IQ) estimated by the short form of the Taiwan Wechsler Adult Intelligence Scale-III; PHQ-9: Patient Health Questionnaire-9; HADS-A: Hospital Anxiety Depression Scale; BFI: Brief Fatigue Inventory; ‡ Significant difference after Bonferroni correction; * p < 0.05.
As for levels of cytokines, IFN-γ, IL-12p70, and IL-17A were significantly lower in the pre-C/T and the post-C/T groups than in the controls (p = 0.001~0.004, Table 1). The plasma concentrations of IL-2 and IL-10 in the post-C/T group were lower than those in the control group (p = 0.016~0.036). IL-13 in the pre-C/T group was also lower than in the controls (p = 0.008). Table 2 showed that after controlling for subjects’ characteristics, mood status, and all other cytokines in ANCOVA, no significant differences in log-transformed values of cytokines were found between the three subgroups except for log- IFN-γ. Correlations between (serum) levels of cytokines and scores of cognitive domains are shown in supplementary Table S1 available online.
Table 2.
Analysis of covariance (ANCOVA) in patients’ characteristics and log-transformed values of cytokines among the three groups.
| Characteristics and Log Transformed Values of Cytokines | Pre-C/T | Post-C/T | Non-Cancer | ANCOVA | ||
|---|---|---|---|---|---|---|
| Cancer Patients | Cancer Patients | Controls | ||||
| (N = 68) | (N = 35) | (N = 30) | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | F | η 2 | p | |
| IQ † | 100.00 (11.37) | 101.14 (13.66) | 106.60 (10.43) | 2.907 | 0.048 | 0.059 |
| PHQ-9 | 5.01 (4.40) | 3.66 (3.13) | 2.07 (2.56) | 5.399 | 0.085 | 0.054 |
| HADS-A | 4.21 (3.74) | 2.37 (2.56) | 1.97 (2.68) | 4.84 | 0.077 | 0.010 * Pre-C/T > control |
| BFI—fatigue severity score | 1.38 (2.24) | 2.18 (2.12) | 2.29 (2.62) | 8.165 | 0.123 | <0.001 Pre-C/T < control Pre-C/T < post-C/T |
| BFI—fatigue interference score | 4.04 (6.23) | 4.94 (8.04) | 0.68 (1.36) | 3.352 | 0.055 | 0.038 Post-C/T >control |
| Cytokines | ||||||
| Log_IFNr | 0.59 (0.47) | 0.67 (0.43) | 1.01 (0.31) | 3.052 | 0.048 | 0.046 * pre C/T < non-cancer |
| Log_IL-2 | −0.01 (3.42) | −0.20 (0.43) | 0.09 (0.40) | 2.612 | 0.041 | 0.078 |
| Log_TNFα | 0.57 (0.25) | 0.65 (0.19) | 0.71 (0.18) | 1.012 | 0.017 | 0.367 |
| Log_IL-4 | 0.78 (0.43) | 0.93 (0.45) | 1.12 (0.37) | 3.193 | 0.051 | 0.045 |
| Log_IL-12 | 0.13 (0.39) | 0.11 (0.45) | 0.41 (0.34) | 0.543 | 0.009 | 0.582 |
| Log_IL-10 | 0.51 (0.40) | 0.31 (0.50) | 0.61 (0.48) | 2.609 | 0.042 | 0.078 |
| Log_IL-13 | 0.15 (0.54) | 0.10 (0.67) | 0.57 (0.35) | 2.123 | 0.034 | 0.124 |
Adjusted for IQ, PHQ-9, HADS-A, fatigue, and significant cytokines; † Significant difference after Bonferroni correction; * p < 0.05.
Results of subjective and objective neuropsychological tests are shown in Table 3. No significant differences were found in FACT-Cog test between the 3 subgroups. The pre-C/T and the post-C/T subgroups had significantly poorer performance than controls on verbal fluency (SFT; p = 0.002, η2 = 0.090).
Table 3.
Mean scores on subjective and objective cognitive evaluations (ANOVA).
| Subjective and Objective Cognitive Assessments | Pre-C/T Cancer Patients (N = 68) |
Post-C/T Cancer Patients (N = 35) |
Non-Cancer Controls (N =30) |
ANOVA | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | p | η2 | Post Hoc Tests ‡ | |
| Functional Assessment of Cancer Therapy Cognitive Scale (FACT-Cog) | 119.26 (10.54) | 118.58 (10.11) | 116.13 (16.25) | 0.485 | 0.011 | |
| Perceived Cognitive Impairment | 66.03 (6.41) | 65.83 (6.25) | 64.50 (9.70) | 0.615 | 0.007 | |
| Comments from Others | 15.71 (0.89) | 15.56 (0.81) | 15.27 (1.82) | 0.201 | 0.024 | |
| Perceived Cognitive Abilities | 22.13 (3.73) | 21.81 (3.48) | 21.30 (4.79) | 0.624 | 0.007 | |
| Impact of Perceived Cognitive Impairments on Quality of Life | 15.39 (1.96) | 15.39 (1.42) | 15.07 (2.16) | 0.714 | 0.005 | |
| Attention function | ||||||
| Digit Span | 11.25 (2.97) | 11.02 (3.38) | 12.01 (2.92) | 0.393 | 0.014 | |
| Color Trails Test 1 | 51.52 (19.99) | 49.26 (22.89) | 47.27 (19.05) | 0.623 | 0.007 | |
| Executive function | ||||||
| Semantic Association of Verbal Fluency | 38.91 (8.22) | 37.33 (8.65) | 44.43 (8.22) | 0.002 * | 0.090 | Control > pre-C/T Control > post-C/T |
| Orthographical Fluency Test | 17.59 (7.71) | 17.60 (7.82) | 20.44 (5.84) | 0.178 | 0.026 | |
| Color Trails Test 2 | 98.20 (34.75) | 98.84 (35.08) | 88.67 (29.77) | 0.380 | 0.014 | |
| Memory function | ||||||
| Word List—Total immediate recall | 9.94 (2.50) | 9.67 (2.77) | 10.93 (2.94) | 0.132 | 0.030 | |
| Word List—Long-delay recall | 10.59 (2.55) | 10.61 (2.53) | 10.97 (2.68) | 0.782 | 0.004 | |
| Word List—Recognition | 11.07 (2.39) | 11.44 (2.27) | 11.23 (2.21) | 0.734 | 0.005 | |
| Visuospatial construction | ||||||
| Block Design | 9.33 (3.00) | 8.75 (3.38) | 9.67 (3.20) | 0.480 | 0.011 | |
| Processing speed | ||||||
| Digit Symbol Substitution | 10.26 (2.73) | 10.36 (2.66) | 11.13 (2.93) | 0.335 | 0.016 | |
| Prospective memory | ||||||
| Event-based | 4.07 (1.32) | 3.87 (0.32) | 3.87 (0.43) | 0.487 | 0.011 | |
| Time-based | 3.34 (0.84) | 3.34 (0.79) | 3.53 (0.73) | 0.517 | 0.010 | |
‡ Significant difference after Bonferroni correction; * p < 0.05.
After adjusting for mood and IQ using ANCOVA, significantly poorer performance in SFT persisted in the pre-C/T (p = 0.027) and post-C/T (p = 0.015) groups compared to controls (Table 4), and significantly better FACT-cog, perceived cognitive impairments or cognitive abilities were found in the pre-C/T group than in the controls. Levels of cognitive performances from other neuropsychological tests were similar between the three groups (Table 4).
Table 4.
Mean scores of subjective and objective cognitive evaluations after adjusting for depression, anxiety, and fatigue (ANCOVA).
| Subjective and Objective Cognitive Assessments | Pre-C/T Cancer Patients (N = 68) |
Post-C/T Cancer Patients (N = 35) |
Non-Cancer Controls (N =30) |
ANCOVA † | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SE) | Mean (SE) | p | η 2 | Post Hoc Tests ‡ | |
| Functional Assessment of Cancer Therapy Cognitive Scale (FACT-Cog) | 119.29 (10.67) | 119.26 (9.40) | 116.13 (16.25) | 0.006 | 0.077 | Pre-C/T > Control |
| Perceived Cognitive Impairment | 66.00 (6.48) | 66.29 (5.71) | 64.50 (9.70) | 0.031 | 0.054 | Pre-C/T > Control |
| Comments from Others | 15.71 (0.90) | 15.60 (0.78) | 15.27 (1.82) | 0.052 | 0.046 | |
| Perceived Cognitive Abilities | 22.21 (3.74) | 21.94 (3.43) | 21.30 (4.79) | 0.019 | 0.061 | Pre-C/T > Control |
| Impact of Perceived Cognitive Impairments on Quality of Life | 15.38 (1.99) | 15.43 (1.42) | 15.07 (2.16) | 0.058 | 0.044 | |
| Attention function | ||||||
| Digit Span | 11.31 (2.85) | 11.06 (3.42) | 12.01 (2.92) | 0.440 | 0.013 | |
| Color Trails Test 1 | 51.36 (19.93) | 48.80 (23.06) | 47.27 (19.05) | 0.623 | 0.007 | |
| Executive function | ||||||
| Semantic Association of Verbal Fluency | 38.99 (8.29) | 37.43 (8.76) | 44.43 (8.22) | 0.011 * | 0.070 | Control > pre-C/T Control > post-C/T |
| Orthographical Fluency Test | 17.79 (7.70) | 17.91 (7.72) | 20.44 (5.84) | 0.708 | 0.005 | |
| Color Trails Test 2 | 97.16 (34.24) | 97.80 (35.02) | 88.67 (29.77) | 0.697 | 0.006 | |
| Memory function | ||||||
| Word List—Total immediate recall | 9.96 (2.52) | 9.69 (2.81) | 10.93 (2.94) | 0.693 | 0.006 | |
| Word List—Long-delay recall | 10.57 (2.58) | 10.69 (2.53) | 10.97 (2.68) | 0.709 | 0.005 | |
| Word List—Recognition | 11.09 (2.42) | 11.43 (2.31) | 11.23 (2.21) | 0.504 | 0.011 | |
| Visuospatial construction | ||||||
| Block Design | 9.43 (2.94) | 8.74 (3.43) | 9.67 (3.20) | 0.086 | 0.038 | |
| Processing speed | ||||||
| Digit Symbol Substitution | 10.26 (2.72) | 10.40 (2.69) | 11.13 (2.93) | 0.975 | 0.000 | |
| Prospective memory | ||||||
| Event-based | 4.13 (1.24) | 3.89 (0.33) | 3.87 (0.43) | 0.115 | 0.034 | |
| Time-based | 3.34 (0.84) | 3.34 (0.80) | 3.53 (0.73) | 0.328 | 0.018 | |
Adjusted for IQ, anxiety, depression, and fatigue. ‡ Significant difference after Bonferroni correction; * p < 0.05.
Table 5 describes results from stepwise multivariate regression models of cytokines, patient characteristics, and mood status as independent variables and covariates, and FACT-Cog, FACT-PCI, FACT-PCA, or SFT as dependent variables for each of the three participant groups. Anxiety was associated with FACT-Cog in controls, and FACT-PCA in the control and post-C/T groups, with higher anxiety scores associated with higher perceived impairments. Similarly, higher depression scores were associated with more perceived impairments. Scores for fatigue were associated with FACT-Cog and FACT-PCI in the pre-C/T and control groups, and with FACT-PCA in the pre-C/T group. Higher age was associated with lower FACT-Cog and FACT-PCA in the post-C/T subgroup, and lower FACT-PCI in the pre- and post-C/T subgroups. The higher the BMI, the lower the FACT-PCI in the post-C/T subgroup. The higher the IQ, the higher the scores of FACT-PCA in the pre-C/T subgroup. The higher the Log IL5, the lower the FACT-PCI in the non-cancer and pre-C/T subgroups. The higher the Log IL10, the better the FACT-PCA in the non-cancer and pre-C/T subgroups. The higher the Log IL13, the lower the FACT-PCA in the pre-C/T subgroup.
Table 5.
Summary of multivariable regression analyses in FACT- Cog total scores, FACT- PCI, FACT- PCA, SFT among the three groups.
| Predictors | Non cancer (n = 30) | Pre-C/T (n = 68) | Post-C/T (n = 35) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 | ΔR2 | p-Value | Confidence Interval (CI) | β | R2 | ΔR2 | p-Value | CI | β | R2 | ΔR2 | p-Value | CI | ||
| FACT-Cog | 0.599 | 0.099 | 0.012 | 0.155 | 0168 | 0.001 | 0.33~1.19 | 0.429 | 0.212 | 0.001 | ||||||
| Age | −0.43 | 0.001 | −0.68~−0.18 | |||||||||||||
| HADS | −2.08 | 0.012 | −3.68~ | −0.49 | ||||||||||||
| PHQ-9 | −1.48 | 0.001 | −2.27~−0.68 | |||||||||||||
| BFI_interference | −7.01 | <0.001 | −10.16~ | −3.85 | −0.73 | −1.13~−0.33 | ||||||||||
| FACT-PCI | 0.569 | 0.084 | 0.025 | 0.334 | 0.058 | 0.019 | 0.397 | 0.078 | 0.044 | |||||||
| Age | 0.16 | 0.011 | 0.04~0.27 | −0.22 | 0.007 | −0.37~−0.06 | ||||||||||
| BMI | −0.40 | 0.044 | −0.78~−0.01 | |||||||||||||
| PHQ-9 | −0.40 | 0.019 | −0.72~−0.07 | −0.71 | 0.008 | −1.23~−0.20 | ||||||||||
| BFI_interference | −4.83 | <0.001 | −6.63~ | −3.03 | −0.36 | 0.005 | −0.61~−0.11 | |||||||||
| Log IL-5 | −7.98 | 0.025 | −14.87~ | −1.08 | −0.73 | −1.13~−0.33 | ||||||||||
| FACT-PCA | 0.443 | 0.115 | 0.021 | 0.227 | 0.069 | 0.017 | 0.340 | 0.144 | 0.010 | |||||||
| Age | −0.17 | 0.001 | −0.27~−0.08 | |||||||||||||
| IQ | 0.01 | 0.005 | 0.03~0.17 | |||||||||||||
| PHQ | −0.23 | 0.017 | −0.41~−0.04 | |||||||||||||
| HADS | −0.76 | 0.010 | −1.32~ | −0.02 | −0.51 | 0.010 | −0.89~−0.13 | |||||||||
| BFI_interference | −1.32 | 0.021 | −2.43~ | −0.21 | ||||||||||||
| Log IL-10 | 3.88 | 0.011 | 0.97~ | 6.79 | 3.66 | 0.002 | 1.43~5.89 | |||||||||
| Log IL-13 | −2.28 | 0.008 | −3.95~−0.62 | |||||||||||||
| SFT | 0.218 | 0.245 | 0.005 | 0.144 | 0157 | 0.001 | 0.458 | 0.077 | 0.036 | |||||||
| Years of education | 1.02 | 0.32~ | 1.72 | 0.76 | 0.33~1.19 | |||||||||||
| IQ | 0.27 | 0.002 | 0.11~0.44 | |||||||||||||
| HADS-A | 1.87 | 0.001 | 0.86~2.87 | |||||||||||||
| PHQ-9 | −0.90 | 0.036 | −1.73~−0.06 | |||||||||||||
| Log IL-13 | −4.94 | 0.008 | −8.46~−1.42 | |||||||||||||
Multivariate stepwise regression adding all variables (including cytokines) into the model. FACT-Cog: Functional Assessment of Cancer Therapy Cognitive Function; FACT-PCI: Perceived Cognitive Impairment; FACT-PCA: Perceived Cognitive Abilities; β: standardized regression coefficients; R2 = R square; ΔR2 = R square change.
For SFT, only education was associated with better performance of SFT in the control and pre-C/T groups. In the post C/T group, four factors were independent predictors of SFT: IQ and anxiety had significantly positive associations with SFT, while depression and log-IL-13 levels had significantly negative associations with SFT, i.e., the higher the log IL13, the poorer the performance on SFT. These four factors explained 7.7% of variance in SFT. Further examinations of effects of each cytokine also revealed that after controlling for all other covariates including significant cytokines, IL-13 was the only cytokine that was significantly negatively associated with SFT (β = −4.86, 95% CI: −8.83~−0.90, p = 0.018).
From the secondary multivariate regression model investigating significant factors associated with SFT among different comparisons, we found that when analyzing the subgroup of cancer vs. non-cancer (n = 136), positive associations were found for SFT and education years (β = 0.76, 95% CI: 0.43~1.10, p < 0.001) and anxiety (β = 0.9, 95% CI: 0.19~0.99, p = 0.005); negative associations were found for the group indicator of ‘cancer vs. non-cancer’ (β = −7.04, 95% CI: −10.34~−3.74, p < 0.001) and log IL-4 (β = −3.31, 95% CI: −6.51~−0.11, p = 0.043). As for the subgroup ‘chemotherapy vs. non-cancer’ (n = 66), SFT was found to be positively correlated with IQ (β= 0.26, 95% CI: 0.10~0.42, p = 0.002) and anxiety (β = 0.83, 95% CI: 0.10~1.57, p = 0.028); and negatively correlated with the group indicator of ‘chemotherapy vs. non-cancer’ (β = −5.92, 95% CI: −9.77~−2.06, p = 0.003). As for the subgroup ‘chemotherapy vs. non-chemotherapy’ (n = 106), SFT was found to be positively correlated with education years (β = 0.70, 95% CI: 0.34~1.07, p < 0.001) and anxiety (β = 0.15, 95% CI: 0.25~1.12, p = 0.002); and negatively correlated with log IL-4 (β = −4.76, 95% CI: −8.19~−1.32, p = 0.007).
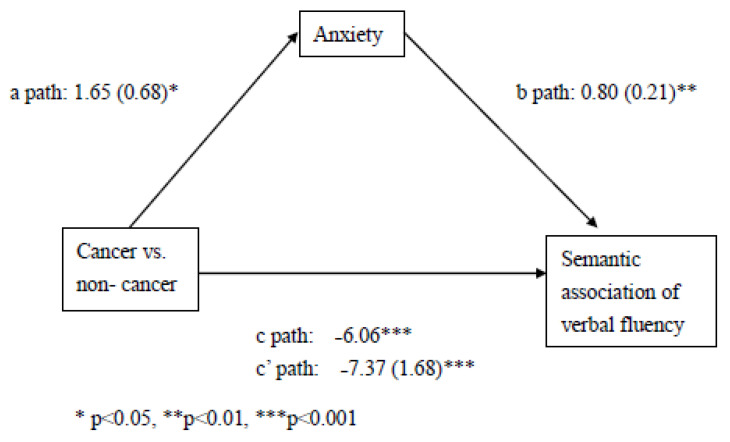
Mediation analyses (Figure 1) showed that anxiety is a partial mediator of SFT and status of cancer vs. non-cancer (HADS scores coefficient = 0.80, t = 3.85, p < 0.001), but the status of chemotherapy vs. non-chemotherapy did not significantly predict SFT (coefficient = −1.58, t = −0.92, p = 0.36); and the status of chemotherapy vs. non-cancer did not predict anxiety (coefficient = 0.48, t = 0.74, p = 0.46).
Figure 1.
Results from mediation analysis for the associations of status of cancer vs. non-cancer (X), semantic association of verbal fluency (Y), partially mediated by anxiety (M).
4. Discussion
Our study is one of the first to investigate the association between cytokines and cognitive function controlling for IQ and psychological symptoms in patients with BC before and after chemotherapy, and with a non-cancer control group. We found most cognitive scores were not associated with cancer status or with cytokine levels. Despite this, a significantly poorer performance in verbal fluency (SFT) was found in the post-C/T group compared to non-cancer controls; and it may be explained by IQ, anxiety, and IL-13 from the multivariate regression model. The higher the log value of IL-13, the poorer the SFT was found in the post-C/T subgroup after further adjusting for significant cytokines. SFT was affected by the status of cancer more than chemotherapy. Significantly better self- perceived cognitive abilities were found in pre-C/T patients than in the controls, and this might be explained by fatigue, depression, anxiety, IL5, 10, or 13. While higher IL-10 was associated with better perceived cognitive ability in pre-C/T and control groups, higher IL-5 and IL-13 were associated with more severe subjective impairment in pre-C/T and control groups.
The result that post-C/T and pre-C/T cancer patients overall had poorer performance in the SFT than in the controls would be consistent with adverse effects of cancer and/or cytotoxic agents on cognitive function, as has been discussed in our previous paper [17] and other studies [4,15]. However, we feel that our findings suggest the poorer performance in SFT may be affected by cancer status than chemotherapy. This is because from our secondary analyses, while chemotherapy vs. non-cancer, and cancer vs. non-cancer were significantly associated with worse SFT, no significant association was found when comparing the chemotherapy vs. non-chemotherapy groups within the BC cases. Adding to the recent meta-analysis suggesting associations of verbal ability and caner status, visuospatial ability and chemotherapy [29], our finding also has some support from scant but relevant literature suggesting that memory impairments in newly diagnosed BC reflect cancer diagnosis rather than chemotherapy [3,10,30].
From our multivariate regression model in the post-C/T group, the finding that higher IL-13 predicted worse SFT may suggest impaired executive function after cancer or chemotherapy. Past studies regarding cognitive domain-specific predictors included associations of sTNFRI and short-term visual memory delayed match to sample test in BC patients receiving chemotherapy [13]. Cheung et al. reported correlations between plasma IL-1β and IL-4 with response speed [11]. Lyon et al. mentioned that there might be multiple relationships among cytokines and domain-specific cognitions, which varied over time [12]. The literature on our finding of IL-13 associations with learning and/or memory is still scarce and inconsistent [31,32], and no previous research has discussed its role in cancer-related cognitive impairment. In the peripheral system, IL-13 may promote allergic inflammation by T-helper type 2 (Th2); or be anti-inflammatory by down-regulating pro-inflammatory cytokines and T-helper type 1. In the central nervous system (CNS), they have been found to be potentially both neuroprotective or neurotoxic [32]. They may be protective and anti-inflammatory by promoting the M2 (or ‘healing’) microglia phenotype to repair neurons [32,33], or stimulate primary astrocytes to produce brain-derived neurotrophic factor associated with cognition [34]. IL-13 may also be pro-inflammatory, causing deaths of neurons sensitive to oxidation during neuro-inflammation [32]. Although no evidence to date has demonstrated IL-13 crossing the blood–brain barrier, rodent models have shown that IL-13 can be produced by microglia and neurons in the CNS, and may be enhanced by peripheral injections neurotoxins [32]. Longitudinal research on roles and interactions of ‘healing’ or ‘harmful’ effects of IL-13 on cognition in BC and chemotherapy may still be needed.
Considering subjective complaints, we found that pre-C/T patients had significantly higher scores in self-perceived cognitions than the controls, and lower IL-5 and IL-13 levels were associated with better perceived function in the FACT-PCI or FACT-PCA, respectively, in pre-C/T patients and controls. On the contrary, higher IL-10 levels were associated with worse subjective function in the FACT-PCA in pre-C/T patients and controls. Although the level of sTNFRII was found to be associated with subjective cognitive complaints in BC patients with or without chemotherapy [10], no previous reports were found in the associations of IL-5 or IL13 and subjective cognitive impairments. Recent research has mentioned IL-10 as an anti-inflammatory cytokine related to Alzheimer disease (AD) [35]. IL-10 is known for reducing immune and inflammatory responses and inhibits the expression of cytokine receptors [36,37]. Although the formation of senile plaque in AD might be associated with activated microglial cells and IL-10 gene polymorphism [38], it remains to be clarified whether anti-inflammatory responses in the brain from IL-10 contribute to neurodegeneration [35,39].
We did not find associations between other cytokines and cognitive declines in the 3 subgroups. Although chemotherapy is generally considered to be immunosuppressive, responses from plasma concentrations, types, or patterns of cytokines may change with time, or have different reactions or interactions [12,40,41]. For instance, it is interesting that all cytokines in the control group were higher than those in the pre-C/T and post-C/T groups. It is possible that the lower cytokine levels in the pre-C/T group compared to controls may be due to immune escape; as Bates et al. described in their review that the development of breast cancer may be related to mechanisms that decrease the ability of immune recognitions to cancer cells, as well as reducing the promotions of immunosuppression. In post-C/T patients, the lower cytokine levels compared to controls may be due to effects from chemotherapy treatments. Lyon et al. described the increase of IL-6 during chemotherapy but decrease afterwards; while continuous decline from baseline over time were for IL-17 [12]. Alterations in different cytokines during inflammatory responses induced by cancer or chemotherapy might be responsible for cognitive deficits in our post-C/T patients [30,42,43]. However, it is important to remember that our samples were relatively small and cytokine levels may be too subject to external influence resulting in loss of signal.
Finally, the status of menopause was not included in the multivariate regression analysis because univariate analysis of menopause and verbal fluency in the post-C/T group was not able to perform when everyone had menopause. Nearly half of these patients had menopause naturally (n = 15), while 15 of them had menopause after chemotherapy, 4 had menopause after hysterectomy, 1 after oophorectomy, and 1 after hormone therapy. No significant correlations were found for reasons of menopause and verbal fluency (p = 0.990). In the subsample that included only non-cancer controls and BC patients without chemotherapy, the status of menopause was not associated with verbal fluency (p = 0.079). Therefore, we believe that impairments in verbal fluency were more likely to be associated with chemotherapy, not the status or reasons of menopause.
Strengths and Limitations
Major strengths of this study are the comprehensive neuropsychological assessments using well-validated batteries and structured ascertainment of IQ, self-perceived, and objective cognitive impairment. The key limitation is the study’s cross-sectional design that restricted the exploration of possible causal processes. Further long-term cohort study investigating changes in cognitive functions before and after chemotherapy are still warranted to control more intrinsic and extrinsic factors to establish relevant causal mechanisms. Second, our numbers of healthy control and post-C/T BC patients may be insufficient to detect small associations between cytokine levels and cognitive performance. Third, this study was conducted in Taiwanese patients and the generalizability to other populations may be restricted. Fourth, our study was still restricted by a limited set of biomarkers, as well as lacking information on some potential confounders, such as diet or physical activity.
5. Conclusions
Our main findings suggest that performance in verbal fluency, at least, might be affected by the presence of BC and mediated by anxiety. Higher levels of cytokines IL-5 and IL-13 were significantly associated with lower subjective cognitive complaints and lower verbal fluency after controlling for IQ and psychological factors. Higher IL-10, on the other hand, was associated with better subjective perceived cognition. Our results indicated that semantic association of verbal fluency and anxiety may be used as important information for providing additional related psychosocial managements in BC patients after chemotherapy. Relevant prophylactic interventions for cognitive preservation associated with regulations in cytokines might also need to be further explored.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13112576/s1, Table S1: Correlations between levels of cytokines and scores of cognitive domains in post-C/T group.
Author Contributions
All authors have contributed significantly, and all authors are in agreement with the content of the manuscript: Conception/design: V.C.-H.C., C.-K.L., B.-S.T., Y.-H.H., S.-I.W.; Collection and/or assembly of data: All authors; Data analysis and interpretation: Han-Pin Hsiao, S.-I.W., and V.C.-H.C.; Manuscript writing-original draft: S.-I.W., and V.C.-H.C.; Final revision and approval of manuscript: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital, and Chang Gung University, Chiayi, Taiwan (CORPG6G0103 and CORPG6G0143). VCHC is part-funded by the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-182-040-MY3, MOST 105-2314-B-182-028, NSC 102-2314-B-040-004-MY3). SIW is part-funded by Department of Medical Research, Mackay Memorial Hospital (MMH-109112, MMH-10914, MMH-108121, MMH-TT-10804, MMH-TH-10804, MMH-108-146). RS is part-funded by: i) the National Institute for Health Research (NIHR) Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London; ii) an NIHR Senior Investigator Award; iii) the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. And the APC was funded by the Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital. All authors have no non-financial interests that may be relevant to the submitted work.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee at Chiayi Chang Gung Memorial Hospital approved this study (IRB number: 201700255B0) on 1 June 2017. Written informed consent was obtained from all participants before entering the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical issues.
Conflicts of Interest
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study. All authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Breast Cancer Incidence and Mortality Worldwide in 2018: Summary. GLOBOCAN/International Agency for Research on Cancer. [(accessed on 21 February 2019)]; Available online: http://globocan.iarc.fr/factsheets/cancers/breast.
- 2.De Angelis R., Sant M., Coleman M.P., Francisci S., Baili P., Pierannunzio D., Trama A., Visser O., Brenner H., Ardanaz E., et al. Cancer survival in Europe 1999-2007 by country and age: Results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 3.Wefel J.S., Saleeba A.K., Buzdar A.U., Meyers C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 4.Reid-Arndt S.A., Hsieh C., Perry M.C. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psycho Oncol. 2010;19:535–544. doi: 10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L.M., Amidi A. Cognitive impairment following hormone therapy: Current opinion of research in breast and prostate cancer patients. Curr. Opin. Support. Palliat. Care. 2017;11:38–45. doi: 10.1097/SPC.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao C., Bernstein L.J., Rich J.B. Executive functioning impairment in women treated with chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017;166:15–28. doi: 10.1007/s10549-017-4376-4. [DOI] [PubMed] [Google Scholar]
- 7.Ahles T.A. Brain vulnerability to chemotherapy toxicities. Psycho Oncol. 2012;21:1141–1148. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermelink K. Chemotherapy and Cognitive Function in Breast Cancer Patients: The So-Called Chemo Brain. J. Natl. Cancer Inst. Monogr. 2015;2015:67–69. doi: 10.1093/jncimonographs/lgv009. [DOI] [PubMed] [Google Scholar]
- 9.Schagen S.B., Muller M.J., Boogerd W., Mellenbergh G.J., van Dam F.S. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J. Natl. Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 10.Patel S.K., Wong A.L., Wong F.L., Breen E.C., Hurria A., Smith M., Kinjo C., Paz I.B., Kruper L., Somlo G., et al. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. J. Natl. Cancer Inst. 2015;107:djv131. doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung Y.T., Ng T., Shwe M., Ho H.K., Foo K.M., Cham M.T., Lee J.A., Fan G., Tan Y.P., Yong W.S., et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 2015;26:1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyon D.E., Cohen R., Chen H., Kelly D.L., McCain N.L., Starkweather A., Ahn H., Sturgill J., Jackson-Cook C.K. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J. Neuroimmunol. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams A.M., Shah R., Shayne M., Huston A.J., Krebs M., Murray N., Thompson B.D., Doyle K., Korotkin J., van Wijngaarden E., et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol. 2018;314:17–23. doi: 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz P.A., Bower J.E., Kwan L., Castellon S.A., Silverman D.H., Geist C., Breen E.C., Irwin M.R., Cole S.W. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav. Immun. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raffa R.B. A proposed mechanism for chemotherapy-related cognitive impairment (’chemo-fog’) J. Clin. Pharm. Ther. 2011;36:257–259. doi: 10.1111/j.1365-2710.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Willik K.D., Koppelmans V., Hauptmann M., Compter A., Ikram M.A., Schagen S.B. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 2018;20:135. doi: 10.1186/s13058-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu Y.H., Chen V.C., Hsieh C.C., Weng Y.P., Hsu Y.T., Hsiao H.P., Wang W.K., Chen H.M., Weng J.C., Wu S.I. Subjective and objective cognitive functionings among patients with breast cancer: Eeffects of chemotherapy and mood symptoms. Breast Cancer. 2021;28:236–245. doi: 10.1007/s12282-020-01168-y. [DOI] [PubMed] [Google Scholar]
- 18.Chen J., Chen H.Y. Wechsler Adult Intelligence Scale. 3rd ed. Chinese Behavioral Science Corp.; Taipei, Taiwan: 2002. (In Chinese) [Google Scholar]
- 19.Yang K.H., Hua M.S. Color Trails Test. Chinese Behavioral Science Corp.; Taipei, Taiwan: 2015. (In Chinese) [Google Scholar]
- 20.Hua M.S., Chang S.H., Chen S.T. Factor structure and age effects with an aphasia test battery in normal Taiwanese adults. Neuropsychology. 1997;11:156–162. doi: 10.1037/0894-4105.11.1.156. [DOI] [PubMed] [Google Scholar]
- 21.Hua M., Chang B.S., Lin K.N., Yang J.M., Lu S.R., Chen S.Y. Wechsler Memory Scale. 3rd ed. Chinese Behavioral Science Corp.; Taipei, Taiwan: 2005. (In Chinese) [Google Scholar]
- 22.Hsu Y., Huang C.F., Tu M.C., Hua M.S. Prospective memory in subjective cognitive decline: A preliminary study on the role of early cognitive marker in dementia. Alzheimer Dis. Assoc. Disord. 2015;29:229–235. doi: 10.1097/WAD.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 23.Wagner L., Sweet J., Butt Z., Lai J.S., Cella D. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy—Cognitive Function Instrument. J. Support. Oncol. 2009;7:W32–W39. [Google Scholar]
- 24.Wang G.L., Hsu S.H., Feng A.C., Chiu C.Y., Shen J.F., Lin Y.J., Cheng C.C. The HADS and the DT for screening psychosocial distress of cancer patients in Taiwan. Psycho Oncol. 2011;20:639–646. doi: 10.1002/pon.1952. [DOI] [PubMed] [Google Scholar]
- 25.Liu S.I., Yeh Z.T., Huang H.C., Sun F.J., Tjung J.J., Hwang L.C., Shih Y.H., Yeh A.W. Validation of Patient Health Questionnaire for depression screening among primary care patients in Taiwan. Compr. Psychiatry. 2011;52:96–101. doi: 10.1016/j.comppsych.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Lin C.C., Chang A.P., Chen M.L., Cleeland C.S., Mendoza T.R., Wang X.S. Validation of the Taiwanese version of the Brief Fatigue Inventory. J. Pain Symptom Manag. 2006;32:52–59. doi: 10.1016/j.jpainsymman.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Ahles T.A., Saykin A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheibel R.S., Valentine A.D., O’Brien S., Meyers C.A. Cognitive dysfunction and depression during treatment with interferon-alpha and chemotherapy. J. Neuropsychiatry Clin. Neurosci. 2004;16:185–191. doi: 10.1176/jnp.16.2.185. [DOI] [PubMed] [Google Scholar]
- 29.Jim H.S., Phillips K.M., Chait S., Faul L.A., Popa M.A., Lee Y.H., Hussin M.G., Jacobsen P.B., Small B.J. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J. Clin. Oncol. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson B., Marks D.L. Pretreatment Cancer-Related Cognitive Impairment—Mechanisms and Outlook. Cancers. 2019;11:687. doi: 10.3390/cancers11050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karo-Atar D., Bitton A., Benhar I., Munitz A. Therapeutic Targeting of the Interleukin-4/Interleukin-13 Signaling Pathway: In Allergy and Beyond. BioDrugs. 2018;32:201–220. doi: 10.1007/s40259-018-0280-7. [DOI] [PubMed] [Google Scholar]
- 32.Mori S., Maher P., Conti B. Neuroimmunology of the Interleukins 13 and 4. Brain Sci. 2016;6:18. doi: 10.3390/brainsci6020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X., Wang H., Sun G., Zhang J., Edwards N.J., Aronowski J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J. Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brombacher T.M., Nono J.K., De Gouveia K.S., Makena N., Darby M., Womersley J., Tamgue O., Brombacher F. IL-13-Mediated Regulation of Learning and Memory. J. Immunol. 2017;198:2681–2688. doi: 10.4049/jimmunol.1601546. [DOI] [PubMed] [Google Scholar]
- 35.Magalhaes C.A., Carvalho M.D.G., Sousa L.P., Caramelli P., Gomes K.B. Alzheimer’s disease and cytokine IL-10 gene polymorphisms: Is there an association? Arq. Neuro Psiquiatr. 2017;75:649–656. doi: 10.1590/0004-282x20170110. [DOI] [PubMed] [Google Scholar]
- 36.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Acuner-Ozbabacan E.S., Engin B.H., Guven-Maiorov E., Kuzu G., Muratcioglu S., Baspinar A., Chen Z., Van Waes C., Gursoy A., Keskin O., et al. The structural network of Interleukin-10 and its implications in inflammation and cancer. BMC Genom. 2014;15(Suppl. 4):S2. doi: 10.1186/1471-2164-15-S4-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma S.L., Tang N.L., Lam L.C., Chiu H.F. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer’s disease. Neurobiol. Aging. 2005;26:1005–1010. doi: 10.1016/j.neurobiolaging.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Kiyota T., Ingraham K.L., Swan R.J., Jacobsen M.T., Andrews S.J., Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyon D.E., Cohen R., Chen H., Kelly D.L., Starkweather A., Ahn H.C., Jackson-Cook C.K. The relationship of cognitive performance to concurrent symptoms, cancer- and cancer-treatment-related variables in women with early-stage breast cancer: A 2-year longitudinal study. J. Cancer Res. Clin. Oncol. 2016;142:1461–1474. doi: 10.1007/s00432-016-2163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroschinsky F., Stolzel F., von Bonin S., Beutel G., Kochanek M., Kiehl M., Schellongowski P. New drugs, new toxicities: Severe side effects of modern targeted and immunotherapy of cancer and their management. Crit. Care. 2017;21:89. doi: 10.1186/s13054-017-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y., Ren Y., Dai Z.J., Wu C.J., Ji Y.H., Xu J. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017;26:421–426. doi: 10.17219/acem/62120. [DOI] [PubMed] [Google Scholar]
- 43.Amidi A., Agerbaek M., Wu L.M., Pedersen A.D., Mehlsen M., Clausen C.R., Demontis D., Borglum A.D., Harboll A., Zachariae R. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11:769–783. doi: 10.1007/s11682-016-9552-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical issues.